Abstract
Purpose
There are few studies on the reconstruction of the middle hepatic vein (MHV) tributaries in split liver transplantation (SLT) using right lobe grafts. This study aimed to compare the outcomes of patients undergoing combined reconstruction of MHV tributaries and the right hepatic vein (RHV) versus those undergoing separate reconstruction.
Methods
Clinical data of 114 patients who underwent SLT from March 2021 to December 2023 were retrospectively collected. We analyzed and compared the postoperative outcomes and reconstructed MHV patency between different modalities of MHV tributaries reconstruction in the right lobe SLT.
Results
Forty-five right lobe grafts were reconstructed with the MHV tributaries and were divided into two groups according to the type of reconstruction. Group 1 (n = 19) received separate reconstruction, while Group 2 (n = 26) received combined reconstruction. The duration of warm ischemia in Group 2 was significantly less than that in Group 1 (47.9 ± 7.8 vs. 59.7 ± 15.6 min, P = 0.002), and the ICU hospitalization time was significantly less in Group 2 than in Group 1 (2 vs. 3 days, P = 0.022). The complete patency rate of the reconstructed MHV at 30 days postoperatively was higher in Group 2 than in Group 1 (96.2% vs. 68.4%, P = 0.031). The rates of postoperative complications were comparable between the two groups.
Conclusion
Combined reconstruction of the MHV tributaries and RHV in SLT improves the rate of complete patency of the reconstructed MHV, reduces warm ischemia time, and shortens the length of ICU stay.
Keywords: Split liver transplantation, Middle hepatic vein, Right lobe, Patency
Introduction
The anatomy of the liver must be appropriately managed in split liver transplantation (SLT) to ensure that both grafts have a complete vascular and biliary supply [1]. The middle hepatic vein (MHV) drains both the right and left lobes of the liver and is usually preserved in the left lobe. Consequently, a lack of MHV drainage causes congestion in the right anterior segment, leading to a decrease in functional graft volume [2]. In contrast, reconstruction of the MHV tributaries can provide more functioning liver tissue to the right lobe, which is physiologically and functionally equivalent to an extended right liver graft [3].
Although techniques for reconstructing the MHV tributaries to restore drainage continue to evolve, the method for optimal outflow reconstruction in right lobe grafts has been inconclusive to date. Most of the past studies were based on living donor liver transplantation, and the materials used to reconstruct the MHV tributaries were mostly expanded polytetrafluoroethylene (ePTFE) due to material shortages and variability within the same study, which would limit the interpretation of the results [4, 5, 6]. Recently, we started to reconstruct the MHV tributaries with iliac vessels in SLT and then anastomosed the reconstructed MHV and the right hepatic vein (RHV) to the inferior vena cava (IVC). This study aimed to assess the feasibility of using iliac vessels for combined MHV tributaries reconstruction in SLT and to compare the postoperative outcomes with separate reconstruction.
Materials and methods
Patients
Clinical data of 114 patients who underwent SLT from March 2021 to December 2023 were retrospectively collected. The exclusion criteria were as follows: (1) patients with left lobe grafts; (2) patients with insufficient clinical data; (3) patients without reconstruction of the MHV tributaries; and (4) patients with extended right liver graft (Fig. 1). Based on the surgical method, the patients were divided into Group 1 and Group 2, with 19 patients in Group 1(MHV tributaries and the RHV anastomosed separately to the IVC) and 26 patients in Group 2(MHV tributaries and the RHV combined to anastomose to the IVC). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All livers were donated from citizens upon death and were not procured from prisoners. This research was approved by the Ethics Committee of The Affiliated Lihuili Hospital of Ningbo University (approval number: KY2024SL239).
Fig. 1.
The patients flow in this study
Variables and definitions
The basic patient data acquired from our center’s liver transplant database included age, sex, indication for transplantation, whether accompanied by liver cancer, model for end-stage liver disease (MELD) score, and Child-Pugh classification. Perioperative indicators included cold ischemia time, warm ischemia time, operation time, intraoperative bleeding, graft weight, graft to recipient weight ratio (GRWR), type of vessel grafts, number of segment 5 (V5), and segment 8 (V8) reconstructions, reconstructed MHV diameter, postoperative liver function test at 1, 3, and 7 days, postoperative complications, ICU hospitalization time, postoperative length of stay, postoperative 7 and 30 days patency of the reconstructed MHV. Cold ischemia time was defined as the interval from the initiation of cold perfusion to the removal of the graft from ice. Warm ischemia time was defined as the interval from aortic cross-clamp (or asystole in non-heart-beating donors) to cold perfusion during retrieval and from removal from ice to establishment of reperfusion after portal vein anastomosis during implantation [7]. Serious complications were defined as complications with a grade ≥ III according to the Clavien‒Dindo classification [8]. Reconstructed MHV occlusion was defined as the absence of blood flow on the venous phase of the abdominal contrast-enhanced computed tomography (CECT). Patency was defined as the presence of blood flow in at least one branch of the reconstructed MHV tributaries, while complete patency was defined as blood flow in all branches of the reconstructed MHV. The patency of the reconstructed MHV was evaluated by daily Doppler ultrasound within the first 3 days after surgery, and abdominal CECT was performed 7 days postoperatively and repeated at 30 days.
Donor operation
First, the gallbladder was resected, the common bile duct was ligated along the superior border of the pancreas, and the right hepatic artery and the right branch of the portal vein were exposed and slung. Intraoperative Doppler ultrasound was used to localize the MHV and its branches; the right hepatic artery and the right branch of the portal vein were clamped, and the splitting lines were marked to the right of the ischemic line. The liver was mobilized by dividing its ligaments, a retrohepatic tunnel was established, and a sling was placed on the left side of the RHV and below the bifurcation of the portal vein to traction the liver. The parenchyma was transected along the right side of the MHV using a Cavitron Ultrasonic Surgical Aspirator (CUSA), and the V5/V8 larger than 5 mm were preserved. After dissection of the parenchyma, cholangiography was performed through the severed end of the common bile duct to define the anatomy of the biliary system, and then the bile duct was dissected. Intravenous heparin was given, and the donor liver was irrigated with University of Wisconsin (UW) solution and moved to the back table. Bilateral iliac vessels were obtained from the donor for backup.
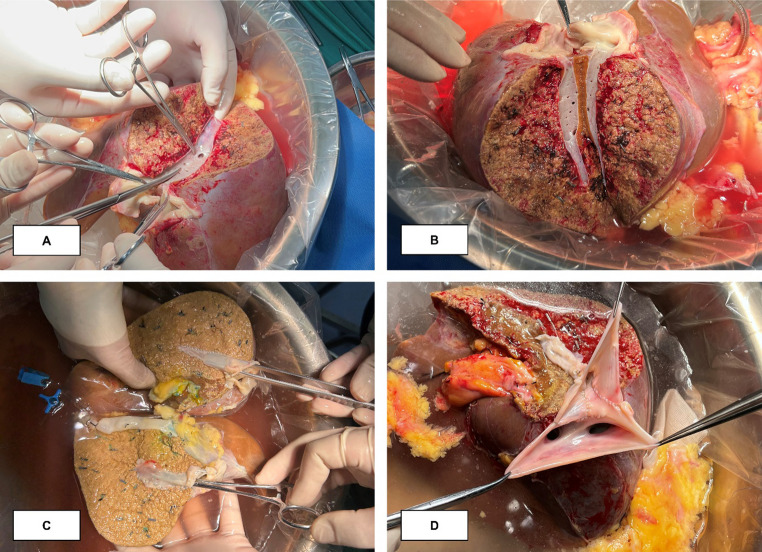
Benching
The MHV tributaries were reconstructed using cadaveric iliac vessels. The reconstruction was performed by creating either one Y-shaped branch (Fig. 2A) or two separate branches (Fig. 2B) using 6 − 0 Prolene. The reconstructed MHV tributaries were then prepared for anastomosis with the IVC. In Group 2, we longitudinally dissected the IVC (Fig. 2C) and used an additional IVC patch to combine the reconstructed MHV and RHV into a triangular outflow tract (Fig. 2D). In two patients, the MHV was split along its center (Fig. 3A) to form two grafts, each containing half of the MHV (Fig. 3B), which were reconstructed into a complete MHV using a venous patch (Fig. 3C), and subsequently reconstructed with the RHV to form a triangular outflow tract (Fig. 3D).
Fig. 2.
Reconstruction of the middle hepatic vein in the right lobe of the liver (A) Reconstruction of the middle hepatic vein with Y-shaped vessel; (B) Reconstruction of the middle hepatic vein with linear vessels; (C) Incision of inferior vena cava; (D) Reconstructed middle hepatic vein and right hepatic vein form a common outflow
Fig. 3.
Reconstruction of the outflow by use of a split middle hepatic vein (A) Clipping the posterior wall of the middle hepatic vein; (B) Acquire two grafts with half of the middle hepatic vein; (C) Reconstruction into two grafts with intact middle hepatic vein; (D) Completion of reconstruction of outflow
Recipient operation
The diseased liver was resected after clamping the IVC with side clamps. The RHV opening of the IVC was dissected longitudinally to form an oval orifice. The iliac vessels and the donor RHV in Group 1 were separately anastomosed to the recipient IVC in an end-to-side fashion (Fig. 4A and B). From the opening of the RHV on the IVC in Group 2, the orifices of the MHV and the left hepatic vein were clipped to the left to form a triangular opening, and the common outflow was anastomosed to the recipient IVC using a 4 − 0 Prolene (Fig. 4C and D). The portal vein was anastomosed end-to-end with 6 − 0 Prolene, the portal vein and IVC were successively opened to restore graft perfusion, and finally, the hepatic artery and bile duct were anastomosed in an end-to-end fashion. Intraoperative Doppler ultrasound was used to detect the graft outflow.
Fig. 4.
Right lobe graft implantation with different types of outflow tract reconstruction (A) Schematic illustration of right lobe graft implantation in Group 1; (B) End-to-side anastomosis of the right hepatic vein to the inferior vena cava; (C) Schematic illustration of right lobe graft implantation in Group 2; (D) Anastomosis of a triangular outflow over the inferior vena cava
Statistical analysis
The data were processed using IBM SPSS Statistics (version 26.0, IBM Corp., Armonk, NY, USA). Student’s t-test was used to analyze normally distributed continuous variables, which were reported as means and standard deviation (SD). The Mann-Whitney U test was used to analyze continuous variables that were not normally distributed. These variables were reported as the median and interquartile range (IQR). Categorical variables were examined using Fisher’s exact test or the Chi-squared test, and they were reported as numbers and percentages. Postoperative liver function curves were plotted using GraphPad Prism software version 9.5. Intergroup differences were deemed statistically significant when P < 0.05.
Results
Of the 45 patients, 41 were male and 4 were female, with a mean age of 54.2 ± 8.2 years. The primary indication for transplantation was chronic hepatitis B infection, accounting for 68.4% and 73.1% of Group 1 and Group 2, respectively. It was accompanied by hepatocellular carcinoma in more than half of the recipients, 73.7% (14/19) in Group 1 and 69.2% (18/26) in Group 2. The baseline demographic characteristics of the two groups are summarized in Table 1. No statistical differences were observed between the baseline data of the two groups (P > 0.05).
Table 1.
Demographic characteristics of the recipients and donors in SLT
| Characteristic | Group1 | Group2 | p-Value |
|---|---|---|---|
| (n = 19) | (n = 26) | ||
| Recipient | |||
| Age, years | 52.6 ± 8.8 | 55.3 ± 7.7 | 0.276 |
| Sex, male: female | 18/1 | 23/3 | 0.841 |
| Indication for transplantation | 0.330 | ||
|
Hepatitis B cirrhosis Hepatitis C cirrhosis Alcoholic liver cirrhosis |
13(68.4) 1(5.3) 4(21.1) |
19(73.1) 1(3.8) 1(3.8) |
|
|
Autoimmune hepatitis Cryptogenic cirrhosis |
1(5.3) 0 |
4(15.4) 1(3.8) |
|
| Accompanying HCC | 14(73.7) | 18(69.2) | 0.745 |
| Child-Pugh classification | 0.671 | ||
| A | 6(31.6) | 11(42.3) | |
| B | 10(52.6) | 10(38.5) | |
| C | 3(15.8) | 5(19.2) | |
|
MELD score Donor |
11(8–15) | 12(7.75–16) | 0.972 |
| Age, years | 39.7 ± 11.6 | 42.4 ± 11.4 | 0.441 |
| Sex, male: female | 17/2 | 24/2 | 1.000 |
Data are presented as mean ± standard deviation or median (interquartile range) or n (%). HCC, hepatocellular carcinoma; MELD, model for end stage liver disease;
Warm ischemia time was significantly longer in Group 1 than in Group 2 (59.7 ± 15.6 vs. 47.9 ± 7.8 min, P = 0.002). Group 1 required a longer duration of ICU stay (3 vs. 2 days, P = 0.022). No statistically significant difference was observed between the two groups in terms of operative time, intraoperative bleeding, graft weight, GRWR, type of vessel grafts, number of V5 and V8 reconstructions, reconstructed MHV diameter, cold ischemia time, serious complications, and hospital stay (Table 2).
Table 2.
Intraoperative characteristics and postoperative parameters of the recipients in SLT
| Characteristic | Group1 | Group2 | p-Value |
|---|---|---|---|
| (n = 19) | (n = 26) | ||
| Operation time, min | 526.1 ± 127.9 | 489.2 ± 88.8 | 0.259 |
| Blood loss, ml | 1000(600–1800) | 900(750–1500) | 0.826 |
| Graft weight, g | 857.6(758–1070) | 952.4(852.3–1032.2) | 0.605 |
| GRWR, % | 1.38 ± 0.29 | 1.43 ± 0.37 | 0.650 |
| Type of vessel grafts | 1.000 | ||
|
Iliac vein graft Iliac artery graft |
4(21.1) 15(78.9) |
5(19.2) 21(80.8) |
|
| No. of V5 reconstructed | 0.373 | ||
|
0 1 2 |
4(21.1) 15(78.9) 0 |
4(15.4) 19(73.1) 3(11.5) |
|
| No. of V8 reconstructed | 0.158 | ||
|
0 1 |
3(15.8) 16(84.2) |
9(34.6) 17(65.4) |
|
| Reconstructed MHV diameter, mm | 9(8–12) | 9(7–10) | 0.384 |
| Warm ischemia time, min | 59.7 ± 15.6 | 47.9 ± 7.8 | 0.002 |
| Cold ischemia time, min | 240(210–330) | 299(251–372.3) | 0.206 |
| ICU stay, days | 3(2–4) | 2(1–3.25) | 0.022 |
| Serious complication | 6(31.6) | 8(30.8) | 0.954 |
| Postoperative length of stay, days | 21(14–32) | 20.5(12–32.75) | 0.954 |
| Patency of the reconstructed MHV, % | |||
| POD 7 | |||
| Patency rate | 18(94.7) | 26(100) | 0.422 |
| Full patency rate | 17(89.5) | 26(100) | 0.173 |
| POD 30 | |||
| Patency rate | 17(89.5) | 25(96.2) | 0.788 |
| Full patency rate | 13(68.4) | 25(96.2) | 0.034 |
Data are presented as mean ± standard deviation or median (interquartile range) or n (%). GRWR, graft to recipient weight ratio; MHV, middle hepatic vein; ICU, intensive care unit;
The incidence of serious postoperative complications in Group 1 was 31.6% (6/19), including one case of intestinal perforation, which was treated with reoperation; two cases of biliary anastomotic stenosis, treated with interventional therapy; one case of gastrointestinal hemorrhage, treated with gastroscopy, but died after complication of multiorgan failure; and two cases of intra-abdominal hemorrhage treated by reoperation. The incidence of serious complications in Group 2 was 30.8% (8/26). Four cases of intra-abdominal hemorrhage were treated surgically, including one case each of bleeding from the vessels of the lesser curvature of the stomach and the greater curvature of the stomach, one case of perforated bleeding from a gastric ulcer combined with cardiac failure that led to the patient’s death and one case of bleeding from the anastomosis of the hepatic artery; one case of gastric retention was treated by endoscopic placement of an enteral nutritional tube; one case of choledochal necrosis that led to a bile leakage was treated by choledochoenteric anastomosis; one case of biliary anastomotic stenosis was treated with interventional therapy; and one case of acute renal failure was treated with hemodialysis.
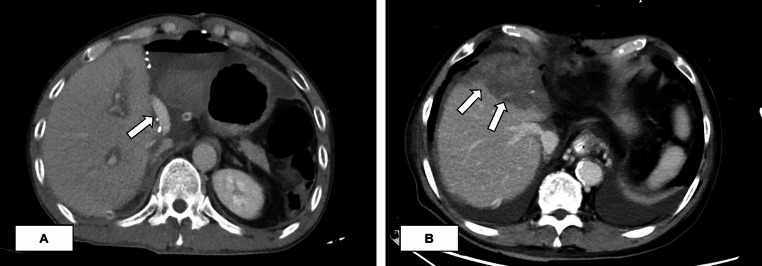
Most liver function indexes peaked on POD 1 and then gradually decreased. POD 1, 3, and 7 aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), and international normalized ratio of prothrombin time (PT-INR) showed no statistically significant differences between the two groups (Fig. 5). The patency rate of reconstructed MHV at POD 7 was 94.7% in Group 1 and 100% in Group 2 (P = 0.422), and the complete patency rate was 89.5% and 100%, respectively (P = 0.173). The patency rate of the reconstructed MHV at POD 30 was 89.5% in Group 1 and 96.2% in Group 2 (P = 0.788), and the complete patency rate was 68.4% and 96.2%, respectively (P = 0.034). In most patients, blood flow was visible in the reconstructed MHV on postoperative CECT (Fig. 6A). In contrast, when the reconstructed MHV was completely obstructed, the right anterior segment often showed severe congestion (Fig. 6B).
Fig. 5.
Postoperative liver function tests in the recipients
Fig. 6.
Reconstructed middle hepatic vein patency assessed by contrast-enhanced CT (A) A patient with reconstructed middle hepatic vein (arrow) patency on CT 30 days after surgery; (B) A patient with reconstructed middle hepatic vein obstruction with right anterior lobe congestion (arrow) 7 days after surgery
Discussion
A right lobe without an MHV often experiences right anterior segment congestion, which can lead to severe graft dysfunction and septic complications [9]. Previously, reconstruction of MHV tributaries to restore drainage was considered time-consuming and added to the complexity of liver transplantation until Lee et al. [10] reconstructed all V5 and V8 larger than 5 mm with favorable results. Current studies have shown that right anterior segment regeneration in right lobe grafts with reconstructed MHV is significant compared to that without MHV tributaries reconstructed [11, 12] and improves the survival of the recipients [13]. However, no consensus exists on the standard technique for outflow reconstruction. In this study, combined reconstruction of MHV tributaries and RHV increases the rate of complete patency of the reconstructed MHV at POD 30 and reduces warm ischemia time, contributing to postoperative recovery. Furthermore, to the best of our knowledge, this is the first study comparing two different outflow reconstruction modalities in SLT using right lobe grafts.
Most centers have adopted separate reconstruction of the RHV and V5/V8 for those who need to reconstruct the MHV tributaries, influenced by living donor liver transplantation. However, some studies have reported that the graft will regenerate in all directions after implantation in the recipient’s right subphrenic, and due to the lack of space in the right subphrenic, the liver will regenerate mainly to the left and ventral side; the enlarged graft will compress the IVC and anastomosis of the RHV from the dorsal side will result in outflow obstruction [14]. Furthermore, an enlarged graft will result in kinking and angulation of the reconstructed MHV tributaries, leading to occlusion of the MHV tributaries. To overcome these problems, Ito et al. [15] sutured the MHV tributaries, the RHV, and the inferior right hepatic vein to a homologous IVC patch and performed a side-to-side anastomosis with the recipient IVC to form a double vena cava. The additional vena cava was able to tolerate compression from liver regeneration and surrounding tissues. Their other approach is to anastomose the reconstructed MHV tributaries to the donor RHV to form a common orifice and then to suture it to a vein patch to form the anterior wall of the IVC. Both techniques use enlargement of the outflow tract to relieve external compression. Kim et al. [16] reconstructed the MHV with an ePTFE before anastomosing it to the RHV and suturing a long saphenous vein patch around the common outflow, which can offset the distortion caused by graft regeneration and effectively expand the outflow tract. In this study, cadaveric iliac vessels were used to reconstruct the MHV tributaries and then anastomosed with the donor RHV to form a common outflow and to make the anastomosis in a triangular shape (Fig. 4D), which is less prone to being twisted and narrowed compared to the original linear anastomosis (Fig. 4B) and without the need for additional patches or artificial vessels. Combined reconstruction of the RHV with MHV tributaries enlarges the outflow, mimicking the natural venous outflow and creating physiologic laminar flow, thereby improving the postoperative prognosis [17]. In the present study, AST and ALT were lower in postoperative Group 2 as compared to Group 1, although the difference was not statistically significant. The ICU stay was also shorter in Group 2, suggesting that combined reconstruction of the outflow may be beneficial for postoperative recovery.
Right lobe graft outflow reconstruction is performed in a narrow and deep space. Excessive anastomosis operations will lead to prolonged warm ischemia time, aggravate ischemia-reperfusion injury, and affect the recovery of graft function in the early postoperative period. Most of the anastomotic procedures in Group 2 were completed on the back table, effectively reducing the warm ischemia time. Our study showed that the warm ischemia time was significantly shorter in Group 2 (47.9 ± 7.8 vs. 59.7 ± 15.6 min, P = 0.002), while the cold ischemia time was not significantly prolonged (240(210–330) vs. 299(251–372.3), P = 0.206). Although the prolongation of cold ischemia time may have resulted in selection bias due to limited sample size showing no statistical difference, we believe this is acceptable. Previous studies have shown that cold ischemia time greater than 10 h is a risk factor for graft loss in split liver transplantation [18].
Early patency of the reconstructed MHV seems to be of greater concern than long-term patency, and 1 to 2 weeks of patency of the reconstructed MHV is sufficient for graft function, as intrahepatic collateral circulation will be established in approximately 1 week postoperatively [10, 19]. The patency rates of the reconstructed MHV at POD 7 were 94.7% and 100% (P = 0.422) in Groups 1 and 2 in this study; the complete patency rates were 89.5% and 100% (P = 0.173), respectively. The patency rates were similar to those reported in other studies [17, 20]. The patency rates at POD 30 were 89.5% and 96.2% (P = 0.565) for Groups 1 and 2, and 68.4% and 96.2% (P = 0.031) for complete patency, respectively. The rate of complete patency was significantly higher in Group 2 than in Group 1 at POD 30. We observed more V5 occlusion in Group 1, probably due to the fact that V5 is usually longer than V8 and undergoes distortion and angulation after liver regeneration, resulting in reduced blood flow and gradual vessel occlusion, whereas in Group 2, the outflow tracts were reconstructed into triangular shapes and enlarged the outflow tracts, thus effectively increasing the rate of complete patency.
As for how to improve the patency of the reconstructed MHV in right lobe transplantation, we also explored the technique of splitting the MHV, which preserves all the tiny branches on the MHV, and theoretically, it seems to be more favorable to the drainage of the right anterior segment of the liver (Fig. 3B); meanwhile, splitting the MHV in this way also makes the reconstructed MHV tributaries and the RHV into a common opening, which may be more favorable to the patency after liver regeneration; however, at present, the sample size is still small, and we will make another comparison in a future study.
There are several limitations to this study. This was a retrospective and small-sample study. Owing to this, the liver function did not show significant differences between the two groups postoperatively. However, split liver transplantation for two adult recipients remains an extremely challenging procedure, even in high-volume transplant centers. This technique requires not only advanced surgical expertise but also strict recipient selection criteria. Further prospective randomized controlled studies are still needed to validate our findings.
Conclusion
In conclusion, combined reconstruction of RHV and MHV tributaries improves the complete patency rate of the reconstructed MHV in the early postoperative period, shortens the warm ischemia time, facilitates postoperative recovery, improves the patient’s prognosis, and provides more choices for the reconstruction of the right lobe graft outflow in SLT.
Author contributions
Z.L. and J.F. participated in the conception and design of the research. All authors participated in the acquisition of the data. Z.L., Y.Y., and H.Z. participated in the analysis and interpretation of the data. Z.L. participated in the writing of the paper. All authors participated in the revision and final approval of the manuscript.
Funding
This work was supported partly by the following funding: Ningbo Public Welfare Science and Technology Plan Project (2024S155), The Ningbo Major Research and Development Plan Project (2024Z179) and Ningbo Top Medical and Health Research Program (2024020818).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reason able request.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The Affiliated Lihuili Hospital of Ningbo University (approval number: KY2024SL239), and individual consent for this retrospective analysis was waived.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Caide Lu, Email: lucaide@nbu.edu.cn.
Jiongze Fang, Email: fangjiongze@gmail.com.
References
- 1.Chaib E, Ribeiro MA Jr., Saad WA, Gama-Rodrigues J (2005) The main hepatic anatomic variations for the purpose of split-liver transplantation. Transplant Proc 37((2):1063–6. 10.1016/j.transproceed.2004.11.054 [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto K, Ogawa K, Tamura K, Ito C, Iwata M, Sakamoto A et al (2022) Importance of reconstruction of middle hepatic vein tributaries of right-lobe grafts in living donor liver transplantation: demonstration of the reconstruction technique. Langenbeck’s Archives Surg 407(4):1585–1594. 10.1007/s00423-021-02398-0 [DOI] [PubMed] [Google Scholar]
- 3.Sugawara Y, Makuuchi M, Sano K, Imamura H, Kaneko J, Ohkubo T et al (2003) Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg 237(2):180–185. 10.1097/01.SLA.0000048444.40498.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borle DP, Pamecha V, Bharathy KGS, Sasturkar SV, Sinha PK, Patidar Y et al (2018) Explant portal vein for reconstructing middle hepatic vein in right lobe living donor liver transplantation-outcome analysis. HPB (Oxford) 20(12):1137–1144. 10.1016/j.hpb.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 5.Thorat A, Jeng LB, Yang HR, Li PC, Li ML, Yeh CC et al (2016) Outflow reconstruction for right liver allograft with multiple hepatic veins: V-plasty of hepatic veins to form a common outflow channel versus 2 or more hepatic vein-to-inferior Vena Cava anastomoses in limited retrohepatic space. Liver Transpl 22(2):192–200. 10.1002/lt.24342 [DOI] [PubMed] [Google Scholar]
- 6.Pomposelli JJ, Akoad M, Khwaja K, Lewis WD, Cheah YL, Verbesey J et al (2012) Evolution of anterior segment reconstruction after live donor adult liver transplantation: a single-center experience. Clin Transpl 26(3):470–475. 10.1111/j.1399-0012.2011.01529.x [DOI] [PubMed] [Google Scholar]
- 7.Halazun KJ, Al-Mukhtar A, Aldouri A, Willis S, Ahmad N (2007) Warm ischemia in transplantation: search for a consensus definition. Transplant Proc 39(5):1329–31. 10.1016/j.transproceed.2007.02.061 [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg. 2004;240(2):205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed]
- 9.Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K et al (2001) Congestion of right liver graft in living donor liver transplantation. Transplantation 71(6):812–814. 10.1097/00007890-200103270-00021 [DOI] [PubMed] [Google Scholar]
- 10.Gyu Lee S, Min Park K, Hwang S, Hun Kim K, Nak Choi D, Hyung Joo S et al (2002) Modified right liver graft from a living donor to prevent congestion1. Transplantation 74(1):54–59. 10.1097/00007890-200207150-00010 [DOI] [PubMed] [Google Scholar]
- 11.Akamatsu N, Sugawara Y, Nagata R, Kaneko J, Aoki T, Sakamoto Y et al (2014) Adult right Living-Donor liver transplantation with special reference to reconstruction of the middle hepatic vein. Am J Transplant 14(12):2777–2787. 10.1111/ajt.12917 [DOI] [PubMed] [Google Scholar]
- 12.Mizuno S, Iida T, Yagi S, Usui M, Sakurai H, Isaji S et al (2006) Impact of venous drainage on regeneration of the anterior segment of right living-related liver grafts. Clin Transplant 20(4):509–516. 10.1111/j.1399-0012.2006.00515.x [DOI] [PubMed] [Google Scholar]
- 13.Guo HJ, Wang K, Chen KC, Liu ZK, Al-Ameri A, Shen Y et al (2019) Middle hepatic vein reconstruction in adult right lobe living donor liver transplantation improves recipient survival. Hepatobiliary Pancreat Dis Int 18(2):125–131. 10.1016/j.hbpd.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Lee SG (2006) Techniques of reconstruction of hepatic veins in living-donor liver transplantation, especially for right hepatic vein and major short hepatic veins of right-lobe graft. J Hepatobiliary Pancreat Surg 13(2):131–138. 10.1007/s00534-005-1019-7 [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Akamatsu N, Tani K, Ito D, Kaneko J, Arita J et al (2016) Reconstruction of hepatic venous tributary in right liver living donor liver transplantation: the importance of the inferior right hepatic vein. Liver Transpl 22(4):410–419. 10.1002/lt.24386 [DOI] [PubMed] [Google Scholar]
- 16.Kim JD, Choi DL, Han YS (2014) Simplified one-orifice venoplasty for middle hepatic vein reconstruction in adult living donor liver transplantation using right lobe grafts. Clin Transpl 28(5):561–568. 10.1111/ctr.12355 [DOI] [PubMed] [Google Scholar]
- 17.Pamecha V, Pattnaik B, Sinha PK, Patil NS, Mohapatra N, Sasturkar SV et al (2021) Single orifice outflow reconstruction: refining the venous outflow in modified right lobe live donor liver transplantation. J Gastrointest Surg 25(8):1962–1972. 10.1007/s11605-020-04776-3 [DOI] [PubMed] [Google Scholar]
- 18.Halac E, Dip M, Quinonez E, Alvarez F, Espinoza JL, Romero P et al (2016) Split liver transplantation: report of right and left graft outcomes from a multicenter Argentinean group. Liver Transpl 22(1):63–70. 10.1002/lt.24338 [DOI] [PubMed] [Google Scholar]
- 19.Yi NJ, Suh KS, Lee HW, Cho EH, Shin WY, Cho JY et al (2007) An artificial vascular graft is a useful interpositional material for drainage of the right anterior section in living donor liver transplantation. Liver Transpl 13(8):1159–1167. 10.1002/lt.21213 [DOI] [PubMed] [Google Scholar]
- 20.Durairaj MS, Shaji Mathew J, Mallick S, Nair K, Manikandan K, Titus Varghese C et al (2021) Middle hepatic vein reconstruction in adult living donor liver transplantation: a randomized clinical trial. Br J Surg 108(12):1426–1432. 10.1093/bjs/znab346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reason able request.