Abstract
An asymmetric Mannich-type reaction of N-protected 2-(cyanomethyl)benzimidazoles with N-benzoyl imines was developed by using chiral phosphoric acid as a chiral Brønsted acid catalyst. Products having vicinal trisubstituted carbon stereogenic centers were formed in a highly diastereo- and enantioselective manner, even though one of the stereogenic centers had an active methine proton. Comprehensive control experiments revealed that high stereoselectivity was achieved through a kinetically controlled process.
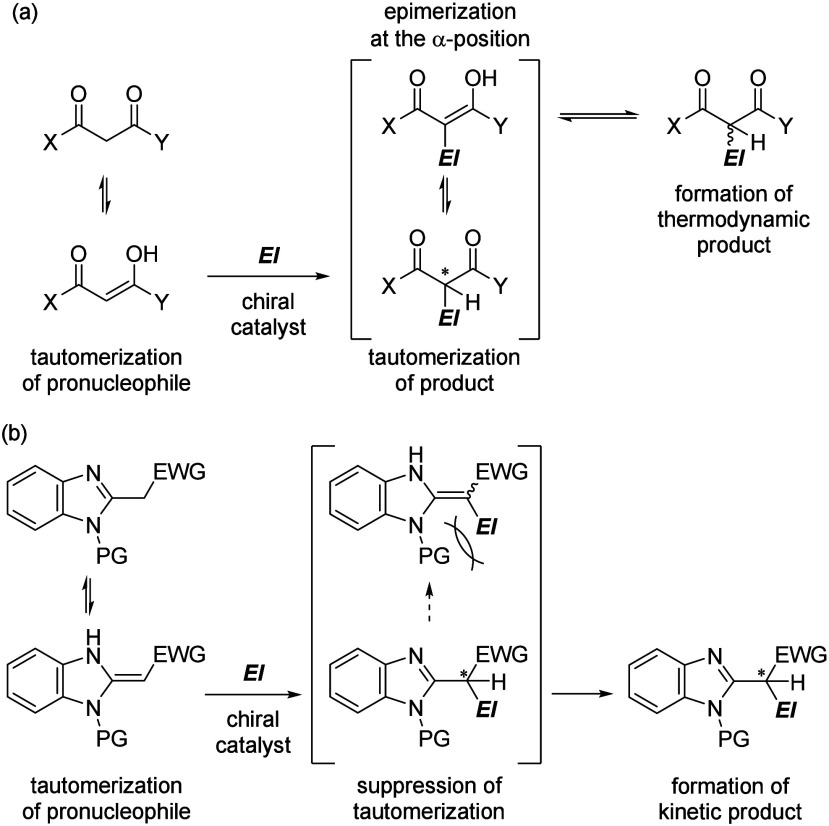
Benzimidazole, a representative azaarene chromophore, is widely found in natural products having pharmaceutical activities. An alkylazaarene derivative, such as 2-alkylbenzimidazole, exhibits unique chemical reactivity owing to the enamine tautomer, which possesses intrinsic nucleophilicity at the α-position. An electron-withdrawing group (EWG) is generally introduced at the α-position to enhance the acidity of the α-proton and the molecular complexity of the nucleophilic addition product. − The EWG-mediated modification facilitates the generation of nucleophilic enamine. Hence, 2-alkylbenzimidazole with an EWG is regarded as an analogue of 1,3-dicarbonyl compounds, which are representative pronucleophiles in organic synthesis. Although 1,3-dicarbonyl compounds are synthetically useful pronucleophiles, one intrinsic drawback associated with the stereoselective reaction of 1,3-dicarbonyl compounds possessing an active methylene moiety with electrophiles ( El ) is the tautomerization of the addition products, which results in epimerization at the α-position and, in general, difficult control of the stereochemical outcome (Figure a). The 2-alkylazaarene derivatives, including benzimidazole analogues, also encounter similar issues. To circumvent this inherent problem, 2-alkylazaarene derivatives having an additional substituent at the α-position are employed. The stereocontrol of a generated tetrasubstituted stereogenic center has drawn much interest as a challenging issue.
1.
Tautomerization of a pronucleophile and formation of a nucleophilic addition product. (a) 1,3-Dicarbonyl compound. (b) 2-Alkylbenzimidazole derivative having an EWG and a PG.
In our continuous efforts to utilize the benzimidazole chromophore in stereoselective reactions, we envisioned the fascinating effects of an additional electron-withdrawing substituent, such as a common protective group (PG), for example, a tert-butoxycarbonyl (Boc) or a benzyloxycarbonyl (Cbz) group, at the nitrogen atom of the 2-alkylbenzimidazole unit (Figure b). Introducing PG enhances the acidity of the α-proton, facilitating the tautomerization of the 2-alkylbenzimidazole pronucleophile and leading to an intriguing steric effect estimated in the nucleophilic addition product. The steric congestion, which originates from A(1,3) strain between El (or EWG) and PG around the tetrasubstituted double bond of the enamine tautomer, will suppress the tautomerization of the addition product.
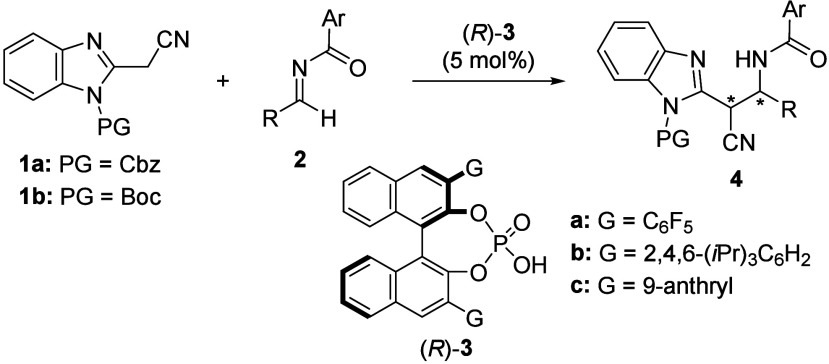
Leveraging the anticipated properties of the 2-alkylbenzimidazole derivative as a pronucleophile, we postulated that a kinetically controlled nucleophilic addition reaction would be feasible. In order to validate the above postulate, we adopted the Mannich-type reaction of N-protected 2-(cyanomethyl)benzimidazoles 1 with N-benzoyl imines 2 using binaphthol-derived chiral phosphoric acid (CPA) as a chiral Brønsted acid catalyst (Scheme ). − Herein, we report that the stereoselective Mannich-type reaction using CPA (R)-3 affords enantioenriched 2-alkylbenzimidazole derivatives 4 having vicinal trisubstituted carbon stereogenic centers in a highly diastereo- and enantioselective manner. Comprehensive control experiments revealed that high stereoselectivity was achieved through a kinetically controlled process.
1. Diastereo- and Enantioselective Mannich-Type Reaction of 2-(Cyanomethyl)benzimidazoles 1 with N-Benzoyl Imines 2 Catalyzed by CPA (R)-3 .
We initiated our investigation by the reaction of 2-(cyanomethyl)benzimidazole 1a, which has a Cbz group on the nitrogen atom, with N-benzoyl imine 2a at 0 °C for 4 h under the influence of CPA (R)-3a having pentafluorophenyl substituents (Table ). When the reaction was conducted in toluene (entry 1) or dichloromethane (entry 2), these commonly used solvents resulted in the quantitative formation of the desired 4aa in a highly enantioselective manner, albeit with low diastereoselectivity. In contrast, the use of THF, which is rarely employed in CPA-catalyzed reactions, improved the diastereoselectivity without any detrimental effect on the enantioselectivity (entry 3). Further screening of the solvents showed that high diastereoselectivity (94/6) was achieved in Et2O, affording 4aa quantitatively with a slight improvement in enantioselectivity (97% ee) (entry 4). The effect of substituents at the 3,3′-positions of CPA was further investigated using (R)-3b and (R)-3c having bulky 2,4,6-triisopropylphenyl and 9-anthryl substituents, respectively (entries 5 and 6). Both catalysts exhibited excellent enantioselectivities (98% ee), although the diastereoselectivity was slightly influenced by the substituent on CPA. The reaction of 1b, which has a Boc protective group, also proceeded smoothly using catalysts 3a and 3b, but the diastereoselectivity decreased (entries 7 and 8). In addition, the enantioselectivity was dependent on catalysts 3a and 3b; the use of 3a resulted in a higher enantioselectivity than the use of 3b, unlike the case of Cbz-protected 1a.
1. Optimization of the Reaction Conditions .
| entry | 1 | (R)-3 | solvent | anti/syn, | ee (%) |
|---|---|---|---|---|---|
| 1 | 1a | (R)-3a | toluene | 60/40 | 96 |
| 2 | 1a | (R)-3a | CH2Cl2 | 64/36 | 95 |
| 3 | 1a | (R)-3a | THF | 85/15 | 95 |
| 4 | 1a | (R)-3a | Et2O | 94/6 | 97 |
| 5 | 1a | (R)-3b | Et2O | 95/5 | 98 |
| 6 | 1a | (R)-3c | Et2O | 91/9 | 98 |
| 7 | 1b | (R)-3a | Et2O | 84/16 | 95 |
| 8 | 1b | (R)-3b | Et2O | 83/17 | 88 |
Reaction conditions: 1 (0.20 mmol), 2a (0.30 mmol, 1.5 equiv), and (R)-3 (5 mol %, 0.01 mmol) in the indicated solvent (2 mL, 0.1 M) under a nitrogen atmosphere unless otherwise specified.
A diastereomeric mixture of 4 was obtained quantitatively in all cases.
The diastereomeric ratio was determined by 1H NMR measurements after short path silica-gel column chromatography.
The enantiomeric excess of the major anti-diastereoisomer was determined by HPLC analysis using a chiral stationary phase column.
A 93% ee for the syn-isomer.
A 60% ee for the syn-isomer.
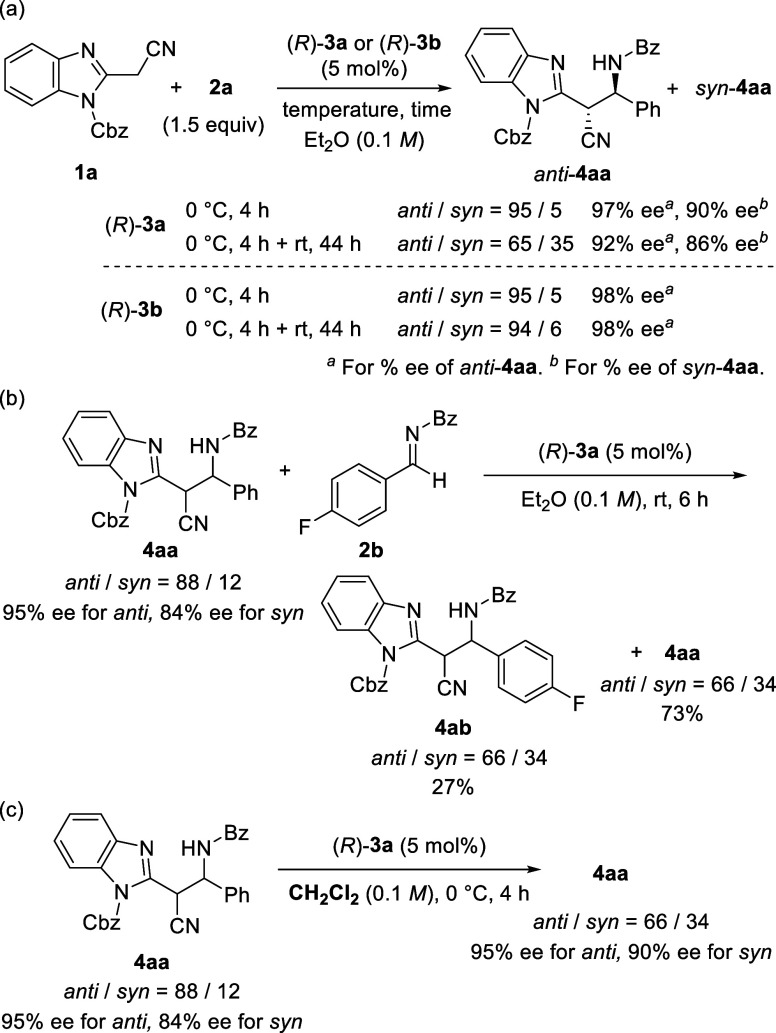
In order to verify the formation of a kinetically controlled product, we monitored the stereoselectivities during the reaction of 1a with 2a (1.5 equiv) in the presence of (R)-3a and (R)-3b (Scheme a). After confirmation of the complete consumption of 1a at 0 °C for 4 h, the reaction temperature was increased to room temperature, and the reaction was continued for an additional 44 h. Neither the enantioselectivity nor the diastereoselectivity was changed by using less acidic (R)-3b. Hence, the tautomerization of formed 4aa seemed to be completely suppressed under the present reaction conditions. In contrast, the use of more acidic (R)-3a resulted in a significant decrease in the diastereoselectivity to 65/35, clearly indicating that epimerization of 4aa occurred. More curiously, a non-negligible decrease in enantioselectivity was also observed in both diastereoisomers, strongly suggesting that the epimerization proceeds not only at the α-position of 4aa but also at the β-position. Consequently, a retro reaction was suspected because tautomerization is responsible only for epimerization at the α-position. Therefore, we performed a control experiment using a 1/1 mixture of product 4aa and imine 2b in the presence of (R)-3a at room temperature for 6 h (Scheme b). As expected, cross product 4ab was formed in 27% yield with 73% recovery of the starting product 4aa. Hence, we confirmed that the retro reaction occurred under the present acidic conditions. It should be emphasized that these epimerization processes were suppressed at 0 °C during the initial 4 h, and hence, initially formed 4aa at 0 °C is regarded as a kinetically controlled product, as postulated (Figure ).
2. Control Experiments: (a) Monitoring the Reaction, (b) Retro Reaction, and (c) Solvent Effect.
As shown in Table , a unique solvent effect was observed in the present reaction. Commonly used solvents, such as toluene and dichloromethane, afforded product 4aa with low diastereoselectivity, despite the high enantioselectivity (entries 1 and 2). We performed a control experiment in dichloromethane using enantiomerically enriched product 4aa [anti/syn = 88 (95% ee)/12 (84% ee)] and (R)-3a to confirm whether a kinetically controlled product could be formed in this commonly used solvent (Scheme c). The diastereoselectivity was markedly decreased to a 66/34 anti/syn ratio, even at 0 °C for 4 h. In contrast, high enantioselectivities were maintained for both diastereoisomers; more importantly, an enhancement of the enantioselectivity from 84% to 90% ee was observed for minor syn-4aa. These results suggest that the epimerization at only the α-position of 4aa, namely, tautomerization, occurred. The enhanced enantioselectivity of syn-4aa stemmed from major anti-4aa having higher enantioselectivity (95% ee) than that of initial syn-4aa (84% ee). In addition, the retro reaction would be excluded from the present epimerization process. On the basis of the above control experiments, we conclude that the use of Et2O is the key to suppressing the tautomerization efficiently.
The optimal conditions for the formation of the kinetically controlled product were identified. Both catalysts (R)-3a and (R)-3b performed efficiently in the reaction of Cbz-protected substrate 1a in Et2O. The scope of the reaction was demonstrated using a series of aryl-substituted imines 2 under the influence of (R)-3a or (R)-3b (see the Supporting Information for details). As shown in Table , a range of imines 2 are suitable for the present reaction, affording desired products 4 quantitatively with high diastereo- and enantioselectivities. The reaction of N-benzoyl imines 2 having a substituent at the para position of the aryl ring, 2b–2f, proceeded well with high stereoselectivities, regardless of the electronic properties of the aryl ring (entries 1–7). The absolute configuration of 4ac was determined to be 1R,2R, indicating anti-relative stereochemistry, as the major stereoisomer using (R)-3a [and also (R)-3b] through single-crystal X-ray analysis of syn-4ac after epimerization at the C1 position of anti-4ac (see the Supporting Information for details). In addition, the reaction on a gram scale (1a, 2.0 mmol; 2d, 3.0 mmol) proceeded smoothly without any undesirable effects on the stereoselectivity, affording 4ad quantitatively, even with a reduced loading of (R)-3a of 2.5 mol % (entry 5). Aryl rings having a substituent at the meta or ortho position, 2g–2i, also underwent the reaction smoothly with good stereoselectivities (entries 8–10). The reaction of 2k, which has a heteroaryl group, 2-thiophenyl, resulted in a decrease in stereoselectivity (entry 12). In contrast, the reaction of 2-naphthyl substrate 2j proceeded well using (R)-3b with excellent stereoselectivity (entry 11). The N-benzoyl group was comparable to those of other aryl rings; the use of 4-bromophenyl 2l and 4-toluyl 2m afforded 4al and 4am, respectively, in a highly enantioselective manner, albeit with a slight decrease in diastereoselectivity (entries 13 and 14). In contrast, the use of N-benzoyl imine 2n having an aliphatic substituent retarded the reaction markedly (entry 15), affording 4an in moderate yield with a significant decrease in enantioselectivity, albeit with relatively high diastereoselectivity. Introducing an ester moiety instead of a cyano group (entry 16) markedly decreased the diastereoselectivity, although the major diastereoisomer exhibited fairly good enantioselectivity. This result emphasizes the crucial role played by a cyano group in achieving high diastereoselectivity.
2. Scope of Substrates Using (R)-3a or (R)-3b .
| entry | (R)-3 | 2: R, Ar | 4 | anti/syn | ee (%) |
|---|---|---|---|---|---|
| 1 | 3b | 2b: 4-FC6H4, Ph | 4ab | >95/5 | 98 |
| 2 | 3b | 2c: 4-ClC6H4, Ph | 4ac | >95/5 | 98 |
| 3 | 3b | 2d: 4-BrC6H4, Ph | 4ad | >95/5 | 98 |
| 4 | 3a | 2d | 4ad | >95/5 | 98 |
| 5 | 3a | 2d | 4ad | >95/5 | 98 |
| 6 | 3a | 2e: 4-CF3C6H4, Ph | 4ae | >95/5 | 99 |
| 7 | 3a | 2f: 4-MeC6H4, Ph | 4af | 94/6 | 99 |
| 8 | 3b | 2g: 3-MeC6H4, Ph | 4ag | >95/5 | 91 |
| 9 | 3a | 2h: 3-FC6H4, Ph | 4ah | 91/9 | 92 |
| 10 | 3a | 2i: 2-FC6H4, Ph | 4ai | >95/5 | 99 |
| 11 | 3b | 2j: 2-naphthyl, Ph | 4aj | >95/5 | 98 |
| 12 | 3b | 2k: 2-thiophenyl, Ph | 4ak | 83/17 | 92 |
| 13 | 3b | 2l: Ph, 4-BrC6H4 | 4al | 94/6 | 99 |
| 14 | 3b | 2m: Ph, 4-MeC6H4 | 4am | 93/7 | 95 |
| 15 | 3a | 2n: cyclohexyl, Ph | 4an | 87/13 | 29, 18 |
| 16 | 3a | 2a: Ph, Ph | 4ca | 73/27 | 90, 81 |
Reaction conditions: 1a (0.20 mmol), 2 (0.30 mmol), and (R)-3 (5 mol %) in Et2O (2 mL, 0.1 M) under a nitrogen atmosphere at 0 °C for 4 h unless otherwise specified.
Diastereomeric mixtures were formed quantitatively, and the diastereomeric ratio (anti/syn) was determined by 1H NMR measurements after short path silica-gel column chromatography.
The enantiomeric excess was determined by HPLC analysis using a chiral stationary phase column.
Gram-scale experiment: 1a (2.0 mmol), 2d (3.0 mmol), and (R)-3a (2.5 mol %, 0.05 mmol) in Et2O (20 mL, 0.1 M).
For 12 h.
A 63% combined yield of diastereoisomers.
Use 1c (0.20 mmol) instead of 1a for 16 h.
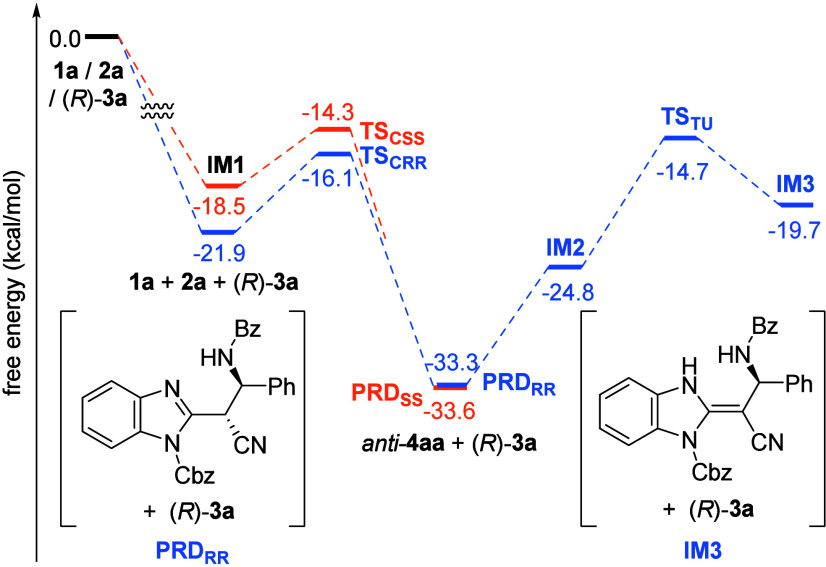
Finally, to gain insight into the reaction profile, we performed density functional theory (DFT) calculations of the reaction of 1a with 2a catalyzed by (R)-3a. Focusing on the (1R,2R)-4aa stereoisomer obtained as the major product, transition state (TS) analysis of C–C bond formation (TS CRR ), namely, the Mannich-type reaction, and the tautomerization (TS TU ) steps was conducted to verify the progress of the retro reaction and the formation of a tautomer. The structural optimization of TSs was performed, taking into account the solvent effect of diethyl ether and using CPCM(ether)/ωB97X-D/def2-SVP, followed by a further single-point energy calculation of these optimized structures at the SMD(ether)/ωB97M-V/def2-SVPD level of theory. The energy diagram for the C–C bond formation and tautomerization steps is shown in Figure (the three-dimensional structures are presented in the Supporting Information). We confirmed that the activation energy for C–C bond formation that gives the product through TS CRR was sufficiently small (ΔG ⧧ = 5.8 kcal/mol), affording (1R,2R)-4aa predominantly (blue line). More importantly, the energy barrier of the backward reaction was not markedly high (ΔG ⧧ = 17.2 kcal/mol), allowing the retro reaction to proceed at room temperature. On the other hand, tautomerization (TS TU ) had a relatively low energy barrier. Still, it was higher (ΔΔG ⧧ = 1.4 kcal/mol) than that of the C–C bond formation step for giving (1R,2R)-4aa, in good agreement with the experimental result that the tautomerization was suppressed at 0 °C. High enantioselectivity was also confirmed by the TS analysis of the pathway to 1S,2S-4aa (TS CSS ) (orange line), which is ΔΔG ⧧ = 1.8 kcal/mol higher than the formation of (1R,2R)-4aa (TS CRR ).
2.
Energy diagram of the reaction of 1a with 2a catalyzed by (R)-3a. The relative free energy (kcal/mol) of the sum of 1a, 2a, and (R)-3a is set to zero.
In conclusion, we demonstrated an asymmetric Mannich-type reaction of N-protected 2-(cyanomethyl)benzimidazoles with N-benzoyl imines catalyzed by chiral phosphoric acid. Taking advantage of the structural features of N-protected 2-(cyanomethyl)benzimidazoles, vicinal trisubstituted carbon stereogenic centers were kinetically controlled in a highly diastereo- and enantioselective manner, even though one of the two stereogenic centers had an active methine proton. DFT calculations characterized the present reaction profile well, which involved not only tautomerization but also retroreaction, both of which were observed under specific reaction conditions. Further studies of other reaction designs utilizing the structural properties of N-protected 2-alkylbenzimidazole derivatives are in progress in our laboratory.
Supplementary Material
Acknowledgments
The computation was performed using the Research Centre for Computational Science, Okazaki, Japan (Project 24-IMS-C110). This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Hybrid Catalysis for Enabling Molecular Synthesis on Demand” (JP17H06447) and a Grant-in-Aid for Transformative Research Areas (A) “Green Catalysis Science for Renovating Transformation of Carbon-Based Resources” (JP23H04908) from MEXT, Japan (M.T.), a Grant-in-Aid for Challenging Research (Exploratory) (JP22K19018) from JSPS (M.T.), and a Grant-in-Aid for Young Scientists (JP19K15552) from JSPS (J.K.).
The data underlying this study are available in the published article and its Supporting Information.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.5c01478.
Experimental details, characterization data, theoretical studies, and 1H, 13C, and 19F NMR spectra and HPLC charts of isolated compounds (PDF)
∇.
J.K.: Graduate School of Pharmaceutical Science, Tohoku University, Aoba-ku, Sendai 980-8578, Japan
H.Y. and L.H. contributed equally to this work. H.Y. contributed design of the work, data curation, formal analysis, and writing of the original draft. L.H. contributed data curation, formal analysis, and investigation (experimental studies). A.T. contributed data curation, formal analysis, and stereochemical assignment. T.S. contributed theoretical calculations and data curation. J.Y. contributed data curation and stereochemical assignment. J.K. contributed formal analysis, theoretical calculations, and review and editing. M.B. contributed project administration, review and editing, and supervision. M.T. contributed conceptualization, design of the work, project administration, review and editing, supervision, and funding acquisition.
The authors declare no competing financial interest.
References
- For reviews of benzimidazole derivatives as pharmerceutical chromophores, see:; a Rajasekhar S., Maiti B., Balamurali M. M., Chanda K.. Synthesis and Medicinal Applications of Benzimidazoles: An Overview. Curr. Org. Synth. 2016;14:40–60. doi: 10.2174/1570179413666160818151932. [DOI] [Google Scholar]; b Patel A., Shah D., Patel N., Patel K., Soni N., Nagani A., Parikh V., Shah H., Bambharoliya T.. Benzimidazole as Ubiquitous Structural Fragment: An Update on Development of its Green Synthetic Approaches. Mini-Rev. Org. Chem. 2021;18:1064–1085. doi: 10.2174/1570193X17999201211194908. [DOI] [Google Scholar]; c Monga J., Ghosh N. S., Rani I., Singh R., Deswal G., Dhingra A. K., Grewal A. S.. Unlocking the Pharmacological Potential of Benzimidazole Derivatives: A Pathway to Drug Development. Curr. Top. Med. Chem. 2024;24:437–485. doi: 10.2174/0115680266283641240109080047. [DOI] [PubMed] [Google Scholar]; d El Alami A., Sdassi H., Bouzikri S.. Review of Synthesis Process of Benzimidazole-heterocycle Hybrid Compounds. Synth. Commun. 2024;54:613–635. doi: 10.1080/00397911.2024.2316718. [DOI] [Google Scholar]
- For Brønsted acid-promoted functionalization at the α-position of azaarene derivatives, see:; a Niu R., Xiao J., Liang T., Li X.. Facile Synthesis of Azaarene-Substituted 3-Hydroxy-2-Oxindoles via Brønsted Acid Catalyzed sp3 C–H Functionalization. Org. Lett. 2012;14:676–679. doi: 10.1021/ol2030982. [DOI] [PubMed] [Google Scholar]; b Wang F. F., Luo C. P., Wang Y., Deng G., Yang L.. Brønsted Acid Promoted Benzylic C–H Bond Functionalization of Azaarenes: Nucleophilic Addition to Aldehydes. Org. Biomol. Chem. 2012;10:8605–8608. doi: 10.1039/c2ob26604k. [DOI] [PubMed] [Google Scholar]; c Li H. Y., Xing L. J., Xu T., Wang P., Liu R. H., Wang B.. An Addition of Benzylic sp3 C–H to Electron-Deficient Olefins. Tetrahedron Lett. 2013;54:858–860. doi: 10.1016/j.tetlet.2012.11.100. [DOI] [Google Scholar]; d Jin J. J., Wang D. C., Niu H. Y., Wu S., Qu G. R., Zhang Z. B., Guo H. M.. Brønsted Acid Catalyzed Synthesis of 1,3-Di(2-Quinolyl)Propane Derivatives via Tandem C(sp3)–H Functionalization. Tetrahedron. 2013;69:6579–6584. doi: 10.1016/j.tet.2013.05.135. [DOI] [Google Scholar]; e Lansakara A. I., Farrell D. P., Pigge F. C.. Brønsted Acid Catalyzed Intramolecular Benzylic Cyclizations of Alkylpyridines. Org. Biomol. Chem. 2014;12:1090–1099. doi: 10.1039/C3OB42039F. [DOI] [PubMed] [Google Scholar]; f Wang F. F., Luo C. P., Deng G., Yang L.. C(sp3)–C(sp3) Bond Formation via Copper/Brønsted Acid Co-Catalyzed C(sp3)–H Bond Oxidative Cross-Dehydrogenative-Coupling (CDC) of Azaarenes. Green Chem. 2014;16:2428–2431. doi: 10.1039/c4gc00038b. [DOI] [Google Scholar]; g Gao X., Zhang F., Deng G., Yang L.. Brønsted Acid Catalyzed Benzylic C–H Bond Functionalization of Azaarenes: Nucleophilic Addition to Nitroso Compounds. Org. Lett. 2014;16:3664–3667. doi: 10.1021/ol501422k. [DOI] [PubMed] [Google Scholar]; h Zhu Z. Q., Bai P., Huang Z. Z.. Dehydrogenative Cross-Coupling Reaction by Cooperative Transition-Metal and Brønsted Acid Catalysis for the Synthesis of β-Quinolinyl α-Amino Acid Esters. Org. Lett. 2014;16:4881–4883. doi: 10.1021/ol502402s. [DOI] [PubMed] [Google Scholar]
- For reviews on enantioselective reactions of azaarene derivatives, see:; a Best D., Lam H. W.. C = N-Containing Azaarenes as Activating Groups in Enantioselective Catalysis. J. Org. Chem. 2014;79:831–845. doi: 10.1021/jo402414k. [DOI] [PubMed] [Google Scholar]; b Meazza M., Rios R.. Enantioselective Synthesis of Alkyl Azaarenes. Asian J. Org. Chem. 2019;8:1800–1812. doi: 10.1002/ajoc.201900363. [DOI] [Google Scholar]
- For 2-alkylazaarene derivatives as the nucleophile for enantioselective reactions, see:; a Trost B. M., Thaisrivongs D. A., Hartwig J.. Palladium-Catalyzed Asymmetric Allylic Alkylations of Polynitrogen-Containing Aromatic Heterocycles. J. Am. Chem. Soc. 2011;133:12439–12441. doi: 10.1021/ja205523e. [DOI] [PubMed] [Google Scholar]; b Vera S., Liu Y., Marigo M., Escudero-Adán E. C., Melchiorre P.. Asymmetric Michael Addition of Nitrobenzyl Pyridines to Enals via Iminium Catalysis. Synlett. 2011;2011:489–494. doi: 10.1055/s-0030-1259518. [DOI] [Google Scholar]; c Fallan C., Lam H. W.. Enantioselective Nickel-Catalyzed Michael Additions of Azaarylacetates and Acetamides to Nitroalkenes. Chem. - Eur. J. 2012;18:11214–11218. doi: 10.1002/chem.201202093. [DOI] [PubMed] [Google Scholar]; d Best D., Kujawa S., Lam H. W.. Diastereo- and Enantioselective Pd(II)-Catalyzed Additions of 2-Alkylazaarenes to N-Boc Imines and Nitroalkenes. J. Am. Chem. Soc. 2012;134:18193–18196. doi: 10.1021/ja3083494. [DOI] [PubMed] [Google Scholar]; e Bastida I., San Segundo M., Lopez R., Palomo C.. Strategy for Stereoselective Metal-free α-Functionalization of 2-Azaaryl Acetates with N-Boc Imines. Chem. - Eur. J. 2017;23:13332–13336. doi: 10.1002/chem.201703748. [DOI] [PubMed] [Google Scholar]; f Li J., Fu Y., Qin C., Yu Y., Li H., Wang W.. Asymmetric Synthesis of Isoquinolinonaphthyridines Catalyzed by a Chiral Brønsted Acid. Org. Biomol. Chem. 2017;15:6474–6477. doi: 10.1039/C7OB01527E. [DOI] [PubMed] [Google Scholar]; g Jiang X., Boehm P., Hartwig J. F.. Stereodivergent Allylation of Azaaryl Acetamides and Acetates by Synergistic Iridium and Copper Catalysis. J. Am. Chem. Soc. 2018;140:1239–1242. doi: 10.1021/jacs.7b12824. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Hu Q., Kondoh A., Terada M.. Enantioselective Direct Mannich-Type Reactions of 2-Benzylpyridine N-Oxides Catalyzed by Chiral Bis(guanidino)iminophosphorane Organosuperbase. Chem. Sci. 2018;9:4348–4351. doi: 10.1039/C8SC00808F. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Wang Y., Wang K., Cao W., Liu X., Feng X.. Diastereo- and Enantioselective 1,6-Conjugate Addition of 2-Azaarylacetamides to Para-Quinone Methides. Org. Lett. 2019;21:6063–6067. doi: 10.1021/acs.orglett.9b02215. [DOI] [PubMed] [Google Scholar]; j Meazza M., Rios R.. Highly Regio- and Enantioselective Organocatalytic γ-Allylic Alkylation of Quinolines. Adv. Synth. Catal. 2021;363:1341–1345. doi: 10.1002/adsc.202001213. [DOI] [Google Scholar]; k Shen Y.-B., Qian H.-L., Yang L., Zhou S., Rao H.-W., Wang Z.-H., You Y., Zhang Y.-P., Yin J.-Q., Zhao J.-Q., Zhang W., Yuan W.-C.. Cu-Catalyzed Direct Asymmetric Mannich Reaction of 2-Alkylazaarenes and Isatin-Derived Ketimines. Org. Lett. 2024;26:1699–1704. doi: 10.1021/acs.orglett.4c00227. [DOI] [PubMed] [Google Scholar]
- For enantioselective construction of tetrasubstituted carbon using azaarene derivatives as nucleophiles, see:; a Izquierdo J., Landa A., Bastida I., López R., Oiarbide M., Palomo C.. Base-Catalyzed Asymmetric α-Functionalization of 2-(Cyanomethyl)azaarene N-Oxides Leading to Quaternary Stereocenters. J. Am. Chem. Soc. 2016;138:3282–3285. doi: 10.1021/jacs.5b13385. [DOI] [PubMed] [Google Scholar]; b Meazza M., Potter M., Pitak M. B., Coles S. J., Mazzanti A., Rios R.. Highly Enantioselective Synthesis of Alkylpyridine Derivatives through a Michael/Michael/Aldol Cascade Reaction. Eur. J. Org. Chem. 2017;2017:719–725. doi: 10.1002/ejoc.201601491. [DOI] [Google Scholar]; c Wang K., Chen C., Liu X., Li D., Peng T., Liu X., Yang D., Wang L.. Enantioselective Reaction between 2-(Cyanomethyl)Azaarenes and N-Boc-Amino Sulfones. Org. Lett. 2018;20:5260–5263. doi: 10.1021/acs.orglett.8b02205. [DOI] [PubMed] [Google Scholar]; d Das A., Joshi H., Singh V. K.. Asymmetric α-Functionalization of 2-Alkyl Azaarenes: Synthesis of Tertiary Fluorides Having Vicinal Stereogenic Centers. Org. Lett. 2021;23:9441–9445. doi: 10.1021/acs.orglett.1c03626. [DOI] [PubMed] [Google Scholar]; e Wang Z.-Q., Liu Z.-C., Huang X., Yin L.. Construction of Halogenated Tetrasubstituted Carbon Centers Through Copper(I)-catalyzed Asymmetric Alkylation of 2-Azaarylesters. Cell Rep. Phys. Sci. 2024;5:101822. doi: 10.1016/j.xcrp.2024.101822. [DOI] [Google Scholar]; f Wen H.-C., Chen W., Li M., Ma C., Wang J.-F., Fu A., Xu S.-Q., Zhou Y.-F., Ni S.-F., Mao B.. Chiral Phosphoric Acid-catalyzed Asymmetric Epoxidation of Alkenyl Aza-heteroarenes Using Hydrogen Peroxide. Nat. Commun. 2024;15:5277. doi: 10.1038/s41467-024-49435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Lapray A., Hiebel M.-A., Oudeyer S., Lohier J.-F., Suzenet F., Brière J.-F.. 3-Alkyl-1,2,4-triazines as Heterocyclic Platforms for Organocatalytic Enantioselective Benzylic C–H Functionalization. Org. Lett. 2025;27:1504–1510. doi: 10.1021/acs.orglett.5c00008. [DOI] [PubMed] [Google Scholar]
- For prevention of tautomerization of 1,3-dicarbonyl derivatives, see:; a Evans D. A., Clark J. S., Metternich R., Novack V. J., Sheppard G. S.. Diastereoselective Aldol Reactions Using β-keto Imide Derived Enolates. A Versatile Approach to the Assemblage of Polypropionate Systems. J. Am. Chem. Soc. 1990;112:866–868. doi: 10.1021/ja00158a056. [DOI] [Google Scholar]; b Evans D. A., Urpi F., Somers T. C., Clark J. S., Bilodeau M. T.. New Procedure for the Direct Generation of Titanium Enolates. Diastereoselective Bond Constructions with Representative Electrophiles. J. Am. Chem. Soc. 1990;112:8215–8216. doi: 10.1021/ja00178a082. [DOI] [Google Scholar]; c Evans D. A., Ng H. P., Clark J. S., Rieger D. L.. Diastereoselective Anti Aldol Reactions of Chiral Ethyl Ketones. Enantioselective Processes for the Synthesis of Polypropionate Natural Products. Tetrahedron. 1992;48:2127–2142. doi: 10.1016/S0040-4020(01)88879-7. [DOI] [Google Scholar]
- a Wang Y. Y., Kanomata K., Korenaga T., Terada M.. Enantioselective Aza Michael-Type Addition to Alkenyl Benzimidazoles Catalyzed by a Chiral Phosphoric Acid. Angew. Chem., Int. Ed. 2016;55:927–931. doi: 10.1002/anie.201508231. [DOI] [PubMed] [Google Scholar]; b Hou L., Kikuchi J., Ye H., Bao M., Terada M.. Chiral Phosphoric Acid-Catalyzed Enantioselective Phospha-Michael-Type Addition Reaction of Diarylphosphine Oxides with Alkenyl Benzimidazoles. J. Org. Chem. 2020;85:14802–14809. doi: 10.1021/acs.joc.0c01840. [DOI] [PubMed] [Google Scholar]; c Hou L., Zhang S., Ma J., Wang H., Jin T., Terada M., Bao M.. Organocatalytic Atroposelective Construction of Axially Chiral Compounds Containing Benzimidazole and Quinoline Rings. Org. Lett. 2023;25:5481–5485. doi: 10.1021/acs.orglett.3c01905. [DOI] [PubMed] [Google Scholar]
- The tautomerization of N-protected 2-cyanomethylbenzimidazoles 1 would generate the E-tautomer as the major geometrical isomer, presumably because of the steric effect and the formation of an intramolecular hydrogen bond between the carbonyl group of the protecting group and α-vinyl hydrogen.
- For reviews on chiral Brønsted acid catalysis, see:; a Doyle A. G., Jacobsen E. N.. Small-Molecule H–Bond Donors in Asymmetric Catalysis. Chem. Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; b Akiyama T., Mori K.. Stronger Brønsted Acids: Recent Progress. Chem. Rev. 2015;115:9277–9306. doi: 10.1021/acs.chemrev.5b00041. [DOI] [PubMed] [Google Scholar]; c James T., van Gemmeren M., List B.. Development and Applications of Disulfonimides in Enantioselective Organocatalysis. Chem. Rev. 2015;115:9388–9409. doi: 10.1021/acs.chemrev.5b00128. [DOI] [PubMed] [Google Scholar]
- For selected reviews on chiral phosphoric acid catalysts, see:; a Terada M.. Binaphthol-Derived Phosphoric Acid as a Versatile Catalyst for Enantioselective Carbon-Carbon Bond Forming Reactions. Chem. Commun. 2008:4097–4112. doi: 10.1039/b807577h. [DOI] [PubMed] [Google Scholar]; b Terada M.. Chiral Phosphoric Acids as Versatile Catalysts for Enantioselective Transformations. Synthesis. 2010;2010:1929–1982. doi: 10.1055/s-0029-1218801. [DOI] [Google Scholar]; c Parmar D., Sugiono E., Raja S., Rueping M.. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014;114:9047–9153. doi: 10.1021/cr5001496. [DOI] [PubMed] [Google Scholar]; d Del Corte X., Martinez De Marigorta E., Palacios F., Vicario J., Maestro A.. An Overview of the Applications of Chiral Phosphoric Acid Organocatalysts in Enantioselective Additions to C = O and C = N Bonds. Org. Chem. Front. 2022;9:6331–6399. doi: 10.1039/D2QO01209J. [DOI] [Google Scholar]; e Betinol I. O., Kuang Y., Mulley B. P., Reid J. P.. Controlling Stereoselectivity with Noncovalent Interactions in Chiral Phosphoric Acid Organocatalysis. Chem. Rev. 2025;125:4184–4286. doi: 10.1021/acs.chemrev.4c00869. [DOI] [PubMed] [Google Scholar]
- For seminal studies of chiral phosphoric acid catalysts, see:; a Akiyama T., Itoh J., Yokota K., Fuchibe K.. Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid. Angew. Chem., Int. Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]; b Uraguchi D., Terada M.. Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation. J. Am. Chem. Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- DFT calculations, see the following. def2-bases:; a Weigend F., Ahlrichs R.. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005;7:3297–3305. doi: 10.1039/b508541a. [DOI] [PubMed] [Google Scholar]; ωB97X-D:; b Chai J.-D., Head-Gordon M.. Long-range Corrected Hybrid Density Functionals with Damped Atom–atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008;10:6615–6620. doi: 10.1039/b810189b. [DOI] [PubMed] [Google Scholar]; ωB97M-V:; c Mardirossian N., Head-Gordon M.. ωB97M-V: A Combinatorially Optimized, Range-separated Hybrid, Meta-GGA Density Functional with VV10 Nonlocal Correlation. J. Chem. Phys. 2016;144:214110. doi: 10.1063/1.4952647. [DOI] [PubMed] [Google Scholar]; CPCM:; d Barone V., Cossi M.. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A. 1998;102:1995–2001. doi: 10.1021/jp9716997. [DOI] [Google Scholar]; SMD:; e Marenich A. V., Cramer C. J., Truhlar D. G.. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B. 2009;113:6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.