Abstract
Background
Diabetes mellitus is a long-term metabolic disease marked by consistently elevated blood glucose levels. Diabetic ketoacidosis is the medical consequence of diabetes mellitus that has the highest attributed fatality rate. Socioeconomically differences affect how long it takes to recover from diabetic ketoacidosis. A few research were carried out in Africa to demonstrate how long diabetic ketoacidosis takes to recover. However, the pooled median recovery time and predictors of diabetic ketoacidosis have not been studied in Africa. Thus, determine the pooled median recovery time and predictors of diabetic ketoacidosis in Africa was the aim of this systematic review and meta-analysis.
Methods
To find available publications, a number of databases were analyzed, including PubMed, Science Direct, Cochrane, Hinari, Google Scholar, grey literature, and articles from various university repository sites. Microsoft Excel version 13 was used to extract and sort the data before exporting it to STATA/MP 17.0 for analysis. The quality of each study was evaluated using the Newcastle-Ottawa Scale. A 95 percent confidence interval Der Simonian random-effects model was employed to investigate the pooled recovery time of diabetic ketoacidosis. Publication bias and heterogeneity were assessed using the Egger's test and I2. Both meta-regression and subgroup analysis were used to determine the potential source of heterogeneity. Statistical significance was defined as P-values below 0.05.
Result
The pooled median recovery time for diabetic ketoacidosis in Africa was 38 h (95 percent CI: 33–43 h), according to this comprehensive review and meta-analysis. Significant heterogeneity is evident when looking at the Galbraith plot with I2 = 100 % (p < 0.001). Research conducted after 2020 revealed that diabetic ketoacidosis has a long recovery time of 40 h (95 percent CI: 3–77 h). However, research with fewer than 300 participants showed that diabetic ketoacidosis recovered more quickly: 18 h (95 percent confidence interval: 12–24 h).
Conclusion
Among patients with diabetic ketoacidosis in Africa, the pooled median recovery time was lengthy. The recovery time from diabetic ketoacidosis was influenced by a number of factors, including the severity of the diabetic ketoacidosis, the delay in starting therapy, and the length of time the patient had diabetes mellitus, and elevated blood glucose levels. Diabetic ketoacidosis recovery time can be shortened by altering these factors.
Keywords: Recovery time, Diabetic ketoacidosis, Predictor, Africa
1. Introduction
A chronic metabolic condition characterized by persistently high blood glucose levels is diabetes mellitus (DM) [1]. This occurs because of the body's inability to produce enough insulin, the cells' poor reaction to the insulin that is produced, or sometimes both [1,2]. A higher risk of stroke, cardiovascular disease, kidney failure, eyesight loss, nerve damage (neuropathy), foot ulcer, and other major long-term issues might result from diabetes mellitus effects on blood vessels, especially the body's large and small blood vessels. And it can also result in a number of short-term consequences, such as diabetic ketoacidosis, hyperosmolar hyperglycemia, and hypoglycemia [1,3].
Diabetic ketoacidosis is a serious and potentially fatal complication of diabetes that primarily affects people with type 1 diabetes, while people with type 2 diabetes can also get it infrequently [4]. It arises when the body breaks down fat for energy instead of using glucose because it lacks enough insulin [4,5]. This process releases acidic molecules into the bloodstream known as ketones, which can accumulate and cause acidosis and other catastrophic health problems [6,7].
The medical consequence of diabetes mellitus with the greatest attributable death rate is diabetic ketoacidosis [8]. Among individuals with diabetes mellitus, the incidence of diabetic ketoacidosis was 263 per 1000 person-years worldwide [9]. Diabetic ketoacidosis death rates vary according on the severity of the illness [10]. In people aged 20 years or older, the mean in-hospital mortality rate is 3.9 % [11]. The death rate may be much greater in extreme problems, particularly those involving coma [12]. In general, based on several factors, diabetic ketoacidosis death rates can range from 1.2 % to higher percentages [13,14].
The effective treatment of diabetic ketoacidosis requires timely diagnosis, comprehensive clinical and biochemical evaluation, and effective care [15]. When blood glucose is less than 200 mg/dL and the following criteria are satisfied, diabetic ketoacidosis is recovered: serum bicarbonate level ≥15 mEq/L, venous pH > 7.3, blood ketones <0.6 mmol/L, and estimated anion gap ≤ of 12 mEq/L [15,16]. Previous studies showed that using standardized protocol for diabetic ketoacidosis management improves diabetic ketoacidosis recovery [16]. Patients with diabetic ketoacidosis should recover completely in a day if they receive the right care. Prolonged recovery from diabetic ketoacidosis can lead to thromboembolic problems, death, cerebral oedema, cardiac arrhythmia, renal failure, coma, lung oedema with respiratory failure, hypoglycemia and hypokalaemia, and thromboembolic complications [17].
Timely detection and treatment of diabetic ketoacidosis lowers the risk of thromboembolic effects, metabolic acidosis, hypophosphatemia, cardiac arrhythmia, hyperchloremia, renal failure, and coma in addition to reducing hypoglycemia and hypokalaemia. Consequently, hospital bed occupancy, length of stay, and the amount of material and human resources needed to manage illness and its complications are all reduced [18,19].
Different studies showed that duration of diabetic ketoacidosis, the duration of diabetes mellitus, comorbidities, the severity of diabetic ketoacidosis, and biochemical markers like blood glucose, serum creatinine, and serum electrolytes all influence how long it takes to recover from diabetic ketoacidosis [19]. With appropriate management patients with diabetic ketoacidosis may recover in 10–36 h depending on its severity [20]. To improve recovery and lower mortality, several nations have adopted or established diabetic ketoacidosis treatment standards, diabetic self-management, education, and other preventative strategies [20].
Different studies conducted in different country showed that regional variation on median recovery time of diabetic ketoacidosis [21,22]. The recovery time for diabetic ketoacidosis varies by geography, according to studies done in different countries [18,21,22]. Africa carried out a few studies to show how long diabetic ketoacidosis takes to recover. Nevertheless, no study on the pooled median recovery and predictors of diabetic ketoacidosis have been conducted in Africa. Therefore, the findings of this systematic review and meta-analysis provide a comprehensive picture of data about diabetic ketoacidosis recovery time at the African level by examining the available single studies conducted in different regions of the continent. Thus, the aim of this systematic review and meta-analysis was to determine the pooled median recovery time and predictors of diabetic ketoacidosis in Africa.
2. Methods
2.1. Study design
Systematic review and meta-analysis.
2.2. Study setting
The major studies included in this systematic review and meta-analysis were carried out among hospitalized diabetic ketoacidosis patients in Africa (South Africa, Kenya, Egypt, and Ethiopia). Because there was so little research's on the recovery time of diabetic ketoacidosis, only five articles were included in this systematic review and meta-analysis.
2.3. Population
All age group of patients with diabetic ketoacidosis.
2.4. Condition
The median recovery time and predictors of diabetic ketoacidosis.
2.5. Outcomes
The primary outcome of this study was pooled median recovery time of diabetic ketoacidosis and the secondary outcome was predictors of recovery time of diabetic ketoacidosis.
2.6. Reporting and registration protocol
The pooled median recovery time and predictors of diabetic ketoacidosis among individuals with diabetes mellitus were estimated by a systematic review and meta-analysis. The PROSPERO database (CRD42025630344) has the protocol for this manuscript registered. According to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA-2020) reporting guideline (S-F1), the results of this systematic review and meta-analysis were reported.
2.7. Databases and search strategies
Many databases were assessed, such as PubMed, Science direct, Hinari, Cochrane, Google Scholar, grey literature, and both published and unpublished articles from different repository sites. The authors searched the relevant articles conducted on recovery time of diabetic ketoacidosis from January 1st, 2015 to December last, 2024. The references of the articles retrieved were also searched to avoid missing any article from different sources. Boolean operators AND combine the keywords Recovery, Time, Diabetic ketoacidosis. The searching term “((Recovery) AND (Time)) AND (Diabetic Ketoacidosis)” were applied on different databases to search articles (S-F2).
2.8. Screening and eligibility of the studies
The “EndNote reference software version 8 citation manager' was used to export all of the articles in order to clean, organize, and eliminate any potential duplications. Each paper was independently examined and assessed by six authors (TL, AS, TK, SB, YA, and TKW) using predefined inclusion criteria based on its title, abstract and relevance. After that, each author (TL, AS, TK, SB, YA, JC, BA, MT, DM, TKW, BM, and DHT) carefully assessed each article's eligibility (inclusion & exclusion). Research on the recovery time of diabetic ketoacidosis in Africa, undertaken between January 1, 2015, and December 30, 2024, was included in both published and unpublished works. Using the Newcastle-Ottawa Scale (NOS) assessment tool (S-F3), all studies were evaluated. Therefore, the study comprised studies that had a true evaluation of outcome, a valid statistical test, exposure ascertainment, and a limited non-response rate. Interventional studies, trials, meta-analyses and systematic reviews, qualitative research, narrative reviews, full-text articles, case reports, and policy comments were not included, though. Moreover, low-quality studies were eliminated from the final analysis following the NOS assessment, which evaluated each study's characteristics. During the process, the authors discussed and resolved any disagreements that came up.
2.9. Data extraction
The data was extracted using a Microsoft Excel-2013 spreadsheet and then the data checked for its completeness and consistence before exported to STATA for analysis. The following information was extracted: the last name of the first author, study year, sample size, study nation, study design, sample size, median recovery time of diabetic ketoacidosis, hazards ratio (HR) with 95 % CI, success, failure, and NOS scale (Table 2). After individually extracting the data and cross-checking it for inconsistencies, the six authors (JC, BA, MT, DM, BM, and DHT) discussed and resolved the data.
Table 2.
Sub-group analysis on the pooled recovery time of diabetic ketoacidosis in the Africa.
| Variables | Subgroup | Studies (n) | Median recovery time of DKA | I2 in percent | P - value |
|---|---|---|---|---|---|
| Country | Ethiopia | 2 | 48 | 99 | <0.001 |
| Other African country | 3 | 32 | 100 | <0.001 | |
| Study year | Before 2020 | 2 | 37 | 99 | <0.001 |
| After 2020 | 3 | 40 | 100 | <0.001 | |
| Sample size | More than 300 | 3 | 52 | 100 | <0.001 |
| Less than 300 | 2 | 18 | 99 | <0.001 | |
| Researches | Published | 3 | 52 | 99 | <0.001 |
| Unpublished | 2 | 18 | 100 | <0.001 |
2.10. Outcome measurement
Recovery time for patients with diabetic ketoacidosis was the outcome variable in this extensive systematic review and meta-analysis. A patient is diagnosed with diabetic ketoacidosis if their blood glucose level is more than 250 mg/dL, their pH is below 7.35, and they have moderate ketonemia (>3 mmol) or a positive urine ketone test result. Diabetic ketoacidosis recovery time is the number of hours that pass between the initial vascular filling used to treat diabetic ketoacidosis in the intensive care unit or emergency room and diabetic ketoacidosis recovery.
2.11. Operational definition
Diabetic ketoacidosis: is an acute life-threatening complication of diabetes mellitus characterized by the triad of hyperglycemia, acidosis, and ketone body in urine analysis that occurs in the presence of very low levels of insulin action [23,24].
2.12. Quality assessment
The results are taken from the extraction sheet by five authors (TL, AS, TK, SB, and YA), and they have been confirmed by the remaining five authors (JC, BA, MT, DM, and DH). Statistical testing, assessment of exposure or risks, comparability, methodological quality, sample representativeness, and statistical testing were the criteria used to assess each study's quality using the Newcastle-Ottawa Scale (NOS). In the final analysis, studies with a score of at least seven or more were considered to be included (Table 2). To decide which research should be considered and included in the analysis, each author conducted their own independent evaluation of the available studies.
2.13. Data processing and analysis
Before being exported to STATA/MP 17.0 for analysis, the data were extracted, sorted, and cleaned in a Microsoft Excel spreadsheet. The pooled median recovery time and CI were estimated using a DerSimonian–Laird random-effects model at a 95 % CI. The predictors of diabetic ketoacidosis among patients in Africa were estimated using a DerSimonian–Laird random-effects model and an inverse-variance fixed model at a 95 % confidence interval (CI). The graphical Galbraith plot and the statistical I2 with its associated p-value were used to evaluate the studies' heterogeneity. A sensitivity analysis was also conducted to examine the potential for an influential study. Additionally, Egger's test (a statistical technique) and a funnel plot (a graphical tool) were utilized for publication bias.
3. Result
3.1. Selection of articles
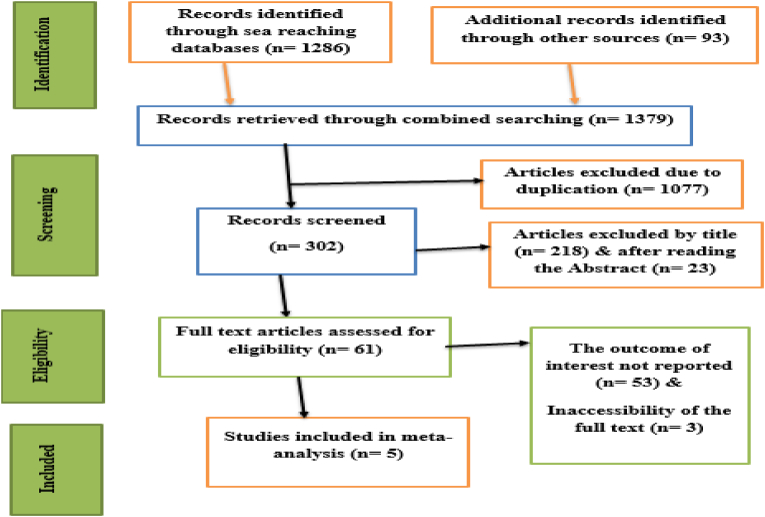
Following several database searches, 1379 articles were discovered. The existence of duplication led to the exclusion of 1077 of these articles. After critically assessing each article's abstract and title, 241 papers were also eliminated from the analysis. 53 studies were also disqualified because they did not meet the inclusion criteria and were considered irrelevant. The inability to obtain the complete text of three articles led to their removal as well. After that, the final analysis included five papers (Fig. 1).
Fig. 1.
PRISMA flow chart of selection of articles for systematic review and meta-analysis of recovery time of diabetic ketoacidosis in Africa.
3.2. Characteristics of the studies and study participants
In this systematic review and meta-analysis, five articles with 1103 study participants were included. The studies were conducted in different country of Africa from January/1/2015 until December 30/2024. Of these studies, two were conducted before 2020 and three were done after 2020. Two studies were carried out in Ethiopia [18,25], one conducted in Kenya [26], one done in South Africa [21] and one conducted in Egypt [22]. The sample size ranged from 42 to 452. In design, all studies were retrospective follow up (Table 1).
Table 1.
Characteristics of included studies on recovery time of diabetic ketoacidosis in the Africa.
| Author | Study year | Country | Study design | Sample size | Median recovery time of DKA | NOS |
|---|---|---|---|---|---|---|
| Temesgen et al. | 2022 | Ethiopia | Retrospective follow up | 452 | 24 | 7 |
| Tefera et al. | 2023 | Ethiopia | Retrospective follow up | 391 | 72 | 7 |
| Kerubo et al. | 2018 | Kenya | Prospective follow up | 42 | 59 | 6 |
| Sabry et al. | 2024 | Egypt | Retrospective follow up | 150 | 15 | 8 |
| Thomas et al. | 2019 | South Africa | Prospective follow up | 68 | 21 | 7 |
3.3. Meta-analysis
3.3.1. Pooled median recovery time of diabetic ketoacidosis
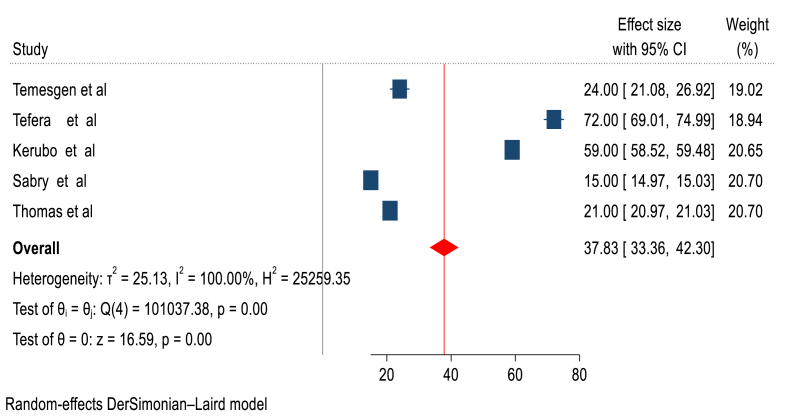
This systematic review and meta-analysis showed that, the pooled median recovery time of diabetic ketoacidosis among patient with diabetic ketoacidosis in Africa was 38 h (95 % CI: 33–43 h) (Figure-2).
Fig. 2.
Forest plot showing the pooled median recovery time of diabetic ketoacidosis in Africa.
3.4. Heterogeneity
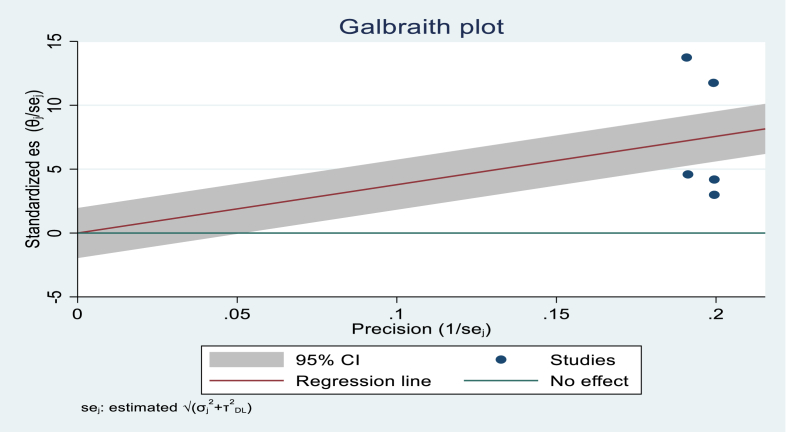
Visual examination of the Galbraith plot reveals the presence of significant heterogeneity (I2 100 %, p < 0.001) (Figure-3).
Fig. 3.
Galbraith plot showing heterogeneity of median recovery time of diabetic ketoacidosis in Africa.
3.5. Subgroup analysis
To identify the source of heterogeneity, sub-group analysis was carried out using the study year, sample size, published and unpublished articles, and the nation where the studies were conducted. According to studies done in Ethiopia, diabetic ketoacidosis patients needed 48 h to recover (95 % CI: 1–95 h). Unpublished studies showed that the recovery period from diabetic ketoacidosis was 52 h (95 % CI 30–73 h). Similarly, studies published after 2020 revealed that diabetic ketoacidosis had a lengthy recovery time of 40 h (95 percent CI: 3–77 h). Research with fewer than 300 participants showed that diabetic ketoacidosis recovered more quickly, taking 18 h (95 percent CI: 12–24 h) (Table 2).
3.6. Publication bias
The asymmetrical distribution of the included papers was evident in the funnel plot, which further demonstrated the likelihood of publication bias. The Egger's test yielded a statistically significant result (p < 0.001). A trim and fill analysis was conducted in order to control for publication bias. Following trim and fill analysis, the bias-adjusted median recovery time for diabetic ketoacidosis was 38 h (95 % CI: 33–43 h). No different result was found by trim and fill analysis, this showed that the publication bias didn't influence the result (median recovery time of diabetic ketoacidosis).
3.7. Meta regression
We computed a meta-regression analysis to detect the possible source of heterogeneity. Accordingly, meta-regression using the moderator like country where the studies were done, published and unpublished article, sample size and study year was done. The result of this meta-regression showed that the country where study conducted had a coefficient of −16.30, a standard error of 4.68, and p-value of 0.001. The publication of the research had the coefficient of −33.90, standard error of 3.95, and the p-value of 0.001. Study year had a coefficient of 3.02, a standard error of 24.61, and a P value of 0.902. The sample size had the coefficient of 33.91, standard error of 3.95, and p value of 0.001. Therefore, country, publication status and sample size were the sources of publication bias, because of their each p-value was 0.05.
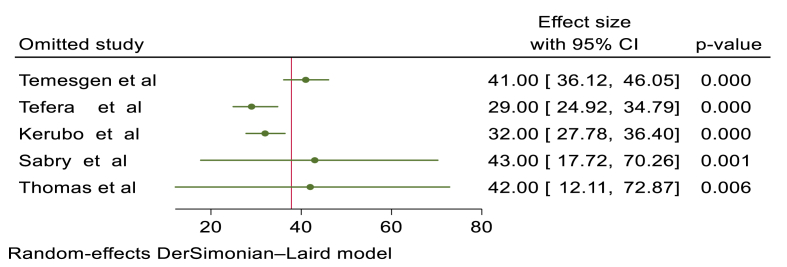
3.8. Sensitivity analysis
A leave-one-point sensitivity analysis conducted using the random-effects model revealed that all of the points were estimates near to the overall 95 % confidence interval (33–43), indicating there was no exaggerated influential study (Figure-4).
Fig. 4.
Sensitivity analysis results of median recovery time of diabetic ketoacidosis.
3.9. Predictors of recovery time of diabetic ketoacidosis
Patients with severe diabetic ketoacidosis recovered from their condition 74 % more slowly than those with mild ketoacidosis (PHR = 0.26, 95 % CI: 0.17–0.35). Compared to patients who stayed with diabetic ketoacidosis for less than 24 h before receiving treatment, those who stayed with the condition for more than 24 h had a 44 % shorter recovery time from the diabetic ketoacidosis (PHR = 0.56, 95 % CI: 0.33–0.80).
The recovery time from diabetic ketoacidosis was 37 % longer for patients with high random blood sugar levels (>500 mg/dl) than for those with low random blood sugar levels (≤500 mg/dl) (PHR = 0.63, 95 % CI 0.51–0.76). Patients with diabetic ketoacidosis who had diabetes mellitus for more than five years experienced a 56 % longer recovery period from their condition than those who had diabetes mellitus for five years or less (PHR = 0.48, 95 % CI: 0.33–0.64). Two primary studies with 843 patients with diabetic ketoacidosis were included in this area of factor analysis (Table 3).
Table 3.
Predictors associated with recovery time of diabetic ketoacidosis.
| Predictors | Number of studies | Sample size | AHR (95 % CI) | P-value | I2 (%) | Heterogeneity test (p-value) | Egger test (p-value | Model used |
|---|---|---|---|---|---|---|---|---|
| Severity of DKA | Two | 843 | 0.26 (0.17–0.35) | <0.001 | 42.54 % | 0.19 | 0.19 | Fixed model |
| Duration of DKA before starting of treatment | Two | 843 | 0.56 (0.33–0.80) | <0.001 | 64.19 % | 0.09 | 0.54 | Random model |
| High random blood sugar level (>500 mg/dl) | Two | 843 | 0.63 (0.51–0.76) | <0.001 | 0 % | 0.85 | 0.85 | Fixed Model |
| Duration of DM | Two | 843 | 0.48 (0.33–0.64) | <0.001 | 66 % | 0.08 | 0.10 | Random model |
4. Discussion
Although this meta-analysis provides useful information about the recovery period from diabetic ketoacidosis in the populations under study, it is constrained by the small sample size (n = 5 studies) and the fact that only data from Ethiopia, Kenya, Egypt, and South Africa were included. As a result, the results might not accurately reflect the wide range of socioeconomic circumstances, healthcare options, and diabetes prevalence found throughout the African continent. Extrapolating our findings across the continent is difficult because of the considerable variation in medical resources, economic stability, cultural practices, and diabetes incidence throughout Africa. Recovery times and diabetes treatment are probably impacted by these various factors [27,28].
Therefore, it is important to recognize that the results of this meta-analysis are most directly applicable to environments that have similar features with the nations that were included. These include areas with comparable access to diabetes education, insulin and other drug availability, dietary trends, and socioeconomic factors that impact access to healthcare. We strongly advise against extending these findings to other African nations without properly taking the local circumstances into account. To increase the generalizability and comprehensiveness of the body of data, future research should place a high priority on broadening the geographic reach of diabetic ketoacidosis recovery studies in Africa through partnerships and primary research in under-represented areas.
This meta-analysis's biggest drawback is the high level of heterogeneity seen in all of the studies included (I2 = 100 %, p < 0.001). A significant amount of the variability cannot be explained, even with our best efforts to find causes of heterogeneity using subgroup analysis and meta-regression on variables including study year, sample size, publication status, and research country. Our pooled effect estimate interpreted carefully due to this ongoing heterogeneity.
The pooled median recovery time for diabetic ketoacidosis patients in Africa was 38 h (95 percent CI: 33–43 h), according to this systematic review and meta-analysis. According to this pooled median recovery time of diabetic ketoacidosis data, the recovery time from diabetic ketosis was longer than the 24-h suggested recovery period. According to international medical guidelines, people with diabetic ketoacidosis diabetic ketoacidosis should recover in about 24 h with the right intervention. This implies that within a day after beginning treatment, the majority of patients should notice a noticeable improvement in their ketone and blood sugar levels [15].
The results of this research are nearly in agreement with those of a South African study that was part of this systematic review and meta-analysis [21]. When compared to research done in Thailand [29] and Australia [30], the results lasted longer. Conversely, it is quicker than a Kenyan research [31]. Since studies indicate that insulin pump therapy has a better clinical outcome than injectable therapy, the variation in the conventional treatment regimen may be the explanation of this differences [32,33]. Low socioeconomic difference could also be the cause, as low educational attainment raises the risk of severe diabetic ketoacidosis, which requires a longer recovery period [33,34].
Patient with sever diabetic ketoacidosis had delayed recovery time of diabetic ketoacidosis than patient with mild diabetic ketoacidosis. The recovery time from severe diabetic ketoacidosis is considerably longer than that of mild or moderate diabetic ketoacidosis [34]. This is because the metabolic imbalance and associated consequences are more severe in those with severe diabetic ketoacidosis. Bicarbonate, blood pH, and blood glucose levels are frequently used to gauge the severity of diabetic ketoacidosis; lower bicarbonate and pH values indicate more severe acidosis, which prolongs recovery [22,34].
The recovery time from diabetic ketoacidosis was longer for patients who stayed with the condition for longer (>24 h) before starting treatment than for those who stayed for shorter (<24 h) before starting treatment. Chronic metabolic abnormalities such as severe dehydration, electrolyte imbalances, and acidosis, which become more ingrained the longer diabetic ketoacidosis persists, can account for this delay [35]. Evidences showed that patients with diabetic ketoacidosis durations longer than 97 h recovered much more slowly than those with shorter durations, and prompt treatment is essential to improving recovery outcomes [16,22].
Patients with diabetic ketoacidosis who had high random blood sugar levels (>500 mg/dl) recovered from their condition more slowly than those who had low random blood sugar levels. Because high glucose concentrations cause greater osmotic diuresis, which leads to more severe electrolyte imbalances and dehydration that take longer to correct with treatment, patients with diabetic ketoacidosis who have a high random blood sugar level (>500 mg/dL) would generally recover more slowly than those with lower blood sugar levels [16]. Therefore, a diabetic ketoacidosis patient with a very high random blood sugar level would need more intensive fluid resuscitation and electrolyte replacement, potentially extending their recovery time compared to a patient with a lower blood sugar level [4,14].
Patients with diabetes mellitus who had been in diabetic ketoacidosis for more than five years recovered from the condition more slowly than those who had been in diabetic ketoacidosis for five years or less. Diabetic ketoacidosis usually takes longer to recover from in patients with diabetes mellitus who have had the condition for more than five years than in those who have had it for less than five years. The reason for this is that long-term diabetes can lead to consequences including increased insulin resistance and advanced vascular damage, which make it harder for the body to regulate blood sugar and reverse the metabolic acidosis associated with diabetic ketoacidosis [36]. In order to recover from diabetic ketoacidosis, a patient with a longer history of diabetes could need more intense therapy and lengthier hospital stays than a patient with a shorter history [36,37].
4.1. Strength and limitation of the study
A comprehensive review of the literature that is specifically focused on a particular condition, the analysis of pooled data using reliable statistical techniques, and a thorough subgroup analysis to pinpoint the sources of heterogeneity.
The reliability of pooled estimates and this research conclusion may be impacted by the notable heterogeneity across included studies, and the small number of studies carried out in Africa may make them non-generalizable. Another constraint that was taken into consideration was the possibility of publishing bias.
5. Conclusion and Recommendation
This pooled median recovery time of diabetic ketoacidosis result showed that the recovery time of diabetic ketosis was prolonged as compared to the recommended one, which was 24 h. International medical guidelines state that with appropriate therapy, diabetic ketoacidosis should be recovered in around 24 h. The pronged median recovery time of diabetic ketoacidosis was reported in Ethiopia, while the fastest recovery time of diabetic ketoacidosis was reported in Egypt. As a result, early treatment and control of high blood sugar are advisable to faster the recovery time of diabetic ketoacidosis.
CRediT authorship contribution statement
Tadios Lidetu: Writing – review & editing, Software, Formal analysis, Data curation, Conceptualization. Simon Birhanu: Writing – review & editing, Conceptualization. Addisu Simachew Asgai: Writing – review & editing. Tsegaamlak Kumelachew Derse: Methodology. Yideg Abinew: Conceptualization. Moges Tadesse: Methodology. Desalegn Mitiku: Conceptualization. Jemberu Chane: Formal analysis, Conceptualization. Banchamilak Adane: Validation, Software. Tadele Kassahun Wudu: Validation, Software, Conceptualization. Betelhem Mekonnen: Data curation, Conceptualization. Desiyalew Habtamu Tamiru: Methodology, Data curation.
Ethical issues
Not applicable.
Human ethics and consent to participate declarations
Not applicable.
Clinical trial number declarations
Not applicable.
Consent for publication
Not applicable.
Funding
Not applicable.
Competing interest
The authors declare that there are no competing interests.
Acknowledgments
We would like to thank all the authors of the primary studies that included in this systematic-review and meta-analysis.
Contributor Information
Tadios Lidetu, Email: tadioslidetu@gmail.com.
Simon Birhanu, Email: abiubirhanu1221@gmail.com.
Addisu Simachew Asgai, Email: addisusimachew29@gmail.com.
Tsegaamlak Kumelachew Derse, Email: tsega441@gmail.com.
Yideg Abinew, Email: yideg18@gmail.com.
Moges Tadesse, Email: moges7045@gmail.com.
Desalegn Mitiku, Email: Desumitiku4@gmail.com.
Jemberu Chane, Email: chanejemberu@gmail.com.
Banchamilak Adane, Email: banchimu4@gmail.com.
Tadele Kassahun Wudu, Email: nabutane2017@gmail.com.
Betelhem Mekonnen, Email: betelhemmekonnen98@gmail.com.
Desiyalew Habtamu Tamiru, Email: desehabt@gmail.com.
ABBREVIATIONS
- CI
Confidence Interval
- DKA
Diabetic Keto-Acidosis
- DM
Diabetes Millitus
- NOS
Newcastle-Ottawa Scale
- PHR
Pooled Hazards Ratio
References
- 1.ElSayed N.A., Aleppo G., Bannuru R.R., Bruemmer D., Collins B.S., Ekhlaspour L., et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes–2024. Diabetes Care. 2024;47 doi: 10.2337/dc24-S008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon J.S., Kang S., Choi J.H., Lee K.A., Moon J.H., Chon S., et al. 2023 clinical practice guidelines for diabetes management in Korea: full version recommendation of the Korean diabetes association. Diabetes & Metabolism Journal. 2024;48(4):546–708. doi: 10.4093/dmj.2024.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel E.D., Gloyn A.L., Evans-Molina C., Joseph J.J., Misra S., Pajvani U.B., et al. Diabetes mellitus—progress and opportunities in the evolving epidemic. Cell. 2024;187(15):3789–3820. doi: 10.1016/j.cell.2024.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran T.T., Pease A., Wood A.J., Zajac J.D., Mårtensson J., Bellomo R., et al. Review of evidence for adult diabetic ketoacidosis management protocols. Front Endocrinol. 2017;8:106. doi: 10.3389/fendo.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyenwe E.A., Kitabchi A.E. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507–521. doi: 10.1016/j.metabol.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Smuel-Zilberberg K., Shalitin S., Yackobovitch-Gavan M., Phillip M., Nimri R. Diabetes ketoacidosis recovery in youth with newly diagnosed and established type 1 diabetes. Pediatr Res. 2022;91(5):1272–1277. doi: 10.1038/s41390-021-01618-z. [DOI] [PubMed] [Google Scholar]
- 7.Vicinanza A., Messaaoui A., Tenoutasse S., Dorchy H. Diabetic ketoacidosis in children newly diagnosed with type 1 diabetes mellitus: role of demographic, clinical, and biochemical features along with genetic and immunological markers as risk factors. A 20-year experience in a tertiary Belgian center. Pediatr Diabetes. 2019;20(5):584–593. doi: 10.1111/pedi.12864. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahimi F., Kutz A., Christ E.R., Szinnai G. Lifetime risk and health-care burden of diabetic ketoacidosis: a population-based study. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.940990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazeli Farsani S., Brodovicz K., Soleymanlou N., Marquard J., Wissinger E., Maiese B.A. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open. 2017;7(7) doi: 10.1136/bmjopen-2017-016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galindo R.J., Zambrano C., Vellanki P. Comment on Desai et al. Health Care Utilization and Burden of Diabetic Ketoacidosis in the U.S. Over the Past Decade: A Nationwide Analysis. Diabetes Care. 2019;42(2):e24. doi: 10.2337/dc18-1424. 2018;41:1631-1638. Diabetes Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akintoye E., Briasoulis A., Egbe A., Dunlay S.M., Kushwaha S., Levine D., et al. National trends in admission and in‐hospital mortality of patients with heart failure in the United States (2001–2014) J Am Heart Assoc. 2017;6(12) doi: 10.1161/JAHA.117.006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma R.C. Epidemiology of diabetes and diabetic complications in China. Diabetologia. 2018;61(6):1249–1260. doi: 10.1007/s00125-018-4557-7. [DOI] [PubMed] [Google Scholar]
- 13.Ramaesh A. Incidence and long-term outcomes of adult patients with diabetic ketoacidosis admitted to intensive care: a retrospective cohort study. J Intensive Care Soc. 2016;17(3):222–233. doi: 10.1177/1751143716644458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhatariya K.K., Glaser N.S., Codner E., Umpierrez G.E. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40. doi: 10.1038/s41572-020-0165-1. [DOI] [PubMed] [Google Scholar]
- 15.Barski L., Golbets E., Jotkowitz A., Schwarzfuchs D. Management of diabetic ketoacidosis. Eur J Intern Med. 2023;117:38–44. doi: 10.1016/j.ejim.2023.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Gosmanov A.R., Gosmanova E.O., Dillard-Cannon E. Management of adult diabetic ketoacidosis. Diabetes, Metab Syndrome Obes Targets Ther. 2014:255–264. doi: 10.2147/DMSO.S50516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaji R., Duraisamy R., Kumar M. Complications of diabetes mellitus: a review. Drug Invent Today. 2019;12(1) [Google Scholar]
- 18.Temesgen D., Miskir Y., Dessie G., Nuru A., Tesema B.B., Azmeraw M., et al. Time to recovery from diabetic ketoacidosis and its predictors among adult diabetic ketoacidosis patients in DEBRE MARKOS referral hospital, north west Ethiopia, 2021: retrospective cohort study. medRxiv. 2022 2022.04. 12.22273779. 19: 13-24. [Google Scholar]
- 19.Gosmanov A.R., Gosmanova E.O., Kitabchi A.E. Endotext; 2021. Hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. [Internet] [Google Scholar]
- 20.Rosenbloom A.L. The management of diabetic ketoacidosis in children. Diabetes ther. 2010;1:103–120. doi: 10.1007/s13300-010-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas S., Mohamed N., Bhana S. Audit of diabetic ketoacidosis management at a tertiary hospital in Johannesburg, South Africa. S Afr Med J. 2019;109(6):407–411. doi: 10.7196/SAMJ.2019.v109i6.13700. [DOI] [PubMed] [Google Scholar]
- 22.Sabry A.A., Alkafafy A.M., Morsy E.Y., Aiad A., Montasser M. Factors affecting time to recovery from diabetic ketoacidosis in adult diabetic patients in Alexandria Main University Hospital. The Egyptian Journal of Internal Medicine. 2024;36(1):99. [Google Scholar]
- 23.Hadgu F.B., Sibhat G.G., Gebretsadik L.G. Diabetic ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes in Tigray, Ethiopia: retrospective observational study. Pediatr Health Med Therapeut. 2019:49–55. doi: 10.2147/PHMT.S207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balmier A., Dib F., Serret-Larmande A., De Montmollin E., Pouyet V., Sztrymf B., et al. Initial management of diabetic ketoacidosis and prognosis according to diabetes type: a French multicentre observational retrospective study. Ann Intensive Care. 2019;9:1–8. doi: 10.1186/s13613-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.TEFERA . ADDIS ABABA University Repository site; 2023. Time to recovery from diabetic ketoacidosis and its predictors among children with type 1 diabetes at selected governmental hospitals in addis ababa, Ethiopia, 2023: retrospective follow-up study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abunga . REPOSITOY SITE; 2015. AN audit of the management of diabetic ketoacidosis at the KENYTTA national hospital. [Google Scholar]
- 27.Sidahmed S., Geyer S., Beller J. Socioeconomic inequalities in diabetes prevalence: the case of South Africa between 2003 and 2016. BMC Public Health. 2023;23(1):324. doi: 10.1186/s12889-023-15186-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastakia S.D., Pekny C.R., Manyara S.M., Fischer L. Diabetes in sub-Saharan Africa–from policy to practice to progress: targeting the existing gaps for future care for diabetes. Diabetes, Metab Syndrome Obes Targets Ther. 2017:247–263. doi: 10.2147/DMSO.S126314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thewjitcharoen Y., Plianpan P., Chotjirat A., Nakasatien S., Chotwanvirat P., Wanothayaroj E., et al. Clinical characteristics and outcomes of care in adult patients with diabetic ketoacidosis: a retrospective study from a tertiary diabetes center in Thailand. Journal of clinical & translational endocrinology. 2019;16 doi: 10.1016/j.jcte.2019.100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M.H., Calder G.L., Santamaria J.D., MacIsaac R.J. Diabetic ketoacidosis in adult patients: an audit of factors influencing time to normalisation of metabolic parameters. Intern Med J. 2018;48(5):529–534. doi: 10.1111/imj.13735. [DOI] [PubMed] [Google Scholar]
- 31.Abunga D.K. University of Nairobi; 2018. An audit of the management of diabetic ketoacidosis at the kenyatta national hospital. [Google Scholar]
- 32.Van den Berghe G., Kitabchi A.E., Fisher J.N. Hyperglycemic crises: diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS) Acute Endocrinology: From Cause to Consequence. 2008:119–147. [Google Scholar]
- 33.French E.K., Donihi A.C., Korytkowski M.T. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. Bmj. 2019;365 doi: 10.1136/bmj.l1114. [DOI] [PubMed] [Google Scholar]
- 34.Lee H.J., Yu H.W., Jung H.W., Lee Y.A., Kim J.H., Chung H.R., et al. Factors associated with the presence and severity of diabetic ketoacidosis at diagnosis of type 1 diabetes in Korean children and adolescents. J Kor Med Sci. 2017;32(2):303–309. doi: 10.3346/jkms.2017.32.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbloom A.L., Hanas R. Diabetic ketoacidosis (DKA): treatment guidelines. Clinical pediatrics. 1996;35(5):261–266. doi: 10.1177/000992289603500506. [DOI] [PubMed] [Google Scholar]
- 36.Alotaibi A., Aldoukhi A., Albdah B., Alonazi J.A., Alseraya A.S., Alrasheed N. Diabetic ketoacidosis treatment outcome and associated factors among adult patients admitted to the emergency department and medical wards at King Abdulaziz Medical City, Riyadh, Saudi Arabia. Cureus. 2020;12(8) doi: 10.7759/cureus.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umpierrez G.E., Davis G.M., ElSayed N.A., Fadini G.P., Galindo R.J., Hirsch I.B., et al. Hyperglycemic crises in adults with diabetes: a consensus report. Diabetes Care. 2024;47(8):1257–1275. doi: 10.2337/dci24-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]