Abstract
The lack of effective therapeutic options for patients suffering from neurological impairments related to acquired brain damage requires novel translational strategies, among which transplantation of neural tissue is receiving strong attention. One of the most recent developments involves the implantation of brain organoid models, derived from embryonic or induced pluripotent stem cells, into damaged rodent cortices. While this approach is gaining popularity, the extent of graft integration within the host tissue remains poorly understood. This review aims to examine whether xenotransplanting organoids into cortical tissue induces functional recovery and plastic adaptation to the damaged implantation sites. Physiological indications of grafted organoid plasticity and integration into the host included viability, corticogenesis, vascularisation, growth, and the development of area-specific morphological identities. The functional integration into host neural circuitry has been probed by tracing of axonal projection growth according to implantation sites, but also through observations of spontaneous, stimulus evoked, and selectively tuned activity of grafted neurons. Finally, some studies also investigated whether the engraftment procedure facilitated behavioural recovery in tasks requiring motor, memory, or reward-seeking functions. Overall, organoid grafts show signs of progressive anatomical, functional, and behaviourally-relevant integration within the damaged host cortices. Yet, further investigation is necessary before this transplantation approach can be actually translated into a robust method to achieve functional restoration in patients suffering from brain damage.
Keywords: Brain damage, Xenotransplantation, Brain organoids, Functional recovery, Organoid transplantation
Introduction
Brain damage, whether resulting from accident or disease, is often a life-threatening condition and, for patients who survive, the remaining lesions profoundly impact their neurophysiological, psychological, and social well-being. Apart from congenital and degenerative neurological impairments, acquired brain injury often results from internal or external incidents [1]. With an estimated prevalence of 0.7% [2], the leading causes for acquired brain damage and its associated neurological disabilities are stroke [3] and traumatic brain injury (TBI) [4]; although less common, similar neurological outcomes can occur postoperatively following glioblastoma resection surgery [5], during which tumour excision may lesion the surrounding cortical tissue. Depending on the severity of cortical damage, neurological deficits persist over long-term periods. Furthermore, the extent of impairment is also affected by pathophysiological mechanisms that are secondary to the primary lesioning of the brain, such as excitotoxicity, free radical damage, blood brain barrier interruptions, and inflammatory reactions [6]. These ancillary responses may lead to further cortical injury and cell death. The bodily reactions to brain injury include evoked angiogenesis, neurogenesis, and synaptogenesis. However, natural brain repair mechanisms only function to a certain extent, hence clinical therapies attempt to enhance these inherent processes to aid the regeneration of neural tissue [7]. Nevertheless, currently available drug-based neurorestorative approaches offer at best a limited recovery. Given the lack of generally efficient therapies, a focus of research has been studying the regenerative potential of transplanted stem cell tissue. Initial studies in which rodent hosts with cortical damage were implanted with embryonic murine cells highlighted the potential of this class of approaches, observing that the grafts physiologically adapted to the novel environment and were functionally integrated to an extent that enabled some restoration of motor and cognitive functions [8, 9]. The scope of this approach was expanded with the use of human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), which have the capacity to be differentiated and patterned into cortical cell classes that are resemblant of those present during embryonic developmental stages [10, 11]. In order to study patient-matched and individualized models of cortical repair, research then shifted to xenotransplanting such tissue and investigate cross-species integration in host cortices [12, 13]. These chimeric models showed similar results in terms of neural adaptation to the rodent hosts, displaying functional integration within the damaged host circuitry and regenerative outcomes in various cortical areas [14]. The prospects of this translational strategy aimed at cortical regeneration was further advanced with novel methods to culture iPSC-derived, self-organised, and multicellular organoid and assembloid brain models [15]. These models attempt to regenerate lesioned brain areas with specifically differentiated and organised cells that mimic regions of the foetal neocortex [16]. The first successful transplantation and functional integration of organoids implanted into the damaged retrosplenial cortex of mice has since attracted attention for further exploration of this cortical repair model [17]. Compared to the in vitro culture environment, transplanted in vivo organoids have shown to be more recapitulative of the brain architecture, with missing cell types supplemented by the host, improved corticogenesis, and synaptic integration with the impaired host neural circuits [18]. This new type of graft may have the potential of outweighing former limitations of cellular implants, mainly due to its structural organisation of cortical cells. More specifically, while injected cells have similar identities to the populations making up an organoid, organoids themselves are characterised by displaying systematised early-development ventricular zone (VZ), subventricular zone (SVZ), and emerging cortical plate-like structures of layers, progenitor cells, and migrating neurons [19]. This fundamental hallmark of regionally patterned organoids highlights their potential advantages as graft candidates, as their increased functional and anatomical complexity may aid in resiliency and restorative capabilities when implanted into a damaged cortical region. Given the novelty and importance of this grafting approach in advancing the realm of regenerative medicine, our objective is to review whether grafting organoids into cortical tissue induces functional recovery and plastic adaptation to the damaged implantation sites. The variation in graft types, hosts subject to implantation, and utilised cortical damage models are summarised in Box 1, which showcases the main methodologies behind the various xenotransplantation procedures. Grafting methods are additionally illustrated in Fig. 1, which also provides an overview of the current report, where we will review the extent of implant-host integration in terms of physiological, functional, and behavioural perspectives. We begin by reviewing physiological aspects regarding the implantation of organoids. The plasticity of grafts within the host environment is discussed with regards to various processes that portray normal tissue function, such as graft viability, cellular composition, structural characteristics, volumetric growth, or vasculature integration post implantation. We then outline whether transplanted organoids show evidence of functional integration within implantation sites. The findings are subdivided between different cortical regions, and functional adaptation is examined in terms of neural connectivity, arrangement of axonal projections and electrophysiological measures of neural activity in engrafted organoids. Finally, we highlight the behavioural outcomes of organoid transplantation. These are summarised across cognitive, motor and sensory related categories of behaviour. By collectively reviewing the physiological, functional, and behavioural features together, we aim to critically evaluate the therapeutic prospects of implanting organoids as a regeneration approach cortical damage.
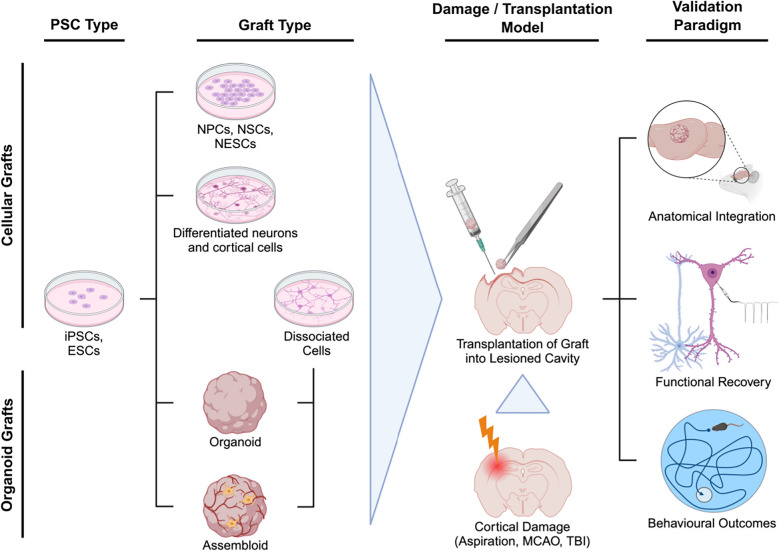
Fig. 1.
Depiction of transplantation model methodologies and approaches to assessing graft integration. Sample tissue selected for engraftment is commonly derived from pluripotent stem cells (PSCs), such as iPSCs or ESCs, through culturing. This enables the generation of cellular grafts like proliferative cells (NPCs, NSCs, NESCs) or various types of neurons and differentiated cortical cells. On the other hand, iPSCs and ESCs may also be cultured into organoids and more advanced assembloids. Furthermore, organoids and assembloids can be dissociated into cells. This is usually conducted to investigate graft integration outcomes of structured organoids/assembloids in comparison to its disassembled and unorganised cellular contents. The cellular or organoid tissue is then selected for engraftment via injection or manual placement into a specific region of the host cortex, depending on the employed transplantation model. The hosts, typically rodents, are usually subject to cortical damage prior to the transplantation. The models of neural damage previously utilized are induced strokes (middle cerebral artery occlusion (MCAO), focal injury stroke), lesioning by aspiration or direct tissue removal, and procedures mimicking TBI. Specifically for organoids, these damage models are used to create cortical cavities to fit the size of grafts for implantation. In general, transplanting grafts into lesioned cortices is conducted for the investigation of cortical regeneration following the engraftment. This regeneration is probed by several validation paradigms, including examining the extents of anatomical integration (survival, growth, vascularisation, structural integrity and cellular composition of the grafts), functional recovery (functional reconstruction via synaptic integration and electrophysiological activity of grafted neurons), or behavioural outcomes of the grafting procedure. Created in BioRender. Ondriš, J. (2025) https://BioRender.com/s34g864
Box 1 Methods of xenotransplanting cortical tissue: Organoids, cells, and hosts
|
Implanting organoid grafts Generally, organoids selected for grafting are derived from ESCs or iPSCs. In some cases, assembloids are chosen for implantation by coculturing organoids with precursors for microglia [20] or vasculature [21, 22]. Transplanting organoids is done by direct implantation into a cortical cavity. This term refers to an ablated region of the cortex, usually the size of the organoid that is thereby implanted. There are several methods of how this cavity is created, each resemblant of some form of cortical damage. One way to ablate the cortex and form a grafting site is by aspirating the cortical area directly prior to the implantation [17, 20, 22–28]. This method may be akin to the type of cortical damage resulting from glioblastoma resection surgery, where the tumour and its proximal area is excised with aspiration [29–31]. A second model of cortical damage, resemblant of TBI, involves direct removal or lesioning of the cortex to form a cavity [21, 32]. A similar way to imitate TBI as a method of creating a graft site is by utilising a biopsy punch [33, 34]. Additionally, TBI is also mimicked by using a controlled cortical impact model (CCI), which is a device that generates lesions with a motorised impactor tip [35]. Other methods, analogous to producing stroke-related cortical damage, are the focal injury stroke model [36] and middle cerebral artery occlusion (MCAO) model [33]. Although utilising different methods, both models aim to mimic neural damage resulting from brain infarctions. The application of CCI, focal injury stroke, and MCAO models is usually conducted a few days prior to the grafting. Conversely, when inducing damage through aspiration or direct tissue removal, implantation sites are usually ablated shortly before the transfer of organoid grafts. A different approach to organoid grafting is via stereotactic sample injection into an early postnatal [37], juvenile [38], or adolescent [39] rodent cortex. Yet, a drawback of the sample injection approach through a syringe is that the precise end-location of the graft may vary in the cortex, given that the implant is not placed within a cavity. Nonetheless, an advantage of this insertion method concerns early postnatal subjects, where creating a precise grafting site via ablation is difficult, and the early-age cortex still experiences ongoing axonal development and innervation |
|
Injecting cell grafts In line with research on transplanting organoids, studies employing cellular grafts usually involve the induction of cortical damage prior to implantations. Yet, a fraction of such studies do not include the generation of neurological impairment prior to the cellular transplantation [12, 38, 40–43]. Nonetheless, previous research utilizing cortical damage models before implanting cellular grafts are resemblant to the ones mentioned in the organoid grafting studies. Hence, details regarding the specific transplantation models are mentioned in Table 2. Interestingly, one previously unmentioned model of TBI entailed injecting ibotenic acid to generate cortical cavities [44]. Furthermore, another study involving cellular grafts aimed to mimic neurodegeneration of dopaminergic neurons resulting from Parkinson’s by injecting 6-hydroxydopamine into the cortex [45]. Following this methodological step, cellular grafts are typically stereotactically injected into the region of interest. Concerning cellular grafts, there is a multitude of available samples to choose from. Although there is an abundance of previous research on implanting cells of murine origin, we mainly focus on transplanted cells derived mainly from human iPSCs or ESCs. Some of the human cells previously grafted include: cortical cells such as neural progenitors/neurons [12, 42, 44, 46], excitatory neurons [43], dopaminergic neurons [41, 45], neural stem cells (NSCs) [47], neuroepithelial stem-cells (NESCs) [13, 48, 49], neural progenitor cells (NPCs) [32], and cells dissociated from organoids [23, 36, 38, 40, 50] |
|
Transplantation hosts The most common species of subjects in transplantation studies are rodents. Irrespectively of the tissue implant type, when transplanting across species the foreign tissue is naturally fought with host immune responses. Generally, previous studies using mice have used breeds that are genetically immunodeficient. The most common types identified in the current review were severe combined immunodeficient (SCID) [24, 35, 38, 39, 50], non-obese diabetic (NOD) -SCID [12, 17, 20, 22, 25, 26, 28, 36, 41, 44, 45], and NOD-SCID gamma (NSG) mice [21, 27, 40, 42, 43]. However, grafting cerebral organoids into the frontoparietal cortex of immunocompetent mice has also been achieved [32]. Conversely, research where tissue was grafted into rats more prevalently employed immunosuppressive drug treatments such as Cyclosporin A [13, 23, 33, 34, 47, 49]. Yet, genetically bred immunodeficiency in rats was also mentioned, with the use of athymic rats [37, 48] |
Plastic anatomical integration of grafts within host cortices
When transplanting an in vitro human iPSC or ESC derived organoid model into a host cortex, it remains paramount that the grafted tissue can anatomically integrate into the novel in vivo environment. This not only concerns the survival of the organoid implant post-transplantation, but also its plastic integration into the damaged cortex to enable a regenerative recovery. The extent of this physiological plasticity may be approached from different angles, such as through assessments of the graft viability, neural cell compositions, structural integrity, volumetric growth, vasculature integration, or other processes related to normal neural tissue function.
Transplanted organoids display favourable signs of viability in the novel host environment
Prior to any assessment of the anatomical fusion between the graft and host site, it is important that the animal and organoid survive the implantation procedure. In terms of transplanted graft viability, implantation of forebrain organoids led to higher graft survival rates (> 80%) compared to transplanted cells that were dissociated from organoids [17, 23]. Regarding the host survival rates, subjects grafted with forebrain organoids showed equivalent survival rates (80–90%) in comparison to ones implanted with dissociated cells. Likewise, studies that transplanted cerebral organoids reported a graft survival rate > 87.5% [24], with the host survival rate being approximately 80% at 60 days post implantation [35], and 85% at 180 days post implantation [28]. Interestingly, long-term monitoring of cerebral organoid vs. dissociated cell transplants into stroke-damaged cortices revealed that although organoid grafts survived and repaired the infarcted tissue, there was only a small number of the implanted dissociated cells alive at 180 days post transplantation [36]. It was also observed that the organoid viability was affected by post-surgery complications of the hosts and differences in cell-lines that were employed to generate the organoid implants [23]. As there appears to be a consistency of high graft and host survival rates among studies that involve transplanting organoids, it appears that organoids are more robust to survival in the novel environment compared to transplanted cellular grafts. Considering the survival rates of assembloid implants, transplanting a vascularised vs. non-vascularised cerebral organoid led to observations showing that, compared to the well-integrated vascularised organoid, the non-vascularised implant did not survive beyond two weeks post transplantation [21]. This presents the beneficial impact of integrated vascularisation on graft viability, which will be discussed in a separate section. However, this study only conducted a single sample replicate per condition. Concerning graft viability, one study investigated the influence of transplanting organoids either directly or 7 days following cortical lesioning from aspiration [24]. Findings revealed that, at three months post implantation, all organoids implanted a week after cortical damage survived, compared to only around 68% of the ones grafted directly after cortical damage. To summarise, organoid and assembloid grafts appear to have high survival rates, which may be influenced by specific time delays between cortical lesioning and sample implantation.
Besides survival, a cellular mechanism that can be considered reflective of the graft robustness to surviving in vivo is the rate of apoptotic cell death. One study demonstrated that the implantation of either younger (55-day) or older (85-day) organoids did not trigger any significant apoptotic reactions, thus irrespectively of the in vitro age of the graft [34]. Furthermore, organoids that survived the transplantation procedure showed a decreasing degree of apoptosis over time, supporting their viability in the new environment [23]. In one case, engrafting MCAO-damaged (Box 1) cortices with organoids was reported to lower the host apoptotic cell death compared to cell death rates in stroke-injured controls [33]. This suggests that the grafts have beneficial effects on improving cortical injury outcomes, like lessening host apoptotic responses to stroke-damage. Interestingly, studies that compared apoptotic cell rates between in vitro and implanted in vivo vascularised organoids demonstrated that the transplanted organoids had a considerably lower number of apoptotic cells compared to the ones in culture [22, 32]. Furthermore, assessing genetic signatures of apoptosis between transplanted organoids and ones in culture also revealed decreased activations of apoptotic pathway genes in the grafted implants [38]. Altogether, while the in vitro organoid age appears not to influence the in vivo viability, transplanted organoids display lower signs of apoptosis compared to ones that remain in culture. This indicates that, in contrast to incubation in vitro, the host cortex is a more viable environment for engrafted organoids.
Another aspect related to graft cell survival is the activity of metabolic pathways. It was previously showed that organoids in culture were prone to display signs of activated metabolic pathways due to the nature of the in vitro environment [40]. This, in turn, caused increased amounts of cellular stress in the organoids, which impaired cell specification types in the process of maturation and differentiation. A recent study compared metabolic stress markers between transplanted organoids and ones cultured in vitro [38]. Results showed that metabolic pathways related to cellular stress were distinctively less activated in transplanted organoids compared to the organoids in vitro. To sum up, grafting organoids appears to result in lower rates of apoptosis and metabolic stress compared to incubating organoids in vitro. These observations support the favourable survival rates of the hosts as well as the organoids implanted into the new environment. Nonetheless, other factors like maturation and cellular differentiation need to be evaluated when considering anatomical integration of grafted tissue.
Grafted organoids mature, establish morphological identities and develop various differentiated cortical cell populations
To determine the extent of anatomical integration between implanted organoids and the host cortex, it is important to assess ways in which the procedure and the new environment affected the development of the grafts post transplantation. One way to probe development is through examination of neural progenitor cells (NPCs), which naturally undergo neurogenesis and differentiate into mature neurons over time. Investigating NPCs present within organoids has revealed that, compared to in vitro conditions, transplanted organoids showed an improved neural maturation and cellular differentiation around 50–65 days post-surgery [17, 35]. Specifically, as indicated by a decrease of NPCs and an increase of mature neurons and astrocytes over time, grafted organoids showed signs of progressive cortical development [26, 33–35]. Conversely, one study found that grafted organoids had increased expressions of progenitor markers relative to organoids in culture, suggesting that the in vivo environment could have boosted proliferation and slowed down maturation [36]. Yet, this study employed the focal stroke injury model, hence the increased proliferation could have been influenced by this factor. Furthermore, the number of proliferative cells is also influenced by the in vitro maturity of the implants prior to the transplantation. Implanting 6-week vs. 10-week cultured organoids demonstrated that the younger grafts had enhanced proliferative cell percentages compared to older organoids when in vivo [24]. In support of this, another study showed that organoids implanted at day 55 in culture displayed elevated signs of neurogenesis, proliferation, and differentiation compared to organoids transplanted at day 85 [34]. In short, transplanting younger organoids results in enhanced neurogenesis and cortical cell differentiation within the host cortex as opposed to prolonged incubation.
This brings to question how the increased implantation-related development might influence the morphological identity and the type of differentiated cells present within grafted organoids. Transcriptomic comparisons have indicated that the developmental state of organoid implants was resemblant of the late fetal period [37]. In addition, area-specific identity, differentiation and cortical development was observed via transcriptomic factors in relation to various cortical regions. These regional identities were attributed to organoids transplanted into motor [33, 34], visual [23], frontal [39], and striatal [38] brain regions. Moreover, the human grafts displayed evidence of synaptogenesis [17, 33, 35], establishing connections to the host neural circuitry. As the specifics of synaptic connectivity depend on the grafting site, this aspect is discussed in more detail in a later section. Remarkably, area-specific identity and plastic integration of organoids grafted into the corpus striatum was evidenced by the presence of epithelium-like cellular structures surrounding the organoid ventricles [38]. These cells also expressed markers related to cerebrospinal fluid secretion, as expected in accordance with their protective function of supporting the blood brain barrier. Together, these findings suggest that the cellular development within an organoid graft is plastic and regionally adapted to the cortical site of implantation through the differentiation of NPCs into area-specific mature cell types. We will next consider the specific identities and compositions of cells in transplanted organoids, as they might reveal more insight into the anatomical integration that occurs post-engraftment.
Single-cell RNA sequencing previously emphasized that grafted organoids were populated by various cell types including neurons, radial glia cells, astrocytes, neural precursor cells and pericyte-like cells necessary for the development of neurovasculature [38]. Focusing specifically on neurons, one study detected glutamatergic neurons with both superficial and deep-layer identity, while there was an absence of GABAergic neurons [37]. Glutamatergic neural identity was also reported within grafted organoids by additional research, without noting the presence of GABAergic neurons [35, 39]. More specifically, one study reported that implanting glia-enriched cortical organoids led to profound maturation of upper-layer excitatory neurons in vivo, with a fraction of about 30% at 5 months post-implantation increasing to nearly 95% upper-layer excitatory neurons at 8 months post-implantation, something which was not seen in pseudo-comparisons with organoids cultured in vitro. Jgamadze et al. [23] further observed that transplanted organoid grafts displayed similar ratios of excitatory and inhibitory neurons compared to the adult human cortex. In contrast, Cao et al. [36] showed that the grafted organoids consisted mainly of mature and immature glutamatergic neurons, with a disproportionately small number of GABAergic neurons. There have been remarks as to why some previous studies may have observed different profiles of excitatory and inhibitory neuron expressions. One explanation is that excitatory neurons emerge from the dorsal pallial cortex, while inhibitory neurons originate and migrate from the subpallial ventral cortex [51]. Given that the organoids selected for grafting are usually of a dorsal cortical identity, this could point to why there is a lack of GABAergic neurons in the grafts post transplantation. As noted in Table 1, there are currently no studies transplanting organoids with ventral identity. Yet, another reason to why there is a varying repertoire of inhibitory neurons could be differences in the employed methods for organoid generation. For example, one study cultured cerebral organoids that developed regional structures with identities of the forebrain, midbrain, and hindbrain [52]. This cerebral organoid model captured the presence and migration of inhibitory neurons from the ventral to the dorsal regions of the model. Hence, while most reports of grafted organoids observe a lack of GABAergic neurons, this could be accounted to the variations in organoid generation methods. In sum, transplanted organoids portray heterogeneous profiles of neuron identities after engraftment.
Table 1.
Overview of organoid graft types, transplantation models, host specifications, cortical regions of implantation, and outcome parameters analysed by previous literature discussed in this review
| Reference | Transplantation Model | Organoid Graft Type | Graft Host | Brain Region | Outcome Parameters |
|---|---|---|---|---|---|
| Jgamadze et al. [23] | Xenotransplantation into aspirated cortical cavity | Human dorsal forebrain organoids | Male young adult rats (8–12 weeks), immunosuppression with cyclosporine A | Border between V1 and V2 | Immunostaining, axonal tracing, extracellular laminar probe electrophysiology, visual stimulation |
| Mansour et al. [17] | Xenotransplantation into aspirated cortical cavity | Human cerebral organoids with forebrain identity | Female NOD-SCID mice (6–10 weeks) | Retrosplenial cortex | Immunostaining, extracellular electrode array electrophysiology, two-photon imaging of calcium and blood flow, optogenetics, behavioural test |
| Wilson et al. [25] | Xenotransplantation into aspirated cortical cavity | Human cortical organoids | Female NOD-SCID mice (8–12 weeks) | Retrosplenial cortex | Immunostaining, extracellular electrode array electrophysiology, two-photon calcium imaging, visual stimulation |
| Schafer et al. [20] | Xenotransplantation into aspirated cortical cavity | Human cortical organoids with forebrain identity assembled with EMPs | Female NOD-SCID mice (6–10 weeks) | Retrosplenial cortex | Immunostaining, transcriptomics |
| Wang et al. [27] | Xenotransplantation into aspirated cortical cavity | Human glia-enriched cortical organoids | Male and female NSG mice (6–12 weeks) | Retrosplenial cortex | Immunostaining, transcriptomics |
| Wang et al. [33] | Xenotransplantation into cortical cavity resulting from MCAO model combined with biopsy punch | Human cerebral organoids | Male rats (no age indicated), immunosuppression with cyclosporine A | Left Motor Cortex | Immunostaining, behavioural tests |
| Wang et al. [34] | Xenotransplantation into cortical cavity resulting from biopsy punch TBI model | Human cerebral organoids | Male rats (no age indicated), immunosuppression with cyclosporine A | Right Motor Cortex | Immunostaining, behavioural tests |
| Cao et al. [36] | Stereotactic injection into cortex damaged by focal injury stroke model | Human cerebral organoids | Male NOD-SCID mice (7–8 weeks) | Somatosensory and motor cortex region | Immunostaining, axonal tracing, whole-cell patch-clamp electrophysiology, optogenetics, behavioural tests |
| Revah et al. [37] | Stereotactic injection into early postnatal cortex | Human cortical organoids | Male and female (3–7 days) athymic (FOXN1–/–) rat pups | S1 | Immunostaining, transcriptomics, MRI, whole-cell patch-clamp/extracellular laminar probe/EEG electrophysiology, fibre photometry, two-photon calcium imaging, optogenetics, behavioural tests |
| Shi et al. [22] | Xenotransplantation into aspirated cortical cavity | Human vascularised (cocultured with HUVECs) or non-vascularised cerebral organoids | NOD-SCID mice (8 weeks), gender not indicated | S1 | Immunostaining, transcriptomics, whole-cell patch-clamp electrophysiology, calcium imaging, two-photon imaging of blood flow |
| Li et al. [26] | Xenotransplantation into aspirated cortical cavity | Human cortical organoids | Male NOD-SCID mice (4–6 weeks) | S1 | Immunostaining, CRISPR/Cas9 gene editing, ultrasound stimulation, transcriptomics, extracellular laminar probe electrophysiology, electrode stimulation, behavioural tests |
| Hu et al. [28] | Xenotransplantation into aspirated cortical cavity | Human brain organoids | Male NOD-SCID mice (4–6 weeks) | S1 | Immunostaining, extracellular laminar probe electrophysiology, behavioural tests |
| Bao et al. [35] | Xenotransplantation into cortical cavity resulting from CCI model lesioning | Human cerebral organoids | Male SCID mice (8 weeks) | Left parietal cortex | Immunostaining, MRI, multi-electrode array electrophysiology, behavioural tests |
| Kitahara et al. [24] | Xenotransplantation into aspirated cortical cavity | Human cerebral organoids | Male and female SCID mice (7 days or 6 weeks) or male cynomolgus monkeys (3 years) immunosuppression with tacrolimus hydrate | Bilateral frontal or parietal cortices (mouse), bilateral M1 (precentral gyrus, monkey) | Immunostaining |
| Daviaud et al. [32] | Xenotransplantation into lesioned cortical cavity resulting from direct removal of brain tissue | Human cerebral organoids | Male and female immunocompetent CD1 mice (8–10 days), no immunosuppressants | Frontoparietal cortex | Immunostaining |
| Dong et al. [39] | Stereotactic injection into the cortex | Human cerebral organoids | SCID mice (8 weeks), gender not indicated | mPFC | Immunostaining, axonal tracing, whole-cell patch-clamp electrophysiology, optogenetics, behavioural tests |
| Huang et al. [38] | Stereotactic injection into the cortex | Premature human cerebral organoids | Female SCID mice (4–5 weeks) | Corpus striatum | Immunostaining, transcriptomics |
| Pham et al. [21] | Xenotransplantation into lesioned cortical cavity resulting from direct removal of brain tissue | Human vascularised cerebral organoids (assembled with iPSC derived endothelial cells) | Male NSG mice (2 months) | Unspecified area of cerebral cortex | Immunostaining |
NOD non-obese diabetic, SCID severe combined immunodeficient, NSG NON-SCID gamma, MRI magnetic resonance imaging, EEG electroencephalogram, V1 primary visual cortex, V2 secondary visual cortex, S1 primary somatosensory cortex, M1 primary motor cortex, (m)PFC (medial) prefrontal cortex, MCAO middle cerebral artery occlusion, TBI traumatic brain injury, CCI controlled cortical impact, EMPs erythromyeloid progenitors, HUVECs human umbilical vein endothelial cells, iPSCs induced pluripotent stem cells
To simplify, methods such as immunochemistry, immunohistochemistry, immunoelectron microscopy and immunofluorescence are collectively referred to as immunostaining
Shifting the focus to other types of cortical cells, grafted organoids also appeared to be populated by astrocytes and oligodendrocytes [17, 32, 33, 36, 39]. Yet, one study argued that these glial cells developed at lower counts compared to the human brain [23]. Another study reported the presence of oligodendrocytes at 7% within the graft, compared to 20% in the surrounding host cortex [25]. Additionally, separate research indicated a specific lack of oligodendrocytes, but normal levels of astrocytes [22, 37]. Furthermore, transplanting organoids into cortical cavities obtained with the CCI, MCAO and focal injury stroke models induced considerably higher glial scaring, which resulted with profuse numbers of astrocytes at the implant-host junction [33, 35] and the infarction core [36]. In addition, implanting glia-enriched cortical organoids resulted in an abundance of human astrocytes within the grafts [27]. Interestingly, this led to further maturation and morphological diversification of the human astrocytes into four specialised subclasses: protoplasmic, interlaminar, fibrous, and varicose projection astrocytes. Comparing the results to findings on implanted organoids, pre-enriching organoids before implantation has led to increased human graft glial and astrocyte maturation. In contrast with transplanted organoids, trans-injected human NPCs appeared to have a less pronounced astrocyte differentiation at 4-weeks post implantation [32]. Overall, these findings indicate significant variability in the differentiated cell types, which might be attributed to differences in the employed implantation and cortical damage models (Tables 1, and 2), but also to the type of graft tissue that is transplanted.
Table 2.
Overview of cellular graft types, transplantation models, host specifications, cortical regions of implantation, and outcome parameters analysed by previous literature discussed in this review. NPCs: neural progenitor cells, ESCs: embryonic stem cells, NESCs: neuroepithelial-like stem cells, NSCs: neural stem cells. All other abbreviations are defined in the legend of Table 1
| Reference | Transplantation Model | Cellular Graft Type | Graft Host | Brain Region | Outcome Parameters |
|---|---|---|---|---|---|
| Jgamadze et al. [23] | Stereotactic injection into aspirated cortical cavity | Cells dissociated from human dorsal forebrain organoids | Male young adult rats (8–12 weeks), immunosuppression with cyclosporine A | Border between V1 and V2 | Immunostaining, axonal tracing, extracellular laminar probe electrophysiology, visual stimulation |
| Cao et al. [36] | Stereotactic injection of grafts into cortex damaged by focal injury stroke model | Cells dissociated from human cerebral organoids | Male NOD-SCID mice (7–8 weeks) | Somatosensory and motor cortex region | Immunostaining, axonal tracing, whole-cell patch-clamp electrophysiology, optogenetics, behavioural tests |
| Huang et al. [38] | Stereotactic injection of organoids or cells into the cortex | Cells dissociated from premature human cerebral organoids | Female SCID mice (4–5 weeks) | Corpus striatum | Immunostaining, transcriptomics |
| Bhaduri et al. [40] | Stereotactic injections of cells into cortex | Cells dissociated from human cortical organoids | Male and female NSG mice (postnatal, 4 days) | PFC or V1 | Immunostaining, transcriptomics |
| Yamagami et al. [50] | Stereotactic injection into aspirated cortical cavity | Cells dissociated from human cerebral organoids | Male and female SCID mice (10 weeks) | Frontal motor cortex | Immunostaining, transcriptomics |
| Daviaud et al. [32] | Xenotransplantation into lesioned cortical cavity resulting from direct removal of brain tissue | NPCs | Male and female immunocompetent CD1 mice (8–10 days), no immunosuppressants | Frontoparietal cortex | Immunostaining |
| Grønning Hansen et al. [46] | Injection of cells into various sites of resected cortex (human) or stereotactic injection into ischemic-lesioned cortical area resulting from the MCAO model (rats) | Human cortical neurons derived from NESCs | Surgically resected human neocortical tissue slices or adult male rats (immunocompetence and precise age not indicated) | Resected middle temporal gyrus (human) or somatosensory cortex (rats) | Immunostaining, axonal tracing, whole-cell patch-clamp electrophysiology, calcium imaging |
| Linaro et al. [42] | Bilateral injections of cells into lateral ventricles of neonatal brain | Cortical cells derived from human ESCs | Male and female immune-deficient (Rag2–/–) or NOD-SCID mice (postnatal, 0–1 days) | Lateral ventricles | Immunostaining, axonal tracing, transcriptomics, whole-cell patch-clamp electrophysiology, widefield/two-photon calcium imaging, behavioural test |
| Espuny-Camacho et al. [44] | Injection of cells into cortical lesions resulting from focal stereotactic injections of ibotenic acid | Cortical progenitors and neurons derived from human ESCs | Male and female NOD-SCID mice (6–8 weeks) | Visual or Motor Cortex | Immunostaining, axonal tracing, whole-cell patch-clamp electrophysiology |
| Steinbeck et al. [45] | Injection of cells into lesioned cortex, Parkinsonian motor-deficit model lesioning with 6-hydroxydopamine | Human mesencephalic dopaminergic neurons derived from ESCs | NOD-SCID mice (2–3 months), gender not indicated | Right striatum | Immunostaining, calcium imaging, whole-cell patch-clamp and extracellular probe electrophysiology, electrode stimulation, behavioural tests |
| Real et al. [43] | Injection of cells into intact cortex | Human cortical excitatory neurons derived from iPSCs | NSG mice (3–4 months), gender not indicated | Somatosensory Cortex | Immunostaining, axonal tracing, transcriptomics, two-photon calcium imaging, whole-cell patch-clamp electrophysiology |
| Espuny-Camacho et al. [12] | Injection of cells into intact cortex | Cortical progenitors and neurons derived from human ESCs | NOD-SCID mice (precise age not indicated), gender not indicated | Motor Cortex | Axonal tracing, transcriptomics, calcium imaging, whole-cell patch-clamp electrophysiology |
| Daadi et al. [47] | Injection of cells into ischemic-lesioned cortical area resulting from the MCAO model | NSCs derived from human ESCs | Male adult rats (precise age not indicated), immunosuppression with cyclosporine A | Striatum | Immunostaining, transcriptomics, whole-cell patch-clamp and extracellular probe electrophysiology, optogenetics, behavioural tests |
| Carlson et al. [41] | Stereotactic injection of cells into intact cortex or transplantation of cells onto brain slice surface | Cortical and dopaminergic neurons derived from human iPSCs | Male NOD-SCID mice (5 weeks) or mouse brain slices | Bilateral striatum or resected cortico-striatal brain slices | Immunostaining, transcriptomics, whole-cell patch-clamp electrophysiology, calcium imaging |
| Palma-Tortosa et al. [48] | Injection of cells into ischemic-lesioned cortical area resulting from the MCAO model | NESCs derived from human iPSCs | Male adult athymic rats (age not indicated) | Cortical region overlapping somatosensory cortex and M1 | Immunostaining, axonal tracing, whole-cell patch-clamp electrophysiology, optogenetics, behavioural test |
| Tornero et al. [49] | Injection of cells into ischemic-injured cortex resulting from the MCAO model | NESCs derived from human iPSCs | Male adult rats (precise age not indicated), immunosuppression with cyclosporine A | Somatosensory cortex | Immunostaining, axonal tracing, extracellular electrophysiology, optogenetics |
| Tornero et al. [13] | Stereotactic injection into ischemic-lesioned cortical area resulting from permanent ligation of middle cerebral artery by suture | NESCs derived from human iPSCs | Rats (gender and age not indicated), immunosuppression with cyclosporine A | Cingulate cortex region (adjacent to the motor cortex) | Immunostaining, transcriptomics, whole-cell patch-clamp electrophysiology, electrode stimulation, behavioural tests |
Apart from the cellular compositions, the integration of grafted organoids can also be marked by the maturation of organoid graft processes, and the extent of their myelination. Myelin is key in the development of a functioning neural network and its presence within grafted organoids could further support plasticity and adapted maturation. While one study showed that myelination was absent [17], another highlighted its presence [33], and Jgamadze et al. [23] further indicated that myelination was less pronounced in grafts compared to the adult human brain. Other studies reported the presence of myelinated fibers originating from the host, but only faintly localized at the graft-host border [22]. Although these observations are less convergent, they suggest that some implanted organoids may develop basic forms of early myelination, demonstrating their progression and plastic integration within the host cortex. Overall, grafted organoids portray signs of maturation, proliferation and establishment of morphological identities, as well as diverse profiles of differentiated cortical cell populations. These aspects support the notion that grafts continue to develop and anatomically integrate following implantation into host sites.
Implanted organoids exhibit limited signs of growth and emergence of neocortical structures
Another factor indicative of proliferation and graft plasticity is growth of the implant. Transplanted organoid grafts exhibit gradual volumetric growth post-transplantation [17, 21, 23]. This growth is attributed to the grafted tissue, as evidenced by the absence of host neurons within the grafts and no observations of infiltrating host neuron migration even after several months post-surgery [23]. The migration of other non-neuronal cell types, such as host microglia, was intermittently reported [17, 37, 38], but their contribution to volumetric growth is likely overshadowed by the graft. Thus, the volumetric growth of implanted organoids could also be accounted to the previously noted increased proliferation post transplantation. However, Davidaud et al. [32] provided contrasting evidence and showed that the organoid graft volume remained stable. Other studies even reported that the cell-count began declining following implantation [33, 34]. Interestingly, while implanting 6-week cerebral organoids presented enormous growth within the host cortex, implanting older 10-week organoids did not lead to such considerable overgrowth [24]. Moreover, implanting the organoids with a 7-day delay post lesioning resulted in an even more pronounced growth. This study also established that the growth of grafts within the host cortex was unaffected by the site of implantation. Furthermore, human neurons within grafted organoids featured more sizeable somas, lengthier dendritic expansions and more extensive arborizations compared to neurons from in vitro cultured organoids [37]. Another factor that was found to influence graft growth was electrical stimulation via a organoid-brain computer-interface (OBCI) system. In a study, researchers found that implanted organoids under OBCI stimulation were larger in volume compared to controls at 60 days post implantation [28]. Finally, one study implanted cortical organoids that had received low-intensity ultrasound (LIUS) treatments during culture [26]. While control organoids demonstrated increased graft area at 2 and 5 months post transplantation, LIUS-treated organoids showed considerably higher growth and proliferation, resulting in overgrowth within the host cortex extending far beyond the implant site. In sum, while reports of implant growth post-transplantation were prevalent, other findings contrasted with this. It therefore appears inconclusive whether the organoids exhibit growth in relation to being transplanted into an in vivo environment. In some cases, transplantation of organoids may have led to overgrowth at the host site; however, this phenomenon was not consistent across the majority of the reviewed studies, which showed variable results. Therefore, overgrowth remains, at the very least, a risk factor in organoid xenotransplantation studies. Nonetheless, implant growth may be influenced by factors like organoid age pre-implantation, time delays between cortical lesioning and grafting, or specific variations in methods between experimental designs.
Other than tissue growth, anatomical plasticity could also be examined by reviewing alterations in the structural organisation and integrity of organoid grafts post implantation. In line with the mid-gestational stage of cortical maturation, in vitro organoids typically exhibit early signs of cortical layers [51]. As previously remarked, this is characterised by early-development VZ, SVZ and cortical plate-like structures of layers [19]. After being transplanted into host cortices, grafted organoids continued to resemble an early-development cortical layer with a sustained morphological identify [17]. It was also repeatedly observed that some grafted organoids exhibited VZ-like structures/rosettes in vivo [17, 22, 32]. One study observed the occurrence of VZ rosette structures 45 days post organoid implantation [36]. This layer organisation is often resemblant of a developing embryonic or foetal neocortical laminar structure, where proliferative cells begin differentiating and migrating to form mature neural circuits [19, 53]. Additionally, the emergence of VZ-like structures was specifically found in grafted organoids, compared to trans-injected dissociated cells where such structures were absent [38]. Nonetheless, in comparison to the fully developed human brain, there is still a lack of classical laminar structure and cellular organisation. This was reported in other studies of grafted organoids, where cellular organisation appeared to form no obvious anatomical lamination [37], or arrangement of specific neurons within expected layers [32]. Another study xenotransplanted more mature organoids with a rudimentary form of layer organisation in vitro. Despite that, laminar organisation disappeared following the transplantation [23]. Briefly put, grafted organoids mostly display signs of premature rudimentary layer organisation. Yet, given their proliferative and developmental state, these structures still lack the specific organization of connections and mature cortical layers that are seen in fully developed cortices. Altogether, findings concerning the implantation of organoids present limited evidence regarding the graft growth and emergence of neocortical structures. While some organoids appear to proliferate and form rudimentary structures, others show signs of stagnation or less integral cellular organisation. Although this highlights some inconclusiveness, other aspects of anatomical graft integration remain to be discussed.
Transplanted organoids are sustained by the host vasculature
The integration of neurovasculature within the grafts is necessary for long-term physiological integration and survival of the foreign tissue. Most studies showed that, after implantation, host vasculature grew and integrated within the grafted organoids [20, 23–25, 27, 28, 32–35, 37]. One study also found that integrated vascularisation greatly influenced the growth and viability of the grafted tissue [17]. Interestingly, pre-engraftment as well as in vivo LIUS treatments of organoids promoted an expansion of vascular areas compared to vascular networks in untreated controls post-transplantation [26]. Similarly, organoid implants receiving electric OBCI stimulation portrayed increased infiltration of host vasculature relative to controls [28]. Moreover, implantation of organoids assembled with rudimentary forms of human vasculature greatly sped up the growth of host vasculature within the grafts [22]. Furthermore, transplantation of organoids assembled with human-iPSC derived endothelial cells also resulted in successful vascularisation of the graft. In this instance, tubular blood vessel capillaries of human origin developed and integrated within the implant, supporting its survival instead of being infiltrated by host vasculature [21]. Gathered, these finding consistently support that grafted organoids are successfully integrated in the vasculature networks of the hosts, supplying the implanted tissue with nutrients and oxygenation, and ultimately further enabling their anatomical adaption and survival. Nonetheless, other aspects leaning on the host environment may influence successful graft integration.
The immunosuppressed host environment responds mildly to exogenous grafts
The integration of tissue in a foreign environment, especially in the case of xenotransplants, is naturally fought with immune responses. Therefore, rodent hosts selected for the organoid implantation procedure (Box 1; Table 1) are either bred from transgenic lines that cause genetic immunodeficiency [17, 20–22, 24–28, 35–39], or are medicated daily with immune suppressants [23, 33, 34]. Nonetheless, as any residual immune responses will be mediated by glial cells, their monitoring remains important when judging how well a given graft has integrated within the host cortical site. Jgamadze et al. [23] compared the density of astrocytes in the implantation site to an intact contralateral hemisphere and to an injury-only control. Observations highlighted that, although the density of astrocytes was higher in the implantation site relative to the baseline of the contralateral hemisphere, astrocytes appeared dispersed, as opposed to forming scar tissue surrounding the graft through astrogliosis. The injury-only control showed higher astrocyte densities compared to the implantation site, suggesting that grafting was less stressful to the host in contrast to regenerating damaged cortices. In another study, LIUS treatments were applied to organoids in vivo following engraftment [26]. Unlike controls, LIUS-treated organoids had reduced graft-to-host borders and a more scattered distribution of glial cells throughout the graft as opposed to developing astroglial scar-like tissue structures. Conversely, another study implanted organoids into cortical cavities created by combining the MCAO-model with a biopsy punch [33]. It was observed that glia scar formation was present and human astrocytes originating from the graft participated in the regenerative process caused by the occlusion damage. This was also reported in the case of organoids transplanted into focal injury stroke damaged cortices, where approximately half of the astrocytes in the infarction core were human [36]. Finally, to investigate human astrocyte reactivity in vivo, researchers injected either saline or a pro-inflammatory cytokine into grafts to induce reactive astrogliosis [27]. They found that the treatment induced heterogeneous reactive responses across astrocyte subpopulations, particularly associated with pro-inflammatory and neurotoxic pathways, alongside reduced metabolic and oxidative stress. Together, it was observed that the host astrocytes conduct their natural function of healing the damaged cortex, and the implantation of an exogenous graft does not appear to foster the formation of a glial barrier of scarring to reject the implant. Rather, it seems that an organoid graft participates in restoring the damage with the involvement of human astrocytes at the site of stroke damage.
In relation to this, neuroinflammatory responses are another host immune process that could hamper the physiological integration of organoids. Organoids grafted into damaged cortices of rats immunosuppressed with cyclosporin A were not observed to elicit neuroinflammatory reactions [34]. A different study, with similarly medicated rats, indicated that markers of activated microglia tended to be higher at the grafting site compared to the contralateral hemisphere, and decreased over time [23]. Hence, the slightly increased microglial response might indicate a mild inflammatory reaction, which was silenced by the immunosuppressive treatments. Conversely, researchers from one study where organoids were transplanted into athymic (generically immunocompromised) rats conducted staining for IBA1 and revealed the occurrence of host microglia within the grafts [37]. Similarly, organoids grafted into genetically immune-deficient mice showed profound migration of host microglia into the grafted tissue [17, 26–28, 38]. Although these findings do not report occurrence of human microglia within the grafts, this can be achieved by transplanting microglia assembloid models [20]. At 8 weeks post implantation, these grafts consistently showed profound expressions of IBA1. With complex branching patterns and mature morphological identities of human microglia, this method of assembloid grafting projects a successful way at integrating human immune cells into foreign cortices. Altogether, observations indicate that host microglia migrate into the organoid grafts mainly in cases where the hosts are transgenically immune-deficient. In contrast, the reported presence of host microglia in rodents immunosuppressed with drug treatments appears to be less pronounced. Trans-injection of NPCs into immunocompetent mice has instead resulted in considerably higher signs of activated host microglia and macrophages with enhanced phagocytic activity [32]. This elevated immune reaction to foreign tissue in immunocompetent mice may serve to further convey the necessity of employing transplantation hosts with compromised immune systems when grafting across species. In summary, responses of recipients to foreign organoid implants are mostly resolved by inducing genetic or drug-maintained host immunodeficiency. The extent of active astrogliosis surrounding the grafts is arguably related to experimental designs or employed cortical damage models. In addition, graft-originating human astrocytes seem to be aiding in cortical repair following stroke damage. With regards to microglia, hosts receiving immunosuppressive treatments appear to result with lower degrees of microglial responses in comparison to employing genetically immunodeficient rodents.
In conclusion, organoid models transplanted into host cortices have demonstrated various signs of anatomical and plastic integration to the novel cortical in vivo environment. Primarily, the adaptation of implanted organoids was evidenced by high graft viability and host survival rates. The viability of implants was influenced by age of the organoids in culture prior to the implantation, suggesting a preference for younger organoids for future transplantations. Grafting also lowered metabolic stress and apoptotic processes compared to in vitro conditions. The physiological extent of plasticity was further demonstrated by signs of graft maturation, proliferation, development of regional morphological identities and differentiation of diverse neural cell compositions post transplantation. Moreover, early signs of VZ-like structural layer organisation conveyed that the implants can sustain a form of rudimentary layer structure that mimics neonatal human cortices, with– however– limited signs of a classical laminar structure. However, in some cases, the grafts had no distinguishable structure or even exhibited signs of stagnation. Nevertheless, most grafted organoids displayed volumetric growth and integration of host vasculature that was able to maintain their survival. Use of genetically immunocompromised hosts gave indications of low immune disturbances within the new host cortex. Yet, future research could further inspect how the host microglia is specifically influenced by different methods of induced host immuno-deficiency. Finally, observations of astrogliosis at sites of implantation were indicative of ongoing restoration of the induced cortical damage, with human graft astrocytes participating in the repair of neural damage. These findings lay the groundwork for positive prospects of organoid grafting as a future application for anatomical regeneration in the recovery of cortical damage. As this is not the sole aspect of a full recovery, the subsequent section delves into investigating whether similar prospects could be reached in terms of functional integration and recovery of damaged neural circuitry.
Functional integration of grafts to host cortices
Beside considering the physiological plasticity and adaptation of grafts to host cortices, it is also essential that the implanted tissue functionally integrates within the host neuronal network to enable a functional recovery of the damaged cortical areas. The level of functional restoration of impaired neural circuitry can be approached by examining the extent of synaptic connectivity between the grafted organoid and host cortex or by analysing the patterns of neuronal activity of the grafted organoids. These functional aspects are regionally-specific to the areas that the organoids are transplanted into (Table 1). Hence, findings will be reviewed across four main categories of cortical systems that have been previously selected as sites for engraftment, namely the higher-order association, motor, somatosensory, and visual systems.
Grafts within higher-order association cortices extend projections throughout host cortices and generate evoked and spontaneous electrophysiological activity
One group of areas that participate in higher-order cognitive functions are association cortices. These regions may be distinguished from primary sensory areas in that they engage in more complex functional aspects of cognition, associating information from other lower-order areas [54]. The extent of functional recovery obtained via organoid implantation into damaged association regions like the frontal or parietal cortices will be assessed by focusing on established synaptic connectivity and electrophysiological activity following engraftment.
Dong et al. [39] implanted multiple cerebral organoids into the mPFC and found that after one month the grafts extended synaptic projections to distal regions such as the lateral hypothalamus and parietal cortex. Optogenetic stimulation of tissue slices during ex vivo whole-cell recordings further established that the implanted organoids not only developed graft-graft neural connectivity, but were also functionally integrated with bidirectional synaptic connections to the host neurons. Electrophysiological activity highlighted that, compared to neurons recorded at 3 months after grafting, those recorded at 5 months post transplantation displayed improved electrophysiological maturity and increased synaptic activity. These findings portray that grafted organoid neurons in the mPFC integrate within the host circuity and generate both spontaneous as well as evoked electrophysiological activity.
Shifting the focus to other association areas, Kitahara et al. [24] implanted cerebral organoids into bilateral aspirated cavities in frontal and parietal cortices. Researchers then investigated how time delays between cortical lesioning and transplantations, age of organoids in culture and location of transplants influenced the growth of axonal projections. While parietal grafts projected axons to neighbouring cortical areas, the cortico-spinal tract and traversed along the corpus callosum (CC), frontal grafts extended additional connections to the striatum. In vitro sample age prior to engraftment also had an impact on axonal growth, with 6-week organoids showing more dense fiber projections to the cortico-spinal tract compared to 10-week organoid grafts. Finally, transplanting 10-week organoids one week after the induced cortical damage resulted in enhanced axonal projections, in contrast to 10-week organoids transplanted directly after lesioning. Although electrophysiological recordings were not conducted, this study showcased valuable effects of organoid age and timing of implantation on the axonal connectivity map of frontal and parietal organoid grafts.
In another study related to higher-order areas, researchers xenotransplanted cerebral organoids or NPCs into the lesioned frontoparietal cortex [32]. Axonal staining revealed that, while NPC grafts appeared to not yet develop any connections at two- or four-weeks post transplantation, transplanted organoids developed axons with distal projections at both experimental timepoints, although without any sort of organised connectivity pattern. The extent of axonal growth within the transplanted organoids was also higher compared to temporally matched organoids in vitro. Even though electrophysiological activity was not reported, this study again presents the benefits of implanting organoid samples, which extend projections that are anatomically required for functional graft-host integration. Finally, one study recorded local field potentials (LFP) 70-days post transplantation via multi-electrode arrays [35]. The cerebral organoid grafts, implanted into damaged left parietal cortices, contained electrophysiologically mature neurons that displayed spontaneous activity patterns and evoked action potentials with stimulation. Furthermore, the grafts extended axonal projections across the CC, but also to proximal areas surrounding the implantation site already at 55 days post implantation.
In summary, organoids grafted into higher-order association areas show extensive axonal growth within the host brains in addition to evoked and spontaneous electrophysiological activity, which appears to mature in later timepoints following engraftment.
Grafts within damaged motor systems form organised networks of connections aligned with their new function
Motor impairments resulting from cortical damage may be one of the most discernible of neurological deficits that affect lives of stoke and TBI patients. Given the relevance of organoid grafting as a possible cortical repair strategy to restore motor actions, functional integration of implants in motor areas has a major clinical potential.
Studies in which cerebral organoids were xenotransplanted into the rat motor cortex showed that human neurons originating from the graft migrated within the host brain [33, 34]. The resettled neurons, largely located in ipsilateral areas, also traversed through the CC into contralateral brain regions, with extensive synaptic integration into the host brain [33]. Another study further localised the bilateral migration of neurons to distal areas from the grafting site such as the thalamus and hippocampus [34]. Although extensive axonal projections are relevant for a functional integration into host circuitry, these studies have not reported the electrophysiological profiles of the grafts.
A more recent study, in which organoids were transplanted into damaged cortical regions including the motor and somatosensory cortices, showed that grafted neurons generated action potentials in response to current stimulation during whole-cell recordings, indicating their electrophysiological maturity [36]. After 180 days post implantation, the grafts not only developed extensive projections to proximate regions such as the secondary somatosensory cortex, but also to more distal ipsilateral and contralateral areas traversing via the CC to the thalamus, striatum, hippocampus and brain stem. These findings suggested of the graft may have participated in restoring motor capabilities, given that the organoids established connections to the host brain circuitry in a similar fashion as is seen in neocortices of mammals. Optogenetic stimulation during whole cell recordings of host and graft neurons additionally revealed that graft neurons were bidirectionally connected with active afferent and efferent projections to host neurons. Moreover, optogenetic stimulation of the graft in vivo elicited LFPs in the ipsilateral striatum and the contralateral cortex. Together, these findings provide evidence that organoids grafted into the damaged motor system are capable of functional integration into the host circuitry, with specific patterns of connectivity that are analogous of supporting their new motor function.
In sum, motor grafts adapt to their sites of implantation and display electrophysiological activity as well as organised projections that are key in the therapeutic context of cortical repair. In contrast to implants in higher-order association cortices, motor grafts develop different configurations of axonal projections, as is expected given the distinct function of this cortical region.
Implants functionally adapt and connect to the somatosensory system and develop the capability of responding to relevant tactile stimuli
As one of the primary sensory areas, the primary somatosensory cortex (S1) is the main cortical domain for processing tactile information. Revah et al. [37] xenotransplanted cerebral organoids into the adult mouse S1. Tracing of efferent projections from the graft exposed dense extensions of axons to the neighbouring ipsilateral S1 and to the ventrobasal nucleus. Mapping of afferent connections revealed that thalamic terminals were present within the implants. When activated optogenetically, these terminals evoked excitatory postsynaptic currents in whole-cell recordings of the human neurons. With similar electrophysiological outcomes, electrical stimulation of regions neighbouring the S1, the internal capsule, and white matter uncovered that these regions also had functional projections to the implant neurons. It was therefore confirmed the graft was functionally integrated within the host circuitry. To further investigate this in vivo, researchers conducted optogenetic stimulation of transplanted organoids at 150 days post implantation. This stimulation elicited rhythmic activity during calcium imaging, as well as synchronous bursts of action potentials in a similar frequency during extracellular recordings. These elicited bursts of activity were observed collectively among ~ 73% of the recorded neurons. Furthermore, bursting neurons portrayed highly correlated activity, which exceeded the degree of correlations among host neurons recorded in non-damaged controls. To test whether neurons were also responsive to tactile stimulation, optotagging was used to record activity of individual graft neurons in response to repeated whisker deflections on the contralateral side to the implanted hemisphere. A prominent increase of firing rates was observed among ~ 54% of the recorded neurons, reaching peak activity at around 650 ms after the whisker stimulation. This response is considerably late compared to expected temporal dynamics in the naïve rodent S1, where whisker stimulation evokes an entire activation of S1 after around 50 ms [55]. Nonetheless, it can be reasoned that the sensory grafts adapted to their respective regional function by receiving suitable functional inputs and displaying activity evoked by tactile stimuli. In sum, these findings point to a functional integration of organoid grafts in the S1, being bidirectionally connected to the host circuity and showing signs of restoring previously impaired sensations as evidenced by elicited activity patterns after tactile stimulation.
Another study compared the outcomes of engrafting cortical organoids that received LIUS treatments during culture prior to transplantation compared to untreated control grafts [26]. While researchers reported increasing graft-to-host synaptic connectivity over time in both groups, LIUS-treated organoids exhibited higher synaptic densities, suggesting enhanced synaptic integration within the host cortex. Axonal tracing of the implants– located in S1– further showed that the highest density of projections was located in the cortex adjacent to the graft, with additional axonal growth observed across the CC and into subcortical regions such as the hippocampus, hypothalamus, amygdala, and bed nucleus of the stria terminalis. Notably, LIUS-pretreated organoids exhibited denser long-range projections to target areas, particularly within S1, compared to controls, suggesting that LIUS boosts extents of functional integration into the host brain. Furthermore, additional in vivo LIUS treatments enhanced graft synaptic connectivity and led to denser axonal projections to the host cortex. The authors next conducted extracellular probe recordings from 3 to 5 months post-transplantation, revealing that both the in vivo LIUS-treated and in vitro LIUS-pretreated organoid grafts exhibited increased spiking activity, informational entropy, and oscillatory signal energy across multiple frequency bands compared to controls. Delivering high-frequency electrical stimulation further enhanced information transfer from grafts to the host brain, indicating the formation of functional graft-host connections. Altogether, these results support that the functional integration of organoids is significantly enhanced when combined with LIUS graft treatments, leading to boosted electrophysiological activity and synaptic connectivity to host circuity.
Building on strategies to enhance functional graft integration, Hu et al. [28] investigated the effects of early- and late-stage electrical stimulations applied via an OBCI system to cortical organoids transplanted into S1. Early electrical stimulation significantly improved electrophysiological activity of the grafts in terms of firing rates, burst dynamics, gamma-band energy, and phase-amplitude coupling compared to unstimulated controls, indicating accelerated functional network maturation. Analysis of axonal projections further confirmed enhanced intra-graft and graft-to-host connectivity of grafts when stimulated via OBCI in later stages of integration. Stimulated organoids exhibited denser axonal projections into the cortex, CC, and subcortical structures, with increased excitatory and inhibitory synapse formation. In sum, these findings demonstrate that while transplanted organoids are able to regionally integrate within host circuitry, OBCI-based stimulation substantially promotes their functional maturity and connectivity within the host networks.
Lastly, one study conducted comparisons between vascularised vs. non-vascularised cerebral organoids implanted into cavities in S1 [22]. Although the authors did not map synaptic connectivity, they established that the grafts were functionally integrated within the host brain with pre- and post-synaptic connections. Whole-cell recordings showed that, compared to non-vascularised organoids, vascularized ones presented higher outward currents as well as enhanced cell capacitance and lower membrane resting potentials. This reflected more extensive growth of processes and increased electrophysiological capabilities, which were indicative of enhanced neural maturity in the assembloid (i.e., vascularized) grafts at 80 days post implantation. Increased functional maturity was further evidenced by more active neurons in the assembloids compared to the non-vascularised grafts. Additionally, this study showcased that the graft neurons received excitatory and inhibitory synaptic potentials, while some also portrayed evidence of bidirectional electrical gap-junction synapses. Finally, calcium imaging revealed spontaneous activity of mature glutamatergic neurons within the implants. In sum, findings indicated the extents of neural development and integrated functional capabilities by the formation of active electrical and chemical receptor connectivity, and the occurrence of spontaneous electrophysiological activity.
Altogether, organoids grafted into a primary sensory area show functional electrophysiological maturity, adapt to damaged host networks, and develop restorative responsiveness to region-specific tactile stimulation. Further, the application of LIUS treatments and OBCI stimulation has facilitated further enhancements of functional graft integration within host cortices. In contrast to the previous cortical areas, where neuronal activity was less investigated, somatosensory grafts displayed evoked activity that is functionally appropriate to the tactile input. In addition, the sensory grafts also developed a different arrangement of neural connections compared to implants in motor and higher-order association cortices.
Visual system grafts integrate into neural networks, respond to visual stimuli, and display evidence of feature-selective neurons
Another sensory ability is housed by the visual system, enabling one of our most necessary of senses. Studies focusing on the visual regions utilised cortical and forebrain organoids for xenotransplantation into the retrosplenial cortex [17, 25] and the border between V1 and V2 [23]. Axonal tracing established that implanted organoids showed clear signs of synaptic integration. Across all transplantations, efferent graft projections mostly terminated at the proximal visual areas surrounding the implant site. More distal projections were also observed, but their density was gradually declining with distance from the implant [17, 23]. Even though most fibers were located in the ipsilateral hemisphere, Mansour et al. [17] also identified projections to contralateral regions and bundles of axons in the CC. Human neurons from the grafts were also observed more distally, migrating through the CC into the contralateral cortex [25]. In one instance, some projections were identified through the midline of the CC, but axons were not found in the contralateral cortex [23]. This points to the graft having developed more ipsilateral efferent connections in this study, as evidenced by observations of distal projections to the hippocampus and motor areas. Yet, a portion of the projections terminated in the thalamic lateral geniculate (LG) and lateral posterior (LP) nuclei. Conversely, tracing afferent connections highlighted that the grafts received ipsilateral inputs primarily from the neighbouring visual areas, while other afferents originated from the hippocampus and thalamus, namely the LG, LP, and ventral lateral nuclei. Additionally, between 2 to 3 months post transplantation, the grafts had approximately 2–4 times the number of connected cells to the contralateral retina compared to the naïve rat visual cortex. This indicates that the grafts were well integrated into the visual network (with the strong connection density being considered a sign of immaturity). Focusing again on ipsilateral distal projections, in addition to observing connections to the hippocampus and thalamus, Mansour et al. [17] also traced efferent axons to the striatum, amigdalar nucleus, and hypothalamus. Human vs. mouse synaptic identity was also tested, revealing bidirectional synaptic connectedness between the grafts and host cortices, predominantly at the tissue junctions [25]. Although the grafts only partly and unexplainably projected to distal cortices such as the motor area, hypothalamus and hippocampus, the connectedness to the retina together with the reciprocal connections with the near visual areas and the thalamus (predominantly visual nuclei) pointed to the conclusion that the grafts were synaptically integrated within the visual network.
To fully restore neurological function following infarctions or brain trauma, organoid grafts should also show signs of undertaking its specialised function. Organoids grafted in the damaged retrosplenial cortex were shown to house glutamatergic neurons that generated spontaneous and synchronised patterns of activity repeatedly over several live calcium imaging sessions [17]. Through extracellular recordings, researchers compared neural activity between early (50-days) and later (115-days) timepoints from the implantation. It was observed that, compared to early recordings, more neurons were active and portrayed a higher synchrony of spontaneous action potentials at later timepoints. In addition, reintroduction or suspension of anaesthesia induced the expected fluctuations in firing rates of neurons in the more mature grafted neural networks. To summarize, the implanted organoids exhibited functional integration and maturation over time, as neurons became more active and showed higher degrees of coordinated spontaneous activity in later timepoints. Finally, to see whether the grafted neurons influenced the LFP in neighbouring host cortices, the implant was optogenetically stimulated. Recordings done in different locations revealed that, in response to optogenetic stimulation, human neurons could evoke LFP oscillations in the 10–20 Hz range. It can therefore be argued that the grafted organoids were not only connected to, but could also exert functional effects within the host circuitry. It remains to be seen, however, whether these functionally connected implants are also capable of processing sensory stimuli, as was the case in somatosensory grafts.
This was actually shown by another study, where organoids were similarly transplanted into the retrosplenial cortex [25]. Already 3-weeks post implantation, the grafted organoid displayed spontaneous multi-unit activity (MUA). In addition, light stimulation in the contralateral eye consistently evoked LFPs and MUA in the nearby host visual cortices, which propagated almost seamlessly to the grafted organoids after stimulus onset. Alike to the former study, evoked LFP activity got stronger as the implant matured. These findings suggest that the visual system implant was sufficiently connected to the host neural network and was excitable by visual stimuli in relation to its grafting site. Further analysis of the LFP showed that the latency of the signal propagation from host visual areas to the retrosplenial graft was resemblant of the one observed in the intact cortex. In terms of oscillatory frequencies, visual stimulation also led to the expected increases of gamma power in the implanted organoid. Finally, induction of anaesthesia was found to cause a more pronounced decrease of MUA in channels located at the graft compared to smaller MUA reductions in channels recording the neighbouring host cortex. This heightened inhibitory effect of anaesthesia in the grafts was reasoned to be due to the early developmental state of the organoids, in which they still lack distal projections and cholinergic terminal inputs. Together, this study showed how an organoid implant is not only functionally integrated, but also actively responds to sensory inputs that are specifically related to the cortical location of its engraftment. In an additional study, in which organoids were implanted between V1 and V2 in rats, extracellular probe recordings showed not only that the graft neurons exhibited spontaneous activity patterns, but also that presentation of visual stimuli during anaesthesia elicited visually-evoked activity within the graft neurons [23]. Additionally, visual stimuli evoked neural activity patterns that resembled those of the naïve rat visual cortex, with similar firing rates, spike amplitudes, and spike widths. Some differences were also noted, namely that the host neurons elicited higher amplitudes of event-related potentials and were more active in response to the stimuli compared to the graft neurons, which in turn featured longer periods of evoked activity following the stimulation. Lastly, graft neurons displayed orientation selectivity upon the presentation of drifting gratings. In comparisons to orientation tuning in the naïve rat neurons, it was observed that the specific distribution of selectively active graft neurons was largely equivalent. Altogether, grafts functionally integrated within the local circuits, showing spontaneous and evoked activity as well as feature selectivity to visual stimuli orientations, signifying that the graft was also integrated within the system level of the visual network.
In conclusion, organoids grafted across higher-order association, motor, somatosensory, and visual cortices developed distinct connectivity patterns with the host. These region-specific arrangements of connections suggest that the organoids have functionally linked to the host networks according to the cortical site of engraftment. In addition, while implants in the motor and association areas showed spontaneous activity that supported their electrophysiological maturity, grafts in the damaged sensory systems were also responsive to environmental stimulation. Furthermore, neurons from organoids grafted in the visual network also adopted region-specific properties such as selective tuning to features of visual stimuli. These factors demonstrate the functional integration of organoids within the host circuitry post-engraftment, regardless of the implantation site, though with differences in the layouts of connections to the host brain. Given that the grafts possibly lead to functional repair across damaged cortical regions, it is expected that this transplantation therapy affects host behaviour. This will be investigated in the next section.
Outcomes of grafting on behaviour
So far, we discussed to what extent grafted organoids anatomically and functionally integrated into the host tissue, and examined which factors influence the degree of integration. We will now turn to examine whether this capability to integrate into the host underlies the potential to restore behavioural functions lost as a consequence of brain damage. We will review this regionally, from behaviour linked to higher-order cognition to motor and sensory-related behaviour, as was done in the previous section.
Grafting organoids recovers and enhances spatial learning and memory
Cognitive functions such as learning and memory are essential in supporting animal behaviour. To address whether organoid implants enable the recovery of these functions, Bao et al. [35] lesioned the left parietal cortices of mice via the CCI model and implanted cerebral organoids to see whether memory and spatial learning would be influenced by the grafting. Mouse behaviour was first assessed using the Morris water maze test, to examine spatial memory capacity of mice in relation to the novel hidden platform. Behaviour was measured by recording the swimming duration, distance, and speed before reaching the platform. Observations indicated that, during training, the visual and motor abilities were equal across mice with grafted organoids and mice subject to CCI lesions with no implants. Yet, during the testing phase, mice engrafted with organoids displayed significantly shorter swimming distances and durations. Overall, compared to the lesioned CCI mice, ones with implanted organoids had enhanced spatial learning capabilities. A second passive avoidance task tested short-term memory to danger situations, previously conditioned through electric shocks in a dark section of a behaviour box. Results showed that organoid-grafted mice avoided the dark section more compared to the CCI lesioned mice. This demonstrated that implanting organoids aided the recovery of short-term memory of mice.
Another category of studies on behavioural outcomes following organoid grafting focused on emotional episodic memory. Dong et al. [39] explored the effects of grafting human cerebral organoids or dissociated cells into the mPFC on mouse behaviour after 6 and 8 weeks post-implantation. Behaviour was evaluated with an open field test and fear conditioning paradigm. Behaviour was compared to a control group where mice received cerebrospinal fluid injections into the same cortical region. To confirm that the transplantation procedure had not interfered with normal behaviour, the open field test was administered to measure explorative motor behaviour between groups. Results highlighted that there was no difference in the quantified parameters of explorative behaviour among control and grafted mice. Mice were then fear-conditioned via auditory tones coupled with electrical shocks, a paradigm that is well known to induce freezing upon learning the association between auditory stimuli and the shock. Mice in the control group had no difference in the percentage of freezing compared to mice injected with dissociated cells. Conversely, mice with organoid grafts showed an elevated percentage of freezing in comparison to controls. These findings suggest that the behavioural manifestation of fear appeared to be intensified by the human organoid grafts.
Altogether, transplanting organoids into the parietal and mPFC cortices of mice presented evidence of enhanced behavioural recovery. This was supported by findings of heightened spatial learning capabilities, increased fear-related short-term memory [35], and elevated freezing responses to fear-inducing conditioned stimuli [39].
Implants in damaged motor-related areas display signs of restoring motor deficits
Neurological damage leading to behavioural motor deficits is a great limit to the potential of one’s survival and quality of life. Organoid grafting in the motor system has thus the potential to aid the recovery of motor behaviour. However, given the current lack of organoid grafting studies addressing this question, this section will also report findings from the more established approach of implanting cell grafts (Box 1).
Within the realm of motor disorders, one study investigated cell grafting as a regenerative therapy approach for Parkinson’s disease by implanting human ESC-derived mesencephalic dopaminergic neurons into the lesioned right striatum in mice [45]. Unilateral lesions were done with 6-hydroxydopamine, to mimic hemi-parkinsonian motor deficits. Over the course of recovery, mice were subject to the amphetamine rotation test, where ipsilateral behavioural circular-rotation movements were considered as indicative of motor damage. Three weeks post-grafting, grafted mice showed no behavioural asymmetry of rotations, while lesioned mice without implanted cells persisted in displaying behavioural ipsilateral rotations during the test. Researchers thereby established that grafting cells enabled a recovery of the induced motor damage. To provide further evidence, mice then participated in a corridor test combined with optogenetics. While control mice without lesions showed no lateralised preference when searching for rewards, lesioned mice preferred searching the corridors on the ipsilateral side to their lesions. Conversely, lesioned mice with grafted mesencephalic dopaminergic neurons behaved similarly to the controls. Furthermore, optogenetic silencing of the grafted cells re-introduced the ipsilateral side preference. Altogether, these findings evidenced that the grafted cells were causally involved in the recovery of sensorimotor deficits resulting from the unilateral Parkinson’s disease model.
Another study explored sensorimotor behaviour in rats following MCAO-related cortical damage [47]. The rats were grafted by injection of NSCs into the lesioned striatum. Motor behaviour was first examined through the cylinder test. Findings indicated that contralateral forelimb use and sensorimotor functions were similar across rats grafted with NSCs compared to lesioned-only vehicle controls. However, when optogenetic stimulation of the implanted NSCs was performed, the grafted rats exhibited improved sensorimotor function and increased movements of forelimbs contralateral to the stroke-induced lesion. Similarly, optogenetic stimulation of the graft increased motor actions and explorative behaviour in an open field test compared to the vehicle-lesioned controls at 2 months post-grafting. In summary, implanted NSCs were functionally integrated within the host cortex, and optogenetically stimulating the grafted cells elicited beneficial effects on the motor-related explorative behaviour. Sensorimotor behaviour in relation to MCAO-induced damage was also investigated by Palma-Tortosa et al. [48], where NESCs were injected into a stroke-injured region encompassing M1 and somatosensory cortices. Behaviour was assessed between control, damage-only and cell transplanted groups with a cylinder test. Findings revealed that while the damage-only group showed less movements in the arm contralateral to the stroke-injured hemisphere, the grafted rats had no imbalance in the number of forelimb movements. However, it was then tested whether this motor recovery resulted from neural activity of the grafts. Optogenetic silencing of the graft cells had no reversing effect on the imbalance of forelimb use in the cylinder test. It was therefore concluded that even though the grafting led to restored motor-deficits, this was not directly caused by the neural activity of the grafted cells. Together, even though it remains inconsistent whether the grafted cells participate in motor recovery through their functional activity, the behavioural outcomes support that the graft can restore motor-deficits in stoke-injured hosts.
In contrast to grafting cells, Cao et al. [36] implanted cerebral organoids into a region spanning the motor and somatosensory cortices which was injured by stroke. Sensorimotor capabilities were examined at multiple timepoints with the cylinder, grid-walking, and adhesive-removal tests across stroke-damaged, grafted and control mice. The cylinder test revealed that grafted mice exhibited less asymmetric forelimb use in comparison to the stroke-damaged mice. Similarly, the grid-walking test highlighted that the implanted mice had a lower occurrence of contralateral motor deficits compared to damage-only mice. Finally, the adhesive-removal task also showed that the grafted mice had touched and removed the adhesive sticker on the paw ipsilateral to their stroke-injury faster than the stroke-damaged mice. Meanwhile, the damage-only group performed poorly on all tests with respect to controls. Furthermore, with the passing of time post implantation, the grafted mice showed progressive improvement in performance that was similar to the controls at later timepoints. Importantly, chemogenetic silencing of the grafts reintroduced motor-deficit indicative performances during all behavioural tests. In sum, this study found that more mature organoid implants in stroke-injured mice can improve and restore sensorimotor deficits in behaviour.
Lastly, evidence of behavioural motor recovery with cerebral organoid grafts was outlined in a study where organoids were transplanted into a cavity in the right rat motor cortex [34]. The cavity was created using a biopsy punch resembling acute TBI. Motor behaviour was quantified through a modified neurological severity score (mNSS) and beam walking test. Already five days post-implantation, rats grafted with cerebral organoids had an elevated mNSS compared to rats with TBI lesions. Moreover, the motor function of the grafted rats recovered to baseline 21 days post-implantation, thereafter being equivalent to control mice. Improvements in behavioural motor function were also observed at a temporally similar rate in the beam walking test for rats with organoid implants compared to TBI-only rats. These findings supported the relevance of grafting organoids as a potential clinical option for TBI.
Overall, grafting cells and organoids into rodents with motor deficits resulting from brain damage has generally been shown to improve motor performance and even enable complete recovery of normal motor behaviour. This showcases the therapeutic potential of neural tissue grafting for the functional recovery of motor-deficits that may result from conditions such as Parkinson’s [45], stroke-damage [36, 47, 48], or TBI [34].
Organoids grafted in the somatosensory system drive reward-seeking behaviour
Motor behaviour is often guided by perceptual information that is sensed from the surroundings, such as tactile and visual information. In spite of the debilitating consequences of damage to sensory cortices (such as blindness), however, organoid grafting in sensory cortices has so far received limited attention, with only one study– to our knowledge– investigating the effects of grafting organoids in the somatosensory system.
Specifically, Revah et al. [37] implanted human cortical organoids via stereotactic injection to the early-postnatal rat S1. Grafted rats showed no behavioural differences across novel-object recognition, fear conditioning and open field tests in comparison to non-transplanted rats. Rats were then trained to lick a spout for rewards while receiving two types of optogenetic stimulations to the grafted organoids, which were infected with a virus during culture to express a channelrhodopsin-2 opsin. Initially, behavioural performance was at chance level. At day 15 in training, rats learned that one of the two optogenetic stimulation wavelengths to the graft was indicative of a reward. This reward-seeking behaviour was evidenced by increased licking behaviour during the rewarded optogenetic trials. The performance was compared to that of rats with grafts not expressing the necessary opsin required for stimulation. This latter control group showed no signs of learning in this operant task. Altogether, this study showed that organoids implanted into early postnatal rat pups not only displayed anatomical signs of functional integration into sensory and motivation-related circuits, but were also capable of modifying behaviour in an optogenetic operant conditioning task. Overall, organoids grafted into a lesioned brain have been shown to integrate into the host to a degree that enables them to influence behaviour and even promote functional recovery. Thus, organoid transplantation has clear potential as a novel therapy for brain damage.
Discussion
Here we analysed the current literature to understand whether grafting organoids into cortical tissue induces plastic adaptation to the damaged implantation sites and, ultimately, functional recovery.
First, we reviewed whether transplanted organoids anatomically and physiologically integrate and adapt to the hosts. The gathered evidence indicated that grafted organoids display normal rates of apoptotic cell death in addition to low cellular metabolic stress. In line with this, most reported transplantations resulted in favourable host and graft survival rates. In terms of anatomical development after transplantation, organoids presented signs of increased maturation, proliferation, and establishment of morphological identities in the respective regions they were transplanted into. Analyses of cellular compositions showcased diverse profiles of differentiated cortical cell populations within the grafts post transplantation, mainly including glutamatergic neurons, astrocytes and NPCs. It was also repeatedly observed that there was a lack of GABAergic neurons and oligodendrocytes. The reason for lower counts of inhibitory neurons across grafted cerebral organoids appeared to be a lack of ventral brain progenitors that usually migrate to dorsal cerebral regions later in development. Furthermore, structural layer organisation was conveyed by early signs of rudimentary layer structures in the form of VZ-like rosettes in some of the implants, mimicking neonatal organization of human cortices. However, in several cases grafted organoids had no obvious distinguishable structure or even exhibited signs of stagnation. Most of the organoids grew larger in volume following engraftment. Moreover, and with high consistency among studies, grafted organoids successfully integrated within the host vasculature networks, enabling anatomical adaption and survival. In terms of recipient immune responses, weak migration of host microglia into the grafted organoids was indicative of low immune disturbances within the new host cortex. This mild immune reaction was most evident in genetically immunocompromised hosts compared to hosts immunosuppressed with drug treatments. Additionally, the host reaction of astrogliosis at sites of damage and implantation appeared to be happening more in relation to restoring the inflicted cortical damage than to surrounding the foreign implant with scar tissue. Regarding astroglial involvement, human astrocytes from implants also elicited signs of aiding the host in the repair of neural damage resulting from strokes. In sum, this accumulated evidence shows that grafted organoids adapt and integrate into the damaged cortical in vivo environments.
Nevertheless, some aspects still need to be improved. The limited development of a classical laminar structure should be of primary concern, yet there are currently no available methods of generating organoids that fully mature with laminar organisation resemblant of a developed adult cortex. Novel research designs could foster further host microglia concentration and migration to the implant site, and study how this is influenced by different methods of induced host immuno-deficiency. This could benefit the current field, by highlighting more evidence on the suitable recipient conditions for lesser immune responses in transplantations of organoids cross-species. Furthermore, the low presence of inhibitory neurons within grafted organoids is another factor that limits the maturation of the grafts. This could be resolved in future studies via utilising organoids [52] or assembloids [56] with both ventral and cerebral identities. Such organoid models might support the ventral to dorsal inhibitory neuron migration, which is less observed in cerebral organoids due to a lack of ventral progenitors [19]. In addition, the available literature provides limited insight into how organoid transplantation impacts host cell proliferation and function, or the extents of such effects. We therefore highlight this as a topic that could be addressed by future research. Finally, the plasticity and physiological integration of grafted organoids has heterogenous outcomes. This variance is likely due to differences in organoid generation protocols across the reviewed studies and remains an open issue. Further efforts to share protocols, as demonstrated by Kelley et al. [57], could promote the standardization of organoid culture, experimental design and transplantation approaches among researchers.
Aside from current limitations and technical challenges that still need to be further addressed, a promising direction for future applications in this field could be related to disease modelling and therapy with the combined use of gene editing technologies and xenotransplantation. This approach could enable the transplantation of genetically edited organoids into host cortices. For example, CRISPR-Cas9 technology was employed in a recent study to generate human autosomal recessive primary microcephaly mutant organoids, which were then used to model the primary microcephaly in vivo within the host mouse cortex [26]. This study demonstrated how such an integrated approach can not only model disease mechanisms but also potentially offer therapeutic solutions, as seen in the rescue of neurodevelopmental defects through targeted LIUS treatments of the mutant grafts.
Is there a lesion method that can be regarded as most suitable for physiological recovery of grafts? Previous findings hinted at the viability of implants in relation to the transplantation damage models. In terms of maturation of cellular organisation, implanting organoids into cortical cavities damaged by aspiration [17, 22], direct removal [32], or stroke-injury [36] led to observing rosette VZ-like structures within the grafts. In contrast, other transplantations into aspirated [23] or undamaged cortices [37] resulted in the absence of clear structural organisation. Therefore, specific types of transplantation models do influence structural organisation of organoids in the host cortex. Furthermore, compared to aspirated cavities [23], TBI and stroke-injury transplantation models induced more pronounced glial scaring, resulting in higher numbers of astrocytes at the implant-host junction [33, 35] and the infarction core [36]. Specifically in stroke-injury models, development of human astrocytes was also noted [36], as well as signs of graft participation in the repair of cortical damage [33]. Host astrocytes were also present within the graft following cortical aspiration, but in a more dispersed manner [23] compared to the examples of TBI and stroke-injury. However, studies typically have idiosyncratic differences in experimental design. To determine the relative benefits of one transplantation model over another in specific physiological aspects of integration, future studies will need to better control for the contribution of different lesion models. Yet, some other aspects seem to be consistently found independently from the employed lesion model. For example, when implanting organoids into aspirated lesions, a 7-day delay before organoid implantation results in higher survival rates and more pronounced growth in volume compared to engrafting directly after lesioning [24]. Additionally, stroke-injury models have been consistently shown to aggravate astrogliosis [33, 35, 36]. In summary, the damage model of cortical aspiration appears to incite less astroglial scaring, whereas direct tissue removal and stroke-injury models seem to yield improved structural integration. However, it is still unclear which lesion model is ultimately more suited to be treated with organoid transplantation. To highlight the current evidence in the field, Table 3 summarizes key advantages and limitations associated with each cortical damage model.
Table 3.
Comparison of cortical damage models. This table presents an overview of commonly used cortical damage models: TBI, MCAO, and aspiration lesion models. Each model is evaluated for its advantages, disadvantages, translational relevance, and reproducibility
| Damage Model | Advantages | Disadvantages |
|---|---|---|
| Traumatic Brain Injury (TBI) |
Reliable and reproducible model of cortical damage High translational relevance due to realistic damage Improved structural integration of grafts Mimics a broad range of injury types and severities |
Formation of glial scaring at the damage site post-implantation Inflammatory response may hinder graft-host integration |
| Middle Cerebral Artery Occlusion (MCAO) |
High translational relevance due to realistic damage Improved structural integration of grafts Availability of well-established protocols for inducing MCAO model damage |
Elevated astrogliosis response at the stroke site Variable and unpredictable cortical damage extent Inflammatory response may hinder graft-host integration |
| Aspiration Lesion |
Less pronounced glial scaring post-implantation Allows for precise control of lesion location and size Surgically less complex approach |
Limited translational relevance May not fully replicate the complex pathology of human cortical injuries |
A related question is whether transplantation of organoids as opposed to cells yields benefits. In the reviewed studies, this was indeed observed. To facilitate a comparison, Table 4 outlines the key benefits and drawbacks of the two major categories of graft types considered. While organoid implants portrayed signs of early neocortical organisation by developing VZ-like rosettes, such structures were absent in trans-injected dissociated cells [38]. Moreover, implanting organoids also led to considerably higher host and graft survival rates compared to transplanted cells [23, 36]. There are also indications of differences in anatomical integration between organoid implant types. Transplanted vascularised assembloids displayed lower rates of apoptotic cell death, while having faster growth and development compared to organoid grafts [22]. In one case, implanting vascularised assembloids resulted in improved graft survival and even the development of human capillaries post implantation, which was not the case in other organoid grafts [21]. Another aspect that induced differences between organoid implants was their in vitro age prior to the transplantation. Implanting organoids that spent less time in culture led to increased cell proliferation and neurogenesis [24, 34], and to the development of more dense fiber projections in the host cortex [24], in contrast to older organoid transplants. In one case, implanting younger organoids also led to graft overgrowth following transplantation, possibly due to the major increase in NPCs compared to older grafts [24]. Yet, the in vitro age of the graft did not seem to affect apoptotic rates [34]. Compared to dissociated cells, organoids demonstrate clear benefits such as increased graft and host survival rates, as well as signs of developing premature structural organisation. Furthermore, vascularised assembloids portray signs of higher viability compared to organoids. The viability of implants is also influenced by the age of the organoids in culture prior to engraftment, suggesting a preference for younger organoids for future transplantations. However, transplanting younger organoids with increased proliferation may not necessarily indicate rapid maturation, which is seen in more older organoids in culture. Overall, whether potential therapeutic effects differ between implanting brain organoids or specific cell types can be mainly linked to the key differences of the two graft types. Organoids display early rudimentary organized structures composed of heterogeneous cell populations, while cellular grafts are more homogenous. The choice between these transplantation methods largely depends on the therapeutic goals and the nature of the injury or disease being treated. Organoids display clear advantages for the repair of complex damage, such as in stroke or TBI, where the restoration of diverse cell types and cortical areas is essential. In contrast, cellular grafts could be more useful for targeted therapies, such as the replacement of specific cell types that are degenerating, like dopaminergic neurons in Parkinson’s disease, a method currently being explored in preclinical studies [58].
Table 4.
Comparison of graft types utilised for implantation and cortical repair. This table summarises key advantages and disadvantages of the two major categories of grafts: organoids/assembloids and cells. The cellular graft category encompasses specific populations from primary cell cultures, such as NPCs, NSCs, NESCs and differentiated neural cells, as well as cellular compositions (i.e., cells dissociated from organoids)
| Graft Type | Advantages | Disadvantages |
|---|---|---|
| Organoids/Assembloids |
High survival rate post-implantation Development of regional morphological cell identities Heterogeneous profiles of differentiated cell types Signs of premature rudimentary layer organisation Evidence of participation in cortical damage restoration Potential for modelling complex neural circuitry in vivo |
Limited control over cell identity and proportion post-implantation Transplantation may lead to graft overgrowth Limited form of classical cortical layer organisation High variability between organoid generation protocols Emerging area of research, requiring further studies to establish a more robust foundation |
| Cells (from multiple sources) |
Lower risk of graft overgrowth Predominantly explored in previous literature, establishing a strong foundation for further research Enables selection of specific graft cell types (not applicable to organoid dissociated cells) Simplified sample culture and scalability for clinical applications (not applicable to organoid dissociated cells) |
Low survival rate post-implantation Homogenous cell identity (not applicable to organoid dissociated cells) Lack of laminar cortical organisation and limited capacity to recapitulate complex brain architecture Reduced functional integration post-implantation |
To assess therapeutic relevance, we next reviewed whether grafted organoids contributed to functional recovery. The organoids implanted into higher-order association areas were marked by extensive axonal growth within the host cortex [24, 32, 35, 39]. Moreover, neurons from these grafts showed evoked and spontaneous electrophysiological activity which matured over time [35, 39]. Similar results were found for motor grafts, which developed organised axonal projections that were characteristic of their sites of implantation [33, 34, 36]. Organoids in the motor system also showed evoked neural activity [36]. Similarly, grafts in the S1 demonstrated functional electrophysiological maturity and were integrated within the host neural networks [22, 26, 28, 37]. Additionally, grafts subjected to LIUS [26] or OBCI [28] stimulation demonstrated increased electrophysiological maturity and functional integration within the host circuits. Sensory grafts also developed responsiveness to tactile stimulation which was region-specific to the engraftment area [37]. Finally, organoids implanted into the visual system, in addition to connecting within the local circuits and exhibiting spontaneous, synchronous as well as evoked patterns of activity [17, 25], displayed tuning to the orientation of visual gratings [23]. However, there is still a lack of electrophysiological or functional in vivo examination in organoids grafted into most cortical regions. To firmly confirm whether functional integration of organoids varies between and is influenced by implantation sites, more complex experimental and electrophysiological investigations are needed. Specifically, we advocate the combination of causal methodologies such as optogenetics with large-scale recording techniques such as two-photon calcium imaging or high-density electrophysiology. In sum, organoids implanted among higher-order association, motor, somatosensory, and visual cortices developed widespread connectivity maps of projections that were arranged in alignment to their cortical sites of implantation. Furthermore, the grafts displayed functional maturity by exhibiting spontaneous neural activity regardless of the engrafted area. In addition, sensory-evoked responses were observed in organoids implanted in sensory cortices, highlighting the potential for functional restoration.
Building upon the observed extent of anatomical plasticity and functional adaptation of the grafted organoids, we wondered whether grafting also promoted behavioural recovery. Transplantation of organoids into higher-order cortices presented evidence of behavioural recovery, namely improved spatial learning capabilities, increased fear-related short-term memory [35], and elevated startle responses to conditioned danger [39]. With regards to motor-deficits, transplanting organoids and cells improved motor and sensorimotor performance and, in some cases, even led to recovery of normal motor behaviour. This supports the therapeutic potential of neural tissue grafting for the restoration of motor-deficits that may result from conditions like Parkinson’s [45], stroke-damage [36, 47, 48], or TBI [34]. Furthermore, organoids that were integrated within the somatosensory and motivation-related circuits showed evidence of modifying behaviour in an optogenetic operant task [37]. Additionally, other promising studies are exploiting pain perception as a behavioural marker to assess the integration of organoids grafted into the somatosensory cortex [26, 28]. In summary, the outcome of grafting organoids across damaged cortical regions appears to have potential for restoring behaviour at the cognitive, motor-action, and sensational levels. However, a large part of the literature only investigated the effects of grafting cells and not organoids. Furthermore, only few cognitive, motor and sensory aspects have so far been studied. Thus, future research should add more evidence to support the prospect of clinically using organoid grafting in relation to promoting the recovery of a wider range of behavioural functions.
While the anatomical, functional and behavioural outcomes of organoid grafting is promising, several ethical and technical considerations must be addressed before clinical translation to human patients is possible. From an ethical standpoint, the use of human-derived brain organoids continues to raise societal, moral, and legal implications that are the subject of active debate in the scientific community [59, 60]. Currently, ethicists are discussing the issues related to the generation of chimeric animals with implanted human tissue, and how morality can be defined in such cases where animals are “biologically humanized” [61]. In addition, another challenge of this field is outlining the way we define the possibility of sentience and consciousness arising in cultured human brain organoids, and whether such networks of active neurons are able to realise or sustain such states of sentience and cognition. Although the field remains cautious, the emergence of complex higher-order cognitive functions in these chimeric models is widely regarded as unlikely within the scientific community [62]. Nonetheless, new regulatory frameworks need to be developed and refined to keep pace with advances in this rapidly expanding field, particularly in addressing the ethical, conceptual and translational boundaries of chimeric rodent models, large-animal experimental studies, or clinical human trials. Such efforts to adapt these frameworks are already underway [63], and continued cross-disciplinary discussion could foster consensus and alignment of ethical guidelines.
Apart from ethical concerns, there are also technical issues in relation to future translational prospects. Standardizing organoid culture protocols [64], ensuring reproducibility of transplantation models across studies and minimizing the risk of uncontrolled growth post-transplantation are some of the challenges that remain with regards to organoid implantation. Efforts to share detailed protocols for experimental design, organoid generation and transplantation methods are underway, and the field should further promote transparency and standardization through wider protocol dissemination [57]. Furthermore, precise control over cell type composition and spatial organisation of brain organoids remains limited, which could influence translational efficacy and safety. Long-term studies are also required to assess the stability, integration, and potential immune response of grafts over longer time periods. Lastly, a significant technical challenge in the clinical application of organoid transplantation is the disparity between the extent of lesions, such as those resulting from stroke, TBI, or glioblastoma resection and the limited size of current in vitro brain organoids. The cavities created by these lesions are often much larger than the organoids can fully fill, raising concerns about their translational potential as an effective recovery method for brain injuries. Moreover, the use of multiple organoids to address this issue remains uncertain, as it is unclear whether this approach would be sufficient to promote complete recovery or lead to effective functional restoration. Addressing these challenges is essential to move from proof-of-concept experiments towards safe and ethically sound clinical applications that are feasible and accepted in practice.
Conclusion
In conclusion, the challenges behind grafting organoids remain rooted in the youth of this tissue grafting therapy approach. With the development of more complex organoids and assembloids that more closely recapitulate the complexity of an adult brain, there is the potential to improve the extent of anatomical integration within damaged cortical sites. By applying state-of-the-art methods for systems neuroscience, coupled with ecologically-relevant behavioural tasks, more evidence will have to be collected on whether the grafted hosts enable functional, behavioural and cognitive recovery following brain damage. Nevertheless, it remains to be seen whether the rodent brain is more permissive to grafting than the human brain, and thus whether the same results will hold when attempting transplantation into the human brain. Furthermore, the perspective of grafting organoids into patients faces significant ethical issues, that are outside the scope of this review. Nevertheless, efforts to make this possible have already started. One preclinical study reported the quality, efficacy and safety of using human iPSC-derived dopaminergic neurons as a cell replacement therapy method for Parkinson’s disease, which enabled clinical trials to commence [58]. Similar clinical trials for Parkinson’s patients were announced with iPSCs in Japan, and human ESCs in the US and Canada [65, 66]. All in all, from the current standpoint and accumulated evidence, we can conclude that transplanting organoids into cortical tissue offers a promising prospect for anatomical, functional, and behavioural recovery following brain damage. Hopefully, with further successes, this organoid grafting method may one day become part of personalized clinical treatments, offering patients with non-fatal acquired brain damage an improved opportunity for recovery from neurological deficits.
Acknowledgements
This study has been supported by the European Union (EIC Pathfinder project NAP, grant agreement 101099310, to JCS and UO).
Author contributions
UO and JO contributed to the conception and design of the article. JO conducted the literature search, drafted the manuscript, and both JO and UO were involved in its editing. UO and JCS provided valuable suggestions and comments during the review process. All authors participated in the final review and approved the submitted version.
Data availability
Not applicable.
Declarations
Conflicts of interest
JCS is founder and CEO of OrganoTherapeutics.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldman L, Siddiqui EM, Khan A et al (2022) Understanding acquired brain injury: a review. Biomedicines 10:2167. 10.3390/biomedicines10092167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mar J, Arrospide A, Begiristain JM et al (2011) The impact of acquired brain damage in terms of epidemiology, economics and loss in quality of life. BMC Neurol 11:46. 10.1186/1471-2377-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankey GJ, Jamrozik K, Broadhurst RJ et al (2002) Long-term disability after first-ever stroke and related prognostic factors in the perth community stroke study, 1989–1990. Stroke 33:1034–1040. 10.1161/01.STR.0000012515.66889.24 [DOI] [PubMed] [Google Scholar]
- 4.Thurman DJ, Alverson C, Dunn KA et al (1999) Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 14:602–615. 10.1097/00001199-199912000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Rahman M, Abbatematteo J, De Leo EK et al (2017) The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg 127:123–131. 10.3171/2016.7.JNS16396 [DOI] [PubMed] [Google Scholar]
- 6.Greve MW, Zink BJ (2009) Pathophysiology of traumatic brain injury. Mt Sinai J Med J Transl Pers Med 76:97–104. 10.1002/msj.20104 [DOI] [PubMed] [Google Scholar]
- 7.Xiong Y, Mahmood A, Choppbfbf M (2010) Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs Lond Engl 11:298–308 [PMC free article] [PubMed] [Google Scholar]
- 8.Dunnett SB, Nathwani F, Björklund A (2000) Chapter 16 the integration and function of striatal grafts. In: Progress in brain research. Elsevier, pp 345–380 [DOI] [PubMed]
- 9.Gage FH, Fisher LJ (1991) Intracerebral grafting: a tool for the neurobiologist. Neuron 6:1–12. 10.1016/0896-6273(91)90116-H [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 11.Tao Y, Zhang S-C (2016) Neural subtype specification from human pluripotent stem cells. Cell Stem Cell 19:573–586. 10.1016/j.stem.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espuny-Camacho I, Michelsen KA, Gall D et al (2013) Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits In Vivo. Neuron 77:440–456. 10.1016/j.neuron.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 13.Tornero D, Wattananit S, Grønning Madsen M et al (2013) Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 136:3561–3577. 10.1093/brain/awt278 [DOI] [PubMed] [Google Scholar]
- 14.Thompson LH, Björklund A (2015) Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol Dis 79:28–40. 10.1016/j.nbd.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 15.Pașca SP, Arlotta P, Bateup HS et al (2022) A nomenclature consensus for nervous system organoids and assembloids. Nature 609:907–910. 10.1038/s41586-022-05219-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camp JG, Badsha F, Florio M et al (2015) Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci 112:15672–15677. 10.1073/pnas.1520760112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour AA, Gonçalves JT, Bloyd CW et al (2018) An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36:432–441. 10.1038/nbt.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varrault A, Journot L, Bouschet T (2019) Cerebral cortex generated from pluripotent stem cells to model corticogenesis and rebuild cortical circuits: In Vitro Veritas? Stem Cells Dev 28:361–369. 10.1089/scd.2018.0233 [DOI] [PubMed] [Google Scholar]
- 19.Eichmüller OL, Knoblich JA (2022) Human cerebral organoids — a new tool for clinical neurology research. Nat Rev Neurol 18:661–680. 10.1038/s41582-022-00723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer ST, Mansour AA, Schlachetzki JCM et al (2023) An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 186:2111-2126.e20. 10.1016/j.cell.2023.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham MT, Pollock KM, Rose MD et al (2018) Generation of human vascularized brain organoids. NeuroReport 29:588–593. 10.1097/WNR.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Sun L, Wang M et al (2020) Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLOS Biol 18:e3000705. 10.1371/journal.pbio.3000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jgamadze D, Lim JT, Zhang Z et al (2023) Structural and functional integration of human forebrain organoids with the injured adult rat visual system. Cell Stem Cell 30:137-152.e7. 10.1016/j.stem.2023.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitahara T, Sakaguchi H, Morizane A et al (2020) Axonal extensions along corticospinal tracts from transplanted human cerebral organoids. Stem Cell Rep 15:467–481. 10.1016/j.stemcr.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson MN, Thunemann M, Liu X et al (2022) Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex. Nat Commun 13:7945. 10.1038/s41467-022-35536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X-H, Guo D, Chen L-Q et al (2024) Low-intensity ultrasound ameliorates brain organoid integration and rescues microcephaly deficits. Brain 147:3817–3833. 10.1093/brain/awae150 [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Zhang L, Novak SW et al (2025) Morphological diversification and functional maturation of human astrocytes in glia-enriched cortical organoid transplanted in mouse brain. Nat Biotechnol 43:52–62. 10.1038/s41587-024-02157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu N, Shi J-X, Chen C et al (2024) Constructing organoid-brain-computer interfaces for neurofunctional repair after brain injury. Nat Commun 15:9580. 10.1038/s41467-024-53858-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagó JR, Pegna GJ, Okolie O et al (2016) Electrospun nanofibrous scaffolds increase the efficacy of stem cell-mediated therapy of surgically resected glioblastoma. Biomaterials 90:116–125. 10.1016/j.biomaterials.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore KM, Graham-Gurysh EG, Bomba HN et al (2020) Impact of composite scaffold degradation rate on neural stem cell persistence in the glioblastoma surgical resection cavity. Mater Sci Eng C 111:110846. 10.1016/j.msec.2020.110846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheets KT, Ewend MG, Mohiti-Asli M et al (2020) Developing implantable scaffolds to enhance neural stem cell therapy for post-operative glioblastoma. Mol Ther 28:1056–1067. 10.1016/j.ymthe.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daviaud N, Friedel RH, Zou H (2018) Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. Eneuro 5:ENEURO.0219-18.2018. 10.1523/ENEURO.0219-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S-N, Wang Z, Xu T-Y et al (2020) Cerebral organoids repair ischemic stroke brain injury. Transl Stroke Res 11:983–1000. 10.1007/s12975-019-00773-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Wang S, Xu T et al (2020) Cerebral organoids transplantation improves neurological motor function in rat brain injury. CNS Neurosci Ther 26:682–697. 10.1111/cns.13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao Z, Fang K, Miao Z et al (2021) Human cerebral organoid implantation alleviated the neurological deficits of traumatic brain injury in mice. Oxid Med Cell Longev 2021:6338722. 10.1155/2021/6338722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao S-Y, Yang D, Huang Z-Q et al (2023) Cerebral organoids transplantation repairs infarcted cortex and restores impaired function after stroke. Npj Regen Med 8:27. 10.1038/s41536-023-00301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Revah O, Gore F, Kelley KW et al (2022) Maturation and circuit integration of transplanted human cortical organoids. Nature 610:319–326. 10.1038/s41586-022-05277-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Huang F, Zhang H et al (2022) In vivo development and single-cell transcriptome profiling of human brain organoids. Cell Prolif 55:e13201. 10.1111/cpr.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong X, Xu S-B, Chen X et al (2021) Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Mol Psychiatry 26:2964–2976. 10.1038/s41380-020-00910-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhaduri A, Andrews MG, Mancia Leon W et al (2020) Cell stress in cortical organoids impairs molecular subtype specification. Nature 578:142–148. 10.1038/s41586-020-1962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson AL, Bennett NK, Francis NL et al (2016) Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds. Nat Commun 7:10862. 10.1038/ncomms10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linaro D, Vermaercke B, Iwata R et al (2019) Xenotransplanted human cortical neurons reveal species-specific development and functional integration into mouse visual circuits. Neuron 104:972-986.e6. 10.1016/j.neuron.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Real R, Peter M, Trabalza A et al (2018) In vivo modeling of human neuron dynamics and Down syndrome. eaau1810 362:1810. 10.1126/science.aau1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espuny-Camacho I, Michelsen KA, Linaro D et al (2018) Human pluripotent stem-cell-derived cortical neurons integrate functionally into the lesioned adult murine visual cortex in an area-specific way. Cell Rep 23:2732–2743. 10.1016/j.celrep.2018.04.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinbeck JA, Choi SJ, Mrejeru A et al (2015) Optogenetics enables functional analysis of human embryonic stem cell–derived grafts in a Parkinson’s disease model. Nat Biotechnol 33:204–209. 10.1038/nbt.3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grønning Hansen M, Laterza C, Palma-Tortosa S et al (2020) Grafted human pluripotent stem cell-derived cortical neurons integrate into adult human cortical neural circuitry. Stem Cells Transl Med 9:1365–1377. 10.1002/sctm.20-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daadi MM, Klausner JQ, Bajar B et al (2016) Optogenetic stimulation of neural grafts enhances neurotransmission and downregulates the inflammatory response in experimental stroke model. Cell Transplant 25:1371–1380. 10.3727/096368915X688533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palma-Tortosa S, Tornero D, Grønning Hansen M et al (2020) Activity in grafted human iPS cell–derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc Natl Acad Sci 117:9094–9100. 10.1073/pnas.2000690117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tornero D, Tsupykov O, Granmo M, et al (2017) Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain aww347. 10.1093/brain/aww347 [DOI] [PubMed]
- 50.Yamagami K, Samata B, Doi D et al (2024) Progranulin enhances the engraftment of transplanted human iPS cell-derived cerebral neurons. Stem Cells Transl Med 13:1113–1128. 10.1093/stcltm/szae066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Lullo E, Kriegstein AR (2017) The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18:573–584. 10.1038/nrn.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lancaster MA, Renner M, Martin C-A et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caviness VS, Takahashi T (1995) Proliferative events in the cerebral ventricular zone. Brain Dev 17:159–163. 10.1016/0387-7604(95)00029-B [DOI] [PubMed] [Google Scholar]
- 54.Teissier A, Pierani A (2021) Wiring of higher-order cortical areas: Spatiotemporal development of cortical hierarchy. Semin Cell Dev Biol 118:35–49. 10.1016/j.semcdb.2021.05.010 [DOI] [PubMed] [Google Scholar]
- 55.Petersen CCH, Grinvald A, Sakmann B (2003) Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured In Vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci 23:1298–1309. 10.1523/JNEUROSCI.23-04-01298.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldassari S, Musante I, Iacomino M et al (2020) Brain organoids as model systems for genetic neurodevelopmental disorders. Front Cell Dev Biol 8:590119. 10.3389/fcell.2020.590119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelley KW, Revah O, Gore F et al (2024) Host circuit engagement of human cortical organoids transplanted in rodents. Nat Protoc 19:3542–3567. 10.1038/s41596-024-01029-4 [DOI] [PubMed] [Google Scholar]
- 58.Kirkeby A, Nelander J, Hoban DB et al (2023) Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson’s disease, STEM-PD. Cell Stem Cell 30:1299-1314.e9. 10.1016/j.stem.2023.08.014 [DOI] [PubMed] [Google Scholar]
- 59.Chen HI, Wolf JA, Blue R et al (2019) Transplantation of human brain organoids: revisiting the science and ethics of brain chimeras. Cell Stem Cell 25:462–472. 10.1016/j.stem.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farahany NA, Greely HT, Hyman S et al (2018) The ethics of experimenting with human brain tissue. Nature 556:429–432. 10.1038/d41586-018-04813-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kataoka M, Gyngell C, Savulescu J, Sawai T (2023) The ethics of human brain organoid transplantation in animals. Neuroethics 16:27. 10.1007/s12152-023-09532-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harary PM, Blue R, Castellanos M et al (2023) Human brain organoid transplantation: ethical implications of enhancing specific cerebral functions in small-animal models. Mol Psychol Brain Behav Soc 2:14. 10.12688/molpsychol.17544.1 [Google Scholar]
- 63.Barnhart AJ, Dierickx K (2023) A tale of two chimeras: applying the six principles to human brain organoid xenotransplantation. Camb Q Healthc Ethics 32:555–571. 10.1017/S0963180123000051 [DOI] [PubMed] [Google Scholar]
- 64.Pașca SP, Arlotta P, Bateup HS et al (2025) A framework for neural organoids, assembloids and transplantation studies. Nature 639:315–320. 10.1038/s41586-024-08487-6 [DOI] [PubMed] [Google Scholar]
- 65.CiRA (2021) Announcement of physician-initiated clinical trials for Parkinson’s disease|News & Events. In: CiRA Cent. IPS Cell Res. Appl. Kyoto Univ. http://www.cira.kyoto-u.ac.jp/e. Accessed 13 Sep 2024
- 66.BlueRock Therapeutics (2021) BlueRock therapeutics in collaboration with memorial sloan kettering cancer center receives IND clearance for DA01 in Parkinson’s disease. In: BlueRock Ther. https://www.bluerocktx.com/bluerock-therapeutics-in-collaboration-with-memorial-sloan-kettering-cancer-center-receives-ind-clearance-for-da01-in-parkinsons-disease/. Accessed 13 Sep 2024
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.