Abstract
Ret/ptc2 is a constitutively active, oncogenic form of the c-Ret receptor tyrosine kinase. Like the other papillary thyroid carcinoma forms of Ret, Ret/ptc2 is activated through fusion of the Ret tyrosine kinase domain to the dimerization domain of another protein. Investigation of requirements for Ret/ptc2 mitogenic activity, using coexpression with dominant negative forms of Ras and Raf, indicated that these proteins are required for mitogenic signaling by Ret/ptc2. Because activation of Ras requires recruitment of Grb2 and SOS to the plasma membrane, the subcellular distribution of Ret/ptc2 was investigated, and it was found to localize to the cell periphery. This localization was mediated by association with Enigma via the Ret/ptc2 sequence containing tyrosine 586. Because Shc interacts with MEN2 forms of Ret, and because phosphorylation of Shc results in Grb2 recruitment and subsequent signaling through Ras and Raf, the potential interaction between Ret/ptc2 and Shc was investigated. The PTB domain of Shc also interacted with Ret/ptc2 at tyrosine 586, and this association resulted in tyrosine phosphorylation of Shc. Coexpression of chimeric proteins demonstrated that mitogenic signaling from Ret/ptc2 required both recruitment of Shc and subcellular localization by Enigma. Because Shc and Enigma interact with the same site on a Ret/ptc2 monomer, dimerization of Ret/ptc2 allows assembly of molecular complexes that are properly localized via Enigma and transmit mitogenic signals via Shc.
The c-Ret protein, a member of the receptor tyrosine kinase (RTK) family of enzymes, was first discovered as the product of a proto-oncogene (51). Germ line mutations in ret are linked to two classes of genetic diseases. Loss of functional Ret results in Hirschsprung’s disease, which is characterized by the absence of enteric autonomic ganglia (2, 15, 43). This has been confirmed by deletion of the ret gene in mice, where, in addition to the enteric neuron abnormalities, there is defective kidney development (49). In contrast, germ line point mutations resulting in constitutively active forms of Ret give rise to the multiple endocrine neoplasia type 2 (MEN2) class of dominantly inherited cancer syndromes (37, 38).
Ret is the kinase through which glia-derived neurotrophic factor (GDNF) and neurturin (NTN) signal (7, 12, 24, 26, 53). GDNF was discovered as a neurotrophic factor for dopaminergic neurons (33) and is a survival factor for motor neurons (21). NTN supports the survival of sympathetic neurons (28). Mice lacking GDNF exhibit a phenotype similar to that of Ret-deficient mice in that they lack enteric neurons and kidneys (35, 40, 44), underscoring the importance of Ret in those developmental processes. Glycosyl-phosphatidylinositol-linked receptors for GDNF and NTN each couple to c-Ret to initiate signal transduction.
In addition to germ line mutations resulting in MEN2, somatic events also lead to activated forms of Ret. Ret-mediated malignant transformation is best characterized in papillary thyroid carcinoma, where chromosomal translocation results in the fusion of the ret locus with other genes (4, 5, 32, 41). Ret/ptc2, encoded by one of the fusion genes, contains 596 residues, with the N-terminal 239 residues derived from the type Iα regulatory subunit of cyclic AMP (cAMP)-dependent protein kinase (RIα) and the C terminus derived from the Ret tyrosine kinase domain. The mitogenic activity of Ret/ptc2 depends on catalytic activity of the kinase domain and constitutive dimerization mediated by the RIα dimerization and docking domain (14). Furthermore, a tyrosine residue at position 586 is absolutely essential for mitogenic activity. This region has been shown to be a docking site for Enigma (13, 57), a 455-amino-acid protein which contains an N-terminal PDZ domain and three C-terminal LIM domains (58). The second of these three LIM domains (LIM2) binds specifically to Ret/ptc2 (56). The corresponding tyrosine residue in MEN2 forms of c-Ret, tyrosine 1062, has been demonstrated to be a docking site for Shc, which contains an N-terminal phosphotyrosine binding domain (PTB) and a C-terminal SH2 domain (1).
We report that both Enigma and Shc bind to Ret/ptc2 at the same site, tyrosine 586, and that both interactions are required for the mitogenic activity of Ret/ptc2. Enigma binds to Ret/ptc2 in a phosphorylation-independent manner through a LIM domain and is anchored to the cell periphery via an N-terminal PDZ domain. Shc binds to Ret/ptc2 through a PTB domain and is then phosphorylated, enabling it to transduce a mitogenic signal through the Ras pathway. These results demonstrate that both autophosphorylation and subcellular localization are required for mitogenic signaling via this intracellular tyrosine kinase. Differential recognition of an unmodified and a modified tyrosine-containing sequence by different protein domains provides for both subcellular localization and assembly of signal transduction complexes. Dimerization via RIα is thus proposed to provide a single Ret/ptc2 complex with the ability to interact with two targets that provide two functions necessary for mitogenic signaling.
MATERIALS AND METHODS
Reagents.
An enhanced chemiluminescence detection kit for Western blots, 5-bromodeoxyuridine (BrdU) labeling reagents, Texas red-labeled streptavidin, and horseradish peroxidase-conjugated anti-mouse immunoglobulin (Ig) and anti-rabbit Ig antibodies were purchased from Amersham (Arlington Heights, Ill.). Biotinylated anti-mouse Ig and anti-rabbit Ig antibodies, as well as fluorescein isothiocyanate (FITC)-conjugated anti-guinea pig Ig and anti-rabbit Ig antibodies, were purchased from Jackson ImmunoResearch (West Grove, Pa.). Anti-hemagglutinin (HA) tag monoclonal antibody was purchased from BAbCo (Berkeley, Calif.), and PY20 monoclonal antibody was from Transduction Laboratories (Lexington, Ky.). The anti-Ret antibody is a rabbit polyclonal antibody raised against a synthetic peptide corresponding to residues 535 to 551 of Ret/ptc2 (14). The β-galactosidase reagent o-nitrophenyl-β-d-galactopyranoside was purchased from Sigma.
DNA constructs.
The mammalian expression plasmids coding for the wild-type and mutant forms of Ret/ptc2 were constructed in pRc/CMV (Invitrogen, La Jolla, Calif.) as described previously (13, 14). Construction of the HA-tagged Enigma expression constructs was also described previously (57). The human Shc cDNA, kindly provided by David Schlaepfer (Salk Institute, La Jolla, Calif.), was subcloned into pRSETb (Invitrogen), and NotI sites were introduced by Kunkel mutagenesis (29) to allow excision of the sequences encoding either full-length Shc, the PTB domain (residues 1 to 213), or the SH2 domain (residues 373 to 479). These fragments were then subcloned into the NotI sites of pGEX, pcDNA3M (57), and pVP16. Dominant negative effects of N17 mutant Ras (8, 17) and the N-terminal epitope (NTE) of Raf (42) have been described, and plasmids expressing these proteins were obtained from Akhelish Pandy (University of Michigan, Ann Arbor). To create the C-terminal fusion of Ret/ptc2 with the Enigma PDZ domain (C′574-PDZ), the Ret/ptc2 cDNA was subcloned into the HindIII and XbaI sites of pcDNA3 (Invitrogen); a linker was added at the XhoI site in the Ret/ptc2 sequence, which created a unique EcoRI site; and then cDNA coding for the N-terminal 279 residues of Enigma was subcloned into the EcoRI and XhoI sites. The plasmid expressing phospholipase Cγ (PLCγ)-Shc was constructed by subcloning cDNA encoding residues 214 to 479 of Shc into pcDNA3M and then splicing a PCR-generated fragment of the PLCγ2 sequence in between the HA tag and the Shc sequences. The fragment of the PLCγ2 gene encodes the N-terminal SH2 domain, which was previously demonstrated to bind Ret/ptc2 (13).
Cell culture and microinjection.
Mouse 10T1/2 fibroblasts were plated in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). The cells were maintained at 37°C in a 10% CO2 atmosphere and split before reaching confluence. For microinjection, cells were plated on glass coverslips and grown to 70% confluence in DMEM plus 10% FBS. The coverslips were then transferred to DMEM containing 0.05% calf serum. After 24 h of incubation in the FBS-free medium, the cells were injected in their nuclei with solutions of injection buffer (20 mM Tris [pH 7.2], 2 mM MgCl2, 0.1 mM EDTA, 20 mM NaCl) containing 100 μg of each expression plasmid DNA per ml and 6 mg of either guinea pig or rabbit IgG (Sigma) per ml. All microinjection experiments were performed with an automatic micromanipulator (Eppendorf, Fremont, Calif.) with glass needles pulled on a vertical pipette puller (Kopf, Tujunga, Calif.).
Mitogenic activity assay.
DNA synthesis was assessed through incorporation of the thymidine analog BrdU and its subsequent detection by immunostaining. Following nuclear microinjection, 0.1% BrdU labeling reagent (Amersham) was added to the starvation medium (DMEM plus 0.05% calf serum), and the cells were incubated for an additional 24 h. Cells were fixed in 95% ethanol–5% acetic acid for 30 min and then washed with phosphate-buffered saline (PBS). Incorporation of BrdU was visualized by successively incubating the fixed cells with mouse anti-BrdU (undiluted), biotinylated donkey anti-mouse IgG (dilution, 1:500), Texas red-labeled streptavidin (dilution, 1:100), and FITC-conjugated anti-rabbit IgG (dilution, 1:100). Cells were scored by fluorescent microscopy for nuclear microinjection (FITC positive) and BrdU incorporation (Texas red positive).
Detection of expressed protein by immunofluorescence.
For observation of expressed protein, 5 h after injection cells were fixed in 3.7% formaldehyde for 5 min and then washed with PBS and incubated with blocking buffer (2% goat serum, 2% bovine serum albumin, 0.1% Triton X-100, and 50 mM glycine, in PBS) for 20 min. For detection of Ret/ptc2 constructs, cells were then incubated successively with rabbit anti-Ret (14) (dilution, 1:500), biotinylated donkey anti-rabbit IgG (dilution, 1:400), Texas red-labeled streptavidin (dilution, 1:100), and FITC-conjugated anti-guinea pig IgG (dilution, 1:100). For detection of HA-tagged Enigma constructs, the same procedure was followed except that the antibodies used in the first two incubations were replaced with mouse monoclonal anti-HA tag antibody (dilution, 1:250) followed by biotinylated donkey anti-mouse IgG (dilution, 1:400). The coinjected samples for confocal microscopy were fixed in the same manner and incubated with the following antibodies: rabbit anti-Ret (1:500), biotinylated donkey anti-rabbit IgG (1:400), Fluorolink Cy5 (Amersham) streptavidin (1:100), mouse monoclonal anti-HA tag antibody (1:250), FITC-conjugated donkey anti-mouse antibody (1:250), and 7-amino-4-methylkoumarin-3-acetic acid-conjugated donkey anti-guinea pig IgG (1:100). Confocal images were collected on an MRC-1024 system (Bio-Rad) attached to an Axiovert 35M (Zeiss AG), with excitation illumination from a krypton-argon laser. Individual images (1,024 by 1,024 pixels) were converted to PICT format and merged as pseudocolor RBG images by using Adobe Photoshop (Adobe Systems). Digital prints were produced with a Fujix Pictography 3000 printer.
Two-hybrid detection of protein-protein interactions.
A yeast two-hybrid system was used to detect interactions (56). Ret/ptc2 cDNA was subcloned into the LexA fusion vector pBTM116 (bait), and point mutants were made by site-directed mutagenesis (29). Human Shc cDNA or PCR-generated fragments were subcloned into pVP16 (prey). These constructs were coexpressed in the L40 strain of Saccharomyces cerevisiae, and single colonies were either plated on solid media containing 100 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml or added to 5-ml liquid cultures for solution assay of β-galactosidase activity (3). Aliquots from these cultures were used to seed fresh cultures, which were grown to an optical density at 600 nm (OD600) of approximately 0.5. Cell pellets from 5 ml of culture were resuspended in 0.5 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol [pH 7.0]). Samples of these resuspensions were diluted 10- to 40-fold (final volume, 1 ml) in Z buffer. Cells were lysed by addition of sodium dodecyl sulfate (SDS) and chloroform followed by vortexing. The chromagenic substrate o-nitrophenyl-β-d-galactopyranoside was added (200 ml of a 4-mg/ml solution), and the reaction was quenched by addition of 0.5 ml of 1 M Na2CO3. Units of activity were calculated as follows: activity = (1,750 × OD420)/[(time)(volume of culture in assay)(OD600 of culture)], where time is in minutes.
GST fusion affinity precipitation.
Two-hybrid system results were verified by using a stably transfected NIH 3T3 cell line expressing an epidermal growth factor receptor (EGFR)-Ret chimeric protein (47). These cells were treated with 100 nM EGF for 10 min before resuspension in lysis buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 10% glycerol, 1% Triton X-100, 1 mM benzamidine, 1 mM tolylsulfonyl phenylalanyl chloromethyl ketone [TPCK], 1 mM Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK], 1 mM phenylmethylsulfonyl fluoride, 1 mM NaVO4). Cleared lysates were incubated for 2 h with approximately 5 μg of glutathione S-transferase (GST) fusion protein bound to glutathione agarose beads (Sigma) in a total volume of 300 μl. The beads were washed four times with lysis buffer, resuspended in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer, boiled, and run on 7.5% gels. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes and probed with either rabbit anti-Ret (14) (1:25,000) or monoclonal antiphosphotyrosine (1:2,500; Transduction Laboratories) antibodies. The GST fusion proteins used were expressed in Escherichia coli from pGEX vectors containing inserts coding for the following sequences: GST (empty vector); GST-ShcSH2 (human Shc SH2 domain, residues 373 to 479); GST-ShcPTB (human Shc PTB domain, residues 1 to 213); and GST-Enigma (human Enigma LIM domains 2 and 3 [the C-terminal 131 residues]).
Cotransfections of 293 cells.
For transfection experiments, 60-mm-diameter dishes of 293 cells were grown in DMEM plus 10% FBS. Once cells reached approximately 40% confluence, they were transfected with 10 μg of each expression construct by calcium phosphate precipitation (9). After transfection, cells were grown for 24 h and then harvested in 0.5 ml of boiling SDS-PAGE sample buffer. Samples (20 μl) of these lysates were run on 12.5% gels and then transferred to PVDF membranes. Blots were incubated for 18 h in TTBS (10 mM Tris [pH 7.5], 100 mM NaCl, 0.1% Tween 20) plus 5% powdered milk and then incubated with antibodies in the same buffer. Primary antibodies used were rabbit anti-Ret (1:25,000 dilution), mouse anti-HA tag monoclonal antibody (1:5,000 dilution), or mouse antiphosphotyrosine monoclonal antibody (1:5,000 dilution), followed by horseradish peroxidase-conjugated secondary antibodies (1:5,000 dilution). Proteins were then detected by an enhanced chemiluminescence kit (Amersham). Affinity precipitation experiments were performed by using the GST fusion LIM domain 2 of Enigma and lysates of 293 cells cotransfected with Ret/ptc2 and Shc expression plasmids. Precipitation conditions were identical to those described for experiments with the EGFR-Ret chimeric receptor.
RESULTS
Mitogenic signaling from Ret/ptc2 is blocked by dominant negative forms of Ras and Raf.
The mitogenic activity of Ret/ptc2 was characterized by a microinjection assay in which serum-starved fibroblasts were injected with expression plasmids and subsequent entry into S phase was assessed by incorporation of the thymidine analog BrdU (14). Injection of a plasmid expressing Ret/ptc2 resulted in over 35% of the injected cells entering S phase (Fig. 1). In contrast, cells coinjected with the Ret/ptc2 construct and one expressing either a dominant negative form of Ras (N17) (8, 17) or of Raf (NTE) (42) responded in a manner that was not significantly different from uninjected controls. The mitogenic activity of Ret/ptc2 thus requires the Ras pathway. Coinjection of a kinase-inactive form of Src had no effect. The inability of activated Ras protein to stimulate more than 35% of injected cells in this type of assay has been observed previously (30).
FIG. 1.
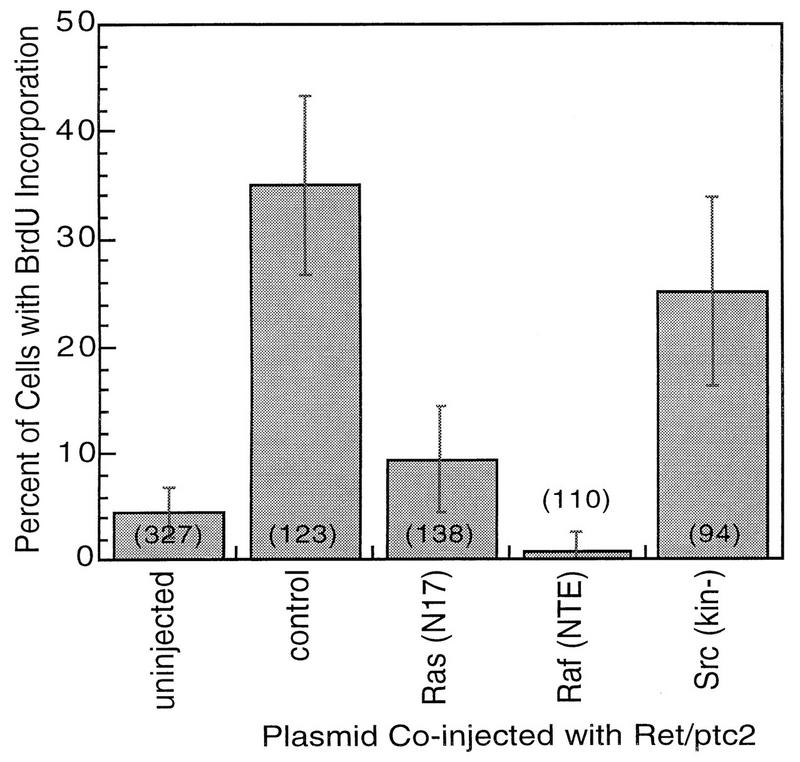
Effect of coexpression with dominant negative Ras or Raf on mitogenic activity of Ret/ptc2. Serum-starved mouse fibroblasts (10T1/2) were microinjected with a mixture of Ret/ptc2 expression plasmid along with one of the following: a control empty plasmid (control); plasmid expressing mutant Ras with serine 17 replaced by asparagine [Ras(N17)]; plasmid expressing the NTE, residues 1 through 290, of Raf-1 [Raf(NTE)]; or plasmid expressing a catalytically inactive form of Src [Src(kin−)]. In each case constructs were injected at 100 μg/ml. Cells were then assessed for entry into S phase by immunofluorescent detection of BrdU incorporation. The fraction of injected cells positive for BrdU incorporation is shown, with error bars displaying the 95% confidence intervals, which were calculated by using the standard error of proportion. The numbers in parentheses are the total numbers of injected cells.
Colocalization of Ret/ptc2 and Enigma.
Because activation of Ras requires recruitment of the guanine nucleotide exchange factor SOS to the plasma membrane (16, 34, 55), the subcellular distribution of Ret/ptc2 was investigated. By using immunofluorescent staining of cells injected with Ret/ptc2 expression constructs (Fig. 2), Ret/ptc2 was found to have a distinctive pattern of localization where much of the cell periphery, including regions which resembled focal adhesions, stained most prominently and filaments resembling the actin cytoskeleton were also visible (Fig. 3A). This pattern was not present in cells expressing a form of Ret/ptc2 that lacked the C-terminal 22 residues, C′574, suggesting that the C terminus of Ret/ptc2 determined the localization of the protein to the plasma membrane (Fig. 3B). Similarly, Y586F mutant Ret/ptc2 lost membrane localization (data not shown). A deletion mutation that eliminated the dimerization domain of Ret/ptc2, a region known to interact with cAMP-dependent kinase-anchoring proteins (22) and to be required for mitogenic activity (14), retained the distribution of full-length Ret/ptc2 (Fig. 3C). Localization is activity independent, because kinase-inactive forms of Ret/ptc2 also localized in a pattern indistinguishable from the wild-type pattern (data not shown).
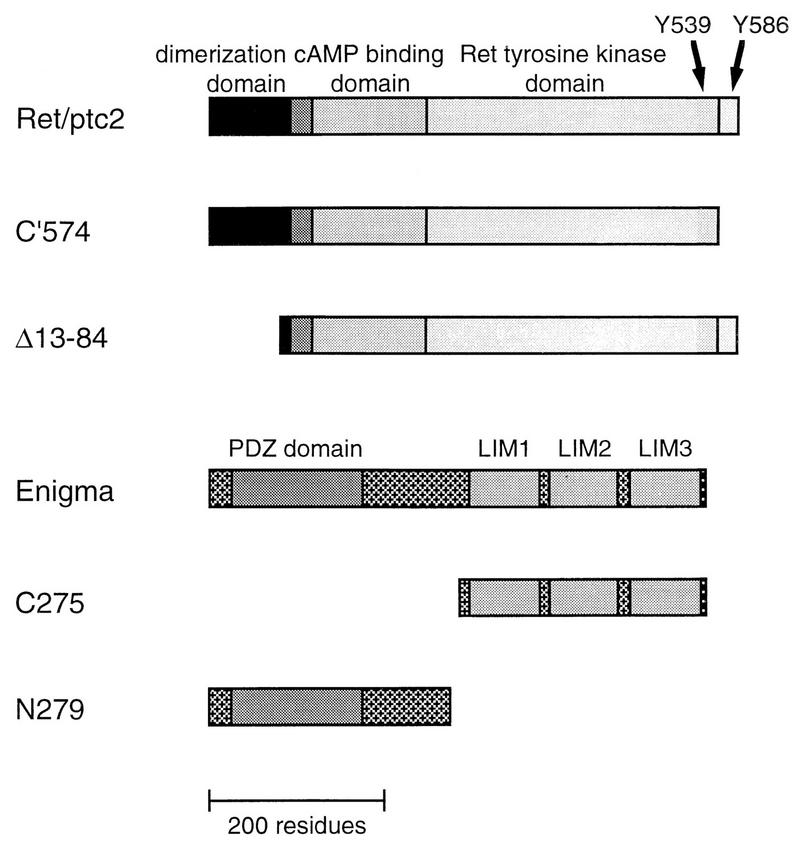
FIG. 2.
Schematic representation of Ret/ptc2 and Enigma constructs. Ret/ptc2 is 596 residues in length, with the N-terminal 236 amino acids from the type Iα regulatory subunit of cAMP-dependent protein kinase (RIα). The dimerization domain of RIα is located within the first 84 residues. Enigma is 455 residues in length, with an N-terminal PDZ domain and three C-terminal LIM domains. An HA tag was added to the amino termini of the Enigma constructs to facilitate detection by anti-HA monoclonal antibodies.
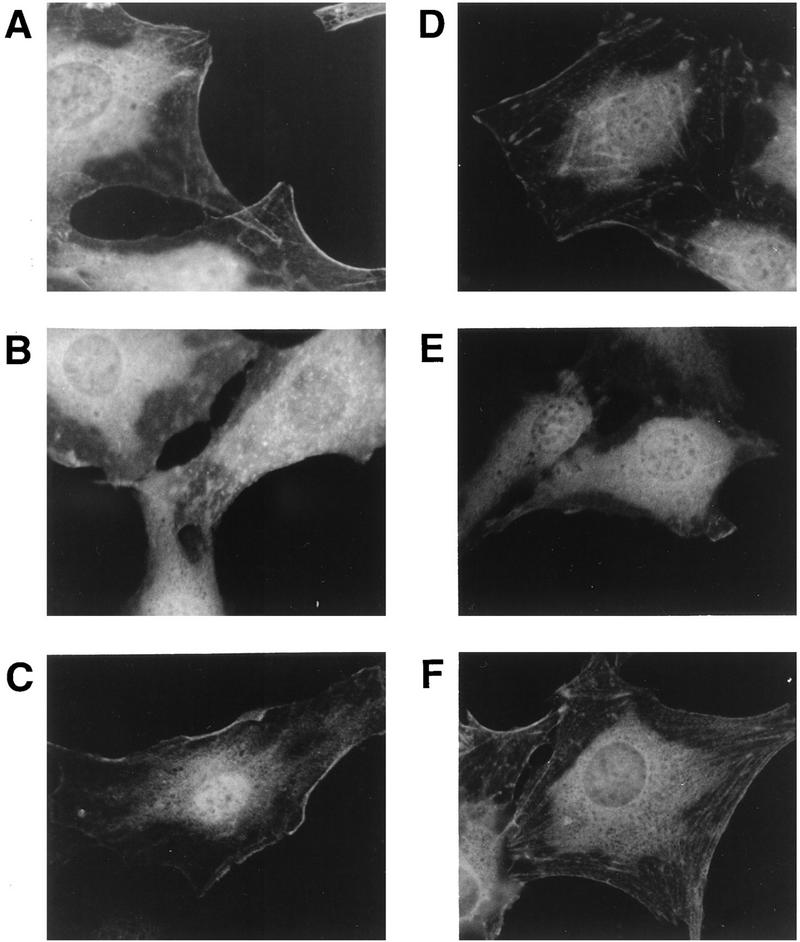
FIG. 3.
Determinants of subcellular localization of Ret/ptc2 and Enigma. (A, B, and C) Sequence requirements for the subcellular targeting of Ret/ptc2. Fibroblasts microinjected with various Ret/ptc2 expression plasmids were fixed 4 h after injection and immunofluorescently stained with anti-Ret antibodies. Constructs injected coded for the following: (A) wild-type Ret/ptc2; (B) C′574, a mutant where the last 22 amino acids are missing, including the Enigma binding site; and (C) Ret/ptc2Δ(13-84), a dimerization domain deletion mutant. (D, E, and F) Sequence requirements for the subcellular targeting of Enigma. Cells injected with constructs expressing HA-tagged forms of Enigma were stained for immunofluorescence with anti-HA tag monoclonal antibodies. Plasmids expressed the following HA-tagged proteins: (D) full-length Enigma; (E) the C-terminal 275 residues of Enigma, which contain the three LIM domains; and (F) the N-terminal 279 residues of Enigma, which contain the PDZ domain.
A potential anchor for Ret/ptc2 is Enigma, a protein previously shown to interact with the C terminus of Ret/ptc2 in an activity-independent manner (13, 57). To test this possibility, the distribution of Enigma was investigated by immunofluorescent staining. Because polyclonal antibodies raised against Enigma lacked the specificity required for this method, plasmids were injected which expressed HA-tagged forms of Enigma that could then be localized with anti-HA monoclonal antibodies (Fig. 2). Full-length Enigma displayed a distribution pattern very similar to that of Ret/ptc2, with pronounced staining of the cell edges and some cytoskeletal components (Fig. 3D). A deletion mutant of Enigma that lacked the PDZ domain exhibited a diffuse, cytoplasmic staining pattern (Fig. 3E), while a mutant containing the PDZ domain but lacking all three LIM domains retained the wild-type distribution (Fig. 3F).
It was previously shown that Enigma interacts with the C terminus of Ret via the second of the C-terminal LIM domains of Enigma (57). These results raised the possibility that Enigma functions as an adapter protein, with the N-terminal PDZ domain of Enigma anchoring an Enigma-Ret/ptc2 complex to a specific subcellular location. To test this possibility, cells were coinjected with plasmids expressing Ret/ptc2 and HA-tagged Enigma and then costained for immunofluorescent confocal microscopy. The two proteins were found to codistribute (Fig. 4).
FIG. 4.
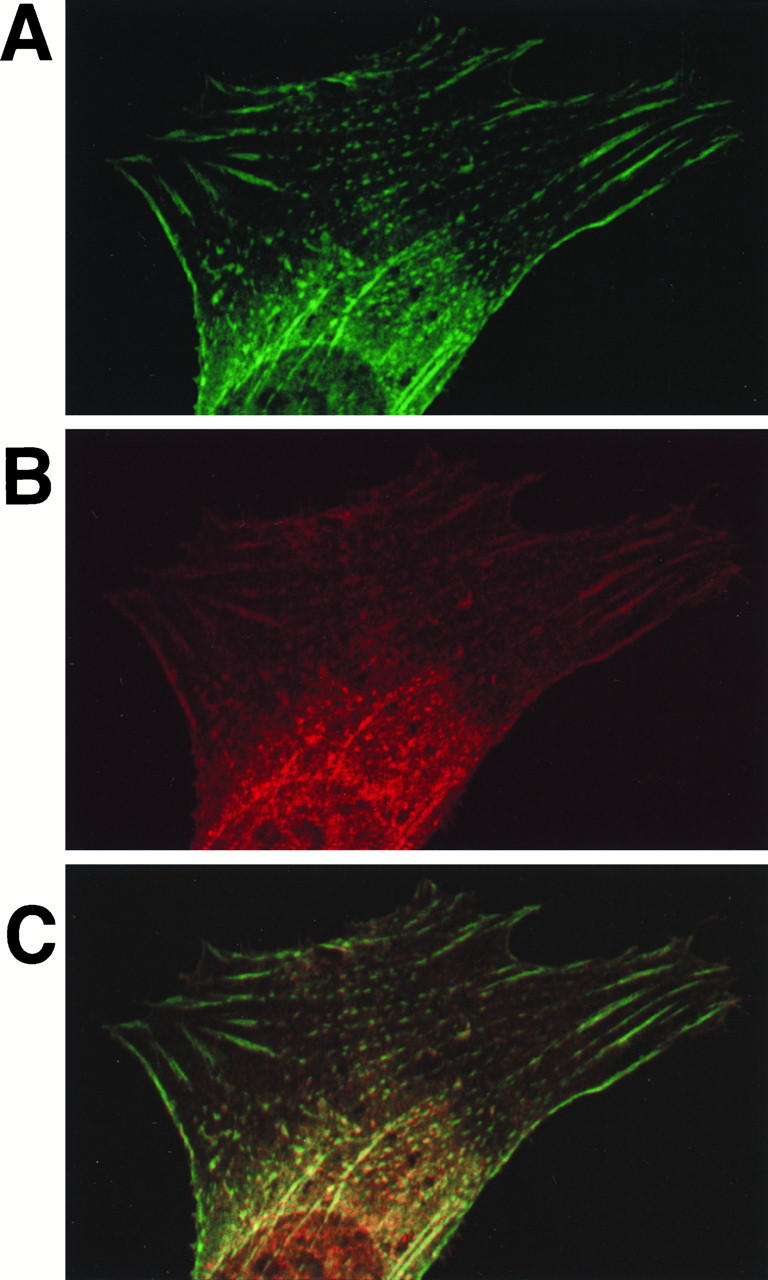
Codistribution of Ret/ptc2 and Enigma. Mouse fibroblasts coinjected with expression plasmids for Ret/ptc2 and HA-tagged Enigma were fixed 4 h after injection, subjected to immunofluorescent staining, and then imaged by confocal microscopy. (A) Enigma distribution shown by fluorescein linked to anti-HA tag monoclonal antibody; (B) Ret/ptc2 distribution shown by fluorescence of Cy-5 linked to anti-Ret antibody; (C) Digital overlay of the two fluorescent signals.
Binding of Shc to Ret/ptc2 through its PTB domain.
The adapter protein Shc binds to activated MEN2 mutant forms of c-Ret (1). Because Shc recruits Grb2 to activated RTKs, resulting in activation of the Ras-Raf pathway, the potential for Shc binding to Ret/ptc2 was investigated. When tested by a yeast two-hybrid approach, Shc was observed to bind to Ret/ptc2 in an interaction that was phosphorylation dependent because a catalytically inactive form of Ret/ptc2, K282R, failed to interact with Shc (Fig. 5). The isolated PTB domain of Shc bound Ret/ptc2, while the SH2 domain alone did not. In addition, mutants of Ret/ptc2 in which tyrosine 586 was eliminated either by point mutation (Y586F) or C-terminal truncation (C′574) failed to interact with Shc. These two-hybrid system results were verified by using a cell line expressing EGFR-Ret chimeric receptors, where stimulation by EGF results in activation of the Ret tyrosine kinase (47). Lysates of these cells were incubated with various GST fusion proteins bound to glutathione agarose. The phosphorylated chimeric receptor bound tightly to the GST-Shc PTB fusion, while no binding to the Shc SH2 domain was detected (Fig. 5D). Binding of the EGFR-Ret chimera to Shc was ligand and autophosphorylation dependent, whereas binding to the LIM2 domain of Enigma was not.
FIG. 5.
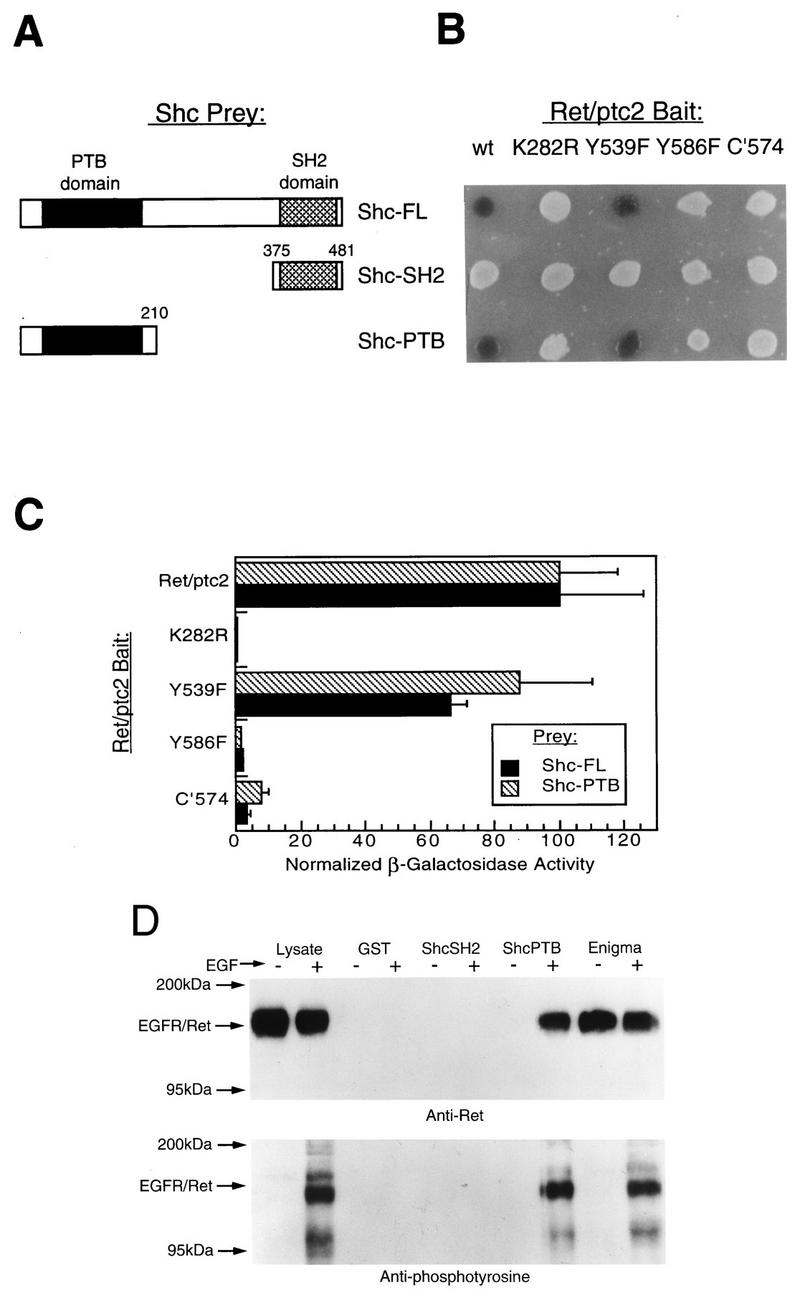
Characterization of the interaction of Shc with Ret/ptc2. The yeast two-hybrid system was used to map the interaction determinants of Ret/ptc2 and Shc by using various Shc-VP16 (Prey) and Ret/ptc2-LexA (Bait) fusion constructs, and results were verified by GST affinity precipitation. (A) Schematic representation of the Shc deletion mutants used. Numbers delineate amino acid sequences included in the constructs. (B) Qualitative β-galactosidase activity of yeast cotransformants. Single colonies were plated on solid media containing X-Gal, and results shown are for 10 h after plating. Bait constructs coded for wild-type Ret/ptc2 (wt), various point mutants, or a C-terminal-truncation mutant (C′574). (C) Solution assay of yeast cotransformant β-galactosidase activity. Cultures of yeast cotransformed with plasmids expressing various Ret/ptc2-LexA and Shc-VP16 fusion proteins were assayed for β-galactosidase activity. Results shown are averages obtained for four assays, with error bars representing the standard deviations. For comparison of results between the full-length (FL) and PTB Shc constructs, normalized values were plotted. The actual activity for full-length Shc with wild-type Ret/ptc2 was 250 U, while the activity for the Shc PTB prey with wild-type Ret/ptc2 was 160 U. Units are per minute, as described for the β-galactosidase solution assay (3). (D) Clonal NIH 3T3 cells expressing an EGFR-Ret chimeric receptor were treated (+) or not treated (−) with 100 nM EGF for 10 min at 37°C before lysis. Aliquots of lysates were incubated with either GST or GST fusion proteins bound to glutathione agarose. The protein fragments expressed as GST fusions were the Shc SH2 and PTB domains and Enigma LIM domains 2 and 3. Western blots of EGFR-Ret that bound to the indicated GST fusion proteins are shown. Gels were run in parallel, blotted to PVDF membranes, and probed with anti-Ret or antiphosphotyrosine antibodies.
Use of chimeric proteins to elucidate the roles of Shc and Enigma.
Both Shc and Enigma binding required tyrosine 586 of Ret/ptc2, a residue that is essential for mitogenic activity (14). We investigated whether either or both of these proteins were required for Ret-induced mitogenesis. The goal was to engineer a form of Ret/ptc2 that would bind Shc and not Enigma and another that would bind Enigma and not Shc. Because the consensus site for Shc PTB domain binding (19, 31, 54) closely resembles the consensus site for Enigma LIM interaction (57), a novel chimeric strategy was devised (Fig. 6A). To test the importance of Enigma anchoring of Ret/ptc2, the unanchored C-terminal-deletion form of Ret/ptc2, C′574, was fused to the N-terminal 279 residues of Enigma to make a chimeric protein referred to as C′574-PDZ. To engineer a form of Shc that would bind to Ret/ptc2 in the absence of tyrosine 586, PLCγ-Shc was constructed; in this fusion the PTB domain of Shc was replaced with the N-terminal SH2 domain of PLCγ. This SH2 domain of PLCγ interacts with tyrosine 539 of Ret/ptc2 (6, 13).
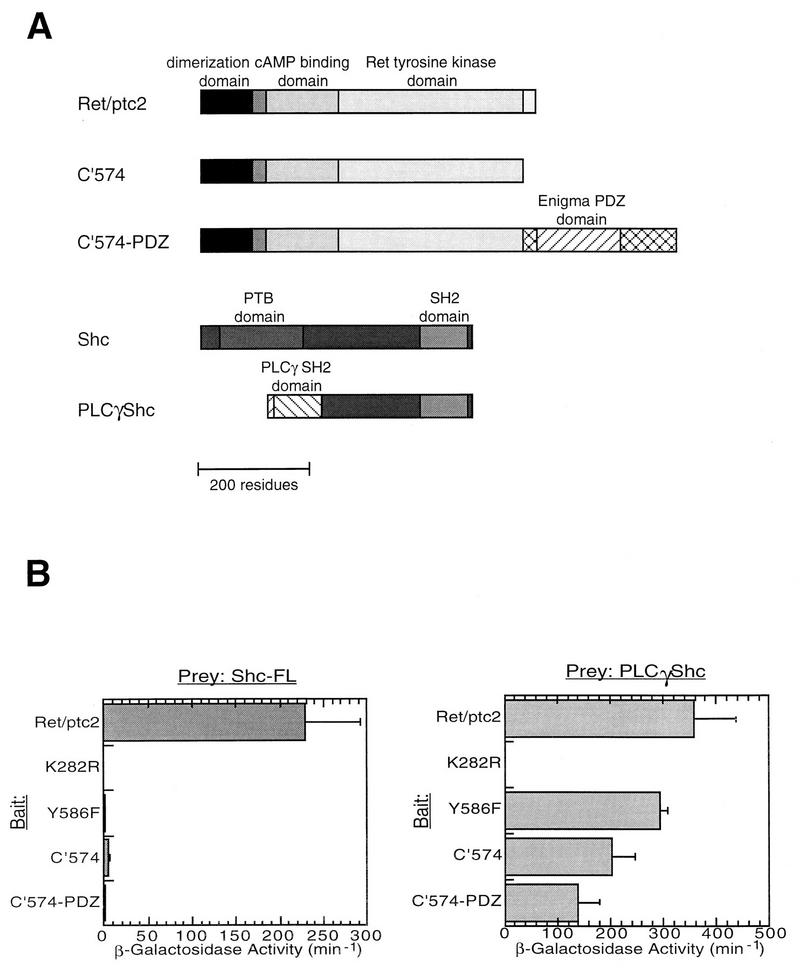
FIG. 6.
Characterization of interactions between Ret/ptc2 and Shc or PLCγ-Shc. The yeast two-hybrid system was used to monitor interactions between various Ret/ptc2-LexA constructs and either Shc-VP16 or PLCγ-Shc-VP16 fusions. (A) Schematic representation of the fusion proteins constructed. C′574 was fused to the N-terminal 279 residues of Enigma to make C′574-PDZ. A fragment of the PLCγ sequence encoding the N-terminal SH2 domain was fused to the C-terminal two-thirds of Shc, replacing the Shc PTB domain (PLCγ-Shc). (B) β-Galactosidase activity of yeast cotransformed with the Ret/ptc2 and Shc constructs. Cultures of yeast cotransformed with plasmids expressing various Ret/ptc2-LexA and either Shc-VP16 or PLCγ-Shc-VP16 fusion proteins were assayed for β-galactosidase activity. Results shown are averages of units of activity from four solution assays (3), with error bars representing the standard deviations.
These chimeric proteins were first characterized in the two-hybrid system and then further characterized in transient transfections. Wild-type Shc interacted with Ret/ptc2 but failed to interact with any of the Ret/ptc2 forms lacking tyrosine 586, including the chimera C′574-PDZ (Fig. 6B, left). The PLCγ-Shc chimeric adapter protein bound to all forms of Ret/ptc2 except the unphosphorylated, kinase-inactive mutant K282R (Fig. 6B, right). This pattern of interaction was confirmed in mammalian cells when the same combinations of expression constructs were cotransfected into embryonic kidney 293 cells. Coexpression of Ret/ptc2 with Shc resulted in Shc tyrosine phosphorylation, while Ret/ptc2 constructs incapable of binding to Shc were also unable to phosphorylate Shc (Fig. 7). In contrast, C′574 and C′574-PDZ were both able to phosphorylate PLCγ-Shc.
FIG. 7.
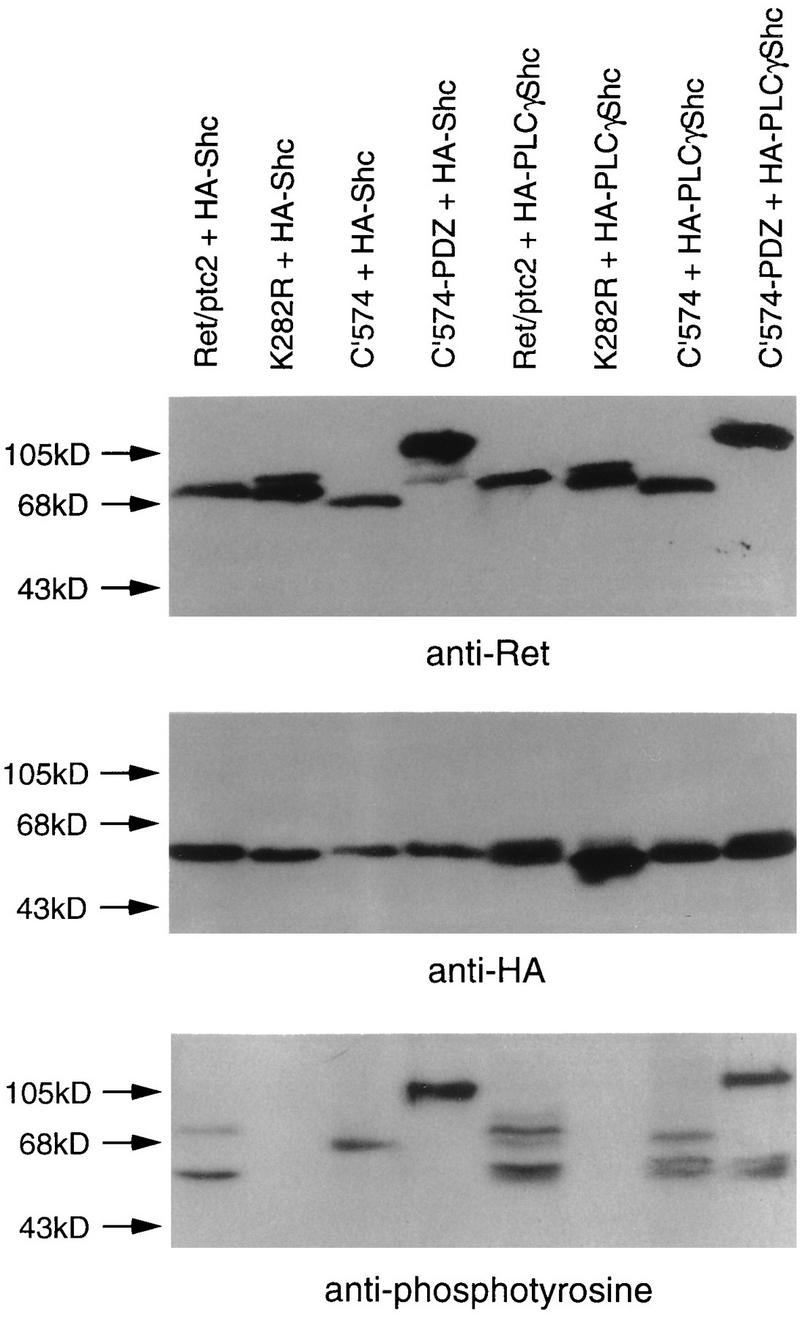
Phosphorylation of Shc and PLCγ-Shc by Ret/ptc2 constructs. Kidney 293 cells were cotransfected with plasmids expressing a form of Ret/ptc2 and either HA-tagged Shc (HA-Shc) or PLCγ-Shc (HA-PLCγShc). The Ret/ptc2 constructs expressed wild-type Ret/ptc2, a kinase-inactive point mutant (K282R), a C-terminal-truncation mutant (C′574), or a mutant in which the C terminus was replaced with the PDZ domain of Enigma (C′574-PDZ). Twenty-four hours after transfection, the cells were harvested, and lysates were subjected to SDS-PAGE. Proteins were then transferred to PVDF membranes and detected with either anti-Ret, anti-HA tag, or antiphosphotyrosine antibodies.
Rescue of the mitogenic activity of Ret/ptc2–C′574-PDZ, but not Ret/ptc2-C′574, by PLCγ-Shc.
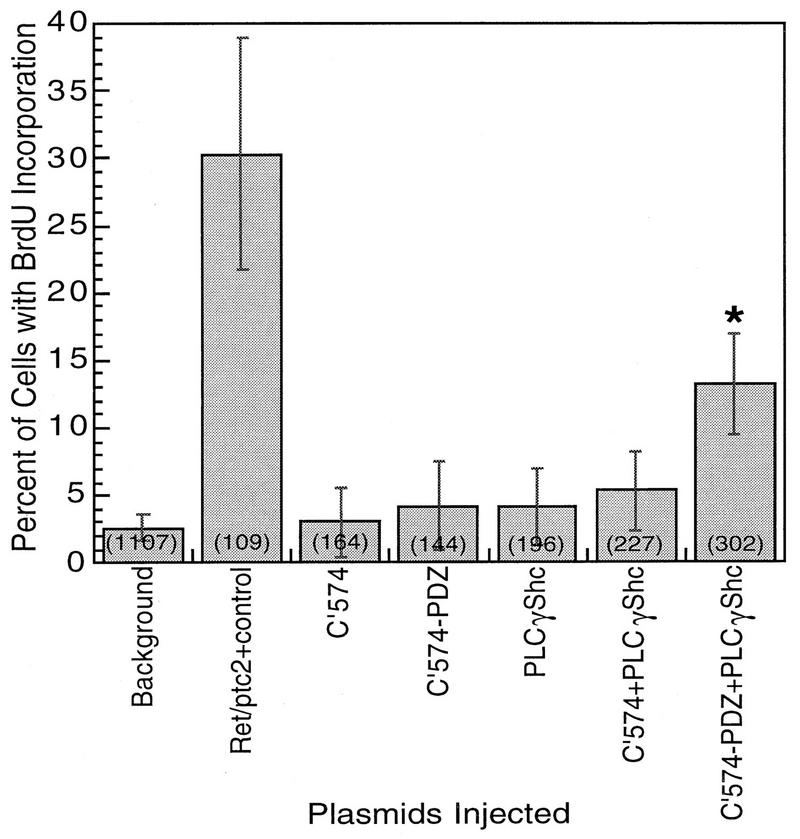
To test which of the interactions are required for Ret/ptc2 mitogenic signaling, the chimeric proteins were evaluated in the mitogenic microinjection assay. As shown previously (13), the mutant C′574 of Ret/ptc2, which does not bind to Enigma and Shc, was not mitogenically active (Fig. 8). Adding the Enigma PDZ domain to make C′574-PDZ failed to increase mitogenic activity (Fig. 8), despite the fact that this chimeric protein was subcellularly localized in the same distribution as that of Enigma and wild-type Ret/ptc2 (data not shown). PLCγ-Shc did not exhibit any mitogenic activity in this assay, and its interaction with C′574 was not sufficient to rescue the mitogenic activity of this nonlocalized form of Ret/ptc2. Only coexpression of PLCγ-Shc with C′574-PDZ resulted in significant stimulation of mitogenic activity (Fig. 8).
FIG. 8.
Mitogenic activity of Ret/ptc2 constructs coexpressed with PLCγ-Shc. Serum-starved mouse fibroblasts (10T1/2) were microinjected with combinations of expression plasmids. In each case, constructs were injected at 100 μg/ml; “background” represents uninjected cells. Cells were then assessed for entry into S phase by immunofluorescent detection of BrdU incorporation. The fraction of injected cells positive for BrdU incorporation is shown, with error bars displaying the 95% confidence intervals, which were calculated by using the standard error of proportion. The numbers in parentheses are the total numbers of injected cells. The asterisk indicates that cells coinjected with plasmids for C′574-PDZ and PLCγ-Shc were significantly above background in BrdU incorporation (P < 0.001).
Affinity precipitation of a Shc and Ret/ptc2 complex with GST-LIM2.
To demonstrate that a complex of a Ret/ptc2 dimer binding to both Shc and Enigma could form, affinity precipitation experiments were carried out with lysates from transfected 293 cells. The cells were cotransfected with constructs expressing HA-tagged Shc and either Ret/ptc2 or Ret/ptc2Δ(13-84). Both Ret/ptc2 and the form lacking the dimerization domain, Ret/ptc2Δ(13-84), bound to Enigma GST-LIM2 fusion protein (Fig. 9, anti-Ret). However, Shc was precipitated only in the presence of dimeric, wild-type Ret/ptc2 (Fig. 9, lower panel). This result demonstrates that a Ret/ptc2 dimer is capable of simultaneously binding to the LIM domain of Enigma and to Shc.
FIG. 9.
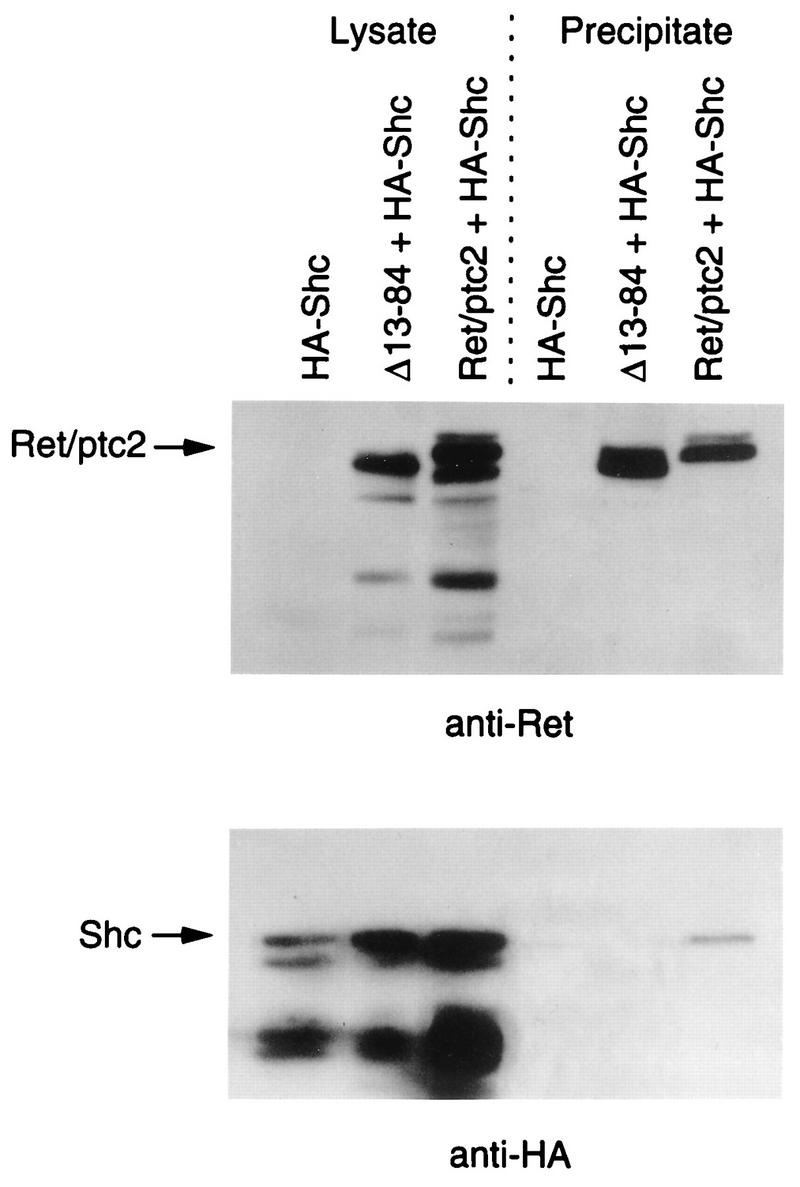
Characterization of the Ret/ptc2-Shc-Enigma complex. Lysates from 293 cells expressing HA-tagged Shc either alone, with wild-type Ret/ptc2, or Ret/ptc2Δ(13-84) were incubated with GST-LIM2 of Enigma bound to glutathione agarose. After extensive washing, the agarose beads were boiled in SDS sample buffer, and bound proteins were resolved by SDS-PAGE. Gels were run in parallel, blotted to PVDF membranes, and probed with anti-Ret or anti-HA antibodies. Lysate samples, run in the first three lanes, were approximately one-fourth of the total amount of lysate used in each incubation.
Effect of tyrosine 586 phosphorylation on Ret/ptc2 interaction with Enigma LIM2.
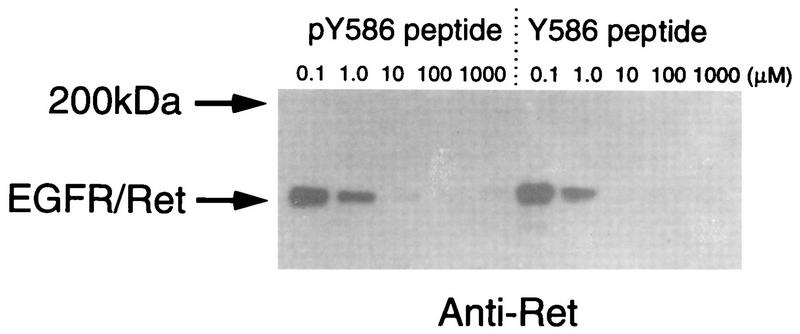
The PTB domain of Shc shows a strict binding preference for phosphorylated tyrosine motifs. To investigate the effect of tyrosine 586 phosphorylation on Enigma binding, peptide competition experiments were performed (Fig. 10). Lysates from cells expressing the chimeric EGFR-Ret receptor were incubated with glutathione agarose beads coated with the Enigma LIM2-GST fusion protein. For competition, the incubation was carried out in the presence of a 20-residue peptide corresponding to the sequence from positions 577 to 596 of Ret/ptc2. The peptide was added at various concentrations and was either phosphorylated (pY586) or not (Y586) at the tyrosine residue corresponding to 586. The phosphorylated and the unphosphorylated versions of the peptide were able to compete with EGFR-Ret binding to GST-LIM2 of Enigma to similar extents, suggesting that, unlike the PTB domain interaction, which requires phosphorylation at this position, the interaction with Enigma was not affected by phosphorylation.
FIG. 10.
Effects of competitor phospho- and dephosphopeptides on the interaction between Ret and Enigma. Lysates from NIH 3T3 cells expressing the EGFR-Ret chimeric receptor were incubated with GST-LIM2 of Enigma bound to glutathione agarose beads. The incubation was carried out in the presence of various concentrations of peptide containing the last 20 residues of Ret/ptc2, residues 577 to 596. The peptide was synthesized to contain either phosphotyrosine 586 (pY586) or tyrosine 586 (Y586). After incubation the beads were washed extensively, and bound EGFR-Ret was visualized by Western blotting with anti-Ret antibody.
DISCUSSION
The Ret/ptc oncogenes are important transforming proteins found in up to one-third of all papillary thyroid carcinomas (39). The causative role of Ret/ptc in cancers is underscored by the finding that targeted expression of the Ret/ptc1 oncogene in transgenic mice results in thyroid carcinomas (23, 45). We have investigated the protein interaction requirements for Ret/ptc2 mitogenic signaling. Guided by the initial result that Ret/ptc2 signals through the Ras pathway, the subcellular localization was determined to see if this non-membrane-spanning form of the c-Ret RTK was positioned to communicate with membrane-anchored Ras. Ret/ptc2 was localized to the plasma membrane via anchoring by Enigma. Furthermore, it was found that Shc was recruited to phosphorylated tyrosine 586 on Ret/ptc2 through binding of its PTB domain. Tyrosine 586 is absolutely required for the mitogenic activity of Ret/ptc2 (14), and because position 586 is also the site of Enigma binding, the relative importance of these interactions was addressed.
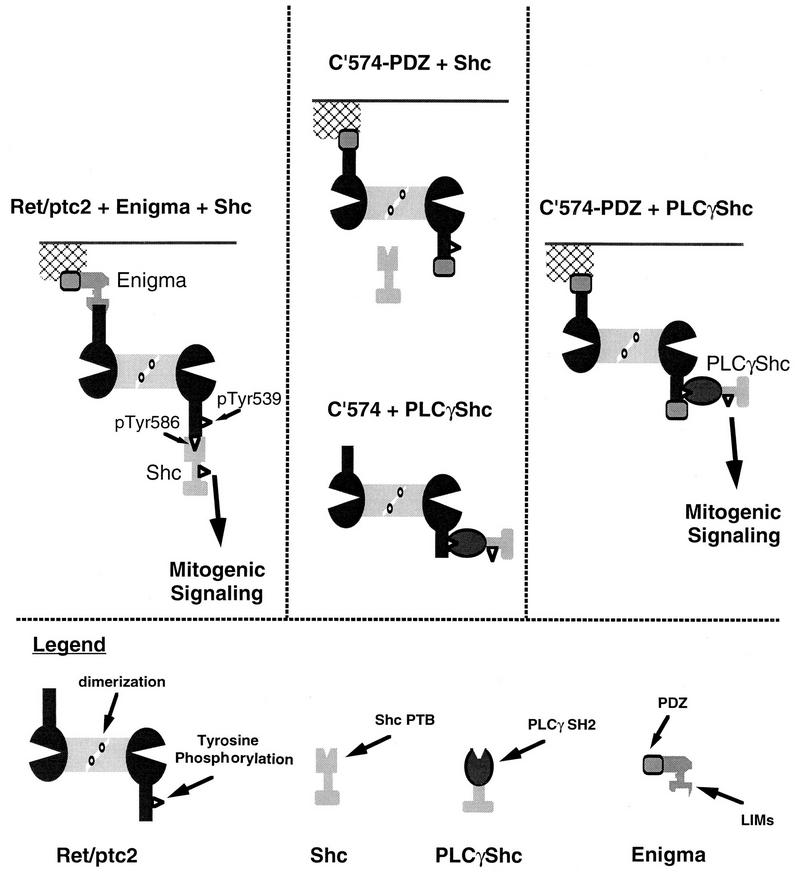
A novel approach employing chimeric forms of Ret/ptc2, Enigma, and Shc was devised to test whether Enigma localization or Shc interaction was important in Ret/ptc2 mitogenic signaling (Fig. 11). Mutants of Ret/ptc2 where the Shc docking site was absent were unable to phosphorylate Shc and were mitogenically inactive. Likewise, a deletion mutant of Ret/ptc2 that lacked an Enigma docking site was diffusely spread throughout the cytoplasm and also lacked mitogenic activity. When the Enigma docking site on Ret/ptc2 was replaced with the PDZ domain of Enigma, Ret/ptc2 was once again targeted to the cell edges, but this construct was unable to bind Shc and remained inactive. Mitogenic activity was restored only when this properly targeted form of Ret/ptc2 was coexpressed with a chimeric form of Shc, PLCγ-Shc, that was capable of interacting with it. From these results we conclude that both subcellular targeting by Enigma and interaction with, followed by phosphorylation of, Shc are required for Ret/ptc2 mitogenic signaling.
FIG. 11.
Proposed model for wild-type Ret/ptc2 mitogenic signaling. The left panel illustrates functions of Enigma and Shc in Ret/ptc2 mitogenic signaling. The center panel summarizes results indicating that functions of either Enigma or Shc alone are not sufficient for Ret/ptc2 activity. The right panel shows restoration of Ret/ptc2 mitogenic activity by reconstitution of both Enigma and Shc functions via chimeric molecules.
A similar requirement for both membrane targeting and tyrosine kinase activity has been demonstrated for cell transformation mediated by v-Src, an oncogenic form of the c-Src nonreceptor tyrosine kinase (10, 25). Subcellular localization of v-Src is dependent on myristoylation at the N terminus. The two functions of subcellular localization and tyrosine kinase signal transduction in Ret/ptc2 are mediated via a single tyrosine-containing sequence located in the C terminus distal to the kinase domain. Dimerization via RIα is proposed to provide a single Ret/ptc2 complex with the ability to interact with two adapter proteins that provide these two necessary functions.
Forms of human papillary thyroid carcinoma oncogenes have been characterized where the tyrosine kinase domain of c-Ret is fused to portions of three other proteins. In the case of Ret/ptc2, the fusion partner is the N-terminal portion of the type Iα regulatory subunit of cAMP-dependent protein kinase (5), which contains a dimerization domain required for mitogenic activity (14). Ret/ptc1 contains the same C-terminal portion of Ret, but it is fused to the N terminus of the H4 gene product (18). Dimerization, in this case mediated by a leucine zipper in H4, is required for the oncogenic activity of Ret/ptc1 (52). The potential of Ret/ptc3 to form dimers has not yet been investigated, but the ELE1 protein involved in the fusion contains a potential coiled-coil motif (46). Since mitogenic activity of the c-Ret kinase can be activated by fusion to different dimerization domains, it is likely that the only role of these fusion events is to provide constitutive dimerization. Subcellular targeting by the C terminus of the Ret tyrosine kinase domain is consistent with the conserved mitogenic potential of this diverse group of Ret/ptc proteins. For many RTKs, it has been demonstrated that ligand-induced dimerization is responsible for catalytic activation and possible trans-phosphorylation, but the present results suggest an additional reason for dimerization. Because the sites of Shc and Enigma docking overlap, an oligomer is required to form a signaling complex where both Shc and Enigma are bound at the same time (Fig. 11). In support of this hypothesis, expression of the C terminus of Enigma, which is capable of binding to but not localizing Ret/ptc2, blocked mitogenic signaling by Ret/ptc2 (13).
In much the same way that Ret/ptc2 is targeted by Enigma and its PDZ domain, the c-Ret receptor tyrosine kinase is likely to be anchored in specific signaling zones along the plasma membrane. The PDZ domain is a recently defined protein-protein interaction motif of approximately 100 amino acids which has been found in a number of proteins, many of which localize near the plasma membrane (20, 48). While the specific target for the Enigma PDZ domain is currently under investigation, the roles of other proteins which contain PDZ domains are being elucidated. The most similar in function to Enigma is LIN-7, a cell junction-associated protein in Caenorhabditis elegans that is required for proper localization of the LET-23 receptor (EGFR) tyrosine kinase (50). Other examples include SAP102 and PSD-95, PDZ domain-containing proteins that link N-methyl-d-aspartic acid receptors to the submembranous cytomatrix and other proteins at postsynaptic densities (27, 36), and GRIP, a PDZ domain protein which may tether glutamate receptors in much the same fashion (11). In this study, the potential requirement of Enigma for proper c-Ret function became evident with Ret/ptc2, a form of c-Ret which is not constrained in the plasma membrane.
ACKNOWLEDGMENTS
We thank Tom Deerinck and Mark Ellisman for expert advice and assistance in confocal microscopy. In addition, we thank P. Paolo Di Fiore, European Institute of Oncology, Milan, Italy, for cells expressing the EGFR-Ret chimera and M. Pierotti, Institute Nationale Tumori, Milan, Italy, for the Ret/ptc2 cDNA.
This research was supported in part by U.S. Army grant AIBS1762 (to S.S.T.) and National Institutes of Health grant DK13149 (to G.N.G.). K.D. was supported by the Markey Charitable Trust as a fellow and by NIH Training Grant NCI T32 CA09523.
REFERENCES
- 1.Asai N, Murakami H, Iwashita T, Takahashi M. A mutation at tyrosine 1062 in MEN2A-Ret and MEN2B-Ret impairs their transforming activity and association with Shc adaptor proteins. J Biol Chem. 1996;271:17644–17649. doi: 10.1074/jbc.271.30.17644. [DOI] [PubMed] [Google Scholar]
- 2.Attie T, Pelet A, Sarda P, Edery P, Eng C, Mulligan L M, Ponder B A J, Munnich A, Lyonnet S. A 7bp deletion of the RET proto-oncogene mutations in familial Hirschsprung’s disease. Hum Mol Genet. 1994;3:1439–1440. doi: 10.1093/hmg/3.8.1439. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 4.Bongarzone I, Butti M G, Coronelli S, Borrello M G, Santoro M, Mondellini P, Pilotti S, Fusco A, Della Porta G, Pierotti M A. Frequent activation of ret protooncogene by fusion with a new activating gene in papillary thyroid carcinomas. Cancer Res. 1994;54:2979–2985. [PubMed] [Google Scholar]
- 5.Bongarzone I, Monzini N, Borrello M G, Carcano C, Ferraresi G, Arighi E, Mondellini P, Della Porta G, Pierotti M A. Molecular characterization of a thyroid tumor-specific transforming sequence formed by the fusion of ret tyrosine kinase and the regulatory subunit RIα of cyclic AMP-dependent protein kinase A. Mol Cell Biol. 1993;13:358–366. doi: 10.1128/mcb.13.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrello M G, Alberti L, Arighi E, Bongarzone I, Battistini C, Bardelli A, Pasini B, Piutti C, Rizzetti M G, Mondellini P, Radice M T, Pierotti M A. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cγ. Mol Cell Biol. 1996;16:2151–2163. doi: 10.1128/mcb.16.5.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buj-Bello A, Adu J, Pinon L G, Horton A, Thompson J, Rosenthal A, Chinchetru M, Buchman V L, Davies A M. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature. 1997;387:721–724. doi: 10.1038/42729. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Szeberenyi J, Cooper G M. Effect of a dominant inhibitory HA-ras mutation on mitogenic signal transduction in NIH 3T3 cells. Mol Cell Biol. 1990;10:5314–5323. doi: 10.1128/mcb.10.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross F R, Garber E A, Pellman D, Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984;4:1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H, O’Brien R J, Fung E T, Lanahan A A, Worley P F, Huganir R L. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 12.Durbec P, Marcos-Gutierrez C V, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Hannu S, Pachnis V. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 13.Durick K, Wu R Y, Gill G N, Taylor S S. Mitogenic signaling by Ret/ptc2 requires association with Enigma via a LIM domain. J Biol Chem. 1996;271:12691–12694. doi: 10.1074/jbc.271.22.12691. [DOI] [PubMed] [Google Scholar]
- 14.Durick K, Yao V J, Borrello M G, Bongarzone I, Pierotti M A, Taylor S S. Tyrosines outside the kinase core and dimerization are required for the mitogenic activity of RET/ptc2. J Biol Chem. 1995;270:24642–24645. doi: 10.1074/jbc.270.42.24642. [DOI] [PubMed] [Google Scholar]
- 15.Edery P, Lyonnet S, Mulligan L M, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fekete C, Ponder B A, Munnich A. Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- 16.Egan S E, Giddings B W, Brooks M W, Buday L, Sizeland A M, Weinberg R A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 17.Feig L A, Cooper G M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grieco M, Santoro M, Berlingieri M T, Melillo R M, Donghi R, Bongarzone I, Pierotti M A, Della Porta G, Fusco A, Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60:557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson T A, He W, Craparo A, Schaub C D, O’Neill T J. Phosphotyrosine-dependent interaction of SHC and insulin receptor substrate 1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol Cell Biol. 1995;15:2500–2508. doi: 10.1128/mcb.15.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison S C. Peptide-surface association: the case of PDZ and PTB domains. Cell. 1996;86:341–343. doi: 10.1016/s0092-8674(00)80105-1. [DOI] [PubMed] [Google Scholar]
- 21.Henderson C E, Phillips H S, Pollock R A, Davies A M, Lemeulle C, Armanini M, Simpson L C, Moffet B, Vandlen R A, Koliatsos V E, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 22.Huang L J, Durick K, Weiner J A, Chun J, Taylor S S. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem. 1997;272:8057–8064. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- 23.Jhiang S M, Sagartz J E, Tong Q, Parker-Thornburg J, Capen C C, Cho J Y, Xing S, Ledent C. Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology. 1996;137:375–378. doi: 10.1210/endo.137.1.8536638. [DOI] [PubMed] [Google Scholar]
- 24.Jing S, Wen D, Yu Y, Hoist P L, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis J, Hu S, Altrock B W, Fox G M. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 25.Kamps M P, Buss J E, Sefton B M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci USA. 1985;82:4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R D, Sherman D, Ho W H, Stone D, Bennett G L, Moffat B, Vandlen R, Simmons L, Gu Q, Hongo J A, Devaux B, Poulsen K, Armanini M, Nozaki C, Asai N, Goddard A, Phillips H, Henderson C E, Takahashi M, Rosenthal A. A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature. 1997;387:717–721. doi: 10.1038/42722. [DOI] [PubMed] [Google Scholar]
- 27.Kornau H C, Schenker L T, Kennedy M B, Seeburg P H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 28.Kotzbauer P T, Lampe P A, Heuckeroth R O, Golden J P, Creedon D J, Johnson E M, Milbrandt J. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- 29.Kunkel T A, Benebek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 30.Kupperman E, Wen W, Meinkoth J L. Inhibition of thyrotropin-stimulated DNA synthesis by microinjection of inhibitors of cellular Ras and cyclic AMP-dependent protein kinase. Mol Cell Biol. 1993;13:4477–4484. doi: 10.1128/mcb.13.8.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laminet A A, Apell G, Conroy L, Kavanaugh W M. Affinity, specificity, and kinetics of the interaction of the SHC phosphotyrosine binding domain with asparagine-X-X-phosphotyrosine motifs of growth factor receptors. J Biol Chem. 1996;271:264–269. doi: 10.1074/jbc.271.1.264. [DOI] [PubMed] [Google Scholar]
- 32.Lanzi C, Borello M G, Bongarzone I, Migliazza A, Fusco A, Grieco M, Santoro M, Gambetta R A, Zunino F, Della Porta G, Pierotti M A. Identification of the product of two oncogenic rearranged forms of the RET proto-oncogene in papillary thyroid carcinomas. Oncogene. 1992;7:2189–2194. [PubMed] [Google Scholar]
- 33.Lin L H, Doherty D H, Lile J D, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 34.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 35.Moore M W, Klein R D, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt L F, Ryan A M, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 36.Muller B M, Kistner U, Kindler S, Chung W J, Kuhlendahl S, Fenster S D, Lau L F, Veh R W, Huganir R L, Gundelfinger E D. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–265. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- 37.Mulligan L M, Eng C, Healey C S, Clayton D, Kwok J B, Gardner E, Ponder M A, Frilling A, Jackson C E, Lehnert H, Neumann H, Thibodeau S, Ponder B. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet. 1994;6:70–74. doi: 10.1038/ng0194-70. [DOI] [PubMed] [Google Scholar]
- 38.Mulligan L M, Kwok J B J, Healey C S, Elsdon M J, Eng C, Gardner E, Love D R, Mole S E, Moore J K, Papi L, Ponder M A, Telenius H, Tunnacliffe A, Ponder B A J. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 39.Pasini B, Ceccherini I, Giovanni R. Ret mutations in human disease. Trends Genet. 1996;12:138–144. doi: 10.1016/0168-9525(96)10012-3. [DOI] [PubMed] [Google Scholar]
- 40.Pichel J G, Shen L, Sheng H Z, Granholm A, Drago J, Grinberg A, Lee E J, Huang S P, Saarma M, Hoffer B J, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 41.Pierotti M A, Santoro M, Jenkins R B, Sozzi G, Bongarzone I, Grieco M, Monzini N, Miozzo M, Herrmann M A, Fusco A, Hay I D, Porta G D, Vecchio G. Characterization of an inversion on the long arm of chromosome 10 juxtaposing D10S170 and RET and creating the oncogenic sequence RET/PTC. Proc Natl Acad Sci USA. 1992;89:1616–1620. doi: 10.1073/pnas.89.5.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pumiglia K, Chow Y H, Fabian J, Morrison D, Decker S, Jove R. Raf-1 N-terminal sequences necessary for Ras-Raf interaction and signal transduction. Mol Cell Biol. 1995;15:398–406. doi: 10.1128/mcb.15.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kaariainen H, Martucciello G. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez M P, Silos-Santiago I, Frisen J, He B, Lira S A, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 45.Santoro M, Chiappetta G, Cerrato A, Salvatore D, Zhang L, Manzo G, Picone A, Portella G, Santelli G, Vecchio G, Fusco A. Development of thyroid papillary carcinomas secondary to tissue-specific expression of the RET/PTC1 oncogene in transgenic mice. Oncogene. 1996;12:1821–1826. [PubMed] [Google Scholar]
- 46.Santoro M, Dathan N A, Berlingieri M T, Bongarzone I, Paulin C, Grieco M, Pierotti M A, Vecchio G, Fusco A. Molecular characterization of RET/PTC3; a novel rearranged version of the RET proto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–516. [PubMed] [Google Scholar]
- 47.Santoro M, Wong W T, Aroca P, Santos E, Mat̆os̆kovā B, Grieco M, Fusco A, Di Fiore P P. An epidermal growth factor receptor/ret chimera generates mitogenic and transforming signals: evidence for a ret-specific signaling pathway. Mol Cell Biol. 1994;14:663–675. doi: 10.1128/mcb.14.1.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saras J, Heldin C H. PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem Sci. 1996;21:455–458. doi: 10.1016/s0968-0004(96)30044-3. [DOI] [PubMed] [Google Scholar]
- 49.Schuchardt A, D’Agati V, Larsson-Plomberg L, Constantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 50.Simske J S, Kaech S M, Harp S A, Kim S K. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell. 1996;85:195–204. doi: 10.1016/s0092-8674(00)81096-x. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi M, Ritz J, Cooper G M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 52.Tong Q, Xing S, Jhiang S M. Leucine zipper-mediated dimerization is essential for the PTC1 oncogenic activity. J Biol Chem. 1997;272:9043–9047. doi: 10.1074/jbc.272.14.9043. [DOI] [PubMed] [Google Scholar]
- 53.Treanor J J S, Goodman L, de Sauvage F, Stone D M, Poulsen K T, Beck C D, Gray C, Armanini M P, Pollock R A, Hefti F, Phillips H S, Goddard A, Moore M W, Buj-Bello A, Davies A M, Asai N, Takahashi M, Vandlen R, Henderson C E, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 54.Trub T, Choi W E, Wolf G, Ottinger E, Chen Y, Weiss M, Shoelson S E. Specificity of the PTB domain of Shc for β turn-forming pentapeptide motifs amino-terminal to phosphotyrosine. J Biol Chem. 1995;270:18205–18208. doi: 10.1074/jbc.270.31.18205. [DOI] [PubMed] [Google Scholar]
- 55.van der Geer P, Hunter T, Lindberg R A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 56.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 57.Wu R Y, Durick K, Songyang Z, Cantley L C, Taylor S S, Gill G N. Specificity of LIM domain interactions with receptor tyrosine kinases. J Biol Chem. 1996;271:15934–15941. doi: 10.1074/jbc.271.27.15934. [DOI] [PubMed] [Google Scholar]
- 58.Wu R Y, Gill G N. LIM domain recognition of a tyrosine-containing tight turn. J Biol Chem. 1994;269:25085–25090. [PubMed] [Google Scholar]