To the Editor:
Shwachman–Diamond syndrome (SDS) is characterized by exocrine pancreatic dysfunction, bone marrow failure with myeloid dysplasia, and predisposition to acute myeloid leukemia (AML).1 There is no known association between SDS and lymphoid malignancies outside of a few case reports.2–4 Here, we present a unique and challenging case of a 27-year-old with SDS diagnosed with relapsed non-Hodgkin lymphoma (NHL) and co-occurring AML.
The patient had a history of neutropenia and splenectomy and was diagnosed with duodenal diffuse large B-cell lymphoma (DLBCL) at age 24, after presenting with nausea, vomiting, abdominal pain, and jaundice. Biopsy of the mass revealed a CD20+ large cell lymphoma, germinal center type, CD10+, PAX5+, CD79a+, BCL-6, MUM-1, and Ki-67 of 90%. Marrow was negative for malignancy, but karyotype was abnormal: 46XY, t(11;16), del(20) (Table 1). He achieved complete remission (CR) with five total cycles of rituximab, cyclophosphamide, daunorubicin, vincristine, and prednisone (R-CHOP). Three years later, he had recurrent fevers, fatigue, and abdominal pain, with computed tomography (CT) revealing a 2.4 × 2.3 cm rounded soft tissue density adjacent to the right common iliac artery/vein. Labs demonstrated pancytopenia with white blood cell count 2100/mm3, absolute neutrophil count 100/mm3, hemoglobin 6.0 g/dL, and platelet count 35,000/mm3, without peripheral blasts.
TABLE 1.
Cytogenetic and molecular features of AML and relapsed DLBCL samples.
| Acute myeloid leukemia | Relapsed diffuse large B-cell lymphoma | |
|---|---|---|
| Phenotype | CD45 (moderate), CD13 (bright), CD33 (moderate), MPO (subset), CD34 (bright), CD117, TdT, CD38, HLA-DR (moderate), CD56, and CD4 (dim-moderate), and negative for CD3 (surface and cytoplasmic), CD19, CD123 | CD20+ large cell lymphoma, germinal center type, CD10+, PAX5+, CD79a+, BCL-6, MUM-1, and Ki-67 of 90% |
| Karyotype | 44,XY,?add(1)(q32),der(5)add(5)(p13)add(5)(q11.2),add(8)(q24.3),−9,del(13)(?q12?q22),add(15)(p11.2),−17,add(18)(p11.2),−20,+mar1[5]/46,XY[14] | 45–46,XY,der(2)t(2;8)(p13;q11.2) add(2)(q36)[14],add(3)(p13),−8,?der(9)add(9)(p11.2)add(9)(q22),add(11)(q23), add(15)(p11.2),add(16)(p12),add(17)(p11.2),+mar[5][cp12]/45–46,idem,−add(17)(p11.2),+add(17)(p12)[cp3] |
| Microarray | 5q deletion (5q14.3→5q35.3), monosomy 9, partial deletion 9p, 8q partial duplication, 17q partial deletion, mosaic loss of Y, and a complex copy number variation of chromosome 21 | N/A |
| FISH | RUNX1 (21q22.11,3–5 copies; 21% of cells) | 1 Copy of chromosome 8 centromere (57% of cells), 4 copies of BCL2 18q21.33 (50% of cells), 3 copies of BCL2 18q21.33 (36% of cells) |
| EGR1 (5q31.2 deletion; 19% of cells) | Burkitt lymphoma and “double-hit” lymphoma negative | |
| Molecular | SBDS c.258+2T>C p.? (gl) | FANCA Y359fs*49 |
| SBDS c.183_184delinsCT p.Lys62* (gl) | TP53 R248W | |
| DNMT3A c.994G>A (VAF 1.2%) | TP53 R280G | |
| TP53 c.742C>T p.R248W (VAF 26.3%) | TP53M246L | |
| TP53 c.736A>C p.M246L (VAF 21.2%) | ||
| TP53 c.13_714dupGT p.K239Vfs*9 (VAF 5.1%) | ||
| TP53 c.838A>G p.R280G (VAF 1.9%) | ||
| TP53 c.637C>T p.R213* (VAF 1.6%) | ||
| TP53 c.818G>A p273H (VAF 0.5%) |
Abbreviations: AML, acute myeloid leukemia; DLBCL, diffuse large B-cell lymphoma.
Bone marrow studies revealed an AML immunophenotype in 45% of cells (Table 1). Genetic studies identified a complex karyotype (Table 1) with fluorescent in situ hybridization (FISH) positive for RUNX1 (21q22.11, three to five copies) and EGR1 (5q31.2 deletion). Microarray demonstrated 5q deletion (5q14.3→5q35.3), partial deletion of 9p and 17q, monosomy 9, 8q partial duplication, mosaic loss of Y, and a complex copy number variation of chromosome 21. Next-generation sequencing (NGS) showed a DNMT3A mutation, multiple TP53 mutations, and germline SBDS mutations (Table 1). Cerebral spinal fluid was negative for malignancy.
Positron emission tomography (PET)–CT demonstrated fluorodeoxyglucose (FDG)-avid wall thickening of the distal ileum, mesenteric, portocaval and popliteal lymph nodes, and focal intramuscular FDG uptake in left posterior chest wall and right thigh consistent with Murphy stage III disease. Biopsy of a mesenteric node (Figure 1) showed CD10+, CD19+, CD20+, Ki-67 (100%) DLBCL. FISH panels for Burkitt lymphoma and “double-hit” lymphoma were negative, but identified one copy of chromosome 8 centromere (57% of cells), four copies of BCL2 18q21.33 (50% of cells), and three copies of BCL2 18q21.33 (36% of cells). Karyotype was complex (Table 1). NGS demonstrated mutations in FANCA: Y359fs*49; TP53: R248W (subclonal), R280G, M246L (subclonal).
FIGURE 1.
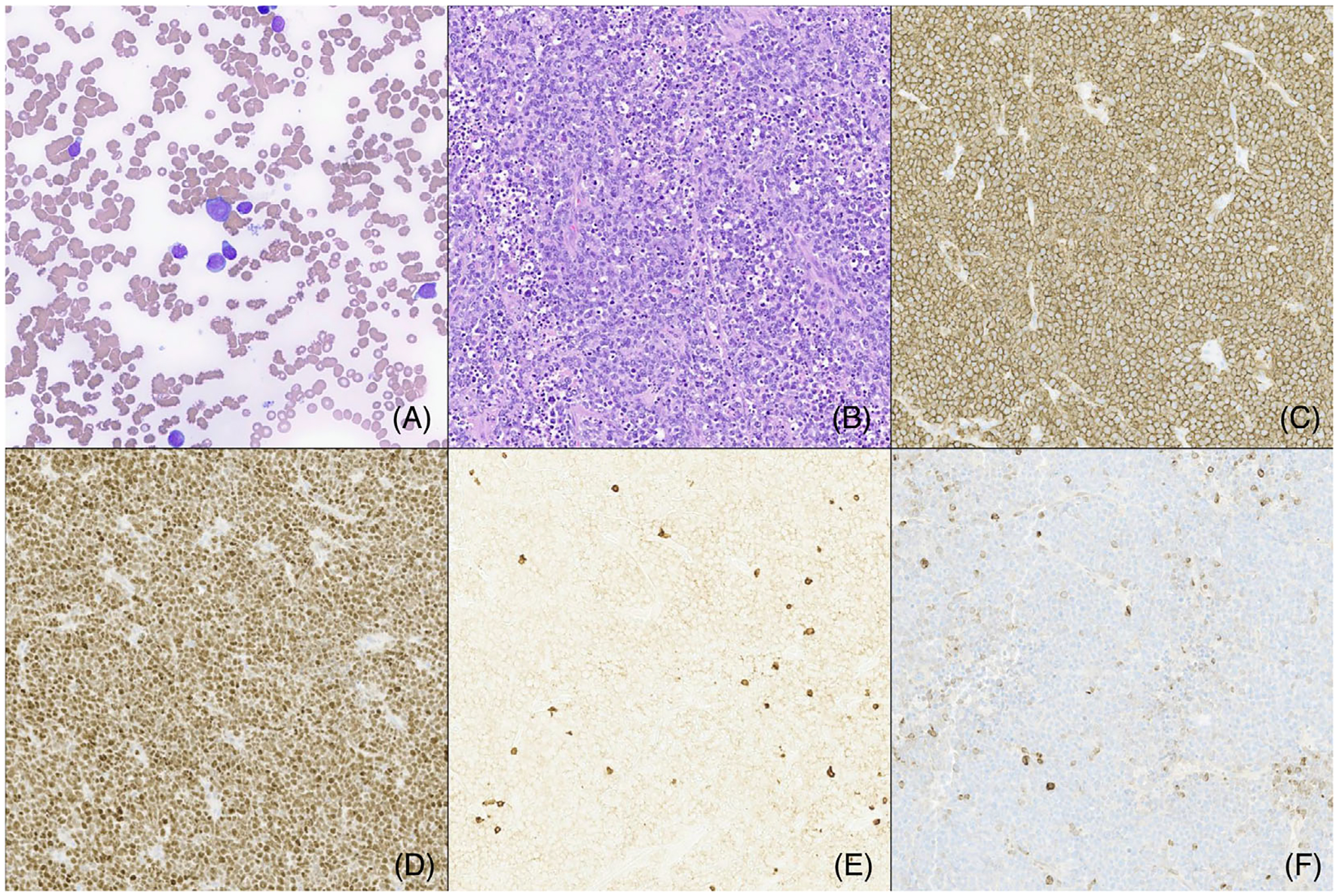
(A) The bone marrow aspirate had scattered blasts that comprised 53% of all cells. (B) The normal mesenteric lymph node architecture was replaced by intermediate-sized cells with irregular nuclear borders, multiple prominent nucleoli, and ample cytoplasm. Apoptotic debris were frequently seen. The tumor cells expressed strong CD20 (C), BCL-6 (D), and CD10 (not shown). They were negative for CD3 (E) and BCL-2 (F).
He received a combination of lymphoid and myeloid-directed chemotherapy, including cyclophosphamide (300 mg/m2) and vincristine (1 mg/m2) on Day 1; methylprednisolone (30 mg/m2 twice daily [BID]) on Days 1–7 (COP); obinutuzumab (1000 mg) on Days 1, 8, 15, 22, and 29; azacitidine (75 mg/m2) on Days 8–14; venetoclax (400 mg) on Days 8–35, and intrathecal cytarabine, methotrexate, and hydrocortisone for central nervous system (CNS)-directed prophylaxis.
Post-Cycle 1 marrow evaluation revealed CR with minimal residual disease (MRD) negativity by flow cytometry and EGR1 FISH positivity in 1% of cells. PET scan was notable for Deauville 4 in the terminal ileum and resolution (Deauville 1) in all other lesions. He received a second cycle of the same chemotherapy, with resultant CR by PET–CT and marrow evaluation (FISH and MRD negative).
Therapy was complicated by Bacillus cereus sepsis with endocarditis, cerebral/cerebellar abscesses, C. difficile colitis, gastric varices, venous thrombosis, and subdural hematomas secondary to anticoagulation.
He received a matched-unrelated donor transplant (human leukocyte antigen [HLA] 10/10 peripheral blood stem cells) with PK-directed busulfan, fludarabine, and anti-thymocyte globulin conditioning followed by immunosuppression with mycophenolate (MMF), cyclosporine A (CSA), and abatacept. He was 98% donor engrafted on post-transplant Day 14, but developed mixed chimerism on post-transplant Day 25 (90% donor), and was rapidly tapered off MMF and CSA. He received a 5-day course of azacitidine (Days 43–47) with resultant greater than 98% chimerism. Repeat bone marrow evaluation (Day 71) was MRD and TP53 negative.
This is the first reported co-occurrence of lymphoid and myeloid malignancies in a patient with underlying marrow failure syndrome. Given the underlying predisposition to myeloid malignancies in SDS, the potential that initial lymphoid-directed therapy exacerbated or accelerated transformation of myeloid disease is likely. SDS patients who develop myelodysplastic syndrome (MDS)/AML have inferior survival attributed to high-risk cytomolecular genetics, lack of standard therapy approach, increased toxicity to chemotherapy, and difficulty reaching curative hematopoietic stem cell transplant (HSCT) due to chemotherapy resistance and therapy-related morbidity/mortality.5 Our therapeutic approach was aimed at combining agents to simultaneously target myeloid and lymphoid cells, while limiting the risk of significant toxicities in order to reach curative HSCT. Venetoclax (an oral BCL2 inhibitor) is an effective, well-tolerated agent in both newly diagnosed and relapsed AML in combination with hypomethylating agents6–9 and other intensive chemotherapies.10–12 Venetoclax has also been utilized in B-lymphoid malignancies including chronic lymphocytic leukemia (CLL)13–15 and B-cell NHL,11,16,17 including DLBCL.11 Based on the safety efficacy of venetoclax in combination with a number of different myeloid and lymphoid chemotherapy back-bones, therapy was augmented with the additions of azacitidine, COP reduction, and obinutuzumab. Obinutuzumab was selected due to prior rituximab exposure, efficacy in upfront and relapse NHL with minimal toxicity,18–20 and published safety data and experience in combination with venetoclax in CLL21,22 and relapsed/refractory (R/R) NHL.23
This case highlights a unique approach to treating co-existing lymphoid and myeloid malignancies with a venetoclax-based chemotherapy regimen in a patient with SDS.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1.Myers KC, Bolyard AA, Otto B, et al. Variable clinical presentation of Shwachman–Diamond syndrome: update from the North American Shwachman–Diamond Syndrome Registry. J Pediatr. 2014;164(164):866–870. doi: 10.1016/j.jpeds.2013.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furutani E, Liu S, Galvin A, et al. Hematologic complications with age in Shwachman–Diamond syndrome. Blood Adv. 2022;6:297–306. doi: 10.1182/bloodadvances.2021005539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verbrugge J, Tulchinsky M. Lymphoma in a case of Shwachman–Diamond syndrome: PET/CT findings. Clin Nucl Med. 2012;37:74–76. doi: 10.1097/RLU.0b013e3182335f1f [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Sadimin E, Drachtman R, Glod J. CNS lymphoma in a patient with Shwachman Diamond syndrome. Pediatr Blood Cancer. 2014;61:564–566. doi: 10.1002/pbc.24743 [DOI] [PubMed] [Google Scholar]
- 5.Myers KC, Furutani E, Weller E, et al. Clinical features and outcomes of patients with Shwachman–Diamond syndrome and myelodysplastic syndrome or acute myeloid leukaemia: a multicentre, retrospective, cohort study. Lancet Haematol. 2020;7:e238–e246. doi: 10.1016/S2352-3026(19)30206-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–629. doi: 10.1056/NEJMoa2012971 [DOI] [PubMed] [Google Scholar]
- 7.Trabal A, Gibson A, He J, et al. Venetoclax for acute myeloid leukemia in pediatric patients: a Texas Medical Center experience. Cancers (Basel). 2023;15:1983. doi: 10.3390/cancers15071983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niswander LM, Chung P, Diorio C, Tasian SK. Clinical responses in pediatric patients with relapsed/refractory leukemia treated with azacitidine and venetoclax. Haematologica. 2023;108:3142–3147. doi: 10.3324/haematol.2022.282637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. doi: 10.1016/S1470-2045(18)30010-X [DOI] [PubMed] [Google Scholar]
- 10.Kadia TM, Reville PK, Borthakur G, et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2021;8:e552–e561. doi: 10.1016/S2352-3026(21)00192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137:600–609. doi: 10.1182/blood.2020006578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahswar R, Beutel G, Klement P, et al. FLA-IDA salvage chemotherapy combined with a seven-day course of venetoclax (FLAVIDA) in patients with relapsed/refractory acute leukaemia. Br J Haematol. 2020;188:e11–e15. doi: 10.1111/bjh.16268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pro B, Leber B, Smith M, et al. Phase II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in combination with rituximab in patients with recurrent B-cell non-Hodgkin lymphoma. Br J Haematol. 2008;143:355–360. doi: 10.1111/j.1365-2141.2008.07353.x [DOI] [PubMed] [Google Scholar]
- 14.O’Brien SM, Cunningham CC, Golenkov AK, et al. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:7697–7702. doi: 10.1200/JCO.2005.02.4364 [DOI] [PubMed] [Google Scholar]
- 15.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826–833. doi: 10.1200/JCO.2016.70.4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelenetz AD. Salles G, Mason KD, et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133:1964–1976. doi: 10.1182/blood-2018-11-880526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2912–2919. doi: 10.1200/JCO.2012.46.9585 [DOI] [PubMed] [Google Scholar]
- 19.Sehn LH, Goy A, Offner FC, et al. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS study. J Clin Oncol. 2015;33:3467–3474. doi: 10.1200/JCO.2014.59.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitolo U, Trneny M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35:3529–3537. doi: 10.1200/JCO.2017.73.3402 [DOI] [PubMed] [Google Scholar]
- 21.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–2236. doi: 10.1056/NEJMoa1815281 [DOI] [PubMed] [Google Scholar]
- 22.Salvaris R, Opat S. An update of venetoclax and obinutuzumab in chronic lymphocytic leukemia. Future Oncol. 2021;17:371–387. doi: 10.2217/fon-2020-0640 [DOI] [PubMed] [Google Scholar]
- 23.Christian BA, Huang Y, Ayyappan S, et al. Results of a phase I study of obinutuzumab, venetoclax, and lenalidomide in relapsed and refractory B-cell non-Hodgkin lymphoma. Blood. 2019;134:4082–4082. doi: 10.1182/blood-2019-126242 [DOI] [Google Scholar]


