Abstract
Objective
This study aims to investigate the interaction between common gene and 5q- chromosome karyotype mutations and the immune microenvironment in myelodysplastic syndromes (MDS), and explore the potential prognostic value of immune markers in MDS.
Methods
A total of 83 MDS patients treated at the Second Hospital of Lanzhou University between January 2019 and April 2024 were enrolled in this study. Patients were divided into mutation and wild-type groups based on gene mutations and the presence of 5q- chromosomal abnormalities. Co-mutations in MDS patients were analyzed. A total of 19 fine immune parameters were measured in the samples using flow cytometry and flow cytometric bead array (CBA) technology. Changes in immune markers between the mutation and wild-type groups were observed, and the correlation between lymphocyte subsets and cytokines in the mutation group was analyzed.
Results
Significant differences in immune markers were observed between the common gene mutation group and the wild-type group in MDS (P < 0.05). Correlations between lymphocyte subsets and cytokines were identified in ASXL1, RUNX1, SF3B1, TET2, TP53, U2AF1, and 5q- mutation groups.
Conclusion
This study investigates the correlation between common MDS mutations and cytokine/lymphocyte subpopulations, preliminarily revealing the interaction between genetic factors and immune markers in myelodysplastic syndrome (MDS), and emphasizes the potential important role of the Th17/Treg axis in the tumor immune microenvironment of MDS, but does not verify the mechanism. The study suggests that fine immune parameters have potential prognostic value in MDS and may guide future MDS treatment strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02845-0.
Keywords: Myelodysplastic syndromes, Gene mutations, Fine immune markers, Immune mechanisms
Introduction
Myelodysplastic syndromes (MDS) are a group of myeloid neoplasms characterized by diverse genotypes and phenotypes, marked by ineffective hematopoiesis and a high risk of progression to acute myeloid leukemia (AML), necessitating risk-adapted treatment strategies [1–3]. MDS patients have been classified into high-risk and low-risk phenotypes according to sequential clinical prognostic scoring systems, which incorporate factors such as morphological abnormalities, clinical hematologic parameters, cytogenetics, and gene mutations.. These scoring systems refine the risk stratification of MDS patients and guide therapeutic management. As our understanding of the genetic basis of MDS pathogenesis advances, MDS classification is already shifting towards a genetic-based categorization, with this trend expected to become even more pronounced in the future [4–6]. Common gene mutations in MDS include SF3B1, IDH1, SETBP1, TET2, ASXL1, SRSF2, RUNX1, U2AF1, TP53, EZH2, and STAG2. There is an interplay between gene mutations and the immune microenvironment in the development and progression of MDS [7–9]. Gene mutations can directly or indirectly influence fine immune parameters in MDS, while fine immune markers, such as cytokines and other immune molecules, can affect gene expression and function by inducing genes involved in inflammation and immune responses. This study aims to explore the potential mechanisms underlying the interaction between common gene mutations in MDS and the immune microenvironment by analyzing differences in fine immune parameters and the correlation between lymphocyte subsets and cytokines associated with common MDS mutations.
Materials and methods
Study subjects
This study included MDS patients treated at the Second Hospital of Lanzhou University from January 2019 to April 2024. Inclusion criteria were: (1) Diagnosis and risk stratification of MDS based on hematological examination and bone marrow aspiration according to the NCCN Guidelines® Insights: Myelodysplastic Syndromes, Version 3.2022 [10]; (2) Newly diagnosed MDS patients who had not received chemotherapy or immunotherapy within the last two months; (3) Age ≥ 18 years; (4) Complete bone marrow examinations, including bone marrow cell morphology, histopathology, flow immunophenotyping, cytogenetics, molecular genetics, and immunological evaluations (lymphocyte subsets, cytokines). Exclusion criteria included: (1) Pregnancy or lactation; (2) Myelodysplasia caused by non-clonal diseases; (3) Poor general condition (ECOG score > 4); (4) Severe dysfunction of major organs such as heart, lung, liver, or kidney, other malignancies; (5) Serious complications; (6) Autoimmune diseases; (7) Secondary MDS due to radiation or chemotherapy for other tumors; (8) Psychiatric disorders or cognitive impairment; (9) Use of immunomodulatory drugs within the last 4 months.
Patients were stratified into relatively low-risk and high-risk groups according to the recommendations of the MDS Expert Panel [8]: low-risk MDS patients were those classified as Low or Intermediate-1 (Int-1) by the International Prognostic Scoring System (IPSS), Very Low, Low, or Intermediate by the Revised IPSS (IPSS-R), or Very Low, Low, or Intermediate by the WHO Prognostic Scoring System (WPSS); whereas high-risk MDS patients were those classified as Intermediate-2 (Int-2) or High by IPSS, Intermediate, High, or Very High by IPSS-R, or High or Very High by WPSS. Furthermore, patients with an IPSS-R score ≤ 3.5 were generally managed as low-risk, while those with a score > 3.5 were managed as high-risk.
Detection of fine immune parameters
Experimental instruments and experimental reagents
An ultra-clean bench, FACS Canto flow cytometer, and flow sampling tubes were purchased from BD Bioscience (USA). Cryogenic refrigerators (4 °C, 20 °C) were sourced from Qingdao Haier Electric Co. (Qingdao, China). Various types of micropipettes were obtained from RAININ Company (USA), while pipette tips were purchased from Eppendorf (Germany). A multi-tube automatic balancing centrifuge was obtained from Thermo Corporation (USA), and disposable sterile pipettes were sourced from Falcon Company (USA). The automatic electrochemical analyzer (cobas e801) was purchased from Roche (Germany). Rapid mixers were provided by Xinkang Medical Equipment Co. (China). APC-labelled Mouse Anti-Human CD4 Antibody, PE-labelled Mouse Anti-Human CD8 Antibody, FITC-labelled Mouse Anti-Human CD3 Antibody, PerCP-labelled Mouse Anti-Human CD45 Antibody, PE-labelled Mouse Anti-Human CD16+ CD56 Antibody, APC-labelled Mouse Anti-Human CD19 Antibody, and Erythrocyte lysate were purchased from BD Bioscience (USA). Phosphate Buffer Solution (PBS, 70-PS0021) was sourced from United Kingdom Biological Co. (UK). Human 12 Cytokines Detection Kit (Flow Fluorescence Method, 20,230,701) was purchased from Hangzhou Sage Biotechnology Co. (Hangzhou, China).
Detection of lymphocyte subsets and treg by flow cytometry
Morning fasting venous blood samples (2 ml) were collected from MDS patients, and 50 μl was added to flow cytometry tubes with corresponding fluorescent monoclonal antibodies, including CD3, CD4, CD8, CD16, CD56, CD19, CD25, and FOXP3+. Data were acquired using a flow cytometer (BD) and analyzed with FACSDiva software (BD, version 6.1). T-cell subsets were defined as follows: total T lymphocytes (CD3+ CD19−), B lymphocytes (CD3-CD19+), NK cells (CD16+ CD56+), CD4+ T cells (CD3+ CD4+), CD8+ T cells (CD3+ CD8+), and regulatory T cells (CD4+ CD25+ FOXP3+), expressed as a proportion of CD4 + cells.
Detection of twelve cytokines by cytometric bead array (CBA)
Peripheral venous blood (2 mL) was collected from fasting subjects each morning into serum tubes, centrifuged at 4 000×g for 10 min, and the resultant serum was harvested for measurement of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, TNF-α, IFN-γ, IFN-α and IL-17A using flow cytometric bead-based capture chip technology. Quantitative calibrators were prepared by transferring the highest-concentration standard into an assay tube, adding 2 mL of sample diluent, and incubating at room temperature for 15 min, then mixing thoroughly; serial 1:2 dilutions were generated across 11 tubes (1:2–1:2048) by transferring 300 µL sequentially through each tube with mixing. Capture beads were prepared by calculating 25 µL per assay (for samples, ten standards, and one negative control), centrifuging the bead suspension at 200×g for 5 min, discarding the supernatant, resuspending in an equal volume of bead buffer, vortexing, and incubating in the dark at room temperature for 15 min; 25 µL of this bead suspension was then added to every calibrator and sample tube. After addition of 25 µL of each diluted calibrator or sample and thorough vortexing, 25 µL of fluorescent detection reagent was added, tubes were gently mixed, and incubated in the dark at room temperature for 2.5 h. Beads were washed once with 1 mL PBS (200×g, 5 min), the supernatant discarded, and the pellet resuspended in 100 µL PBS before analysis on the flow cytometric bead-based capture chip system.
Collection of MDS gene mutations and grouping
Gene mutation testing was performed by Hyster Laboratories or Goldwyn Medical, including 60 common MDS-related genes. Mutations were detected using next-generation sequencing (NGS) and panel sequencing. Bone marrow samples (2 ml) were collected to isolate mononuclear cells, and genomic DNA was extracted. Capture probe library construction technology was used to capture all target genes, with sequencing performed on Illumina platforms (NovaSeq6000/HiSeq2500). The average sequencing depth was 2000×. Gene mutations were recorded, excluding nonsense mutations. Patients were grouped into mutation (MT) and wild-type (WT) groups based on the presence of specific gene mutations. Due to limitations in sample size, when grouping individual gene mutation groups and wild-type groups, some patients may have mutations in other genes. Therefore, when multiple gene mutations are present, the immune response cannot be distinguished based on which gene is responsible. This is a limitation of this article: when multiple gene mutations are present, the immune response cannot be distinguished based on which gene is responsible. However, some genes often co-occur with mutations in other genes, which is a clinical characteristic of these genes. Therefore, we believe that this can to some extent reflect the issue.
Follow-up
Follow-up was conducted via review of inpatient and outpatient medical records and telephone interviews. The follow-up period ended on October 1, 2024. Progression-free survival (PFS) was defined as the time from the start of treatment to disease progression or death. Overall survival (OS) was defined as the time from diagnosis to death from any cause or last follow-up.
Statistical analysis
Statistical analyses were conducted using R 4.2.3.
Survival analysis for OS and PFS was performed using the Kaplan–Meier method, with differences between groups assessed by the Log-rank test. A P value of less than 0.05 was considered statistically significant.
Gene co-mutation analysis was carried out using the ComplexHeatmap package in R. Gene mutation data were processed, and Pearson correlation matrices were computed to assess the relationships between different mutations. P values were calculated for each correlation, and statistically significant correlations were annotated as follows: *P < 0.05, **P < 0.01, ***P < 0.001. Heatmaps were generated to provide a visual representation of these correlations and their statistical significance.
Scatter plots were created using the ggplot2 and ggpubr packages, with linear regression trend lines (geom_smooth function) fitted to the data. The plots display correlation coefficients (r values) and associated p-values. The strength of the correlations was categorized as follows: strong correlation (r > 0.7), moderate correlation (r = 0.3–0.7), and weak correlation (r = 0–0.3), with statistical significance indicated by P < 0.05.
For a comprehensive analysis of immunological and hematological parameters, violin plots with overlaid boxplots were created to compare the distribution of parameters between the two groups using ggplot2, and statistical significance was assessed using t-tests. The plots were annotated with significance levels to indicate statistical differences between groups: *P < 0.05, **P < 0.01, ***P < 0.001.
For each immune parameter, group comparisons between MT and WT groups were performed using independent two-sample t-tests. Bar plots depicting the means ± standard error (SE) for each group were created using ggplot2, with individual data points overlaid as scatter points. The plots were annotated with significance levels to indicate statistical differences between groups: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Clinical characteristics of patients
A total of 83 newly diagnosed MDS patients were included in this study, comprising 34 relatively low-risk patients and 49 relatively high-risk patients. Among these, 31 patients had no gene mutations, 26 patients had one gene mutation, 16 patients had two gene mutations, 10 patients had three or more gene mutations==. The basic characteristics of the MDS patients are summarized in Table 1.
Table 1.
Patient characteristics
| Relatively low-risk patients | Relatively high-risk patients | Total | |
|---|---|---|---|
| Number of cases | 34 | 49 | 83 |
| Age | 53.5 (18–84) | 59 (18–86) | 55 (18–86) |
| Gender | |||
| Male | 17 | 20 | 37 |
| Female | 17 | 29 | 46 |
| WBC (×109/L) | 2.74 ± 0.24 | 2.94 ± 0.30 | 2.86 ± 0.20 |
| NE% | 43.62 ± 3.56 | 44.12 ± 2.77 | 43.92 ± 2.17 |
| LY% | 38.94 ± 3.94 | 39.43 ± 2.54 | 39.23 ± 2.19 |
| MO% | 6.29 ± 0.77 | 9.19 ± 0.88 | 7.99 ± 0.62 |
| Number of mutations n (%) | |||
| 0 | 19 | 12 | 31 |
| 1 | 8 | 18 | 26 |
| 2 | 5 | 11 | 16 |
| ≥ 3 | 2 | 8 | 10 |
| 5q- chromosome karyotype mutations | 3(All are isolated del(5q)) | 5(complex aberrant) | 8 |
Frequency of common gene mutations
The frequencies of gene mutations and 5q- chromosomal abnormalities among the 83 patients are shown in Fig. 1. The results indicated that ASXL1 mutations were observed in 17 cases(20.48%), U2AF1 in 12 cases (14.46%), SF3B1 in 11 cases(13.25%), TP53 in 10 cases(12.05%), TET2 in 9 cases(10.84%), RUNX1 in 6 cases(7.23%), IDH1 in 5 cases(6.02%), BCOR in 4 cases(4.82%), SETBP1 in 4 cases(4.82%), SRSF2 in 4 cases(4.82%), EZH2 in 3 cases(3.61%), STAG2 in 3 cases(3.61%), and 5q- chromosomal mutations in 8 cases(9.64%). Patients were grouped based on the number of common MDS gene mutations: 31 patients had no mutations, 26 patients had one mutation, 16 patients had two mutations, 6 patients had three mutations, and 4 patients had three or more mutations.
Fig. 1.
Frequencies of common gene mutations in MDS patients. A The frequencies of MDS common gene mutations and 5q- chromosome karyotype in 83 patients were analyzed. B Frequency of the number ofcommon mutations in MDS
Efficacy and survival analysis
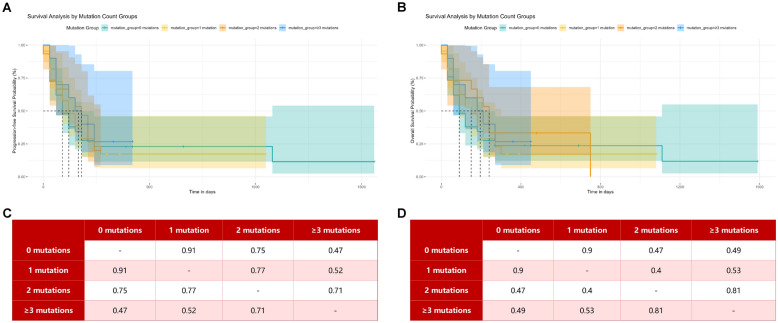
By the follow-up date, 7 of the 83 MDS patients were lost to follow-up, 58 had died, and 18 were still alive. Among the 76 patients who were followed up, the median overall survival (OS) time was 5 months (range 0–53 months), and the median progression-free survival (PFS) time was 4 months (range 0–52 months). The 1-year OS rate was 13.16% (10/76), and the 1-year PFS rate was 9.21% (7/76). Kaplan–Meier survival curve analyses comparing common gene mutation groups and 5q- mutation groups versus wild-type groups are shown in Fig. 2. Kaplan–Meier survival curve analyses grouped by the number of common MDS gene mutations are shown in Fig. 3.
Fig. 2.

Kaplan–Meier survival curve analysis of some common genes and 5q- in MDS between the mutation group and the wild group. A, B PFS and OS between the SF3B1 gene mutation group and the wild group. C, D PFS and OS between the ASXL1 gene mutation group and the wild group. E, F PFS and OS between the U2AF1 gene mutation group and the wild group. G, H PFS and OS between the TP53 gene mutation group and the wild group. I, J: PFS and OS between the RRUNX1 gene mutation group and the wild group. K, L: PFS and OS between the TET2 gene mutation group and the wild group. M, N PFS and OS between the IDH gene mutation group and the wild group. O, P PFS and OS between the 5q- mutation group and the wild group
Fig. 3.
Kaplan–Meier survival analysis was performed after grouping MDS patients with different number of gene mutations. A PFS survival analysis of patients with different number of mutations. B OS survival analysis of patients with different number of mutations. C P value of PFS survival analysis between groups. D P value of OS survival analysis between groups
Co-mutations of common MDS genes
The gene correlation heatmap (Fig. 4) reveals co-mutations among several genes: RUNX1 with ZRSR2 and TET2; TP53 with WT1; IDH1 with SRSF2; ASXL1 with ZRSR2, SRSF2, and U2AF1; U2AF1 with SETBP1; BCOR with DNMT3A, STAG2, and EZH2; EZH2 with STAG2 and SETBP1; SETBP1 with STAG2; and DNMT3A with KRAS. Notably, strong co-mutations were observed between RUNX1 and ZRSR2, IDH1 and SRSF2, ASXL1 and SRSF2, U2AF1, and DNMT3A and KRAS.
Fig. 4.

Gene correlation heatmap. Heatmap shows Pearson correlation coefficients between variables. Statistical significance was assessed by two‑tailed Pearson’s test and is indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
Differential analysis of immune and hematological parameters between relatively low-risk and relatively high-risk MDS patients
The comparative analysis of immunological and hematological parameters between relatively low-risk and relatively high-risk MDS patients (Fig. 5) revealed that the relatively high-risk group exhibited significantly higher levels of Tregs (8.23 ± 0.46% vs. 6.58 ± 0.47%; P < 0.05), monocyte percentage (9.16 ± 0.88% vs. 5.94 ± 0.71%; P < 0.01) and monocyte count (0.31 ± 0.05 × 109/L vs. 0.18 ± 0.02 × 109/L; P < 0.05). Given that only a few parameters reached statistical significance in this comparison, we therefore decided to further explore whether common gene mutations in MDS influence patients’ immune profiles.
Fig. 5.
Violin plots of immunological and haematological parameters in relatively low-risk versus high-risk MDS patients. Panels A–S show key immunophenotypic and cytokine markers; panels T–AC show routine complete blood-count indices. Low-risk patients are plotted in pink and high-risk in blue; each violin’s central box spans the interquartile range with the median. Statistical significance was determined by two-tailed Student’s t-test (*P < 0.05; **P < 0.01); NS, not significant
Differential analysis of fine immune markers in patients with different numbers of gene mutations
We conducted a differential analysis of immune indicators across patients with different numbers of gene mutations. We found that there were fewer statistically significant indicators. The levels of IFN-α in patients with 0 gene mutations were higher than in those with 1 gene mutation (0 mutations: mean = 1.64 ± 0.46, 1 mutation: mean = 0.21 ± 0.08, P < 0.05). Similarly, the levels of IL-6 in patients with 0 gene mutations were higher than in those with 1 gene mutation (0 mutations: mean = 15.84 ± 4.58, 1 mutation: mean = 3.41 ± 0.45, P < 0.05). We further analyzed the immune indicators between the gene mutation groups and the wild-type groups (Fig. 6).
Fig. 6.
Differential analysis of fine immune markers in patients with different numbers of gene mutations. Data presented as mean ± SEM; statistical significance denoted by *P < 0.05, **P < 0.01, ***P < 0.001
Analysis of differences in immune markers between MT and WT groups of common mutated genes in MDS
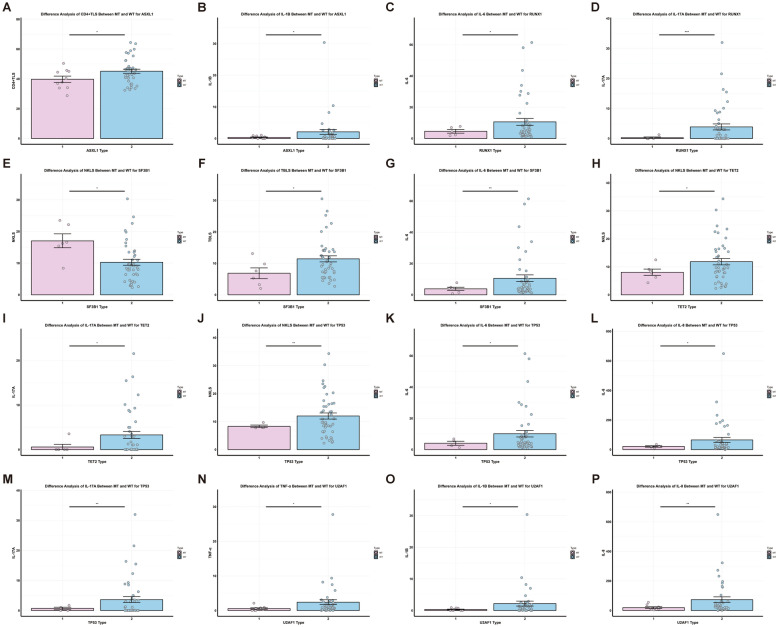
In the ASXL1 mutation group, the proportion of CD4+ T cells (39.74 ± 2.10% vs. 45.12 ± 1.35%, P < 0.05) and IL-1β levels (0.38 ± 0.13 pg/ml vs. 2.10 ± 0.80 pg/ml, P < 0.05) were lower than those in the wild-type group, as shown in Fig. 7. The RUNX1 mutation group had lower levels of IL-6 (4.64 ± 1.20 pg/ml vs. 10.65 ± 2.17 pg/ml, P < 0.05) and IL-17A (0.25 ± 0.25 pg/ml vs. 3.87 ± 0.99 pg/ml, P < 0.001) compared to the wild-type group. The SF3B1 mutation group showed a higher proportion of NK cells (17.04 ± 2.21% vs. 10.27 ± 0.94%, P < 0.05) but lower total B cells (6.84 ± 1.70% vs. 11.43 ± 0.96%, P < 0.05) and IL-6 levels (3.83 ± 1.01 pg/ml vs. 10.60 ± 2.17 pg/ml, P < 0.01) compared to the wild-type group (Fig. 7). In the TET2 mutation group, both the NK cell proportion (8.07 ± 1.13% vs. 11.83 ± 1.13%, P < 0.05) and IL-17A levels (0.59 ± 0.59 pg/ml vs. 3.29 ± 0.78 pg/ml, P < 0.05) were lower than in the wild-type group (Fig. 7).
Fig. 7.
The differences of fine immune indexes between the wild type and the mutant type of some common mutated genes in MDS. MT-mutant type, WT-wild type. A, B ASXL1 gene mutation. C, D RUNX1 gene mutation. E–G SF3B1 gene mutation. H, I TET2 gene mutation. J–M TP53 gene mutation. N–P U2AF1 gene mutation. Data presented as mean ± SEM; statistical significance denoted by *P < 0.05, **P < 0.01, ***P < 0.001
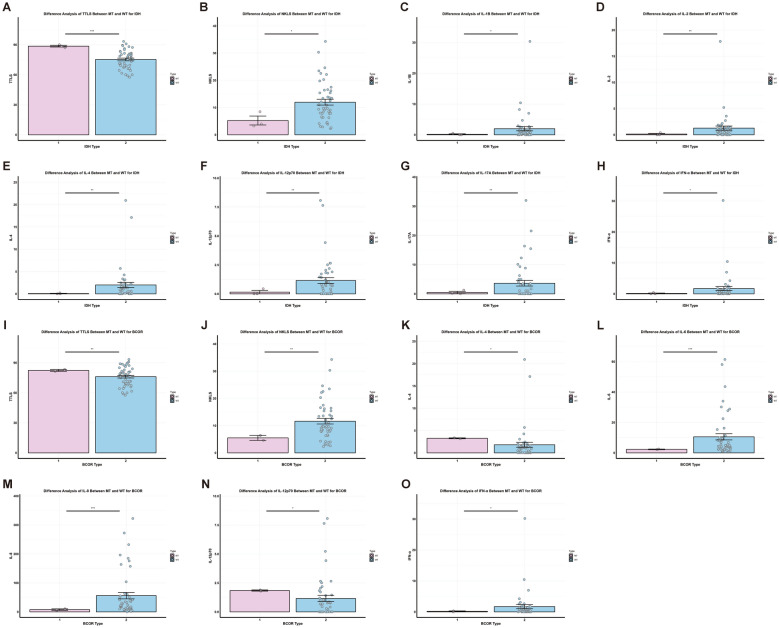
The TP53 mutation group had lower NK cell proportions (8.31 ± 0.46% vs. 12.00 ± 1.09%, P < 0.01), IL-6 (4.07 ± 1.30 pg/ml vs. 10.17 ± 2.06 pg/ml, P < 0.05), IL-8 (21.44 ± 5.10 pg/ml vs. 65.33 ± 16.71 pg/ml, P < 0.05), and IL-17A (0.69 ± 0.42 pg/ml vs. 3.64 ± 0.97 pg/ml, P < 0.01) levels compared to the wild-type group (Fig. 8). The U2AF1 mutation group exhibited lower levels of TNF-α (0.57 ± 0.22 pg/ml vs. 2.40 ± 0.72 pg/ml, P < 0.05), IL-1β (0.31 ± 0.13 pg/ml vs. 2.19 ± 0.79 pg/ml, P < 0.05), and IL-8 (19.54 ± 5.78 pg/ml vs. 73.45 ± 19.01 pg/ml, P < 0.01) than the wild-type group (Fig. 8). In the IDH1 mutation group, the total T cell proportion was higher (88.53 ± 0.87% vs. 75.24 ± 1.34%, P < 0.001), whereas the NK cell proportion (5.25 ± 1.64% vs. 11.98 ± 1.06%, P < 0.05), IFN-α (0.14 ± 0.14 pg/ml vs. 1.70 ± 0.68 pg/ml, P < 0.05), IL-1β (0.16 ± 0.16 pg/ml vs. 1.98 ± 0.69 pg/ml, P < 0.05), and IL-2 (0.14 ± 0.14 pg/ml vs. 1.29 ± 0.39 pg/ml), IL-4 (0.07 ± 0.07 pg/ml vs. 1.99 ± 0.56 pg/ml), IL-12p70 (0.15 ± 0.15 pg/ml vs. 1.15 ± 0.25 pg/ml), and IL-17A (0.47 ± 0.40 pg/ml vs. 3.62 ± 0.95 pg/ml) levels (all P < 0.01) were lower than in the wild-type group (Fig. 8). For the BCOR mutation group, the total T cell proportion (82.40 ± 1.00% vs. 76.11 ± 1.38%, P < 0.01), IL-4 (2.85 ± 0.42 pg/ml vs. 3.05 ± 1.35 pg/ml, P < 0.05), and IL-12p70 (1.39 ± 0.46 pg/ml vs. 1.33 ± 0.31 pg/ml, P < 0.05) levels were higher, while the NK cell proportion (5.50 ± 0.90% vs. 11.62 ± 1.04%, P < 0.01), IL-6 (2.32 ± 0.20 pg/ml vs. 10.55 ± 2.04 pg/ml, P < 0.001), IL-8 (7.76 ± 2.82 pg/ml vs. 56.21 ± 11.09 pg/ml, P < 0.001), and IFN-α (0.16 ± 0.16 pg/ml vs. 1.70 ± 0.66 pg/ml, P < 0.05) were lower compared to the wild-type group (Fig. 8).
Fig. 8.
The differences of fine immune indexes between the wild type and the mutant type of IDH2 and BCOR in MDS. MT-mutant type, WT-wild type. A-H: IDH2 gene mutation. I-O: BCOR gene mutation. Data presented as mean ± SEM; statistical significance denoted by *P < 0.05, **P < 0.01, ***P < 0.001
In the SETBP1 mutation group, IL-5 (1.00 ± 0.12 pg/ml vs. 0.85 ± 0.15 pg/ml, P < 0.01) and IL-12p70 (1.32 ± 0.55 pg/ml vs. 1.33 ± 0.31 pg/ml, P < 0.05) levels were higher, whereas the total T cell proportion (76.37 ± 5.49% vs. 76.20 ± 1.20%, P < 0.001), total B cell proportion (6.90 ± 2.59% vs. 10.90 ± 0.72%, P < 0.05), IL-1β (0.93 ± 0.76 pg/ml vs. 4.13 ± 2.32 pg/ml, P < 0.05), IL-17A (1.36 ± 0.87 pg/ml vs. 4.31 ± 1.12 pg/ml, P < 0.05), and TNF-α (1.11 ± 0.58 pg/ml vs. 4.61 ± 2.51 pg/ml, P < 0.05) were lower than in the wild-type group (Fig. 9). The 5q- mutation group had lower levels of IL-1β (0.44 ± 0.28 pg/ml vs. 2.02 ± 0.72 pg/ml, P < 0.05), IL-2 (0.34 ± 0.21 pg/ml vs. 1.33 ± 0.41 pg/ml, P < 0.05), IL-4 (0.50 ± 0.31 pg/ml vs. 2.03 ± 0.59 pg/ml, P < 0.05), IL-8 (25.18 ± 5.72 pg/ml vs. 65.89 ± 17.08 pg/ml, P < 0.05), IL-17A (0.94 ± 0.61 pg/ml vs. 3.68 ± 0.99 pg/ml, P < 0.05), and IFN-α (0.11 ± 0.07 pg/ml vs. 1.79 ± 0.71 pg/ml, P < 0.05) compared to the wild-type group (Fig. 9).
Fig. 9.
The differences of fine immune indexes between the wild type and the mutant type of SETBP1 and 5q- in MDS. MT-mutant type, WT-wild type. A-G: SETBP1 gene mutation. H-M: 5q- mutation. Data presented as mean ± SEM; statistical significance denoted by *P < 0.05, **P < 0.01, ***P < 0.001
Correlation analysis between lymphocyte subsets and cytokines
The correlation analysis between lymphocyte subsets and cytokines (Table 2) revealed the following significant associations: In the ASXL1 mutation group, CD4 + T cells were positively correlated with IL-5, IL-10, and IL-12p70, while NK cells were positively correlated with IL-1β (P < 0.05). In the RUNX1 mutation group, NK cells were positively correlated with IFN-α, IFN-γ, IL-1β, IL-2, and TNF-α, and Treg cells were positively correlated with IFN-α, IL-1β, IL-2, and TNF-α. CD8 + T cells were positively correlated with IL-5, IL-10, and IL-12p70, while total B cells were negatively correlated with IFN-α, IFN-γ, IL-1β, and IL-2 (P < 0.05).
Table 2.
Correlation analysis between lymphocyte subsets and cytokines in different gene mutations
| lymphocyte subsets | cytokines | P value | r value |
|---|---|---|---|
| ASXL1 | |||
| CD4 + TLs | IL-5 | 0.041 | 0.62 |
| CD4 + TLs | IL-10 | 0.009 | 0.74 |
| CD4 + TLs | IL-12p70 | 0.012 | 0.72 |
| NKTLs | IL-1β | 0.039 | 0.63 |
| RUNX1 | |||
| CD8 + TLs | IL-4 | 0.032 | -0.85 |
| CD8 + TLs | IL-5 | 0.003 | -0.96 |
| CD8 + TLs | IL-12p70 | 0.04 | -0.83 |
| NK TLs | IFN-α | 0.002 | 0.96 |
| NK TLs | IFN-γ | 0.003 | 0.96 |
| NK TLs | IL-1β | 0.001 | 0.98 |
| NK TLs | IL-2 | 0.001 | 0.97 |
| NK TLs | TNF-α | 0.043 | 0.83 |
| TBLs | IFN-α | 0.023 | -0.87 |
| TBLs | IFN-γ | 0.008 | -0.92 |
| TBLs | IL-1β | 0.012 | -0.91 |
| TBLs | IL-2 | 0.008 | -0.92 |
| Treg | IFN-α | 0.019 | 0.88 |
| Treg | IL-1β | 0.025 | 0.87 |
| Treg | IL-2 | 0.02 | 0.88 |
| Treg | TNF-α | 0.02 | 0.88 |
| SF3B1 | |||
| CD8 + TLs | IL-6 | 0.04 | 0.78 |
| TET2 | |||
| NK TLs | IL-8 | 0.012 | 0.86 |
| TP53 | |||
| CD4 + TLs | TNF-α | 0.05 | 0.88 |
| NK TLs | IL-1β | 0.049 | -0.88 |
| NK TLs | IL-4 | 0.03 | -0.91 |
| Treg | IL-2 | 0.024 | 0.93 |
| Treg | IL-4 | 0.029 | 0.92 |
| Treg | IL-5 | 0.023 | 0.93 |
| U2AF1 | |||
| CD4 + TLs | IFN-α | 0.007 | 0.78 |
| CD4 + TLs | IL-2 | 0.01 | 0.76 |
| CD4 + TLs | IL-5 | 0.046 | 0.64 |
| CD4 + TLs | IL-12p70 | 0.018 | 0.72 |
| CD4 + TLs | TNF-α | 0.039 | 0.66 |
| Treg | IL-6 | 0.04 | 0.65 |
| 5q- | |||
| NK TLs | TNF-α | 0.04 | -0.83 |
In the SF3B1 mutation group, CD8 + T cells were positively correlated with IL-6 (P < 0.05). In the TET2 mutation group, NK cells were positively correlated with IL-8 (P < 0.05). In the TP53 mutation group, CD4+ T cells were positively correlated with TNF-α, and Treg cells were positively correlated with IL-2, IL-4, and IL-5, whereas NK cells were negatively correlated with IL-1β and IL-4 (P < 0.05).
For the U2AF1 mutation group, CD4+ T cells were positively correlated with IFN-α, IL-2, IL-5, IL-12p70, and TNF-α, while Treg cells were positively correlated with IL-6 (P < 0.05). In the 5q- mutation group, NK cells were negatively correlated with TNF-α (P < 0.05).
Discussion
Gene mutations in MDS are associated with prognosis, but there is a lack of studies on how common MDS mutations and 5q- chromosomal abnormalities affect fine immune markers. This study included 83 newly diagnosed MDS patients and performed Kaplan–Meier survival analysis for PFS and OS. We analyzed co-mutations of common MDS mutations and conducted differential analysis of fine immune parameters in mutation groups, including ASXL1, RUNX1, SF3B1, TET2, TP53, U2AF1, IDH1, BCOR, SETBP1, and 5q-, compared to wild-type groups. We also explored correlations between lymphocyte subsets and cytokines in the ASXL1, RUNX1, SF3B1, TET2, TP53, U2AF1, and 5q- mutation groups.
Survival analysis revealed no statistically significant differences in OS and PFS among groups (P > 0.05). Possible reasons include: (1) insufficient sample size and limited numbers of patients in certain gene mutation groups, particularly those with multiple co-mutations; (2) individualized treatment regimens optimized for each patient, showing good efficacy across various MDS subtypes; (3) some patients did not receive further treatment after diagnosis for personal reasons. The potential impact of different therapeutic regimens on immune parameters and prognosis was not controlled or adjusted for in this study. Future research should incorporate treatment-adjusted models or stratified analyses, provided that an adequate sample size is available, to further elucidate the role of therapeutic interventions. Continued follow-up is required for a more comprehensive evaluation of treatment efficacy.An international retrospective cohort study involving 2,043 MDS patients found co-mutations of SRSF2 with ASXL1, RUNX1, IDH1, and EZH2 [11], consistent with our findings of strong co-mutations between SRSF2, ASXL1, and IDH1. Retrospective analysis of 234 MDS patients by Wang H et al. [12] showed that U2AF1 mutations were positively associated with co-mutations in ASXL1, RUNX1, and SETBP1, with U2AF1-mutated patients often progressing to AML when co-mutated with ASXL1, and increased relapse risk with RUNX1 mutations. Our study confirmed the co-mutation of U2AF1 with ASXL1 and SETBP1. Additionally, we identified co-mutations between RUNX1 and ZRSR2, TET2, which have been previously reported [13]. These studies highlight the impact of common MDS gene mutations and co-mutations on clinical characteristics and prognosis, yet no research has specifically investigated their influence on immune markers.
It should be acknowledged that MDS patients often present with pancytopenia affecting all three lineages. In our cohort, the mean WBC count was 2.86 ± 0.20 × 109/L—below the normal reference range—yet the lymphocyte percentage remained within normal limits (39.23 ± 2.19%). Importantly, our analyses of immune‐parameter differences compared the proportions of lymphocyte subsets rather than absolute counts. Because relative lymphocyte percentages were maintained, we believe the risk of distortion in our correlation analyses due to overall leukopenia or lymphopenia is minimal.
First, we conducted a differential analysis of immune indicators in relatively high-risk and low-risk MDS patients, as well as in patients with different numbers of gene mutations. We found that there were fewer statistically significant indicators, but it is noteworthy that we observed a higher proportion of Tregs in high-risk patients compared to low-risk patients. David A. Sallman et al. found that in TP53-mutated cases, highly immune-suppressive regulatory T cells (Tregs) (i.e., ICOS high/PD-1−) and myeloid-derived suppressor cells (PD-1 low) were significantly expanded, suggesting that an increased Treg proportion is associated with poor prognosis. Shahram Y. Kordasti et al.'s research revealed a significant correlation between an increased number of CD4+ Tregs and MDS subgroups, including marrow blasts of 5% or more (P < 0.001), higher International Prognostic Scoring System (IPSS) scores (P < 0.001), and disease progression (P < 0.001), indicating that CD4+ Treg expansion is a characteristic of high-risk MDS and progression to an aggressive disease subtype. Given the limited number of positive indicators, we further explored the immune markers between the mutation groups and wild-type groups for common gene mutations.
ASXL1 mutations are associated with poor prognosis and promote the progression of MDS when co-mutated with other genes, often accompanied by anemia and thrombocytopenia [14]. Our findings suggest that ASXL1 mutations are linked to reduced CD4+ T cell proportions and lower IL-1β levels, indicating that ASXL1 may contribute to immune suppression within the tumor microenvironment, accelerating MDS progression to AML. IL-1β's role in cancer is controversial, with evidence showing both pro-tumor and anti-tumor potentials depending on the tumor type and model [15–18]. IL-1β can exhibit anti-tumor potential by activating group 3 innate lymphoid cells (ILC3) or triggering CD8+ T cell anti-tumor responses [19], but elevated IL-1β has also been linked to tumor progression [20]. This study found decreased IL-1β levels in patients with ASXL1, U2AF1, IDH1, SETBP1, and 5q- mutations, and positive correlations between NK cells and IL-1β in the ASXL1 and RUNX1 mutation groups, suggesting that reduced IL-1β is associated with poor prognosis in MDS and may have anti-tumor effects, warranting further mechanistic exploration.
IL-17A, a signature cytokine of the Th17 cell subset, plays crucial roles in autoimmune disease, inflammation, and cancer, and is vital in host defense against infections [21–23]. Our study observed reduced IL-17A expression in RUNX1, TET2, TP53, IDH1, SETBP1 mutations, and 5q- chromosomal abnormalities, suggesting that decreased IL-17A is linked to poor prognosis in MDS. IL-17A's protective role extends beyond Th17 cells, being produced by CD8 T cells, γδ T cells, natural killer T cells, and innate lymphoid cells [21]. The lower expression of IL-17A in high-risk MDS mutations supports the notion that MDS is an immunosuppressive disease, with IL-17A levels correlating positively with prognosis. Increased tumor-associated IL-17 has been shown to enhance survival rates in ovarian cancer patients [24]. Th17 and Treg cells maintain an immune equilibrium regulated by the TGF-β/IL-2 and IL-6 cytokine axis [25]. Th17 cytokines, such as IL-1β and IL-6, can induce Treg cells to produce IL-17 [26, 27]. In the RUNX1, TP53, IDH1, and 5q- mutation groups, we also observed decreased IL-6 or IL-1β levels, suggesting that reduced cytokine expression may inhibit Treg production of IL-17A. Th17 and Treg cells can interconvert under the influence of cytokines [25, 28–30], highlighting the importance of the Th17/Treg axis in the MDS tumor microenvironment, with implications for risk stratification and prognosis. Further exploration of the Th17/Treg balance in MDS is a critical area of future research, as modulating this axis could refine immunotherapy strategies.
NK cells possess anti-tumor capabilities, with their deficiency closely linked to hematologic malignancies [31–33]. Our findings indicated that reduced NK cell proportions are associated with poor prognosis mutations such as TP53, TET2, IDH1, and BCOR, whereas higher NK cell proportions were observed in favorable prognosis mutations like SF3B1, suggesting NK cells are a potential prognostic marker in MDS. Cytokines such as IL-2, IL-12, IL-15, and IL-18 promote NK cell proliferation and activation, with IL-12 stimulating IFN-γ production and IL-18 enhancing FASL expression and TNF production in NK cells [31]. Reduced NK cell proportions and decreased IL-2 and IL-12p70 levels in IDH1 mutations, and decreased IL-2 in the 5q- chromosomal group, further support the involvement of NK cells and related cytokines in the MDS immune microenvironment.
The limitations of this study include the small sample size, and the presence of co-mutations introduced confounding factors in the analysis of immune markers. Future studies should increase the sample size to better understand co-mutations and immune marker differences in MDS. Further investigations into the relationship between fine immune parameters and common MDS gene mutations, as well as their prognostic value, are needed. Expanding the analysis to broader lymphocyte subpopulations and their secreted cytokines could deepen our understanding of the interaction between common MDS mutations and the immune microenvironment, providing guidance for treatment strategies. A significant limitation of this study is the lack of initial multiple testing correction when analyzing immune parameter differences across gene mutations. Subsequent corrections for multiple comparisons notably reduced the statistical significance of most observed associations. Thus, these findings should be interpreted cautiously as exploratory.
This study explored the relationship between MDS gene mutations and fine immune parameters, exploring associations between common MDS mutations and immune markers. Results indicated that prognostic genes affect immune cells and cytokines, with poor prognosis genes linked to unfavorable immune markers. The Th17/Treg axis plays a critical role in modulating the MDS tumor immune microenvironment. Fine immune parameters have prognostic value in MDS and may guide treatment strategies.
Conclusion
This study explores the associations between common MDS gene mutations and fine immune markers, suggesting potential links between poor prognosis mutations and immune profile alterations. However, the causal and prognostic significance of these associations requires further validation.. MDS genetic characteristics may influence prognosis by affecting the tumor immune microenvironment, with the Th17/Treg axis and related cytokines playing significant roles. Future studies should integrate fine immune parameter and genetic testing to improve MDS prognosis assessment and guide treatment strategies, while further research is needed to elucidate specific immune mechanisms in MDS.
Supplementary Information
Author contributions
Li Lijuan: Conceptualization. Zheng Hanxue: Writing—original draft. Li Lijuan, Zhang Liansheng, Meng Zilu: Writing—review & editing. Li Lijuan and Zhang Liansheng: Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (82360029), the Commissioned Project of National Clinical Medicine Research Center for Hematological System Diseases (2021WWA01), the Clinical Medical Research Center of Hematological Diseases in Gansu Province (21JR7RA435), the Natural Science Foundation of Science and Technology Program of Gansu Province (21JR7RA394) and the Natural Science Foundation of Science and Technology Program of Gansu Province (21JR11RA104).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Lanzhou University Second Hospital (Approval No. 2024A-1037), and all procedures were performed in accordance with the guidelines outlined in the Declaration of Helsinki. Informed consent was obtained from all individual participants or their legal guardians included in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liansheng Zhang and Lijuan Li have contributed equally to this work.
Contributor Information
Liansheng Zhang, Email: zhanglsh@lzu.edu.cn.
Lijuan Li, Email: doctorlilj@163.com.
References
- 1.Li H, Hu F, Gale RP, et al. Myelodysplastic syndromes. Nat Rev Dis Prim. 2022;8(1):74. [DOI] [PubMed] [Google Scholar]
- 2.Sekeres MA, Taylor J. Diagnosis and treatment of myelodysplastic syndromes: a review. JAMA. 2022;328(9):872–80. [DOI] [PubMed] [Google Scholar]
- 3.Dezern AE, Greenberg PL. The trajectory of prognostication and risk stratification for patients with myelodysplastic syndromes. Blood. 2023;142(26):2258–67. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(8):1307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arber DA, Orazi A, Hasserjian RP, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gera K, Chauhan A, Castillo P, et al. Vaccines: a promising therapy for myelodysplastic syndrome. J Hematol Oncol. 2024;17(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanagal-Shamanna R, Beck DB, Calvo KR. Clonal hematopoiesis, inflammation, and hematologic malignancy. Annu Rev Pathol. 2024;19:479–506. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Sevilla JJ, Colla S. T-cell dysfunctions in myelodysplastic syndromes. Blood. 2024;143(14):1329–43. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg PL, Stone RM, Al-Kali A, et al. NCCN guidelines® insights: myelodysplastic syndromes, version 3.2022. J Natl Compr Canc Netw. 2022;20(2):106–17. [DOI] [PubMed] [Google Scholar]
- 11.Bersanelli M, Travaglino E, Meggendorfer M, et al. Classification and personalized prognostic assessment on the basis of clinical and genomic features in myelodysplastic syndromes. J Clin Oncol. 2021;39(11):1223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Guo Y, Dong Z, et al. Differential U2AF1 mutation sites, burden and co-mutation genes can predict prognosis in patients with myelodysplastic syndrome. Sci Rep. 2020;10(1):18622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Zhou F, Cai X, et al. Mutational landscape of patients with acute myeloid leukemia or myelodysplastic syndromes in the context of RUNX1 mutation. Hematology. 2020;25(1):211–8. [DOI] [PubMed] [Google Scholar]
- 14.Medina EA, Delma CR, Yang FC. ASXL1/2 mutations and myeloid malignancies. J Hematol Oncol. 2022;15(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bent R, Moll L, Grabbe S, et al. Interleukin-1 beta-A friend or foe in malignancies? Int J Mol Sci. 2018;19(8):2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14(5):408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Macfadyen JG, Thuren T, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–42. [DOI] [PubMed] [Google Scholar]
- 18.Wong CC, Baum J, Silvestro A, et al. Inhibition of IL1β by canakinumab may be effective against diverse molecular subtypes of lung cancer: an exploratory analysis of the CANTOS trial. Cancer Res. 2020;80(24):5597–605. [DOI] [PubMed] [Google Scholar]
- 19.Bruchard M, Geindreau M, Perrichet A, et al. Recruitment and activation of type 3 innate lymphoid cells promote antitumor immune responses. Nat Immunol. 2022;23(2):262–74. [DOI] [PubMed] [Google Scholar]
- 20.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–8. [DOI] [PubMed] [Google Scholar]
- 21.Iwakura Y, Ishigame H, Saijo S, et al. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–62. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SC, Caveney NA, Yen M, et al. Organizing structural principles of the IL-17 ligand-receptor axis. Nature. 2022;609(7927):622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song M, Liang J, Wang L, et al. IL-17A functions and the therapeutic use of IL-17A and IL-17RA targeted antibodies for cancer treatment. Int Immunopharmacol. 2023;123: 110757. [DOI] [PubMed] [Google Scholar]
- 24.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S, Takaku M, Zou L, et al. Reversing SKI-SMAD4-mediated suppression is essential for T(H)17 cell differentiation. Nature. 2017;551(7678):105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mailer RK, Joly AL, Liu S, et al. IL-1β promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci Rep. 2015;5:14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinshaw DC, Benavides GA, Metge BJ, et al. Hedgehog signaling regulates treg to Th17 conversion through metabolic rewiring in breast cancer. Cancer Immunol Res. 2023;11(5):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downs-Canner S, Berkey S, Delgoffe GM, et al. Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+) T(reg) cells are a source of tumour-associated T(reg) cells. Nat Commun. 2017;8:14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discov. 2022;21(8):559–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor RP. T cells reinforce NK cell-mediated ADCC. Blood. 2024;143(18):1786–7. [DOI] [PubMed] [Google Scholar]
- 33.Cózar B, Greppi M, Carpentier S, et al. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.