Abstract
5-Aminopentanol (5-AP) is a valuable amino alcohol with potential applications in polymer synthesis and bioplastics. Conventional production methods rely on petroleum-based feedstocks and metal catalysts, which raise environmental and sustainability concerns. In this study, a de novo biosynthetic pathway for 5-AP production from l-lysine was developed in Escherichia coli. The engineered pathway consisted of lysine decarboxylase 2 (LdcC), putrescine aminotransferase (PatA), and tested aldehyde reductase (YahK, YihU, YqhD). Among the tested reductases, aldehyde reductase exhibited the highest catalytic efficiency, producing 44.5 ± 2.6 mM of 5-AP (0.44 ± 0.03 mol5 − AP/moll−lysine). The replacement of the expression system with a T7-based dual-plasmid platform, pET24ma::ldcC, and pCDFDuet-1::yqhD::patA co-transformed into E. coli, increased the production to 60.7 ± 5.8 mM, accompanied by reduced cadaverine accumulation. Further enhancement was achieved by increasing the gene dosage of PatA, leading to 68.5 ± 4.2 mM 5-AP and reduced by 40% in cadaverine levels. Cadaverine is a precursor in the production of 5-AP, and its accumulation is an important factor in the limitation of conversion to 5-AP. Intracellular cofactor regeneration is expected to cause an indirect supply of α-KG, a cofactor, to enhance conversion to 5-AP. To support intracellular cofactor regeneration, glucose supplementation and increased aeration were applied, resulting in a final titer of 78.5 ± 1.2 mM 5-AP and improved precursor utilization. This study is the first report of selective microbial 5-AP production and highlights the importance of PatA expression in pathway optimization. The newly established l-lysine (C6) valorization process which converts l-lysine to high-value materials such as 1,5-PDO, glutarate, and 5-AP offers a promising route for the sustainable biosynthesis of amino alcohols, laying the groundwork for future improvements through enzyme engineering and metabolic design.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40643-025-00904-6.
Keywords: 5-Aminopentanol, l-Lysine (C6) valorization process, Whole-cell bioconversion, Escherichia coli, Novel bioplastic monomer
Introduction
The increasing environmental pollution caused by the excessive production and use of petroleum-based plastics has led to a growing interest in the development of bioplastics (Millican and Agarwal 2021; MacLeod et al. 2021). Non-biodegradable plastics, which cannot be degraded into CO2 through natural remediation processes, have accumulated over time, leading to various plastic waste problems. Furthermore, microplastics, generated through physicochemical degradation without further breakdown into CO2, have caused serious detrimental impacts on ecosystems, human health, and climate change (Akdogan and Guven 2019; Amobonye et al. 2021; Zhang et al. 2022). These issues have highlighted the urgent need for the development of more environmentally friendly plastics, such as bio-based polymers. Accordingly, various plastics, including polyesters and polyamides, have been successfully developed using cadaverine and glutaric acid produced by recombinant microorganisms. These bioplastics are synthesized from renewable resources, such as glucose and l-lysine. Recently, as part of these research efforts, a new type of biodegradable plastic, polyurea ester (PUE), has been proposed (Tang et al. 2016; Dziewior et al. 2024).
PUE is emerging as a promising candidate to address the poor mechanical properties that have limited conventional biodegradable polymers. Its production begins with the synthesis of di(hydroalkyl)urea via the reaction of amino alcohols, such as 4-aminobutanol, 5-aminopentanol (5-AP), and 6-aminohexanol with urea, followed by polymerization with dimethyl esters (e.g., dimethyl succinate, adipate, or sebacate) to form PUE. The production of PUE requires amino alcohols like 5-AP as key precursors for prepolymer synthesis. Given these advantages, there is growing interest in the biological production of amino alcohols such as 5-AP. This approach offers a promising route toward sustainable and environmentally friendly PUE production by reducing reliance on petroleum-based processes and utilizing bio-based substrates and biological methods (López-Garzón et al. 2014; Prabowo et al. 2020; Wang et al. 2023).
5-AP is a linear C5 chemical with an amino group at one end and a hydroxyl group at the other. Traditionally, its production has relied on chemical methods, using furfural as substrate and Ni-based catalysts, such as NiCo/Al₂O₃ and Ni-Mg₃AlOx2, to facilitate the reaction (Li et al. 2020; Yang et al. 2024). Although this method offers high conversion efficiency, the use of metal-based catalysts raises environmental and human health concerns, emphasizing the need for a more sustainable production route (Egorova and Ananikov 2016; Das et al. 2019). Conventionally, 5-AP is utilized in the chemical synthesis of δ-valerolactam, a monomer or precursor for various polymers such as nylon-5 (Reddy et al. 1994; Nova et al. 2010; Von Tiedemann et al. 2020). More recently, 5-AP has gained attention as a monomer for di(hydroxyalkyl)urea, which can be polymerized with diacids, such as dimethyl succinate, dimethyl adipate, and dimethyl sebacate, to produce a novel polymer, PUE. Despite its expanding applications, there have been no reports on the selective biosynthesis of 5-AP with high conversion efficiency from renewable resources such as glucose and l-lysine.
An l-lysine (C6) valorization process which converts l-lysine to high-value materials such as 1,5-PDO, glutarate, and 5-AP was successfully developed for producing C5 building blocks using a whole-cell bioconversion approach. This includes the biological production of compounds such as cadaverine, glutaric acid, and 1,5-pentanediol. The valorization of cadaverine, a decarboxylated diamine derivative of l-lysine, has been reported to reach titers exceeding 2 M in Escherichia coli strains expressing l-lysine decarboxylases, such as lysine decarboxylase 1 (CadA) and lysine decarboxylase 2 (LdcC) (Oh et al. 2015; Kim et al. 2019). Glutaric acid production, another successfully developed l-lysine valorization process, can be achieved through a sequential enzymatic reaction involving lysine monooxygenase, δ-aminovaleramidase, 5-aminovalerate aminotransferase, and glutarate semialdehyde dehydrogenase. Initial studies achieved a glutaric acid production yield of 1.7 g/L through the whole-cell bioconversion of l-lysine using an E. coli strain (Rohles et al. 2016). More recently, the yield was substantially improved, with over 70 g/L of glutaric acid produced using E. coli (Wang et al. 2021). 1,5-Pentanediol (1,5-PDO) has also been reported to be produced via whole-cell bioconversion by implementing a novel biosynthetic route from l-lysine to 1,5-PDO in an engineered E. coli strain, achieving a concentration of 4.03 mM. To enable this biosynthetic route, CadA, 4-aminobutyrate aminotransferase, NADPH-dependent reductase, and glutamate dehydrogenase were introduced into E. coli. This engineering strategy enabled the successful synthesis of 1,5-PDO through the sequential conversion of l-lysine via decarboxylation, cadaverine transamination, and glutaraldehyde reduction, with cofactor regeneration further supporting and enhancing 1,5-PDO production (Hua et al. 2024).
Despite the successful biosynthesis of various C5 chemicals, including cadaverine (as a diamine), glutaric acid (as a diacid), and 1,5-pentanediol (as a dialcohol), the selective biological production of 5-AP as an amino alcohol has not yet been reported. In this study, a selective and efficient biosynthetic pathway for 5-AP production from l-lysine was established, representing the first reported microbial route with a high conversion yield. A de novo biosynthetic pathway was constructed to enable the stepwise conversion of l-lysine to 5-AP. Among several candidates, a reductase responsible for converting 5-aminopentanal to 5-aminopentanol was identified and incorporated into the system. To improve production efficiency, key reaction parameters including temperature, pH, and concentrations of cofactors and co-substrates were systematically evaluated. Based on these analyses, the transamination step catalyzed by PatA was identified as a major bottleneck. To address this, the gene copy number of the patA was increased and α-ketoglutarate (α-KG) was supplemented, leading to enhanced flux toward 5-AP via more efficient cadaverine conversion.
Materials and methods
Chemicals
Chemicals used in this study, including l-lysine (≥ 98%), cadaverine (≥ 95%), 5-aminopentanol (≥ 92%), α-KG (≥ 99%), isopropyl β-D-1-thiogalactopyranoside (IPTG; ≥99%), pyridoxal-5-phosphate (≥ 99%), NADPH, tetrasodium salt (≥ 93%), imidazole (≥ 99%), potassium phosphate dibasic (≥ 98%), kanamycin, and ampicillin, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tris-HCl (pH 8.5) was purchased from LPS Solutions (Daejeon, Korea). High salt lysogenic broth and spectinomycin were purchased from Duchefa Biochemie (Haarlem, Netherlands) and Tokyo Chemical Industry (Tokyo, Japan), respectively. Diethyl ethoxymethylenemalonate (97%) and methanol (≥ 99.9%) were purchased from Tokyo Chemical Industry and Sigma-Aldrich. Water, sulfuric acid (≥ 99.999%), 1 M sodium acetate (pH 4.6), and acetonitrile were purchased from Samchun Chemicals (Seoul, Republic of Korea), Sigma-Aldrich, Biosolution (Seoul, Republic of Korea), and Avantor (Pennsylvania, USA), respectively, and were used in the preparation of the high-performance liquid chromatography (HPLC) mobile phase.
Plasmids and bacterial strains
E. coli DH5α was used for genetic manipulation, while BL21(DE3) was employed for whole-cell catalyst preparation. DNA manipulation was performed according to the standard protocols. For the initial biosynthetic route construction of 5-AP biosynthesis, the customized vector pKM212 was used to generate pKM212::ldcC::patA::yahK, pKM212::ldcC::patA::yihU, and pKM212::ldcC::patA::yqhD. After digestion with the restriction enzymes specified in the primer list, the constructs were assembled using the primers listed in Supplementary Table 2. Additionally, the derivative vector pET24ma, which was modified from pET24a by replacing f1 ori with p15A ori, was used to construct pET24ma::ldcC by ligating the amplified ldcC gene. The gene products were amplified using the primers listed in Supplementary Tables 2 and subsequently digested with the restriction enzymes listed in Supplementary Table 2 (Shin et al. 2018). Gibson Assembly® Master Mix from New England Biolabs (E2611L) was used for assembling pCDFDuet-1::yqhD::patA, and pET21b(+)::patA. Commercial vectors and genes, pCDFDuet-1, pET21b(+), yqhD, and patA were amplified (Gibson et al. 2009). The information about all genes used in this study is listed in Supplementary Table (1) The amplified vectors and genes were assembled according to the manufacturer’s instructions. The primers used to amplify the vectors and genes are listed in Supplementary Table (2) The constructed vectors are listed in Supplementary Table (3) The plasmid maps are provided in Supplementary Fig. 1.
Fig. 1.
Strategies for developing a biosynthetic system for 5-AP production from l-lysine. (a) Schematic representation of the whole-cell bioconversion pathway from l-lysine to 5-AP. (b) Overview of the engineering strategies employed in this study for constructing the 5-AP biosynthesis platform
Whole-cell biocatalyst preparation and reaction
For the heterologous expression of enzymes to prepare biocatalysts, seed culture was performed using the recombinant E. coli strains listed in Supplementary Table 3 at 37 °C and 200 rpm overnight. The seed culture (4 mL) was transferred to 100 mL of culture medium and incubated at 30 °C with shaking at 200 rpm for 18–24 h. When the optical density (OD600) reached 0.6, 0.05 mM isopropyl β-D-1-thiogalactopyranoside was added to the culture medium, and the cells were further cultivated at 16 °C with shaking at 200 rpm for 24 h. The biocatalysts were harvested by centrifugation at 4 °C and 4000 rpm for 20 min, followed by two washes with 1 M Tris-HCl buffer (pH 8.5). For the preparation of the whole-cell bioconversion reaction, reaction mixtures were prepared in 5 mL of 500 mM Tris-HCl buffer (pH 8.5), containing 100 mM l-lysine, α-KG (20–100 mM), NADPH (0 or 10 mM), and 0.1 mM pyridoxal-5-phosphate. When necessary, glucose (0–4%), mannitol (2%), and sorbitol (2%) were added to the reaction mixtures. The standard whole-cell bioconversion reactions were carried out in 50-mL falcon tubes at pH 6.5 and 30 °C for 48 h (OD600 = 40). Sampling was conducted every 24 h. After each sampling, the samples were centrifuged to obtain a culture medium containing l-lysine, cadaverine, and 5-AP.
Analytic method
Cell density was measured by determining OD600 using a spectrophotometer (X-ma 1200 V; Human Corporation, Seoul, Republic of Korea). The concentration of α-KG was quantified using HPLC (Nexera, Shimadzu, Tokyo, Japan) equipped with a refractive index detector and an Aminex HPX-87 H column (300 × 7.8 mm, Bio-Rad, California, USA), using a flow rate of 0.6 mL/min at 60 °C. Sulfuric acid (4 mM) was used as the mobile phase. The concentrations of l-lysine, cadaverine, and 5-AP were determined using HPLC with a Capcell Pak C18 UG (Agilent ZORBA × SD-C18 column, California, USA) and the diethyl ethoxymethylenemalonate derivatization method. Potassium borate buffer, methanol, distilled water, diethyl ethoxymethylenemalonate, and sample were mixed together. For 2 h, the samples were heated at 70 °C (Kim et al. 2015). Peaks for each chemical were identified at a wavelength of 284 nm using a variable wavelength detector. Peaks were separated through gradient elution with mobile phases consisting of 25 mM sodium acetate buffer (pH 4.8) as buffer A and 100% acetonitrile as buffer B, using the following gradient program: 0–2 min, 20–25% buffer B; 2–32 min, 25–60% buffer B; and 30–40 min, 60–20% buffer B. The flow rate was set to 1 mL/min, and the column oven temperature was maintained at 40 °C (Cha et al. 2022, 2023). Conversion yield and selectivity were calculated using the following equations, respectively.
 |
 |
Results and discussion
Development of 5-AP biosynthetic pathway from l-lysine
5-AP is typically produced through chemical conversion processes using both petroleum-based and biomass-derived substrates, such as olefins and furfural. Although these methods have demonstrated efficient production, they pose significant environmental concerns owing to the use of metal catalysts like NiCo/Al₂O₃ and Ni-Mg₃AlOx2 (Pelckmans et al. 2017; Li et al. 2020; Yang et al. 2024). Recently, environmentally friendly biological methods for 5-AP synthesis from glucose have been developed using E. coli expressing CadA, PatA, and alcohol dehydrogenase (YjgB). However, because the system was originally designed for 1,5-pentanediol production, 5-AP was only produced as an intermediate, resulting in a low titer (1.5 g/L) and poor selectivity (Ma et al. 2025). To establish an environmentally friendly and more selective biological platform for 5-AP production, a biosynthetic pathway starting from l-lysine was developed in this study. This design was guided by the mechanistic similarity between the l-lysine-to-5-AP route and the established conversion of putrescine to 4-aminobutanol, which led to the incorporation of cadaverine as a key intermediate (Figure. 1a). As shown in Fig. 1, ldcC and patA, which encode lysine decarboxylase 2 and diamine aminotransferase, respectively, were introduced into the host strain. To screen for the most efficient reductase for the conversion of 5-aminopentanal to 5-AP, three candidate enzymes (yahK, yihU, and yqhD) were individually introduced into the pKM212::patA::ldcC backbone, resulting in the constructs pKM212::yahK::patA::ldcC, pKM212::yihU::patA::ldcC, and pKM212::yqhD::patA::ldcC (Figure. 1b). Each plasmid was then transformed into E. coli BL21(DE3) cells for gene overexpression, resulting in E. coli APYahK, E. coli APYihU, and E. coli APYqhD. Whole-cell bioconversion was performed using the recombinant strains E. coli APYahK, E. coli APYihU, and E. coli APYqhD with 100 mM l-lysine and 100 mM α-KG in 500 mM Tris-HCl buffer (pH 6.5) at 30 °C, resulting in 5-AP production of 15.6 ± 2 mM, 2.2 ± 0.2 mM, and 44.5 ± 0.03 mM, respectively (Fig. 2a). The conversion profile of YqhD revealed that l-lysine was completely consumed within 6 h, with the concurrent formation of cadaverine (71.4 ± 0.4 mM) and 5-AP (17.7 ± 1.2 mM), followed by the gradual depletion of cadaverine and a peak in 5-AP concentration at 36 h (44.5 ± 2.6 mM), after which a slight decrease was observed (Fig. 2b). Accordingly, YqhD was identified as the most effective enzyme in the biosynthetic pathway with the highest 5-AP conversion yield (0.44 ± 0.03 mol5 − AP/moll−lysine). This observation aligns with previous reports demonstrating the superior catalytic performance of YqhD over other reductases in the production of C4 and C5 compounds, such as γ-hydroxybutyrate and 1,5-pentanediol. In the case of γ-hydroxybutyrate production, YqhD was reported to be more than twice as effective as other reductases, including YjgB, YahK, and YihU (Zhu et al. 2024). Similarly, for 1,5-pentanediol production, YqhD exhibited approximately 1.5-fold higher activity than other reductases such as YjgB and YahK (Jarboe 2011; Cen et al. 2022). Taken together, these results demonstrate that the newly constructed biosynthetic pathway from l-lysine to 5-AP was functionally active.
Fig. 2.
Screening of efficient reductases for the reduction of 5-aminopentanal. (a) Comparison of 5-AP production from l-lysine using E. coli APYahK, E. coli APYihU, and E. coli APYqhD, expressing different reductases at 36 h. Beige bars: concentration of l-lysine at 0 h, yellow diamond bars: concentration of residual l-lysine at 36 h, blue ladder bars: concentration of cadaverine at 36 h, orange diagonal bars: concentration of 5-AP at 36 h (b) Time-course profile of 5-AP production from l-lysine in E. coli APYqhD
Enhanced 5-AP production through adjustment of the expression system
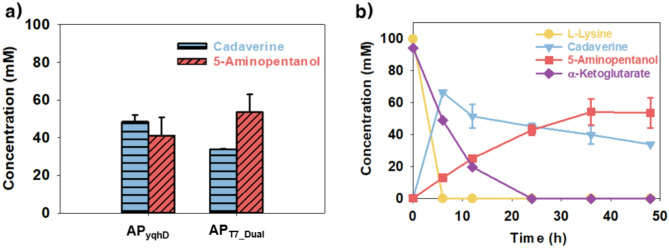
Despite the successful conversion of l-lysine to 5-AP using E. coli APYqhD, the yield was limited to 0.44 ± 0.03 mol5 − AP/moll−lysine. To address this limitation, the Tac promoter–based custom vector pKM212 was replaced with T7 promoter–driven expression vectors (pCDFDuet-1 and pET24ma), as the T7 system is widely used for the high-level expression of recombinant proteins supported by bacteriophage T7 RNA polymerase (Lozano Terol et al. 2021). Using this system, a dual-enzyme expression vector (pCDFDuet-1::yqhD::patA) and a single-enzyme expression vector (pET24ma::ldcC) were constructed and co-transformed into E. coli BL21(DE3), resulting in E. coli APT7_Dual harboring both plasmids (Fig. 1b). To compare 5-AP production efficiency, whole-cell bioconversion was conducted using E. coli APYqhD and E. coli APT7_Dual under the same reaction conditions (100 mM l-lysine and 100 mM α-KG in 500 mM Tris-HCl buffer at pH 6.5 and 30 °C). As a result, E. coli APYqhD and APT7_Dual produced 44.5 ± 2.6 and 60.7 ± 5.8 mM 5-AP, respectively (Fig. 3a). Although both strains completely consumed l-lysine within 6 h and showed similar initial trends, E. coli APT7_Dual continued to convert cadaverine even after 24 h, leading to a final 5-AP concentration of 60.7 ± 5.8 mM at 36 h, higher than that observed for E. coli APYqhD (Fig. 3b). Additionally, cadaverine accumulation was reduced in the E. coli APT7_Dual strain, with 40 ± 0.9 mM cadaverine accumulating, compared with 55 ± 2.5 mM in the E. coli APYqhD strain. These findings indicate that the change in the expression system contributed to enhanced 5-AP production, with E. coli APT7_Dual achieving a 1.36-fold improvement over E. coli APYqhD under the tested conditions. However, cadaverine accumulated partially unconverted at 48 h, indicating that the overall pathway had not yet reached full efficiency and may benefit from further optimization of the reaction conditions and pathway components (Fig. 3b).
Fig. 3.
Enhancing conversion efficiency through modification of the expression system. (a) Effect of expression system modification on 5-AP production from l-lysine using E. coli APT7_Dual at 36 h. (b) Time-course profile of 5-AP production and cadaverine accumulation in E. coli APT7_Dual
Evaluation of reaction conditions and pathway limitations in 5-AP biosynthesis
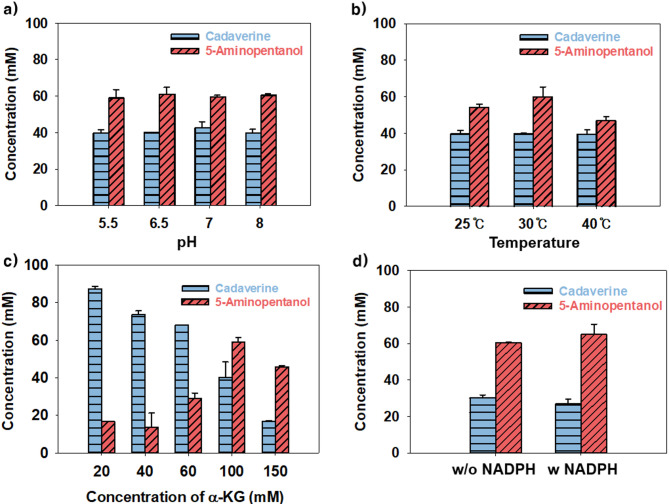
To investigate the cause of residual cadaverine observed in the previous experiments, whole-cell bioconversion was carried out using E. coli APT7_Dual under varying reaction conditions, including pH, temperature, α-KG concentration, and NADPH supplementation (Fig. 4). Within the tested pH range (5.5 to 8.0), 5-AP production showed no significant variation, and temperature had only a modest effect, with 30 °C giving slightly higher titers than other conditions (Fig. 4a and b). In the case of α-KG concentration, 100 mM was found to be optimal, while higher concentrations led to a decrease in 5-AP production (Fig. 4c). Supplementation with 10 mM NADPH did not result in a significant increase in 5-AP production, indicating that cofactor addition alone was insufficient to improve the overall pathway efficiency (Fig. 4d). These optimal values coincided with the standard conditions used in this study, suggesting that the earlier bioconversion assays had already been conducted under near-optimal conditions. Figure 3b indicates that, although the reaction was conducted under favorable conditions, a substantial amount of cadaverine accumulated at the end of the process. Because cadaverine is the direct precursor of 5-AP, this observation suggests that the downstream steps, particularly the conversion of cadaverine to 5-aminopentanal and subsequently to 5-AP, were limiting under the tested conditions. These reactions are catalyzed by PatA and YqhD, which utilize α-KG and NADPH, respectively. Given that both cofactors were sufficiently supplied, this limitation was presumed to arise from inadequate enzymatic activity rather than substrate availability. Among the two enzymes, these PatA-mediated transamination step was considered the primary bottleneck, because it governs the flux from cadaverine to 5-aminopentanal, a key intermediate in the pathway. To address this limitation, the next set of experiments evaluated whether enhanced PatA expression could reduce cadaverine accumulation, thereby improving 5-AP production.
Fig. 4.
Identification of bottlenecks in whole-cell bioconversion. Effect of reaction conditions and cofactors on 5-AP production including (a) temperature, (b) pH, (c) concentration of α-KG, and (d) supplementation of NADPH. w/o NADPH shows the same result as the previous condition as a control, and w NADPH indicates the results when 10 mM of NADPH was supplied
Effect of PatA expression on the 5-AP production
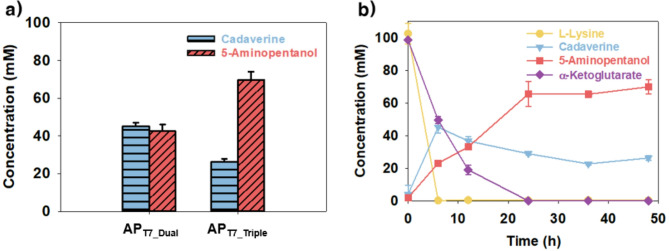
Because adjustments to the reaction conditions did not significantly improve 5-AP production, the PatA-mediated transamination step was suspected to be a bottleneck, likely due to insufficient enzyme expression, which limited the conversion of cadaverine to 5-aminopentanal in the l-lysine-to-5-AP pathway. This hypothesis is supported by a previous study that reported that 4-aminobutanol production was improved by repositioning the patA gene within the plasmid and adding an N-terminal 6×His tag to increase expression levels (Prabowo et al. 2020). To address this issue, the effect of increasing the patA gene copy number was investigated (Fig. 5). A separate plasmid, pET21b(+)::patA, was constructed and co-transformed into E. coli APT7_Dual, resulting in a new strain, E. coli APT7_Triple (Fig. 1b). Whole-cell bioconversion using this strain yielded 68.5 ± 4.2 mM of 5-AP from 100 mM l-lysine and 100 mM α-KG in 500 mM Tris-HCl buffer at 30 °C and pH 6.5, corresponding to a 68.5 ± 4.2% yield at 24 h. This was 1.6-fold higher than that produced by E. coli APT7_Dual at 24 h (42.8 ± 2.8 mM) (Fig. 5a, b). Cadaverine accumulation was also significantly reduced, with the residual concentration decreasing to 28.5 ± 0.9 mM, which was 0.63-fold of the level observed in E. coli APT7_Dual at 24 h (44.7 ± 1.3 mM) (Fig. 5a, b). In terms of molar conversion, the yield improved from 6.07 ± 0.58 mol5 − AP/moll−lysine in E. coli APT7_Dual to 6.85 ± 0.42 mol5 − AP/moll−lysine in E. coli APT7_Triple. These results suggest that increased PatA expression, achieved by co-expressing patA from an additional plasmid, improved the efficiency of cadaverine conversion and utilization of α-KG. Collectively, these findings confirmed the importance of the PatA-catalyzed step as a key control point in the overall biosynthetic pathway. The resulting strain, E. coli APT7_Triple, represents a significantly improved host for 5-AP production compared with earlier constructs.
Fig. 5.
Effect of increasing the patA gene copy number. (a) Comparison of 5-AP production and cadaverine accumulation by E. coli APT7_Dual and E. coli APT7_Triple at 24 h. (b) Time-course profile of 5-AP production showing improved conversion by E. coli APT7_Triple
Enhancing 5-AP production via carbon source supplementation
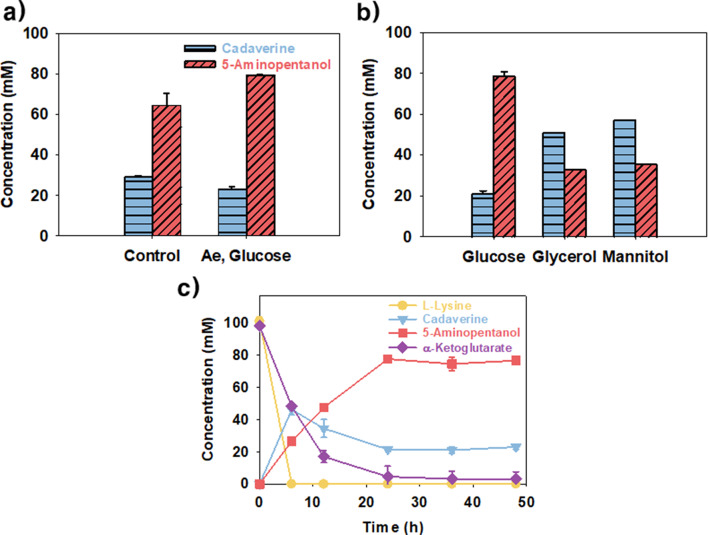
Previous studies have reported that direct supplementation of α-KG is insufficient to sustain the transamination, as α-KG is rapidly metabolized through the citric acid cycle, limiting its availability for aminotransferase activity (Wang et al. 2017; Cha et al. 2022). To address this, we evaluated the effect of indirectly supplying α-KG through glucose metabolism to enhance 5-AP production. Furthermore, to enhance citric acid cycle activity and α-KG generation, aeration was increased by replacing the cap of the Falcon tube with a perforated parafilm. When the reaction was performed using the E. coli APT7_Triple strain under 30 °C in Tris-HCl buffer (pH 6.5) conditions with the supplementation of 120 mM glucose, 78.5 ± 1.2 mM 5-AP was successfully synthesized, accompanied by a reduction in cadaverine accumulation to 20 ± 0.9 mM (Fig. 6a and c). Compared with the reaction without aeration and glucose supplementation, which resulted in 68.5 ± 4.2 mM 5-AP at 24 h, production increased by 1.14-fold. Based on these results, we replaced glucose with glycerol and mannitol, which are known to enhance the reducing power (Murarka et al. 2008; Ortjohann and Schönheit 2024). Contrary to our expectations, 5-AP production did not improve. Only 32.8 ± 0.2 and 35.5 ± 0.1 mM 5-AP were synthesized when using glycerol and mannitol as carbon sources, respectively. Additionally, cadaverine accumulation increased, with 51.0 ± 0.1 and 57.0 ± 0.1 mM of cadaverine accumulating when fed with glycerol and mannitol, respectively (Fig. 6b). In a previous study, 5-AP was reported as an intermediate and was produced at a low yield of 17.5%, suggesting poor selectivity (Ma et al. 2025). In contrast, this study demonstrates a highly selective 5-AP production system, achieving 79.7 ± 1.2% selectivity and a 78.5 ± 1.2% yield by systematic engineering shown in Table 1. Taken together, we established a system for the selective production of 5-AP, although the PatA-mediated transamination remains incomplete and still offers room for further improvement. Moving forward, systematic engineering strategies aimed at enhancing both the catalytic efficiency and expression level of PatA will be critical for developing a more efficient production platform, ultimately enabling industrial application with high product selectivity and concentration.
Fig. 6.
Indirect supply of α -KG through carbon source metabolism. (a) Effect of increased aeration and glucose supplementation on 5-AP production. Control shows the condition without glucose, Ae, glucose indicates the condition with 120 mM glucose and aeration simultaneously. (b) Comparison of 5-AP production using different carbon sources (glucose, glycerol, and mannitol). (c) Time-course profile of 5-AP production and cadaverine accumulation in response to 2% glucose supplementation
Table 1.
Comparison of 5-aminopentanol production capability
| Chemically 5-AP production | ||||||
|---|---|---|---|---|---|---|
| Substrate | Catalyst | Conversion (%) | Conversion selectivity(%) | Ref | ||
| 2-hydoxytetrahydropyran | Ni2CO1/Al2O3 | 100 | 79 | Yang et al. 2024 | ||
| 2-hydoxytetrahydropyran | 40Ni-LDO | 100 | 93.1 | Li et al. 2020 | ||
| Bio-based 5-AP production | ||||||
| Strains | Production method | Substrate (mM) | Product (mM) | Conversion yield (%) | Co-factors (mM) | Ref |
| ML21 | Flask fermentation | Glucose (83.2) | 5-AP (14.5) | 17.5 | Ma et al. 2025 | |
| E. coli AP T7_Triple | Whole-cell bioconversion | l-lysine (100) | 5-AP (78.5) | 78.5 | α-KG (100), PLP (0.1) NADPH (10) Glucose (122) | This study |
Conclusion
In this study, the first selective biological production pathway of 5-AP from l-lysine was successfully established in E. coli. Among the tested enzymes, YqhD was identified as the most effective reductase, and its combination with LdcC and PatA enabled the initial biosynthesis of 5-AP. The transition to a T7-based dual-plasmid expression system and increasing the copy number of patA gene significantly improved the production efficiency while reducing cadaverine accumulation. Additional enhancement was achieved through glucose supplementation and improved aeration, which supported cofactor regeneration via central metabolism. In contrast, alternative carbon sources such as glycerol and mannitol were less effective. Overall, this l-lysine-based biosynthetic strategy offers a scalable and environmentally friendly route for 5-AP production. A summary of 5-AP conversion yield under different reaction conditions and strains is provided in Table 1. This study is expected to contribute to the future development of microbial platforms for the sustainable and selective production of 5-AP, facilitating its applications in biopolymer synthesis as a monomer of PUE and as a precursor of δ-valerolactam for nylon-5 polymerization. These polymers have potential for usage in biomedical applications, packaging, and the fiber industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
BWL: data curation, methodology, analysis, and validation. Writing original draft. HTK: conceptualization, data curation, analysis, and validation. Writing original draft, review, and editing. HGK: conceptualization, data curation, analysis, and validation. Writing original draft, review, and editing. KJY: data curation, methodology, analysis, and validation. GK: data curation, methodology, analysis, and validation. YJJ: data curation, methodology, analysis, and validation. HGC: data curation, methodology, analysis, and validation. YJ: visualization. YHY: conceptualization and supervision. SHP: conceptualization, supervision, and funding acquisition. Writing original draft, review and editing. KP: conceptualization, supervision, and funding acquisition. Writing original draft, review and editing.
Funding
This study was supported by the R&D Program of MOTIE/KEIT (20014350, 20015041, 20018337, and 20018132). This study was supported by the cooperative Research Program for Agriculture Science and Technology Development (PJ0170820) through the Rural Development Administration (2022R1A4A1033015).
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Byung Wook Lee, Hee Taek Kim and Hyun Gi Koh contributed equally to this work.
Contributor Information
See-Hyoung Park, Email: shpark74@hongik.ac.kr.
Kyungmoon Park, Email: pkm2510@hongik.ac.kr.
References
- Akdogan Z, Guven B (2019) Microplastics in the environment: A critical review of current Understanding and identification of future research needs. Environ Pollut 254:113011 [DOI] [PubMed] [Google Scholar]
- Amobonye A, Bhagwat P, Raveendran S et al (2021) Environmental impacts of microplastics and nanoplastics: a current overview. Front Microbiol 12:768297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen X, Liu Y, Zhu F et al (2022) Metabolic engineering of Escherichia coli for high production of 1,5-pentanediol via a cadaverine-derived pathway. Metab Eng 74:168–177. 10.1016/j.ymben.2022.10.012 [DOI] [PubMed] [Google Scholar]
- Cha H-G, Kim HT, Park S-H et al (2022) Development of a glutaric acid production system equipped with Stepwise feeding of monosodium glutamate by whole-cell bioconversion. Enzym Microb Technol 159:110053 [DOI] [PubMed] [Google Scholar]
- Cha H-G, Kim HT, Park S-H et al (2023) Enhanced production of glutaric acid by biocatalyst-recycled bioconversion process integrated with in situ product recovery by adsorption. Enzym Microb Technol 171:110307 [DOI] [PubMed] [Google Scholar]
- Das KK, Reddy RC, Bagoji IB et al (2019) Primary concept of nickel toxicity – an overview. J Basic Clin Physiol Pharmacol 30:141–152. 10.1515/jbcpp-2017-0171 [DOI] [PubMed] [Google Scholar]
- Dziewior CS, Godwin K, Judge NG et al (2024) Poly (ester urea) s: synthesis, material properties, and biomedical applications. Prog Polym Sci 156:101866 [Google Scholar]
- Egorova KS, Ananikov VP (2016) Which metals are green for catalysis?? Comparison of the toxicities of ni, cu, fe, pd, pt, rh, and Au salts. Angew Chem Int Ed 55:12150–12162. 10.1002/anie.201603777 [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang R-Y et al (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345 [DOI] [PubMed] [Google Scholar]
- Hua W, Liang B, Zhou S et al (2024) An integrated cofactor and co-substrate recycling pathway for the biosynthesis of 1,5-pentanediol. Microb Cell Fact 23:132. 10.1186/s12934-024-02408-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarboe LR (2011) YqhD: a broad-substrate range aldehyde reductase with various applications in production of biorenewable fuels and chemicals. Appl Microbiol Biotechnol 89:249–257. 10.1007/s00253-010-2912-9 [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim HJ, Shin J-H et al (2015) Application of diethyl ethoxymethylenemalonate (DEEMM) derivatization for monitoring of lysine decarboxylase activity. J Mol Catal B: Enzymatic 115:151–154 [Google Scholar]
- Kim HT, Baritugo K-A, Oh YH et al (2019) High-level conversion of l-lysine into cadaverine by Escherichia coli whole cell biocatalyst expressing Hafnia alvei l-lysine decarboxylase. Polymers 11:1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tian J, Liu H et al (2020) Efficient Synthesis of 5-Amino-1-pentanol from Biomass-Derived Dihydropyran over Hydrotalcite-Based Ni–Mg3 AlO x Catalysts. ACS Sustainable Chem Eng 8:6352–6362. 10.1021/acssuschemeng.0c00394 [Google Scholar]
- López-Garzón CS, van der Wielen LAM, Straathof AJJ (2014) Green upgrading of succinate using dimethyl carbonate for a better integration with fermentative production. Chem Eng J 235:52–60. 10.1016/j.cej.2013.09.017 [Google Scholar]
- Lozano Terol G, Gallego-Jara J, Sola Martínez RA et al (2021) Impact of the expression system on Recombinant protein production in Escherichia coli BL21. Front Microbiol 12:682001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Xie C, Zhang Y et al (2025) Design an Energy-Conserving pathway for efficient biosynthesis of 1,5‐Pentanediol and 5‐Amino‐1‐Pentanol. Biotech Bioeng 122:445–451. 10.1002/bit.28875 [DOI] [PubMed] [Google Scholar]
- MacLeod M, Arp HPH, Tekman MB, Jahnke A (2021) The global threat from plastic pollution. Science 373:61–65. 10.1126/science.abg5433 [DOI] [PubMed] [Google Scholar]
- Millican JM, Agarwal S (2021) Plastic pollution: A material problem?? Macromolecules 54:4455–4469. 10.1021/acs.macromol.0c02814 [Google Scholar]
- Murarka A, Dharmadi Y, Yazdani SS, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74:1124–1135. 10.1128/AEM.02192-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nova A, Balcells D, Schley ND et al (2010) An Experimental– Theoretical study of the factors that affect the switch between Ruthenium-Catalyzed dehydrogenative amide formation versus amine alkylation. Organometallics 29:6548–6558. 10.1021/om101015u [Google Scholar]
- Oh YH, Kang K-H, Kwon MJ et al (2015) Development of engineered Escherichia coli whole-cell biocatalysts for high-level conversion of l-lysine into cadaverine. J Ind Microbiol Biotechnol 42:1481–1491 [DOI] [PubMed] [Google Scholar]
- Ortjohann M, Schönheit P (2024) Sugar alcohol degradation in archaea: uptake and degradation of mannitol and sorbitol in Haloarcula hispanica. Extremophiles 28:48. 10.1007/s00792-024-01365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelckmans M, Renders T, Van de Vyver S, Sels BF (2017) Bio-based amines through sustainable heterogeneous catalysis. Green Chem 19:5303–5331 [Google Scholar]
- Prabowo CPS, Shin JH, Cho JS et al (2020) Microbial production of 4-amino‐1‐butanol, a four‐carbon amino alcohol. Biotech Bioeng 117:2771–2780. 10.1002/bit.27438 [DOI] [PubMed] [Google Scholar]
- Reddy BN, Kulkarni SJ, Subrahmanyam M (1994) Zeolite catalysed intramolecular cyclization of 5-amino-1-pentanol to piperidine bases. Appl Catal A 119:23–32 [Google Scholar]
- Rohles CM, Gießelmann G, Kohlstedt M et al (2016) Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate. Microb Cell Fact 15:154. 10.1186/s12934-016-0553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Joo JC, Lee E et al (2018) Characterization of a Whole-Cell biotransformation using a constitutive lysine decarboxylase from Escherichia coli for the High-Level production of cadaverine from industrial grade l-Lysine. Appl Biochem Biotechnol 185:909–924. 10.1007/s12010-018-2696-4 [DOI] [PubMed] [Google Scholar]
- Tang D, Chen Z, Correa-Netto F et al (2016) Poly (urea ester): A family of biodegradable polymers with high melting temperatures. J Polym Sci Part A: Polym Chem 54
- Von Tiedemann P, Anwar S, Kemmer-Jonas U et al (2020) Synthesis and solution processing of Nylon‐5 ferroelectric thin films: the renaissance of Odd‐Nylons? Macro Chem Phys 221:1900468. 10.1002/macp.201900468 [Google Scholar]
- Wang J, Wu Y, Sun X et al (2017) De Novo biosynthesis of glutarate via α-Keto acid carbon chain extension and decarboxylation pathway in Escherichia coli. ACS Synth Biol 6:1922–1930. 10.1021/acssynbio.7b00136 [DOI] [PubMed] [Google Scholar]
- Wang J, Gao C, Chen X, Liu L (2021) Engineering the Cad pathway in Escherichia coli to produce glutarate from l-lysine. Appl Microbiol Biotechnol 105:3587–3599. 10.1007/s00253-021-11275-1 [DOI] [PubMed] [Google Scholar]
- Wang Y-G, Han X-C, Gu B et al (2023) Insight into the green route to dimethyl succinate by direct esterification of bio-based disodium succinate using CO2 and CH3OH. Chin J Chem Eng 64:188–195. 10.1016/j.cjche.2023.05.017 [Google Scholar]
- Yang J, Zhang J, Benassi E et al (2024) NiCo/Al2O3 nanocatalysts for the synthesis of 5-amino-1-pentanol and 1, 5-pentanediol from biomass-derived 2-hydroxytetrahydropyran. Green Chem Eng 5:119–131 [Google Scholar]
- Zhang W, Liu X, Liu L et al (2022) Effects of microplastics on greenhouse gas emissions and microbial communities in sediment of freshwater systems. J Hazard Mater 435:129030 [DOI] [PubMed] [Google Scholar]
- Zhu F, Qin N, Cen X et al (2024) Metabolic engineering of Corynebacterium glutamicum for the production of the Four-Carbon platform chemicals γ-Hydroxybutyrate and γ-Butyrolactone. ACS Synth Biol 13:3754–3764. 10.1021/acssynbio.4c0060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.