Abstract
Objective
Osteoarthritis (OA) is a degenerative joint disease characterized by cartilage degradation, causing severe pain and disability. Recent studies suggest that miR-450a-5p may regulate inflammatory pathways in OA. This study aimed to elucidate the role of miR-450a-5p in OA, providing a potential therapeutic target for the clinical treatment.
Methods
Cartilage tissues were collected from OA patients undergoing knee replacement surgery, and CHON-001 cells were treated with interleukin (IL)-1β to induce an OA model in vitro. Real-time quantitative polymerase chain reaction was used to detect the miR-450a-5p expression, and Western blot determined the lipopolysaccharide-induced tumor necrosis factor (TNF)-α factor (LITAF) expression. The targeting relationship between LITAF and miR-450a-5p was verified by dual-luciferase reporter assay. Cell proliferation and apoptosis were assessed using the Cell Counting Kit-8 assay and flow cytometry, respectively. Levels of IL-6, IL-10, and TNF-α were measured via enzyme-linked immunosorbent assay. In addition, Western blot was employed to detect the expressions of matrix metalloproteinase-3 (MMP-3), collagen III, and aggrecan in extracellular matrix (ECM).
Results
MiR-450a-5p expression was significantly down-regulated in OA tissues and IL-1β-induced CHON-001 cells (~60%), while LITAF expression was markedly increased (~1.8-fold). There was a negative correlation between miR-450a-5p and LITAF in OA tissues (r = −0.596, P < 0.01). MiR-450a-5p directly targeted and inhibited LITAF expression. Its overexpression promoted chondrocyte proliferation, reduced apoptosis and inflammatory cytokines, and mitigated ECM degradation.
Conclusions
MiR-450a-5p inhibited LITAF expression, thereby attenuating apoptosis, inflammation, and ECM degradation in chondrocytes. It may serve as a promising therapeutic target for OA.
Keywords: osteoarthritis, chondrocytes, IL-1β, LITAF, miR-450a-5p, CHON-001
Introduction
Osteoarthritis (OA) is the most common joint disease in the elderly, leading to chronic pain, stiffness, and disability. 1 As reported previously, OA affected 12% of the population over 25 years old and 50% of the population over 65 years old. 2 The main characteristics of OA include chronic inflammation, progressive destruction of articular cartilage, and subchondral sclerosis. 3 The development of OA has been demonstrated to be related to age, previous joint injury, obesity, genetics, gender, and joint shape. 4 However, there are still no effective disease-modifying therapies due to the limited understanding of OA pathogenesis. Currently, joint replacement is the main clinical treatment for advanced OA. 5
Chondrocytes, the only resident cells in joint cartilage, play a crucial role in maintaining the dynamic balance between anabolism and catabolism in ECM. Risk factors such as abnormal mechanical stress and pro-inflammatory cytokines (like interleukin [IL]-1β) have been proved to reduce chondrocytes and degrade the ECM in cartilage, as evidenced by increased levels of matrix metalloproteinases (MMPs) and decreased levels of collagen III and aggrecan. 6 Despite efforts to understand the pathological process of OA have increased, the molecular mechanisms remain elusive. Therefore, identifying novel drug targets to develop more effective treatment options is of high medical necessity.
Recently, microRNAs (miRNAs) have been recognized as one of the epigenetic mechanisms in gene expression regulation. 7 Innovative targets of OA, such as genes involved in OA development, have been uncovered through genetic networks, epigenetics, and miRNA-based approaches. 8 The miRNAs are a group of conserved small RNAs, consisting of 20 to 22 nucleotides, which regulate the post-transcriptional expression of mRNAs by binding to the 3’-untranslated region, leading to mRNA denaturation and inhibition of mRNA translation. 9 Several miRNAs have been identified to be associated with OA pathogenesis. For example, miR-27a promotes the autophagy and apoptosis in IL-1β-treated chondrocytes via the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. 10 In addition, miR-29b-3p promotes chondrocyte apoptosis by targeting progranulin, thereby promoting OA development. 11 Another study demonstrated that miR-140-5p affects chondrocyte proliferation, apoptosis, and inflammation by targeting high mobility group box 1 in OA. 12
MiR-450a-5p regulates a variety of biological responses and has been implicated in the pathogenesis of various diseases. It is often abnormally expressed in malignancies, where it influences processes such as cell proliferation, apoptosis, autophagy, and angiogenesis.13,14 For instance, miR-450a-5p was shown to modulate autophagy by targeting the estimated glomerular filtration rate, enhancing the sensitivity of glioma cells to gefitinib in chemotherapy. 15 Low expression of miR-450a-5p has been reported to be associated with poor prognosis in colorectal cancer and other diseases, indicating its potential regulatory effect on cancer stem cell properties and angiogenesis. 16 Moreover, recent studies have highlighted its role in regulating inflammatory signaling pathways and cellular stress responses. 17 Given that chronic inflammation, matrix degradation, and cell death are central to the pathophysiology of OA, it is conceivable that miR-450a-5p may influence OA progression by modulating similar mechanisms in chondrocytes. However, its specific role in OA remains unclear. Therefore, this study aimed to elucidate the biological function and regulatory targets of miR-450a-5p in OA, thereby providing a new therapeutic target for clinical treatment.
Lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α factor (LITAF) is an important transcription factor that activates the transcription of inflammatory cytokines induced by LPS, such as TNF-α. 18 Briefly, LITAF interacts with the signal transducer and activator of transcription 6 (STAT6), forming a complex that translocates into the nucleus, where it binds to the promoter regions of cytokines and mediates the expression of inflammatory cytokines (such as TNF-α, IL-6, and IL-10). 19 A recent study has shown that LITAF, an endolysosomal protein, is not only active against bacterial toxins and Gasdermin D (a terminal effector of pyroptosis) but also mediates cell resistance to pore-forming toxins, leading to cell death. 20 Importantly, LITAF has been implicated in the regulation of inflammatory responses in various diseases such as arthritis. Dysregulation of LITAF expression can lead to an exacerbated inflammatory response, contributing to the degradation of cartilage and progression of OA.18,21
To investigate the potential role of miR-450a-5p and LITAF in OA, we collected cartilage tissues from OA patients and established an IL-1β-induced chondrocyte injury model in CHON-001 cells. The purpose of this study was to explore the regulatory roles of miR-450a-5p and LITAF in OA progression, providing a theoretical basis for the treatment of OA.
Methods
Clinical Sample Collection
Cartilage tissues, used for RNA extraction and subsequent experiments to analyze miR-450a-5p and LITAF expression, were collected from 2 distinct groups of participants at the Affiliated Wuxi Fifth Hospital of Jiangnan University between January 2020 and March 2021. The OA group consisted of 20 patients undergoing knee replacement surgery due to OA, diagnosed based on clinical symptoms and radiographic evidence. The normal group comprised 20 individuals without OA who underwent traumatic amputation surgery and had no history of joint diseases. All participants provided informed consent voluntarily, and the study procedures were approved by the research ethics committee of Affiliated Wuxi Fifth Hospital of Jiangnan University (Ethics Review No. 20191201). Demographic details, including age, gender distribution, and any relevant clinical characteristics, were recorded for both groups to ensure comparability and minimize potential confounders (Supplementary Table 1).
Cell Culture and Transfection
Human chondrocyte cell line CHON-001 (ATCC) was cultured in a complete Dulbecco’s Modified Eagle Medium (DMEM, Solarbio, Beijing) containing 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin-streptomycin solution (Beyotime, Guangzhou) in an incubator at 37°C with 5% CO2. An in vitro OA cell model was constructed by treating CHON-001 cells for 12 hours with IL-1β (10 ng/ml, PeproTech, USA). 22
Cells at passages 3 to 5 were used for experiments. When the cell confluence reached 70% to 80%, negative control (NC) mimics, miR-450a-5p mimics, NC plasmid, and LITAF overexpression plasmid were transfected into the cells using Lipofectamine 2000 (Invitrogen, USA). After transfection, the cells were collected for subsequent experiments.
Real-Time Quantitative Polymerase Chain Reaction
Total RNA from cells or tissues was extracted using Trizol reagent (Thermo Fisher Scientific) and quantified with the NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, USA). Then, the extracted RNA was reverse transcribed into cDNA by PrimeScript RT Master Mix (Thermo Fisher Scientific) according to the manufacturer’s protocol. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) was performed using the SYBR Green kit (Applied Biosystems, USA) on an ABI 7500 Real-Time PCR System (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference. Each experiment was performed in 6 replicates. The procedures were shown as follows: pre-denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds, annealing at 60°C for 1 minute, and extension at 72°C for 30 seconds. The relative expression of the target genes was calculated using the 2−ΔΔ Ct method. 23 The primer sequences are shown in Table 1.
Table 1.
The Primer’s Information.
| Gene | Primer sequence (5’-3’) | Product Size (bp) | Annealing temperature (°C) | Cycle number | Ref. |
|---|---|---|---|---|---|
| miR-450a-5p | F: CGATCGGTTTTGCGATGTGTTCC R: ATCCAGTGCAGGGTCCGAGG |
31 | 60.3 | 40 | RID-899C7JAK013 |
| LITAF | F: TCCTTCGTATTATACCCAGCCA R: GTGCTGCACGTAGACCGTC |
76 | 60.1 | 40 | RID-8BZVB4V5013 |
| GAPDH | F: CATCACTGCCACCCAGAAGACTG R: ATGCCAGTGAGCTTCCCGTTCAG |
101 | 60.2 | 40 | RID-899SABFV013 |
LITAF = lipopolysaccharide-induced tumor necrosis factor-α factor; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
CCK-8 Assay
Cell proliferation was determined using CCK-8 regent (Beyotime, #C0037) based on the manufacturer’s instructions. 24 Briefly, cells (2 × 104 cells/well) were seeded in a 96-well plate and subjected to the following treatments. The cells in the control group received no additional treatment. The cells in the IL-1β group were treated with IL-1β (10 ng/ml) for 12 hours. The cells in the IL-1β + NC mimics group were transfected with NC mimics and treated with IL-1β (10 ng/ml) for 12 hours. The cells in the IL-1β + miR-450a-5p group were transfected with miR-450a-5p mimics and treated with IL-1β for 12 hours. Subsequently, 10 µl of CCK-8 solution was added to each well on days 0, 1, 2, and 3 after the indicated treatment, and the cells were incubated at 37°C for 2 hours. The absorbance at 450 nm was measured using a microplate reader. All experiments were biologically repeated at least 3 times.
Flow Cytometry
The treated CHON-001 cells were harvested using trypsin and washed twice with pre-cooled sterile phosphate-buffered saline (PBS). The cell concentration was adjusted to 5 × 105 cells/ml. Then, 200 μl of cell suspension was incubated with 10 μl of Annexin V-FITC (Beyotime, #C1062M) and 10 μl of propidium iodide solution (20 mg/l) for 10 minutes at room temperature in the dark. Upon adding 500 μl of PBS, the cell apoptosis was determined via flow cytometry (BD Biosciences, LSRFortessa, Beijing). The apoptosis index was calculated as follows: Apoptosis index = number of apoptotic cells / (number of apoptotic cells + number of normal cells) × 100%. 25
Enzyme-Linked Immunosorbent Assay
The cultured cells were centrifuged for 10 minutes to collect the supernatants. Then, the levels of TNF-α (#ab181421), IL-6 (#ab178013), and IL-18 (#ab215539) were measured using corresponding enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, UK), following the manufacturer’s protocol. Finally, the optical density at 450 nm (OD450) was determined. 24
Western Blot Analysis
Cells were collected, and the total protein was extracted using cell lysis buffer. 24 The protein concentration was measured with the BCA Protein Assay Kit (Beyotime, #P0012). Equal amounts of protein (20 μg) were mixed with 5× sample loading buffer, boiled, and denatured. Upon separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the proteins were transferred onto the polyvinylidene fluoride (PVDF) membranes (Millipore, USA). The membranes were blocked with 5% skimmed milk for 1 hour, incubated with primary antibodies overnight at 4°C. After washing 3 times, the membranes were incubated with the secondary antibodies at room temperature for 1 hour. Following washing, the membranes were supplemented with chemiluminescence reagent and visualized using a gel imaging system. Image J software was used to analyze the signals of protein bands, and GAPDH was used as an internal reference. The primary and secondary antibodies, all purchased from Abcam, were used as follows: MMP-3 (1:1000, #ab52915), collagen III (1:1000, #ab7778), aggrecan (1:1000, #ab3778), LITAF (1:1000, #ab187533), GAPDH (1:2500, #ab9485), and goat anti-rabbit IgG H&L (HRP) (1:5000, #ab6721).
Dual Luciferase-Reporter Assay
The interaction between miR-450a-5p and LITAF was assessed by luciferase analysis. 26 Specifically, the cells were transfected with dual-luciferase reporter vectors containing wild-type (WT) or mutant-type (MUT) binding sites of LITAF and miR-450a-5p, as well as miR-450a-5p mimics, using Lipofectamine 2000 (Invitrogen). After 24 hours of transfection, the luciferase signal was detected using the dual-luciferase reporter assay kit (Beyotime, #RG027).
Data Analysis
SPSS 26.0 was used for 1-way analysis of variance and independent samples t-test analysis, and GraphPad Prism 9.0 was employed for plotting. An independent t-test was used for comparison between 2 groups, and a 1-way analysis of variance for comparison among multiple groups. The results were represented as mean ± standard deviation, the Pearson correlation coefficient was used to analyze the expression correlation, and P < 0.05 was considered statistically significant.
Results
Reduced Expression of miR-450a-5p in Cartilage Tissues and Interleukin-1β-Induced CHON-001 Cells
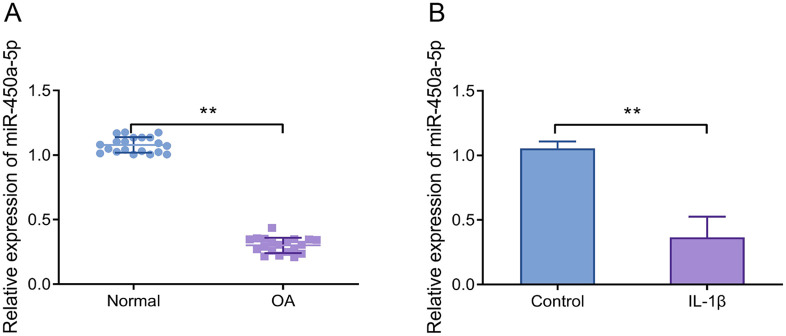
The expression level of miR-450a-5p was significantly reduced in the cartilage tissues obtained from OA patients compared to normal controls ( Fig. 1A ). Statistical analyses revealed no significant demographic differences between the OA and normal groups (Supplementary Table 1). Besides, at the cellular level, the expression level of miR-450a-5p was markedly reduced in IL-1β-treated CHON-001 cells ( Fig. 1B ). The results suggested that miR-450a-5p expression was related to the inflammatory environment of the organism or cells.
Figure 1.
MiR-450a-5p expression is significantly elevated in cartilage tissues and IL-1β-induced chondrocytes. (A) RT-qPCR was used to detect the miR-450a-5p expression level of cartilage tissues in OA patients and non-OA patients, **P < 0.01, normal group versus OA group. (B) RT-qPCR was performed to measure the miR-450a-5p expression level in IL-1β-treated CHON-001 cells, **P < 0.01. IL-1β = interleukin-1β; OA = osteoarthritis; RT-qPCR = real-time quantitative polymerase chain reaction.
MiR-450a-5p Inhibits Apoptosis, Inflammation, and Extracellular Matrix Degradation in Interleukin-1β-Induced CHON-001 Cells
To verify the function of miR-450a-5p, CHON-001 cells were transfected with miR-450a-5p mimics. RT-qPCR results confirmed successful up-regulation of miR-450a-5p ( Fig. 2A ). CCK-8 and flow cytometry analyses showed that, compared with the control group, cell proliferation was significantly reduced and apoptosis was remarkably increased in the IL-1β group. In contrast, the IL-1β + miR-450a-5p group exhibited a notable elevation in cell proliferation and a significant reduction in apoptosis compared to the IL-1β + NC mimics group ( Fig. 2B and C ).
Figure 2.
MiR-450a-5p inhibits IL-1β-induced apoptosis, inflammation, and extracellular matrix degradation in chondrocytes. (A) The mRNA levels of miR-450a-5p were examined by RT-qPCR, **P < 0.01, miR-450a-5p group versus NC mimics group; (B) cell proliferation at 0, 24, 48, and 72 hours was detected by CCK-8 assay. (C) Flow cytometry was performed to assess the apoptosis level of CHON-001 cells; (D) ELISA was used to determine the levels of IL-6, IL-18, and TNF-α; (E) Western blot was utilized to detect the protein expression levels of MMP-3, collagen III, and aggrecan; **P < 0.01, IL-1β group versus control group; ##P < 0.01, IL-1β + miR-450a-5p group versus IL-1β + NC mimics group. IL-1β = interleukin-1β; ECM = extracellular matrix; RT-qPCR = real-time quantitative polymerase chain reaction; CCK-8 = cell counting kit-8; ELISA = enzyme-linked immunosorbent assay; IL = interleukin; TNF-α = tumor necrosis factor-α; MMP-3 = matrix metalloproteinase-3.
In addition, up-regulation of miR-450a-5p level could moderate the release of cellular inflammatory factors. In contrast to the control group, the levels of IL-6, IL-18, and TNF-α were considerably raised in the IL-1β group. Overexpression of miR-450a-5p inhibited the increase of these cytokines induced by IL-1β ( Fig. 2D ). Western blot analysis revealed that, in comparison with the control group, the protein expression level of MMP-3 in the IL-1β group was notably increased, while collagen III and aggrecan protein levels were significantly reduced. These effects were partially reversed by overexpression of miR-450a-5p ( Fig. 2E ). Collectively, miR-450a-5p overexpression could reverse IL-1β-induced apoptosis, inflammation, and ECM degradation in chondrocytes.
Lipopolysaccharide-Induced Tumor Necrosis Factor-α Factor Acts as a Target Gene of miR-450a-5p
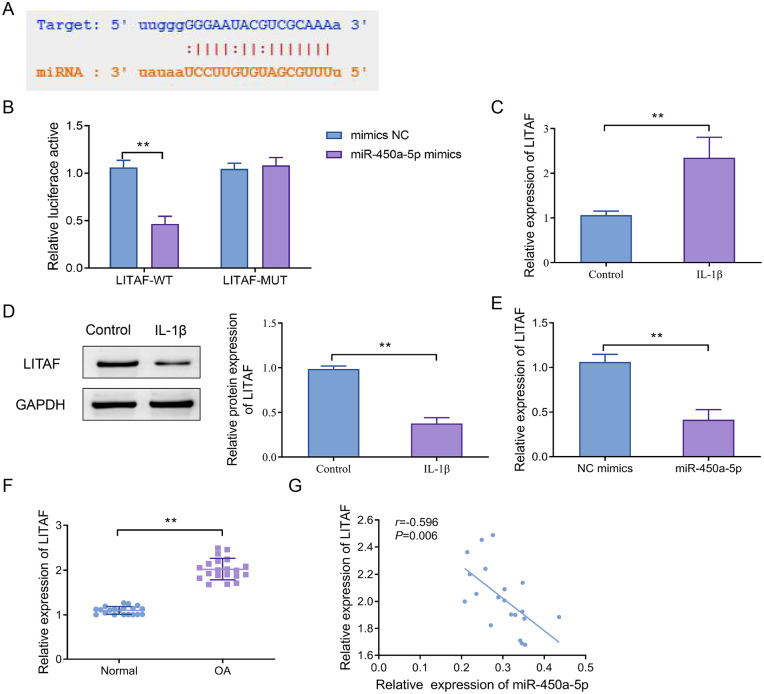
The interaction site between miR-450a-5p and LITAF was predicted by bioinformatics tools ( Fig. 3A ). To confirm this interaction, we constructed recombinant luciferase reporter gene vectors containing WT or MUT binding sites of LITAF and co-transfected them with miR-450a-5p mimics into cells. The results disclosed that miR-450a-5p mimics significantly inhibited the luciferase activity of the LITAF-WT vector (P < 0.01), whereas this effect was not observed in the LITAF-MUT vector ( Fig. 3B ). RT-qPCR and Western blot results revealed that LITAF expression was markedly up-regulated after IL-1β stimulation ( Fig. 3C and D ). Overexpression of miR-450a-5p remarkably suppressed LITAF expression ( Fig. 3E ). Furthermore, in clinical samples, LITAF expression was considerably elevated in the OA group in contrast to the normal group ( Fig. 3F ). Moreover, the Pearson correlation analysis disclosed that the expression of miR-450a-5p was negatively correlated with LITAF expression in OA tissues ( Fig. 3G ). The above results indicated that miR-450a-5p could bind to LITAF and inhibit its expression.
Figure 3.
LITAF serves as a direct target of miR-450a-5p. (A) The target sequences for miR-450a-5p binding to LITAF were predicted; (B) a dual-luciferase reporter assay was used to validate the binding of miR-450a-5p to LITAF, **P < 0.01; (C) RT-qPCR was utilized to detect the LITAF mRNA level in CHON-001 cells; (D) Western blot was adopted to determine the protein level of LITAF in CHON-001 cells, **P < 0.01; (E) the expression level of LITAF was measured after transfection with miR-450a-5p, **P < 0.01; (F) RT-qPCR was used to detect the LITAF mRNA level in cartilage tissues of OA patients, **P < 0.01; (G) the correlation between the expression of miR-450a-5p and LITAF was assessed by the Pearson analysis. LITAF = lipopolysaccharide-induced tumor necrosis factor-α factor; RT-qPCR = real-time quantitative polymerase chain reaction; OA = osteoarthritis.
Lipopolysaccharide-Induced Tumor Necrosis Factor-α Factor Reduces the Protective Effect of miR-450a-5p on Interleukin-1β-Induced CHON-001 cells
To determine whether miR-450a-5p regulated IL-1β-induced apoptosis, inflammation, and ECM degradation by inhibiting LITAF, we co-overexpressed miR-450a-5p and LITAF in CHON-001 cells. Western blot analysis showed that LITAF protein levels were significantly increased (~1.8-fold) in the miR-450a-5p + LITAF group compared to the miR-450a-5p + vector group ( Fig. 4A ). Functionally, compared with the NC mimics + vector group, the miR-450a-5p + vector group showed significantly higher cell proliferation (~2.0-fold) and reduced apoptosis (~50% decrease), while these effects were reversed by LITAF co-overexpression ( Fig. 4B–D ).
Figure 4.
LITAF reduces the protective effect of miR-450a-5p on IL-1β-induced chondrocytes. (A) Western blot was used to detect the changes in LITAF protein expression; (B) the proliferation ability of CHON-001 cells was measured by CCK-8; (C–D) the apoptosis level of CHON-001 cells; (E) ELISA was adopted to determine the levels of IL-6, IL-18, and TNF-α; (F–G) Western blot and quantitative statistical analysis for the protein expression levels of MMP-3, collagen III, and aggrecan, **P < 0.01 versus NC mimics + vector group, ##P < 0.01, versus miR-450a-5p + vector group. TNF-α = tumor necrosis factor-α; LITAF = lipopolysaccharide-induced TNF-α factor; IL = interleukin; CCK-8 = cell counting kit-8; ELISA = enzyme-linked immunosorbent assay; MMP-3 = matrix metalloproteinase-3.
ELISA results revealed that levels of IL-6, IL-18, and TNF-α were considerably decreased in the miR-450a-5p + vector group compared to the NC mimics + vector group, while these levels were significantly increased in the miR-450a-5p + LITAF group in comparison with the miR-450a-5p + vector group ( Fig. 4E ). Similarly, miR-450a-5p overexpression alleviated ECM degradation, as shown by reduced MMP-3 and increased collagen III and aggrecan protein levels, whereas LITAF co-expression counteracted these effects ( Fig. 4F and G ).
Overall, the above results collectively suggested that overexpression of LITAF reversed the ameliorative effects of miR-450a-5p on IL-1β-induced apoptosis, inflammation, and ECM degradation in CHON-001 cells.
Discussion
Abnormal regulation of miRNA level is closely associated with a variety of diseases, including OA. 27 Various miRNAs have been reported to regulate the development of OA. For example, microRNA-142-3p inhibits chondrocyte death and inflammation in OA by inhibiting the nuclear factor kappa B (NF-κB) pathway regulated by HMGB1, 28 and miR-210 also inhibits this pathway through targeting death receptor 6. 29 The findings underscore the critical role of miRNA-regulated signaling networks in OA. In this study, we found that miR-450a-5p expression was significantly reduced in OA cartilage and IL-1β-treated chondrocytes in contrast to the normal controls, suggesting its role in inhibiting OA progression. Given the multifaceted roles of miRNAs in cellular processes, we speculated that miR-450a-5p could interact with other miRNAs that target similar pathways or genes involved in OA. For instance, miR-140 is another miRNA known to be involved in cartilage homeostasis and OA. 30 It targets ADAMTS-5, a key enzyme responsible for cartilage degradation. It is conceivable that miR-450a-5p may work in conjunction with miR-140 to modulate cartilage matrix components and inflammation. Similarly, miR-34a, which targets the SIRT1 gene and is involved in apoptosis and senescence of chondrocytes, might interact with miR-450a-5p in regulating these processes. 31 Therefore, further research is required to elucidate the precise mechanisms and interactions between miR-450a-5p and other miRNAs.
IL-1β is known to play a key role in inducing cartilage degeneration. 32 It affects the ECM of articular cartilage, which mediates various biological responses for cartilage recovery and homeostasis. 33 Persistent ECM damage can impair joint function and accelerate OA development. 34 IL-1β modulates ECM composition by reducing levels of structural proteins like collagen III and aggrecan, 35 while promoting the production of MMPs such as MMP-1, MMP-3, and MMP-13, leading to cartilage degradation. 36 In our study, overexpression of miR-450a-5p in IL-1β-stimulated CHON-001 cells reversed these deleterious effects by enhancing cell proliferation, reducing apoptosis, and regulating cytokine secretion and ECM degradation markers such as IL-6, IL-18, TNF-α, MMP-3, collagen III, and aggrecan. This highlights the therapeutic potential of miR-450a-5p in OA. Modulation of miR-450a-5p expression, either via gene therapy or small molecule delivery, may alleviate inflammation and protect cartilage. Thus, future studies should focus on the role of miR-450a-5p in different OA models and investigate its interaction with other miRNAs and signaling cascades. However, despite its therapeutic potential, miRNA-based strategies face several translational challenges, including limited in vivo stability, off-target effects, and the difficulty of achieving sustained and targeted delivery to articular cartilage.
TNF-α, an important cytokine mediator of immunomodulation and inflammation, plays a pro-inflammatory and pro-apoptotic role in a variety of cell types. 37 LITAF, a transcription factor, enhances TNF-α gene expression and has been linked to the transcription of various inflammatory cytokines (such as TNF, monocyte chemoattractant protein 1 [MCP-1], and IL-10). 38 Dysregulation of LITAF expression contributes to chronic inflammation and has been implicated in cancers including pancreatic and gastric cancer. 39 For example, miR-1 has been shown to inhibit gastric cancer cell growth by targeting LITAF, 40 and miRNA-106 was reported to affect prostate cancer radioresistance via LITAF regulation. 41 In our study, we demonstrated that miR-450a-5p directly targets LITAF by binding its 3’UTR, thereby inhibiting its expression. This was validated by dual-luciferase reporter assay and supported by increased LITAF expression in OA tissues and IL-1β-treated chondrocytes. Moreover, simultaneous overexpression of miR-450a-5p and LITAF showed that LITAF could reverse the protective effects of miR-450a-5p. Mechanistically, LITAF has been shown to interact with STAT6, facilitating its nuclear translocation and transcriptional activation of inflammatory genes, including TNF-α. 42 In addition, LITAF may enhance NF-κB pathway activation, which is known to upregulate matrix-degrading enzymes such as MMP-3. 43 Therefore, knockdown of LITAF may downregulate both TNF-α and MMP-3 by attenuating these signaling cascades, thereby reducing inflammation and cartilage degradation in OA.
Our findings align with previous research highlighting the role of miRNAs in OA pathogenesis, such as miR-27 and miR-140, which modulate inflammatory pathways and cartilage degradation processes. 44 Like these miRNAs, miR-450a-5p appears to play a protective role against inflammation through its regulatory effects on LITAF. This discovery not only reinforces the importance of miR-450a-5p in OA but also distinguishes our findings by detailing the specific mechanisms through which miR-450a-5p acts. This could explain discrepancies with previous studies that may arise from different experimental conditions or focus on other miRNAs. Further validation of these results is necessary to consolidate miR-450a-5p’s role as a potential therapeutic target in OA.
However, we acknowledged several limitations in this study. First, our study was limited to a single human chondrocyte cell line and primarily relies on in vitro experiments. Therefore, in vivo animal experiments and more human chondrocyte cell lines should be taken into consideration in the future. Second, the sample size of this study was small, and future studies should include larger samples and validation in clinical samples. In addition, investigating the role of miR-450a-5p at different time points could provide a more comprehensive understanding.
In summary, our study provides an understanding of miRNA roles in OA, highlighting miR-450a-5p’s capacity to influence disease progression by targeting key molecular pathways. Future research should expand on the intricate network of miRNA interactions to fully elucidate their potential in OA treatment strategies.
Conclusion
To sum up, miR-450a-5p is significantly downregulated in OA tissues and has the ability to target and inhibit LITAF expression. This regulation helps suppress chondrocyte apoptosis, inflammation, and ECM degradation ( Fig. 5 ). Therefore, miR-450a-5p may be a promising potential target for the treatment of OA. Future studies should evaluate the in vivo efficacy of miR-450a-5p and develop targeted delivery strategies while clarifying its interactions with other miRNAs and signaling pathways to define its therapeutic potential.
Figure 5.
The diagram of potential mechanism by which miR-450a-5p targets and inhibits LITAF in OA.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035251344478 for Mir-450a-5p Ameliorates IL-1β-Induced Chondrocyte Apoptosis, Inflammation, and Extracellular Matrix Degradation by Down-Regulating LITAF by Guo-feng Jia, Wei Tan and Xu Han in CARTILAGE
Footnotes
Acknowledgments and Funding: The authors would like to thank the patients for their support in publishing this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: G.-f.J., W.T., and X.H. contributed to concepts, design, data analysis, statistical analysis, manuscript preparation, data acquisition, and manuscript editing.
Ethics Approval and Consent to Participate: All participants were informed and voluntarily signed the informed consent. All study procedures were approved by the research ethics committee of Affiliated Wuxi Fifth Hospital of Jiangnan University.
Consent of Publication: Not applicable.
ORCID iD: Xu Han  https://orcid.org/0009-0002-2960-6818
https://orcid.org/0009-0002-2960-6818
Availability of Data and Materials: The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2. Ye H, He Y, Zheng C, Wang F, Yang M, Lin J, et al. Type 2 diabetes complicated with heart failure: research on therapeutic mechanism and potential drug development based on insulin signaling pathway. Front Pharmacol. 2022;13:816588. doi: 10.3389/fphar.2022.816588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160-7. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yunus MHM, Nordin A, Kamal H. Pathophysiological perspective of osteoarthritis. Medicina (Kaunas). 2020;56(11):614. doi: 10.3390/medicina56110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11(1):35-44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16(3):6093-112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arif KMT, Elliott EK, Haupt LM, Griffiths LR. Regulatory mechanisms of epigenetic miRNA relationships in human cancer and potential as therapeutic targets. Cancers (Basel). 2020;12(10):2922. doi: 10.3390/cancers12102922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reynard LN, Barter MJ. Osteoarthritis year in review 2019: genetics, genomics and epigenetics. Osteoarthritis Cartilage. 2020;28(3):275-84. doi: 10.1016/j.joca.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 9. Lu X, Lin J, Jin J, Qian W, Weng X. Hsa-miR-15a exerts protective effects against osteoarthritis by targeting aggrecanase-2 (ADAMTS5) in human chondrocytes. Int J Mol Med. 2016;37(2):509-16. doi: 10.3892/ijmm.2015.2446. [DOI] [PubMed] [Google Scholar]
- 10. Cai C, Min S, Yan B, Liu W, Yang X, Li L, et al. MiR-27a promotes the autophagy and apoptosis of IL-1beta treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging (Albany NY). 2019;11(16):6371-84. doi: 10.18632/aging.102194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Chen L, Li Q, Wang J, Jin S, Zheng H, Lin J, et al. MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J Cell Mol Med. 2017;21(12):3347-59. doi: 10.1111/jcmm.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Shen S, Li Z, Li W, Weng X. MIR-140-5p affects chondrocyte proliferation, apoptosis, and inflammation by targeting HMGB1 in osteoarthritis. Inflamm Res. 2020;69(1):63-73. doi: 10.1007/s00011-019-01294-0. [DOI] [PubMed] [Google Scholar]
- 13. Koperski L, Kotlarek M, Swierniak M, Kolanowska M, Kubiak A, Gornicka B, et al. Next-generation sequencing reveals microRNA markers of adrenocortical tumors malignancy. Oncotarget. 2017;8(30):49191-200. doi: 10.18632/oncotarget.16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Yao L, Liu F, Hong J, Chen L, Zhang B, et al. Characterization of microRNA expression in serous ovarian carcinoma. Int J Mol Med. 2014;34(2):491-8. doi: 10.3892/ijmm.2014.1813. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Yang L, Liao F, Wang W, Wang ZF. MiR-450a-5p strengthens the drug sensitivity of gefitinib in glioma chemotherapy via regulating autophagy by targeting EGFR. Oncogene. 2020;39(39):6190-202. doi: 10.1038/s41388-020-01422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Chen S, Zhuo L, Zhu Y, Zheng H. Regulation of cancer stem cell properties, angiogenesis, and vasculogenic mimicry by miR-450a-5p/SOX2 axis in colorectal cancer. Cell Death Dis. 2020;11(3):173. doi: 10.1038/s41419-020-2361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kundu M, Basu J. The role of microRNAs and long non-coding RNAs in the regulation of the immune response to mycobacterium tuberculosis infection. Front Immunol. 2021;12:687962. doi: 10.3389/fimmu.2021.687962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zou J, Guo P, Lv N, Huang D. Lipopolysaccharide-induced tumor necrosis factor-alpha factor enhances inflammation and is associated with cancer (Review). Mol Med Rep. 2015;12(5):6399-404. doi: 10.3892/mmr.2015.4243. [DOI] [PubMed] [Google Scholar]
- 19. Liu G, Li Z, Yang M, Lin L, Liu J, Chen M. Functional characterization of a putative lipopolysaccharide-induced TNF-alpha factor (LITAF) from blood clam Tegillarca granosa in innate immunity. Fish Shellfish Immunol. 2020;97:390-402. doi: 10.1016/j.fsi.2019.12.051. [DOI] [PubMed] [Google Scholar]
- 20. Stefani C, Bruchez AM, Rosasco MG, Yoshida AE, Fasano KJ, Levan PF, et al. LITAF protects against pore-forming protein-induced cell death by promoting membrane repair. Sci Immunol. 2024;9(91):eabq6541. doi: 10.1126/sciimmunol.abq6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coaccioli S, Sarzi-Puttini P, Zis P, Rinonapoli G, Varrassi G. Osteoarthritis: new insight on its pathophysiology. J Clin Med. 2022;11(20):6013. doi: 10.3390/jcm11206013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Baicalin alleviates IL-1beta-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed Pharmacother. 2018;99:184-90. doi: 10.1016/j.biopha.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 23. Zhao F, Maren NA, Kosentka PZ, Liao YY, Lu H, Duduit JR, et al. An optimized protocol for stepwise optimization of real-time RT-PCR analysis. Hortic Res. 2021;8(1):179. doi: 10.1038/s41438-021-00616-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi J, Cao F, Chang Y, Xin C, Jiang X, Xu J, et al. Long non-coding RNA MCM3AP-AS1 protects chondrocytes ATDC5 and CHON-001 from IL-1beta-induced inflammation via regulating miR-138-5p/SIRT1. Bioengineered. 2021;12(1):1445-56. doi: 10.1080/21655979.2021.1905247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wani FA, Bhardwaj S. Evaluating the importance of apoptotic index, mitotic index and turnover index in premalignant and malignant lesions of cervix. J Open J Pathol. 2015;5(2):29-37. [Google Scholar]
- 26. Li Y, Wang P, Wu LL, Yan J, Pang XY, Liu SJ. miR-26a-5p inhibit gastric cancer cell proliferation and invasion through mediated Wnt5a. Onco Targets Ther. 2020;13:2537-50. doi: 10.2147/OTT.S241199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nugent M. MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthritis Cartilage. 2016;24(4):573-80. doi: 10.1016/j.joca.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Ju J, Ni B, Wang H. The emerging role of miR-506 in cancer. Oncotarget. 2016;7(38):62778-88. doi: 10.18632/oncotarget.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan S, Wang M, Zhao J, Zhang H, Zhou C, Jin L, et al. MicroRNA-34a affects chondrocyte apoptosis and proliferation by targeting the SIRT1/p53 signaling pathway during the pathogenesis of osteoarthritis. Int J Mol Med. 2016;38(1):201-9. doi: 10.3892/ijmm.2016.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li SH, Wu QF. MicroRNAs target on cartilage extracellular matrix degradation of knee osteoarthritis. Eur Rev Med Pharmacol Sci. 2021;25(3):1185-97. doi: 10.26355/eurrev_202102_24821. [DOI] [PubMed] [Google Scholar]
- 31. Yan S, Dong W, Li Z, Wei J, Han T, Wang J, et al. Metformin regulates chondrocyte senescence and proliferation through microRNA-34a/SIRT1 pathway in osteoarthritis. J Orthop Surg Res. 2023;18(1):198. doi: 10.1186/s13018-023-03571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Xu H, Li X, Chen H, Zhang H, Zhu X, et al. Cucurbitacin E reduces IL-1beta-induced inflammation and cartilage degeneration by inhibiting the PI3K/Akt pathway in osteoarthritic chondrocytes. J Transl Med. 2023;21(1):880. doi: 10.1186/s12967-023-04771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Y, Liu S, Huang J, Guo W, Chen J, Zhang L, et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459. doi: 10.1155/2014/648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan Z, Ji L. Hck promotes IL-1beta-induced extracellular matrix degradation, inflammation, and apoptosis in osteoarthritis via activation of the JAK-STAT3 signaling pathway. Adv Rheumatol. 2024;64(1):88. doi: 10.1186/s42358-024-00427-2. [DOI] [PubMed] [Google Scholar]
- 35. Yang B, Kang X, Xing Y, Dou C, Kang F, Li J, et al. Effect of microRNA-145 on IL-1beta-induced cartilage degradation in human chondrocytes. FEBS Lett. 2014;588(14):2344-52. doi: 10.1016/j.febslet.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 36. Mukherjee A, Das B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater Biosyst. 2024;13:100090. doi: 10.1016/j.bbiosy.2024.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mahdavi Sharif P, Jabbari P, Razi S, Keshavarz-Fathi M, Rezaei N. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine. 2020;130:155066. doi: 10.1016/j.cyto.2020.155066. [DOI] [PubMed] [Google Scholar]
- 38. Huang C, Chen D, Zhu H, Lv S, Li Q, Li G. LITAF enhances radiosensitivity of human glioma cells via the FoxO1 pathway. Cell Mol Neurobiol. 2019;39(6):871-82. doi: 10.1007/s10571-019-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Turan NN, Moshal KS, Roder K, Baggett BC, Kabakov AY, Dhakal S, et al. The endosomal trafficking regulator LITAF controls the cardiac Nav1.5 channel via the ubiquitin ligase NEDD4-2. J Biol Chem. 2020;295(52):18148-59. doi: 10.1074/jbc.RA120.015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen YC, Wu CC, Tu YT, Chen YR, Lee MC, Tsai KW. Involvement of the microRNA-1-LITAF axis in gastric cancer cell growth and invasion. Anticancer Res. 2020;40(11):6247-56. doi: 10.21873/anticanres.14645. [DOI] [PubMed] [Google Scholar]
- 41. Hoey C, Ray J, Jeon J, Huang X, Taeb S, Ylanko J, et al. miRNA-106a and prostate cancer radioresistance: a novel role for LITAF in ATM regulation. Mol Oncol. 2018;12(8):1324-41. doi: 10.1002/1878-0261.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang C, Qiao X, Jin Y, Yang W, Yu Z, Wang L, et al. An LPS-induced TNF-alpha factor involved in immune response of oyster Crassostrea gigas by regulating haemocytes apoptosis. Fish Shellfish Immunol. 2024;148:109513. doi: 10.1016/j.fsi.2024.109513. [DOI] [PubMed] [Google Scholar]
- 43. Li WJ, Zhou WP, Li XY, Jiang XL, Deng YC, Shen J, et al. LITAF promotes atherosclerotic plaque formation by stimulating the NF-kappaB inflammatory pathway. Curr Med Sci. 2023;43(6):1201-5. doi: 10.1007/s11596-023-2802-x. [DOI] [PubMed] [Google Scholar]
- 44. Malemud CJ. MicroRNAs and osteoarthritis. Cells. 2018;7(8):92. doi: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035251344478 for Mir-450a-5p Ameliorates IL-1β-Induced Chondrocyte Apoptosis, Inflammation, and Extracellular Matrix Degradation by Down-Regulating LITAF by Guo-feng Jia, Wei Tan and Xu Han in CARTILAGE