Abstract
Fluorosis is a worldwide public health problem, in which the heart is an important target organ. However, studies on its toxicological mechanism in embryonic development are limited. This study assessed the toxicity of sodium fluoride (NaF) toward zebrafish embryos. We determined the mortality, hatching rate, phenotypic malformation, heart function, and morphology of zebrafish embryos after exposure to NaF. Subsequently, the molecular mechanism was revealed using high-throughput RNA sequencing analysis. The expression levels of key genes for heart development were detected using quantitative real-time reverse transcription PCR. The 50% lethal concentration (LC50) value of NaF toward zebrafish embryos at 96 h post-fertilization was 335.75 mg/L. When the concentration of NaF was higher than 200 mg/L, severe deformities, such as pericardial edema, yolk sac edema, spine curvature, shortened body length, reduced head area, and eye area, were observed. The heart rate of the embryos exposed to NaF decreased in a dose-dependent fashion. The distance between the sinus venosus and bulbus arteriosus was significantly increased in the NaF-exposed group compared with that in the control group. The stroke volume and cardiac output decreased significantly in the NaF groups. Compared with the control group, the expression levels of Gata4, Tbx5a, Hand2, Tnnt2c, Nppa, and Myh6 were significantly increased in the NaF-treated group. Through transcriptome sequencing, 1354 differentially expressed genes (DEGs) were detected in the NaF (200 mg/L) treated groups, including 1253 upregulated genes and 101 downregulated genes. Gene ontology functional analysis and Kyoto Encyclopedia of Genes and Genomes pathway analyses of the DEGs showed that cardiac-related pathways, such as actin cytoskeleton regulation, Jak-Stat, PI3k-Akt, and Ras, were activated in the NaF-exposed group. This study revealed the underlying mechanism of fluoride-induced cardiac morphological and functional abnormalities and provides clues for the clinical prevention and treatment of fluorosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12011-024-04381-4.
Keywords: Sodium fluoride, Zebrafish, Developmental abnormalities, Cardiotoxicity, RNA-seq
Background
Fluoride is widely present in the environment as a constituent of organic or inorganic substances, and plays a key role in humans and animals [1, 2]. In unpolluted surface water, the content of fluoride is 0.01–0.3 mg/L. However, fluoride can accumulate in surface water and groundwater because of both natural and man-made processes, such as the discharge of fluoride ions in domestic and industrial wastewater, the overuse of pesticides and fertilizers, the differentiation of natural fluorine-containing minerals, and volcanic eruptions [3]. The concentration of fluoride in the groundwater in fluorosis areas in China is 2.3–8 mg/L, and the concentration of fluoride in boiled water is higher than 5 mg/L, reaching as high as 45 mg/L. In industrial wastewater, the concentration of fluoride is about 96.8 mg/L; however, extreme levels of up to 3000–5000 mg/L have been reported [1]. Low concentrations of fluoride can prevent dental caries and help bone growth; however, high concentrations can cause not only dental and skeletal fluorosis, but also effects on the nervous system, cardiovascular system, and immune system [4–6].
The heart is the power organ of the circulatory system and is a target organ of fluorosis. Epidemiological surveys show that the detection and mortality rates of heart disease in residents in fluorosis areas are significantly higher than those in non-fluorosis areas [7]. An epidemiological survey of 26 patients with fluorosis showed that an excessive intake of fluoride caused a decrease in myocardial contractility [8]. In addition, excessive intake of fluoride has been associated with electrocardiographic changes and heart enlargement in humans [9], and peripheral vascular disease, ventricular diastolic dysfunction, and carotid atherosclerosis [10]. In vivo experiments have shown that excessive intake of fluoride can lead to reduced cardiac output and pathological changes in the heart tissue, such as extensive inflammatory cell infiltration and nuclear agglutination, muscle fiber damage, and mitochondrial abnormalities [11, 12]. These pathological changes are similar to those observed in myocardial hypertrophy and cardiomyopathy [13]. The above research results suggest that excessive intake of fluoride leads to defects in the structure and function of the heart, ultimately inducing a range of cardiovascular diseases. However, the mechanism by which fluoride causes cardiovascular disease in humans remains to be determined.
Zebrafish (Danio rerio), a small tropical freshwater teleost, is an ideal model organism to study vertebrate congenital heart disease [14]. Its features of short generation time, high fecundity, rapid development, and transparency during early developmental make it easy to carry out rapid toxicity assessment in vivo [15, 16]. In addition, zebrafish have similar cardiac developmental processes, cardiac function, and heart disease characteristics to humans [17]. It has been reported that homologs of about 70% of human genes can be found in zebrafish, and more than 80% of proteins that cause human diseases have homologs in zebrafish [18]. However, there has been no report on the effect of fluorine on zebrafish cardiac function and its regulatory mechanism.
In this study, we explored the cardiac teratogenicity and developmental toxicity of NaF using the zebrafish embryo model. The mechanism of NaF toxicity in the zebrafish embryonic heart was analyzed by transcriptomic analysis. This study revealed the mechanism of the cardiotoxicity caused by fluorosis and provided research data for the prevention and treatment of cardiac injury caused by fluorosis.
Methods
Chemicals
NaF (purity ≥ 99%, CAS: 7681–49-4) was purchased from Sigma (St. Louis, MO, USA), dissolved in egg water (0.54 g sea salt + 9 L double distilled water + 100 μL, 0.2% Methylene Blue) to reach stock solution of 20 g/L, and then stored at − 20 °C. The NaF exposure solution was prepared by adding an appropriate volume of the stock solution to egg water.
Animal Maintenance and NaF Exposure
Wild-type zebrafish (Tu) were maintained at 28.5 °C under a day-night cycle of 14-h light/10-h dark. Embryos were collected from a spawning box in which two males and two females were housed overnight. Embryos were gathered within 1 h post-fertilization (hpf) and maintained at 28.5 °C in egg water. Normally developing embryos were selected for subsequent experiments. Serial dilutions (0 mg/L, 100 mg/L, 200 mg/L, 300 mg/L) of NaF in egg water were prepared before each experiment. Embryos (6 hpf) were distributed into six-well plates (NEST, Shanghai, China) and incubated with 5 mL per well of NaF solution at the designated concentrations at 28.5 °C in a biochemical incubator. Zebrafish embryos treated with fresh egg water served as the control group. To maintain the NaF concentration, the exposure solution was renewed daily.
Cardiac Malformation and Functional Assessment
The larvae were randomly selected at 120 hpf and anesthetized using 150 mg/L tricaine, and then fixed in 3% methylcellulose (Sigma) to capture lateral-view images. The morphology of the embryos was observed and photographed using a ZEISS Discovery.V20 microscope (ZEISS, Oberkochen, Germany) equipped with a Nikon camera (Tokyo, Japan). The length of sinus venosus (SV) and bulbus arteriosus (BA) was defined as the SV-BA distance, which were measured from digital images of whole-mount embryos, as previously described [19]. The heart rate (HR) was also determined under the ZEISS Discovery.V20 microscope. We measured the long axis length (a) and short axis length (b) between the myocardial borders of the ventricles at diastole and systole, respectively. The formula: volume = 4/3πab2 [20] was used to calculate the end-diastolic volume (EDV) and the end-systolic volume (ESV). To measure the HR, we counted the number of heart contractions in 15-s intervals. The stroke volume (SV) was calculated using the equations SV = EDV-ESV, and cardiac output (CO) was calculated as CO = SV × HR [21].
Isolation of RNA and Sequencing
In the control group and the 200 mg/L NaF-treated group, 60 larval zebrafish at 120 hpf were employed to extract RNA for RNA sequencing (RNA-seq), and each group was composed of three biological replicates (n = 3). Consequently, 360 larvae were used for six RNA-seq samples. The experiment was conducted by Sangon Biotech (Shanghai, China). Total RNA was extracted using TRIzol (Sangon Biotech). The concentration of the total RNA was determined using a Qubit2.0 fluorometer (Invitrogen, Paisley, UK). Agarose gel electrophoresis was used to detect RNA integrity and genome contamination. After mRNA isolation, fragmentation, double-stranded cDNA synthesis, end repair, A-tailing, adaptor ligation, and library amplification, the RNA samples were finally transformed into a library suitable for sequencing on the Illumina® platform (Hieff NGS™ MaxUp Dual-mode mRNA Library Prep Kit for Illumina®, YEASEN, Shanghai, China). Wayne diagram was performed using the online tool (https://bioinformatics.psb.ugent.be/webtools/Venn/). The Database for Annotation, Visualization, and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/, version DAVID 2021) provides a comprehensive set of functional annotation tools for investigators to understand the biological significance of a list of genes. The results of functional enrichment analysis and volcano plot were visualized by by R software package ggplot2. The heat map was visualized by by R software package (pheatmap).
Total RNA Isolation
Total RNA Kit I (OMEGA: R6834-01) was used for total RNA isolation. Collect 60 zebrafish embryos/group, then add TRK Lysis Buffer and Lyse, after homogenizing, add equal volume alcohol, washing three times, centrifuging 12,000 g for 2 min, add 50 µL Nuclease-free Water, centrifuge at top speed for 2 min, and store eluted RNA at − 70 °C.
Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) Analysis
To verify the RNA-seq data, we extracted total RNA from 120-hpf larvae and quantified it using a Nano Drop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The RNA was reverse transcribed into cDNA (RevertAid First Strand cDNA Synthesis Kit, Thermo Fisher Scientific), and the qPCR step of the qRT-PCR protocol was carried out using SYBR Green Master Mix (Takara, Dalian, China) on a CFX 96 instrument (BIO-RAD, Hercules, CA, USA). The relative mRNA expression levels were normalized using the 2−ΔΔCT method, with gapdh (encoding glyceraldehyde-3-phosphate dehydrogenase) as the housekeeping gene. Three separate samples were tested three times. We using the online tool (https://bioinfo.ut.ee/primer3-0.4.0/) to design primer sequences. The design parameter is that primer size from 18 to 25 bp, primer Tm from 57.0 to 63 °C, primer GC% from 40 to 60, and product size from 100 to 200 bp. The sequences of the primers used are shown in Table 1.
Table 1.
Primers used for different genes in Danio rerio
| Primer name | GenBank Number | Product length | Sequence |
|---|---|---|---|
| Gapdh-forward/reverse | NM_001115114.1 | 130 bp | 5¢-tccatctttgacgctggtgctg-3¢/5¢-actccttggaggccatgtgtgc-3¢ |
| Rac1a-forward/reverse | NM_199771.1 | 141 bp | 5¢-gcgagatgacaaggacaccatcg-3¢/5¢- ttaaggccgcgttgggttaagg-3¢ |
| Cdc42l-forward/reverse | NM_199865.3 | 147 bp | 5¢-cccgagagtggggagaagctgt-3¢/5¢-gcgcttcttgggtttggtctca-3¢ |
| Socs3b-forward/reverse | NM_213304.1 | 128 bp | 5¢-cccccaccggtacaagaccttc-3¢/5¢- tcagcagagtgctggcctcctt-3¢ |
| Hsp90b1-forward/reverse | NM_198210.2 | 149 bp | 5¢-tgctctggttgccagtcagtatgg-3¢/5¢- tgatgagtggatgtttggggttga-3¢ |
| Pak2a-forward/reverse | XM_005171263.4 | 100 bp | 5¢-tgttgctactggccaagaggttgc-3¢/5¢- cagctccttcatcaccaggatctca-3¢ |
| Nppa-forward/reverse | NM_198800.3 | 112 bp | 5¢-atggccgggggactaattctga-3¢/5¢-tgagcttggccatgttgctgtc-3¢ |
| Tnnt2c-forward/reverse | NM_181499.3 | 112 bp | 5¢-cctgacggggagaaggtggact-3¢/5¢-cctccttctgcctggtggtgaa-3¢ |
| Stat3-forward/reverse | XM_021469347.1 | 138 bp | 5¢-cacaacctgcaggacatcaggaa-3¢/5¢-tccattcaggtcctgggacagc-3¢ |
| C3a.3-forward/reverse | XM_021475507.1 | 143 bp | 5¢-gcggtatgcaaacacccctcag-3¢/5¢-cgtcctcgttgttctttggtttgc-3¢ |
| C6-forward/reverse | NM_200638.2 | 132 bp | 5¢-gaatgcgggattgagcctttga-3¢/5¢-gcgcccacagtctctctcatca-3¢ |
| F9b-forward/reverse | NM_001040310.1 | 124 bp | 5¢-cacgcttcagcgggaagaactg-3¢/5¢-ccctcggcacagtcacacactg-3¢ |
| Fgb-forward/reverse | NM_212774.1 | 135 bp | 5¢-ggagccgaggacacaaagcaaa-3¢/5¢-tggtttgactgcatcgctctga-3¢ |
| F5-forward/reverse | NM_001328531.1 | 122 bp | 5¢-tggacagcaaagaaccggacaa-3¢/5¢-gtccacgcaatgtaggcccaag-3¢ |
| Hand2-forward/reverse | NM_131626.3 | 125 bp | 5¢-cgccaaagaagaaaggcgaaaga-3¢/5¢-gcttcagctccaatgcccaaac-3¢ |
| Gata4-forward/reverse | NM_131236.2 | 114 bp | 5¢-gaggagacgcgacccatcaaga-3¢/5¢- ggatccgcttggagagcccata-3¢ |
| Tbx5a-forward/reverse | XM_021475995.1 | 142 bp | 5¢-acctgctgcctcagtccagctc-3¢/5¢- tgatcatcctcgctggaggaactc-3¢ |
| Myh6-forward/reverse | NM_198823.1 | 121 bp | 5¢-tagcgggttggctggtgaagaa-3¢/5¢-ccgccgtctgatcctgcataac-3¢ |
Ethical Statement
The study was carried out in compliance with the Guide for the Care and Use of Laboratory Animals. All experiments were approved by the Committee on the Ethics of Guizhou University of Traditional Chinese Medicine, 20240418128.
Statistical Analysis
The results are reported as the means ± SEM (standard error of mean). All data were analyzed using SPSS 23 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism7 software (GraphPad Software Inc., La Jolla, CA, USA). The significant differences between the exposure groups and control group were subsequently analyzed statistically using one-way analysis of variance (ANOVA) followed by Dunnett’s test using SPSS 23. A value of p < 0.05 was considered statistically significant.
Results
The Lethal Effect of Sodium Fluoride
Exposure to various concentrations of NaF showed different toxicities toward the embryos. The mortality rate was used to calculate the LC50 at 96 hpf using Kow’s method, which showed that 96 h-LC50 for NaF exposure was 335.75 mg/L, with a 95% confidence interval of 296.48–380.23 mg/L (Table 2). Therefore, we used 100 mg/L, 200 mg/L, and 300 mg/L of NaF for subsequent experiments.
Table 2.
Effect of NaF exposure on the mortality rate of zebrafish embryos (means ± SEM)
| Concentration | Mortality rate, % | Mortality rate, % | Mortality rate, % |
|---|---|---|---|
| 72 hpf | 96 hpf | 120 hpf | |
| Control | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 100 mg/L | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 200 mg/L | 1.67 ± 1.67 | 11.67 ± 1.67 | 15.00 ± 2.89 |
| 300 mg/L | 13.33 ± 1.67 | 21.67 ± 1.67 | 38.33 ± 4.41 |
Developmental Toxicity of Sodium Fluoride Toward Zebrafish Embryos or Larvae
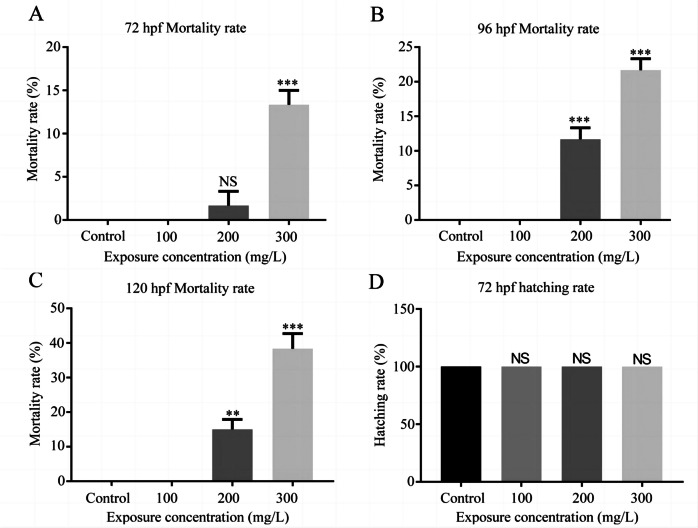
All NaF-treated embryos and control embryos survived at 24 and 48 hpf. However, there were significant differences in the mortality rate between the NaF-treated (200 and 300 mg/L) and the control groups at 72, 96, and 120 hpf (Fig. 1A–C). In the control group, and the 100, 200, and 300 mg/L NaF groups, the hatching rates at 72 hpf were 100% (Fig. 1D and Table S1).
Fig. 1.
Mortality and hatching rate of zebrafish after exposure to NaF. A Mortality rate of 72-hpf embryos exposed to NaF. B Mortality rate of 96-hpf embryos exposed to NaF. C Mortality rate of 120-hpf embryos exposed to NaF (n = 20 for each concentration, replicated thrice). D Hatching rate of 72-hp embryos exposed to NaF. Data are presented as means ± SEM (n = 60 for each concentration, replicated thrice). *p value < 0.05, **p value < 0.01, ***p value < 0.001
Effects of Fluoride Poisoning on Zebrafish Embryos
Severely deformed morphology was observed in the NaF-treated zebrafish embryos at 120 hpf, such as pericardial edema, yolk sac edema, and spinal curvature (Fig. 2A). In addition, compared with that in the control group, the deformity rates observed in the 200 and 300 mg/L groups were significantly increased in a dose-dependent manner (Fig. 2B). Pericardial edema was the most common malformation in the 200 and 300 mg/L treatment groups, based on the number of deformities in each group (Fig. 2C).
Fig. 2.
Malformation of zebrafish larvae exposed to different NaF doses at 120 hpf. A Malformation rate caused by different dosage of NaF in 120-hpf zebrafish larvae. B Representative images of the malformation in sodium fluoride-treated larvae (arrows indicate abnormalities): yolk sac edema (YSE), pericardial edema (PE), and spinal curvature (SC). C The prevalence rate of malformations in zebrafish larvae exposed to NaF at 120 hpf. Data are presented as the mean ± SEM (n = 10 embryos for each concentration, replicated thrice). NS, not significant; p value > 0.05; **p value < 0.01; ***p value < 0.001
Toxic Effects of Fluorosis on Juvenile Zebrafish
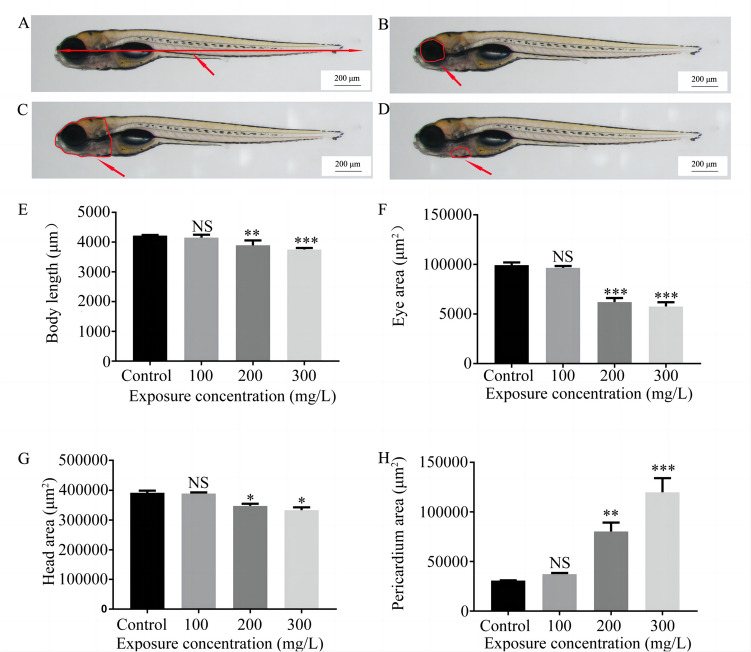
The body length, head area, eye area, and pericardium area of each group of larvae were measured at 120 hpf to evaluate the developmental toxicity of NaF toward larvae. The obvious morphological changes were shortening of the body length (Fig. 3A), decreased head area (Fig. 3B) and eye area (Fig. 3C), and increased pericardium area (Fig. 3D). All of these phenotypes were dose-dependent.
Fig. 3.
Phenotypes of 120-hpf larvae exposed to different NaF concentrations. A Body length. B Head area. C Eye area. D Pericardium area. Data are presented as means ± SEM (n = 10 with each concentration replicated thrice). **p value < 0.01, ***p value < 0.001
Morphological Effects of Sodium Fluoride on the Cardiac System in Zebrafish
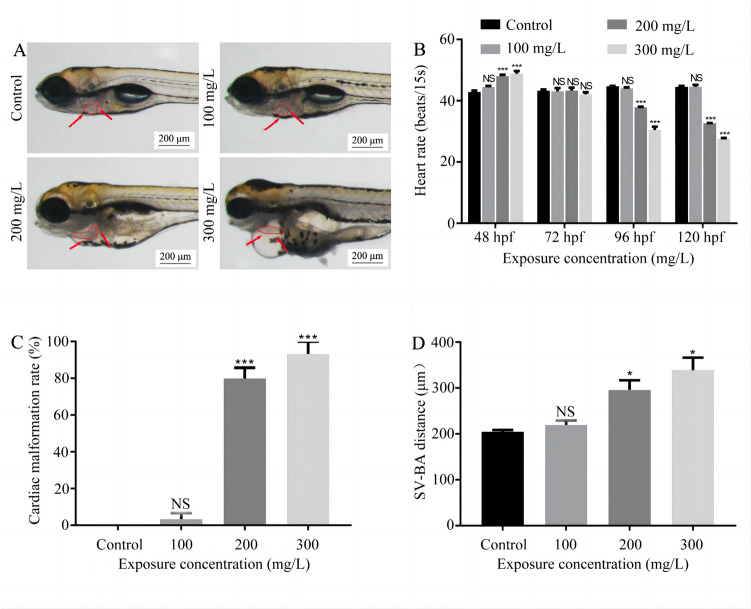
We evaluated alterations to the heart morphology induced by NaF at 120 hpf. A series of cardiac malformations appeared in a dose-dependent manner, including pericardial edema and abnormal cardiac loop (Fig. 4A). To further assess the effects of NaF exposure on heart function, we recorded the HR of larvae at 48, 72, 96, and 120 hpf. The results showed that the HR increased after NaF treatment at 48 hpf, while NaF treatment had no significant effect on the HR at 72 hpf. At 96 and 120 hpf, the 200 and 300 mg/L NaF-treated groups had significantly lower HRs (Fig. 4B). At 120 hpf, in the control group, the hearts were S-Shaped with no visible pericardial edema (Fig. 4C). However, the hearts of the NaF-exposed group were string-like and elongated. Treatment with NaF also induced an increased in the SV-BA distance (Fig. 4D).
Fig. 4.
Effect of NaF on the heart rate and morphology of zebrafish embryos. A Representative images of the alteration to the heart morphology induced by NaF in zebrafish embryos at 120 hpf. (V: ventricle; A: atrium). B Heart rate of 48, 72, 96, and 120 hpf zebrafish embryos exposed to NaF (n = 10, with each concentration replicated thrice). C Rate of cardiac malformation at 120 hpf. D Quantification of the SV-BA distance as a measure of NaF-induced morphological alteration of zebrafish embryo hearts at 120 hpf. Data are the mean ± SEM (n = 10, with three replicates for each concentration). *p < 0.05, ***p < 0.001
Functional Effects of Sodium Fluoride on the Cardiac System in Zebrafish
To explore the changes in cardiac function caused by NaF exposure, we examined the EDV, ESV, stroke volume, and CO of larvae at 120 hpf. The NaF-treated larvae showed a significant decrease in EDV in the 300 mg/L group and an increase in the ESV in the 100 mg/L and 200 mg/L groups; however, the ESV was significantly decreased in the 300 mg/L group (Fig. 5A). The stroke volume was significantly decreased in the 200 and 300 mg/L groups (Fig. 5B). The CO was significantly decreased in all three NaF-exposed groups (Fig. 5C).
Fig. 5.
Cardiac function in 120 hpf zebrafish larvae exposed to NaF. A Volume of the ventricle at end-diastole (EDV) and end-systole (ESV). B Stroke volume. C Cardiac output. Data are the mean ± SE (n = 5, with three replicates for each concentration). *p value < 0.05, **p value < 0.01, ***p value < 0.001
Differential Gene Expression Caused by Sodium Fluoride
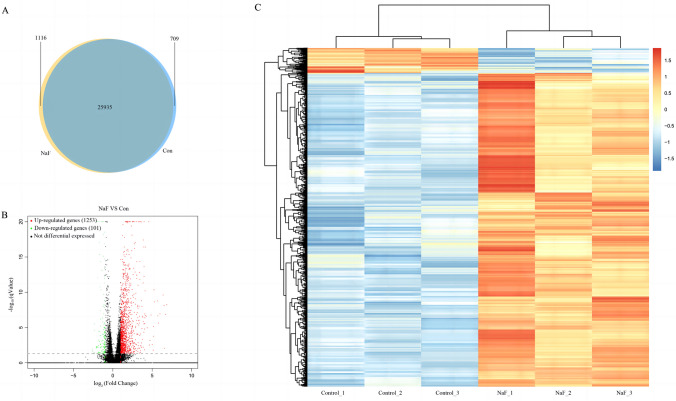
RNA-seq analysis was used to unravel the underlying mechanisms of NaF toxicity in zebrafish. After sequencing, we first evaluated the quality of sequencing data using FastQC, followed by Trimmomatic analysis to obtain clean data (Table S2). HISAT2 was used to compare the clean data of the samples with the reference genome and count the mapping information (Table S3). We found that 1116 (4.02%) genes were specifically expressed and 709 (2.55%) genes were absent in the NaF exposure group compared with the control group (Fig. 6A). Zebrafish differentially expressed genes (DEGs) were selected using DEGseq, with the filter conditions of qValue < 0.05 and |log2FoldChange|> 1. Compared with the control group, the NaF (200 mg/L) exposure group had 1253 upregulated genes and 101 downregulated genes (Fig. 6B). A heatmap of significant DEGs upon NaF treatment is shown in Fig. 6C.
Fig. 6.
Identification of differentially expressed genes (DEGs) and their heatmap in zebrafish after exposure to NaF. A Venn diagram of the total number of identified genes in the NaF exposure and control groups. B Volcano plot constructed using fold-change values and adjusted p values (p adj). Red: upregulated, Blue: downregulated. C The lower row of the heat map consists of the detailed configuration of the left three normal samples and the right three NaF exposure samples. Red indicates upregulated genes, and blue indicates downregulated genes
Cardiac Development-Related Gene Expression
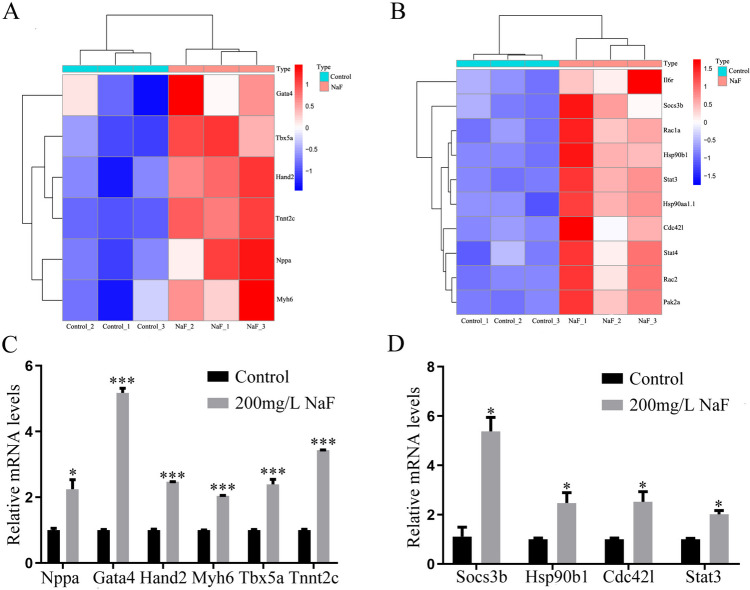
Based on the RNA-seq results, the expression of genes related to cardiac development, such as, Gata4 (encoding GATA binding protein 4), Tbx5a (encoding T-box transcription factor 5A), Hand2 (encoding heart and neural crest derivatives expressed 2), Tnnc2 (encoding troponin C2, fast skeletal type), Nppa (encoding natriuretic peptide A), and Myh6 (encoding myosin heavy chain 6) were significantly increased after exposure to NaF (Fig. 7A, B). The RT-qPCR results showed that the expression level of Nppa, Gata4, Tbx5a, Hand2, Tnnc2, and Myh6 increased significantly in the NaF-treated group compared with that in the control group (Fig. 7C), which agreed with the RNA-seq results.
Fig. 7.
Expression levels of cardiac development-related genes and enrichment pathways related to cardiotoxicity. A Heat map of cardiac development-related genes. B Heat map showing the upregulated genes for pathways related to cardiotoxicity. C Relative mRNA expression level of cardiac development-related genes from the results of RNA-Seq, as analyzed using qRT-PCR. D Relative mRNA expression level of the upregulated genes for pathways related to cardiotoxicity from the results of RNA-Seq, as analyzed using qRT-PCR. The data are presented as the mean ± SEM (n = 3, with three replicates for each concentration). *p value < 0.05, ***p value < 0.001
GO Functional and KEGG Pathway Analyses of DEGs
Gene Ontology enrichment analysis was used to reveal the biological functions of all the DEGs. A histogram of DEG GO enrichment can intuitively reflect the number of genes in the molecular function (MF), cellular component (CC), and biological process (BP) categories. We selected the top 30 significantly enriched GO terms (18 BP, 6 CC, and 6 MF) (Fig. 8A). In organisms, the core signal transduction and biochemical metabolism pathways involving DEGs can be preliminarily identified through KEGG enrichment analysis. Our results showed that the DEGs were enriched in regulation of actin cytoskeleton, Jak-STAT, PI3K-Akt, Rap1, Focal adhesion, Ras, MAPK, and cAMP signaling pathways. To investigate the potential connections between these signaling pathways and NaF-induced cardiac dysplasia, we tested the expression levels of the core genes of these signaling pathways. The heat map results showed that expression levels of these core genes were significantly upregulated (Fig. 8B).
Fig. 8.
Alterations to gene expression after exposure to NaF at 200 mg/L. A GO enrichment histogram; the ordinate shows the 30 most significant enriched GO terms, and the abscissa shows the number of DEGs associated with each term. Blue indicates biological processes, green indicates cellular components, and red represents molecular function. B KEGG enrichment scatter plot for the DEGs; the vertical axis represents the name of the pathway and the horizontal axis represents the gene ratio. The gene ratio refers to the ratio of the number of DEGs in this pathway term. The size of the dot indicates the number of DEGs in this pathway, and the color of the dot corresponds to different Q value ranges. The closer the color is to red, the more significant the enrichment
Expression of Genes Associated with the Complement and Coagulation Cascade Pathway
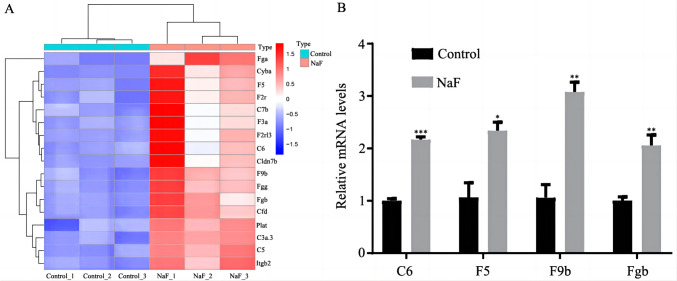
The KEGG results showed that “complement and coagulation cascades” was the most enriched pathway. To investigate the potential connection between this pathway and NaF-induced cardiac teratogenicity, we analyzed the expression levels of c6 (encoding complement C6), f5 (encoding coagulation factor V), f9b (encoding coagulation factor IXb), and fgb (encoding fibrinogen beta chain). According to the RNA-seq results, the expression levels of c6, f5, f9b, and fgb were significantly increased, as shown in the heat map (Fig. 9A). Moreover, the RT-qPCR results were similar to the trend observed using RNA-Seq, indicating the reliability of the RNA-Seq data (Fig. 9B). Thus, NaF leads to cardiac defects partially by activating the complement and coagulation cascade pathway.
Fig. 9.
The effect of NaF (200 mg/L) exposure on the complement and coagulation cascade pathway activation-related genes in 120 hpf zebrafish larvae. A Heatmaps showing the up- and downregulated genes detected by RNA-seq. Three independent biological samples per group are represented. Red indicates upregulated genes, and blue indicates downregulated genes. B Relative mRNA expression level of genes related to the complement and coagulation cascade pathway, as assessed using RT-qPCR. Data are presented as the mean ± SEM (n = 3, with three replicates for each concentration). *p value < 0.05, ** p value < 0.01, ***p value < 0.001
Discussion
Epidemiological survey results show that compared with those in residents in low-fluoride areas, the rates of heart disease and mortality of residents in high-fluoride areas are significantly higher [13]. However, the molecular mechanism of fluoride-induced heart disease is unknown. We utilized the zebrafish larvae as a model to assess the toxic effects of NaF on cardiac development. Our findings suggest that NaF exposure causes cardiac developmental malformations, including pericardial edema, and cardiac structural and functional impairment.
The heart is the first organ to form and plays a critical role during the development of embryos. The zebrafish heart is mature and functional at 72 hpf [22–24], and its morphological structure and function are similar to the human heart during the early stage of development [25]. In recent years, zebrafish have been widely used as a vertebrate model to research human cardiovascular disease [26]. In addition, they are widely used for environmental toxicity detection and drug toxicity safety evaluation [27, 28]. The heart must undergo a series of complex processes as it matures, and any defect in these processes can lead to congenital heart disease [29]. The evaluation of NaF o is necessary for safety and zebrafish is a well-accepted animal model for researching “predictive toxicology” [30]. Early embryos are often used to assess toxicity during embryonic development [31]. Embryos (6 hpf) were distributed into six-well plates and incubated with NaF at the designated concentrations in this study. Zebrafish exposed to NaF exhibited severe heart malformation, such as pericardial edema, cardiac loop deformity, and an increased SV-BA distance. These cardiac morphological abnormalities are similar to the toxic effects of other pollutants, such as prothioconazole [32], triadimefon [33], and pyrimethanil [34]. A correctly looping heart is critical for the formation of a normal cardiac structure [35]. Previous reports indicated that HR is a key parameter in evaluating heart function [32, 36]. Our results showed that NaF exposure decreased the HR at 96 hpf and 120 hpf. These results suggested that NaF decreases the HR at the late stage of development. Overall, we hypothesized that NaF exposure leads to cardiac malformation in zebrafish.
Our results suggested that NaF might be toxic to heart development. As the NaF concentration increased, the severity of heart teratogenicity increased. However, little study has explored the mechanism of NaF-induced cardiotoxicity. To explore potential environmental health risks and mechanism induced by NaF, we conducted RNA-seq analysis in zebrafish larvae. In zebrafish, cardiac morphogenesis is regulated by critical genes, including Gata4, Tbx5a, Hand2, Tnnt2c, Nppa, and Myh6. In cardiac morphogenesis, Gata4 plays a critical role [37] and is a key gene in regulating cardiomyocyte proliferation and cardiac septal development [38]. The expression level of Gata4 and the distance of SV-BA were significantly increased in the NaF-treated groups. It is suggested that NaF-treated may lead to abnormal cardiac septal development. Transcription factor Tbx5a is vital for cardiac morphogenesis [39]. In zebrafish, the cardiac phenotype of Tbx5a deficiency was similar to Holt-Oram syndrome [40]. Interestingly, hyperactivity of Tbx5a also causes cardiac defects [41]. Transcription factor Hand2 is an important regulator of embryonic cardiac development and promotes cardiac reprogramming and ventricular cardiomyocyte expansion [42, 43]. The precise expression level of Hand2 is essential for normal heart morphogenesis and function [44, 45]. Cardiac troponin T (Tnnt2c) regulates the interaction between myosin and actin, and plays an important role in myofbrillogenesis in zebrafish [46, 47]. Atrial natriuretic peptide (Nppa) expression in the early-developing heart shows a restricted and dynamic pattern [48]. Nppa is a key and sensitive marker for the developing heart. For example, the patterning of Nppa expression is altered in congenital heart disease [49]. In addition, the upregulation of Nppa expression causes cardiac deformity [50]. In the developing heart, Myh6 is highly expressed and mutations of Myh6 lead to atrial septal defects and cardiomyopathy [51]. Our results showed that the expression level of the critical genes (Gata4, Tbx5a, Hand2, Tnnc2, Nppa, and Myh6) were significantly increased in the NaF-treated groups, indicating that NaF exposure causes cardiac dysmorphogenesis through these key genes.
In addition, we used GO and KEGG to analyse the potential mechanisms of cardiac deformity caused by NaF exposure. The results of bioinformatic analysis offer novel insights into the underlying mechanism. After exposure to NaF, activation of the Ras signaling pathway was detected in the 120 hpf zebrafish, indicating that NaF might lead to heart development deformity through Ras signaling. Both the Ras and Rho (RhoA, Rac1, and Cdc42) subfamilies belong to the small G protein superfamily, which regulates many cellular responses, and overexpression of RhoA promotes lethal heart failure. Rac1 and Cdc42 regulate the actin/myosin cytoskeleton in many cells [52]. Our results showed that the expression level of Cdc42l was increased in the NaF exposure group at 120 hpf, further suggesting that NaF activates the Ras signaling pathway.
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway can transduce numerous of cytokines, such as tumor necrosis factor (TNF), interleukin (IL) 1β, IL-6, and transforming growth factor beta (TGFβ), and then regulates a series of physiological and pathophysiological processes, including cell differentiation, cell proliferation and apoptosis, immune responses, and inflammation [53]. Our results showed that the expression level of Stat3 was significantly increased in the NaF-treated groups. This suggests that NaF activates inflammatory signaling pathways. IL-6 might mediate heart failure by activating the JAK/STAT signaling pathway. Suppressor of cytokine signaling (SOCS) regulates the JAK/STAT signaling pathway, and their interaction with other inflammatory factors might lead to heart failure. IL-6-like cytokines, JAK/STAT signaling, and SOCS play key roles in the regulation of heart failure [54]. In this study, the expression level of Socs3b was significantly increased in the NaF-treated groups, it is further indicated that activation the JAK/STAT pathway play a critical role in NaF-induced cardiac deformity.
Complement cascade and blood coagulation are two major contributors to the first line of defense against infection. The complement system plays a critical role in innate and adaptive immunity [55]. In addition to the detection and removal of pathogens [56], the complement system also participates in many other life activities, such as the removal of immune complexes, the promotion of angiogenesis, tissue regeneration, and lipid metabolism [57]. Therefore, the abnormal activation and expression of the complement system in the body will unbalance homeostasis, resulting in a variety of autoimmune and inflammation-related diseases [58]. It was reported that the complement and coagulation cascade pathway is associated with the risk of heart failure, myocardial infarction, and cardiovascular-related mortality in patients with established coronary heart disease [59]. The above studies suggest that the complement and coagulation cascade pathway play a critical role in cardiovascular disease by mediating inflammation. Our results showed that the complement and coagulation cascade pathway was the most enriched pathway in the NaF-treated group. This indicated that NaF might induce cardiac malformation through the complement and coagulation cascade pathway. In the present study, the expression levels of c3a.3, c5, c6, and c7b were significantly increased in the NaF exposure group as a result of the strong immune toxicity of NaF. The expression levels of f3a, f5, f9b, and f2r were significantly increased in the NaF exposure group. The F2r gene, also known as par1, contributes to inflammatory responses. Studies indicated that Par1 activation increased inflammation [60]. To sum up, we suspected that these factors could affect the autoimmune response of zebrafish, leading to inflammation and apoptosis, and thus might initiate the sequential process of heart development.
Conclusion
In conclusion, this study found that NaF exposure induces numerous structural and functional malformations during zebrafish development. Our comprehensive analysis of DEGs and their associated enriched pathways revealed a complicated relationship between the effects on the immune system and the cardiac system. NaF is excessively used and frequently detected; therefore, a more comprehensive understanding of its toxic effects is important to improve the risk assessment and supervision of such environmental pollution. This study provides some clues for the clinical prevention and treatment of patients with fluorosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
WFQ, LY, TDX, FC, and WS conceived of and designed the study. With full access to all data, they take responsibility for the integrity of the data, the accuracy of the data analysis, and the writing of the report. YJL, TTT, and TMX critically revised the report. HYW, YJL, and TTT performed the statistical analyses. All the authors contributed to the data acquisition and analyses. All the authors have reviewed and approved of the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 82060079, 81670290); the Natural Science Foundation of Guizhou Province (grant numbers QianKeHe Support [2022]181, ZK(2021)357, Qiankehe Cooperation Platform Talents [2021] Postdoctoral Station 007); the Research Project of Education Department of Guizhou Province (grant number QianJiaoJi [2023]037, QianJiaoJi (2022)219); the Natural Science Foundation of Guiyang City (grant numbers [2019]9–2, [2022]4–3-2, [2022]4–3-10); and the Subject Excellent Reserve Talent Project (grant number gyfyxkrc-2023–14). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data Availability
No datasets were generated or analyzed during the current study.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feiqing Wang, Fa Chen, and Wen Song contributed equally to this work.
Contributor Information
Dongxin Tang, Email: tangdongxintcm@163.com.
Yang Liu, Email: ly7878@163.com.
References
- 1.Jianjie C, Wenjuan X, Jinling C (2016) Fluoride caused thyroid endocrine disruption in male zebrafish (Danio rerio). Aquat Toxicol 171:48–58 [DOI] [PubMed] [Google Scholar]
- 2.Li M, Cao J, Chen J et al (2016) Waterborne fluoride exposure changed the structure and the expressions of steroidogenic-related genes in gonads of adult zebrafish (Danio rerio). Chemosphere 145:365–375 [DOI] [PubMed] [Google Scholar]
- 3.Camargo JA (2003) Fluoride toxicity to aquatic organisms: a review. Chemosphere 50(3):251–264 [DOI] [PubMed] [Google Scholar]
- 4.DeyBhowmik A, Shaw P, Mondal P et al (2021) Calcium and vitamin D supplementation effectively alleviates dental and skeletal fluorosis and retain elemental homeostasis in mice. Biol Trace Elem Res 199(8):3035–3044 [DOI] [PubMed] [Google Scholar]
- 5.Adali MK, Varol E, Aksoy F et al (2013) Impaired heart rate recovery in patients with endemic fluorosis. Biol Trace Elem Res 152(3):310–315 [DOI] [PubMed] [Google Scholar]
- 6.Perumal E, Paul V, Govindarajan V et al (2013) A brief review on experimental fluorosis. Toxicol Lett 223(2):236–251 [DOI] [PubMed] [Google Scholar]
- 7.Fihmonov SN, Panev NI, Korotenko OY et al (2016) Evaluation of risk factors in atherosclerosis in workers with chronic fluorine intoxication. Med Tr Prom Ekol 5:6–11 [PubMed] [Google Scholar]
- 8.Yuan ZW (1990) A study of left ventricular function of workers exposed to fluorine. Zhonghua Yu Fang Yi Xue Za Zhi [Chin J Prevent Med] 24(4):217–219 [PubMed] [Google Scholar]
- 9.Flora SJ, Pachauri V, Mittal M et al (2011) Interactive effect of arsenic and fluoride on cardio-respiratory disorders in male rats: possible role of reactive oxygen species. Biometals: Int J Role Metal Ions Biol Biochem Med 24(4):615–628 [DOI] [PubMed] [Google Scholar]
- 10.Yan X, Dong N, Hao X et al (2019) Comparative transcriptomics reveals the role of the toll-like receptor signaling pathway in fluoride-induced cardiotoxicity. J Agric Food Chem 67(17):5033–5042 [DOI] [PubMed] [Google Scholar]
- 11.Kiyuna LA, Albuquerque RPE, Chen CH et al (2019) Targeting mitochondrial dysfunction and oxidative stress in heart failure: challenges and opportunities. Free Radic Biol Med 129:155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HW, Liu J, Zhao J et al (2018) Ca(2+) metabolic disorder and abnormal expression of cardiac troponin involved in fluoride-induced cardiomyocyte damage. Chemosphere 201:564–570 [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Yan X, Xu G et al (2020) ITRAQ-based proteomics reveals the potential mechanism of fluoride-induced myocardial contraction function damage. Ecotoxicol Environ Saf 197:110605 [DOI] [PubMed] [Google Scholar]
- 14.Driever W, Solnica-Krezel L, Schier AF et al (1996) A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123:37–46 [DOI] [PubMed] [Google Scholar]
- 15.Han L, Xia Q, Zhang L et al (2019) Induction of developmental toxicity and cardiotoxicity in zebrafish embryos/larvae by acetyl-11-keto-beta-boswellic acid (AKBA) through oxidative stress. Drug Chem Toxicol 1–8. 10.1080/01480545.2019.1663865 [DOI] [PubMed]
- 16.Gu J, Wang H, Zhou L et al (2020) Oxidative stress in bisphenol AF-induced cardiotoxicity in zebrafish and the protective role of N-acetyl N-cysteine. Sci Total Environ 731:139190 [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Gao Q, Feng Z et al (2021) Protective effects of spermidine and melatonin on deltamethrin-induced cardiotoxicity and neurotoxicity in zebrafish. Cardiovasc Toxicol 21(1):29–41 [DOI] [PubMed] [Google Scholar]
- 18.Molina B, Chavez J, Grainger S (2020) Zebrafish models of acute leukemias: current models and future directions. Wiley Interdiscip Rev Dev Biol e400. 10.1002/wdev.400 [DOI] [PMC free article] [PubMed]
- 19.Antkiewicz DS, Burns CG, Carney SA et al (2005) Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci: Off J Soc Toxicol 84(2):368–377 [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Huang W, Dahme T et al (2008) Depletion of zebrafish essential and regulatory myosin light chains reduces cardiac function through distinct mechanisms. Cardiovasc Res 79(1):97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Zhang Y, Chen M et al (2019) Exposure to low-level metalaxyl impacts the cardiac development and function of zebrafish embryos. J Environ Sci 85:1–8 [DOI] [PubMed] [Google Scholar]
- 22.Fishman MC, Chien KR (1997) Fashioning the vertebrate heart: earliest embryonic decisions. Development 124(11):2099–2117 [DOI] [PubMed] [Google Scholar]
- 23.Stainier DY, Lee RK, Fishman MC (1993) Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 119(1):31–40 [DOI] [PubMed] [Google Scholar]
- 24.Stainier DY (2001) Zebrafish genetics and vertebrate heart formation. Nat Rev Genet 2(1):39–48 [DOI] [PubMed] [Google Scholar]
- 25.Thisse C, Zon LI (2002) Organogenesis–heart and blood formation from the zebrafish point of view. Science 295(5554):457–462 [DOI] [PubMed] [Google Scholar]
- 26.Li H, Trager LE, Liu X et al (2022) lncExACT1 and DCHS2 regulate physiological and pathological cardiac growth. Circulation 145(16):1218–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong X, Zhang L, Zha J (2022) Toxicity of waterborne vortioxetine, a new antidepressant, in non-target aquatic organisms: from wonder to concern drugs? Environ Pollut 304:119175 [DOI] [PubMed]
- 28.Lu J, Wang W, XU W et al (2022) Induction of developmental toxicity and cardiotoxicity in zebrafish embryos by Emamectin benzoate through oxidative stress. Sci Total Environ 825:154040 [DOI] [PubMed]
- 29.Beis D, Bartman T, Jin SW et al (2005) Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132(18):4193–4204 [DOI] [PubMed] [Google Scholar]
- 30.Sipes NS, Padilla S, Knudsen TB (2011) Zebrafish: as an integrative model for twenty-first century toxicity testing. Birth Defects Res C Embryo Today 93(3):256–267 [DOI] [PubMed] [Google Scholar]
- 31.He L, Chen Y, Hu Z et al (2021) Evaluation of 3,4,4,9-trichlorocarbanilide to zebrafish developmental toxicity based on transcriptomics analysis. Chemosphere 278:130349 [DOI] [PubMed]
- 32.Sun Y, Cao Y, Tong L et al (2020) Exposure to prothioconazole induces developmental toxicity and cardiovascular effects on zebrafish embryo. Chemosphere 251:126418 [DOI] [PubMed]
- 33.Liu HC, Chu TY, Chen LL et al (2017) The cardiovascular toxicity of triadimefon in early life stage of zebrafish and potential implications to human health. Environ Pollut 231(Pt 1):1093–1103 [DOI] [PubMed] [Google Scholar]
- 34.Meng Y, Zhong K, Xiao J et al (2020) Exposure to pyrimethanil induces developmental toxicity and cardiotoxicity in zebrafish. Chemosphere 255:126889 [DOI] [PubMed]
- 35.Ramasubramanian A, Nerurkar NL, Achtien KH et al (2008) On modeling morphogenesis of the looping heart following mechanical perturbations. J Biomech Eng 130(6):061018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson WA, Shvartsburd Z, Vijayan MM (2022) The antidepressant venlafaxine perturbs cardiac development and function in larval zebrafish. Aquat Toxicol 242:106041 [DOI] [PubMed]
- 37.Holtzinger A, Evans T (2005) Gata4 regulates the formation of multiple organs. Development 132(17):4005–4014 [DOI] [PubMed] [Google Scholar]
- 38.Ang YS, Rivas RN, Ribeiro AJS et al (2016) Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell 167(7):1734–49 e22 [DOI] [PMC free article] [PubMed]
- 39.Ahn DG, Kourakis MJ, Rohde LA et al (2002) T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature 417(6890):754–758 [DOI] [PubMed] [Google Scholar]
- 40.Garrity DM, Childs S, Fishman MC (2002) The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development 129(19):4635–4645 [DOI] [PubMed] [Google Scholar]
- 41.Tong X, Zu Y, Li Z et al (2014) Kctd10 regulates heart morphogenesis by repressing the transcriptional activity of Tbx5a in zebrafish. Nat Commun 5:3153 [DOI] [PubMed]
- 42.Mcfadden DG, Barbosa AC, Richardson JA et al (2005) The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development 132(1):189–201 [DOI] [PubMed] [Google Scholar]
- 43.Song K, Nam YJ, Luo X et al (2012) Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485(7400):599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vandusen NJ, Casanovas J, Vincentz JW et al (2014) Hand2 is an essential regulator for two Notch-dependent functions within the embryonic endocardium. Cell Rep 9(6):2071–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchihashi T, Maeda J, Shin CH et al (2011) Hand2 function in second heart field progenitors is essential for cardiogenesis. Dev Biol 351(1):62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang W, Zhang R, Xu X (2009) Myofibrillogenesis in the developing zebrafish heart: a functional study of tnnt2. Dev Biol 331(2):237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parmacek MS, Solaro RJ (2004) Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis 47(3):159–176 [DOI] [PubMed] [Google Scholar]
- 48.Zeller R, Bloch KD, Williams BS et al (1987) Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev 1(7):693–698 [DOI] [PubMed] [Google Scholar]
- 49.Bruneau BG (2011) Atrial natriuretic factor in the developing heart: a signpost for cardiac morphogenesis. Can J Physiol Pharmacol 89(8):533–537 [DOI] [PubMed] [Google Scholar]
- 50.Kim YC, Lee SR, Jeon HJ et al (2020) Acute toxicities of fluorene, fluorene-1-carboxylic acid, and fluorene-9-carboxylic acid on zebrafish embryos (Danio rerio): molecular mechanisms of developmental toxicities of fluorene-1-carboxylic acid. Chemosphere 260:127622 [DOI] [PubMed]
- 51.Jin SC, Homsy J, Zaidi S et al (2017) Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet 49(11):1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clerk A, Sugden PH (2000) Small guanine nucleotide-binding proteins and myocardial hypertrophy. Circ Res 86(10):1019–1023 [DOI] [PubMed] [Google Scholar]
- 53.Igaz P, Toth S, Falus A (2001) Biological and clinical significance of the JAK-STAT pathway; lessons from knockout mice. Inflamm Res: Off J Eur Histamine Res Soc 50(9):435–441 [DOI] [PubMed] [Google Scholar]
- 54.Terrell AM, Crisostomo PR, Wairiuko GM et al (2006) Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock 26(3):226–234 [DOI] [PubMed] [Google Scholar]
- 55.Song WC, Sarrias MR, Lambris JD (2000) Complement and innate immunity. Immunopharmacology 49(1–2):187–198 [DOI] [PubMed] [Google Scholar]
- 56.Ricklin D, Lambris JD (2013) Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol 190(8):3831–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricklin D, Hajishengallis G, Yang K et al (2010) Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11(9):785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan BP, Harris CL (2015) Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discovery 14(12):857–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Zhang Y, Liu YM et al (2020) Uncovering the protective mechanism of Huoxue Anxin Recipe against coronary heart disease by network analysis and experimental validation. Biomed Pharmacother = Biomed Pharmacother 121:109655 [DOI] [PubMed]
- 60.Tang X, Ma X, Cao J et al (2022) The influence of temperature on the antiviral response of mIgM(+) B lymphocytes against Hirame novirhabdovirus in flounder (Paralichthys olivaceus). Front Immunol 13:802638 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.