The range in serum estradiol concentration in transdermal estradiol users in the real world is wide, and there is substantial interindividual variation among women using the same dose. Up to one in four women have subtherapeutic estradiol levels despite using the highest licensed dose and may benefit from an off-label dose for therapeutic effect.
Key Words: Dose customization, Hormone therapy, Interindividual variation, Menopause, Personalized care, Transdermal estradiol
Abstract
Objectives
The aims of the study are to explore the range and variation in serum estradiol concentration, and to estimate the prevalence of “poor absorption” (women using licensed estradiol doses with subtherapeutic levels), in perimenopausal and postmenopausal women using transdermal estradiol in the real world.
Methods
This is a cross-sectional analysis in a specialist menopause clinic in the UK.
Results
Serum samples were obtained from 1,508 perimenopausal and postmenopausal women. A total of 61.87% were using licensed doses. The median estradiol concentration was 355.26 pmol/L (interquartile range 198.44-646.15 pmol/L). A reference interval for the whole cohort was defined as 54.62-2,050.55 pmol/L. There was substantial interindividual variation across the dose range. Variance was greater in younger women (P = 0.002) and gel users (P = 0.002). There was a trend toward greater variance in women using higher doses, but the association failed to reach statistical significance (P = 0.074). One in four women (24.84%) using the highest licensed dose had subtherapeutic levels (<200 pmol/L). Older women (≥50 y) and patch users were more likely to have low levels (odds ratio 1.77, 95% confidence interval 1.22-2.62, P = 0.003; and odds ratio 1.51, 95% confidence interval 1.18-1.95, P = 0.001, respectively).
Conclusions
The reference interval for perimenopausal and postmenopausal women using on-label and off-label doses of transdermal estradiol in the real world is wide, and there is considerable interindividual variation. The number of estradiol users with low estradiol levels (<200 pmol/L) is higher than previously recognized. Measurement of serum estradiol can be helpful to identify women who may benefit from an off-label dose. Dose customization is key to ensure that all women can reap the benefits of HT.
Around 15% of women aged 45 to 64 in England are currently prescribed hormone therapy (HT) to alleviate menopausal symptoms.1 For most women, transdermal 17β-estradiol with or without micronized progesterone is the gold standard HT regimen with few if any proven risks.2,3
The optimal plasma estradiol concentration for relief of menopausal symptoms and prevention of bone loss is 220-550 pmol/L (60-150 pg/mL).4 Levels of 220 pmol/L (60 pg/mL) relieve hot flashes in 50% of women and prevent bone resorption; 100% elimination of hot flashes and bone accretion occurs when levels approximate 400 pmol/L (100 pg/mL).5,6
Early studies reported a linear, positive correlation between transdermal estradiol dose and mean plasma estradiol concentration, with attainment of therapeutic levels using patches delivering up to 100 mcg/d, or up to four pumps of estradiol gel daily (3 mg/d).7,8 However, studies that reported a predictable dose-response relationship were typically small, with sample sizes ranging from just 11 to 50 postmenopausal women.7-9 Mean estradiol levels can be misleading because there is substantial interindividual and intraindividual variation in estradiol pharmacokinetics, resulting in up to 10-fold differences in estradiol levels between women using the same dose patch or gel.6,9,10 Because there is no “one size fits all” dose that achieves therapeutic blood levels in all women, menopause guidelines recommend that the dose is individualized to ensure that all women can reap the short and long-term health benefits associated with hormone replacement.11-13
Limited data suggest that up to 20% of women are “poor absorbers” of transdermal estradiol.4,6 It is essential that ‘poor absorbers’ with subtherapeutic levels are prescribed higher doses to relieve symptoms and mitigate risks associated with chronic estrogen deficiency. For some women, therapeutic levels will only be achieved using off-label doses. However, a recent safety alert in the UK warned against prescribing estrogen “in doses higher than the upper limit listed in the individual Summary of Product Characteristics (SmPC) as these limits are informed by the results of clinical trials, to ensure patient safety.”14 In fact, early dose finding studies were focused on establishing pharmacokinetic parameters and/or clinical efficacy—they were not designed to assess long-term safety.5,9,15,16
There are no safety data in women using high (off-label) estradiol doses. However, as many women using high doses are “poor absorbers” with normal estradiol levels, it is highly unlikely that they will be at greater risk of harm than “good absorbers” who achieve normal levels using on-label doses. Indeed, failing to prescribe a dose sufficient to elevate serum estradiol levels into the therapeutic range is more likely to cause harm because women will continue to experience distressing symptoms, and they will not benefit from estrogen's bone-,17 cardio-,18 neuro-,19 and breast-20 protective effects.
A recent UK guideline concerning the management of unscheduled bleeding in women using HT has caused further confusion by recommending that women using high off-label estrogen doses should also receive a high off-label progestogen dose, to reduce the risk of endometrial cancer.21 However, routinely prescribing high progestogen doses may cause side effects that limit adherence and is not necessary if women are “poor absorbers” and not exposed to unopposed estrogen. Indeed, increased use of HT in recent years,22 including “increasing numbers of women using high estrogen doses,”14 has not been associated with an increased incidence of endometrial cancer.21
Failing to emphasize that there is a paucity of safety data to inform dose recommendations, failing to acknowledge that the “lowest effective dose” might be an off-label dose for many women, and deliberately discouraging clinicians from prescribing off-label doses by exaggerating the potential (theoretical) risks of “overtreatment” while understating the (evidence-based) harms of undertreatment, has resulted in considerable anxiety and confusion for general practitioners (GPs) and women using HT in the UK. Many GPs no longer prescribe off-label doses, some have refused to prescribe HT altogether, and many women are turning to private menopause specialists because they are unable to access the care they need in the National Health Service.
The aims of this study were three-fold: 1) to measure the range in serum estradiol concentration in a real-world clinic cohort of perimenopausal and postmenopausal women treated with transdermal estradiol; 2) to explore the association between serum estradiol concentration and estradiol dose, estradiol formulation, and age/menopause status; and 3) to estimate the number (%) of perimenopausal and postmenopausal women with subtherapeutic levels despite using the highest licensed dose, who may require an off-label dose for clinical effect (“poor absorbers”). It is hoped that this will lead to an improved understanding of transdermal estradiol pharmacokinetics and enable clinicians to deliver more personalized, high quality menopause care.
METHODS
Study design and population
This was a single-center, UK-based, cross-sectional study. The Newson Health Clinic, established in 2018, is the largest specialist menopause clinic in the UK. In 2023, there were on average 3,359 menopause consultations per month, including 1,142 new patient consultations. All patient data is recorded in a secure, web-based clinic management system (Semble Ltd, UK23). Consequently, Newson Health has access to a unique, large, electronic dataset that can be used for clinical research and audit purposes.
All women attending the Newson Health clinic between 1st May and 31st July 2023 were included if they had been using transdermal estradiol (patch, gel, or spray) for ≥3 months and had at least one serum estradiol level recorded in the study period.
Data collection
Characteristics of the study population (age, menopause status, estradiol dose, and formulation) and laboratory test results were extracted from the medical records.
Equivalent dose estimation
The British Menopause Society 'Women's Health Concern HT Types, Doses and Regimens' factsheet was used to calculate equivalent doses of different estradiol formulations (Supplemental Table 1, http://links.lww.com/MENO/B327).24 Estradiol dose was categorized using the “number of pumps equivalent” or “PE.” For example, women using four pumps of 0.06% gel daily (0.75 mg estradiol per actuation), or a 100-mcg patch twice weekly, were both included in the “four pumps equivalent” (4 PE) dose category.
Serum estradiol concentration
Serum estradiol levels are not routinely monitored in women prescribed HT. Blood tests were undertaken for the following reasons: to determine if estradiol levels were in the physiological range in women with a suboptimal clinical response; when there were menopausal symptoms that were difficult to distinguish from side effects such as bleeding or headaches; and/or at the patient's request for any reason.
Women consulted online or in-person. Blood tests were accessed via Nationwide Pathology, a private pathology provider with 250 phlebotomy clinics across the UK. Samples were either couriered or sent in tracked prepaid envelopes to Nationwide Pathology, Leicestershire, UK. Upon arrival, samples were processed immediately using the Atelica IM Enhanced Estradiol (eE2) assay as per the manufacturer's instructions.
The Atelica IM analyzer was calibrated twice daily using standard controls and underwent monthly external quality assurance. Detectable concentrations ranged from 40.95 to 10,410.00 pmol/L. The intra-assay coefficient of variation was 2.7% to 7% and the uncertainty of measurement was 5.58%.
Research ethics and consent
Informed consent was obtained from all participants for their data to be used for the purpose of research and audit. Ethical approval for the study was granted by the UCL Research Ethics Committee (UCL REC ID: 9093.008).
Statistical analysis
All analyses were performed in the statistical software package R version 4.4.1.25
Descriptive statistics were used to summarize patient demographics and outcome variables. Continuous variables were summarized using mean (SD), median (interquartile range [IQR]) and range, and categorical variables as frequency (%). Reference ranges for serum estradiol concentration were defined as the interval between the 2.5th and 97.5th percentile. Bootstrapping was used to calculate the 90% confidence intervals (CI) for the lower and upper limits.26 Levene's test was used to compare the variance in different subgroups (<50 y vs >50 y, perimenopausal vs postmenopausal, gel vs patch users).
A linear model was fitted to the log-transformed estradiol concentration (outcome variable) to test for an association with estradiol dose, estradiol formulation (gel and patch), and age (<50 and >50 y). Menopause status (perimenopausal vs postmenopausal) was not included as it was found to be associated with age (P ≤ 0.001) and including both may have caused issues with multicollinearity. Age was chosen over menopause status as it is less subjective and likely to be more accurate (women do not always know their menopause status, and notes are not always updated when women transition from perimenopause to postmenopause). Regarding formulation, women using estradiol spray, or a combination of patch/spray or spray/gel were excluded as the sample sizes were small (n = 1, n = 2, and n = 27, respectively). Estradiol dose was treated as a categorical variable to allow for nonlinearity, and because it was not possible to extrapolate beyond the measured dose range. One and seven PE were excluded from the model due to insufficient data points (n = 29 and 33 women, respectively), and > 8 PE was excluded from the model due to being a heterogeneous group. Interactions between dose and age, and dose and formulation, were considered but found to be nonsignificant and excluded from the final model. Women with estradiol concentrations at the lower (n = 22) or upper (n = 1) limit of detection were excluded.
Estimated mean percentage change in estradiol concentration and 95% CIs were reported for each estradiol dose relative to 2 PE, for older women (≥50 y) relative to younger women, and for patch users relative to gel users. Pairwise comparisons were calculated using the R package ggeffects27 and P values were adjusted for multiple comparisons using the false discovery rate.
Logistic regressions were used to model the probability of either a high or low serum estradiol concentration and to test for associations between high or low estradiol concentrations and estradiol dose, estradiol formulation (gel vs patch), and age (<50 vs >50 y). The variables included were the same as those included in the linear model, but dose was categorized as on-label vs off-label because the number of women with high and low levels in each dose category was small. The results are reported as odds ratios with 95% CI.
RESULTS
A total of 9,941 menopause consultations were conducted between 1st May and 31st July 2023. A total of 1,508 women (15.17%) were eligible for inclusion in the whole study cohort. Participant demographics are presented in Table 1; the study Consolidated Standards of Reporting Trials (CONSORT) diagram is provided in Supplemental Figure 1 (http://links.lww.com/MENO/B327). The mean age was 55.03 years (SD 5.93 y). The median age was 55 years (IQR 52-58 y). Twenty-two percent of clinic attendees were perimenopausal, 54% were postmenopausal, and 23% were unsure of their menopause status (eg, women using a Mirena coil or posthysterectomy may not have known their menopause status).
Table 1.
Participant demographics
| Variable | Category | N | % |
|---|---|---|---|
| Age | <50 y | 242 | 16.05 |
| ≥50 y | 1,266 | 83.95 | |
| Diagnosis | Perimenopause | 337 | 22.35 |
| Menopause | |||
| - Premature ovarian insufficient (POI)a | 16 | 1.06 | |
| - Early menopauseb | 12 | 0.80 | |
| - Menopause aged 45–55 y | 715 | 47.41 | |
| - Late menopause >55 y | 8 | 0.53 | |
| - Surgical menopause | 62 | 4.11 | |
| Unsure | 358 | 23.74 | |
| Estradiol Formulation | Gel | 696 | 46.15 |
| Patch | 666 | 44.16 | |
| Patch and gel | 116 | 7.69 | |
| Patch and spray | 1 | 0.07 | |
| Spray | 27 | 1.79 | |
| Spray and gel | 2 | 0.13 | |
| Estradiol dose equivalent (PE)c | 1 | 29 | 1.92 |
| 2 | 187 | 12.40 | |
| 3 | 250 | 16.58 | |
| 4 | 467 | 30.97 | |
| 5 | 162 | 10.74 | |
| 6 | 171 | 11.34 | |
| 7 | 33 | 2.19 | |
| 8 | 111 | 7.36 | |
| 8+ | 85 | 5.64 | |
| Unknownd | 13 | 0.86 |
N, number; POI, premature ovarian insufficiency; PE, pump equivalents.
aPOI is menopause under the age of 40 years.
bEarly menopause is menopause between the ages of 40 and 45 years.
cDose is expressed as number of pumps equivalent (PE).
dThirteen women were using an unknown dose. These women were included in the whole cohort analysis for reference range, but excluded from subanalyses.
A total of 1,002 women (90.32%) were using estradiol in the form of a patch or gel. One hundred sixteen women (7.69%) were using both a patch and gel. Thirty women (1.99%) were using the spray either alone (n = 27) or in combination with the patch (n = 1) or gel (n = 2).
Almost two-thirds of women (61.87%) were using licensed doses of up to four pumps gel daily or equivalent (4 PE); just over a third (37.27%) were using higher off-label doses. The median dose was four PE (IQR 3.00-6.00).
The distribution of serum estradiol concentration was positively skewed with a mean concentration of 544.59 pmol/L (SD 682.55 pmol/L) and a median concentration of 355.26 pmol/L (IQR 198.44-646.15 pmol/L) (Supplemental Fig. 2, http://links.lww.com/MENO/B327). The reference range (the interval between the 2.5th and 97.5th centile) for the whole cohort was 54.62-2,050.55 pmol/L. When stratified by menopause status, the reference range for perimenopausal transdermal estradiol users was 88.54-3,151.62 pmol/L, and the range for postmenopausal transdermal estradiol users was 42.47-1,817.27 pmol/L. The reference ranges in women using only licensed doses (1-4 PE) were as follows: perimenopausal women 84.50-3,100.88 pmol/L and postmenopausal women 40.95-1,664.54 pmol/L (Supplemental Table 2, http://links.lww.com/MENO/B327).
Interindividual variation in serum estradiol concentration
Median estradiol levels increased from 191.30 pmol/L (IQR 100.62-303.37 pmol/L) in women using one PE to 334.32 pmol/L (IQR 202.12-567.85 pmol/L) in women using four PE (the maximum licensed dose), and 505.30 pmol/L (IQR 275.28-907.40 pmol/L) in women using eight PE (Supplemental Table 3, http://links.lww.com/MENO/B327).
There was considerable interindividual variation in serum estradiol concentration (Fig. 1). Low (<2.5th centile) and high (>97.5th centile) levels were observed in every dose category excluding 7 PE (n = 33). Variance was greater in younger versus older women (P = 0.002), perimenopausal versus postmenopausal women (P < 0.0001), and gel versus patch users (P = 0.002). There was a trend toward greater variance in women using higher doses, but the trend failed to reach statistical significance at the 5% level (P = 0.074) (Supplemental Table 4, http://links.lww.com/MENO/B327).
FIG. 1.
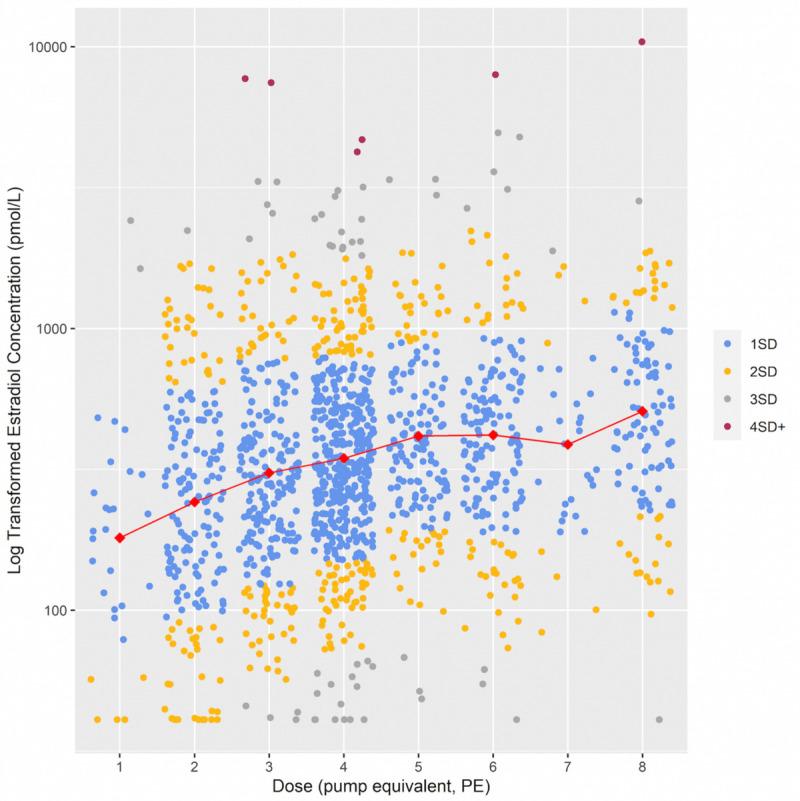
Relationship between log transformed serum estradiol concentration and estradiol dose for the whole cohort (N = 1,508). Dose was treated as a categorical variable to allow for nonlinearity, and because it was not possible to extrapolate beyond the licensed dose range. For example, one pump equivalent (PE) equates to one pump of 0.06% gel daily, or a 25-mcg patch twice weekly. Red diamonds represent the mean serum estradiol concentration per dose category. Blue, yellow, gray, and red dots represent levels within one, two, three, and four standard deviations from the mean, respectively. The log transformed model approximates a normal distribution with 69.4% of residuals within one SD of the mean, 95.5% within 2 SD, and 99.6% within 3 SD of the mean. The data points have been jittered for ease of visualization.
Association between serum estradiol concentration and dose, age, and estradiol formulation
Statistical modeling revealed a positive association between dose and estradiol concentration (Supplemental Table 4, http://links.lww.com/MENO/B327). For example, compared with women using two PE, estradiol concentration was estimated to be on average 37.6% higher in women using four PE (95% CI 19.0%-58.9%, P < 0.001) and 98.3% higher in women using 8 PE (95% CI 60.4%-145.2%, P < 0.001).
However, the predicted differences in estradiol concentration between each dose pairwise comparison revealed that whilst the difference in estradiol concentration between women using on-label versus off-label doses was statistically significant, there was no evidence of a difference between adjacent dose categories (2 vs 3 PE, 3 vs 4 PE, 4 vs 5 PE, 5 vs 6 PE, and 6 vs 8 PE) (Supplemental Table 5, http://links.lww.com/MENO/B327).
Serum estradiol levels were estimated to be 27.7% lower in older (≥50 y) versus younger women (95% CI 18.0%-36.3%, P < 0.001), and 25.7% lower in women using patches compared with women using gel (95% CI 18.4%-32.3%, P < 0.001). The predicted difference in estradiol concentration between women <50 years versus women ≥50 years was 138.27 pmol/L (95% CI 77.84-198.71 pmol/L, P < 0.001), and between gel and patch users was 126.74 pmol/L (95% CI 85.01-168.48 pmol/L, P < 0.001) (Supplemental Tables 4 and 6, http://links.lww.com/MENO/B327).
The relationship between serum estradiol concentration and age, estradiol dose, and formulation, as predicted by the model, is illustrated in Figure 2.
FIG. 2.
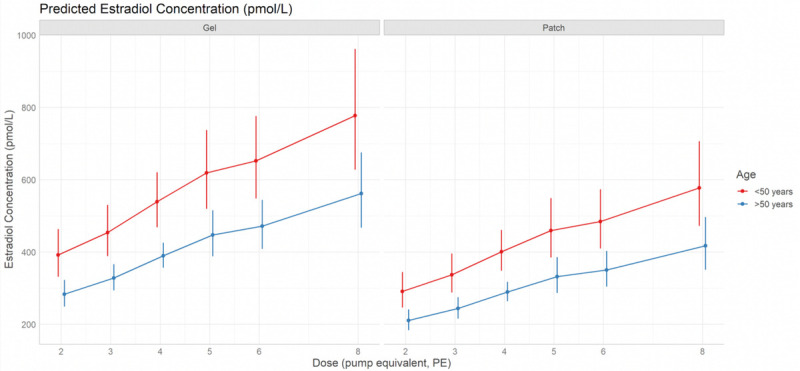
Predicted estradiol concentration (95% confidence interval) according to estradiol dose, age and formulation using a linear model. The predicted values (plus 95% confidence intervals) of estradiol concentration (pmol/L) according to estradiol dose (number of pump equivalents, PE), age (<50 or ≥50 y) and estradiol formulation (patch or gel) from the linear model of log-transformed estradiol concentration. Gel users are shown in the left-hand plot, patch users are represented in the right-hand plot. Predicted values for women ≥50 years are shown in blue (n = 1,266, 83.95%); predicted values in younger women (<50 y) are shown in red (n = 242, 16.05%). The larger confidence intervals in women <50 years reflect a greater degree of uncertainty due to the smaller sample size. The plots demonstrate that at any given dose, predicted estradiol levels are greatest for women ≥50 years using estradiol gel. Whilst the predicted values increase with dose, the confidence intervals associated with off-label doses are larger and overlap, indicating a greater degree of uncertainty in the off-label dose range. One and seven PE were excluded from the model as there were insufficient data points (29 and 33 women respectively). > 8 PE was excluded from the model due to being a heterogeneous group.
Estradiol concentration: low and high levels
Low (subtherapeutic) estradiol levels were defined clinically as <200 pmol/L.4-6 In the whole cohort (N = 1,508), 378 women (25.07%) had low levels. Two hundred ninety-six of 933 women (31.73%) using licensed doses (1-4 PE) had estradiol levels of <200 pmol/L, including 116 of 467 women (24.84%) using the highest licensed dose (4 PE), compared with 82 of 562 women (14.59%) using off-label doses (Fig. 3, Supplemental Table 7, http://links.lww.com/MENO/B327).
FIG. 3.
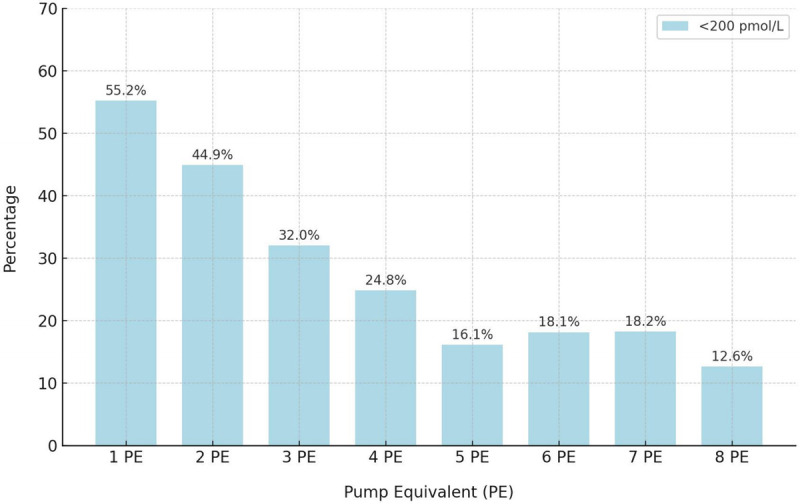
The percentage of women with low estradiol levels (<200 pmol/L) in each dose category. Dose (number of pump equivalents, PE) was treated as a categorical variable. The number of women with low or subtherapeutic levels (<200 pmol/L) decreases as the dose increases, but up to 1 in four women using the maximum licensed dose (4 PE) have low levels, and around 1 in 6 women using high off-label doses (5–8 PE) have low levels.
There is no clinical threshold above which estradiol levels are known to be harmful. Using the upper cutoff of 2,051 pmol/L, which, by definition, includes 2.5% of the cohort (n = 37), 18 of 933 women (1.93%) using on-label doses had estradiol levels of >2,051 pmol/L, compared with 19 of 562 women using off-label doses (3.38%) (Supplemental Table 7, http://links.lww.com/MENO/B327). The difference was not statistically significant (P = 0.11).
Logistic regression modeling was used to test the associations between low and high estradiol levels with age, estradiol dose (on-label vs off-label), and estradiol formulation. Women using on-label doses (1-4 PE) were 2.73 times more likely to have a low level compared with women using off-label doses (≥5 PE) (odds ratio [OR] 2.73, 95% CI 2.04-3.70, P < 0.001). Older women (≥50 y) were 1.77 times more likely to have subtherapeutic levels compared with younger women (OR 1.77, 95% CI 1.22-2.62, P = 0.003). Patch users were 1.51 times more likely to have subtherapeutic levels compared with gel users (OR 1.51, 95% CI 1.18-1.95, P = 0.001).
Conversely, younger women (<50 y) and gel users were more likely to have high levels compared with older women (≥50 y) and patch users, although the difference was not statistically significant at the 5% level (OR 2.16, 95% CI 0.93-4.65, P = 0.06; and OR 2.00, 95% CI 0.96-4.41, P = 0.07, respectively). There was no evidence of an association between a high estradiol concentration and on vs off-label dose (P = 0.14).
Supplemental Table 8 (http://links.lww.com/MENO/B327) summarizes the characteristics of women with high levels (n = 38). Four PE was the dose most frequently prescribed for women with high levels (n = 11). The medical records of seven women with estradiol concentrations >4,000 pmol/L were reviewed. All were clinically well at the time of blood sampling. The highest level was observed in a postmenopausal woman using an estradiol spray (1.53-mg estradiol per spray), six sprays daily (10,410 pmol/L, the upper limit of detection). This participant did not reattend for a repeat measurement during the study interval but was noted to have a serum level of 550.3 pmol/L 5 months earlier while using the same dose/formulation. Three perimenopausal gel users had levels >7,000 pmol/L. One woman with a level of 7,969.9 pmol/L was advised to reduce her dose from six PE to four PE. A repeat test 6 weeks later revealed a level of 232.37 pmol/L. Two women using three PE of gel had levels >7,000 pmol/L; the dose was unaltered and repeat tests during the study interval revealed levels of 96.05 pmol/L and 132 pmol/L. The highest level recorded in a patch user was 4,238.9 pmol/L in a postmenopausal woman using a 100-mcg patch (4 PE) twice weekly. The dose was unaltered and a repeat test 1 week later revealed an estradiol level of 80.68 pmol/L.
DISCUSSION
The aim of this study was to measure the range and interindividual variation in serum estradiol concentration in women treated with transdermal estradiol, and to estimate the prevalence of “poor absorbers” (women using licensed doses with subtherapeutic levels). To our knowledge, this is the largest study to measure hormone levels in women using transdermal estradiol, and the first to include both perimenopausal and postmenopausal women, using both licensed and off-label doses, in a real-world clinic setting.
The median estradiol level was 355.26 pmol/L (IQR 198.44-646.15 pmol/L) in keeping with data that suggest the optimal level for symptom relief is around 400 pmol/L for most women.4,5
The reference range for serum estradiol concentration in perimenopausal and postmenopausal HT users attending a private menopause clinic was 54.62-2,050.55 pmol/L (14.88-558.53 pg/mL). This is wider than the physiological reference range in premenopausal women (110-1,300 pmol/L or 30-350 pg/mL).10 In premenopausal women, preovulatory estradiol levels typically peak at 550 to 1,300 pmol/L,10 but values of up to 2,750 pmol/L have been reported.28 Estradiol levels can be up to 30% higher in perimenopausal women due to dysregulation of the hypothalamic-pituitary-ovarian axis and/or luteal-out-of-phase events.29,30 An additive effect will be observed in women using exogenous estrogen (HT). This accounts for the wide range in serum estradiol concentration in the study cohort, especially in perimenopausal women (88.54-3,151.62 pmol/L), and illustrates the importance of not relying on blood tests to diagnose perimenopause or to guide HT (estradiol) dose decisions.
There was a positive association between dose and mean estradiol concentration, with higher levels typically observed in women using higher doses. However, there was considerable interindividual variation across the dose range, with both low (<2.5th centile) and high (>97.5th centile) levels observed in every dose category. This is consistent with previous studies that have demonstrated up to a 10-fold difference in serum estradiol concentration in women using the same dose patch or gel.10 Consequently, dose-adjacent ranges (eg, levels in women using 3 PE compared with women using 2 or 4 PE) overlapped and did not significantly differ. This emphasizes the importance of tailoring estradiol dose to the individual according to clinical symptoms, because dose does not reliably predict serum estradiol concentration, which usually correlates with therapeutic response.
Numerous factors affect transdermal estradiol absorption in the real world. Different estradiol formulations and brands have distinctive physicochemical properties that influence the rate at which estradiol is absorbed.31-33 Biological factors such as age, ethnicity, and differences in skin adiposity, hydration, blood flow (capillary density), and temperature, influence absorption and bioavailability.9,34 Absorption may be increased in individuals with inflammatory skin conditions such as eczema.34 Up to 20% of patch users report local skin reactions35,36 that are usually mild but may reduce adhesion (decreased absorption) or increase skin permeability (increased absorption) and contribute to interindividual variability.34 The amount of estradiol absorbed also varies according to the site of application (eg, abdomen vs buttock)37 and the surface area over which gel is applied.6,10 Washing the skin within an hour of gel application has been shown to reduce mean serum estradiol concentration by up to 22%.38 Timing of blood sampling relative to time since application matters because there is significant fluctuation between peak and trough levels.10 For example, mean serum estradiol levels in postmenopausal women using a 50-mcg estradiol patch have been shown to decrease by 50% over a 72-hour period.8
Interindividual differences in estradiol distribution, metabolism, and excretion contribute to variation in serum estradiol concentration in HT users.35,36 Factors, such as diet, physical activity, stress, smoking, caffeine, and alcohol consumption, influence estradiol pharmacokinetics and contribute to marked intraindividual fluctuations in estradiol levels in women using a constant daily dose.10,39 Circadian variation in dermal blood flow contributes to intraindividual variation since dermal blood flow and absorption are higher in the evening.32,40
In the study cohort, estradiol levels were estimated to be on average 38.4% higher (95% CI 22.0%-56.9%) in younger women (<50 y) and varied significantly more than levels in older women. This is consistent with higher, fluctuating, endogenous estrogen levels in perimenopausal women and contributes to intraindividual and interindividual variation in serum estradiol concentration in a real-world clinic cohort.
Serum estradiol concentration was also significantly higher, and variance was significantly greater, in women using gel (median estradiol concentration in women using gel: 378.47 pmol/L, IQR 203.20-691.87 pmol/L; median estradiol concentration in women using patches: 322.64 pmol/L, IQR 181.42-551.19 pmol/L). This is consistent with previous studies demonstrating similar bioavailability but greater fluctuation and higher peak levels in gel users.8,41,42 In a randomized, cross-over study that compared estradiol levels in 24 postmenopausal women using 1.0 mg estradiol gel versus a 50-mcg patch, peak levels varied 11-fold with the gel versus 7-fold with the patch.42 Variance is likely to be greater in our cohort because, unlike studies in which the surface area of gel application has been tightly controlled, the surface area of application is highly variable in the real world (patch users are less susceptible because the surface area is fixed). Additionally, skin contamination may result in spuriously high levels in gel users.43
Almost a third of women (32%) using licensed doses (1-4 PE) had subtherapeutic serum estradiol levels (<200 pmol/L). The odds of having a low level were significantly higher among older women (≥50 y) and patch users. Overall, approximately one in two women using one or two PE, one in three women using three PE, and one in four women using four PE (the highest licensed dose) had low levels. This suggests that up to one in four women may need a higher off-label dose to achieve therapeutic levels. This is higher than the 5%-20% prevalence reported previously,6 possibly because our data was collected in the real world and is therefore subject to greater variation. Prescribing off-label doses when clinically indicated is consistent with menopause guidelines that do not set arbitrary limits on estrogen dose, but recommend that the dose is individualized to achieve treatment goals (symptom relief and/or bone protection).11-13 Notably, although the odds of having a low level were 2.73-fold higher in women using licensed doses, around one in six women using off-label doses (≥5 PE) also had subtherapeutic levels. These women may be more likely to benefit from a change in formulation, but patient preference is key to optimize adherence, and HT stock shortages may limit choice.44
We defined a high level as >2,051 pmol/L based on the reference range observed in the whole cohort (2.5% of women had levels >2,051 pmol/L). It should be emphasized that this is an arbitrary threshold that has no clinical meaning and was used purely to interrogate the relationship between dose and high levels. There was no evidence that use of off-label versus on-label doses increased the odds of a high level (P = 0.14). Higher levels were more frequently observed in younger women with higher, fluctuating endogenous estradiol levels, and in gel users with higher peak levels versus patch users, but the associations failed to reach significance at the 5% level.
Some women attending a specialist clinic use high doses to achieve high levels for therapeutic effect. For example, psychological symptoms in the menopause transition are common and can be effectively treated with HT +/− additional psychiatric support.11,45-47 Limited evidence suggests that some women with severe depressive symptoms need higher estradiol levels for symptom relief.48,49 Occasionally, high doses are used to suppress ovulation in perimenopausal women with severe symptoms resulting from excessive, unpredictable hormone fluctuations. In younger women with distressing cyclical symptoms, cycle suppression is usually achieved using a combined contraceptive pill.50 Most combined oral contraceptives contain 30-mcg ethinyl estradiol, which is roughly dose-equivalent to a 300-mcg 17β-estradiol patch twice weekly, or 12 pumps 17β-estradiol gel daily.51 If using HT to suppress ovulation in perimenopausal women, 2x 100-mcg estradiol patches (200 mcg) twice weekly are usually sufficient,48 but higher doses may be needed in younger women and/or poor absorbers.
Seven women (0.46%) were observed to have very high estradiol levels (>4,000 pmol/L). All the women were clinically well, and all had normal or low levels when the blood test was repeated, suggesting that the results were erroneous. Gel contamination due to prior application at the site of venepuncture may cause spuriously high levels in clinical practice.43 False elevation of estradiol may also occur when there are analytical errors, for example, due to the presence of cross-reacting substances that compete with estradiol for antibody binding (eg, prednisolone, cortisol), or interfering substances that impact other components of the immunoassay (eg, heterophile antibodies, autoantibodies, biotin).52-54 This highlights the importance of repeating a blood test before adjusting the dose in women without symptoms of estrogen excess (such as nausea, headaches, mastalgia, bleeding).
Strengths and limitations
The present study has several strengths. First, in contrast to previous studies with small sample sizes, this study included 1,508 women. Second, the use of real-world data enhance the generalizability of our results. To our knowledge, this is the only study that has included both perimenopausal and postmenopausal women, and the only study to include women using both licensed and off-label doses. Use of real-world data also accounts for factors known to influence both interindividual and intraindividual variation, including adherence and imperfect use, and provides a unique insight into the range of estradiol levels encountered in clinical practice. Third, this study was conducted in a single center and serum estradiol concentrations were measured in a single lab using the same, validated immunoassay kit, allowing for direct comparison of serum estradiol concentrations in women using different formulations and doses. Our study also has several limitations. First, perimenopausal women (women <50 y) were included but the sample size was small (n = 242, 16% of the study cohort). Consequently, there is greater uncertainty in the results obtained for younger women and more research is needed to accurately define the reference range for serum estradiol in younger women using transdermal estradiol. Statistical modeling accounts for smaller sample sizes, so this does not affect the results obtained in subgroup comparisons. Second, 95% of Newson Health Clinic attendees are White British and, compared with the general population, women who access private healthcare are less deprived and less likely to experience ill-health. Ethnicity, healthy lifestyle behaviors (such as smoking and physical activity), comorbidities and polypharmacy, influence estradiol pharmacokinetics and serum levels. This limits the generalizability of our findings. Further, women attending a menopause clinic, especially those having blood tests, are more likely to experience difficult symptoms and/or have issues with absorption compared with women managed in primary care. As such, their dose requirements may be different, and the prevalence of “poor absorbers” in the background population may be lower. Third, time of recorded dose and blood sampling may have differed by up to 3 months and it is possible that the dose recorded may not have always been the dose used at the time of sampling. Moreover, while compliance with transdermal HT is generally good, up to 25% of women may not take their HT as prescribed.55 Poor or suboptimal adherence may have resulted in underestimation of the upper limits of the observed reference ranges, and overestimation of the prevalence of ‘poor absorbers.’ Fourth, estimation of dose equivalence and the use of immunoassay to measure serum estradiol concentration limit the accuracy of our results. Although more accurate quantification of the relationship between estradiol dose and level would be interesting, it would be of limited clinical value because, as we have shown, interindividual variation is substantial and dose should be clinically guided. Furthermore, gold standard mass spectrometry-based techniques are not widely available, and our immunoassay-based results more accurately reflect real-world conditions. Finally, a one off serum estradiol level is not a reliable reflection of total body exposure (the area under the plasma drug concentration-time curve), or local estradiol levels in target tissues, and therefore does not predict clinical efficacy or safety. However, our findings are clinically relevant because if HT users with persistent menopausal symptoms are found to have a low estradiol level, a higher (+/− off-label) dose can be safely tried, which is reassuring for both women and clinicians.
CONCLUSIONS
Our study provides novel insight into the range and interindividual variation in serum estradiol concentration in perimenopausal and postmenopausal women using transdermal estradiol in a real-world setting. Our data suggest that up to one in four women may need off-label doses to achieve therapeutic levels. Blood tests are not needed to diagnose menopause in women over the age of 45, because the diagnosis is clinical; and blood tests are not helpful to diagnose perimenopause, because estrogen levels fluctuate considerably. Blood tests cannot be used to inform dose decisions when levels are in the therapeutic range, because there is substantial intraindividual variation in serum estradiol concentration and clinical effect (pharmacodynamics). However, blood tests can be useful when the clinical response is suboptimal, especially in women using high licensed doses (4 PE), to determine whether a change in formulation or an off-label dose is likely to be of benefit. A blood test is also helpful when women with no or minimal menopausal symptoms want HT for bone protection, to confirm that levels are therapeutic (>200 pmol/L).
Improved understanding of the substantial interindividual variation in estradiol pharmacokinetics facilitates dose customization based on clinical symptoms. Measurement of serum estradiol is useful to identify and guide dose customization in women who absorb transdermal estradiol poorly. Personalized care is key to prevent the immediate and long-term harms of estrogen deficiency.
Acknowledgments
We thank the study participants for granting permission for us to use their data for the purpose of this study. We are grateful to Ahmed Kamel for his assistance with data analysis.
Footnotes
Funding/support: None reported.
Financial disclosure/conflicts of interest: Lynsey McColl has an ongoing relationship with Newson Health Ltd, via her company Select Statistical Services Ltd providing statistical analysis and support as a consultant. The other authors have nothing to disclose.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
Contributor Information
Daniel Reisel, Email: dan.reisel@newsonhealth.co.uk.
Aini Kamal, Email: aini.kamal@newsonhealth.co.uk.
Amy Neville, Email: amy.neville@newsonhealth.co.uk.
Lynsey McColl, Email: lynsey@select-statistics.co.uk.
Rebecca Lewis, Email: rebecca.lewis@newsonhealth.co.uk.
Louise Newson, Email: louise.newson@newsonhealth.co.uk.
REFERENCES
- 1.Department of Health and Social Care. Caulfield M, Barclay S. Hundreds of thousands of women experiencing menopause symptoms to get cheaper HRT. Available at: http://www.gov.uk/government/news/hundreds-of-thousands-of-women-experiencing-menopause-symptoms-to-get-cheaper-hormone-replacement-therapy. Accessed March 17, 2023.
- 2.Ruan X, Mueck AO. Primary choice of estrogen and progestogen as components for HRT: a clinical pharmacological view. Climacteric 2022;25:443–452. doi: 10.1080/13697137.2022.2073811 [DOI] [PubMed] [Google Scholar]
- 3.Levy B, Simon JA. A contemporary view of menopausal hormone therapy. Obstet Gynecol 2024;144:12–23. doi: 10.1097/AOG.0000000000005553 [DOI] [PubMed] [Google Scholar]
- 4.de Lignieres B. Hormone replacement therapy: clinical benefits and side-effects. Maturitas 1996;23(Suppl):S31–S36. doi: 10.1016/s0378-5122(96)90012-2 [DOI] [PubMed] [Google Scholar]
- 5.Steingold KA Laufer L Chetkowski RJ, et al. Treatment of hot flashes with transdermal estradiol administration. J Clin Endocrinol Metab 1985;61:627–632. doi: 10.1210/jcem-61-4-627 [DOI] [PubMed] [Google Scholar]
- 6.Armston A, Wood P. Hormone replacement therapy (oestradiol-only preparations): can the laboratory recommend a concentration of plasma oestradiol to protect against osteoporosis? Ann Clin Biochem 2002;39(Pt 3):184–193. doi: 10.1258/0004563021902107 [DOI] [PubMed] [Google Scholar]
- 7.Balfour JA, Heel RC. Transdermal estradiol. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of menopausal complaints. Drugs 1990;40:561–582. doi: 10.2165/00003495-199040040-00006 [DOI] [PubMed] [Google Scholar]
- 8.Scott RT, Jr., Ross B, Anderson C, Archer DF. Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol. Obstet Gynecol 1991;77:758–764. [PubMed] [Google Scholar]
- 9.Farahmand S, Maibach HI. Transdermal drug pharmacokinetics in man: Interindividual variability and partial prediction. Int J Pharm 2009;367(1-2):1–15. doi: 10.1016/j.ijpharm.2008.11.020 [DOI] [PubMed] [Google Scholar]
- 10.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875 [DOI] [PubMed] [Google Scholar]
- 11.Hamoda H, Panay N, Pedder H, Arya R, Savvas M. The British Menopause Society & Women's Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod Health 2020;26:181–209. doi: 10.1177/2053369120957514 [DOI] [PubMed] [Google Scholar]
- 12.Baber RJ Panay N Fenton A, IMS Writing Group . 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric 2016;19:109–150. doi: 10.3109/13697137.2015.1129166 [DOI] [PubMed] [Google Scholar]
- 13.The Hormone Therapy Position Statement of the North American Menopause Society" Advisory Panel . The 2022 hormone therapy position statement of the North American Menopause Society. Menopause 2022;29:767–794. doi: 10.1097/GME.0000000000002028 [DOI] [PubMed] [Google Scholar]
- 14.British Menopause Society . Joint BMS FSRH RCGP RCOG SfE and RCN Women's Health Forum safety alert. April 2, 2023. Available at: https://thebms.org.uk/2023/04/joint-bms-fsrh-rcgp-rcog-sfe-and-rcn-womens-health-forum-safety-alert/ Accessed December 12, 2023. [DOI] [PubMed]
- 15.Naunton M, AFY AH, JRBJ B, Archer DF. Estradiol gel: review of the pharmacology, pharmacokinetics, efficacy, and safety in menopausal women. Menopause 2006;13:517–527. doi: 10.1097/01.gme.0000191881.52175.8c [DOI] [PubMed] [Google Scholar]
- 16.de Lignieres B, Vincens M. Differential effects of exogenous oestradiol and progesterone on mood in post-menopausal women: individual dose/effect relationship. Maturitas 1982;4:67–72. doi: 10.1016/0378-5122(82)90021-4 [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, Jiang X, Sun Y, Shu W. Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials. Menopause 2016;23:461–470. doi: 10.1097/GME.0000000000000519 [DOI] [PubMed] [Google Scholar]
- 18.Hodis HN, Mack WJ. Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease: it is about time and timing. Cancer J 2022;28:208–223. doi: 10.1097/PPO.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nerattini M Jett S Andy C, et al. Systematic review and meta-analysis of the effects of menopause hormone therapy on risk of Alzheimer's disease and dementia. Front Aging Neurosci 2023;15. doi: 10.3389/fnagi.2023.1260427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlebowski RT Aragaki AK Pan K, et al. Randomized trials of estrogen-alone and breast cancer incidence: a meta-analysis. Breast Cancer Res Treat 2024;206:177–184. doi: 10.1007/s10549-024-07307-9 [DOI] [PubMed] [Google Scholar]
- 21.Manley K Hillard T Clark J, et al. Management of unscheduled bleeding on HRT: a joint guideline on behalf of the British Menopause Society, Royal College Obstetricians and Gynaecologists, British Gynaecological Cancer Society, British Society for Gynaecological Endoscopy, Faculty of Sexual and Reproductive Health, Royal College of General Practitioners and Getting it Right First Time. Post Reprod Health 2024;30:95–116. doi: 10.1177/20533691241254413 [DOI] [PubMed] [Google Scholar]
- 22.Alsugeir D, Wei L, Adesuyan M, Cook S, Panay N, Brauer R. Hormone replacement therapy prescribing in menopausal women in the UK: a descriptive study. BJGP Open 2022;6:BJGPO.2022.0126. doi: 10.3399/BJGPO.2022.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semble . Semble Technology Limited. London, UK: Available at: https://www.semble.io. Accessed August 12, 2024. [Google Scholar]
- 24.Women's Health Concern . HRT - types, doses and regimens. September 2021. Available at: https://www.womens-health-concern.org/wp-content/uploads/2022/11/27-WHC-FACTSHEET-HRT-Doses-NOV2022-A.pdf. Accessed December 12, 2023.
- 25.R Core Team (2023) . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing: Available at: https://www.R-project.org/. Accessed July 10, 2024. [Google Scholar]
- 26.Finnegan D. Reference Intervals. R package version 1.3.1. 2024. Available at: https://cran.r-project.org/web/packages/referenceIntervals/index.html. Accessed July 10, 2024.
- 27.Lüdecke D. ggeffects: Tidy data frames of marginal effects from regression models. J Open Source Softw 2018;3:772. doi: 10.21105/joss.00772 [DOI] [Google Scholar]
- 28.Jameson J, Dr Groot L. Endocrinology: adult and pediatric. In: Chapter 155: Endocrine Testing. 6th ed,. Philadelphia, PA: Elsevier Health Sciences; 2010: ISBN 978-1-4160-5583-9. [Google Scholar]
- 29.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev 1998;19:397–428. doi: 10.1210/edrv.19.4.0341 [DOI] [PubMed] [Google Scholar]
- 30.Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab 2021;106:1–15. doi: 10.1210/clinem/dgaa764 [DOI] [PubMed] [Google Scholar]
- 31.Harrison LL, Harari D. An evaluation of bioequivalence of two 7-day 17beta-estradiol transdermal delivery systems by anatomical site. J Clin Pharmacol 2002;42:1134–1141. doi: 10.1177/009127002401382740 [DOI] [PubMed] [Google Scholar]
- 32.Rohr UD, Nauert C, Stehle B. 17Beta-estradiol delivered by three different matrix patches 50 microg/day: a three way cross-over study in 21 postmenopausal women. Maturitas 1999;33:45–58. doi: 10.1016/s0378-5122(99)00039-0 [DOI] [PubMed] [Google Scholar]
- 33.Reginster JY Albert A Deroisy R, et al. Plasma estradiol concentrations and pharmacokinetics following transdermal application of Menorest 50 or Systen (Evorel) 50. Maturitas 1997;27:179–186. doi: 10.1016/s0378-5122(97)00027-3 [DOI] [PubMed] [Google Scholar]
- 34.Singh I, Morris AP. Performance of transdermal therapeutic systems: effects of biological factors. Int J Pharm Investig 2011;1:4–9. doi: 10.4103/2230-973X.76721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Electronic medicines compendium. Evorel 100 patches. Last reviewed October 3, 2022. Available at: https://www.medicines.org.uk/emc/product/10932/smpc. Accessed December 12, 2023.
- 36.Electronic medicines compendium. Estradot 100 patches. Last reviewed June 15, 2022. Available at: https://www.medicines.org.uk/emc/product/7225/smpc. Accessed December 12, 2023.
- 37.Taggart W, Dandekar K, Ellman H, Notelovitz M. The effect of site of application on the transcutaneous absorption of 17-beta estradiol from a transdermal delivery system (Climara). Menopause 2000;7:364–369. doi: 10.1097/00042192-200007050-00010 [DOI] [PubMed] [Google Scholar]
- 38.Solvay pharmaceuticals . EstroGel (estradiol gel) 0.06% [package insert]. Marietta, GA: United Pharmaceuticals Inc, a Solvay Pharmaceuticals Company; 2004: Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21166_estrogel_lbl.pdf. Accessed November 26, 2023 [Google Scholar]
- 39.Kraemer GR Kraemer RR Ogden BW, et al. Variability of serum estrogens among postmenopausal women treated with the same transdermal estrogen therapy and the effect on androgens and sex hormone binding globulin. Fertil Steril 2003;79:534–542. doi: 10.1016/s0015-0282(02)04755-6 [DOI] [PubMed] [Google Scholar]
- 40.Rohr UD, Ehrly AM, Kuhl H. Plasma profiles of transdermal 17 beta-estradiol delivered by two different matrix patches. A four-way cross-over study in postmenopausal women. Arzneimittelforschung 1997;47:761–767 [PubMed] [Google Scholar]
- 41.Jarvinen A, Nykanen S, Paasiniemi L. Absorption and bioavailability of oestradiol from a gel, a patch and a tablet. Maturitas 1999;32:103–113. doi: 10.1016/s0378-5122(99)00021-3 [DOI] [PubMed] [Google Scholar]
- 42.Järvinen A, Bäckström A, Elfström C, Viitanen A. Comparative absorption and variability in absorption of estradiol from a transdermal gel and a novel matrix-type transdermal patch. Maturitas 2001;38:189–196. doi: 10.1016/s0378-5122(00)00222-x [DOI] [PubMed] [Google Scholar]
- 43.Vihtamaki T, Luukkaala T, Tuimala R. Skin contamination by oestradiol gel–a remarkable source of error in plasma oestradiol measurements during percutaneous hormone replacement therapy. Maturitas 2004;48:347–353. doi: 10.1016/S0378-5122(03)00043-4 [DOI] [PubMed] [Google Scholar]
- 44.Wise J. Why are there shortages of HRT and other drugs in the UK? BMJ 2022;377:o1183. doi: 10.1136/bmj.o1183 [DOI] [PubMed] [Google Scholar]
- 45.Ancelin ML, Scali J, Ritchie K. Hormonal therapy and depression: are we overlooking an important therapeutic alternative? J Psychosom Res 2007;62:473–485. doi: 10.1016/j.jpsychores.2006.12.019 [DOI] [PubMed] [Google Scholar]
- 46.Behrman S, Crockett C. Severe mental illness and the perimenopause. BJPsych Bull 2023:1–7. doi: 10.1192/bjb.2023.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institute for Health and Care Excellence. CKS. Menopause: Hormone replacement therapy (HRT) . Last revised in September 2022. Available at: https://cks.nice.org.uk/topics/menopause/prescribing-information/hormone-replacement-therapy-hrt/. Accessed December 12, 2023.
- 48.Studd JW. A guide to the treatment of depression in women by estrogens. Climacteric 2011;14:637–642. doi: 10.3109/13697137.2011.609285 [DOI] [PubMed] [Google Scholar]
- 49.Garnett T, Studd JW, Henderson A, Watson N, Savvas M, Leather A. Hormone implants and tachyphylaxis. Br J Obstet Gynaecol 1990;97:917–921. doi: 10.1111/j.1471-0528.1990.tb02447.x [DOI] [PubMed] [Google Scholar]
- 50.American College of Obstetricians and Gynecologists’ Committee on Clinical Consensus-Gynecology . General approaches to medical management of menstrual suppression: acog clinical consensus no. 3. Obstet Gynecol 2022;140:528–541. doi: 10.1097/AOG.0000000000004899 [DOI] [PubMed] [Google Scholar]
- 51.Mashchak CA Lobo RA Dozono-Takano R, et al. Comparison of pharmacodynamic properties of various estrogen formulations. Am J Obstet Gynecol 1982;144:511–518. doi: 10.1016/0002-9378(82)90218-6 [DOI] [PubMed] [Google Scholar]
- 52.Sturgeon CM, Viljoen A. Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem 2011;48(Pt 5):418–432. doi: 10.1258/acb.2011.011073 [DOI] [PubMed] [Google Scholar]
- 53.Atkins P, Mattman A, Thompson D. Falsely elevated serum estradiol due to heterophile antibody interference: a case report. Arch Endocrinol Metab, 2021;65:237–241. doi: 10.20945/2359-3997000000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol 2014;14:237. doi: 10.1186/1472-6890-14-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serfaty D de Reilhac P Eschwege E, et al. Compliance with hormone replacement therapy in menopausal women: results of a two-year prospective French study comparing transdermal treatment with fixed oral combination therapy [in French]. Gynecol Obstet Fertil 2003;31:525–533. doi: 10.1016/s1297-9589(03)00130-9 [DOI] [PubMed] [Google Scholar]


