Abstract
Background
Toxoplasma gondii is a widespread parasite that can infect almost all vertebrate species including humans, causing variable clinical symptoms from asymptomatic infection to serious diseases. Though extensive research has been done in recent decades, the prevention and control of T. gondii continue to present substantial challenges. Herbal medicines have long been a rich source of chemical entities and may provide new avenues for drug discovery against T. gondii. Thus, this study was performed to investigate the anti-T. gondii effect of two monomers, beta, beta-dimethylacrylshikonin (DMAS) and isobutyrylshikonin (IBS), extracted from the roots of a widely distributed and used medical plant.
Methods
The cytotoxicity of DMAS and IBS on Vero cells was evaluated using the MTT assay, and the toxicity in mice was assessed on the basis of the changes of body weight combined with the histopathologic examinations on spleen, liver, and kidney. The effects of DMAS and IBS on mice against T. gondii acute infection were evaluated by combining survival curves with splenic histopathologic examination. Ultrastructural change in T. gondii tachyzoites post co-incubation in vitro was observed by electron microscopy. ACT1-quantitative polymerase chain reaction (qPCR) was conducted to quantify T. gondii tachyzoites, including proliferation and the inhibitory efficacy of DMAS and IBS. Invasion and attachment, intracellular proliferation, and parasitophorous vacuole viability evaluations were conducted to assess the effects on the asexual life cycle of T. gondii. In addition, untargeted metabolomics analysis was performed to clarify the underlying mechanisms by which DMAS and IBS act against this parasite.
Results
Both DMAS and IBS, with higher half-maximal cytotoxic concentration (CC50) values, exhibited concentration-dependent cytotoxicity in Vero cells and significantly inhibited the intracellular proliferation of T. gondii in vitro, showing lower half-maximal inhibitory concentration (IC50) values and higher selectivity index (SI) values. DMAS showed a statistically more potent effect than IBS, but both were not significantly more potent than that of pyrimethamine (PM). The tachyzoites exhibited severe ultrastructural damage following treatment with DMAS or IBS. Metabolomics analysis indicated that this abnormal biological lesion was caused by the disruptions in purine and pyrimidine metabolism pathways in T. gondii, with mechanisms likely differing from that of PM. In vivo, a dose of 1.5 mg/kg of DMAS showed no significant toxicity in Kunming (KM) mice, with no significant pathological damage or weight loss. At this dosage, both DMAS and IBS significantly alleviated the splenic hyperemia and statistically prolonged the survival times of T. gondii-infected mice.
Conclusions
This study demonstrated that DMAS and IBS have an inhibitory effect on T. gondii infection in vitro and in vivo, probably associated with the disruption of nucleotide metabolism in the parasite. These results highlight that the two monomers, in particular DMAS, hold promise as a potential therapeutic medicine for toxoplasmosis.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-025-06865-1.
Keywords: Toxoplasma gondii; Beta, beta-dimethylacrylshikonin; Isobutyrylshikonin; Anti-infection; Metabolomics
Background
Toxoplasma gondii, the obligate intracellular Apicomplexan protozoan responsible for the common parasitic disease toxoplasmosis, has been continuously researched for more than 100 years [1]. Over 300 genotypes of T. gondii have been identified, including strains belonging to clonal lineages, such as RH and GT1 (type I); PRU, PTG, and ME49 (type II); and CTG and VEG (type III) strains [2]. Though both clonal lineages and other strains of T. gondii can infect virtually all vertebrate species, including humans, the clonal lineages are still standard for uncovering the epidemiological characteristics and pathogenic mechanisms of this parasite. It has been verified that the virulence of T. gondii, especially in vivo, is closely associated with the immune state of hosts and the genotype of infecting strains [3, 4]. Generally, humans are extensively affected by the oocysts in feline feces, the bradyzoites in tissue cysts, and the tachyzoites of T. gondii through ingestion of raw or undercooked meats and vegetables, consumption of contaminated water, or congenitally exposed from an infected mother, and exhibit distinct clinical symptoms ranging from asymptomatic states in immune-competent individuals to serious diseases, especially in immunocompromised patients and fetuses [4, 5].
Despite the substantial efforts that have been made to prevent and control this parasite, including the identification of vaccine candidates and evaluation of therapeutic compounds including pyrimethamine (PM) and sulfadiazine, these interventions have shown limited effectiveness, durability, and safety [6–8]; therefore, it remains pressing for further research to identify more effective and safer treatments. As a gift, plants have existed on the planet for many thousand years, and a number of them, including Chinese herbal medicines, have been recognized for their therapeutic properties for human diseases, e.g., artemisinin against malaria [9]. Though some plants and their natural products have limited efficacy in complex human illnesses, such as cancer, diabetes, encephalopathy, and autoimmune disorders, they still continuously serve as an important treasury for chemical entities to sustain drug discovery in the clinic [10]. Chinese herbal medicines, which have evolved over millennia through empirical practices aimed at immune modulation and pathogen control, contain abundant complex compositions and important biomedical information, awaiting further elucidation through advanced scientific techniques and modern research approaches [11].
In recent years, metabolomics, especially when combined with liquid chromatography/mass spectrometry (LC/MS) and advanced data conversion and multivariant analyses, has become an increasingly valuable tool for identifying potential biomarkers and understanding the mechanisms of pathogen infection, including those caused by T. gondii and other parasites such as Trypanosoma cruzi [12, 13]. Therefore, we conducted research by using T. gondii PRU tachyzoites (type II, a common genotype causing human infection) to assess the therapeutic potential of two herbal monomers, namely beta, beta-dimethylacrylshikonin (DMAS) and isobutyrylshikonin (IBS), derived from Lithospermum erythrorhizon (Boraginaceae), a plant mainly distributed in East Asia, and a satisfactory result was initially demonstrated in vitro [4, 14]. To further elucidate the mechanisms underlying their activity against T. gondii, we employed untargeted ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS), alongside potency evaluations in vitro and in Kunming (KM) mice (a frequently used animal model susceptible to T. gondii infection [15, 16]), to compare their effects with those of PM. This study aimed to evaluate the therapeutic potential of DMAS and IBS derived from L. erythrorhizon against T. gondii in vitro and in vivo, and to explore the underlying mechanisms of their anti-T. gondii activity.
Methods
Mice
Six-week-old female KM mice (33–35 g) purchased from SPF (Beijing) Biotechnology Co., Ltd., were raised in well-ventilated cages with ad libitum access to food and sterilized water. Mice were maintained under standard condition at a temperature of 18–22 °C, a relative humidity of 50–60%, and a 12 h light/dark cycle with a day–night reversed pattern.
Parasites, host cells, and monomers
The tachyzoites of the T. gondii PRU strain (type II) was used in the present study. Proliferative African green monkey kidney cells (Vero; ATCC, Maryland, USA) were used for maintaining T. gondii, as well as for cytotoxicity and potency evaluation of monomers in vitro, electron microscopy, and untargeted metabolomics analysis. Confluent monolayer human foreskin fibroblasts (HFF; ATCC) were used for assessing the effects of monomers on the asexual life cycle of T. gondii. CF-1 mouse embryonic fibroblasts (MEFs; CoBioer Biosciences Co., Ltd., Nanjing, China) were used for evaluation of parasitophorous vacuole (PV) viability. These parasites and host cells were stored at the Guangxi Key Laboratory of Brain and Cognitive Neuroscience in Guilin Medical University [17–19]. Two monomers, DMAS (HPLC ≥ 98%; Solarbio® Life Sciences, Beijing, China) and IBS (HPLC ≥ 98%; Yuanye Bio-Technology, Shanghai, China), along with PM (Sigma-Aldrich, Basel, Switzerland), were each dissolved using cell culture grade dimethyl sulfoxide (DMSO, Solarbio® Life Sciences) to make 10 mg/mL stock solutions, according to the manufacturers′ guidance. The chemical structures of DMAS and IBS are shown in Additional file 1: Fig. S1.
Cytotoxicity assay of monomers on Vero cells
Cytotoxicity of DMAS, IBS, PM, and DMSO was assessed on Vero cells using the MTT assay. Briefly, following three generations of growth, Vero cells (~5.6 × 104 cells/mL, 90 μL/well) were plated into 96-well culture plates (Thermo Fisher Scientific, MA, USA) and incubated at 37 °C in a 5% CO2 atmosphere overnight. A top gradient of 0.4 mg/mL (~1.08 mM for DMAS, ~1.12 mM for IBS, and ~1.61 mM for PM) was prepared by diluting 3.2 μL of each drug stock solution (10 mg/mL) into 76.8 μL of Dulbecco’s modified Eagle medium (DMEM, Gibco, CA, USA). This solution was serially diluted twofold in separate Eppendorf (EP) tubes using blank DMEM as the diluent. Blank DMEM served as the zero gradient. The DMSO control group was prepared with a top gradient of 4% (v/v) of DMSO/DMEM, the content of which was the same as that of the drug experimental groups. After incubation, 10 μL of each dilution (including the zero gradient) was added into the 96-well plates, and the cells were incubated in a cell incubator for 32 h. Then, 50 μL 1 × MTT (Jiancheng, Nanjing, China) was added and mixed with soft shaking, and the cells were continuously incubated for 4 h. After removing the supernatant, 150 μL of DMSO was added to dissolve the formed formazan using an oscillator. The absorbance was measured at 570 nm using a microplate reader (Bio-Rad, CA, USA). Subsequently, half-maximal cytotoxic concentrations (CC50) of the drugs were calculated. All the experiments, including the DMSO control, were performed in triplicate.
Monomer potency on Vero cells
After thawing and subsequently passaging three generations in T25 cell flasks (Thermo Fisher Scientific), Vero cells (~5 × 103 cells/mL, 0.5 mL/well) were plated into 24-well culture plates (Thermo Fisher Scientific) and incubated at 37 °C in an atmosphere of 5% CO2/air for 24 h using DMEM containing 10% fetal bovine serum (FBS, Invitrogen, CA, USA). Afterward, the cells/well were infected with the purified PRU tachyzoites (MOI = 1). MOI indicates multiplicity of infection. Two hours later, the cells were washed three times using blank medium, and 1 mL of 2% FBS/DMEM containing 10 μg/mL DMAS, IBS, PM, or isopycnic DMSO was added. FBS/DMEM (2%) served as a control. After incubation for 72 h in a cell incubator with an interval of 4 h for static culture, the cells were collected for DNA extraction. The number of PRU tachyzoites calculated by ACT1-qPCR (based on a previous study [20]) was used to evaluate the potency of the monomers in comparison with PM. All the experiments, including controls, were performed in triplicate.
Determination of half-maximal inhibitory concentration (IC50)
The inhibitory efficacy of DMAS, IBS, and PM on T. gondii was assessed by determining their IC50 values, and the experiments were performed as outlined above, following the procedure of monomer potency on Vero cells with some modifications. In brief, the Vero cells in 24-well plates were infected with the purified T. gondii PRU tachyzoites (MOI = 1). Two hours later, the well was washed and filled with 1 mL of 2% FBS/DMEM containing five different gradients of DMAS, IBS, or PM. The five gradient concentrations were 10, 7.5, 5.63, 4.22, and 3.16 μg/mL for IBS; 8, 4, 2, 1, and 0.5 μg/mL for DMAS; and 1, 0.5, 0.25, 0.13, and 0.06 μg/mL for PM, respectively. Blank DMEM served as the zero gradient. After 72 h of incubation, the DNA extraction and ACT1-qPCR were performed to quantify tachyzoites [20]. The experiments were conducted in triplicate. Moreover, the selectivity index (SI), calculated as the ratio of CC50 to IC50 (CC50/IC50), was used to measure the therapeutic window of drugs in the assay system [21, 22].
Toxicity evaluation of monomers in vivo
Female KM mice were adapted to the standard animal housing for 3 days before treatment. On the basis of a previous report [23], mice were administered intraperitoneal injections of 0.1 mL of 1.5 mg/kg DMAS, IBS, PM, or isopycnic vehicle once daily for 3 days. The vehicle was composed of DMSO, PEG300, Tween-80, and saline (Solarbio® Life Sciences). Another three mice without any treatment served as control. At 1 day post the last injection, body weight changes of the mice, including the control group, were measured, and the histopathologic analyses of spleen, liver, and kidney were performed, based on H&E staining, to evaluate the toxicity of DMAS, IBS, and PM in vivo.
Efficacy of anti-T. gondii infection in KM mice
Following adaptation, mice were infected with 5 × 104 T. gondii PRU tachyzoites per mouse through intraperitoneal injection. At 6 h post-infection, the mice were separated into five groups (ten mice per group) and were administered intraperitoneal treatment once daily for 3 days with 0.1 mL of 1.5 mg/kg DMAS, IBS, PM, isopycnic vehicle, or without any treatment (control). At 1 day post the final administration, three mice from each group were euthanized for splenic histopathologic analysis. The remaining seven mice in each group were monitored to record the survival time until all experimental animals had reached their humane endpoints.
Invasion and attachment
All of the T. gondii tachyzoites collected from HFF cells at 2 h post-infection were used to evaluate the impact of DMAS, IBS, and PM on the invasion and attachment of this parasite. In brief, 5 mL of 2% FBS/DMEM containing 2.7 × 105 purified tachyzoites of the T. gondii PRU strain, along with 35 μg of DMAS, IBS, or PM, or isopycnic DMSO, were added into a HFF cell-plated T25 cell flask (MOI = 1). Blank DMEM served as control. At 2 h post-infection, the cultures were digested with 0.25% trypsin (Solarbio® Life Sciences), collected by centrifugation at 2500 rpm for 5 min, and softly washed three times using sterile phosphate-buffered saline (PBS). Then, the cultures were equally divided into two parts: one part was crushed using a 27G needle to calculate the total number of parasites, including both intracellular and extracellular tachyzoites, while the other part was left intact to assess only the extracellular tachyzoites. To quantify the parasites, a HRP-linked T. gondii polyclonal antibody (incubation with cultures at a 1:250 dilution at 37 °C for 1 h, Invitrogen) and a 3,3',5,5'-tetramethylbenzidine (TMB) substrate color development kit (mlbio, shanghai, China) were used, according to the manufacturers′ protocol, to generate the standard curves based on OD450 values. All experiments were performed in triplicate and completed within 4 h of initiation.
Intracellular proliferation
To evaluate effect of the monomers on the intracellular proliferation of the T. gondii PRU strain, the number of tachyzoites in a PV formed in the HFF cells was counted at 48 h post-infection, according to previous protocols [18, 24] with some modifications. In brief, HFF cells growing on glass coverslips in a 24-well plate were co-incubated with the same number of parasites (MOI = 1) and 7 μg/mL of DMAS, IBS, PM, or isopycnic DMSO at 37 °C in an atmosphere of 5% CO2/air for 48 h. After incubation, the coverslips were stained with Giemsa dye (Solarbio® Life Sciences) and the number of tachyzoites in at least 50 PVs was counted in each experiment. All experiments were performed in triplicate.
Evaluation of parasitophorous vacuole viability
To assess the effect of DMAS, IBS, and PM on the egress of T. gondii tachyzoites in vitro, a PV viability assay was conducted according to a previous report [19]. CF-1 MEF cells were seeded into a 24-well plate at a density of 1 × 105 cells per well and reached confluence overnight. The MEF cells were then stimulated with 200 IU/mL interferon (IFN)-γ (Dalian Bergolin Biotechnology Co., Ltd., China) 24 h prior to infection, and the cells without IFN-γ served as the non-activated control. MEF cells in all wells were infected with the same number of T. gondii PRU tachyzoites (MOI = 1), and egress plaques (EPs) were let to develop for 4 days. At 4 h before the end of experiment, 7 μg/mL of DMAS, IBS, PM, or isopycnic DMSO was added to evaluate their impact on PV viability. Blank DMEM served as control. The number of EPs in at least ten random fields of each well was counted microscopically. The percentage of PV viability was calculated by comparing the number of EPs in the IFN-γ-activated group to those in the non-activated control group.
Electron microscope observations
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to assess the effects of monomers on the structural morphology and internal ultrastructure of T. gondii tachyzoites on Vero cells. In brief, the Vero cells were grown on glass coverslips in a 24-well plate for SEM or in a T25 cell flask for TEM analysis, and were co-incubated with the same number of T. gondii PRU tachyzoites (MOI = 1) and 7 μg/mL DMAS or IBS, 2 μg/mL PM, or isopycnic DMSO for 36 h at 37 °C in an atmosphere of 5% CO2/air. Blank DMEM served as the control. The SEM and TEM protocols were conducted according to our previous report [25].
Sample preparation for untargeted metabolomics
In brief, Vero cells, revived by three serial generations, were plated into T75 cell flasks (Thermo Fisher Scientific). After incubation for 24 h in a cell incubator, the cells were infected with same number of the purified wild-type PRU tachyzoites (MOI = 1). Two hour later, the cells were washed and 10 mL of 2% FBS/DMEM containing 0.1% (v/v) of DMSO and 1% (m/v) of DMAS or IBS were added. The FBS/DMEM containing isopycnic DMSO but without monomer served as control. The incubation conditions were the same as that for monomer potency evaluation. Following incubation, the cells were collected using a cell scraper, totally removed into a new 15 mL EP tube, and stored at −80 °C for the following LC–MS/MS analysis. The experiments, including DMSO control, were performed in sextuplicate.
Metabolite extraction
The sample was transferred into a new EP tube three times using 1 mL of methanol (67-56-1; CNW Technologies, Germany), acetonitrile (75-05-8; CNW Technologies), and water (2:2:1). After vortexing for 30 s, the sample was homogenized thrice with porcelain beads using a TissueLyser (Shanghai Jingxin Industrial Development Co., Ltd., China) at 45 Hz for 4 min and an Ultrasonic Apparatus (Leidebang Electronics [Shenzhen] Co., Ltd., China) for 5 min under ice water incubation. Afterward, the sample was stored at −20 °C for 1 h and centrifuged at 12,000 rpm at 4 °C for 15 min to precipitate proteins. The supernatant (800 μL) was collected into a new tube and dried in a vacuum concentrator without heating. The extracts were redissolved in 100 μL of acetonitrile and water (1:1). After vortex mixing for 30 s and sonication for 10 min with an ice bath, the sample was again centrifuged at 12,000 rpm at 4 °C for 15 min. In total, 10 μL of supernatant was taken from each tube and pooled as the quality control (QC) sample, and 60 μL was transferred into a fresh LC/MS glass vial for the further UHPLC-QTOF-MS analysis. QC samples were analyzed in quintuplicate.
LC–MS/MS analysis
LC–MS/MS analyses were performed using an UHPLC system (1290; Agilent Technologies, CA, USA) equipped with an ACQUITY UPLC BEH Amide column (1.7 μm, 2.1 mm × 100 mm; Waters, MA, USA) coupled to Triple TOF 6000 mass spectrometer (QTOF, AB Sciex, MA, USA). The flow rate was 500 μL/min, and the mobile phase consisted of solvent A (25 mM NH4OAc and 25 mM NH4OH in water, pH = 9.75) and solvent B (acetonitrile), and was carried out with an elution gradient as follows: 5% A + 95% B for 0, 0.5, 9.1, and 12 min; 35% A + 65% B for 7 min; and 60% A + 40% B for 8 and 9 min. The injection volume was 2 μL for both positive electrospray ionization (ESI+) and negative electrospray ionization (ESI−) modes. The QTOF mass spectrometer was used to acquire MS/MS spectra based on an information-dependent basis (IDA) during the LC/MS experiment. In this mode, the acquisition software (Analyst TF 1.7; AB Sciex) was used to continuously evaluate the full scan survey MS data as it collects and triggers the acquisition of MS/MS spectra according to the preselected criteria. In each cycle, twelve kinds of precursor ions, the intensity of which were greater than 100, were chosen for fragmentation at 30 eV collision energy (CE) and 15 MS/MS events with product ions were accumulated per 50 ms. ESI source conditions were set as follows: 60 psi both for ion source gas 1 and 2; 35 psi for curtain gas; 650 ℃ for source temperature; 5000 V in ESI+ or −4000 V in ESI− for ion spray voltage floating (ISVF). QC samples were used to evaluate the stability of instruments during the whole acquisition process.
Data preprocessing and annotation
All MS raw data files (.wiff) were converted to mzXML format using the ProteoWizard software. The retention time (RT) correction, peak recognition, extraction, integration and alignment were performed using the R package XCMS (Version 3.2). A data matrix consisting of RT, mass-to-charge ratio (m/z) values, and peak intensity was generated. Peak annotation was performed using the R package CAMERA after XCMS data processing. To filter a single peak, only the areas with no more than 50% missing (null) values were retained, both for a single group and for all groups. The missing value in the original data was filled with half of the minimum value, and the normalization was performed using the total ion current (TIC), i.e., the area summation of all the peaks in samples.
Metabolite identification and KEGG pathway enrichment
Principal component analysis (PCA), an unsupervised pattern recognition statistical method based on multidimensional data, was performed to preliminarily assess the overall metabolic differences among groups, and the variability within each group, for all samples including QCs. The correlation of different samples was evaluated using a heatmap with an index Spearman’s rank correlation (SRC) [26]. Orthogonal partial least squares discriminant analysis (OPLS-DA) was performed using the R package ROPLS (Version 3.3.2) to obtain more reliable information on the correlations among experimental groups and the inter-group differences of metabolites [27]. Three predictive parameters, R2X, R2Y, and Q2Y were calculated, where R2X and R2Y represent the explanation rate of the model for X and Y matrices, respectively, and Q2Y indicates the predictive ability of the model. The reliability of model was verified by alignment analysis.
The in-house MS2 database was applied to identify the metabolites in monomer-treated and control cell samples, with m/z values and retention times (RT) serving as criteria to distinguish the differential metabolites [28]. The variable importance in projection (VIP) from the OPLS-DA model, combined with the fold change (FC) and the P-value using Student’s t-test, were used to identify the differentially expressed metabolites between different groups [8]. The data was converted to log2 values, and the cluster analysis (CA) based on heatmaps and volcano plots was performed to reflect the disturbed metabolic state in DMAS- or IBS-treated cells in comparison with the control group [29]. The identified differential metabolites were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.kegg.jp/kegg/) to ascertain the enriched metabolic pathways. The mutual correlations of differential metabolites and different KEGG pathways between DMAS- or IBS-treated samples and the control group were revealed using two-way Venn diagrams [30].
Statistical analysis
The data in this study, including curve fitting and calculation of CC50, IC50, and SI values, were analyzed using SPSS18.0 Data Editor (SPSS Inc., IL, USA) and GraphPad Prism 8 software (GraphPad Software LLC, CA, USA). Student’s t-tests were used to evaluate the toxicity and potency of monomers in vitro and in vivo in comparison with the controls, and to identify the differential metabolites among different cell samples. The data would be considered as statistically different if P < 0.05, or significantly different if P < 0.01 or < 0.001. In this study, the P-values combined with VIP thresholds were used to identify the differential metabolic products (DMPs) as a P-value < 0.05 and VIP > 1. For the three predictive parameters (R2X, R2Y, and Q2Y) in OPLS-DA, the closer they are to 1, the more stable and reliable the model is. Specifically, the OPLS-DA model would be considered effective if Q2Y > 0.5, and excellent if Q2Y > 0.9.
Results
Cytotoxicity and potency of DMAS and IBS in vitro
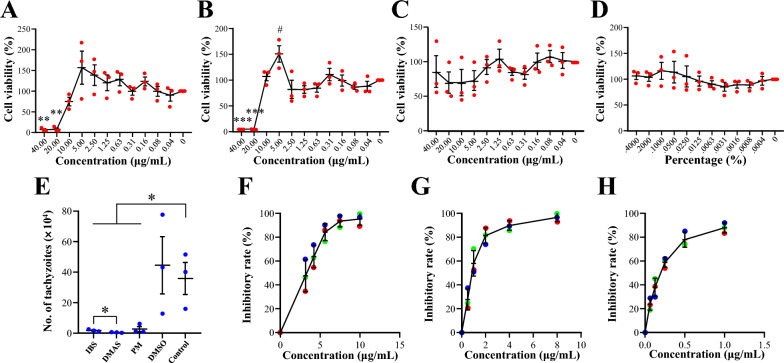
As shown in Fig. 1A–D, statistically different cytotoxicity was detected at concentrations of 40 μg/mL and 20 μg/mL for both DMAS and IBS, when compared with the blank control (i.e., zero gradient). Interestingly, the suitably lower rather than higher concentrations, such as 5 μg/mL for DMAS, in the study were found to be more beneficial for the growth of host Vero cells. No statistical difference was found for other detected concentrations of DMAS and IBS, and all gradients of PM and isopycnic DMSO in controls. Afterward, the CC50 values of the drugs were calculated, and higher threshold levels emerged for DMAS and IBS with no statistical differences (11.92 ± 1.79 μg/mL or 32.18 ± 4.84 μM for DMAS, and 14.77 ± 2.67 μg/mL or 41.20 ± 7.44 μM for IBS), whereas they were significantly lower when compared with PM (Table 1). These results confirmed the cytotoxicity profiles of the concentrations of DMAS, IBS, PM, and DMSO controls used on Vero cells.
Fig. 1.
Cytotoxicity and potency analyses for IBS and DMAS compared with PM in vitro. Monomer cytotoxicity was demonstrated using percentages of cell viability compared with the blank control (zero gradient). Host Vero cells were treated with IBS (A), DMAS (B), PM (C), or DMSO (D) to calculate the CC50 values of drugs. The solid red dots indicate the data from three independent experiments and the short black lines indicate the average values. Compared with zero gradient, the statistical difference was marked with * (down) or # (up). One * or # indicates P < 0.05, two indicates P < 0.01, and three indicates P < 0.001. E Monomer potency against T. gondii PRU tachyzoites at the drug concentration of 10 μg/mL. *P < 0.05. Inhibitory curves of IBS (F), DMAS (G), and PM (H) were drawn for calculating their IC50 values against T. gondii infection
Table 1.
CC50, IC50, and SI values of IBS, DMAS, and PM calculated using different drug concentrations on Toxoplasma gondii PRU tachyzoites in vitro
| Drug | Top concentrations of drugs (μg/mL) | Dilution ratios | Values of CC50* | Values of IC50* | Values of SI | ||||
|---|---|---|---|---|---|---|---|---|---|
| For CC50 | For IC50 | For CC50 | For IC50 | μg/mL | μM | μg/mL | μM | ||
| IBS | 40 | 10 | 1:2 | 3:4 | 14.77 ± 2.67A | 41.20 ± 7.44a | 3.63 ± 0.51A | 10.13 ± 1.42a | 4.07 |
| DMAS | 8 | 1:2 | 11.92 ± 1.79A | 32.18 ± 4.84a | 1.32 ± 0.11B | 3.56 ± 0.29b | 9.04 | ||
| PM | 1 | > 40.00B | > 160.83b | 0.30 ± 0.04C | 1.22 ± 0.16c | > 131.29 | |||
*The data are shown as the mean ± S.D. The statistical difference between any two groups is marked by superscript capital or lowercase letters, and the different letters indicate a P-value of < 0.05
On the basis of the cytotoxicity data, the final concentration of 10 μg/mL was therefore chosen for evaluating the monomer potency of DMAS and IBS, as well as PM and DMSO controls, against wild-type T. gondii PRU tachyzoites, given that DMAS has a larger relative molecular weight than IBS and PM. In Fig. 1E, the data showed that at the concentration of 10 μg/mL, DMAS, IBS, and PM significantly inhibited the in vitro growth of T. gondii PRU tachyzoites compared with the blank control. Notably, the potency of DMAS for T. gondii infection appeared to be superior to that of IBS. Subsequently, IC50 and SI values of DMAS and IBS against PRU tachyzoites were examined and compared with that of PM, revealing that DMAS had a lower IC50 value and a higher SI value than IBS, though both of them seemed inferior to PM (Fig. 1F–H; Table 1), illustrating our prior speculations.
Toxicity and potency of DMAS and IBS in vivo
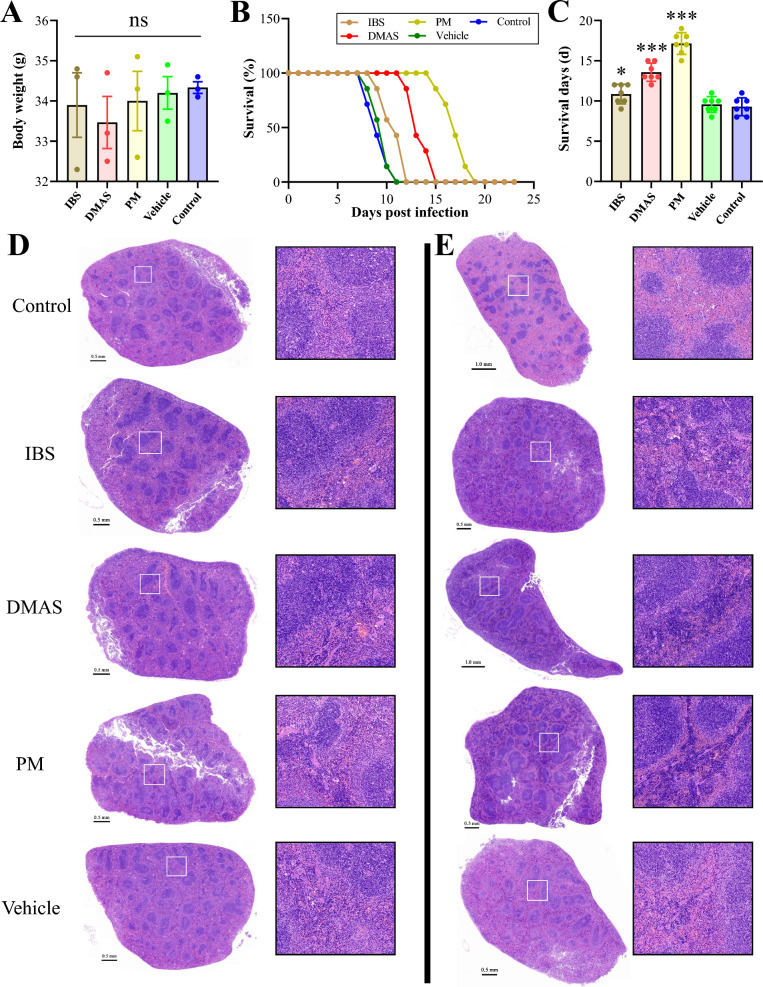
To evaluate the toxicity and potency of DMAS and IBS in vivo, a transparent injection was made by diluting the stock solution of DMAS, IBS, or PM previously dissolved in DMSO (10 mg/mL) into PEG300, Tween-80, and saline, and used for intraperitoneal injections into KM mice at a dose of 1.5 mg/kg once daily for 3 days. At 6 h prior to the first injection, the mice were intraperitoneally infected with 5 × 104 purified T. gondii PRU tachyzoites for potency evaluation. Changes in body weight and the histopathologic examinations on the spleen, liver, and kidney via H&E staining were performed 1 day after the final drug injection, and the survival time was recorded daily until all infected mice had reached their humane endpoints.
The data indicated that the protocol of administration performed in this study had no significantly negative effects on the mice, with no change of body weights (Fig. 2A) and no obvious discernible differences in the histopathologic examinations of the tissues of the spleen (Fig. 2D), the liver, and the kidneys (Additional file 2: Fig. S2). For monomer potency against T. gondii infection in vivo, the results revealed that both DMAS and IBS prolonged the survival times of infected mice in comparison with the vehicle and control groups (Fig. 2B, C), showing greater improvement and modification of hyperemia conditions caused by T. gondii PRU acute infections in spleens than that seen in controls (Fig. 2E).
Fig. 2.
Toxicity and potency assays for IBS and DMAS in comparison with PM in vivo. (A) Body weight of KM mice after drug administration. ns means no significance. (B) Survival curves and (C) survival days of KM mice infected with PRU tachyzoites. *P < 0.05, and ***P < 0.001. (D, E) Splenic histopathologic examination via H&E staining. (D) The monomer toxicity on spleen. (E) Splenic examination post-PRU acute infection and drug administration. Panels on the right are magnified versions of the boxed areas in images on the left
Impacts of DMAS and IBS on the asexual life cycle of T. gondii tachyzoites
To evaluate the effects of DMAS and IBS on the asexual life of T. gondii in vitro, three key experiments were performed, i.e., invasion and attachment, intracellular proliferation, and egress. For invasion and attachment analysis based on ELISA, cells co-incubated with purified PRU tachyzoites and DMAS, IBS, PM, or isopycnic DMSO were collected at 2 h post-infection. Blank DMEM served as control. Standard curves were constructed from six concentration gradients of purified T. gondii PRU tachyzoites to correlate the number of parasites with OD450 values. As the data shows in Fig. 3A–H, the best fit function for the relationship between the logarithm of the number of parasites and OD450 values was the trinomial function (Fig. 3F) with R2 = 0.9993, which was used to calculate the absolute number of intracellular PRU tachyzoites at 2 h post-infection in experimental groups. No statistical difference was detected between experimental groups (Fig. 3I).
Fig. 3.
Impact of IBS and DMAS on the asexual life cycle of Toxoplasma gondii PRU tachyzoites in vitro. Several kinds of curves with correlation index R2 were fitted between the number of tachyzoites and OD450 values. They are the (A) exponential function, (B) linear function, (C) logarithmic function, (D) power function, (E) quadratic function, (F) trinomial function, and (G) quadrinomial function, respectively. (H) The scatter diagram between the logarithm of the number of tachyzoites and OD450 values. (I) Invasion and attachment analysis based on curve fitting. (J) Intracellular proliferation analysis and (K) egress experiments based on PV viability evaluation. The statistical differences are marked using *P < 0.05, **P < 0.01, ***P < 0.001. ns indicates no significance
For evaluating the effects of monomers on the intracellular proliferation of T. gondii, the number of tachyzoites in PVs formed at 48 h post-infection were counted using an oil immersion lens. The data showed that both DMAS and IBS significantly inhibited the intracellular proliferation of T. gondii PRU tachyzoites, although their effects were slightly weaker than PM (Fig. 3J). For the egress assay based on PV viability evaluation, no significant difference was observed among all the experimental groups (Fig. 3K). These results confirmed that DMAS and IBS disturbed the cell division of T. gondii, though their mechanisms may differ from that of PM due to their distinct chemical structures (Additional file 1: Fig. S1).
Ultrastructural changes of T. gondii tachyzoites post co-incubation with DMAS and IBS
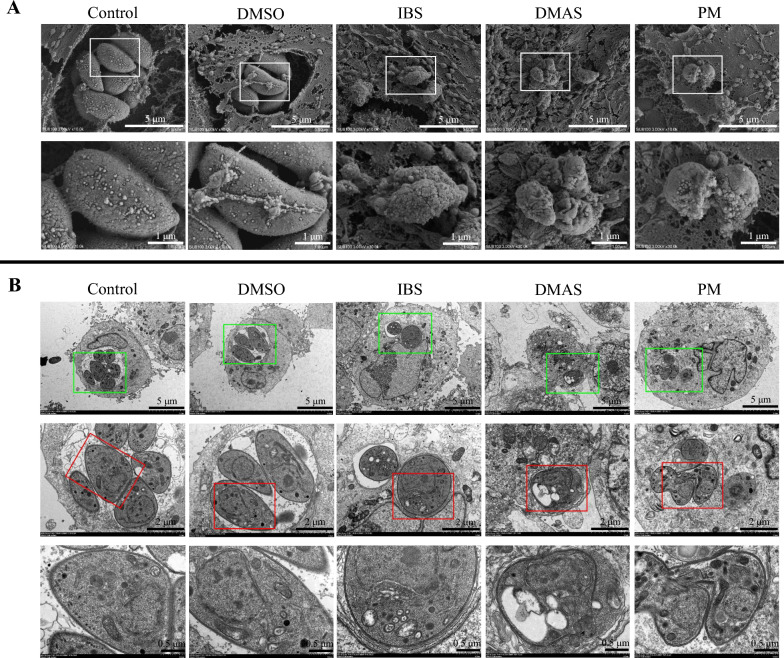
To further explore the potential mechanism of action of DMAS and IBS on T. gondii, ultrastructural changes of surface and internal structures of T. gondii PRU tachyzoites were observed by SEM and TEM at 36 h post co-incubation with DMAS, IBS, PM, or DMSO. As shown in Fig. 4A, the tachyzoites in the isopycnic DMSO and control groups exhibited normal morphology with smooth surfaces. At 36 h post co-incubation with DMAS, IBS, and PM, the surfaces of surviving T. gondii tachyzoites became shrunken, twisted, deformed, and concave. The DMAS-treated group exhibited a rougher surface compared with the IBS-treated group, while the PM-treated group showed a distinct response, characterized by conglutination. These changes suggested that the T. gondii PRU tachyzoites, especially post-treatment with DMAS, may have lost their ability to proliferate in host cells, which can be further revealed by TEM analysis.
Fig. 4.
Ultrastructural alterations induced by IBS or DMAS compared with PM treatment in T. gondii PRU tachyzoites. Examination by (A) scanning electron microscopy and (B) transmission electron microscopy. Lower images are the magnified versions of the upper images, revealing significant ultrastructural damage on the surface and the intramembrane of T. gondii tachyzoites
TEM analysis (Fig. 4B) under the same experimental condition as SEM, revealed that tachyzoites in host cells treated with isopycnic DMSO or blank DMEM (control) exhibited characteristic tapered or taper-like cross-sections, with clear visibility of key organelles, including the conoid, nuclei, mitochondria, and dense granules. In contrast, after 36 h post-treatment with DMAS or IBS, the tachyzoites became nearly round and markedly vacuolar, especially in DMAS-treated host cells, and their internal key organelles were ruthlessly pinched into a corner and had become indistinct. Post-PM treatment, the presence of a leaf-like nuclei of host cell and a mitotic tachyzoite within the same field suggested that although PM inhibited intracellular proliferation of T. gondii PRU tachyzoites, some of them were still surviving under the treatment conditions.
Cell metabolic profiles
We next analyzed the total ion chromatograms from three experimental groups involved in DMAS, IBS, and control (without monomer), and recorded a stable RT without peaks drifts in both ESI+ and ESI− modes. Representative TIC chromatograms of cell samples in vitro were within a 7.5 min window (Additional file 3: Fig. S3A–F). In addition, five QCs run throughout the entire analysis are shown in Additional file 4: Fig. S4. The correlation of cell samples was evaluated by the index SRC (Additional file 5: Fig. S5), and PCA analyses, including 3D score plots (Additional file 3: Fig. S3G, H) and ichnographies separately compared with control (Fig. 5A), were both applied to assess the metabolic difference among groups, the variability within each group, and the LC–MS stability and repeatability. The data indicated that the detection system used in the study was stable, and the cell samples were well-separated and distinguished from each other. File conversion, RT correction, and peak recognition, extraction, integration, and alignment were subsequently carried out. As a result, 2890 and 1449 metabolites were determined in each sample profile in ESI+ or ESI− mode, respectively (Additional file 6: Table S1 and Additional file 7: Table S2).
Fig. 5.
PCA ichnography, OPLS-DA score, volcano plots, heatmaps and two-way Venn diagrams post-monomer treatment in comparison with control (Ctrl). (A) PCA analyses between monomer-treated samples and control group in ESI+ and ESI−, respectively. (B) OPLS-DA score plot analyses between monomer-treated samples and control group. DMAS versus Ctrl (R2X = 0.583, R2Y = 1, and Q2Y = 0.976 in ESI+ ; R2X = 0.669, R2Y = 1, and Q2Y = 0.99 in ESI−), IBS versus Ctrl (R2X = 0.619, R2Y = 0.998, and Q2Y = 0.977 in ESI+ ; R2X = 0.678, R2Y = 1, and Q2Y = 0.988 in ESI−), and x- and y-axes indicate PC1 and PC2, respectively. (C) Volcano plots of all the metabolites marked with color points. x- and y-axes indicate log2FC and −log10(P-value in Student’s t-test), respectively. The point size indicates VIP values in the OPLS-DA model. The upregulated, downregulated, and unchanged metabolites were respectively colored with red, green, and black. (D) Heatmaps of the differential metabolites. (E) Venn diagrams showing the common and unique differential metabolites between DMAS versus Ctrl and IBS versus Ctrl, respectively. (F) Venn diagrams showing the common and unique different KEGG pathways between DMAS versus Ctrl and IBS versus Ctrl, respectively
To minimize interference of heterogeneous peaks and detection system errors, as well as to obtain more reliable information on correlation among groups and the inter-group difference of metabolites, the OPLS-DA model combined with the ROPLS was established for the following analysis, which would be further confirmed post-R2 and Q2 permutations, as shown in Additional file 8: Fig. S6. In general, the OPLS-DA model was considered effective and reliable if Q2Y > 0.5, or excellent if Q2Y > 0.9. As shown in Fig. 5B, the OPLS-DA score plots can clearly distinguish the experimental groups (DMAS and IBS) from the control samples both in ESI+ and ESI− modes.
Identification of differential metabolites
A total of 1348 differential metabolic products (DMPs) in ESI+ (604 downregulated DMPs and 744 upregulated DMPs), as well as 779 DMPs in ESI− (208 downregulated DMPs and 571 upregulated DMPs), were identified in the comparison between the DMAS-treated group and the control group. Moreover, a total of 1402 DMPs in ESI+ (675 downregulated DMPs and 727 upregulated DMPs), as well as 745 DMPs in ESI− (241 downregulated DMPs and 504 upregulated DMPs), were identified in the comparison between the IBS-treated group and the control group (Fig. 5C). The DMPs were further clustered and analyzed using a heatmap (Fig. 5D). By searching the mass-based metabolomic database, the top 10 up- and downregulated DMPs in both comparisons (DMAS-treated group versus control group, and IBS-treated group versus control group) in both ESI+ and ESI− modes were identified and shown in Additional file 9: Fig. S7. Excluding larger numbers of unnamed and inconsequential metabolites, the downregulated DMPs displayed a more important significance: such as 2’-deoxyuridine, deoxyuridine, and deoxyinosine in the DMAS-treated group versus control group in ESI−; pseudouridine in the IBS treated group versus control group in ESI−; and N6-methyladenine in both comparisons in ESI+ and ESI− modes.
As shown in Fig. 5E, we identified 889 DMPs in ESI+ and 545 DMPs in ESI− that were shared between the two comparisons (DMAS-treated group versus control group, and IBS-treated group versus control group). Notably, 13 DMPs were inversely expressed during the experiments (Table 2), including l-tryptophan, N-acetyl-D-glucosamine, acetylcarnitine, l-serine, maltotriose, ergothioneine, phosphorylcholine, and stachyose in ESI+ mode, and palmitic acid, taurocholate, 2-hydroxybutanoic acid, α, α-trehalose, and maltotriose in ESI− mode.
Table 2.
Inverse expression list of metabolites shared by DMAS and IBS compared with control group
| Mode | KEGG ID | Metabolite name | RT (s) | Inversely expressed metabolites# | Metabolic pathways | |
|---|---|---|---|---|---|---|
| DMAS versus control | IBS versus control | |||||
| ESI+ | C00078 | l-Tryptophan | 231.683 | ↓ | ↑ | Mineral absorption; glucosinolate biosynthesis; biosynthesis of alkaloids derived from shikimate pathway; biosynthesis of plant hormones; phenylalanine, tyrosine and tryptophan biosynthesis; tryptophan metabolism |
| C00140 | N-Acetyl-D-glucosamine | 237.914 | ↓ | ↑ | Phosphotransferase system (PTS); amino sugar and nucleotide sugar metabolism; ABC transporters | |
| C02571 | Acetylcarnitine | 286.026 | ↑ | ↓ | Insulin resistance | |
| C00065 | l-Serine | 369.480 | ↓ | ↑ | Vancomycin resistance; mineral absorption; sulfur metabolism; cysteine and methionine metabolism; methane metabolism; ABC transporters; carbon metabolism; sphingolipid signaling pathway | |
| C01835 | Maltotriose | 428.145 | ↑ | ↓ | ABC transporters | |
| C05570 | Ergothioneine | 446.290 | ↑ | ↓ | Histidine metabolism | |
| C00588 | Phosphorylcholine | 455.456 | ↓ | ↑ | Glycerophospholipid metabolism; choline metabolism in cancer | |
| C01613 | Stachyose | 470.065 | ↑ | ↓ | Galactose metabolism | |
| ESI− | C00249 | Palmitic acid | 43.129 | ↑ | ↓ | Cutin, suberine and wax biosynthesis; biosynthesis of unsaturated fatty acids; fatty acid biosynthesis |
| C05122 | Taurocholate | 181.587 | ↑ | ↓ | Taurine and hypotaurine metabolism | |
| C05984 | 2-hydroxybutanoic acid | 185.675 | ↑ | ↓ | Propanoate metabolism | |
| C01083 | α, α-Trehalose | 427.858 | ↑ | ↓ | Starch and sucrose metabolism | |
| C01835 | Maltotriose | 428.152 | ↑ | ↓ | ABC transporters | |
#↑, upregulated; ↓, downregulated
Enrichment of the changed KEGG pathways and biological impact
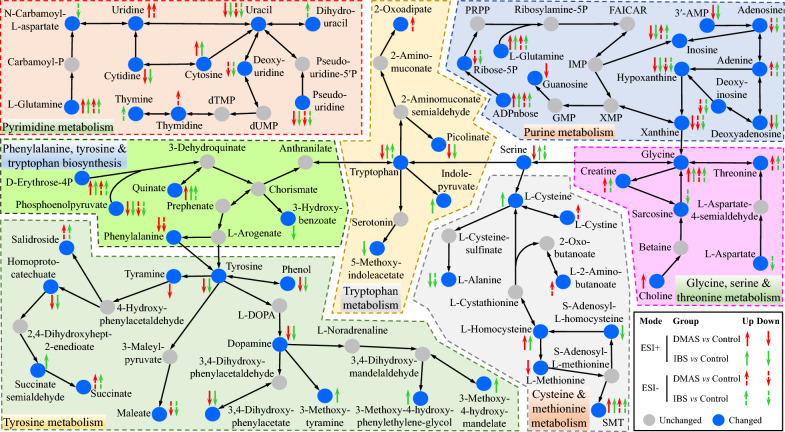
When comparing the KEGG pathway enrichment between the DMAS-treated group versus control group, and the IBS-treated group versus control group, the larger number of differential metabolites were mainly involved in 103 and 100 changed KEGG pathways (CKPs) in ESI+ mode, and 84 and 78 CKPs in ESI− mode, respectively (Fig. 5F), with 76 and 61 CKPs shared between the two comparisons. Of these, several metabolic pathways, such as pyrimidine metabolism; cysteine and methionine metabolism; phenylalanine, tyrosine and tryptophan biosynthesis; purine metabolism; glycine, serine and threonine metabolism; tryptophan metabolism; and tyrosine metabolism, showed a larger number of significantly differential metabolites (Fig. 6 and Additional file 10: Fig. S8). Of note, purine metabolism (ko00230) and pyrimidine metabolism (ko00240) pathways were directly affected by DMAS and IBS, while other altered metabolic pathways appeared to be secondary or additive effects.
Fig. 6.
Pathway analysis of differential metabolites comparing DMAS and IBS with control. Differential metabolites involved in purine metabolism (ko00230); pyrimidine metabolism (ko00240); glycine, serine, and threonine metabolism (ko00260); cysteine and methionine metabolism (ko00270); tyrosine metabolism (ko00350); tryptophan metabolism (ko00380); and phenylalanine, tyrosine, and tryptophan biosynthesis (ko00400) are shown based on the KEGG database. SMT, S-methyl-5′-thioadenosine; PRPP, phosphoribosyl pyrophosphate; L-DOPA, levodopa; FAICAR, 5-formamidoimidazole-4-carboxamide ribotide
Discussion
As a gift of nature and an important treasury for chemical entities, plants have potential therapeutic properties for treating a wide array of diseases of animals and human beings, and some of them, including Artemisia annua, have been widely studied and verified for their medicinal efficacy against various diseases [9, 10]. Confronting the current challenges posed by toxoplasmosis caused by T. gondii, which lacks an ideal vaccine or effective therapeutic agents, medicinal plants may offer a promising alternative against T. gondii infections. Thus, in the present study, we focused on two herbal monomers, DMAS and IBS, extracted from L. erythrorhizon, to evaluate their potential against tachyzoite infection of the T. gondii PRU strain (type II), a common genotype responsible for human toxoplasmosis. A series of experiments, including toxicity and potency analyses, electron microscopy observations, and untargeted metabolomics, provided promising results both in vitro and in vivo.
For cytotoxicity evaluation in vitro, we found that higher concentrations of DMAS and IBS exhibited detrimental effects on host cell growth; however, appropriate concentrations, such as 5 μg/mL of DMAS in this study, showed beneficial effects on host cell growth, supporting the notion that herbal medicines and their monomers often exhibit concentration-dependent effects [31–35]. These findings, alongside higher CC50 values of DMAS and IBS, also provide valuable information for subsequent potency experiments in vitro. Besides, treatments with PM, isopycnic DMSO, and lower doses of DMAS and IBS revealed no significant impacts on the growth of host cells, suggesting that DMAS and/or IBS, especially post-toxicity elimination, such as by chemical modification or de novo synthesis, could help widen the extent of their application.
Afterward, the potency of DMAS and IBS against T. gondii PRU tachyzoites was examined in vitro. The results indicated that both DMAS and IBS can significantly inhibit the growth of T. gondii, with no statistical differences when compared with isopycnic PM. These results are promising, not only because the experiments revealed the efficacy of DMAS, a known anti-tumor drug, as an anti-T. gondii infection property [23, 36], but also because they highlight the potential dual therapeutic properties of these compounds. To further assess the potency of anti-T. gondii infection in vitro, values of IC50 and SI of the two monomers were measured and compared with PM [21, 22]. The data indicated that DMAS and IBS exhibited lower IC50 values, with DMAS showing a statistically more potent effect than IBS, but both were not significantly superior when compared with PM.
To evaluate the toxicity and potency of DMAS and IBS in vivo, female KM mice were used in this study. Previous research has demonstrated that 1.5 mg/kg DMAS through intraperitoneal injection has no significant impact on mice [23]. On the basis of the results of previous report and the above cytotoxicity analysis in vitro, the dose and route for administration was confirmed, and the isopycnic vehicle composed of DMSO, PEG300, Tween-80, and saline, and the mice without any treatment served as controls. Analysis of body weight changes and histopathology on spleen, liver, and kidney tissue further confirmed that 1.5 mg/kg DMAS, IBS, or PM did not cause significant toxicity in mice, which was consistent with a previous report [23]. Notably, potency analysis in vivo showed that treatment with DMAS, IBS, and PM through intraperitoneal injections significantly prolonged the survival times of T. gondii PRU-infected mice and modified the splenic hyperemia conditions caused by Toxoplasma acute infection, affirming the anti-T. gondii efficacy of DMAS and IBS both in vitro and in vivo. Despite the higher IC50 values of DMAS and IBS compared with PM, the promising efficacy of DMAS and IBS in vivo highlighted the potential of these herbal monomers as effective therapeutic agents against T. gondii infections. This is especially significant because DMAS, a known anti-tumor drug, not only exhibits anti-T. gondii infection but also underscores the therapeutic versatility of compounds derived from traditional herbal medicine, such as L. erythrorhizon, offering an alternative approach for combating toxoplasmosis.
To assess the effects of DMAS and IBS on the asexual life cycle of T. gondii tachyzoites, several experiments were performed, including invasion and attachment, intracellular proliferation, and egress assays. According to the previous descriptions, the majority of T. gondii tachyzoites, including the wild-type PRU strain used in the study, are capable of completing the invasion tasks at 2 h post-infection through gliding and attachment, and any sluggish tachyzoites that fail to invade can be effectively removed by washing, if necessary [37, 38]. Therefore, the effects of DMAS and IBS on invasion and attachment of T. gondii can be assessed at 2 h post-infection, because any significant disruption in the biological process would likely result in a measurable statistical difference at this time point. Also, the test method was replaced by an ELISA assay, which offers a more efficient approach compared with indirect immunofluorescence analysis, which require at least four kinds of primary and secondary antibodies and a considerably longer processing time [37].
The results of correlation analysis between the number of tachyzoites and values of OD450 revealed that a trinomial function with higher R2 = 0.9993 provided the optimal fit for the tachyzoite–OD450 relationship, outperforming both a log-transformed model that lacked regularity in its scatter distribution and a quartic polynomial that, despite achieving R2 = 1, produced biologically implausible negative predictions. On the basis of the best fit trinomial function, the absolute number of T. gondii tachyzoites at 2 h post-infection was calculated and no difference was detected, suggesting that DMAS, IBS, and PM have no significant impact on the invasion and attachment of T. gondii in vitro. Both DMAS and IBS influenced the intracellular proliferation of T. gondii tachyzoites in host cells without a close association with PV viability, similar to the PM control.
To further uncover the underlying mechanism through which monomers might act as anti-T. gondii infection agents, the surface and internal ultrastructure of T. gondii tachyzoites at 36 h post co-incubation were investigated by SEM and TEM observations. In SEM, compared with the isopycnic DMSO and blank control, the surfaces of surviving T. gondii tachyzoites became shrunken, twisted, deformed, and concave, with a rougher surface, especially post-DMAS treatment compared with IBS. Moreover, in the PM-treated group, conglutination was observed. The TEM results revealed that post co-incubation, the tachyzoites became markedly vacuolar, especially after DMAS treatment, and their key organelles including nuclei, mitochondria, and dense granules were pinched into a corner, becoming indistinct. In contrast, a rare phenomenon was observed under PM treatment, where a leaf-like nuclei of host cell and a mitotic tachyzoite (still surviving under the effective PM concentration) were captured within the same field of view. These data confirmed the above findings and suggested that DMAS and/or IBS possess different mechanisms from PM against T. gondii infection, both in vitro and in vivo. In addition, the mechanism of PM, including anti-infection properties, has been nearly clarified [39–41]. Thus, differing from previous reports focusing on signaling pathways involved by DMAS [23, 36], an untargeted metabolomics assay was performed in the present study to elucidate the mechanisms underlying the effects of both DMAS and IBS [42].
In this study, a total of 2890 metabolites were detected in ESI+ , and 1449 metabolites were detected in ESI− mode, showing that the number of differential metabolites in ESI+ was greater than that in ESI− mode when comparing DMAS versus control and IBS versus control. In spite of that, the differential metabolites identified in ESI− mode, including 2′-deoxyuridine, deoxyuridine, deoxyinosine, pseudouridine, and N6-methyladenine, which exhibited greater than twofold downregulation, still display an unignorable biological impact. These metabolites were mainly involved in or closely associated with purine metabolism (ko00230) and pyrimidine metabolism (ko00240) pathways, because other differential metabolite ions in the two pathways appeared to be additive or secondary effects. Noteworthily, T. gondii is auxotrophic for several nutrients, and the absence of arginine, tryptophan, or purine will severely restrict the growth of this parasite [43]. Obviously, it is not the key mechanism as nutrients such as arginine and tryptophan, especially in IBS-treated cell samples, were abundant.
In addition, 13 differential metabolites were inversely expressed in DMAS in comparison with IBS, which might be caused by their distinct side chains (i.e., beta, beta-dimethylacryloyl in DMAS and isobutyryl in IBS), suggesting a potential focus for future studies. As a gift of the Earth, L. erythrorhizon, named as Zicao in traditional Chinese herbal medicine, contains numerous bioactive components including DMAS and IBS, as well as shikonin, isovalerylshikonin, deoxyshikonin, acetylshikonin, (2-methylbutyryl) shikonin, lithospermic acid, and lithospermoside. These bioactive components have been reported to be associated with wound healing, antioxidant properties, and anti-inflammatory effects [44, 45]. The present study revealed that DMAS and IBS have anti-T. gondii potency in vitro and in vivo, probably associated with the disruption of T. gondii nucleotide metabolism. However, further research is necessary by utilizing more novel technologies such as multi-omics [46], and exploring other monomers of L. erythrorhizon, to identify the drug targets of this plant against T. gondii infections, which will contribute to the prevention and control of toxoplasmosis.
Conclusions
In the study, the toxicity, including cytotoxicity on host cells, and the potency of anti-T. gondii infection of DMAS and IBS, two monomers with similar chemical structures derived from L. erythrorhizon, were evaluated in comparison with PM in vitro and in vivo. In addition, untargeted metabolomics combined with SEM and TEM analyses were further performed to reveal the mechanisms underlying their effects. The data indicated that DMAS and IBS have anti-T. gondii properties both in vitro and in vivo, with DMAS showing superior efficacy to IBS. Notably, higher concentrations of the two monomers were found to be markedly toxic to host cells and was not good for host cell growth, which was further confirmed by the untargeted metabolomics assay, and the results suggested that the inhibitory process was associated with purine and pyrimidine metabolism pathways, different from the signaling pathway alterations previously reported. SEM and TEM analyses revealed significant alterations in the surface and internal ultrastructure of T. gondii tachyzoites following treatment with the two monomers. In summary, this study highlights DMAS and IBS derived from L. erythrorhizon as promising anti-T. gondii candidates, particularly DMAS after toxicity elimination. Future research should focus on exploring key enzymes or proteins involved in purine and pyrimidine metabolism pathways in T. gondii tachyzoites that interact with these monomers.
Supplementary Information
Additional file 1: Figure S1. The chemical structures of DMAS (CID: 156594098) and IBS (CID: 479500).
Additional file 2: Figure S2. The histopathologic examinations in the tissues of liver (A) and kidney (B) post T. gondii PRU acute infection and drug administration to evaluate the toxicity of DMAS, IBS and PM in vivo.
Additional file 3: Figure S3. Representative total ion current (TIC) chromatograms and PCA 3D score plots of T. gondii-infected cells. Representative TIC chromatograms of control (A & D), DMAS (B & E), and IBS (C & F) in ESI+ mode (A, B & C) and ESI- mode (D, E & F). PCA 3D score plots in ESI+ mode (G) and ESI- mode (H).
Additional file 4: Figure S4. Representative total ion current (TIC) chromatograms of five QC samples in ESI+ mode (A) and ESI- mode (B).
Additional file 5: Figure S5. Correlation of the T. gondii-infected cell samples revealed using heat maps in ESI+ (A) and ESI- (B). The Spearman’s rank correlation (SRC) was used to assess the biological duplication in the study, with a closer square of SRC to 1 indicating a stronger correlation between the different samples.
Additional file 6: Table S1. Details and KEGG pathway annotations of the metabolites identified in DMAS vs control and IBS vs control in ESI+ mode. ID is the serial number of metabolites. MS1 and MS2 indicate the primary and secondary mass spectroscope, respectively. Type is the matching type and ppm is the abbreviation for part per million. The different KEGG pathways were annotated in the last column.
Additional file 7: Table S2. Details and KEGG pathway annotations of the metabolites identified in DMAS vs control and IBS vs control in ESI- mode. ID is the serial number of metabolites. MS1 and MS2 indicate the primary and secondary mass spectroscope, respectively. Type is the matching type and ppm is the abbreviation for part per million. The different KEGG pathways were annotated in the last column.
Additional file 8: Figure S6. Confirmation for the OPLS-DA score plots in ESI+ (A & B) and ESI- (C &D). (A & C) DMAS vs control (pR2Y = 0.025, pQ2 = 0.01 ESI+; pR2Y = 0.02, pQ2 = 0.02 ESI-); (B & D) IBS vs control (pR2Y = 0.005, pQ2 = 0.005 ESI+; pR2Y = 0.005, pQ2 = 0.005 ESI-). The blue and red horizontal lines indicate R2 and Q2 in original model, and their values post permutation are marked with corresponding color diamond points, respectively. The points on or under the horizontal line, that is, the values post permutation no more than that in original model, suggest the employed model is efficient and useful.
Additional file 9: Figure S7. The top 10 up- and down-regulated differentially metabolic products in DMAS vs control and IBS vs control in both ESI+ and ESI- modes.
Additional file 10: Figure S8. Statistics of KEGG pathway enrichments of the differential metabolites during monomer treatment in comparison with control. Rich factor is the ratio of the differential metabolites in a given pathway to the total number of metabolites in that pathway, and a higher rich factor suggests a greater degree of enrichment. The size of bubbles in the figure represents the number of significantly differential metabolites (NM) enriched to the corresponding pathway. q value indicates the adjusted p value.
Acknowledgements
We thank the Biomarker Technologies Corporation (http://www.bmkgene.com/) for technical assistance in metabolomics analyses.
Abbreviations
- CA
Cluster analysis
- CC50
Half-maximal cytotoxic concentration
- CE
Collision energy
- CKP
Changed KEGG pathway
- DMAS
Beta, beta-dimethylacrylshikonin
- DMEM
Dulbecco’s modified Eagle medium
- DMP
Differential metabolic product
- ELISA
Enzyme-linked immunosorbent assay
- EPs
Egress plaques
- ESI
Electrospray ionization
- FC
Fold change
- HFF
Human foreskin fibroblast
- IBS
Isobutyrylshikonin
- IC50
Half-maximal inhibitory concentration
- ISVF
Ion spray voltage floating
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LC/MS
Liquid chromatography/mass spectrometry
- MEF
Mouse embryonic fibroblast
- MOI
Multiplicity of infection
- MTT
3-(4,5-Dimethylthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OPLS-DA
Orthogonal partial least squares discriminant analysis
- PCA
Principal component analysis
- PM
Pyrimethamine
- PTS
Phosphotransferase system
- PV
Parasitophorous vacuole
- QC
Quality control
- RT
Retention time
- SEM
Scanning electron microscope
- SI
Selectivity index
- SRC
Spearman’s rank correlation
- TEM
Transmission electron microscope
- TIC
Total ion current
- UHPLC-QTOF-MS
Untargeted ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry
- Vero
African green monkey kidney cell
- VIP
Variable importance in the projection
Author contributions
Z.Y.L., X.Q.Z., W.B.Z., and H.T.G. conceived and designed this study, and critically revised the manuscript. H.T.G. performed the experiments, analyzed the data, and drafted the manuscript. L.W., B.Z., S.C.X., and W.B.Z. participated in the implementation of the study. X.Q.Z. and Z.Y.L. supervised the project. All authors reviewed and approved the final version of the manuscript.
Funding
The study was kindly supported by the Natural Science Fund of Guangxi Zhuang Autonomous Region (grant no. 2025GXNSFHA069261), the Basic Ability Improvement Project for Guangxi Young- and Middle-Aged Teacher Research in University (grant no. 2025KY0536), the Research Project for Disease Control and Prevention in Guangxi (grant no. GXJKKJ24C009), the Fourth Phase Training Program of Guangxi One Thousand Young- and Middle-Aged Core Teachers in University (grant no. 2020-58), the Research Fund of Shanxi Province for Introduced High-level Leading Talents (grant no. RFSXIHLT202101), and the Special Research Fund of Shanxi Agricultural University for High-level Talents (grant no. 2021XG001). The funders had no role in the study design, data analysis, data interpretation, and the writing of this report.
Availability of data and materials
The datasets supporting the findings of this article are included within the paper and its supplementary materials. The metabolomics data has been deposited in Mendeley Data (https://data.mendeley.com/ preview/z2h3dk3n9f).
Declarations
Ethics approval and consent to participate
All the animals were strictly handled in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China, following established good animal practices. The animal experiments performed in this study were approved by the Experimental Animal Ethics Committee of Guilin Medical University (approval no. GLMC202307169).
Consent for publication
Not applicable.
Competing interests
Xing-Quan Zhu is a Subject Editor for Parasites & Vectors and was not involved in the peer review of this paper. The authors declare no other competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wen-Bin Zheng, Email: wenbinzheng1@126.com.
Xing-Quan Zhu, Email: xingquanzhu1@hotmail.com.
Zhong-Yuan Li, Email: lizhongyuan@glmc.edu.cn.
References
- 1.Elsheikha HM, Marra CM, Zhu XQ. Epidemiology, pathophysiology, diagnosis, and management of cerebral toxoplasmosis. Clin Microbiol Rev. 2021;34:e00115-e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo H, Tan J, He Y, Yuan S, Jin K, Li Z. In vitro virulence contrast of seven genetically distinct Toxoplasma gondii isolates after rejuvenation in vivo. Acta Parasitol. 2024;69:227–32. [DOI] [PubMed] [Google Scholar]
- 3.Lima TS, Lodoen MB. Mechanisms of human innate immune evasion by Toxoplasma gondii. Front Cell Infect Microbiol. 2019;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milne G, Webster JP, Walker M. Toxoplasma gondii: an underestimated threat? Trends Parasitol. 2020;36:959–69. [DOI] [PubMed] [Google Scholar]
- 6.Wang JL, Zhang NZ, Li TT, He JJ, Elsheikha HM, Zhu XQ. Advances in the development of anti-Toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trend Parasitol. 2019;35:239–53. [DOI] [PubMed] [Google Scholar]
- 7.Węglińska L, Bekier A, Trotsko N, Kaproń B, Plech T, Dzitko K, et al. Inhibition of Toxoplasma gondii by 1,2,4-triazole-based compounds: marked improvement in selectivity relative to the standard therapy pyrimethamine and sulfadiazine. J Enzyme Inhib Med Chem. 2022;37:2621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou CX, Gan Y, Elsheikha HM, Chen XQ, Cong H, Liu Q, et al. Sulfadiazine sodium ameliorates the metabolomic perturbation in mice infected with Toxoplasma gondii. Antimicrob Agent Chemother. 2019;63:e00312-e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu Y. Artemisinin-a gift from traditional Chinese medicine to the world (Nobel Lecture). Angew Chem Int Ed Engl. 2016;55:10210–26. [DOI] [PubMed] [Google Scholar]
- 10.Li FS, Weng JK. Demystifying traditional herbal medicine with modern approach. Nat Plant. 2017;3:17109. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Xie Y, Guo M, Rosner MH, Yang H, Ronco C. Nephrotoxicity and Chinese herbal medicine. Clin J Am Soc Nephrol. 2018;13:1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colas RA, Ashton AW, Mukherjee S, Dalli J, Akide-Ndunge OB, Huang H, et al. Trypanosoma cruzi produces the specialized proresolving mediators resolvin D1, resolvin D5, and resolvin E2. Infect Immun. 2018;86:e00688-e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhai B, He JJ, Xie SC, Qiu Y, Miao Z, Liu Y, et al. Metabolomics study of cat small intestine during the early stage of Toxoplasma gondii oocyst formation identifies potential biomarkers. Vet Parasitol. 2022;309:109764. [DOI] [PubMed] [Google Scholar]
- 14.Le TT, Kang TK, Lee WB, Jung SH. Antiallergic effects of N, N-dicoumaroylspermidine isolated from Lithospermum erythrorhizon on mast cells and ovalbumin-induced allergic rhinitis. Int J Mol Sci. 2022;23:10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JL, Li TT, Elsheikha HM, Chen K, Cong W, Yang WB, et al. Live attenuated Pru:Δcdpk2 strain of Toxoplasma gondii protects against acute, chronic, and congenital toxoplasmosis. J Infect Dis. 2018;218:768–77. [DOI] [PubMed] [Google Scholar]
- 16.Wang JL, Elsheikha HM, Zhu WN, Chen K, Li TT, Yue DM, et al. Immunization with Toxoplasma gondii GRA17 deletion mutant induces partial protection and survival in challenged mice. Front Immunol. 2017;8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li ZY, Liang X, Guo HT, Tan J, Zhu XQ, Liu Q. Toxoplasma invasion delayed by TgERK7 eradication. Parasitol Res. 2020;119:3771–6. [DOI] [PubMed] [Google Scholar]
- 18.Li ZY, Wang ZD, Huang SY, Zhu XQ, Liu Q. TgERK7 is involved in the intracellular proliferation of Toxoplasma gondii. Parasitol Res. 2016;115:3419–24. [DOI] [PubMed] [Google Scholar]
- 19.Fox BA, Guevara RB, Rommereim LM, Falla A, Bellini V, Pètre G, et al. Toxoplasma gondii parasitophorous vacuole membrane-associated dense granule proteins orchestrate chronic infection and GRA12 underpins resistance to host gamma interferon. mBio. 2019;10:e00589-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo HT, Chen ZB, Li ZY. Screening and identification of primers for internal control used in ACT1-qPCR analysis of Toxoplasma gondii. Chin J Parasitol Parasit Dis. 2018;36:375–80. [Google Scholar]
- 21.Esharkawy ER, Almalki F, Hadda TB. In vitro potential antiviral SARS-CoV-19- activity of natural product thymohydroquinone and dithymoquinone from Nigella sativa. Bioorg Chem. 2022;120:105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges BS, Bueno GP, Tomiotto-Pellissier F, Figueiredo FB, Soares Medeiros LC. In vitro anti-Leishmania activity of triclabendazole and its synergic effect with amphotericin B. Front Cell Infect Microbiol. 2023;12:1044665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao ZJ, Zhang YY, Fan YY, Jin SJ, Yan J, Zheng XW, et al. β, β-Dimethylacrylshikonin exerts antitumor activity via Notch-1 signaling pathway in vitro and in vivo. Biochem Pharmacol. 2012;84:507–12. [DOI] [PubMed] [Google Scholar]
- 24.Lamarque MH, Roques M, Kong-Hap M, Tonkin ML, Rugarabamu G, Marq JB, et al. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat Commun. 2014;5:4098. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Zhai B, Wang C, Elsheikha HM, Guo H, Zheng XN, et al. Glabridin exhibits potent inhibitory effects against Toxoplasma gondii in vitro and in vivo. Parasit Vectors. 2024;17:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Wang C, Peng S, Zhu X, Zhang Z, Zhao Y, et al. Pivotal interplays between fecal metabolome and gut microbiome reveal functional signatures in cerebral ischemic stroke. J Transl Med. 2022;20:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong X, Zhu Z, Wei Y, Ngo D, Zhang R, Du M, et al. Plasma insulin-like growth factor binding protein 7 contributes causally to ARDS 28-day mortality: evidence from multistage Mendelian randomization. Chest. 2021;159:1007–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, He JJ, Hou JL, Zhou CX, Elsheikha HM, Zhu XQ. Ultra performance liquid chromatography-tandem mass spectrometry-based metabolomics reveals metabolic alterations in the mouse cerebellum during Toxoplasma gondii infection. Front Microbiol. 2020;11:1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D, Mu D, Cheng M, Dou Y, Zhang X, Feng Z, et al. Differences in lipidomics may be potential biomarkers for early diagnosis of pancreatic cancer. Acta Cir Bras. 2020;35:e202000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong SR, Lee TH, Cheong SK, Lim YM. Geographical factor influences the metabolite distribution of house edible bird’s nests in Malaysia. Front Nutr. 2021;8:658634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leem K, Park SY, Lee DH, Boo YM, Cho KH, Lim J, et al. Effects of Jaoga-Yukmiwon (R), a Korean herbal medicine, on chondrocyte proliferation and longitudinal bone growth in adolescent male rats. Phytother Res. 2003;17:1113–6. [DOI] [PubMed] [Google Scholar]
- 32.Udalamaththa VL, Jayasinghe CD, Udagama PV. Potential role of herbal remedies in stem cell therapy: proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandlik Ingawale DS, Namdeo AG. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J Diet Suppl. 2021;18:183–226. [DOI] [PubMed] [Google Scholar]
- 34.Wu YY, Xu YM, Lau ATY. Epigenetic effects of herbal medicine. Clin Epigenetics. 2023;15:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar S, Singh B, Singh R. Catharanthus roseus (L.) G. Don: a review of its ethnobotany, phytochemistry, ethnopharmacology and toxicities. J Ethnopharmacol. 2022;284:114647. [DOI] [PubMed] [Google Scholar]
- 36.Shen XJ, Wang HB, Ma XQ, Chen JH. β, β-Dimethylacrylshikonin induces mitochondria dependent apoptosis through ERK pathway in human gastric cancer SGC-7901 cells. PLoS ONE. 2012;7:e41773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang ZW, Wang M, Sun LX, Elsheikha HM, Lei CL, Wang JL, et al. Trx4, a novel thioredoxin protein, is important for Toxoplasma gondii fitness. Parasit Vectors. 2024;17:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu XJ, Gao J, Zheng XN, Elsheikha HM, Li TT, Kou YJ, et al. The splicing factor SR2 is an important virulence factor of Toxoplasma gondii. Front Microbiol. 2023;14:1302512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heppler LN, Attarha S, Persaud R, Brown JI, Wang P, Petrova B, et al. The antimicrobial drug pyrimethamine inhibits STAT3 transcriptional activity by targeting the enzyme dihydrofolate reductase. J Biol Chem. 2022;298:101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paiboonrungruang C, Xiong Z, Lamson D, Li Y, Bowman B, Chembo J, et al. Small molecule screen identifies pyrimethamine as an inhibitor of NRF2-driven esophageal hyperplasia. Redox Biol. 2023;67:102901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Su Q, Chen K, Wu Q, Ren J, Tang W, et al. Pyrimethamine upregulates BNIP3 to interfere SNARE-mediated autophagosome-lysosomal fusion in hepatocellular carcinoma. J Pharm Anal. 2024;14:211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloehn J, Lunghi M, Varesio E, Dubois D, Soldati-Favre D. Untargeted metabolomics uncovers the essential lysine transporter in Toxoplasma gondii. Metabolites. 2021;11:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Augusto L, Amin PH, Wek RC, Sullivan WJ Jr. Regulation of arginine transport by GCN2 eIF2 kinase is important for replication of the intracellular parasite Toxoplasma gondii. PLoS Pathog. 2019;15:e1007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo C, He J, Song X, Tan L, Wang M, Jiang P, et al. Pharmacological properties and derivatives of shikonin-a review in recent years. Pharmacol Res. 2019;149:104463. [DOI] [PubMed] [Google Scholar]
- 45.Kang TK, Le TT, Kim KA, Kim YJ, Lee WB, Jung SH. Roots of Lithospermum erythrorhizon promotes retinal cell survival in optic nerve crush-induced retinal degeneration. Exp Eye Res. 2021;203:108419. [DOI] [PubMed] [Google Scholar]
- 46.Singh P, Lonardi S, Liang Q, Vydyam P, Khabirova E, Fang T, et al. Babesia duncani multi-omics identifies virulence factors and drug targets. Nat Microbiol. 2023;8:845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The chemical structures of DMAS (CID: 156594098) and IBS (CID: 479500).
Additional file 2: Figure S2. The histopathologic examinations in the tissues of liver (A) and kidney (B) post T. gondii PRU acute infection and drug administration to evaluate the toxicity of DMAS, IBS and PM in vivo.
Additional file 3: Figure S3. Representative total ion current (TIC) chromatograms and PCA 3D score plots of T. gondii-infected cells. Representative TIC chromatograms of control (A & D), DMAS (B & E), and IBS (C & F) in ESI+ mode (A, B & C) and ESI- mode (D, E & F). PCA 3D score plots in ESI+ mode (G) and ESI- mode (H).
Additional file 4: Figure S4. Representative total ion current (TIC) chromatograms of five QC samples in ESI+ mode (A) and ESI- mode (B).
Additional file 5: Figure S5. Correlation of the T. gondii-infected cell samples revealed using heat maps in ESI+ (A) and ESI- (B). The Spearman’s rank correlation (SRC) was used to assess the biological duplication in the study, with a closer square of SRC to 1 indicating a stronger correlation between the different samples.
Additional file 6: Table S1. Details and KEGG pathway annotations of the metabolites identified in DMAS vs control and IBS vs control in ESI+ mode. ID is the serial number of metabolites. MS1 and MS2 indicate the primary and secondary mass spectroscope, respectively. Type is the matching type and ppm is the abbreviation for part per million. The different KEGG pathways were annotated in the last column.
Additional file 7: Table S2. Details and KEGG pathway annotations of the metabolites identified in DMAS vs control and IBS vs control in ESI- mode. ID is the serial number of metabolites. MS1 and MS2 indicate the primary and secondary mass spectroscope, respectively. Type is the matching type and ppm is the abbreviation for part per million. The different KEGG pathways were annotated in the last column.
Additional file 8: Figure S6. Confirmation for the OPLS-DA score plots in ESI+ (A & B) and ESI- (C &D). (A & C) DMAS vs control (pR2Y = 0.025, pQ2 = 0.01 ESI+; pR2Y = 0.02, pQ2 = 0.02 ESI-); (B & D) IBS vs control (pR2Y = 0.005, pQ2 = 0.005 ESI+; pR2Y = 0.005, pQ2 = 0.005 ESI-). The blue and red horizontal lines indicate R2 and Q2 in original model, and their values post permutation are marked with corresponding color diamond points, respectively. The points on or under the horizontal line, that is, the values post permutation no more than that in original model, suggest the employed model is efficient and useful.
Additional file 9: Figure S7. The top 10 up- and down-regulated differentially metabolic products in DMAS vs control and IBS vs control in both ESI+ and ESI- modes.
Additional file 10: Figure S8. Statistics of KEGG pathway enrichments of the differential metabolites during monomer treatment in comparison with control. Rich factor is the ratio of the differential metabolites in a given pathway to the total number of metabolites in that pathway, and a higher rich factor suggests a greater degree of enrichment. The size of bubbles in the figure represents the number of significantly differential metabolites (NM) enriched to the corresponding pathway. q value indicates the adjusted p value.
Data Availability Statement
The datasets supporting the findings of this article are included within the paper and its supplementary materials. The metabolomics data has been deposited in Mendeley Data (https://data.mendeley.com/ preview/z2h3dk3n9f).