Abstract
Background
Obsessive–compulsive disorder (OCD) is a debilitating neuropsychiatric illness characterized by obsessions and compulsions that are distressing, impair function, and are time-consuming, especially in severe cases. Up to 40% of people with OCD have treatment-refractory OCD and experience inadequate response to multiple trials and combinations of treatments. Neurosurgery is an important treatment option for people with severe, treatment-refractory OCD but is typically invasive. Magnetic resonance-guided focused ultrasound (MRgFUS) is a noninvasive technology that is used to perform neurosurgery. We conducted a health technology assessment of MRgFUS neurosurgery for people with severe, treatment-refractory OCD, which included an evaluation of effectiveness, safety, the budget impact of publicly funding MRgFUS neurosurgery, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence published since 2013. We assessed the risk of bias of each included study using the Joanna Briggs Institute's Critical Appraisal Checklist for Case Series, and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic literature search of the economic evidence. We estimated the 5-year budget impact of publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario. Owing to a lack of comparative clinical evidence, we did not conduct a primary economic evaluation. To contextualize the value of MRgFUS neurosurgery, we spoke to people with treatment-refractory OCD who underwent the procedure, as well as those on the waitlist.
Results
We included 2 studies in the clinical evidence review. In these small case series, MRgFUS neurosurgery led to improvements in OCD symptoms, quality of life, and patient functioning, as well as treatment response for many but not all patients (GRADE: Very low). In a minority of cases, the procedure could not be successfully performed due to skull factors (GRADE: Very low). MRgFUS neurosurgery was also found to have a favourable safety profile (GRADE: Very low). No cases of re-treatment were reported (GRADE: Very low). No studies compared MRgFUS neurosurgery with other neurosurgeries.
Due to the lack of comparative clinical evidence, the cost-effectiveness of MRgFUS neurosurgery could not be determined. Our budget impact analysis found that publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario would cost an additional $1.9 million over 5 years.
Patients reported the negative impacts that OCD had on their day-to-day activities, work and school, social life and family relationships, and mental health. The 6 participants who underwent MRgFUS neurosurgery commented on the positive impact that it had on their OCD symptoms, mental health, and quality of life.
Conclusions
MRgFUS neurosurgery may be an effective and generally safe treatment option for severe, treatment-refractory OCD, but the evidence is very uncertain. The cost-effectiveness of MRgFUS neurosurgery could not be determined given the lack of comparative clinical evidence. Publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario would result in an additional cost of $1.9 million over 5 years. Patients and care partners emphasized the negative impact of OCD in their lives and highlighted the importance of having access to MRgFUS neurosurgery as a treatment option for treatment-refractory OCD.
Objective
This health technology assessment evaluates the effectiveness and safety of magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery for people with treatment-refractory obsessive–compulsive disorder (OCD). It also evaluates the budget impact of publicly funding MRgFUS neurosurgery and the experiences, preferences, and values of people with treatment-refractory OCD.
Background
Health Condition
Obsessive–compulsive disorder (OCD) is a debilitating neuropsychiatric illness characterized by obsessions (recurrent, persistent intrusive thoughts or images that cause marked anxiety) and/or compulsions (repetitive behavioural or mental rituals performed in response to obsessions) that cause distress and functional impairment and are time-consuming.1,2 Diagnosis often occurs in late childhood to early adolescence or in early adulthood, with most cases diagnosed before 30 years of age.2–4 The symptoms of OCD tend to be episodic but chronic,5 and additional psychiatric comorbidities (e.g., depression) are common.2,4
The lifetime prevalence of OCD for adults globally is approximately 1% to 2%, though it is likely underdiagnosed.2,6 An analysis of data from the 2012 Canadian Community Health Survey - Mental Health measured the prevalence of OCD among people in Canada 15 years of age or older to be 0.93% (95% confidence interval, 0.75%-1.11%).7 Symptoms, especially when severe, profoundly interfere with and negatively impact an individual's personal, social, and work lives, as well as those of their care partners.2 The prevalence of suicide attempts among people with OCD has been estimated to be around 15%.8 The severity of OCD symptoms is most commonly assessed by the Yale-Brown Obsessive–Compulsive Scale (Y-BOCS),9 which scores severity as ranging from subclinical (i.e., Y-BOCS total severity score of 0 to 7), to mild (8 to 15), moderate (16 to 23), severe (24 to 31), and extreme (32 to 40) (scores above 15 are clinically significant).10 In a US national survey, about half of people with OCD reported severe disability from their illness and another third reported moderate disability.11
Clinical Need and Population of Interest
The primary treatment for OCD is cognitive behavioural therapy (CBT) with OCD-specific protocols and exposure and response prevention (ERP) and pharmacotherapy (i.e., selective serotonin reuptake inhibitors [SSRIs]), delivered independently or in combination.2 Although these constitute the most effective, evidence-based treatments,4 about half of patients with OCD (30%-60%) do not experience partial or full response to first-line interventions (partial response and full response are defined as >25% to <35% reduction and ≥35% reduction in Y-BOCS total severity score, respectively).12–16 Second-line or adjunctive pharmacologic agents (e.g., tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, antipsychotics) may be trialled to augment primary treatment in cases with poor treatment response.17 Various types of transcranial magnetic stimulation (TMS) (such as repetitive transcranial magnetic stimulation [rTMS] or deep transcranial magnetic stimulation [dTMS]) appear promising.18,19 Some TMS devices hold regulatory approval for the treatment of OCD in select jurisdictions (e.g., US, some European countries, Canada); however, these treatments are currently not accessible in Canada.19
Other interventions, including transcranial direct current stimulation and electroconvulsive therapy, are generally not recommended, owing to a lack of compelling evidence for their use in treating OCD.2,18–21 Despite the best combination of treatments, as many as 20% to 40% of people with OCD do not respond to second-line, third-line, or more treatments and remain severely ill and extremely impaired by their symptoms; this is referred to as treatment-refractory OCD. 5,14
It is challenging to quantify how many people with OCD have treatment-refractory OCD, in part because less than half of people with OCD seek treatment.2 There is also a lack of an operational definition for treatment-refractory or intractable disease22; however, the concepts are related and encompass elements of little or no symptom improvement or worsening symptoms, despite adequate trials of the best available and acceptable noninvasive treatments.5,22–24
Current Neurosurgery Options
Neurosurgery for severe, treatment-refractory OCD can use ablation (therapeutically destroying neural tissue and creating a lesion at the target brain structure or circuit) or deep brain stimulation (DBS; using electrical impulses to stimulate and disrupt brain activity in the target area). For both types of neurosurgery, the intention is to alter the structure and function of the brain to improve OCD symptoms by disrupting the underlying neural pathways.14,17 The pathophysiology of OCD is not fully understood; however, the connections between the orbitofrontal cortex, anterior cingulate cortex, thalamus, and basal ganglia (i.e., cortico-striato-thalamo-cortical circuits), as well as dysfunction in serotonergic and glutamatergic systems, are strongly implicated.4,7,23,25,26 DBS and ablative neurosurgery are stereotactic neurosurgeries, utilizing an external frame attached to the patient's head to enable very precise localization of brain targets, typically bilaterally (on both sides of the brain). The therapeutic effects of these procedures appear after a lag time of weeks or months; research suggests that the therapeutic effects may last for years after surgery.27
Neurosurgery for OCD should be provided only at specialty medical centres with an experienced multidisciplinary team and the necessary equipment.24,28–30 Table 1 summarizes some of the considerations for any neurosurgery for OCD from organizations and entities that specialize in neurosurgery. Although treatment-refractory OCD is estimated to occur in a sizeable proportion of treatment-seeking people, a very small number fulfill additional eligibility criteria (e.g., duration of illness, functional impairment, acute suicidality, medical comorbidities) and are candidates for neurosurgery.17 A longitudinal study on OCD in the US estimated that around 0.6% of treatment-seeking people with OCD may meet surgical eligibility criteria (i.e., severity, functional impairment, failure of sufficient treatment trials) for 1 type of neurosurgery, DBS.17 A neurosurgeon in Ontario estimated, based on their clinical practice, that between 1 in 1,000 and 1 in 100 people with OCD who seek treatment could be refractory to nonsurgical treatments and be surgical candidates (A. Lozano, MD, PhD, virtual communication, June 6, 2023). Another neurosurgeon in Ontario estimated that one-third of people with severe, treatment-refractory OCD who would be eligible for intensive treatments (e.g., day programs or residential treatment) would be surgical candidates (N. Lipsman, MD, PhD, email communication, July 19, 2023).
Table 1:
Summary of Considerations for Determining Eligibility and Suitability for Neurosurgical Intervention for Patients With OCD
| Entity | Eligibility guidance | Additional considerations |
|---|---|---|
| Committee for Neurosurgery for Psychiatric Disorders, part of the World Society for Stereotactic and Functional Neurosurgery (WSSFN) and the European Society for Stereotactic and Functional Neurosurgery (ESSFN)a Consensus Guidelines24 |
|
|
| Indian Psychiatric Society,31 Indian Society for Stereotactic and Functional Neurosurgery, and The Neuromodulation Society Consensus Criteria 201929 |
|
|
| Royal College of Psychiatrists Position Statement 2017 (UK)28 |
|
|
Abbreviations: GAF, Global Assessment of Functioning; OCD, obsessive–compulsive disorder; Y-BOCS, Yale-Brown Obsessive–Compulsive Scale.
Partnering with the working group “Deep Brain Stimulation in Psychiatry: Guidance for Responsible Research and Application”, the Psychiatric Neurosurgery Committee of the American Society for Stereotactic and Functional Neurosurgery (ASSFN), the Latin American Society for Stereotactic and Functional Neurosurgery (SLANFE), the Asian-Australasian Society for Stereotactic and Functional Neurosurgery (AASSFN), and the World Psychiatric Association (WPA).
Lack of efficacy or disabling side effects.
For example, pharmacotherapy and behavioural therapy. As outlined by Visser-Vanderwalle et al30: “insufficient response to, at minimum: 2 selective serotonin reuptake inhibitors (SSRIs) at the maximum tolerated dose for at least 12 weeks; clomipramine at a maximum tolerated dosage for at least 12 weeks; 1 augmentation trial with an antipsychotic for at least 8 weeks, in combination with one of the aforementioned drugs; and a complete trial of exposure-based cognitive behavioural therapy (CBT) confirmed by a psychotherapist.”
Includes systematic treatment trials not discontinued prematurely due to mild side effects as follows: at least 3 months of ≥2 SSRIs and clomipramine, plus augmentation with at least 1 antipsychotic for at least 8 weeks and adequate trial of exposure and response prevention (ERP) CBT (≥20 sessions) or inability to tolerate the anxiety caused by therapy.29
The established surgeries have been found, mainly in small and often single-arm studies, to be clinically effective and safe for the treatment of OCD.19,32 The surgeries offer different advantages and disadvantages resulting from the various approaches and mechanisms of action. Neurosurgery for OCD is not curative and should be part of a treatment plan that includes ongoing pharmacotherapy and/or psychotherapy to help patients manage their symptoms.
Ablative Neurosurgery
Ablative neurosurgery for psychiatric indications has existed since the 1940s.14 There are a few surgical procedures that specify different neural pathways or targets, including cingulotomy (targeting the anterior cingulate cortex and cingulum), subcaudate tractotomy (targeting fibres below the caudate nucleus), limbic leucotomy (a combination of cingulotomy and subcaudate tractotomy), and anterior capsulotomy (targeting the anterior limb of the internal capsule [ALIC]).4,23 The most frequently performed procedures are capsulotomy and cingulotomy.23
Radiofrequency Ablation
Radiofrequency ablation (RFA) is the most established technique, with RFA anterior capsulotomy having the longest history of use. This type of open surgery involves a skin incision, cranial access through a burr hole in the skull, the insertion of very fine probes through the brain to the target area, and thermal ablation (permanent ablation of target tissue using heat). The lesion created is precise and develops immediately. This surgery is typically conducted under general anesthesia, but can be done under conscious sedation, and is associated with risks of invasive (open) surgery (e.g., infection, stroke, brain bleeding, brain swelling, wound complications, anesthesia complications) and may not be suitable for people on anticoagulant therapy. Clinical experts advised that RFA neurosurgery is the standard of care for patients with treatment-refractory OCD and is approved and available (covered by hospital global budget) in Canada. A 2016 systematic review found that RFA cingulotomy resulted in short- and long-term treatment response (partial or full) in 38% to 63% of patients with treatment-refractory OCD at 12 to 24 months after the procedure.13 The rates of surgical complications (e.g., intracranial infection) are low (e.g., 1%-2%23), but transient cognitive effects or other adverse effects can occur in around 20% of cases (e.g., subjective memory issues, urinary incontinence).13 Serious or permanent adverse effects following RFA capsulotomy or cingulotomy, such as memory or cognitive deficits, seizures, or personality changes, occur in around 2% to 5% of patients.13,23
Stereotactic Radiosurgery
Anterior capsulotomy can also be performed using stereotactic radiosurgery (focused radiation). Gamma Knife radiosurgery is a technique that delivers focused radiation through the intact skull to the target area of the brain. Unlike in RFA, where the lesion is created via craniotomy (opening the skull) immediately and precisely at the time of surgery, in radiosurgery the lesion develops gradually at the target site over time following surgery, taking up to several weeks or months to fully form. The irradiated area undergoes inflammatory changes and demyelination (damage of the insulating layer around nerves) of white matter tracts, with longer-term effects arising from the inability to produce certain neurotransmitters, thereby disrupting the targeted circuits.27 For radiosurgical capsulotomy, the procedures are completed within a couple of hours, and the radiation dose delivered can range from 120 to 200 gray (Gy).27 Gamma Knife stereotactic radiosurgery devices are approved by Health Canada (e.g., Leksell Gamma Knife, licence number 14773), and local experts advise that they are available and covered by hospital global budget for lesional neurosurgery in Canada. Gamma Knife radiosurgery for OCD may result in complete or partial response for about 60% to 70% of individuals.23,27 Although craniotomy is not required and therefore this procedure does not hold the risks of open surgery, individuals are exposed to ionizing radiation, and the volume of the lesion is less predictable owing to the nature of radiation. As a result, the biological response to focused radiation can be idiosyncratic and difficult to predict, with some patients having no lesions and others having very large lesions. Transient adverse effects occur in most cases and can include vertigo, nausea or vomiting, and mild headaches.13 Longer-term or permanent adverse effects can occur in 5% to 20% of people after Gamma Knife neurosurgery and may include radiation-induced cysts in the brain (which may be asymptomatic or cause neurological sequelae), weight gain, headaches, and loss of interest.13,27,33
Deep Brain Stimulation
DBS involves the semipermanent implantation of probes into the brain that are attached to a battery-operated neurostimulator (pulse generator) implanted subcutaneously (under the skin), near the clavicle (collarbone), chest, or belly.34 The surgery is typically done in 2 stages: one surgery for the probes and another to implant the pulse generator. The optimal target for DBS in OCD is not yet known; however, common targets include the ALIC white matter tract, nucleus accumbens, subthalamic nucleus, and bed nucleus of the stria terminalis.35 The advantages of DBS over ablative neurosurgery are its ability to be tailored to each individual and its potential reversibility.36 The stimulation settings (i.e., amplitude, frequency, pulse width) can be adjusted to minimize side effects while maximizing the therapeutic effect on symptoms.37,38 Probe implantation requires craniotomy, and one or both stages of the surgery may be done under general anesthesia, which bring unlikely but possible complications related to both procedures (e.g., infection, hemorrhage). In addition, there is device maintenance required over time (e.g., battery changes) and potential device-related complications (e.g., device malfunction, infection, pain at implantation site).34 This surgery is not appropriate for people at elevated risk of infection, those taking anticoagulant therapy, or those with certain behaviours (e.g., skin picking, head banging).32 In 2009, the US Food and Drug Administration (FDA) granted a Humanitarian Device Exemption (HDE 50003) to DBS in the ALIC for OCD, and in the same year, DBS for OCD was granted a CE mark in Europe.39 According to clinical experts in this field in Ontario, DBS for OCD may be offered off-label under compassionate grounds and with clinical trial or philanthropic funds. DBS was first used for psychiatric indications in the late 1990s and has been demonstrated to be effective and cost-effective for treatment-refractory OCD, with studies reporting that around 60% to 75% of patients experience clinical response (i.e., Y-BOCS score reduced by ≥35%)40,41 and incremental cost-effectiveness ratios (ICERs) in UK-, Korea-, and Netherlands-based analyses are around $35,000 USD to $65,000 USD per quality-adjusted life-year (QALY) gained relative to nonsurgical therapy.41
Health Technology Under Review
Magnetic resonance-guided focused ultrasound (MRgFUS) is a thermal ablation technology consisting of a special ultrasound transducer helmet, a specialized control console, and real-time magnetic resonance imaging (MRI). It is a noninvasive technique for ablative neurosurgery that uses sound waves to generate precise, targeted lesions in the key brain circuits implicated in OCD. Ultrasound waves are emitted through the intact skull, and when they converge at the focal point, the target brain tissue is heated and ablated (focal coagulative necrosis). The patient's head must be completely shaved and have a stereotactic frame attached. During the procedure, real-time MRI provides detailed images of the brain, allowing for a high degree of precision and minimizing the risk of damage to surrounding tissue.42 Real-time feedback of thermal data throughout the procedure allows the clinical team to precisely adjust the location and temperature parameters.42
MRgFUS can be used to perform capsulotomy instead of a traditional open surgical approach. The procedure takes 2.5 to 4 hours and is completed entirely in the MRI suite, with patients awake. Although an anesthetist is typically present, it is done without the need for general anesthesia. Because MRgFUS takes place inside an MRI machine, this procedure cannot be done if an individual has contraindications to MRI such as incompatible implanted medical devices or body size. The manufacturer notes that the device should not be used for people with substance abuse disorders, renal disease, pregnancy, contrast agent allergies, or cerebrovascular disease.42 Ultimately, it is the joint decision of the neurosurgeon and psychiatrist whether it is appropriate to offer MRgFUS to a patient. In addition, a high skull-density ratio (≥0.40, assessed by brain computed tomography) is key to achieving therapeutic temperature (i.e., to ablate tissue) and is associated with lesion efficacy,43 although a sufficient lesion may still be achieved in patients with less favourable skull densities. MRgFUS forgoes the risks associated with open surgery and provides an option for people who cannot have general anesthesia. It can also provide a treatment option for people with traditional surgical contraindications and people who find invasive procedures, ionizing radiation, or the associated risks unacceptable.
Regulatory Information
The Exablate Neuro (or Exablate 4000; Insightec Ltd) is the only commercially available MRgFUS system for neurological indications. It consists of a piezoceramic helmet transducer with a phased array of 1,024 rays (at 650 Hz) and specialized algorithms to ensure that the beams reach the target, and is compatible with 1.5T and 3T MRI machines.42 The device holds active Health Canada licences as a Class III device (licence numbers 96969 and 103423), as well as regulatory approval from several other jurisdictions. In Canada, OCD is not an approved intended use for the Exablate Neuro; the Exablate Neuro is currently intended for use in the unilateral treatment of refractory essential tremor (thalamotomy) in patients 22 years of age or older.44 Thalamotomy with MRgFUS has been recommended and publicly funded for essential tremor in Ontario since 2018.45,46 Internationally, the intended uses are mainly for movement disorders (e.g., essential tremor, Parkinson's disease) and neuropathic pain (Appendix 1, Table A1). South Korea is the only jurisdiction that mentions behavioural disorders when describing the intended uses of the device.
Ontario, Canadian, and International Context
Ontario and Canada
MRgFUS neurosurgery for OCD requires highly specialized, multidisciplinary clinical teams. At the time of writing, we are aware of 4 sites in Canada with the expertise and equipment required to perform MRgFUS neurosurgery: 1 in Calgary, Alberta; 1 in Montreal, Quebec; and 2 in Toronto, Ontario. One of the sites in Ontario has treated patients with movement disorders, including essential tremor, Parkinson's disease, and dystonia (A. Lozano, MD, PhD, virtual communication, June 6, 2023). The other site in Ontario has assessed many patients with severe, treatment-refractory OCD who are eligible for surgical intervention and has treated some carefully selected patients with MRgFUS, first in the context of a phase I research study (n = 12),47 and subsequent patients have received treatment with support from philanthropic funds. The Exablate Neuro is being used (off-label) under humanitarian and compassionate grounds, obviating the need for an operating room procedure and the risks of open surgery (N. Lipsman, MD, PhD, virtual communication, July 22, 2022).
Referrals for potential candidates for MRgFUS neurosurgery in Ontario can be sent by any physician, including a family doctor, but in most cases a treating psychiatrist makes the referral. Individuals then undergo screening by a multidisciplinary team. The screening process aims to determine the suitability and safety of MRgFUS for an individual patient. People with severe, treatment-refractory OCD must have a primary diagnosis of OCD and are carefully screened for comorbidities that may make them ineligible (e.g., substance abuse disorder, psychosis).
If initial eligibility is met, the individual then undergoes psychiatric assessment by 2 independent psychiatrists, who must independently conclude and agree that it is appropriate to proceed with a neurosurgical consultation. If the individual is deemed a suitable candidate for MRgFUS neurosurgery by the neurosurgeon, the procedure is scheduled, and the individual undergoes preoperative assessment, including brain imaging, bloodwork, neuropsychologic assessment, and other appropriate tests. This is similar to the process for any psychiatric neurosurgery. The time from referral to MRgFUS procedure is estimated to be 6 to 8 months (A. Baskaran, PhD, virtual communication, May 26, 2023). Since 2017, the 1 site in Ontario that offers MRgFUS neurosurgery has provided treatment for 32 carefully selected patients with OCD from Ontario and across Canada (as of December 14, 2023), with about 1 patient per month undergoing MRgFUS (A. Baskaran, PhD, email communication, December 14, 2023). The volumes to date are based on a balance of the need for MRgFUS with the site's capacity to treat based on clinical and human resources (e.g., MRgFUS suite availability, MRI time, surgeon time and scheduling) (N. Lipsman, MD, PhD, virtual communication, May 26, 2023).
The University of Calgary-affiliated site in Alberta lists essential tremor and OCD (bilateral capsulotomy) as indications for MRgFUS neurosurgery.48 According to the Focused Ultrasound Foundation, this is provided in the context of clinical research.49 The Montreal Neurological Institute and Hospital site treats only essential tremor with MRgFUS.49
International
Most international guidelines do not provide any recommendations about the use of MRgFUS for treatment-refractory OCD, except the 2023 guideline from the World Federation of Societies of Biological Psychiatry18 (see Table 2). In 2021, the National Evidence-based Healthcare Collaborating Agency in South Korea assessed the safety and effectiveness of MRgFUS for neurosurgical indications, including treatment-refractory OCD.50 The clinical systematic review results were examined by the New Health Technology Assessment (nHTA) Committee in South Korea, which concluded that further research was required to determine safety and effectiveness in improving symptoms.50 Ablative neurosurgery with MRgFUS for OCD is available at Yonsei University Health System, Severance Hospital in South Korea.51 The National Institute for Health and Care Excellence (NICE) guideline has not been updated since 2005 and does not recommend ablative neurosurgery for severe, chronic, treatment-resistant OCD unless requested by the patient.21 All more-recent guidelines state that DBS or ablative neurosurgeries can be considered as last resorts for treatment-refractory OCD (Table 2).
Table 2:
Summary of Recommendations for Neurosurgeries for Treatment-Refractory OCD
| Entity (jurisdiction) | Recommendation | Source(s) |
|---|---|---|
| American Psychiatric Association (US) | Ablative neurosurgery for severe, treatment-refractory OCD is rarely indicated and, along with DBS, should be performed only at sites with expertise in both OCD and these treatment approaches | American Psychiatric Association, 200752,53 |
| Canadian clinical practice guidelines (Canada) |
Ablative neurosurgery Capsulotomy or cingulotomy may be effective in reducing symptoms in patients with severe, treatment-refractory OCD; however, these treatments are usually considered last resorts |
Katzman et al, 20142 |
|
DBS DBS may improve symptoms and functionality in up to two-thirds of patients with highly treatment-refractory OCD |
Katzman et al, 20142 | |
| Congress of Neurological Surgeons and the American Society for Stereotactic and Functional Neurosurgery (US) |
DBS Bilateral subthalamic nucleus DBS is recommended for medically refractory OCD, above best medical management; bilateral nucleus accumbens or BNST DBS may also be used |
Staudt et al, 202154 |
| Indian Psychiatric Society (India) |
Ablative neurosurgery/DBS Can be recommended for carefully selected patients with treatment-refractory OCD after discussing the pros and cons |
Janardhan Reddy et al, 201731 |
| NICE (UK) |
Ablative neurosurgery Not recommended for severe, chronic, treatment-resistant OCD unless a patient requests it; many considerations and due processes |
NICE, 20 0521,a |
|
DBS DBS for chronic, severe, treatment-resistant OCD in adults should be done only in the context of research |
NICE, 202155 | |
| Royal College of Psychiatrists Position Statement (UK) |
Ablative neurosurgery Anterior capsulotomy and anterior cingulotomy are considered part of acceptable, safe, effective, and established clinical practice in the UK for chronic, otherwise treatment-refractory OCD |
Royal College of Psychiatrists, 201728 |
|
DBS Should be considered investigational and be used only within a research protocol that has full ethics approval |
Royal College of Psychiatrists, 201728 | |
| World Federation of Societies of Biological Psychiatry (International) |
Ablative neurosurgery RFA, Gamma Knife, and MRgFUS should be restricted to carefully selected patients with treatment-refractory OCD |
Bandelow et al, 202318 |
|
DBS DBS should be restricted to carefully selected patients with treatment-refractory OCD |
Bandelow et al, 202318 |
Abbreviations: BNST, bed nucleus of the stria terminalis; DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; NICE, National Institute for Health and Care Excellence; OCD, obsessive–compulsive disorder; RFA, radiofrequency ablation.
Evidence update completed in 2013; no new evidence was identified relevant to intensive or inpatient treatments for OCD.20
Individual Preferences for Neurosurgery
Whether a person receives ablative neurosurgery or DBS depends on many factors beyond medical and psychiatric suitability. For example, there may be a strong preference for a single ablative procedure or a minimally invasive approach with radiosurgery, or varying acceptability of implants in the body. There is also consideration of accessibility to highly specialized centres, given that DBS requires many follow-up visits after surgery to learn how to use the device and adjust the settings.32
People who underwent neurosurgical intervention, such as DBS, for treatment-refractory OCD have reported marked improvement in symptoms and satisfaction with their procedure.56 In feedback collected from patients, they noted that their symptoms and function improve considerably, they are satisfied with DBS as a treatment, and they rate their quality of life as better following surgery and finetuning of DBS settings.56 People with OCD who underwent DBS recommended improving the awareness and availability of DBS as a treatment option.56 Some patients and members of the medical community may be hesitant about neurosurgical treatment for OCD; this stems from other historical psychiatric surgeries (e.g., lobotomy) that do not resemble modern neurosurgical techniques.32
Equity Context
It is well documented that people with OCD experience a variety of complex issues and are far more likely than the general population to report needing psychological help and not receiving it.7 People with severe OCD experience stigma and discrimination in addition to severe illness, which compounds poor quality of life, social exclusion, and low self-esteem.57,58 In Canada, people with OCD are more likely to have lower income or live in rural areas, and are less likely to be employed.7 There is also a slightly higher prevalence of OCD among females than males (1.04% versus 0.81%).7 Although the prevalence of OCD is slightly higher among females and there are some socioeconomic and demographic trends among people with OCD compared with the general population, no specific subgroup or population was identified that would likely benefit more from this intervention over another.
Therefore, if MRgFUS neurosurgery for treatment-refractory OCD is funded and implemented in Ontario, it is not expected that it would contribute to inequality among people with different equity factors; rather, it is expected that public funding could improve equity through improved access for people who do not want or cannot undergo invasive ablative surgery, or who are waiting long durations with substantial distress and disability due to surgical wait times and backlog. Public funding and implementation will expand access to a last-line treatment option that can positively impact patient quality of life.
Expert Consultation
We engaged with clinical experts in the specialty areas of neurosurgery and psychiatry to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD 42023457743), available at crd.york.ac.uk/PROSPERO.
Clinical Evidence
Research Question
What are the effectiveness and safety of magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery for the treatment of people with treatment-refractory obsessive–compulsive disorder (OCD)?
Methods
Clinical Literature Search
We performed a clinical literature search on August 14, 2023, to retrieve studies published from January 1, 2013, until the search date, given that the phase I trials of the Exablate Neuro were published in 2013. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the National Health Service Economic Evaluation Database (NHS EED), and APA PsycInfo.
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.59
We created database auto-alerts in MEDLINE, Embase, and APA PsycInfo and monitored them until November 14, 2023. We also performed a targeted grey literature search of the International HTA Database, the websites of health technology assessment organizations and regulatory agencies, and clinical trial and systematic review registries, following a standard list of sites developed internally. See Appendix 2 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published since January 1, 2013
-
Comparative (randomized or nonrandomized) or single-arm cohort studies, case series, and case reports
-
∘
Case reports were excluded if case series were identified, given that the latter have greater potential for statistical power and external validity to inform conclusions and evidence-based recommendations
-
∘
-
Systematic reviews (including meta-analyses and health technology assessments that include a systematic review) that match our research question and inclusion criteria
-
∘
Systematic reviews must clearly report literature search methods, including (at a minimum) information about the databases searched, search terms, and search dates
-
∘
Systematic reviews that have a broader scope must also report separate patient characteristics, methods, results, and critical appraisal for our population, intervention, comparators, and outcomes (PICO) in sufficient detail
-
∘
Exclusion Criteria
Editorials, commentaries, narrative reviews, conferences abstracts, posters, letters, and preprints
Non-English full-text reports and publications
Studies with broader or diverse populations from which participant characteristics, methods, and results for those with OCD cannot be extracted
Proof-of-concept, feasibility, or technical validation studies (i.e., not clinical application)
Animal and in vitro studies
Participants
Inclusion Criteria
Adults (≥18 years old) with severe, treatment-refractory OCD, as defined by the study (e.g., treatment failures, severity according to Yale-Brown Obsessive–Compulsive Scale [Y-BOCS] total severity score, Clinical Global Impression [CGI] score, clinical judgement of patients’ functional limitations)
Exclusion Criteria
Mild or moderate OCD, presence of psychotic symptoms in the past or present, active severe substance abuse, comorbid dementia or neurodegenerative disorders
Interventions
Inclusion Criteria
-
MRgFUS psychiatric neurosurgery, with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
-
∘
Anterior capsulotomy, anterior cingulotomy, caudate tractotomy, or limbic leukotomy (bilateral or unilateral)
-
∘
Exclusion Criteria
Other brain targets or neurosurgeries
Comparators
Inclusion Criteria
Radiofrequency ablation (RFA), with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
Gamma Knife radiosurgery, with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
Deep brain stimulation (DBS), with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
No comparator
Exclusion Criteria
Nonsurgical treatments (e.g., deep transcranial magnetic stimulation [dTMS], pharmacotherapy, cognitive behavioural therapy [CBT], exposure and response prevention [ERP] therapy, intensive inpatient treatment)
Outcome Measures
-
OCD symptoms, for example:
-
∘
Change in Y-BOCS or Clinical Global Impression - Improvement (CGI-I) scores
-
∘
Partial or complete response (e.g., >25% to <35% reduction or ≥35% reduction Y-BOCS score, respectively)
-
∘
Clinical remission (e.g., ≥55% improvement of Y-BOCS score, Y-BOCS score < 7)
-
∘
Adverse effects and events
Neurocognitive changes (e.g., personality changes)
Technical failure (e.g., unable to achieve therapeutic temperature, patient cannot tolerate head frame or sonications during procedure)
Follow-up interventions or re-treatment
Patient quality of life (e.g., functionality)
Patient satisfaction
Literature Screening
Two reviewers screened titles and abstracts to assess the eligibility of a sample of 100 citations to validate the inclusion and exclusion criteria. Ninety-percent agreement was reached prior to discussing conflicts, after which 100% agreement was confirmed. One reviewer then screened all remaining citations using Covidence systematic review management software60 and obtained the full texts of studies that appeared eligible for the review, according to the inclusion criteria. The same reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists of all identified systematic reviews and all included studies for any additional relevant studies not identified through the search, and consulted clinical experts for feedback on omissions regarding pivotal studies. Citation flow and reasons for exclusion for full-text articles are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.61
Data Extraction
We extracted relevant data on study design and characteristics; risk-of-bias items; results; and population, intervention, comparator, outcome, time, and setting (PICOTS) using a data form to collect information on the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, whether the study compared 2 or more groups)
Outcomes (e.g., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, time points at which the outcomes were assessed)
Where multiple articles reported on the same outcomes and patients, we extracted data from the most comprehensive and recent publication(s), supplemented with information from others as needed.
Equity Considerations
We used the PROGRESS-Plus framework to help explicitly consider health equity in our health technology assessment.62 PROGRESS-Plus is a health equity framework used to identify population and individual characteristics across which health inequities may exist. These characteristics include place of residence; race or ethnicity, culture, or language; gender or sex; disability; occupation; religion; education; socioeconomic status; social capital; and other key characteristics (e.g., age) that may stratify health opportunities and outcomes. Potential equity issues related to the research question were not evident during scoping. However, we report the available characteristics of participants in the included studies (e.g., PROGRESS-Plus categories).
Statistical Analysis
For cases in which information about people with OCD was combined with that of other patient populations, we calculated measures of central tendency and descriptive statistics (e.g., mean, standard deviation) for people with OCD only from the data available in the published articles and supplementary materials.
One reviewer assessed for the presence and extent of statistical, methodological, and clinical heterogeneity and considered this when interpreting the results.63 We did not perform a meta-analysis given the very low number of studies and methodological diversity between studies.64,65 Therefore, results are summarized using structured tabulation66 and narrative summaries.
Critical Appraisal of Evidence
We assessed risk of bias using the Joanna Briggs Institute's Critical Appraisal Checklist for Case Series67 (Appendix 3).
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.68 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence.
Results
Clinical Literature Search
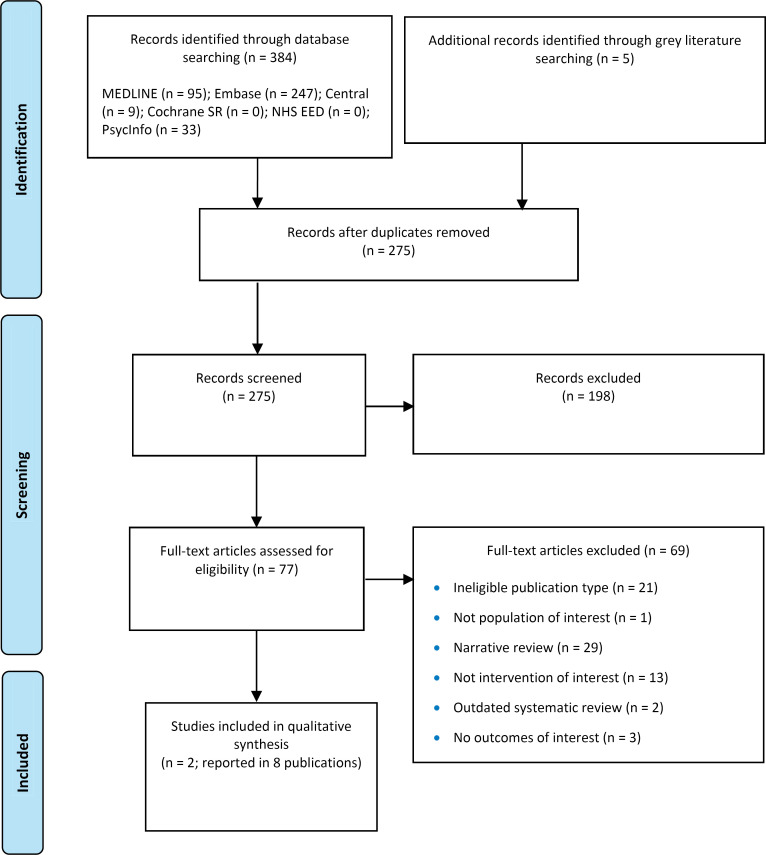
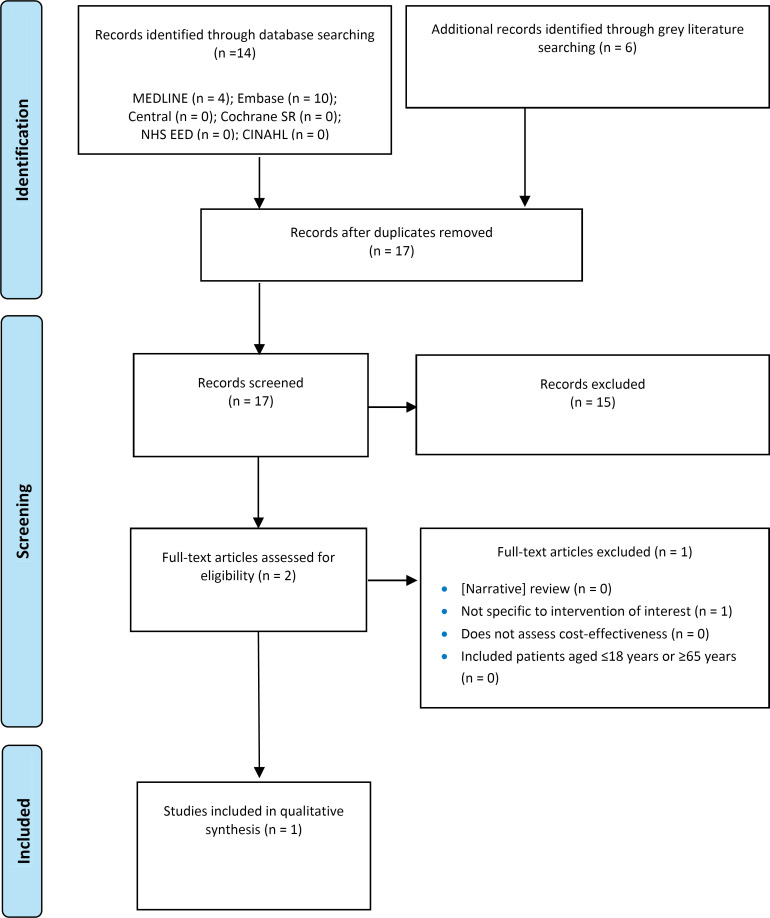
The systematic search of the clinical literature yielded 275 citations published between January 1, 2013, and August 14, 2023, including grey literature and after duplicates were removed. We did not identify any additional eligible studies from other sources, including database alerts (monitored until November 14, 2023). We identified 3 systematic reviews14,69,70 that were ineligible; specifically, they did not match our research question, did not provide adequate literature search details, or the literature search was outdated (e.g., missing the most recent studies published). As per our protocol, single case reports were excluded given that larger case series were identified. One case report71 was also subsumed in a publication of a case series72; therefore, the individual report was excluded. See Appendix 4 for a list of selected studies excluded after full-text review. We included 2 primary studies (case series) reported in 8 publications.72–79 Figure 1 presents the PRISMA flow diagram for the clinical literature search.
Figure 1: PRISMA Flow Diagram - Clinical Systematic Review.
PRISMA flow diagram showing the clinical search strategy. The search of the clinical literature yielded 275 citations published between January 1, 2013, and August 14, 2023, including grey literature searches and after duplicates were removed. We screened the abstracts of the 275 identified studies and excluded 198. We assessed the full text of 77 articles and excluded a further 69. In the end, we included 2 articles in the qualitative synthesis.
Abbreviations: Cochrane SR, the Cochrane Database of Systematic Reviews; NHS EED, National Health Service Economic Evaluation Database; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Page et al.61
Characteristics of Included Studies
We included 2 studies that examined MRgFUS capsulotomy in patients with severe, treatment-refractory OCD. One study was conducted in Canada72 and the other in South Korea,79 and both registered protocols on clinical trial registries.
The study conducted in South Korea published 2 articles on subsets of study participants, including preliminary clinical findings78 and an analysis of neural oscillation patterns after the procedure.73 However, no outcome data from these publications are reported, given that it was only a subset of all patient data and another publication reported outcomes of the entire case series.79 The Canadian researchers reported results of the clinical trial on MRgFUS for OCD along with a second trial of the same intervention for patients with treatment-refractory major depressive disorder.72 Therefore, we separated and reported only on the characteristics, procedural information, analysis, and results of patients with OCD as available from the published article and supplementary materials.
The clinical and demographic characteristics of study participants are presented in Table 3. These open-label case series prospectively followed patients with treatment-refractory OCD who did not respond to multiple trials of pharmacologic and cognitive-behavioural therapies and underwent MRgFUS capsulotomy. To be eligible for MRgFUS, individuals were required to have a primary diagnosis of OCD with a minimum duration of 3 to 5 years and a Y-BOCS score of 28 or greater (i.e., severe OCD). There was 1 exception to this score cut-off in the Canadian study; the authors state that 1 person was treated outside the clinical trial, on humanitarian grounds, given that they were substantially impaired despite not meeting the Y-BOCS criteria (i.e., score of 23 arising from their OCD manifesting with minimal compulsions).72
Table 3:
Characteristics of Studies Included in the Clinical Systematic Review
| Author, year, country | Study design | Participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study type | Procedure (brain target) | Follow-up | Outcomes of interest | Population | Sample size, N (n with psychiatric comorbidity) | Duration of OCD, mean (range), y | Age, mean (SD), y | Sex, M:F | |
| Davidson et al, 2020,72,74–76 Canada | Prospective, open-label case series | Bilateral MRgFUS capsulotomy using Exablate Neuro (ALIC) | 6-12 moa | Y-BOCS score Adverse events Neurocognitive test results Technical failure Quality of life Follow-up interventions |
Primary diagnosis of OCD (DSM-V) and minimum illness duration of 5 y, Y-BOCS score ≥ 28 and refractory to
|
6a (4a) | 14.5 (6-24) Median, 13 | 31 (4.7b) Median, 31 | 3:3 |
| Kim et al, 20 18,73,77–79 South Korea | Prospective, open-label case series | Bilateral MRgFUS capsulotomy using Exablate Neuro (ALIC) | 2 y | Y-BOCS score CGI score Global functioning Adverse events Technical failure Follow-up interventions | Primary diagnosis of OCD (DSM-IV) and more than 5 y of symptoms and dysfunction, Y-BOCS score ≥ 28 and refractory to
|
11 (7) | 14.6 (9-24) Median, 13 | 32 (8.1) Median, 34 | 5:6 |
Abbreviations: ALIC, anterior limb of the internal capsule; CBT, cognitive behavioural therapy; CGI, Clinical Global Impression; DSM, Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, 4th edition; DSM-V, 5th edition); ERP, exposure and response prevention; F, female; M, male; MRgFUS, magnetic resonance-guided focused ultrasound; OCD, obsessive–compulsive disorder; SD, standard deviation; SSRI; selective serotonin reuptake inhibitor; Y-BOCS, Yale-Brown Obsessive–Compulsive Scale.
Number of patients with OCD. Nine patients with OCD were enrolled; 1 was excluded after consent but before MRgFUS. Of the 8 patients with OCD for whom MRgFUS was attempted, the procedure was not completed in 2 cases, and information on the characteristics of these 2 patients is not available.72 One additional patient was treated after March 2019 but before December 2019 and had not reached 6 months of follow-up as of September 2019 when analysis was conducted.74
Calculated from data on 6 patients with OCD available in the publication and its published supplementary material.72
MRgFUS neurosurgery was performed in a 3T MRI using the Exablate Neuro system (Insightec Ltd, Haifa, Israel). The surgical target was the anterior limb of the internal capsule (ALIC), and capsulotomy was performed bilaterally. In the Canadian study, the procedure lasted 3 to 4 hours72 and was performed under conscious sedation (e.g., low-dose propofol or dexmedetomidine74) to ensure patients were more comfortable, especially during the high-energy sonications. Nasal oxygen was also provided to patients during the procedure, and all patients underwent postoperative imaging to examine the lesion. In the South Korean study, patients were fully awake and responsive throughout the 5- to 7-hour MRgFUS procedure, underwent postprocedure MRI scans, and were monitored as inpatients for 24 hours, then every 2 to 4 weeks at outpatient psychiatric clinic visits.78
Neither study provided CBT or ERP therapy as a co-intervention after MRgFUS neurosurgery, but in 1 study, patients were reminded of the techniques they had learned by psychiatrists at follow-up visits during the study.78 In both studies, patients were required to be on a stable medication regimen (i.e., no changes) for at least 30 days prior to MRgFUS capsulotomy. In the South Korean study,79 all patients continued to take their previous medication regimen and dosage throughout the entire 2-year study period,77 whereas in the Canadian study, although patients were encouraged to continue on the same medications and doses in the postoperative period, medication changes occurred at the discretion of the treating psychiatrist; only 2 of the participants did not have any medication changes during the follow-up period.72
There was no information reported in either study about study participants’ race, ethnicity, culture, place of residence, socioeconomic status, occupation or work arrangements, gender identity, sexual orientation, religion, educational level, social capital, or disabilities.
Risk of Bias in the Included Studies
The detailed results of the risk-of-bias assessment are presented in Appendix 3, Table A2.67 There were some unclear aspects related to the selection of participants, complete inclusion of cases,72,79 and inclusion of consecutive cases.72 The demographic characteristics of study participants were partially unclear.72,79
OCD Symptoms
Y-BOCS Score
The studies assessed the severity of OCD symptoms at baseline and throughout follow-up. Both studies72,79 assessed the Y-BOCS score of most or all patients at 1, 3, 6, and 12 months after the procedure, and Kim et al79 assessed Y-BOCS additionally at 1 week and 24 months. In the study by Davidson et al,72 a Y-BOCS score was not available for all 6 patients at each follow-up time point; 1 patient was treated outside of the clinical trial and another had not yet reached 12 months of follow-up (Table 4).
Table 4:
Y-BOCS Score Over Time Before and After MRgFUS Capsulotomy
| Author, year | Country | Y-BOCS score, mean, SD (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 wk | 1 mo | 3 mo | 6 mo | 12 mo | 24 mo | ||
| Davidson et al, 202072 | Canada | 33, 7.6 (6) | NA | 29.8, 3.1 (5) | 28.6, 5.7 (5) | 25.8, 8.6 (6) | 20, 2.1 (4) | NA |
| Kim et al, 201879 | South Korea | 34.4, 2.3 (11) | 30.3,a 4.3 (11) | 28.2,b 4.6 (11) | 25.9,b 4.2 (11) | 23.6,b 4.5 (11) | 21.8,b 4.8 (11) | 21.3,b 6.2 (11) |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not assessed at this time point; SD, standard deviation; Y-BOCS, Yale-Brown Obsessive–Compulsive Scale.
Statistically significant change from baseline (P = .035) in post hoc analysis with Bonferroni correction.79
Statistically significant change from baseline (P < .05) in post hoc analysis with Bonferroni correction.79
The numeric value of the Y-BOCS scores decreased at every follow-up in both studies (Table 4), indicating an improvement in OCD symptoms. Kim et al79 analyzed the change in Y-BOCS score with a linear mixed model for repeated measures and found a statistically significant decrease in mean scores over the follow-up period (P < .001). Statistically significant improvement from baseline was seen as early as 1 week after the procedure (P = .03), and scores continued to improve at every follow-up visit (statistically significant, P < .05 for all; post hoc analysis with Bonferroni correction).79
Using a linear mixed model for repeated measures, Kim et al79 reported that Y-BOCS scores decreased over the 24-month follow-up; this decrease was statistically significant (P < .001). Compared with baseline, improvements in OCD symptoms were seen starting as early as 1 week after the procedure (P = .035) and at each time point thereafter (P < .05 for all, in the post hoc analysis for changes from baseline with Bonferroni corrections).79
The reductions in mean Y-BOCS scores (Table 4) translated into changes in symptom severity10 from extreme at baseline, to severe during approximately the first 6 months, to moderate at the end of follow-up.
Davidson et al72 reported the mean change in Y-BOCS score before and after the procedure. This was analyzed at the last available follow-up for each patient; for 4 patients, this was at 12 months, and for the other 2 patients, this was at 6 months, as they had not yet reached 12 months since MRgFUS capsulotomy. Across the patients with OCD, the mean percent change in Y-BOCS score at last follow-up was a statistically significant reduction of 33.3% (P = .03; Table 5).72 The percent change (reductions) from baseline to the 6-, 12-, and 24-month follow-ups in the study by Kim et al79 were numerically similar to those in the study by Davidson et al72 (Table 5).
Table 5:
Change in Y-BOCS Score from Baseline After MRgFUS Capsulotomy
| Author, year | Time point | Mean change from baseline, % (SD) | P value |
|---|---|---|---|
| Davidson et al, 202072 | Last follow-upa | -33.3 (NR) | .03 |
| Kim et al, 201879 | 6 mo | -31.1 (13.3) | NR |
| 12 mo | -36.1 (15.3) | NR | |
| 24 mo | -37.8 (18.9) | NR |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NR, none reported; SD, standard deviation; Y-BOCS, Yale-Brown Obsessive–Compulsive Scale.
For 4 patients, this was at 12 months, and for the other 2 patients, this was at 6 months, as they had not yet reached 12 months since MRgFUS capsulotomy.72
The GRADE quality of the evidence was rated as Very low, given limitations related to imprecision (Appendix 3, Table A3).
CGI Score
One study79 also measured OCD symptoms using the CGI scale, a common clinical research tool administered by an experienced clinician to quantify and track a patient's psychiatric illness.80 The CGI scale consists of 2 items: severity (CGI-S) and improvement (CGI-I). CGI-S reflects an assessment of psychopathology over the past week (rated from 1 to 7, with higher scores reflecting greater severity), whereas CGI-I reflects on improvements since a treatment was administered (rated from 1 to 7, with lower scores reflecting improvement and higher scores reflecting worsening).80 The descriptions of each score for CGI-S and CGI-I are listed in the footnotes of Table 6. Each CGI item is a single question, assessed at baseline and subsequent visits. Given that CGI-I is assessed relative to pretreatment, there is no baseline score.
Table 6:
CGI Scores Over 24-Month Follow-Up After MRgFUS Capsulotomy
| Scale | CGI scores, mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 wk | 1 mo | 3 mo | 6 mo | 12 mo | 24 mo | P valuea | |
| CGI-Sb | 6.1 (0.2) | 5.6 (0.2) | 5.4 (0.3) | 4.5 (0.2) | 4.4 (0.2) | 4.0 (0.2) | 3.9 (0.3) | .001 |
| CGI-Ic | — | 3.4 (0.2) | 3.2 (0.3) | 2.7 (0.2) | 2.5 (0.2) | 2.0 (0.3) | 2.1 (0.3) | <.001 |
Abbreviations: CGI, Clinical Global Impression; CGI-I, Clinical Global Impression - Improvement; CGI-S, Clinical Global Impression - Severity; MRgFUS, magnetic resonance-guided focused ultrasound; SD, standard deviation.
Analyzed using a linear mixed model for repeated measures across the 24-month follow-up period.
Scores reflect the severity of mental illness the patient is experiencing at a given time (1, normal, not at all ill; 2, borderline; 3, mildly ill; 4, moderately ill; 5, markedly ill; 6, severely ill; 7, among the most extremely ill patients).80
CGI was assessed at baseline, 1 week, and 1, 3, 6, 12, and 24 months after MRgFUS capsulotomy. The mean baseline CGI-S score was about 6 (i.e., severely ill). As shown in Table 6, CGI-I scores showed a statistically significant decrease over 24 months of follow-up (P < .001), with mean scores of approximately 2 (i.e., much improved) at the last follow-up.79 Similarly, a statistically significant reduction was seen for CGI-S scores after MRgFUS capsulotomy, with a mean score at 24 months of approximately 4 (i.e., moderately ill; P = .001).79
The GRADE quality of the evidence was rated as Very low, given limitations related to imprecision (Appendix 3, Table A3).
Treatment Response
More than half of the participants in the studies experienced complete or partial treatment response (Table 7); however, the definition of response differed between studies. Davidson et al72 defined treatment response considering the Y-BOCS score, whereas Kim et al79 considered both Y-BOCS and CGI scores to define response.
Table 7:
Treatment Response After MRgFUS Capsulotomy
| Author, year | Time point | Responders, % (n) | Partial responders, % (n) | Remission, % (n) | Nonresponders, % (n) |
|---|---|---|---|---|---|
| Davidson et al, 202072 | Last follow-upa | 66.7 (4) | NR | NR | 33.3 (2) |
| Kim et al, 201879 | 12 mo | 54.5 (6)b | 27.3 (3)c | - | 18.2 (2) |
| 24 mo | 54.5 (6) | 18.2 (2) | 9.1 (1)d | 18.2 (2) |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NR, not reported.
For 4 patients, this was 12 at months, and for the other 2 patients, this was at 6 months, as they had not yet reached 12 months since MRgFUS capsulotomy.72
Defined as ≥35% reduction from baseline Y-BOCS score and much or very much improved on CGI—I (i.e., rating of 1 or 2).79
Defined as 25% to 35% reduction from baseline Y—BOCS score and minimally improved on CGI—I (i.e., rating ≥ 3).79
Defined as Y-BOCS score ≤ 12 and normal or borderline mentally ill on CGI-S (i.e., rating of 1 or 2).79
In the study by Davidson et al,72 4 patients (66.7%) met the criteria for complete response (≥35% reduction in Y-BOCS score) at last follow-up. The remaining 2 patients did not meet the criteria for partial response. In aggregate, the mean reduction at last follow-up (-33.3%; Table 5) did not meet the predefined criteria for complete response but qualified as partial response (>25% to <35% reduction in Y-BOCS score).
In the study by Kim et al,79 patients’ standard Y-BOCS score reductions and CGI scores were both considered in the categorization of responders (≥35% reduction in Y-BOCS score, and CGI-I score of 1 or 2), partial responders (25% to 35% reduction in Y-BOCS score, and CGI-I score of ≥3), or remission (Y-BOCS score of ≤12, and CGI-S score of 1 or 2). Nearly 55% (n = 6) of those who underwent MRgFUS capsulotomy were considered responders at the 12-month follow-up, and about 27% (n = 3) were partial responders. At 24 months after the procedure, 1 person (9.1%) was in remission, while the proportion of responders remained the same at nearly 55% (n = 6) and about 18% of participants (n = 2) were partial responders.
The GRADE quality of the evidence was rated as Very low, given limitations related to imprecision (Appendix 3, Table A3).
Adverse Events
Both studies commented on the absence of serious adverse events or persistent physical, neurological, or psychological complications.72,79 Davidson et al72 reported that no serious treatment-related adverse events occurred during the study, and that there were no suicide attempts during follow-up.
Kim et al79 reported that none of the adverse events observed in previously published studies on RFA capsulotomy — fatigue, urinary incontinence, seizures, and behavioural changes (e.g., hypomania, personality changes, emotional blunting, indifference, or carelessness) — occurred in any patient during the 24 months of follow-up after MRgFUS capsulotomy. They also commented that no mania or hypomania; impulsivity, disinhibition, or executive dysfunction; or alcohol consumption was reported by patients or observed by their caregivers and psychiatrists over the study period.
The procedure was well tolerated by patients, with nearly two-thirds experiencing some transient adverse effect. The presence of physical or neurological adverse effects during the procedure was assessed before and after each sonication (range 23 to 26 sonications, lasting 10 to 31 seconds each) by both a neurosurgeon (i.e., lateralizing symptoms and signs) and a psychiatrist (i.e., mood changes, decreases in cognition).78
Nonserious adverse effects occurred in 4 of 6 patients with OCD (66.6%) who underwent MRgFUS capsulotomy in the study by Davidson et al.72 The most common nonserious adverse effects were swelling (n = 2, 33.3%), redness or pain at the stereotactic head frame pin site (n = 2, 33.3%), and mild headaches (n = 3, 50%). These effects lasted from less than 24 hours to 5 days, and no intervention was reportedly needed to resolve them72 (Table 8).
Table 8:
Nonserious Adverse Events During and After MRgFUS Capsulotomy
| Adverse event reported | n (%) | Details |
|---|---|---|
| Davidson et al, 202072 | ||
| Headache | 3 (50%) | Duration <24 h to 5 d and resolved without intervention |
| Pin-site swelling | 2 (33.3%) | |
| Pin-site erythema | 2 (33.3%) | |
| Kim et al, 201879 | ||
| Headache - short, periodic, mild during procedure at high-temperature sonications | 7 (63.6%) | Resolved spontaneously or after 1 dose of analgesic medication |
| Nausea, vomiting, dizziness during procedure | 5 (45.5%) | Resolved at end of procedure or after 1 dose of antiemetic medication |
| Increased anxiety | 3 (27.3%) | Resolved after 1 dose of benzodiazepine |
| Stomach upset | 2 (18.2%) | Resolved with 1 dose of H2 blocker medication |
| Transient warm sensation in brain during high sonications | 1 (9.1%) | None reported |
Abbreviations: H2, histamine type 2 receptor; MRgFUS, magnetic resonance-guided focused ultrasound.
Kim et al79 similarly reported transient nonserious adverse effects that resolved spontaneously, at completion of the procedure, or with a single dose of medication. The most common nonserious adverse effects were headache (n = 7, 63.6%), vestibular symptoms (e.g., nausea or vomiting; n = 5, 45.5%), anxiety (n = 3, 27.3%), stomach upset (n = 2, 18.2%), and transient warm sensation in the brain (n = 1, 9.1%).79 The study also reported that there were no statistically significant changes in body weight during the 24-month follow-up period.
The GRADE quality of the evidence was rated as Very low, given limitations related to imprecision (Appendix 3, Table A3).
Neurocognitive Changes
Both studies administered a series of neuropsychological tests during the study.
In the Canadian case series,75 5 of 6 patients with OCD underwent baseline and postoperative neuropsychological assessment including verbal learning, visuospatial memory, executive function, frontal systems behaviour, and symbol-digit testing. A series of tests assessing intellectual function, executive function, episodic memory, and processing speed was administered at baseline and at 6 and 12 months after the procedure. The Wechsler Test of Adult Reading (WTAR) was measured only once and determined that baseline intellectual functioning was average to high-average. Tests administered after MRgFUS capsulotomy included the California Verbal Learning Test, second edition (CVLT-II; comprising 4 scores: total recall, delayed free recall, delayed cued recall, and delayed recognition discrimination); the Brief Visuospatial Memory Test - Revised (BVMT-R; immediate and delayed recall scales); the Symbol Digit Modalities Test (SDMT); the Frontal Systems Behavior Scale (FrSBe; self-report version, including total, apathy, disinhibition, and dysexecutive scores, with lower scores representing fewer behavioural symptoms); the Delis-Kaplan Executive Function System (D-KEFS) sorting test (correct sorts and description scores); and the Iowa Gambling Task (IGT). To minimize practise effects on the repeated tests, different versions (alternate forms) of the CVLT-II, D-KEFS sorting test, and BVMT-R were used at measurement time points; therefore, scores were standardized using published normative data for analysis.
In their statistical analysis, Davidson et al75 combined the results of 5 patients who had OCD with the results of 5 patients who had treatment-refractory major depressive disorder to determine the change in test scores after MRgFUS capsulotomy from baseline. A change of ≥2 standard deviations (SD) on each neuropsychological test was considered clinically meaningful. Davidson et al75 report that there were no negative effects of MRgFUS capsulotomy on cognitive or behavioural function, and potentially some modest improvements in apathy and executive function for patients with OCD and patients with major depressive disorder. Among the 5 patients with OCD, 1 exhibited improved performance of ≥2 SD on at least 1 score at 6 months, and 4 patients showed improvement of ≥2 SD on at least 1 measure at 12 months. None of the patients with OCD experienced a clinically meaningful decline (i.e., ≥2 SD from baseline) on any neuropsychological test score at the 6- or 12-month follow-up after MRgFUS capsulotomy. As there were no statistical analyses of the neuropsychological test results for only the patients with OCD, we calculated the mean scores over the study period for the patients with OCD from available data in the report by Davidson et al75 to provide a descriptive overview (Appendix 5, Table A4).
In the South Korean study,79 an analogous series of neuropsychological tests was conducted at baseline and at 6, 12, and 24 months after MRgFUS capsulotomy (Table 9). The tests assessed intellectual function (Wechsler Adult Intelligence Scale, Korean version [K-WAIS]), memory (memory quotients of the Rey-Kim Memory Test), executive function (Controlled Oral Word Association Test [COWAT] and Korean Colour Word Stroop Test [Stroop]), and attention (Digit Span test). All 11 study participants completed the tests at baseline and at the 6-month follow-up. However, only 8 participants completed the neuropsychological tests at 12 months, and 10 participants completed them at the 24-month followup. Changes in neuropsychological function over the study period were statistically analyzed using a linear mixed model for repeated measures, as well as with post hoc analyses of change from baseline for each variable at each time point, with Bonferroni correction.
Table 9:
Neuropsychological Function Before and After MRgFUS Capsulotomy
| Test | Function(s) measured | Baseline, mean (SD) (n = 11) | 6 mo, mean (SD) (n = 11) | 12 mo, mean (SD) (n = 8) | 24 mo, mean (SD) (n = 10) | P value |
|---|---|---|---|---|---|---|
| Wechsler Adult Intelligence Scale, Korean version (K-WAIS) | Cognitive ability | 90.9 (19.3) | 93.5 (19.7) | 95.9 (20.7) | 95.5 (14.0) | >.05 |
| Memory quotients of the Rey-Kim Memory Test | Memory | 94.4 (13.9) | 103.3 (13.3) | 110.3 (13.7) | 110.1 (14.8) | <.001 |
| Controlled Oral Word Association Test (COWAT) | Verbal fluency - semantic | 19.5 (6.4) | 18.3 (5.8) | 18.3 (6.6) | 17.4 (3.9) | >.05 |
| Verbal fluency - phonemic | 35.5 (15.0) | 38.2 (20.6) | 41.1 (14.3) | 36.6 (7.0) | >.05 | |
| Korean Colour Word Stoop Test | Cognitive processing |
1.25 (0.32) | 1.30 (0.48) | 1.22 (0.45) | 1.16 (0.28) | >.05 |
| Digit Span test | Memory | 10.2 (2.3) | 10.1 (2.5) | 11.3 (2.8) | 11.4 (2.2) | >.05 |
Abbreviations: OCD, obsessive–compulsive disorder; MRgFUS, magnetic resonance-guided focused ultrasound; SD, standard deviation. Source: Kim et al.79
No statistically significant changes were observed in executive function, intellectual function, or attention after MRgFUS capsulotomy.79 Memory quotient scores improved over 24 months of follow-up (statistically significant, P < .001); however, practise effects cannot be ruled out as a potential contributor to this finding. There were no statistically significant changes in the other neuropsychological tests assessed after the procedure (i.e., K-WAIS, COWAT, Stroop, and Digit Span; Table 9).
The GRADE quality of the evidence was rated as Very low, given limitations related to risk of bias and imprecision (Appendix 3, Table A3).
Technical Failure
The information available on technical failure pertained to the inability to complete the procedure (i.e., create a therapeutic lesion), as opposed to issues with the technology (e.g., device malfunction). MRgFUS capsulotomy could not be completed in 2 of the 8 people with treatment-refractory OCD (25%) enrolled in the Canadian clinical trial (see Figure 2 in the report by Davidson et al72). This was reportedly due to an inability to sufficiently heat the target area of the brain because of individual skull factors (e.g., skull-density ratio) hindering the transmission of ultrasound. Given that the procedure could not be completed in these patients, there are no outcome data or information for these 2 participants.
A preliminary report78 from the South Korean clinical trial on the first 6 patients with OCD (recruited between March 2012 and August 2013) noted 1 technical failure due to insufficient temperature rise related to skull factors (e.g., density, thickness). This 1 technical failure for OCD was reported alongside 3 technical failures for essential tremor, where the mean skull-density ratio of this group of cases combined was 0.3 and maximal temperature was less than 45°C. The final publication of the entire case series79 reported and displayed in their patient flow that all people enrolled and eligible completed the procedure; therefore, it is unclear when the technical failure for OCD reported in the earlier publication occurred and how it was counted.
The GRADE quality of the evidence was rated as Very low, given limitations related to risk of bias and imprecision (Appendix 3, Table A3).
Follow-Up Interventions and Re-treatment
No subsequent interventions or re-treatment after MRgFUS capsulotomy were reported for patients with OCD in either study.72,79 The GRADE quality of the evidence was rated as Very low, given limitations related to imprecision (Appendix 3, Table A3).
Quality of Life and Functionality
Quality of Life Enjoyment and Satisfaction
Davidson et al72 assessed quality of life preoperatively and postoperatively via the Quality of Life Enjoyment and Satisfaction Questionnaire as a secondary outcome. This self-reported tool captures a person's degree of enjoyment and satisfaction across various aspects of daily functioning (e.g., leisure time activities, physical health, social relationships, subjective feelings, general activities).81 Higher scores indicate greater enjoyment and satisfaction.81 One participant treated on humanitarian or compassionate grounds (i.e., outside of the clinical trial eligibility) did not have a baseline measurement, so complete data are available for only 5 of 6 participants with OCD (83.3%). There was no statistical testing for the before-and-after comparison; however, quality-of-life scores after the procedure were numerically higher than at baseline (Table 10). The GRADE quality of the evidence was rated as Very low, given limitations related to imprecision (Appendix 3, Table A3).
Table 10:
Quality of Life Before and After MRgFUS Capsulotomy
| Author, year | Baseline Q-LES-Q | Follow-up Q-LES-Q | ||
|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | |
| Davidson et al, 202072 | 32.2 (11.6) | 5a | 41.8 (15.1) | 5a |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; Q-LES-Q Quality of Life Enjoyment and Satisfaction Questionnaire; SD, standard deviation.
One participant was treated on humanitarian or compassionate grounds (i.e., outside of the clinical trial) and did not have a baseline Q-LES-Q measurement. This patient's score after MRgFUS capsulotomy was 61.72
Psychosocial and Occupational Functioning
Kim et al79 assessed psychosocial and occupational functioning using the Global Assessment of Functioning (GAF) scale. The GAF scale is a non-disease-specific clinician assessment of the severity of mental illness symptoms and their impact on the psychological, social, and occupational functioning of a patient.82,83 The score ranges from 0 to 100, with higher scores indicating better functioning.82,83 All study participants had psychosocial dysfunction (i.e., GAF of ≤50 at the start of the study; mean, 35.8). GAF was administered at baseline and at 3, 6, 12, and 24 months after MRgFUS capsulotomy and was documented to have statistically significant improvement across the study period (Table 11). Statistically significant improvements from baseline were seen as early as 3 months after the procedure (P < .001, with Bonferroni correction).79 The GRADE quality of the evidence was rated as Very low, given limitations related to imprecision (Appendix 3, Table A3).
Table 11:
GAF Scores Over 24-Month Follow-Up After MRgFUS Capsulotomy
| Author, year | GAF score, mean (SD) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 mo | 6 mo | 12 mo | 24 mo | P valuea | |
| Kim et al, 201879 | 35.8 (4.98) | 44.3 (3.6) | 48.0 (7.1) | 53.9 (10.8) | 56.0 (10.3) | <.001 |
Abbreviations: GAF, Global Assessment of Functioning; MRgFUS, magnetic resonance-guided focused ultrasound; SD, standard deviation.
Linear mixed model for repeated measures to analyze change in scores across the 24-month follow-up period.
Ongoing Studies
We identified 1 future study in the US that will assess the effectiveness and safety of bilateral MRgFUS capsulotomy for treatment-refractory OCD.84 The “Sonication-based OCD Neurosurgical Intervention Via Capsulotomy (SONIC)” study is planned to begin in June 2024 and involve 2 stages: first, a case series of people with severe, treatment-refractory OCD (planned recruitment, n = 10), and subsequently, people with moderate-to-severe, treatment-refractory OCD (planned recruitment, n = 56). In stage 1, participants with severe OCD will receive MRgFUS capsulotomy and be followed with best medical care for 12 months. The investigators will then submit an Investigational Device Exemption supplement to the US Food and Drug Administration (FDA) and, if approved, the second stage of the study will proceed. Stage 2 will involve a period of randomization for participants with moderate-to-severe OCD to MRgFUS capsulotomy or sham, with follow-up and crossover to MRgFUS capsulotomy for nonresponders assigned to the sham group. The study will use the Exablate Neuro MRgFUS system and technical protocol based on that used in the South Korean and Canadian studies included in this review. Data collection on primary outcomes is forecasted to be completed in June 2030, and study completion in 2032.
The research group of the included study by Davidson et al72 is preparing a manuscript reporting on an additional 6 patients with severe, treatment-refractory OCD who received MRgFUS capsulotomy in their clinical trial. The manuscript will report outcomes for all 12 patients and with longer follow-up (C. Hamani, MD, PhD; B. Davidson, MD; and N. Lipsman, MD, PhD; email communication, December 7, 2023).
Discussion
Our systematic review identified 2 small case series that examined MRgFUS in 17 patients. Very low certainty evidence suggests that MRgFUS capsulotomy may lead to clinically meaningful improvement in OCD symptoms for people with severe, treatment-refractory OCD. The severity of OCD symptoms in the studies improved on average from extreme at baseline to moderate after MRgFUS capsulotomy. The beneficial effects materialized over the weeks and months following the procedure, and most but not all patients experienced partial or complete treatment response, despite variable definitions of treatment response between studies. In addition, though adverse effects during or immediately following the procedure were common, all were mild and transient. The studies did not document any issues with conscious sedation, serious or persistent adverse events, or declines in neurocognitive function after MRgFUS capsulotomy. Importantly, quality of life and functioning improved for patients.
It is difficult to contextualize the effects of MRgFUS within other neurosurgeries for OCD given the absence of comparative studies. The rate of treatment response observed after MRgFUS capsulotomy in our review was in a range similar to that reported in published studies of capsulotomy performed with RFA or Gamma Knife (i.e., approximately 50%-60%),13,23,27 which represents a substantial change in the trajectory of a severe, treatment-refractory illness. Capsulotomy with MRgFUS is noninvasive and uses sound waves, thereby avoiding the risks of open surgery or ionizing radiation required by other capsulotomy techniques. The observed safety profile of MRgFUS capsulotomy differs from those of RFA and Gamma Knife capsulotomy, after which up to 5% and 20% of patients, respectively, can experience serious adverse events (e.g., infection, hemorrhage, brain cyst) or declines in neuropsychological function.13,23,27,33 It has been posited that the favourable adverse-effect profile of MRgFUS capsulotomy may be partly attributed to the ability to create smaller, more precise lesions with MRgFUS technology.75 It is not possible for all patients to undergo MRgFUS neurosurgery successfully. Treatment failure, due to the inability to sufficiently heat the target area and create a therapeutic lesion, is sometimes precluded by individual anatomical factors such as skull density and thickness. In capsulotomy, the target is deeper in the brain than the target of movement disorders (e.g., ventral intermediate nucleus), and heating efficacy at the target is decreased as a result of the skull heating during the procedure.76 The limitations of focused ultrasound depth penetration are the reason that capsulotomy is the only ablative neurosurgery that can be done with current technology of MRgFUS, as it is not possible to reach deeper targets (e.g., for cingulotomy).74 Treating clinicians must highlight the possibility of MRgFUS failure to patients when eliciting informed consent.76
The positive effects of MRgFUS capsulotomy appeared durable after the procedure for 6, 12, or 24 months, and no latent adverse events appeared in the studies. Side effects occurred peri-operatively (within days of the procedure) and improved quickly, whereas the beneficial effects accrued and sustained over time. No instances of re-treatment occurred, likely as bilateral lesions were successfully created for all study participants in whom MRgFUS was possible. Among the patients with treatment-refractory major depressive disorder reported by Davidson et al,76 1 case of re-treatment (i.e., second-side lesion creation) with MRgFUS was completed without adverse events and with some subsequent clinical improvement.
Though some patients in 1 study had changes in their medication after MRgFUS capsulotomy, this is not particularly relevant. OCD is a chronic condition and requires ongoing, comprehensive treatment, including ERP and/or pharmacotherapy. As with all neurosurgeries for treatment-refractory OCD, MRgFUS neurosurgery is not intended to be curative. Rather, neurosurgery may change brain response to medication or enable improved participation in - and, thereby, effectiveness of - ERP therapy.85
Equity Considerations
There was little information in the included studies about PROGRESS-Plus62 characteristics, across which health inequities may exist. Therefore, we cannot comment on who was or was not represented in the available evidence. Improvements in both capacity and coordination of the spectrum treatment for OCD are required to facilitate equitable access for patients. Factors such as geography, variable or poor access to primary and tertiary mental health care, and the stigmatization of mental illness and its surgical treatments may contribute to inequities in access. Neurosurgery for OCD, including MRgFUS neurosurgery, requires a referral from a physician, typically a treating psychiatrist. At present, there is no clear treatment or referral pathway for patients with OCD in Ontario to facilitate access to psychiatrists or neurosurgical consultation for those with severe, treatment-refractory disease.
Strengths and Limitations
To our knowledge, this is the first systematic review of the effect of MRgFUS neurosurgery for severe, treatment-refractory OCD. The body of evidence consists of case series; therefore, we cannot compare the effectiveness or safety with that of other neurosurgeries. Case series studies lack statistical power and are at increased risk of bias given that there is no control group.67 This is unsurprising in this field of clinical research, as despite decades of neurosurgery for severe, treatment-refractory OCD, there remain no studies comparing DBS with ablative neurosurgery.19 Several reasons may contribute to this, including the higher cost of DBS, preference by providers and patients for reversible treatment, and that DBS and ablative neurosurgery may not both be suitable for the same population.32 Clinical trials of neurosurgery for OCD are challenged by ethical considerations, the difficulty in recruiting sufficient numbers of participants (partly due to low numbers of referrals for surgery), and a latency of years to observe outcomes.32 The forthcoming data on MRgFUS neurosurgery for OCD that we are aware of (see Ongoing Studies) does not compare MRgFUS with other surgical methods for capsulotomy.
We aspired to meta-analyze the data on treatment response; however, this was not appropriate due to differing outcome definitions. In addition, the small sample sizes and low number of studies posed challenges for meaningful quantitative synthesis.86 As a strength, the 2 included case series were preplanned, prospective, and applied clear inclusion criteria, follow-up, and measurement of the condition and outcomes. It was unclear whether the series represent consecutive and complete inclusion of cases at each centre, which could contribute to the reliability of a case series.67 There is considerable uncertainty about the effect estimates due to limitations in the quality of the body of evidence (Appendix 3, Table A3). However, MRgFUS neurosurgery is reserved for people with OCD who are extremely disabled by their condition and have not responded to all other treatment options. OCD symptoms tend to remain stable over time,87,88 and remission is rare over the long term.89 Untreated, severe OCD is associated with an elevated risk of suicide,8 reduced quality of life,90 and caregiver burnout91 and invariably results in chronic disability.11
Conclusions
There is considerable uncertainty; however, the evidence suggests that MRgFUS capsulotomy for severe, treatment-refractory OCD:
-
May improve OCD symptoms (GRADE: Very low) and result in treatment response (GRADE:
Very low)
May have a favourable safety profile (GRADE: Very low); no occurrences of serious or persistent adverse events were reported
May have little to no effect on neurocognitive function (GRADE: Very low)
May have a technical failure rate of up to 25% (GRADE: Very low)
May improve quality of life (GRADE: Very low)
May improve patient functioning (GRADE: Very low)
May not require re-treatment or follow-up interventions; however, the evidence is very uncertain, as no occurrences were reported (GRADE: Very low)
Economic Evidence
Research Question
What is the cost-effectiveness of magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery compared with radiofrequency ablation (RFA), Gamma Knife radiosurgery, and deep brain stimulation (DBS) for the treatment of people with treatment-refractory obsessive–compulsive disorder (OCD)?
Methods
Economic Literature Search
We performed an economic literature search on August 14, 2023, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE, Embase, and APA PsycInfo and monitored them until February 16, 2024. We also performed a targeted grey literature search following a standard list of websites developed internally, which includes the International HTA Database and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Literature Search, above, for further details on methods used. See Appendix 2 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Cost-benefit analyses, cost-effectiveness analyses, cost-utility analyses, cost-consequence analyses, or cost analyses
Exclusion Criteria
Narrative reviews, letters, editorials, case reports, commentaries, abstracts, posters, and unpublished studies
Population
Adults (≥18 years old) with severe, treatment-refractory OCD, as defined by the study
Interventions
MRgFUS neurosurgery, with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
Comparators
RFA, with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
Gamma Knife radiosurgery, with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
DBS, with or without co-interventions (such as psychotherapy and/or pharmacotherapy)
No comparator
Outcome Measures
Costs
Health outcomes (e.g., partial or complete response, change in Yale-Brown Obsessive–Compulsive Scale [Y-BOCS] score, quality-adjusted life-years [QALYs])
Incremental costs
Incremental effectiveness
Incremental cost-effectiveness ratios (ICERs)
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Microsoft Excel92 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. The same reviewer then examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, ICERs)
We contacted study authors to provide clarification as needed.
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.93 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into 2 sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be applicable.
Results
Economic Literature Search
The economic literature search yielded 17 citations, including grey literature results and after removing duplicates, published from database inception until August 14, 2023. We identified no additional eligible studies from other sources, including database alerts (monitored until February 16, 2024). In total, we identified 1 study (a threshold analysis) that met our inclusion criteria. Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 2: PRISMA Flow Diagram - Economic Systematic Review.
PRISMA flow diagram showing the economic systematic review. The economic literature search yielded 14 citations published between database inception and August 14, 2023. We included 6 additional studies from other sources. After removing duplicates, we screened the abstracts of the 17 identified studies and excluded 15. We assessed the full text of 2 articles and excluded a further 1. In the end, we included 1 article in the qualitative synthesis.
Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health Literature; Cochrane SR, the Cochrane Database of Systematic Reviews; NHS EED, National Health Service Economic Evaluation Database; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Page et al.61
Overview of Included Economic Studies
We identified 1 economic study investigating the cost-effectiveness of MRgFUS neurosurgery for the treatment of patients with OCD.94 Kumar et al94 investigated the cost-effectiveness of MRgFUS neurosurgery compared with radiofrequency capsulotomy, a type of RFA, for people with treatment-refractory OCD. A summary of the included study is provided in Table 12.
Table 12:
Characteristics of Studies Included in the Economic Systematic Review
| Author, year, country, intervention, comparator | Analysis | Study population | Results | |||||
|---|---|---|---|---|---|---|---|---|
| Technique | Design (model) | Approach or perspective | Time horizon (discount rate) | Health outcomes | Costs | Cost-effectiveness | ||
| Kumar et al, 2019,94 US | Threshold analysis | Decision tree | Societal | 1 y (N/A) | Patients with treatment-refractory OCD considered suitable candidates for surgery | — | Currency, cost year: USD, 2017 | ICER not reported; study concluded that MRgFUS neurosurgery was cost-effective under a range of possible values, and results were most sensitive to cost, effectiveness, and complication rate of MRgFUS neurosurgery. PSA was not reported. |
| I: MRgFUS neurosurgery | — | — | — | — | NR | NR | ||
| C: RFA | 0.212 QALYs | $24,099 USD | ||||||
Abbreviations: C, comparator; I, intervention; ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; N/A, not applicable; NR, not reported; OCD, obsessive–compulsive disorder; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year; RFA, radiofrequency ablation.
Kumar et al94 conducted a threshold analysis to estimate the necessary cost and clinical parameters for MRgFUS to conclude that MRgFUS neurosurgery is cost-effective compared with RFA. The authors stated that this decision was made due to the limited clinical data evaluating MRgFUS neurosurgery for OCD, as only 1 case series of 4 patients published by Jung et al78 was available at the time of their analysis.
The analysis used a decision tree over a 1-year time horizon from a US societal perspective.94 Effectiveness was defined as a reduction in Y-BOCS score. The effectiveness of RFA was derived from an unpublished meta-regression of observational data in which change in Y-BOCS score was converted to mean improvement in utility. The costs for RFA and MRgFUS neurosurgery were derived from Medicare reimbursement rates.
The study estimated the 1-year cost of RFA to be $24,099 USD with 0.212 QALYs per patient.94 Rather than reporting a single threshold value for the cost and clinical parameters of MRgFUS neurosurgery, a 3-way sensitivity analysis was graphically reported for utility of MRgFUS ranging from 0 to 0.5 QALYs, cost from $10,000 USD to $25,000 USD, and 10%, 20%, or 30% probability of complications. For most combinations of values of the variables identified, MRgFUS neurosurgery was cost-effective, and the authors concluded that MRgFUS neurosurgery was cost-effective under a wide range of values. However, there was no justification for the range of values used in the sensitivity analysis and a willingness-to-pay threshold was not provided.
Applicability of the Included Studies
Appendix 6, Table A5 provides the results of the quality appraisal checklist for economic evaluations applied to the included studies. One study94 was included and was deemed not applicable to our research question. Although the study included the population, intervention, and comparator of interest, it is an early cost-effectiveness analysis based on theoretical data inputs and assumptions, as there was no clinical study comparing MRgFUS neurosurgery with RFA. The authors conducted a threshold analysis to determine when MRgFUS neurosurgery would be considered cost-effective compared with RFA.
There were no studies applicable to our research question, so no methodological quality assessment was applied.
Discussion
Kumar et al94 conducted a threshold analysis to determine the cost and clinical parameters of MRgFUS neurosurgery required for it to be cost-effective compared with RFA. The authors acknowledged the lack of data on MRgFUS neurosurgery and did not provide any sources or justification for MRgFUS neurosurgery clinical input data.
Strengths and Limitations
We conducted a thorough review of the economic literature to examine the cost-effectiveness of MRgFUS neurosurgery for severe, treatment-refractory OCD. We were limited in our conclusions about the cost-effectiveness of MRgFUS neurosurgery by the paucity of evidence identified.
Conclusions
We identified 1 study conducted by Kumar et al94 that we deemed not applicable to our research question. Kumar et al94 conducted a threshold analysis because of limited clinical data on MRgFUS neurosurgery. They concluded that MRgFUS neurosurgery could be cost-effective for a range of input values but acknowledged that their model is not a substitute for randomized controlled trials directly comparing strategies and that data on long-term efficacy and complications could impact cost-effectiveness findings.
Primary Economic Evaluation
Although 2 trials examining magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery in patients with severe, treatment-refractory obsessive–compulsive disorder (OCD) have been completed and published, the studies are limited by small sample sizes and were conducted without comparator arms (see Strengths and Limitations in Clinical Evidence).47,74,79,95 Due to the lack of comparative clinical evidence, we did not conduct a primary economic evaluation.
Budget Impact Analysis
Research Question
What is the potential 5-year budget impact for the Ontario Ministry of Health of publicly funding magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery for people with treatment-refractory obsessive–compulsive disorder (OCD)?
Methods
Analytic Framework
We estimated the budget impact of publicly funding MRgFUS neurosurgery using the cost difference between 2 scenarios: (1) current clinical practice without public funding for MRgFUS neurosurgery (the current scenario) and (2) anticipated clinical practice with public funding for MRgFUS neurosurgery (the new scenario). Figure 3 presents the budget impact model schematic.
Figure 3: Schematic Model of Budget Impact.
Flow chart describing the model for the budget impact analysis. Based on the size of the population of interest, we created 2 scenarios: the current scenario, which would explore the distribution of treatment strategies, resource use, and total costs without public funding for MRgFUS neurosurgery; and the new scenario, which would explore the distribution of treatment strategies, resource use, and total costs with public funding for MRgFUS neurosurgery. The budget impact would represent the difference in costs between the 2 scenarios.
Key Assumptions
We made the following assumptions:
Interventions were performed in hospitals with existing infrastructure (e.g., magnetic resonance imaging [MRI] suite); therefore, capital and fixed costs for equipment were not included.
Any adjunct pharmacotherapy remained constant before and after treatment.
Adverse events occurred within the first year of treatment; thus, costs were incurred in the same year as treatment.
Only costs of serious adverse events resulting in hospitalization were included.
Deep brain stimulation (DBS) for OCD is available only through research and is not publicly funded; therefore, it incurred no costs from the Ministry of Health perspective.
Population of Interest
The size of the population of interest (people in Ontario with severe, treatment-refractory OCD) was estimated based on published epidemiological data and expert opinion.
We used population projections from the Ontario Ministry of Finance to estimate the adult population (≥18 years old) in Ontario for 2024 to 2028.96
We applied the lifetime prevalence of OCD among people in Canada estimated from the 2012 Canadian Community Health Survey - Mental Health, (0.93%; 95% confidence interval, 0.75%-1.11%) to estimate the number of people in Ontario with OCD.7 We estimated the distribution of OCD severity based on data from an epidemiological study in the US that measured severity of OCD using the Yale-Brown Obsessive–Compulsive Scale (Y-BOCS).6 Nearly one-third (30.7%) of people with OCD were classified as having severe OCD (defined in the study as Y-BOCS score > 30). We applied this estimate to determine the number of eligible patients based on severity. Treatment rates for OCD were low; the same US study found that about 31% of people with severe OCD reported receiving OCD-specific treatment.6
Ontario Health's quality standard for OCD recommends a stepped-care approach beginning with the least intensive, efficacious treatment.97 Primary treatment includes pharmacotherapy and psychotherapy.2 However, for some people with OCD, primary treatment is insufficient and their OCD is treatment-refractory, meaning that there is insufficient response to or disabling side effects with at least first- and second-line therapy including at least 1 trial with adjuvant antipsychotic medication and at least 1 complete trial of cognitive behavioural therapy (CBT).30 In the literature, estimates of the percentage of people with OCD who have treatment-refractory OCD vary, with one source reporting 30% to 40%.14
Insightec Ltd (Haifa, Israel), currently the only manufacturer of MRgFUS neurosurgery equipment, notes that people exhibiting behaviours consistent with substance abuse are contraindicated for the MRgFUS neurosurgical procedure.98 Therefore, we removed approximately 11% (95% confidence interval, 5.35%-15.93%) of people with OCD from our population of interest, as this represents the percentage of people with OCD who also met the criteria for substance abuse or dependence in the last 12 months.7
People with severe, treatment-refractory OCD are potential candidates for MRgFUS neurosurgery; however, access to mental health care is limited. In the Canadian 2022 Mental Health and Access to Care Survey, about 49% of people who met diagnostic criteria for a mood, anxiety, or substance use disorder in the previous 12 months reported talking to any health professional about their mental health in the past year, and that percentage decreased to about 13% when asked about psychiatrists specifically.99 An expert on OCD estimated that only about 50% of people have access to secondary or tertiary OCD-specialized psychiatrists (P. M. A. Richter, MD, email communication, October 9, 2023). Potential candidates for MRgFUS neurosurgery are screened by 2 psychiatrists who must independently conclude that the patient is a candidate for MRgFUS neurosurgery before a surgical referral is made. Criteria for screening may include checking for medical comorbidities, treatment history, duration of illness, and functional impairment. A clinical expert estimated that one-third of people referred for MRgFUS neurosurgery are medically and psychiatrically eligible for surgery (N. Lipsman, MD, PhD, email communication, December 14, 2023). In one site's experience with offering MRgFUS neurosurgery, 85% of people who are suitable candidates for surgery accepted the treatment (N. Lipsman, MD, PhD, email communication, December 14, 2023).
Once a patient is deemed to be a candidate for MRgFUS neurosurgery, there are additional criteria to ensure that the procedure will be successful, including a minimum skull-density ratio to ensure adequate heating to create a lesion. Boutet et al100 reported skull-density ratios of patients with movement disorders who were candidates for MRgFUS thalamotomy, a type of ablative neurosurgery that targets a different location in the brain than capsulotomy. They found that a skull-density ratio of ≥0.4 was optimal and reported that 21 out of 136 patients (15%) failed to meet that criterion.100 Although Davidson et al74 suggest that MRgFUS capsulotomy may require a higher skull-density ratio cut-off of ≥0.45 due to the necessity of reaching a deeper brain structure, the proportion of patients who met this cut-off was not reported. In our reference case, we used the percentage of patients with a skull density ratio of ≥0.4 (85%) from the study by Boutet et al.100
Table 13 summarizes the process of estimating our population of interest: the number of people in Ontario with severe, treatment-refractory OCD who are candidates for MRgFUS neurosurgery.
Table 13:
Process of Estimating the Population of Interest
| Variable | Year 1 (2024) | Year 2 (2025) | Year 3 (2026) | Year 4 (2027) | Year 5 (2028) |
|---|---|---|---|---|---|
| Ontario population ≥ 18 years olda | 12.8 million | 13.0 million | 13.3 million | 13.5 million | 13.7 million |
| People with OCD (0.93%)7 | 118,721 | 121,314 | 123,435 | 125,167 | 127,006 |
| Y-BOCS severity score > 30 (30.7%)6 | 36,447 | 37,243 | 37,895 | 38,426 | 38,991 |
| Treatment-seeking (30.9%)6 | 11,262 | 11,508 | 11,709 | 11,874 | 12,048 |
| Treatment-refractory (35%)14 | 3,942 | 4,028 | 4,098 | 4,156 | 4,217 |
| Without comorbid substance use disorder (89%)7 | 3,508 | 3,585 | 3,647 | 3,699 | 3,753 |
| Access to secondary or tertiary OCD-specialized psychiatrist (50%) | 1,754 | 1,792 | 1,824 | 1,849 | 1,876 |
| Referred for MRgFUS neurosurgery (10%) | 175 | 179 | 182 | 185 | 188 |
| Medically and psychiatrically eligible for MRgFUS neurosurgery (33%) | 58 | 59 | 60 | 61 | 62 |
| Interest and consent to MRgFUS neurosurgery (85%) | 49 | 50 | 51 | 52 | 53 |
| Favourable skull density (85%)100 | 42 | 43 | 43 | 44 | 45 |
Note: Numbers may be inexact due to rounding.
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; OCD, obsessive–compulsive disorder; Y-BOCS, Yale-Brown Obsessive–Compulsive Scale.
Data for ages 20 years and older were used, as data on ages 18 years and older were unavailable.
Current Intervention Mix
Canadian guidelines have scant recommendations on treatment options for people with OCD who are refractory to pharmacotherapy and psychotherapy.2 Options for severe, treatment-refractory OCD include ablative neurosurgical options and DBS. There are 3 different methods that can be used for ablative neurosurgery: radiofrequency ablation (RFA), Gamma Knife radiosurgery, and MRgFUS neurosurgery (the intervention of interest).
There are only 2 sites in Ontario that have the equipment and expertise to offer any of these procedures. RFA and Gamma Knife radiosurgery are provided for patients with treatment-refractory OCD and are covered by the hospitals’ global budgets. MRgFUS neurosurgery and DBS are being used off-label under humanitarian exemptions and are paid for by philanthropic or clinical trial funding (N. Lipsman, MD, PhD, email communication, October 9, 2023). Currently, there is only 1 site in Ontario that offers MRgFUS neurosurgery or Gamma Knife radiosurgery and 2 sites in Ontario that offer DBS or RFA for people with treatment-refractory OCD (IntelliHealth Ontario, August 4, 2023).
Although the population of interest was estimated at around 40 people per year, the number of people receiving these treatments in Ontario appears to be much lower, likely owing to a lack of awareness of treatment options or a lack of an established referral pathway for severe, treatment-refractory OCD (P. M. A. Richter, MD, email communication, October 9, 2023). A study conducted in Quebec that surveyed psychiatry residents and psychiatrists identified several barriers to referring patients for neurosurgery, including lack of knowledge on the technical aspects of the procedures, efficacy, potential side effects, eligibility criteria, availability, and referral process.101 Other barriers included fear of irreversible consequences and patient or family resistance to the procedures.
We used the IntelliHealth Ontario portal to obtain the number of documented Gamma Knife radiosurgery and RFA procedures in the Canadian Institute for Health Information (CIHI) Discharge Abstract Database for Ontario from 2019 to 2022 (Table 14). Procedures were identified using the Canadian Classification of Health Interventions (CCI) codes as shown in Appendix 7, Table A6, with a main diagnosis of OCD identified by International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada (ICD-10-CA) codes (F42; see Appendix 7, Table A7). In Ontario, an average of about 4 patients with OCD received Gamma Knife radiosurgery (45%) or RFA (55%) annually from 2019 to 2022.
Table 14:
Volume of Current Interventions
| Procedurea | Number of procedures performed | % | |||||
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | Total | Annual average, excluding 2020 | ||
| Gamma Knife radiosurgery | <6 | 0 | <6 | <6 | <6 | 1.7 | 45 |
| RFA | <6 | 0 | <6 | <6 | 6 | 2.0 | 55 |
| Total | <6 | 0 | <6 | <6 | 11 | 3.7 | 100 |
Abbreviations: RFA, radiofrequency ablation.
Procedures were identified using the following Canadian Classification of Health Interventions (CCI) codes: Gamma Knife radiosurgery was 1AE27JX or 1AN27JX; and RFA was 1AE59SEGX, 1AE59SZGX, 1AE59SZAW, 1AE59SEAW, 1AN59SEAW, 1AN59SEGX, 1AN59SZAW, or 1AN59SZGX. Source: IntelliHealth Ontario, Inpatient Discharges, 2019-2022, accessed June 22, 2023.
As DBS is not funded by the Ontario Ministry of Health and we are taking the Ministry of Health perspective for our analysis, we did not include treatment with DBS in our reference case. We conducted a scenario analysis assuming public funding of DBS for both the procedure and device.
Uptake of the New Intervention and New Intervention Mix
Because MRgFUS neurosurgery does not share the risks of RFA associated with open surgery or the risks of Gamma Knife radiosurgery stemming from ionizing radiation, we conducted the reference case analysis assuming that publicly funded MRgFUS neurosurgery would replace these treatments (N. Lipsman, MD, PhD, email communication, October 9, 2023).
We assumed that all procedures would take place at a large urban teaching hospital with the necessary neurosurgical and psychiatric expertise required to diagnose, treat, and manage patients. Currently, neurosurgical treatment for patients with severe, treatment-refractory OCD largely takes place at 1 site located in Toronto, Ontario. We assumed that in the new scenario, this site would continue to treat patients and would offer MRgFUS neurosurgery to all patients to fully replace RFA; however, a small proportion of patients would be unable to receive MRgFUS neurosurgery due to unamenable skull factors or contraindications to MRI. We assumed that these patients would receive Gamma Knife radiosurgery instead. Therefore, in the new scenario, we assumed that 85% of patients who would usually be treated with RFA or Gamma Knife radiosurgery would instead be treated with MRgFUS neurosurgery, and the remaining 15% would be treated with Gamma Knife radiosurgery (Table 15). We included a scenario analysis in which patients not eligible for MRgFUS neurosurgery in the new scenario would receive RFA instead (Appendix 7, Table A13).
Table 15:
Volume of Treatments in the Current and New Scenarios
| Scenario | Year 1 (2024) | Year 2 (2025) | Year 3 (2026) | Year 4 (2027) | Year 5 (2028) | Total |
|---|---|---|---|---|---|---|
| Current scenario | ||||||
| Gamma Knife radiosurgery | 2 | 2 | 2 | 2 | 2 | 10 |
| RFA | 2 | 2 | 2 | 2 | 2 | 10 |
| No surgery (expanded population) | 12 | 15 | 18 | 21 | 24 | 90 |
| New scenario | ||||||
| Gamma Knife radiosurgery, 15% of original population | 1 | 1 | 1 | 1 | 1 | 5 |
| RFA, 0% | 0 | 0 | 0 | 0 | 0 | 0 |
| MRgFUS neurosurgery, 85% of original population + expansion | 15 | 18 | 21 | 24 | 27 | 105 |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; RFA, radiofrequency ablation.
Since 2017, 1 site in Ontario has been treating patients with OCD using MRgFUS neurosurgery as part of a clinical trial through research and philanthropic funding; thus, it is not captured in the administrative data reported in Table 14. The site has treated 32 patients using MRgFUS neurosurgery since 2017, with current annual patient volume closer to 9 to 12 patients (N. Lipsman, MD, PhD, email communication, November 8, 2023). To understand whether this represented a replacement of other types of neurosurgeries, we examined administrative data in Ontario from 2002 to 2022. Prior to the availability of MRgFUS neurosurgery, treatment volumes were consistently no more than 4 patients per year. Thus, we assumed that the 12 patients treated using MRgFUS neurosurgery represented an expansion of the treated population and not a replacement of previously used treatments.
In our reference case, we assumed that this expansion of the population treated with MRgFUS neurosurgery would continue, beginning with the current volume of 12 additional patients in year 1 and linearly increasing to 24 patients in year 5 as awareness spreads, referral patterns are established, and patients and referring physicians are more accepting of a noninvasive surgical option (Table 15). We conducted scenario analyses with different uptake and treatment patterns (Appendix 7, Table A13).
Resources and Costs
Our budget impact analysis includes only costs associated with the health technology from the Ministry of Health perspective:
Costs of the surgical procedures
Costs of adverse events
Ongoing monitoring costs
Adjunct medication costs
We reported costs in 2023 Canadian dollars and sourced all costs from Ontario data. For instances in which costs were taken from sources not reported in 2023 dollars, we used the all-items Consumer Price Index from Statistics Canada to adjust costs to 2023 dollars.102 No discounting was applied. We sourced costs for professional services from the Ontario Schedule of Benefits for Physician Services.103 We based hospital costs on patient-level costing sourced from the IntelliHealth Ontario portal. We reported costs for DBS in the upcoming tables, but they were only used in a scenario analysis.
A schematic of the included costs by cohort and year is shown in Figure 4.
Figure 4: Schematic Model of Costs Included in Budget Impact Analysis.
Diagram describing the included costs for each cohort and year of the budget impact analysis. The sum of the columns indicates the total annual costs calculated for each of the current and new scenarios.
Summary of Per-Patient Costs
An overview of the average cost per patient is presented in Table 16. For the first year, average costs ranged from $19,734 for MRgFUS neurosurgery to $743 for patients who did not receive any surgical treatment. Ongoing monitoring and adjunct medication costs are incurred annually in subsequent years as depicted in Figure 4.
Table 16:
Total Per-Patient Costs
| Cost type | MRgFUS neurosurgery, $a | RFA, $a | Gamma Knife radiosurgery, $a | No surgical treatment, $a |
|---|---|---|---|---|
| Surgical procedure | 18,768 | 11,866 | 7,084 | 0 |
| Ongoing monitoringb | 223 | 223 | 223 | 0 |
| Adjunct medicationsb | 743 | 743 | 743 | 743 |
| Adverse events | 0 | 288 | 1,200 | 0 |
| Total cost per patient | 19,734 | 13,120 | 9,249 | 743 |
Note: Numbers may be inexact due to rounding.
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; RFA, radiofrequency ablation.
All costs are reported in 2023 Canadian dollars.
Costs are incurred annually.
Procedure Costs
Table 17 presents the cost for each surgical procedure. The total mean cost per procedure was $18,768 for MRgFUS neurosurgery, $11,866 for RFA, and $7,084 for Gamma Knife radiosurgery.
Table 17:
Cost Inputs for Surgical Procedures
| Resource item | MRgFUS neurosurgery, $a | RFA, $a | Gamma Knife radiosurgery, $a | DBS, $a,b | Data source |
|---|---|---|---|---|---|
| Preprocedure | |||||
| OHIP professional fees | |||||
| Neurosurgery, special surgical consultation | 163 | 163 | 163 | 163 | Schedule of Benefits103 (A935) |
| Psychiatry consultation | 223 | 223 | 223 | 223 | Schedule of Benefits103 (A195) |
| MRI scan - professional fee | 73 | 73 | 73 | 73 | Schedule of Benefits103 (X421) |
| CT scan - professional fee | 65 | 65 | 65 | 65 | Schedule of Benefits103 (X401) |
| Diagnostic procedure costs | |||||
| MRI scan - procedure cost | 1,397 | 1,397 | 1,397 | 1,397 | IntelliHealth Ontarioc (CCI code: 3.AN.40.^^) |
| CT scan - procedure cost | 856 | 856 | 856 | 856 | IntelliHealth Ontarioc (CCI code: 3.AN.20^^) |
| Total preprocedure cost | 2,776 | 2,776 | 2,776 | 2,776 | |
| Periprocedure | |||||
| OHIP professional fees | |||||
| Physician fees | 2,040d | 2,040d | 811 | 2,040d | Schedule of Benefits103 (N124, X313) |
| Surgical assistant fees | 388 | 388 | 0 | 613 | Calculated, see Appendix 7, Table A10 |
| Anesthesiologist fees | 573 | 573 | 0 | 852 | Calculated, see Appendix 7, Table A11 |
| Inpatient costs | |||||
| Device cost | N/A | N/A | N/A | 19,100 | Ontario hospital currently conducting the proceduree |
| Average total cost per patient | 11,240 | 4,338f | 1,746g | 27,557h | Ontario hospital currently conducting the proceduree; IntelliHealth Ontarioi; see Appendix 7, Table A8 |
| Total periprocedure cost | 14,241 | 7,339 | 2,557 | 50,162 | |
| Postprocedure | |||||
| OHIP professional fees | |||||
| Neurosurgery - repeat consultation | 58 | 58 | 58 | 58 | Schedule of Benefits103 (A046) |
| Psychiatry consultation | 223 | 223 | 223 | 223 | Schedule of Benefits103 (A195) |
| MRI scan - professional fee | 73 | 73 | 73 | 73 | Schedule of Benefits103 (X421) |
| Diagnostic procedure costs | |||||
| MRI scan - procedure cost | 1,397 | 1,397 | 1,397 | 1,397 | IntelliHealth Ontarioc (CCI code: 3.AN.40.AA) |
| Total postprocedure cost | 1,751 | 1,751 | 1,751 | 1,751 | |
| Total cost per procedure | 18,768 | 11,866 | 7,084 | 56,407 | |
Note: Numbers may be inexact due to rounding.
Abbreviations: CCI, Canadian Classification of Health Interventions; CT, computed tomography; DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; MRI, magnetic resonance imaging; N/A, not applicable; OHIP, Ontario Health Insurance Plan; RFA, radiofrequency ablation.
All costs are reported in 2023 Canadian dollars.
For scenario analyses only.
Accessed June 26, 2023.
This service may be included in an existing insured service or may require its own fee code. Final interpretation of the Schedule of Benefits occurs between the Ministry of Health and the Ontario Medical Association.
Email communication, July 28, 2023.
CCI procedure code: 1AN59SEAW, 1AN59SEGX for OCD diagnosis; weighted average of cases from 2017 to 2019, inflated to 2023 Canadian dollars.
CCI procedure code: 1AE27JX, 1AN27JX for OCD diagnosis; weighted average of cases from 2019 to 2022, inflated to 2023 Canadian dollars.
CCI procedure code: 1AE53SEJA, 1AE53SZJA, 1AN53SEJA for OCD diagnoses; weighted average of cases from 2012 to 2019, inflated to 2023 Canadian dollars.
Accessed July 5, 2023.
We divided procedure costs into preprocedure, periprocedure, and postprocedure costs.
Preprocedure costs include the costs of physician appointments and imaging. We assumed that before undertaking the procedure, all patients would undergo a specialty neurosurgery consultation and a psychiatric consultation. We also assumed that all patients would undergo an MRI and computed tomography (CT) scan prior to surgery.
Periprocedure costs include the inpatient hospital costs (e.g., nursing, radiology, pharmacy, other support services, overhead), physician fees, surgical assistant fees, and anesthesiologist fees. We obtained physician fees from the Ontario Schedule of Benefits for Physician Services.103 There are no existing fee codes for MRgFUS, RFA, or DBS for the treatment of OCD, so based on consultations with clinical experts, we applied fee code N124, which is for functional stereotaxy for the treatment of movement disorders. This service may be included in an existing insured service or may require its own fee code. Final interpretation of the Schedule of Benefits occurs between the Ministry of Health and the Ontario Medical Association. We estimated inpatient hospital costs using patient-level data from IntelliHealth Ontario by searching for the relevant CCI codes and ICD-10-CA diagnostic codes for OCD (Appendix 7, Table A8). For MRgFUS neurosurgery, inpatient costs were provided by an Ontario hospital currently conducting the procedure (email communication, July 28, 2023).
We excluded capital and fixed costs of MRgFUS equipment purchase, installation, and maintenance in the reference case and assumed that the procedure is performed only in hospitals with pre-existing infrastructure. Currently, there are 2 sites in Ontario with the infrastructure and multidisciplinary expertise required to perform MRgFUS neurosurgery that use equipment that was either donated or purchased by the hospital. The Ministry of Health would be providing funding for operational costs only. Both sites are currently using MRgFUS neurosurgery to treat movement disorders, and 1 site is also treating patients with treatment-refractory OCD. We conducted a scenario analysis that includes capital and fixed costs of the MRgFUS equipment (see Appendix 7, Table A9).
Postprocedure costs include the cost of a follow-up MRI, repeat consultation with a neurosurgeon, and psychiatry consultation.
Cost of Adverse Events
We estimated the costs of managing adverse events by multiplying the expected frequency of adverse events (Table 18) by the management cost per hospitalized adverse event (Table 19) and the percentage of cases that are treated in hospital (Table 20). We included only serious adverse events that required hospitalization. The cost of managing adverse events averaged per person was $0 for MRgFUS neurosurgery, $288 for RFA, and $1,200 for Gamma Knife radiosurgery (Table 20).
Table 18:
Frequency of Adverse Events
| Event | Percentage of patients | Source(s) | ||
|---|---|---|---|---|
| Reference case | Plausible range minimum | Plausible range maximum | ||
| MRgFUS neurosurgery | ||||
| None | N/A | N/A | N/A | Clinical review |
| RFA | ||||
| Infection | 1.6% (1/64) | 0.0% | 4.8% (1/21) | Montoya et al, 2002113; Sheth et al, 2013108 (reference case) |
| Cerebral hemorrhage | 8.1% (3/37) | 0.0% | 8.1% (3/37) | Gong et al, 2018107; Liu et al, 2008105; Liu et al, 2017104 (reference case); Zhan et al, 2014106 |
| Gamma Knife radiosurgery | ||||
| Perilesional edema | 12.5% (1/8) | 0.0% | 12.5% (1/8) | Gupta et al, 2019110; Lopes et al, 20 1433 (reference case); Peker et al, 2020111; Rasmussen et al, 2018109 |
| Brain cyst | 12.5% (1/8) | 2.5% (1/40) | 9.1% (5/55) | |
| DBSa | ||||
| Infection (surgically treated) | 2.0% | N/A | N/A | Gadot et al, 2022112 |
| Hardware malfunction | 8.0% | N/A | N/A | |
| Seizure (generalized tonic-clonic) | 2.4% | N/A | N/A | |
| Cerebral hemorrhage (with sequalae) | 0.8% | N/A | N/A | |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; N/A, not applicable; RFA, radiofrequency ablation.
For scenario analysis only.
Table 19:
Management Cost per Adverse Event Hospitalization
| Adverse event | ICD-10-CA diagnosis code | Case mix group number, description | Hospitalization cost, $a | Physician cost, $a | Total cost, $a | Source(s) |
|---|---|---|---|---|---|---|
| Infection | T81.4 (infection following a procedure, not elsewhere classified) | 650, multisystemic/unspecified site infection with intervention 32, infection/inflammation of central nervous system except meningitis | 18,054 | 2,677 (using a ratio of 0.15 for physician costs to total costs for the given case mix groups) |
20,731 | CIHI patient cost estimator114; IntelliHealth Ontariob |
| Cerebral hemorrhage | S061 (traumatic cerebral edema)c | 782, postoperative hemorrhage | 10,419 | 3,935 (using a ratio of 0.38 for physician costs to total costs for the given case mix group) |
14,354 | CIHI patient cost estimator114; IntelliHealth Ontariob |
| Brain cyst | G930 (cerebral cyst)c | 782, postoperative hemorrhage | 5,321 | 2,010 (using a ratio of 0.38 for physician costs to total costs for the given case mix group) |
7,331 | CIHI patient cost estimator114; IntelliHealth Ontariob |
| Perilesional edema | S061 (traumatic cerebral edema)c | 782, postoperative hemorrhage | 10,419 | 3,935 (using a ratio of 0.38 for physician costs to total costs for the given case mix group) |
14,354 | CIHI patient cost estimator114; IntelliHealth Ontariob |
| Seizure (generalized tonic-clonic) | N/A | 40, seizure disorder except status epilepticus | 5,403 | 1,343 | 6,746 | CIHI patient cost estimator114 |
Note: Numbers may be inexact due to rounding.
Abbreviations: CIHI, Canadian Institute for Health Information; ICD-10-CA, International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada; N/A, not applicable.
All costs are reported in 2023 Canadian dollars.
Accessed November 9, 2023.
Only cases with a main procedure of MRI or CT scan were selected to estimate the average cost.
Table 20:
Costs of Managing Adverse Event Hospitalizations
| Adverse event | Reference case, $a | Plausible range minimum, $a | Plausible range maximum, $a | Treatment assumptions | Source(s) |
|---|---|---|---|---|---|
| MRgFUS neurosurgery | |||||
| No adverse events reported | N/A | N/A | N/A | Clinical review | |
| Average total per-person cost of managing adverse events | 0.00 | 0.00 | 0.00 | ||
| RFA | |||||
| Infection | 32.39 | 0.00 | 98.72 | 10% of patients require hospitalization; the remaining 90% of patients are treated with antibiotics as outpatientsb | CIHI patient cost estimator114; IntelliHealth Ontario1; Montoya et al, 2002113; Sheth et al, 2013108 |
| Cerebral hemorrhage | 256.05 | 0.00 | 256.05 | 22% (8/37) of cases are symptomatic116 and are hospitalized for close monitoring and blood pressure controlb | CIHI patient cost estimator114; Gong et al, 2018107; Horisawa et al, 2021116; IntelliHealth Ontarioc; Liu et al, 2008105; Liu et al, 2017104; Zhan et al, 2014106 |
| Average total per-person cost of managing adverse events | 288.44 | 0.00 | 354.77 | ||
| Gamma Knife radiosurgery | |||||
| Brain cyst | 302.39 | 60.48 | 302.39 | 33% of cysts are symptomatic,109 require hospitalization, and are managed with inpatient observationb | CIHI patient cost estimator114; Gupta et al, 2019110; IntelliHealth Ontarioc; Lopes et al, 20 1433; Peker et al, 2020111; Rasmussen et al, 2018109 |
| Perilesional edema | 897.15 | 0.00 | 897.15 | 50% of patients are treated in the hospitalb | CIHI patient cost estimator114; Gupta et al, 2019110; IntelliHealth Ontarioc; Lopes et al, 20 1433; Peker et al, 2020111; Rasmussen et al, 2018109 |
| Average total per-person cost of managing adverse events | 1,199.53 | 60.48 | 1,199.53 | ||
| DBS | |||||
| Infection (surgically treated) | 414.61 | N/A | N/A | All surgically treated infections are managed in the hospital | CIHI patient cost estimator114; Gadot et al, 2022112; IntelliHealth Ontarioc |
| Hardware malfunction | 2,979.57 | N/A | N/A | All hardware malfunctions require a reoperation117 | Fenoy and Simpson, 2014117; Gadot et al, 2022112 |
| Seizure (generalized tonic-clonic) | 161.91 | N/A | N/A | All seizure disorders require 1 hospitalization | CIHI patient cost estimator114; Gadot et al, 2022112 |
| Cerebral hemorrhage (with sequalae) | 114.83 | N/A | N/A | All cerebral hemorrhage with sequalae require hospitalization | CIHI patient cost estimator114; Gadot et al, 2022112; IntelliHealth Ontarioc |
| Average total per-person cost of managing adverse events | 3,670.94 | N/A | N/A | ||
Note: Numbers may be inexact due to rounding.
Abbreviations: CIHI, Canadian Institute for Health Information; DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; N/A, not applicable; RFA, radiofrequency ablation.
All costs are reported in 2023 Canadian dollars.
B. Davidson, MD, email communication, November 8, 2023.
Accessed November 9, 2023.
The reporting of the frequency of adverse events varied widely across studies. In the absence of meta-analyses, we prioritized studies that were more recent, had larger sample sizes, had longer follow-up time, and used capsulotomy (Table 18). For RFA, we used the frequency of adverse events reported by Liu et al104 in their 2017 study of 37 patients with OCD treated with RFA. We reviewed additional studies that reported adverse events in patients with OCD treated with RFA and used the minimum and maximum reported values among all 4 publications to calculate the plausible range.105–107 The frequency of infection for RFA was taken from a study of over 60 patients treated in the US.108 The frequency of adverse events associated with Gamma Knife radiosurgery was taken from a 2014 randomized controlled trial comparing 12-month outcomes of 8 patients who received Gamma Knife radiosurgery with 8 patients who received a sham procedure (i.e., simulated Gamma Knife radiosurgery using the same equipment).33 The estimates for the plausible range of frequencies were the minimum and maximum reported values among 3 other studies.109–111 Although only used for scenario analyses, in Table 17, we present the frequency of adverse events for DBS taken from a recent systematic review and meta-analysis that summarized 34 studies on DBS for treating patients with OCD.112
The hospitalization costs of adverse events were taken from IntelliHealth Ontario inpatient discharge data by ICD-10-CA diagnosis code. Physician costs are not included in the IntelliHealth Ontario data, and the specific physician fee codes used during an adverse event hospitalization are challenging to estimate, as they may differ for each patient depending on the care required. To estimate the physician costs, we adopted the method used in a previously published health technology assessment in which the ratio of the physician costs to hospital costs was estimated using the CIHI patient cost estimator.114,115 We then obtained physician costs by multiplying the calculated ratio by the hospital costs obtained from IntelliHealth Ontario. When costing data could not be obtained from IntelliHealth Ontario, we used the CIHI patient cost estimator, which included both hospitalization and physician fees. The details for the costing approach are presented in Table 19.
The total cost of managing adverse events was averaged per person by multiplying the frequency of adverse events (Table 18) by the cost of managing the adverse event in the hospital (Table 19) and the percentage of cases that would be managed for that adverse event in the hospital (Table 20).
Ongoing Monitoring Costs
We assumed that anyone who underwent a neurosurgical procedure would have an annual psychiatry consultation for at least 5 years (Table 21).
Table 21:
Ongoing Monitoring Costs Following Surgical Procedures
| Resource item | Unit cost, $a | Number of visits per year | Total cost, $a | Data source and comments |
|---|---|---|---|---|
| MRgFUS neurosurgery | ||||
| Psychiatry consultation | 222.50 | 1 | 222.50 | Schedule of Benefits103 (A195) |
| RFA | ||||
| Psychiatry consultation | 222.50 | 1 | 222.50 | Schedule of Benefits103 (A195) |
| Gamma Knife radiosurgery | ||||
| Psychiatry consultation | 222.50 | 1 | 222.50 | Schedule of Benefits103 (A195) |
| DBS | ||||
| First year following surgery | ||||
| Clinical programming of deep brain stimulator, 2 implantation sites | 343.55 | 1 | 343.55 | Schedule of Benefits103 (G547, G549) |
| Subsequent years following surgery | ||||
| Clinical programming of deep brain stimulator, 2 implantation sites | 343.55 | 2 | 687.10 | Schedule of Benefits103 (G547, G549) |
Note: Numbers may be inexact due to rounding.
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; RFA, radiofrequency ablation.
All costs are reported in 2023 Canadian dollars.
Adjunct Medication Costs
Although we assumed that there would be no changes to medication usage in the reference case, we describe the process of estimating medication costs for scenario analyses. We ran a scenario analysis in which medications are reduced for all types of neurosurgeries such that medication costs decrease by 25%,109,118 but there are no changes in medication usage or cost for those who do not receive neurosurgery.
We estimated the cost of medications for the patients with OCD reported in the 2020 publication by Davidson et al72 (Appendix 7, Table A12). Medications were costed using the Ontario Drug Benefit Formulary119 with the reported amount paid for by the Ministry of Health plus an 8% markup.120
The average annual cost of medications was almost $900 and ranged from $0 to approximately $1,850. In Ontario, medication costs are covered via the Ontario Drug Benefit (ODB) program for qualifying patients. Ontarians may be eligible for ODB if they are 65 years or older, 24 years or younger with no private insurance, living in long-term care, receiving benefits from Ontario Works or the Ontario Disability Support Program (ODSP), or enrolled in the Trillium Drug Program.121
We estimated the proportion of the population with medication coverage using the mean age reported in the clinical trials and reports of disability coverage. The mean age of patients with OCD in the clinical trials was less than 65 years (Table 3). The MRgFUS neurosurgery clinical trials did not report demographic characteristics that could inform whether participants were receiving benefits (e.g., socioeconomic status, disability status), such as those from Ontario Works or ODSP. However, Lee et al122 conducted a trial on 5 cases of DBS for patients with treatment-refractory OCD in Ontario between 2010 and 2015, and they reported that 80% of patients were receiving disability benefits. Therefore, we assumed that 80% of patients receiving neurosurgery for OCD are eligible for ODB and included their medication costs in the Ministry of Health perspective. We applied this percentage to the average cost of medications calculated from the publication by Davidson et al72 and added dispensing fees, assuming that medications would be dispensed 4 times per year with an average dispensing fee of $10, giving an average per-person annual medication cost of $743.
Internal Validation
The secondary health economist (HAT) conducted formal internal validation. This process included checking for errors and ensuring the accuracy of parameter inputs and equations in the budget impact analysis.
Analysis
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. We also conducted sensitivity analyses to explore how the results are affected by varying input parameters and model assumptions. We used Microsoft Excel to code the budget impact analysis model.92
We conducted the following sensitivity or scenario analyses:
Scenario 1, population of interest: We used the population of interest estimated by epidemiological parameters (Table 13) as the total population to represent an upper bound on the volume of the intervention. For the current scenario, we assumed that the number of people accessing RFA and Gamma Knife radiosurgery stays the same and the remaining people receive no surgical treatment (Appendix 7, Table A13).
Scenario 2, increased expansion of MRgFUS neurosurgery: We assumed that the population would expand by an additional 12 nonsurgically treated people receiving MRgFUS neurosurgery in year 1, increasing linearly to 30 additional people in year 5 (Appendix 7, Table A13).
Scenario 3, steady but slower expansion of MRgFUS neurosurgery: We assumed that the population would expand by an additional 12 nonsurgically treated people per year accessing MRgFUS neurosurgery (Appendix 7, Table A13).
Scenario 4, steady but increased expansion of MRgFUS neurosurgery: We assumed that the population would expand by an additional 24 nonsurgically treated people per year accessing MRgFUS neurosurgery (Appendix 7, Table A13).
Scenario 5, decreased Gamma Knife radiosurgery: We explored a scenario in which MRgFUS technology has improved and obviates the need for Gamma Knife radiosurgery by reducing the percentage of people receiving Gamma Knife radiosurgery each year in the new scenario from 15% to 0% (Appendix 7, Table A13).
Scenario 6, patients not eligible for MRgFUS neurosurgery receive RFA: We assumed that people who are not eligible for MRgFUS neurosurgery in the new scenario will receive RFA (Appendix 7, Table A13).
Scenario 7, public funding includes capital costs (average patient volume): We included capital expenditures of MRgFUS neurosurgery in the total cost of MRgFUS neurosurgery (Appendix 7, Table A9).
Scenario 8, public funding includes capital costs (low patient volume): We included capital expenditures of MRgFUS neurosurgery in the total cost of MRgFUS neurosurgery, assuming an annual caseload of 60 patients (48 with essential tremor and 12 with OCD).
Scenario 9, public funding includes capital costs (high patient volume): We included capital expenditures of MRgFUS neurosurgery in the total cost of MRgFUS neurosurgery, assuming an annual caseload of 78 patients (48 with essential tremor and 30 with OCD).
Scenario 10, medication costs decrease by 25%: We explored the impact of a reduction in medication usage after neurosurgery by reducing the medication costs for people who received neurosurgery by 25% (Appendix 7, Table A12).
Scenario 11, re-treatment for MRgFUS neurosurgery with Gamma Knife radiosurgery: We examined the impact of 10% of patients treated with MRgFUS neurosurgery requiring re-treatment with Gamma Knife radiosurgery due to failure to create a lesion with MRgFUS neurosurgery. To calculate the cost of re-treatment, we estimated the number of patients who fail treatment by multiplying the number of patients treated with MRgFUS neurosurgery by the failure rate of MRgFUS neurosurgery and then multiplying the resulting number of patients by the procedure cost of Gamma Knife radiosurgery.
Scenario 12, re-treatment for MRgFUS neurosurgery with RFA: We examined the impact of 10% of patients treated with MRgFUS neurosurgery requiring re-treatment with RFA due to failure to create a lesion with MRgFUS neurosurgery. To calculate the cost of re-treatment, we estimated the number of patients who fail treatment by multiplying the number of patients treated with MRgFUS neurosurgery by the failure rate of MRgFUS neurosurgery and then multiplying the resulting number of patients by the procedure cost of RFA.
Scenario 13, decreased inpatient procedure cost of MRgFUS neurosurgery: We examined the impact of decreasing the inpatient procedure costs of MRgFUS neurosurgery by 20% from $11,240 to $8,992, making the total procedure cost $16,520 instead of the reference case value, $18,768.
Scenario 14, increased inpatient procedure cost of MRgFUS neurosurgery: We examined the impact of increasing the inpatient procedure costs of MRgFUS neurosurgery by 20% from $11,240 to $13,488, making the total procedure cost $21,016 instead of the reference case value, $18,768.
Scenario 15, decreased inpatient procedure cost of RFA: We examined the impact of lower inpatient procedure costs for RFA by using the lower 95% confidence interval estimate from the IntelliHealth Ontario data (Appendix 7, Table A8), $1,256, rather than the mean, $4,338, making the total procedure cost $8,784 instead of the reference case value, $11,866.
Scenario 16, increased inpatient procedure cost of RFA: We examined the impact of higher inpatient procedure costs for RFA by using the upper 95% confidence interval estimate from the IntelliHealth Ontario data (Appendix 7, Table A8), $7,420, rather than the mean, $4,338, making the total procedure cost $14,945 instead of the reference case value, $11,866.
Scenario 17, decreased inpatient procedure cost of Gamma Knife radiosurgery: We examined the impact of lower inpatient procedure costs for Gamma Knife radiosurgery by using the lower 95% confidence interval estimate from the IntelliHealth Ontario data (Appendix 7, Table A8), $1,583, rather than the mean, $1,746, making the total procedure cost $6,921 instead of the reference case value, $7,084.
Scenario 18, increased inpatient procedure cost of Gamma Knife radiosurgery: We examined the impact of higher inpatient procedure costs for Gamma Knife radiosurgery by using the upper 95% confidence interval estimate from the IntelliHealth Ontario data (Appendix 7, Table A8), $1,908, rather than the mean, $1,746, making the total procedure cost $7,247 instead of the reference case value, $7,084.
Scenario 19, lower estimate of RFA adverse event costs: We examined the impact of the cost of adverse events of RFA by calculating costs using the upper estimates for the plausible range of frequency of adverse events (Tables 18 and 20).
Scenario 20, upper estimate of RFA adverse event costs: We examined the impact of the cost of adverse events of RFA by calculating costs using the lower estimates for the plausible range of frequency of adverse events (Tables 18 and 20).
Scenario 21, lower estimate of Gamma Knife radiosurgery adverse event costs: We examined the impact of the cost of adverse events of Gamma Knife radiosurgery by calculating costs using the upper estimates for the plausible range of frequency of adverse events (Tables 18 and 20).
Scenario 22, no anesthesiologist costs for MRgFUS neurosurgery: We examined the impact of eliminating the cost of an anesthesiologist for neurosurgical procedures that do not require general anesthesia.
Scenario 23, alternate fee code for Gamma Knife radiosurgery: We examined the impact of physician fees for Gamma Knife radiosurgery being compensated using fee code N124 and including costs for a surgical assistant and anesthesiologist. The total procedure cost increased from $7,084 to $9,610.
Scenario 24, public funding of DBS and replacement of DBS by MRgFUS neurosurgery: We examined the impact of simultaneous public funding of DBS and replacement of DBS by MRgFUS neurosurgery in the new scenario. We estimated that 2 people per year would receive DBS under public funding in the current scenario based on historical volumes of neurosurgery for severe, treatment-refractory OCD and assumed that they would receive MRgFUS neurosurgery in the new scenario. The costs of DBS are reported in Tables 17, 20, and 21.
Scenario 25, increase in physician fees: We examined the impact of a 4% increase in all physician fees.
Results
Reference Case
We estimated that publicly funding MRgFUS neurosurgery for severe, treatment-refractory OCD would incur an additional $251,601 in year 1 and increase to an additional $494,174 in year 5, for a total budget impact of $1,861,100 over the next 5 years (Table 22). The budget increase is mainly a result of more patients accessing treatment. In the new scenario, 110 patients would receive neurosurgical treatment over 5 years, whereas in the current scenario, only 20 patients receive neurosurgery, meaning 90 additional people would access neurosurgical treatment. The largest component of the budget impact is the additional cost of the surgical procedure, which accounts for $1,816,582 of the 5-year budget impact and represents about 98% of the total budget. Over the 5 years, there would also be increased costs of $53,400 for monitoring patients. Replacing RFA and Gamma Knife radiosurgery with MRgFUS neurosurgery (a noninvasive procedure that does not use ionizing radiation) would result in fewer serious adverse events and save $8,882 in adverse event costs over 5 years. Results stratified by intervention are presented in Appendix 7, Table A14.
Table 22:
Budget Impact Analysis Results - Reference Case
| Scenario | Budget impact, $a | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Totalb,c | |
| Current scenario | ||||||
| Surgical procedure costs | 37,900 | 37,900 | 37,900 | 37,900 | 37,900 | 189,498 |
| Monitoring costs | 890 | 1,780 | 2,670 | 3,560 | 4,450 | 13,350 |
| Adjunct medication costs | 11,888 | 26,006 | 42,352 | 60,928 | 81,732 | 222,906 |
| Adverse event costs | 2,976 | 2,976 | 2,976 | 2,976 | 2,976 | 14,880 |
| Total cost of current scenario | 53,654 | 68,661 | 85,898 | 105,363 | 127,058 | 440,634 |
| New scenario | ||||||
| Surgical procedure costs | 288,607 | 344,911 | 401,216 | 457,520 | 513,825 | 2,006,079 |
| Monitoring costs | 3,560 | 7,788 | 12,683 | 18,245 | 24,475 | 66,750 |
| Adjunct medication costs | 11,888 | 26,006 | 42,352 | 60,928 | 81,732 | 222,906 |
| Adverse event costs | 1,200 | 1,200 | 1,200 | 1,200 | 1,200 | 5,998 |
| Total cost of new scenario | 305,255 | 379,904 | 457,450 | 537,893 | 621,232 | 2,301,733 |
| Budget impactb,c | ||||||
| Surgical procedure costs | 250,707 | 307,012 | 363,316 | 419,621 | 475,925 | 1,816,582 |
| Monitoring costs | 2,670 | 6,008 | 10,013 | 14,685 | 20,025 | 53,400 |
| Adjunct medication costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse event costs | -1,776 | -1,776 | -1,776 | -1,776 | -1,776 | -8,882 |
| Total budget impact | 251,601 | 311,243 | 371,552 | 432,529 | 494,174 | 1,861,100 |
All costs are reported in 2023 Canadian dollars.
Negative costs indicate savings.
Results may appear inexact due to rounding.
Sensitivity Analysis
The results of the 25 scenario analyses are presented in Table 23. Compared with the reference case, scenarios in which the costs of RFA or Gamma Knife radiosurgery increased, cost of MRgFUS neurosurgery decreased, medications costs were reduced, or treatment volume was less than the reference case resulted in a lower budget impact. Scenarios in which costs of RFA or Gamma Knife radiosurgery decreased, cost of MRgFUS neurosurgery increased, capital or re-treatment costs for MRgFUS neurosurgery were included, or treatment volume was greater than the reference case resulted in a higher budget impact compared with the reference case.
Table 23:
Budget Impact Analysis Results - Scenario Analyses
| Scenario | Budget impact, $a | %changec | Total number of surgically treated patients in new scenario | |||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Totalb | |||
| Reference case | 251,601 | 311,243 | 371,552 | 432,529 | 494,174 | 1,861,100 | - | 110 |
| Scenario 1, population of interest | 745,359 | 772,804 | 781,482 | 809,150 | 837,041 | 3,945,836 | 112% | 217 |
| Scenario 2, increased expansion of MRgFUS neurosurgery | 251,601 | 350,710 | 432,685 | 536,026 | 622,232 | 2,193,253 | 18% | 126 |
| Scenario 3, steady but slower expansion of MRgFUS neurosurgery | 251,601 | 252,042 | 250,254 | 246,236 | 239,990 | 1,240,123 | -33% | 80 |
| Scenario 4, steady but increased expansion of MRgFUS neurosurgery | 488,405 | 500,432 | 510,231 | 517,800 | 523,140 | 2,540,008 | 36% | 140 |
| Scenario 5, decreased Gamma Knife radiosurgery | 262,086 | 321,728 | 382,037 | 443,014 | 504,659 | 1,913,524 | 3% | 110 |
| Scenario 6, patients not eligible for MRgFUS neurosurgery receive RFA | 255,472 | 315,114 | 375,423 | 436,401 | 498,045 | 1,880,455 | 1% | 110 |
| Scenario 7, public funding includes capital costs (average patient volume) | 420,664 | 514,119 | 608,242 | 703,031 | 798,489 | 3,044,546 | 64% | 110 |
| Scenario 8, public funding includes capital costs (low patient volume) | 437,571 | 534,407 | 631,911 | 730,082 | 828,920 | 3,162,890 | 70% | 110 |
| Scenario 9, public funding includes capital costs (high patient volume) | 394,655 | 482,908 | 571,828 | 661,416 | 751,671 | 2,862,477 | 54% | 110 |
| Scenario 10, medication costs decrease by 25% | 248,629 | 304,741 | 360,964 | 417,298 | 473,741 | 1,805,373 | -3% | 110 |
| Scenario 11, 10% of patients treated with MRgFUS neurosurgery require re-treatment with Gamma Knife radiosurgery | 262,226 | 311,243 | 371,552 | 432,529 | 494,174 | 1,871,725 | 1% | 110 |
| Scenario 12, 10% of patients treated with MRgFUS neurosurgery require re-treatment with RFA | 269,400 | 311,243 | 371,552 | 432,529 | 494,174 | 1,878,899 | 1% | 110 |
| Scenario 13, decreased inpatient procedure cost of MRgFUS neurosurgery | 217,881 | 270,779 | 324,344 | 378,577 | 433,478 | 1,625,058 | -13% | 110 |
| Scenario 14, increased inpatient procedure cost of MRgFUS neurosurgery | 285,321 | 351,707 | 418,761 | 486,482 | 554,870 | 2,097,141 | 13% | 110 |
| Scenario 15, decreased inpatient procedure cost of RFA | 257,765 | 317,407 | 377,716 | 438,693 | 500,338 | 1,891,919 | 2% | 110 |
| Scenario 16, increased inpatient procedure cost of RFA | 245,437 | 305,079 | 365,389 | 426,366 | 488,010 | 1,830,280 | -2% | 110 |
| Scenario 17, decreased inpatient procedure cost of Gamma Knife radiosurgery | 255,179 | 314,821 | 375,131 | 436,108 | 497,752 | 1,878,991 | 1% | 110 |
| Scenario 18, increased inpatient procedure cost of Gamma Knife radiosurgery | 251,438 | 311,080 | 371,390 | 432,367 | 494,011 | 1,860,286 | 0%d | 110 |
| Scenario 19, lower estimate of RFA adverse event costs | 252,178 | 311,820 | 372,129 | 433,106 | 494,751 | 1,863,984 | 0%d | 110 |
| Scenario 20, upper estimate of RFA adverse event costs | 251,468 | 311,110 | 371,420 | 432,397 | 494,041 | 1,860,436 | 0%d | 110 |
| Scenario 21, lower estimate of Gamma Knife radiosurgery adverse event costs | 252,740 | 312,382 | 372,691 | 433,669 | 495,313 | 1,866,795 | 0%d | 110 |
| Scenario 22, no anesthesiologist costs for MRgFUS neurosurgery | 243,004 | 300,926 | 359,517 | 418,774 | 478,700 | 1,800,921 | -3% | 110 |
| Scenario 23, alternate fee code for Gamma Knife radiosurgery | 249,075 | 308,771 | 369,026 | 430,004 | 491,648 | 1,848,470 | -1% | 110 |
| Scenario 24, public funding of DBS and replacement of DBS by MRgFUS neurosurgery | 168,730 | 214,068 | 257,845 | 300,061 | 340,715 | 1,281,419 | -31% | 120 |
| Scenario 25, increase in physician fees | 253,638 | 313,879 | 374,814 | 436,444 | 498,768 | 1,877,543 | 1% | 110 |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; RFA, radiofrequency ablation.
All costs are reported in 2023 Canadian dollars.
Results may appear inexact due to rounding.
Percent change calculated as the difference in the total budget impact of the scenario analysis and the total budget impact of the reference case divided by the total budget impact of the reference case.
Percent change was very small (<0.5%).
Overall, treatment volume (the number of treated patients in the new scenarios) resulted in the largest changes to the budget impact. In the reference case, the number of patients treated with neurosurgery in the new scenario was 110 over 5 years. For scenarios 1 to 4, the number of surgically treated patients in the new scenario ranged from 80 (scenario 3, steady but slower expansion of MRgFUS neurosurgery) to 217 (scenario 1, population of interest). The 5-year budget impact ranged from $1,240,123 (scenario 3) to $3,945,836 (scenario 1).
Discussion
In this budget impact analysis, we estimated the costs required to publicly fund MRgFUS neurosurgery for adults with severe, treatment-refractory OCD. We found that publicly funding this intervention would result in a $1.9 million increase in the budget over 5 years for 110 people to receive neurosurgical treatment over the same 5-year period.
In our reference case, we assumed that the number of people surgically treated over 5 years increases from 20 to 110 with public funding of MRgFUS neurosurgery. MRgFUS is the only neurosurgical option that is noninvasive and does not use ionizing radiation, eliminating the potential risks related to general anesthesia and open surgery in RFA and the potential risks related to exposure to ionizing radiation in Gamma Knife radiosurgery.33,104–107,109–111 We assumed that this would lead to more interest and acceptance of a surgical option. MRgFUS neurosurgery is performed in an MRI suite rather than an operating room; thus, it would not use limited operating room time. Additionally, the procedure requires a 1-night hospital stay, unlike RFA in which hospital stays ranged from 1 to 3 days (see Appendix 7, Table A8).
Only 2 sites in Ontario currently have the equipment necessary to perform these interventions. Through consultation with clinical experts, we do not foresee a capacity issue; however, there may be geographical inequity, as people living farther from the sites would be required to travel for the procedure.
Strengths and Limitations
Our analysis was strengthened by continuous engagement with clinical experts currently performing MRgFUS neurosurgery who confirmed and helped inform key model parameters and assumptions. Additionally, we ran extensive scenario analyses to determine the impact of our assumptions and uncertainty in key parameters.
The following limitations should be noted when interpreting the findings of this analysis. Inpatient procedure costs for RFA and Gamma Knife radiosurgery were sourced from administrative data, but there were few procedures to treat OCD, so there was a lot of uncertainty in the costs. The inpatient procedure costs were likely underestimated. When administrative data were used to estimate the inpatient procedure cost of MRgFUS neurosurgery, the cost was much lower than the value provided directly from a centre currently performing the procedure. We believe that the cost estimate for MRgFUS neurosurgery provided from the centre is most accurate, and we used it in our analysis. Similar estimates for RFA and Gamma Knife radiosurgery were not available, and we used the best available evidence. In our sensitivity analyses, the inpatient procedure costs for RFA and Gamma Knife radiosurgery were demonstrated to have little effect on the budget impact due to the low number of RFA and Gamma Knife procedures.
Our analysis did not account for potential changes in health care use such as doctor's visits, emergency department visits, or hospitalizations. Furthermore, many of the costs of OCD fall on the patient and are not within the Ministry of Health perspective, such as psychotherapy and lost workdays.
Conclusions
We expect public funding of MRgFUS neurosurgery for treatment-refractory OCD in Ontario to result in an additional increase in budget of $1.9 million and 110 patients receiving neurosurgical treatment over 5 years.
Patient volume and potential public funding of capital costs had the largest impact on the budget.
Preferences and Values Evidence
Objective
The objective of this analysis was to explore the underlying values, needs, and priorities of those who have lived experience of obsessive–compulsive disorder (OCD), as well as the preferences and perceptions of patients, family, and care partners of magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery for the treatment of people with treatment-refractory OCD.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other care partners, and the person's personal environment. Engagement also provides insight into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).123–125 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are important to consider and to understand the impact of the technology in people's lives, we may speak directly with people who live with a given health condition, including those with experience of the technology or intervention we are exploring.
For this analysis, we examined the preferences and values of people with lived experience of OCD via direct engagement. The initiative was led by the Patient and Public Partnering team at Ontario Health, and direct engagement with eligible participants was completed through telephone interviews.
Direct Patient Engagement
Methods
Partnership Plan
The partnership plan for this health technology assessment focused on consultation to examine the experiences of people with OCD and those of their families and other care partners. We engaged people via telephone interviews.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people with OCD, their journey to diagnosis, and the experiences of their families and care partners.126 The sensitive nature of exploring people's experiences of a health condition and their quality of life further supported our choice of methodology.
Participant Outreach
We used an approach called purposive sampling,127–130 which involves actively reaching out to people with direct experience of the health condition and health technology or intervention being reviewed.
We approached the MRgFUS neurosurgery centre at a hospital in Toronto, Ontario, in an effort to engage with patients who have undergone or are on the waitlist for the procedure.
Inclusion Criteria
We sought to speak with adults with lived experience of OCD who underwent or may undergo MRgFUS neurosurgery. People did not need to have direct experience with MRgFUS neurosurgery in order to participate.
Exclusion Criteria
We did not set exclusion criteria for participants who otherwise met the inclusion criteria.
Participants
For this project, we spoke to a total of 14 participants. Nine of the 14 participants were diagnosed with OCD, and the other 5 participants were care partners of people with OCD. Of the 9 patients who were interviewed, 6 had experience with MRgFUS neurosurgery and 3 were on the waitlist.
Approach
At the beginning of the interview, we explained the role of our organization, the purpose of this health technology assessment, the risks of participation, and how participants’ personal health information would be protected. We gave this information to participants both verbally and in a letter of information (Appendix 8) if requested. We then obtained participants’ verbal consent before starting the interview. With participants’ consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 30 to 60 minutes. The interview was semistructured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.131 Questions focused on the impact of OCD on quality of life, the journey to diagnosis, experience with MRgFUS neurosurgery, and the impact of MRgFUS neurosurgery. Please see Appendix 9 for our interview guide.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze interview transcripts. This approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.132,133 We used the qualitative data analysis software program NVivo134 to identify and interpret patterns in the data. The patterns we identified allowed us to describe the impact of OCD on the patient's life and decision-making factors for MRgFUS neurosurgery.
Results
Living With OCD
Participants with OCD described experiencing different symptoms, including obsessive, intrusive thoughts that trigger distress as well as compulsive behaviours performed in an attempt to stop the obsessive thoughts or decrease distress. These symptoms were persistent and impacted different aspects of their daily lives.
My physical compulsions are very minimized, but my mental compulsions are way through the roof and very debilitating. I call them spikes. So, if I have an OCD spike, [if I have a] random intrusive thought roll in, my brain goes, “Oh God, that's horrible. We need to focus on that,” even though, logically, I know that it's not a problem.
I started having intrusive song lyrics playing in my head 24/7. It's a common experience for anyone from time to time to get a song stuck in their head, right? Like an earworm, but mine were super intrusive, super constant, very loud. It was almost as if I had this kind of crazy stereo in my head that just wouldn't shut up. It was really horrible.
I'm struggling with contamination issues. Symptoms are hand washing and ruminations.
My school desk always had to be perfectly organized, and if someone moved something, I had to do a lot of reorganizing stuff.
Most participants with OCD started showing symptoms as a child and were formally diagnosed with OCD as a child or adolescent.
My OCD journey started when I was around 6, but I didn't get formally diagnosed until I was around 11 or 10.
I remember things really started to show in terms of my OCD when I started grade 9 in 2008. I was 14 years old at the time.
Impact on Day-to-Day Life
Participants described the effect that OCD had on their day-to-day life, including difficulty performing activities such as eating, showering, leaving the house, and doing household chores.
Sometimes he'll go 3 or 4 days without getting up to even eat or drink or do anything.
Leaving the house or my apartment on my own is impossible because OCD just completely takes over.
I stopped showering every day and say, “Oh, well, it doesn't matter if you don't do it. Just go to work anyway.” I wouldn't brush my teeth every day. I sleep on the couch instead of in my bed.
It was so hard to [fold] laundry. Usually, I just [keep my washed clothes in] the dryer machine. Other times, there are periods where it [laundry] would be on my bed for months at a time.
Participants with OCD also mentioned that it takes them longer to complete tasks that would otherwise be completed in shorter periods owing to their tendency to repeat the same task multiple times before feeling satisfied.
I remember I spent like 40 minutes loading and unloading [the] dishwasher. I just couldn't get it right. Then I broke down calling my mom.
I have problems reading where I'll read the sentence in a paragraph and then I lose where I am in the sentence and then I have to start over again at the beginning of the sentence.
Impact on Work and School
Participants explained that their OCD symptoms contributed to decreased productivity and performance at work, as well as repercussions in their career path. Most of them had to quit their jobs altogether because their symptoms were persistent and prevented them from working.
Currently [I'm in my 20s], I'm not working. I'm unable to work mostly because of my OCD.
She lost her job as a dental hygienist because she couldn't do that work anymore.
If I worked, I would basically not be able to work really well because I have to repeat tasks and it would take me forever to do it.
He was working his way up [in his career]. But the OCD just kept him from progressing. It [OCD] was always there, nagging at him. There were various times where he would spend hours sitting in the car because he couldn't leave his vehicle.
Participants also mentioned that OCD negatively affected their pursuit of education, such that it took them longer to complete or couldn't complete their program. In turn, this also impacted their ability to pursue a career.
It took me 6 years to do a 2-year program because I'm used to repeating things.
I had to drop out of school.
Impact on Social Life and Family Relationships
Participants’ social life and family relationships were also impacted by their OCD symptoms. Participants explained that they had limited social interactions with their friends and families due to their symptoms. This made it difficult to engage with others and form relationships. Some mentioned having social anxiety.
He has no social life. He does not talk to his friends. He does not talk to family members. He barely talks to me. It's very severe.
I would say it impacts his social life immensely. He has social anxiety as well, so a lot of his mental compulsions are around social interactions.
I have social anxiety. When I go out to meet new people, a lot of the time I become too anxious and I can't come up with anything to say back to the person, so I create awkward situations.
Participants mentioned that OCD affected their family dynamics and strained their relationships with family members and friends.
It [OCD] changed my family dynamics. Before I was diagnosed, I had good relationship[s] with my sister and my parents, but as I got diagnosed and I became more severe as time went by, my parents have to focus on me a lot and that caused my sister to be quite lonely.
[OCD has] strained friendships, [it has] strained relationships. When my OCD symptoms really started to deteriorate, [it affected my relationships].
Impact on Mental Health
Mental health was emphasized as being substantially impacted by OCD. Participants reported that their mental health disorders, including anxiety and depression, were associated with their OCD. Furthermore, most participants reported struggling with suicidal thoughts.
Along with his OCD, he's been diagnosed with having major depressive disorder.
I have a major problem with depression and anxiety.
I also developed a lot of suicidal ideation and suicidal thoughts.
I feel there's sometimes no hope for me, that I'm going to be like this forever, and a lot of times, I feel like committing suicide because of that.
Some participants experienced lack of sleep, which further exacerbated their mental health problems.
I think OCD is the driver which really contributed to my lack of sleep and depression.
I'm always going through my list and it's hard to sleep. I haven't slept for the last 3 days.
Impact on Care Partners
Care partners, which are mostly family members of people with OCD, reported the substantial impact that caring for someone with OCD has on different aspects of their lives, including social, mental, professional, and financial aspects. Care partners spoke about feeling socially isolated due to their overwhelming care duties. They also reported increased anxiety and stress leading to repercussions in their career. Furthermore, most care partners mentioned the financial burden that comes with taking care of a loved one with OCD, as they become the sole financial provider.
I have no family or friends that come into the house anymore because my son basically sleeps on the couch in the dining room.
His suicide attempts also negatively impacted all of us … I've been clinically diagnosed with PTSD.
I had a nervous breakdown about a year and a half ago, and I'm now out of work. I had to quit my job.
If he gets the surgery, we'll be going down to Toronto, and this will be on my dime. So, financially it's been a lot because I try and help him with food payments and buying him whatever he needs.
Patients also reflected the reliance they had on their care partners (family members) due to their OCD symptoms.
My parents had to once again take care of me as if I was a child and dedicate a lot of their time to support me and keep me safe, given the suicidal ideation I was having.
It puts a lot of stress on my dad because he can't always be there for me because he's busy with work.
Treatment
Participants spoke about their journey of trying different treatment options for OCD, including medications, cognitive behavioural therapy (CBT), exposure and response prevention (ERP) therapy, magnetic seizure therapy, and repetitive transcranial magnetic stimulation (rTMS). However, their OCD symptoms did not improve after exhausting these treatment options, leading them to consider MRgFUS neurosurgery. Participants also commented on the long time that it took to get a referral for MRgFUS neurosurgery and the difficulty to find treatment.
I explored all the traditional medication combos that are used to treat OCD, including the goldstandard heavy hitters like clomipramine, but that was not making a meaningful difference.
I tried CBT, ERP, mindfulness practices with different psychologists, and rTMS… CBT hasn't really worked for me.
I tried magnetic seizure therapy. It was still experimental at the time. And I remember I did that a couple times before, but I stopped because I had a bad experience.
The wait times were extremely long to even get an initial appointment [for MRgFUS neurosurgery].
It was difficult to get treatment for my son because he's a case that seems to be somewhat unique [treatment-resistant OCD] compared to the general OCD population.
MRgFUS Neurosurgery
Awareness About MRgFUS Neurosurgery
Participants highlighted the lack of awareness about MRgFUS neurosurgery as a treatment option for treatment-refractory OCD. Most participants mentioned that they found out about MRgFUS neurosurgery through research or word of mouth. They noted having to self-advocate to get a referral to the MRgFUS program.
I think not a lot of people know about it [MRgFUS neurosurgery] and that it's even an option. For people with treatment-resistant OCD who are not getting relief from medication, it can be really life-saving treatment.
I heard about FUS [MRgFUS] actually from a friend. I was an inpatient with them, so they suffer as well with OCD. I heard from them about the treatment because I reached out to them.
I was researching about the OCD program, I found something called focused ultrasound, and I also read about patient stories online … They [patients treated with MRgFUS] have gotten better. So, when I went to [the hospital], I mentioned the focused ultrasound to the psychiatrist I was seeing.
I asked his [my son's] psychiatrist to look into neurosurgery, which is where we found the FUS clinical trial. So, it was over a year we were trying to access that treatment and finally got it this past June.
Another participant mentioned hesitating to undergo MRgFUS neurosurgery due to the unfamiliarity of neurosurgery as a treatment option for a psychiatric condition like OCD.
It seemed a bit daunting at first. It seemed kind of scary. It wasn't something that I immediately said, “Oh, I need this,” because I was thinking, “Oh my gosh, surgery for a mental health condition. That seems a bit far-fetched.”
Decision-Making for MRgFUS Neurosurgery
Participants were motivated to seek neurosurgical treatment for their OCD after exhausting most of the traditional treatment options. They reported feeling desperate to find a relief from their condition and considered MRgFUS neurosurgery as their last resort.
We have explored almost every option that we can possibly think of or researched.
The reason I reached out to [the hospital] [to get MRgFUS neurosurgery] is because I was very desperate to find a solution.
I want to do absolutely everything I can to get my neurons in order … even if the focused ultrasound fails, I'm willing to go in for deep brain stimulation.
One participant mentioned that testimonies from people who had positive experiences with MRgFUS neurosurgery motivated them to seek treatment.
Another thing that really propelled me to undergo focused ultrasound is the people who have undergone it said that they've seen progress, and it's helped them. So, I told myself that I'll never know if I will get any benefit from it if I don't undergo this procedure.
Experience With MRgFUS Neurosurgery
Most participants who underwent MRgFUS neurosurgery reported having a positive experience with little to no side effects and a short recovery time.
I would say it's [the surgery experience is] pretty positive. I didn't have any side effects from it. They said your head [was] supposed to hurt for a few days. I didn't have that.
Surgery was fine. There were no side effects from it. It was just a day surgery, and I slept overnight at the hospital. It was fairly painless, except for just a few moments during which there was some pain but tolerable.
Patients reported having to take time off from work for recovery after surgery.
He was working at the time. So, he took time off work. The recommendation from the doctor was a week to 2 weeks postsurgery, depending on the headaches.
I was able to get to work right away; they told me to take a few days off, so I just worked like 2 days after.
Participants who were on the waitlist for MRgFUS neurosurgery mentioned that they felt hopeful waiting for their surgery and viewed it as a last resort. Some participants reported feeling nervous and scared thinking about their upcoming surgery.
The focused ultrasound has been my lifeline mentally. It gives me some hope.
I honestly don't know where we go from here. It's a place you get to at the end of the line, and by the end, you've exhausted your financial resources.
I'm also a little bit nervous about having a brain surgery because it's my first time and I'm afraid something might be wrong.
I'm also worried if the surgery itself will be painful, even though I know that they will be basically turning off my brain.
Impact of MRgFUS Neurosurgery
OCD Symptoms
Participants who underwent MRgFUS neurosurgery reported the impact it had on their OCD symptoms. The effectiveness of MRgFUS neurosurgery varied from patient to patient, but most participants described a lessening of their OCD symptoms following surgery.
That horrible kind of loud, intrusive, constant loops that were playing in my head gradually subsided.
Immediately after the surgery, like for a few months after, his compulsion seemed to lessen.
My OCD symptoms have been really minimal and nonexistent and not impacting my day-today life.
However, some participants who underwent MRgFUS neurosurgery have not yet seen positive changes in their OCD symptoms and expressed disappointment.
There was no impact aside from the 3 or 4 good days. It's now 2 months … but it's very disheartening.
Unfortunately, she hasn't got better from it. But I understand it's supposed to take some time before it kicks in.
Mental Health
Participants also reported an improvement in their mental health following MRgFUS neurosurgery. Some mentioned that their depression and anxiety symptoms lessened, while others noted improvement in their sleep.
After surgery, he definitely had improved mood.
It is kind of like a factory reset in terms of his depressive thoughts.
There's a bit of a diminishing in anxiety and depression.
I do notice that my sleep is a bit better. Before, I had a lot of trouble sleeping and relaxing to go to sleep.
Concurrent Treatments
Many participants mentioned that following MRgFUS neurosurgery, they had lowered their dosage of certain medications that are used to treat their OCD or associated depression and anxiety, or stopped taking these medications altogether. Others reported that the lessening of their OCD symptoms after surgery allowed them to have effective therapy sessions.
The goal is eventually to eliminate lorazepam. And we've been doing that successfully so far.
We did reduce one of his medications, so he's no longer on risperidone; his psychologist ended his sessions.
I was able to lower some of it [medication], and I was able to actually come off one of them.
I'm able to do exposure therapy much better than I could before because before I was so anxious about doing exposures, but I would say it's much better now.
Quality of Life
Participants highlighted the positive impact that MRgFUS neurosurgery had on their quality of life. They emphasized the importance of regaining their independence to perform day-to-day activities with little to no support needed from care partners. Some participants mentioned that they were able to go back to school or work following their treatment.
While I was so ill, my parents were taking care of me as a dependent, but after treatment, I was able to get on my own feet and become an independent functioning adult.
I don't have problems with doing activities of daily living like cooking. And [I] can shower and stuff because it doesn't take me as long to do so. I have better social relationships. I'm not disappearing from tasks and people. I do extracurricular activities as well.
I was doing full-time co-op for school and I'm doing school at the same time, so I'm balancing a part-time job as well as school. I'm pretty good for the most part right now.
I was able to start work, so I started teaching at a private school, and then I went back to school, which is what I'm doing now.
Participants also reported that MRgFUS neurosurgery was a life-saving treatment for them at the end of their treatment journey and emphasized the importance of having access to MRgFUS neurosurgery for treatment-refractory OCD.
I had the procedure, and it really saved my life and transformed my life.
It really saved my life and turned my life around.
I would want to emphasize that when we were looking for treatment for our son, if this surgery wasn't available, we would have hit the end of the road.
For some people who are drug-resistant, this might be literally their last option, so I just feel it's very important to have these options open.
Barriers
Participants spoke about the barriers that they faced while trying to access treatment for OCD. They highlighted transportation, cost of treatment, and difficulty navigating the health care system as the main barriers.
Transportation
Because the MRgFUS neurosurgery centre is in a hospital in Toronto, patients who live outside the Greater Toronto Area had to travel long distances to their appointments. Participants reported travelling from other cities in Ontario, as well as out of province, to access treatment. Additionally, some patients travelled with their care partners and had to seek accommodation in a hotel or with relatives near Toronto.
I had to commute from [another city] to Toronto. My mom drove me, and she stayed at a hotel while I had to stay overnight out of hospital posttreatment.
We travelled to Toronto from [another province] for that appointment. We stayed in a hotel for 2 nights and then we were fortunate enough to have family who lived just 2 hours outside of Toronto, so we stayed with them.
I think one of the biggest obstacles for me is that I need someone's help in order to get to the hospital. And thankfully, I always had my dad who drove me to the hospital in Toronto.
Cost of Treatment
Participants reported the financial burden that they faced while paying out of pocket for their OCD treatment, including medication and therapy costs. Since most patients were not able to work and generate income, care partners had to share the financial burden.
The cost of his medication has been a lot… and there's the cost for his therapy which is $250 every time he goes. He used to go 3 times a month, but now just to save money, he's going once a month.
We flew home the day after his surgery, and we've had 1 trip for [a] follow-up appointment so far. So that's another 2 nights - the hotel and round-trip plane tickets. So, it's been costly, but worth it.
I was doing therapy for a bit, but I don't think it helped enough and it's too big of an expense to pay out of pocket.
Challenges in Navigating the Health Care System
Participants reported that they had difficulty finding the right treatment for their condition. They mentioned that after exhausting multiple treatment options for their OCD, getting a referral to the MRgFUS program was a challenge and required a lot of self-advocacy. One participant noted the long wait time to get an initial appointment for MRgFUS neurosurgery.
I think the biggest barrier was finding the treatment. Getting him to surgery was a bit of an effort and a push. It wasn't like we went to a doctor and then he said, “Oh, here's your referral,” and then you move along the process - we had to navigate the health care system a lot ourselves.
If we didn't ask certain questions or reach out to certain contacts at [the hospital], I don't know if this ever would have happened. So, it was just less organic in receiving the treatment.
The wait times were extremely long to even get an initial appointment. That's something that was difficult to navigate just because of how severe my symptoms were and how desperate I was for some relief.
Some participants who underwent MRgFUS neurosurgery reported that they felt a lack of support from their health care team following surgery.
They didn't help me navigate the system in finding someone [psychiatrist] to see … maybe that's why 6 months after surgery, I had a flare-up in my OCD symptoms.
I think after the surgery, I felt like I was on my own in terms of finding a regular psychiatrist I could see. They said after the surgery, it is important to maintain a regimen of therapy.
There were some barriers because the psychiatrist that I was seeing … doesn't specialize in OCD, so he was limited in terms of medications that he could suggest.
Preferences and Values Evidence Discussion
All participants had lived experience of OCD or were a family member or care partner of someone with OCD. Participants reported the negative impacts that OCD had on their day-to-day activities, work and school, social life and family relationships, and mental health. They spoke about the journey to manage their condition, the various treatment options that they had explored, and their experience with MRgFUS neurosurgery. Most participants had undergone MRgFUS neurosurgery, while some were on the waitlist to undergo this procedure. Participants also highlighted the importance of expanding access to neurosurgical treatment options such as MRgFUS neurosurgery for people with treatment-refractory OCD.
Our analysis was limited by a lack of geographic representation among participants, most of whom lived in southern Ontario; however, both urban and rural perspectives were provided, and 1 out-of-province participant was included.
Preferences and Values Evidence Conclusions
Participants spoke about the impact of living with OCD. They reflected on their experience undergoing MRgFUS neurosurgery. Most participants who underwent MRgFUS neurosurgery commented on the positive impact that it had on their OCD symptoms, mental health, and quality of life. Those who were on the waitlist expressed their hopeful desire to get relief from their condition following surgery. All patients and care partners emphasized the importance of having access to MRgFUS neurosurgery as a treatment option for treatment-refractory OCD. They regarded MRgFUS neurosurgery as a last resort after exhausting multiple treatment options.
Conclusions of the Health Technology Assessment
Based on 2 small case series, the evidence suggests that MRgFUS neurosurgery for severe, treatment-refractory OCD may improve OCD symptoms, quality of life, and patient functioning, and lead to treatment response for many but not all patients. MRgFUS neurosurgery was also found to have a favourable safety profile. In a minority of cases, the procedure could not be successfully performed owing to skull factors, and no cases of re-treatment were reported. However, the evidence is very uncertain due mainly to limitations in study design, sample size, and statistical power.
The cost-effectiveness of MRgFUS neurosurgery is unknown. There are no directly applicable published data on cost-effectiveness. Due to the lack of comparative clinical evidence, we did not conduct a primary economic evaluation. We estimate that the budget impact of publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario would be an additional $1.9 million over 5 years.
Most patients who underwent MRgFUS neurosurgery commented on the positive impact that it had on their OCD symptoms, mental health, and quality of life. All patients and care partners emphasized the importance of having access to MRgFUS neurosurgery as a treatment option for treatment-refractory OCD.
Acknowledgments
This report was developed by a multidisciplinary team from Ontario Health. The primary clinical epidemiologist was Alexis Schaink, the secondary clinical epidemiologist was Myra Wang, the primary medical librarian was Caroline Higgins, the secondary medical librarian was Corinne Holubowich, the primary health economist was Hailey Saunders, the secondary health economist was Hong Anh Tu, and the patient engagement analyst was Samrawit Lemma.
The medical editor was Kesiah Stoker. Others involved in the development and production of this report were Molly Li, Claude Soulodre, Susan Harrison, Sarah McDowell, Chunmei Li, Jigna Mistry, Andrée Mitchell, Charles de Mestral, and Nancy Sikich.
We would like to thank the following people for lending their expertise to the development of this report:
Anusha Baskaran, Sunnybrook Health Sciences Centre
Allison Bethune, Sunnybrook Health Sciences Centre
Daniel Blumberger, The Centre for Addiction and Mental Health
Benjamin Davidson, Sunnybrook Health Sciences Centre
Peter Giacobbe, Sunnybrook Health Sciences Centre
Clement Hamani, Sunnybrook Health Sciences Centre
Nir Lipsman, Sunnybrook Health Sciences Centre
Andres Lozano, Alan and Susan Hudson Chair in Neurosurgery at University Health Network and University of Toronto
Carrie MacCready, Sunnybrook Health Sciences Centre
Sean Nestor, Sunnybrook Health Sciences Centre
Jennifer Rabin, Harquail Centre for Neuromodulation, Sunnybrook Research Institute
Peggy M. A. Richter, Frederick W. Thompson Anxiety Disorders Centre, Sunnybrook Health Sciences Centre
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
Citation
Ontario Health. Magnetic resonance-guided focused ultrasound neurosurgery for treatment-refractory obsessive–compulsive disorder: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2025 May;25(1):1-123. Available from: hqontario.ca/evidence-to-improve-care/health-technology-assessment/reviews-and-recommendations/magnetic-resonance-guided-focused-ultrasound-neurosurgery-for-treatment-refractory-obsessive–compulsive-disorder
Abbreviations
- ALIC:
anterior limb of the internal capsule
- BVMT-R:
Brief Visuospatial Memory Test - Revised
- CBT:
cognitive behavioural therapy
- CCI:
Canadian Classification of Health Interventions
- CGI:
Clinical Global Impression
- CGI-I:
Clinical Global Impression - Improvement
- CGI-S:
Clinical Global Impression - Severity
- CIHI:
Canadian Institute for Health Information
- COWAT:
Controlled Oral Word Association Test
- CT:
computed tomography
- CVLT-II:
California Verbal Learning Test, second edition
- DBS:
deep brain stimulation
- D-KEFS:
Delis-Kaplan Executive Function System
- dTMS:
deep transcranial magnetic stimulation
- ERP:
exposure and response prevention
- FDA:
Food and Drug Administration (US)
- FrSBe:
Frontal Systems Behavior Scale
- GAF:
Global Assessment of Functioning
- GRADE:
Grading of Recommendations Assessment, Development, and Evaluation
- HDE:
Humanitarian Device Exemption
- ICD-10-CA:
International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada
- ICER:
incremental cost-effectiveness ratio
- IGT:
Iowa Gambling Task
- K-WAIS:
Wechsler Adult Intelligence Scale, Korean version
- MRgFUS:
magnetic resonance-guided focused ultrasound
- MRI:
magnetic resonance imaging
- NHS EED:
National Health Service Economic Evaluation Database
- NICE:
National Institute for Health and Care Excellence
- OCD:
obsessive–compulsive disorder
- ODB:
Ontario Drug Benefit
- ODSP:
Ontario Disability Support Program
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QALY:
quality-adjusted life-year
- RFA:
radiofrequency ablation
- rTMS:
repetitive transcranial magnetic stimulation
- SD:
standard deviation
- SDMT:
Symbol Digit Modalities Test
- SSRI:
selective serotonin reuptake inhibitor
- TMS:
transcranial magnetic stimulation
- WTAR:
Wechsler Test of Adult Reading
- Y-BOCS:
Yale-Brown Obsessive–Compulsive Scale
Glossary
- Adverse effect:
An adverse effect (or adverse reaction) is an undesired harmful or noxious effect resulting from a preventive, diagnostic, or therapeutic procedure.135
- Adverse event:
An adverse event is an undesired harmful or noxious event temporally associated with a preventive, diagnostic, or therapeutic procedure that may present during or after, but is not necessarily causally related.135
- Base case:
In economic evaluations, the base case is the “best guess” scenario, including any assumptions, considered most likely to be accurate. In health technology assessments conducted by Ontario Health, the reference case is used as the base case.
- Budget impact analysis:
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- CE mark:
A CE (Conformité Européenne) mark is a requirement for medical devices to be marketed and sold in Europe.136 A CE mark reflects that the device complies with all legally mandated essential requirements.
- Cost-effective:
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness analysis:
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of 2 or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost-utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost-utility analysis:
A cost-utility analysis is a type of economic evaluation used to compare the benefits of 2 or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost-utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Decision tree:
A decision tree is a type of economic model used to assess the costs and benefits of 2 or more alternative health care interventions. Each intervention may be associated with different outcomes, which are represented by distinct branches in the tree. Each outcome may have a different probability of occurring and may lead to different costs and benefits.
- Deterministic sensitivity analysis:
Deterministic sensitivity analysis is an approach used to explore uncertainty in the results of an economic evaluation by varying parameter values to observe the potential impact on the cost-effectiveness of the health care intervention of interest. One-way sensitivity analysis accounts for uncertainty in parameter values one at a time, whereas multiway sensitivity analysis accounts for uncertainty in a combination of parameter values simultaneously.
- Discounting:
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Ontario Health use an annual discount rate of 1.5% for both future costs and future benefits.
- Equity:
Unlike the notion of equality, equity is not about treating everyone the same way.137 It denotes fairness and justice in process and in results. Equitable outcomes often require differential treatment and resource redistribution to achieve a level playing field among all individuals and communities. This requires recognizing and addressing barriers to opportunities for all to thrive in our society.
- Exposure and response prevention (ERP):
Exposure and response prevention (ERP) is an effective type of therapy specifically for people with OCD.138 It is a type of cognitive behavioural therapy.
- Health inequity:
Health inequities are avoidable inequalities in health between groups of people within countries and between countries.139 These inequities arise from inequalities within and between societies. Social and economic conditions and their effects on people's lives determine their risk of illness and the actions taken to prevent them becoming ill or treat illness when it occurs.
- Humanitarian Device Exemption (HDE):
A Humanitarian Device Exemption (HDE) is a specific type of marketing approval from the US Food and Drug Administration (FDA) for a medical device intended for small patient populations.140 This type of approval is exempt from certain effectiveness requirements and subject to certain profit and use restrictions.
- Incremental cost:
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER):
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Ministry of Health perspective:
The perspective adopted in economic evaluations determines the types of costs and health benefits to include. Ontario Health develops health technology assessment reports from the perspective of the Ontario Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- Multiway sensitivity analysis:
A multiway sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying a combination of model input (i.e., parameter) values simultaneously between plausible extremes to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- One-way sensitivity analysis:
A one-way sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying 1 model input (i.e., a parameter) at a time between its minimum and maximum values to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- Probabilistic analysis:
A probabilistic analysis (also known as a probabilistic sensitivity analysis) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY):
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost-utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by 1 quality-adjusted life-year.
- Reference case:
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- (Treatment) Refractory:
Denotes that a person has no or inadequate improvement in their condition with many other trials and combinations of the best available treatment. Refractory OCD can occur at any severity; however, neurosurgery is reserved for people who have severe illness, owing to the profound disabling nature of the condition.
- Scenario analysis:
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis:
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Societal perspective:
The perspective adopted in an economic evaluation determines the types of costs and health benefits to include. The societal perspective reflects the broader economy and is the aggregation of all perspectives (e.g., health care payer and patient perspectives). It considers the full effect of a health condition on society, including all costs (regardless of who pays) and all benefits (regardless of who benefits).
- Threshold analysis:
A variant on deterministic sensitivity analysis, which involves changing the value of 1 or more parameters until the output of interest crosses some threshold that is considered to have decision relevance.
- Time horizon:
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Utility:
A utility is a value that represents a person's preference for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value:
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost-utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
Appendices
Appendix 1: International Regulatory Status of the Exablate Neuro
Table A1:
International Regulatory Status of the Exablate Neuro MRgFUS System
| Jurisdiction(s) | Intended use(s) | Additional information about intended use(s) |
|---|---|---|
| Canada,a Singapore |
|
|
| Argentina, Australia, Brazil, Chile, EU, India, Israel, Kazakhstan, Philippines, Thailand |
|
|
| South Korea |
|
|
| China |
|
|
| Japan |
|
|
| Russia |
|
|
| Taiwan |
|
|
| US (FDA) |
|
|
Abbreviations: DBS, deep brain stimulation; FDA, Food and Drug Administration; MR, magnetic resonance.
Same information for Canadian regulatory status received from Health Canada (email communication, August 2022).
Source: Insightec Regulatory Approvals44 as of 5 July 2023.
Appendix 2: Literature Search Strategies
Clinical Evidence Search
Search date: August 14, 2023
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database, APA PsycInfo
Database segments: EBM Reviews - Cochrane Central Register of Controlled Trials <July 2023>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to August 9, 2023>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2023 Week 32>, Ovid MEDLINE(R) ALL <1946 to August 11, 2023>, APA PsycInfo <1967 to August Week 1 2023>
Search Strategy:
-
1
exp Obsessive–Compulsive Disorder/ (83745)
-
2
(anankastic personalit* or (compulsi* adj3 (neuros#s or obsessi* or personalit*)) or hoarding* or (obsessive adj3 (disorder* or neuros#s or personalit*)) or OCD or OCPD or trOCD).ti,ab,kf. (89840)
-
3
or/1-2 (117135)
-
4
Magnetic Resonance Imaging, Interventional/ (4086)
-
5
Ultrasonography, Interventional/ (36631)
-
6
Ultrasonic Therapy/ (19833)
-
7
High-Intensity Focused Ultrasound Ablation/ (7043)
-
8
(((focus* adj3 (ultrasound* or ultra sound* or ultrasonograph* or ultra sonograph*)) or FUS) and (MRI or MR or MRI-guide* or MR-guide* or magnet* resonance* or high-frequenc* or high-intensit*)).ti,ab,kf. (14915)
-
9
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU*).ti,ab,kf. (2708)
-
10
(insightec* or exablate*).ti,ab,kf. (396)
-
11
(phased array* or (piezoceramic* adj3 (helmet* or transducer*)) or (transducer adj3 helmet*)).ti,ab,kf. (7573)
-
12
Neurosurgical Procedures/ or Psychosurgery/ or Neurosurgery/ or Ablation Techniques/ (159399)
-
13
(neuro* surg* or neurosurg* or psychiatric surg* or psycho* surg* or psychosurg* or ablat* or capsulotom* or cingulotom* or leukotom* or tractotom*).ti,ab,kf. (547408)
-
14
or/12-13 (621820)
-
15
Magnetic Resonance Imaging/ or Ultrasonography/ (1586646)
-
16
(magnet* resonance* or mri or mri-guide* or mr-guide* or ultrasound* or ultrasonograph* or high-frequency or high-intensity).ti,ab,kf. (2885348)
-
17
or/15-16 (3494536)
-
18
14 and 17 (95746)
-
19
or/4-11,18 (167031)
-
20
3 and 19 (577)
-
21
exp Animals/ not Humans/ (16694649)
-
22
20 not 21 (488)
-
23
limit 22 to english language [Limit not valid in CDSR; records were retained] (469)
-
24
limit 23 to yr=“2013 -Current” (283)
-
25
24 use medall,coch,cctr,cleed (104)
-
26
exp obsessive compulsive disorder/ (83745)
-
27
(anankastic personalit* or (compulsi* adj3 (neuros#s or obsessi* or personalit*)) or hoarding* or (obsessive adj3 (disorder* or neuros#s or personalit*)) or OCD or OCPD or trOCD).tw,kw,kf. (91482)
-
28
or/26-27 (118412)
-
29
interventional magnetic resonance imaging/ (4047)
-
30
interventional ultrasonography/ (33979)
-
31
exp ultrasound therapy/ (38296)
-
32
(((focus* adj3 (ultrasound* or ultra sound* or ultrasonograph* or ultra sonograph*)) or FUS) and (MRI or MR or MRI-guide* or MR-guide* or magnet* resonance* or high-frequenc* or high-intensit*)).tw,kw,kf,dv. (14988)
-
33
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU*).tw,kw,kf,dv. (2722)
-
34
(insightec* or exablate*).tw,kw,kf,dv. (667)
-
35
(phased array* or (piezoceramic* adj3 (helmet* or transducer*)) or (transducer adj3 helmet*)).tw,kw,kf,dv. (7604)
-
36
neurosurgery/ or psychosurgery/ or ablation therapy/ or capsulotomy/ or tractotomy/ (127807)
-
37
(neuro* surg* or neurosurg* or psychiatric surg* or psycho* surg* or psychosurg* or ablat* or capsulotom* or cingulotom* or leukotom* or tractotom*).tw,kw,kf,dv. (549304)
-
38
or/36-37 (601003)
-
39
nuclear magnetic resonance imaging/ or ultrasound/ (1199730)
-
40
(magnet* resonance* or mri or mri-guide* or mr-guide* or ultrasound* or ultrasonograph* or high-frequency or high-intensity).tw,kw,kf,dv. (2901916)
-
41
or/39-40 (3325924)
-
42
38 and 41 (90753)
-
43
or/29-35,42 (168286)
-
44
28 and 43 (593)
-
45
(exp animal/ or nonhuman/) not exp human/ (11875088)
-
46
44 not 45 (582)
-
47
limit 46 to english language [Limit not valid in CDSR; records were retained] (560)
-
48
limit 47 to yr=“2013 -Current” (372)
-
49
48 use emez (247)
-
50
exp obsessive compulsive disorder/ (83745)
-
51
(anankastic personalit* or (compulsi* adj3 (neuros#s or obsessi* or personalit*)) or hoarding* or (obsessive adj3 (disorder* or neuros#s or personalit*)) or OCD or OCPD or trOCD).ti,ab,id,hw. (104855)
-
52
or/50-51 (118045)
-
53
(((focus* adj3 (ultrasound* or ultra sound* or ultrasonograph* or ultra sonograph*)) or FUS) and (MRI or MR or MRI-guide* or MR-guide* or magnet* resonance* or high-frequenc* or high-intensit*)).ti,ab,id,hw. (18380)
-
54
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU*).ti,ab,id,hw. (2567)
-
55
(insightec* or exablate*).ti,ab,id,hw. (449)
-
56
(phased array* or (piezoceramic* adj3 (helmet* or transducer*)) or (transducer adj3 helmet*)).ti,ab,id,hw. (7425)
-
57
neurosurgery/ or psychosurgery/ or lesions/ or tractotomy/ (102861)
-
58
(neuro* surg* or neurosurg* or psychiatric surg* or psycho* surg* or psychosurg* or ablat* or capsulotom* or cingulotom* or leukotom* or tractotom*).ti,ab,id,hw. (643773)
-
59
or/57-58 (648090)
-
60
magnetic resonance imaging/ or ultrasound/ (1357492)
-
61
(magnet* resonance* or mri or mri-guide* or mr-guide* or ultrasound* or ultrasonograph* or high-frequency or high-intensity).ti,ab,id,hw. (4219256)
-
62
or/60-61 (4219256)
-
63
59 and 62 (110567)
-
64
or/53-56,63 (127371)
-
65
52 and 64 (623)
-
66
(animal not human).po. (376878)
-
67
65 not 66 (623)
-
68
limit 67 to english language [Limit not valid in CDSR; records were retained] (599)
-
69
limit 68 to yr=“2013 -Current” (374)
-
70
69 use psyb (33)
-
71
25 or 49 or 70 (384)
-
72
71 use medall (95)
-
73
71 use emez (247)
-
74
71 use cctr (9)
-
75
71 use coch (0)
-
76
71 use cleed (0)
-
77
71 use psyb (33)
-
78
remove duplicates from 71 (276)
Economic Evidence Search
Search date: August 14, 2023
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database, APA PsycInfo
Database segments: EBM Reviews - Cochrane Central Register of Controlled Trials <July 2023>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to August 9, 2023>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2023 Week 32>, Ovid MEDLINE(R) ALL <1946 to August 11, 2023>, APA PsycInfo <1967 to August Week 1 2023>
Search Strategy:
-
1
exp Obsessive–Compulsive Disorder/ (83745)
-
2
(anankastic personalit* or (compulsi* adj3 (neuros#s or obsessi* or personalit*)) or hoarding* or (obsessive adj3 (disorder* or neuros#s or personalit*)) or OCD or OCPD or trOCD).ti,ab,kf. (89840)
-
3
or/1-2 (117135)
-
4
Magnetic Resonance Imaging, Interventional/ (4086)
-
5
Ultrasonography, Interventional/ (36631)
-
6
Ultrasonic Therapy/ (19833)
-
7
High-Intensity Focused Ultrasound Ablation/ (7043)
-
8
(((focus* adj3 (ultrasound* or ultra sound* or ultrasonograph* or ultra sonograph*)) or FUS) and (MRI or MR or MRI-guide* or MR-guide* or magnet* resonance* or high-frequenc* or high-intensit*)).ti,ab,kf. (14915)
-
9
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU*).ti,ab,kf. (2708)
-
10
(insightec* or exablate*).ti,ab,kf. (396)
-
11
(phased array* or (piezoceramic* adj3 (helmet* or transducer*)) or (transducer adj3 helmet*)).ti,ab,kf. (7573)
-
12
Neurosurgical Procedures/ or Psychosurgery/ or Neurosurgery/ or Ablation Techniques/ (159399)
-
13
(neuro* surg* or neurosurg* or psychiatric surg* or psycho* surg* or psychosurg* or ablat* or capsulotom* or cingulotom* or leukotom* or tractotom*).ti,ab,kf. (547408)
-
14
or/12-13 (621820)
-
15
Magnetic Resonance Imaging/ or Ultrasonography/ (1586646)
-
16
(magnet* resonance* or mri or mri-guide* or mr-guide* or ultrasound* or ultrasonograph* or high-frequency or high-intensity).ti,ab,kf. (2885348)
-
17
or/15-16 (3494536)
-
18
14 and 17 (95746)
-
19
or/4-11,18 (167031)
-
20
3 and 19 (577)
-
21
exp Animals/ not Humans/ (16694649)
-
22
20 not 21 (488)
-
23
22 use coch,cleed (0)
-
24
Economics/ (291937)
-
25
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (149018)
-
26
Economic Aspect/ or exp Economic Evaluation/ (554452)
-
27
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw,kf. (1495945)
-
28
exp “Cost”/ (691065)
-
29
(cost or costs or costing or costly).ti. (349448)
-
30
cost effective*.tw,kw,kf. (479466)
-
31
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog* or increment*)).ab,kw,kf. (340690)
-
32
Monte Carlo Method/ (83819)
-
33
(decision adj1 (tree* or analy* or model*)).tw,kw,kf. (76255)
-
34
(markov or markow or monte carlo).tw,kw,kf. (192519)
-
35
Quality-Adjusted Life Years/ (55958)
-
36
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw,kf. (123229)
-
37
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw,kf. (225057)
-
38
or/24-37 (3160071)
-
39
22 and 38 (13)
-
40
39 use medall,cctr (4)
-
41
23 or 40 (4)
-
42
exp obsessive compulsive disorder/ (83745)
-
43
(anankastic personalit* or (compulsi* adj3 (neuros#s or obsessi* or personalit*)) or hoarding* or (obsessive adj3 (disorder* or neuros#s or personalit*)) or OCD or OCPD or trOCD).tw,kw,kf. (91482)
-
44
or/42-43 (118412)
-
45
interventional magnetic resonance imaging/ (4047)
-
46
interventional ultrasonography/ (33979)
-
47
exp ultrasound therapy/ (38296)
-
48
(((focus* adj3 (ultrasound* or ultra sound* or ultrasonograph* or ultra sonograph*)) or FUS) and (MRI or MR or MRI-guide* or MR-guide* or magnet* resonance* or high-frequenc* or high-intensit*)).tw,kw,kf,dv. (14988)
-
49
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU*).tw,kw,kf,dv. (2722)
-
50
(insightec* or exablate*).tw,kw,kf,dv. (667)
-
51
(phased array* or (piezoceramic* adj3 (helmet* or transducer*)) or (transducer adj3 helmet*)).tw,kw,kf,dv. (7604)
-
52
neurosurgery/ or psychosurgery/ or ablation therapy/ or capsulotomy/ or tractotomy/ (127807)
-
53
(neuro* surg* or neurosurg* or psychiatric surg* or psycho* surg* or psychosurg* or ablat* or capsulotom* or cingulotom* or leukotom* or tractotom*).tw,kw,kf,dv. (549304)
-
54
or/52-53 (601003)
-
55
nuclear magnetic resonance imaging/ or ultrasound/ (1199730)
-
56
(magnet* resonance* or mri or mri-guide* or mr-guide* or ultrasound* or ultrasonograph* or high-frequency or high-intensity).tw,kw,kf,dv. (2901916)
-
57
or/55-56 (3325924)
-
58
54 and 57 (90753)
-
59
or/45-51,58 (168286)
-
60
44 and 59 (593)
-
61
(exp animal/ or nonhuman/) not exp human/ (11875088)
-
62
60 not 61 (582)
-
63
Economics/ (291937)
-
64
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (149018)
-
65
Economic Aspect/ or exp Economic Evaluation/ (554452)
-
66
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw,kf. (1495945)
-
67
exp “Cost”/ (691065)
-
68
(cost or costs or costing or costly).ti. (349448)
-
69
cost effective*.tw,kw,kf. (479466)
-
70
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog* or increment*)).ab,kw,kf. (340690)
-
71
Monte Carlo Method/ (83819)
-
72
(decision adj1 (tree* or analy* or model*)).tw,kw,kf. (76255)
-
73
(markov or markow or monte carlo).tw,kw,kf. (192519)
-
74
Quality-Adjusted Life Years/ (55958)
-
75
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw,kf. (123229)
-
76
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw,kf. (225057)
-
77
or/63-76 (3160071)
-
78
62 and 77 (15)
-
79
78 use emez (11)
-
80
exp obsessive compulsive disorder/ (83745)
-
81
(anankastic personalit* or (compulsi* adj3 (neuros#s or obsessi* or personalit*)) or hoarding* or (obsessive adj3 (disorder* or neuros#s or personalit*)) or OCD or OCPD or trOCD).ti,ab,id,hw. (104855)
-
82
or/80-81 (118045)
-
83
(((focus* adj3 (ultrasound* or ultra sound* or ultrasonograph* or ultra sonograph*)) or FUS) and (MRI or MR or MRI-guide* or MR-guide* or magnet* resonance* or high-frequenc* or high-intensit*)).ti,ab,id,hw. (18380)
-
84
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU*).ti,ab,id,hw. (2567)
-
85
(insightec* or exablate*).ti,ab,id,hw. (449)
-
86
(phased array* or (piezoceramic* adj3 (helmet* or transducer*)) or (transducer adj3 helmet*)).ti,ab,id,hw. (7425)
-
87
neurosurgery/ or psychosurgery/ or lesions/ or tractotomy/ (102861)
-
88
(neuro* surg* or neurosurg* or psychiatric surg* or psycho* surg* or psychosurg* or ablat* or capsulotom* or cingulotom* or leukotom* or tractotom*).ti,ab,id,hw. (643773)
-
89
or/87-88 (648090)
-
90
magnetic resonance imaging/ or ultrasound/ (1357492)
-
91
(magnet* resonance* or mri or mri-guide* or mr-guide* or ultrasound* or ultrasonograph* or high-frequency or high-intensity).ti,ab,id,hw. (4219256)
-
92
or/90-91 (4219256)
-
93
89 and 92 (110567)
-
94
or/83-86,93 (127371)
-
95
82 and 94 (623)
-
96
(animal not human).po. (376878)
-
97
95 not 96 (623)
-
98
economics/ or economy/ (405741)
-
99
pharmacoeconomics/ or health care economics/ (237355)
-
100
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (1454271)
-
101
exp “costs and cost analysis”/ (740078)
-
102
cost*.ti. (374690)
-
103
cost effective*.tw. (472203)
-
104
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog* or increment*)).ab. (321117)
-
105
markov chains/ (30432)
-
106
(decision adj1 (tree* or analy* or model*)).tw. (73708)
-
107
(markov or markow or monte carlo).tw. (186506)
-
108
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (121775)
-
109
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (220768)
-
110
or/98-109 (3057736)
-
111
97 and 110 (14)
-
112
111 use psyb (0)
-
113
41 or 62 or 112 (583)
-
114
41 or 79 or 112 (15)
-
115
limit 114 to english language [Limit not valid in CDSR; records were retained] (14)
-
116
115 use medall (4)
-
117
115 use emez (10)
-
118
115 use cctr (0)
-
119
115 use coch (0)
-
120
115 use cleed (0)
-
121
115 use psyb (0)
-
122
remove duplicates from 115 (11)
Grey Literature Search
Performed on: August 18-21, 2023
Websites searched: Alberta Health Evidence Reviews, Alberta Health Services, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Université de Québec-Université Laval, Health Technology Assessment Database, Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Centers for Medicare & Medicaid Services Technology Assessments, Veterans Affairs Health Services Research and Development, Institute for Clinical and Economic Review, Oregon Health Authority Health Evidence Review Commission, Washington State
Health Care Authority Health Technology Reviews, National Institute for Health and Care Excellence (NICE), National Health Service England, Healthcare Improvement Scotland, Health Technology Wales, Ireland Health Information and Quality Authority Health Technology Assessments, Australian Government Medical Services Advisory Committee, Australian Safety and Efficacy Register of New Interventional Procedures -Surgical (ASERNIP-S), Italian National Agency for Regional Health Services (AGENAS), Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Swedish Agency for Health Technology Assessment and Assessment of Social Services, Ministry of Health Malaysia Health Technology Assessment Section, Tuft's Cost-Effectiveness Analysis Registry, PROSPERO, EUnetHTA, ClinicalTrials.gov
Keywords used: obsessive, obsession, compulsive, compulsion, mental health, psychiatric, OCD, hoarding, MR-guided, magnetic resonance, ultrasound, MRgFUS, HIFU, ablation, surgery, neurosurgical, neurosurgery, psychosurgery
Clinical results (included in PRISMA): 5 Economic results (included in PRISMA): 6
Ongoing health technology assessments (PROSPERO/EUnetHTA): 1 Ongoing randomized clinical trials (ClinicalTrials.gov): 7
Appendix 3: Critical Appraisal of Clinical Evidence
Table A2:
Risk of Biasa Among Case Series of MRgFUS Capsulotomy for Treatment-Refractory OCD
| Author, year | Inclusion criteria | Measurement of condition | Valid methods to identify condition | Consecutive cases included | Complete inclusion of cases | Demographics reporting | Clinical information reporting | Outcomes or follow-up | Site or clinic reporting | Appropriate statistical analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Davidson et al, 202072 | Yb | Yc | Yd | Ue | Ue | Uf | Yg | Yh | Ui | Yj |
| Kim et al, 201879 | Yb | Yc | Yd | Yk | Uk | Uf | Yg | yl | Ui | Ym |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; N, no; OCD, obsessive–compulsive disorder; U, unclear; Y, yes.
Risk of bias was assessed using the Joanna Briggs Institute's Critical Appraisal Checklist for Case Series.67
Explicit inclusion and exclusion criteria were defined and published in protocol.
OCD diagnosis was defined according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition, DSM-IV, or 5th edition, DSM-V).
Severity of OCD was based on existing definitions of Yale-Brown Obsessive–Compulsive Scale (Y-BOCS) scores, in addition to standard diagnostic criteria.
Patients referred to the centre were assessed to determine whether they met inclusion criteria and, if so, were offered treatment. One patient with OCD was excluded after enrolment but prior to treatment (because they withdrew consent; see Supplementary Figure 2 in the report by Davidson et al72).
Age, sex, and time period were reported, but no information about participants’ other demographics was provided (e.g., education, geographic region, ethnicity). gClear reporting of relevant clinical information of the participants.
All participants were followed after the procedure for at least 6 months, some up to 12 months, with symptoms, complications, and adverse events assessed and documented.
Clinical information on population is sufficiently detailed to judge comparability or differences to other researchers’ populations. However, there were partial demographic data, which may or may not be sufficient for this purpose.
Nonparametric tests were used for analysis, which is appropriate given the unknown distribution of the data due to small sample size.
Authors state that participants were recruited from a patient pool at the site and assessed for eligibility. Fourteen patients were assessed for eligibility; 2 did not meet inclusion criteria and 1 declined to participate.
All participants were followed after the procedure for 24 months, with symptoms, complications, and adverse events assessed and documented. Participants lost to follow-up were reported clearly.
Analysis was done with a linear mixed model for repeated measures with unstructured covariance matrix, and post hoc analysis included Bonferroni correction for multiple comparisons.
Table A3:
GRADE Evidence Profile for the Effect of MRgFUS Capsulotomy for Treatment-Refractory OCD
| Number of studies (design) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| OCD symptoms: Y-BOCS score | |||||||
| 2 (case series)72,79 | No serious limitationsa | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetectedc | None | ⊕ Very low |
| OCD symptoms: treatment response | |||||||
| 2 (case series)72,79 | No serious limitationsa | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetectedc | None | ⊕ Very low |
| OCD symptoms: CGI scores | |||||||
| 1 (case series)79 | No serious limitationsa | Noned | No serious limitations | Serious limitations (-1)b | Undetectede | None | ⊕ Very low |
| Adverse events | |||||||
| 2 (case series)72,79 | No serious limitationsa | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetectedc | None | ⊕ Very low |
| Neurocognitive changes | |||||||
| 2 (case series)72,79 | Serious limitations (-1)f | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetectedc | None | ⊕ Very low |
| Technical failure | |||||||
| 2 (case series)72,79 | Serious limitations (-1)g | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetectedc | None | ⊕ Very low |
| Follow-up interventions and re-treatment | |||||||
| 2 (case series)72,79 | No serious limitationsa | No serious limitations | No serious limitations | No serious limitationsh | Undetectedc | None | ⊕ Very low |
| Quality of life | |||||||
| 1 (case series)72 | No serious limitationsa | Noned | No serious limitations | Serious limitations (-1)b | Undetectede | None | ⊕ Very low |
| Psychosocial and occupational functioning | |||||||
| 1 (case series)79 | No serious limitationsa | Noned | No serious limitations | Serious limitations (-1)b | Undetectede | None | ⊕ Very low |
Abbreviations: CGI, Clinical Global Impression; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MRgFUS, magnetic resonance-guided focused ultrasound; OCD, obsessive–compulsive disorder; Y-BOCS, Yale-Brown Obsessive–Compulsive Scale.
See risk-of-bias assessment in Appendix 3, Table A2.
Very small sample sizes and optimal information size criteria not met; therefore, uncertainty remains in the precision of estimates.
Cannot definitively assess presence or absence of publication bias because the evidence is derived from 2 studies.
Cannot be evaluated, as there is a single study.
Cannot definitively assess presence or absence of publication bias because the evidence is derived from a single study.
Both studies had missing data for 1 or more patients for this outcome.
Two publications of the study (Kim et al79 and Jung et al77) report apparently conflicting information about treatment failures for the same study (i.e., 1 treatment failure versus none).
No re-treatment or subsequent interventions occurred in either study.
Appendix 4: Selected Excluded Studies - Clinical Evidence
For transparency, we provide a list of selected studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary reason for exclusion |
|---|---|
| Davidson B, Eapen-John D, Mithani K, et al. Lesional psychiatric neurosurgery: meta-analysis of clinical outcomes using a transdiagnostic approach. J Neurol Neurosurg Psychiatry. 2022;93(2):207-15. | Results for OCD and MRgFUS not presented separately (systematic review) |
| Stieglitz LH, Oertel MF, Accolla EA, et al. Consensus Statement on High-Intensity Focused Ultrasound for Functional Neurosurgery in Switzerland. Front Neurol. 2021;12:722762. | Guideline - no outcomes of interest |
| Lai Y, Wang T, Zhang C, et al. Effectiveness and safety of neuroablation for severe and treatment-resistant obsessive–compulsive disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2020;45(5):356-69. | Outdated, missing most recent studies (systematic review) |
| Kinfe T, Stadlbauer A, Winder K, Hurlemann R, Buchfelder M. Incisionless MR-guided focused ultrasound: technical considerations and current therapeutic approaches in psychiatric disorders. Expert Rev Neurother. 2020;20(7):687-96. | No information on search dates, key words (systematic review) |
| Pepper J, Zrinzo L, Hariz M. Anterior capsulotomy for obsessive–compulsive disorder: a review of old and new literature. J Neurosurg. 2020;133(5):1595-604. | Outdated, missing most recent studies (systematic review) |
| McGovern RA, Sheth SA. Role of the dorsal anterior cingulate cortex in obsessive–compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery. J Neurosurg. 2017;126(1):132-47. | No outcomes of interest (systematic review) |
| Piper RJ, Hughes MA, Moran CM, Kandasamy J. Focused ultrasound as a non-invasive intervention for neurological disease: a review. Br J Neurosurg. 2016;30(3):286-93. | Wrong study type (narrative review) |
| Davidson B, Hamani C, Rabin JS, et al. Magnetic resonance-guided focused ultrasound capsulotomy for musical obsessions. Biol Psychiatry. 2021;90(10):e49-e50. | Duplicate report (case data included in case series) |
Appendix 5: Neuropsychological Tests in Patients With OCD
Table A4:
Overview of Neuropsychological Test Results at Baseline and Follow-Up After MRgFUS Capsulotomy in 5 People with Treatment-Refractory OCD
| Test | Function(s) measured | Baseline, mean (SD)a | 6 mo, mean (SD)a | 12 mo, mean (SD)a |
|---|---|---|---|---|
| Wechsler Test of Adult Reading (WTAR) | Intellectual functioning | 103.4 (5.46) | NAb | NAb |
| California Verbal Learning Test, second edition (CVLT-II) | Memory - total recall | 52.4 (9.45) | 53.6 (9.21) | 50.6 (10.24) |
| Memory - delayed free recall | -0.1 (1.08) | -0.5 (0.87) | 0.6 (1.19) | |
| Memory - delayed cued recall | 0.1 (0.65) | 0.2 (0.57) | 0.4 (0.82) | |
| Memory - delayed recognition discrimination | 0.4 (0.65) | 0.3 (0.27) | 0.4 (0.65) | |
| Brief Visuospatial Memory Test - Revised (BVMT-R) | Memory - immediate recall | 37.4 (15.36) | 45.8 (8.44) | 47 (15.3) |
| Memory - delayed recall | 39.6 (15.5) | 47.2 (5.76) | 50.8 (13.79) | |
| Delis-Kaplan Executive Function System (D-KEFS) sorting test | Executive function - correct sorts | 8.8 (1.64) | 11 (1) | 11.8 (3.27) |
| Executive function - descriptive score | 9.2 (1.30) | 10.8 (1.3) | 11 (3.39) | |
| Symbol Digit Modalities Test (SDMT), oral version | Cognitive processing | -1.36 (0.63) | -0.96 (0.31) | -0.9 (0.51) |
| Iowa Gambling Task (IGT) total score | Cognitive function | 44.2 (10.78) | 48.67 (11.93)c | 51 (5.35)d |
| Frontal Systems Behavior Scale (FrSBe), self-report version | Apathy, disinhibition, executive dysfunction - total score | 7.8 (17.81) | 58 (12.43) | 57.6 (16.47) |
| Disinhibition score | 51.6 (17.60) | 47.4 (15.04) | 40.8 (8.41) | |
| Apathy score | 80.8 (20.68) | 66 (13.55) | 65.6 (25.51) | |
| Dysexecutive score | 73 (19.30) | 61.4 (8.68) | 61.2 (15.90) |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not assessed; OCD, obsessive–compulsive disorder; SD, standard deviation.
Calculated for patients with OCD from individual patient data from Supplementary Table 2 in the report by Davidson et al.75
Assessed only to determine baseline intellectual functioning.
n = 3.
n = 4.
Source: Davidson et al.75
Appendix 6: Results of the Applicability Checklist for Studies Included in the Economic Literature Review
Table A5:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of MRgFUS Neurosurgery
| Author, year, country | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of QALYs? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| Kumar et al, 2019,94 US | Yes, patients with treatment-refractory OCD | Yes, includes MRgFUS neurosurgery and RFA | Partially | No, stated societal but only hospital costs of procedure and some complications were included | Partially, only treatment effects for the comparator were included | N/A; time horizon, 1 y | Yes | No, only hospital costs were included | Not applicable |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “N/A” (not applicable).
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; OCD, obsessive–compulsive disorder; QALY, quality-adjusted life-year; RFA, radiofrequency ablation; Y-BOCS, Yale-Brown Obsessive–Compulsive Scale.
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Appendix 7: Supplementary Economic Tables
Table A6:
CCI Codes for Intervention and Comparators
| Intervention category | CCI code | Long description |
|---|---|---|
| MRgFUS neurosurgery | 1AE59JAAZ | Destruction, thalamus and basal ganglia using ultrasound, external approach |
| RFA | 1AE59SEGX | Destruction, thalamus and basal ganglia using device NEC, burr hole approach |
| 1AE59SZAW | Destruction, thalamus and basal ganglia using radiofrequency probe, open approach | |
| 1AE59SZGX | Destruction, thalamus and basal ganglia using device NEC, open approach | |
| 1AE59SEAW | Destruction, thalamus and basal ganglia using radiofrequency probe, burr hole approach | |
| 1AN59SEAW | Destruction, brain with radiofrequency probe, burr hole approach | |
| 1AN59SEGX | Destruction, brain with device NEC, burr hole technique | |
| 1AN59SZAW | Destruction, brain with radiofrequency probe, craniotomy flap technique for access | |
| 1AN59SZGX | Destruction, brain with device NEC, open approach | |
| Gamma Knife radiosurgery | 1AE27JX | Radiation, thalamus and basal ganglia using focused beam [e.g., Gamma Knife, CyberKnife stereotactic radiosurgery] |
| 1AN27JX | Radiation, brain using focused beam [e.g., Gamma Knife, CyberKnife stereotactic radiosurgery] | |
| DBS | 1AE53SEJA | Implantation of internal device, thalamus and basal ganglia of electrodes [e.g., recording, stimulating] using burr hole approach |
| 1AE53SZJA | Implantation of internal device, thalamus and basal ganglia of electrodes [e.g., recording, stimulating] using open approach | |
| 1AN53SEJA | Implantation of internal device, brain of electrodes [e.g., recording, stimulating] using burr hole approach | |
| 1AN53SZJA | Implantation of internal device, brain of electrodes [e.g., recording, stimulating] (craniotomy flap technique for access) |
Abbreviations: CCI, Canadian Classification of Health Interventions; DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; NEC, not elsewhere classified; RFA, radiofrequency ablation.
Table A7:
ICD-10-CA Codes for OCD Diagnoses
| ICD-10-CA code | ICD-10-CA description |
|---|---|
| F420a | Predominantly obsessional thoughts or ruminations |
| F421a | Predominantly compulsive acts (obsessional rituals) |
| F422a | Mixed obsessional thoughts and acts |
| F428 | Other obsessive–compulsive disorders |
| F429 | Obsessive–compulsive disorder, unspecified |
Abbreviations: ICD-10-CA, International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada; OCD, obsessive–compulsive disorder.
Code was included in search, but no cases were identified.
Table A8:
Inpatient Costs
| Procedure | CCI codes | Diagnosis code(s) | Years | Number of cases | Mean cost, $a | Standard deviation, $a | Mean length of stay, d | 95% confidence interval, $a,b |
|---|---|---|---|---|---|---|---|---|
| RFA | 1AN59SEAW, 1AN59SEGX | F429 | 2017-2022 | 8 | 4,327 | 3,677 | 1.6 | 1,256-7,420 |
| Gamma Knife radiosurgery | 1AE27JX, 1AN27JX | F428, F429 | 2019-2022 | 5 | 1,741 | 131 | 1.0 | 1,583-1,908 |
| DBS | 1AE53SEJA, 1AESZJA, 1AN53SEJA | F428, F429 | 2012-2019 | 7 | 27,557 | 8,792 | 1.7 | NA |
Abbreviations: CCI, Canadian Classification of Health Interventions; DBS, deep brain stimulation; NA, not available; RFA, radiofrequency ablation.
All costs are reported in 2023 Canadian dollars.
95% confidence intervals were calculated from the IntelliHealth Ontario data assuming that the data followed a t-distribution owing to small sample sizes.
Source: IntelliHealth Ontario, accessed July 5, 2023.
Table A9:
Capital and Fixed Costs of MRgFUS Neurosurgery
| Resource item | Cost, $a |
|---|---|
| Insightec Exablate Neuro system and equipment | 2,370,207 |
| Installation | 218,788 |
| Annual depreciation | |
| Equipment depreciation (Insightec Exablate Neuro) | 517,799b |
| Equipment depreciation (head frame) | 13,370 |
| Annual service contract (echo focusing system) | 60,775 |
| Annual service contract (Insightec Exablate Neuro) | 151,936 |
| Total fixed cost per year | 743,880 |
| Annual caseload | 66c |
| Useful life (years) | 5 |
| Average fixed cost per case | 11,271 |
Note: Numbers may be inexact due to rounding.
All costs are reported in 2023 Canadian dollars.
Insightec equipment and installation cost divided by useful life.
Includes 48 essential tremor cases and an average of 18 OCD cases annually.
Table A10:
Surgical Assistant Fees
| Procedure | MRgFUS neurosurgery | RFA | Gamma Knife radiosurgery | DBS | Source |
|---|---|---|---|---|---|
| Average surgery treatment time, h | 3 | 3 | N/A | 4.5 | Expert opiniona |
| Number of basic units | 9 | 9 | N/A | 9 | Schedule of Benefits103 (N124) |
| Number of time unitsb | 22 | 22 | N/A | 40 | Calculated based on average surgery length |
| Total number of units | 31 | 31 | N/A | 49 | |
| Unit price, $c | 12.51 | 12.51 | 12.51 | 12.51 | Schedule of Benefits103 (N124) |
| Total cost, $c | 388 | 388 | N/A | 613 |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; N/A, not applicable; RFA, radiofrequency ablation.
N. Lipsman, MD, PhD, email communication, December 18, 2023.
Time units are calculated according to rules in the Schedule of Benefits as follows: 1 unit per 15-minute increment in the first hour, 2 units per 15-minute increment after the first hour up to 2.5 hours, and 3 units per 15-minute increment after 2.5 hours.
All costs are reported in 2023 Canadian dollars.
Table A11:
Anesthesiologist Fees
| Procedure | MRgFUS neurosurgery | RFA | Gamma Knife radiosurgery | DBS | Source |
|---|---|---|---|---|---|
| Average surgery treatment time, h | 3 | 3 | N/A | 4.5 | |
| Number of basic units | 11 | 11 | N/A | 11 | Schedule of Benefits103 (N124) |
| Number of time unitsa | 26 | 26 | N/A | 44 | Calculated based on average surgery length |
| Total number of units | 37 | 37 | N/A | 55 | |
| Unit price, $b | 15.49 | 15.49 | N/A | 15.49 | Schedule of Benefits103 (N124) |
| Total cost, $b | 573 | 573 | N/A | 852 |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; N/A, not applicable; RFA, radiofrequency ablation.
Time units are calculated according to rules in the Schedule of Benefits as follows: 1 unit per 15-minute increment in the first hour, 2 units per 15-minute increment after the first hour up to 1.5 hours, and 3 units per 15-minute increment after 1.5 hours.
All costs are reported in 2023 Canadian dollars.
Table A12:
Cost of Adjunct Medications
| Medication | Daily dose, mg/d | Unit cost, $/mg | Cost per dose, $a | Annual cost, $ | Source |
|---|---|---|---|---|---|
| Patient 1b | |||||
| Amitriptyline | 300 | 0.0031 | 0.924 | 337.26 | ODB Formulary119,c |
| Citalopram | 40 | 0.00333 | 0.1332 | 48.62 | ODB Formulary119,c |
| Risperidone | 1.5 | 1.1176 | 1.6764 | 611.89 | ODB Formulary119,c |
| Total annual cost | 997.76 | ||||
| Patient 2b | |||||
| Sertraline | 250 | 0.0061 | 1.5160 | 553.34 | ODB Formulary119,c |
| Risperidone | 2 | 1.1176 | 2.2352 | 815.85 | ODB Formulary119,c |
| Lorazepam | 1 | 0.0718 | 0.0718 | 26.21 | ODB Formulary119,c |
| Clonazepam | 1.5 | 0.0836 | 0.1254 | 45.77 | ODB Formulary119,c |
| Total annual cost | 1,441.17 | ||||
| Patient 3b | |||||
| Paroxetine | 40 | 0.01625 | 0.65 | 237.25 | ODB Formulary119,c |
| Clonazepam | 1 | 0.0836 | 0.0836 | 30.51 | ODB Formulary119,c |
| Memantine | 10 | 0 | 0 | 0.00 | ODB Formulary119,c |
| Total annual cost | 276.76 | ||||
| Patient 4b | |||||
| Clomipramine | 300 | 0.0126 | 3.7746 | 1,377.73 | ODB Formulary119,d |
| Desvenlafaxine | 200 | 0 | 0 | 0.00 | ODB Formulary119,d |
| Lurasidone | 40 | 0.030625 | 1.225 | 447.13 | ODB Formulary119,d |
| Lorazepam | 1 | 0.0718 | 0.0718 | 26.21 | ODB Formulary119,c |
| Total annual cost | 1,851.06 | ||||
| Patient 5b | |||||
| Nil | 0.00 | ||||
| Total annual cost | 0.00 | ||||
| Patient 6b | |||||
| Fluoxetine | 60 | 0.016555 | 0.9933 | 362.55 | ODB Formulary119,d |
| Nortriptyline | 20 | 0.02995 | 0.599 | 218.64 | ODB Formulary119,d |
| Clonazepam | 2.25 | 0.0836 | 0.1881 | 68.66 | ODB Formulary119,c |
| Trazodone | 50 | 0.001108 | 0.0554 | 20.22 | ODB Formulary119,d |
| Lorazepam | 4 | 0.0718 | 0.2872 | 104.83 | ODB Formulary119,c |
| Total annual cost | 774.90 | ||||
| Average annual medications costse | 928.78 | ||||
Abbreviation: ODB, Ontario Drug Benefit.
Unit costs represent the cost paid by the ODB Program (Ministry of Health) plus an 8% markup. If the unit cost is $0, none of the cost of the medication is paid for by the ODB Program.
Medications at time of MRgFUS neurosurgery for patients with OCD as reported in Supplementary Table 2 in the report by Davidson et al.72
Accessed October 24, 2023.
Accessed November 2, 2023.
Includes $10 dispensing fee, 4 times per year.
Table A13:
Volume of Treatments in Selected Scenario Analyses
| Scenario | Year 1 (2024) | Year 2 (2025) | Year 3 (2026) | Year 4 (2027) | Year 5 (2028) | Total |
|---|---|---|---|---|---|---|
| Scenario 1, population of interest | ||||||
| Current scenario | ||||||
| Gamma Knife radiosurgery | 2 | 2 | 2 | 2 | 2 | 10 |
| RFA | 2 | 2 | 2 | 2 | 2 | 10 |
| No surgery (expanded population) | 38 | 39 | 39 | 40 | 41 | 197 |
| New scenario | ||||||
| Gamma Knife radiosurgery, 15% of original population | 1 | 1 | 1 | 1 | 1 | 5 |
| RFA, 0% | 0 | 0 | 0 | 0 | 0 | 0 |
| MRgFUS neurosurgery, 85% of original population + expansion | 41 | 42 | 42 | 43 | 44 | 212 |
| Scenario 2, increased expansion of MRgFUS neurosurgery | ||||||
| Current scenario | ||||||
| Gamma Knife radiosurgery | 2 | 2 | 2 | 2 | 2 | 10 |
| RFA | 2 | 2 | 2 | 2 | 2 | 10 |
| No surgery (expanded population) | 12 | 17 | 21 | 26 | 30 | 106 |
| New scenario | ||||||
| Gamma Knife radiosurgery, 15% of original population | 1 | 1 | 1 | 1 | 1 | 5 |
| RFA, 0% | 0 | 0 | 0 | 0 | 0 | 0 |
| MRgFUS neurosurgery, 85% of original population + expansion | 15 | 20 | 24 | 29 | 33 | 121 |
| Scenario 3, steady but slower expansion of MRgFUS neurosurgery | ||||||
| Current scenario | ||||||
| Gamma Knife radiosurgery | 2 | 2 | 2 | 2 | 2 | 10 |
| RFA | 2 | 2 | 2 | 2 | 2 | 10 |
| No surgery (expanded population) | 12 | 12 | 12 | 12 | 12 | 60 |
| New scenario | ||||||
| Gamma Knife radiosurgery, 15% of original population | 1 | 1 | 1 | 1 | 1 | 5 |
| RFA, 0% | 0 | 0 | 0 | 0 | 0 | 0 |
| MRgFUS neurosurgery, 85% of original population + expansion | 15 | 15 | 15 | 15 | 15 | 75 |
| Scenario 4, steady but increased expansion of MRgFUS neurosurgery | ||||||
| Current scenario | ||||||
| Gamma Knife radiosurgery | 2 | 2 | 2 | 2 | 2 | 10 |
| RFA | 2 | 2 | 2 | 2 | 2 | 10 |
| No surgery (expanded population) | 24 | 24 | 24 | 24 | 24 | 120 |
| New scenario | ||||||
| Gamma Knife radiosurgery, 15% of original population | 1 | 1 | 1 | 1 | 1 | 5 |
| RFA, 0% | 0 | 0 | 0 | 0 | 0 | 0 |
| MRgFUS neurosurgery, 85% of original population + expansion | 27 | 27 | 27 | 27 | 27 | 135 |
| Scenario 5, decreased Gamma Knife radiosurgery | ||||||
| Current scenario | ||||||
| Gamma Knife radiosurgery | 2 | 2 | 2 | 2 | 2 | 10 |
| RFA | 2 | 2 | 2 | 2 | 2 | 10 |
| No surgery (expanded population) | 12 | 15 | 18 | 21 | 24 | 90 |
| New scenario | ||||||
| Gamma Knife radiosurgery, 15% of original population | 0 | 0 | 0 | 0 | 0 | 0 |
| RFA, 0% | 0 | 0 | 0 | 0 | 0 | 0 |
| MRgFUS neurosurgery, 85% of original population + expansion | 16 | 19 | 22 | 25 | 28 | 110 |
| Scenario 6, patients not eligible for MRgFUS neurosurgery receive RFA | ||||||
| Current scenario | ||||||
| Gamma Knife radiosurgery | 2 | 2 | 2 | 2 | 2 | 10 |
| RFA | 2 | 2 | 2 | 2 | 2 | 10 |
| No surgery (expanded population) | 12 | 15 | 18 | 21 | 24 | 90 |
| New scenario | ||||||
| Gamma Knife radiosurgery, 0% | 0 | 0 | 0 | 0 | 0 | 0 |
| RFA, 15% of original population | 1 | 1 | 1 | 1 | 1 | 5 |
| MRgFUS neurosurgery, 85% of original population + expansion | 15 | 18 | 21 | 24 | 27 | 105 |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; RFA, radiofrequency ablation.
Table A14:
Detailed Budget Impact Analysis Results - Reference Case
| Current scenario | Year 1, $a | Year 2, $a | Year 3, $a | Year 4, $a | Year 5, $a | Total, $a |
|---|---|---|---|---|---|---|
| MRgFUS neurosurgery | ||||||
| Surgical procedure costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Monitoring costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adjunct medication costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse event costs | 0 | 0 | 0 | 0 | 0 | 0 |
| RFA | ||||||
| Surgical procedure costs | 23,732 | 23,732 | 23,732 | 23,732 | 23,732 | 118,660 |
| Monitoring costs | 445 | 890 | 1,335 | 1,780 | 2,225 | 6,675 |
| Adjunct medication costs | 1,486 | 2,972 | 4,458 | 5,944 | 7,430 | 22,291 |
| Adverse event costs | 577 | 577 | 577 | 577 | 577 | 2,884 |
| Gamma Knife radiosurgery | ||||||
| Surgical procedure costs | 14,168 | 14,168 | 14,168 | 14,168 | 14,168 | 70,838 |
| Monitoring costs | 445 | 890 | 1,335 | 1,780 | 2,225 | 6,675 |
| Adjunct medication costs | 1,486 | 2,972 | 4,458 | 5,944 | 7,430 | 22,291 |
| Adverse event costs | 2,399 | 2,399 | 2,399 | 2,399 | 2,399 | 11,995 |
| No (surgical) treatment | ||||||
| Surgical procedure costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Monitoring costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adjunct medication costs | 8,916 | 20,062 | 33,436 | 49,039 | 66,872 | 178,325 |
| Adverse event costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 53,654 | 68,661 | 85,898 | 105,363 | 127,058 | 440,634 |
| New scenario | Year 1, $a | Year 2, $a | Year 3, $a | Year 4, $a | Year 5, $a | Total, $a |
|---|---|---|---|---|---|---|
| MRgFUS neurosurgery | ||||||
| Surgical procedure costs | 281,523 | 337,827 | 394,132 | 450,437 | 506,741 | 1,970,660 |
| Monitoring costs | 3,338 | 7,343 | 12,015 | 17,355 | 23,363 | 63,413 |
| Adjunct medication costs | 11,145 | 24,520 | 40,123 | 57,956 | 78,017 | 211,761 |
| Adverse event costs | 0 | 0 | 0 | 0 | 0 | 0 |
| RFA | ||||||
| Surgical procedure costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Monitoring costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adjunct medication costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse event costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Gamma Knife radiosurgery | ||||||
| Surgical procedure costs | 7,084 | 7,084 | 7,084 | 7,084 | 7,084 | 35,419 |
| Monitoring costs | 223 | 445 | 668 | 890 | 1,113 | 3,338 |
| Adjunct medication costs | 743 | 1,486 | 2,229 | 2,972 | 3,715 | 11,145 |
| Adverse event costs | 1,200 | 1,200 | 1,200 | 1,200 | 1,200 | 5,998 |
| No (surgical) treatment | ||||||
| Surgical procedure costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Monitoring costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adjunct medication costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse event costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 305,255 | 379,904 | 457,450 | 537,893 | 621,232 | 2,301,733 |
| Budget impact | Year 1, $a | Year 2, $a | Year 3, $a | Year 4, $a | Year 5, $a | Total, $a |
|---|---|---|---|---|---|---|
| MRgFUS neurosurgery | ||||||
| Surgical procedure costs | 281,523 | 337,827 | 394,132 | 450,437 | 506,741 | 1,970,660 |
| Monitoring costs | 3,338 | 7,343 | 12,015 | 17,355 | 23,363 | 63,413 |
| Adjunct medication costs | 11,145 | 24,520 | 40,123 | 57,956 | 78,017 | 211,761 |
| Adverse event costs | 0 | 0 | 0 | 0 | 0 | 0 |
| RFA | ||||||
| Surgical procedure costs | -23,732 | -23,732 | -23,732 | -23,732 | -23,732 | -118,660 |
| Monitoring costs | -445 | -890 | -1,335 | -1,780 | -2,225 | -6,675 |
| Adjunct medication costs | -1,486 | -2,972 | -4,458 | -5,944 | -7,430 | -22,291 |
| Adverse event costs | -577 | -577 | -577 | -577 | -577 | -2,884 |
| Gamma Knife radiosurgery | ||||||
| Surgical procedure costs | -7,084 | -7,084 | -7,084 | -7,084 | -7,084 | -35,419 |
| Monitoring costs | -223 | -445 | -668 | -890 | -1,113 | -3,338 |
| Adjunct medication costs | -743 | -1,486 | -2,229 | -2,972 | -3,715 | -11,145 |
| Adverse event costs | -1,200 | -1,200 | -1,200 | -1,200 | -1,200 | -5,998 |
| No (surgical) treatment | ||||||
| Surgical procedure costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Monitoring costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Adjunct medication costs | -8,916 | -20,062 | -33,436 | -49,039 | -66,872 | -178,325 |
| Adverse event costs | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 251,601 | 311,243 | 371,552 | 432,529 | 494,174 | 1,861,100 |
Note: Numbers may be inexact due to rounding.
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; RFA, radiofrequency ablation.
All costs are reported in 2023 Canadian dollars.
Appendix 8: Letter of Information
Appendix 9: Interview Guide
Key Messages
What Is This Health Technology Assessment About?
Obsessive–compulsive disorder (OCD) is a mental illness characterized by obsessions (recurring intrusive thoughts or images that cause anxiety) and/or compulsions (repetitive behavioural or mental rituals done in response to obsessions). For people living with OCD, the symptoms are very distressing and can take up many hours of the day, especially with severe OCD, which can prevent them from working or even caring for themselves.
Specialized therapy or medications can improve the symptoms of OCD. However, some people with OCD try many different types and combinations of these treatments with no meaningful improvement; this is considered treatment-refractory OCD. Neurosurgery (brain surgery) may be an option for people who have severe, treatment-refractory OCD. These surgeries are usually invasive (involve opening the skull) and can either ablate (destroy) or use electrical impulses to interrupt the brain circuits associated with OCD symptoms. Magnetic resonance-guided focused ultrasound (MRgFUS) is a technology that can be used to perform noninvasive (incisionless) neurosurgery for people with severe, treatment-refractory OCD and avoids the surgical risks of invasive procedures.
This health technology assessment looked at how safe and effective MRgFUS neurosurgery is for people with treatment-refractory OCD. It also looked at the budget impact of publicly funding MRgFUS neurosurgery and the experiences, preferences, and values of people with treatment-refractory OCD.
What Did This Health Technology Assessment Find?
MRgFUS neurosurgery may be an effective and generally safe treatment option for severe, treatment-refractory OCD, but the evidence is very uncertain.
We were unable to determine the cost-effectiveness of MRgFUS neurosurgery. We estimated that publicly funding MRgFUS neurosurgery for people with treatment-refractory OCD in Ontario would result in a total cost increase of $1.9 million over 5 years.
Patients who underwent MRgFUS neurosurgery commented on the positive impact that it had on their OCD symptoms, mental health, and quality of life. All patients and care partners emphasized the importance of having access to MRgFUS neurosurgery as a treatment option for treatment-refractory OCD.
Contributor Information
Ontario Health (Quality):
Alexis Schaink, Myra Wang, Caroline Higgins, Corinne Holubowich, Hailey Saunders, Hong Anh Tu, and Samrawit Lemma
About Us
We are an agency created by the Government of Ontario to connect, coordinate, and modernize our province's health care system. We work with partners, providers, and patients to make the health system more efficient so everyone in Ontario has an opportunity for better health and well-being.
Equity, Inclusion, Diversity and Anti-Racism
Ontario Health is committed to advancing equity, inclusion and diversity and addressing racism in the health care system. As part of this work, Ontario Health has developed an Equity, Inclusion, Diversity and Anti-Racism Framework, which builds on existing legislated commitments and relationships and recognizes the need for an intersectional approach.
Unlike the notion of equality, equity is not about sameness of treatment. It denotes fairness and justice in process and in results. Equitable outcomes often require differential treatment and resource redistribution to achieve a level playing field among all individuals and communities. This requires recognizing and addressing barriers to opportunities for all to thrive in our society.
For more information about Ontario Health, visit OntarioHealth.ca.
References
- (1).American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington: The Association; 2013. [Google Scholar]
- (2).Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive–compulsive disorders. BMC Psychiatry. 2014; 14(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).DynaMed. Obsessive–compulsive disorder (OCD) [Internet]. EBSCO Information Services; c2023. [cited 2023 Jul 5]. Available from: https://www.dynamed.com/condition/obsessive–compulsive-disorder-ocd [Google Scholar]
- (4).Hirschtritt ME, Bloch MH, Mathews CA. Obsessive–compulsive disorder: advances in diagnosis and treatment. JAMA. 2017; 317(13):1358–67. [DOI] [PubMed] [Google Scholar]
- (5).Kuhne F, Ay DS, Marschner L, Weck F. The heterogeneous course of OCD - a scoping review on the variety of definitions. Psychiatry Res. 2020; 285:112821. [DOI] [PubMed] [Google Scholar]
- (6).Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive–compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010; 15(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Osland S, Arnold PD, Pringsheim T. The prevalence of diagnosed obsessive compulsive disorder and associated comorbidities: a population-based Canadian study. Psychiatry Res. 2018; 268:137–42. [DOI] [PubMed] [Google Scholar]
- (8).Pellegrini L, Maietti E, Rucci P, Casadei G, Maina G, Fineberg NA, et al. Suicide attempts and suicidal ideation in patients with obsessive–compulsive disorder: a systematic review and meta-analysis. J Affect Disord. 2020; 276:1001–21. [DOI] [PubMed] [Google Scholar]
- (9).Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989; 46(11):1006–11. [DOI] [PubMed] [Google Scholar]
- (10).Wootton BM, Tolin DF. Obsessive–compulsive disorder. In: Friedman HS, editor. Encyclopedia of mental health. 2nd ed. Oxford: Academic Press; 2016. p. 227–31. [Google Scholar]
- (11).National Institute of Mental Health. Statistics: obsessive–compulsive disorder (OCD) [Internet]. c2023. [cited 2023 Jul 5]. Available from: https://www.nimh.nih.gov/health/statistics/obsessive–compulsive-disorder-ocd
- (12).Albert U, Marazziti D, Di Salvo G, Solia F, Rosso G, Maina G. A systematic review of evidence-based treatment strategies for obsessive–compulsive disorder resistant to first-line pharmacotherapy. Curr Med Chem. 2018; 25(41):5647–61. [DOI] [PubMed] [Google Scholar]
- (13).Brown LT, Mikell CB, Youngerman BE, Zhang Y, McKhann GM, 2nd, Sheth SA. Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive–compulsive disorder: a systematic review of observational studies. J Neurosurg. 2016; 124(1):77–89. [DOI] [PubMed] [Google Scholar]
- (14).Lai Y, Wang T, Zhang C, Lin G, Voon V, Chang J, et al. Effectiveness and safety of neuroablation for severe and treatment-resistant obsessive–compulsive disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2020; 45(5):356–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lewin AB, De Nadai AS, Park J, Goodman WK, Murphy TK, Storch EA. Refining clinical judgment of treatment outcome in obsessive–compulsive disorder. Psychiatry Res. 2011; 185(3):394–401. [DOI] [PubMed] [Google Scholar]
- (16).Mataix-Cols D, Fernandez de la Cruz L, Nordsletten AE, Lenhard F, Isomura K, Simpson HB. Towards an international expert consensus for defining treatment response, remission, recovery and relapse in obsessive–compulsive disorder. World Psychiatry. 2016; 15(1):80–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Garnaat SL, Greenberg BD, Sibrava NJ, Goodman WK, Mancebo MC, Eisen JL, et al. Who qualifies for deep brain stimulation for OCD? Data from a naturalistic clinical sample. J Neuropsychiatry Clin Neurosci. 2014; 26(1):81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Bandelow B, Allgulander C, Baldwin DS, Costa D, Denys D, Dilbaz N, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive–compulsive and posttraumatic stress disorders - Version 3. Part II: OCD and PTSD. World J Biol Psychiatry. 2023; 24(2):118–34. [DOI] [PubMed] [Google Scholar]
- (19).Fineberg NA, Hollander E, Pallanti S, Walitza S, Grunblatt E, Dell'Osso BM, et al. Clinical advances in obsessive–compulsive disorder: a position statement by the International College of Obsessive–Compulsive Spectrum Disorders. Int Clin Psychopharmacol. 2020; 35(4):173–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).National Institute for Health and Care Excellence. Obsessive–compulsive disorder. Evidence Update September 2013. Evidence Update 47 [Internet]. Manchester (UK): The Institute; 2013. [cited 2023 Jul 5]. Available from: https://www.nice.org.uk/guidance/cg31/documents/cg31-obsessive–compulsive-disorder-ocd-and-body-dysmorphic-disorder-bdd-evidence-update2 [Google Scholar]
- (21).National Institute for Health and Care Excellence. Obsessive–compulsive disorder and body dysmorphic disorder: treatment. CG31 [Internet]. 2005. [cited 2023 Jul 5]. Available from: https://www.nice.org.uk/guidance/cg31
- (22).Pallanti S, Hollander E, Bienstock C, Koran L, Leckman J, Marazziti D, et al. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol. 2002; 5(2):181–91. [DOI] [PubMed] [Google Scholar]
- (23).Etherington LA, Matthews K, Akram H. New directions for surgical ablation treatment of obsessive compulsive disorder. Curr Top Behav Neurosci. 2021; 49:437–60. [DOI] [PubMed] [Google Scholar]
- (24).Nuttin B, Wu H, Mayberg H, Hariz M, Gabriels L, Galert T, et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry. 2014; 85(9):1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Goodman WK, Storch EA, Sheth SA. Harmonizing the neurobiology and treatment of obsessive–compulsive disorder. Am J Psychiatry. 2021; 178(1):17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Karas PJ, Lee S, Jimenez-Shahed J, Goodman WK, Viswanathan A, Sheth SA. Deep brain stimulation for obsessive compulsive disorder: evolution of surgical stimulation target parallels changing model of dysfunctional brain circuits. Front Neurosci. 2018; 12:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Martinez-Alvarez R. Radiosurgery for behavioral disorders. Prog Neurol Surg. 2019; 34:289–97. [DOI] [PubMed] [Google Scholar]
- (28).Royal College of Psychiatrists. Statement on Neurosurgery for Mental Disorder (NMD), also known as Psychiatric Neurosurgery. Position statement CERT 05/17 [Internet]. London: The College; 2017. [cited 2023 Jul 5]. Available from: https://www.rcpsych.ac.uk/docs/default-source/about-us/who-we-are/ectcommittee-vns-dbs-ablative-neurosurgery-statement-feb17.pdf?sfvrsn=eba0287a_2 [Google Scholar]
- (29).Doshi PK, Arumugham SS, Bhide A, Vaishya S, Desai A, Singh OP, et al. Indian guidelines on neurosurgical interventions in psychiatric disorders. Indian J Psychiatry. 2019; 61(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Visser-Vandewalle V, Andrade P, Mosley PE, Greenberg BD, Schuurman R, McLaughlin NC, et al. Deep brain stimulation for obsessive–compulsive disorder: a crisis of access. Nat Med. 2022; 28(8):1529–32. [DOI] [PubMed] [Google Scholar]
- (31).Janardhan Reddy YC, Sundar AS, Narayanaswamy JC, Math SB. Clinical practice guidelines for obsessive–compulsive disorder. Indian J Psychiatry. 2017; 59(Suppl 1):S74–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Müller S, van Oosterhout A, Bervoets C, Christen M, Martínez-Álvarez R, Bittlinger M. Concerns about psychiatric neurosurgery and how they can be overcome: recommendations for responsible research. Neuroethics. 2022; 15(1):6. [Google Scholar]
- (33).Lopes AC, Greenberg BD, Canteras MM, Batistuzzo MC, Hoexter MQ, Gentil AF, et al. Gamma ventral capsulotomy for obsessive–compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2014; 71(9):1066–76. [DOI] [PubMed] [Google Scholar]
- (34).MedlinePlus. Deep brain stimulation [Internet]. National Library of Medicine; c2023. [updated 2021 Oct 25; cited 2023 Jul 5]. Available from: https://medlineplus.gov/ency/article/007453.htm
- (35).Winter L, Saryyeva A, Schwabe K, Heissler HE, Runge J, Alam M, et al. Long-term deep brain stimulation in treatment-resistant obsessive–compulsive disorder: outcome and quality of life at four to eight years follow-up. Neuromodulation. 2021; 24(2):324–30. [DOI] [PubMed] [Google Scholar]
- (36).Suetens K, Nuttin B, Gabriels L, Van Laere K. Differences in metabolic network modulation between capsulotomy and deep-brain stimulation for refractory obsessive–compulsive disorder. J Nucl Med. 2014; 55(6):951–9. [DOI] [PubMed] [Google Scholar]
- (37).Dougherty DD. Deep brain stimulation: clinical applications. Psychiatr Clin North Am. 2018; 41(3):385–94. [DOI] [PubMed] [Google Scholar]
- (38).Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019; 15(3):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Menchon JM, Real E, Alonso P, Aparicio MA, Segalas C, Plans G, et al. A prospective international multi-center study on safety and efficacy of deep brain stimulation for resistant obsessive–compulsive disorder. Mol Psychiatry. 2021; 26(4):1234–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Alonso P, Cuadras D, Gabriels L, Denys D, Goodman W, Greenberg BD, et al. Deep brain stimulation for obsessive–compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS One. 2015; 10(7):e0133591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Pinckard-Dover H, Ward H, Foote KD. The decline of deep brain stimulation for obsessive–compulsive disorder following FDA Humanitarian Device Exemption approval. Front Surg. 2021; 8:642503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Insightec Ltd. Exablate Neuro [Internet]. c2023. [cited 2023 Jul 5]. Available from: https://insightec.com/exablate-neuro/
- (43).Chang KW, Park YS, Chang JW. Skull factors affecting outcomes of magnetic resonance-guided focused ultrasound for patients with essential tremor. Yonsei Med J. 2019; 60(8):768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Insightec Ltd. Regulatory approvals [Internet]. c2023. [cited 2023 Jul 5]. Available from: https://insightec.com/regulatory-approvals/
- (45).Government of Ontario. News release: Ontario expanding life-changing treatment for patients with essential tremors [Internet]. 2019. [cited 2023 Dec 15]. Available from: https://news.ontario.ca/en/release/52984/ontario-expanding-life-changing-treatment-for-patients-with-essential-tremors
- (46).Health Quality Ontario. Magnetic resonance-guided focused ultrasound neurosurgery for essential tremor: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2018 May; 18(4):1–141. Available from: https://hqontario.ca/Portals/0/documents/evidence/reports/hta-magnetic-esonance-guided-1804.pdf [PMC free article] [PubMed] [Google Scholar]
- (47).Sunnybrook Health Sciences Centre. Trial of MR-guided focused ultrasound (MRgFUS) bilateral capsulotomy for the treatment of refractory obsessive–compulsive disorder (OCD). ClinicalTrials.gov identifier: NCT03156335. Updated 2023 Dec 5. Accessed 2023 Apr 15. Available from: https://clinicaltrials.gov/study/NCT03156335.
- (48).University of Calgary. MR guided focused ultrasound (MRgFUS) [Internet]. c2023. [cited 2023 Dec 15]. Available from: https://cumming.ucalgary.ca/research/MRgFUS/clinical/what-mrgfus
- (49).Focused Ultrasound Foundation. Treatment sites: Canada [Internet]. c2023. [cited 2023 Dec 12]. Available from: https://www.fusfoundation.org/the-technology/treatment-sites/?country=Canada
- (50).National Evidence-based Healthcare Collaborating Agency Center for New Health Technology Assessment. Magnetic resonance imaging guided high-intensity ultrasound focusing [brain] 2021. Contract No.: HTA-2021-65.
- (51).Focused Ultrasound Foundation. Treatment sites: Yonsei University Health System, Severance Hospital [Internet]. c2023. [cited 2023 Dec 15]. Available from: https://www.fusfoundation.org/the-technology/treatment-sites/001F000000hbKe7IAE/
- (52).American Psychiatric Association. Practice guideline for the treatment of patients with obsessive–compulsive disorder [Internet]. Arlington (VA): The Association; 2007. [cited 2023 Jul 5]. Available from: https://psychiatryonline.org/pb/assets/raw/sitewide/practiceguidelines/guidelines/ocd.pdf [PubMed] [Google Scholar]
- (53).Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB, American Psychiatric A. Practice guideline for the treatment of patients with obsessive–compulsive disorder. Am J Psychiatry. 2007; 164(7 Suppl):5–53. [PubMed] [Google Scholar]
- (54).Staudt MD, Pouratian N, Miller JP, Hamani C, Raviv N, McKhann GM, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines for Deep Brain Stimulations for Obsessive–Compulsive Disorder: Update of the 2014 Guidelines. Neurosurgery. 2021; 88(4):710–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).National Institute for Health and Care Excellence. Deep brain stimulation for chronic, severe, treatment resistant obsessive–compulsive disorder in adults. IPG 693 [Internet]. 2021. [cited 2023 Jul 5]. Available from: https://www.nice.org.uk/guidance/ipg693
- (56).Acevedo N, Castle DJ, Bosanac P, Groves C, Rossell SL. Patient feedback and psychosocial outcomes of deep brain stimulation in people with obsessive–compulsive disorder. J Clin Neurosci. 2023; 112:80–5. [DOI] [PubMed] [Google Scholar]
- (57).El-Badri S, Mellsop G. Stigma and quality of life as experienced by people with mental illness. Australas Psychiatry. 2007; 15(3):195–200. [DOI] [PubMed] [Google Scholar]
- (58).Kaur R, Garg R, Raj R. Quality of life among patients with obsessive compulsive disorder: impact of stigma, severity of illness, insight, and beliefs. Ind Psychiatry J. 2023; 32(1):130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016; 75:40–6. [DOI] [PubMed] [Google Scholar]
- (60).Covidence systematic review software [Computer program]. Melbourne (Australia): Veritas Health Innovation. Available from https://www.covidence.org/. [Google Scholar]
- (61).Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol [Internet]. 2021. [cited 2021 May 27]; 134: 178–89. Available from: https://www.sciencedirect.com/science/article/pii/S0895435621000731 [DOI] [PubMed] [Google Scholar]
- (62).Evidence for equity: PROGRESS-Plus [Internet]. London: Cochrane Collaboration; c2023. [cited 2023 Oct 6]. Available from: https://methods.cochrane.org/equity/projects/evidence-equity/progress-plus [Google Scholar]
- (63).Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses [Internet]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. 2021. Available from: https://training.cochrane.org/handbook/current/chapter-10
- (64).Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009; 172(1):137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Valentine JC, Pigott TD, Rothstein HR. How many studies do you need?: A primer on statistical power for meta-analysis. Journal of Educational and Behavioral Statistics. 2010; 35:215–47. [Google Scholar]
- (66).McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. 2022. Available from: https://training.cochrane.org/handbook/current/chapter-12
- (67).Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020; 18(10):2127–33. [DOI] [PubMed] [Google Scholar]
- (68).Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): Grade Working Group; 2013. [cited 2017 Dec]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. [Google Scholar]
- (69).Davidson B, Eapen-John D, Mithani K, Rabin JS, Meng Y, Cao X, et al. Lesional psychiatric neurosurgery: meta-analysis of clinical outcomes using a transdiagnostic approach. J Neurol Neurosurg Psychiatry. 2022; 93(2):207–15. [DOI] [PubMed] [Google Scholar]
- (70).Pepper J, Zrinzo L, Hariz M. Anterior capsulotomy for obsessive–compulsive disorder: a review of old and new literature. J Neurosurg. 2019:1–10. [DOI] [PubMed]
- (71).Davidson B, Hamani C, Rabin JS, Meng Y, Richter MA, Giacobbe P, et al. Magnetic resonance-guided focused ultrasound capsulotomy for musical obsessions. Biol Psychiatry. 2021; 90(10):e49–e50. [DOI] [PubMed] [Google Scholar]
- (72).Davidson B, Hamani C, Rabin JS, Goubran M, Meng Y, Huang Y, et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: clinical and imaging results from two phase I trials. Mol Psychiatry. 2020; 25(9):1946–57. [DOI] [PubMed] [Google Scholar]
- (73).Chang JG, Kim DW, Jung HH, Chang WS, Kim CH, Kim SJ, et al. Evaluation of changes in neural oscillation after bilateral capsulotomy in treatment refractory obsessive–compulsive disorder using magnetoencephalogram. Asian J Psychiatr. 2023; 82:103473. [DOI] [PubMed] [Google Scholar]
- (74).Davidson B, Hamani C, Huang Y, Jones RM, Meng Y, Giacobbe P, et al. Magnetic resonance-guided focused ultrasound capsulotomy for treatment-resistant psychiatric disorders. Oper Neurosurg (Hagerstown). 2020; 19(6):741–9. [DOI] [PubMed] [Google Scholar]
- (75).Davidson B, Hamani C, Meng Y, Baskaran A, Sharma S, Abrahao A, et al. Examining cognitive change in magnetic resonance-guided focused ultrasound capsulotomy for psychiatric illness. Transl Psychiatry. 2020; 10(1):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Davidson B, Mithani K, Huang Y, Jones RM, Goubran M, Meng Y, et al. Technical and radiographic considerations for magnetic resonance imaging-guided focused ultrasound capsulotomy. J Neurosurg. 2020; 135(1):291–9. [DOI] [PubMed] [Google Scholar]
- (77).Jung HH, Chang WS, Rachmilevitch I, Tlusty T, Zadicario E, Chang JW. Different magnetic resonance imaging patterns after transcranial magnetic resonance-guided focused ultrasound of the ventral intermediate nucleus of the thalamus and anterior limb of the internal capsule in patients with essential tremor or obsessive–compulsive disorder. J Neurosurg. 2015; 122(1):162–8. [DOI] [PubMed] [Google Scholar]
- (78).Jung HH, Kim SJ, Roh D, Chang JG, Chang WS, Kweon EJ, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive–compulsive disorder: a proof-of-concept study. Mol Psychiatry. 2015; 20(10):1205–11. [DOI] [PubMed] [Google Scholar]
- (79).Kim SJ, Roh D, Jung HH, Chang WS, Kim CH, Chang JW. A study of novel bilateral thermal capsulotomy with focused ultrasound for treatment-refractory obsessive–compulsive disorder: 2-year follow-up. J Psychiatry Neurosci. 2018; 43(5):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007; 4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- (81).Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993; 29(2):321–6. [PubMed] [Google Scholar]
- (82).American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 2000. [Google Scholar]
- (83).Aas IH. Guidelines for rating Global Assessment of Functioning (GAF). Ann Gen Psychiatry. 2011; 10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Brigham and Women's Hospital. Sonication-based OCD neurosurgical intervention via capsulotomy (SONIC). ClinicalTrials.gov identifier: NCT06131502. Updated 2023 Nov 18. Accessed 2023 Nov 20. Available from: https://clinicaltrials.gov/study/NCT06131502.
- (85).Mantione M, Nieman DH, Figee M, Denys D. Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive–compulsive disorder. Psychol Med. 2014; 44(16):3515–22. [DOI] [PubMed] [Google Scholar]
- (86).von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol. 2015; 15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Mataix-Cols D, Rauch SL, Baer L, Eisen JL, Shera DM, Goodman WK, et al. Symptom stability in adult obsessive–compulsive disorder: data from a naturalistic two-year follow-up study. Am J Psychiatry. 2002; 159(2):263–8. [DOI] [PubMed] [Google Scholar]
- (88).Pinto A, Mancebo MC, Eisen JL, Pagano ME, Rasmussen SA. The Brown Longitudinal Obsessive Compulsive Study: clinical features and symptoms of the sample at intake. J Clin Psychiatry. 2006; 67(5):703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Bloch MH, Green C, Kichuk SA, Dombrowski PA, Wasylink S, Billingslea E, et al. Long-term outcome in adults with obsessive–compulsive disorder. Depress Anxiety. 2013; 30(8):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Subramaniam M, Soh P, Vaingankar JA, Picco L, Chong SA. Quality of life in obsessive–compulsive disorder: impact of the disorder and of treatment. CNS Drugs. 2013; 27(5):367–83. [DOI] [PubMed] [Google Scholar]
- (91).Ramos-Cerqueira AT, Torres AR, Torresan RC, Negreiros AP, Vitorino CN. Emotional burden in caregivers of patients with obsessive–compulsive disorder. Depress Anxiety. 2008; 25(12):1020–7. [DOI] [PubMed] [Google Scholar]
- (92).Microsoft Excel [Computer program]. Redmond (WA): Microsoft Corporation. Available from https://www.microsoft.com/en-ca/microsoft-365/excel. [Google Scholar]
- (93).National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd ed. Appendix I: Quality appraisal checklist - economic evaluations [Internet]. London: The Institute; 2012 Sep 26. [cited 2016. Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [PubMed] [Google Scholar]
- (94).Kumar KK, Bhati MT, Ravikumar VK, Ghanouni P, Stein SC, Halpern CH. MR-guided focused ultrasound versus radiofrequency capsulotomy for treatment-refractory obsessive–compulsive disorder: a cost-effectiveness threshold analysis. Front Neurosci. 2019; 13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).InSightec. ExAblate treatment of medication refractory obsessive compulsive disorder. ClinicalTrials.gov identifier: NCT01986296. Updated 2020 Apr 13. Accessed 2023 Aug 10. Available from: https://clinicaltrials.gov/study/NCT01986296·
- (96).Ontario Ministry of Finance. Population projections [Internet]. Toronto (ON): King's Printer for Ontario; 2023. [cited 2023 Nov 23]. Available from: https://data.ontario.ca/dataset/population-projections [Google Scholar]
- (97).Health Quality Ontario. Obsessive–compulsive disorder: care in all settings [Internet]. Toronto: Queen's Printer for Ontario; 2020. Available from: https://www.hqontario.ca/Portals/0/documents/evidence/quality-standards/qs-obsessive–compulsive-disorder-quality-standard-en.pdf [Google Scholar]
- (98).Insightec Ltd. Safety information [Internet]. c2023. [cited 2023 Aug 14]. Available from: https://insightec.com/safety-information/
- (99).Stephenson E. Mental disorders and access to mental health care. Ottawa (ON): Statistics Canada; 2023. [Google Scholar]
- (100).Boutet A, Gwun D, Gramer R, Ranjan M, Elias GJB, Tilden D, et al. The relevance of skull density ratio in selecting candidates for transcranial MR-guided focused ultrasound. J Neurosurg. 2019; 132(6):1785–91. [DOI] [PubMed] [Google Scholar]
- (101).Cormier J, Iorio-Morin C, Mathieu D, Ducharme S. Psychiatric neurosurgery: a survey on the perceptions of psychiatrists and residents. Can J Neurol Sci. 2019; 46(3):303–10. [DOI] [PubMed] [Google Scholar]
- (102).Consumer Price Index, annual average, not seasonally adjusted. Table 18-10-0005-01 [Internet]. Ottawa (ON): Statistics Canada; 2023. [updated 2023 Jan 17; cited 2023 Jun 6]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501 [Google Scholar]
- (103).Schedule of Benefits Physician Services Under the Health Insurance Act. Toronto (ON): Ontario Ministry of Health; 2023. [Google Scholar]
- (104).Liu HB, Zhong Q, Wang W. Bilateral anterior capsulotomy for patients with refractory obsessive–compulsive disorder: a multicenter, long-term, follow-up study. Neurol India. 2017; 65(4):770–6. [DOI] [PubMed] [Google Scholar]
- (105).Liu K, Zhang H, Liu C, Guan Y, Lang L, Cheng Y, et al. Stereotactic treatment of refractory obsessive compulsive disorder by bilateral capsulotomy with 3 years follow-up. J Clin Neurosci. 2008; 15(6):622–9. [DOI] [PubMed] [Google Scholar]
- (106).Zhan S, Liu W, Li D, Pan S, Pan Y, Li Y, et al. Long-term follow-up of bilateral anterior capsulotomy in patients with refractory obsessive–compulsive disorder. Clin Neurol Neurosurg. 2014; 119:91–5. [DOI] [PubMed] [Google Scholar]
- (107).Gong F, Li P, Li B, Zhang S, Zhang X, Yang S, et al. A study of cognitive function in treatment-refractory obsessive–compulsive disorder treated with capsulotomy. J Neurosurg. 2018; 128(2):583–95. [DOI] [PubMed] [Google Scholar]
- (108).Sheth SA, Neal J, Tangherlini F, Mian MK, Gentil A, Cosgrove GR, et al. Limbic system surgery for treatment-refractory obsessive–compulsive disorder: a prospective long-term follow-up of 64 patients. J Neurosurg. 2013; 118(3):491–7. [DOI] [PubMed] [Google Scholar]
- (109).Rasmussen SA, Noren G, Greenberg BD, Marsland R, McLaughlin NC, Malloy PJ, et al. Gamma ventral capsulotomy in intractable obsessive–compulsive disorder. Biol Psychiatry. 2018; 84(5):355–64. [DOI] [PubMed] [Google Scholar]
- (110).Gupta A, Shepard MJ, Xu Z, Maiti T, Martinez-Moreno N, Silverman J, et al. An International Radiosurgery Research Foundation multicenter retrospective study of gamma ventral capsulotomy for obsessive compulsive disorder. Neurosurgery. 2019; 85(6):808–16. [DOI] [PubMed] [Google Scholar]
- (111).Peker S, Samanci MY, Yilmaz M, Sengoz M, Ulku N, Ogel K. Efficacy and safety of gamma ventral capsulotomy for treatment-resistant obsessive–compulsive disorder: a single-center experience. World Neurosurg. 2020; 141:e941–e52. [DOI] [PubMed] [Google Scholar]
- (112).Gadot R, Najera R, Hirani S, Anand A, Storch E, Goodman WK, et al. Efficacy of deep brain stimulation for treatment-resistant obsessive–compulsive disorder: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2022. [DOI] [PubMed]
- (113).Montoya A, Weiss AP, Price BH, Cassem EH, Dougherty DD, Nierenberg AA, et al. Magnetic resonance imaging-guided stereotactic limbic leukotomy for treatment of intractable psychiatric disease. Neurosurgery. 2002; 50(5):1043–9; discussion 9-52. [DOI] [PubMed] [Google Scholar]
- (114).Canadian Institute for Health Information. Patient cost estimator [Internet]. c2023. [cited 2023 Nov 16]. Available from: https://www.cihi.ca/en/patient-cost-estimator
- (115).Ontario Health. Mechanical thrombectomy for acute and subacute blocked arteries and veins in the lower limbs: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2023 Jan; 23(1):1–244. Available from: https://hqontario.ca/Portals/0/Documents/evidence/reports/hta-mechanical-thrombectomy-for-acute-and-subacute-blocked-arteries-and-veins-in-the-lower-limbs.pdf [PMC free article] [PubMed] [Google Scholar]
- (116).Horisawa S, Fukui A, Nonaka T, Kawamata T, Taira T. Radiofrequency ablation for movement disorders: risk factors for intracerebral hemorrhage, a retrospective analysis. Oper Neurosurg (Hagerstown). 2021; 21(3):143–9. [DOI] [PubMed] [Google Scholar]
- (117).Fenoy AJ, Simpson RK, Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014; 120(1):132–9. [DOI] [PubMed] [Google Scholar]
- (118).Graat I, Mocking R, Figee M, Vulink N, de Koning P, Ooms P, et al. Long-term outcome of deep brain stimulation of the ventral part of the anterior limb of the internal capsule in a cohort of 50 patients with treatment-refractory obsessive–compulsive disorder. Biol Psychiatry. 2021; 90(10):714–20. [DOI] [PubMed] [Google Scholar]
- (119).Ontario Ministry of Health. Ontario Drug Benefit Formulary [Internet]. Toronto (ON): King's Printer for Ontario; 2023. [updated 2023 Jul 31; cited 2023 Nov 15]. Available from: https://www.formulary.health.gov.on.ca/formulary/ [Google Scholar]
- (120).Ontario Ministry of Health. Ontario Drug Programs Reference Manual. Revision 9 [Internet]. Toronto (ON): Queen's Printer for Ontario; 2019. [updated 2023 Oct 10; cited 2023 Nov 15]. Available from: https://files.ontario.ca/moh-odp-reference-manual-en.pdf [Google Scholar]
- (121).Ontario Ministry of Health. Get coverage for prescription drugs [Internet]. Toronto (ON): Queen's Printer for Ontario; 2016. [updated 2023 Jan 30; cited 2023 Sep 1]. Available from: https://www.ontario.ca/page/get-coverage-prescription-drugs [Google Scholar]
- (122).Lee DJ, Dallapiazza RF, De Vloo P, Elias GJB, Fomenko A, Boutet A, et al. Inferior thalamic peduncle deep brain stimulation for treatment-refractory obsessive–compulsive disorder: a phase 1 pilot trial. Brain Stimul. 2019; 12(2):344–52. [DOI] [PubMed] [Google Scholar]
- (123).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011; 4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (124).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012; 5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (125).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario-final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015 Apr. [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (126).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (127).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (128).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (129).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (130).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (131).Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA_KFacey_Jun13.pdf [Google Scholar]
- (132).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990; 13(1):3–21. [Google Scholar]
- (133).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (134).NVivo qualitative data analysis software. QSR International (Doncaster, Victoria, Australia). Available at: https://www.qsrinternational.com/nvivo/home.
- (135).Porta MS. A dictionary of epidemiology. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- (136).CE marking [Internet]. European Union; 2024. [cited 2024 Feb]. Available from: https://europa.eu/youreurope/business/product-requirements/labels-markings/ce-marking/index_en.htm [Google Scholar]
- (137).Ontario Health's equity, inclusion, diversity and anti-racism framework [Internet]. Toronto (ON): Ontario Health; 2022. [cited 2023 Mar 22]. Available from: https://www.ontariohealth.ca/sites/ontariohealth/files/2020-12/Equity%20Framework.pdf [Google Scholar]
- (138).Hezel DM, Simpson HB. Exposure and response prevention for obsessive–compulsive disorder: a review and new directions. Indian J Psychiatry. 2019; 61(Suppl 1):S85–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).World Health Organization. Social determinants of health: key concepts [Internet]. Geneva: The Organization; 2013 May 7. [cited 2022 Mar 22]. Available from: https://www.who.int/newsroom/questions-and-answers/item/social-determinants-of-health-key-concepts [Google Scholar]
- (140).Humanitarian Device Exemption [Internet]. US Food and Drug Administration; 2022. [updated 2022 Oct 3; cited 2024 Feb]. Available from: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/humanitarian-device-exemption [Google Scholar]