Abstract
Psychomotor retardation, characterized by slowing of speech, thoughts, and a decrease of movements, is frequent in patients with major depressive disorder (MDD). However, its neurobiological correlates are still poorly understood. This study aimed to explore if cerebral blood flow (CBF) and resting state functional connectivity (rs-FC) of the motor network are altered in patients with MDD and if these changes are associated with psychomotor retardation. Thirty-six right-handed patients with depression and 19 right-handed healthy controls (HC) that did not differ regarding age and sex underwent arterial spin labelling (ASL) and resting-state functional MRI (rs-fMRI) scans. Psychomotor retardation was assessed with the motoric items of the core assessment of psychomotor change (CORE) questionnaire. Patients with MDD had more pronounced psychomotor retardation scores than HC. Patients with MDD had reduced CBF in bilateral cingulate motor area (CMA) and increased resting-state functional connectivity (rs-FC) between the cluster in the CMA and a cluster localized in bilateral supplementary motor areas (SMA). Furthermore, increased rs-FC between the CMA and the left SMA was associated with more pronounced psychomotor retardation. Our results suggest that reduced perfusion of the CMA and increased rs-FC between the CMA and the SMA are associated with psychomotor retardation in patients with depression.
Keywords: Depression, Psychomotor retardation, Arterial spin labelling, Functional MRI
Introduction
Psychomotor disturbances are frequent in major depressive disorder (MDD). The Diagnostic and Statistical Manual (DSM-5) lists the following items as related to psychomotor disturbances: psychomotor retardation/ agitation and loss of interest in previously enjoyed activities. Psychomotor retardation is more frequent than psychomotor agitation [1, 2]. It is characterized by a slowing of speech, thoughts, and a decrease of movements [3]. Psychomotor retardation including decreased physical activity has been repeatedly reported in patients with MDD and even in patients with remitted depression [1, 4]. Psychomotor retardation is particularly pronounced in melancholic depression [5, 6], a subtype of depression with more marked biological alterations [7, 8] and presumably greater reduction in symptom severity following pharmacological antidepressive treatment [9]. Furthermore, psychomotor retardation may predict treatment response to electroconvulsive therapy (ECT) [10]. Although psychomotor retardation is frequently encountered in depression, few studies have targeted the behavioral assessment or neurobiological underpinnings of this phenomenon. Studies using wrist actigraphy reported links to daily activities and symptom ratings [11]. Furthermore, reduced physical activity in subjects with MDD was associated with alterations in cerebral resting perfusion or white matter pathways in core components of the motor system, particularly in premotor areas such as cingulate motor area (CMA) and supplementary motor area (SMA) [12–17].
Motor behaviour underlies a network that primarily comprises the basal ganglia, the cerebellum, the primary and the supplementary motor cortex (SMA) [18–20]. Further regions that are essential for motivated and goal directed behaviour are the prefrontal cortex (PFC) and the anterior cingulate cortex (ACC). Both the PFC and the ACC have strong links to limbic brain regions (e.g. amygdala, hippocampus) [21, 22]. The ACC plays an important role for action-outcome learning, specifically the CMA integrates reward outcome information, action information and it contains projections to premotor cortical areas [21, 22]. Structural and functional alterations in PFC, ACC, CMA and SMA have been reported repeatedly in patients with depression [7, 13, 14, 23]. Functionally, these disturbances may lead to lack of motivation and drive consequently resulting in psychomotor retardation [12, 15, 24, 25].
Resting-state cerebral blood flow offers insights on locally altered states of brain metabolism [26]. Brain areas with tonically altered neural activity are expected to exert distinct patterns of functional connectivity with other brain areas [27]. It was the aim of this analysis to extend knowledge on the neurobiology of psychomotor retardation in MDD using a multimodal functional magnetic resonance imaging (fMRI) approach. First, we aimed to investigate if there are alterations of cerebral blood flow (CBF) in the motor network in patients with MDD. We hypothesized reduced CBF in motor areas in patients with MDD, as psychomotor retardation is a frequent sign in MDD. Second, we aimed to explore, if these alterations in CBF are associated with changes in resting-state functional connectivity (rs-FC) between areas of reduced CBF and regions within the motor network. Third, we hypothesized that more pronounced changes in CBF and in rs-FC within the motor network are associated with higher ratings of psychomotor retardation.
Methods
Participants
Thirty-six currently depressed individuals were recruited at the University Hospital of Psychiatry and Psychotherapy in Bern, Switzerland. Participants of the present study have also been included in previous reports [7, 23, 28–31]. Inclusion criteria for this study were a diagnosis of major depressive disorder (MDD) according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [32], a completed assessment of the core assessment of psychomotor change (CORE) questionnaire [33], age between 18 and 65 years and right-handedness as assessed using the Edinburgh Handedness Inventory [34]. We used the Mini International Neuropsychiatric Interview (MINI) to screen for psychiatric comorbidities [35] and the structured clinical interview for DSM-IV Axis II (SCID-II) to screen for personality disorders [36], which were exclusion criteria to participate in the study. Furthermore, we excluded participants with neurological disorders and with contraindications to perform an MRI-scan (e.g. claustrophobia). We used the 21-item Hamilton rating scale for depression (HAMD) [37] and the 21-item self-report Beck Depression Inventory (BDI-II) [38] to assess depression severity. To assess psychomotor retardation, we used a sum score of the items 3 (postural slumping), 10 (body immobility (amount, not speed)), 13 slowed movement (speed, not amount) and 15 (delay in motor activity), which comprises all motoric CORE-items besides those that are related to language. Out of the 36 patients 32 were on antidepressive medication (selective serotonin reuptake inhibitors (SSRI): n = 7; serotonin noradrenaline reuptake inhibitors (SNRI): n = 13; bupropion: n = 3; tricyclic antidepressants: n = 8; tranylcypromine: n = 1). Augmentation strategies were applied in 17 patients, thirteen with lithium and four with atypical antipsychotics. Furthermore, two patients took zolpidem for sleep induction.
We included 19 right-handed healthy controls (HC). Assessment for HC was identical to the patient group. Inclusion criteria were the absence of any present or past psychiatric disorder as assessed with the MINI. All participants provided written informed consent. The study was approved by the local Ethics Committee of Bern (KEK Bern; BASEC-number: 2017 − 00731).
MRI acquisition
We acquired structural and functional brain data with a 3 Tesla MRI scanner (Magnetom Prisma, Siemens, Erlangen, Germany) using a 64-channel head and neck coil at the Swiss Institute for Translational and Entrepreneurial Medicine (SITEM) in Bern.
For acquiring anatomical data, we used a 3D T1-weighted MP2RAGE sequence with the following parameters: FOV = 256 × 256 mm2, matrix = 256 × 256, slices = 256, voxel resolution = 1 × 1 × 1 mm3, TR/TE = 5000/2.98 ms, TI = 700 ms and T2 = 2500 ms.
For calculating cerebral blood flow (CBF) we acquired data using a pulsed arterial spin labeling (PASL) sequence with PICORE Q2TIPS technique [39, 40]. Basic parameters for the PASL sequence were the following: FOV = 230 × 230 mm2, matrix = 64 × 64, slices = 22, voxel resolution = 3.6 × 3.6 × 6.0 mm3, TR/TE = 3300/13 ms, flip angle = 90° and PICORE Q2T perfusion mode. In total, we acquired 90 pairs of label/control volumes in the axial direction with a tagging bolus duration (TI1) of 700 ms saturation and an inversion time (TI2) of 2200 ms, whereas during TI2, the labeled blood perfuses the brain tissues, resulting in a decrease of the MR signal.
We acquired an 8-minute continuous resting-state BOLD fMRI scan with the condition ‘eyes closed’ to measure functional connectivity between brain areas. Image acquisition based on echo planar imaging (EPI) with the following parameters: 480 volumes with 48 slices per volume, FOV = 230 × 230 mm2, matrix = 94 × 94, voxel resolution = 2.4 × 2.4 × 2.4 mm3, TR = 1000 ms, and TE = 30 ms.
For computation of a field map, we acquired a magnitude and phase images with a double-echo spoiled gradient echo sequence with the following parameters: TR = 500 ms, TE = 4.92/7.38 ms, voxel resolution = 2.4 × 2.4 × 2.4 mm3, flip angle = 60°.
Data analyses
Calculation of cerebral blood flow (CBF)
For calculation of cortical cerebral blood flow ( ), we analysed PASL data (180 label and control images) using in house MATLAB scripts (MATLAB R2023a, MathWorks, USA). Initially, we corrected the raw PASL volumes to minimize the impact of movement artifacts. Subsequently, we applied a field map correction to all the realigned volumes. Finally, we computed the CBF time series using the following formula:
), we analysed PASL data (180 label and control images) using in house MATLAB scripts (MATLAB R2023a, MathWorks, USA). Initially, we corrected the raw PASL volumes to minimize the impact of movement artifacts. Subsequently, we applied a field map correction to all the realigned volumes. Finally, we computed the CBF time series using the following formula:  , where
, where  is the difference signal (control – labelled),
is the difference signal (control – labelled),  the equilibrium brain tissue magnetization and
the equilibrium brain tissue magnetization and  the decay time for labelled blood at 3 Tesla [41]. For improvement of signal-to-noise ratio, we calculated the average CBF for the time series. We computed the average CBF for the time series to improve the signal-to-noise ratio. Then, we co-registered these averaged maps to structural T1-weighted images and isolated CBF within grey matter using binary masks derived using the DL+DiReCT (individual space, AsegAtlas) [42, 43]. Finally, we used SPM12 software (http://www.fil.ion.ucl.ac.uk/spm) to normalize individual grey matter CBF to standard Montreal Neurological Institute space. Normalized volumes were smoothed with a 6-mm full-width at half maximum (FWHM) kernel to reduce partial volume effects. A whole-brain two-sample t-test was performed to compare regional CBF between groups. Age and sex were included as covariates, cluster forming threshold was set to p-value < 0.001 and to identify significant clusters we used a family wise error (FWE) correction of p-value < 0.05.
the decay time for labelled blood at 3 Tesla [41]. For improvement of signal-to-noise ratio, we calculated the average CBF for the time series. We computed the average CBF for the time series to improve the signal-to-noise ratio. Then, we co-registered these averaged maps to structural T1-weighted images and isolated CBF within grey matter using binary masks derived using the DL+DiReCT (individual space, AsegAtlas) [42, 43]. Finally, we used SPM12 software (http://www.fil.ion.ucl.ac.uk/spm) to normalize individual grey matter CBF to standard Montreal Neurological Institute space. Normalized volumes were smoothed with a 6-mm full-width at half maximum (FWHM) kernel to reduce partial volume effects. A whole-brain two-sample t-test was performed to compare regional CBF between groups. Age and sex were included as covariates, cluster forming threshold was set to p-value < 0.001 and to identify significant clusters we used a family wise error (FWE) correction of p-value < 0.05.
Calculation of functional connectivity (FC)
We analysed resting-state fMRI using the CONN 21a toolbox [44]. Pre-processing steps included the realignment and co-registration of EPI volumes to T1-weighted MP2RAGE, segmentation, and normalization to MNI space, and smoothing using an FWHM kernel of 8 mm. We applied band-pass filtering (0.008–0.09 Hz) to remove physiological signals and regress nuisance variables of each 5-time series within segmented white matter and cerebrospinal fluid and of 12 realignment parameters. Scrubbing outlier volumes with global BOLD signal or framewise displacement (FD) higher than the 95th percentile was performed using the Artefact Detection Tools (ART) toolbox implemented in CONN. For every subject, we averaged FD and DVARS, which is the spatial root mean square of the BOLD signal after temporal differencing [45].
We used a-posteriori the binary mask of the significant cluster of the CBF group differences for computation of seed-based rs-FC maps (first-level). Second-level between group analyses of the FC-maps were corrected with regressors age, sex, mean FD, and mean DVARS. We used a voxel threshold of p < 0.01 and a cluster correction with a false discovery rate (FDR) correction of p < 0.05.
Correlational analyses
Within patients, we performed for CBF maps and for seed-based (seed: a-posteriori binary mask) rs-FC maps whole-brain linear regression analysis with the psychomotor retardation sum score of the items 3, 10, 13 and 15 of the CORE. We used a voxel threshold of p < 0.01 and a two-side cluster correction with a family wise error (FWE) correction of p < 0.05. CBF maps were corrected for age and sex, rs-FC-maps were corrected with regressors age, sex, mean FD and mean DVARS.
Statistical analyses of clinical and demographic data
The Statistical Package for Social Sciences SPSS 28.0 (SPSS, Inc., Chicago, Illinois) was used for analyses of clinical characteristics and demographics between patients and HC using independent t-tests or χ2 tests as appropriate for continuous and dichotomous data. To estimate the effect size of significant findings, we calculated Cohen’s d using the following formula:  , where
, where  is the ratio of the difference in a number’s estimated value from its assumed value to its standard error in a significant cluster and
is the ratio of the difference in a number’s estimated value from its assumed value to its standard error in a significant cluster and  is the number of subjects included in the analysis [46].
is the number of subjects included in the analysis [46].
Results
Participant demographic and clinical characteristics
Patients and HC did not differ regarding age and sex (see Table 1 for clinical and demographic characteristics). Six patients met criteria for double-depression for many years (range 8 to 27 years).
Table 1.
Demographics
| Patients | Controls | Statistics | |
|---|---|---|---|
| Age (years) | 43.8 ± 12.7 | 42.1 ± 13.4 | p = 0.64 |
| Sex (female/ male) | 19/17 | 8/11 | p = 0.57 |
| Duration of current episode (months) | 12.6 ± 10.5 | N/A | N/A |
| Number of episodes | 3.2 ± 2.1 | N/A | N/A |
| HAMD-total | 22.0 ± 5.6 | 0.7 ± 1 | p < 0.001 |
| BDI-total | 27.6 ± 9.2 | 1.6 ± 2.4 | p < 0.001 |
| CORE-total | 12.6 ± 7.8 | 0.2 ± 0.8 | p < 0.001 |
| CORE (psychomotor retardation) | 3.6 ± 2.8 | 0.1 ± 0.3 | p < 0.001 |
Abbreviations: N/A: not applicable
Group differences in cerebral blood flow (CBF)
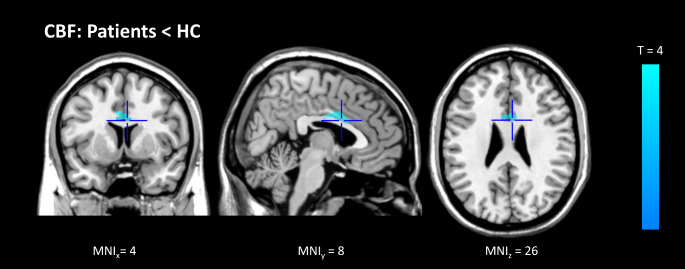
In the whole-brain group comparison, we found a large cluster in the bilateral cingulate cortex, mainly localized in the CMA, with lower CBF in patients compared to controls (MNI-coordinates (x, y, z): 4, 8, 26, cluster (number of voxels): 674, p-FWE: 0.009, T = 4.24, Cohen’s d = 0.57, medium effect size). See Fig. 1.
Fig. 1.
Group difference in regional CBF in grey matter in standardized MNI space
Blue represents significant relative hypoperfusion in the bilateral CMA within patients
Group differences in functional connectivity (FC)
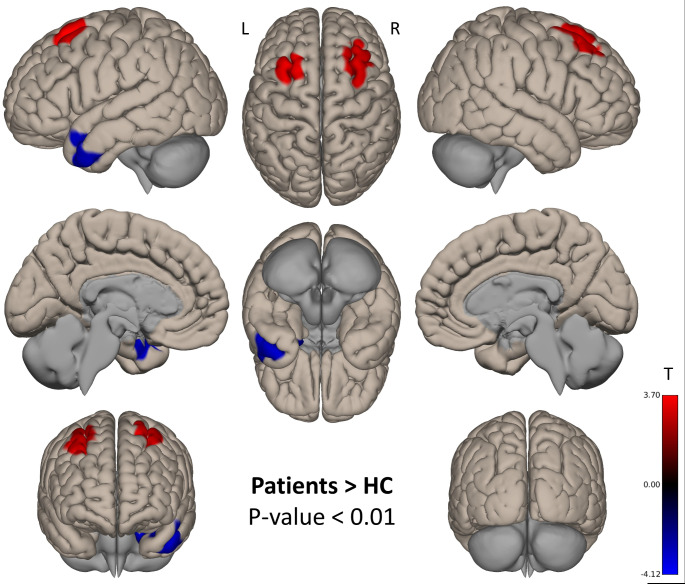
After using the significant CBF cluster localized in the CMA as a binary seed mask, we found that patients had increased rs-FC in contrast to HC in bilateral superior and middle frontal gyrus (Cohen’s d = 0.50, medium effect size), localized in the lateral part of the SMA. In the opposite direction, HC showed increased rs-FC in the left temporal pole (Cohen’s d = 0.56, medium effect size). See Table 2; Fig. 2.
Table 2.
Group difference in seed based rs-FC of the cingulate motor area
| Comparison | Area | Hemisphere | Cluster (voxels) | MNI (x y z) |
p-FWE |
|---|---|---|---|---|---|
| Patients > HC | Middle and superior frontal gyrus | R | 503 | 32 40 48 | 0.000668 |
| Middle and superior frontal gyrus | L | 474 | -26 20 58 | 0.002082 | |
| Patients < HC | Temporal pole | L | 728 | -44 0 34 | 0.000052 |
Abbreviations: L: left hemisphere; R: right hemisphere
Fig. 2.
Group differences in seed-based rs-FC of the cingulate motor area
Positive contrasts (patients > HC) are displayed in red, negative contrasts (patients < HC) are displayed in blue
Correlational analyses
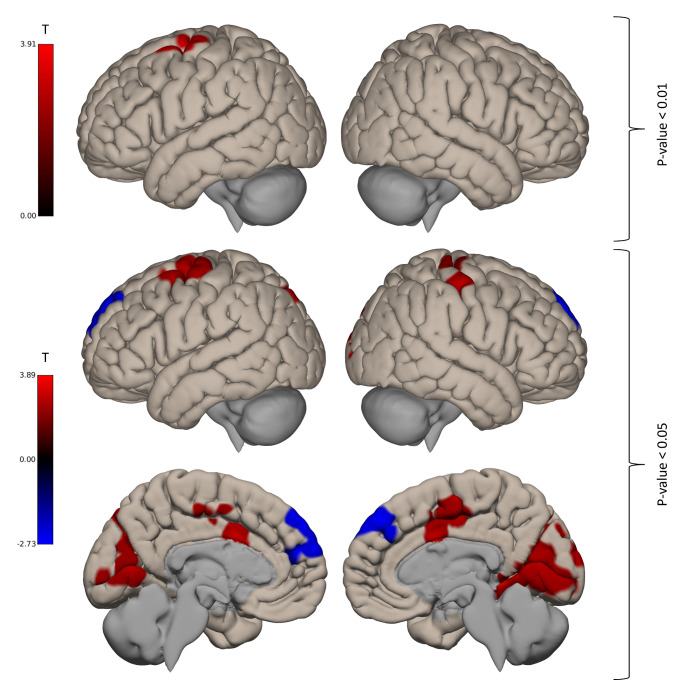
Within patients, we found a positive correlation between psychomotor retardation and rs-FC between the CMA (a-posteriori binary mask) and a cluster in the left hemisphere localised in the precentral gyrus, middle and superior frontal gyrus (MNI-coordinates (x, y, z): -26 -8 46, cluster (number of voxels): 455, p-FWE: 0.0031, Cohen’s d = 0.65, moderate effect size). No significant correlation was found for CBF and CORE motor retardation. See Fig. 3.
Fig. 3.
Correlation of psychomotor retardation and seed-based rs-FC of the CBF cluster within patients
Positive correlations are displayed in red, negative correlations are displayed in blue. For visualization purpose, we also show results with a voxel threshold of p < 0.05
Discussion
This study explored if alterations of local CBF are associated with rs-FC and psychomotor retardation in patients with MDD. In patients with MDD, we found reduced CBF in bilateral CMA, a segment of the ACC that is essential for the planning and initiation of movements. However, the severity of psychomotor retardation in patients failed to correlate with CMA CBF. Furthermore, there was an increase of rs-FC between the cluster with reduced CBF in the CMA and bilateral SMA in patients with depression. In addition, within patients increased rs-FC between the CMA-cluster and a cluster in the left SMA was associated with more pronounced psychomotor retardation.
Our results suggest a core role of the CMA, a segment of the ACC, for the pathophysiology of depression. The ACC integrates information from the limbic system, in particular the rostral ACC that receives input from the amygdala, the hippocampus, and the ventral striatum [47] regions that are heavily implicated in the neurobiology of depression. The CMA integrates information from the anterior and the posterior cingulum and projects to the SMA, a region that is essential for the initiation and inhibition of motor behaviour [21, 22, 48]. Our finding of reduced CBF in the CMA complements a previous ASL study pointing to a negative association between CBF in the ACC and apathy in depression [49]. Another study demonstrated a specific role of CMA perfusion for physical activity in MDD [14]. Psychomotor retardation in depression has also been associated with altered perfusion patterns in the insula, frontal brain regions, and the SMA [14, 16]. Overall, findings of increases and decreases of CBF in depression have been reported in widespread brain region and its functional relevance remains to be elucidated [50].
The lower CBF in the CMA indicates altered brain metabolism in this area, suggesting aberrant flow of information from and to this brain region. Indeed, in addition to our finding of reduced CBF in the CMA, we found increased rs-FC between the CMA and bilateral superior and middle frontal gyri, localized in the lateral parts of the SMA in patients with MDD. Increases of rs-FC between the CMA and the left SMA were associated with more pronounced psychomotor retardation in patients. Psychomotor retardation is thought to arise from multiple alterations within the cerebral motor network, including components such as CMA, SMA, primary motor cortex (M1), thalamus, cerebellum, and basal ganglia. In fact, prior unimodal work has shown alterations in these components or white matter pathways connecting them [1, 2, 12–15, 24]. In depression with psychomotor retardation rs-FC was increased between thalamus and M1/SMA, SMA and M1, as well as between thalamus and putamen [1]. In line with these connectivity patterns, the current study provides information on CBF and connectivity in the CMA. Results suggest that psychomotor retardation cannot be simply attributed to reduced perfusion in the CMA alone but rather to alterations of a connectivity within the motor circuitry. Aberrant coupling between the CMA and the SMA probably relates to lack of drive and reduced daily activity, features that are commonly observed in patients with depression [4, 11]. Neural activity in the motor circuit is altered in patients with psychomotor disturbance [51]. The net behavioral effect of retardation may arise from lack of activity in some components or from concurrent increases of activity within the circuit. The interactions between circuit components can have inhibitory and excitatory effects that are balanced within the system. In depression, we may assume that this balance is disturbed. However, the exact mechanism is yet to be unravelled. Still, research in schizophrenia with psychomotor slowing suggests some transdiagnostic commonalities, particularly relating to SMA and cerebellar connectivity [52–55]. Finally, the alterations in cortical motor areas and the dense connectivity within the motor circuit hold promise for non-invasive brain stimulation techniques to ameliorate retardation or slowing [1, 56] .
Finally, our study has some limitations. First, sample size is relatively small. Much larger sample sizes may be required to substantiate reproducible results [57]. However, we took great care to reduce the risk of false positive results by checking for outliers and by applying a stringent correction for multiple comparisons. The effect sizes (Cohen’s d > 0.5) also support the credibility of our findings. Second, most patients were medicated with antidepressants which may affect motor behaviour and measures of CBF, and rs-FC. Third, no objective measures of motor activity (e.g. actigraphy) were available allowing for assessment of spontaneous motor activity also outside the interview situation. Fourth, our finding of reduced CBF in the CMA is followed up by rs-FC analyses that are linked to psychomotor retardation. However, CBF reductions in the CMA did not correlate with psychomotor retardation. Therefore, this finding does not imply specificity regarding psychomotor retardation. Fifth, we chose a data driven approach (CBF reductions) to investigate functional networks associated with psychomotor retardation. Thus, identified networks related to psychomotor retardation are far from complete and further structures relevant to motor behaviour (e.g. basal ganglia, cerebellum) are not considered in our analyses [1, 2].
In conclusion, our analysis suggests that reduced perfusion of the CMA and increased rs-FC between the CMA and the SMA are associated with psychomotor retardation in patients with MDD. This finding is highly plausible, given the integrative role of the ACC for emotion processing and action planning and the frequently reported alterations of structure and function of the ACC in patients with depression [7, 58, 59]. Furthermore, the CMA has a critical role within the motor circuit.
Acknowledgements
This work was supported by the Robert Enke Foundation (to Tobias Bracht and Sebastian Walther), by the Novartis Foundation for Medical Biological Research (#19C203) (to Tobias Bracht and Sebastian Walther) by the Swiss Life Foundation (to Tobias Bracht), and by the Adrian et Simone Frutiger Foundation (to Tobias Bracht). Niklaus Denier also received a postdoc grant from the Adrian et Simone Frutiger Foundation.
Funding
Open access funding provided by University of Bern
Declarations
Competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sebastian Walther and Niklaus Denier these two authors contributed equally.
References
- 1.Wuthrich F, Lefebvre S, Mittal VA, Shankman SA, Alexander N, Brosch K et al (2024) The neural signature of psychomotor disturbance in depression. Mol Psychiatry 29(2):317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther S, Bernard JA, Mittal VA, Shankman SA (2019) The utility of an RDoC motor domain to understand psychomotor symptoms in depression. Psychol Med 49(2):212–216 [DOI] [PubMed] [Google Scholar]
- 3.Schrijvers D, Hulstijn W, Sabbe BG (2008) Psychomotor symptoms in depression: a diagnostic, pathophysiological and therapeutic tool. J Affect Disord 109(1–2):1–20 [DOI] [PubMed] [Google Scholar]
- 4.Wuthrich F, Nabb CB, Mittal VA, Shankman SA, Walther S (2022) Actigraphically measured psychomotor slowing in depression: systematic review and meta-analysis. Psychol Med 52(7):1208–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracht T, Horn H, Strik W, Federspiel A, Schnell S, Hofle O et al (2014) White matter microstructure alterations of the medial forebrain bundle in melancholic depression. J Affect Disord 155:186–193 [DOI] [PubMed] [Google Scholar]
- 6.Parker G, Hadzi-Pavlovic D, Austin MP, Mitchell P, Wilhelm K, Hickie I et al (1995) Sub-typing depression, I. is psychomotor disturbance necessary and sufficient to the definition of melancholia? Psychol Med 25(4):815–823 [DOI] [PubMed] [Google Scholar]
- 7.Mertse N, Denier N, Walther S, Breit S, Grosskurth E, Federspiel A et al (2022) Associations between anterior cingulate thickness, cingulum bundle microstructure, melancholia and depression severity in unipolar depression. J Affect Disord 301:437–444 [DOI] [PubMed] [Google Scholar]
- 8.Denier N, Walther S, Schneider C, Federspiel A, Wiest R, Bracht T (2020) Reduced tract length of the medial forebrain bundle and the anterior thalamic radiation in bipolar disorder with melancholic depression. J Affect Disord 274:8–14 [DOI] [PubMed] [Google Scholar]
- 9.Imai H, Noma H, Furukawa TA (2021) Melancholic features (DSM-IV) predict but do not moderate response to antidepressants in major depression: an individual participant data meta-analysis of 1219 patients. Eur Arch Psychiatry Clin Neurosci 271(3):521–526 [DOI] [PubMed] [Google Scholar]
- 10.van Diermen L, Vanmarcke S, Walther S, Moens H, Veltman E, Fransen E et al (2019) Can psychomotor disturbance predict ect outcome in depression? J Psychiatr Res 117:122–128 [DOI] [PubMed] [Google Scholar]
- 11.Razavi N, Horn H, Koschorke P, Hugli S, Hofle O, Muller T et al (2011) Measuring motor activity in major depression: the association between the Hamilton Depression Rating Scale and actigraphy. Psychiatry Res 190(2–3):212–216 [DOI] [PubMed] [Google Scholar]
- 12.Bracht T, Federspiel A, Schnell S, Horn H, Hofle O, Wiest R et al (2012) Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS ONE 7(12):e52238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walther S, Hugli S, Hofle O, Federspiel A, Horn H, Bracht T et al (2012) Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol Dis 47(1):13–19 [DOI] [PubMed] [Google Scholar]
- 14.Walther S, Hofle O, Federspiel A, Horn H, Hugli S, Wiest R et al (2012) Neural correlates of disbalanced motor control in major depression. J Affect Disord 136(1–2):124–133 [DOI] [PubMed] [Google Scholar]
- 15.Cantisani A, Koenig T, Stegmayer K, Federspiel A, Horn H, Muller TJ et al (2016) EEG marker of inhibitory brain activity correlates with resting-state cerebral blood flow in the reward system in major depression. Eur Arch Psychiatry Clin Neurosci 266(8):755–764 [DOI] [PubMed] [Google Scholar]
- 16.Cantisani A, Stegmayer K, Bracht T, Federspiel A, Wiest R, Horn H et al (2016) Distinct resting-state perfusion patterns underlie psychomotor retardation in unipolar vs. bipolar depression. Acta Psychiatr Scand 134(4):329–338 [DOI] [PubMed] [Google Scholar]
- 17.Bracht T, Steinau S, Federspiel A, Schneider C, Wiest R, Walther S (2018) Physical activity is associated with left corticospinal tract microstructure in bipolar depression. Neuroimage Clin 20:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31(2–3):236–250 [DOI] [PubMed] [Google Scholar]
- 19.Mittal VA, Bernard JA, Northoff G (2017) What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull 43(5):949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walther S, Mittal VA (2022) Motor Behavior is relevant for understanding mechanism, bolstering prediction, and improving treatment: a transdiagnostic perspective. Schizophr Bull 48(4):741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolls ET (2019) The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct 224(9):3001–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolls ET (2023) Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Struct Funct 228(5):1201–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bracht T, Mertse N, Walther S, Ludi K, Breit S, Federspiel A et al (2022) Link between structural connectivity of the medial forebrain bundle, functional connectivity of the ventral tegmental area, and anhedonia in unipolar depression. Neuroimage Clin 34:102961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantisani A, Koenig T, Horn H, Muller T, Strik W, Walther S (2015) Psychomotor retardation is linked to frontal alpha asymmetry in major depression. J Affect Disord 188:167–172 [DOI] [PubMed] [Google Scholar]
- 25.Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M (2021) All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatry 26(1):92–102 [DOI] [PubMed] [Google Scholar]
- 26.Madsen SS, Lindberg U, Asghar S, Olsen KS, Moller K, Larsson HBW et al (2023) Reproducibility of cerebral blood flow, oxygen metabolism, and lactate and N-acetyl-aspartate concentrations measured using magnetic resonance imaging and spectroscopy. Front Physiol 14:1213352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JJ, Jann K, Wang DJ (2015) Characterizing resting-state brain function using arterial spin labeling. Brain Connect 5(9):527–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bracht T, Denier N, Wallimann M, Walther S, Mertse N, Breit S et al (2022) Hippocampal volume and parahippocampal cingulum alterations are associated with avoidant attachment in patients with depression. J Affect Disorders Rep. ;10
- 29.Bracht T, Walther S, Breit S, Mertse N, Federspiel A, Meyer A et al (2023) Distinct and shared patterns of brain plasticity during electroconvulsive therapy and treatment as usual in depression: an observational multimodal MRI-study. Transl Psychiatry 13(1):6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denier N, Walther S, Breit S, Mertse N, Federspiel A, Meyer A et al (2023) Electroconvulsive therapy induces remodeling of hippocampal co-activation with the default mode network in patients with depression. Neuroimage Clin 38:103404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denier N, Breit S, Soravia LM, Walther S, Mertse N, Krone L et al (2024) Low sleep quality in major depressive disorder is associated with thinning and decreased functional connectivity of the insular cortex: insular cortex and sleep quality in depression. J Affect Disorders Rep. ;16
- 32.APA. Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM–5; American Psychiatric Association [APA] (2013) 2013
- 33.Parker G, Hadzi-Pavlovic D, Melancholia (1996) A disorder of Movement and Mood - A phenomenological and neurobiological review. Cambridge University Press, Cambridge [Google Scholar]
- 34.Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113 [DOI] [PubMed] [Google Scholar]
- 35.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998) The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 quiz 4–57 [PubMed]
- 36.Wittchen HU, Zaudig M, Fydrich T (1997) Strukturiertes klinisches interview für DSM-IV. Hogrefe, Göttingen [Google Scholar]
- 37.Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6(4):278–296 [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown G (1996) Beck Depression Inventory -II. APA PsyTests
- 39.Luh WM, Wong EC, Bandettini PA, Hyde JS (1999) QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med 41(6):1246–1254 [DOI] [PubMed] [Google Scholar]
- 40.Wong EC, Buxton RB, Frank LR (1998) Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med 39(5):702–708 [DOI] [PubMed] [Google Scholar]
- 41.Federspiel A, Müller TJ, Horn H, Kiefer C, Strik WK (2006) Comparison of spatial and temporal pattern for fMRI obtained with BOLD and arterial spin labeling. J Neural Transm (Vienna) 113(10):1403–1415 [DOI] [PubMed] [Google Scholar]
- 42.Rebsamen M, Rummel C, Reyes M, Wiest R, McKinley R (2020) Direct cortical thickness estimation using deep learning-based anatomy segmentation and cortex parcellation. Hum Brain Mapp 41(17):4804–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355 [DOI] [PubMed] [Google Scholar]
- 44.Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2(3):125–141 [DOI] [PubMed] [Google Scholar]
- 45.Afyouni S, Nichols TE (2018) Insight and inference for DVARS. NeuroImage 172:291–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1):4–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walther S, Alexaki D, Weiss F, Baumann-Gama D, Kyrou A, Nuoffer MG et al (2024) Psychomotor slowing in psychosis and inhibitory repetitive transcranial magnetic stimulation: a Randomized Clinical Trial. JAMA Psychiatry [DOI] [PMC free article] [PubMed]
- 49.Batail JM, Corouge I, Combes B, Conan C, Guillery-Sollier M, Verin M et al (2023) Apathy in depression: an arterial spin labeling perfusion MRI study. J Psychiatr Res 157:7–16 [DOI] [PubMed] [Google Scholar]
- 50.Wang YM, Yang ZY (2022) Aberrant pattern of cerebral blood flow in patients with major depressive disorder: a meta-analysis of arterial spin labelling studies. Psychiatry Res Neuroimaging 321:111458 [DOI] [PubMed] [Google Scholar]
- 51.Wuthrich F, Lefebvre S, Mittal VA, Shankman SA, Alexander N, Brosch K et al (2023) The neural signature of psychomotor disturbance in depression. Mol Psychiatry [DOI] [PMC free article] [PubMed]
- 52.Moussa-Tooks AB, Beermann A, Manzanarez Felix K, Coleman M, Bouix S, Holt D et al (2024) Isolation of distinct networks driving action and cognition in psychomotor processes. Biol Psychiatry [DOI] [PMC free article] [PubMed]
- 53.Lefebvre S, Gehrig G, Nadesalingam N, Nuoffer MG, Kyrou A, Wuthrich F et al (2024) The pathobiology of psychomotor slowing in psychosis: altered cortical excitability and connectivity. Brain 147(4):1423–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV (2017) Aberrant hyperconnectivity in the Motor System at Rest is linked to Motor abnormalities in Schizophrenia Spectrum disorders. Schizophr Bull 43(5):982–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walther S, Schappi L, Federspiel A, Bohlhalter S, Wiest R, Strik W et al (2017) Resting-state hyperperfusion of the supplementary motor area in Catatonia. Schizophr Bull 43(5):972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walther S, Alexaki D, Weiss GS, Vladimirova F, Stegmayer I (2020) K, Inhibitory Repetitive Transcranial Magnetic Stimulation to treat Psychomotor slowing: a transdiagnostic, mechanism-based Randomized double-blind controlled trial. Schizophrenia Bull Open. ;1(1)
- 57.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS et al (2022) Reproducible brain-wide association studies require thousands of individuals. Nature 603(7902):654–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bracht T, Linden D, Keedwell P (2015) A review of white matter microstructure alterations of pathways of the reward circuit in depression. J Affect Disord 187:45–53 [DOI] [PubMed] [Google Scholar]
- 59.Rolls ET, Cheng W, Gong W, Qiu J, Zhou C, Zhang J et al (2019) Functional connectivity of the Anterior Cingulate Cortex in Depression and in Health. Cereb Cortex 29(8):3617–3630 [DOI] [PubMed] [Google Scholar]