Abstract
Keratoconus (KC) is a prevalent ectatic corneal disease and the leading cause of corneal transplantation globally. Despite evidence of mitochondrial abnormalities in KC, the underlying mechanisms remain unclear. Our aim was to investigate the role of mitochondrial dysfunction in this pathological condition. Based on transcriptomics datasets of KC, mitochondria-related differentially expressed genes (mDEGs) were identified and analyzed for potential functional pathways, protein-protein interaction (PPI), and gene regulatory networks. Hub genes were further screened and validated by multiple machine learning (ML) algorithms, followed by a comprehensive visualization of single-cell atlas and immune landscape. Additionally, bioinformatic results were validated through quantitative PCR, Western blot, and transcriptomics analysis in an in vitro KC model based on matrix stiffness using human stromal keratocytes. In total, 104 mDEGs were identified, enriched in pathways related to oxidative stress, apoptotic mitochondrial changes, ferroptosis, and inflammatory responses. Nine characteristic genes (CYP24A1, ACSL4, ACADL, HELZ2, AMT, DEPTOR, TUBA1A, TYMS, and ACSL5) were selected and validated using multiple ML models. Single cell sequencing data highlighted ACSL4 as the most promising biomarker, primarily expressed in corneal stromal cells (CSCs). Immune infiltration analysis revealed that ACSL4 was positively associated with monocytes and negatively correlated with eosinophils in KC. In cellular experiments, ACSL4 expression was significantly upregulated in response to decreased substrate stiffness, suggesting its critical role in KC development. These findings suggest a mitochondrial-related molecular mechanism implicated in KC pathogenesis. The identified pivotal biomarker ACSL4 provides a novel framework for future mechanistic and therapeutic studies of KC.
Keywords: Keratoconus, Mitochondrial dysfunction, ACSL4, Corneal stromal cells, Single cell transcriptome
Graphical Abstract

1. Introduction
Keratoconus (KC) is a progressive corneal disorder characterized by stromal thinning, structural weakening of the anterior limiting membrane, and asymmetric conical protrusion of the cornea [1], [2]. These morphological changes exacerbate corneal aberrations, often resulting in reduced visual acuity and functional disability [3]. It is a leading indication for corneal transplant surgery worldwide, with a reported prevalence of 1:2000 in the general population and higher rates among young adults [4]. The multifactorial etiology of KC includes both environmental and genetic factors, with a family history, eye rubbing, eczema, asthma, and allergy identified as risk factors [4]. Various therapeutic strategies have been developed to manage KC, including the use of corrective lenses, corneal collagen cross-linking (CXL), and intracorneal inserts [4], [5]. While corneal transplantation remains the gold standard for advanced cases, its limitations—such as prolonged recovery times, graft rejection risks, and dependence on donor tissue availability—highlight the need for less invasive alternatives [6]. Given the global scarcity of viable donor corneas, it is critical to elucidate the molecular mechanisms and pathophysiological pathways driving KC progression. Such insights could unveil novel therapeutic targets, paving the way for innovative non-surgical treatments that address the disease’s underlying causes rather than merely mitigating symptoms.
Emerging evidence highlights the multifactorial pathogenesis of KC, involving genetic susceptibility, immune dysregulation, and oxidative stress [7]. Studies indicate that KC corneas exhibit elevated oxidative stress biomarkers, such as extracellular superoxide dismutase (EC-SOD), catalase, peroxynitrite, and malondialdehyde (MDA), alongside diminished antioxidant enzyme activity. This imbalance compromises the cornea’s ability to neutralize reactive oxygen species (ROS) and protect against oxidative damage [8]. Moreover, oxidative stress has been reported to induce autophagy dysregulation in the corneal epithelial cells of KC patients, leading to cellular damage and dysfunction [9]. While the precise mechanisms underlying ROS-antioxidant imbalance remain unclear, mitochondrial dysfunction is hypothesized to play a central role, potentially driving disease progression through disrupted redox homeostasis [10], [11].
Mitochondria are critical regulators of cellular metabolism and energy production, also serve as primary sources of ROS via oxidative phosphorylation [12]. In KC, corneal tissues exhibit marked mitochondrial abnormalities, including reduced membrane potential and elevated mitochondrial DNA (mtDNA) damage [13]. Reactive nitrogen species (RNS) and ROS further exacerbate mtDNA damage, disrupting oxidative phosphorylation and contributing to extracellular matrix destabilization—a hallmark of KC pathology [13]. Notably, KC fibroblasts demonstrate an inherent hypersensitive response to oxidative stressors in vitro, accompanied by mitochondrial dysfunction and mtDNA damage [14]. In addition, KC patients exhibit a significant increase in the mean mtDNA content of peripheral blood leukocytes, along with potentially pathogenic nonsynonymous mtDNA mutations, indicating systemic mitochondrial involvement in the disease [15], [16]. These findings collectively implicate mitochondrial dysfunction as a key driver of KC development. However, systematic investigations into its mechanistic role remain limited, highlighting the need for deeper exploration to identify novel therapeutic targets.
In this study, we investigated the potential role of mitochondrial dysfunction in KC pathogenesis through integrated bulk and single cell RNA-seq analysis. Based on transcriptomic datasets GSE77938 and GSE151631, we identified mitochondrial-associated differentially expressed genes (DEGs) and analyzed their roles in functional pathways, protein-protein interaction (PPI) networks, and regulatory networks involving microRNAs (miRNAs) and transcription factors (TFs). Hub genes were validated by multiple machine learning (ML) algorithms, with cell-type-specific expression patterns visualized through single-cell RNA sequencing (HRA000728 dataset). Immune landscape profiling further mapped inflammatory signatures in KC. In addition, hub genes were validated using an in vitro KC model. Our integrative approach highlights potential therapeutic targets and advances understanding of mitochondrial mechanisms in KC. A schematic overview of the study design is provided in Fig. 1.
Fig. 1.
Schematic illustration of the present study. This study integrates transcriptomic datasets (GSE77938, GSE151631) to identify mitochondrial-associated genes in keratoconus (KC). Machine learning and single-cell RNA-seq (HRA000728) prioritized hub gene ACSL4, validated in vitro under varying substrate stiffness. Immune profiling mapped inflammatory signatures, highlighting immune cell infiltration.
2. Material and methods
2.1. Cell culture and treatment
The human stromal keratocyte cell line (HTK cells) was generously provided by Dr. James Jester of the University of California Irvine. HTK cells were maintained in DMEM/F12 medium (Corning, USA) supplemented with 10 % fetal bovine serum (Gibco, USA) at 37°C in a 5 % CO2 humidified incubator. For experimental treatments, cells were digested with 0.25 % trypsin and seeded on six-well culture plates coated with substrates of defined stiffness (Young’s modulus: 32 kPa and 64 kPa; Advanced Biomatrix, USA) at an initial density of 5 × 105/well, as previously described [17]. HTK Cells plated on normal tissue culture plastic (TCP) plates were used as control.
2.2. RNA sequencing
Total RNA was extracted from HTK cells grown under three conditions (32 kPa, 64 kPa, and TCP) using a commercial RNA extraction kit (TianGen, China), following the manufacturer’s protocol. RNA sequencing was performed by the Newcore Biotech (Shanghai, China) using Illumina-based protocols. Raw read counts were processed and normalized using R (v4.0.2) with the limma (v3.42.2) and edgeR (v3.28.1) packages for differential gene expression analysis.
2.3. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from different subsets of HTK cells on 6-well plates using the total RNA extraction kit (TianGen, China). RNA was reverse transcribed into complementary DNA (cDNA) using the PrimeScript RT reagent Kit (Takara, USA). Using the Hieff SYBR Green Master Mix (Yeasen, China), quantitative PCR (qPCR) was then performed on an ABI Prism 7000 instrument (Applied Biosystems, USA). The Pfaffl method was applied to determine the relative quantification of target mRNA expression levels. Each sample was detected in three replicates. The paired primer sequences are listed in Table S1.
2.4. Western blot
Total cellular protein was extracted and quantified using a BCA kit (Abiowell, China). The protein solution was mixed with loading buffer (Abiowell, China) and denatured by heating. After cooling, the sample was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, the proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The membrane was then blocked with 5 % non-fat milk. Primary antibodies against ACSL4 (Bioss, China; #bsm-62432R) or GAPDH (Beyotime, China; #AF5009) were incubated at 4 °C for 16 h, followed by incubation with HRP-conjugated secondary antibodies at room temperature for 1 h. Protein bands were visualized using an ECL chemiluminescence kit (Abiowell, China) and imaged using a ChemiDoc Imaging System (Bio-Rad, USA).
2.5. Bulk and single cell transcriptomics data source
Two publicly available bulk RNA-seq datasets (GSE77938 and GSE151631) were retrieved from the Gene Expression Omnibus (GEO) database. GSE77938 was designated as the metadata set consisting of 25 KC and 25 donor-matched normal corneas [18]. GSE151631 was used as a validation dataset, which included 19 samples of KC and 7 health control [19]. Raw data were normalized and analyzed using the “edgeR” package. The Integrated Mitochondrial Protein Index (IMPI) of the MitoMiner database (http://mitominer.mrc-mbu.cam.ac.uk/) provides 1626 human genes that encode mitochondrially localized proteins [20].
Single cell data were requested from the Genome Sequence Archive (GSA) database under accession code HRA000728 [21]. It contains corneal cells from three central cornea tissues (without endothelium) of KC patients after deep anterior lamellar keratoplasty (DALK) surgeries and four control samples from healthy donors.
2.6. Analysis of functional pathways, PPI, and multifactor regulatory network
DEGs between KC and healthy corneas were identified in the metadata set GSE77938 using thresholds of |log2 fold change (FC)| > 1.5 or < 0.67 and p < 0.05. A volcano plot was generated for DEGs visualization by “volcano3D” R package. Mitochondria-related DEGs (mDEGs) were filtered via Venn graph using Jvenn, a plug-in for the jQuery Javascript library. These mDEGs were then imported to the STRING database (http://string-db.org/) for PPI network and further visualized using Cytoscape (v3.8.2) [22].
Gene ontology (GO) was conducted via the R package “clusterProfiler”, including biological processes (BP), cellular components (CC), and molecular functions (MF). Enrichment analyses of Kyoto encyclopedia of genes and genomes (KEGG) were performed using the KOBAS data source (http://kobas.cbi.pku.edu.cn/genelist/) [23]. The statistical significance was determined using the False Discover Rate (FDR) < 0.05, p < 0.05, and count ≥ 2 thresholds. The results were graphically presented using the R package “ggplot2”.
A multifactor regulatory network integrating transcription factors (TFs) and microRNAs (miRNAs) was constructed using predictions from miRanda (v3.3a) [24], TargetScan (v7.2) [25], and Enrichr (https://maayanlab.cloud/Enrichr/) [26].
2.7. Machine learning algorithms
mDEGs with ≥ 2 nodes in the PPI network were selected for ML modeling. The least absolute shrinkage and selection operator (LASSO) regression is a widely utilized method that has demonstrated success in removing insignificant variables through the penalization of their corresponding regression coefficients [27]. It was applied for dimensionality reduction to identify the valuable predictive genes and their coefficients were determined by the best penalty parameter lambda (λ) related to the 10-fold cross validation using the “glmnet” R package. A risk score was calculated using the corresponding regression coefficients and the expression levels of the candidate genes. Another algorithm, the Support Vector Machine (SVM)-Recursive Feature Elimination (RFE) algorithm was used to further refine predictive genes using the “caret” R package [28].
To validate the predictive mitochondria-related gene signature, a Support Vector Machine (SVM) model was utilized. The training sessions for the SVM model were conducted using the metadata set GSE77938, and the predictive effectiveness of the hub genes was subsequently evaluated in the validation set GSE151631. The receiver operating characteristic (ROC) analysis was employed to assess the model's ability to differentiate KC from normal samples, with results visualized using the “ggplot2” package in R. The area under curve (AUC) was calculated to determine the accuracy of the diagnostic signature.
2.8. Analyses of single cell transcriptome data
The original single-cell transcriptomic data (3 KC samples and 4 control samples) was downloaded from the GSA database (accession number: HRA000728) [21]. Cells with fewer than 200 or more than 10,000 detected genes, as well as those with fewer than 100 or more than 30,000 total counts, were excluded. Additionally, cells with more than 20 % mitochondrial genes and more than 3 % hemoglobin genes were filtered out using the “PercentageFeatureSet” function in R. The “NormalizeData” function was then applied to normalize the gene expression data. Highly variable genes were identified using the “FindVariableFeatures” function from the Seurat package in R. Sample integration and batch elimination were performed via the “RunHarmony” function, with a dimensionality of 35. Cells were clustered using the “FindClusters” and “FindNeighbors” functions (resolution = 0.1), after scaling all genes with the “ScaleData” function and identifying anchors through “PCA” dimensionality reduction. Subsequently, two dimensionality reduction methods were employed including t-distributed stochastic neighbor embedding (tSNE) and uniform manifold approximation and projection (UMAP), via Seurat in R [29], [30]. The “FindAllMarkers” function was implemented to compute the DEGs between distinct clusters and/or cell types.
2.9. Analyses of immune infiltration landscape and immune correlation
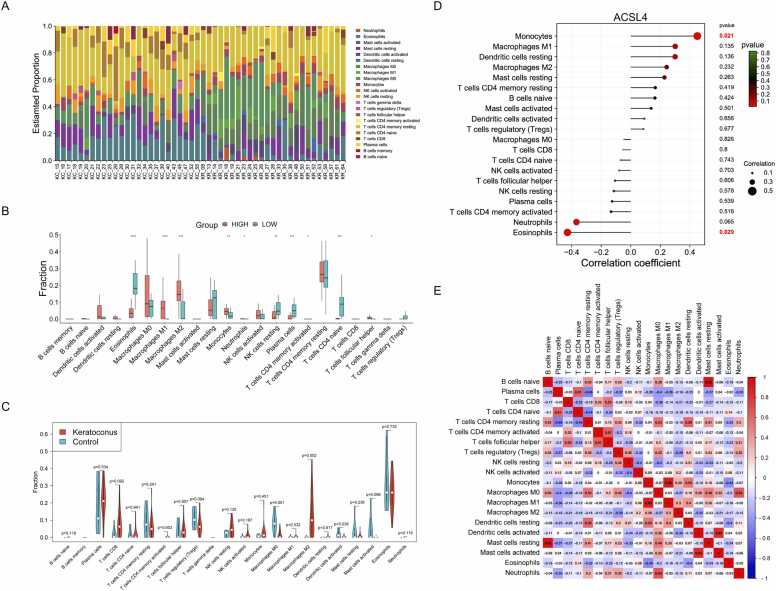
Single sample gene set enrichment analysis (ssGSEA) was utilized to analyze specific immune expression in each sample in the metadata set GSE77938. The proportion of the 22 types of immune cells in the immune infiltration landscape of each sample was evaluated via the CIBERSORT algorithm in R software. The 22 distinct immune cell types include B cells memory, B cells naïve, dendritic cells activated, dendritic cells resting, eosinophils, macrophages M0, macrophages M1, macrophages M2, mast cells activated, mast cells resting, monocytes, neutrophils, natural killer (NK) cells activated, NK cells resting, plasma cells, T cells CD4 memory activated, T cells CD4 memory resting, T cells CD4 naïve, T cells CD8, T cells follicular helper, T cells gamma delta, and T cells regulatory (Tregs). Using a LASSO-based risk score, we categorized the KC patients into high- and low-risk groups based on the median value. Gene set variation analysis (GSVA) was conducted to assess the relative abundance of immune cells between different risk groups and between normal and KC patients, utilizing the R packages “ggplot2”, “ggpubr” and “GSVA”. Correlation analysis of the 20 types of immune cells was demonstrated with matrix diagrams in R package “corrplot”. Furthermore, Pearson’s correlation analysis was conducted to examine the association between ACSL4 and the levels of 20 types of infiltrating immune cells in KC.
2.10. Statistics
Statistical analyses were conducted using R software (v4.1.2) and GraphPad Prism (v8.0). Data were presented as the mean ± standard deviation (SD). The Pearson correlation method was employed to analyze statistical correlations. Differences between two groups were assessed using the two-sample Wilcoxon test and Student’s t-test. The Kruskal-Wallis test was utilized to evaluate gene expression levels across three independent groups. Benjamini-Hochberg method was adopted for adjusted p value. P < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3. Results
3.1. Characterization of mitochondria-related DEGs and functional explorations in KC
A total of 3436 DEGs were successfully identified from the metadata dataset GSE77938. Specifically, 2910 DEGs were found to be downregulated, while 526 DEGs were upregulated in KC vs. normal cornea samples (Fig. 2A). Following the intersection of Venn diagram, a total of 104 DEGs were found to be associated with mitochondria based on the MitoMiner database (Fig. 2B, Table S2). The PPI network was constructed based on these mDEGs using the STRING database, followed by visualization in Cytoscape software (Fig. 2C). The network diagram consisted of 104 nodes and 134 edges representing protein interactions, with 33 mDEGs exhibiting degree ≥ 2, namely, LRRK2, CMPK2, TYMS, IFIT3, IFI27, IFI6, LAP3, SNCA, TUBB3, XAF1, ACADL, ACSL5, ALDH1L1, ANXA1, CYP27A1, HELZ2, REM1, STAR, TUBA1A, ACSL4, ACTN1, ALDH2, AMT, BBC3, BCAT1, CYP24A1, DEPTOR, GK, GPAT2, MGST1, NOX4, SOD2, and VIM (Fig. 2D).
Fig. 2.
Analyses of mitochondria-related differentially expressed genes (mDEGs) and protein-protein interaction (PPI) in keratoconus (KC). (A) Volcano plot showing 3436 DEGs in the KC dataset GSE77938. (B) Venn graph showing 104 overlapping mDEGs in KC based on the MitoMiner database. (C) PPI network of the mDEGs using the STRING tool. (D) PPI network drawn by the Cytoscape plug-in CytoHubba. The larger size of the node indicates the corresponding gene with a greater log2 Fold Change (FC).
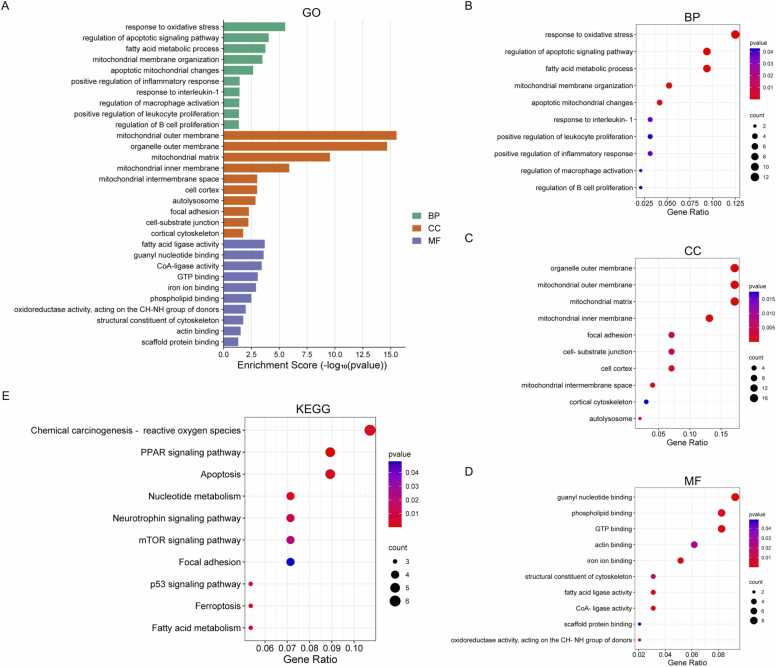
To identify key biological pathways linked to mitochondrial dysfunction in KC, we performed a comprehensive GO analysis on mDEGs (Fig. 3A). BP terms prominently implicated response to oxidative stress, regulation of apoptotic signaling pathway, fatty acid metabolic process, mitochondrial membrane organization, apoptotic mitochondrial changes, positive regulation of inflammatory response, response to interleukin-1, regulation of macrophage activation, positive regulation of leukocyte proliferation, and regulation of B cell proliferation (Fig. 3B, Table S3). CC enrichment highlighted mitochondrial substructures (outer membrane, matrix, inner membrane) alongside cytoskeletal and adhesion-related regions (focal adhesion, cell-substrate junctions) (Fig. 3C, Table S4). MF analysis revealed roles in fatty acid ligase activity, guanyl nucleotide binding, CoA-ligase activity, GTP binding, iron ion binding, phospholipid binding, oxidoreductase activity acting on the CH-NH group of donors, structural constituent of cytoskeleton, actin binding, and scaffold protein binding (Fig. 3D, Table S5). KEGG pathway analysis further underscored mitochondrial involvement, with enriched pathways implicating redox imbalance (chemical carcinogenesis-reactive oxygen species), programmed cell death (apoptosis, ferroptosis), metabolic dysregulation (fatty acid and nucleotide metabolism), and stress-responsive signaling cascades (mTOR, p53, neurotrophin, and PAR pathways) (Fig. 3E, Table S6). Collectively, these findings demonstrated that multiple pathways closely related to mitochondrial dysfunction, including oxidative stress, apoptosis, ferroptosis, and various immune responses, play a collective role in the pathogenesis of KC.
Fig. 3.
Functional analyses of the mitochondria-related differentially expressed genes (mDEGs). (A) Gene Ontology (GO) analysis of the mDEGs including (B) biological process (BP), (C) cellular component (CC), and (D) molecular function (MF). (E) Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the mDEGs.
3.2. Screening and validation of hub genes via multiple machine learning models
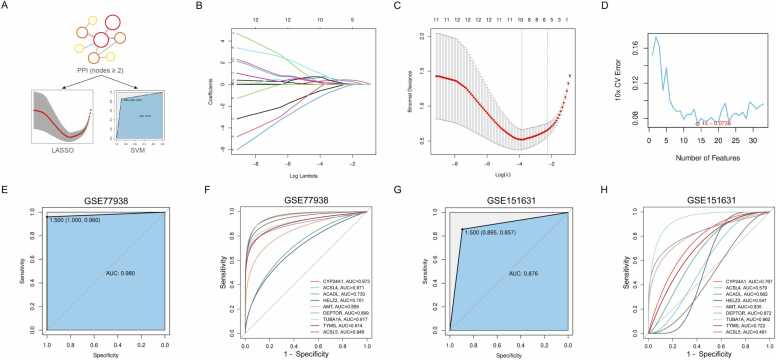
Two ML algorithms LASSO and SVM-RFE were used to screen for candidate biomarkers among 33 DEGs exhibiting a degree ≥ 2 in the PPI network (Fig. 4A). The LASSO model, optimized via 10-fold cross-validation (optimal log(λ) = 9) (Fig. 4B), identified nine key genes: CYP24A1, ACSL4, ACADL, HELZ2, AMT, DEPTOR, TUBA1A, TYMS, and ACSL5 (Fig. 4C). In addition, the SVM-RFE algorithm prioritized 14 genes with best accuracy and minimal classification error, namely ACTN1, ACADL, AMT, DEPTOR, HELZ2, VIM , CMPK2, ACSL5, CYP24A1, TUBA1A, SNCA, ACSL4, IFIT3, and LRRK2 (Fig. 4D).
Fig. 4.
Screening and validation of hub genes via multiple machine learning (ML) models. (A) Schematic diagram of the ML screening process. (B) The penalty graph of the characteristic variable coefficients by least absolute shrinkage and selection operator (LASSO) algorithm. (C) The LASSO coefficient curve of 9 genes. Two dotted lines λ indicate lambda.min (left) and lambda.1se (right), and the λ between these two values is considered appropriate. (D) Fourteen diagnostic genes screened by support vector machine-recursive feature elimination (SVM-RFE) algorithm. (E) Validation of the 9 gene signature diagnostic of keratoconus (KC) in the metadata set GSE77938, with (F) individual receiver operating characteristic (ROC) curve drawn for each gene. (E) Validation of the 9 gene signature diagnostic of KC in the validation set GSE151631, with (F) individual receiver operating characteristic (ROC) curve drawn for each gene.
Validation of the LASSO-derived nine-gene signature in the GSE77938 cohort using SVM demonstrated high accuracy in discriminating KC from control samples (AUC = 0.980) (Fig. 4E), with CYP24A1 (AUC = 0.973), ACSL4 (AUC = 0.971), and ACSL5 (AUC = 0.949) exhibiting the highest diagnostic accuracy (Fig. 4F). This gene signature retained strong predictive performance in the independent GSE151631 validation dataset (AUC = 0.857), confirming its reliability (Fig. 4G,H). Collectively, these results establish CYP24A1, ACSL4, ACADL, HELZ2, AMT, DEPTOR, TUBA1A, TYMS, and ACSL5 as hub genes with significant potential for distinguishing KC patients from healthy individuals, warranting further mechanistic investigation.
3.3. Analysis of TFs-miRNAs-mRNAs regulatory network
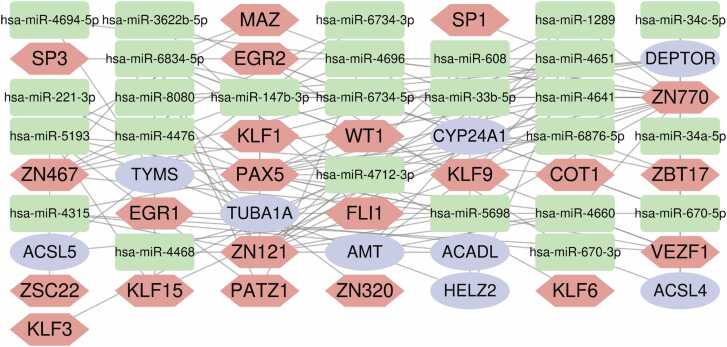
Recent advances in understanding gene regulation have highlighted the critical roles of TFs and miRNAs in controlling transcriptional and post-transcriptional processes [31], [32]. To explore regulatory mechanisms underlying KC, we constructed a multifactor interaction network based on the nine hub genes, integrating predictions from multiple databases. As shown in Fig. 5, this network comprises 9 mRNAs (CYP24A1, ACSL4, ACADL, HELZ2, AMT, DEPTOR, TUBA1A, TYMS, and ACSL5), 26 miRNAs (hsa-miR-1289, hsa-miR-4712–3p, hsa-miR-4641, hsa-miR-608, hsa-miR-4468, hsa-miR-34a-5p, hsa-miR-4660, hsa-miR-147b-3p, hsa-miR-8080, hsa-miR-6834–5p, hsa-miR-4651, hsa-miR-4694–5p, hsa-miR-6876–5p, hsa-miR-4315, hsa-miR-5698, hsa-miR-5193, hsa-miR-4696, hsa-miR-34c-5p, hsa-miR-670–5p, hsa-miR-670–3p, hsa-miR-33b-5p, hsa-miR-3622b-5p, hsa-miR-221–3p, hsa-miR-4476, hsa-miR-6734-3p, and hsa-miR-6734–5p), 22 TFs (ZN770, ZN121, ZN467, MAZ, PAX5, KLF15, SP1, PATZ1, VEZF1, EGR1, ZSC22, EGR2, KLF9, KLF1, KLF3, ZBT17, FLI1, ZN320, WT1, KLF6, SP3, and COT1).
Fig. 5.
Multifactor gene regulatory network integrating the 9 mitochondria-related differentially expressed genes (mDEGs), and their corresponding microRNAs (miRNAs) and transcription factors (TFs). The green squares stand for miRNAs, red hexagons for TFs, and purple circles for mDEGs.
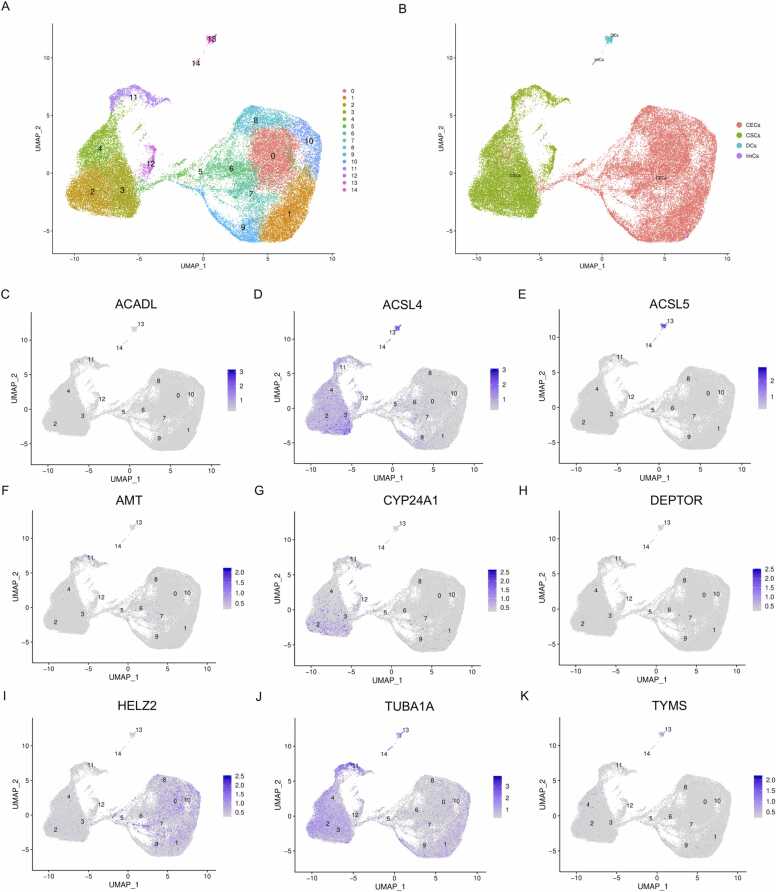
3.4. Visualization of hub genes at the single cell level
Single cell data for KC were acquired from the GSA database (ID: HRA000728), comprising three KC and four healthy donor corneas. UMAP dimensionality reduction revealed 14 distinct cell clusters (Fig. 6A), which were annotated into four major cell populations: corneal epithelial cells (CECs), corneal stromal cells (CSCs), dendritic cells (DCs), and immune cells (ImCs) (Fig. 6B). Expression patterns of nine candidate hub genes (ACADL, ACSL4, ACSL5, AMT, CYP24A1, DEPTOR, HELZ2, TUBA1A, and TYMS) were mapped across these clusters (Fig. 6C–K, S1–S9). Specifically, the expression levels of ACADL (Fig. 6C and S1), ACSL5 (Fig. 6E and S3), AMT (Fig. 6F and S4), CYP24A1 (Fig. 6G and S5), DEPTOR (Fig. 6H and S6), and TYMS (Fig. 6K and S9) were minimal in all cell clusters. In contrast, ACSL4 exhibited pronounced enrichment in CSCs (Fig. 6D and S2). Additionally, HELZ2 localized predominantly in CECs (Fig. 6I and S9), while TUBA1A was expressed in both CSCs and CECs (Fig. 6J and S8). Given the hallmark features of KC, including stromal thinning and dysfunction [33], [34], [35], HELZ2 and TUBA1A—both highly expressed in epithelial cells—were deemed unsuitable as stromal-specific biomarkers. This prioritized ACSL4, an enriched gene in CSCs, as the most promising biomarker for KC. Its expression profile was further validated through tSNE visualization (Fig. S10).
Fig. 6.
Single-cell transcriptional profiling of keratoconus (KC) samples, revealing hub gene expression across distinct corneal cell populations. (A,B) Uniform manifold approximation and projection (UMAP) visualization identified 14 cell clusters (A), annotated into four major cell types: corneal epithelial cells (CECs), stromal cells (CSCs), dendritic cells (DCs), and immune cells (ImCs) (B). (C–K) Expression patterns of hub genes, including (C) ACADL, (D) ACSL4, (E) ACSL5, (F) AMT, (G) CYP24A1, (H) DEPTOR, (I) HELZ2, (J) TUBA1A, and (K) TYMS were mapped across cell clusters.
3.5. Characteristics of immune infiltration landscape and immune correlation
Given the functional evidence implicating immune dysregulation in KC pathogenesis, we systematically profiled immune cell infiltration and pathway activity using the GSE77938 dataset. Comparative analysis of 22 immune cell types revealed distinct proportions and enrichment scores between KC and healthy corneas (Fig. 7A and S11). Stratifying KC patients into high- and low-risk groups further uncovered significant differences in the infiltration of eosinophils, macrophages M1, macrophages M2, monocytes, neutrophils, NK cells resting, plasma cells, T cells CD4 memory activated, T cells CD4 naïve, and T cells follicular helper (Fig. 7B). Furthermore, KC samples exhibited reduced infiltration of M0 macrophages and activated dendritic cells, alongside elevated M2 macrophage levels, compared to controls (Fig. 7C). Notably, ACSL4 expression correlated positively with monocyte abundance but inversely with eosinophil infiltration, suggesting its immunomodulatory role (Fig. 7D). Correlation network analysis identified neutrophils, activated dendritic cells, M0 macrophages, and CD4+ memory T cells as central hubs coordinating immune crosstalk in KC (Fig. 7E). These findings indicated the interplay between stromal biomarkers like ACSL4 and immune remodeling in KC progression.
Fig. 7.
Immune infiltration landscape and immune correlation analysis in keratoconus (KC). (A) Stacked bar chart of the relative proportions of 22 infiltrated immune cells in KC and control patients. (B) Differences in 22 types of immune cells between high- (red) and low-risk (green) groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (C) Differences in 22 types of immune cells between KC (red) and normal (green) samples. (D) Correlation analysis between ACSL4 and 20 immune cells in KC. (E) Correlation matrix of the enrichment scores of 20 immune cell subtypes in KC. Red indicates positive correlations while blue indicates negative correlations. The darker the color the stronger the correlation. The correlation scores were presented on criteria of p < 0.05.
3.6. In vitro validation using cellular model of KC
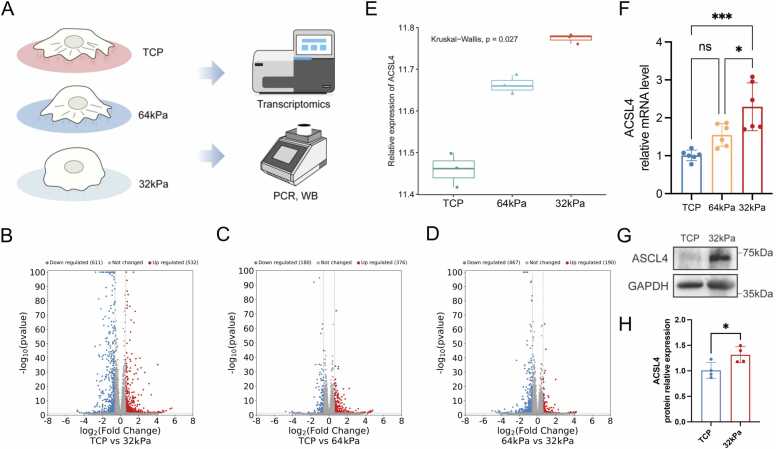
KC is characterized by progressive structural and compositional alterations in the corneal stroma and extracellular matrix (ECM), resulting in reduced tissue stiffness—a critical factor driving the irregular corneal morphology central to the disease pathogenesis [36], [37]. To explore the role of biomechanical cues in KC, we analyzed ACSL4 expression using single-cell RNA sequencing and found that ACSL4 was highly expressed in KC stromal cells. To further dissect stiffness-dependent transcriptional changes, HTK cells were cultured on substrates of varying stiffness (32 kPa, 64 kPa, and TCP), followed by transcriptomic, qRT-PCR, and Western blot validation (Fig. 8A).
Fig. 8.
Expression of ACSL4 in an in vitro keratoconus (KC) model. (A) Schematic diagram of the in vitro validation. In brief, HTK cells were cultured in different culture plates, including normal tissue culture plastic (TCP), and culture plates with Young’s modulus of 64 kPa and 32 kPa, followed by PCR and transcriptomic analysis. (B–D) Volcano plot showing (B) 1143 differentially expressed genes (DEGs) in 32 kPa vs. TCP group, (C) 556 DEGs in 64 kPa vs. TCP group, and (D) 657 DEGs in 32 kPa vs. 64 kPa group. (E) Relative ACSL4 expression in TCP, 64 kPa, and 32 kPa groups. Kruskal–Wallis test was used for significance estimation. (F) Relative mRNA expression of ACSL4 expression in TCP, 64 kPa, and 32 kPa groups. ns, no significance. (G) Western blot and (H) relative protein expression of ACSL4 in TCP and 32 kPa groups. ns, no significance. *p < 0.05, ***p < 0.001.
Transcriptomic profiling identified 1143 DEGs in 32 kPa vs. TCP group (Fig. 8B), 556 DEGs in 64 kPa vs. TCP group (Fig. 8C), and 657 DEGs in 32 kPa vs. 64 kPa group (Fig. 8D). Heatmap visualization confirmed distinct transcriptional profiles across substrate conditions (Fig. S12 A-C). Additionally, the expression levels of the 9 hub genes were analyzed (Table S7-S9). The results showed that several hub genes were differentially expressed, including 4 genes (ACSL4, TYMS, TUBA1A, DEPTOR) in 32 kPa vs. TCP group (Table S7), 5 genes (ACSL4, CYP24A1, TYMS, TUBA1A, HELZ2) in 64 kPa vs. TCP group (Table S8), and 4 genes (ACSL4, TYMS, TUBA1A, HELZ2) in 64 kPa vs. 32 kPa group (Table S9). Notably, ACSL4 emerged as uniquely consistent, showing significant upregulation with decreasing substrate stiffness in all comparisons (Fig. 8E), a trend validated by qRT-PCR (Fig. 8F) and Western blot (Fig. 8G,H; Original Western blot bands shown in Fig. S13). These findings position ACSL4 as a potential mechanosensitive regulator in KC development, linking biomechanical stress to mitochondrial dysfunction.
To clarify substrate stiffness-dependent pathways in KC, we performed functional enrichment analyses across substrate groups (32 kPa, 64 kPa, TCP) (Fig. 9, Table S10-S15). GO terms in all comparisons (32 kPa vs. TCP, 64 kPa vs. TCP, 64 kPa vs. 32 kPa) highlighted inflammatory regulation, oxidative stress response, MAP kinase signaling, lipid metabolism, ECM remodeling, and immune activation, including leukocyte proliferation and T cell activation (Fig. 9A,C,E; Tables S10–S12). KEGG pathway analysis further implicated apoptosis, focal adhesion, ferroptosis, cellular senescence, TNF/NF-κB signaling, and immune-mediated processes (e.g., Th17 differentiation, NK cell cytotoxicity) (Fig. 9B,D,F; Tables S13–S15). Collectively, these pathways suggested the intricate interplay between biomechanical stimuli, redox imbalance, and immune dysregulation in KC, providing valuable mechanistic insights into stromal degeneration and disease development.
Fig. 9.
Functional analyses of the in vitro keratoconus (KC) model. (A) Gene Ontology (GO) including biological process (BP), cellular component (CC), and molecular function (MF), and (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis for HTK cells cultured in 32 kPa vs. TCP group. (C) GO and (D) KEGG analysis for HTK cells cultured in 64 kPa vs. TCP group. (E) GO and (F) KEGG analysis for HTK cells cultured in 32 kPa vs. 64 kPa group.
4. Discussion
While mitochondrial abnormalities have been implicated in KC, their mechanistic role in disease pathogenesis remains poorly understood. Through integrative bulk/single-cell transcriptomics and in vitro validation, this study establishes mitochondrial dysfunction as a key driver of KC pathology, supported by four major findings: (1) KC corneas exhibit significant alterations in mitochondrial-associated gene expression, indicative of impaired mitochondrial metabolism and structural integrity; (2) Mitochondrial-linked pathways are prominently dysregulated in KC, including ROS imbalance, membrane disorganization, apoptosis, and ferroptosis; (3) Machine learning and single-cell analyses identified ACSL4, a ferroptosis-related gene, as a critical biomarker. Its expression correlates with immune cell infiltration, bridging metabolic stress and inflammatory responses in KC; (4) ACSL4 expression decreases with substrate stiffness (32 kPa, 64 kPa, and TCP) in stromal cells, suggesting mechanosensitive regulation may lead to mitochondrial dysfunction. These insights position mitochondrial health as central to KC pathogenesis, with ACSL4 emerging as a promising therapeutic target to restore biomechanical and immune homeostasis.
The relationship between ferroptosis and mitochondrial dysfunction is intricate and multifaceted, involving key factors such as lipid metabolism, and iron homeostasis. Recent research has established that acyl-CoA synthetase long-chain family member 4 (ACSL4) serves as a pivotal link between lipid metabolism, ferroptosis, and mitochondrial integrity. ACSL4 catalyzes the conversion of long-chain fatty acids into acyl-CoA derivatives, essential for various metabolic pathways, including lipid synthesis and degradation. The activation of polyunsaturated fatty acids (PUFAs) by ACSL4 can lead to increased lipid peroxidation, a hallmark of ferroptosis [38]. Elevated production of ROS during this process has the potential to compromise mitochondrial integrity and impede ATP production, further exacerbating mitochondrial dysfunction [39]. In cancers such as non-small cell lung cancer (NSCLC) and triple-negative breast cancer (TNBC), ACSL4 is implicated in metabolic reprogramming, where alterations in fatty acid metabolism support tumor growth and survival. This metabolic adaptation can lead to further mitochondrial dysfunction as cells respond to new metabolic demands [40]. Moreover, emerging evidence suggests that ACSL4 expression can be upregulated in response to inflammatory stimuli, resulting in increased production of pro-inflammatory cytokines and activation of cell death pathways, which further contribute to mitochondrial damage and dysfunction. In neurodegenerative disorders such as Parkinson's disease and autoimmune encephalitis, increased expression of ACSL4 may exacerbate neuroinflammation and compromise mitochondrial health [41]. Inhibiting ACSL4 may offer a therapeutic strategy to reduce lipid peroxidation and oxidative stress, potentially mitigating ferroptosis and protecting mitochondrial function. A deeper understanding of the intricate mechanisms involving ACSL4 is essential for developing therapeutic approaches aimed at reducing inflammation and preserving mitochondrial health across various disease contexts.
The integration of ML in this study, through LASSO regression, SVM, and SVM-RFE algorithms, facilitated robust identification of mitochondrial-associated biomarkers. As biotechnological tools such as ML become essential to precision medicine, it is critical to train clinicians and researchers in these methodologies [42]. ML enhances biomarker discovery and improves predictive modeling for personalized therapeutic strategies. Future initiatives should prioritize interdisciplinary training programs to bridge the gap between computational biology and clinical practice. Beyond biomarker ACSL4, our multifactor regulatory network further identified miRNAs (e.g., hsa-miR-34a-5p, hsa-miR-221–3p) and TFs (e.g., SP1, KLF9) as potential regulators of KC pathogenesis. TFs and miRNAs are increasingly recognized for their roles in immune-mediated ocular disorders [43], [44]. For instance, hsa-miR-34a-5p regulates inflammatory responses in diabetic retinopathy, while SP1 modulates corneal epithelial repair [45], [46]. These findings suggest that targeting miRNA-TF networks could provide novel avenues for modulating immune-metabolic crosstalk in KC.
KC is traditionally characterized as a non-inflammatory eye disease, primarily because the corneas of KC patients typically do not exhibit inflammation-related manifestations. However, this study uncovers a complex immune microenvironment in KC, marked by significant infiltration of diverse immune cells, including eosinophils, macrophages M1, macrophages M2, monocytes, neutrophils, NK cells resting, plasma cells, T cells CD4 memory activated, T cells CD4 naïve, and T cells follicular helper. Strikingly, ACSL4 expression correlated positively with monocyte abundance but inversely with eosinophil levels, suggesting its regulatory role in immune crosstalk during KC pathogenesis. Recent studies have indicated that there may be underlying immune responses or subtle inflammatory processes involved in the development of KC. It is particularly noteworthy that patients with allergic ocular diseases exhibit a higher KC risk, suggesting a potential link between ocular immune dysregulation and KC [47]. Research has shown altered immune cell populations in the ocular surface and circulatory system of KC patients, including activated neutrophils, NK cells, and gamma delta T cells, indicating an immune landscape that may contribute to disease progression [48]. Furthermore, elevated levels of various inflammatory factors, such as IL-6, IL-10, and MMP-9, have been identified in the cornea and tears of KC patients [49]. Some of these factors are unique to KC, indicating specific inflammatory pathways involved in its pathogenesis. Interestingly, infiltrating monocytes may play a crucial role in the development of KC by enhancing inflammatory responses and promoting neovascularization and profibrotic effects, which contribute to corneal scarring and further degradation of corneal structure [50]. Eosinophils, particularly in patients with concurrent allergic conditions, may also significantly influence KC pathogenesis through the release of various mediators, including cytokines, chemokines, and granules that can affect local inflammation and tissue remodeling [47]. Notably, in the lungs, elevated levels of ACSL4 have been shown to enhance eosinophil activity by inhibiting the p38 MAPK pathway in asthma, which affects airway hyperreactivity and remodeling. Understanding these intricate immune interactions provides valuable insights into the pathogenesis of KC and opens avenues for potential therapeutic strategies targeting immune modulation in this condition. Future research could further explore the specific mechanisms by which ACSL4 influences immune cell behavior and its implications for treatment.
Notably, our results revealed a mechanobiological interplay between ACSL4 expression and corneal tissue dynamics in KC. In vitro modeling of KC demonstrated consistency in ACSL4 expression under varying substrate stiffness conditions (32 kPa, 64 kPa, and TCP), with expression levels negatively correlating with increased mechanical rigidity. This suggests that ECM stiffness directly modulates ferroptosis susceptibility via ACSL4 regulation. These findings align with emerging evidence linking matrix stiffness to the regulation of oxidative stress, mitochondrial dysfunction, and ferroptotic cell death [51]. Recent evidence suggests that ferroptosis is sensitive to cell density and the mechanical characteristics of the environment, with regulation via the Hippo pathway, which affects Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). YAP/TAZ can upregulate ACSL4, promoting the synthesis of polyunsaturated fatty acids (PUFAs) in phospholipids (PUFA-PL), thereby rendering cellular membranes more susceptible to oxidative damage [52]. Concurrently, YAP/TAZ upregulates transferrin receptor (TFRC) expression, which increases exogenous iron uptake. This process is essential factor for ferroptosis induction, further linking mechanical properties to cell death and mitochondrial dysfunction pathways [53]. The YAP/ACSL4 axis also plays a significant role in modulating immune cell function and the inflammatory response. By regulating ACSL4, YAP can modulate macrophage polarization and inflammatory mediator production, substantially affecting immune cell behavior across various disease conditions. Therapeutic strategies aimed at modulating the Hippo pathway or its downstream effects on ACSL4 presents a promising therapeutic strategy, potentially alleviating fibrosis and corneal degeneration in KC and related disorders.
There are several limitations to our work. First, while ACSL4 was validated at mRNA/protein levels in HTK cells under varying substrate stiffness, our study does not establish whether its upregulation is a cause or consequence of KC. Comprehensive in vivo studies, such as siRNA knockdown, overexpression studies, or ferroptosis-related assays (e.g., GPX4/SLC7A11 modulation) and CRISPR-based knockdown in animal models, are needed to clarify its mechanistic role. Second, due to restricted access to clinical KC patient samples, our analysis relied on public datasets from GEO and GSA databases, which may introduce selection bias. Larger prospective cohorts are needed to validate the current findings. We are also establishing a local KC registry to include cornea samples, tear cytokine profiles, corneal imaging, and longitudinal clinical data, which will enhance future translational relevance. Third, while ACSL4 is a ferroptosis marker, the study's focus is on mitochondrial dysfunction rather than direct assays of ferroptosis. Future work should explore ferroptosis in KC stromal cells to contextualize ACSL4’s role. Fourth, the immune infiltration landscape was assessed using transcriptomic data, which provides insights into gene expression but does not establish direct cause-and-effect relationships with immune responses in KC development. Further in-depth studies are warranted to explore the underlying immune mechanisms of ACSL4 in KC. Finally, to maximize clinical utility, we recognize the need for user-friendly tools, such as webservers or applications, that will allow clinicians and researchers to explore our datasets, validate biomarkers, and predict KC risk. This will be a key focus of our future research efforts.
5. Conclusion
To summarize, this study revealed a mitochondrial-related molecular mechanism underlying KC pathogenesis and identified ACSL4 as a pivotal biomarker. Based on transcriptomic datasets, nine characteristic genes were validated using multiple ML models. Single cell sequencing data further identified ACSL4 as the most promising biomarker, which was expressed primarily in CSCs. Immune infiltration analysis revealed a comprehensive immune microenvironment in KC in which ACSL4 participated. Furthermore, ACSL4 expression was significantly upregulated in response to increased substrate stiffness in HTK cells, suggesting its potential role in KC development. Future work should prioritize functional experiments to clarify whether ACSL4 directly drives KC pathogenesis or serves as a correlative marker of disease severity.
CRediT authorship contribution statement
Yuchen Cai: Formal analysis, Funding acquisition, Writing – original draft. Tianyi Zhou: Software, Data curation, Formal analysis. Xueyao Cai: Formal analysis, Writing – review & editing. Wenjun Shi: Writing – review & editing. Hao Sun: Supervision, Methodology, Writing – review & editing. Yao Fu: Project administration, Supervision, Funding acquisition, Conceptualization.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82070919, 82271041, and 82471039), Program of Shanghai Academic/Technology Research Leader (22XD1401800), Shanghai Key Clinical Specialty, Shanghai Eye Disease Research Center (2022ZZ01003), Biomaterials and Regenerative Medicine Institute Cooperative Research Project, Shanghai Jiao Tong University School of Medicine (2022LHA06), Shanghai Post-doctoral Excellence Program (2024409), and Postdoc Initiative Fund of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (202401056).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We extend our sincere gratitude to the GEO and GSA repositories for the KC datasets, as well as to Shanghai Newcore Biotech for conducting the RNA Sequencing and subsequent bioinformatics analyses.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.csbj.2025.05.013.
Contributor Information
Hao Sun, Email: b24008@sh9hospital.org.cn.
Yao Fu, Email: fuyao@sjtu.edu.cn.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Data availability
All data is available from the corresponding authors upon request.
References
- 1.Gomes J.A.P., Tan D., Rapuano C.J., Belin M.W., Ambrósio R., Guell J.L., et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34:359–369. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 2.Jones-Jordan L.A., Walline J.J., Sinnott L.T., Kymes S.M., Zadnik K. Asymmetry in keratoconus and vision-related quality of life. Cornea. 2013;32:267–272. doi: 10.1097/ICO.0b013e31825697c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson A.E., Hayes S., Hardcastle A.J., Tuft S.J. The pathogenesis of keratoconus. Eye Lond Engl. 2014;28:189–195. doi: 10.1038/eye.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santodomingo-Rubido J., Carracedo G., Suzaki A., Villa-Collar C., Vincent S.J., Wolffsohn J.S. Keratoconus: an updated review. Contact Lens Anterior Eye. 2022;45 doi: 10.1016/j.clae.2021.101559. [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh R., Ong Z.Z., Rampat R., Alió Del Barrio J.L., Barua A., Ang M., et al. Management of keratoconus: an updated review. Front Med. 2023;10 doi: 10.3389/fmed.2023.1212314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alió Del Barrio J.L., Bhogal M., Ang M., Ziaei M., Robbie S., Montesel A., et al. Corneal transplantation after failed grafts: options and outcomes. Surv Ophthalmol. 2021;66:20–40. doi: 10.1016/j.survophthal.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Dammak A., Pastrana C., Martin-Gil A., Carpena-Torres C., Peral Cerda A., Simovart M., et al. Oxidative stress in the anterior ocular diseases: diagnostic and treatment. Biomedicines. 2023;11:292. doi: 10.3390/biomedicines11020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navel V., Malecaze J., Pereira B., Baker J.S., Malecaze F., Sapin V., et al. Oxidative and antioxidative stress markers in keratoconus: a systematic review and meta-analysis. Acta Ophthalmol (Copenh) 2021;99:e777–e794. doi: 10.1111/aos.14714. [DOI] [PubMed] [Google Scholar]
- 9.Yıldız E., Aydemir D., Zibandeh N., Kuşan E., Gümüş K., İlhan Saraç Ö., et al. Investigation of mitophagy biomarkers in corneal epithelium of keratoconus patients. Curr Eye Res. 2022;47:661–669. doi: 10.1080/02713683.2022.2025846. [DOI] [PubMed] [Google Scholar]
- 10.Atilano S.R., Coskun P., Chwa M., Jordan N., Reddy V., Le K., et al. Accumulation of mitochondrial DNA damage in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1256–1263. doi: 10.1167/iovs.04-1395. [DOI] [PubMed] [Google Scholar]
- 11.Vallabh N.A., Romano V., Willoughby C.E. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion. 2017;36:103–113. doi: 10.1016/j.mito.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao X.-D., Chen Z.-L., Qu M.-L., Zhao X.-W., Li S.-X., Chen P. In: Mishmar D., editor. Vol. 11. 2016. Decreased integrity, content, and increased transcript level of mitochondrial DNA are associated with keratoconus. (PLOS ONE). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chwa M., Atilano S.R., Hertzog D., Zheng H., Langberg J., Kim D.W., et al. Hypersensitive response to oxidative stress in keratoconus corneal fibroblasts. Invest Opthalmol. Vis Sci. 2008;49:4361. doi: 10.1167/iovs.08-1969. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Amero K.K., Kondkar A.A., Azad T.A., Sultan T., Kalantan H., Al-Muammar A.M. Keratoconus is associated with increased copy number of mitochondrial DNA. Mol Vis. 2014;20:1203–1208. [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Amero K.K., Azad T.A., Kalantan H., Sultan T., Al-Muammar A.M. Mitochondrial sequence changes in keratoconus patients. Invest Ophthalmol Vis Sci. 2014;55:1706–1710. doi: 10.1167/iovs.14-13938. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Liu J., Li J., Li Y., Sun J., Deng Y., et al. Substrate stiffness can affect the crosstalk between adipose derived mesenchymal stem cells and macrophages in bone tissue engineering. Front Bioeng Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1133547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabza M., Karolak J.A., Rydzanicz M., Szcześniak M.W., Nowak D.M., Ginter-Matuszewska B., et al. Collagen synthesis disruption and downregulation of core elements of TGF-β, Hippo, and Wnt pathways in keratoconus corneas. Eur J Hum Genet. 2017;25:582–590. doi: 10.1038/ejhg.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinde V., Hu N., Mahale A., Maiti G., Daoud Y., Eberhart C.G., et al. RNA sequencing of corneas from two keratoconus patient groups identifies potential biomarkers and decreased NRF2-antioxidant responses. Sci Rep. 2020;10:9907. doi: 10.1038/s41598-020-66735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A.C., Robinson A.J. MitoMiner v4.0: an updated database of mitochondrial localization evidence, phenotypes and diseases. Nucleic Acids Res. 2019;47:D1225–D1228. doi: 10.1093/nar/gky1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dou S., Wang Q., Zhang B., Wei C., Wang H., Liu T., et al. Single-cell atlas of keratoconus corneas revealed aberrant transcriptional signatures and implicated mechanical stretch as a trigger for keratoconus pathogenesis. Cell Discov. 2022;8:66. doi: 10.1038/s41421-022-00397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin C.-H., Chen S.-H., Wu H.-H., Ho C.-W., Ko M.-T., Lin C.-Y. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(4) doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie C., Mao X., Huang J., Ding Y., Wu J., Dong S., et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enright A.J., John B., Gaul U., Tuschl T., Sander C., Marks D.S. MicroRNA targets in drosophila. Genome Biol. 2003;5 doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal V., Bell G.W., Nam J.-W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs.:38. [DOI] [PMC free article] [PubMed]
- 26.Xie Z., Bailey A., Kuleshov M.V., Clarke D.J.B., Evangelista J.E., Jenkins S.L., et al. Gene set knowledge discovery with enrichr. Curr Protoc. 2021;1 doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Angelo G.M., Rao D., Gu C.C. Combining least absolute shrinkage and selection operator (LASSO) and principal-components analysis for detection of gene-gene interactions in genome-wide association studies. BMC Proc. 2009;3(7) doi: 10.1186/1753-6561-3-s7-s62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanz H., Valim C., Vegas E., Oller J.M., Reverter F. SVM-RFE: selection and visualization of the most relevant features through non-linear kernels. BMC Bioinforma. 2018;19:432. doi: 10.1186/s12859-018-2451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz-Papkovich A., Anderson-Trocmé L., Gravel S. A review of UMAP in population genetics. J Hum Genet. 2021;66:85–91. doi: 10.1038/s10038-020-00851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W., Cerise J.E., Yang Y., Han H. Application of t-SNE to human genetic data. J Bioinform Comput Biol. 2017;15:1750017. doi: 10.1142/S0219720017500172. [DOI] [PubMed] [Google Scholar]
- 31.Cheng C., Alexander R., Min R., Leng J., Yip K.Y., Rozowsky J., et al. Understanding transcriptional regulation by integrative analysis of transcription factor binding data. Genome Res. 2012;22:1658–1667. doi: 10.1101/gr.136838.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tafrihi M., Hasheminasab E. MiRNAs: biology, biogenesis, their web-based tools, and databases. Micro Shariqah U Arab Emir. 2019;8:4–27. doi: 10.2174/2211536607666180827111633. [DOI] [PubMed] [Google Scholar]
- 33.Foster J.W., Shinde V., Soiberman U.S., Sathe G., Liu S., Wan J., et al. Integrated stress response and decreased ECM in cultured stromal cells from keratoconus corneas. Invest Ophthalmol Vis Sci. 2018;59:2977–2986. doi: 10.1167/iovs.18-24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dou S., Liu X., Shi W., Gao H. New dawn for keratoconus treatment: potential strategies for corneal stromal regeneration. Stem Cell Res Ther. 2023;14:317. doi: 10.1186/s13287-023-03548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty R., Mahendran K., Joshi P.D., Jeyabalan N., Jayadev C., Das D. Corneal stromal regeneration-keratoconus cell therapy: a review. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2023;261:3051–3065. doi: 10.1007/s00417-023-06064-7. [DOI] [PubMed] [Google Scholar]
- 36.Thomasy S.M., Leonard B.C., Greiner M.A., Skeie J.M., Raghunathan V.K. Squishy matters - Corneal mechanobiology in health and disease. Prog Retin Eye Res. 2024;99 doi: 10.1016/j.preteyeres.2023.101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vellara H.R., Patel D.V. Biomechanical properties of the keratoconic cornea: a review. Clin Exp Optom. 2015;98:31–38. doi: 10.1111/cxo.12211. [DOI] [PubMed] [Google Scholar]
- 38.Gan B. ACSL4, PUFA, and ferroptosis: new arsenal in anti-tumor immunity. Signal Transduct Target Ther. 2022;7:128. doi: 10.1038/s41392-022-01004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merkel M., Goebel B., Boll M., Adhikari A., Maurer V., Steinhilber D., et al. Mitochondrial reactive oxygen species formation determines ACSL4/LPCAT2-mediated ferroptosis. Antioxidants. 2023;12:1590. doi: 10.3390/antiox12081590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan J., Bode A.M., Luo X. ACSL family: The regulatory mechanisms and therapeutic implications in cancer. Eur J Pharmacol. 2021;909 doi: 10.1016/j.ejphar.2021.174397. [DOI] [PubMed] [Google Scholar]
- 41.Chen F., Kang R., Liu J., Tang D. The ACSL4 network regulates cell death and autophagy in diseases. Biology. 2023;12:864. doi: 10.3390/biology12060864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keane P.A., Topol E.J. AI-facilitated health care requires education of clinicians. Lancet Lond Engl. 2021;397:1254. doi: 10.1016/S0140-6736(21)00722-4. [DOI] [PubMed] [Google Scholar]
- 43.Shao D., He S., Ye Z., Zhu X., Sun W., Fu W., et al. Identification of potential molecular targets associated with proliferative diabetic retinopathy. BMC Ophthalmol. 2020;20:143. doi: 10.1186/s12886-020-01381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Y., Zhou T., Chen J., Cai X., Fu Y. Uncovering the role of transient receptor potential channels in pterygium: a machine learning approach. Inflamm Res. 2023;72(3):589–602. doi: 10.1007/s00011-023-01693-4. [DOI] [PubMed] [Google Scholar]
- 45.Zaniolo K., Gingras M.-E., Audette M., Guérin S.L. Expression of the gene encoding poly(ADP-ribose) polymerase-1 is modulated by fibronectin during corneal wound healing. Invest Ophthalmol Vis Sci. 2006;47:4199–4210. doi: 10.1167/iovs.06-0176. [DOI] [PubMed] [Google Scholar]
- 46.Desjardins P., Le-Bel G., Ghio S.C., Germain L., Guérin S.L. The WNK1 kinase regulates the stability of transcription factors during wound healing of human corneal epithelial cells. J Cell Physiol. 2022;237:2434–2450. doi: 10.1002/jcp.30698. [DOI] [PubMed] [Google Scholar]
- 47.Seth I., Bulloch G., Vine M., Outmezguine J., Seth N., Every J., et al. The association between keratoconus and allergic eye diseases: A systematic review and meta-analysis. Clin Exp Ophthalmol. 2023;51:01–16. doi: 10.1111/ceo.14215. [DOI] [PubMed] [Google Scholar]
- 48.D’Souza S., Nair A.P., Sahu G.R., Vaidya T., Shetty R., Khamar P., et al. Keratoconus patients exhibit a distinct ocular surface immune cell and inflammatory profile. Sci Rep. 2021;11 doi: 10.1038/s41598-021-99805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niu X., Xu M., Zhu J., Zhang S., Yang Y. Identification of the immune-associated characteristics and predictive biomarkers of keratoconus based on single-cell RNA-sequencing and bulk RNA-sequencing. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1220646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X., Liu C., Cui Z., Huang Y., Luo Q., Chen S., et al. Integrative transcriptomics analysis and experimental validation reveal immunomodulatory patterns in keratoconus. Exp Eye Res. 2023;230 doi: 10.1016/j.exer.2023.109460. [DOI] [PubMed] [Google Scholar]
- 51.Ke W., Liao Z., Liang H., Tong B., Song Y., Li G., et al. Stiff substrate induces nucleus pulposus cell ferroptosis via YAP and N-cadherin mediated mechanotransduction. Adv Health Mater. 2023;12 doi: 10.1002/adhm.202300458. [DOI] [PubMed] [Google Scholar]
- 52.Magesh S., Cai D. Roles of YAP/TAZ in ferroptosis. Trends Cell Biol. 2022;32:729. doi: 10.1016/j.tcb.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J., Minikes A.M., Gao M., Bian H., Li Y., Stockwell B.R., et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Data Availability Statement
All data is available from the corresponding authors upon request.