Abstract
Cancer is one of the major diseases threatening human health in the world. According to the latest global cancer statistics from the International Agency for Research on Cancer (IARC), there were approximately 20 million new cancer cases and 10 million cancer deaths worldwide. Amidst this global health concern, branched chain amino acids have emerged as key players, playing an important role in the occurrence and development of cancer. In certain malignancies like colorectal cancer, the average level of BCAA in tumor tissues is twice that in normal tissues. BCAA metabolism is intricately associated with the progression of multiple tumors and is modulated by diverse enzymes, including BCAT, BCKDH, and BCKDK. The metabolism of BCAA involves multiple enzymes and biochemical processes via signaling pathways such as PI3K/AKT/mTOR and AMPK/mTOR, etc. In addition, mTOR inhibitors show potential value in cancer treatment by regulating the metabolism and signaling pathways of tumor cells, which provides a new direction for anticancer efforts. Simultaneously, BCAAs are closely associated with tumor immunity, including NK cells, CD4+ T cells, and CD8+ T cells. At present, the research on BCAA metabolism and related enzymes and signaling pathways is still in progress, aiming at identifying new targets and ideas for cancer therapy, and combined therapy will become an important trend in the future. In this review, we discuss the role and mechanisms of BCAA metabolism in human cancer.
Keywords: BCAA, Metabolic reprogramming, BCAT, Cancer, mTOR
Introduction
Currently, cancer stands as one of the primary causes of human mortality globally, consistently attracting the dedicated efforts of numerous scientific researchers in the pursuit of a deeper understanding [1]. Metabolic disorder is one of the key signs of tumor biological characteristics, such as abnormal lactate and amino acid metabolism. Although previous studies have extensively documented the regulatory function of abnormal lactate metabolism in different cancers, the abnormal metabolism of amino acids in tumors, particularly branched chain amino acids (BCAA), remains a promising avenue that needs further in-depth investigation in future research.
BCAAs play a pivotal role in the development and occurrence of malignancies [2, 3], and profoundly affect the tumor microenvironment (TME) [4]. BCAAs, including valine, isoleucine, and leucine, are essential amino acids mainly obtained through dietary intake [5]. In recent years, BCAAs have become an increasingly common focus in tumor research, and some studies have shown that the increasing intake of BCAA in mice can inhibit the growth and metastasis of breast cancer (BC) [6]. In those suffering from colorectal cancer (CRC), the increased intake of BCAA in the diet is positively correlated with the increase of death risk [7], and BCAA may also be related to the occurrence of pancreatic ductal adenocarcinoma (PDAC). BCAA transaminase 2 (BCAT2) is an indispensable enzyme for BCAA decomposition, and its acetylation can inhibit the catabolism of BCAA and the growth of pancreatic cancer [8, 9]. Currently, it has been firmly established that the metabolic process of BCAA is closely associated with the progression of various tumors. As depicted in Fig. 1, with the continuous deepening of research, it has been discovered that BCAA metabolism not only directly governs tumor growth but also bears a relation to tumor immunity and drug resistance. Consequently, elucidating the metabolism of BCAA in tumors is of utmost significance.
Fig. 1.
Metabolism of BCAAs in various tumors in humans. The figure showcases the metabolic processes of BCAA within diverse human tumors, aiming to reveal the unique features of these processes in different tumor contexts
Compounds, polymers, and nanomedicines, as well as metabolic abnormalities, are also associated with the sensitivity of tumors to chemotherapy [10–12]. Valine is an essential amino acid for the occurrence and development of gastric cancer (GC) [13, 14]. An imbalanced content of amino acids in the body can enhance the sensitivity of cells to chemotherapy [10, 15]. Future research directions could focus on delving into the molecular mechanisms underlying the abnormal metabolism of BCAA and their relationships with tumor development and chemosensitivity, providing a solid theoretical foundation for the development of more precise and efficient anti-cancer drugs and treatment regimens [16]. Meanwhile, it is necessary to further optimize the design and preparation processes of compounds, polymers, and nanomedicines to enhance their targeting ability, bioavailability, and safety.
This review systematically focuses on the metabolism of BCAA within tumors and also comprehensively explores the roles of diverse metabolic enzymes and signaling pathways in tumor occurrence and development.
Metabolic recombination in tumors
TME signifies the substantial and characteristic alterations in the metabolic pathways of cancer cells during their growth and proliferation. These alterations actively contribute to tumor development and metastasis. Metabolic reprogramming in tumors is a characteristic feature that enables cancer cells to adapt to their rapid growth and proliferation needs. Metabolic reprogramming meets the increased dietary needs, regulates many pathogenic mechanisms in tumors, and leads to the malignancy of tumors. This metabolic reprogramming is one of the important mechanisms by which tumor cells adapt to the harsh microenvironment and support their uncontrolled growth [17]. BCAA plays a crucial part in tumor reprogramming, and its metabolism can supply acetyl-CoA, thus facilitating the TCA cycle in tumor cells.
The abnormal metabolism of BCAA plays a pivotal role in tumor metabolic reprogramming. In the tumorigenic environment, alterations in BCAA metabolism, such as abnormal changes in related enzyme activities, drive the metabolic reprogramming process. This provides the necessary energy and materials for tumor cell growth and proliferation, thereby promoting tumor development. BCAA can function as a source of energy and precursors for biosynthesis in this process. Tumor metastasis, as a critical stage of tumor development, is intertwined with the overall tumor progression. Abnormal metabolism of BCAA can enhance the migration and invasion capabilities of tumor cells by altering their energy supply and metabolic microenvironment. The metabolic reprogramming can alter the TME to promote metastasis and affect the expression patterns of metastasis-related genes, such as the PI3K/AKT/mTOR pathway [18, 19]. Among them, BCAA will not only supply necessary substances but also regulate signaling pathways to support tumor development.
Studies indicate that blocking the specific exogenous amino acids on which tumors depend will lead to amino acid starvation, growth stagnation, and ultimately triggers apoptosis [20]. With the ongoing and in-depth research on amino acids, it has been increasingly recognized that BCAA, in particular, play a pivotal role in the development of many tumors. Currently, the mechanism of BCAA involved in tumor metabolic reprogramming is intricate and still needs to be clarified. In the future, it is anticipated that more comprehensive and profound research will further clarify the specific metabolic pathways and key regulatory nodes in different tumor types.
Previous studies have shown that BCAA plays a key role in regulating the function of immune cells and tumor growth [21]. The damage of BCAA degradation leads to the accumulation of BCAA in CD8+ T cells, leading to high activity of CD8+ T cells and enhancing antitumor immunity. The accumulation of BCAAs promotes the effector function and antitumor immunity of CD8+ T cells by reprogramming glucose metabolism [22], making BCAA a supplementary component for the clinical efficacy of anti-PD-1 immunotherapy for tumors.
BCAA metabolism has a significant role in tumor metabolic reprogramming. Targeting BCAA metabolism may offer a potential approach for cancer treatment. Understanding the role of BCAAs in the metabolic reprogramming of tumors is crucial for us to explore the intricate mechanisms underlying cancer development. The precise manipulation of BCAA metabolic pathways holds the promise of crafting more effective therapeutic strategies for cancer treatment.
Metabolism of branched-chain amino acids
Cancer metastasis is the primary cause of cancer death, which needs the support of metabolic reprogramming. The metabolic reprogramming of BCAA meets the increased nutritional requirements, promotes the transformation of tumors, and leads to tumor deterioration. The metabolism of BCAA not only furnishes a nitrogen source for tumors but also generates carbon skeletons. These carbon skeletons can fuel the energy demands for oxidative phosphorylation and the TCA cycle, playing a pivotal role in maintaining the energy metabolism balance within tumor cells [23]. From a nutritional perspective, it is undeniable that BCAA facilitate tumor progression. Consequently, the expression of relevant metabolic enzymes can partially modulate tumor development. Studies have shown that branched-chain keto-acid dehydrogenase kinase (BCKDK), which regulates the rate-limiting enzyme in the BCAA catabolic pathway, promotes the proliferation of tumor cells [24, 25]. Metabolic alterations of BCAA are a distinct feature of various cancers and have a significant impact on maintaining tumor proliferation and invasiveness [26]. It affects the provision of energy and biosynthetic precursors mentioned above, the regulation in signal pathways, and some growth-related regulatory factors in TME, such as VEGF. All these functions establish the special position of BCAA in tumors.
The metabolism of BCAA has been studied in a variety of tumors (Fig. 1), including hepatocellular carcinoma (HCC), BC, PDAC, and non-small cell lung cancer (NSCLC), etc. [27–31] (Table 1). Tumor cells usually have a relatively high ability to obtain BCAA. In addition, the latest research has found that the metabolism of BCAA and its transporter protein (SLC7A5) might be associated with the development of thyroid cancer [32]. Numerous studies have indicated that the level of BCAA appears abnormal during the development of some cancers. Among them, the research on the metabolism of BCAA focuses more on BCAA aminotransferase (BCAT). BCAT is an enzyme that initiates the catabolism of BCAA, it exerts a crucial role in the metabolism of BCAA [33]. Previous studies have indicated that the regulatory role of BCAT is often decisive in BCAA metabolism. Due to the high metabolic demands of tumor cells, the expression of BCAT in tumor cells is often elevated.
Table 1.
BCAA alterations and related proteins in human cancers
| Cancer type | BCAA levels | Protein levels | Effect on cancer progression | References |
|---|---|---|---|---|
| PDAC | N.D | BCAT2↑ | Tumor growth | [8] |
| ↑ | BCAT2↑ BCKDHA↑ | Tumor growth | [50] | |
| ↑ | BCAT2 mutation | Tumor growth | [9] | |
| ↑ | BCAT2↑ | Tumor growth | [51] | |
| HCC | ↑ | Hyperactivation of mTOR | Tumor growth | [27] |
| N.D | BCAT1↑ | Tumor growth | [36] | |
| ↑ | BCKDH↓ | Tumor growth | [56] | |
| BC | ↑ | N.D | Tumor shrinkage | [6] |
| N.D | BCAT1↑ | Tumor growth | [31] | |
| N.D | BCKDK↑ | Tumor growth | [62] | |
| NSCLC | ↑ | BCKDHA↓ BCKDK↑ | Tumor growth | [30] |
| ↑ | BCKDK↑ | Tumor growth | [63] | |
| GBM | N.D | BCAT1↑ | Tumor growth | [39] |
| N.D | BCAT1↑ BCKDK↑ | Tumor growth | [38] | |
| GC | N.D | BCAT1↑ | Tumor growth | [40] |
| N.D | BCAT1↑ | Tumor growth | [73] | |
| OSCC | N.D | BCKDH↑ | Tumor growth | [58] |
| ccRCC | N.D | BCAT1↑ | Tumor growth | [99] |
↑, increase. ↓, decrease. N.D., not determined. PDAC, pancreatic ductal adenocarcinoma
HCC hepatocellular carcinoma, BC breast cancer, NSCLC non-small cell lung cancer, GBM glioblastoma multiforme, GC gastric cancer, OSCC oral squamous cell carcinoma, CcRCC clear cell renal cell carcinoma
Metabolic enzymes related to BCAAs
BCAT1
BCAT has been considered a prognostic cancer marker and is very important in the development of cancer. Typically, the upregulation of BCAT1 expression has been correlated with more aggressive cancer progression, garnering significant scientific interest over the past several years [34–36]. A comprehensive and in-depth exploration of the mechanism of BCAT1 in cancer is of great significance for achieving a more profound understanding of both BCAA metabolism and cancer development. At the same time, BCAT1 provides a solid theoretical basis for the development of cancer diagnosis and treatment.
BCAT1 is capable of catalyzing the transamination reaction of BCAA. The high expression of BCAT1 can result in an enhanced catabolism of BCAA, providing energy and precursors of biosynthesis for tumor cells, thus promoting the growth and proliferation of tumor cells. In various tissues, the regulation of plasma BCAAs and its catabolic defects are mainly caused by the changes in the enzymatic activity of the first two enzymes, namely BCAT and branched-chain α-keto acid dehydrogenase (BCKD) [37]. To sum up, BCAT1 exerts significant effects in various types of cancers. By catalyzing the initial step of BCAA decomposition, BCAT1 affects cell proliferation, regulates the interaction of intracellular molecules, and drives the growth, migration, invasion and proliferation of tumors, which provides a new perspective and direction for cancer diagnosis and treatment.
Some studies indicate that BCAT1 facilitates the initial phase of BCAA degradation and is highly upregulated in cells that are resistant to anti-estrogen treatment. BCAT1 expression can be detected in primary BC and positively correlates with an elevated Ki-67 proliferation index. In triple-negative breast cancer and glioblastoma multiforme (GBM), BCAT1 plays an important role in tumor migration, invasion, and proliferation [38, 39]. The abnormal expression and functional alteration of BCAT1 in multiple cancer types suggest its crucial significance in the process of tumorigenesis and development, laying a foundation for further exploration of its specific mechanism of action in other cancers.
In the research on GC, the mutation of alanine at codon 61 (BCAT1E61A) caused by glutamic acid of BCAT1 leads to its increased function. BCAT1E61A's higher enzymatic activity can promote the catabolism of BCAA and accelerate cell growth and metastasis, and BCAT1 can also activate the PI3K/AKT/mTOR pathway to contribute to the development of tumors [40]. Moreover, BCAT1 directly interacts with RhoC, resulting in an increase in RhoC activity. The activity of RhoC plays an important role in the development and metastasis of various cancers, such as GC and bladder cancer [41–43]. The latest research indicates that the transcription factor EB (TFEB) is highly expressed in pancreatic cancer and regulates BCAT1 to modulate the catabolism of BCAA, thereby partially inhibiting the proliferation of pancreatic cancer cells, providing a new direction for the treatment of targeted metabolic reprogramming of pancreatic cancer [44]. Accumulating evidence has demonstrated that BCAT1, functioning as a key promoter of tumor metastasis and proliferation, holds paramount significance not only in the early detection of tumors but also plays a pivotal role at the treatment phase [45]. These research findings indicate that the regulatory role of BCAT1 in GC and pancreatic cancer brings new hope for cancer metabolic targeted therapy. Moreover, it further highlights the importance of BCAT1 in tumor research and provides ideas for subsequent related research in more cancer types.
In the BCAT family, BCAT has two isoforms, BCAT1 and BCAT2, which are encoded by their genes. BCAT1 mainly functions in the cytoplasm, while BCAT2 is mainly located in the mitochondria. BCAT2 is widely expressed, whereas BCAT1 is only expressed in certain organs like the brain, etc. Given the unique intracellular localization and ubiquitous expression characteristics of BCAT2, further investigation is warranted to deeply explore its functional mechanism in BCAA metabolism, which differs from that of BCAT1.
BCAT2
BCAT1 and BCAT2 exert disparate functions in the metabolic pathway of BCAA. BCAT1 is predominantly localized in the cytoplasm. Its function lies in catalyzing the transfer of the amino group from BCAA to α-keto acids, thereby yielding the corresponding α-keto acids (BCKAs) and ammonia. This process represents the initial step in the catabolism of BCAA. The generated α-keto acids can undergo further metabolism to partake in energy-generating processes or other biosynthetic pathways. BCAT2 mainly functions in the mitochondria, particularly in organs with high metabolic activity, such as the liver and kidneys. The function of BCAT2 is analogous to BCAT1, both catalyzing the transamination reaction between BCAAs and αKG, producing BCKAs and glutamic acid [46]. In the metabolism of BCAA, αKG accepts the amino groups removed from BCAT1 or BCAT2 by BCAAs and is converted into glutamic acid (Fig. 2).
Fig. 2.
Role of BCAA-metabolizing enzymes BCAT1 and BCAT2. This figure illustrates the crucial functions of the BCAA metabolizing enzymes BCAT1 and BCAT2
After BCAT1 and BCAT2 catalyze their reactions, BCAAs are converted into corresponding BCKAs, which are primarily processed by the BCKD to produce acetyl-CoA, subsequently entering the TCA cycle. These processes are crucial steps in the metabolic pathway of BCAA, ensuring that the catabolism of amino acids can proceed efficiently in the mitochondria, providing cells with energy and intermediate products required for biosynthesis [47].
In melanoma (MM), BCAT2 has been identified as a carcinogenic factor and promotes the progression of melanoma [48]. Compared with other tumors, BCAT2 is more extensively studied in PDAC. PDAC is known for its high invasiveness, early metastasis tendency, lack of specific symptoms, and difficulty in treatment. Numerous studies have demonstrated the important role of BCAT2 in the occurrence of PDAC [49–51]. Furthermore, BCAT2 may play a certain role in the progression of prostate cancer and could serve as a potential marker for developing new therapeutic targets. [52]. Additionally, another group of researchers concluded through proteomic analysis that the expression of BCAT2 is associated with a poor prognosis of prostate cancer [53]. Apart from the aforementioned tumor types, studies in intrahepatic cholangiocarcinoma (ICC) were also found that the metabolism of BCAA and the expression of BCAT affect the development of ICC, providing a potential target for subsequent BCAA research [54]. During the growth and proliferation procedures of various tumors, BCAT2 participates in different occurrence and development pathways. Inhibiting the proliferation of some tumors by gene knockout of BCAT2 has made certain progress [55]. Future research should further investigate the mechanism of BCAT2 in different tumors and its interaction with other molecules, thus fully understanding the role of BCAT2 in tumors related to BCAA metabolism.
Given the specific and discrepant expression of BCAT1 and BCAT2 in tumor cells, they hold promise as diagnostic markers for certain malignancies. Detecting the expression levels of BCAT1 and BCAT2 in tumor tissues or blood can assist the early diagnosis of tumors. Moreover, their expression levels are linked to tumor prognosis, making them potential prognostic indicators. Developing inhibitors targeting BCAT1 and BCAT2 is a promising therapeutic strategy, as these inhibitors can disrupt tumor cell energy supply and biosynthesis pathways by inhibiting BCAA metabolism, thereby suppressing cell growth and proliferation. They can also serve as potential targets for tumor treatment in the future, and the combined use with some drugs, such as anti-PD-1 drugs, holds great prospects in the future. However, current research on BCAT1 and BCAT2 is still in its early stages, and further in-depth studies on their biological functions and mechanisms as well as their clinical application value in tumors.
BCKDH and BCKDK
On the other hand, branched-chain keto acid dehydrogenase (BCKDH) and BCKDK are also key enzymes regulating BCAA metabolism. The BCKDH kinase mainly acts on the BCKDH complex in mitochondria and is responsible for catalyzing the final step of BCAA metabolism, converting α-keto acid into branched-chain acyl coenzyme A. The BCKDH kinase inhibits the activity of the BCKDH complex by phosphorylating the E1α subunit of the BCKDH complex, thereby reducing the catabolism of BCAA [56, 57]. Clarifying the roles of BCKDH and BCKDK in BCAA metabolism is crucial for understanding BCAA metabolism and its regulation mechanism in tumors.
For example, in the study of oral squamous cell carcinoma (OSCC), it is reported that BCKDH is overexpressed in OSCC and affects the tumor process [58]. Moreover, the knockdown of BCKDHA can also significantly inhibit the proliferation of PDAC cells [50, 59]. Many discoveries have also been made in the study of the interaction between BCKDK and MEK/ERK signal pathway. The BCKDK-MEK1 axis may contribute to the development of CRC [60], and BCKDK promotes the proliferation and migration of ovarian cancer by activating the MEK/ERK signaling pathway [61]. Moreover, downregulating the expression of BCKDK can inhibit the migration of BC cells [62]. At the same time, downregulating BCKDK will also reduce the in vitro proliferation of NSCLC cells and aggravate apoptosis [63]. The metabolism of BCAA regulated by BCKDK and its function in different tumor types have been verified. Besides, the latest research has found that the modification of BCKDK enhances the anticancer efficacy of CAR-T cells.
The two enzymes, BCKDH and BCKDK, play regulatory roles in the middle and downstream of BCAA metabolism. At present, their regulatory effects on BCAA metabolism have been found in a variety of tumors. It has been discovered that BCKDK can enhance MEK/ERK signal transduction, a crucial pathway driving the growth and proliferation of cancer cells. BCKDH and BCKDK have emerged as an appealing therapeutic target for BCAA-related diseases and cancers [64, 65]. Similar to BCAT, the expression levels or activities of BCKDH and BCKDK may potentially serve as markers for the diagnosis or prognosis of certain types of tumors. Additionally, targeting BCKDH and BCKDK holds promise as a cancer treatment approach. Hence, further research is required to fully clarify their functions and explore their potential as therapeutic targets and biomarkers for tumors.
The regulation of BCAA metabolism by BCAA-metabolizing enzymes in tumors is a crucial part of tumor metabolic reprogramming. These enzymes facilitate BCAA metabolism to meet the unique demands of tumor cells through precise regulatory mechanisms. During tumorigenesis, BCAA metabolizing enzymes are often over-expressed. As research progresses, BCAA-metabolizing enzymes could potentially assist in tumor diagnosis and provide a theoretical basis for developing drugs that target these enzymes to inhibit tumor progression.
Signaling pathways of BCAA metabolism
In the field of BCAA metabolism, while there has been relatively extensive research on related metabolic enzymes, the exploration of signal transduction pathways is also of utmost significance. BCAA metabolism is intricately associated with three major signal pathways: the mTOR pathway, the PI3K/AKT pathway, and the AMPK pathway. These pathways are highly interconnected functionally and regulate BCAA metabolism through various dimensions, including nutrient sensing and energy homeostasis. The aberrant regulation of these pathways is closely intertwined with the onset and progression of various diseases, particularly tumors. The mTOR pathway has received significant research attention due to its central role in coordinating BCAA-related metabolic processes and its profound implications for disease pathogenesis.
mTOR is a serine/threonine protein kinase that forms two different mTOR complexes, namely mTORC1 and mTORC2. mTORC1 is mainly composed of the following components: mTOR is the core kinase used to phosphorylate downstream targets; Raptor is the binding partner of mTOR and helps recruit and phosphorylate downstream targets; mLST8, also known as GβL, is related to the stability and activity of mTORC1. PRAS40 can act as an inhibitor of mTORC1 but can also be phosphorylated and activated by mTORC1 under certain conditions; Deptor can inhibit the activity of mTORC1. mTORC2 is mainly composed of mTOR, Rictor, mSin1, Protor-1, mLST8, and Sik3. Rictor, as a binding partner, is crucial for the formation and activity of mTORC2; mSin1 helps maintain the stability and activity of mTORC2; Protor and Sik3 are involved in the regulation of mTORC2. The mTOR pathway plays a very critical role in tumors, including promoting tumor cell proliferation and invasion, inhibiting cell apoptosis, and regulating the metabolic reprogramming of tumor cells so that they can use nutrients more effectively and promote the rapid growth of tumors [66–68]. Abnormal mTOR metabolism has already been regarded as a hallmark feature of tumorigenesis.
BCAAs, especially leucine, are the main activators of mTOR [69]. Therefore, some researchers evaluated the growth dependence of osteosarcoma on leucine and found that blocking leucine uptake by using the leucine structural analog N-acetylleucinamide (NALA) will cause the downregulation of mTORC1 and thus affect tumor development [70]. In non-tumorigenic human mammary epithelial cells, BRCA1 mutation results in a notable increase in BCAA levels. Leucine can promote protein translation through mTOR and succinyl coenzyme A. For isoleucine, the latest research has found that high-dose isoleucine can significantly inhibit tumor growth and reduce tumor burden, which is independent of mTORC1 activation [71]. The growth factor pathway mainly involves PI3K/Akt and Ras/Raf/MEK/ERK, while the energy state pathway is caused by the activation of AMPK, including glucose and LKB1 (liver kinase B1). There is also a nutrient sensing pathway that is mainly mediated by activation through nutrients such as amino acids and glucose [72]. BCAA is closely related to mTOR, and the mTOR pathway interacts with multiple signaling pathways to perform complex regulatory functions in tumors.
In various tumors, the PI3K-AKT-mTOR signaling axis is frequently in an abnormally active state. The PI3K signaling pathway serves as a crucial upstream regulatory route for mTOR activation. The activated PI3K phosphorylates AKT, leading to the activation of mTORC1 and initiating a cascade of responses that promote tumor growth, as shown in Fig. 3. Studies have shown that BCAT1 and CDK12 can activate the PI3K/AKT/mTOR pathway and participate in the progression of GC [73, 74]. The dysregulation of mTOR often occurs in various tumors, and there are multiple reasons for the abnormal activation of mTOR [75]. Including the PI3K/AKT/PTEN and insulin/IGF-1 signaling pathways mentioned earlier [76, 77], as well as epigenetic changes such as the TME and methylation, which may cause abnormal activation of mTOR [78, 79]. The activation of mTOR can activate protein synthesis and promote the cell cycle and cell proliferation, thereby promoting tumor growth. It also facilitates angiogenesis to supply nutrients to the tumor and utilizes glucose, lipids, and amino acid metabolism to provide essential nutrients for tumor growth while inhibiting apoptosis and promoting cell survival [80–82]. Multiple factors can lead to the abnormal activation of mTOR, which plays multifaceted promoting roles in BCAA metabolism. Currently, there are still many challenges in precisely targeting the abnormal activation of mTOR to influence BCAA metabolism and thus intervene in tumor progression. Therefore, the development of mTOR inhibitors has great potential to become anticancer drugs.
Fig. 3.
The role of mTOR in BCAA signaling pathway. This figure elaborates on how mTOR participates in and influences the BCAA signaling pathway
Currently, structurally diverse mTOR inhibitors are in clinical trials, and further research is still needed in the next step [83]. mTOR inhibitors have been proven to be able to induce autophagy, promote tumor cell death, and make individual tumors sensitive to other therapeutic agents. The first-generation mTOR inhibitors are primarily rapamycin and its derivatives. It was found inhibited the immune system by blocking the activation and proliferation of T cells and was used to prevent organ rejection in kidney transplant patients at that time. TSC1 (Tuberous Sclerosis Complex 1) and TSC2 (Tuberous Sclerosis Complex 2) are two tumor suppressor genes. The TSC1 and TSC2 tumor suppressor genes encode proteins that form the TSC complex, which functions as a GTPase activating protein (GAP) in cells and inhibits the activity of Rheb to regulate mTORC1. Rheb is a small GTPase. When it is in the GTP-bound state, it can activate mTORC1. The TSC1/TSC2 complex promotes the GTPase activity of Rheb, making it change from the GTP-bound state to the GDP-bound state, thereby inhibiting the activity of mTORC1 (Fig. 3). S6K is a downstream effector of the mTORC1 signaling pathway and is directly regulated by mTORC1. The triggering of S6K can promote protein synthesis and cell growth.
In tumors, the mutation or inactivation of TSC1 or TSC2 will lead to the loss of TSC complex function, which in turn leads to the continuous activation of mTORC1 by Rheb, making S6K continuously phosphorylated and activated [84]. The ability of rapamycin to inhibit the phosphorylation and activation of S6K indicates that it may have the property of inhibiting cancer growth [85, 86]. The efforts of pharmaceutical personnel have led to the development of a variety of related drugs. Later, researchers collectively called them rapalogs, including everolimus temsirolimus, and sirolimus (rapamycin), etc. [87, 88] (Fig. 4). First-generation mTOR inhibitors have drawbacks such as limitations in their mechanism of action, induction of feedback activation, and limited anti-tumor activity. To overcome these problems, the development of second-generation mTOR inhibitors has become a necessity.
Fig. 4.
The function of first-generation mTOR inhibitors
Nevertheless, these drugs have some adverse reactions, such as causing inflammation, stroke, infarction, and drug resistance. They are only significantly effective against mTORC1 and have relatively little impact on mTORC2 [89]. Later, researchers developed dual mTORC1/mTORC2 inhibitors and combined them with some drugs, which showed good therapeutic effects [90, 91]. These are called second-generation mTOR inhibitors, such as AZD2014, OSI-027, INK128, etc. However, second-generation mTOR inhibitors still need further research and development, and their clinical application effects and safety still need to be verified.
Targeting mTOR inhibitors for cancer treatment is a good therapeutic prospect. It has multi-target effects and better therapeutic effects on certain tumors. Besides, combination therapy will become an important trend in the future. The combined use of mTOR inhibitors and other cancer treatment methods has shown great potential [92–94]. However, the side effects of the drugs, alongside drug resistance, are drawbacks that cannot be overlooked. Additionally, mTOR plays a pivotal role in normal cell growth, metabolism, and immune regulation. Therefore, the application of mTOR inhibitors may affect the function of normal cells and cause some adverse reactions [95]. In conclusion, mTOR inhibitors have broad prospects in cancer treatment. Future research will be dedicated to developing more efficient and more specific drugs, optimizing combination therapy regimens, overcoming drug resistance, regulating the tumor immune microenvironment, and realizing precision medicine treatment, bringing more hope and better therapeutic effects to cancer patients.
The metabolism of BCAA in tumor cells is precisely regulated by multiple signal pathways, among which mTOR is the most crucial regulator. The research on the signal pathways of BCAA metabolism provides potential targets for tumor treatment. However, the complex interactive network among these signal pathways and continuous research is still needed to fully comprehend their interconnections.And there is huge potential to develop new treatments based on this.
BCAA metabolism and tumor immunity
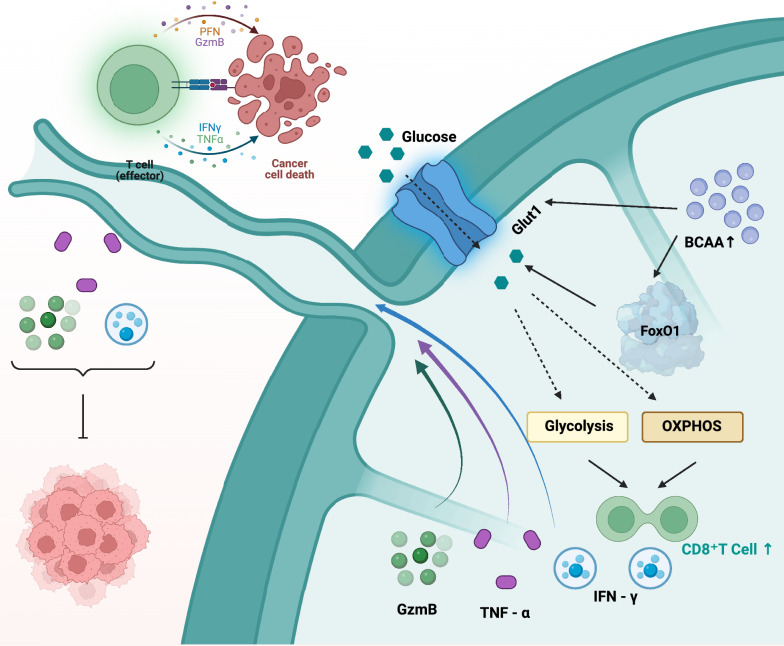
BCAA metabolism is closely associated with tumor immunity. It can regulate the differentiation and activity of immune cells [96]. Studies have reported that in mice with impaired BCAA degradation, BCAA accumulates in CD8+ T cells, resulting in high-activity CD8+ T cells and enhanced anti-tumor immunity [22] (Fig. 5). Besides, BCAA can also support the activities of neutrophils and NK cells. Moreover, it can regulate the differentiation ability of monocytes, all of which indicate the potential of BCAA in immunity.
Fig. 5.
The accumulation of BCAAs promote anti-tumor effects. This figure demonstrates how the increased accumulation of BCAA can trigger certain biological responses that contribute to inhibiting tumor growth and exerting anti-tumor effects
After BCAA supplementation, the hyperfunction of CD8+ T cells was reproduced, and a synergistic effect with anti-PD-1 was observed, indicating that BCAAs can serve as a supplementary factor for the clinical efficacy of anti-PD-1 immunotherapy in NSCLC patients. Immunological analysis revealed a significant inverse relationship between the secretion of chemokines associated with CD8+ T cells and BCAT2 [97]. Subsequently, it was discovered that the combination of BCAT2 deficiency and anti-PD-1 antibody treatment exhibited a synergistic effect in vivo, indicating the potential of BCAT2-based combined therapy. Moreover, excessive activation of PI3K/mTOR is related to lymphocyte depletion and wound-healing immune landscapes in uterine leiomyosarcoma [98], which contributes to tumor immune evasion. Conversely, inhibition of PI3K/mTOR can induce remodeling of the TME and promote adaptive anti-tumor immune responses.
Similarly, two clusters related to BCAA metabolism with distinct prognostic and immune-infiltration characteristics were identified in Clear cell renal cell carcinoma (ccRCC) [99]. RT-qPCR results demonstrated that BCAT1 was overexpressed in ccRCC tissues and cell lines. BCAT1 has a tumor-promoting function in ccRCC, which is closely associated with immunosuppressive cells and checkpoints, and promotes the proliferation and metastasis of ccRCC cells. The BCAA metabolism signature provides a new perspective for ccRCC immunotherapy targets, demonstrating the close relationship between BCAA metabolism and tumor immunity. These findings provide a theoretical basis for further exploring new immunotherapy targets in ccRCC and also lay a foundation for studying the relationship between metabolism and immunity in other tumor types.
Apart from the metabolic enzymes of BCAA, mTOR, as an important protein kinase in BCAA metabolism, also plays a crucial role in immune regulation. Leucine, one of the major activators of mTOR, can influence T-cell differentiation and cytokine production through binding to SLC7A5. Therefore, it is necessary to strengthen the research on the regulatory mechanism of mTOR on tumor immunity [100] in BCAA metabolism [100]. It is expected to provide new targets and strategies for tumor immunotherapy.
BCAA metabolism is closely associated with tumor immunology. BCAA accumulate in CD8+ T cells, enhancing their activity and boosting anti-tumor immunity. BCAA supplementation can reproduce the hyperfunction of CD8+ T cells and show a synergistic effect with anti PD-1 immunotherapy in NSCLC patients. Moreover, BCAA metabolism is related to the secretion of chemokines associated with CD8+ T cells. BCAT2, a key enzyme in BCAA metabolism, is inversely related to the secretion of these chemokines. The combination of BCAT2 deficiency and anti-PD-1 antibody treatment exhibits a synergistic effect in vivo. In addition, mTOR, an important protein kinase in BCAA metabolism, also plays a crucial role in immune regulation. Leucine, one of the major activators of mTOR, can influence T cell differentiation and cytokine production through binding to SLC7A5. Overall, BCAA metabolism has a significant impact on tumor immunity, and further exploration of this relationship may provide new targets and strategies for tumor immunotherapy.
In summary, whether the BCAA content directly affects the abundance of intracellular T cells or influences tumor immunity through the expression of BCAA-related metabolic enzymes and changes in signaling pathways, it offers new insights into tumor immunological research. In the future, it is expected to further explore the specific mechanisms of BCAA metabolism in different tumor types, develop targeted drugs for BCAA metabolism pathways to regulate immune microenvironment, and enhance the efficacy of tumor immunotherapy. At the same time, the combined application of novel treatment strategies targeting BCAA metabolism with existing tumor immunotherapies (such as immune checkpoint inhibitors, adoptive cell therapy, etc.) should be explored.
Dietary BCAA and cancer progression
Oral administration of BCAA, as the most direct means of regulating BCAA metabolism, has attracted great attention in past research. In mice with HCC, supplementation with BCAA may suppress the development of HCC [101]. One potential mechanism is the inhibition of TGF-β1 signaling via the mTOR pathway.
This conclusion, however, needs further research to verify, and the situation in humans may be different. The accumulation of BCAAs in human body may lead to the onset of some tumors and even the death of patients. This can be alleviated by restricting BCAA intake in the diet [51]. Likewise, in studies on PDAC, it has been found that targeting BCAT2 to regulate the metabolism of BCAAs and controlling the intake of BCAAs to influence the development of PDAC are of great significance [7].
Similarly, high-level dietary BCAA intake resulting from BCAT2 deficiency promotes the occurrence of CRC [102]. It was discovered that the accumulation of BCAA promotes the chronic activation of mTORC1, thus mediating the carcinogenic effect of BCAA. However, the relationship between BCAA and tumor development is complex. High levels of BCAA in the diet may have both promoting and inhibitory effects on tumors. For instance, in studies on BC, the genetic defect in BCAA catabolism caused by knockout of Pp2cm leads to BCAA accumulation. This indicates that high BCAA levels inhibit the growth and metastasis of BC, highlighting the potential of increasing BCAA dietary intake in BC treatment [5, 103]. Therefore, comprehensive research is still needed to determine whether controlling the intake of BCAA in the diet has a therapeutic effect on certain types of human cancers.
At the same time, intestinal microflora also has a significant impact on the metabolism of the body [104, 105]. CRC is a typical example. Clostridium symbiosum can selectively enrich in the tumor tissues. Specifically, through the mechanism of anaerobic fermentation, Clostridium symbiosum generates a large amount of BCAAs, including valine, leucine, and isoleucine [106]. After being absorbed in the intestine, these BCAAs promptly participate in the metabolic processes of tumor cells, thereby powerfully promoting the growth process of tumor cells. In gastrointestinal cancer, there are also studies indicating that the gut microbiota and its metabolites can regulate the development of cancer. This research area holds broad prospects in both diagnosis and treatment [107, 108].
The gut microbiota and diet stand as two pivotal factors influencing the levels of BCAAs in tumor patients. The gut microbiota synthesizes or consumes BCAAs through its metabolic activities. In contrast, diet serves as an exogenous source of BCAAs for the body. These two elements interact with each other, jointly shaping the BCAA levels in tumor patients and, in turn, exerting an impact on the occurrence and development of tumors.
Conclusion, challenges, and perspectives
BCAA possess significant significance in the occurrence and progression of tumors. The metabolism of BCAA is linked to numerous tumors, and its metabolic process is regulated by a variety of enzymes, including BCAT, BCKDH, and BCKDK. BCAT1 and BCAT2 have potential value in the diagnosis and treatment of tumors, and BCKDH and BCKDK have gradually become the focus of research as well. The mTOR pathway plays a crucial role in tumors, and mTOR inhibitors are anticipated to become anti-cancer drugs, yet they encounter issues such as side effects and drug resistance. Additionally, BCAA metabolism has a strong connection with tumor immunity.
BCAA play a complex role in tumor metabolism, with dietary BCAA exerting both promoting and inhibiting effects on tumors. In some tumors, BCAA supplementation supports cell proliferation by supplying energy and building blocks, while in others, BCAA supplementation inhibits tumor development, potentially through mechanisms like triggering cell-cycle arrest or enhancing immune-mediated anti-tumor responses. The underlying causes of these opposing effects remain unclear, mainly due to tumor-specific genetic features and microenvironment differences. This dual-characteristic distinguishes our research from traditional one-way studies. There is an urgent need for in-depth research to clarify the role of BCAA in tumor development and treatment and to pioneer new concepts for nutritional intervention in oncology, which is crucial for developing better cancer treatments.
BCAT1, a critical enzyme in BCAA metabolism, and BCKDK, which phosphorylates and inactivates BCKDH in BCAA catabolism, are both significant targets for cancer research. Suppression of BCAT1 can interfere with BCAA metabolism, thus triggering an energy crisis of tumor cells and effectively inhibiting their growth. When combined with immunotherapy, BCAT1 inhibitors may break immune tolerance. By reducing BCAA levels in the TME, the activity of immune cells, such as T cells can be enhanced, improving their ability to recognize and attack tumor cells. Meanwhile, high BCKDK expression in some tumors enables cells to adapt to the stress of chemotherapy drugs, leading to resistance [109]. Understanding how BCKDK contributes to drug resistance can guide the development of BCKDK inhibitors. Developing highly specific BCAT1 and BCKDK inhibitors could lead to more targeted and effective anti-cancer drugs with fewer side effects on normal cells. These inhibitors could also reverse drug resistance, making previously unresponsive tumor cells sensitive to chemotherapy again, thus expanding treatment options for cancer patients.
A comprehensive exploration of the metabolic mechanism of BCAA in tumors and the development of targeted drugs for related enzymes and signal pathways will help to break the limitations of existing drugs. Simultaneously, it is of great significance for clinical practice [110, 111]. We will continue to study the role of precise dietary intervention of BCAA in cancer treatment, clarify its therapeutic efficacy for specific cancers, and initiate a new model of diet-assisted cancer treatment. In addition, in-depth study on the metabolic mechanism of BCAA in different tumor types is helpful to develop precise targeted drugs, regulate tumor immune microenvironment and improve the efficacy of tumor immunotherapy. Combining metabolic regulation with immunotherapy is a great progress in cancer treatment strategy. In summary, BCAA metabolism has great potential in tumor research. We focus on accurate and integrated innovative research, which is expected to provide completely new targets and concepts for cancer treatment and bring new hope for cancer treatment.
Acknowledgements
The authors acknowledge the use of Biorender, which is used to create schematic Figures 1–5.
Abbreviations
- BCAA
Branched chain amino acid
- TME
Tumor microenvironment
- CRC
Colorectal cancer
- PDAC
Pancreatic ductal adenocarcinoma
- BCAT2
Branched chain amino acid transaminase 2
- BCKDK
Branched-chain keto-acid dehydrogenase kinase
- HCC
Hepatocellular carcinoma
- BC
Breast cancer
- NSCLC
Non-small cell lung cancer
- BCAT
BCAA aminotransferase
- BCKD
Branched-chain α-keto acid dehydrogenase
- GBM
Glioblastoma multiforme
- GC
Gastric cancer
- TFEB
Transcription factor EB
- ICC
Intrahepatic cholangiocarcinoma
- BCKDH
Branched-chain keto acid dehydrogenase
- OSCC
Oral squamous cell carcinoma
- NALA
N-acetylleucinamide
- TSC1
Tuberous Sclerosis Complex 1
- TSC2
Tuberous Sclerosis Complex 2
- GAP
GTPase activating protein
- BMS
BCAA metabolism signature
- ccRCC
Clear cell renal cell carcinoma
Author contributions
All authors listed have made substantial, direct, and intellectual contributions to the work and have approved it for publication.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82203377 to Yanwei Lu; 82473238 to Haibo Zhang), Zhejiang Natural Science Foundation of China (Grant number: LQ22H160036 to Yanwei Lu; LY24H160022 to Haibo Zhang). Zhejiang Health Science and Technology Project (2025KY031 to Haibo Zhang).
Availability of data and materials
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
As this manuscript pertains to a review article, so conventional requirements of ethical approval and participant consent are not applicable in this context.
Consent for publication
All authors grant their consent for the publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao Xiong, Ruiqi Liu and Keke Xu have contributed equally to this study.
Contributor Information
Hailong Sheng, Email: zea974606755@163.com.
Yanwei Lu, Email: luyanwei@hmc.edu.cn.
Haibo Zhang, Email: zhbdoctor@163.com.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Sideris GA, Tsaramanidis S, Vyllioti AT, Njuguna N. The role of branched-chain amino acid supplementation in combination with locoregional treatments for hepatocellular carcinoma: systematic review and meta-analysis. Cancers (Basel). 2023;15(3):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Wang W, Zhu F, Duan Q. The role of branched chain amino acids metabolic disorders in tumorigenesis and progression. Biomed Pharmacother. 2022;153: 113390. [DOI] [PubMed] [Google Scholar]
- 4.Ericksen RE, Han W. Give and take: competition for BCAAs in the tumour microenvironment. Nat Metab. 2020;2(8):657–8. [DOI] [PubMed] [Google Scholar]
- 5.Hou Y, Wu G. Nutritionally essential amino acids. Adv Nutr. 2018;9(6):849–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi R, Yao C, Chen S, Liu Y, He Y, Zhang J, et al. Elevated BCAA suppresses the development and metastasis of breast cancer. Front Oncol. 2022;12: 887257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long L, Yang W, Liu L, Tobias DK, Katagiri R, Wu K, et al. Dietary intake of branched-chain amino acids and survival after colorectal cancer diagnosis. Int J Cancer. 2021;148(10):2471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JT, Yin M, Wang D, Wang J, Lei MZ, Zhang Y, et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat Cell Biol. 2020;22(2):167–74. [DOI] [PubMed] [Google Scholar]
- 9.Lei MZ, Li XX, Zhang Y, Li JT, Zhang F, Wang YP, et al. Acetylation promotes BCAT2 degradation to suppress BCAA catabolism and pancreatic cancer growth. Signal Transduct Target Ther. 2020;5(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali I, Wani WA, Saleem K, Wesselinova D. Syntheses, DNA binding and anticancer profiles of L-glutamic acid ligand and its copper(II) and ruthenium(III) complexes. Med Chem. 2013;9(1):11–21. [DOI] [PubMed] [Google Scholar]
- 11.Ali I, Wani WA, Saleem K, Hsieh M-F. Anticancer metallodrugs of glutamic acid sulphonamides: in silico, DNA binding, hemolysis and anticancer studies. RSC Adv. 2014;4(56):29629–41. [Google Scholar]
- 12.Ali I, Wani WA, Khan A, Haque A, Ahmad A, Saleem K, et al. Synthesis and synergistic antifungal activities of a pyrazoline based ligand and its copper(II) and nickel(II) complexes with conventional antifungals. Microb Pathog. 2012;53(2):66–73. [DOI] [PubMed] [Google Scholar]
- 13.Ali I, Wani WA, Haque A, Saleem K. Glutamic acid and its derivatives: candidates for rational design of anticancer drugs. Future Med Chem. 2013;5(8):961–78. [DOI] [PubMed] [Google Scholar]
- 14.Ali I, Aboul-Enein HY, Ghanem A. Enantioselective toxicity and carcinogenesis. Curr Pharm Anal. 2005;1(1):109–25. [Google Scholar]
- 15.Ali I, Wani WA, Saleem K, Haque A. Thalidomide: a banned drug resurged into future anticancer drug. Curr Drug Therapy. 2012;7(1):13–23. [Google Scholar]
- 16.Ali I, Alsehli M, Scotti L, Scotti MT, Tsai S-T, Yu R-S, et al. Progress in polymeric nano-medicines for theranostic cancer treatment. Polymers. 2020;12(3):598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Hong JH, Huang Y, Liu S, Yin J, Deng P, et al. EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in ovarian cancer. Mol Cancer. 2023;22(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Song W, Gao Y, Zhang Y, Zhao Y, Hao S, et al. The Role of Tumor Metabolic Reprogramming in Tumor Immunity. Int J Mol Sci. 2023;24(24):17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karami Fath M, Ebrahimi M, Nourbakhsh E, Zia Hazara A, Mirzaei A, Shafieyari S, et al. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol Res Pract. 2022;237: 154010. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes HS, Silva Teixeira CS, Fernandes PA, Ramos MJ, Cerqueira NM. Amino acid deprivation using enzymes as a targeted therapy for cancer and viral infections. Expert Opin Ther Pat. 2017;27(3):283–97. [DOI] [PubMed] [Google Scholar]
- 21.Yu Z, Qiu B, Zhou H, Li L, Niu T. Characterization and application of a lactate and branched chain amino acid metabolism related gene signature in a prognosis risk model for multiple myeloma. Cancer Cell Int. 2023;23(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao CC, Sun RM, Yang Y, Zhou HY, Meng ZW, Chi R, et al. Accumulation of branched-chain amino acids reprograms glucose metabolism in CD8+ T cells with enhanced effector function and anti-tumor response. Cell Rep. 2023;42(3): 112186. [DOI] [PubMed] [Google Scholar]
- 23.Mayers JR, Torrence ME, Danai LV, Papagiannakopoulos T, Davidson SM, Bauer MR, et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science. 2016;353(6304):1161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Lin Y, Tang Y, Jiang M, Chen X, Chen H, et al. MAZ-mediated up-regulation of BCKDK reprograms glucose metabolism and promotes growth by regulating glucose-6-phosphate dehydrogenase stability in triple-negative breast cancer. Cell Death Dis. 2024;15(7):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang K, Xu C, Sun H, Xuan Z, Liu Y, Li J, et al. Branched-chain keto-acid dehydrogenase kinase regulates vascular permeability and angiogenesis to facilitate tumor metastasis in renal cell carcinoma. Cancer Sci. 2023;114(11):4270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suh EH, Hackett EP, Wynn RM, Chuang DT, Zhang B, Luo W, et al. In vivo assessment of increased oxidation of branched-chain amino acids in glioblastoma. Sci Rep. 2019;9(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Wang F, Yan G, Tong Y, Guo W, Li S, et al. CPT1A loss disrupts BCAA metabolism to confer therapeutic vulnerability in TP53-mutated liver cancer. Cancer Lett. 2024;595: 217006. [DOI] [PubMed] [Google Scholar]
- 28.Biswas D, Slade L, Duffley L, Mueller N, Dao KT, Mercer A, et al. Inhibiting BCKDK in triple negative breast cancer suppresses protein translation, impairs mitochondrial function, and potentiates doxorubicin cytotoxicity. Cell Death Discov. 2021;7(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falcone M, Maddocks ODK. The KRAS-BCAA-BCAT2 axis in PDAC development. Nat Cell Biol. 2020;22(2):139–40. [DOI] [PubMed] [Google Scholar]
- 30.Xue M, Xiao J, Jiang W, Wang Y, Zuo D, An H, et al. Loss of BCAA catabolism enhances Rab1A-mTORC1 signaling activity and promotes tumor proliferation in NSCLC. Transl Oncol. 2023;34: 101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibrahim SL, Abed MN, Mohamed G, Price JC, Abdullah MI, Richardson A. Inhibition of branched-chain alpha-keto acid dehydrogenase kinase augments the sensitivity of ovarian and breast cancer cells to paclitaxel. Br J Cancer. 2023;128(5):896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YH, Yoon SJ, Kim M, Kim HH, Song YS, Jung JW, et al. Integrative multi-omics analysis reveals different metabolic phenotypes based on molecular characteristics in thyroid cancer. Clin Cancer Res. 2024;30(4):883–94. [DOI] [PubMed] [Google Scholar]
- 33.Papathanassiu AE, Ko JH, Imprialou M, Bagnati M, Srivastava PK, Vu HA, et al. BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases. Nat Commun. 2017;8:16040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivanand S, Vander Heiden MG. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell. 2020;37(2):147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian L, Li N, Lu XC, Xu M, Liu Y, Li K, et al. Enhanced BCAT1 activity and BCAA metabolism promotes RhoC activity in cancer progression. Nat Metab. 2023;5(7):1159–73. [DOI] [PubMed] [Google Scholar]
- 36.Li GS, Huang HQ, Liang Y, Pang QY, Sun HJ, Huang ZG, et al. BCAT1: a risk factor in multiple cancers based on a pan-cancer analysis. Cancer Med. 2022;11(5):1396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimou A, Tsimihodimos V, Bairaktari E. The critical role of the branched chain amino acids (BCAAs) catabolism-regulating enzymes, branched-chain aminotransferase (BCAT) and branched-chain α-keto acid dehydrogenase (BCKD), in human pathophysiology. Int J Mol Sci. 2022;23(7):4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Peng H, Zhou M, Bao L, Wang C, Cai F, et al. Targeting BCAT1 Combined with α-Ketoglutarate Triggers Metabolic Synthetic Lethality in Glioblastoma. Cancer Res. 2022;82(13):2388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z, Gu Z, Wang L, Guan Y, Lyu Y, Zhang J, et al. Nuclear translocation of LDHA promotes the catabolism of BCAAs to sustain GBM cell proliferation through the TxN antioxidant pathway. Int J Mol Sci. 2023;24(11):9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu X, Zhan PP, Sun LX, Yu L, Liu J, Sun LC, et al. BCAT1 activates PI3K/AKT/mTOR pathway and contributes to the angiogenesis and tumorigenicity of gastric cancer. Front Cell Dev Biol. 2021;9: 659260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bueno De Paiva L, Aline Bernusso V, Machado-Neto JA, Traina F, Ridley AJ, Olalla-Saad ST, et al. Effects of RhoA and RhoC upon the sensitivity of prostate cancer cells to glutamine deprivation. Small GTPases. 2021;12(1):20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Wang J, Zhou K, Lv J, Wang L, Gao S, et al. Cytotoxic necrotizing factor 1 promotes bladder cancer angiogenesis through activating RhoC. FASEB J. 2020;34(6):7927–40. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Li Y, Fan L, Wang B, Liu W, Cui J, et al. LncRNA THUMPD3-AS1 promotes invasion and EMT in gastric cancer by regulating the miR-1297/BCAT1 pathway. iScience. 2023;26(9): 107673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T, Hu Q, Li B, Fan G, Jing D, Xu J, et al. Transcription factor EB reprograms branched-chain amino acid metabolism and promotes pancreatic cancer progression via transcriptional regulation of BCAT1. Cell Prolif. 2024;27: e13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laven-Law G, Kichenadasse G, Young GP, Symonds EL, Winter JM. BCAT1, IKZF1 and SEPT9: methylated DNA biomarkers for detection of pan-gastrointestinal adenocarcinomas. Biomarkers. 2024;29(4):194–204. [DOI] [PubMed] [Google Scholar]
- 46.Patrick M, Gu Z, Zhang G, Wynn RM, Kaphle P, Cao H, et al. Metabolon formation regulates branched-chain amino acid oxidation and homeostasis. Nat Metab. 2022;4(12):1775–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knerr I, Colombo R, Urquhart J, Morais A, Merinero B, Oyarzabal A, et al. Expanding the genetic and phenotypic spectrum of branched-chain amino acid transferase 2 deficiency. J Inherit Metab Dis. 2019;42(5):809–17. [DOI] [PubMed] [Google Scholar]
- 48.Tian Y, Ma J, Wang H, Yi X, Wang H, Zhang H, et al. BCAT2 promotes melanoma progression by activating lipogenesis via the epigenetic regulation of FASN and ACLY expressions. Cell Mol Life Sci. 2023;80(11):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Z, Zheng J, Liu J, Yang X, Chen K. Novel branched-chain amino acid-catabolism related gene signature for overall survival prediction of pancreatic carcinoma. J Proteome Res. 2022;21(3):740–6. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Cho YR, Kim JH, Kim J, Nam HY, Kim SW, et al. Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism. Exp Mol Med. 2019;51(11):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li JT, Li KY, Su Y, Shen Y, Lei MZ, Zhang F, et al. Diet high in branched-chain amino acid promotes PDAC development by USP1-mediated BCAT2 stabilization. Natl Sci Rev. 2021;9(5): nwab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu ZY, Huang RH. Integrating single-cell RNA-sequencing and bulk RNA-sequencing data to explore the role of mitophagy-related genes in prostate cancer. Heliyon. 2024;10(9): e30766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong B, Xu JY, Huang Y, Guo J, Dong Q, Wang Y, et al. Integrative proteogenomic profiling of high-risk prostate cancer samples from Chinese patients indicates metabolic vulnerabilities and diagnostic biomarkers. Nat Cancer. 2024;5(9):1427–47. [DOI] [PubMed] [Google Scholar]
- 54.Kitagawa A, Osawa T, Noda M, Kobayashi Y, Aki S, Nakano Y, et al. Convergent genomic diversity and novel BCAA metabolism in intrahepatic cholangiocarcinoma. Br J Cancer. 2023;128(12):2206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antanavičiūtė I, Mikalayeva V, Ceslevičienė I, Milašiūtė G, Skeberdis VA, Bordel S. Transcriptional hallmarks of cancer cell lines reveal an emerging role of branched chain amino acid catabolism. Sci Rep. 2017;7(1):7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss M, Hettrich S, Hofmann T, Hachim S, Günther S, Braun T, et al. Mitolnc controls cardiac BCAA metabolism and heart hypertrophy by allosteric activation of BCKDH. Nucleic Acids Res. 2024;52(11):6629–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson D, Eichler SJ, Yiew NKH, Colca JR, Cho K, Patti GJ, et al. Mitochondrial pyruvate carrier inhibition initiates metabolic crosstalk to stimulate branched chain amino acid catabolism. Mol Metab. 2023;70: 101694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimm M, Calgéer B, Teriete P, Biegner T, Munz A, Reinert S. Targeting thiamine-dependent enzymes for metabolic therapies in oral squamous cell carcinoma? Clin Transl Oncol. 2016;18(2):196–205. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Z, Achreja A, Meurs N, Animasahun O, Owen S, Mittal A, et al. Tumour-reprogrammed stromal BCAT1 fuels branched-chain ketoacid dependency in stromal-rich PDAC tumours. Nat Metab. 2020;2(8):775–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian Q, Yuan P, Quan C, Li M, Xiao J, Zhang L, et al. Phosphorylation of BCKDK of BCAA catabolism at Y246 by Src promotes metastasis of colorectal cancer. Oncogene. 2020;39(20):3980–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Yu D, Li L, Xiao J, Zhu Y, Liu Y, et al. BCKDK promotes ovarian cancer proliferation and migration by activating the MEK/ERK signaling pathway. J Oncol. 2022;22(2022):3691635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu C, Yang K, Xuan Z, Li J, Liu Y, Zhao Y, et al. BCKDK regulates breast cancer cell adhesion and tumor metastasis by inhibiting TRIM21 ubiquitinate talin1. Cell Death Dis. 2023;14(7):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Xiao J, Jiang W, Zuo D, Wang X, Jin Y, et al. BCKDK alters the metabolism of non-small cell lung cancer. Transl Lung Cancer Res. 2021;10(12):4459–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.East MP, Laitinen T, Asquith CRM. BCKDK: an emerging kinase target for metabolic diseases and cancer. Nat Rev Drug Discov. 2021;20(7):498. [DOI] [PubMed] [Google Scholar]
- 65.Bowman CE, Neinast MD, Jang C, Patel J, Blair MC, Mirek ET, et al. Off-target depletion of plasma tryptophan by allosteric inhibitors of BCKDK. bioRxiv [Preprint]. 2024 Mar 10:2024.03.05.582974. [DOI] [PMC free article] [PubMed]
- 66.Marafie SK, Al-Mulla F, Abubaker J. mTOR: its critical role in metabolic diseases, cancer, and the aging process. Int J Mol Sci. 2024;25(11):6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe R, Wei L, Huang J. mTOR signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med. 2011;52(4):497–500. [DOI] [PubMed] [Google Scholar]
- 68.Basnet R, Bahadur Basnet B, Gupta R, Basnet T, Khadka S, Shan AM. Mammalian target of rapamycin (mTOR) signalling pathway-a potential target for cancer intervention: a short overview. Curr Mol Pharmacol. 2024;17(1): e310323215268. [DOI] [PubMed] [Google Scholar]
- 69.Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122(3):489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin SB, Reiche WS, Fifelski NA, Schultz AJ, Stanford SJ, Martin AA, et al. Leucine and branched-chain amino acid metabolism contribute to the growth of bone sarcomas by regulating AMPK and mTORC1 signaling. Biochem J. 2020;477(9):1579–99. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Ou Y, Luo R, Wang J, Wang D, Guan J, et al. SAR1B senses leucine levels to regulate mTORC1 signalling. Nature. 2021;596(7871):281–4. [DOI] [PubMed] [Google Scholar]
- 72.Ma L, Zhang R, Li D, Qiao T, Guo X. Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chem Biol Interact. 2021;1(349): 109659. [DOI] [PubMed] [Google Scholar]
- 73.Xu Y, Yu W, Yang T, Zhang M, Liang C, Cai X, et al. Overexpression of BCAT1 is a prognostic marker in gastric cancer. Hum Pathol. 2018;75:41–6. [DOI] [PubMed] [Google Scholar]
- 74.Gao LZ, Wang JQ, Chen JL, Zhang XL, Zhang MM, Wang SL, et al. CDK12 promotes the proliferation, migration, and angiogenesis of gastric carcinoma via activating the PI3K/AKT/mTOR signaling pathway. Appl Biochem Biotechnol. 2023;195(11):6913–26. [DOI] [PubMed] [Google Scholar]
- 75.Zanini S, Renzi S, Giovinazzo F, Bermano G. mTOR pathway in gastroenteropancreatic neuroendocrine tumor (GEP-NETs). Front Endocrinol (Lausanne). 2020;16(11): 562505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng L, Li B, Xi Y, Cai M, Tian Z. Aerobic exercise and resistance exercise alleviate skeletal muscle atrophy through IGF-1/IGF-1R-PI3K/Akt pathway in mice with myocardial infarction. Am J Physiol Cell Physiol. 2022;322(2):C164–76. [DOI] [PubMed] [Google Scholar]
- 77.García-Mato Á, Cervantes B, Rodríguez-de la Rosa L, Varela-Nieto I. IGF-1 controls metabolic homeostasis and survival in HEI-OC1 auditory cells through AKT and mTOR signaling. Antioxidants (Basel). 2023;12(2):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen G, Chen H, Ren S, Xia M, Zhu J, Liu Y, et al. Aberrant DNA methylation of mTOR pathway genes promotes inflammatory activation of immune cells in diabetic kidney disease. Kidney Int. 2019;96(2):409–20. [DOI] [PubMed] [Google Scholar]
- 79.Torrence ME, MacArthur MR, Hosios AM, Valvezan AJ, Asara JM, Mitchell JR, et al. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. Elife. 2021;1(10): e63326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Luo M, Wang F, Tong Y, Li L, Shu Y, et al. AMPK induces degradation of the transcriptional repressor PROX1 impairing branched amino acid metabolism and tumourigenesis. Nat Commun. 2022;13(1):7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan LY, Qin X, Li L, Zhou J, Zhou M, Li X, et al. Overexpression of LINC00037 represses cervical cancer progression by activating mTOR signaling pathway. J Cell Physiol. 2019;234(8):13353–60. [DOI] [PubMed] [Google Scholar]
- 82.Murugan AK, Liu R, Xing M. Identification and characterization of two novel oncogenic mTOR mutations. Oncogene. 2019;38(26):5211–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Divé I, Klann K, Michaelis JB, Heinzen D, Steinbach JP, Münch C, et al. Inhibition of mTOR signaling protects human glioma cells from hypoxia-induced cell death in an autophagy-independent manner. Cell Death Discov. 2022;8(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lai M, Zou W, Han Z, Zhou L, Qiu Z, Chen J, et al. Tsc1 regulates tight junction independent of mTORC1. Proc Natl Acad Sci U S A. 2021;118(30): e2020891118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liao WT, Chiang YJ, Yang-Yen HF, Hsu LC, Chang ZF, Yen JJY. CBAP regulates the function of Akt-associated TSC protein complexes to modulate mTORC1 signaling. J Biol Chem. 2023;299(12): 105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Göktuna Sİ. IKBKE inhibits TSC1 to activate the mTOR/S6K pathway for oncogenic transformation. Turk J Biol. 2018;42(4):268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pal SK, Figlin RA. Treatment options in metastatic renal cell carcinoma: focus on mTOR inhibitors. Clin Med Insights Oncol. 2010;4:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blagosklonny MV. Rapamycin for longevity: opinion article. Aging (Albany NY). 2019;11(19):8048–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee L, Ito T, Jensen RT. Everolimus in the treatment of neuroendocrine tumors: efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin Pharmacother. 2018;19(8):909–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Srivastava RK, Li C, Khan J, Banerjee NS, Chow LT, Athar M. Combined mTORC1/mTORC2 inhibition blocks growth and induces catastrophic macropinocytosis in cancer cells. Proc Natl Acad Sci U S A. 2019;116(49):24583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Z, Fan Q, Luo X, Lou K, Weiss WA, Shokat KM. Brain-restricted mTOR inhibition with binary pharmacology. Nature. 2022;609(7928):822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao X, Zhao F, Wang Y, Ma X, Chai H, Han J, et al. Discovery of novel hybrids of mTOR inhibitor and NO donor as potential anti-tumor therapeutics. Bioorg Med Chem. 2023;15(91): 117402. [DOI] [PubMed] [Google Scholar]
- 93.Rostamzadeh D, Haghshenas MR, Samadi M, Mojtahedi Z, Babaloo Z, Ghaderi A. Immunosuppressive effects and potent anti-tumor efficacy of mTOR inhibitor everolimus in breast tumor-bearing mice. Iran J Allergy Asthma Immunol. 2022;21(3):287–99. [DOI] [PubMed] [Google Scholar]
- 94.Cai Z, Wang J, Li Y, Shi Q, Jin L, Li S, et al. Overexpressed Cyclin D1 and CDK4 proteins are responsible for the resistance to CDK4/6 inhibitor in breast cancer that can be reversed by PI3K/mTOR inhibitors. Sci China Life Sci. 2023;66(1):94–109. [DOI] [PubMed] [Google Scholar]
- 95.Bhin J, Yemelyanenko J, Chao X, Klarenbeek S, Opdam M, Malka Y, et al. MYC is a clinically significant driver of mTOR inhibitor resistance in breast cancer. J Exp Med. 2023;220(11): e20211743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Q, Zhu X, Huang P, Li C, Han L, Han Y, et al. BCKDK modification enhances the anticancer efficacy of CAR-T cells by reprogramming branched chain amino acid metabolism. Mol Ther. 2024;32(9):3128–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cai Z, Chen J, Yu Z, Li H, Liu Z, Deng D, et al. BCAT2 shapes a noninflamed tumor microenvironment and induces resistance to anti-PD-1/PD-L1 immunotherapy by negatively regulating proinflammatory chemokines and anticancer immunity. Adv Sci (Weinh). 2023;10(8): e2207155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Wispelaere W, Annibali D, Tuyaerts S, Messiaen J, Antoranz A, Shankar G, et al. PI3K/mTOR inhibition induces tumour microenvironment remodelling and sensitises pS6high uterine leiomyosarcoma to PD-1 blockade. Clin Transl Med. 2024;14(5): e1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng J, Liu Y, Wang J, Shi J, Li L, Jiang X, et al. Integrated single-cell and bulk characterization of branched chain amino acid metabolism-related key gene BCAT1 and association with prognosis and immunogenicity of clear cell renal cell carcinoma. Aging (Albany NY). 2024;16(3):2715–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aghapour SA, Torabizadeh M, Bahreiny SS, Saki N, Jalali Far MA, Yousefi-Avarvand A, et al. Investigating the dynamic interplay between cellular immunity and tumor cells in the fight against cancer: an updated comprehensive review. IJBC. 2024;16(2):84–101. [Google Scholar]
- 101.Takegoshi K, Honda M, Okada H, Takabatake R, Matsuzawa-Nagata N, Campbell JS, et al. Branched-chain amino acids prevent hepatic fibrosis and development of hepatocellular carcinoma in a non-alcoholic steatohepatitis mouse model. Oncotarget. 2017;8(11):18191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang ZR, Jiang S, Han JX, Gao Y, Xie Y, Chen J, et al. Deficiency of BCAT2-mediated branched-chain amino acid catabolism promotes colorectal cancer development. Biochim Biophys Acta Mol Basis Dis. 2024;1870(2): 166941. [DOI] [PubMed] [Google Scholar]
- 103.Şahin TÖ, Yilmaz B, Yesilyurt N, Cicia D, Szymanowska A, Amero P, et al. Recent insights into the nutritional immunomodulation of cancer-related microRNAs. Phytother Res. 2023;37(10):4375–97. [DOI] [PubMed] [Google Scholar]
- 104.Güler MS, Arslan S, Ağagündüz D, Cerqua I, Pagano E, Berni Canani R, et al. Butyrate: a potential mediator of obesity and microbiome via different mechanisms of actions. Food Res Int. 2025;199: 115420. [DOI] [PubMed] [Google Scholar]
- 105.Açar Y, Ağagündüz D, De Cicco P, Capasso R. Flavonoids: their putative neurologic roles, epigenetic changes, and gut microbiota alterations in Parkinson’s disease. Biomed Pharmacother. 2023;168: 115788. [DOI] [PubMed] [Google Scholar]
- 106.Ren YM, Zhuang ZY, Xie YH, Yang PJ, Xia TX, Xie YL, et al. BCAA-producing Clostridium symbiosum promotes colorectal tumorigenesis through the modulation of host cholesterol metabolism. Cell Host Microbe. 2024;32(9):1519-1535.e7. [DOI] [PubMed] [Google Scholar]
- 107.Ağagündüz D, Çelik E, Cemali Ö, Bingöl FG, Özenir Ç, Özoğul F, et al. Probiotics, live biotherapeutic products (LBPs), and gut-brain axis related psychological conditions: implications for research and dietetics. Probiotics Antimicrob Proteins. 2023;15(4):1014–31. [DOI] [PubMed] [Google Scholar]
- 108.Ağagündüz D, Cocozza E, Cemali Ö, Bayazıt AD, Nanì MF, Cerqua I, et al. Understanding the role of the gut microbiome in gastrointestinal cancer: a review. Front Pharmacol. 2023;14:1130562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rajabi L, Ebrahimdoost M, Mohammadi SA, Soleimani Samarkhazan H, Khamisipour G, Aghaei M. Aqueous and ethanolic extracts of Moringa oleifera leaves induce selective cytotoxicity in Raji and Jurkat cell lines by activating the P21 pathway independent of P53. Mol Biol Rep. 2025;52(1):102. [DOI] [PubMed] [Google Scholar]
- 110.Aghaei M, Khademi R, Bahreiny SS, Saki N. The need to establish and recognize the field of clinical laboratory science (CLS) as an essential field in advancing clinical goals. Health Sci Rep. 2024;7(8): e70008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saki N, Haybar H, Aghaei M. Subject: motivation can be suppressed, but scientific ability cannot and should not be ignored. J Transl Med. 2023;21(1):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.