Abstract
Seminolipids are testis-specific ether glycolipids that are important for spermatogenesis. The fatty alcohol (ether-linked alkyl moiety) in ether lipids is generated from an acyl-CoA by fatty acyl-CoA reductase (FAR). To date, the diversity of the alkyl and acyl moieties in seminolipids, the specific stage of spermatogenesis during which seminolipids are produced, and the FAR isozyme (FAR1 or FAR2) involved in the synthesis of the alkyl moieties have remained largely unclear. Here, we demonstrated that Far1 is expressed in the mouse testis via quantitative RT-PCR analysis, whereas Far2 was barely detectable. In situ hybridization and quantitative RT-PCR analysis of spermatogenic cells separated via FACS revealed that Far1 is expressed in spermatogonia, spermatocytes, and spermatids. We generated Far1 KO mice and found that male Far1 KO mice were infertile. In these mice, sperms were absent in the epididymides and the testes were small, with multinucleated cells and vacuoles in the seminiferous tubules. LC-MS/MS analysis showed that the vast majority of seminolipids (>90%) in WT mouse testes contained C16:0 in both the alkyl and the acyl moieties. Seminolipids were present in all subclasses of spermatogenic cells in WT mice, but they were absent in Far1 KO mice. Instead, the production of nonether, diacyl-type sulfogalactosyl lipids (sulfogalactosyl diacylglycerols) was induced in Far1 KO mice. In conclusion, the alkyl and acyl moieties of seminolipids in the testis are low in diversity, and Far1 is essential for seminolipid synthesis and spermatogenesis.
Keywords: spermatogenesis, mass spectrometry, gene knockout, mouse, lipid, ether lipid, seminolipid, fatty acyl-CoA reductase
Sperms have unique properties not found in other cells, including a haploid genome, the ability to move with the aid of a flagellum, and the capacity to fertilize eggs. To acquire these properties, sperms are produced via complex, spatiotemporally regulated processes involving the differentiation and maturation of spermatogenic cells. The production and functioning of spermatogenic cell- and sperm-specific proteins and lipids are essential for these processes.
The testis is composed of seminiferous tubules, which produce sperms, and interstitial connective tissue containing Leydig cells, which produce sex hormones (1). Sperms produced in the seminiferous tubules are transported via the efferent ductule of the testis to the epididymis, where they become highly motile and acquire the ability to fertilize. The epithelium in the seminiferous tubule contains spermatogenic cells and Sertoli cells, which form a blood–testis barrier and support spermatogenesis physically and nutritionally (1, 2). Spermatogenic cells are further subdivided into spermatogonia, spermatocytes, and spermatids. The spermatogonium, located on the basal lamina of the seminiferous epithelium, is a stem cell that undergoes somatic division to self-replicate or differentiate into spermatocytes. Spermatocytes differentiate into haploid spermatids via meiotic division. The morphology of the spermatid is initially round, but it elongates as the acrosome and flagellum are formed. During the division of spermatogenic cells, cytokinesis is incomplete, resulting in the cells’ cytoplasm being connected by intercellular bridges (3). At the stage when the elongated spermatids release residual bodies, the spermatids separate and are released as sperms into the lumen of the seminiferous tubules. The residual bodies released are subsequently phagocytosed by Sertoli cells.
Seminolipids are lipids that exist specifically in spermatogenic cells (4). In mammals, most of the glycolipids are sphingolipids (glycosphingolipids), but seminolipids belong to the glycerolipids. A seminolipid has an alkyl moiety, an acyl moiety, and a sulfogalactose moiety at the sn-1, sn-2, and sn-3 positions, respectively (Fig. 1) (5). The synthesis of alkyl moieties in ether lipids, including seminolipids, occurs in the peroxisomes (6, 7). The fatty alcohol constituting the alkyl moiety of a seminolipid is produced by fatty acyl-CoA reductase (FAR), which reduces a fatty acyl-CoA to a fatty alcohol. In the final two steps of seminolipid synthesis, galactose is added to 1-alkyl-2-acyl-glycerol by ceramide galactosyltransferase (CGT), generating 3-galactosyl-1-alkyl-2-acyl-glycerol. Subsequently, sulfate is introduced into the galactose group of 3-galactosyl-1-alkyl-2-acyl-glycerol by cerebroside sulfotransferase (CST). Both Cgt KO mice and Cst KO mice lack seminolipids in their testes and exhibit defects in spermatogenesis (8, 9), demonstrating that seminolipids are indispensable for spermatogenesis.
Figure 1.
Synthetic pathway of seminolipids. In the first reaction, glycerone phosphate (dihydroxyacetone phosphate) O-acyltransferase (GNPAT) esterifies a fatty acid to the sn-1 position of glycerone-3-phosphate. This fatty acid is then replaced by a fatty alcohol, which is linked to the sn-1 position via an ether bond, to form 1-alkyl-glycerone-3-phosphate. This reaction is catalyzed by alkyl glycerone phosphate synthase (AGPS) and the fatty alcohol is generated from fatty acyl-CoA by the fatty acyl-CoA reductase (FAR). There are two FAR isozymes, FAR1 and FAR2, in mammals. 1-Alkyl-glycerone-3-phosphate undergoes reduction of a carbonyl group to a hydroxy group, esterification of a fatty acid to the sn-2 position, and removal of a phosphate group to form 1-alkyl-2-acyl-glycerol. Finally, a seminolipid is formed by the attachment of a galactose to 1-alkyl-2-acyl-glycerol by ceramide galactosyltransferase (CGT) and subsequent sulfation of the galactose moiety by cerebroside sulfotransferase (CST).
In mammals, there are two FAR isozymes: FAR1 and FAR2 (10). In mice, Far1 is expressed in various tissues and organs, with the highest levels in the preputial glands, followed by the kidney, testis, and brain (10). In contrast, Far2 is expressed in a relatively tissue- or organ-specific manner; its expression levels are highest in the eyelids (meibomian glands), followed by the skin, small intestine, and brain (10). FAR1 exhibits activity toward C16 to 18 acyl-CoAs, whereas FAR2 is active toward acyl-CoAs with a wider range of chain lengths (10, 11). Although it has been reported that the seminolipid species with C16:0 in both the alkyl and the acyl moieties (O-C16:0/C16:0, where O- indicates ether-linked) is abundant (12, 13, 14), the detailed composition of seminolipids in the testis remains undetermined. Furthermore, it remains unclear which FAR isozyme (FAR1 or FAR2) is involved in the synthesis of seminolipids and during which stages of spermatogenesis seminolipids are synthesized. Mutations in FAR1 cause the inherited disease CSPSD (cataracts, spastic paraparesis, and speech delay) (15, 16, 17, 18); however, spermatogenesis in patients with CSPSD has not been reported. During the preparation for this study, Far1 KO mice were generated and reported to exhibit a severe reduction in plasmalogen levels in the testis and, similarly to Cgt KO and Cst KO mice, impaired spermatogenesis (19). However, seminolipids were not measured in that study, so the relative contributions of Far1 and Far2 in the synthesis of seminolipids were not determined.
In this study, we examined the expression of Far1 and Far2, the alkyl/acyl composition of seminolipids, and the abundance of seminolipids at different stages of spermatogenesis in the testes of WT mice. We also independently generated Far1 KO mice and analyzed their fertility, testicular histology, and lipid profile to reveal the role of Far1 in seminolipid synthesis and spermatogenesis.
Results
Far1 is expressed in the testis
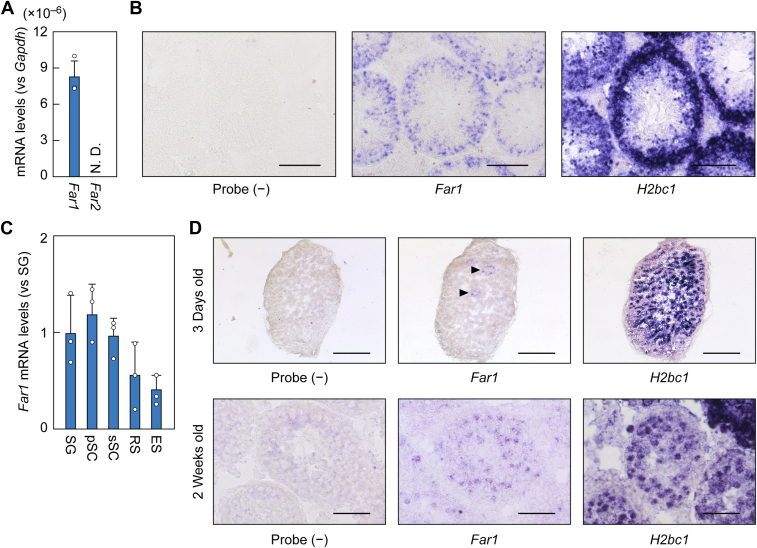
To examine the contribution of the FAR1 and FAR2 isozymes to the synthesis of seminolipids, we first analyzed the mRNA levels of Far1 and Far2 in the testes of WT mice via quantitative RT-PCR, using specific primers for each gene (11), and found that only Far1, and not Far2 mRNA, was expressed in the testes (Fig. 2A and Fig. S1). We then examined the distribution of Far1 mRNA in the testes via in situ hybridization. The control experiment, which had no probe, generated no signals, but hybridization with the Far1 probe-stained cells lining the basal lamina and extending to the luminal center of the seminiferous tubules (Fig. 2B). This staining pattern was similar to that observed for the testis-specific histone gene H2bc1, which is expressed in spermatogenic cells, indicating that Far1 is expressed in spermatogenic cells. Far1 staining was not observed in the interstitial connective tissue outside the seminiferous tubules, where the Leydig cells exist.
Figure 2.
Far1 is expressed in the testis.A, total RNAs were extracted from the testes of 8-week-old C57BL/6 mice and subjected to quantitative RT-PCR using specific primers for Far1, Far2, or the housekeeping gene Gapdh. Values presented are means + SD of each mRNA quantity relative to that of Gapdh (n = 3). B, fresh frozen sections were prepared from the testes of 6-month-old C57BL/6 mice and subjected to in situ hybridization using digoxigenin-labeled antisense RNA probes against Far1 or H2bc1. Probe (−) represents a negative control experiment without probes. Scale bars represent 100 μm. C, spermatogenic cells prepared from the testes of 8-week-old C57BL/6 mice were fractionated into spermatogonia (SG), primary spermatocytes (pSC), secondary spermatocytes (sSC), round spermatids (RS), and elongated spermatids (ES) by FACS. Total RNAs extracted from each fraction were subjected to quantitative RT-PCR using specific primers for Far1 or the housekeeping gene Gapdh. Values presented are means + SD of Far1 mRNA quantities relative to Gapdh (n = 3). D, fresh frozen sections were prepared from the testes of 3-day-old and 2-week-old C57BL/6 mice and subjected to in situ hybridization using digoxigenin-labeled antisense RNA probes against Far1 or H2bc1. Probe (−) represents a negative control experiment without probes. Scale bars represent 400 μm and 50 μm for 3-day-old and 2-week-old mice, respectively. N. D., not detected.
Next, to examine when Far1 is expressed during the differentiation of spermatogenic cells, we isolated the spermatogonia, spermatocytes, round spermatids, and elongated spermatids from the testes of adult WT mice via FACS and measured the levels of Far1 mRNA via quantitative RT-PCR. Far1 was expressed in all spermatogenic cells (Fig. 2C). The mRNA levels in the spermatogonia and spermatocytes were approximately double those in the round and elongated spermatids.
During postnatal testis development, the spermatogenic cells in the seminiferous tubules at postnatal day 3 consist mostly of gonocytes, the precursors of spermatogonia, and include a small number of spermatogonia (20). By postnatal week 2, spermatogonia and spermatocytes constitute about 80% and 20% of spermatogenic cells, respectively (21). Thus, cell types are limited at these stages and can be identified histologically. We examined Far1 expression at these stages using in situ hybridization. At postnatal day 3, Far1 was expressed in only a small number of putative spermatogonia (Fig. 2D, arrowheads), and at postnatal week 2, Far1 was expressed in the spermatogonia and spermatocytes in the peripheral and central regions, respectively, in the seminiferous tubules (Fig. 2D). These results indicate that the expression of Far1 starts when the spermatogonia are derived from gonocytes and continues during differentiation into spermatocytes and spermatids.
1-Alkyl and 2-acyl composition of seminolipids in the testis
Seminolipids in the testis consist predominantly of species with C16:0 as both their alkyl and their acyl moieties (O-C16:0/C16:0) (12, 13, 14). Smaller quantities of other species with C14:0 to 18:0 alkyl or acyl moieties are also present (12, 13). To date, seminolipids other than the O-C16:0/C16:0 species have been analyzed via LC-MS, fast atom bombardment MS, and imaging MS (12, 13, 14). However, LC-MS and fast atom bombardment MS cannot distinguish between alkyl and acyl moieties in the sn-1 and sn-2 positions, respectively, and imaging MS has a poor quantitation capability. Thus, the precise alkyl/acyl composition of seminolipids has not yet been clarified. To address this issue, we analyzed seminolipids via LC-MS/MS, which can distinguish species with alkyl/acyl moieties of various chain lengths and degrees of unsaturation. In the analysis, the alkyl or acyl moiety was fixed at C16:0, whereas the other moiety was variable, with chain lengths of C14 to 26 and 0 to 6 double bonds (Fig. 3A). In total, 11 seminolipid species were identified (Figs. 3B and S2). Consistent with previous reports (12, 13, 14), O-C16:0/C16:0 was the most abundant species, comprising 91% of the total seminolipids (Fig. 3B). The next most abundant species were O-C18:1/C16:0 (3%), O-C18:0/C16:0 (2%), and O-C16:0/C14:0 (2%). The following species were present in trace quantities (<1%): O-C16:0/C15:0, O-C16:0/C16:1, O-C16:0/C17:0, O-C16:0/C18:1, O-C14:0/C16:0, O-C15:0/C16:0, and O-C16:1/C16:0.
Figure 3.
1-Alkyl and 2-acyl composition of seminolipids in the testis.A, the structure of a seminolipid. The ranges of chain lengths and degrees of unsaturation in 1-alkyl and 2-acyl moieties, analyzed via LC-MS/MS, are indicated by black and red dashed rectangles, respectively. B, lipids were extracted from the testes of 8-week-old C57BL/6 mice and seminolipids were quantified via LC-MS/MS. The ratios of 11 species detected in the analysis are presented. C, spermatogenic cells prepared from the testes of 8-week-old C57BL/6 mice were fractionated into spermatogonia (SG), primary spermatocytes (pSC), secondary spermatocytes (sSC), round spermatids (RS), and elongated spermatids (ES) by FACS. Lipids were extracted from each fraction, and seminolipids were quantified via LC-MS/MS. Values presented are means + SD of total seminolipids in the indicated cell fractions (n = 3).
Next, to determine the stage of spermatogenesis at which seminolipids are present, testes from WT mice were dispersed and spermatogonia, primary and secondary spermatocytes, and round and elongated spermatids were separated via FACS. Lipids were extracted from each spermatogenic cell population and seminolipids were quantified via LC-MS/MS. The levels of seminolipids in spermatogonia and spermatocytes were similar and approximately double those in spermatids (Fig. 3C). The levels of seminolipids correlated well with Far1 mRNA expression (Fig. 2C).
Impaired spermatogenesis in Far1 KO mice
We produced Far1 KO mice using the CRISPR-Cas9 system to investigate the role of FAR1 in seminolipid synthesis and spermatogenesis. The guide RNA was designed to target the sequence downstream of the start codon in exon 3. The resulting Far1 KO allele had a 22 bp deletion that encompassed the start codon (Fig. 4A). To obtain homozygous Far1 KO mice, heterozygous Far1 KO mice were interbred, and the offspring were genotyped at 3 weeks of age. The proportion of homozygous KO mice (4.0%) was lower than the 25% expected under Mendel’s law (Table 1). Quantitative RT-PCR analysis of Far1 and Far2 in the testes of 8-week-old male WT and Far1 KO mice revealed similar Far1 mRNA levels in WT and Far1 KO mice, excluding the possibility of the nonsense-mediated mRNA decay of Far1 mRNA in Far1 KO mice (Fig. 4B). Far2 mRNAs were detected in neither WT nor Far1 KO mice; there was thus no compensatory increase in Far2 expression in Far1 KO mice. To examine whether some homozygous Far1 KO mice had died during embryonic development, we genotyped embryos at embryonic day 18.5 (E18.5) and found that the proportion of homozygous KO mice was 25%, as expected under Mendel’s law (Table 2). We next examined the survival rate of WT and homozygous Far1 KO mice (hereafter referred to as Far1 KO mice) after birth. Of 12 newborn mice of each genotype, 11 WT (92%) and six Far1 KO mice (50%) were alive day of birth, and nine WT (75%) and one KO mouse (8%) were alive on the following day (Fig. 4C). Thus, the Far1 KO mice exhibited high early postnatal lethality, but not embryonic lethality. Production of more offspring resulted in some Far1 KO mice that lived for up to 4 weeks after birth, with some of those even surviving more than 1 year.
Figure 4.
Impaired spermatogenesis in Far1 KO mice.A, generation of Far1 KO mice using the CRISPR-Cas9 system. The gene structure of Far1 (coding regions and untranslated regions in black and white, respectively) is shown, along with the nucleotide sequences of WT and Far1 KO mice around the guide RNA sequence (blue) and the protospacer-adjacent motif sequence (red). The box indicates a start codon. B, total RNAs were extracted from the testes of 8-week-old male WT and Far1 KO mice and subjected to quantitative RT-PCR using specific primers for Far1, Far2, or the housekeeping gene Gapdh. Values presented are means + SD of each mRNA quantity relative to Gapdh (n = 3). C, newborn WT and Far1 KO mice (n = 12 for each genotype) were monitored at the indicated ages and the survival rates were calculated. D, the appearance and body weights of 8-week-old male WT and Far1 KO mice. Values presented are means + SD (n = 3). E, the appearance and weights of the testes of 8-week-old male WT and Far1 KO mice. Values presented are means + SD (n = 3). Statistically significant differences are indicated (Welch’s t test; ∗∗p < 0.01). Scale bar represents 3 mm. F, the appearance of epididymides of 8-week-old male WT and Far1 KO mice. Scale bar represents 3 mm. G and H, images of paraffin sections of the epididymides (G) and testes (H) from 7-month-old WT and Far1 KO mice stained with H&E. Scale bars represent 50 μm. Black dashed ovals: examples of differentiating spermatogonia, spermatocytes, and spermatids, which were aligned from the basal lamina toward the center of the seminiferous tubules; black arrowheads: Sertoli cells; white arrowheads: multinucleated cells; asterisks: vacuoles. I and J, total RNAs extracted from the testes of 8-week-old male WT and Far1 KO mice were subjected to quantitative RT-PCR using specific primers for Ddx4 (pan-spermatogenic cell marker), Wt1 (Sertoli cell marker), Ccna1 and Hsp70-2 (primary spermatocyte markers), and Prm1 and Hspa1l (spermatid markers), or the housekeeping gene Gapdh. Values presented are means + SD of mRNA quantities relative to Gapdh (n = 3). Statistically significant differences are indicated (Welch’s t test; ∗p < 0.05, ∗∗p < 0.01). N. D., not detected.
Table 1.
Number of Far1+/+, Far1+/−, and Far1−/− mice at 3 weeks after birth
| Genotype | Far1+/+ | Far1+/− | Far1−/− | Total |
|---|---|---|---|---|
| Male | 41 | 117 | 7 | 165 |
| Female | 55 | 97 | 6 | 158 |
| Total | 96 | 214 | 13 | 323 |
| Ratio | 29.7% | 69.3% | 4.0% | 100% |
Table 2.
Number of Far1+/+, Far1+/−, and Far1−/− mice at embryonic day 18.5
| Genotype | Far1+/+ | Far1+/− | Far1−/− | Total |
|---|---|---|---|---|
| Number | 37 | 54 | 31 | 122 |
| Ratio | 30.3% | 44.3% | 25.4% | 100% |
Adult male Far1 KO mice were normal in appearance (Fig. 4D). They had smaller body weights than WT mice, but this difference was not statistically significant (Fig. 4D). The testes of Far1 KO mice were smaller than those of WT mice, with weights that were 40% of WT mouse testes (Fig. 4E). In WT mice, the epididymis appeared white because it contained mature sperms (22), but in Far1 KO mice, it was rather translucent (Fig. 4F). The fertility status of male WT, heterozygous Far1 KO, and Far1 KO mice was examined by crossing them with female WT mice. Crossing with male WT or heterozygous Far1 KO mice resulted in pregnancy and the delivery of offspring within one and a half months (Table 3). In contrast, crossing with male Far1 KO mice did not result in pregnancy even after more than 3 months, and no offspring were produced. These results indicate that the male Far1 KO mice are infertile.
Table 3.
Number of offsprings obtained by mating male Far1+/+, Far1+/−, and Far1−/− mice with WT female mice
| Genotype of male mice | Far1+/+ | Far1+/− | Far1−/− |
|---|---|---|---|
| Number of male mice mated | 4 | 2 | 2 |
| Number of deliveries | 4 | 2 | 0 |
| Mean number of offspring per litter | 10.0 | 15.5 | 0 |
Histological analyses were performed on the epididymides and testes of WT and Far1 KO mice by staining paraffin sections with H&E. The epididymides of WT mice were full of sperms, whereas those of Far1 KO mice contained none (Fig. 4G). In the seminiferous tubules in the testes of WT mice, spermatogonia, spermatocytes, and spermatids were readily observed, aligned from the basal lamina toward the center of the seminiferous tubules (Fig. 4H, black dashed ovals). In contrast, in Far1 KO mice, the cell positions were disorganized, and multinucleated cells were present in the seminiferous tubules (Fig. 4H, white arrowheads), suggesting impaired cytokinesis in the spermatogenic cells. In addition, there were vacuoles in the seminiferous tubules (Fig. 4H, asterisks). Sertoli cells were present only near or attached to the basal lamina of the seminiferous tubules in WT mice, while in Far1 KO mice, they were also ectopically present, adjacent to vacuoles in the center of the tubule (Fig. 4H, black arrowheads).
The mRNA levels of pan-spermatogenic cell marker Ddx4 (DEAD box polypeptide 4) (23) and Sertoli cell marker Wt1 (Wilms tumor 1) (24) in the testes were examined via quantitative RT-PCR. The levels of Ddx4 were reduced in Far1 KO mice relative to WT mice, while the Wt1 levels were comparable between the two groups (Fig. 4I). Next, to examine the differentiation of spermatogenic cells, we also quantified the mRNA levels of spermatogonium cell markers Ccna1 (Cyclin A1) (25) and Hsp70-2 (heat shock protein 70) (26) and spermatocyte markers Prm1 (Protamine 1) (27) and Hspa1l (Heat shock protein family A member 1 like) (28). In Far1 KO mice, the mRNA levels of Ccna1 were significantly reduced to 43% of those in WT mice (Fig. 4J). The mRNA levels of Hsp70-2 showed a similar reduction, although that decrease was not statistically significant. The mRNA levels of Prm1 and Hspa1l in Far1 KO mice were reduced to less than 5% of those in WT mice. Since the Ccna1 and Hsp70-2 genes are highly expressed in spermatogonia during the prophase of meiosis I, in which they are important (26, 29), these results indicate that, in Far1 KO mice, the spermatocytes fail to complete meiosis I, resulting in the impaired differentiation of spermatocytes into spermatids. Combined, these results demonstrate that male Far1 KO mice are infertile due to impaired spermatogenesis in the testes.
Impaired production of seminolipids in Far1 KO mice
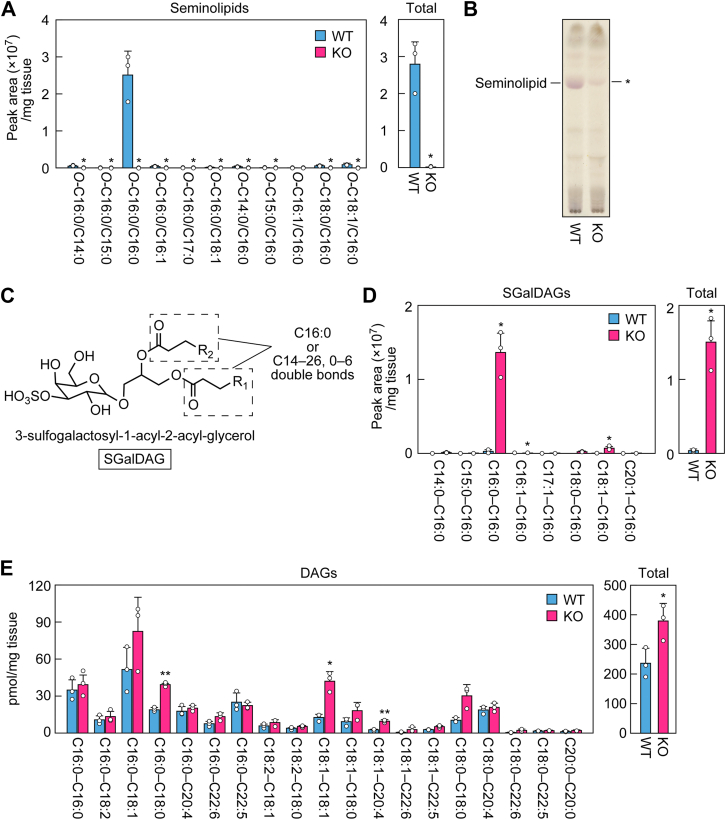
To investigate the contribution of FAR1 to seminolipid synthesis, we extracted lipids from the testes of WT and Far1 KO mice and analyzed the seminolipids using LC-MS/MS. In the Far1 KO mice, all seminolipid species were essentially absent (Fig. 5A). Lipids were then separated via TLC, and glycolipids were detected using orcinol sulfate staining. The seminolipid band detected in WT mice was not entirely absent in the Far1 KO mice but remained faintly detectable (Fig. 5B). This apparent discrepancy between the LC-MS/MS and TLC analyses (Figure 5, A and B) may be explained by the presence of other lipids that were structurally similar to seminolipids in Far1 KO mice. We hypothesized that these lipids may have been sulfogalactosyl-diacylglycerols (3-sulfogalactosyl-1-acyl-2-acyl-glycerols; SGalDAGs), which have a 1-acyl moiety instead of a 1-alkyl moiety as in seminolipids (Fig. 5C). To test this hypothesis, we scraped the band from the TLC plate, extracted the lipids, and subjected them to LC-MS/MS analysis. Based on the structural analogy of seminolipids, we applied the following settings for the LC-MS/MS analysis: one acyl moiety was set as C16:0 while the other was allowed to vary, with chain lengths of C14 to 26 and 0 to 6 double bonds (Fig. 5C). This analysis revealed that SGalDAGs were present in trace quantities in the testes of WT mice but substantially more abundant in Far1 KO mice (42-fold the quantities in WT mice; Figs. 5D and S3). The most abundant species was C16:0–C16:0, which comprised 99% and 91% of total SGalDAGs in WT and Far1 KO mice, respectively. We hypothesized that the almost exclusive presence of C16:0–C16:0 SGalDAG species may result from the abundance of C16:0–C16:0 diacylglycerol (DAG), which we assume to be the precursor for the SGalDAG. To test this, we examined the acyl-chain composition of DAGs in the testes of the WT and Far1 KO mice. We measured DAGs with C16:0, C18:0, C18:1, C18:2, C20:4, C22:5, or C22:6 in one of their two acyl chains. In contrast to SGalDAGs, there were many DAG species with a different combination of these acyl chains; C16:0–C18:1 was the most abundant species, followed by C16:0–C16:0 in WT mice (Fig. 5E). In Far1 KO mice, C16:0–C18:1 was also the most abundant species, and C16:0–C16:0 was the fourth most abundant species. The levels of C16:0–C16:0 species were comparable between WT and Far1 KO mice. On the other hand, the levels of C16:0–C18:0, C18:1–C18:1, and C18:1–C20:4 species and the total DAG levels were higher in Far1 KO mice than in WT mice. Thus, the almost exclusive presence of C16:0–C16:0 SGalDAG species does not reflect the acyl composition of DAGs. In conclusion, seminolipids are almost absent in Far1 KO mice, but there is a compensatory increase in SGalDAGs, whose acyl composition is similar to the acyl/alkyl composition of seminolipids but not to the acyl composition of DAGs.
Figure 5.
Large decrease in seminolipids and compensatory increase in SGalDAGs in Far1 KO mice.A, lipids were extracted from the testes of 8-week-old male WT and Far1 KO mice and seminolipids were quantified via LC-MS/MS. Values presented are means + SD (n = 3). Statistically significant differences are indicated (Welch’s t test; ∗p < 0.05). B, lipids extracted from the testes of 8-week-old male WT and Far1 KO mice were separated via TLC and glycolipids were detected via orcinol sulfate staining. C, structure of SGalDAG (3-sulfogalactosyl-1-acyl-2-acyl-glycerol). The ranges of chain lengths and degrees of unsaturation in 1-acyl and 2-acyl moieties (dotted rectangles), analyzed via LC-MS/MS, are indicated. D and E, lipids were extracted from the testes of 8-week-old male WT and Far1 KO mice, and SGalDAGs and DAGs were quantified via LC-MS/MS. The quantity of each SGalDAG (D) and DAG (E) species and their total quantities are presented. Values presented are means + SD (n = 3). Statistically significant differences are indicated (Welch’s t test; ∗p < 0.05, ∗∗p < 0.01).
Changes in the quantities of sulfatides, sphingomyelins, and ceramides in Far1 KO mice
The enzymes that catalyze the last two steps in the synthesis of seminolipids, CGT and CST, are also involved in the synthesis of sulfatides (Fig. 6A), the sulfated glycosphingolipids that are abundant in the brain, especially in myelin (9, 30). Total lipids from the testis and brain of WT mice were separated via TLC and glycolipids were detected using orcinol sulfate staining. In the brain, nonhydroxy and 2-hydroxy forms of sulfatides and galactosylceramides were the predominant glycolipids (Fig. 6B). However, these glycolipids were not detected in the testes. To determine whether sulfatides were indeed not absent in the testes, lipids extracted from the testes and brains as control tissues from WT and Far1 KO mice were subjected to LC-MS/MS, which is more sensitive than TLC. This analysis revealed that the testes of WT mice contained trace quantities of the nonhydroxy form of sulfatides (0.13% of those in the brain; Figs. 6C and S4). The fatty acid moiety of the testis sulfatides was mostly C16:0 (Fig. 6C). In Far1 KO mice, the total quantity of sulfatides was 2.3 times higher than that in WT mice. The mRNA levels of Cgt and Cst, examined via quantitative RT-PCR, were not significantly different between WT and Far1 KO mice (Fig. 6D) and thus were not correlated with the increases in the quantity of C16:0 sulfatide in Far1 KO mice.
Figure 6.
Changes in the quantities of sulfatides, sphingomyelins, and ceramides in the testes of Far1 KO mice.A, synthetic pathway of sulfatide from ceramide. B, lipids were extracted from the brain (10-week-old, female) and testis (7-week-old, male) of C57BL/6 mice and separated via TLC. Glycolipids were detected via orcinol sulfate staining. C, lipids were extracted from the testes of 7-week-old male WT and Far1 KO mice and the nonhydroxy forms of sulfatides were quantified via LC-MS/MS. Values presented are means + SD of sulfatide species containing the indicated acyl moiety (n = 3). Statistically significant differences are indicated (Welch’s t test; ∗p < 0.05). D, total RNAs were extracted from the testes of 8-week-old male WT and Far1 KO mice and subjected to quantitative RT-PCR using specific primers for Cgt, Cst, or the housekeeping gene Gapdh. Values presented are means + SD of each mRNA quantity relative to Gapdh (n = 3). E and F, lipids were extracted from the testes of 8-week-old male WT and Far1 KO mice, and ceramides (E) and sphingomyelins (F) were quantified via LC-MS/MS. Values presented are means + SD of ceramide/sphingomyelin species containing the indicated acyl moiety (n = 3). Statistically significant differences are indicated (Welch’s t test; ∗p < 0.05, ∗∗p < 0.01). G, total RNAs were extracted from the testes of 8-week-old male WT and Far1 KO mice and subjected to quantitative RT-PCR using specific primers for Cers3, Elovl4, or the housekeeping gene Gapdh. Values presented are means + SD of mRNA quantities relative to Gapdh (n = 3). GalCer, galactosylceramide; 2-OH, 2-hydroxy; N. D., not detected.
In the testis, polyunsaturated fatty acids (PUFAs) such as C30:5 and C30:6 are found in the acyl moieties of sphingolipids such as ceramides and sphingomyelins (30). The ceramide synthase CERS3 is involved in the synthesis of these sphingolipids, and Cers3 KO mice exhibit a deficiency in spermatogenesis (31). We measured ceramides and sphingomyelins in the testes of WT and Far1 KO mice using LC-MS/MS. In the Far1 KO mice, the levels of many ceramides and sphingomyelins containing ≥C28 PUFAs were significantly lower than in WT mice (Fig. 6, E and F). Conversely, the levels of ceramides containing C16–C24 saturated or monounsaturated fatty acids were higher. To reveal the possible mechanisms responsible for reducing the levels of ceramides and sphingomyelins containing ≥C28 PUFAs, we examined the mRNA levels of Cers3 and Elovl4, the latter of which is involved in the synthesis of ≥C28 PUFAs, via quantitative RT-PCR. The mRNA levels of Cers3 and Elovl4 were similar in WT and Far1 KO mice (Fig. 6G). Therefore, the reduced levels of ceramides and sphingomyelins containing ≥C28 PUFAs were not caused by reduced expression levels of Cers3 or Elovl14. Rather, these decreases may have been due to the lower number of differentiated spermatogenic cells. The same mechanism could also explain the increased levels of ceramides containing C16–C24 fatty acids. Alternatively, the increases may have resulted in part from the accumulation of the FAR1 substrate, C16:0 acyl-CoA, since it is incorporated into ceramides directly or after conversion to C18–C24 acyl-CoAs via elongation or to monounsaturated acyl-CoAs via desaturation in Far1 KO mice.
Discussion
It has not previously been determined which of the two mammalian FAR isozymes, FAR1 or FAR2, is involved in the synthesis of seminolipids in the testis. In this study, we observed that Far1 was expressed in spermatogenic cells and Far2 was not expressed in the testis (Fig. 2). Far1 KO mice exhibited a loss of seminolipids in the testes, reduced numbers of spermatogenic cells and impaired spermatogenesis in the seminiferous tubules, reduced testicular weights, an absence of sperms in the epididymides, and male infertility (Figs. 4 and 5; Table 3). Far1 is therefore essential for the synthesis of seminolipids, which are involved in spermatogenesis.
During our analyses of Far1 KO mice, another group also generated Far1 KO mice and reported the results of their analyses (19). Similar to the present study, they reported that these Far1 KO mice exhibited partial postnatal lethality, reduced testicular weights, an absence of sperms, the presence of multinucleated spermatogenic cells, and reduced mRNA levels of genes expressed in spermatogonia and spermatocytes. However, while the authors of that study used LC-MS to show that plasmalogens (representatives of ether lipids) were almost absent, they did not analyze seminolipids. In this study, we found that seminolipids were absent in the testes of Far1 KO mice (Fig. 5A).
Fatty alcohols produced by FAR1 are used for synthesizing ether lipids, including seminolipids, plasmalogens, and platelet-activating factors, as well as nonether lipids such as wax esters. So far, no studies have demonstrated the production of platelet-activating factors or wax esters in the testis or their involvement in spermatogenesis. The Agps KO mice, which lack the alkyl glycerone-phosphate synthase involved in ether lipid synthesis in general but not in nonether lipid synthesis (Fig. 1), exhibit spermatogenesis defects like those in Far1 KO mice (32). Thus, it is unlikely that nonether lipids produced via a FAR1-dependent mechanism are involved in spermatogenesis. Cgt KO and Cst KO mice exhibited a loss of seminolipids, deficient spermatogenesis with multinucleated spermatogenic cells, and male infertility (8, 9); phenotypes similar to those in Far1 KO mice. Cgt and Cst are involved in the synthesis of seminolipids but no other ether lipids or wax esters; thus, seminolipids were identified as the ether lipids that are most important for spermatogenesis.
It has been established that O-C16:0/C16:0 is the most abundant seminolipid species in the testis (12, 13, 14). However, the diversity and detailed composition of the alkyl and acyl moieties, including chain lengths and degrees of unsaturation, in seminolipids have remained unknown. In this study, through comprehensive analyses using LC-MS/MS, we revealed the precise alkyl and acyl chain composition of seminolipids (Fig. 3B). In addition, we examined SGalDAGs, which increased in Far1 KO mice to compensate for the loss of seminolipids, and sulfatides in the testis, and found that the predominant acyl moieties in these lipids were also C16:0 (Fig. 5D). In contrast, the acyl chain composition of ceramides and DAGs, precursors for the synthesis of sulfatides and SGalDAGs, respectively, was not limited to C16:0 but included multiple species (Figs. 5E and 6E). Considering that the most abundant acyl moieties in sulfatides in the brain are C24:1, C24:0, and C22:0 (Fig. S4) (33, 34), there appears to be a testis-specific mechanism that promotes the selective enrichment of C16:0 fatty acid into sulfatides. A possible mechanism is that the substrate specificity of CGT is altered in favor of ceramide containing C16:0 fatty acid due to the unique membrane environment of the testis, which is enriched in PUFA-containing sphingolipids. Similarly, in such a membrane environment, not only C16:0 ceramide but also O-C16:0/C16:0 1-alkyl-2-acyl-glycerol and C16:0–C16:0 DAG, which may have similar three-dimensional structures to C16:0 ceramide, may be recognized as substrates by CGT to generate O-C16:0/C16:0 seminolipid and C16:0–C16:0 SGalDAG, respectively.
We observed multinucleated spermatogenic cells in the testes of Far1 KO mice (Fig. 4H). Therefore, it is possible that seminolipids are important for the formation and maintenance of intercellular bridges between spermatogenic cells and that their deficiency causes the arrest of cytokinesis. The O-C16:0/C16:0 seminolipid is widely distributed in the seminiferous tubules of WT mice, as demonstrated by imaging MS (13). Immunohistochemical analysis using an antibody that recognizes sulfated glycolipids, including seminolipids, stained intercellular bridges in the seminiferous tubules (35). These observations suggest that seminolipids are components of intercellular bridges. The uniformity of seminolipids, which are composed almost entirely of saturated alkyl and acyl chains of the same carbon number (C16), may be necessary for the formation and maintenance of intercellular bridges by conferring intercellular bridge membranes with the properties of tight packing, uniform thickness, and low fluidity. The Far1 KO mice had increased levels of C16:0–C16:0 SGalDAG in the testes (Fig. 5D) but nevertheless exhibited multinuclear spermatogenic cells (Fig. 4H), suggesting the importance of the O-C16:0 moiety in seminolipids or that there was an insufficient quantity of SGalDAGs to compensate for the loss of seminolipids.
Multinucleated spermatogenic cells in seminiferous tubules have also been observed in mice with KO of the genes involved in the synthesis of glycosphingolipids containing ultra-long-chain fatty acids (≥C26). These genes include the ceramide synthase Cers3 and the glucosylceramide synthase Ugcg (31). Seminolipids may therefore cooperate with glycosphingolipids containing ultra-long-chain fatty acids in the formation and maintenance of intercellular bridges.
Sertoli cells perform multiple functions, such as providing physical support for spermatogenic cells, maintaining spermatogonia and their differentiation into spermatids, and forming the blood–testis barrier (2). In the Far1 KO mice, cell positions were disorganized, and some Sertoli cells were ectopically located in the seminiferous tubules, adjacent to vacuoles (Fig. 4H). The WT spermatogonia transplanted into the seminiferous tubules of Cst KO mice, which lack seminolipids in their testes and exhibit defects in spermatogenesis, generated colonies, and proceeded with spermatogenesis via meiosis in the presence of Cst KO Sertoli cells, demonstrating that seminolipids are necessary for spermatogonia but not for Sertoli cells (36). Thus, the ectopic localization of Sertoli cells may have been caused by secondary effects due to abnormalities in spermatogenesis. The vacuoles are likely to have been produced via the apoptosis of spermatogenic cells (19). Since Sertoli cells are attached to the spermatogenic cells, they may have migrated along with the dead spermatogenic cells.
In summary, we found that FAR1 is involved in seminolipid synthesis and spermatogenesis in the testis. Most of the Far1 KO mice died within a few days after birth, probably due to impaired production of plasmalogens—ether lipids that are abundant in the brain. Mutations in FAR1 cause the inherited disease CSPSD, which causes neurological symptoms (15, 16, 17, 18). Far1 KO mice may be useful not only for elucidating the mechanism of spermatogenesis but also for investigating the pathogenic mechanisms of these neurological symptoms.
Experimental procedures
Mice
Far1 KO mice were generated using the CRISPR-Cas9 system as follows. The guide RNA was designed to target the 20 bases adjacent to the protospacer-adjacent-motif sequence in exon 3 of Far1. A pair of oligonucleotides (5′-CACCGTCTTCCCCTCGTAGTATTCT-3′ and 5′-AAACAGAATACTACGAGGGGAAGAC-3′) containing the targeted sequence was annealed and cloned into the BbsI site of the CRISPR/Cas9 vector pX330-U6-Chimeric_BB-CBh-hSpCas9 (Addgene). The Far1-targeting plasmid was injected into fertilized eggs of C57BL/6J mice, and the injected eggs were transferred to the uteri of female C57BL/6J mice. To determine the genotypes of the offspring, genomic DNA from their tails was prepared and subjected to PCR using a pair of primers (5′-GGGATCCGTGAGTGATTTGTCTGATATGATCC-3′ and 5′-AATGCTTCACAAAATCCACACAAGC-3′) to amplify the DNA fragments containing the target sequence. The amplified DNA fragments were analyzed via agarose gel electrophoresis and Sanger sequencing. A founder mouse with a 22 bp deletion in exon 3 of Far1 was obtained and crossed with C57BL/6J mice to establish a heterozygous Far1 KO mouse line. Homozygous Far1 KO mice were obtained by mating male and female heterozygous Far1 KO mice. All the mice were housed under specific pathogen-free conditions at a room temperature of 23 ± 1 °C and a humidity of 50 ± 10%, with a 12 h light and 12 h dark cycle and water and food (Rodent Diet CE-2; CLEA Japan) available ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Hokkaido University.
Quantitative RT-PCR
After the testes of 8-week-old male mice had been dissected, they were immediately immersed in RNAlater Stabilization Solution (Thermo Fisher Scientific) and stored for ≥ 24 h at 4 °C. Total RNA was isolated using TRIzol Reagent (Thermo Fisher Scientific) and converted to first-strand cDNA using the PrimeScript II 1st Strand cDNA Synthesis Kit (Takara Bio), following the manufacturer’s instructions. Real-time quantitative PCR was performed using the first-strand cDNA, KOD SYBR qPCR Mix (Toyobo), and gene-specific primer pairs (Table 4) on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories). The mRNA levels were normalized with respect to Gapdh.
Table 4.
Oligonucleotide primers used in quantitative RT-PCR
| Name | Nucleotide sequence (5′ to 3′) |
|---|---|
| Gapdh-F | GAACGGGAAGCTCACTGGCATGGCC |
| Gapdh-R | TGTCATACCAGGAAATGAGCTTGAC |
| Far1-F | GATAATGTCAATATGTTAATGAACC |
| Far1-R | TCAGTATCTCATAGTGCTGGATGCTCG |
| Far2-F | TCCATGCTGGAGTATTTCATCAACC |
| Far2-R | TTGAACAAGGGACAAATGAAGAACC |
| Ddx4-F | CTGTCAGACGCTCAACAGGA |
| Ddx4-R | CGCTGTATTCAACGTGTGCT |
| Wt1-F | TCCGGTCAGCATCTGAAACC |
| Wt1-R | GAGCTGGTCTGAGCGAGAAA |
| Ccna1-F | GCTAATCGCCCAGACAGAGAAGAA |
| Ccna1-R | CCCCATGGTCAGAGAGCACTTTC |
| Hsp70-2-F | CAGACGCAGACCTTCACTACCTACTC |
| Hsp70-2-R | TTTTGTCCTGCTCGCTAATCTTGCC |
| Prm1-F | TCACAGGTTGGCTGGCTCGACCCAGG |
| Prm1-R | ATTGGCAGGTGGCATTGTTCCTTAGC |
| Hsc70t-F | TCCAAACTGGATCGAAGGCGTAGAG |
| Hsc70t-R | AGATCTCCTCTGGGTAGAAGGCTTTC |
| Cgt-F | CACTGCCAGAAGATCTGCAGAGGTG |
| Cgt-R | GAGCTTAGTGTTGTTTCCTAGGTTC |
| Cst-F | ATGACTCTGCTGCCAAAGAAGCCC |
| Cst-R | TGCGTCTTCATGAACACAATATCTCG |
| Cers3-F | CTGGCTTCCTCCAACAATAAAGTGG |
| Cers3-R | TCAAGTTACACTTCTTTGCCAGTCC |
| Elovl4-F | GAGGAAGAAAAACAACCAAGTCTCC |
| Elovl4-R | AATTTACTCTCCTTTTGGCTTCCCG |
In situ hybridization
To construct antisense RNA probes, Far1 and H2bc1 cDNAs were amplified via PCR using the following primer pairs: for Far1, 5′-ATGGTTTCAATCCCAGAATACTACG-3′ and 5′-ACTACTTCATCAATATGCTTTCG-3′; and for H2bc1, 5′-ATGCCGGAGGTGGCGGTAAAGGGTG-3′ and 5′-TCACTTGGAGCTGGTGTACTTGGTG-3′. The amplified DNA fragments were cloned into the pGEM-T Easy Vector (Promega), and digoxigenin-labeled RNA probes were synthesized using the DIG RNA labeling mix (Merck) and SP6 RNA polymerase (Merck).
In situ hybridization was performed as follows. Testes isolated from male mice at 3 days, 2 weeks, and 6 months after birth were frozen in Tissue-Tek OCT compound (Sakura Finetek Japan) at −80 °C. Samples were cut into 20 μm sections using a cryostat (CM3050S; Leica Biosystems), attached to glass slides, fixed with 10% formaldehyde in PBS, and then hybridized with a digoxigenin-labeled Far1 or H2bc1 RNA probe. After washing, the hybridized probe was detected using alkaline phosphatase–conjugated anti-digoxigenin Fab fragments (Merck), followed by signal development for 6 to 24 h in a solution containing nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Merck). The samples were covered with glass coverslips using CC/Mount (Merck). Images were captured using a DM5000 B light microscope (Leica Biosystems) equipped with a DFC295 digital color camera (Leica Biosystems).
Separation of spermatogenic cells by FACS
The testes dissected from 12-week-old male mice were cut into small pieces and incubated with 1 mg/ml collagenase type I (Fujifilm Wako Pure Chemical) and 0.5 units of DNase type I (Nippon Gene) in Dulbecco’s modified Eagle medium for 20 min at 33 °C with gentle rotation to digest extracellular matrices and DNA released from dead cells. The samples were then incubated with 0.25% trypsin (Fujifilm Wako Pure Chemical) for 20 min at 33 °C to disperse cells, and the reaction was terminated by adding 10% fetal bovine serum. After filtration through a 70 μm cell strainer, spermatogenic cells were stained with Hoechst 33342 solution (6 μg/million cells; Dojindo Laboratories) for 30 min at 33 °C, followed by staining with 1 μg/ml propidium iodide solution (BioLegend) for 10 min at room temperature. The samples were centrifuged (400g, 10 min, 4 °C), and the resulting pellets were suspended in PBS containing 2.5% fetal bovine serum, filtered through a 35 μm cell strainer, and sorted using the Cell Sorter SH800 (SONY). After excluding propidium-iodide–positive dead cells, spermatogenic cells were sorted into five major populations (spermatogonia, primary spermatocytes, secondary spermatocytes, round spermatids, and elongated spermatids) based on the intensity of two different wavelengths of fluorescence (Hoechst blue and Hoechst red) emitted by Hoechst33342 as described previously (37). Hoechst blue and Hoechst red were detected using 450/50 nm and 665/30 nm bandpass filters, respectively.
Lipid extraction
Each testis dissected from the 8-week-old male mice was homogenized in 2.5 ml of chloroform/methanol/formic acid (100:200:1, v/v) containing 1 nmol of the C16:0 ceramide standard labeled with nine deuterium (d9), N-palmitoyl(d9) D-erythro-sphingosine (Avanti Research). The homogenate was centrifuged (1500g, 3 min, room temperature) and separated into supernatant and a pellet. The supernatant was recovered, and the pellet was subjected to a second extraction with 2.5 ml of chloroform/methanol/formic acid (100:200:1, v/v) and centrifuged as above. The supernatants from both extractions were combined. The combined sample was mixed with 3 ml of chloroform and 5.4 ml of water, vigorously mixed, and centrifuged (1500g, 3 min, room temperature). The resulting lower (organic) phase was recovered and dried.
For the alkaline hydrolysis of ester-linked lipids, the lipid sample, dissolved in 450 μl of chloroform/methanol (1:2, v/v), was mixed with 11.25 μl of 4 M potassium hydroxide in methanol and incubated for 1 h at 37 °C. The sample was neutralized by adding 11.25 μl of 4 M formic acid, followed by vigorous mixing. The sample was then mixed with 150 μl of chloroform and 270 μl of water, followed by centrifugation (20,600g, 5 min, room temperature). The resulting organic phase was recovered and dried.
Brains dissected from 6–10-week-old female mice were chopped into small pieces. Ten milligrams of tissue was suspended in 450 μl of chloroform/methanol (1:2, v/v) in a tube containing zirconia beads (SARSTEDT) and vigorously mixed (4500 rpm, 1 min, 4 °C, repeated twice) using a Micro Smash MS-100 (TOMY Seiko). The homogenate was centrifuged (20,600g, 5 min, room temperature) and separated into supernatant and a pellet. The supernatant was recovered and the pellet was subjected to a second extraction with 450 μl of chloroform/methanol (1:2, v/v) and centrifuged as above. The combined sample was mixed with 300 μl of chloroform and 540 μl of water, vigorously mixed, and centrifuged (20,600g, 5 min, room temperature). The resulting organic phase was recovered and dried.
Each population of spermatogenic cells, sorted as above, was centrifuged (400g, 10 min, 4 °C), and the pellet was suspended in 400 μl of chloroform/methanol (1:1, v/v) containing 10 pmol of the d9-C16:0 ceramide standard. After 180 μl of water had been added, the sample was vigorously mixed for 1 min and centrifuged (20,600g, 5 min, room temperature). The resulting organic phase was recovered and dried.
LC-MS/MS analysis
For LC-MS/MS analysis, LC-coupled, triple quadrupole mass spectrometers (Xevo TQ-S and Xevo TQ-XS; Waters) were used. For the analyses of seminolipids, SGalDAGs, and sulfatides, the samples were dissolved in chloroform/methanol (1:2, v/v) and separated using a YMC-Triart C18 metal-free reversed-phase column (1.9 μm particle size, 2.1 mm inner diameter, 50 mm length; YMC, Kyoto Japan). The column temperature was set at 55 °C. The flow rate was set to 0.25 ml/min in a binary gradient system using mobile phase A (methanol/acetonitrile/water [1:1:3, v/v] containing 5 mM ammonium formate) and mobile phase B (2-propanol/water [49:1, v/v] containing 5 mM ammonium formate). The gradient steps were as follows: 0 to 1 min, 0% B; 1 to 5 min, linear gradient to 50% B; 5 to 25 min, linear gradient to 95% B; 25 to 25.1 min, step to 0% B; and 25.1 to 30 min, 0% B.
For the analysis of ceramides, sphingomyelins, and DAGs, the samples dissolved in chloroform/methanol (1:2, v/v) were separated using an ACQUITY UPLC CSH C18 reversed-phase column (1.7 μm particle size, 2.1 mm inner diameter, 100 mm length; Waters). The LC flow rate was 0.3 ml/min in a binary gradient system using mobile phase C (acetonitrile/water [3:2, v/v] containing 5 mM ammonium formate) and mobile phase D (acetonitrile/2-propanol [1:9, v/v] containing 5 mM ammonium formate). The gradient steps were as follows: 0 min, 40% D; 0 to 18 min, linear gradient to 100% D; 18 to 23 min, 100% D; 23 to 23.1 min, step to 40% D; and 23.1 to 25 min, 40% D.
Electrospray ionization was performed using the parameters listed in Table S1. MS/MS analysis was performed in multiple reaction monitoring mode using the m/z values of the precursor (Q1) and product (Q3) ions specific to each lipid species and optimized collision energies (Tables S2−S10). Data analysis was performed using the MassLynx software (Waters). The quantity of each seminolipid and SGalDAG species was presented as peak area because their standards were not commercially available. The quantity of each sulfatide, ceramide, sphingomyelin, and DAG species was calculated from its peak area as its ratio to the value of the corresponding C17:0 sulfatide (external standard; Avanti Research), d9-C16:0 ceramide (internal standard), d9-C18:1 sphingomyelin (external standard; Avanti Research), and C15:0/d7-C18:1 DAG (internal standard; Avanti Research), respectively.
H&E staining
The testes and epididymides of the 8-week-old WT and Far1 KO mice were fixed with 3.7% formaldehyde in 0.1 M PBS (pH 7.4) for >24 h at 4 °C. The fixed tissues were dehydrated, embedded in paraffin, cut into 4 μm-thick sections, deparaffinized, rehydrated, and stained with hematoxylin and eosin using an automated staining system (Tissue Tek DRS 2000; Sakura Finetek) as described previously (38). Bright-field images were captured using a Leica DM5000 B microscope equipped with a DFC295 digital color camera (Leica Microsystems).
Lipid analysis by TLC
Lipids (1 mg from the brain and 3–5 mg from the testis) were separated via TLC (Silica Gel 60 TLC plate, Merck) with methyl acetate/2-propanol/chloroform/methanol/0.25% calcium chloride in water (25:25:25:10:9, v/v) as the solvent system. The TLC plate was dried, sprayed with orcinol sulfate reagent (0.2% orcinol in 2.1 M aqueous sulfuric acid), dried again, and then heated to 100 °C to detect glycolipids.
Data availability
All data generated or analyzed during this study are contained within the article.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
A. T., T. N., K. O., and T. T. investigation; T. N. and T. S. writing–original draft; T. S. and A. K. supervision; T. S. and A. K. funding acquisition; A. K. writing–review and editing; A. K. project administration; A. K. conceptualization.
Funding and additional information
This work was supported by a grant from the Terumo Life Science Foundation (grant number; 24-Ⅲ4018 to A. K.) and by KAKENHI (grant numbers: JP22H04986 to A. K. and JP23K24020 to T. S.) from the Japan Society for the Promotion of Science (JSPS).
Reviewed by members of the JBC Editorial Board. Edited by George M. Carman
Footnotes
Present address for Takayuki Sassa: Department of Life Science, Kyushu Sangyo University, 2-3-1 Matsukadai, Higashi-ku, Fukuoka 813 to 8503, Japan.
Contributor Information
Takayuki Sassa, Email: sassat@ip.kyusan-u.ac.jp.
Akio Kihara, Email: kihara@pharm.hokudai.ac.jp.
Supporting information
References
- 1.Ross M.H., W. Pawlina . 5th ed. Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2006. Histology: A Text and Atlas With correlated cell and molecular biology. [Google Scholar]
- 2.O'Donnell L., Smith L.B., Rebourcet D. Sertoli cells as key drivers of testis function. Semin. Cell. Dev. Biol. 2022;121:2–9. doi: 10.1016/j.semcdb.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Braun R.E., Behringer R.R., Peschon J.J., Brinster R.L., Palmiter R.D. Genetically haploid spermatids are phenotypically diploid. Nature. 1989;337:373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- 4.Ishizuka I. Chemistry and functional distribution of sulfoglycolipids. Prog. Lipid. Res. 1997;36:245–319. doi: 10.1016/s0163-7827(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 5.Ishizuka I., Suzuki M., Yamakawa T. Isolation and characterization of a novel sulfoglycolipid, ‘seminolipid,’ from boar testis and spermatozoa. J. Biochem. 1973;73:77–87. [PubMed] [Google Scholar]
- 6.Braverman N.E., Moser A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Watschinger K., Werner E.R. Orphan enzymes in ether lipid metabolism. Biochimie. 2013;95:59–65. doi: 10.1016/j.biochi.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto H., Tadano-Aritomi K., Tokumasu A., Ito K., Hikita T., Suzuki K., et al. Requirement of seminolipid in spermatogenesis revealed by UDP-galactose: ceramide galactosyltransferase-deficient mice. J. Biol. Chem. 2000;275:22623–22626. doi: 10.1074/jbc.C000200200. [DOI] [PubMed] [Google Scholar]
- 9.Honke K., Hirahara Y., Dupree J., Suzuki K., Popko B., Fukushima K., et al. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4227–4232. doi: 10.1073/pnas.032068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng J.B., Russell D.W. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. J. Biol. Chem. 2004;279:37789–37797. doi: 10.1074/jbc.M406225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuka K., Sawai-Ogawa M., Kihara A. Formation of fatty alcohols-components of meibum lipids-by the fatty acyl-CoA reductase FAR2 is essential for dry eye prevention. FASEB J. 2022;36 doi: 10.1096/fj.202101733R. [DOI] [PubMed] [Google Scholar]
- 12.Attar M., Kates M., Bou Khalil M., Carrier D., Wong P.T., Tanphaichitr N. A Fourier-transform infrared study of the interaction between germ-cell specific sulfogalactosylglycerolipid and dimyristoylglycerophosphocholine. Chem. Phys. Lipids. 2000;106:101–114. doi: 10.1016/s0009-3084(00)00147-x. [DOI] [PubMed] [Google Scholar]
- 13.Goto-Inoue N., Hayasaka T., Zaima N., Setou M. The specific localization of seminolipid molecular species on mouse testis during testicular maturation revealed by imaging mass spectrometry. Glycobiology. 2009;19:950–957. doi: 10.1093/glycob/cwp089. [DOI] [PubMed] [Google Scholar]
- 14.Kongmanas K., Xu H., Yaghoubian A., Franchini L., Panza L., Ronchetti F., et al. Quantification of seminolipid by LC-ESI-MS/MS-multiple reaction monitoring: compensatory levels in Cgt+/– mice. J. Lipid. Res. 2010;51:3548–3558. doi: 10.1194/jlr.D010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almuqbil M., AbuMelha A., Albokhari D. Milder presentation of autosomal dominant fatty acyl CoA reductase 1-related syndrome: report of the first Middle Eastern patient and review of the literature. Clin. Case. Rep. 2022;10 doi: 10.1002/ccr3.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alshenaifi J., Ewida N., Anazi S., Shamseldin H.E., Patel N., Maddirevula S., et al. The many faces of peroxisomal disorders: lessons from a large Arab cohort. Clin. Genet. 2019;95:310–319. doi: 10.1111/cge.13481. [DOI] [PubMed] [Google Scholar]
- 17.Buchert R., Tawamie H., Smith C., Uebe S., Innes A.M., Al Hallak B., et al. A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am. J. Hum. Genet. 2014;95:602–610. doi: 10.1016/j.ajhg.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferdinandusse S., McWalter K., Te Brinke H., L I.J., Mooijer P.M., Ruiter J.P.N., et al. An autosomal dominant neurological disorder caused by de novo variants in FAR1 resulting in uncontrolled synthesis of ether lipids. Genet. Med. 2021;23:740–750. doi: 10.1038/s41436-020-01027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan B., Yuan S., Mayernik L., Yap Y.T., Moin K., Chung C.S., et al. Disrupted intercellular bridges and spermatogenesis in fatty acyl-CoA reductase 1 knockout mice: a new model of ether lipid deficiency. FASEB J. 2023;37 doi: 10.1096/fj.202201848R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goertz M.J., Wu Z., Gallardo T.D., Hamra F.K., Castrillon D.H. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Invest. 2011;121:3456–3466. doi: 10.1172/JCI57984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montoto L.G., Arregui L., Sanchez N.M., Gomendio M., Roldan E.R. Postnatal testicular development in mouse species with different levels of sperm competition. Reproduction. 2012;143:333–346. doi: 10.1530/REP-11-0245. [DOI] [PubMed] [Google Scholar]
- 22.Papaioannou V.E., Behringer R. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York: 2004. Mouse phenotypes: a handbook of mutation analysis. [Google Scholar]
- 23.Tanaka S.S., Toyooka Y., Akasu R., Katoh-Fukui Y., Nakahara Y., Suzuki R., et al. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes. Dev. 2000;14:841–853. [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Q.S., Wang X.N., Wen Q., Zhang Y., Chen S.R., Zhang J., et al. Wt1 deficiency causes undifferentiated spermatogonia accumulation and meiotic progression disruption in neonatal mice. Reproduction. 2014;147:45–52. doi: 10.1530/REP-13-0299. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney C., Murphy M., Kubelka M., Ravnik S.E., Hawkins C.F., Wolgemuth D.J., et al. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Dix D.J., Allen J.W., Collins B.W., Poorman-Allen P., Mori C., Blizard D.R., et al. HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development. 1997;124:4595–4603. doi: 10.1242/dev.124.22.4595. [DOI] [PubMed] [Google Scholar]
- 27.Caldwell K.A., Handel M.A. Protamine transcript sharing among postmeiotic spermatids. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2407–2411. doi: 10.1073/pnas.88.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Xie W., Yao Y., Zhu Y., Zhou J., Cui Y., et al. The heat shock protein family gene Hspa1l in male mice is dispensable for fertility. PeerJ. 2020;8 doi: 10.7717/peerj.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nickerson H.D., Joshi A., Wolgemuth D.J. Cyclin A1-deficient mice lack histone H3 serine 10 phosphorylation and exhibit altered aurora B dynamics in late prophase of male meiosis. Dev. Biol. 2007;306:725–735. doi: 10.1016/j.ydbio.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coetzee T., Fujita N., Dupree J., Shi R., Blight A., Suzuki K., et al. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 31.Rabionet M., Bayerle A., Jennemann R., Heid H., Fuchser J., Marsching C., et al. Male meiotic cytokinesis requires ceramide synthase 3-dependent sphingolipids with unique membrane anchors. Hum. Mol. Genet. 2015;24:4792–4808. doi: 10.1093/hmg/ddv204. [DOI] [PubMed] [Google Scholar]
- 32.Liegel R.P., Ronchetti A., Sidjanin D.J. Alkylglycerone phosphate synthase (AGPS) deficient mice: models for rhizomelic chondrodysplasia punctate type 3 (RCDP3) malformation syndrome. Mol. Genet. Metab. Rep. 2014;1:299–311. doi: 10.1016/j.ymgmr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isokawa M., Sassa T., Hattori S., Miyakawa T., Kihara A. Reduced chain length in myelin sphingolipids and poorer motor coordination in mice deficient in the fatty acid elongase Elovl1. FASEB Bioadv. 2019;1:747–759. doi: 10.1096/fba.2019-00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pintado-Sierra M., Garcia-Alvarez I., Bribian A., Medina-Rodriguez E.M., Lebron-Aguilar R., Garrido L., et al. A comprehensive profiling of sulfatides in myelin from mouse brain using liquid chromatography coupled to high-resolution accurate tandem mass spectrometry. Anal. Chim. Acta. 2017;951:89–98. doi: 10.1016/j.aca.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 35.Fujiwara Y., Ogonuki N., Inoue K., Ogura A., Handel M.A., Noguchi J., et al. t-SNARE Syntaxin2 (STX2) is implicated in intracellular transport of sulfoglycolipids during meiotic prophase in mouse spermatogenesis. Biol. Reprod. 2013;88:141. doi: 10.1095/biolreprod.112.107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Hayashi Y., Cheng X., Watanabe T., Wang X., Taniguchi N., et al. Testis-specific sulfoglycolipid, seminolipid, is essential for germ cell function in spermatogenesis. Glycobiology. 2005;15:649–654. doi: 10.1093/glycob/cwi043. [DOI] [PubMed] [Google Scholar]
- 37.Gaysinskaya V., Bortvin A. Flow cytometry of murine spermatocytes. Curr. Protoc. Cytom. 2015;72:7.44.1–7.44.24. doi: 10.1002/0471142956.cy0744s72. [DOI] [PubMed] [Google Scholar]
- 38.Sassa T., Ohno Y., Suzuki S., Nomura T., Nishioka C., Kashiwagi T., et al. Impaired epidermal permeability barrier in mice lacking Elovl1, the gene responsible for very-long-chain fatty acid production. Mol. Cell. Biol. 2013;33:2787–2796. doi: 10.1128/MCB.00192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are contained within the article.