Abstract
We mapped the elements that mediate termination of transcription downstream of the chicken βH- and βA-globin gene poly(A) sites. We found no unique element and no segment of 3′-flanking DNA to be significantly more effective than any other. When we replaced the native 3′-flanking DNA with bacterial DNA, it too supported transcription termination. Termination in the bacterial DNA depended on a functional poly(A) signal, which apparently compelled termination to occur in the downstream DNA with little regard for its sequence. We also studied premature termination by poorly processive polymerases close to the promoter. The rate of premature termination varied for different DNA sequences. However, the efficiencies of poly(A)-driven termination and promoter-proximal premature termination varied similarly on different DNAs, suggesting that poly(A)-driven termination functions by returning the transcription complex to a form which resembles a prior state of low processivity. The poly(A)-driven termination described here differs dramatically from the poly(A)-assisted termination previously described for the simian virus 40 (SV40) early transcription unit. In the SV40 early transcription unit, essentially no termination occurs downstream of the poly(A) site unless a special termination element is present. The difference between the βH-globin and SV40 modes of termination is governed by sequences in the upstream DNA. For maximum efficiency, the βH-globin poly(A) signal required the assistance of upstream enhancing sequences. Moreover, the SV40 early poly(A) signal also drove termination in βH-globin style when it was placed in a βH-globin sequence context. These studies were facilitated by a rapid, improved method of run-on transcription analysis, based on the use of a vector containing two G-free cassettes.
Our understanding of transcription termination by eukaryotic RNA polymerase II is steadily increasing as more terminators from protein-coding genes are characterized. One theme that has emerged from the characterization of several transcription units is the requirement of a functional poly(A) signal for efficient termination to proceed (11, 32, 36, 66). In many cases, if any part of the poly(A) signal is damaged by mutation, transcription termination is impaired (11, 15, 36, 66). In other cases, however, a correlation between the efficiency of polyadenylation and the efficiency of termination is not apparent (7, 17, 61). One interpretation of poly(A)-dependent termination is that some aspect of the 3′-end processing reaction is involved in potentiating termination by the polymerase. Possibly the 3′-end cleavage event activates a termination factor (11, 51). Alternatively, assembly of the cleavage-polyadenylation complex at the polymerase surface may signal termination (41).
The nature of the actual termination event potentiated by the poly(A) signal is unclear. Logan et al. (36) have suggested that interaction with the poly(A) signal returns the transcriptional apparatus to a prior state of low processivity. Thus altered, the polymerase dissociates stochastically from the template. This model is based on the observation that successful transcription of eukaryotic pre-mRNA-coding genes requires not only the initiation of transcription at the promoter but also the establishment of a processive elongation complex. The commencement of elongation without the establishment of processivity results in premature termination of transcription within the first several hundred base pairs downstream of the promoter (8, 28, 33, 40, 42, 47–49, 52, 53, 67, 69). The model of Logan et al. (36) is consistent with the behavior of transcription units in which transcription terminates heterogeneously, beginning immediately downstream of the poly(A) site (1, 22). However, no candidate Logan-type termination region has yet been definitively characterized.
On the other hand, not explained by the model of Logan et al. (36) are the many instances in which transcription proceeds for great distances past the poly(A) site with little decrease in processivity (12, 27, 39, 44). In the well-characterized case of a chimeric plasmid containing the simian virus 40 (SV40) early transcription unit (10, 11), little termination occurs beyond the poly(A) site unless a special termination element is encountered. However, a functional poly(A) signal is required in order for the downstream termination element to be fully effective (10, 11). Thus, efficient termination in this case is potentiated by a functional poly(A) signal but is realized only upon encountering the additional downstream terminator.
Downstream terminator elements may not be uncommon and apparently take a variety of forms. The one described by Connelly and Manley (10, 12) is the protein binding site, CCAAT, probably bound by CP1. Ashfield et al. (2, 3) have also characterized a protein binding site (for MAZ) that acts as a downstream terminator. Additional downstream terminators that probably act at the level of DNA sequence per se rather than as protein binding sites have previously been described (16, 60). How these elements act is unknown, but their collaboration with poly(A) sites has led to the suggestion that they are pause sites, whose role is to delay the polymerase until polyadenylation has had sufficient time to occur (11, 51). Indeed, independent evidence suggests that one of these elements is in fact a pause site (16).
Although the downstream elements that have been characterized so far depend on functional upstream poly(A) signals for full efficiency, in at least one case, the CCAAT box, the signal is partially effective on its own. Moreover, duplicating the element increases its effectiveness when no poly(A) site is present (12). Thus, as has previously been suggested (14, 55), it is possible that some terminators of processive RNA polymerase II transcription do not require the assistance of a poly(A) signal.
In the present work, we characterized extensively the elements responsible for termination of chicken βH-globin gene transcription. We found that termination of transcription for this gene is best described by the model of Logan et al. (36) and thus differs from that of most genes so far characterized. Termination was poly(A) dependent, and no additional downstream termination element was required. Moreover, termination appeared to commence promptly after the poly(A) site; no significant span of processive transcription intervened between the poly(A) signal and the region of termination. In comparison to the previous work described above, these results suggest that the following two modes of transcription termination exist:poly(A)-driven, terminator-independent termination and poly(A)-assisted, terminator-dependent termination. We therefore carried out a functional comparison of the 3′-flanking regions of the βH-globin and SV40 early transcription units. The comparison confirmed the inferred difference between the two termination modes and showed moreover that the poly(A)-driven mode of the βH-globin gene depends on the presence of an upstream termination enhancer which is not present in the SV40 early region.
MATERIALS AND METHODS
Plasmids pORgf3, pORgf3·2, pORgf7, p<ABCDEF>, p<ABCDE>, pAsv<BsvL>, p<3>1·2, and pHyb.
The 376-bp G-free segment for pORgf3 was obtained as a BamHI-EcoRI fragment from the original G-free construct, pC2AT (56), and inserted into the multiple cloning site of pBluescript SKII+ (Stratagene) at the SmaI site in the G-free orientation with respect to the T7 promoter. The multiple cloning site with embedded G-free cassette was excised by BssHII digestion and inserted into the HindIII site of pOR4 (13). This is just downstream of the SV40 origin so that G-free transcription is driven by the SV40 early promoter (21). For pORgf3·2 (Fig. 1A), the 261-bp G-free segment was obtained as a HindIII-BglII fragment from pRL542 (37) and inserted into the HindIII site of pBluescript SKII+ in the G-free orientation with respect to the T3 promoter. This was excised from the multiple cloning site as a BamHI-SalI fragment and inserted into the BstXI site downstream of the 376-bp cassette of pORgf3. For the 750-bp G-free cassette in pORgf7, a 374-bp G-free SstI-SmaI fragment from pORgf3 was reinserted into the SmaI site of pORgf3 to tandemize the G-free cassette.
FIG. 1.
Vectors for studying transcription termination and representative experiments to illustrate their use. (A) pORgf3·2, the principal transient-expression plasmid used in this work to study transcription termination. “3·2” signifies a 376-nucleotide cassette, followed by a 261-nucleotide cassette. Insertion derivatives of pORgf3·2 are named as follows for insert X in the upstream multiple cloning site and insert Y between the cassettes: pORgf3·2:X<Y>. For brevity, we often abbreviate this as pX<Y>3·2 or simply pX<Y>. (B) Comparison of terminating and nonterminating inserts by G-free cassette analysis. The uncorrected readthrough values shown are the postsequence/presequence (Post/Pre) ratios normalized for C content (175 and 112) in the long and short cassettes, respectively. For reasons we do not yet understand, there is some variation in the postsequence/presequence ratio for any given plasmid from one transfection to another. This source of experimental uncertainty can be substantially reduced by including in each set of transfections a standard reference plasmid against which all transfectants are normalized. The normalization given here was to pORgf3·2. (C) pHyb, a conventional plasmid for studying termination by run-on transcription and hybridization analysis. (D) Comparison of terminating and nonterminating inserts by hybridization analysis. The uncorrected readthrough is calculated for C contents of 107 and 203 for pre- and postsequences, respectively. Normalization was to pHyb<neo> itself.
The βH 3′-flanking region in p<ABCDEF> was reassembled from the inserts in pCBG11 and pCBG13 (63) as follows. First, DNA was assembled in the ClaI-SalI-XhoI-ApaI-KpnI portion of the pBluescript SKII+ multiple cloning site by inserting a Bsp1286I fragment (A) into the SalI site (with regeneration of SalI sites flanking fragment A) and an MboII fragment (BCDE) into the ApaI site to give the intermediate pABCDE. Then the ensemble containing fragments A, B, C, D, and E was transferred as a ClaI-KpnI fragment into the BamHI site of pORgf3·2 to give p<ABCDE>. Finally, an F-containing BstXI-XbaI fragment from p<DEF> (Table 1) (BstXI is in segment D, and XbaI is in the vector) was used to replace the homologous segment of p<ABCDE>, thus generating p<ABCDEF>. This construction route introduces some multiple cloning site nucleotides (including the XhoI site) between segments A and B and also results in the repetition of 35 chicken nucleotides at the A-B junction.
TABLE 1.
Plasmid clones (not described in Materials and Methods) used in this work
| Plasmid | Distance (bp)
|
Construction | |
|---|---|---|---|
| Between cassettes | From poly(A) site to postcassette | ||
| p<> | 96 | Same as pORgf3·2 (see Materials and Methods) | |
| p<neo> | 588 | 495-bp BglII-RsaI fragment from the neomycin resistance gene of pSV2neo (57) inserted into the BamHI site of p<> | |
| p<ABCD> | 2,098 | 1,330 | As for p<ABCDE> except for a ClaI-SstI fragment inserted into p<> |
| p<ABC> | 1,578 | 806 | BamHI segment of p<ABCDE>inserted into the BamHI site of p<> |
| p<AB> | 941 | 173 | As for p<ABCDE> except for a ClaI-AflIII fragment inserted into p<> (with regeneration of BamHI sites) |
| p<ABC1> | 1,275 | 511 | AflIII-RsaI fragment from p<ABC> inserted into the XbaI site of p<AB> |
| p<ABC2> | 1,260 | 492 | RsaI-XbaI fragment from p<ABC> inserted into the XbaI site of p<AB> |
| p<ABDEF> | 1,781 | 1,013 | ABC segment removed from p<ABCDEF> with BamHI and replaced with the AB BamHI fragment from p<AB> |
| p<DEF> | 866 | SalI fragment from pCBG12.8 (50) inserted into the BamHI site of p<> | |
| p<D> | 605 | SalI-SstI fragment from pCBG12.8 (previously inserted into the BamHI site of p<> in the reverse orientation) re-excised with SmaI and reinserted into the BamHI site of p<> in the native orientation | |
| p<BD> | 843 | 699 | KpnI-BglII PCR fragment extending from −124 to +137 on the map (Fig. 2) joined to a BamHI-KpnI PCR fragment extending from 799 to 1262 on the map by means of compatible sticky-end ligation between the BglII and BamHI sites, with the resulting KpnI fragment blunted into the BamHI site of p<> |
| p<ABD> | 1,453 | 685 | As for p<D> except with the SmaI fragment inserted into the XbaI site of p<AB> |
| pAB<DEF> | 866 | 1,399 | ClaI-AflIII segment of pABCDE inserted into the KpnI site of p<DEF> |
| pAB<neo> | 588 | 1,121 | SmaI fragment of pAB<DEF> replaced with the SmaI fragment from p<neo> |
| p<ABneo> | 1,487 | 719 | SmaI fragment from p<neo> inserted into the XbaI site of p<AB> |
| p<A> | 655 | SmaI fragment of p<> replaced with a SalI fragment from pAB<neo> | |
| pAB<> | 46 | 579 | neo fragment removed from pAB<neo> with SmaI |
| pAB<cat> | 601 | 1,134 | SmaI fragment of pAB<neo> replaced with cat coding sequences (the HindIII-NcoI fragment) from pRSVcat (24) |
| p<cat> | 601 | SalI fragments containing segment AB deleted from pAB<cat> | |
| p<ABcat> | 1,500 | 732 | HindIII-NcoI fragment of pRSVcat inserted into the XbaI site of p<AB> |
| pAP<cat> | 601 | 1,059 | XhoI fragment of pAB<cat> replaced by the SV40 early poly(A) signal (indicated by capital letters) contained in the following oligonucleotide and its complement: 5′-cccggttaACAAATAAAGCATTTTTTTCACTGCATTCTAGTTGTGGTTTGTggatccccgt (11) |
| pAPΔ<cat> | 594 | 1,052 | As for pAP<cat>, except with the segment coding for the U-rich element (underlined in pAP<cat> deleted |
| pAPrev<cat> | 601 | As for pAP<cat>, with the oligonucleotide inserted in reverse orientation | |
| pAP<neo> | 588 | 1,047 | As for pAP<cat>, with pAB<neo> as the starting construct |
| pA<cat> | 601 | As for pAP<cat>, with no poly(A) signal insertion | |
| p<lac> | 545 | PvuII fragment containing lac and multiple cloning site sequences from pBluescript SKII+ (Stratagene) inserted into the BamHI site of p<> | |
| pAB<lac> | 492 | 1,027 | SmaI fragment of pAB<neo> replaced with the PvuII fragment from pBluescript SKII+ |
| p<ABlac> | 1,393 | 625 | PvuII fragment from pBluescript SKII+ inserted into the XbaI site of p<AB> |
| pD<C2> | 358 | XbaI fragment containing segment D excised from p<D> and inserted into the upstream XhoI site of p<D> to give pD<D>, with the RsaI-XbaI segment of p<ABC> used to replace the SmaI segment of pD<D> | |
| pDAB<C2> | 358 | 862 | ClaI-AflIII segment of pABCDE inserted into the HindIII site of pD<C2> |
| pD<BC2> | 636 | 506 | BC2XhoI-EcoRI fragment of p<ABC2> used to replace the SmaI segment of pD<D> (see pD<C2>) |
| pDA<C2> | 358 | SalI fragment containing segment A (see Materials and Methods) inserted into the HindIII site of pD<C2> | |
| pDA<BC2> | 636 | 506 | As for pDA<C2> except with insertion into pD<BC2> |
| pD<> | 46 | SmaI fragment deleted from pD<D> (see pD<C2>) | |
| pD<A> | 655 | BamHI-SstI fragment from pABCDE inserted into the ApaI site of p<A> | |
| p(AB)sv<cat> | 601 | 1,078 | SalI fragments of pAB<cat>, containing the βH-globin AB region, replaced by the NcoI-BamHI fragment of pRSVcat, containing a comparable region from SV40 |
| p<WXYZ> | 2,389 | 1,906 | Chicken HindIII-BamHI fragment from pCBG15 (63) inserted into the BamHI site of p<> |
| p<W> | 627 | 144 | ApaI-SstI segment of p<WXYZ> inserted at the BamHI site of p<> |
| p<WX> | 1,220 | 737 | SstI-PvuII segment of p<WXYZ> inserted at the XbaI site of p<W> |
| p<WY> | 1,263 | 780 | ApaLI-BglI segment of p<WXYZ> inserted at the XbaI site of p<W> |
| p<WZ> | 1,272 | 789 | BglI-XbaI segment of p<WXYZ> inserted at the XbaI site of p<W> |
| p<WYZ> | 1,927 | 1,444 | ApaLI-XbaI segment of p<WXYZ> inserted at the XbaI site of p<W> |
| pORgf1·2 | 109 | HindIII-XbaI fragment of pORgf3·2 containing the 376-bp G-free cassette replaced with an SstI-ApaI fragment from pRL542 containing a 130-bp G-free cassette | |
| p<neo>1·2 | 588 | SmaI fragment of pORgf1·2 replaced with the SmaI fragment from p<neo> | |
| pAB<neo>1·2 | 588 | 869 | SmaI fragment from p<AB> inserted at the XhoI site of p<neo>1·2 |
For pAsv<BsvL>, two G-free cassettes (indicated by < and >) were inserted into pRSVcat (24) upstream and downstream of the poly(A) signal. The upstream cassette, inserted at the HpaI site, was 261 bp long and was obtained as an EcoRI-HphI fragment from pORgf3·2. For the downstream cassette, a 130-bp G-free sequence (an ApaI-BamHI fragment from pRL542 embedded in a multiple cloning site) with additional flanking restriction sites (SpeI and BamHI upstream and ClaI, PstI, and NheI downstream) was inserted at the BamHI site. This gave pAsv<Bsv>, for which the distance from the poly(A) site to the downstream cassette is 61 bp. A 400-bp spacer (the 400-bp rung of a size marker ladder [Life Technologies]) was then inserted into the SpeI site, bringing the distance from the poly(A) site to the downstream cassette to 469 bp.
For p<3>1·2, a 380-bp G-free sequence was obtained from pORgf7 by PCR (primers, 5′-CCCGGATCCATCCCCAATCATAAAATTATCTC and 5′-CCCTCTAGAGATTGAAAGAGGGGAGGAAGG), trimmed with BamHI and XbaI, and inserted into BamHI- and XbaI-digested pRSVgf1·2. The latter clone is a derivative of pORgf1·2 (Table 1) in which the SphI-AvrII fragment has been replaced by an NdeI-HindIII fragment from pRSVcat. For technical reasons, a 63-bp XhoI-XbaI fragment from p<ABlac> was then inserted into the BamHI site between the 130- and 380-bp G-free cassettes.
pHyb (Fig. 1C) was constructed by inserting a HindIII-BamHI fragment from pRSVcat into the HindIII site of pOR4. The BalI site in the middle of the HindIII-BamHI fragment was converted into a KpnI site by linker ligation and provided the insertion site for foreign DNA. The presequence, delimited by the HindIII and KpnI sites, and postsequence, delimited by the KpnI and BamHI sites, were inserted into both M13mp18 and M13mp19 in order to generate the necessary single-strand-specific probes for hybridization analysis.
Confirmation of plasmid constructs.
All constructs used in this work were confirmed by restriction mapping with at least three different digests. Selected plasmids were also subjected to further confirmation as follows. The presence of the G-free cassettes in pORgf3·2 (Fig. 1A) and in p<neo> and p<ABCDE> (Table 1) was confirmed by using a T7 promoter situated 5′ of the first G-free cassette to generate G-free transcripts in the presence of T1 RNase. Sequencing was used to confirm the 5′ ligation junction of the upstream cassette in pORgf3·2. To verify that the plasmids in a given preparation constituted a homogeneous population (i.e., contained no admixed deletion variants), plasmids pORgf3·2, p<neo>, p<ABC>, p<ABD>, p<ABCDE>, and p<ABDEF> were cut with restriction enzymes to isolate the two G-free cassettes on separate restriction fragments and then analyzed by gel electrophoresis. The ethidium bromide staining pattern was recorded by digital photography and analyzed with NIH Image (version 1.60) to verify that the cassette-containing fragments within each plasmid were present in equal molar ratio.
Transfection and isolation of nuclei.
For our early work (Fig. 1D), we used DEAE-dextran transfections into COS cells by the method of Kaufman (30), except that there was no chloroquine treatment. For most of the work reported here, we used calcium phosphate transfections by the method of Ausubel et al. (4), except that COS cells were incubated at 37°C under 5% CO2 at all times. The DNA-calcium phosphate mix was incubated at room temperature for exactly 20 min before being added to cells. After 20 to 24 h, cells were rinsed twice with 5 ml of serum-free Dulbecco modified Eagle medium and replated with 10 ml of Dulbecco modified Eagle medium containing 10% fetal bovine serum. For our most recent experiments, we used Lipofectamine (Gibco BRL) for transfection, employing the protocol supplied by the manufacturer. Six micrograms of DNA and 18 μl of Lipofectamine were used on cells that were 50 to 80% confluent in a 60-mm-diameter tissue culture dish. In our experience, Lipofectamine gives the most reliable results because of the improved signal-to-noise ratio that results from the higher transfection efficiency. In all cases, nuclei were isolated 44 to 48 h after transfection by the method of Ausubel et al. (4). During the final step, nuclei were washed with nuclei storage buffer containing 1 mM dithiothreitol and resuspended in a final volume of 40 μl of nuclei storage buffer (except for the hybridization run-on procedure, where the final volume was 100 μl). Nuclei were stored at −70°C for up to several months.
Nuclear run-on transcription and hybridization.
Run-on transcription was performed by the method of Ausubel et al. (4). The final RNA sample was resuspended in hybridization buffer (0.25 M Na2HPO4, 0.25 M NaH2PO4, 1 mM EDTA, 1% bovine serum albumin, 7% sodium dodecyl sulfate [SDS]). M13 single-stranded DNAs containing sequences complementary to the pre- and postsequence transcripts of pHyb were prepared by infecting log-phase JM109 cells with M13 clones. The supernatant of the overnight culture was collected, and the phage were precipitated with 3.2% polyethylene glycol 8000–2.4% NaCl and then resuspended in 10 mM Tris–1 mM EDTA (pH 8) for the isolation of DNA by phenol-chloroform extraction.
The Nytran membrane for blotting was soaked first in deionized water and then in 8× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Together with two pieces of similarly prewet filter paper, the membrane was assembled into the slot blot apparatus. Wells were washed twice with 8× SSC, and then 10 μg of single-stranded DNA in 250 μl of 8× SSC was applied to wells. The blot was washed twice with 250 μl of 8× SSC, and DNA was immobilized on the membrane by UV cross-linking. The blot was prehybridized in hybridization buffer overnight at 65°C, and then the sample was applied to the blot and hybridized overnight at 65°C. The blot was washed twice with 2× SSC–0.1% SDS at 65°C for 15 min and twice with 0.1× SSC–0.1% SDS at 65°C for 15 min. Finally, the blot was wrapped in Saran Wrap on wet Whatman 3MM filter paper for exposure to X-ray film. The resulting image was quantitated by densitometry and captured for publication by using an AGFA Arcus II scanner with FotoLook 95 (version 2.0) software.
G-free nuclear run-on transcription assay.
Forty microliters of nuclei (105 nuclei/μl) was mixed with 40 μl of 2× transcription buffer to give final concentrations of 5 mM Tris-Cl (pH 8), 2.5 mM MgCl2, 300 mM NH4SO4, 0.5 mM ATP, 0.5 mM UTP, 2.5 mM dithiothreitol, 1,000 U of T1 RNase, 32 U of RNase inhibitor, and 30 μCi of [α-32P]CTP and incubated at 37°C for 45 min. The reaction was then brought up to 2.5 mM CTP (nonradioactive) and 0.025 mM GTP for a 12-min cold chase. The chase is necessary to maximize the yield of cassette transcripts. Apparently, the concentration of CTP (nanomolar levels) at the labeling step is insufficient to permit quantitative elongation to the ends of the cassettes within an experimentally reasonable length of time. The cold chase presumably allows all cassette transcripts to reach full length for accurate quantitation by gel electrophoresis.
The reaction was stopped by the addition of 150 μl of 0.5 M NaCl–50 mM MgCl2–2 mM CaCl2–10 mM Tris-Cl (pH 7.4) containing 30 U of RNase-free DNase I. RNA was purified by using TRIzol (Gibco/BRL) according to the instructions on the product information sheet. The final sample was resuspended in 35 μl of 7 M urea and was heated to 70°C before being loaded onto a 7 M urea–6% polyacrylamide gel. The gel was run at 530 V for 5 to 6 h, dried, wrapped, and exposed to a phosphor screen overnight. G-free bands were quantitated with a PhosphorImager (Molecular Dynamics). The gel image was scanned with a resolution of 176 μm per pixel and analyzed by using ImageQuant software under the Peak Finder mode. The entire width of the lane was used for quantitation. The baseline and peak assignments were determined automatically with the following parameters: noise, 0.5; sensitivity, 4; kernel, 10 (with additional minor manual adjustments). All data presented in this report are averages of different G-free analyses (usually three or four) from separate transfections.
RESULTS
G-free cassette assay for transcription termination.
The method used is based on transfection, followed by run-on transcription with a plasmid such as pORgf3·2 (Fig. 1A). This plasmid contains two G-free cassettes (56) of different lengths arranged in tandem and separated by a multiple cloning site into which foreign DNA can be inserted. When they are transcribed in the G-free orientation, G-free cassettes give rise to transcripts devoid of G residues and immune to digestion by the G-specific nuclease, T1. After transfection, any transcription termination that occurs between the two cassettes in vivo leads to a lower polymerase density in the downstream member of the duo. The relative polymerase densities in the two cassettes can be determined by isolating nuclei, carrying out run-on transcription in the presence of T1 RNase, running the surviving G-free transcripts from the two cassettes on a gel, quantitating the densities of the two G-free bands, and establishing their ratio. A similar rationale has previously been applied to the study of premature termination in vitro in nuclear extracts (33, 37).
To illustrate the use of pORgf3·2 in measuring transcription termination, the results of a typical experiment are shown in Fig. 1B. The ABCDE region of the chicken βH-globin gene transcription unit (Fig. 2) encompasses the 3′ end of the gene itself and flanking sequences (CDE) in which transcription termination is known to occur in vivo (50). The ABCDE region was inserted between the two cassettes of pORgf3·2 and transfected into COS cells in parallel with a control plasmid containing a prokaryotic DNA insert (neo, a segment from the neomycin resistance gene). By our convention, we refer to these plasmids as p<ABCDE> and p<neo>, where < and > denote the pre- and postcassettes respectively. We call the two cassettes and the region between them collectively the cassette window. Figure 1B, lane 1, shows that with neo in the cassette window, there was little effect on the abilities of polymerases to read through from one cassette into the next (“uncorrected readthrough”). Moreover, when the p<neo> results were normalized to the results (not shown) of a parallel transfection with pORgf3 · 2 lacking any insert, there was no termination that is attributable to the prokaryotic DNA at all (normalized readthrough) (Fig. 1B). In contrast, Fig. 1B, lane 2, shows that with the ABCDE region of the βH-globin gene in the cassette window, virtually none of the polymerases that transited the first cassette succeeded in reaching the second. These results confirm both the validity of the assay and the functionality of the βH-globin gene terminator in the pORgf3·2 background.
FIG. 2.
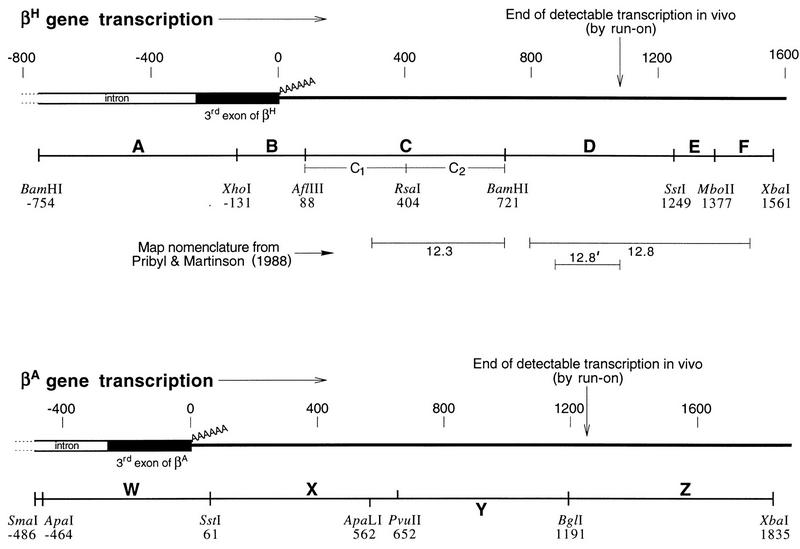
Maps of the βH- and βA-globin gene transcription termination regions drawn to scale and calibrated in base pairs. The run-on transcription endpoints indicated are those of Pribyl and Martinson (50) for βH-globin and of Villeponteau et al. (63) for βA-globin. The βH-globin map is of the DNA insert in clone p<ABCDEF>. Relevant restriction sites are shown. All are native chicken sites, except for the flanking BamHI and XbaI sites and the internal XhoI site. The βA-globin map is of the DNA insert in p<WXYZ>. Restriction sites shown are native chicken sites, except for the flanking SmaI and XbaI sites. The lettered segments in subclones used in this work (e.g., in Fig. 5 through 7) correspond to the lettered segments shown below the corresponding map, although for some constructs (where described) we used segments of slightly differing lengths. The exact cloning strategies for all clones are described in Materials and Methods and Table 1.
We emphasize that nuclear run-on transcription was not used here to study the process of transcription termination within the isolated nuclei. It is a nascent transcript pulse-labeling procedure used for locating and counting polymerases (31, 46). When nuclei are isolated, the elongating polymerases stall for lack of nucleotides, remaining at the positions they occupied in vivo just prior to nuclear isolation. When nuclei are pulsed with [α-32P]-labeled nucleotides, each polymerase resumes elongation for a short distance, generating a radioactively labeled transcript tag that signifies its location. Under the high-salt run-on conditions used here, all polymerases are believed to resume elongation in vitro, regardless of whether they were elongating or pausing in vivo, and the results obtained agree with those of other methods for locating and counting polymerases (31, 46).
In conventional run-on procedures, the labeling step is followed by hybridization of the in vitro-labeled RNA to various probes that have been blotted on membranes. The results of an experiment illustrating this approach are shown in Fig. 1D. The experiment was exactly parallel to that of Fig. 1B except that the DNA segments ABCDE and neo were inserted into pHyb (Fig. 1C) and run-on transcripts were quantitated by slot blot hybridization rather than by G-free analysis. Figure 1D shows that there was strong hybridization of the run-on RNA to the postsequence probe for the neo insert but poor hybridization to the postsequence probe for the ABCDE insert. These results confirm that the ABCDE region is a strong terminator of transcription, which is consistent with the G-free analysis (Fig. 1B).
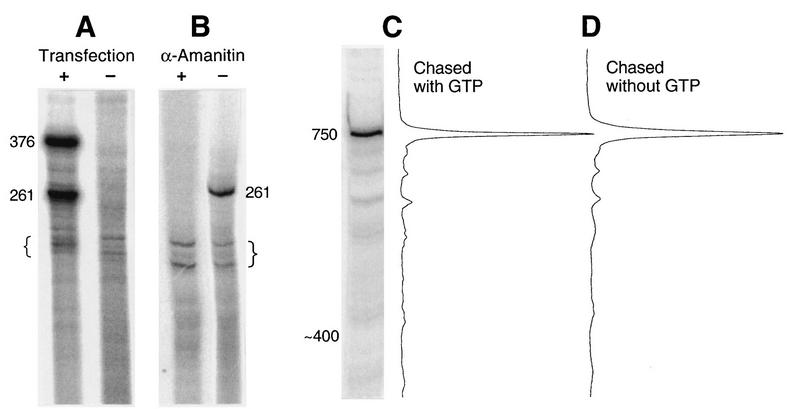
It is evident from Fig. 1B that gel lanes from the G-free procedure exhibited some background. Concerned that the background may have been due to nonspecific degradation of G-free transcripts, we checked several parameters. First, we found that the background was of cellular, not plasmid, origin because it was present whether cells had been transfected with a plasmid or not (Fig. 3A). Second, the transcription of G-free RNA is α-amanitin sensitive, whereas the transcription of background RNA is α-amanitin and 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole-resistant (Fig. 3B and unpublished observations). Therefore, G-free transcription is due to RNA polymerase II, whereas background transcription is not. Finally, in contrast to G-free RNA, the efficiency of labeling of background RNA did not depend on a cold-CTP chase (data not shown), suggesting that the background does not arise from long tracts of genomic G-free sequence. Nevertheless, the background pattern was fairly consistent, notably the cluster of bands indicated by brackets in Fig. 3A and B. The T1 resistance of background RNA may reflect special structural characteristics that protect this RNA from the T1 nuclease in our procedure.
FIG. 3.
(A) The T1-resistant background is of cellular origin. Cells were either transfected with pORgf3·2 (+) or left untransfected (−) and then processed for G-free analysis in the usual way. (B) G-free transcription is α-amanitin sensitive, but the cellular background is α-amanitin resistant. Cells were transfected with pAsv<Bsv>, and the subsequent nuclear preparation was divided in two. Each part was then subjected to run-on transcription in the presence (+) or absence (−) of α-amanitin at 0.12 μg/ml. The region of the gel exhibiting the 261-nucleotide cassette transcript is shown. pAsv<Bsv> does not contain a 376-bp cassette. (C) Survival of 750-nucleotide G-free transcripts in the standard G-free run-on procedure. Both the image of the gel lane and its associated scan are shown. The transfecting plasmid was pORgf7. (D) Effect of omitting GTP from the cold chase in the standard G-free run-on procedure. An aliquot of nuclei from the same nuclear preparation as that used for panel B was subjected to run-on transcription in parallel with panel B, except that GTP was omitted from the cold chase. Braces indicate a cluster of background bands referred to in the text.
Although the controls described above made clear that the T1-resistant background was not indicative of wholesale degradation of G-free transcripts, a small amount of differential degradation of the two G-free cassette transcripts due to their difference in target size was not excluded. To evaluate this possibility, we carried out the G-free assay with pORgf7, which contains a single 750-bp cassette. A comparison of the cassette signal to the background, which is of cellular origin, provides an estimate of the efficiency of transfection and of cassette RNA recovery. The gel lane in Fig. 3C and its associated scan reveal a strong cassette signal relative to background, indicating that the 750-nucleotide cassette transcript is not very susceptible, if at all, to loss despite its size. It is therefore unlikely that size-dependent preferential degradation of the smaller (376- and 261-nucleotide) cassette transcripts occurs to any significant extent. Nevertheless, to remove the possibility of this or any other type of systematic error in our analysis, we routinely normalized all data to an appropriate reference plasmid transfected in parallel with the experimental ones (see the legend to Fig. 1B).
For the work reported here, our routine procedure was to include GTP along with CTP in the cold chase as a precaution for cassettes that may harbor multiple polymerases. Since a polymerase cannot exit a cassette in the absence of GTP, multiple polymerases give rise in the absence of GTP to a polymerase stack in which only the lead polymerase bears a full-length cassette transcript (59). By chasing in the presence of GTP, stalled polymerases would be allowed to resume elongation and each cassette transcript would reach full length. The excess cold CTP present in the chase prevents the labeling of non-G-free transcripts during this step. However, more recently, we have found that GTP is actually dispensable. Identical results were obtained with and without GTP in the chase (Fig. 3C and D and data not shown). Since the results shown in Fig. 3C and D were obtained with a 750-bp cassette, they indicate that most of the elongating polymerases on these plasmids are spaced more than 750 bp apart.
Among the practical advantages of G-free analysis over hybridization is, of course, the elimination of hybridization, washing, and preparation of hybridization probes and blots. In addition, G-free analysis yields a better signal than does conventional hybridization analysis because all of the RNA for each cassette appears as a single band on a gel, whereas in hybridization analysis, the signal is limited by the efficiency of hybridization. These factors combine to reduce turnaround time for G-free analysis to a fraction of that required for conventional methods.
To determine whether the G-free procedure yields results that are consistent with those of the hybridization method of analysis, we made a direct comparison of the two. As shown in Fig. 4, both methods yield comparable results for a variety of DNA inserts which vary in termination effectiveness. Therefore, due to the practical and theoretical advantages (see Discussion) of the G-free procedure, including the possibility of more meaningful quantitation of the results, we chose to carry out all subsequent work by the new method.
FIG. 4.
Comparison of the G-free and hybridization methods of transcription termination analysis. Readthrough values were normalized to that of p<neo>. Error bars show standard deviations, and the number of independent measurements taken for each point is given below.
Deletion analysis of the βH-globin gene transcription termination region.
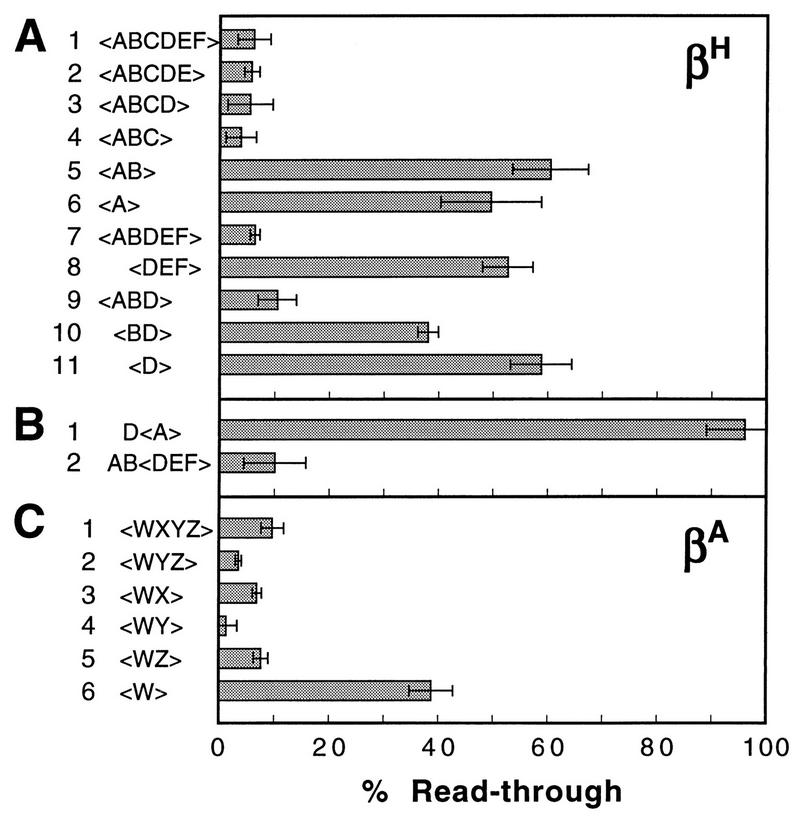
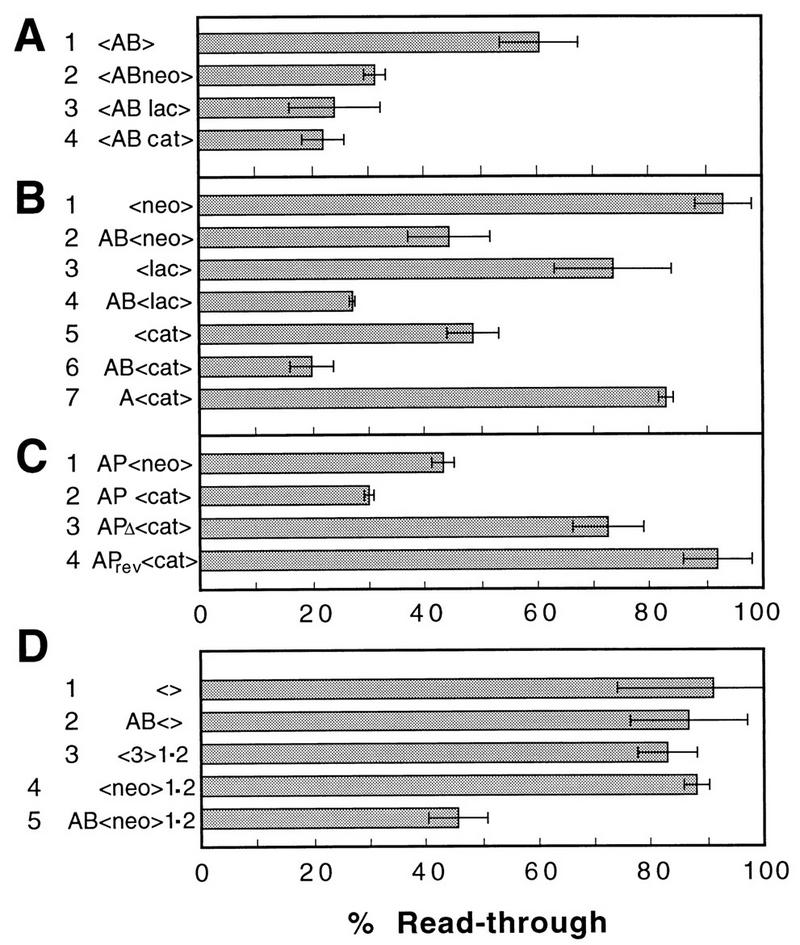
The ABCDEF region of the βH-globin transcription unit (Fig. 2) encompasses the 3′ end of the gene itself (AB) and the termination region (CDEF). In order to locate the elements required for transcription termination, we carried out a deletion analysis by progressively removing DNA from the 3′ end of the region and then assaying for termination efficiency in pORgf3·2. Figure 5A shows that the complete region, ABCDEF (lane 1), acted as an effective terminator when it was placed in the cassette window of pORgf3·2. Sequential removal of segments F, E, and D had no effect on termination efficiency (Fig. 5A, lanes 2 through 4). However, the removal of segment C drastically reduced termination efficiency (Fig. 5A, lanes 5 and 6). Apparently half of the overall termination in ABCDEF occurs within segment C.
FIG. 5.
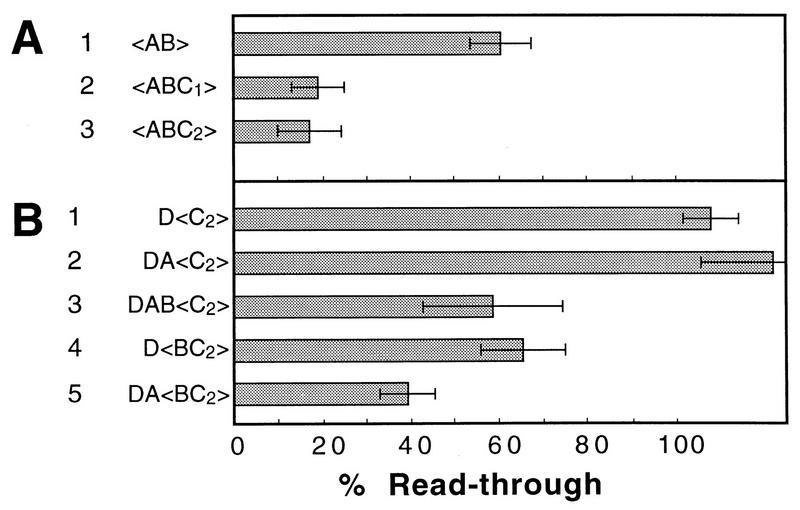
Dissection of the βH- and βA-globin transcription termination regions. The percent readthrough was determined by the G-free run-on procedure, and values were normalized to that of pD<>. (A) Deletion analysis of the βH-globin region. (B) Modular rearrangements of the βH-globin region. (C) Deletion analysis of βA-globin. Error bars show standard deviations.
To determine whether segment C contains a special transcription terminator, we deleted segment C alone from the region to give ABDEF. Termination remained efficient (Fig. 5A, lane 7). Therefore, segment C is not uniquely required for efficient termination, indicating that there is functional redundancy in the βH-globin termination region as previously reported for the murine βmaj-globin gene (60). Further deletion analysis showed that AB was required for efficient termination (Fig. 5A, lane 8) but that EF was not (lane 9). Thus, either segment C or D can support efficient termination (lanes 4 and 9) but not in the absence of AB (lane 8). Moreover, both segments A and B are required for efficient termination; segment B alone is only partially effective in potentiating termination in segment D (Fig. 5A, lanes 9 through 11).
Thus, the complete terminator is apparently tripartite. Elements in both segments A and B were necessary for maximum efficiency (Fig. 5A, lanes 9 through 11), and downstream DNA was required (lanes 4 and 9 versus lane 5). The element in segment B is presumably the poly(A) signal, whereas that in segment A is an unidentified termination enhancer. The enhancer could be the 3′ splice site contained in segment A (Fig. 2), which would be expected to enhance termination by increasing the efficiency of polyadenylation (26, 35, 45, 64), or it could be a relative of another recently described enhancer of termination (17). An investigation into the nature of each of these three elements is described in more detail below.
None of the constructs (Fig. 5A) allowed complete readthrough. This is what would be expected of a typical RNA polymerase II transcription unit, where the promoter dispatches both highly processive and poorly processive polymerases to the template downstream (see the introduction). Thus, about half of the polymerases entering the cassette window (located only about 165 bp downstream of the promoter in pORgf3·2) were apparently of the poorly processive type, failing to traverse the DNA insert even when it was taken from the body of the βH-globin gene (i.e., segment A) (Fig. 5A, lane 6).
If the failure to exceed 50 to 60% readthrough (Fig. 5A) reflects premature termination by a separate class of polymerases, then eliminating that class of polymerases before they enter the cassette region should allow the remaining polymerases to register complete readthrough. We used segments A and D to test this prediction. Neither segment A nor D contains a poly(A) site, yet neither allowed more than 50 to 60% readthrough (Fig. 5A, lanes 6 and 11). We then modified construct p<A> by inserting segment D between the promoter and the cassette window. As expected, those polymerases that did traverse D were also capable of traversing A so that nearly 100% readthrough was recorded (Fig. 5B, lane 1). We regularly obtained this type of result with various modular rearrangements of DNA segments in our system (e.g., Fig. 6B; compare lane 7 with lane 5); therefore, we conclude that this reflects the presence of about 50% poorly processive polymerases in the promoter-proximal regions of our constructs.
FIG. 6.
Transcription termination by RNA polymerase II in prokaryotic DNA. (A) Standard constructs with the βH-globin poly(A) site. (B) Split constructs with the βH-globin poly(A) site. (C) Split constructs with the SV40 poly(A) site. For panels A through C, normalization was to pD<>. (D) Various arrangements of G-free sequences. The plasmids in lanes 1 and 2 were pORgf3·2 itself (also called p<>) and its AB derivative, respectively. The plasmids in lanes 3 through 5 were derivatives of pORgf1·2. For this panel, each plasmid was first normalized to its homologous parent transfected in parallel and then this value was multiplied by the readthrough ratio (p<>/pD<>). Thus, the value for lane 2 was normalized to that of pORgf3·2 and the value for lane 1 was normalized to itself. The values for lanes 3 through 5 were normalized to that for pORgf1·2. The readthrough value for pD<> was assumed to reflect complete readthrough of both cassettes. Multiplying by the p<>/pD<> ratio allowed direct comparison to all of the other data in this report. Error bars show standard deviations.
Although the AB segment was required to potentiate efficient termination (Fig. 5A; compare lanes 7 and 8), when it was placed by itself in the cassette window, it appeared to be no more effective than was segment A alone (Fig. 5A, lanes 5 and 6). The poly(A) site in segment AB lies very close to the end of that DNA segment. Presumably with segment AB alone between the cassettes there is insufficient DNA downstream of the poly(A) site to capture the termination process within the cassette window. We therefore made construct pAB<DEF> to see whether the presence of segment AB outside the window would lead to termination downstream within the window, in the DEF segment of DNA. This expectation was confirmed. The placement of segment AB upstream of the cassette window in pAB<DEF> potentiated termination downstream in DEF (Fig. 5B, lane 2). Note that termination in the <DEF> cassette window was potentiated simply by adding segment AB upstream of the cassette window in p<DEF>, without any modification to the <DEF> window region itself. We refer to constructs such as pD<A> and pAB<DEF> as split constructs.
βA-Globin gene transcription termination region.
The properties of the βA-globin gene transcription termination region resemble those of the βH-globin gene. Figure 5C shows the results of a deletion analysis of the WXYZ region (Fig. 2) of the βA-globin gene. Any combination of segments X, Y, and Z can be deleted with no impairment of termination, provided that at least one such segment remains. However, deletion of the complete XYZ region, leaving only segment W, which contains the poly(A) signal, substantially impairs termination. Thus, as for the βH-globin gene, there is redundancy of termination potential in the 3′-flanking region of the βA-globin gene.
Prokaryotic DNA can support poly(A)-dependent termination.
Since poly(A)-dependent termination appears to occur somewhat promiscuously in the 3′-flanking DNA of the βH- and βA-globin genes, we wondered whether prokaryotic DNA would also support termination. Figure 6A shows that three arbitrarily selected segments of prokaryotic DNA supported substantial amounts of transcription termination when they were appended to the βH-globin AB segment, which contains the poly(A) site. To confirm that transcription termination indeed is potentiated in prokaryotic DNA and does not occur within segment AB itself, we removed segment AB from the cassette window and placed it upstream, yielding split constructs (Fig. 6B). Figure 6B, lane 1, shows that neo DNA alone was relatively transparent to the passage of polymerases. Even poorly processive polymerases got through for the most part. However, when segment AB was placed upstream of the cassette window, neo became a respectable terminator (Fig. 6B, lane 2). Note that the 44% termination registered by pAB<neo> did not include any poorly processive polymerases being dislodged, since most of these were removed by the upstream A segment and never reached the cassettes (see above). Thus, the even-numbered lanes in Fig. 6B record the actual percentages of processive polymerases that succumbed to poly(A)-dependent termination. Accordingly, it can be seen that all three examples of prokaryotic DNA supported termination potentiated by the AB segment upstream. Figure 6B, lane 7, shows that the presence of the B segment was required for termination to be potentiated. Since the poorly processive polymerases are removed by the A region, most of the polymerases that survive region A also read through cat. Hence, pA<cat> registered more readthrough (Fig. 6B, lane 7) than did p<cat>, its parent (lane 5).
We wanted to be sure that genuine poly(A)-dependent termination occurred in prokaryotic DNA and that it was not an effect peculiar to the βH-globin B fragment. Therefore, we replaced fragment B in some of the constructs described above with a synthetic DNA fragment containing a 43-bp sequence (P) taken from the core poly(A) signal of the SV40 early transcription unit (11). Lanes 1 and 2 of Fig. 6C show that the core poly(A) signal from SV40 supported essentially the same level of termination in prokaryotic DNA as did the B fragment of the βH-globin gene. To further evaluate the poly(A) dependence of termination in prokaryotic DNA, we mutated the SV40 poly(A) signal by deleting its downstream U-rich element. This mutation substantially weakens the poly(A) signal, seriously impairing its ability to direct 3′-end processing and to potentiate termination in its native context (11). Fig. 6C, lane 3, shows that this weakened poly(A) signal (PΔ) was also seriously impaired in its ability to direct termination in prokaryotic DNA. Finally, Fig. 6C, lane 4, shows (as expected) that when the poly(A) signal was inactivated by inversion, essentially no termination occurred. These results firmly establish that prokaryotic DNA supports genuine poly(A)-dependent termination in our system. This contrasts with the report of Tantravahi et al. (60), who observed no termination in prokaryotic (cat) DNA when it was placed downstream of the mouse βmaj-globin poly(A) region.
Postprocessive trans-poly(A) polymerases resemble preprocessive polymerases.
One model for poly(A)-dependent termination, originally proposed by Logan et al. (36), is that the elongation complex is returned to a state that resembles its original preprocessive form. Since DNA sequences vary in how permissive they are to elongation, a prediction of this model is that promoter-proximal preprocessive polymerases resemble trans-poly(A) postprocessive polymerases in their ability to negotiate different DNA sequences. [We refer to polymerases that have crossed the poly(A) site as trans-poly(A) polymerases.] The results in Fig. 6B confirm this prediction. Figure 6B, lanes 1, 3, and 5, show that the prokaryotic DNA segments neo, lac, and cat decreased in their permissivity to elongation of promoter-proximal polymerases in that order. Similarly, lanes 2, 4, and 6 of Fig. 6B show that the permissivities to elongation of trans-poly(A) polymerases decreased in the same order.
We refer to permissive sequences like neo as being smooth and to poorly permissive ones like cat as being bumpy. This emphasizes that the sequence features responsible for these differences in permissivity are likely to be mundane, multiple, and minor. It is unlikely, for example, that the cat DNA segment, taken from the coding region of a prokaryotic gene, would contain any sequence elements of special significance to transcription termination in a eukaryotic system. Therefore, the similar responses to bumpiness of both promoter-proximal and trans-poly(A) polymerases suggest that the latter are converted to a state that is capable of terminating in response to rather general features of DNA sequence.
Termination is negligible within G-free cassette sequences.
Since prokaryotic DNA dislodges poorly processive polymerases and supports poly(A)-dependent termination, we decided to evaluate polymerase processivity and termination within the artificial G-free cassette sequences themselves (Fig. 3C and D and 6D). Cassette-mediated termination could conceivably occur both in vivo, thereby decreasing the magnitude of the cassette signal, and in vitro (during the run-on procedure), thereby decreasing the clarity of the cassette signal by smearing the band to lower molecular weights. Figure 3C and D show that there was no detectable termination during the run-on procedure in vitro since even a 750-bp-long segment of G-free DNA yielded a clear, sharp, discrete band.
Figure 6D, lanes 1 through 3, shows that cassette-mediated termination in vivo also was minimal. The construct in Fig. 6D, lane 1, pORgf3·2, provided information on the amount of termination by poorly processive polymerases, whereas the construct in lane 2 provided an estimate of the amount of poly(A)-dependent termination by processive polymerases. In both cases, the effect was only about 10%. Specifically, this means that there is only about 10% excess cassette-mediated termination in the postcassette in vivo. (Excess cassette-mediated termination in the precassette in vivo is invisible in this procedure except as a general decrease in overall signal.) To obtain an additional estimate of the amount of termination that actually occurs within G-free cassette DNA in vivo, we constructed p<3>1·2 (Fig. 6D, lane 3). This construct contains 380 bp of G-free cassette DNA (imbedded in 166 bp of multiple cloning site DNA) as the insert in its cassette window. The construct was also made with a shortened precassette (130 bp), thus moving the insert closer to the promoter to maximize exposure to poorly processive polymerases. Figure 6D, lane 3, shows that inserted G-free cassette DNA and increased proximity to the promoter resulted in only a small additional increment in termination. Thus, G-free cassette DNA is relatively permissive to the passage of polymerases.
Cassette-mediated termination in the postcassette introduces, at worst, a correctable systematic overestimate of termination, which is significant only for inserts that are poor terminators. Because the results discussed above show that the effect is small, we did not attempt to correct for this effect in these studies.
In addition to the controls described immediately above relevant to the postcassette, we also wished to test directly our premise that variations in termination within the precassette are invisible in this procedure (see above). We therefore measured termination across neo of poorly processive (<neo>) and processive but poly(A)-potentiated (AB<neo>) polymerases by using the shortened (130-bp) cassette in the pre- sequence position. The results (Fig. 6D, lanes 4 and 5) were virtually identical to those previously obtained with the 376-bp precassette (Fig. 6B, lanes 1 and 2). Taken together, these data show that the G-free cassette procedure for measuring termination is quantitatively reliable.
Termination commences promptly after the poly(A) site.
It has previously been suggested that after the poly(A) site of the mouse βmaj-globin gene, there is an obligatory spacing requirement of about 500 bp before termination can begin (60). Indeed, in many transcription units, the polymerase may traverse several kilobases downstream of the poly(A) site with little or no termination at all (10, 44). Since in some of our constructs termination appeared to occur efficiently with distances between the poly(A) site and the downstream cassette that were close to the minimum mentioned above (Fig. 5A, lane 9; Fig. 6A, lane 3) (see Table 1 for distances), we decided to address this issue. For this purpose, we prepared p<ABC1> and p<ABC2>, which contain the upstream and downstream halves, respectively, of the βH-globin 3′-flanking segment C. In both of these constructs, the distance between the poly(A) site and the downstream cassette is 500 bp (Table 1). Figure 7A, lanes 2 and 3, shows that both constructs exhibited efficient termination, suggesting that any spacing requirement is either small or absent. Moreover, both constructs exhibited similar levels of termination, reinforcing the view that there is no specific DNA sequence requirement for termination in this system.
FIG. 7.
Termination and enhanced termination promptly after the poly(A) site. (A) Termination downstream of the poly(A) site. (B) Termination enhancer in the βH-globin region. Normalization was to pD<>. Error bars show standard deviations.
To confirm that termination in the C subfragment constructs (Fig. 7A) is poly(A) dependent, we prepared split constructs (Fig. 7B, lanes 1 through 3) in which C2 alone was placed in the cassette window. Segment D was placed in front of the window in each of these constructs to remove poorly processive polymerases. Figure 7B, lane 1, shows that, as expected, the processive polymerases in pD<C2> read through subfragment C2 without terminating. The same was true for pDA<C2> (Fig. 7B, lane 2). However, when segment B, which contains the poly(A) site, was placed upstream of the cassette window to give pDAB<C2> (Fig. 7B, lane 3), about half of the processive polymerases terminated between the cassettes. This result was comparable to that for p<ABC2>, in which about two-thirds of the polymerases leaving B terminated while traversing C2 (Fig. 7A; compare lanes 1 and 3).
Unfortunately, since the βH-globin 3′-flanking region lacks a discrete terminator, we cannot check directly for a minimum spacing requirement simply by moving a terminator element progressively closer to the poly(A) site. Moreover, since a specific terminator element is absent, termination occurs gradually over several hundred base pairs of DNA (44a). Therefore, in addition to any spacing after the poly(A) site that may be required before the termination process can be initiated (60), a sufficient length of DNA must be present prior to the downstream cassette in order to capture a sufficient number of termination events within the cassette window for reliable measurement. Within these limitations, we pursued further the question of the spacing requirement in the following three ways. First, we carried out a variation of the direct test (Fig. 7A) by inserting B just upstream of C2 in pD<C2> to give pD<BC2>. A comparison of lane 4 to lane 1 of Fig. 7B shows that despite a distance of only about 500 bp between the poly(A) site and the downstream cassette, significant termination occurred. Second, noting that segment A enhanced poly(A)-dependent termination (Fig. 5A; compare lanes 9 and 10) but did not itself potentiate any termination (Fig. 6B, lane 7, and C, lane 4), we placed segment A in front of the cassette window of pD<BC2> to give pDA<BC2>. A comparison of lanes 4 and 5 in Fig. 7B shows that the poly(A)-dependent termination in these constructs was enhanced, once again despite a distance of only 500 bp. For our third test, we reasoned that if there is a spacing requirement for poly(A)-dependent termination in subfragment C2, then moving the poly(A) site next to subfragment C2 from farther upstream should reduce termination efficiency. To test this prediction, we compared termination across subfragment C2 in pDAB<C2> (Fig. 7B, lane 3) with that in pDA<BC2> (lane 5), in which the poly(A) site is closer to subfragment C2 by more than 350 bp. It is clear that moving the poly(A) site closer to subfragment C2 had no detrimental effect on termination and, if anything, may have caused termination efficiency to increase. Based on a total of four independent comparisons, we therefore conclude that any spacing requirement for poly(A)-dependent termination in the chicken βH-globin gene is either small or absent.
SV40 early transcription unit lacks a termination enhancer and fails to induce termination in downstream DNA.
The results described so far reveal the βH-globin system to be dramatically different from most of the other poly(A)-dependent terminators that have been characterized. For example, poly(A)-dependent termination after the SV40 early transcription unit does not commence immediately downstream of the poly(A) site. Rather, transcription continues with scarcely any decrease until a special termination element is encountered (10). Thus, both βH-globin transcription termination and SV40 early transcription termination are poly(A) dependent, but SV40 early termination does not occur without an additional termination signal in the downstream DNA. In contrast, βH-globin termination begins soon after the poly(A) site and requires no additional downstream signal.
Among the features of the βH-globin and SV40 early transcription units which may contribute to the contrasting modes of termination are the following: (i) the polyadenylation signal, (ii) the 3′-terminal exon sizes (the size of the final βH-globin exon [Fig. 2] is less than half the average for terminal exons [6], whereas the size of the last SV40 early exon is more than three times the average [9], and (iii) the promoters (transcription of the βH-globin constructs in our experimental system is driven [ironically] by the SV40 early promoter, whereas transcription of the SV40 early region in the constructs of Connelly and Manley [10] is driven by the adenovirus late promoter). We show below that none of these differences in the structure of the two transcription units is responsible for their differences in termination mechanism.
We can already rule out the poly(A) signals themselves as being responsible for the terminator-dependent (i.e., SV40) versus terminator-independent (i.e., βH-globin) mechanisms. Figure 6C shows that in the presence of the A fragment from βH-globin, the SV40 early poly(A) signal potentiated termination in prokaryotic DNA without the need of any additional downstream information. Therefore, the SV40 early poly(A) signal can potentiate terminator-independent transcription termination in the appropriate sequence background.
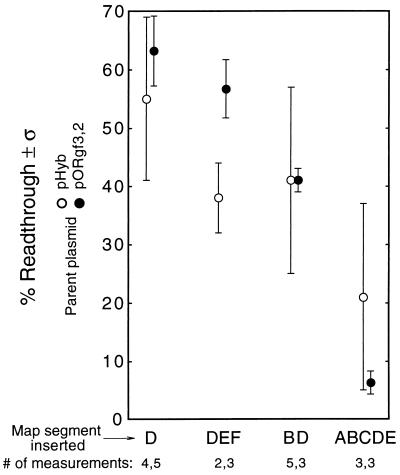
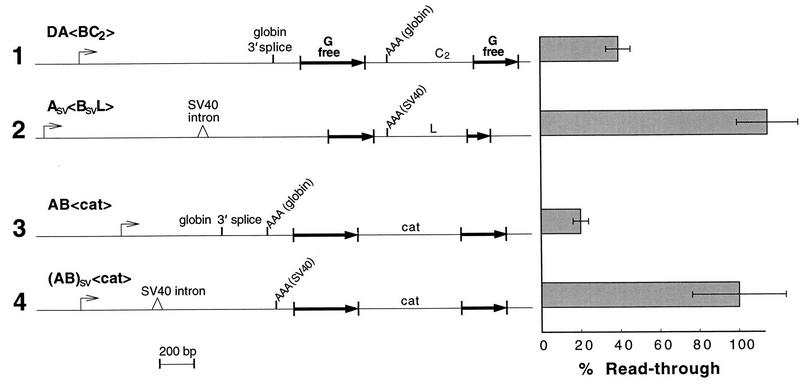
To assess the possible role of exon size in directing termination, we took advantage of pRSVcat (24). This expression vector is based on a derivative of the SV40 early transcription unit in which the size of the terminal exon has been reduced by more than 1.3 kb (25). We inserted a G-free cassette upstream of the SV40 early poly(A) site of pRSVcat, and downstream we inserted some spacer DNA plus another G-free cassette. These insertions were well away from the sequences required for full polyadenylation activity (11). The resulting plasmid was pAsv<BsvL> (Fig. 8, lane 2), a construct analogous to pDA<BC2> (Fig. 7B, lane 5, and 8, lane 1). Figure 8, lane 2, shows that pAsv<BsvL> exhibited complete readthrough, in contrast to pDA<BC2>, which exhibited substantial termination (Fig. 8, lane 1).
FIG. 8.
Chicken βH-globin and SV40 early transcription units demonstrate two modes of transcription termination. Lanes 1 and 3 are identical to lane 5 of Fig. 7B and lane 6 of Fig. 6B, respectively. Normalization for lane 2 was to pAsv<Bsv>. All other lanes were normalized to pD<>. Error bars show standard deviations.
Finally, we asked whether the particular mode of transcription termination is promoter dependent. To do this, we moved the abbreviated SV40 early transcription unit from pRSVcat into one of our own constructs by replacing the AB portion of pAB<cat> (Fig. 6B, lane 6) with the comparable segment of SV40 early DNA to give p(AB)sv<cat>. Figure 8, lane 4, shows that p(AB)sv<cat> exhibited complete readthrough, in contrast to pAB<cat>, which exhibited efficient termination (Fig. 8, lane 3). The failure of p(AB)sv<cat> to terminate was not a result of the failure of the SV40 poly(A) site to function in the context of our G-free constructs since replacing segment B alone of pAB<cat> with a PCR copy of the SV40 poly(A) site gave substantial termination (pAP<cat>; Fig. 6C, lane 2). The difference between the SV40 and βH-globin mechanisms of termination is therefore determined by some property of the upstream A portions of these two transcription units. We have already shown that the A region of the βH-globin gene contains an enhancer of termination. Apparently, SV40 lacks this enhancer and depends instead on a downstream element (a CCAAT box in the constructs of Connelly and Manley [10, 12]) to effect the actual termination event.
DISCUSSION
We used an improved method of transcription run-on analysis to characterize the transcription termination elements of the chicken βH-globin gene. We have shown that transcription termination for this gene is poly(A) dependent and that it requires a termination enhancer upstream of the poly(A) site for maximum efficiency. No additional downstream termination signal is required; even prokaryotic DNA downstream of the poly(A) site supports termination.
This mode of RNA polymerase II termination differs dramatically from that reported for the SV40 early and mouse βmaj-globin genes, in which no detectable termination downstream of the poly(A) site occurs, even for several kilobases in the case of SV40, until special termination elements are encountered (10, 12, 60). We have established that these differences are real by using our methods of measurement to confirm the lack of termination downstream of the poly(A) site for SV40 and, conversely, by showing that the same prokaryotic sequence (cat) that fails to support termination in the mouse globin system does so efficiently in the chicken βH-globin system. We conclude that there are at least two distinct types of poly(A)-dependent termination. In poly(A)-assisted termination (the SV40 type), the poly(A) signal renders the polymerase susceptible to a downstream termination signal but does not otherwise impart a significant decrease in processivity to the polymerase. In poly(A)-driven termination (the βH-globin type), the poly(A) signal goes beyond assisting downstream termination and actually revokes the processivity of the transcription complex, thus guaranteeing rapid, signal-independent termination on almost any downstream DNA with little regard for sequence.
G-free cassette analysis of run-on transcription.
The studies reported here were facilitated by a new method of run-on transcription analysis that makes use of G-free cassettes (56). As described above, there are considerable practical advantages to the G-free method, not the least of which are convenience and turnaround time. In addition, the inherent modularity of the method facilitates the rearrangement of functional modules, as we did throughout this work in order to gain new insights into the interactions among different elements.
There are also substantial theoretical advantages to the G-free procedure. In conventional run-on procedures, the perceived locations of polymerases vary with the duration of the in vitro elongation step because polymerases can move both into and out of probe regions during elongation (58). This effect is unimportant only if polymerase densities and elongation rates are uniform across the entire region being assayed, a condition not commonly fulfilled in transcription termination studies. For G-free analysis, the 32P labeling step in the run-on procedure is carried out in the absence of GTP so that no upstream polymerases can invade the cassettes in vitro and no polymerases in the cassettes can leave. Hence, the signal for each cassette arises strictly from the polymerases that are trapped within the cassette at the time of nuclear isolation. Moreover, since elongation rates within the two cassettes are likely to be essentially the same, we assume that the cassettes act as unbiased receptacles for polymerases in vivo.
An additional theoretical advantage of the G-free procedure is that only genuine termination, not pausing, is predicted to give a decrease in the postcassette/precassette ratio. To illustrate, consider a strong pause sequence inserted at the KpnI site of the conventional hybridization vector pHyb (Fig. 1C). Any stacking of polymerases behind a transcription complex immobilized by pausing would result in increased polymerase density on the upstream presequence. The resulting increase in run-on transcript production from this region would then decrease the postsequence/presequence hybridization ratio, a result indistinguishable from termination. In contrast, similar stacking of polymerases into the precassette of the G-free vector pORgf3·2 (Fig. 1A) would reduce rather than increase the precassette signal because run-on transcription for the G-free procedure, unlike that for a conventional hybridization assay, is carried out in the absence of GTP. Any polymerases accumulated over a pause region in inserted DNA would therefore remain immobilized during the run-on step and physically prevent any polymerases that are stacked back into the cassette from yielding full-length cassette transcripts (59). Consequently, the postcassette/precassette ratio for the assay would increase rather than decrease. This scenario illustrates that in the G-free assay, only termination can reduce the postcassette/precassette ratio. We therefore regard any reduction in this ratio to be indicative of genuine termination.
Poly(A)-driven transcription termination.
The main conclusion from this work is that the 3′ end of the chicken βH-globin gene directs RNA polymerase II to terminate transcription beginning directly downstream of the poly(A) site, without the need for any further termination signals in the downstream DNA. This mode of termination is consistent with the model for poly(A)-dependent termination proposed by Logan et al. (36). The key feature of this model is that an event triggered by the poly(A) signal causes the processive transcription elongation complex to revert to a state of poor processivity. An obligatory correlate of the model is that termination downstream of the poly(A) site is a consequence simply of destabilization of the elongation complex and does not require any additional termination signal in the downstream DNA.
The results shown in Fig. 5 and 6 support the model of Logan et al. (36). Figure 5 shows that for both the βH- and βA-globin genes, virtually any segment of the 3′-flanking DNA can support termination downstream of their respective poly(A) sites. The implication that termination for these genes may, in fact, be signal independent downstream of the poly(A) site was confirmed by the results shown in Fig. 6, which demonstrated that even randomly selected segments of prokaryotic DNA supported termination.
The prokaryotic DNA segments neo, lac, and cat varied in the effectiveness with which they supported the imposed poly(A)-driven termination (Fig. 6A, lanes 2 through 4, and B, lanes 2, 4, and 6). This suggests that among the influences which increase the probability of termination of destabilized elongation complexes are some rather general features of DNA sequence. If the trans-poly(A) poorly processive transcription complexes resemble the promoter-proximal poorly processive transcription complexes, as suggested by Logan et al. (36), then one would expect these two poorly processive versions of the transcription complex to respond similarly to these general DNA sequence features. Figure 6B shows that this was indeed the case, with promoter-proximal complexes exhibiting decreasing stabilities on prokaryotic DNAs in the same order (neo, lac, and cat [lanes 1, 3, and 5, respectively]) as did trans-poly(A) transcription complexes (lanes 2, 4, and 6, respectively). This is predicted by the model of Logan et al. (36) but is not expected by (though not inconsistent with) other models (11, 51).
If transcription terminates principally because of general destabilization of the transcription complex (loss of processivity) rather than in response to a specific termination signal, then one would expect the polymerase density after the poly(A) site to decay stochastically to zero. The rate of this decay would determine the distance required for termination to reach completion. Thus, the length of a DNA fragment (rather than any specific signal it might contain) and general features of DNA sequence (as yet undefined) are the principal operative parameters that govern termination efficiency in an assay for this type of termination. Accordingly, p<ABC> (Fig. 5A, lane 4), with 800 bp between the poly(A) site and the postcassette (Table 1), terminated more effectively than did p<ABC1> and p<ABC2> (Fig. 7A, lanes 2 and 3, respectively), with only 500 bp between the poly(A) site and the postcassette. Indeed, 700 to 800 bp appears to be a generally sufficient length of 3′-flanking DNA within which to allow complete termination to occur (Fig. 5A, lanes 4 and 9, and C, lanes 2, 4, and 5). This is consistent with the results of genomic run-on transcription of the βH- and βA-globin genes in chick erythrocyte nuclei (Fig. 2) when the fact that run-on transcripts can extend several hundred base pairs past the positions of stalled polymerases is taken into account (58). Of course, it is not surprising that the 3′-flanking DNAs of the βH- and βA-globin genes, though they lack specific termination signals, were somewhat more efficient than was the prokaryotic DNA we tested in supporting transcription termination (compare Fig. 5 and 6).
Termination enhancer.
For maximum efficiency, βH-globin termination must be enhanced by an upstream element in segment A of the βH-globin map (Fig. 5A, lanes 9 and 10, and 7B, lanes 4 and 5). This element did not by itself potentiate termination (Fig. 6B, lanes 5 and 7, and 7B, lanes 1 and 2) but acted only through a downstream poly(A) signal (Fig. 6B, lanes 6 and 7, and C, lanes 2 and 4, and 7B, lanes 2 and 3). This element can legitimately be regarded as an enhancer because it operated similarly on two unrelated poly(A) sites (Fig. 6B, lane 6, and C, lane 2) and because it functioned well even when its distance from the poly(A) site was increased by an insertion of more than 375 bp of foreign DNA (Fig. 7B, lane 5).
We have not yet tested specifically the possibility that the element in segment A acts by enhancing polyadenylation. Such an effect could be ascribed to the 3′ splice site coded for by segment A (26, 35, 45, 64), or to the presence of a genuine polyadenylation enhancer (23, 38). However, a deletion analysis of segment A did not implicate the splice site (data not shown) and replacement of the SV40 early poly(A) signal of pRSVcat (the HpaI-BamHI fragment) with the βH-globin poly(A) signal (segment B) led to no significant loss of chloramphenicol acetyltransferase activity in expression assays, showing that the βH-globin poly(A) site is not dependent on a special enhancer for its activity (34a). These results are consistent with those for the only other upstream enhancer of termination so far identified (17), which has little discernible effect on polyadenylation efficiency but contributes strongly to transcription termination.
Poly(A)-assisted transcription termination.
Connelly and Manley, studying the SV40 early transcription unit, were the first to provide a complete description of the elements required for poly(A)-dependent termination (10–12). They showed that for an SV40-adenovirus recombinant transcription unit, both a functional poly(A) signal and a downstream terminator are required to ensure complete transcription termination. In contrast to the βH-globin system described here, they showed that there is essentially no termination downstream of the poly(A) site, as measured by run-on transcription, unless the terminator is present (10, 12). Moreover, an S1 protection assay for genome-length transcripts within nuclear RNA showed that the average polymerase continued for more than 4 kb past the poly(A) site when the terminator was removed. Thus, the SV40 early poly(A) site can assist a downstream element in terminating transcription but is ineffective in directing termination on its own. The nature of the interaction between the poly(A) signal and the transcription apparatus in poly(A)-assisted termination is thus quite different from that in the poly(A)-driven mode of termination, which is characteristic of the βH-globin gene.
A casual survey of reported run-on transcription patterns suggests that the poly(A)-assisted style of termination is fairly common. Poly(A)-assisted termination is indicated whenever transcription continues for a considerable distance (e.g., from 500 bp up to several kilobases) past the poly(A) site with little or no decline (5, 10, 18–20, 27, 29, 34, 39, 43, 44, 54, 60, 62, 68). Subsequent termination may then be precipitous (10, 20, 34, 39, 62) or gradual (5, 27, 29, 60, 62), depending on the nature or presence of any terminator. On the other hand, poly(A)-driven termination cannot be unambiguously identified in the absence of experiments that demonstrate efficient DNA sequence-independent termination downstream of the poly(A) site. However, candidate poly(A)-driven terminators are indicated by transcription units whose transcription goes into decline immediately after the poly(A) site (1, 22, 65). Nevertheless, when a confirmed termination element exists immediately downstream of a poly(A) site (2, 7, 16), the nature of the poly(A) dependence of termination remains obscure in the absence of further experiments.
cis-acting elements, not the poly(A) signal itself, govern the mode of poly(A) dependence.
The most thoroughly characterized of the poly(A)-assisted class of transcription terminators is the one that contains the SV40 early poly(A) signal (10–12). In the absence of an auxiliary downstream termination element, polymerases cross the SV40 early poly(A) signal and continue for at least several kilobases with no detectable termination. Nevertheless, we found that a PCR copy of the same signal directed immediate, signal-independent termination of the poly(A)-driven type when it was placed in the context of chicken βH-globin upstream sequences (Fig. 6C). Evidently, the SV40 poly(A) signal, limited to a facilitating function in its native context (10, 12) (Fig. 8, lanes 2 and 4), became the final instrument of termination when it was placed in a chicken background (Fig. 6C).
The converse experiment for two different immunoglobulin poly(A) signals has previously been reported. Thus, a DNA segment, designated XX, containing the immunoglobulin γ2a poly(A) signal, drove substantial downstream termination, whereas the deletion variant SphX, lacking only a small segment of upstream sequence, exhibited complete readthrough (17). Similarly, a DNA segment, designated μmδ, containing the immunoglobulin M membrane-form μ chain poly(A) signal directed efficient downstream termination, whereas an upstream substitution variant, 3′SPδ, did not (61). In both of these cases, it was verified that polyadenylation efficiency was not altered. Therefore, the upstream sequences are genuine termination elements, not merely modulators of polyadenylation efficiency.
Mutations within the poly(A) signal region can also influence termination efficiency, as illustrated by a recent study of yeast (7). Thus, pNU, containing the poly(A) signal from the ura4 gene of Schizosaccharomyces pombe, directed efficient termination apparently at a discrete element that was about 200 bp downstream of the poly(A) cleavage site. In contrast, pNUM, bearing several mutations in the vicinity of the poly(A) signal, terminated only gradually throughout a region that extended an additional 400 bp downstream. However, pNU and pNUM exhibited equivalent polyadenylation efficiencies (7).
Taken together, all of these results indicate that the reported correlation between poly(A) site strength and termination efficiency (15) is valid only within a particular mode of poly(A) dependence. Any changes in the cis-acting elements that govern the mode of poly(A) dependence alter the apparent relationship between poly(A) site strength and transcription termination.
ACKNOWLEDGMENTS
We thank E. Erickson for carrying out some preliminary work at the inception of this study, N. Nguyen for constructing some of the plasmids used and for communicating unpublished results, and R. Landick for providing plasmid pRL542.
This work was supported by NIH grant GM50863.
REFERENCES
- 1.Affolter M, Ruiz-Carrillo A. Transcription unit of the chicken histone H5 gene and mapping of H5 pre-mRNA sequences. J Biol Chem. 1986;261:11496–11502. [PubMed] [Google Scholar]
- 2.Ashfield R, Enriquez-Harris P, Proudfoot N J. Transcriptional termination between the closely linked human complement genes C2 and factor B: common termination factor for C2 and c-myc? EMBO J. 1991;10:4197–4207. doi: 10.1002/j.1460-2075.1991.tb04998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashfield R, Patel A J, Bossone S A, Brown H, Campbell R D, Marcu K B, Proudfoot N J. MAZ-dependent termination between closely spaced human complement genes. EMBO J. 1994;13:5656–5667. doi: 10.1002/j.1460-2075.1994.tb06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley & Sons; 1988. [Google Scholar]
- 5.Baker C C, Noe J S. Transcriptional termination between bovine papillomavirus type 1 (BPV-1) early and late polyadenylation sites blocks late transcription in BPV-1-transformed cells. J Virol. 1989;63:3529–3534. doi: 10.1128/jvi.63.8.3529-3534.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berget S M. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 7.Birse C E, Lee B A, Hansen K, Proudfoot N J. Transcriptional termination signals for RNA polymerase II in fission yeast. EMBO J. 1997;16:3633–3643. doi: 10.1093/emboj/16.12.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blau J, Xiao H, McCracken S, O’Hare P, Greenblatt J, Bentley D. Three functional classes of transcriptional activation domain. Mol Cell Biol. 1996;16:2044–2055. doi: 10.1128/mcb.16.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carswell S, Alwine J C. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol Cell Biol. 1989;9:4248–4258. doi: 10.1128/mcb.9.10.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly S, Manley J L. A CCAAT box sequence in the adenovirus major late promoter functions as part of an RNA polymerase II termination signal. Cell. 1989;57:561–571. doi: 10.1016/0092-8674(89)90126-8. [DOI] [PubMed] [Google Scholar]
- 11.Connelly S, Manley J L. A functional mRNA polyadenylation signal is required for transcription termination by RNA polymerase II. Genes Dev. 1988;2:440–452. doi: 10.1101/gad.2.4.440. [DOI] [PubMed] [Google Scholar]
- 12.Connelly S, Manley J L. RNA polymerase II transcription termination is mediated specifically by protein binding to a CCAAT box sequence. Mol Cell Biol. 1989;9:5254–5259. doi: 10.1128/mcb.9.11.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLucia A L, Deb S, Partin K, Tegtmeyer P. Functional interactions of the simian virus 40 core origin of replication with flanking regulatory sequences. J Virol. 1986;57:138–144. doi: 10.1128/jvi.57.1.138-144.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dressler G R, Fraser N W. DNA sequences downstream of the adenovirus type 2 fiber polyadenylation site contain transcription termination signals. J Virol. 1987;61:2770–2776. doi: 10.1128/jvi.61.9.2770-2776.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwalds-Gilbert G, Prescott J, Falck-Pedersen E. 3′ RNA processing efficiency plays a primary role in generating termination-competent RNA polymerase II elongation complexes. Mol Cell Biol. 1993;13:3472–3480. doi: 10.1128/mcb.13.6.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enriquez-Harris P, Levitt N, Briggs D, Proudfoot N J. A pause site for RNA polymerase II is associated with termination of transcription. EMBO J. 1991;10:1833–1842. doi: 10.1002/j.1460-2075.1991.tb07709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaspohler J A, Boczkowski D, Hall B L, Milcarek C. The 3′-untranslated region of membrane exon 2 from the γ2a immunoglobulin gene contributes to efficient transcription termination. J Biol Chem. 1995;270:11903–11911. doi: 10.1074/jbc.270.20.11903. [DOI] [PubMed] [Google Scholar]
- 18.Ford J P, Hsu M T. Transcription pattern of in vivo-labeled late simian virus 40 RNA: equimolar transcription beyond the mRNA 3′ terminus. J Virol. 1978;28:795–801. doi: 10.1128/jvi.28.3.795-801.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser N W, Nevins J R, Ziff E, Darnell J E., Jr The major late adenovirus type-2 transcription unit: termination is downstream from the last poly(A) site. J Mol Biol. 1979;129:643–656. doi: 10.1016/0022-2836(79)90474-1. [DOI] [PubMed] [Google Scholar]
- 20.Frayne E G, Leys E J, Crouse G F, Hook A G, Kellems R E. Transcription of the mouse dihydrofolate reductase gene proceeds unabated through seven polyadenylation sites and terminates near a region of repeated DNA. Mol Cell Biol. 1984;4:2921–2924. doi: 10.1128/mcb.4.12.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fromm M, Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1:457–481. [PubMed] [Google Scholar]
- 22.Giardina C, Lis J T. Polymerase processivity and termination on Drosophila heat shock genes. J Biol Chem. 1993;268:23806–23811. [PubMed] [Google Scholar]
- 23.Gilmartin G M, Hung S L, DeZazzo J D, Fleming E S, Imperiale M J. Sequences regulating poly(A) site selection within the adenovirus major late transcription unit influence the interaction of constitutive processing factors with the pre-mRNA. J Virol. 1996;70:1775–1783. doi: 10.1128/jvi.70.3.1775-1783.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorman C M, Merlino G T, Willingham M C, Pastan I, Howard B H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci USA. 1982;79:6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunderson S I, Vagner S, Polycarpou-Schwarz M, Mattaj I W. Involvement of the carboxyl terminus of vertebrate poly(A) polymerase in U1A autoregulation and in the coupling of splicing and polyadenylation. Genes Dev. 1997;11:761–773. doi: 10.1101/gad.11.6.761. [DOI] [PubMed] [Google Scholar]
- 27.Hagenbüchle O, Wellauer P K, Cribbs D L, Schibler U. Termination of transcription in the mouse α-amylase gene Amy-2a occurs at multiple sites downstream of the polyadenylation site. Cell. 1984;38:737–744. doi: 10.1016/0092-8674(84)90269-1. [DOI] [PubMed] [Google Scholar]
- 28.Hair A, Morgan G T. Premature termination of tubulin gene transcription in Xenopus oocytes is due to promoter-dependent disruption of elongation. Mol Cell Biol. 1993;13:7925–7934. doi: 10.1128/mcb.13.12.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwamoto S, Eggerding F, Falck-Pederson E, Darnell J E., Jr Transcription unit mapping in adenovirus: regions of termination. J Virol. 1986;59:112–119. doi: 10.1128/jvi.59.1.112-119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman R J. Vectors used for expression in mammalian cells. Methods Enzymol. 1990;185:487–511. doi: 10.1016/0076-6879(90)85041-l. [DOI] [PubMed] [Google Scholar]
- 31.Krumm A, Meulia T, Brunvand M, Groudine M. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 1992;6:2201–2213. doi: 10.1101/gad.6.11.2201. [DOI] [PubMed] [Google Scholar]
- 32.Lanoix J, Acheson N H. A rabbit β-globin polyadenylation signal directs efficient termination of transcription of polyomavirus DNA. EMBO J. 1988;7:2515–2522. doi: 10.1002/j.1460-2075.1988.tb03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J M, Greenleaf A L. Modulation of RNA polymerase II elongation efficiency by C-terminal heptapeptide repeat domain kinase I. J Biol Chem. 1997;272:10990–10993. doi: 10.1074/jbc.272.17.10990. [DOI] [PubMed] [Google Scholar]
- 34.LeMeur M A, Galliot B, Gerlinger P. Termination of the ovalbumin gene transcription. EMBO J. 1984;3:2779–2786. doi: 10.1002/j.1460-2075.1984.tb02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Liu, D. H., and S. J. Kim. Unpublished data.
- 35.Liu X, Mertz J E. Sequence of the polypyrimidine tract of the 3′-terminal 3′ splicing signal can affect intron-dependent pre-mRNA processing in vivo. Nucleic Acids Res. 1996;24:1765–1773. doi: 10.1093/nar/24.9.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan J, Falck-Pedersen E, Darnell J E, Jr, Shenk T. A poly(A) addition site and a downstream termination region are required for efficient cessation of transcription by RNA polymerase II in the mouse βmaj-globin gene. Proc Natl Acad Sci USA. 1987;84:8306–8310. doi: 10.1073/pnas.84.23.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.London L, Keene R G, Landick R. Analysis of premature termination in c-myc during transcription by RNA polymerase II in a HeLa nuclear extract. Mol Cell Biol. 1991;11:4599–4615. doi: 10.1128/mcb.11.9.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou H, Gagel R F, Berget S M. An intron enhancer recognized by splicing factors activates polyadenylation. Genes Dev. 1996;10:208–219. doi: 10.1101/gad.10.2.208. [DOI] [PubMed] [Google Scholar]
- 39.Maa M C, Chinsky J M, Ramamurthy V, Martin B D, Kellems R E. Identification of transcription stop sites at the 5′ and 3′ ends of the murine adenosine deaminase gene. J Biol Chem. 1990;265:12513–12519. [PubMed] [Google Scholar]
- 40.Maderious A, Chen-Kiang S. Pausing and premature termination of human RNA polymerase II during transcription of adenovirus in vivo and in vitro. Proc Natl Acad Sci USA. 1984;81:5931–5935. doi: 10.1073/pnas.81.19.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson S D, Wickens M, Bentley D L. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- 42.Meulia T, Krumm A, Groudine M. Distinct properties of c-myc transcriptional elongation are revealed in Xenopus oocytes and mammalian cells and by template titration, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), and promoter mutagenesis. Mol Cell Biol. 1993;13:5647–5658. doi: 10.1128/mcb.13.9.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore B B, Tan J, Lim P L, Tucker P W, Yuan D. Regulatory elements necessary for termination of transcription within the Ig heavy chain gene locus. Nucleic Acids Res. 1993;21:1481–1488. doi: 10.1093/nar/21.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nevins J R, Darnell J E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978;15:1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- 44a.Nguyen, N. Unpublished data.
- 45.Niwa M, Rose S D, Berget S M. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 1990;4:1552–1559. doi: 10.1101/gad.4.9.1552. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien T, Lis J T. Rapid changes in Drosophila transcription after an instantaneous heat shock. Mol Cell Biol. 1993;13:3456–3463. doi: 10.1128/mcb.13.6.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okamoto H, Sheline C T, Corden J L, Jones K A, Peterlin B M. Trans-activation by human immunodeficiency virus Tat protein requires the C-terminal domain of RNA polymerase II. Proc Natl Acad Sci USA. 1996;93:11575–11579. doi: 10.1073/pnas.93.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parada C A, Roeder R G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 49.Plet A, Eick D, Blanchard J M. Elongation and premature termination of transcripts initiated from c-fos and c-myc promoters show dissimilar patterns. Oncogene. 1995;10:319–328. [PubMed] [Google Scholar]
- 50.Pribyl T M, Martinson H G. Transcription termination at the chicken βH-globin gene. Mol Cell Biol. 1988;8:5369–5377. doi: 10.1128/mcb.8.12.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proudfoot N J. How RNA polymerase II terminates transcription in higher eukaryotes. Trends Biochem Sci. 1989;14:105–110. doi: 10.1016/0968-0004(89)90132-1. [DOI] [PubMed] [Google Scholar]
- 52.Rasmussen E B, Lis J T. Short transcripts of the ternary complex provide insight into RNA polymerase II elongational pausing. J Mol Biol. 1995;252:522–535. doi: 10.1006/jmbi.1995.0517. [DOI] [PubMed] [Google Scholar]
- 53.Roberts S, Bentley D L. Distinct modes of transcription read through or terminate at the c-myc attenuator. EMBO J. 1992;11:1085–1093. doi: 10.1002/j.1460-2075.1992.tb05147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohrbaugh M L, Johnson III J E, James M D, Hardison R C. Transcription unit of the rabbit β1 globin gene. Mol Cell Biol. 1985;5:147–160. doi: 10.1128/mcb.5.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato K, Ito R, Baek K H, Agarwal K. A specific DNA sequence controls termination of transcription in the gastrin gene. Mol Cell Biol. 1986;6:1032–1043. doi: 10.1128/mcb.6.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sawadogo M, Roeder R G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci USA. 1985;82:4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Southern P J, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1:327–341. [PubMed] [Google Scholar]
- 58.Strobl L J, Eick D. Hold back of RNA polymerase II at the transcription start site mediates down-regulation of c-myc in vivo. EMBO J. 1992;11:3307–3314. doi: 10.1002/j.1460-2075.1992.tb05409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szentirmay M N, Sawadogo M. Transcription factor requirement for multiple rounds of initiation by human RNA polymerase II. Proc Natl Acad Sci USA. 1991;88:10691–10695. doi: 10.1073/pnas.88.23.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tantravahi J, Alvira M, Falck-Pedersen E. Characterization of the mouse βmaj globin transcription termination region: a spacing sequence is required between the poly(A) signal sequence and multiple downstream termination elements. Mol Cell Biol. 1993;13:578–587. doi: 10.1128/mcb.13.1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tisch R, Kondo N, Hozumi N. Parameters that govern the regulation of immunoglobulin δ heavy-chain gene expression. Mol Cell Biol. 1990;10:5340–5348. doi: 10.1128/mcb.10.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandenbergh D J, James-Pederson M, Hardison R C. An apparent pause site in the transcription unit of the rabbit α-globin gene. J Mol Biol. 1991;220:255–270. doi: 10.1016/0022-2836(91)90011-t. [DOI] [PubMed] [Google Scholar]
- 63.Villeponteau B, Landes G M, Pankratz M J, Martinson H G. The chicken β globin gene region. Delineation of transcription units and developmental regulation of interspersed DNA repeats. J Biol Chem. 1982;257:11015–11023. [PubMed] [Google Scholar]
- 64.Wassarman K M, Steitz J A. Association with terminal exons in pre-mRNAs: a new role for the U1 snRNP? Genes Dev. 1993;7:647–659. doi: 10.1101/gad.7.4.647. [DOI] [PubMed] [Google Scholar]
- 65.Weintraub H, Larsen A, Groudine M. α-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981;24:333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- 66.Whitelaw E, Proudfoot N. α-Thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3′ end processing in the human α2 globin gene. EMBO J. 1986;5:2915–2922. doi: 10.1002/j.1460-2075.1986.tb04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams R D, Lee B A, Jackson S P, Proudfoot N J. Activation domains of transcription factors mediate replication dependent transcription from a minimal HIV-1 promoter. Nucleic Acids Res. 1996;24:549–557. doi: 10.1093/nar/24.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu M, Barnard M B, Rose S M, Cockerill P N, Huang S Y, Garrard W T. Transcription termination and chromatin structure of the active immunoglobulin kappa gene locus. J Biol Chem. 1986;261:3838–3845. [PubMed] [Google Scholar]
- 69.Yankulov K Y, Pandes M, McCracken S, Bouchard D, Bentley D L. TFIIH functions in regulating transcriptional elongation by RNA polymerase II in Xenopus oocytes. Mol Cell Biol. 1996;16:3291–3299. doi: 10.1128/mcb.16.7.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]