Abstract
Cystic fibrosis (CF) is an inherited condition that leads to multiorgan dysfunction, especially in the respiratory, gastrointestinal, and reproductive tracts, with associated conditions including persistent pulmonary infection, liver disease, pancreatic insufficiency, and infertility. Historically, people with CF (pwCF) suffered a shortened lifespan due to complications of the condition, namely respiratory. The emphasis on center-based, multidisciplinary care and the widespread introduction of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy has resulted in pwCF living longer and healthier lives. Now they may encounter some of the health and social issues associated with growing older, which previously were not a typical experience for this population. In this article, we review relevant health issues for the aging CF population, including complications that arise from the condition itself, issues encountered due to treatment, and general conditions associated with aging that may manifest earlier or differently in pwCF. We discuss the recommendations for screening and treatment of relevant conditions, and considerations for the integration of healthcare professionals across disciplines into the care of this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40266-025-01207-3.
Key Points
| People with cystic fibrosis are living longer with an ever-growing number entering the geriatric age group. |
| The implementation of center-based care and the widespread use of CFTR modulators has contributed to the increased life expectancy, necessitating a renewed focus on diseases traditionally associated with aging and not experienced by this patient population. |
| Recommendations for screening for chronic conditions, such as diabetes and bone disease, among others, differ in this population, and there are additional considerations for drug–drug interactions related to CFTR modulators and other chronic CF-related therapies. |
Introduction
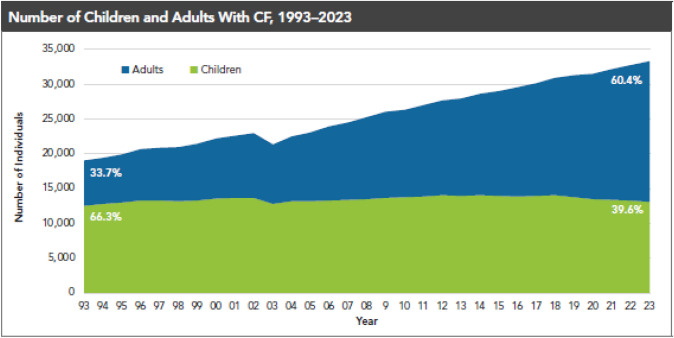
Cystic fibrosis (CF) is an inherited autosomal recessive condition with an estimated population of nearly 40,000 children and adults living with CF in the USA and more than 160,000 people with CF (pwCF) worldwide [1]. CF is caused by defects in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, an epithelial surface channel that governs chloride and bicarbonate conductance in various organs, including the sweat glands and the respiratory, gastrointestinal, and reproductive tracts. Dysfunction or absence of the CFTR channel leads to a range of adverse health consequences, including bronchiectasis with persistent pulmonary infection, pancreatic insufficiency, liver disease, and infertility, among others [2]. These contribute to considerable comorbidities requiring chronic medications and therapies, as well as frequent hospitalizations to address acute CF-related complications. Historically, CF was also associated with early mortality (Fig. 1), which improved incrementally with center-based specialty care and medications approved to treat the consequences of CF complications.
Fig. 1.
The adult CF population is growing. The number of children (< 18 years) and adults (≥ 18 years) reported in the Cystic Fibrosis Foundation Patient Registry (1). The pediatric population has remained fairly stable over time with patients transitioning into adulthood at a rate similar to new diagnoses at birth, but the adult population has grown steadily and is predicted to continue
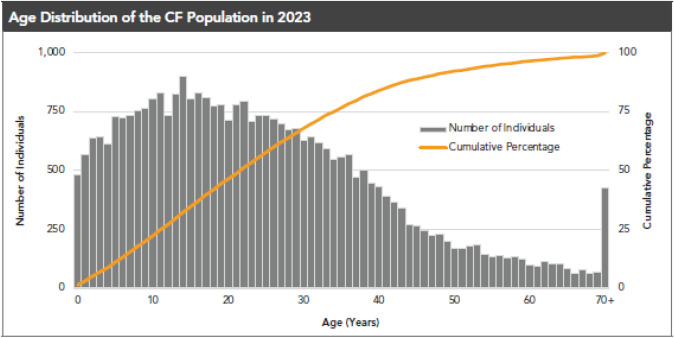
In addition to the advent of center-based care, the development of CFTR modulators, small molecules that address the basic defect in CF by increasing the quantity and function of CFTR protein at the cell surface [3], has led to remarkable improvements in the health of pwCF. There are fewer symptoms (e.g., cough and sputum production), fewer events that lead to hospitalization, and a marked increase in the predicted survival of pwCF [4–6]. The result has been an increase in the number of adults with CF, reaching parity with the pediatric population in ~2014. Adults now represent a majority of the CF population (Fig. 1). In addition, there is an increasing number of pwCF reaching into later adult years (Fig. 2) with ~ 25% of pwCF over the age of 40 years [7]. These observations suggest a need for healthcare providers to have greater knowledge of CF and its complications to best address this aging population.
Fig. 2.
People with CF are living longer and age distribution is changing. The Cystic Fibrosis Patient Registry shows that people with CF (pwCF) are living longer and the predicted survival has increased considerably. Approximately 25% of pwCF are > 35 years of age, with many living into their 60s (1)
What follows is a summary of relevant healthcare conditions for an aging CF population. Some of these are known sequelae of CF, others are related to the consequences of CF treatments, and there are issues relevant to all adults (not just those with CF) but not previously a common experience or expectation for pwCF. Finally, we will comment on how care can be best provided for the adult with CF, addressing the roles of the CF center as well as primary care providers or consultants who may encounter pwCF.
CF-Related Complications
Bronchiectasis and Persistent Airway Infection
The natural history of pulmonary disease in pwCF starts with the defect in CFTR activity, resulting in a reduction of airway surface liquid and impaired mucociliary clearance. This leads to retention of phlegm in the airways and creates a vulnerable environment for chronic bacterial infection. The ensuing inflammatory response is exaggerated, leading to a greater amount of phlegm in the airways, causing obstruction and injury, leading to the development of bronchiectasis. Typical daily symptoms include cough with sputum production; there are also periods of worsening with increased pulmonary and systemic symptoms and reduced lung function, deemed pulmonary exacerbations [8]. The result is a progressive loss of lung function and reduced survival. Typical therapies to manage airways disease include regular use of airway clearance therapies [9] and aerosolized treatments including hypertonic saline, dornase alfa, and antibiotics. These symptomatic therapies addressed the symptoms associated with the downstream consequences of reduced CFTR activity.
The introduction of CFTR modulators, which unlike the symptomatic therapies address the underlying defect, have led to better clearance of airway phlegm, fewer symptoms, improved lung function, and fewer pulmonary exacerbations for many pwCF [4, 5]. The bronchiectasis does not reverse, nor does the persistence of infection, although the burden of infection is significantly lessened such that some patients may no longer grow a pathogen in their respiratory cultures. However, many patients will continue to suffer pulmonary exacerbations. CFTR modulators may not be effective for some because of unresponsive mutations, often in people from minority groups [10, 11], or because of adverse reactions to the medications [12]. Thus, there is a heterogeneous population of pwCF in terms of their eligibility for CFTR modulators as well as their tolerance of them.
Some pwCF will have improvement in symptoms and lung function in response to CFTR modulators such that it is hard to appreciate the benefit of other CF-approved aerosolized therapies. Short-term trials have demonstrated no apparent adverse response to stopping these therapies [13], but there remain pwCF who will continue to benefit from them. Those pwCF with advanced lung disease and those who cannot use the CFTR modulators may still benefit from their chronic therapies.
Although registry data suggest that infection rates decreased with increasing age and there are fewer hospitalizations in older populations (age > 60 years) [7], we must be cautious with our interpretation of these data. Because CF patients generally had an earlier mortality, only those pwCF with milder manifestations of disease would survive into older years. Now that many pwCF are benefiting from CFTR modulators, we will see many more patients living into later decades, but it is not yet known whether they will have similar outcomes to those with inherently milder disease. For example, imaging of adult patients with a good clinical response to CFTR modulators demonstrate persistent bronchiectasis (Supplementary Fig. 1); it remains to be seen what happens to pwCF who are started on CFTR modulators early in life, potentially delaying the development of bronchiectasis and slowing the development of more severe impairment.
Pancreatic Insufficiency
Most pwCF have pancreatic insufficiency and will need supplemental digestive enzymes. Although this status has been shown to change in pediatric subjects in response to CFTR modulators, this does not seem to be the case in adult pwCF, and they will continue to need enzyme replacement to properly digest their food. However, the CFTR modulators may normalize the pH in the gastrointestinal tract, making the digestive enzymes more effective, perhaps contributing to the development of obesity seen in pwCF [14]. This could alter symptoms in response to this change in the intestinal milieu, but the dose of enzymes should not be decreased as a response; these are typically titrated to the size and content of the meals to assure sufficient enzymes to digest the food [15].
Abdominal discomfort is a common symptom reported by pwCF. There can be many etiologies (e.g., cholecystitis, pancreatitis) but the most common is fecal obstruction of the colon (also known as constipation). An abdominal x-ray is most helpful in these cases with evidence of a significant amount of stool without signs of complete bowel obstruction (i.e., air–fluid levels). The recommended approach consists of osmotic laxatives to clear the bowels, increased hydration, and consideration for a maintenance bowel regimen [16].
Cystic-Fibrosis-Related Diabetes (CFRD)
CFRD is a common complication of CF, affecting up to 20% of teenagers and half of adults over the age of 50 years [17]. Although the pathogenesis of CFRD is complex and not fully understood, it is largely driven by insulin insufficiency through progressive damage to pancreatic tissue, including insulin-producing beta cells. However, there is no direct correlation between the degree of pancreatic fibrosis and development or severity of diabetes, suggesting alternative mechanisms such as a direct influence of CFTR on beta cells and a contribution of insulin resistance [18, 19]. Risk factors for developing CFRD include severe genotype, exocrine pancreatic insufficiency, liver disease, systemic corticosteroid use, and nocturnal continuous enteral feedings [17, 18]. CFRD is associated with increased mortality and greater decline in lung function over time, as well as the development of microvascular complications [20].
Typical diabetes features such as polyuria, polydipsia, and ketoacidosis are rare, thus screening is important to detect disease. The recommended screening for CFRD is a 2-h oral glucose tolerance test (OGTT), with guidance for interpretation outlined in guidelines [20]. Alternative diagnostic recommendations exist for CFRD diagnosis during acute illness and for patients on continuous enteral feeding [20]. Hemoglobin A1c is thought to underestimate the degree of hyperglycemia in CFRD, and thus is not recommended as a single test for screening. Continuous glucose monitoring has been proposed as an alternative screening tool for CFRD, however, to date there are no specific guidelines for its use in screening or treatment monitoring [21].
Management of CFRD is similar to Type 1 DM and includes insulin as the therapy of choice [20]. To date, oral agents used for type 2 diabetes have not proven efficacious for CFRD, but there may be a role for glucagon-like peptide (GLP) agonists or dipeptidyl peptidase-4 inhibitors [17, 22–24]. Microvascular complications, including retinopathy, neuropathy, and nephropathy, are seen in CFRD, albeit at lower rates than in type 1 or 2 DM [25]; annual screening with eye exam, foot exam, and urine albumin excretion is recommended beginning 5 years after the diagnosis, or if the time of diagnosis is not known, when fasting hyperglycemia is documented [18]. The CF Foundation has recommended inclusion of an endocrinologist as an essential partner to a CF center [26].
Cystic-Fibrosis-Related Bone Disease
Bone disease is another common extrapulmonary complication of CF. Prior to the development of CFTR modulators, the prevalence of osteoporosis in young adults was 23.5%, and the prevalence of osteopenia was 38% [27]. Annualized losses in bone mineral density in young adults with CF are similar to postmenopausal women [28]. Rates of low bone density and fragility fractures are even higher in patients awaiting lung transplantation, and if severe, can preclude eligibility for transplant [29]. With an aging CF population we expect complications from bone disease to become a more prominent issue, although the impact of CFTR modulators is unclear.
The pathogenesis of CF bone disease is multifactorial. It arises from an interplay of factors, including the inability to achieve peak bone mass in early adulthood, impaired fat-soluble vitamin absorption, and delayed puberty. Medication use, including glucocorticoids and proton pump inhibitors, may also play a role, along with the impact of recurrent infections, chronic inflammation, and comorbid disease states, including liver disease and diabetes. CFTR may also directly impact bone homeostasis and remodeling [28, 30].
The recommended screening for CF-related bone disease is dual-energy x-ray absorptiometry (DXA) for all adults (age 18 years and older) and especially if undernourished, with poor lung function (i.e., FEV1 < 50% predicted), glucocorticoids > 5 mg/day for > 90 days/year, or history of fracture. For interpretation in adults 18–30 years, T- and Z-scores are equivalent, whereas T-scores should be used for those 30 years or older [28]. The World Health Organization (WHO) classification for osteopenia (T-score less than − 1 and greater than − 2.5 SD) and osteoporosis (T score less than or equal to − 2.5 SD) are also used in CF-related bone disease [31]. Treatment recommendations as well as the frequency of repeat DXA are determined by T- and Z-scores (see Table 1) [28]. The treatment of CF-related bone disease is controversial. Guidelines recommend treatment of osteoporosis with bisphosphonates, which act by reducing bone resorption and may be given orally or intravenously (IV) [32]. In a 2023 Cochrane review, bisphosphonates were shown to increase bone mineral density at the lumbar spine and hip in adults. The impact of highly effective modulator therapy on the natural history of bone disease is not yet known, however, several ongoing observational studies are seeking to evaluate this question.
Nutrition and Obesity
In previous decades, pwCF were generally undernourished because of their pancreatic insufficiency as well as increased caloric usage given their underlying lung disease, persistent infection, and excess inflammation. Attention to nutritional health had been a priority and pwCF increased their weight and body mass index (BMI), and this is likely a major contributor to improved survival over time. It is well established that lung health is associated with nutritional status [33, 34] and the CFF established guidance for a target BMI for male (23 kg/m2) and female individuals (22 kg/m2) in response to these observations [35].
Over the last two decades there has been a reduction in the number of pwCF categorized as undernourished (i.e., BMI < 18 kg/m2); however, an analysis of the CF Registry showed a steady increase in the proportion of pwCF who meet the criteria for overweight and obese [14, 36]. Factors associated with obesity included milder CFTR mutations, pancreatic sufficiency, milder lung impairment, and the use of CFTR modulators [14, 36]. At the time of that analysis, relatively fewer pwCF were prescribed CFTR modulators; now that most pwCF are taking CFTR modulators, there is even greater interest on the impact this will have on the prevalence of obesity in this population. Proposed mechanisms of weight gain related to CFTR modulators include reduced resting energy expenditure, improved nutrient absorption, and increased caloric intake [37] on a background of generally poor diet quality, which includes high saturated fat and low fiber intake [38]. Long-term (1 year) of exposure to modulators, particularly elexacaftor/tezacaftor/ivacaftor (ETI), has been associated with increased rates of metabolic syndrome, defined by abnormalities in fasting glucose, HDL cholesterol, triglycerides, waist circumference, and hypertension [39].
Pharmacologic therapies, including GLP-1 receptor agonists, have been increasingly prescribed for weight management in the general population [40], and there is interest in the effectiveness and safety of these medications in pwCF. One small study of pwCF treated with GLP-1 agonists showed associations with weight loss, as well as improved glycemic control in the subset of patients with CFRD[41]. Larger studies are needed to confirm this finding. Overweight and obesity are epidemic in the USA, with an estimated prevalence of 75% in adults in the general population[42]. Overnutrition is a problem in both pwCF and the general population that is projected to increase over time [42], with associated complications including type 2 diabetes, cardiovascular disease, hypertension, obstructive sleep apnea, and certain types of cancer [43].
Liver Disease
Liver disease affects up to 40% of pwCF with a broad array of manifestations. CFTR is present along the apical surface of cholangiocytes in bile ducts and plays a role in bile acid production and homeostasis. CFTR dysfunction leads to biliary stasis and fibrosis, with subsequent cirrhosis and portal hypertension [44]. Not all liver disease in pwCF stems from CFTR dysfunction; other contributing factors include medication toxicity, nutritional deficiencies, pancreatic dysfunction, recurrent infections, and hepatic congestion [44]. Excessive alcohol use has also been identified in the adult population [45]. The majority of cases of serious liver disease with cirrhosis and portal hypertension manifest before the age of 18 years, and 75% of liver transplants in pwCF are in children [46]. However, since pwCF are living longer and may be experiencing more conditions associated with metabolic dysfunction, as detailed above, there is the potential for increased incidence of liver disease later in life.
Liver disease in cystic fibrosis has been broadly defined as CF hepatobiliary disease (CFHBI), with advanced CF liver disease (aCFLD) representing a subset with more severe disease (Table 2). For all adults, annual screening for CFHBI with labs (including total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyltransferase, and platelet count) and physical examination to evaluate for hepatosplenomegaly are recommended [47]. Adults should have a baseline abdominal ultrasound (US) to assess the liver and spleen [47]. Hepatic steatosis is a common finding in pwCF, but is not hypothesized to be directly related to CFTR dysfunction; other factors including nutritional factors as well as obesity have been implicated [44]. Liver elastography is useful to detect early fibrosis and is recommended annually in all adults with identified CFHBI [47]. There are no specific treatments for CFHBI. Ursodeoxycholic acid (UDCA) is a bile acid that has been used in cholestatic liver conditions, including CF-related liver disease. Given conflicting data regarding its efficacy, and no robust evidence that it prevents aCFLD, routine use is not recommended in pwCF [47, 48].
Hepatotoxicity is common adverse effect of CFTR modulators, and monitoring of liver function tests is recommended at baseline, every month for the first 6 months of therapy, and periodically thereafter [49]. There is a box warning for drug-induced liver injury and liver failure for both elexacaftor/tezacaftor/ivacaftor (ETI, or Trikafta) and the new, once-daily modulator vanzacaftor/tezacaftor/deutivacaftor (Alyftrek). Modulators can be safe to use in CFHBI and even after liver transplantation, but should be used cautiously in patients with aCFLD, particularly in the setting of decompensated or advanced disease; these patients should be managed in close conjunction with a hepatologist and a pharmacist [47, 49].
Complications Associated with Treatment of CF
Consequences of Medications
PwCF are likely to have been treated with aminoglycosides throughout their lives. Pseudomonas aeruginosa is one of the most common pathogens overall, and especially in adults or those with more advanced lung impairment. Current strategies to address P. aeruginosa include eradication at first identification in respiratory cultures and suppression of the bacteria in those with chronic infection [50, 51]. Aerosolized tobramycin has been commonly used for both of these circumstances, either as a limited duration (for eradication) or continuous therapy (for suppression). In addition, pwCF are subject to intermittent events of clinical worsening called pulmonary exacerbations; these are often manifest as increased cough and sputum production, loss of weight, increased fatigue, and a reduction in pulmonary function [8, 52]. Oftentimes these are treated with systemic antibiotics, which may also include aminoglycosides such as tobramycin. Intravenous or inhaled amikacin may be used as part of treatment of nontuberculous mycobacterial infection, which affects approximately 12–25% of pwCF [53].
The most common toxicities of aminoglycosides include nephrotoxicity and ototoxicity, including cochleotoxicity and vestibulotoxicity [54, 55]. These can occur acutely or could be a result of the cumulative effect of many courses of antibiotics. Symptoms of ototoxicity may include tinnitus, sensorineural hearing loss, or vertigo/balance issues, which can be permanent [55]. Therapeutic drug monitoring is recommended for systemic aminoglycoside use [56] but acute effects on hearing can be detected even with careful dose monitoring [57]. The prevalence of hearing loss in the CF population varies widely in registry data and studies, but is likely underappreciated and associated with cumulative exposure [58–60].
Given the potential to receive ototoxic medications, including aminoglycosides, macrolides, and glycopeptides such as vancomycin, the CF Foundation recommends a baseline hearing study for all adults and children with CF, and annual monitoring in pwCF who continue to receive ototoxic medications [55]. Careful medication reconciliation can help minimize the use of other potential ototoxic medications, including nonsteroidal antiinflammatory drugs (NSAIDs) and diuretics, when possible, especially if intravenous aminoglycosides are being used [61].
The majority of eligible pwCF take CFTR modulators, including elexacaftor/tezacaftor/ivacaftor (Trikafta), tezacaftor/ivacaftor (Symdeko), and lumacaftor/ivacaftor (Orkambi). Of these, Trikafta is the most commonly prescribed [58]. Pharmacologic interactions are possible between the CFTR modulators and other agents that are metabolized in the liver by cytochrome P450 family of enzymes; additional interactions are possible through effects on drug transport proteins [62]. Recent comprehensive reviews on drug–drug interactions with modulators are available [62, 63], and a pharmacist should be engaged when starting new medications on pwCF.
Complications Related to Vascular Access
PwCF who are treated with systemic antibiotics for pulmonary exacerbations may have vascular access, including peripherally inserted central catheters (PICCs), placed to make home intravenous therapy easier. Many pwCF will have had multiple devices placed over their lifetime or may have a totally implantable venous access device (TIVAD), e.g. a port, placed for recurrent use [64]. Complications of PICC lines include insertion site pain, thrombosis, and infection. Historically, larger diameter and multi-lumen PICCs were associated with higher complication rates, including deep venous thrombosis (DVT) [65]. Prolonged pain after insertion and catheter obstruction were also common, affecting up to 20% of patients [66]. Patient-associated risk factors for complications have been identified, including poor nutritional status and multiple line placements [64]. A recent prospective, observational study showed that placement of single lumen, smaller diameter (e.g., ≤ 4.5 French) PICCs under ultrasound guidance has low rates of vascular and infectious complications in pwCF [67].
The reduction in pulmonary exacerbations attributed to CFTR modulators should reduce the need for indwelling catheters, and removal of TIVADs should be considered to avoid potential long-term complications. It is also important to have knowledge of catheter usage, as this may inform evaluation of unusual symptoms (e.g., edema in an extremity) or planning for a surgical procedure.
Issues that pwCF May Get to Experience that are More Typical of Adulthood
Since most, if not all, children with CF will age into adulthood, there should be planning on how to live a long life, much as for any other child. Transitions from adolescence, young adulthood, and older adulthood have long been discussed in the CF literature and attention is placed on assisting families with preparing their child with CF for eventual independence. Although much of this has focused on the health care issues as they move into an adult clinic, there is also discussion about relationships, as well as greater emphasis on sexual issues, education, and career planning, among others.
Reproductive Health and Parenthood
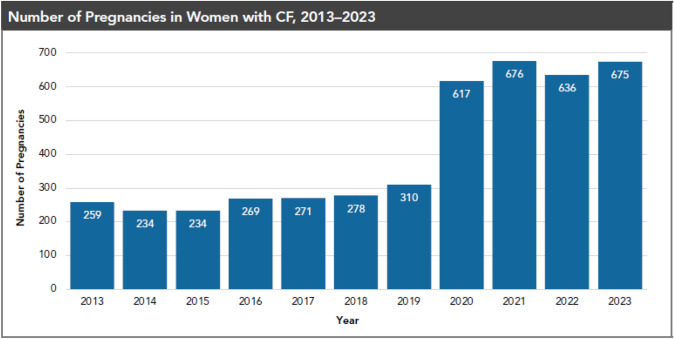
Almost all male and many female individuals with cystic fibrosis experience infertility. Historically, the presence of subfertility in female individuals with CF has been estimated to be around 35%, which is higher than the rate of 5–15% in the general population [68]. Dysfunction of CFTR in the female reproductive tract causes dehydrated cervical mucus, as well as an altered intrauterine milieu that may be inhospitable for fertilization. In addition, nutritional deficiencies and chronic inflammation may contribute to anovulation [69]. Since the introduction of modulators in 2019, there has been a significant increase in pregnancy rates in female individuals with CF (Fig. 3). Overall, studies do not demonstrate a significant impact of pregnancy on maternal health with regard to survival, decline in lung function, or rates of pulmonary exacerbations [70]. The Maternal and Fetal Outcomes in the Era of Modulators (MAYFLOWERS) study is an ongoing multicenter observational trial that is examining the impact of pregnancy in CF in the era of widespread modulator use [71]. Gestational diabetes is common and management is similar to women without CF [70]. Female individuals with CF should be counseled about the possibility for pregnancy and options for contraception.
Fig. 3.
Rising numbers of pregnancies in women with CF. The reported pregnancies in the CF Foundation Patient Registry (1) showed a marked increase following the introduction of elexacaftor/tezacaftor/ivacaftor
There remain uncertainties regarding pregnancy in CF as most female pwCF who become pregnant will also be on some medications. Some of these drugs are known to be safe (e.g., beta-lactams, dornase), while others are likely to be safe (e.g., macrolides), and there is limited guidance on medications during pregnancy [72]. There is a growing experience of CFTR modulators during pregnancy that suggests they are likely to be safe, balancing the risk of stopping these medications with the potential risk for continuance [73].
Most male individuals with cystic fibrosis are infertile because of congenital bilateral absence of the vas deferens, leading to a lack of sperm in the ejaculate fluid. Diagnosis can be confirmed with both semen analysis showing azoospermia, as well as with physical examination and scrotal ultrasound. Male individuals with CF produce normal sperm and can father biological children with assisted reproductive technology, which includes sperm retrieval through testicular or epididymal aspiration/biopsy and subsequent intrauterine insemination or in vitro fertilization [74].
While some pwCF may choose to pursue pregnancy, other options for family building exist and can be explored. These options include adoption, step-parenthood, foster care, and surrogacy [75].
Menopause
Menopause is defined as the cessation of menstrual periods for 12 consecutive months, with an average age of onset of 51 years in the general population [76]. A total of 80–90% of women will experience symptoms with menopause, including vasomotor symptoms such as night sweats and hot flashes, cognitive and mood changes, and urogenital issues. Menopause also influences long-term health with impacts on bone loss, the development of cardiovascular disease, and cognitive function [76]. There are few data on menopause in female individuals with CF, although one study found a median age of onset of menopause to be earlier (48.5 years) than in the general population. A significant proportion of female individuals reported worsening of CF-related symptoms during and after menopause [77].
Medications used to treat menopausal symptoms include hormone-replacement therapy (HRT), including both estrogen and progesterone therapy, selective-serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), gabapentin, and fezolinetant. HRT is recommended for women younger than 60 years or who are within 10 years of menopause with no contraindications. Special considerations that may preclude safe HRT use in women with CF include history of venous thromboembolic disease or liver disease. Caution is recommended for the CFTR modulator lumacaftor-ivacaftor, which can reduce efficacy of HRT and interact with SSRIs.
Cancer Screening
As the life expectancy of pwCF increases, we anticipate increased rates of cancer in this population, particularly among older patients. Registry data have signaled an increased number of multiple types of cancer in pwCF, including gastrointestinal, renal, thyroid, testicular, and lymphoma [78, 79]. Of these, digestive tract cancers, particularly colorectal cancer, have been of the highest concern, and screening guidelines for pwCF differ from the general population.
An elevated risk of gastrointestinal tract cancer in pwCF has been well established [80]. Proposed mechanisms behind this include CFTR dysfunction in the GI tract leading to impaired epithelial homeostasis, nutritional factors including high fat diet, and gut dysbiosis [81]. Rates are also higher in patients with more severe genotypes and following solid organ transplant [82]. The median age of onset of colorectal cancer in pwCF is 20–30 years younger than in the general population [81, 83]. Therefore, the CF Colorectal Cancer Screening Consensus guidelines recommend screening colonoscopy beginning at age 40 years, with rescreening every 5 years assuming negative examinations [81]. If adenomas are discovered, the next colonoscopy should occur in 3 years. In pwCF who have undergone an organ transplant, colonoscopy is recommended starting at age 30 years and within 2 years of transplant. To date, there is insufficient evidence to support less invasive modes of screening, including fecal immunochemical tests (FIT) or computed tomography (CT) colonography [81]. The median colorectal cancer screening rate among the eligible CF population hovers around 30%, highlighting the need for increased awareness and implementation of this recommendation [58].
Retrospective studies have suggested an increased risk of human papilloma virus (HPV)-related dysplasia and high rates of abnormal pap smears in women with CF [84]. Patients who have undergone lung transplantation are at even higher risk for cervical dysplasia and cervical cancer [85]. In one study, transplantation was associated with 4.5-fold higher risk of HPV; non-transplanted women had higher rates of abnormal cervical cytology, but similar rates of high-risk HPV compared with the general population [86]. These data highlight the importance of cervical cancer screening and HPV vaccination in the CF population.
Increased rates of testicular cancer have been seen in registry studies and is more common in severe genotypes. There are currently no specific guidelines for screening for testicular cancer in the CF population, however, providers should be aware of this association [87].
Cardiovascular Disease and Dyslipidemia
Historically, cardiovascular disease has not been a significant comorbidity in pwCF. This has been attributed to low prevalence cardiovascular risk factors in this population (e.g., hypertension, overweight and obesity, and tobacco use). In addition, the earlier mortality perhaps prevented pwCF from living long enough to develop cardiovascular disease. However, the observation regarding obesity prevalence noted above has generated greater concern about complications related to dyslipidemia and cardiovascular disease.
A multinational, retrospective cohort study found that pwCF have a higher risk of major adverse cardiac events (MACE), including myocardial infarction, left-sided heart failure, and atrial fibrillation, compared with a matched, non-CF population. Additionally, the risk of MACE in people with CF was on par with other inflammatory conditions that portend a higher risk for cardiovascular disease, including human immunodeficiency virus (HIV), systemic lupus erythematosus, and rheumatoid arthritis. MACE were associated with diabetes and hypertension, as well as disease-specific risk factors including lung function and intravenous antibiotic use. This study included data from the years 2016–2020, and thus does not reflect the full impact of CFTR modulator therapy [88]. Providers should be cognizant of the potential for cardiovascular disease in pwCF, however, there are no specific screening or treatment recommendations in this population.
CFTR is present in vascular smooth muscle cells and endothelial cells, however, its physiological role is not fully understood. Lower rates of hypertension in the CF population have been attributed to salt losses from CFTR dysfunction [89]. The prevalence of hypertension is 2–11% in adults with CF, which is lower than the general population rates in the UK and USA, which range from 25 to 48% [88, 90]. This prevalence may change with widespread use of modulators, which can increase blood pressure. In one case series of 79 patients who were started on modulator therapy with elexacaftor-tezacaftor-ivacaftor (ETI), 4 developed hypertension requiring antihypertensive therapy. Patients who developed hypertension had preexisting metabolic conditions, including CFRD and liver disease [91]. Treatment for hypertension in CF should follow standard age-based guidelines and targets.
Abnormal lipid metabolism is another evolving topic in the care of pwCF. Historically, hypertriglyceridemia was more common in pwCF compared with the general population, possibly related to nutrient malabsorption or chronic inflammation; conversely, cholesterol and LDL levels were lower in pwCF [92]. These metabolic parameters may be impacted by treatment with modulators, which has implications for other related health conditions. In one study, treatment with ETI increased total, HDL, and LDL cholesterol in patients with CFRD [93]. Another study showed that overweight and obese pwCF had higher total and LDL cholesterol levels compared with normal/underweight pwCF [36].
Screening for lipid disorders with an annual lipid profile is strongly recommended in all men age 35 years or older and in women age 45 years or older with risk factors for coronary heart disease (CHD) [94]. Earlier screening in men age 20–35 years and women age 20–45 years with other risk factors for CHD is also recommended [94]. An annual lipid profile is recommended for patients with CF-related diabetes and pancreatic exocrine sufficiency or if any of the following risk factors are present: obesity, family history of CHD, or immunosuppressive therapy following transplantation [20]. Treatment of dyslipidemia should follow standard guidelines [95], however drug–drug interactions, particularly with regards to potential hepatotoxicity, should be considerations in the CF population.
Planning for Work, Financial Independence, and Retirement
PwCF are living not only longer but overall healthier lives and should be encouraged to develop educational and vocational goals. Currently, many adults are pursuing bachelor’s or other advanced degrees [58], and a significant proportion of pwCF have part-time or full-time jobs [96]. In many individuals, the diagnosis of CF affects career choice [97], and most are engaged in non-manual occupations, including clerical, professional, or managerial positions [96]. PwCF may face unique barriers impacting their ability to work, including the need for time off due to illness [97]. The CF Foundation provides guidance related to workplace accommodations and benefits [98], and social workers are available as a core part of the CF team to offer guidance [2]. Projected increases in survival owing to modulator therapy are substantial [6], and more pwCF will enter the retirement years. Appropriate financial planning should be promoted in younger people with CF, and guidance provided to older pwCF who may not have anticipated needing a pension or retirement savings [99].
The CF Care Model
The benefit of the CFTR modulators has changed the health of pwCF dramatically, such that now many are looking to reduce the frequency of office visits and some of their treatments. There is recent guidance on how this should impact the CF center care team and how the team provides care [26]. The CF center remains essential, but primary care providers (PCPs) should be engaged as key partners in the delivery of care. This will require increased communication between pwCF, their families, the CF center and the PCP so that all will know who should be contacted for different health questions and to assure that complications could be detected early to allow for appropriate intervention.
The role of the pharmacist as a member of the core CF team has been made clear [100], but this role should be highlighted given the potential for the additional comorbidities described here and the potential for drug–drug interactions.
It is also critical to recognize that not all pwCF are benefiting from the CFTR modulators. Some do not have CFTR mutations that are responsive to the modulators and others are not able to tolerate the medications due to adverse effects. We should anticipate even greater heterogeneity in the health status of our patients, and although CF-related complications are common, they may manifest differently in patients, thus an individualized care plan is needed for all.
Conclusions
PwCF are experiencing longer and healthier lives due to modulator therapy and standardized, center-based care. Despite this, complications do occur, which may be related to the underlying disease, the treatments, or just by virtue of people living longer. There remains heterogeneity in this population with regard to the severity of lung disease and the presence of CF-related comorbidities. Metabolic complications, including weight gain, hypertension, and lipid disorders, which have historically been of low concern in this population, may become more prominent over time. The model of care is evolving in response to the changing health of our patients, and that should include greater partnership with PCPs.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by the Carolinas Consortium. Dr. Flume is supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under grant no. UL1 TR001450. Dr. Sullivan receives funding from the Cystic Fibrosis Foundation. Dr. Mingora receives funding from the Cystic Fibrosis Foundation.
Conflicts of interest
Dr. Flume has grant support from Boeringher-Ingelheim, Cystic Fibrosis Foundation Therapeutics, National Institutes of Health, and Vertex Pharmaceuticals, Inc, and provides consultation to Sionna and Vertex Pharmaceuticals, Inc.
Availability of data and material
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed substantially to the writing of the manuscript.
References
- 1.Guo J, Garratt A, Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros. 2022;21(3):456–62. [DOI] [PubMed] [Google Scholar]
- 2.Ong T, Ramsey BW. Cystic fibrosis: a review. JAMA. 2023;329(21):1859–71. [DOI] [PubMed] [Google Scholar]
- 3.Jia S, Taylor-Cousar JL. Cystic fibrosis modulator therapies. Annu Rev Med. 2023;74:413–26. [DOI] [PubMed] [Google Scholar]
- 4.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez A, Daly C, Vega-Hernandez G, MacGregor G, Rubin JL. Elexacaftor/tezacaftor/ivacaftor projected survival and long-term health outcomes in people with cystic fibrosis homozygous for F508del. J Cyst Fibros. 2023;22(4):607–14. [DOI] [PubMed] [Google Scholar]
- 7.Ostrenga JS, Robinson K, Brown AW, Goss CH, Cromwell EA. Aging with CF: characteristics of people with CF aged 40 and older in the United States. J Cyst Fibros. 2025;24(1):183–6. [DOI] [PubMed] [Google Scholar]
- 8.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flume PA, Robinson KA, O’Sullivan BP, Finder JD, Vender RL, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54(4):522–37. [PubMed] [Google Scholar]
- 10.McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol. 2021;56(6):1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai M, Hine C, Whitehouse JL, Brownlee K, Charman SC, Nagakumar P. Who are the 10%? Non eligibility of cystic fibrosis (CF) patients for highly effective modulator therapies. Respir Med. 2022;199: 106878. [DOI] [PubMed] [Google Scholar]
- 12.Dagenais RVE, Su VCH, Quon BS. Real-world safety of CFTR modulators in the treatment of cystic fibrosis: a systematic review. J Clin Med. 2020;10(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer-Hamblett N, Ratjen F, Russell R, Donaldson SH, Riekert KA, Sawicki GS, et al. Discontinuation versus continuation of hypertonic saline or dornase alfa in modulator treated people with cystic fibrosis (SIMPLIFY): results from two parallel, multicentre, open-label, randomised, controlled, non-inferiority trials. Lancet Respir Med. 2023;11(4):329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szentpetery S, Fernandez GS, Schechter MS, Jain R, Flume PA, Fink AK. Obesity in cystic fibrosis: prevalence, trends and associated factors data from the US cystic fibrosis foundation patient registry. J Cyst Fibros. 2022;21(5):777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freswick PN, Reid EK, Mascarenhas MR. Pancreatic enzyme replacement therapy in cystic fibrosis. Nutrients. 2022;14(7):1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham JM, Taylor CJ. Cystic fibrosis & disorders of the large intestine: DIOS, constipation, and colorectal cancer. J Cyst Fibros. 2017;16(Suppl 2):S40–9. [DOI] [PubMed] [Google Scholar]
- 17.Putman MS, Norris AW, Hull RL, Rickels MR, Sussel L, Blackman SM, et al. Cystic fibrosis-related diabetes workshop: research priorities spanning disease pathophysiology, diagnosis, and outcomes. Diabetes. 2023;72(6):677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granados A, Chan CL, Ode KL, Moheet A, Moran A, Holl R. Cystic fibrosis related diabetes: pathophysiology, screening and diagnosis. J Cyst Fibros. 2019;18(Suppl 2):S3-s9. [DOI] [PubMed] [Google Scholar]
- 19.Kelly A, Moran A. Update on cystic fibrosis-related diabetes. J Cyst Fibros. 2013;12(4):318–31. [DOI] [PubMed] [Google Scholar]
- 20.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan CL, Ode KL, Granados A, Moheet A, Moran A, Hameed S. Continuous glucose monitoring in cystic fibrosis—a practical guide. J Cyst Fibros. 2019;18(Suppl 2):S25-s31. [DOI] [PubMed] [Google Scholar]
- 22.Geyer MC, Sullivan T, Tai A, Morton JM, Edwards S, Martin AJ, et al. Exenatide corrects postprandial hyperglycaemia in young people with cystic fibrosis and impaired glucose tolerance: a randomized crossover trial. Diabetes Obes Metab. 2019;21(3):700–4. [DOI] [PubMed] [Google Scholar]
- 23.Santhakumar A, Lewis F, Pickles J, Winterbottom H, Punt S, Beynon J, et al. Role for DPP4 inhibitor therapy in cystic fibrosis related diabetes: a single centre experience. J Cyst Fibros. 2024;23(5):853–6. [DOI] [PubMed] [Google Scholar]
- 24.Gnanapragasam H, Mustafa N, Bierbrauer M, Andrea Providence T, Dandona P. Semaglutide in cystic fibrosis-related diabetes. J Clin Endocrinol Metab. 2020;105(7):2341. [DOI] [PubMed] [Google Scholar]
- 25.Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla C, Moran A. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care. 2007;30(5):1056–61. [DOI] [PubMed] [Google Scholar]
- 26.Goetz DM, Brown RF, Filigno SS, Bichl SL, Nelson AL, Merlo CA, et al. Cystic fibrosis foundation position paper: redefining the CF care model. J Cyst Fibros. 2024;23:1055. [DOI] [PubMed] [Google Scholar]
- 27.Paccou J, Zeboulon N, Combescure C, Gossec L, Cortet B. The prevalence of osteoporosis, osteopenia, and fractures among adults with cystic fibrosis: a systematic literature review with meta-analysis. Calcif Tissue Int. 2010;86(1):1–7. [DOI] [PubMed] [Google Scholar]
- 28.Aris RM, Merkel PA, Bachrach LK, Borowitz DS, Boyle MP, Elkin SL, et al. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90(3):1888–96. [DOI] [PubMed] [Google Scholar]
- 29.Cairoli E, Eller-Vainicher C, Morlacchi LC, Tarsia P, Rossetti V, Pappalettera M, et al. Bone involvement in young adults with cystic fibrosis awaiting lung transplantation for end-stage respiratory failure. Osteoporos Int. 2019;30(6):1255–63. [DOI] [PubMed] [Google Scholar]
- 30.Anabtawi A, Le T, Putman M, Tangpricha V, Bianchi ML. Cystic fibrosis bone disease: pathophysiology, assessment and prognostic implications. J Cyst Fibros. 2019;18(Suppl 2):S48-s55. [DOI] [PubMed] [Google Scholar]
- 31.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffery TC, Chang AB, Conwell LS. Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database Syst Rev. 2023;1(1):Cd002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr. 2000;137(3):374–80. [DOI] [PubMed] [Google Scholar]
- 34.Sharma R, Florea VG, Bolger AP, Doehner W, Florea ND, Coats AJ, et al. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax. 2001;56(10):746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–9. [DOI] [PubMed] [Google Scholar]
- 36.Harindhanavudhi T, Wang Q, Dunitz J, Moran A, Moheet A. Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: a single-center analysis. J Cyst Fibros. 2020;19(1):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey J, Krick S, Fontaine KR. The changing landscape of nutrition in cystic fibrosis: the emergence of overweight and obesity. Nutrients. 2022;14(6):1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greaney C, Doyle A, Drummond N, King S, Hollander-Kraaijeveld F, Robinson K, Tierney A. What do people with cystic fibrosis eat? Diet quality, macronutrient and micronutrient intakes (compared to recommended guidelines) in adults with cystic fibrosis-a systematic review. J Cyst Fibros. 2023;22(6):1036–47. [DOI] [PubMed] [Google Scholar]
- 39.Ratti GA, Smith H, Mirfakhraee S, Reisch J, Cohen L, Jain R, Finklea JD. Development of metabolic syndrome in people with cystic fibrosis one year after exposure to elexacaftor-tezacaftor-ivacaftor. J Cyst Fibros. 2024;24:47. [DOI] [PubMed] [Google Scholar]
- 40.Elmaleh-Sachs A, Schwartz JL, Bramante CT, Nicklas JM, Gudzune KA, Jay M. Obesity management in adults: a review. JAMA. 2023;330(20):2000–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S, Jain R, Mirfakhraee S. Glucagon-like-peptide-1 agonist therapy in adults with cystic fibrosis. J Cyst Fibros. 2024;24:40. [DOI] [PubMed] [Google Scholar]
- 42.National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990–2021, and forecasts up to 2050. Lancet. 2024. [DOI] [PMC free article] [PubMed]
- 43.Michos ED, Lopez-Jimenez F, Gulati M. Role of glucagon-like peptide-1 receptor agonists in achieving weight loss and improving cardiovascular outcomes in people with overweight and obesity. J Am Heart Assoc. 2023;12(11): e029282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dana J, Debray D, Beaufrère A, Hillaire S, Fabre M, Reinhold C, et al. Cystic fibrosis-related liver disease: clinical presentations, diagnostic and monitoring approaches in the era of CFTR modulator therapies. J Hepatol. 2022;76(2):420–34. [DOI] [PubMed] [Google Scholar]
- 45.Lowery EM, Afshar M, West N, Kovacs EJ, Smith B, Joyce C. Self-reported alcohol use in the cystic fibrosis community. J Cyst Fibros. 2020;19(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung DH, Narkewicz MR. Cystic fibrosis-related cirrhosis. J Cyst Fibros. 2017;16(Suppl 2):S50-s61. [DOI] [PubMed] [Google Scholar]
- 47.Sellers ZM, Assis DN, Paranjape SM, Sathe M, Bodewes F, Bowen M, et al. Cystic fibrosis screening, evaluation, and management of hepatobiliary disease consensus recommendations. Hepatology. 2024;79(5):1220–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng K, Ashby D, Smyth RL. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst Rev. 2017;9(9):Cd000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eldredge JA, Oliver MR, Ooi CY. Cystic fibrosis liver disease in the new era of cystic fibrosis transmembrane conductance regulator (CFTR) modulators. Paediatr Respir Rev. 2024;50:54–61. [DOI] [PubMed] [Google Scholar]
- 50.Mogayzel PJ Jr, Naureckas ET, Robinson KA, Brady C, Guill M, Lahiri T, et al. Cystic Fibrosis Foundation pulmonary guideline. Pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc. 2014;11(10):1640–50. [DOI] [PubMed] [Google Scholar]
- 51.Mogayzel PJ Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–9. [DOI] [PubMed] [Google Scholar]
- 52.Flume PA, Mogayzel PJ Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–8. [DOI] [PubMed] [Google Scholar]
- 53.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(Suppl 1):i1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madaule J, Valenzuela F, Mittaine M, Gallois Y, Baladi B, Murris M, et al. Exploration of the relationship between cumulative exposure to tobramycin and ototoxicity in patients with cystic fibrosis. J Cyst Fibros. 2023;22(5):944–8. [DOI] [PubMed] [Google Scholar]
- 55.Kimple AJ, Senior BA, Naureckas ET, Gudis DA, Meyer T, Hempstead SE, et al. Cystic Fibrosis Foundation otolaryngology care multidisciplinary consensus recommendations. Int Forum Allergy Rhinol. 2022;12(9):1089–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pyle-Eilola AL. Chapter 8—guidelines for monitoring vancomycin, aminoglycosides, and other antibiotics. In: Dasgupta A, editor. Therapeutic drug monitoring. 2nd ed. New York: Academic Press; 2024. p. 197–215. [Google Scholar]
- 57.Harruff EE, Kil J, Ortiz MGT, Dorgan D, Jain R, Poth EA, et al. Ototoxicity in cystic fibrosis patients receiving intravenous tobramycin for acute pulmonary exacerbation: ototoxicity following tobramycin treatment. J Cyst Fibros. 2021;20(2):288–94. [DOI] [PubMed] [Google Scholar]
- 58.Cystic Fibrosis Foundation Patient Registry. Bethesda, Maryland: ©2024 Cystic Fibrosis Foundation; 2023.
- 59.Elson EC, Meier E, Oermann CM. The implementation of an aminoglycoside induced ototoxicity algorithm for people with cystic fibrosis. J Cyst Fibros. 2021;20(2):284–7. [DOI] [PubMed] [Google Scholar]
- 60.Zettner EM, Gleser MA. Progressive hearing loss among patients with cystic fibrosis and parenteral aminoglycoside treatment. Otolaryngol Head Neck Surg. 2018;159(5):887–94. [DOI] [PubMed] [Google Scholar]
- 61.Rizk HG, Lee JA, Liu YF, Endriukaitis L, Isaac JL, Bullington WM. Drug-induced ototoxicity: a comprehensive review and reference guide. Pharmacotherapy. 2020;40(12):1265–75. [DOI] [PubMed] [Google Scholar]
- 62.Purkayastha D, Agtarap K, Wong K, Pereira O, Co J, Pakhale S, Kanji S. Drug-drug interactions with CFTR modulator therapy in cystic fibrosis: focus on Trikafta®/Kaftrio®. J Cyst Fibros. 2023;22(3):478–83. [DOI] [PubMed] [Google Scholar]
- 63.Gavioli EM, Guardado N, Haniff F, Deiab N, Vider E. A current review of the safety of cystic fibrosis transmembrane conductance regulator modulators. J Clin Pharm Ther. 2021;46(2):286–94. [DOI] [PubMed] [Google Scholar]
- 64.May TL, Gifford AH, Lahiri T, Black A, Trang J, Cornell AG, et al. Complications of long and intermediate term venous catheters in cystic fibrosis patients: a multicenter study. J Cyst Fibros. 2018;17(1):96–104. [DOI] [PubMed] [Google Scholar]
- 65.Mermis JD, Strom JC, Greenwood JP, Low DM, He J, Stites SW, Simpson SQ. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–10. [DOI] [PubMed] [Google Scholar]
- 66.Dupont C, Gouya H, Panzo R, Hubert D, Correas JM, Agrario L, et al. Complications of peripherally inserted central catheters in adults with cystic fibrosis or bronchiectasis. J Vasc Access. 2015;16(3):245–9. [DOI] [PubMed] [Google Scholar]
- 67.Gifford AH, Hinton AC, Jia S, Nasr SZ, Mermis JD, Lahiri T, et al. Complications and practice variation in the use of peripherally inserted central venous catheters in people with cystic fibrosis: the Prospective Study of Peripherally Inserted Venous Catheters in People With Cystic Fibrosis Study. Chest. 2023;164(3):614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shteinberg M, Lulu AB, Downey DG, Blumenfeld Z, Rousset-Jablonski C, Perceval M, et al. Failure to conceive in women with CF is associated with pancreatic insufficiency and advancing age. J Cyst Fibros. 2019;18(4):525–9. [DOI] [PubMed] [Google Scholar]
- 69.Hughan KS, Daley T, Rayas MS, Kelly A, Roe A. Female reproductive health in cystic fibrosis. J Cyst Fibros. 2019;18(Suppl 2):S95-s104. [DOI] [PubMed] [Google Scholar]
- 70.Jain R, Kazmerski TM, Zuckerwise LC, West NE, Montemayor K, Aitken ML, et al. Pregnancy in cystic fibrosis: review of the literature and expert recommendations. J Cyst Fibros. 2022;21(3):387–95. [DOI] [PubMed] [Google Scholar]
- 71.Jain R, Magaret A, Vu PT, VanDalfsen JM, Keller A, Wilson A, et al. Prospectively evaluating maternal and fetal outcomes in the era of CFTR modulators: the MAYFLOWERS observational clinical trial study design. BMJ Open Respir Res. 2022;9(1). [DOI] [PMC free article] [PubMed]
- 72.Montemayor K, Tullis E, Jain R, Taylor-Cousar JL. Management of pregnancy in cystic fibrosis. Breathe (Sheff). 2022;18(2): 220005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elijah J, Fitzgerald LJ, Phan H. Use of CFTR modulators in special populations, part 1: pregnancy and lactation. Pediatr Pulmonol. 2023;58(12):3377–85. [DOI] [PubMed] [Google Scholar]
- 74.Yoon JC, Casella JL, Litvin M, Dobs AS. Male reproductive health in cystic fibrosis. J Cyst Fibros. 2019;18(Suppl 2):S105–10. [DOI] [PubMed] [Google Scholar]
- 75.Kazmerski TM, West NE, Jain R, Uluer A, Georgiopoulos AM, Aitken ML, Taylor-Cousar JL. Family-building and parenting considerations for people with cystic fibrosis. Pediatr Pulmonol. 2022;57(Suppl 1):S75-s88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gatenby C, Simpson P. Menopause: physiology, definitions, and symptoms. Best Pract Res Clin Endocrinol Metab. 2024;38(1): 101855. [DOI] [PubMed] [Google Scholar]
- 77.Prochownik K, Jain R, Taylor-Cousar JL, Lavage DR, Stransky OM, Thomas HN, Kazmerski TM. Menopause in people with cystic fibrosis. Menopause. 2023;30(4):401–5. [DOI] [PubMed] [Google Scholar]
- 78.Johannesson M, Askling J, Montgomery SM, Ekbom A, Bahmanyar S. Cancer risk among patients with cystic fibrosis and their first-degree relatives. Int J Cancer. 2009;125(12):2953–6. [DOI] [PubMed] [Google Scholar]
- 79.Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013;105(2):122–9. [DOI] [PubMed] [Google Scholar]
- 80.Neglia JP, Wielinski CL, Warwick WJ. Cancer risk among patients with cystic fibrosis. J Pediatr. 1991;119(5):764–6. [DOI] [PubMed] [Google Scholar]
- 81.Hadjiliadis D, Khoruts A, Zauber AG, Hempstead SE, Maisonneuve P, Lowenfels AB. Cystic fibrosis colorectal cancer screening consensus recommendations. Gastroenterology. 2018;154(3):736-45.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoskins B, Wasuwanich P, Scheimann AO, Karnsakul W. Screening strategy for gastrointestinal and hepatopancreatobiliary cancers in cystic fibrosis. World J Gastrointest Oncol. 2021;13(9):1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW 3rd, Lowenfels AB. Cancer risk in nontransplanted and transplanted cystic fibrosis patients: a 10-year study. J Natl Cancer Inst. 2003;95(5):381–7. [DOI] [PubMed] [Google Scholar]
- 84.Rousset-Jablonski C, Reynaud Q, Nove-Josserand R, Ray-Coquard I, Mekki Y, Golfier F, Durieu I. High proportion of abnormal pap smear tests and cervical dysplasia in women with cystic fibrosis. Eur J Obstet Gynecol Reprod Biol. 2018;221:40–5. [DOI] [PubMed] [Google Scholar]
- 85.Thornton C, Somayaji R, Chu A, Parkins MD. Human papillomavirus (HPV) and cervical dysplasia in adult female cystic fibrosis (CF) lung transplant recipients. Thorax. 2022;77(6):625–7. [DOI] [PubMed] [Google Scholar]
- 86.Rousset-Jablonski C, Mekki Y, Denis A, Reynaud Q, Nove-Josserand R, Durupt S, et al. Human papillomavirus prevalence, persistence and cervical dysplasia in females with cystic fibrosis. J Cyst Fibros. 2023;22(3):505–14. [DOI] [PubMed] [Google Scholar]
- 87.Maisonneuve P, Lowenfels AB. Cancer in cystic fibrosis: a narrative review of prevalence, risk factors, screening, and treatment challenges: adult cystic fibrosis series. Chest. 2022;161(2):356–64. [DOI] [PubMed] [Google Scholar]
- 88.Frost F, Nazareth D, Fauchier L, Wat D, Shelley J, Austin P, et al. Prevalence, risk factors and outcomes of cardiac disease in cystic fibrosis: a multinational retrospective cohort study. Eur Respir J. 2023;62(4):2300174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lieberman J, Rodbard S. Low blood pressure in young adults with cystic fibrosis: an effect of chronic salt loss in sweat? Ann Intern Med. 1975;82(6):806–8. [DOI] [PubMed] [Google Scholar]
- 90.Valman HB, France NE. The vas deferens in cystic fibrosis. Lancet. 1969;2(620):566–7. [DOI] [PubMed] [Google Scholar]
- 91.Gramegna A, De Petro C, Leonardi G, Contarini M, Amati F, Meazza R, et al. Onset of systemic arterial hypertension after initiation of elexacaftor/tezacaftor/ivacaftor in adults with cystic fibrosis: a case series. J Cyst Fibros. 2022;21(5):885–7. [DOI] [PubMed] [Google Scholar]
- 92.Figueroa V, Milla C, Parks EJ, Schwarzenberg SJ, Moran A. Abnormal lipid concentrations in cystic fibrosis. Am J Clin Nutr. 2002;75(6):1005–11. [DOI] [PubMed] [Google Scholar]
- 93.Petersen MC, Begnel L, Wallendorf M, Litvin M. Effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. J Cyst Fibros. 2022;21(2):265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Screening for lipid disorders in adults: recommendation statement. Am Fam Physician. 2009;80(11):1273–4. [PubMed]
- 95.Chou R, Cantor A, Dana T, Wagner J, Ahmed AY, Fu R, Ferencik M. Statin use for the primary prevention of cardiovascular disease in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328(8):754–71. [DOI] [PubMed] [Google Scholar]
- 96.Leso V, Romano R, Santocono C, Caruso M, Iacotucci P, Carnovale V, Iavicoli I. The impact of cystic fibrosis on the working life of patients: a systematic review. J Cyst Fibros. 2022;21(2):361–9. [DOI] [PubMed] [Google Scholar]
- 97.Targett K, Bourke S, Nash E, Murphy E, Ayres J, Devereux G. Employment in adults with cystic fibrosis. Occup Med (Lond). 2014;64(2):87–94. [DOI] [PubMed] [Google Scholar]
- 98.Ehrenkranz NJ, Nerenberg DE, Shultz JM, Slater KC. Intervention to discontinue parenteral antimicrobial therapy in patients hospitalized with pulmonary infections: effect on shortening patient stay. Infect Control Hosp Epidemiol. 1992;13(1):21–32. [DOI] [PubMed] [Google Scholar]
- 99.Gramegna A, Addy C, Allen L, Bakkeheim E, Brown C, Daniels T, et al. Standards for the care of people with cystic fibrosis (CF); planning for a longer life. J Cyst Fibros. 2024;23(3):375–87. [DOI] [PubMed] [Google Scholar]
- 100.Brown RF, Close CT, Mailes MG, Gonzalez LJ, Goetz DM, Filigno SS, et al. Cystic fibrosis foundation position paper: redefining the cystic fibrosis care team. J Cyst Fibros. 2024;23(6):1045–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.