Graphical abstract
Keywords: Sensory neuron, Spinal motoneuron, Regeneration-associated genes, Axonal injury, Transcriptome, Microenvironment
Highlights
-
•
Genome-wide molecular comparison of injury response between motor- and sensory neurons.
-
•
Similar injury state between motor- and sensory neurons, but a delayed response in motoneurons.
-
•
A conserved regeneration-associated module between motor- and sensory neurons.
-
•
Knockdown of Rnf122 significantly inhibit axon regrowth in vitro.
-
•
Immune niches led to large molecular divergence between motor- and sensory neurons upon injury.
Abstract
Introduction
Motor neurons differ from sensory neurons in aspects including origins and surrounding environment. Understanding the similarities and differences in molecular response to peripheral nerve injury (PNI) and regeneration between sensory and motor neurons is crucial for developing effective drug targets for CNS regeneration. However, genome-wide comparisons of molecular changes between sensory and motor neurons following PNI remains limited.
Objectives
This study aims to investigate genome-wide convergence and divergence of injury response between sensory and motor neurons to identify novel drug targets for neural repair.
Methods
We analyzed two large-scale RNA-seq datasets of in situ captured sensory neurons (SNs) and motoneurons (MNs) upon PNI, retinal ganglion cells and spinal cord upon CNS injury. Additionally, we integrated these with other related single-cell level datasets. Bootstrap DESeq2 and WGCNA were used to detect and explore co-expression modules of differentially expressed genes (DEGs).
Results
We found that SNs and MNs exhibited similar injury states, but with a delayed response in MNs. We identified a conserved regeneration-associated module (cRAM) with 274 shared DEGs. Of which, 47% of DEGs could be changed in injured neurons supported by single-cell resolution datasets. We also identified some less-studied candidates in cRAM, including genes associated with transcription, ubiquitination (Rnf122), and neuron-immune cells cross-talk. Further in vitro experiments confirmed a novel role of Rnf122 in axon growth. Analysis of the top 10% of DEGs with a large divergence suggested that both extrinsic (e.g., immune microenvironment) and intrinsic factors (e.g., development) contributed to expression divergence between SNs and MNs following injury.
Conclusions
This comprehensive analysis revealed convergent and divergent injury response genes in SNs and MNs, providing new insights into transcriptional reprogramming of sensory and motor neurons responding to axonal injury and subsequent regeneration. It also identified some novel regeneration-associated candidates that may facilitate the development of strategies for axon regeneration.
Introduction
Neural injury is prevalent in clinic settings, often caused by trauma, neurodegenerative diseases, or other factors, leading to significant neurological deficits. This is particularly evident in neurotrauma within the central nervous system (CNS), such as spinal cord injury (SCI). Unlike the CNS, where damaged axon has limited regenerative ability, severed axon in the peripheral nerve system (PNS) can spontaneously regenerate, as exemplified by the sciatic nerve.
The sciatic nerve is mixed nerve containing both sensory axons and motor axons. The somas of sensory axons are located in the dorsal root ganglion (DRG) within the PNS, while the somas of motor axons are located in the gray matter of the ventral horn of the spinal cord within the CNS. When the rodent sciatic nerve is crushed, it affects both sensory and motor neurons, enabling robust axon regeneration and functional recovery. This makes it an ideal injury model for studying conserved cellular and molecular processes during the nerve regeneration. DRG sensory neurons possess a unique duality, with peripheral regeneration-competent axons in the PNS and central regeneration-incompetent axons projecting into the dorsal spinal cord. Extensive studies have explored regenerative genetic basis of DRG sensory neurons at multiple levels, including transcriptional, epigenetic, and cellular heterogeneity on a genome-wide scale [1], [2], [3]. Sensory neurons arise from the neural crest, while motoneurons and other CNS neurons, such as retina ganglion cells, originate from the neural tube. They also differ in anatomy, surrounding environment, growth requirements, and also response to axonal injury [4]. Understanding their molecular similarities and differences in responding to axonal injury may give novel insights into manipulating for CNS injury, such as SCI. However, genome-wide studies in comparing molecular response after injury between motoneurons and sensory neurons are still limited.
Axonal injury triggers a rapid increase in axonal calcium concentration, which retrogradely signal to the cell body, inducing cell body responses and sterile inflammatory [5]. Our pioneer works used laser capture microdissection (LCM) coupled with high-coverage RNA sequencing (LCM-seq) to decipher the transcriptional profiles of rat spinal motoneurons and DRG neurons following sciatic nerve crush [6], [7]. LCM technology allows for in situ capturing of target cells without tissue dissociation, preserving the cross-talk information between target cells and their microenvironment [6]. These two large-scale, high-coverage, and similar time-series LCM-seq datasets, generated using common laboratory workflows, provide approximate single-soma resolution and precise transcriptional information, facilitating the comparison and exploration of convergent and divergent injury responses between sensory and motor neurons. Considering the mixture of LCM captured samples, including target cells and their microenvironment, we termed these two datasets as SNs (sensory neuron and its niches) and MNs (motoneuron and its niches) respectively.
Approximately 50 % of sensory and motor neurons are axotomized upon sciatic nerve injury. Atf3, a marker for injured sensory and motor neurons following peripheral nerve injury, enables the distinction between ‘injured’ and ‘intact’ neurons. In this study, we focused on transcriptional changes in injured neurons and systematically compare the convergent and divergent injury response genes between sensory and motor neurons. The available of single-cell level RNA-seq datasets of mouse DRG, along with previously integrated bulk RNA-seq datasets of rat and mouse DRG following peripheral nerve injury, allows us to identify and infer reliable genes changed in injured neurons [8], [9], [10]. Additionally, we compared these findings with CNS injury datasets, including bulk RNA-seq of spinal cord and sorted retinal ganglion cells (RGCs), as well as developing spinal cord, motoneurons and sensory neurons, to infer expression changes of candidates following CNS injury and development [11], [12], [13], [14], [15]. This comprehensive analysis aims to provide a genome-wide perspective on the convergent and divergent transcriptional reprogramming of sensory and motor neurons in response to axonal injury and subsequent regeneration. We also aim to identify some novel regeneration-associated candidates that may facilitate the development of neural repair.
Materials and methods
Animals
Adult male Sprague-Dawley rats (8 weeks) weighting 200–220 g were obtained from the Experimental Animal Center of Nantong University. All animal procedures were performed in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committees of Nantong University.
Animal surgery and tissue preparation
The rat sciatic nerve crush model was established as previously described [6]. Following the surgery, rats were anesthetized using a composite anesthetic at time points: 0 h (namely naïve), 12 h, 1d, 3d, and 7d post-surgery, and then cervical dislocation was performed. We collected L4/L5 DRG and corresponding ventral spinal cord from the ipsilateral side of the injured nerve, and then placed in cryogenic tubes and frozen in liquid nitrogen, then stored in an ultra-low temperature refrigerator at −80 °C.
Total RNA extraction and qPCR
Total RNA was extracted from the harvested L4/L5 DRG and spinal cord using TRIzol (Sigma, USA) respectively according to the manufacture’s protocol. The RNA quality and concentration were measured with a NanoDrop 2000 spectrophotometer. cDNA synthesis was performed using the PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara, Japan). Quantitative PCR (qPCR) reactions were performed using the FastStart Essential DNA Green Master (Roche, Switzerland). Gadph was used as the endogenous control. Gene expression levels were calculated based on the 2-ΔΔCT method. The primer sequences for Tnik, Stmn4, Flrt3, Tph2, Phldb3 and Rnf122 were listed below:
Tnik: F- TCCAATGGGGAGAAATGCCC; R- ACCTGGTGGTCTCTTAAAATGC.
Stmn4: F- ACCCTCGCAGCCTATAAGGA; R- TCCAGTCAGCACTGCCTTTC.
Flrt3: F- CCCCACAACCACCCTCAATC; R- AGTTCCGTGAGAACAGGGAC.
Tph2: F- GTGACGCTGAATCCACCTGA; R- CCTTGAATCCTGGGTGGTCG.
Phldb3: F- CCTCCCGACCTTTGTCTGTC; R- GGGTGAAGCACAGGGTTTCT.
Rnf122: F- GCACCCATTCCAGTGGTGTA; R- AAGCACCACCTCCTTGTAGC.
Primary DRG neuron culture and neurite outgrowth in vitro by siRNA on microfluidic device and glass slide
DRG tissues were collected and washed twice with PBS, then digested with collagenase (3 mg/ml) at 37℃ for 90 min, followed by digestion with 0.25 % trypsin for 10 min at 37℃. After gentle trituration, the dissociated cells were plated on poly-L-lysine coated glass and cultured in an incubator at 37℃. For siRNA transfection, a mixture of siRNA diluent solution (siRnf122 or siControl) and RNAi transfection reagent was prepared according to the manufacturer’s protocol.
For assessment of neurite outgrowth of DRG neurons in the sterile microfluidic devices, about 3 × 104 cells were added to the upper left well. The siRNA mixture was immediately added after 4 h planting. After 8 h, the neuronal medium was replaced and replenished every 48 h. After 4 days in culture, axons could reach across to the right axon chambers. Fixation was performed on 4 days later, and then subjected to immunofluorescence staining.
For assessment of neurite outgrowth of DRG neurons in the culture dish, about 3 × 104 cells were planted in 24-well plates. siRNA transfection was performed according to the manufacture’s protocol after 4 h. After 8 h, the neuronal medium was replaced and replenished every 48 h. After 4 days, fixation and immunofluorescence staining were performed.
The primary anti-Tuj1 (ab78078, Abcam) antibody was used for visualization of neuronal soma and axons. We used the NeuronJ implemented in ImageJ for the measurement of the longest neurite in each DRG neuron. The siRNA sequences for Rnf122 were listed as following:
Sense: GCUGGAUGAAUUGGUGUAATT; antisense: UUACACCAAUUCAUCCAGCTT.
In situ hybridization
The Digoxigenin (DIG)-labeled cDNA probe for rat Rnf122 was synthesized using the DIG RNA Labeling Kit (Roche). The rat L4/L5 DRG tissues at 3 days post-SNC were collected, digested with proteinase K, fixed with 4 % paraformaldehyde and dehydrated for DRG cryosections. The cryosections were prehybridized for 2 h and then hybridized with DIG-labeled probe for 12–16 h at 68℃ in a humidified chamber. The sections were blocked with AP-conjugated Fab anti-DIG antibody (11093274910, Roche) overnight at 4℃. Following staining was carried out using 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (BCIP-NBT, Roche). The probe sequences for Rnf122 were listed as following:
Rnf122: F-ACGAGCAACUCUGACCUUGA; R- GCAUUACUGUACCCUCCCGA.
Clustering analysis, injury and cell-type scores across DRG neurons
To make our two previous large-scale LCM-seq datasets [6], [7] comparable, we employed a similar method to re-analyze the SNs dataset. The clustering results of MNs were retrieved from our previous study [6]. First, samples with low-expression of the neuronal marker Rbfox3 (FPKM<1) were discarded and standard analysis was performed using Seura [16] (V4). A sample with a normalized expression of Atf3 (≥1) was labelled as an injured neuron. We classified samples based on the clustering results and Atf3 expression as previously described [6].
To estimate the activity of a given gene set, we used the AUCell R package [17] and calculated the AUC score. The injury-related gene set was selected based on robust up-regulation in both SNs and MNs at 12 h and 1d in state3 respectively. The gene set of markers for DRG neuron subtypes was retrieved from the supplementary materials [3].
Identification of robust differentially expressed genes
To identify robust DEGs, we employed a similar method as we analyzed for MNs [6] in SNs. Briefly, we first performed a bootstrap DESeq2 method by randomly selected the same sample number from the naïve and isolated distinct states of samples collected from the injured timepoints and repeated each comparison with 100 times. For a test, the gene with an adjusted P-value < 0.05 and |log2fold-change|≥log21.5 was selected as a DEG. Then, we summarized and calculated a DEG frequency for all tests. DEGs with frequency ≥ 50 were defined as robust. Roust DEGs in MNs were retrieved from our previous study [6] and filtered using the same criterion.
Co-expression analysis and visualization
Weighted correlation network analysis (WGCNA) [18] was used to explore co-expression modules of DEGs identified in MNs and SNs respectively. A soft power of 4 was chose to build the adjacency matrix, and one-step network construction was employed for module detection in MNs and SNs respectively. We calculated shared gene number among modules from MNs and SNs and performed overlap test using GeneOverlap R package. NGenomeSyn was used for visualization of module relationship between MNs and SNs [19]. Functional enrichment for each module was conducted using the clusterProfiler [20]. Circular visualization of modules and other attributes (e.g., labeled transcription factors, expression pattern) was plotted using the Circos program [21]. Protein-protein interaction (PPI) were retrieved from the STRING database [22] and visualized using Gephi [23]. Heatmap of gene expression was plotted using the ComplexHeatmap [24] or ggplot2 package implemented in the R language.
Expression pattern divergence analysis
To estimate expression divergence, we employed two metrics: Euclidean distance (ED) and Pearson’s correlation (r). We also calculated coefficient of variation (CV) to estimate fluctuation of gene expression change in MNs and SNs upon injury. To avoid bias caused by abnormal high expression of genes in several samples, we calculated the upper quartile (Q1) expression in each group (naïve, 1d, 3d and 7d) at injured states (state2 and state3 for 1d, 3d and 7d; state 1 for the naïve) for a DEG and discarded DEGs with row maximum Q1 < 10. To avoid tissue-specific bias, we converted the expression values into relative fold-change compared to the naïve in MNs and SNs respectively. To avoid extremely low expression (∼0) in the naïve, we added a pseudo value (0.1) to each value (Q1 + 0.1 as the expression for each gene). Then, we calculated CV for a gene in MNs and SNs respectively, and calculated r and ED for each gene pair between MNs and SNs.
Other public dataset analysis
Datasets of single-cell or single-nucleus RNA sequencing (scRNA-seq or snRNA-seq) of mouse DRG neurons were retrieved from the Gene Expression Omnibus (GEO) database under accessions, GSE71453 [9] and GSE154659 [10]. A single-cell dataset of rat spinal cord upon spinal cord injury was retrieved from Yao et al [25]. Standard Seurat analysis was employed. Original cell-type metadata for GSE154659 was used for snRNA-seq from mouse DRG. The expression profiles of regenerating and surviving retinal ganglion cells (RGCs) in PTEN knockout mice and injured RGCs were retrieved from the GEO database under accessions GSE206626 [26] and GSE184547 [12]. Datasets of rat spinal cord during development and injury were retrieved from previous studies [11], [15]. Datasets of mouse spinal motoneurons and DRG neurons during development were retrieved from the GEO database under accessions GSE198767 [13] and GSE66128 [14]. Gene quantification from raw data and differential expression analysis were performed as previously described [8] using DESeq2 [27]. Differential expression results of bulk RNA-seq of mouse and rat DRG were retrieved from our previous study [8].
Results
Similar injury states in sensory neurons and motoneurons with delayed response in motoneurons
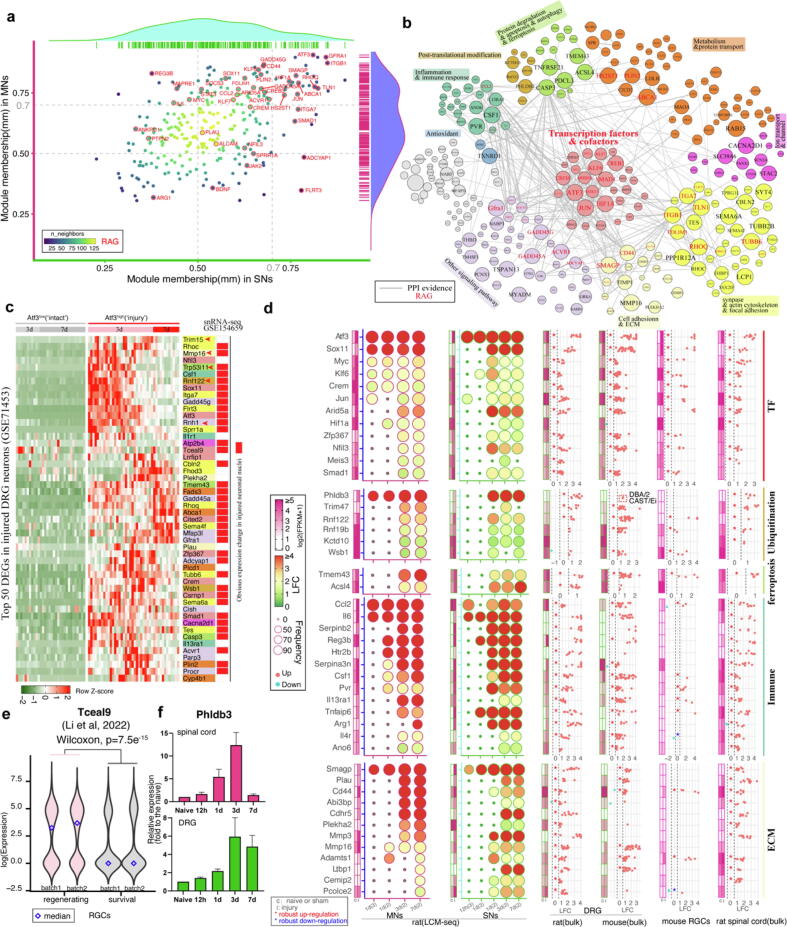
We have elucidated the neuronal injury states in motoneurons following sciatic nerve crush (SNC) from early injury to regenerating stages (naïve, 0.5 h, 3 h, 12 h, 1d, 3d, and 7d) [6]. Concurrently, we conducted LCM-seq on rat DRG sensory neurons at comparable timepoints (naïve, 3 h, 6 h, 12 h, 1d, 3d and 7d) independently [7] (Fig. 1a and 1b). For DRG neurons, samples with low Rbfox3 (NeuN) expression (<1) were excluded, leaving 692 samples for analysis. We used Atf3 expression and clustering analysis to determine the injury state for each sample. The clustering distribution was similar to that observed in MNs, with cluster2 including part of samples from 3d and 7d which exhibited high expression of Atf3 (designed as state2). However, we observed an increased number of samples from 1d (47 out of 99, ∼47 %) in cluster2 compared to MNs (6 out of 98, ∼6%). Additionally, 29 % of samples from 1d (29 out of 99) and 47 % of samples from 12 h (48 out of 102) showed Atf3 expression in cluster1 (designed as state3), whereas few samples from 12 h in MNs did using the same criterion (Fig. 1c and 1d, state of samples with low Atf3 expression was designed as state1). Next, we employed a similar robust DEGs detection method but adopted a stricter screening criterion as used in Zhang et al [6]. To estimate the injury score, we screened four injury markers (Atf3, Il6, Gadd45a, Gadd45g) that were commonly induced at 12 h in SNs and 1d in MNs, then calculated the AUC scores. The result showed a similar distribution of injury scores with Atf3 expression (Fig. 1e and 1f), suggesting a delay of response in injured MNs. Given our method of sample collection, the captured sensory neurons were predominantly large diameter A-fiber sensory neurons. We confirmed this by calculating the AUC scores of sensory neuron subtype-specific markers [3]. Both the AUC scores and the expression of classical cell-type markers indicated high activity of Nefh+ neurons (Fig. S1), supporting that the previously captured sensory neurons were primarily large diameter A-fiber sensory neurons.
Fig. 1.
Estimation of injured motoneurons and sensory neurons from captured samples using LCM-seq with the uniform analysis. Schematic representation of the two experimental paradigms (a) and time-points (b). Clustering and injured state inference for SNs (c) and MNs (d). The UMAP projection of MNs was directly retrieved from our previous study [6] and replotted. e, f, Distribution of injury scores across different injury timepoints in SNs and MNs based on conserved injured-related genes.
Comparison and exploration of robust DEGs and co-expression modules in/between SNs and MNs
We employed a bootstrap strategy to screen DEGs by comparing each injury state at each injured timepoint against the naïve. Most DEGs were found in state2 (‘injury and regenerating’) at 1d, 3d and 7d in both SNs and MNs (Fig. 2a). In state1 (“intact”) at 7d in MNs, over 200 DEGs were identified, of which up-regulated genes (e.g., Gpr84, P2ry6, Aif1) were primarily involved in inflammation and immune response. In contrast, in state1 at 7d in SNs, nearly all 10 up-regulated genes (e.g., Atf3, Sprr1a) were injury-induced in neurons but had much lower expression than in injured neurons (7d at state2, Additional file 1: Fig. S2). We focused on DEGs at 12 h, 1d, 3d and 7d in the state2 and state3 in SNs and MNs and counted the shared DEGs between neighbor timepoints in SNs and MNs, or between SNs and MNs. There was a large overlap of DEGs at 3d and 7d in/between MNs and SNs (Fig. 2b).
Fig. 2.
Robust differentially expressed genes and co-expression modules of injured neurons in SNs and MNs. a, Summary of robust DEGs in SNs and MNs. b, Summary of shared DEG numbers within or between SNs and MNs across injured time points. c, Co-expression modules in SNs. d, Co-expression modules in MNs. Colorful bar plots showed top significant enriched terms for genes in the corresponding module. e, Four significant overlapping modules between SNs and MNs. The ‘Synteny’ plot showed the shared DEGs in each module from SNs (top) and MNs (bottom). The number of genes in significant overlapping modules was shown.
We then performed co-expression module analysis to explore these DEGs in SNs and MNs respectively. In SNs, 1,477 DEGs were divided into 7 modules, and in MNs, 2,649 DEGs were divided into 6 modules (Fig. 2c and 2d). We found 11 well-known regeneration-associated transcription factors (e.g., Myc, Klf6, Atf3, Smad1) in the turquoise module in SNs and the blue module in MNs (Fig. 2c and 2d). In the SNs turquoise module (748 genes, 50.6 %), most genes showed up-regulation following nerve injury and were involved in terms including “wound healing”, “response to transforming growth factor beta”, and “response to cAMP”. In the SNs blue module (157 genes), up-regulated genes were involved in terms of “actin filament organization” and “tissue regeneration”, while down-regulated genes were involved in terms of “synapse assembly”, “cell junction assembly” and “transmission of nerve impulse” (Fig. 2c). In the SNs yellow module (83 genes), most genes were related to inflammation and immune response, including “antigen processing and presentation”, “leukocyte mediated immunity” and “regulation of immune effector process”. Genes in other four SNs modules (red, black, brown and green) mostly presented down-regulation following nerve injury and were related to terms including “regulation of membrane potential”, “neurofilament cytoskeleton organization” and “mitochondrial respiratory chain complex assembly”.
In MNs, we found two large modules: turquoise (931 genes, 35.1 %) and blue (723 genes, 27.3 %), with most genes showing up-regulation after injury. Genes in the turquoise module were significantly enriched in terms related to inflammation and immune response, including “myeloid leukocyte activation”, “positive regulation of cytokine production” and “adaptive immune response” (Fig. 2d). Re-analysis of a single-cell RNA-seq dataset of rat spinal cord after spinal cord injury[25] showed that most genes with a high module membership (mm > 0.8, 113 genes) in the turquoise module were highly or specifically expressed in microglia/macrophages (Fig. S3). Genes in the blue module were significantly enriched in terms related to axon injury and regeneration (Fig. 2d). We next estimated module conservation between SNs and MNs based on shared genes in their modules and performed an overlap test. Four significant overlap module pairs were detected (SNs-MNs): turquoise-blue (274 genes), yellow-turquoise (49 genes), black-yellow (27 genes) and red-red (10 genes) (Fig. 2c-2e). These overlaps may give molecular clues regarding the conserved injury response in SNs and MNs.
Shared genes from the regeneration-associated module between SNs and MNs in response to the injury
We explored the shared injury response genes in the conserved regeneration-associated module pairs (Fig. 2e) between SNs and MNs by integrating with other related datasets. These datasets included public data on spinal cord (or DRG) development and injury at bulk and/or single-cell levels, and retinal ganglion cells (RGCs) following optic nerve crush [8], [10], [11], [15], [25].
Known RAGs and transcription-associated genes in the conserved co-expression module. In the conserved module of “turquoise ∼ blue”, 274 shared genes were significantly enriched in terms of “wound healing”, “inflammatory response”, and “regulation of cell morphogenesis”. We manually checked these genes against the PubMed database for those validated regeneration-associated genes (RAGs) with experimental evidence of roles in axon regrowth. Forty-three RAGs (15.6 %) have been confirmed with the roles in axon regrowth, including recently reported axon regrowth-suppressor Ptpn2 [28] and axon regrowth-promoter Plin2 [26] (Fig. 3a). Most of these RAGs (42 out of 43 genes) showed a relatively high module membership (mm > 0.5) in the SNs module (turquoise) and/or MNs module (blue, Fig. 3a). Next, we constructed a co-expression network and extracted common interaction of these 274 genes between SNs and MNs, retrieving evidence of protein–protein interaction from the STRING database [22] (Fig. 3b). By combing gene annotation and literatures, we grouped these genes into several major functional classes: gene transcription, metabolism, cytoskeleton, apoptosis, post-translation modification and immune response (Fig. 3b). Known RAGs with a high connection were most related to gene transcription (e.g., Atf3, Sox11, Jun, Smad1), lipid (e.g., Plin2, Abca1), focal adhesion (e.g., Itgb1, Tln1, Pdlim1), and cell adhesion (e.g., Cd44, Smagp [29], Fig. 3b).
Fig. 3.
Expression of shared genes from the conserved regeneration-associated module in different datasets. a, Correlation of 274 shared genes with the blue module (MNs) and the turquoise module (SNs). Red circles and texts indicated validated regeneration-associated genes (RAGs) in vitro and/or in vivo based on literature evidences. b, Major functional classes of 274 shared genes. The size of the nodes depicts connection degree and only interactions supported by protein–protein interaction (PPI) in the STRING database were shown. Red-labelled genes indicated literature-evidenced RAGs. The color of the nodes indicated distinct functional classes divided by manual review of literatures and functional annotation. c, Top 50 up-regulated shared genes in GSE71453 [9] and GSE154659 [3]. The color for genes is the same as those in b panel. d, Expression of genes of interest in datasets of LCM-seq of rat SNs and MNs, and log-transformed fold-change in bulk RNA-seq of rat and mouse dorsal root ganglion (DRG), mouse retinal ganglion cells (RGCs) and rat spinal cord tissue after peripheral nerve injury and optic nerve crush and spinal cord injury, respectively. e, Expression of Tceal9 in PTEN knock-out mouse RGCs according to the study of Li et al [26]. f, qPCR validation of Phldb3 in tissues of rat DRG and the ventral horn of the spinal cord. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Given that laser-captured samples include target cells and their micro-environment and lack of single-cell resolution datasets for rat sensory neurons or motoneurons following peripheral nerve injury, we re-analyzed public datasets of scRNA-seq or snRNA-seq of mouse DRGs after sciatic nerve injury[9], [10]. We found that 124 orthologous genes (∼47 %) also showed significant up-regulation in injured mouse DRG neurons (Atf3high) at 3d and/or 7d post-injury, with most showing pronounced expression in injured neuronal nuclei, such as Atf3, Sox11, Sprr1a, and Gadd45a (Figs. S4 and S5; the top 50 DEGs in injured neurons were shown in Fig. 3c). Notably, Tceal9 (Wbp5), which normally decreases in neurons during development, showed a significant up-regulation in injured neurons (3 ∼ 5 times in SNs and 1.5 ∼ 2.35 times in RGCs), with a particularly marked increase in MNs (10 ∼ 13 times) (Fig. S6). Interestingly, compared to surviving RGCs, Tceal9 also exhibited significant up-regulation in regenerating RGCs from PTEN knockout mice (P=7.5e-15, Fig. 3e).
Genes associated with cross-talk between injured neurons and their microenvironment. We identified five less-studied genes ranked at the top along with Atf3 and Sox11: Trim15, Mmp16, Tp53i11 (Trp53i11 in mice), Rnf122, and Rnh1 (Fig. 3c). Six genes associated with ubiquitylation, including Rnf122, Wsb1 and Kctd10, were upregulated in injured neuron, with Rnf122 showing the most significant change (Fig. 3d). Serval genes involved in microglia activation and proliferation were identified in injured neurons, including Csf1, Reg3b, Il6, and Ccl2 (Fig. 3d). We also observed significant upregulation of Pvr and Serpina3n, which regulate other immune cells (e.g., T cells [30], natural killer (NK) cells [31]). High expression of PVR on hepatocytes could increase crosstalk with TIGIT on NK cells to avoid overactivation of NK cells, promoting liver regeneration [31]. We found that a NK cell marker Klrk1 (NKG2D) was dramatically up-regulated around injured sensory and motor neurons (Fig. S7), suggesting NK cells infiltration. Il4r and Il13ra1, which form a heterodimer receptor and activated by IL4 or IL13, were co-upregulated after axonal injury with Il13ra1 showing a larger change (Fig. 3d), suggesting the activation of IL13-IL13RA1 signaling. IL13-IL13RA1 could activate the Jak/Stat signaling pathway, which is beneficial for axon regeneration. Indeed, we observed significant upregulation of Jak2 and Stat3 in SNs and MNs after axonal injury (Fig. 3b).
Metabolism-associated genes in injured neurons. Three genes (Gch1, Spr, and Dhfr) involved in tetrahydrobiopterin (BH4) cofactor production were identified, with Gch1 showing the highest up-regulation in both SNs and MNs (Fig. 4a and 4b). BH4 has antioxidant activity and antagonizes ferroptotic cell death [32]. Inhibiting ferroptosis could effectively ameliorate iron dysfunction, redox stabilization, neuronal remodeling and functional recovery after trauma [33]. Both pro- and anti-ferroptosis genes (Acsl4 and Tmem43) and iron-associated genes (Hamp, Steap1, and Cyb561) were up-regulated after axonal injury (Fig. 3d and Fig. 4a). Lipid dynamics of the axon membranes for growth and extension is important for regeneration. Genes associated with cholesterol biosynthesis (Dhcr24) and transport (Ldlr and Abca1) were upregulated in injured neurons after PNI. However, both Dhcr24 and Ldlr showed down-regulation in RGCs and spinal cord after central nerve injury (Fig. 4a). Overexpression of Dhcr24 exerts neuroprotection under acute inflammation condition [34]. In addition, we also found genes related to lipid droplet (Plin2), lipid transport (C2cd2), and highly unsaturated fatty acid biosynthesis (Fads3) that showed a significant up-regulation in injured neurons (Fig. 3c and Fig. 4a). Fads3 could increase docosahexaenoic acid (DHA) level during the brain development [35]. Several studies have shown that DHA has a role of neuroprotection against spinal cord and brain injury [36], [37]. Plin2, a coat protein and stabilization of lipid droplet, has been validated as a pro-regenerative factor in RGCs after injury [26]. Genes related to glucose metabolism (e.g., Pgm2, Pfkfb3, Acss2), amino acid metabolism (e.g., Hpd), and nucleotide metabolism (Uck2) were also upregulated (Fig. 4a). Acss2, important for histone acetylation [38], was significantly upregulated in injured neurons after PNI, unlike its downregulation in the spinal cord and minimal change in RGCs after injury (Fig. 4a and Fig. S8). Investigation of epigenomic signatures related to axonal regenerative ability demonstrated enhanced histone acetylation in DRG with peripheral axon injury but not with central axon injury [1].
Fig. 4.
Expression of metabolism, protein/ion transport and cytoskeleton-associated genes from the shared regeneration-associated module in diverse datasets. a, Expression of target genes in MNs and SNs, and other datasets. b, Illustration of genes involved in folate biosynthesis (upper) and protein transport (lower). c, Expression of Ap1s1 in regenerating and surviving retinal ganglion cells. d, Illustration of genes involved in ion transport, synapse, and cytoskeleton.
Transport and cytoskeleton associated genes in injured neurons. We focused on shared genes related to axonal transport, cytoskeleton remodeling and synapse, which are crucial for axon regrowth and functional recovery. The initial injury signal, calcium influx, triggers various cell autonomous mechanisms required for successful axon growth [39]. We identified three calcium-associated genes (Cacna2d1, Atp2b4 and Stac2) that showed little change or down-regulation in RGCs and/or spinal cord after injury but significant up-regulation in injured neurons after PNI (Fig. 4a and 4d). A calcium-activated lipid scramblase, Ano6 (Tmem16F), was significantly up-regulated in SNs and MNs, and injured neurons/neuronal nucleus post-PNI (Fig. 3d and Fig. S5). Membrane trafficking through endosomes is important for the repair and regeneration of severed axon. We identified seven genes associated with membrane trafficking, including three small GTPase (Rab15, Rab33b, Rab9a) and a clathrin-associated gene (Ap1s1, Fig. 4a and 4b). Most of these genes showed down-regulation or little changes post-SCI (Fig. 4a). Specifically, we found significant up-regulation of Ap1s1 in regenerating RGCs compared to surviving RGCs after injury (Fig. 4c). For genes associated with cytoskeleton and synapse, we found six genes that showed up-regulation in SNs and MNs after PNI but little change or down-regulation in RGCs and spinal cord after injury, including synapse-related genes (Erc2, Cbln2, Tprg1l), actin-binding (Ehbp1), actin filament polymerization (Fhod3), and microtubule (Mtus2, Fig. 4a). Two genes encoding β-tubulin, related to microtubule dynamics and growth cone, showed dramatic changes in SNs and MNs after PNI: Tubb6 and neuronal Tubb2b (Fig. 4a). Knockdown of Tubb6 could inhibit axon regrowth and knockout of neuronal specific Tubb3 (Tuj1) decreased growth cone microtubule dynamics and neurite outgrowth [28], [40]. We also found two up-regulated genes encoding microtubule plus end tracking proteins: Mapre1 (EB1) and Mtus2 (TIP150 [41], Fig. 4d). EB1 is a marker for growing microtubules and mutation of EB1 in nematodes displayed reduced mechanosensory PLM neurons regrowth [28], [42]. Of the actin cytoskeleton related genes, Sprr1a, an epithelial differentiation gene, had extremely low expression during development but was induced in damaged nerve and soma, including sensory and motor neurons and RGCs (Fig. 4a). Focal adhesions (FAs) are sub-cellular structures containing integrins and FA molecules, such as talin, vinculin, paxillin and focal adhesion kinase, and mediating cell-matrix adhesion. We found nine genes annotated with the term of “focal adhesion”, including integrins (Itga7, Itgb1), and FA molecules (Tln1, Tes, Trim15, Fig. 3b). Six out of nine genes have been reported with roles in axon regeneration, including Pdlim1, Flrt3, Itgb1, Itga7, Tln1 and the recently identified Map2k1 [43]. We found up-regulation of Pdlim1, a regulator of focal adhesion, in DRG and spinal cord upon PNI and/or SCI, but was low expressed in RGCs (Fig. 4a), suggesting a distinct regulation of focal adhesion in RGCs.
Several genes showed a rat-preference injury response with low expression or little change in mice post-PNI, including genes involved in neuronal iron homeostasis (Hamp [44], Rasd1 [45]), microglia activation (Reg3b), M2 macrophage polarization (Serpinb2, Htr2b [46]), matrix metalloproteinase (Mmp3), and metabolism (Hpd, Dhfr and Spr, Fig. 3d and Fig. 4a). Some of these genes, such as Hamp, Rasd1, Reg3b, and Mmp3, support neuronal survival [44], [45], [47], [48]. Additionally, Phldb3, a less-studied gene, was significantly up-regulated in rat injured SNs and MNs at 1d, 3d and 7d, generally consistent with qPCR and bulk RNA-seq result (Fig. 3d and 3f). Phldb3 could bind MDM2, facilitating MDM2-mediated ubiquitination and degradation of p53, promoting proliferation and reducing apoptosis [49]. Interestingly, Phldb3 was also up-regulated in two mouse strains (DBA/2 and CAST/Ei) with higher regenerative capacity than C57BL/6 at 5d post-PNI [50], despite low expression levels (<1, Fig. 3d), suggesting its importance in neural injury and repair.
Functional examination of Rnf122 in axon regrowth
Next, we selected a less-studied gene, Rnf122, in the conserved regeneration-associated module, for experimental validation of its expression and function in axon regrowth. Considering the major composition of sensory neurons in dorsal root ganglion and well-established in vitro assay for monitoring neurite growth in adult DRG neuron, we performed experiments on DRG neurons. qPCR result showed obviously increased expression of Rnf122 in DRG following axonal injury compared to the sham-operated group at the tissue-level (Fig. 5a). RNA in situ hybridization result showed that Rnf122 was predominantly expressed in DRG neurons (Fig. 5b). Following, we constructed siRNA inference to knock down Rnf122 expression and confirmed a significant reduction in Rnf122 level after siRNA transfection using qPCR (Student’s t-test; p < 0.05, Fig. 5c). By in vitro assay experiment, we showed that knocking down Rnf122 expression in cultured DRG neurons could significantly reduce axonal length (Student’s t-test; p < 0.01, Fig. 5d and 5e). Besides, we also employed a microfluidic device that separates axons from the soma (Fig. 5f). The result also showed a significant inhibition of axon regrowth in the Rnf122 siRNA group (Student’s t-test; p < 0.01, Fig. 5g and 5h). Taken together, these results confirmed a novel role of Rnf122 in axon regrowth in vitro.
Fig. 5.
Functional examination of Rnf122 in axon regrowth. a, qPCR validate of Rnf122 expression in rat DRG upon sciatic nerve crush (SNC). b, In situ hybridization of Rnf122 in rat DRG at 3d post-SNC. c, siRNA inference efficiency confirmed by qPCR. Representative immunofluorescence staining assessed axon regrowth in cultured DRG neurons on cell slides after Rnf122 knockdown (d) and longest neurite length statistics (e). Seven longest neurite length were measured in each condition. f, Schematic diagram of the microfluidic device. Representative immunofluorescence staining assessed axon regrowth in cultured DRG neurons on the microfluidic device after Rnf122 knockdown (g) and longest neurite length statistics (h). Soma and axons were labelled with anti-Tuj1 antibody. Approximately 43 ∼ 70 DRG neurons were measured in each condition. Data was shown as mean + s.e.m. *p < 0.05, **p < 0.01.
Expression divergence between sensory and motor neurons responding to the injury
We estimated the divergence of molecular response to axonal injury from SNs and MNs using Pearson’s correlation (r), Euclidean distance (ED), and coefficient of variation (CV) for 2,615 DEG pairs and ranked based on the ED values. Most DEGs in both MNs and/or SNs exhibited small variation (CV), high correlation (r) and small divergent distance (ED), such as Atf3 and Gap43 (Fig. 6a and 6b, Table S1). Focusing on the top 10 % of DEGs with large divergence (ED), these DEGs were significantly enriched in terms of “inflammatory response”, “positive regulation of cytokine production”, and “response to wounding” (Fig. 6c). Analysis of the expression level and patterns of these top-ranked genes revealed that most were injury-induced in neurons or expressed in non-neuronal cells (e.g., microglia/macrophage), indicating distinct expression change against injury between SNs and MNs, especially in MNs (Table S1). We next focused on 23 DEGs with large divergence, including 8 in MNs and 15 in SNs (Fig. 6d). Most of these DEGs with dramatic changes in SNs were also supported by bulk RNA-seq datasets of rat and/or mouse DRG upon PNI (Fig. 6d). We chose four genes (Flrt3, Stmn4, Tph2 and Tnik) for qPCR validation of expression change in the tissues of rat DRG and ventral spinal cord. The result showed a larger change of these four genes in DRG than in spinal cord upon axonal injury compared to the sham-operated group, which was generally consistent with the sequencing data, though that were validated at tissue-level (Fig. 6g). The result of Flrt3 is also consistent with the previous report [51].
Fig. 6.
Divergence of expression patterns upon axonal injury estimated between SNs and MNs. a, Coefficient of variation (CV) of 2,615 DEG pairs in SNs and MNs, Pearson’s correlation (circle color) and Euclidean distance (ED, circle size) between SNs and MNs. b, Density distribution of ED. Red shaded area highlighted the top 10 % DEGs with large ED. c, Significant enriched functional terms of the top 10 % DEGs with large ED. d, Expression of divergent candidates in MNs, SNs and other datasets. e, Functional class of some divergent candidates from panel d. f, Expression of Ngfr in mouse developing spinal motoneurons and DRG neurons and rat spinal cord tissue, and expression in SNs and MNs. g. qPCR validation of four divergent candidates in rat spinal cord and DRG. h, Expression of Tll1 in SNs and MNs. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Among these divergent candidates, five genes (Tph2, Csrp3, Calb1 and Crisp3) showed both species- and tissue-preference, four genes showed tissue-preference (Mcub, Trhr, Syne4 and Kcns1), and three genes (Flrt3, Stmn4 and Tnik) showed dramatic upregulation in DRG neurons upon axonal injury. Three genes, Mcub, Scn9a and Kcns1, were involved in ion transport (Fig. 6e). Mcub, which regulates mitochondrial Ca2+ uniporter (MCU) to avoid Ca2+ overload, was induced in adult mouse heart and exerts cardiac protection after ischemia–reperfusion injury [52]. We found a low expression (<1) of Mcub under normal condition and were induced with a dramatic up-regulation in both spinal cord (including MNs) and RGCs after nerve injury (Fig. 6d). Conversely, Mcub had higher expression in mouse and rat DRG under normal conditions, but showed little change after injury (Fig. 6d), indicating tissue-preference of Mcub in both homeostasis and injury condition. Mcub could be expressed in a wide range of cell types, including neurons and immune cells (e.g., microglia and macrophage). Mcub could also promote a phenotypic switch from pro-inflammatory to anti-inflammatory in macrophage, supporting satellite cell differentiation critical for skeletal muscle regeneration after injury [53]. We also found another gene, Ngfr, a low-affinity nerve growth receptor for Ngf and other neurotrophins (e.g., Bdnf), presenting a similar expression signature as Mcub (Fig. 6d and 6f). Expression of Ngfr was gradually decreased during development and extremely low expressed (<1) in adult in both bulk tissue of spinal cord and spinal motoneurons, while highly expressed in DRG neurons and little change during development even in adult (Fig. 6f). Ngfr was also low expressed in RGCs under normal condition and little change upon injury (Fig. 6d). Expression signature of Ngfr in hypoglossal motoneurons in development and nerve injury had been well investigated and confirmed [54]. Tll1, a member of the peptidase M12 family, processes procollagen C-propeptides and is involved in extracellular matrix organization. Tll1 could be expressed in Schwann cells and regulates the correct spatial and temporal assembly of Ranvier nodes [55]. We found low and rare expression of Tll1 in mouse DRG neurons according to scRNA-seq and snRNA-seq, but it showed expression in bulk RNA-seq and SNs (Fig. S9), while high expression in neuron (including motoneurons) from mouse lumbar spinal cord [56]. This suggested a distinct major cell-types of Tll1 in DRG (Schwann cell) and spinal cord (neuron). Additionally, Tll1 showed an opposite expression pattern in between MNs and SNs (r = -0.89) with increased expression in MNs while down-regulation or little change in both rat and mouse DRG post-injury (Fig. 6a, 6d and 6h). Tll1 was low expression and little change in bulk or scRNA-seq of rat spinal cord post-SCI. We also observed expression of Tll1 in RGCs but was decreased upon injury (Fig. 6d).
Three genes related with cytoskeleton, Stmn4, Csrp3 and Flnc, showed dramatic change in SNs and high expression in injured mouse DRG neurons and nuclei, except for Csrp3 which was expressed in rat SNs (Fig. 6d). Csrp3 was initially regarded as a muscle-specific protein and was later confirmed to be induced in rat DRG neurons and RGCs, not in mice upon axotomy, and its expression in axotomized RGCs correlated with their ability to regenerate injured axons [57]. However, Csrp3 showed relatively low expression (<1) in rat MNs and spinal cord upon PNI and SCI. These evidences suggested that induction of Csrp3 in nerve injury presented not only species-preference but also neuronal types preference. Stmn4, Flrt3 and Tnik showed similar expression signature with a large change in DRG and RGCs but showed a little change in MNs upon injury. Flrt3 has been investigated with roles in promoting neurite growth and neuropathic pain in sensory neurons upon injury [51], [58]. Tnik, a member of the Ste20 family kinase, along with Map4k4 and Mink1, regulate dule leucine zipper kinase (DLK) initiation which is required for stress-induced JNK signaling in neurons [59]. Additionally, two genes, Slc7a6os and Tph2, showed relatively large changes in rat SNs or opposite expression patterns between rat MNs and SNs (Fig. 6d). Slc7a6os, a homolog with Saccharomyces cerevisiae polypeptide Iwr1, may participate in RNA pol II nuclear localization and play a critical role in CNS development by defining areas in zebrafish [60]. Tph2, the rate-limiting enzyme for synthesis of neuronal 5-HT and a marker for serotonergic neurons, showed a significant up-regulation in rat SNs but down-regulation or little change in rat MNs and rat spinal cord respectively, while extremely low expression in mouse DRG (Fig. 6d and 6g). Tph2 was also low expressed in rat spinal cord tissue and down-regulation after SCI (Fig. 6d).
Discussion
In this work, we presented a comprehensive analysis of the molecular response of sensory neurons and motoneurons to axonal injury, revealing both convergent and divergent gene expression patterns and identifying a conserved regeneration-associated module. By integration analysis of expression profiles from related datasets, including sorted regenerating retinal ganglion cells and spinal cord after central nerve injury, we highlighted several novel regeneration-associated candidates (Rnf122, Ap1s1, Pvr, Tceal9 and Il13ra1) within the module. Using in vitro experiments, we confirmed a novel role of Rnf122 in regulating axon regrowth. The major points were summarized in Fig. 7.
Fig. 7.
Summary of convergent and divergent transcriptional signatures of MNs and SNs responding to sciatic nerve crush (SNC). The soma of lumbar DRG sensory neuron resides in the PNS while the soma of lumbar spinal motoneuron is located in the CNS, and their fibers converge and act as a component of the sciatic nerve. Under physiological conditions, DRG neuron somas are enveloped by satellite glial cells. Following SNC, injury signals (e.g., increase of intracellular calcium ion concentration) propagate retrogradely to the somas of both sensory and motor neurons, triggering cell body responses in the injured neurons and altering the extrinsic environment (e.g., immune cell infiltration, resident microglia proliferation and migration). Shared genes within conserved regeneration-associated modules were involved in intrinsic growth capability (e.g., gene transcription, cytoskeleton) and mediating cross-talk between injured neurons and their environment (e.g., immune cells). The immune microenvironment contributed to the largest divergent expression patterns between SNs and MNs. Additionally, some intrinsic factors, such as genes exhibiting cell type specificity or varying degrees of injury response, also drive divergence in gene expression changes between SNs and MNs.
Our pioneer work of large-scale and high-coverage sequencing of in situ captured spinal motoneurons as well as DRG neurons and their minimal microenvironment after axonal injury in comparable timepoints, provided a critical and valuable resource for comparison and exploration of injury response between motoneurons and sensory neurons [6], [7]. More importantly, our captured neurons are large soma, especially for DRG neurons with large- and small-size sensitize differently to injury [3], which is able to reduce influence caused by different neuronal cell types. Atf3, an early injury-induced molecule in sensory neurons and spinal motoneurons, is required for injured neuron to transform into a regenerative state [3], [6]. Our analysis showed two clusters, three injury states, and significant overlap of differentially expressed genes compared to the naïve in both MNs and SNs after injury. Notably, samples from 1d post-injury in both MNs and SNs spanned two clusters and three injury states, indicating that 1d was a potential key timepoint for transitioning from a “pre-regeneration” state to a “regenerating” state. These suggested similar transcriptional reprogramming in response to axonal injury between sensory neurons and motoneurons. However, Atf3 expression was evident as early as 12 h in sensory neurons, while it appeared at 1d in motoneurons after injury at our investigated timepoints. Additionally, a large percentage of DRG neurons (47 %) were in state2 (“injury and regenerating”) at 1d, while only 6 % of motoneurons were. These findings suggested a delayed response in motoneurons during the first week after injury. Comparative studies of regeneration across different neuronal subtypes have been recently conducted using reporter transgenic mice. These studies showed greater axonal regeneration in motoneurons than proprioceptors (large-diameter sensory neurons) at 7d after injury [61], [62]. However, our results for the first time indicated that somas of sensory neurons initiated molecular response to injury earlier than motoneurons.
Sensory neurons are enveloped by satellite glial cells under the physiological condition. Both injured DRG neurons and motoneurons were surrounded by microglia/macrophages, but injured motoneurons showed massive proliferation and migration around the soma [6], [63]. Consistent with this phenomenon, we observed a dramatic change in the genes expressed in microglia/macrophages in both “intact” MNs and “injured” MNs, while there was little change in “intact” SNs and moderate change in “injured” SNs. Additionally, we detected a large module (931 genes, turquoise module) with genes highly or specially expressed in microglia/macrophage in the spinal cord, while a small module (83 genes, yellow module) was observed in SNs. These findings reflected a large difference in the immune microenvironment around injured motor and sensory neurons.
Understanding the distinct molecular responses of CNS and PNS neurons to injury is essential for developing effective CNS regeneration strategies [64]. Identifying divergent intrinsic molecules, including neuronal cell-types preference (e.g., Kcns1, Trhr), distinct expression baseline in the adult (Mcub, Ngfr), expression in adult or injury-induced (Flrt3, Stmn4, Tnik, Tph2; Csrp3), and distinct cell-types (Tll1), may provide promising targets for CNS regeneration. For example, Mcub, which exhibits mitochondrial protection by inhibiting Ca2+ overload caused by injury, showed low expression in both MNs and RGCs, but higher expression in DRG neurons under the normal condition. However, only MNs showed dramatic up-regulation upon axonal injury, while RGCs showed low expression. Given its role in cellular protection in cardiomyocyte against ischemic injury [52], it is worth investigation whether overexpression of Mcub could promote CNS regeneration, such as in RGCs. Another example is Tll1, an extracellular matrix remodeling metalloprotease, which showed significant up-regulation in MNs but down-regulation or low expression in DRG neurons, RGCs and spinal cord tissue upon injury. BMP1-like proteinases, including Tll1, are beneficial for skin wound healing and regulates zebrafish cardiac morphogenesis [65], [66]. It remains to be experimentally investigated whether the significant upregulation of Tll1 in MNs after axonal injury could benefit axon regeneration or be applied in CNS regeneration. We also observed genes that showed distinct developmental and injury response transcriptional signatures, such as Ngfr, reflecting intrinsic differences between CNS and PNS neurons.
Although we identified some divergent candidates and noted a significant difference in the immune microenvironment, the transcriptional programming of injured motoneurons and sensory neurons is similar. This includes the activation of regeneration-associated transcription factors related to stress response (e.g., Atf3) and development (e.g., Sox11), induction of genes involved in regulating extracellular matrix (Cd44, Mmp16) and immune cells (Il6, Reg3b) as well as other known regeneration-associated genes (e.g., Sprr1a) within our identified conserved regeneration-associated module. Pathological protein aggregation, oxidative stress, and inflammation are several hallmarks of neurodegenerative diseases, including spinal cord injury and Alzheimer diseases [67]. Genes associated with autophagy, antioxidant, and anti-inflammation (e.g., Il33) were also identified in the module, which may provide molecular targets for developing small molecules in the treatment of nerve injury and neurodegenerative diseases [68], [69], [70]. Recently, the activation of innate immune response in injured neurons to promote axon regeneration, such as IL17 receptor A signaling, has also been reported [28], [71]. Of the identified conserved candidates, Pvr and Il13ra1 have been reported to promote regeneration of other tissues by inhibiting NK cells overactivation and through cross-talk with group 2 innate lymphoid cells in liver and gut epithelium, respectively [31], [72], but their role in nerve injury remains unexplored. Accumulation of cytotoxic NK cells has been detected in post-mortem amyotrophic lateral sclerosis (ALS) motor cortex and spinal cord, contributing to motor neuron degeneration [73]. Co-culture of DRG neurons with activated NK cells significantly reduces neurite growth and increases the fragmentation of neurites in DRG neurons [74]. Additionally, we observed a significant up-regulation of NK cells marker in both injured sensory neurons and motoneurons, indicating the presence of activated NK cells. Therefore, we hypothesized that the upregulation of Pvr expression in injured neurons may protect the soma from neurotoxic NK cells, thereby promoting axon regeneration. Furthermore, integrating with neuronal development and other related injury datasets, highlighted some less-studied genes, including Tceal9, Ap1s1, and Rnf122. The significant up-regulation of Tceal9 and Ap1s1 in regenerating RGCs compared to surviving RGCs, suggested a potential role in regeneration. Rnf122, an E3 ubiquitin ligase, has been shown to be expressed in various immune cells, with preferential expression in macrophages and is implicated in regulating antiviral innate immunity [75]. However, the expression and role of Rnf122 in neural tissues has not been described yet. We found significant up-regulation of Rnf122 in both injured sensory neurons and motoneurons, where it co-expressed with key regeneration-associated regulators, Atf3 and Sox11. Our in vitro experiments showed significant inhibition of axon regrowth when treated with siRNAs targeting Rnf122, indicating its novel role in regulation axon regrowth. However, in vivo experiments and the molecular mechanism of Rnf122 in regulating axon regeneration require further investigation.
Our study has several notable limitations. First, a limited sample size of RNA-Seq datasets was analyzed. Second, we only focused on several specific neuronal types, sensory neurons, spinal motoneurons and RGCs, after injury. Genome-wide similar and divergent molecular response against injury in cellular heterogeneity among these categories, especially in sensory neurons which include up to 9 subtypes [3], should be explored in the future. Third, our analyzed LCM-Seq datasets of sensory neurons and motoneurons were not at single-cell resolution, which included individual neuronal somas and their minimal microenvironment. Due to limited single-cell level datasets of rat DRG and/or spinal cord after peripheral nerve injury, our major results were inferred from single-cell level datasets of mouse DRG after injury, which may cause some bias. Furthermore, differences in gene expression response to injury between mice and rats were observed, which may provide additional insights or raise questions about the applicability of the findings to humans. Although we have identified a series of regeneration-associated candidates, here we have only confirmed a novel role of Rnf122 in axon regrowth. Our next steps will involve experimental exploration of additional candidates in nerve injury and regeneration. Last, our analyzed spinal motoneurons are lower motor neurons. Although the somas of spinal motoneurons are locate in the CNS, their long axons extend into the PNS to connect with peripheral muscles, allowing them to control muscle contraction and movement. As an important component of the PNS, spinal motoneurons were also classified as peripheral neurons. Therefore, we also analyzed the expression profiling of other CNS neurons, RGCs, and spinal cord after CNS injury to aid in the interpretation. Upper motoneurons are primarily located in the motor cortex of the brain, and their axons extend down to synapse with spinal motoneurons. Future comparative studies on the molecular response across sensory neurons, lower motoneurons and upper motoneurons after injury may enhance our understanding of the molecular similarities and differences in regeneration between CNS neurons and PNS neurons.
Conclusions
In summary, this study elucidates the molecular similarity and difference between sensory neurons and motoneurons responding to axonal injury, highlighting some novel regeneration-associated candidates. Future research should focus on validating these findings across different injury models and exploring the therapeutic potential of targeting these candidates to promote CNS regeneration. Understanding these mechanisms will be pivotal in developing new strategies for neural repair and functional recovery following nerve injury.
Data availability statement
The RNA-Seq datasets used in this study are freely available from the public databases and accessions are described in the Method Section. The other data that support the findings of this study are available from the corresponding author upon request.
Compliance with ethics requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
All animal procedures were performed in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committees of Nantong University (Approval no. P20230313-016).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 32130060, 32171352), the Natural Science Foundation of Jiangsu Province (BK20202013), the Jiangsu Provincial Key Medical Center and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the ‘Qing-Lan Project’ of Colleges in Jiangsu Province (2024) and the Shuangchuang Doctor program of Jiangsu Province (Grant No. JSSCBS20211127).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2024.07.008.
Contributor Information
Jian Yang, Email: dna2009@ntu.edu.cn.
Xiaosong Gu, Email: nervegu@ntu.edu.cn.
Lian Xu, Email: xulian@ntu.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Palmisano I., Danzi M.C., Hutson T.H., Zhou L., McLachlan E., Serger E., et al. Epigenomic signatures underpin the axonal regenerative ability of dorsal root ganglia sensory neurons. Nat Neurosci. 2019;22:1913–1924. doi: 10.1038/s41593-019-0490-4. [DOI] [PubMed] [Google Scholar]
- 2.Avraham O., Feng R., Ewan E.E., Rustenhoven J., Zhao G., Cavalli V. Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair. Elife. 2021;10 doi: 10.7554/eLife.68457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renthal W., Tochitsky I., Yang L., Cheng Y.C., Li E., Kawaguchi R., et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron. 2020;108(128–144):e129. doi: 10.1016/j.neuron.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheah M., Fawcett J.W., Haenzi B. Differential regenerative ability of sensory and motor neurons. Neurosci Lett. 2017;652:35–40. doi: 10.1016/j.neulet.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Rishal I., Fainzilber M. Axon-soma communication in neuronal injury. Nat Rev Neurosci. 2014;15:32–42. doi: 10.1038/nrn3609. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Xu L, Li X, Chen Z, Chen J, Zhang T, Gu X, Yang J: Deciphering the dynamic niches and regeneration-associated transcriptional program of motoneurons following peripheral nerve injury. iScience 2022, 25:104917. [DOI] [PMC free article] [PubMed]
- 7.Zhao L.-L., Zhang T., Huang W.-X., Guo T.-T., Gu X.-S. Transcriptional regulatory network during axonal regeneration of dorsal root ganglion neurons: laser-capture microdissection and deep sequencing. Neural Regen Res. 2023;18:2056–2066. doi: 10.4103/1673-5374.366494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L., Chen Z., Li X., Xu H., Zhang Y., Yang W., et al. Integrated analyses reveal evolutionarily conserved and specific injury response genes in dorsal root ganglion. Sci Data. 2022;9:666. doi: 10.1038/s41597-022-01783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu G., Huang K., Hu Y., Du G., Xue Z., Zhu X., et al. Single-cell RNA-seq reveals distinct injury responses in different types of DRG sensory neurons. Sci Rep. 2016;6:31851. doi: 10.1038/srep31851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renthal W., Tochitsky I., Yang L., Cheng Y.C., Li E., Kawaguchi R., et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron. 2020;108:128–144.e129. doi: 10.1016/j.neuron.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu B., Yao C., Wang Y., Mao S., Wang Y., Wu R., et al. The landscape of gene expression and molecular regulation following spinal cord hemisection in rats. Front Mol Neurosci. 2019;12:287. doi: 10.3389/fnmol.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian F., Cheng Y., Zhou S., Wang Q., Monavarfeshani A., Gao K., et al. Core transcription programs controlling injury-induced neurodegeneration of retinal ganglion cells. Neuron. 2022;110(2607–2624):e2608. doi: 10.1016/j.neuron.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel T., Hammelman J., Aziz S., Jang S., Closser M., Michaels T.L., et al. Transcriptional dynamics of murine motor neuron maturation in vivo and in vitro. Nat Commun. 2022;13:5427. doi: 10.1038/s41467-022-33022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tedeschi A., Dupraz S., Laskowski C.J., Xue J., Ulas T., Beyer M., et al. The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron. 2016;92:419–434. doi: 10.1016/j.neuron.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Yang J., Zhao L., Yi S., Ding F., Yang Y., Liu Y., et al. Developmental temporal patterns and molecular network features in the transcriptome of rat spinal cord. Engineering. 2021;7:1592–1602. [Google Scholar]
- 16.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., 3rd, Zheng S., Butler A., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(3573–3587):e3529. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aibar S., Gonzalez-Blas C.B., Moerman T., Huynh-Thu V.A., Imrichova H., Hulselmans G., et al. SCENIC: single-cell regulatory network inference and clustering. Nat Methods. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He W., Yang J., Jing Y., Xu L., Yu K., Fang X. NGenomeSyn: an easy-to-use and flexible tool for publication-ready visualization of syntenic relationships across multiple genomes. Bioinformatics. 2023;39 doi: 10.1093/bioinformatics/btad121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb) 2021;2 doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szklarczyk D., Gable A.L., Nastou K.C., Lyon D., Kirsch R., Pyysalo S., et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastian M, Heymann S, Jacomy M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proceedings of the International AAAI Conference on Web and Social Media 2009.
- 24.Gu Z., Eils R., Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 25.Yao C., Cao Y., Wang D., Lv Y., Liu Y., Gu X., et al. Single-cell sequencing reveals microglia induced angiogenesis by specific subsets of endothelial cells following spinal cord injury. FASEB J. 2022;36:e22393. doi: 10.1096/fj.202200337R. [DOI] [PubMed] [Google Scholar]
- 26.Li L., Fang F., Feng X., Zhuang P., Huang H., Liu P., et al. Single-cell transcriptome analysis of regenerating RGCs reveals potent glaucoma neural repair genes. Neuron. 2022;110(2646–2663):e2646. doi: 10.1016/j.neuron.2022.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Yang C., Wang X., Miao J., Chen W., Zhou Y., et al. Driving axon regeneration by orchestrating neuronal and non-neuronal innate immune responses via the IFNgamma-cGAS-STING axis. Neuron. 2022 doi: 10.1016/j.neuron.2022.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandran V., Coppola G., Nawabi H., Omura T., Versano R., Huebner E.A., et al. A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron. 2016;89:956–970. doi: 10.1016/j.neuron.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vicuna L., Strochlic D.E., Latremoliere A., Bali K.K., Simonetti M., Husainie D., et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med. 2015;21:518–523. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bi J., Zheng X., Chen Y., Wei H., Sun R., Tian Z. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology. 2014;60:1389–1398. doi: 10.1002/hep.27245. [DOI] [PubMed] [Google Scholar]
- 32.Kraft V.A.N., Bezjian C.T., Pfeiffer S., Ringelstetter L., Muller C., Zandkarimi F., et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q.S., Jia Y.J. Ferroptosis: a critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen Res. 2023;18:506–512. doi: 10.4103/1673-5374.350187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martiskainen H., Paldanius K.M.A., Natunen T., Takalo M., Marttinen M., Leskela S., et al. DHCR24 exerts neuroprotection upon inflammation-induced neuronal death. J Neuroinflammation. 2017;14:215. doi: 10.1186/s12974-017-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J.Y., Qin X., Liang A., Kim E., Lawrence P., Park W.J., et al. Fads3 modulates docosahexaenoic acid in liver and brain. Prostaglandins Leukot Essent Fat Acids. 2017;123:25–32. doi: 10.1016/j.plefa.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun E., Zhang J., Deng Y., Wang J., Wu Q., Chen W., et al. Docosahexaenoic acid alleviates brain damage by promoting mitophagy in mice with ischaemic stroke. Oxid Med Cell Longev. 2022;2022:3119649. doi: 10.1155/2022/3119649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip P.K., Bowes A.L., Hall J.C.E., Burguillos M.A., Ip T.H.R., Baskerville T., et al. Docosahexaenoic acid reduces microglia phagocytic activity via miR-124 and induces neuroprotection in rodent models of spinal cord contusion injury. Hum Mol Genet. 2019;28:2427–2448. doi: 10.1093/hmg/ddz073. [DOI] [PubMed] [Google Scholar]
- 38.Mews P., Donahue G., Drake A.M., Luczak V., Abel T., Berger S.L. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature. 2017;546:381–386. doi: 10.1038/nature22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mar F.M., Bonni A., Sousa M.M. Cell intrinsic control of axon regeneration. EMBO Rep. 2014;15:254–263. doi: 10.1002/embr.201337723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latremoliere A., Cheng L., DeLisle M., Wu C., Chew S., Hutchinson E.B., et al. Neuronal-specific TUBB3 is not required for normal neuronal function but is essential for timely axon regeneration. Cell Rep. 2018;24(1865–1879):e1869. doi: 10.1016/j.celrep.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams G., Jr., Zhou J., Wang W., Wu H., Quan J., Liu Y., et al. The Microtubule plus end tracking protein TIP150 interacts with cortactin to steer directional cell migration. J Biol Chem. 2016;291:20692–20706. doi: 10.1074/jbc.M116.732719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L., Wang Z., Ghosh-Roy A., Hubert T., Yan D., O'Rourke S., et al. Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron. 2011;71:1043–1057. doi: 10.1016/j.neuron.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boato F., Guan X., Zhu Y., Ryu Y., Voutounou M., Rynne C., et al. Activation of MAP2K signaling by genetic engineering or HF-rTMS promotes corticospinal axon sprouting and functional regeneration. Sci Transl Med. 2023;15(eabq6885) doi: 10.1126/scitranslmed.abq6885. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y.F., Zhang C., Yang G., Qian Z.M., Zhang M.W., Ma J., et al. Hepcidin protects neuron from hemin-mediated injury by reducing Iron. Front Physiol. 2017;8:332. doi: 10.3389/fphys.2017.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheah J.H., Kim S.F., Hester L.D., Clancy K.W., Patterson S.E., 3rd, Papadopoulos V., et al. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de las Casas-Engel M, Dominguez-Soto A, Sierra-Filardi E, Bragado R, Nieto C, Puig-Kroger A, Samaniego R, Loza M, Corcuera MT, Gomez-Aguado F, et al: Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol 2013, 190:2301-2310. [DOI] [PubMed]
- 47.Yong V.W. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 48.Tam J., Rosenberg L., Maysinger D. Islet-neogenesis-associated protein enhances neurite outgrowth from DRG neurons. Biochem Biophys Res Commun. 2002;291:649–654. doi: 10.1006/bbrc.2002.6497. [DOI] [PubMed] [Google Scholar]
- 49.Chao T., Zhou X., Cao B., Liao P., Liu H., Chen Y., et al. Pleckstrin homology domain-containing protein PHLDB3 supports cancer growth via a negative feedback loop involving p53. Nat Commun. 2016;7:13755. doi: 10.1038/ncomms13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omura T., Omura K., Tedeschi A., Riva P., Painter M.W., Rojas L., et al. Robust axonal regeneration occurs in the injured CAST/Ei mouse CNS. Neuron. 2015;86:1215–1227. doi: 10.1016/j.neuron.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada M., Fujita Y., Hayano Y., Hayakawa H., Baba K., Mochizuki H., et al. Increased expression of fibronectin leucine-rich transmembrane protein 3 in the dorsal root ganglion induces neuropathic pain in rats. J Neurosci. 2019;39:7615–7627. doi: 10.1523/JNEUROSCI.0295-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huo J., Lu S., Kwong J.Q., Bround M.J., Grimes K.M., Sargent M.A., et al. MCUb induction protects the heart from postischemic remodeling. Circ Res. 2020;127:379–390. doi: 10.1161/CIRCRESAHA.119.316369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feno S., Munari F., Reane D.V., Gissi R., Hoang D.H., Castegna A., et al. The dominant-negative mitochondrial calcium uniporter subunit MCUb drives macrophage polarization during skeletal muscle regeneration. Sci Signal. 2021;14(eabf3838) doi: 10.1126/scisignal.abf3838. [DOI] [PubMed] [Google Scholar]
- 54.Wood S.J., Pritchard J., Sofroniew M.V. Re-expression of nerve growth factor receptor after axonal injury recapitulates a developmental event in motor neurons: differential regulation when regeneration is allowed or prevented. Eur J Neurosci. 1990;2:650–657. doi: 10.1111/j.1460-9568.1990.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 55.Eshed-Eisenbach Y., Devaux J., Vainshtein A., Golani O., Lee S.J., Feinberg K., et al. Precise spatiotemporal control of nodal Na(+) channel clustering by bone morphogenetic protein-1/tolloid-like proteinases. Neuron. 2020;106(806–815):e806. doi: 10.1016/j.neuron.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Matson K.J.E., Russ D.E., Kathe C., Hua I., Maric D., Ding Y., et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat Commun. 2022;13:5628. doi: 10.1038/s41467-022-33184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin E., Leibinger M., Gobrecht P., Hilla A., Andreadaki A., Fischer D. Muscle LIM protein is expressed in the injured adult CNS and promotes axon regeneration. Cell Rep. 2019;26(1021–1032):e1026. doi: 10.1016/j.celrep.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 58.Robinson M., Parsons Perez M.C., Tebar L., Palmer J., Patel A., Marks D., et al. FLRT3 is expressed in sensory neurons after peripheral nerve injury and regulates neurite outgrowth. Mol Cell Neurosci. 2004;27:202–214. doi: 10.1016/j.mcn.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Larhammar M., Huntwork-Rodriguez S., Rudhard Y., Sengupta-Ghosh A., Lewcock J.W. The Ste20 family kinases MAP4K4, MINK1, and TNIK converge to regulate stress-induced JNK signaling in neurons. J Neurosci. 2017;37:11074–11084. doi: 10.1523/JNEUROSCI.0905-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benini A., Cignarella F., Calvarini L., Mantovanelli S., Giacopuzzi E., Zizioli D., et al. slc7a6os gene plays a critical role in defined areas of the developing CNS in zebrafish. PLoS One. 2015;10:e0119696. doi: 10.1371/journal.pone.0119696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolívar S., Sanz E., Ovelleiro D., Zochodne D.W., Udina E. Neuron-specific RNA-sequencing reveals different responses in peripheral neurons after nerve injury. Elife. 2024;12 doi: 10.7554/eLife.91316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolívar S., Udina E. Preferential regeneration and collateral dynamics of motor and sensory neurons after nerve injury in mice. Exp Neurol. 2022;358 doi: 10.1016/j.expneurol.2022.114227. [DOI] [PubMed] [Google Scholar]
- 63.Jager S.E., Pallesen L.T., Richner M., Harley P., Hore Z., McMahon S., et al. Changes in the transcriptional fingerprint of satellite glial cells following peripheral nerve injury. Glia. 2020;68:1375–1395. doi: 10.1002/glia.23785. [DOI] [PubMed] [Google Scholar]
- 64.Varadarajan S.G., Hunyara J.L., Hamilton N.R., Kolodkin A.L., Huberman A.D. Central nervous system regeneration. Cell. 2022;185:77–94. doi: 10.1016/j.cell.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muir A.M., Massoudi D., Nguyen N., Keene D.R., Lee S.J., Birk D.E., et al. BMP1-like proteinases are essential to the structure and wound healing of skin. Matrix Biol. 2016;56:114–131. doi: 10.1016/j.matbio.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlantoni C., Allanki S., Kontarakis Z., Rossi A., Piesker J., Gunther S., et al. Tie1 regulates zebrafish cardiac morphogenesis through Tolloid-like 1 expression. Dev Biol. 2021;469:54–67. doi: 10.1016/j.ydbio.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 67.Wilson D.M., 3rd, Cookson M.R., Van Den Bosch L., Zetterberg H., Holtzman D.M., Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186:693–714. doi: 10.1016/j.cell.2022.12.032. [DOI] [PubMed] [Google Scholar]
- 68.Uddin M.S., Stachowiak A., Mamun A.A., Tzvetkov N.T., Takeda S., Atanasov A.G., et al. Autophagy and Alzheimer's disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci. 2018;10:04. doi: 10.3389/fnagi.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grewal A.K., Singh T.G., Sharma D., Sharma V., Singh M., Rahman M.H., et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111729. [DOI] [PubMed] [Google Scholar]
- 70.Kabir M.T., Rahman M.H., Shah M., Jamiruddin M.R., Basak D., Al-Harrasi A., et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112610. [DOI] [PubMed] [Google Scholar]
- 71.Enamorado M., Kulalert W., Han S.J., Rao I., Delaleu J., Link V.M., et al. Immunity to the microbiota promotes sensory neuron regeneration. Cell. 2023 doi: 10.1016/j.cell.2022.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu P., Zhu X., Wu J., He L., Lu T., Wang Y., et al. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol. 2019;20:183–194. doi: 10.1038/s41590-018-0297-6. [DOI] [PubMed] [Google Scholar]
- 73.Garofalo S., Cocozza G., Porzia A., Inghilleri M., Raspa M., Scavizzi F., et al. Natural killer cells modulate motor neuron-immune cell cross talk in models of amyotrophic lateral sclerosis. Nat Commun. 2020;11:1773. doi: 10.1038/s41467-020-15644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davies A.J., Kim H.W., Gonzalez-Cano R., Choi J., Back S.K., Roh S.E., et al. Natural killer cells degenerate intact sensory afferents following nerve injury. Cell. 2019;176(716–728):e718. doi: 10.1016/j.cell.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W., Jiang M., Liu S., Zhang S., Liu W., Ma Y., et al. RNF122 suppresses antiviral type I interferon production by targeting RIG-I CARDs to mediate RIG-I degradation. PNAS. 2016;113:9581–9586. doi: 10.1073/pnas.1604277113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-Seq datasets used in this study are freely available from the public databases and accessions are described in the Method Section. The other data that support the findings of this study are available from the corresponding author upon request.