Abstract
In this study, we designed and synthesized a new non-volatile solid additive FcF10 by integrating two pentafluorophenyl (C6F5) groups into the cyclopentadienyl (CP) rings of ferrocene (Fc) through ester linkages. The FcF10 with a three-dimensional (3D) framework facilitated morphological optimization in the PM6:Y6 system through a combination of π···π, F···π, and F···F interactions between the CP and C6F5 rings in FcF10 and the C6F2 rings in Y6. The FcF10-incorporated (3.75 wt %) PM6:Y6-based solar cell device achieved a higher power conversion efficiency (PCE) of 17.00%, with a V oc of 0.85 V, a J sc of 27.35 mA cm–2, and an FF of 73.29%, compared to the pristine PM6:Y6 device. These improvements are attributed to the formation of a favorable active layer morphology, which enhances exciton dissociation and charge transport while reducing bimolecular and trap-assisted recombination. The FcF10 additive facilitates non-covalent interactions with Y6, such as F···F, F···π, and π···π interactions between the Cp and C6F5 rings in FcF10 and the FIC end groups in Y6. These supramolecular interactions improve molecular stacking and crystallinity within the Y6 domain, as evidenced by red-shifted Y6 absorption, reduced π–π stacking d-spacing, and increased coherence lengths of Y6. Furthermore, the PM6:Y6:FcF10 device demonstrates superior thermal stability, retaining 88% of its initial PCE after prolonged thermal annealing at 85 °C. Overall, the incorporation of FcF10 achieves an optimized and stable donor–acceptor morphology, offering a promising approach for high-performance and thermally stable organic photovoltaics.
Keywords: solid additives, nonfullerene acceptors, morphology control, organic solar cells, non-volatile additives, perfluorophenyl derivatives

Introduction
Organic solar cells (OSCs) have gained significant attention as promising solar energy converters due to their lightweight nature, low production cost, mechanical flexibility, and potential for large-area fabrication. − To achieve commercial viability, it is essential to combine high power conversion efficiency (PCE) with long-term operational stability. Recent advancements in Y-series nonfullerene acceptors (NFAs) have propelled OSCs to PCEs exceeding 19% when paired with compatible donor materials. − These breakthroughs underscore the practical potential of OSCs in energy applications. Alongside the development of new active materials, morphology control emerges as a pivotal strategy for further optimizing both the PCE and device stability. Bulk heterojunction (BHJ) devices, characterized by an optimal donor–acceptor interfacial area, are widely adopted for their ability to facilitate efficient charge separation. Achieving the desired morphology in the active layer has been a primary focus of research, with extensive efforts devoted to microstructure regulation. Among the various factors influencing blend nanostructures, intrinsic intermolecular interactions between donor and acceptor molecules, as well as their relative solubility in the processing solvent play a critical role. Various approaches have been developed to manipulate phase separation and domain behavior in active layers, including multi-component blending systems, thermal/solvent annealing, − and additive treatments. − Among these, the incorporation of additives has emerged as an effective and cost-efficient strategy for enhancing the performance of organic solar cells.
Liquid additives, such as 1,8-diiodooctane (DIO), diphenyl ether (DPE), and 1-chloronaphthalene (CN), are widely used due to their high boiling points and selective solubility, which enable precise control over donor–acceptor phase separation and the formation of an optimized bicontinuous network. However, the high boiling points can leave residual traces in the active layer, leading to potential morphological degradation, reduced reproducibility, and compromised stability. − Solid additives, a promising alternative to liquid additives, can be categorized into two types based on their presence in the active layer: volatile solid additives and non-volatile solid additives. Unlike liquid additives, solid additives can effectively stabilize the morphology of the active layer, thereby enhancing the device stability. Their working mechanisms include strengthening donor–acceptor interactions, such as hydrogen bonding, , π–π stacking, halogen bonding, and quadrupole moment interactions. They also inhibit crystal nucleation and growth, , reduce intermolecular adsorption energy, , and increase the free volume within donor and/or acceptor materials. In recent years, various volatile solid additives have been described to enhance the efficiency and stability of OSCs, leading to significant advancements in device performance. The function of volatile solid additives is to improve active layer bicontinuous morphology by enhancing intermolecular π–π interactions and crystallinity of polymers and NFA nanodomains, leading to enhanced exciton dissociation and charge transfer. − In 2020, Sun and co-workers introduced ferrocene (Fc) as a volatile solid additive in PM6:Y6 blends, achieving a PCE of 17.4%, which significantly surpassed the 15.5% PCE of devices without additives and the 16.5% PCE achieved with the CN solvent additive. The enhanced efficiency with ferrocene was attributed to increased molecular crystallinity, improved charge transport, and reduced rate of charge recombination. Ferrocene-treated OSCs also demonstrated superior photostability compared with CN-processed devices. Compared to volatile additives, non-volatile additives have demonstrated multifunctional roles in OSCs, acting as molecular locks, morphological controllers, and stacking promoters. These additives stabilize the active layer morphology, suppress phase separation, improve thermal stability, and enhance both device performance and fabrication reproducibility. We recently developed a non-volatile additive, bis(perfluorophenyl)pimelate (BF7), featuring two perfluorophenyl (C6F5) groups linked to a linear aliphatic chain via ester linkages. The hand-grab-like C6F5 moieties of BF7, retained in the film after processing, form supramolecular physical attractions with fullerene derivatives, suppressing their aggregation. As a result, [6,6]-phenyl-C61-butyric acid methyl ester-based solar cells with BF7 incorporation demonstrated not only improved efficiency but also exceptional thermal stability. For Y6-based OSCs, PM6:Y6:BF7 devices achieved a PCE of 17.01%, compared to 15.16% for those without BF7. Moreover, the BF7-enhanced PCE maintained 95% of its peak performance after 72 h of annealing at 100°C. This improvement arises from preferential F−π supramolecular interactions between the C6F5 moieties of BF7 and the difluorophenyl (C6F2)-based FIC end groups of Y6. These interactions optimize the active layer morphology by creating fractal-like network structures with ideal Y6 nanocrystallite size and orientation, leading to enhanced thermal stability.
In this study, we combined the advantages of Fc and BF7 by designing a new non-volatile solid additive, FcF10, which was synthesized by integrating two pentafluorophenyl (C6F5) groups into the cyclopentadienyl (CP) rings of Fc through ester linkages. The FcF10 with the three-dimensional (3D) framework facilitated morphological optimization in the PM6:Y6 system through a combination of π···π, F···π, and F···F interactions between the CP and C6F5 rings in FcF10 and the C6F2 rings in Y6, leading to a significant enhancement in device performance. The FcF10-incorporated (3.75 wt %) PM6:Y6 device achieved a PCE increase from 15.34 to 17.00%, driven by an improved fill factor (FF) of 73.29% and a short-circuit current density (J sc) of 27.35 mA/cm2. Ultraviolet–visible (UV–Vis) spectroscopy, space-charge-limited current (SCLC) measurements, charge recombination analysis, atomic force microscopy (AFM), and grazing-incidence wide-angle X-ray scattering (GIWAXS) have been utilized to investigate the morphological and electrical effects introduced by FcF10.
Experimental Section
Synthesis of FcF10
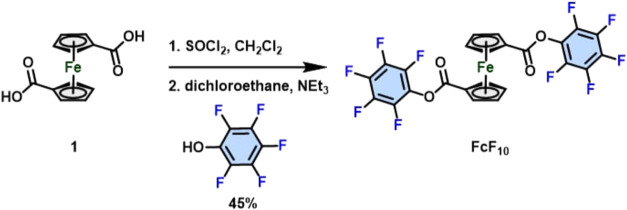
A solution of thionyl chloride (0.12 mL) in dichloromethane (2 mL) was added dropwise to a solution of 1,1′-ferrocenedicarboxylic acid (200.0 mg, 0.73 mmol) of freshly dried dichloromethane (3 mL) at 0 oC. The solution was first stirred for 2 h at 0 oC and then for 6 h at room temperature. After the completion of the reaction, solvent was evaporated to give the intermediate 1,1′-ferrocenedicarbonyl chloride without purification. A solution of pentafluorobenzyl alcohol (295.5 mg, 1.61 mmol) and triethylamine (0.24 mL) in dichloroethane (2 mL) was added to the acid chloride solution in dichloroethane (3 mL). The reaction mixture was refluxed for 12 h. The product was extracted with dichloromethane and water for 3 times. The organic collection layer was dried over anhydrous MgSO4. After the solvent was removed under reduced pressure, the residue was purified by column chromatography on silica gel (dichloromethane/hexane 1/3) to afford an orange solid of 199 mg (45%). 1H NMR (400 MHz, CDCl3) δ 5.11 (m, 4H), 4.73 (m, 4H). 13C NMR (100 MHz, CDCl3) δ: 166.64, 141.34, 139.54, 137.97, 125.02, 74.99, 72.80, 69.17. HRMS (FD, C24H8O4F10Fe): calcd, 605.9617; found 605.9607.
Results and Discussion
The synthesis of FcF10 is depicted in Scheme . 1,1′-Ferrocenedicarboxylic acid was reacted with thionyl chloride to form a Fc-COCl intermediate, which was immediately reacted with 2,3,4,5,6-pentafluorophenol to yield FcF10 in a 45% yield. The detailed synthetic procedure, mass spectrometry, and 1H NMR and 13C NMR of new compounds are shown in the Experimental Section and Supporting Information.
1. Synthesis of FcF10 .
UV–Vis absorption spectra were employed to illustrate the effect of intermolecular interactions upon addition of FcF10 to PM6 and Y6. Figure displays the normalized solid-state absorption spectra of pristine PM6 and Y6 films, both with and without 3.75 wt % FcF10 additive. Additionally, we compared the absorption spectra before and after thermal annealing at 100°C. In the case of PM6, the pristine thin film exhibited a λmax at 612 nm, with no significant shifts observed after adding FcF10 (614 nm). After thermal annealing, the spectra showed no notable change compared with those without annealing, indicating that FcF10 did not affect the donor domain. Conversely, the Y6 film showed a red shift in λmax from 808 to 819 nm and a decreased shoulder at 750 nm upon the addition of FcF10. After thermal annealing, the λmax further red-shifted by 8 to 827 nm. These absorption spectra results suggest that the addition of FcF10 might promote increased J-aggregation in Y6 with tightened molecular packing.
1.
UV–Vis absorption spectra of (a) PM6 and (b) Y6 thin films with and without adding FcF10 and thermal annealing treatment at 100 °C.
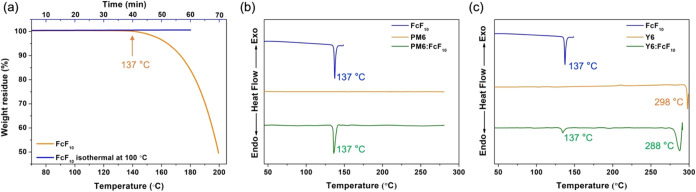
Thermal properties were evaluated by using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). First, we measured the weight loss of FcF10 under increasing temperatures with a ramp rate of 10 °C/min, as shown in Figure a. The results indicate that the weight of FcF10 begins to decrease at 137 °C, suggesting the onset of sublimation. Additionally, DSC measurements in Figure b revealed a strong endothermic peak at 137 °C, consistent with the TGA results. To confirm that FcF10 acts as a non-volatile solid additive in the device processing, we conducted an isothermal measurement at 100 °C (the thermal annealing temperature). After 1 h at 100 °C, FcF10 showed nearly no weight loss, implying its stability under thermal annealing conditions.
2.
(a) TGA and isothermal measurements at 100 °C of FcF10. DSC measurements of (b) FcF10, pristine PM6, and PM6 with FcF10; (c) FcF10, pristine Y6, and Y6 with FcF10 with a ramping rate of 10 °C/min.
To further investigate the effect of FcF10 on the morphology of PM6 and Y6, we blended FcF10 (3.75 wt %) into the polymer donor PM6 or acceptor Y6. As shown in Figure c, we observed that PM6 with FcF10 exhibit only a weak endothermic peak at 137 °C, corresponding to the sublimation temperature of FcF10. This observation suggests that FcF10 does not significantly affect the morphology of the PM6 domain. In contrast, Figure c shows that the melting temperature (T m) of Y6 decreased from 298 to 288 °C upon the addition of FcF10, while the characteristic peak of FcF10 remained unchanged. These results suggest that FcF10 primarily influences the stacking and interactions among the Y6 molecules. It is speculated that FcF10 tends to localize at the interfaces between Y6-rich domains, acting as a three-dimensional spacer that weakens inter-domain interactions and leads to a slightly lower T m.
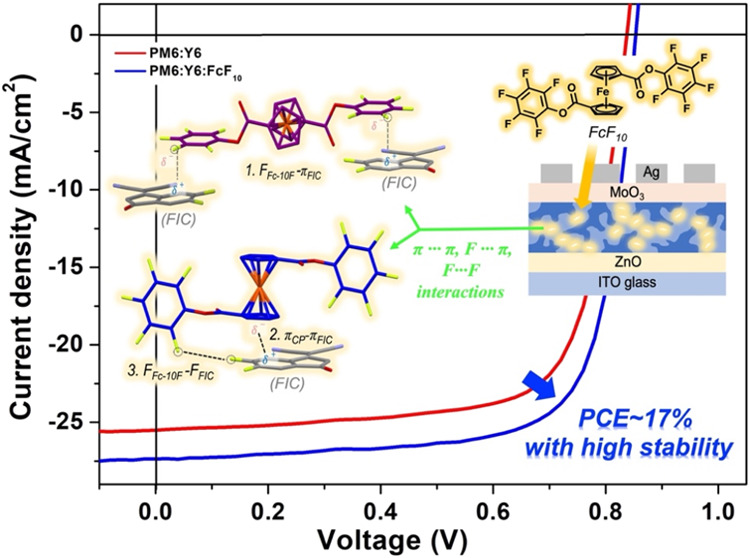
To investigate the photovoltaic properties of devices incorporating FcF10 as an additive, inverted configuration OSCs with an ITO/ZnO/active layer/MoO3/Ag architecture were fabricated using PM6 and Y6 as the active layer materials. Detailed fabrication methods are described in the Supporting Information. The characteristics, J–V curves, and external quantum efficiency (EQE) spectra of these devices are presented in Table and Figure . The reference device, consisting of a PM6:Y6 blend with 0.6 vol % of CN, exhibited a V oc of 0.84 V, a J sc of 25.50 mA cm–2, and an FF of 71.74%, resulting in a PCE of 15.34%, consistent with values reported in the literature. To optimize FcF10 content, its weight ratio was screened from 0.625 to 5 wt % relative to PM6, with the device performance summarized in Table S1. Compared to the reference device, the optimized device with 3.75 wt % of FcF10 showed a slightly higher V oc of 0.85 V, notable increase in J sc to 27.35 mA cm–2, and a slight improvement in FF to 73.29%, enhancing the PCE to 17.00%. The comparable V oc values between devices with and without FcF10 suggest that FcF10 does not alter the energy levels of PM6 or Y6. The observed improvements in J sc and FF are primarily attributed to enhanced molecular ordering, improved charge transport, and reduced recombination rather than changes in energy level offsets. Further EQE measurements, shown in Figure b, indicated higher EQE values around 650 and 830 nm regions, aligning with the maximum characteristic absorption regions of PM6 and Y6. This enhancement suggests that FcF10 improves the PM6 and Y6 domains and optimizes charge carrier kinetics, leading to concurrent improvements in J sc and FF. Additionally, the red-shifted absorption spectrum of the FcF10-modified PM6 blend broadens the absorption range, contributing to higher carrier generation and improved J sc. To further investigate charge transport, hole-only devices (ITO/PEDOT/active layer/Au) and electron-only devices (Al/active layer/Al) were fabricated to evaluate hole and electron mobilities using the space-charge-limited current (SCLC) model (Figure S1). As shown in Figure S1 and Table , the hole and electron mobilities for the pristine PM6:Y6 blend were estimated at 1.12 × 10–4 cm2 V–1 s–1 and 1.34 × 10–4 cm2 V–1 s–1, respectively. In contrast, the PM6:Y6 blend with FcF10 exhibited enhanced mobilities of 2.14 × 10–4 cm2 V–1 s–1 for holes and 2.53 × 10–4 cm2 V–1 s–1 for electrons. The improvement in hole and electron mobilities in the FcF10-modified blend suggests that the inclusion of FcF10 enhances intermolecular stacking within the p- and n-type domains, facilitating charge carrier transport. This improvement in mobility is consistent with the observed increases in J sc and FF in the devices, as it enables more efficient charge extraction and reduced recombination.
1. Characteristics of PM6:Y6-Based Devices with and without Optimized FcF10 Weight Content .
| FcF10 (wt %) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | μh (cm2 V–1 s–1) | μe (cm2 V–1 s–1) | μh/μe ratio |
|---|---|---|---|---|---|---|---|
| NA | 0.84 (0.83 ± 0.01) | 25.50 (25.42) (25.84 ± 0.60) | 71.74 (70.50 ± 1.61) | 15.34 (15.18 ± 0.21) | 1.12 × 10–4 | 1.34 × 10–4 | 0.84 |
| 3.75 | 0.85 (0.85 ± 0.01) | 27.35 (26.72)(26.87 ± 0.43) | 73.29 (72.63 ± 0.86) | 17.00 (16.53 ± 0.31) | 2.14 × 10–4 | 2.53 × 10–4 | 0.85 |
The average value was calculated from 10 devices.
J sc calculated by EQE. μh = hole mobility, μe = electron mobility.
3.
(a) J–V curves and (b) EQE spectra of PM6:Y6-based devices with and without FcF10.
To investigate the influence of FcF10 on charge recombination in the device, the dependence of J sc and V oc on light intensity (P light) was studied. The relationship between J sc and P light can be described by the power-law equation J sc ∝ (P light)α, where α indicates the degree of bimolecular recombination. As shown in Figure a, the extracted α values for devices without and with FcF10 were 0.947 and 0.973, respectively, indicating that bimolecular recombination was effectively reduced in the FcF10-processed device under short-circuit conditions. Furthermore, the relationship between V oc and P light helps analyze trap-assisted recombination within the devices. By the examination of the slope of V oc versus the natural logarithm of P light, the recombination mechanism can be clarified. As shown in Figure b, OSCs with only the CN additive exhibited a slope of 1.19 kT/q, while devices with FcF10 as an additive had a slope of 1.08 kT/q. These results suggest that the use of FcF10 effectively suppresses trap-assisted recombination, contributing to improved device performance.
4.
Plots of (a) dependences of J sc and (b) V oc on light intensity P light, and (c) J ph against V eff for the PM6:Y6-based devices with or without FcF10.
To investigate the factors contributing to the enhanced J sc and FF, measurements of the saturation current density J sat and exciton dissociation probability (η diss) of optimized devices were performed. The photocurrent density (J ph) versus effective voltage (V eff) curves are shown in Figure c. Here, J ph represents the difference between J L and J D, where J L and J D denote the current density under illumination and in the dark, respectively. V eff is defined as the difference between V 0 (the voltage at which J ph is zero) and V app (the applied bias voltage). It was observed that J ph reached J sat when V eff approached 2 V, suggesting that all of the excitons in the device were dissociated into free carriers and collected by the electrodes. The J sat values for devices without and with FcF10 were 27.13 and 27.34 mA cm–2, respectively, indicating that FcF10 enhances the photon harvesting capability of the PM6:Y6 blend. Under short-circuit conditions, PM6:Y6 and PM6:Y6:FcF10 devices exhibited η diss values of 95.28 and 95.97%, respectively, and charge collection probabilities (η coll) of 80.39 and 81.91%, respectively. These results demonstrate that FcF10 aids in charge extraction, contributing to the observed improvements in J sc and FF.
Single-crystal structures and crystallographic data of FcF10 are shown in Figure a and Table S2. The detailed single-crystal measurement is included in the Supporting Information. It is interesting to observe that the planes of two C6F5 rings are oriented almost perpendicularly to the plane of two CP rings in a FcF10 molecule. A unit cell in the single-crystal structure consists of three FcF10 molecules that self-assemble into two types of center-offset plane-to-plane π–p stacking modes. In the first mode, the C6F5 ring of one FcF10 molecule (purple) aligns parallel in center-offset stacking with another C6F5 ring of an adjacent FcF10 molecule (black). This interaction is reinforced by the electronegative peripheral F atoms of the C6F5 ring interacting with the electropositive center of the adjacent C6F5 ring (F FcF10–πFcF10) with a plane-to-plane distance of 3.502 Å. In the second arrangement, regarded as the πFcF10–πCP mode, the electropositive center of the C6F5 ring of FcF10 (purple) aligns parallel and center-offset with the electronegative center of the CP ring of an adjacent FcF10 molecule (blue) with a shorter plane-to-plane distance of 3.486 Å.
5.
(a) Single-crystal structure and intermolecular packing of FcF10. (b) Molecular packing in a unit cell of FcF10, showing two center-offset stacking modes via π···π, F···π, and F···F interactions.
Additionally, Figure b illustrates that the fluorine atom on the para position of the C6F5 ring of the central FcF10 molecule (purple) engages in F···F interactions with the fluorine atom on the ortho position of adjacent molecules (blue) (para-F-ortho-F). This F···F interaction, with a short contact of 2.820 Å, aligns the molecules directionally, causing the planes of the CP (blue) and PFB (purple) rings to be slightly non-parallel.
Similarly, we propose that the terminal difluorophenyl moeity of FIC, the end group of Y6, can form analogous molecular interactions with FcF10, including interactions between (1) the electronegative F atom of FcF10 and the electropositive difluorophenyl ring of FIC (F FcF10 – πFIC) and (2) vice versa (F FIC – πFcF10), (3) the Cp ring of FcF10 and the difluorophenyl ring of FIC (πCP – πFIC), and (4) F atoms on FcF10 and FIC (F FcF10 – F FIC). These intermolecular interactions could not only optimize nanocrystalline domain formation but also stabilize the morphology, enhancing the device stability.
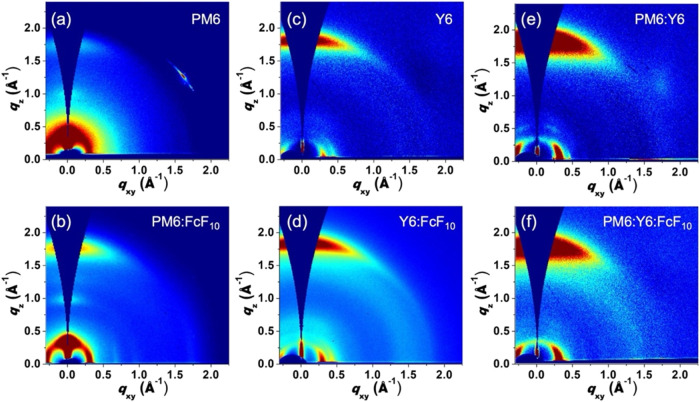
The intermolecular π–π stacking distances and nanocrystalline domain sizes of molecular arrangements in thin films were evaluated by using grazing-incidence wide-angle X-ray scattering (GIWAXS). We systematically investigated three systems: PM6 and Y6 neat films, the PM6:Y6 blend films, and their FcF10-incorporated counterparts. A summary of the GIWAXS data and related details is provided in Table . The GIWAXS two-dimensional (2D) image of the neat PM6 film displays a diffraction peak at q z =1.75 Å–1 along the out-of-plane direction, corresponding to PM6’s π–p stacking d-spacing of 3.59 Å (Figure a). After the addition of FcF10, the peak at q z =1.75 Å–1 remains unchanged in the PM6:FcF10 blend (Figure b). However, the peak becomes narrower and more intense, indicating FcF10 induces an increase in coherence length (CL) of PM6 π–π stacking domain from 22.32 to 24.12 Å. For Y6 (Figure c,d), the incorporation of FcF10 shifted the π–π stacking signal peak in the out-of-plane direction from q z = 1.802 to 1.812 Å–1, corresponding to a decrease in the π–p stacking d-spacing from 3.49 to 3.45 Å. Moreover, the CL of Y6 increased from 24.09 to 25.16 Å, indicating improved crystallinity and the formation of larger crystal grains upon FcF10 addition. The closer stacking of Y6 molecules might be ascribed to the non-covalent interactions such as F···F, F···π, and π···π interactions between Y6 and FcF10. The selective effect of FcF10 on Y6 rather than on PM6 arises from the chemical structure of the two materials. Y6 contains terminal difluorobenzene (FIC) units capable of engaging in strong F···F, F···π, and π–π interactions with the perfluorinated moieties of FcF10. In contrast, PM6 lacks such reactive end groups, resulting in minimal interaction with FcF10 and negligible influence on its stacking behavior. For the PM6:Y6 blend system shown in Figure e,f, the active layer showed an increase in CL from 19.13 to 20.94 Å upon FcF10 addition, suggesting larger molecular grain sizes. Notably, the π–π stacking and d-spacing of the PM6:Y6 blend remained constant at 3.53 Å. The corresponding one-dimensional (1D) line-cut profiles of GIWAXS diffraction patterns of these thin films are shown in Figure S2. This improved structural arrangement likely facilitates carrier mobility by minimizing carrier scattering at grain boundaries, aligning with the observed increases in electron and hole mobility from space-charge-limited current (SCLC) measurements.
2. GIWAXS Data of PM6, Y6, PM6:Y6, and the FcF10-Incorporated Counterparts.
| in plane |
out
of plane |
|||||
|---|---|---|---|---|---|---|
| materials | q xy (Å–1) | d-spacing (Å) | q z (Å–1) | d-spacing (Å) | FWHM (Å–1) | CL (Å) |
| PM6 | 0.28 | 22.39 | 1.75 | 3.59 | 0.25 | 22.32 |
| PM6:FcF10 | 0.29 | 21.59 | 1.75 | 3.59 | 0.23 | 24.12 |
| Y6 | 0.29 | 22.00 | 1.80 | 3.49 | 0.24 | 24.09 |
| Y6:FcF10 | 0.29 | 22.00 | 1.82 | 3.45 | 0.23 | 25.16 |
| PM6:Y6 | 0.30 | 21.00 | 1.78 | 3.53 | 0.30 | 19.13 |
| PM6:Y6:FcF10 | 0.29 | 21.48 | 1.78 | 3.53 | 0.27 | 20.94 |
d-spacing = 2π/q.
CL =2 kπ/q.
6.
2D GIWAXS patterns of the thin films of (a) PM6, (b) PM6:FcF10, (c) Y6, (d) Y6:FcF10, (e) PM6:Y6, and (f) PM6:Y6:FcF10.
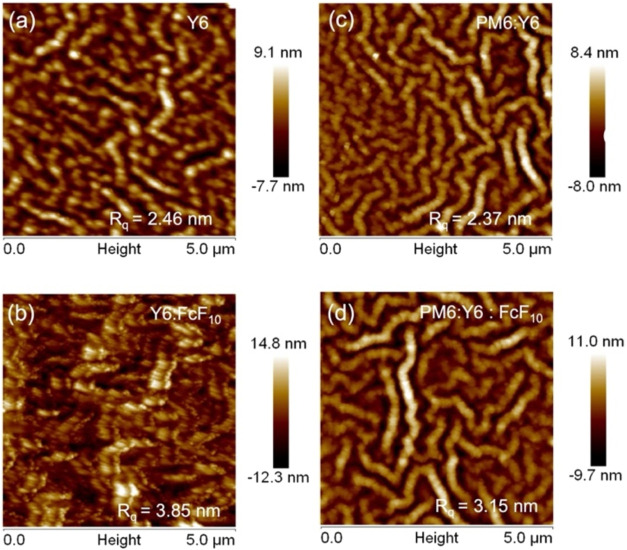
The surface morphology of the active layers was examined using atomic force microscopy (AFM), as illustrated in Figure , which presents AFM height images of pristine Y6 and PM6:Y6 blend films along with their FcF10-modified counterparts. The incorporation of FcF10 resulted in an increase in surface root-mean-square roughness (R q) from 2.46 to 3.85 nm for the Y6 film. The notable increase in R q for the Y6 film corresponds to enhanced crystallinity, as evidenced by the increased CL observed in the GIWAXS analysis. This phenomenon suggests that intermolecular interactions induced by FcF10, such as F FcF10···πFIC, πCP···πFIC, and F FcF10···F FIC have a greater influence on the molecular stacking of Y6. This observation aligns with the DSC and UV–Vis spectroscopy results. Additionally, the PM6:Y6 blend system exhibited an increase in R q from 2.37 to 3.15 nm upon FcF10 addition, suggesting that FcF10 promotes a more favorable morphology for phase separation.
7.
AFM height images of the (a) Y6, (b) Y6:FcF10, (c) PM6:Y6, and (d) PM6:Y6:FcF10 thin films.
The thermal stability of the devices was evaluated by monitoring their performance upon storage at 85 °C, addressing the high-temperature challenges typically faced by OSCs during prolonged exposure. As shown in Figure , after prolonged annealing at 85 °C for 360 h, the PM6:Y6 device with FcF10 demonstrated superior thermal stability, retaining 88% of its initial PCE compared to 81% for the device without FcF10. These findings indicate that FcF10 not only enhances the performance but also strengthens the device stability. This improvement is associated with the preferential supramolecular interactions between the perfluorophenyl moieties of FcF10 and the difluorophenyl-based FIC end groups of Y6, leading to a stabilized morphology of the active layer and enhanced thermal stability.
8.

Normalized performance of the PM6:Y6 and PM6:Y6:FcF10 devices as a function of time at 85 °C.
Compared to other additives, FcF10 exhibits characteristics similar to the non-volatile additive BF7 due to the presence of perfluorophenyl units that engage in strong non-covalent interactions with fluorinated acceptors Y6. Unlike volatile ferrocene (Fc), which evaporates during annealing, FcF10 remains in the active layer. Its ferrocene-based core introduces a three-dimensional structure that not only promotes crystalline packing but also serves as a spacer between Y6 domains, regulating morphology and enhancing its long-term stability.
Conclusions
In conclusion, this study demonstrates that the incorporation of FcF10 as a non-volatile solid additive significantly enhances both the performance and thermal stability of PM6:Y6-based OSCs. The PM6:Y6 device with 3.75 wt % of FcF10 achieved a higher PCE of 17.00%, with a V oc of 0.85 V, a J sc of 7.35 mA cm–2, and an FF of 73.29%, outperforming the reference device with a PCE of 15.4%. These improvements are attributed to the formation of a favorable active layer morphology, which enhances exciton dissociation and charge transport while reducing bimolecular and trap-assisted recombination. The FcF10 additive facilitates non-covalent interactions with Y6, such as F···F, F···π, and π···π interactions between the Cp and C6F5 rings in FcF10 and the FIC end groups in Y6. These supramolecular interactions improve molecular stacking and crystallinity within the Y6 domain, as evidenced by red-shifted Y6 absorption, reduced π–π stacking d-spacing, and increased coherence lengths of Y6, corroborated by UV–Vis spectroscopy, DSC analysis, GIWAXS, and AFM measurements. Furthermore, thermal stability tests at 85 °C demonstrate the ability of FcF10 in maintaining device performance, with an 88% retention of the initial PCE after prolonged thermal annealing.
Supplementary Material
Acknowledgments
This work is supported by the National Science and Technology Council, Taiwan (Grant No. 113-2221-E-A49-001, 113-2113-M-A49-015-MY3, and 113-2113-M-018-005-MY3) and the Ministry of Education, Taiwan (SPROUT ProjectCenter for Emergent Functional Matter Science, National Yang Ming Chiao Tung University). We acknowledge financial support from Austria’s Agency for Education and Internationalisation (OEAD) within the S&T Cooperation project No. TW04/2021. GIWAXS measurements were conducted at the TPS 25A beamline of the National Synchrotron Radiation Research Center (NSRRC), and we extend our special thanks to Dr. Jhih-Min Lin and Dr. Yi-Wei Tsai for their assistance with GIWAXS data processing. We gratefully acknowledge the support of Prof. Sariciftci for our collaboration between Austria and Taiwan, as well as his valuable discussions.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.5c04989.
#.
C.-L.T. and H.-C.L. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Organic Photovoltaics; Brabec, C. ; Dyakonov, V. ; Parisi, J. ; Sariciftci, N. S. , Eds.; Springer Verlag: Berlin, 2003. [Google Scholar]
- Sun, S.-S. ; Sariciftci, N. S. . Organic Photovoltaics: Mechanisms, Materials and Devices; CRC Press (Taylor & Francis Group): Boca Raton, FL, 2005. [Google Scholar]
- Heeger, A. ; Sariciftci, N. S. ; Namdas, E. . Semiconducting and Conducting Polymers; Oxford University Press: Oxford, 2010. [Google Scholar]
- Cheng Y.-J., Yang S.-H., Hsu C.-S.. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009;109:5868–5923. doi: 10.1021/cr900182s. [DOI] [PubMed] [Google Scholar]
- Li Y.. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012;45:723–733. doi: 10.1021/ar2002446. [DOI] [PubMed] [Google Scholar]
- Brabec C. J., Sariciftci N. S., Hummelen J. C.. Plastic Solar Cells. Adv. Funct. Mater. 2001;11:15–26. doi: 10.1002/1616-3028(200102)11:1<15::AID-ADFM15>3.0.CO;2-A. [DOI] [Google Scholar]
- Tang C. W.. Two-Layer Organic Photovoltaic Cell. Appl. Phys. Lett. 1986;48:183–185. doi: 10.1063/1.96937. [DOI] [Google Scholar]
- Thompson B. C., Frechet J. M.. Polymer-Fullerene Composite Solar Cells. Angew. Chem. Int. Ed. 2008;47:58–77. doi: 10.1002/anie.200702506. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhan X.. Fused-Ring Electron Acceptors for Photovoltaics and Beyond. Acc. Chem. Res. 2021;54:132–143. doi: 10.1021/acs.accounts.0c00575. [DOI] [PubMed] [Google Scholar]
- Yu G., Gao J., Hummelen J. C., Wudl F., Heeger A. J.. Polymer Photovoltaic Cells: Enhanced Efficiencies Via a Network of Internal Donor-Acceptor Heterojunctions. Science. 1995;270:1789–1791. doi: 10.1126/science.270.5243.1789. [DOI] [Google Scholar]
- Zhang G., Zhao J., Chow P. C. Y., Jiang K., Zhang J., Zhu Z., Zhang J., Huang F., Yan H.. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018;118:3447–3507. doi: 10.1021/acs.chemrev.7b00535. [DOI] [PubMed] [Google Scholar]
- Huang K.-H., Tseng C.-C., Tsai C.-L., Xue Y.-J., Lu H.-C., Lu C.-F., Chang Y.-Y., Huang C.-L., Hsu I.-J., Lai Y.-Y., Zheng Y.-P., Jiang B.-H., Chen C.-P., Chien S.-Y., Jeng U.-S., Hsu C.-S., Cheng Y.-J.. Highly Crystalline Selenium-Substituted C-Shaped Ortho-Benzodipyrrole-Based a-D-a-Type Nonfullerene Acceptor Enabling Solution-Processed Single-Crystal-Like Thin Films for Air-Stable, High-Mobility N-Type Transistors. Adv. Funct. Mater. 2024:2419176. doi: 10.1002/adfm.202419176. [DOI] [Google Scholar]
- Wang Y.-B., Tsai C.-L., Xue Y.-J., Jiang B.-H., Lu H.-C., Hong J.-C., Huang Y.-C., Huang K.-H., Chien S.-Y., Chen C.-P., Cheng Y.-J.. Fluorinated and Methylated Ortho-Benzodipyrrole-Based Acceptors Suppressing Charge Recombination and Minimizing Energy Loss in Organic Photovoltaics. Chem. Sci. 2025;16:3259–3274. doi: 10.1039/D4SC07146H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q., Ma R., Bi Z., Liao X., Wu B., Zhang S., Su W., Fang J., Zhao C., Yan C., Chen K., Li Y., Gao C., Li G., Ma W.. 19.28% Efficiency and Stable Polymer Solar Cells Enabled by Introducing an Nir-Absorbing Guest Acceptor. Adv. Funct. Mater. 2023;33:2211385. doi: 10.1002/adfm.202211385. [DOI] [Google Scholar]
- Zhu L., Zhang M., Xu J., Li C., Yan J., Zhou G., Zhong W., Hao T., Song J., Xue X., Zhou Z., Zeng R., Zhu H., Chen C.-C., MacKenzie R. C. I., Zou Y., Nelson J., Zhang Y., Sun Y., Liu F.. Single-Junction Organic Solar Cells with over 19% Efficiency Enabled by a Refined Double-Fibril Network Morphology. Nat. Mater. 2022;21:656–663. doi: 10.1038/s41563-022-01244-y. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang Y., Bi P., Chen Z., Qiao J., Li J., Wang W., Zheng Z., Zhang S., Hao X., Hou J.. Binary Organic Solar Cells with 19.2% Efficiency Enabled by Solid Additive. Adv. Mater. 2023;35:2301583. doi: 10.1002/adma.202301583. [DOI] [PubMed] [Google Scholar]
- Zhou M., Liao C., Duan Y., Xu X., Yu L., Li R., Peng Q.. 19.10% Efficiency and 80.5% Fill Factor Layer-by-Layer Organic Solar Cells Realized by 4-Bis(2-Thienyl)Pyrrole-2,5-Dione Based Polymer Additives for Inducing Vertical Segregation Morphology. Adv. Mater. 2023;35:2305240. doi: 10.1002/adma.202305240. [DOI] [PubMed] [Google Scholar]
- Xu X., Jing W., Meng H., Guo Y., Yu L., Li R., Peng Q.. Sequential Deposition of Multicomponent Bulk Heterojunctions Increases Efficiency of Organic Solar Cells. Adv. Mater. 2023;35:2208997. doi: 10.1002/adma.202208997. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Wang J., Bi P., Ren J., Wang Y., Yang Y., Liu X., Zhang S., Hou J.. Tandem Organic Solar Cell with 20.2% Efficiency. Joule. 2022;6:171–184. doi: 10.1016/j.joule.2021.12.017. [DOI] [Google Scholar]
- Ma R., Jiang X., Fu J., Zhu T., Yan C., Wu K., Müller-Buschbaum P., Li G.. Revealing the Underlying Solvent Effect on Film Morphology in High-Efficiency Organic Solar Cells through Combined Ex Situ and in Situ Observations. Energy Environ. Sci. 2023;16:2316–2326. doi: 10.1039/D3EE00294B. [DOI] [Google Scholar]
- Xue Y.-J., Lai Z.-Y., Lu H.-C., Hong J.-C., Tsai C.-L., Huang C.-L., Huang K.-H., Lu C.-F., Lai Y.-Y., Hsu C.-S., Lin J.-M., Chang J.-W., Chien S.-Y., Lee G.-H., Jeng U. S., Cheng Y.-J.. Unraveling the Structure–Property–Performance Relationships of Fused-Ring Nonfullerene Acceptors: Toward a C-Shaped Ortho-Benzodipyrrole-Based Acceptor for Highly Efficient Organic Photovoltaics. J. Am. Chem. Soc. 2024;146:833–848. doi: 10.1021/jacs.3c11062. [DOI] [PubMed] [Google Scholar]
- Li G., Shrotriya V., Huang J., Yao Y., Moriarty T., Emery K., Yang Y.. High-Efficiency Solution Processable Polymer Photovoltaic Cells by Self-Organization of Polymer Blends. Nat. Mater. 2005;4:864–868. doi: 10.1038/nmat1500. [DOI] [Google Scholar]
- Ma W., Yang C., Gong X., Lee K., Heeger A. J.. Thermally Stable, Efficient Polymer Solar Cells with Nanoscale Control of the Interpenetrating Network Morphology. Adv. Funct. Mater. 2005;15:1617–1622. doi: 10.1002/adfm.200500211. [DOI] [Google Scholar]
- Guo X., Cui C., Zhang M., Huo L., Huang Y., Hou J., Li Y.. High Efficiency Polymer Solar Cells Based on Poly(3-Hexylthiophene)/Indene-C70 Bisadduct with Solvent Additive. Energy Environ. Sci. 2012;5:7943–7949. doi: 10.1039/c2ee21481d. [DOI] [Google Scholar]
- Park J. H., Kim J. S., Lee J. H., Lee W. H., Cho K.. Effect of Annealing Solvent Solubility on the Performance of Poly(3-Hexylthiophene)/Methanofullerene Solar Cells. J. Phys. Chem. C. 2009;113:17579–17584. doi: 10.1021/jp9029562. [DOI] [Google Scholar]
- Gasparini N., Salleo A., McCulloch I., Baran D.. The Role of the Third Component in Ternary Organic Solar Cells. Nat. Rev. Mater. 2019;4:229–242. doi: 10.1038/s41578-019-0093-4. [DOI] [Google Scholar]
- Guo X., Zhang M., Ma W., Zhang S., Hou J., Li Y.. Effect of Solvent Additive on Active Layer Morphologies and Photovoltaic Performance of Polymer Solar Cells Based on Pbdttt-C-T/Pc71bm. RSC Adv. 2016;6:51924–51931. doi: 10.1039/C6RA06020J. [DOI] [Google Scholar]
- Lee J. K., Ma W. L., Brabec C. J., Yuen J., Moon J. S., Kim J. Y., Lee K., Bazan G. C., Heeger A. J.. Processing Additives for Improved Efficiency from Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2008;130:3619–3623. doi: 10.1021/ja710079w. [DOI] [PubMed] [Google Scholar]
- Peet J., Kim J. Y., Coates N. E., Ma W. L., Moses D., Heeger A. J., Bazan G. C.. Efficiency Enhancement in Low-Bandgap Polymer Solar Cells by Processing with Alkane Dithiols. Nat. Mater. 2007;6:497–500. doi: 10.1038/nmat1928. [DOI] [PubMed] [Google Scholar]
- Zhang M., Guo X., Ma W., Ade H., Hou J.. A Polythiophene Derivative with Superior Properties for Practical Application in Polymer Solar Cells. Adv. Mater. 2014;26:5880–5885. doi: 10.1002/adma.201401494. [DOI] [PubMed] [Google Scholar]
- Oh J., Jung S., Jeong M., Lee B., Lee J., Cho Y., Lee S. M., Chen S., Zhang Z.-G., Li Y., Yang C.. Ring-Perfluorinated Non-Volatile Additives with a High Dielectric Constant Lead to Highly Efficient and Stable Organic Solar Cells. J. Mater. Chem. C. 2019;7:4716–4724. doi: 10.1039/C9TC00762H. [DOI] [Google Scholar]
- Jhuo H.-J., Sharma S., Chen H.-L., Chen S.-A.. A Nonvolatile Morphology Regulator for Enhancing the Molecular Order in the Active Layer and Power Conversion Efficiency of Polymer Solar Cells. J. Mater. Chem. A. 2018;6:8874–8879. doi: 10.1039/C8TA01739E. [DOI] [Google Scholar]
- Tsai C.-L., Hung K.-E., Lu H.-C., Tseng C.-C., Cao F.-Y., Cheng Y.-J.. Volatile and Non-Volatile Additives for Polymer Solar Cells from Fullerene to Non-Fullerene Systems. J. Polym. Res. 2023;30:404. doi: 10.1007/s10965-023-03784-6. [DOI] [Google Scholar]
- Yuan J., Zhang Y., Zhou L., Zhang G., Yip H.-L., Lau T.-K., Lu X., Zhu C., Peng H., Johnson P. A., Leclerc M., Cao Y., Ulanski J., Li Y., Zou Y.. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule. 2019;3:1140–1151. doi: 10.1016/j.joule.2019.01.004. [DOI] [Google Scholar]
- Chang L., Lademann H. W. A., Bonekamp J.-B., Meerholz K., Moulé A. J.. Effect of Trace Solvent on the Morphology of P3ht:Pcbm Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2011;21:1779–1787. doi: 10.1002/adfm.201002372. [DOI] [Google Scholar]
- de Villers B. J. T., O’Hara K. A., Ostrowski D. P., Biddle P. H., Shaheen S. E., Chabinyc M. L., Olson D. C., Kopidakis N.. Removal of Residual Diiodooctane Improves Photostability of High-Performance Organic Solar Cell Polymers. Chem. Mater. 2016;28:876–884. doi: 10.1021/acs.chemmater.5b04346. [DOI] [Google Scholar]
- Classen A., Heumueller T., Wabra I., Gerner J., He Y., Einsiedler L., Li N., Matt G. J., Osvet A., Du X., Hirsch A., Brabec C. J.. Revealing Hidden Uv Instabilities in Organic Solar Cells by Correlating Device and Material Stability. Adv. Energy Mater. 2019;9:1902124. doi: 10.1002/aenm.201902124. [DOI] [Google Scholar]
- Cheng P., Yan C., Lau T.-K., Mai J., Lu X., Zhan X.. Molecular Lock: A Versatile Key to Enhance Efficiency and Stability of Organic Solar Cells. Adv. Mater. 2016;28:5822–5829. doi: 10.1002/adma.201600426. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Bao C., Cui S., Zhong P., Zhang K., Zhu W., Liu Y.. Boosting the efficiency of PTB7-Th:PC71BM polymer solar cells via a low-cost halogen-free supramolecular solid additive. J. Mater. Chem. C. 2020;8:16551–16560. doi: 10.1039/D0TC04096G. [DOI] [Google Scholar]
- Yu R., Yao H., Xu Y., Li J., Hong L., Zhang T., Cui Y., Peng Z., Gao M., Ye L., Tan Z. A., Hou J.. Quadrupole Moment Induced Morphology Control Via a Highly Volatile Small Molecule in Efficient Organic Solar Cells. Adv. Funct. Mater. 2021;31:2010535. doi: 10.1002/adfm.202010535. [DOI] [Google Scholar]
- Bao S., Yang H., Fan H., Zhang J., Wei Z., Cui C., Li Y.. Volatilizable Solid Additive-Assisted Treatment Enables Organic Solar Cells with Efficiency over 18.8% and Fill Factor Exceeding 80% Adv. Mater. 2021;33:2105301. doi: 10.1002/adma.202105301. [DOI] [PubMed] [Google Scholar]
- Yang W., Luo Z., Sun R., Guo J., Wang T., Wu Y., Wang W., Guo J., Wu Q., Shi M., Li H., Yang C., Min J.. Simultaneous Enhanced Efficiency and Thermal Stability in Organic Solar Cells from a Polymer Acceptor Additive. Nat. Commun. 2020;11:1218. doi: 10.1038/s41467-020-14926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Cai J., Guo C., Li D., Du B., Zhuang Y., Cheng S., Wang L., Liu D., Wang T.. Simultaneously Enhanced Efficiency and Operational Stability of Nonfullerene Organic Solar Cells via Solid-Additive-Mediated Aggregation Control. Small. 2021;17:2102558. doi: 10.1002/smll.202102558. [DOI] [PubMed] [Google Scholar]
- Cai J., Wang H., Zhang X., Li W., Li D., Mao Y., Du B., Chen M., Zhuang Y., Liu D., Qin H.-L., Zhao Y., Smith J. A., Kilbride R. C., Parnell A. J., Jones R. A. L., Lidzey D. G., Wang T.. Fluorinated solid additives enable high efficiency non-fullerene organic solar cells. J. Mater. Chem. A. 2020;8:4230–4238. doi: 10.1039/C9TA13974E. [DOI] [Google Scholar]
- Fan H., Yang H., Wu Y., Yildiz O., Zhu X., Marszalek T., Blom P. W. M., Cui C., Li Y.. Anthracene-Assisted Morphology Optimization in Photoactive Layer for High-Efficiency Polymer Solar Cells. Adv. Funct. Mater. 2021;31:2103944. doi: 10.1002/adfm.202103944. [DOI] [Google Scholar]
- Yu R., Yao H., Hong L., Qin Y., Zhu J., Cui Y., Li S., Hou J.. Design and application of volatilizable solid additives in non-fullerene organic solar cells. Nat. Commun. 2018;9:4645. doi: 10.1038/s41467-018-07017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Ryu H. S., Han L., Cai Y., Duan X., Wei D., Woo H. Y., Sun Y.. High-efficiency organic solar cells enabled by an alcohol-washable solid additive. Sci. China Chem. 2021;64:2161–2168. doi: 10.1007/s11426-021-1121-y. [DOI] [Google Scholar]
- Song X., Zhang K., Guo R., Sun K., Zhou Z., Huang S., Huber L., Reus M., Zhou J., Schwartzkopf M., Roth S. V., Liu W., Liu Y., Zhu W., Müller-Buschbaum P.. Process-Aid Solid Engineering Triggers Delicately Modulation of Y-Series Non-Fullerene Acceptor for Efficient Organic Solar Cells. Adv. Mater. 2022;34:2200907. doi: 10.1002/adma.202200907. [DOI] [PubMed] [Google Scholar]
- Dong M., Chen S., Hong L., Jing J., Bai Y., Liang Y., Zhu C., Shi T., Zhong W., Ying L., Zhang K., Huang F.. 19.0% Efficiency Binary Organic Solar Cells Enabled by Using a Building Block as Solid Additive. Nano Energy. 2024;119:109097. doi: 10.1016/j.nanoen.2023.109097. [DOI] [Google Scholar]
- Xiao M., Meng Y., Tang L., Li P., Tang L., Zhang W., Hu B., Yi F., Jia T., Cao J., Xu C., Lu G., Hao X., Ma W., Fan Q.. Solid Additive-Assisted Selective Optimization Strategy for Sequential Deposited Active Layers to Construct 19.16% Efficiency Binary Organic Solar Cells. Adv. Funct. Mater. 2024;34:2311216. doi: 10.1002/adfm.202311216. [DOI] [Google Scholar]
- Ye L., Cai Y., Li C., Zhu L., Xu J., Weng K., Zhang K., Huang M., Zeng M., Li T., Zhou E., Tan S., Hao X., Yi Y., Liu F., Wang Z., Zhan X., Sun Y.. Ferrocene as a highly volatile solid additive in non-fullerene organic solar cells with enhanced photovoltaic performance. Energy Environ. Sci. 2020;13:5117–5125. doi: 10.1039/D0EE02426K. [DOI] [Google Scholar]
- Liao M.-H., Tsai C.-E., Lai Y.-Y., Cao F.-Y., Wu J.-S., Wang C.-L., Hsu C.-S., Liau I., Cheng Y.-J.. Morphological Stabilization by Supramolecular Perfluorophenyl-C60 Interactions Leading to Efficient and Thermally Stable Organic Photovoltaics. Adv. Funct. Mater. 2014;24:1418–1429. doi: 10.1002/adfm.201300437. [DOI] [Google Scholar]
- Tsai C.-L., Chan T.-H., Lu H.-C., Huang C.-L., Hung K.-E., Lai Y.-Y., Cheng Y.-J.. Synthesis of Angular-Shaped Naphthodithiophenediimide and Its Donor–Acceptor Copolymers as Nonvolatile Polymer Additives for Organic Solar Cells. J. Mater. Chem. A. 2023;11:7572–7583. doi: 10.1039/D3TA00519D. [DOI] [Google Scholar]
- Hung K. E., Tsai C. E., Chang S. L., Lai Y. Y., Jeng U. S., Cao F. Y., Hsu C. S., Su C. J., Cheng Y. J.. Bispentafluorophenyl-Containing Additive: Enhancing Efficiency and Morphological Stability of Polymer Solar Cells Via Hand-Grabbing-Like Supramolecular Pentafluorophenyl-Fullerene Interactions. ACS Appl. Mater. Interfaces. 2017;9:43861–43870. doi: 10.1021/acsami.7b13426. [DOI] [PubMed] [Google Scholar]
- Hung K. E., Lin Y. S., Xue Y. J., Yang H. R., Lai Y. Y., Chang J. W., Su C. J., Su A. C., Hsu C. S., Jeng U. S., Cheng Y. J.. Non-Volatile Perfluorophenyl-Based Additive for Enhanced Efficiency and Thermal Stability of Nonfullerene Organic Solar Cells Via Supramolecular Fluorinated Interactions. Adv. Energy Mater. 2022;12:2103702. doi: 10.1002/aenm.202103702. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.