Abstract
The transcription factor E2F1 is believed to be involved in the regulated expression of the DNA replication genes. To gain insights into the transcriptional activation function of E2F1, we looked for proteins in HeLa nuclear extracts that bind to the activation domain of E2F1. Here we show that DDB, a putative DNA repair protein, associates with the activation domain of E2F1. DDB was identified as a heterodimeric protein (48 and 127 kDa) that binds to UV-damaged DNA. We show that the UV-damaged-DNA binding activity from HeLa nuclear extracts can associate with the activation domain of E2F1. Moreover, the 48-kDa subunit of DDB, synthesized in vitro, binds to a fusion protein of E2F1 depending on the C-terminal activation domain. The interaction between DDB and E2F1 can also be detected by coimmunoprecipitation experiments. Immunoprecipitation of an epitope-tagged DDB from cell extracts resulted in the coprecipitation of E2F1. In a reciprocal experiment, immunoprecipitates of E2F1 were found to contain DDB. Fractionation of HeLa nuclear extracts also revealed a significant overlap in the elution profiles of E2F1 and DDB. For instance, DDB, which does not bind to the E2F sites, was enriched in the high-salt fractions containing E2F1 during chromatography through an E2F-specific DNA affinity column. We also observed evidence for a functional interaction between DDB and E2F1 in living cells. For instance, expression of DDB specifically stimulated E2F1-activated transcription. In addition, the transcriptional activation function of a heterologous transcription factor containing the activation domain of E2F1 was stimulated by coexpression of DDB. Moreover, DDB expression could overcome the retinoblastoma protein (Rb)-mediated inhibition of E2F1-activated transcription. The results suggest that this damaged-DNA binding protein can function as a transcriptional partner of E2F1. We speculate that the damaged-DNA binding function of DDB, besides repair, might serve as a negative regulator of E2F1-activated transcription, as damaged DNA will sequester DDB and make it unavailable for E2F1. Furthermore, the binding of DDB to damaged DNA might be involved in downregulating the replication genes during growth arrest induced by damaged DNA.
E2F1 is the most-studied member of the E2F family of transcription factors. E2F1 binds the consensus E2F site (TTTCGCGC) as a heterodimer in conjunction with DP1, and it stimulates transcription both in vitro and in vivo (3, 13, 20–22, 31). Several genes that are essential for DNA replication and S-phase entry have been shown to be transcriptionally activated by overexpression of E2F1. Included are genes expressing dihydrofolate reductase (4, 26, 56), ribonucleotide reductase, PCNA, DNA polymerase α, thymidine kinase, cyclin E, cyclin A, and E2F1, as well as cdc2 (8, 42, 44). This is consistent with the observation that overexpression of E2F1 induces quiescent cells to enter S phase (2, 30).
The activity and levels of E2F1 are regulated very tightly during the progression of the cell cycle. Expression of E2F1 increases late in G1 phase (56). It has been shown that the E2F sites in the E2F1 promoter are responsible for its cell cycle-regulated expression (26, 29). The activity of E2F1 is regulated by cell cycle-regulatory proteins such as the retinoblastoma protein (Rb) and cyclin A. The transcriptional activation domain of E2F1 at the C-terminal region contains a binding site for the Rb tumor suppressor protein (21, 31). E2F1 was cloned based on its ability to interact with Rb (21, 31). It is generally believed that binding of Rb to the C-terminal activation domain of E2F1 converts an activator to a repressor (20, 54, 65, 66). Both in vitro and in vivo studies have confirmed the notion that Rb binding leads to a reduction in E2F1-activated transcription (1, 13, 24). The N-terminal region of E2F1 contains a consensus cyclin A-binding motif (37, 38, 67). It has been shown that, in vitro, binding of cyclin A-cdk2 to E2F1 results in the phosphorylation of the DP1 subunit (13). DP1 phosphorylated in this manner is unable to remain associated with E2F1, which leads to a loss in the DNA binding activity of E2F1 (13, 37, 38, 67). The binding of cyclin A-cdk2 to E2F1 is important for progression through S phase, as mutation in the cyclin A-binding site causes an arrest in S phase followed by apoptosis (38).
The elaborate regulatory mechanisms imposed upon E2F1 emphasize the importance of E2F1 and related factors in cell cycle progression. Because E2F1 is a transcription factor, it is likely that it exerts most of its biological function by altering the expression of target genes. Therefore, an analysis of the protein partners that participate in E2F1-activated transcription will be important in understanding the molecular mechanism underlying the cell cycle-regulatory function of E2F1. It is noteworthy that in several E2F1-activated genes, the E2F-binding sites overlap with the transcription initiation site. It is possible that in these circumstances E2F1 acts as a transcriptional-initiator protein. Studies on the transcription activation domain of E2F1 indicated that it could interact with the basal transcription factor TATA-binding protein in vitro (18). Studies by the same group of researchers also provided indirect evidence for a role of the transcriptional-coactivator protein p300 in the mechanism by which E2F1 activates transcription (60).
We observed that the UV-damaged-DNA binding (DDB) protein associates with the activation domain of E2F1. DDB was originally identified as a cellular activity that binds UV-damaged DNA (7, 27, 33, 50, 61). DDB activity was also found to be missing in cells from a subset (20%) of xeroderma pigmentosum complementation group E (XP-E) patients (7). The DDB activity is associated with a heterodimer of two polypeptides, which migrate in sodium dodecyl sulfate (SDS)-polyacrylamide gels as 41-kDa (p48DDB) and 127-kDa (p125DDB) species (12, 28, 33, 43). Moreover, microinjection of the DDB proteins complements the repair deficiencies in XP-E cell strains lacking the DDB activity (34). Interestingly, p125DDB was also cloned by yeast two-hybrid screening as a protein that interacts with the hepatitis B virus X protein, which is a potent activator of transcription (40). However, a role for DDB in hepatitis B virus X protein-mediated transcriptional activation has yet to be established.
Here we show that the DDB activity in HeLa cell nuclear extracts can bind to the activation domain of E2F1. During fractionation of the extracts through ion-exchange columns and DNA affinity columns, a significant portion of E2F1 copurifies with the DDB activity. Using recombinant proteins, we show that the p48DDB subunit efficiently interacts with the activation domain of E2F1. We also provide evidence that E2F1 coimmunoprecipitates with p48DDB. In transient transfection assays, expression of DDB (the heterodimer of p48DDB and p125DDB) specifically stimulates E2F1-activated transcription. The transactivation function of a Gal4 fusion protein containing the activation domain of E2F1 was also stimulated by the expression of DDB. Moreover, coexpression of DDB overcomes Rb repression of E2F1-activated transcription. These results are consistent with the notion that DDB is a part of the mechanism by which E2F1 activates transcription.
MATERIALS AND METHODS
Cell culture.
HeLa cells were grown in suspension in spinner bottles (Bellco). Cell volume was doubled when cell density reached 106/ml. All other cells were grown on 10-cm dishes in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum in a 5% CO2-supplemented atmosphere.
Binding of HeLa nuclear proteins to GST fusion proteins containing the activation domain of E2F1.
HeLa cell nuclear extracts were prepared according to a previously described procedure (9). After dialysis for 12 to 16 h, the extracts were subjected to centrifugation at 100,000 × g for 60 min. The supernatant was used for binding experiments. Thirty milliliters of extracts (200 mg) were incubated with 0.5 ml of glutathione (GSH)-Sepharose beads containing 5 mg of glutathione S-transferase (GST) or a GST fusion protein containing the C-terminal activation domain of E2F1. The incubation was carried out on a nutator for 30 min at 4°C. The beads were then collected by centrifugation. The beads were washed extensively with buffer containing 150 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 0.5% Nonidet P-40 (NP-40). The bound proteins were eluted with the same buffer containing 1% SDS.
Immunoexpression screening of cDNA library.
A Uni-ZAP human primary fibroblast cDNA library [oligo(dT) primed] was screened by using a polyclonal antiserum according to a previously described procedure (50a).
Assay of the E2F-binding and DDB activity.
E2F-DNA binding assays were carried out as described previously (55). DDB assays were carried out essentially by the method of Hwang and Chu (27). Briefly, a pyrimidine-rich, 148-bp DNA fragment (HindIII/PvuII) of the bacterial chloramphenicol gene was cut out for use as a probe. This fragment was labeled with [α-32P]dATP by using the Klenow fragment of DNA polymerase I, and then it was subjected to UV irradiation at 10,000 J/m2. Samples were then assayed for DDB activity by electrophoretic mobility shift assay. Reaction mixtures consisted of buffer U (12 mM HEPES [pH 7.9], 60 mM KCl, 5 mM MgCl2, 0.6 mM EDTA, 1 mM dithiothreitol [DTT], 12% glycerol) with 0.2 ng of the probe (described above) and 1 μg of salmon sperm DNA as a nonspecific competitor in a total volume of 30 μl. Competitions were performed with 20 ng of unlabeled chloramphenicol acetyltransferase (CAT)-DNA fragment, either untreated or treated with UV radiation (10,000 J/m2). Reaction mixtures were incubated for 20 min at room temperature. Eight microliters was then loaded onto a 4% nondenaturing polyacrylamide gel in TGE buffer (50 mM Tris [pH 8.5], 380 mM glycine, 2 mM EDTA) and resolved for 2.5 h at 300 V at 4°C.
Fractionation of HeLa nuclear extracts.
Nuclear extract was prepared from HeLa cells as described previously (68). Four hundred milligrams of extract was fractionated on a 40-ml heparin-Sepharose column (Sigma), equilibrated in buffer HE (20 mM HEPES [pH 7.9], 0.2 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 0.1 mM EDTA, 10% glycerol) containing 0.1 M KCl. The column was then washed in buffer HE containing 0.1 M KCl and was eluted first in a 0.25 M KCl step, followed by a 10-bed volume gradient from 0.25 to 0.75 M KCl in buffer HE. Fractions active in the E2F-DNA binding assay were pooled, dialyzed against buffer QE (20 mM Tris [pH 7.5], 0.2 mM DTT, 0.1 mM PMSF, 0.1 mM EDTA, 10% glycerol, 0.1% NP-40) containing 0.1 M KCl, and subjected to Q-Sepharose chromatography. The Q-Sepharose column was eluted in a 10-bed volume buffer QE gradient (0.1 to 0.55 M KCl). Fractions were analyzed for E2F activity, and peak fractions were pooled and dialyzed against buffer AE (20 mM HEPES [pH 7.9], 0.2 mM DTT, 0.1 mM PMSF, 0.1 mM EDTA, 10% glycerol, 0.1% NP-40) containing 0.1 M KCl. E2F and mutant DNA affinity columns were prepared according to the method of Chellappan et al. (6). One-milliliter affinity columns were loaded in the presence of 50 μg of salmon sperm DNA to prevent binding of nonspecific DNA-binding proteins. Following washing in buffer AE containing 0.1 M KCl, the columns were eluted in steps of 0.3 and 0.6 M KCl in buffer AE.
Recombinant plasmids.
pGEX-E2F1(356-437) was created by PCR amplification of E2F1 cDNA encoding residues 356 to 437. The fragment was cloned into the pGEX-2T vector (Pharmacia) by using the BamHI and EcoRI restriction site-engineered PCR product. PGEX-E2F1(1-437), pGEX-E2F1(1-417), and pGEX-E2F1(1-363) were created by subcloning the inserts from pBS(RSV)GAL4-E2F1(1-437), -(1-417), and -(1-363), respectively (15). Inserts were cut at the 3′ end with XbaI, blunt ended, and then cut at the 5′ end with BamHI. The inserts were then cloned into pGEX5X-3 cut with BamHI and SmaI. pT7-125 and pT7-48 were created by PCR amplification of p125 and p48 cDNA (kind gifts of G. Chu and S. Linn, respectively). KpnI (5′) and XbaI (3′) restriction sites and T7 tag (5′) sequences (57) were engineered into the primers to facilitate cloning. Sequences of primers are as follows: p125 upstream primer, ggggtaccaccatggctagcatgactggtggacagcaaatgggtatgtcgtacaactacgtg; p48 upstream primer, ggggtaccaccatggctagcatgactggtggacagcaaatgggtatggctcccaagaaacgc; p125 downstream primer, ggtctagaggatccgagttagctcct; p48 downstream primer, ggtctagacttccgtgctctggcttc. PCR fragments were then cloned into pCDNA3 (Invitrogen) cut with KpnI and XbaI.
Immunoprecipitation and Western blot.
Cells were harvested after DNA transfection. The harvested cells were washed twice with phosphate-buffered saline, and were suspended in lysis buffer (60 μl for cells from one 100-mm-diameter dish) that contained 20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 0.1% NP-40, 1 mM DTT, 0.5 mM PMSF, and 10% glycerol. After incubation at 4°C for 30 min, the lysates were centrifuged at 13,000 × g for 10 min. The supernatants were used for the immunoprecipitation experiments.
Agarose-linked T7 antibody (Novagen) and protein A-Sepharose-bound hemagglutinin (HA) antibody (Santa Cruz) were further cross-linked by using dimethylpimelimidate (DMP). The antibody-containing beads were washed with 0.2 M Na-borate (pH 9.0) and resuspended in 1 ml of Na-borate containing 5 mg of DMP/ml. The incubation with DMP was carried out at room temperature for 30 min. The beads were then washed with 0.2 M ethanolamine (pH 8.0), followed by blocking with ethanolamine (0.2 M; pH 8.0) for 2 h at room temperature. The beads were then washed with buffer W (20 mM Tris-HCl [pH 7.8], 100 mM NaCl, 0.1% NP-40, 1 mM EDTA) and used in immunoprecipitation experiments.
Cell lysates (0.5 mg) were incubated with beads containing covalently bound antibodies for 2 h at 4°C. The beads were then collected by centrifugation. Precipitates were washed three times with 1 ml of buffer W. The bound proteins were subjected to Western blot analysis. Western blotting was performed by using anti-rabbit and anti-mouse Fab fragments conjugated to horseradish peroxidase (Amersham) and Pierce Supersignal detection reagents according to the manufacturers’ instructions.
DNA transfection and CAT assays.
Transient transfections were carried out by the calcium phosphate method as previously described (41). Twenty micrograms of DNA was used per 100-mm-diameter plate. Each experiment was controlled for transfection efficiency by including 1 μg of pCMV–β-galactosidase in each transfection and then normalizing for β-galactosidase activity. CAT assays were performed by the xylene extraction method of Seed and Sheen (53).
RESULTS
DDB activity associates with the activation domain of E2F1.
To identify proteins that associate with the activation domain of E2F1, a GST fusion protein containing the activation domain of E2F1 immobilized onto GSH-Sepharose beads was used to affinity select proteins from HeLa-cell nuclear extracts as described in Materials and Methods. Briefly, HeLa nuclear extracts (9) were dialyzed overnight followed by centrifugation at 100,000 × g for 1 h. The supernatant was incubated with beads containing the activation domain of E2F1. After an extensive wash, proteins bound to the beads were eluted with buffer containing 1% SDS. A comparison of proteins bound to GST and GST–E2F1(363-437) is shown in Fig. 1A. Three polypeptides of 350 to 400, 127, and 40 to 45 kDa (Fig. 1) were very reproducibly observed to be enriched by the activation domain of E2F1. We decided to analyze the 127-kDa polypeptide because the purified preparation of GST–E2F1(363-437) fusion protein often contained a contaminant protein of 45 kDa. SDS gel-purified 127-kDa polypeptide was used to raise rabbit antiserum. The antiserum recognized multiple polypeptides, including a 127-kDa polypeptide, in HeLa nuclear extract (data not shown). Expression screening of a human fibroblast cDNA library using an antiserum that recognized the 127-kDa polypeptide resulted in isolation of five different cDNA clones. Partial DNA sequencing revealed that three clones corresponded to uncharacterized genes, and one clone corresponded to fibulin (data not shown). Clone SH1 corresponded to the 127-kDa subunit of the DDB activity (12, 28). A comparison of DNA sequences from the 5′ and 3′ ends of clone SH1 with DDB1 (p125DDB) is shown in Fig. 1B. The 5′ end of clone SH1 corresponded to the coding region of p125DDB, whereas the 3′ end of SH1 corresponded to the 3′ end of p125DDB. Because that was the only clone that corresponded to a 127-kDa protein, we carried out a detailed analysis of the DDB activity for its ability to interact with E2F1.
FIG. 1.

(A) Identification of HeLa nuclear proteins that associate with the activation domain of E2F1. HeLa nuclear extracts (0.2 g/30 ml) were incubated with GSH-Sepharose beads (0.5 ml) containing 5 mg of either GST alone or GST fused with the activation domain of E2F1 [GST–E2F1(363-437)] as described in Materials and Methods. After a wash, the bound proteins were eluted with 0.5 ml of buffer containing 1% SDS. Fifty microliters of the eluted material was subjected to SDS-8% polyacrylamide gel electrophoresis followed by staining with silver reagents. Results from a typical experiment are shown. Bands indicated with arrows were seen more reproducibly than the others. (B) The 5′ and 3′ ends of clone SH1 are homologous to DDB1, which encodes the 127-kDa subunit of DDB(p125DDB). A comparison of DNA sequences from the 5′ and the 3′ ends of clone SH1 with the sequences of DDB1 cDNA is shown. Numbers represent nucleotide positions in DDB1 cDNA.
First, we assayed for the presence of the DDB activity in the E2F1 affinity-selected protein fractions from HeLa nuclear extract. A specific gel retardation assay for DDB described by Hwang and Chu was used (27). Briefly, a 148-bp DNA fragment derived from the bacterial CAT gene cDNA was labeled in the presence of [α-32P]dATP and Klenow enzyme. The labeled DNA was subjected to UV irradiation (10,000 J/m2). The UV-irradiated, labeled DNA was used as a probe in a gel retardation assay as described in Materials and Methods. GST-E2F1 bound to GSH-Sepharose beads was incubated with HeLa nuclear extracts. After a thorough wash of the beads with buffer containing 20 mM Tris · HCl, 150 mM NaCl, 0.5% NP-40, and 1 mM EDTA, the bound proteins were eluted with the same buffer containing 1 M KCl. An aliquot of the protein fraction bound to GST-E2F1 or GST alone was analyzed for the presence of DDB. We observed that a significant part of the DDB activity in the loading material (approximately 30%) was specifically retained by the GST-E2F1-containing beads (Fig. 2, left panel). The GST beads, on the other hand, did not retain any detectable amount of DDB. Consistent with a previous report (27), we observed two gel-shifted complexes of DDB, which were specifically competed by UV-irradiated DNA. To further investigate whether the binding involved the activation domain of E2F1, binding of DDB to C-terminal deletion mutants was compared. The activation domain of E2F1 has been mapped to residues 363 to 437 (21, 31). We observed that a deletion mutant lacking the activation domain [E2F1(1-363)] was severely impaired in binding to DDB (Fig. 2, left panel). Moreover, a fusion protein containing the activation domain alone was able to bind DDB (Fig. 2, right panel), suggesting that the activation domain is involved in binding to DDB.
FIG. 2.
DDB associates with the activation domain of E2F1. GST fusion proteins (200 μg) containing full-length E2F1 (GST-E2F1), a mutant E2F1 [GST–E2F1(1-363)] harboring a C-terminal deletion that deletes the activation domain, or a mutant E2F1 containing only the activation domain [GST–E2F1(363-437)] were immobilized onto GSH-Sepharose beads. Beads containing the E2F1 proteins were incubated with HeLa nuclear extracts (Nuc. Ext.; 0.1 mg) for 30 min at 4°C. Beads were collected by centrifugation and extensively washed with buffer containing 150 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 0.5% NP-40. The bound proteins were eluted with buffer containing 1 M KCl. An aliquot of the eluted material was subjected to a DDB assay as described in Materials and Methods. Where indicated, unlabeled probe DNA (20 ng) before (Non-UV Comp.) or after (UV Comp.) UV treatment was included in the reaction mixtures. Numbers above lanes in the right panel represent assays of subsequent elutions.
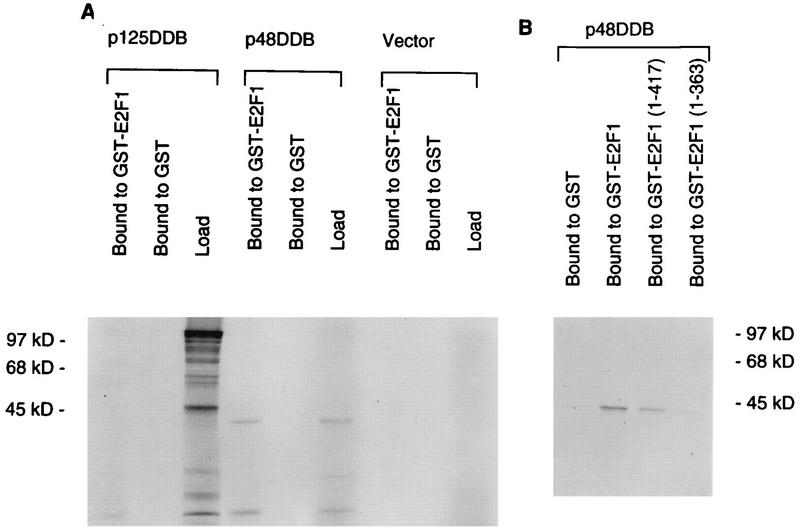
The DDB activity was shown to purify with two polypeptides: a 41-kDa (p48DDB) and a 127-kDa (p125DDB) polypeptide (12, 28, 33, 43). pCDNA3 clones containing the cDNAs corresponding to the two polypeptides were used to obtain 35S-labeled proteins in reticulocyte lysates. The 35S-labeled p48DDB and p125DDB proteins were used to assay for their ability to bind GST fusion proteins containing E2F1 or two C-terminal deletion mutants of E2F1 in GST pull-down assays. As can be seen in Fig. 3, the p48DDB subunit quantitatively bound to E2F1, whereas only a trace of p125DDB bound to E2F1 under the assay conditions. Moreover, it was clear that the binding of p48DDB to E2F1 depended upon the activation domain of E2F1, because the C-terminal mutant [E2F1(1-363)] lacking the activation domain failed to bind p48DDB (Fig. 3B).
FIG. 3.
The recombinant p48 subunit of DDB associates with the activation domain of E2F1. The p48 and the p125 subunits of DDB were transcribed and translated in vitro by using T7 RNA polymerase and reticulocyte lysate. The translation was performed in the presence of [35S]methionine. The translated products (2 μl) were incubated with 1 μg of the indicated GST fusion proteins containing full-length E2F1 (A) or C-terminal deletions (B). GSH-Sepharose beads (10 μl) were added to each of the incubation mixtures, followed by incubation on a nutator for 30 min at 4°C. The beads were collected by centrifugation and were extensively washed with buffer NETN. The bound proteins were eluted with SDS gel loading buffer and were subjected to SDS–10% polyacrylamide gel electrophoresis followed by autoradiography.
Because DDB activity, but not recombinant p125DDB, interacted with E2F1, we wanted to confirm that p48DDB is indeed a part of DDB activity and that it interacts with p125DDB. Bacterially produced recombinant p48DDB and p125DDB were inactive in binding to UV-irradiated DNA (data not shown). We investigated whether overexpression of p48DDB in mammalian cells increases DDB activity in the cell extracts. An HA epitope-tagged p48DDB was expressed in the NIH 3T3 mouse fibroblast cell line. These cells contain a low level of endogenous DDB. Extracts of the HA-p48DDB-transfected cells were assayed for DDB activity. A plasmid expressing another HA-tagged protein, HA-hnRNP K, was used as a control (39). Clearly, the extracts from the HA-p48DDB-transfected cells contained a much higher level of DDB activity (Fig. 4A). Moreover, addition of a monoclonal antibody against HA in the DNA-binding reaction produced a supershifted complex (Fig. 4A). To obtain further evidence, the extracts of the transfected cells were also immunoprecipitated with HA antibody. The immunoprecipitated proteins were released by incubation with the HA-tagged peptide and were subjected to DDB assay. The immunoprecipitates from HA-p48DDB-transfected cell extracts contained DDB activity (Fig. 4B). These results are consistent with those of a recent study by Nichols et al. (43), which demonstrated that p48DDB is a part of DDB activity. To confirm that p48DDB interacts with p125DDB, 35S-labeled p125DDB was incubated with bacterially produced T7 epitope-tagged (57) p48DDB. A monoclonal antibody against the T7 epitope coimmunoprecipitated 35S-labeled p125DDB from a mixture of T7-p48DDB and 35S-labeled p125DDB (Fig. 4C). These results are consistent with those of previous studies which indicated that p48DDB and p125DDB are components of DDB activity (12, 28, 33, 43). Taken together, these results also suggest that the p48DDB subunit of DDB interacts with the activation domain of E2F1. It is possible that p125DDB interacts with E2F1 through p48DDB, and that could be the reason why it was pulled down by GST-E2F1. However, we cannot rule out the possibility that a posttranslationally modified p125DDB also interacts with the activation domain of E2F1.
FIG. 4.
p48DDB is a component of DDB activity. (A) NIH 3T3 cells were transfected with plasmids expressing HA-tagged hnRNP K or p48DDB. Extracts (10 μg) of the transfected cells were subjected to a DDB assay as described in Materials and Methods. Where indicated, 1 μg of a monoclonal antibody against HA (HA-ab) or T7 (Control-ab) was added in the reaction mixture. (B) Extracts from NIH 3T3 cells transfected with HA-p48DDB or HA-hnRNP K expression plasmid were immunoprecipitated (IP) with a monoclonal antibody against HA. The immunoprecipitated proteins were released by incubation with 1 mg of HA-tagged peptide/ml. An aliquot of the released proteins was subjected to a DDB assay as described in Materials and Methods. (C) 35S-labeled p125DDB (without an epitope tag) was incubated with bacterially produced and partially purified T7-tagged p48DDB (see Materials and Methods). The incubation mixture was immunoprecipitated with a monoclonal antibody against T7 epitope (T7-ab). The immunoprecipitated material was analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography.
Evidence for an in vivo interaction between E2F1 and DDB.
To obtain evidence for an in vivo interaction between DDB and E2F1, we looked for a coimmunoprecipitation of E2F1 with p48DDB and p125DDB. The coimmunoprecipitation experiments were performed with U2OS osteosarcoma and C33A cervical-carcinoma cells. We obtained very similar results with the two cell lines (data not shown). The data from U2OS cells are shown. Cells were transfected with plasmids expressing HA-tagged E2F1 and T7 epitope-tagged subunits of DDB (p48DDB and p125DDB). The extracts of the transfected cells were subjected to immunoprecipitation with antibodies against the epitopes. In order to immunoprecipitate p48DDB and p125DDB, an agarose-linked monoclonal antibody against the T7 epitope (Novagen) that was further cross-linked as described in Materials and Methods was used. The immunoprecipitates were washed and eluted with SDS gel loading buffer as described in Materials and Methods. The eluted proteins were subjected to a Western blot assay to detect the presence of E2F1. The blot was probed with a monoclonal antibody against E2F1. As can be seen in Fig. 5 (left panel), the T7 epitope antibody specifically coprecipitated E2F1 from the extracts of cells expressing T7-DDB. Extracts of cells expressing both subunits of DDB consistently exhibited a higher level of coprecipitation of E2F1 than did extracts of cells expressing the individual subunits of DDB. This was also observed in the reciprocal experiment in which the extracts were immunoprecipitated with the HA antibody to precipitate HA-E2F1. The HA antibody was cross-linked to protein A-Sepharose beads as described in Materials and Methods. A Western blot assay of the HA immunoprecipitate with T7 antibody detected the presence of p48DDB only when the extracts from cells expressing both subunits were used (Fig. 5, right panel). In other experiments, we did detect coprecipitation of T7-p48DDB with HA antibody from cells expressing HA-E2F1 and T7-p48DDB; however, that was consistently much less compared to those extracts containing both subunits of DDB (data not shown). It is also noteworthy that cells expressing the T7 epitope-tagged p125DDB subunit alone exhibited detectable coprecipitation of E2F1 with the T7 antibody (Fig. 5, left panel). However, we were unable to detect coprecipitation of p125DDB with the HA antibody in a reproducible manner. It is likely that the interaction of p125DDB is sensitive to the washing steps during immunoprecipitation. Nevertheless, the coimmunoprecipitation of p48DDB with E2F1 provided evidence for an in vivo interaction between E2F1 and DDB.
FIG. 5.
Coimmunoprecipitation of DDB with E2F1. U2OS cells were transfected with a plasmid expressing HA-tagged E2F1 (4 μg) alone or in combination with plasmids expressing T7-tagged p48DDB (4 μg), p125DDB (8 μg), or a combination of the two. Extracts (0.5 mg) from transfected cells were immunoprecipitated (IP) with T7 antibody (T7-ab) covalently linked to agarose beads as described in Materials and Methods. The immunoprecipitates were analyzed by Western blotting with E2F1 antibody (E2F1-ab; left panel). Extracts (0.5 mg) were also immunoprecipitated with HA antibody (HA-ab) covalently linked to protein A-Sepharose beads. The precipitates obtained with HA antibody were analyzed for the presence of DDB by using a Western blot assay and the T7 antibody (right panel).
To obtain further evidence for an in vivo interaction between E2F1 and DDB activity, we investigated copurification of the two during fractionation of cell extracts. The procedure for purification of the E2F-DNA binding activity from HeLa cell nuclear extracts has been described before (68). Therefore, we fractionated HeLa nuclear extracts and looked for copurification of E2F1 and DDB. The column fractions were assayed for DDB and E2F1 or E2F-DNA binding activity. Extracts were subjected to heparin-agarose chromatography and Q-Sepharose chromatography, and the columns were eluted with KCl gradients (68). A significant part of the DDB activity coeluted with the E2F-DNA binding activity from both columns (data not shown). Approximately 35% of the total DDB activity in the HeLa nuclear extracts was enriched in the Q-Sepharose fractions containing the E2F-DNA binding activity (data not shown). The Q-Sepharose-purified E2F-containing fractions were subjected to an E2F-specific DNA affinity chromatography. E2Fs can be brought to a high degree of purity (approximately 1,000-fold purification) by using E2F site-containing DNA affinity columns (68). We generated two affinity resins: one containing an oligomeric wild-type E2F-cognate element, and another containing an oligomeric mutant E2F-cognate element. The monomeric wild-type sequence corresponded to 40 bp between −30 and −70 of the adenovirus E2 promoter (49), whereas the mutant contained substitutions of the CGCG sequences within the E2F consensus with AAAA in the same 40-bp DNA. The resins were prepared side by side, and care was taken to avoid exposure to UV light. Because we planned to compare binding, care was also taken to obtain approximately equal levels of cross-linking to the activated Sepharose resin (see Materials and Methods).
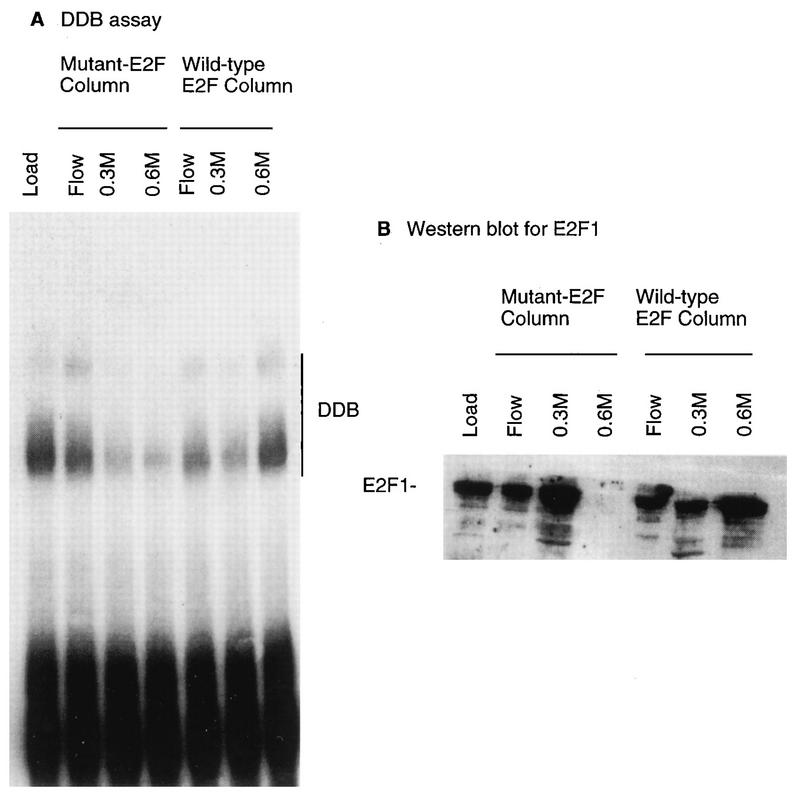
The Q-Sepharose fractions containing the bulk of the E2F activity were pooled and dialyzed, and equal amounts were subjected to the two DNA affinity columns. The columns were eluted successively by 0.1, 0.3, and 0.6 M KCl. The column fractions were assayed for E2F activity and DDB activity. For each of the elutions, the bulk of the activities were detected in one column volume. The fractions from each of the elutions were pooled, and an assay is shown in Fig. 6. A gel retardation assay was used to show the elution profile of the DDB activity from the two columns (Fig. 6, left panel), and a Western blot assay was used to show the elution of E2F1 from the two columns (Fig. 6, right panel). As expected, a significant portion of E2F1 in the loading material was specifically eluted from the wild-type column during the 0.6 M KCl elution, even after a five-column volume wash with buffer containing 0.3 M KCl. The 0.6 M eluate from the mutant column, on the other hand, contained very little E2F1. Interestingly, DDB activity exhibited an elution profile very similar to that of E2F1 from the wild-type E2F affinity column. As was the case with E2F1, a significant part of the DDB activity was eluted only during a 0.6 M KCl elution of the wild-type E2F site-containing column. This result was reproduced by using affinity resins made in three different batches (data not shown). Because we did not detect any E2F site-specific DNA binding by DDB (data not shown), these results are consistent with the notion that the high-affinity binding of DDB to the wild-type E2F site-containing resin is due to its interaction with E2F1. The 0.3 M eluate from the mutant E2F site-containing column contained higher amounts of E2F1 than of DDB. It is possible that nonspecific DNA binding by E2F1 destabilizes the interaction between E2F1 and DDB during chromatography through the mutant E2F column. This is not surprising, because nonspecific DNA binding might alter conformation or involve regions of E2F1 that are important for a stable interaction with DDB.
FIG. 6.
Copurification of DDB and E2F1 through an E2F-DNA affinity column. HeLa nuclear extracts were fractionated through a heparin-agarose and a Q-Sepharose column as described in Materials and Methods (also in reference 68). The Q-Sepharose fractions containing the E2F and DDB activities were subjected to chromatography through a wild-type E2F–DNA or a mutant E2F–DNA affinity column. The columns were eluted successively with 0.1 (Flow), 0.3, and 0.6 M KCl-containing buffer. Fractions from each elution that were active for E2F-DNA binding activity were pooled. Aliquots of the pooled fractions were assayed for DDB in gel retardation assays as described in Materials and Methods (A). The fractions were also assayed for E2F1 by a Western blot assay using a monoclonal antibody against E2F1 (B). The blot was developed with enhanced chemiluminescence.
Expression of DDB specifically stimulates E2F1-activated transcription and overcomes Rb inhibition of E2F1-activated transcription.
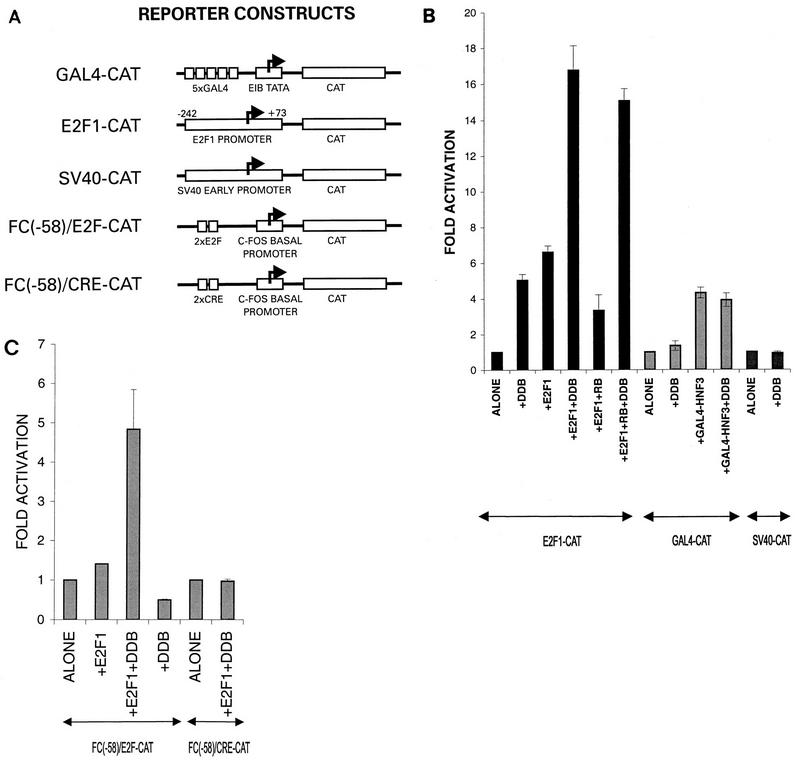
In order to investigate the functional significance of the interaction between E2F1 and DDB, the effect of DDB on E2F1-activated transcription was studied. In order to avoid a negative regulation of Rb, an Rb−/− cell line, C33A, was employed to carry out the transcription studies. Several reporter CAT genes, including one with a naturally occurring E2F1-regulated promoter, were used (Fig. 7A). E2F1 is an autoregulated gene, and it contains E2F1-binding sites near its transcription initiation sites (26, 29). We observed that the expression of DDB stimulated transcription from the E2F1 promoter (Fig. 7B). The simian virus 40 early promoter and a Gal4 site-containing promoter were unaffected by the expression of DDB under similar assay conditions (Fig. 7B). Consistent with the findings of previous studies (26, 29), expression of E2F1 stimulated transcription from the E2F1 promoter-containing reporter gene (Fig. 7B). In addition, coexpression of Rb reduced the E2F1-activated transcription of this reporter gene (Fig. 7B). More interestingly, we observed that the coexpression of DDB with E2F1 reproducibly produced much greater stimulation of the E2F1 site-containing reporter gene. The extent of stimulation obtained by the simultaneous expression of E2F1 and DDB (16- to 18-fold) was consistently greater than what was expected from an additive effect (5- and 6-fold), suggesting that E2F1 and DDB could cooperate to stimulate transcription from an E2F1-regulated promoter.
FIG. 7.
DDB cooperates with E2F1 to stimulate transcription from E2F1-regulated promoters and overcomes Rb inhibition. (A) Schematic diagram of the reporter genes used in transcription studies. E2F1-CAT and FC(−58)/E2F–CAT contain E2F-binding sites in their promoter regions. (B) C33A cells were transfected with the indicated reporter genes (5 μg). Where indicated, the transfection mixture also contained plasmids expressing E2F1 (0.5 μg), the DDB subunits (5 μg each), a combination of E2F1 (0.5 μg) and DDB (5 μg each), or Gal4-HNF3 (1 μg). A plasmid expressing the Rb gene (2 μg) was also included in the indicated transfection mixtures. DNA transfection was carried out by the Ca-phosphate precipitation method as described in Materials and Methods. A plasmid expressing β-galactosidase was included to control for the transfection efficiencies. Averages of fold activation of the CAT gene activity from three independent experiments are shown. (C) C33A cells were transfected with the indicated CAT reporter genes [FC(−58)/E2F–CAT or FC(−58)/CRE-CAT] (1 μg) along with plasmids expressing E2F1 (0.25 μg) alone, DDB (2 μg each) alone, or a combination of E2F1 (0.25 μg) and DDB (2 μg each). A plasmid expressing β-galactosidase was included to control for the transfection efficiencies. Averages of fold activation of the CAT gene activity from three independent experiments are shown. SV40, simian virus 40.
The cooperation between E2F1 and DDB was also observed in studies with a chimeric promoter, FC(−58)/E2F, that contains E2F sites as the only upstream elements (39). This promoter is much weaker than the E2F1 promoter, and expression of E2F1 or DDB alone had very little effect (Fig. 7C). However, coexpression of E2F1 and DDB caused a significant stimulation of transcription (Fig. 7C). The stimulatory effect is not nonspecific, because a comparable promoter containing CRE sites instead of E2F sites was not stimulated by the coexpression of E2F1 and DDB. The C-terminal activation domain of E2F1 has been shown to be involved in the degradation of E2F1 by the ubiquitin-proteasome pathway (25). Because DDB binds to this activation domain, the transcriptional stimulatory effect could be a result of stabilization of the E2F1 protein. Although we cannot rule out a stabilization effect of DDB, we were unable to detect any significant alteration of the steady-state level of the E2F1 protein by the coexpression of DDB (data not shown).
DDB, like Rb, interacts with the activation domain of E2F1. Therefore, we investigated the effects of coexpression of DDB on Rb inhibition. Expression of Rb inhibited E2F1-activated transcription from the E2F1-CAT reporter gene (Fig. 7B). Coexpression of DDB efficiently overcame the Rb repression of E2F1-activated transcription (Fig. 7B), suggesting the possibility that Rb and DDB compete for overlapping sites within the activation domain of E2F1. DDB, E2F1, and Rb proteins were expressed by using the cytomegalovirus promoter, and expression of DDB did not alter the steady-state levels of E2F1 and Rb (data not shown).
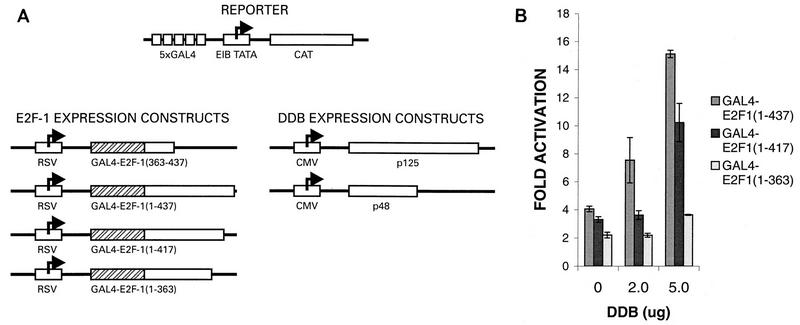
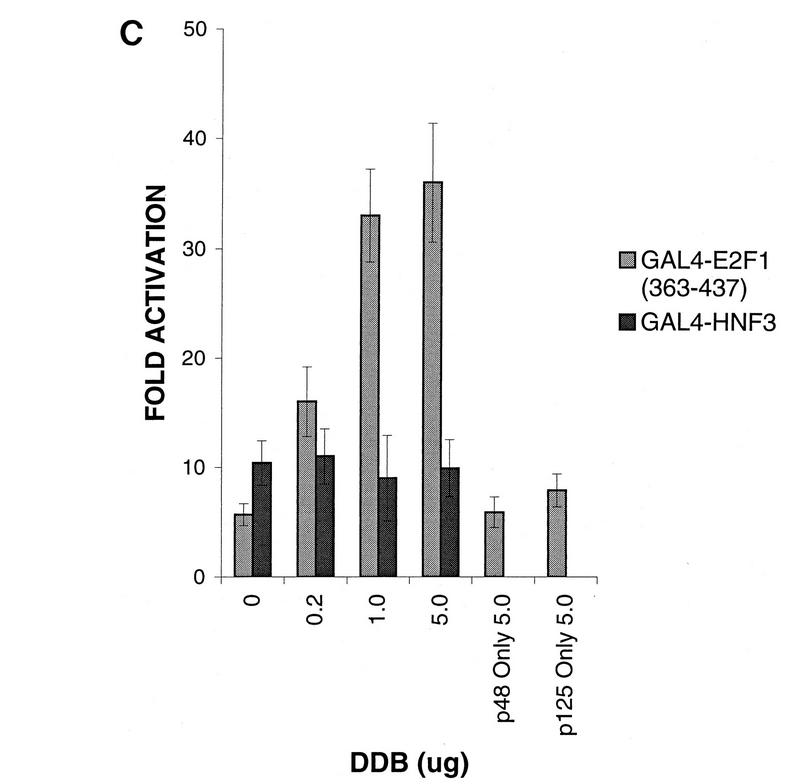
To confirm that the transcriptional stimulatory activity of DDB depends on an interaction with the activation domain of E2F1, we used Gal4 fusion constructs containing E2F1 or E2F1 mutants harboring deletions in the C-terminal activation domain and a reporter CAT gene containing five Gal4-binding sites (Fig. 8A). The DNA transfection mixtures also contained a plasmid that expresses β-galactosidase to control for the transfection efficiencies. Averages of the transfection results from six independent experiments are summarized in Fig. 8B. As reported by others (47), transcriptional activation by E2F1 was dependent upon the activation domain in the C-terminal region. The construct Gal4–E2F1(1-363) exhibited reduced transcriptional stimulatory activity compared to the wild-type E2F1 construct (Fig. 8B). More interestingly, coexpression of p48DDB and p125DDB reproducibly stimulated transcription activated by the wild-type and the E2F1(1-417) constructs. The extent of stimulation varied between three- and fivefold and was reproducible. To see whether the activation domain of E2F1 alone could support the stimulatory effects of DDB, a Gal4 fusion construct [Gal4–E2F1(363-437)] containing the E2F1 residues between 363 and 437 was also analyzed in transfection assays. A Gal4-HNF3 construct was used as a control for specificity. Coexpression of DDB resulted in stimulation of transcription driven by the Gal4–E2F1(363-437) fusion protein but not in stimulation of transcription driven by the Gal4-HNF3 fusion protein (Fig. 8C). Coexpression of any one subunit of DDB had no reproducible effect. Moreover, expression of a fusion protein of Gal4-p48DDB in conjunction with p125DDB had no detectable effect on the reporter gene containing Gal4-binding sites (data not shown), suggesting that DDB needs to interact with the E2F1 activation domain in order to carry out its stimulatory function. Taken together, these results clearly suggest a role of DDB in the transcriptional-activation function of E2F1.
FIG. 8.
Transcriptional stimulatory function of DDB depends upon an interaction with the activation domain of E2F1. (A) Schematic diagram of the Gal4 fusion constructs and the Gal4 site-containing gene used in transfection studies. (B) A plasmid (0.5 μg) expressing Gal4 fused with wild-type E2F1 or with one of the indicated C-terminal deletion mutants was cotransfected with DDB expression constructs (5 μg of each of the plasmids expressing p48DDB and p125DDB) and the Gal4-binding site-containing reporter gene (5 μg) into C33A cells by using the Ca-phosphate precipitation method as described in Materials and Methods. A plasmid expressing β-galactosidase was included to control for the transfection efficiencies. The CAT gene activity was measured by a previously described procedure, and the results were normalized on the basis of the β-galactosidase values. Averages of fold activation from six independent experiments are shown. (C) A plasmid (1 μg) expressing Gal4 fused with the E2F1 activation domain [Gal4-E2F1(363-437)] or the transcription factor HNF3 (Gal4-HNF3) was transfected into C33A cells along with the indicated amounts of the DDB expression plasmids and the Gal4 site-containing reporter genes. A plasmid expressing β-galactosidase was included to control for the transfection efficiencies. Averages of fold activation of the normalized CAT gene activity from six independent experiments are shown.
DISCUSSION
The transcriptional-activation function of E2F1 is important for the expression of several genes that are essential for DNA replication and cell cycle progression (8, 42, 44, 56). The activation domain of E2F1 is also a target of the tumor suppressor Rb (15, 20). Therefore, analysis of the protein partners of the E2F1 activation domain will be important in understanding the mechanism by which E2F1 stimulates the expression of cell cycle-regulated genes. Moreover, these studies will potentially provide further insights into the mechanism of Rb repression. To this end, we have shown that DDB protein interacts with the activation domain of E2F1 and stimulates E2F1-activated transcription.
DDB was identified as an activity that binds UV-damaged DNA. UV irradiation causes formation of cyclobutane dimers in pyrimidines within DNA. In normal human cells, these dimers are efficiently removed and repaired. Patients suffering from xeroderma pigmentosum are deficient in repairing the damage induced by UV irradiation. The deficiency arises from defects in the enzymes involved in the DNA repair process. Several complementation groups of xeroderma pigmentosum (XP) have been characterized. Two groups identified DDB activity as a potential candidate for the complementation group XP-E because 20% of XP-E patients were found to lack DDB activity (7). Moreover, microinjections of purified DDB protein could complement the repair defect in cells lacking DDB activity (34). However, these results were contradicted by another study which indicated that the replication factor RP-A complements in vitro repair deficiencies of XP-E patients (32). Experiments carried out in this study raised questions about any role of DDB activity in the nucleotide excision repair process (32). p48DDB and p125DDB were found in highly purified preparations of DDB activity from HeLa cell extracts (12, 28, 33, 43). However, it is not clear whether these two proteins are sufficient for the activity because the recombinant proteins, made in bacteria or synthesized in vitro, are inactive in binding to UV-damaged DNA (data not shown). Consistent with the observation of Nichols et al. (43), we observed that expression of p48DDB or p125DDB in mammalian cells resulted in an increase in DDB activity (Fig. 4 and data not shown). Moreover, we observed evidence for an interaction between p48DDB and p125DDB (Fig. 4). Thus, this study also confirmed the notion that p48DDB and p125DDB are components of DDB activity. E2F1 is not an essential component of the DDB activity because purification of DDB from HeLa nuclear extracts through a damaged-DNA affinity column produced active DDB that is free of E2F1 (data not shown).
We observed that DDB activity, as well as the p48DDB subunit of DDB activity, could interact with the activation domain of E2F1. p48DDB is interesting because it possesses an extensive sequence homology with a 48-kDa Rb-binding protein (46), which is also a subunit of the chromatin assembly factor CAF-1 (62). p48DDB, like the 48-kDa Rb-binding protein, contains the WD motif (data not shown) (Protein Motifs database), which has been shown to be involved in protein-protein interaction (36). Thus, it is not surprising that the p48DDB subunit of DDB activity is able to interact with E2F1. The homology with the 48-kDa subunit of the chromatin assembly factor CAF-1 may also reflect a role for p48DDB in altering chromatin structure, which might be important in the mechanism by which DDB protein stimulates E2F1-activated transcription.
p48DDB and p125DDB were the only proteins that copurified with DDB activity (12, 28, 33, 43). Therefore, it is likely that these proteins are involved in repairing UV-damaged DNA. The observation that these putative repair proteins associate with the activation domain of E2F1 and stimulate E2F1-activated transcription is not without precedence because there are examples of repair proteins playing a role in RNA polymerase II transcription. For example, the basal transcription factor TFIIH has been shown to copurify with the repair proteins ERCC2 (XPD-complementing activity) and ERCC3 (XPB-complementing activity) (10, 11, 14, 16, 51, 52, 58, 63). ERCC2 and ERCC3 possess DNA helicase activity and are believed to be involved in nucleotide excision repair (10, 11, 16, 17, 19, 51). These proteins were also shown to be functional subunits of the transcription factor TFIIH because addition of specific antibodies against these DNA repair proteins inhibited activity of TFIIH in mRNA transcription (10). These observations are also consistent with the fact that transcriptionally active genes are repaired more efficiently than inactive genes (19). Cells from patients suffering from the hereditary disease Cockayne syndrome are defective in the repair of transcriptionally active genes (references 23 and 51 and references therein). Interestingly, the Cockayne syndrome genes, CS-A and CS-B, have been shown to encode for proteins that might be involved in RNA polymerase II transcription (23). In this context it is noteworthy that p125DDB was also shown to interact with the hepatitis B virus X transcriptional-transactivator protein (40).
The interactions of putative repair proteins with the activation domain of E2F1 is also interesting in light of the observation that this transcription factor is involved in the expression of genes essential for DNA replication and cell division (8, 42, 44, 56). Several of these genes, such as dhfr and c-myc, have been shown to be efficiently repaired after DNA damage by UV irradiation (45, 48, 59). And survival of mammalian cells after UV irradiation correlates with the repair of these essential genes (5). Moreover, for the dhfr gene (which is an E2F1-regulated gene), it was shown that the transcribed strand is repaired more efficiently than the nontranscribed strand (48). It is therefore tempting to speculate that E2F1 recruits DDB to carry out two functions: repairing of the template DNA and activation of transcription. It is possible that during initiation E2F1 recruits DDB, which becomes a part of the elongation complex, and that once a damage site is encountered, DDB stimulates assembly of repair complexes. Clearly, further studies will be required to test this hypothesis.
It is also possible that DDB activity plays a regulatory role in UV-damaged cells. It was shown that cells exposed to UV irradiation are arrested at G1 phase (reference 35 and references therein). It is possible that DDB plays a role in this UV-induced growth arrest. Damaged DNA might sequester DDB and make it unavailable for the E2F1-activated transcription. This would reduce expression of genes necessary for progression through the S phase. It is generally believed that p53 plays a major role in growth arrest induced by UV irradiation (35, 64). p53-mediated growth arrest involves an increased synthesis of p21Cip1 followed by accumulation of the underphosphorylated, active Rb (35). The active form of Rb generates repressor complexes with the E2F family factors, which would shut down expression of genes necessary for progression into S phase. Other regulatory mechanisms may be present to control the activity of the transcriptional partner of the E2F family of factors. Sequestration of an essential partner by damaged DNA itself would be an extremely efficient regulation in this regard.
ACKNOWLEDGMENTS
We thank Stuart Linn (University of California, Berkeley) and Gilbert Chu (Stanford University) for the DDB cDNA clones. We also thank W. Kaelin, Jr. (Dana-Farber Cancer Institute) for the Gal4-E2F1 constructs and R. Costa (University of Illinois at Chicago) for the Gal4-HNF3 construct. The E2F1-CAT reporter gene plasmid was a kind gift of J. R. Nevins (Howard Hughes Medical Institute, Duke University Medical Center). We are also grateful to D. M. Livingston (Dana-Farber Cancer Institute) for the E2F1-cDNA.
This work was supported by a grant from the American Cancer Society (ACS BE-219A) to P.R.
REFERENCES
- 1.Arroyo M, Raychaudhuri P. Retinoblastoma-repression of E2F-dependent transcription depends on the ability of the retinoblastoma protein to interact with E2F and is abrogated by the adenovirus E1A oncoprotein. Nucleic Acids Res. 1992;20:5947–5954. doi: 10.1093/nar/20.22.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asano M, Nevins J R, Wharton R P. Ectopic E2F expression induces S phase and apoptosis in Drosophila imaginal discs. Genes Dev. 1996;10:1422–1432. doi: 10.1101/gad.10.11.1422. [DOI] [PubMed] [Google Scholar]
- 3.Bandara L R, Lam E W, Sorensen T S, Zamanian M, Girling R, La Thangue N B. DP-1: a cell cycle-regulated and phosphorylated component of transcription factor DRTF1/E2F which is functionally important for recognition by pRb and the adenovirus E4 orf 6/7 protein. EMBO J. 1994;13:3104–3114. doi: 10.1002/j.1460-2075.1994.tb06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake M, Azizkhan J C. Transcription factor E2F is required for efficient expression of the hamster dihydrofolate reductase gene in vitro and in vivo. Mol Cell Biol. 1989;9:4994–5002. doi: 10.1128/mcb.9.11.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohr V A, Okumoto D S, Hanawalt P C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci USA. 1986;83:3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 7.Chu G, Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988;242:564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- 8.DeGregori J, Kowalik T, Nevins J R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dignam J D, Lebovitz R M, Roeder R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drapkin R, Reardon J T, Ansari A, Huang J C, Zawel L, Ahn K, Sancar A, Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994;368:769–772. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- 11.Drapkin R, Sancar A, Reinberg D. Where transcription meets repair. Cell. 1994;77:9–12. doi: 10.1016/0092-8674(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 12.Dualan R, Brody T, Keeney S, Nichols A F, Admon A, Linn S. Chromosomal localization and cDNA cloning of the genes (DDB1 and DDB2) for the p127 and p48 subunits of a human damage-specific DNA binding protein. Genomics. 1995;29:62–69. doi: 10.1006/geno.1995.1215. [DOI] [PubMed] [Google Scholar]
- 13.Dynlacht B D, Flores O, Lees J A, Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 14.Feaver W J, Svejstrup J Q, Bardwell L, Bardwell A J, Buratowski S, Gulyas K D, Donahue T F, Friedberg E C, Kornberg R D. Dual roles of a multiprotein complex from S. cerevisiae in transcription and DNA repair. Cell. 1993;75:1379–1387. doi: 10.1016/0092-8674(93)90624-y. [DOI] [PubMed] [Google Scholar]
- 15.Flemington E K, Speck S H, Kaelin W G., Jr E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc Natl Acad Sci USA. 1993;90:6914–6918. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedberg E C. Relationships between DNA repair and transcription. Annu Rev Biochem. 1996;65:15–42. doi: 10.1146/annurev.bi.65.070196.000311. [DOI] [PubMed] [Google Scholar]
- 17.Habraken Y, Sung P, Prakash S, Prakash L. Transcription factor TFIIH and DNA endonuclease Rad2 constitute yeast nucleotide excision repair factor 3: implications for nucleotide excision repair and Cockayne syndrome. Proc Natl Acad Sci USA. 1996;93:10718–10722. doi: 10.1073/pnas.93.20.10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagemeier C, Cook A, Kouzarides T. The retinoblastoma protein binds E2F residues required for activation in vivo and TBP binding in vitro. Nucleic Acids Res. 1993;21:4998–5004. doi: 10.1093/nar/21.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanawalt P C. Transcription-coupled repair and human disease. Science. 1994;266:1957–1958. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- 20.Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13:6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helin K, Lees J A, Vidal M, Dyson N, Harlow E, Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992;70:337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- 22.Helin K, Wu C L, Fattaey A R, Lees J A, Dynlacht B D, Ngwu C, Harlow E. Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation. Genes Dev. 1993;7:1850–1861. doi: 10.1101/gad.7.10.1850. [DOI] [PubMed] [Google Scholar]
- 23.Henning K A, Li L, Iyer N, McDaniel L D, Reagan M S, Legerski R, Schultz R A, Stefanini M, Lehmann A R, Mayne L V, Friedberg E C. The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell. 1995;82:555–564. doi: 10.1016/0092-8674(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 24.Hiebert S W, Chellappan S P, Horowitz J M, Nevins J R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann F, Martelli F, Livingston D M, Wang Z. The retinoblastoma gene product protects E2F1 from degradation by the ubiquitin-proteasome pathway. Genes Dev. 1996;10:2949–2959. doi: 10.1101/gad.10.23.2949. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao K M, McMahon S L, Farnham P J. Multiple DNA elements are required for the growth regulation of the mouse E2F1 promoter. Genes Dev. 1994;8:1526–1537. doi: 10.1101/gad.8.13.1526. [DOI] [PubMed] [Google Scholar]
- 27.Hwang B J, Chu G. Purification and characterization of a human protein that binds to damaged DNA. Biochemistry. 1993;32:1657–1666. doi: 10.1021/bi00057a033. [DOI] [PubMed] [Google Scholar]
- 28.Hwang B J, Liao J C, Chu G. Isolation of a cDNA encoding a UV-damaged DNA binding factor defective in xeroderma pigmentosum group E cells. Mutat Res. 1996;362:105–117. doi: 10.1016/0921-8777(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 29.Johnson D G, Ohtani K, Nevins J R. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- 30.Johnson D G, Schwarz J K, Cress W D, Nevins J R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 31.Kaelin W G, Jr, Krek W, Sellers W R, DeCaprio J A, Ajchenbaum F, Fuchs C S, Chittenden T, Li Y, Farnham P J, Blanar M A, et al. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992;70:351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 32.Kazantsev A, Mu D, Nichols A F, Zhao X, Linn S, Sancar A. Functional complementation of xeroderma pigmentosum complementation group E by replication protein A in an in vitro system. Proc Natl Acad Sci USA. 1996;93:5014–5018. doi: 10.1073/pnas.93.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keeney S, Chang G J, Linn S. Characterization of a human DNA damage binding protein implicated in xeroderma pigmentosum E. J Biol Chem. 1993;268:21293–21300. [PubMed] [Google Scholar]
- 34.Keeney S, Eker A P, Brody T, Vermeulen W, Bootsma D, Hoeijmakers J H, Linn S. Correction of the DNA repair defect in xeroderma pigmentosum group E by injection of a DNA damage-binding protein. Proc Natl Acad Sci USA. 1994;91:4053–4056. doi: 10.1073/pnas.91.9.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 36.Komachi K, Redd M J, Johnson A D. The WD repeats of Tup1 interact with the homeo domain protein alpha 2. Genes Dev. 1994;8:2857–2867. doi: 10.1101/gad.8.23.2857. [DOI] [PubMed] [Google Scholar]
- 37.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin W G, Jr, Livingston D M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 38.Krek W, Xu G, Livingston D M. Cyclin A-kinase regulation of E2F-1 DNA binding function underlies suppression of an S phase checkpoint. Cell. 1995;83:1149–1158. doi: 10.1016/0092-8674(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 39.Lee M H, Mori S, Raychaudhuri P. trans-Activation by the hnRNP K protein involves an increase in RNA synthesis from the reporter genes. J Biol Chem. 1996;271:3420–3427. doi: 10.1074/jbc.271.7.3420. [DOI] [PubMed] [Google Scholar]
- 40.Lee T H, Elledge S J, Butel J S. Hepatitis B virus X protein interacts with a probable cellular DNA repair protein. J Virol. 1995;69:1107–1114. doi: 10.1128/jvi.69.2.1107-1114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morosov A, Phelps W C, Raychaudhuri P. Activation of the c-fos gene by the HPV16 oncoproteins depends upon the cAMP-response element at −60. J Biol Chem. 1994;269:18434–18440. [PubMed] [Google Scholar]
- 42.Nevins J R. A link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 43.Nichols A F, Ong P, Linn S. Mutations specific to the xeroderma pigmentosum group E Ddb− phenotype. J Biol Chem. 1996;271:24317–24320. doi: 10.1074/jbc.271.40.24317. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani K, DeGregori J, Nevins J R. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci USA. 1995;92:12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen L N, Orren D K, Bohr V A. Gene-specific and strand-specific DNA repair in the G1 and G2 phases of the cell cycle. Mol Cell Biol. 1995;15:3731–3737. doi: 10.1128/mcb.15.7.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian Y-W, Wang Y-C J, Hollinsworth R E J, Jones D, Ling N, Lee E Y-H P. A retinoblastoma-binding protein related to a negative regulator of Ras in yeast. Nature. 1993;364:648–652. doi: 10.1038/364648a0. [DOI] [PubMed] [Google Scholar]
- 47.Qin X Q, Livingston D M, Ewen M, Sellers W R, Arany Z, Kaelin W G., Jr The transcription factor E2F-1 is a downstream target of RB action. Mol Cell Biol. 1995;15:742–755. doi: 10.1128/mcb.15.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rampino N J, Bohr V A. Rapid gene-specific repair of cisplatin lesions at the human DUG/DHFR locus comprising the divergent upstream gene and dihydrofolate reductase gene during early G1 phase of the cell cycle assayed by using the exonucleolytic activity of T4 DNA polymerase. Proc Natl Acad Sci USA. 1994;91:10977–10981. doi: 10.1073/pnas.91.23.10977. . (Erratum, 92:5249, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raychaudhuri P, Bagchi S, Neill S D, Nevins J R. Activation of the E2F transcription factor in adenovirus-infected cells involves E1A-dependent stimulation of DNA-binding activity and induction of cooperative binding mediated by an E4 gene product. J Virol. 1990;64:2702–2710. doi: 10.1128/jvi.64.6.2702-2710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reardon J T, Nichols A F, Keeney S, Smith C A, Taylor J S, Linn S, Sancar A. Comparative analysis of binding of human damaged DNA-binding protein (XPE) and Escherichia coli damage recognition protein (UvrA) to the major ultraviolet photoproducts: T[c,s]T, T[t,s]T, T[6-4]T, and T[Dewar]T. J Biol Chem. 1993;268:21301–21308. [PubMed] [Google Scholar]
- 50a.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 51.Sancar A. DNA excision repair. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- 52.Schaeffer L, Roy R, Humbert S, Moncollin V, Vermeulen W, Hoeijmakers J H, Chambon P, Egly J M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993;260:58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- 53.Seed B, Sheen J Y. A simple phase-extraction assay for chloramphenicol acetyltransferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 54.Sellers W R, Rodgers J W, Kaelin W G., Jr A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc Natl Acad Sci USA. 1995;92:11544–11548. doi: 10.1073/pnas.92.25.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shiyanov P, Bagchi S, Adami G, Kokontis J, Hay N, Arroyo M, Morozov A, Raychaudhuri P. p21 disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996;16:737–744. doi: 10.1128/mcb.16.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slansky J E, Li Y, Kaelin W G, Farnham P J. A protein synthesis-dependent increase in E2F1 mRNA correlates with growth regulation of the dihydrofolate reductase promoter. Mol Cell Biol. 1993;13:1610–1618. doi: 10.1128/mcb.13.3.1610. . (Erratum, 13:7201.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Studier F W, Rosenberg A H. Genetic and physical mapping of the late region of bacteriophage T7 DNA by use of cloned fragments of T7 DNA. J Mol Biol. 1981;153:503–525. doi: 10.1016/0022-2836(81)90405-8. [DOI] [PubMed] [Google Scholar]
- 58.Svejstrup J Q, Wang Z, Feaver W J, Wu X, Bushnell D A, Donahue T F, Friedberg E C, Kornberg R D. Different forms of TFIIH for transcription and DNA repair: holo-TFIIH and a nucleotide excision repairosome. Cell. 1995;80:21–82. doi: 10.1016/0092-8674(95)90447-6. [DOI] [PubMed] [Google Scholar]
- 59.Tang M S, Pao A, Zhang X S. Repair of benzo(a)pyrene diol epoxide- and UV-induced DNA damage in dihydrofolate reductase and adenine phosphoribosyltransferase genes of CHO cells. J Biol Chem. 1994;269:12749–12754. [PubMed] [Google Scholar]
- 60.Trouche D, Kouzarides T. E2F1 and E1A(12S) have a homologous activation domain regulated by RB and CBP. Proc Natl Acad Sci USA. 1996;93:1439–1442. doi: 10.1073/pnas.93.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Assendelft G B, Rigney E M, Hickson I D. Purification of a HeLa cell nuclear protein that binds selectively to DNA irradiated with ultra-violet light. Nucleic Acids Res. 1993;21:3399–3404. doi: 10.1093/nar/21.15.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verreault A, Kaufman P D, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Svejstrup J Q, Feaver W J, Wu X, Kornberg R D, Friedberg E C. Transcription factor b (TFIIH) is required during nucleotide-excision repair in yeast. Nature. 1994;368:74–76. doi: 10.1038/368074a0. [DOI] [PubMed] [Google Scholar]
- 64.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 65.Weintraub S J, Chow K N, Luo R X, Zhang S H, He S, Dean D C. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 66.Weintraub S J, Prater C A, Dean D C. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992;358:259–261. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- 67.Xu M, Sheppard K A, Peng C Y, Yee A S, Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol. 1994;14:8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yee A S, Raychaudhuri P, Jakoi L, Nevins J R. The adenovirus-inducible factor E2F stimulates transcription after specific DNA binding. Mol Cell Biol. 1989;9:578–585. doi: 10.1128/mcb.9.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]