Abstract
The minute virus of mice, an autonomous parvovirus, requires entry of host cells into the S phase of the cell cycle for its DNA to be amplified and its genes expressed. This work focuses on the P4 promoter of this parvovirus, which directs expression of the transcription unit encoding the parvoviral nonstructural polypeptides. These notably include protein NS1, necessary for the S-phase-dependent burst of parvoviral DNA amplification and gene expression. The activity of the P4 promoter is shown to be regulated in a cell cycle-dependent manner. At the G1/S-phase transition, the promoter is activated via a cis-acting DNA element which interacts with phase-specific complexes containing the cellular transcription factor E2F. It is inhibited, on the other hand, in cells arrested in G1 due to contact inhibition. This inhibitory effect is not observed in serum-starved cells. It is mediated in cis by cyclic AMP response elements (CREs). Unlike serum-starved cells, confluent cells accumulate the cyclin-dependent kinase inhibitor p27, suggesting that the switch from CRE-mediated activation to CRE-mediated repression involves the p27 protein. Accordingly, plasmid-driven overexpression of p27 causes down-modulation of promoter P4 in growing cells, depending on the presence of at least two functional CREs. No such effect is observed with two other cyclin-dependent kinase inhibitors, p16 and p21. Given the importance of P4-driven synthesis of protein NS1 in parvoviral DNA amplification and gene expression, the stringent S-phase dependency of promoter P4 is likely a major determinant of the absolute requirement of the minute virus of mice for host cell proliferation.
Cell cycle progression is coupled to the phase-dependent transcription of certain genes required for phase-specific metabolic activities. The varying transcription rate of such genes can be ascribed to various transcription factors whose activity is differentially regulated throughout the cell cycle. The best studied of these is E2F. In G1 and late S/G2, E2F is down-regulated by the so-called pocket proteins (pRB, p107, and p130). At these points in the cycle, the pocket proteins are in a hypophosphorylated state and can interact directly with E2F (reviewed recently in reference 26). As a result, E2F fails to induce and can even repress transcription of its target genes (4). Yet at the G1/S-phase transition, E2F exerts a strong activating effect. At this time, phosphorylation of pocket proteins by cyclin–cyclin-dependent kinase (cdk) complexes dissociates the complexes (34). cdk inhibitors (CKIs) such as p27KIP1, p16, and p21 suppress E2F-mediated promoter activation (44, 48, 62). Other factors, notably members of the ATF/cyclic AMP response element (CRE)-binding protein (CREB) and Sp1 transcription-factor families, which interact, respectively, with CREs and GC boxes, may also contribute to cell-cycle-dependent gene transcription. Activation of the adenovirus E2 promoter by G1 cyclins is mediated by both CRE- and GC-box-binding proteins (46). Furthermore, recent data suggest that a CRE motif is involved in S-phase activation (13) and G1-phase repression (60) of cyclin A gene expression. Results conflicting with this finding were obtained by other authors (44), however, and so the involvement of other factors besides E2F in cell cycle-dependent transcription remains to be unraveled.
In this study, we used the minute virus of mice (prototype strain, MVMp), an autonomous parvovirus, as a model for investigating cell cycle regulation of promoter function. This choice was based on the low genetic complexity of parvoviruses and on the fact that their life cycle is highly dependent on host cell factors, notably ones transiently expressed during the S phase of the cell cycle (42, 49, 54). Unlike other DNA viruses, parvoviruses fail to induce resting cells to enter the S phase (54). Consequently, their multiplication is delayed until the host cells enter on their own a round of genomic DNA replication. MVMp virions contain a linear, single-stranded DNA genome of about 5 kb, comprising two overlapping transcription units. The focus of this study is P4, the early promoter of MVMp. P4 directs expression of the parvoviral nonstructural (NS) proteins, the major one being NS1, a multifunctional DNA-binding protein required for replication of the parvoviral DNA. NS1 also transactivates the second parvoviral promoter (P38), thereby controlling the viral capsid genes (9, 27). The activity of the P4 promoter is modulated in cis via motifs known to bind a variety of cellular transcription factors (Fig. 1), including CREs, a GC box, and a putative E2F-binding site (16). Since the burst of parvoviral gene expression occurs as host cells enter the S phase (11), we have investigated whether the activity of promoter P4 is differentially regulated in the course of the cell cycle.
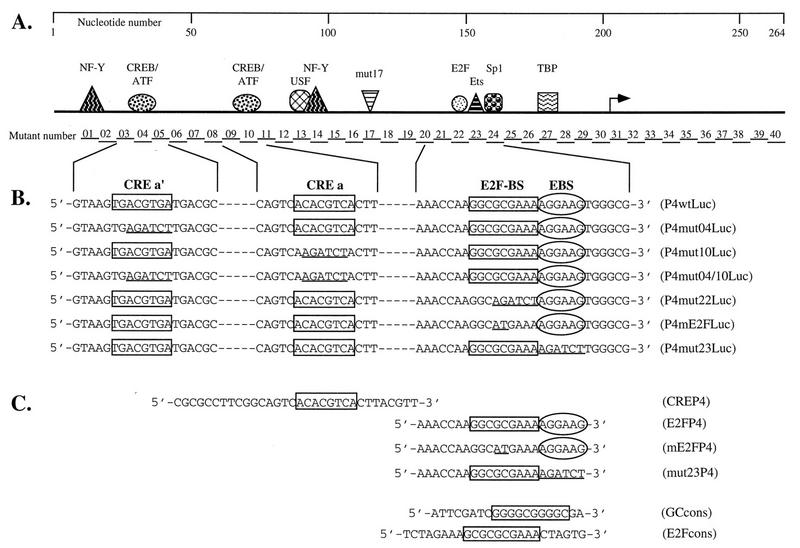
FIG. 1.
Schematic representation of the MVMp P4 promoter, of the mutants derived from it, and of the oligonucleotides used in this study. (A) The upper panel depicts the P4 promoter from nt 1 to the translation initiation site at nt 261 (numbering according to Astell et al. [2]). The arrow indicates the transcription initiation site. Symbols represent transcription factors shown to interact with specific P4 promoter sequence elements: TBP, TATA-box-binding protein (1, 16); Sp1, GC-box-binding proteins (1, 17); Ets, Ets family of transcription factors (17); E2F, E2F-binding-site-specific protein complexes (reference 16 and this study); mut17, as yet unidentified proteins binding to and activating promoter P4 via the DNA element mutated in P4mut17 (15); NF-Y, Y-box-binding protein (18); USF, E-box-binding protein (19); CREB/ATF, CRE-binding proteins (36). The 40 contiguous BglII substitutions introduced in mutants P4mut01 to P4mut40, respectively, are located along the promoter. (B and C) The sequence elements analyzed in this study are framed and aligned beneath the line diagram of the promoter. Underlined sequences indicate mutations introduced in the various elements. (B) P4 promoter constructs driving expression of the luciferase reporter gene. (C) Oligonucleotides used as probes or competitors in electrophoretic mobility assays.
The results presented here show that the P4 promoter of MVMp is indeed activated at the G1/S-phase transition, principally via the proximal E2F-binding site. Our in vitro data suggest that at this stage, the E2F-binding motif binds the so-called free form of E2F. We have further uncovered a novel pathway of transcriptional up- and down-regulation, mediated by CREs previously shown to constitute binding sites for ATF/CREB family transcription factors (36): when present in at least two copies, the P4 CREs mediate promoter activation in growing and serum-starved cells but promoter repression in contact-inhibited cells. The switch from activation to repression can be triggered by overexpression of CKI p27KIP1, a known mediator of contact inhibition-induced G1 arrest, while CKIs p16 and p21 have no detectable effect. The new regulatory system thus clearly differs from the above-mentioned E2F-pocket protein system.
MATERIALS AND METHODS
Cell cultures and cell synchronization.
The established lines of Fisher rat fibroblasts (FR3T3) and Swiss mouse embryo fibroblasts (NIH 3T3) were grown at 37°C in an atmosphere containing 5% CO2 in Dulbecco’s modified Eagle’s minimum essential medium supplemented with 10% aseptic donor calf serum (DCS) and 1% sodium pyruvate. The established A9 cell line was grown in minimum essential medium supplemented with 5% aseptic fetal calf serum.
Cells were synchronized in phase G0/G1 by culturing them for 72 h in Dulbecco’s modified Eagle’s medium supplemented with 0.5% DCS (serum starvation) or by keeping them for 72 h at high density (contact inhibition). Synchronization in phase G2 was obtained by incubating the cells in 2 mM thymidine for 12 h, after which the thymidine was removed and the cells were treated with nocodazole (50 ng/ml) for 14 h.
Plasmids.
Plasmid P4wtLuc expressing the firefly luciferase gene under the control of the MVMp P4 promoter was obtained as follows. First the HindIII-EcoRI fragment of plasmid pLucDSS (18), containing the luciferase gene and its genuine polyadenylation signal, was cloned into the pBSK− vector (Stratagene). This yielded plasmid pBSK-Luc. The 38-bp HindIII-XbaI fragment of pBSK-Luc was then replaced with an oligonucleotide of identical sequence except that the bases surrounding the ATG codon were modified to create an NcoI restriction site (CCATGG). Subsequently, the ScaI-NcoI fragment of plasmid pP4Cat (52), containing promoter P4 and downstream leader sequences up to the ATG codon, was inserted in front of the firefly luciferase gene, thus generating plasmid P4wtLuc.
Linker-scanning mutants of promoter P4 were obtained by site-directed mutagenesis. To this end, the palindromic P4 promoter was excised from plasmid P4wtLuc and cut in two, yielding one fragment containing the left arm of the palindrome from nucleotide (nt) +1 (downstream from the P4wtLuc plasmid XbaI site) of the MVM genome to the AflIII site (nt 75) and a second fragment containing the right arm of the palindrome from the AflIII site to the XbaI site at the very beginning of the luciferase gene. This separation avoided competition between mutation-bearing oligonucleotides and the complementary sequence of the palindrome during the annealing step of mutagenesis. After 3′-recessed end filling by means of the Klenow fragment of Escherichia coli DNA polymerase I, P4 fragments were cloned into the blunted SalI site of the pAlter vector (Promega), allowing site-directed mutagenesis with the Altered Sites System (Promega) according to the manufacturer’s recommendations. For this purpose, we used 46-mer oligonucleotides in which the BglII recognition motif (AGATCT) was substituted for a P4 promoter sequence of equivalent length. Subsequently, the mutated left and right P4 fragments were substituted for the equivalent wild-type ScaI-AflIII and AflIII-NcoI fragments of plasmid P4wtLuc, respectively, thus generating a linker-scanning series of full-length mutated P4 promoters. The resulting plasmids were named P4mutxLuc (Fig. 1A), x varying from 1 to 40 in contiguous mutants spanning the whole promoter from left to right.
The luciferase gene was placed under the thymidine kinase (tk) promoter of herpes simplex virus type 1 (HSV-1). This was done by replacing in plasmid pBLCat4 (28) the AvaI-EcoRI fragment containing the chloramphenicol acetyltransferase (cat) gene with the NcoI-EcoRI fragment of pLucDSS (18), containing the luciferase gene. This yielded plasmid HSVtkLuc.
Deletion of the BamHI-BglII fragment from P4mut26Luc yielded the minimal promoter construct TATALuc, retaining only the TATA box and downstream region of promoter P4 (including the transcription start site and leader sequence). Synthetic oligonucleotides flanked by BamHI and BglII restriction sites and containing either a P4 promoter-derived CRE motif, a consensus GC box, or a mutated P4 promoter-derived CRE motif were phosphorylated and self-ligated. Monomers, dimers, and trimers of each oligonucleotide were isolated on a 5% polyacrylamide gel and substituted for the upstream P4 region between the BamHI and BglII sites of P4mut26Luc. The minimal reporter constructs obtained in this way were named CRExLuc, GCxLuc, CREmxLuc, where x varies from 1 to 3.
DNA transfection and reporter protein analysis.
Growing cells were transfected by calcium phosphate coprecipitation (7). Serum-starved and contact-inhibited cells were transfected with the help of the DOTAP reagent (Boehringer Mannheim) in the presence of 0.5% DCS, as recommended by the manufacturer. In some experiments, the Polybrene-dimethyl sulfoxide (30%) shock method (6, 22) was used to transfect serum-starved cells. Cultures (5 × 105 cells per 60-mm-diameter petri dish) were cotransfected with 1 μg of reporter construct and 1 μg of plasmid pBLCat4 used as a standard. In some assays, the DNA inoculum also included a p27KIP1-expressing effector plasmid (40) or the corresponding empty vector (pX) as a control. The total amount of inoculated DNA was adjusted to 6 μg per transfection by addition of salmon sperm DNA. In transient expression assays, CAT or luciferase activity was measured 48 h posttransfection in samples containing equal amounts of total protein from whole-cell extracts. CAT activities were determined according to the phase extraction protocol (45). Luciferase activities were measured with a Berthold luminometer (3). Only luciferase activity readings at least threefold above background were considered significant.
To generate stable transfectants, cells were cotransfected with 0.1 μg of plasmid pSV2Neo (51) and a 10-fold molar excess of the plasmid of interest. Pools of stable transfectants were selected for their resistance to G418 (500 μg/ml) over a 14-day period.
Flow cytometry.
Cells were harvested by trypsinization, washed with phosphate-buffered saline (PBS), and fixed for 5 min at room temperature in paraformaldehyde (2%) and lysolecithin (20 μg/ml). The cells were then washed twice in PBS and fixed in 80% methanol; 105 cells were transferred to an Eppendorf tube, washed in PBS, and resuspended in 500 μl of PBS containing RNase A (0.1 mg/ml). After incubation for 30 min at 37°C, propidium iodide was added (500 μl of a 50-μg/ml solution). Analysis by flow cytometry (fluorescence-activated cell sorting [FACS]) was performed with a Becton Dickinson FACSort. Cell cycle profiles were determined by using the CellFIT cell cycle analysis software package (Becton Dickinson).
For determining the cell cycle distribution of transfected cells, vector pXNS1404-405 (57) expressing an inactive parvoviral NS1 protein was used along with the effector plasmid (1:1 molar ratio) to cotransfect the cells. These were harvested, fixed, and incubated with the NS1-specific rabbit antiserum Sp8 (16). NS1-positive cells were detected with the help of fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G. After propidium iodide staining, 106 events were acquired on the FACSort, and cell cycle distribution of fluorescein isothiocyanate-positive cells was analyzed as described above.
Cell protein extraction and gel retardation assays.
Extractions were performed as described by Kumar and Chambon (23), with previously described modifications (5). Briefly, FR3T3 cells were harvested with a rubber policeman, collected in ice-cold PBS, and washed twice. The pellet was resuspended in 1.5 volumes of lysis buffer (20 mM HEPES [pH 7.9], 0.4 M NaCl, 25% glycerol, 1 mM EDTA, 2.5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride). After incubation on ice for 20 min, the lysate was frozen at −70°C, thawed on ice, and vigorously vortexed. After centrifugation (18,000 × g, 10 min, 4°C), the supernatant was recovered, frozen in liquid nitrogen, and used as whole-cell protein extract in gel retardation assays.
The DNA oligonucleotides used as probes or unlabeled competitors are depicted in Fig. 1. Double-stranded oligonucleotide probes were end labeled to a high specific activity by using the Klenow fragment of E. coli DNA polymerase I. About 250 pmol of labeled probe was incubated in the absence or presence of a 100-fold molar excess of unlabeled competitor oligonucleotide for 10 min at room temperature with 0.5 μg salmon sperm DNA, 2 mM MgCl2, 10% glycerol, and whole-cell extract (3 to 6 μg of protein) in a final volume of 10 μl. For supershift assays, the extracts were first incubated for 1 h on ice with antibodies (1 μl) prior to addition of oligonucleotide. Antibodies were purchased from Dianova (cdk2) and from Santa Cruz (p130 and p107) or were a generous gift from M. Pagano (cyclin A). Following incubation, the samples were loaded onto a 4.5% nondenaturing polyacrylamide gel. Electrophoresis was performed in 0.3× Tris-borate-EDTA for 90 min at 4°C.
Protein analysis by Western blotting.
Total protein was extracted from 105 cells as described above and fractionated by electrophoresis on a 12% polyacrylamide gel containing 1% sodium dodecyl sulfate. Proteins were transferred onto a Hybond ECL membrane (Amersham) in a Trans-Blot semidry electrophoretic transfer cell (Bio-Rad) (200 mA for 1 h) according to the manufacturer’s instructions. The membranes were incubated first for 1 h in blocking buffer (5% powdered milk, 1% sodium caseinate) and then for 1 h with antibodies diluted in blocking buffer. Monoclonal antibodies specifically recognizing CKI p27 (Transduction Laboratories) were used at 1:1,000 dilution, and polyclonal antisera raised against either cyclin A (generous gift from M. Pagano), rat CREB in its nonphosphorylated or phosphorylated (both from Upstate Biotechnology Inc.) state, human CKI p16 (Pharmingen), or human CKI p21 (Transduction Laboratories) were used at 1:2,500 dilution. Membranes were washed three times for 5 min each in PBS containing 0.1% Tween 20 and then further incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit immunoglobulin G (1:1,000 dilution in blocking buffer; Dianova). All incubations were performed at room temperature. Immunocomplexes were detected with ECL reagent (Amersham) according to the manufacturer’s instructions.
RESULTS
MVMp P4 promoter activity is host cell cycle dependent.
The focus of this study was the regulation of the MVMp P4 promoter. Since a burst of viral gene expression is observed in the S phase (54), the question was: is the activity of the early MVM P4 promoter subject to cell cycle-dependent regulation? To monitor P4 activity through the different phases of the host cell cycle, we stably transfected FR3T3 rat fibroblasts with a P4-driven firefly luciferase gene (P4wtLuc [Fig. 1]). To avoid positional effects, we used pools of stably transfected cells throughout the study.
FR3T3 cells were chosen for the ease with which they are synchronized following serum starvation or contact inhibition. Both modes of synchronization were used to obtain cell populations arrested in G0/G1, allowing analysis of events occurring at the G1/S-phase transition upon release from growth arrest.
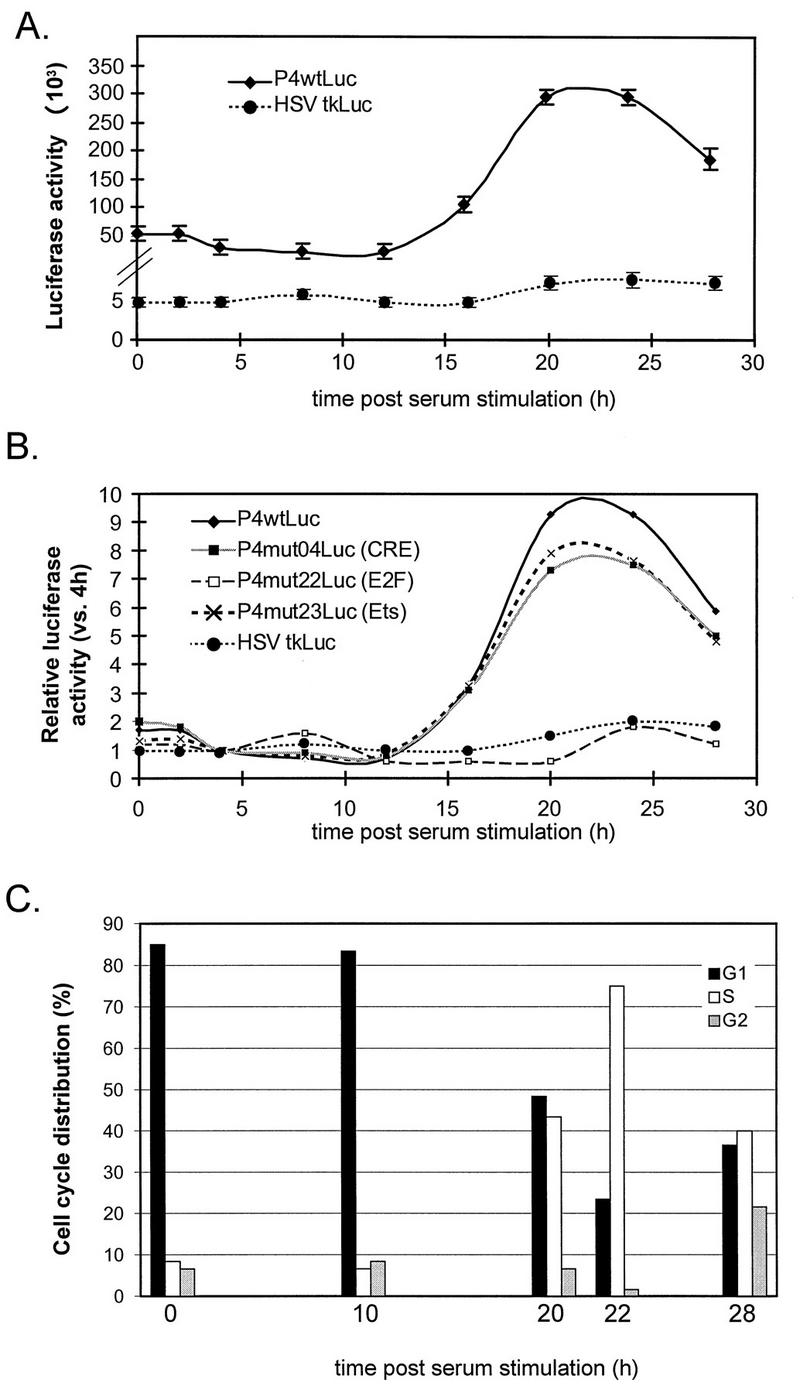
After serum starvation for 72 h in medium containing 0.5% DCS, stable FR3T3 transfectants were released from growth arrest by increasing the DCS concentration to 20%. Every 4 h postrelease, we analyzed the cells’ cell cycle distribution and measured their luciferase activity. Up to 90% of the cells in serum-starved populations were found to be arrested in G0. Some 20 h after release, 50% of the cells were found to have left G1 and entered the S phase (Fig. 2C). Some P4 promoter activity was detected during the first 15 h after serum addition, while the cells were still mostly in G1 (Fig. 2A), but this activity increased about 10-fold as the cells entered the S phase (Fig. 2A and B). The same experiment was performed on a control pool of FR3T3 cells stably transfected with a reporter gene driven by the cell cycle-independent HSV-1 minimal tk promoter. These cells showed little change in luciferase activity as the cells transited from the G1 phase of the cell cycle to the S phase (Fig. 2A and B).
FIG. 2.
Cell cycle-dependent activity of promoter P4 in cells synchronized by serum starvation. Pools of FR3T3 cells stably transfected with reporter construct P4wtLuc, P4mutLuc, or HSVtkLuc were synchronized by serum starvation. After release from the block, luciferase activities were measured at 4-h intervals. (A) Absolute luciferase activities achieved by P4wtLuc and HSVtkLuc are given in arbitrary light units, after correction for the number of cells and the background of the luminometer. Averages of six independent experiments are shown with standard deviation bars. (B) Relative luciferase activities achieved by P4wtLuc, derived mutants (P4mut04/22/23-Luc), or HSVtkLuc. Each activity level is expressed as the ratio of the value measured for the construct concerned at the indicated time to the value measured 4 h postrelease. (C) Cell cycle distribution of stably transfected FR3T3 cultures at different times (0, 10, 20, 22, and 28 h) after release from serum starvation, as determined by FACS analysis.
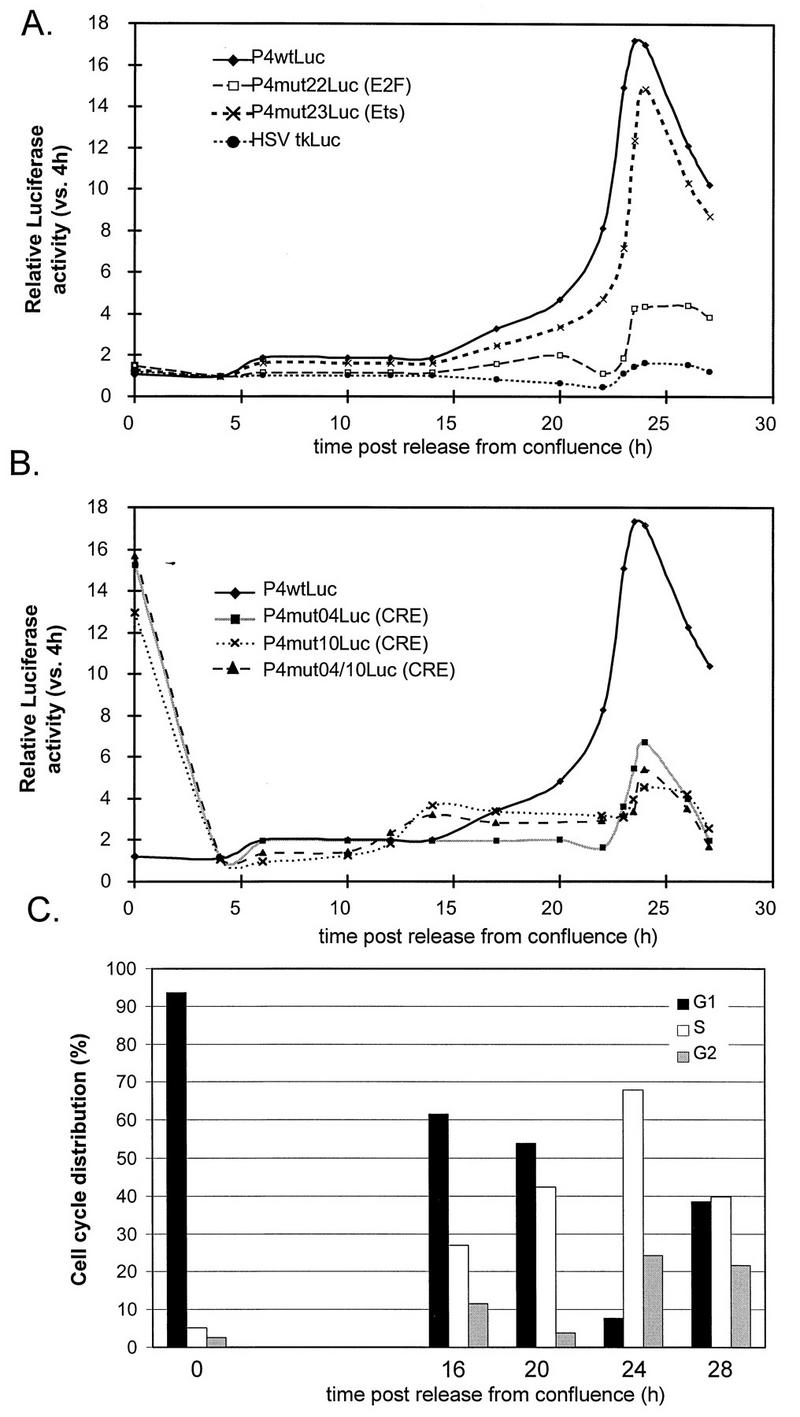
Due to contact inhibition, FR3T3 cells stably transfected with the wild-type P4 promoter construct became arrested in G1 when grown at high density (Fig. 3C). After subculturing, the cells were analyzed every 4 h to determine their cell cycle distribution and luciferase activity. Under these conditions, the cells synchronously entered a new cell cycle (Fig. 3C) and showed an approximately 16-fold increase in promoter P4 activity at the G1/S transition (Fig. 3A and B). Stably transfected control cells containing the reporter gene under the control of the HSV-1 minimal tk promoter again displayed little induction of promoter activity.
FIG. 3.
Cell cycle-dependent activity of promoter P4 in cells synchronized by contact inhibition. Pools of FR3T3 cells stably transfected with a reporter construct (P4wtLuc, a derived mutant [P4mutxLuc], or HSVtkLuc) were synchronized by contact inhibition. After release from the block, luciferase activities were measured at 4-h or shorter intervals. (A and B) Relative luciferase activities for a given construct are expressed as ratios of the values measured at the indicated time versus 4 h postrelease. (C) Cell cycle distribution of stably transfected FR3T3 cultures at different times (0, 16, 20, 24, and 28 h) after release from confluence, as determined by FACS analysis.
Around 25 h after release from either type of growth arrest, a decrease in P4 promoter activity was detected, correlating with the appearance of cells in G2 (Fig. 2 and 3). Since the cells became desynchronized later after release, it was necessary to confirm that P4 is indeed down-regulated during G2. To this end, G2-arrested cells were obtained by adding nocodazole, a drug that disturbs microtubule polymerization without affecting transcriptional processes (8). G2-blocked cells exhibited the same luciferase activity as cells released from nocodazole-imposed arrest and allowed to enter G1 (data not shown). We conclude that promoter P4 activity is differentially regulated in the course of the cell cycle, being higher in the S phase than in phases G1 and G2. These same cell cycle-specific variations in P4 promoter activity were demonstrated in P4wtLuc-transfected NIH 3T3 and A9 mouse cells synchronized by serum starvation (data not shown).
A cis-acting E2F-like binding site mediates S-phase activation of promoter P4.
The P4 promoter can interact with a variety of cellular transcription factors (1, 16, 17, 19, 36) (Fig. 1). To see whether S-phase P4 activation involves known transcription factor-binding sites or perhaps other, as yet unidentified sequences, we constructed a series of BglII linker-scanning P4 mutants, each driving the luciferase reporter gene. In each mutant construct, the BglII recognition motif (AGATCT) was substituted for a P4 promoter sequence of equal length. The whole P4 promoter and immediate downstream region were scanned with consecutive substitutions spanning nt 19 to 25 in mutant 1 (P4mut01Luc) up to nt 255 to 260 in mutant 40 (P4mut40Luc) (Fig. 1). We then generated pools of FR3T3 cells stably transfected with the different mutant P4 reporter constructs. In these cells, luciferase activity was assayed at 4-h intervals after release from serum starvation or contact inhibition.
As shown in Fig. 2B, all of the P4 mutants (illustrated for P4mut04Luc and P4mut23Luc) but one (P4mut22Luc) retained the ability of the wild-type promoter to be activated during the S phase following release from serum starvation. In P4mut22Luc, which exhibited similar luciferase activities during G1 and S, the BglII recognition motif has replaced a sequence resembling an E2F-binding site (59). To further test the involvement of this putative E2F-binding element in increasing P4 promoter activity during the S phase, we used another P4 promoter construct (P4mE2FLuc [Fig. 1]) carrying point mutations previously shown to inactivate genuine E2F-binding sites (35). This mutant also exhibited comparable activities in G1 and S (data not shown), indicating that the E2F-like element is indeed important in cell cycle-dependent regulation of the P4 promoter and is probably a functional E2F-binding site.
Further confirmation of the involvement of this site came from experiments conducted in cells released from contact inhibition, where the above-mentioned P4mut22 derivative again exhibited little activation in the S phase compared to the wild-type promoter (Fig. 3A). Interestingly, two other sites besides the E2F-like element were found to impair S-phase P4 induction after release from contact inhibition. These additional sites correspond to substitutions mut04 and mut10 (Fig. 3B), which affect known CRE motifs (36). Each of these mutations taken independently has the same effect on the cell cycle regulation of the P4 promoter as both mutations combined (Fig. 3B). The different behaviors in G1 of contact inhibition- and serum starvation-synchronized cells may reflect the fact, discussed below, that the CREs of promoter P4 exert opposite effects in contact-inhibited and serum-starved cells.
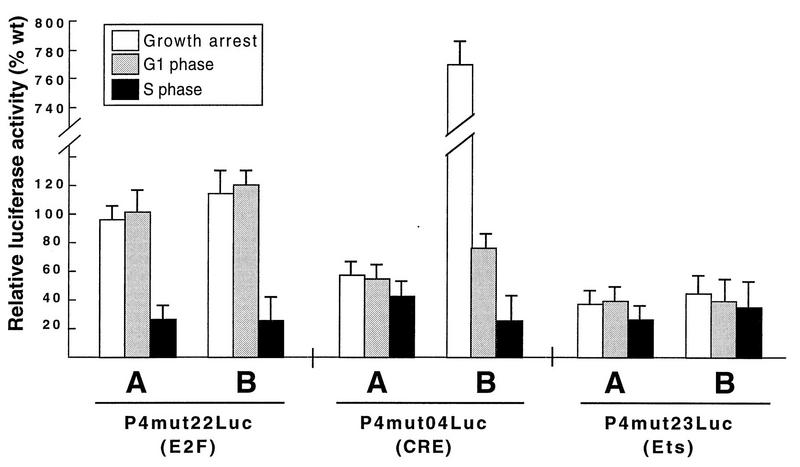
Experiments with stably transfected FR3T3 cells, like those just described, are suitable for determining changes in the activity of a given P4 promoter construct during the cell cycle, but for lack of an internal standard, they are unsuitable for comparing different promoter derivatives to determine their strengths. It is not possible, in such experiments, to determine whether the S-phase increase in P4 promoter activity mediated by the E2F-like site is due to stimulation of transcription in the S phase, inhibition in G1, or both. To address this question, we used a standardized transient transfection protocol to compare wild-type and mutant P4 promoter constructs in G1- and S-phase cells. Serum-starved FR3T3 cells were cotransfected with a wild-type or mutant reporter construct (all constructs were used in equal amounts) plus pBL4Cat, used as an internal standard to determine transfection efficiency. The cells were then released into the mitotic cycle. Luciferase activities were determined 4 and 20 h postrelease and adjusted for differences in transfection efficiency. As shown in Fig. 4 (P4mut22Luc panel), mutant P4 constructs with an altered E2F-like site behaved like wild-type constructs in arrested cells and during the G1 phase following release but displayed less reporter gene expression than the wild type during the S phase. This was true in both starvation-synchronized and contact inhibition-synchronized cells and indicates that the E2F-like motif acts specifically in the S phase to activate promoter P4. As a control, a P4 promoter construct mutated in the Ets-binding site (P4mut23Luc) was analyzed in the same way. As illustrated in Fig. 4 (P4mut23Luc panel), the mut23 substitution impaired P4 functioning to similar extents in G1- and S-phase cells, confirming that the Ets element is involved in overall P4 promoter activation (17), independently of the point in the mitotic cycle (see above).
FIG. 4.
Comparison of wild-type and mutant forms of promoter P4: activities during different phases of the cell cycle. FR3T3 cultures were growth arrested by serum starvation (A) or contact inhibition (B), transfected with equal amounts of wild-type or mutant (mut04, mut22, or mut23) P4 promoter-driven luciferase gene constructs, and released into the cell cycle. Transient expression assays were carried out by measuring luciferase activities prior to release from the block (growth arrest) and at different intervals thereafter (corresponding to the G1 and S phases [Fig. 2C and 3C]). Levels of mutant-P4-driven luciferase gene expression are shown as percentages of the values determined for the wild-type promoter at the same time points.
The P4 E2F-like motif interacts with different E2F-containing protein complexes in the course of the cell cycle.
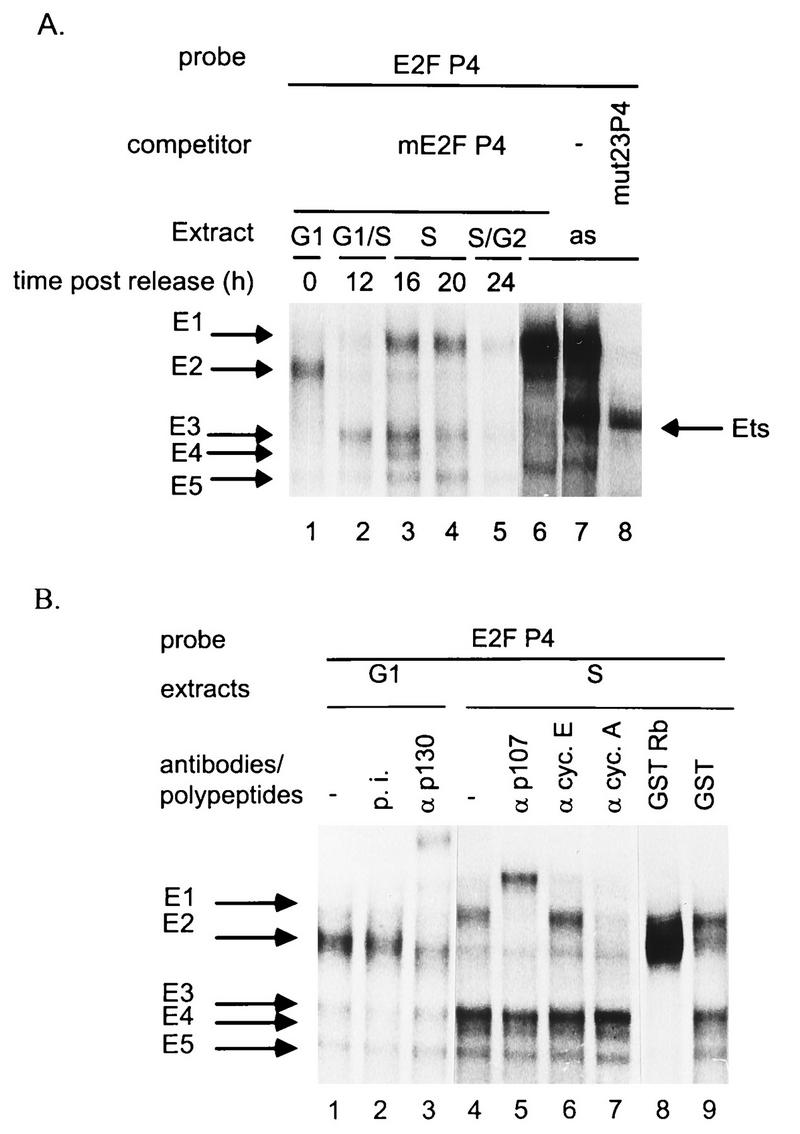
The E2F-like element of P4 lies in the proximal region of the promoter, immediately upstream from the Ets- and Sp1-binding sites (1, 17). Recently, in vivo and in vitro footprinting experiments have demonstrated its interaction with cell proteins (16). To identify these proteins, we performed gel retardation assays with crude extracts of asynchronous FR3T3 cells, using a radiolabeled oligonucleotide probe (E2FP4 [Fig. 1C]) matching a region of the P4 promoter encompassing the E2F-like and Ets motifs. As illustrated in Fig. 5A, two major and several minor protein-DNA complexes were detected (lane 7). These complexes all proved specific, since their formation was inhibited by the homologous competitor oligonucleotide but not by heterologous competitors (data not shown). The protein constituent of the lower major complex has been shown to belong to the Ets family of transcription factors (17). Formation of this complex was specifically suppressed by the mE2F competitor (Fig. 5A, lane 6) containing an Ets-binding motif but no functional E2F recognition site (17) (Fig. 1C). We therefore included the mE2F competitor in all further assays so as to detect only E2F-specific DNA-protein complexes.
FIG. 5.
Association of FR3T3 cell proteins with the P4 promoter E2F-binding site. Whole FR3T3 cell extracts were incubated with 32P-end-labeled oligonucleotide E2FP4 containing the E2F-like motif of promoter P4 (Fig. 1). Specific DNA-protein complexes (arrows) were separated by electrophoresis and revealed by autoradiography. The free probe ran out of the gel. (A) Extracts were prepared from FR3T3 cultures at various intervals after release from confluence (corresponding to the indicated phases of the cell cycle) and supplemented with a 100-fold molar excess of unlabeled mE2FP4 competitor oligonucleotide. Asynchronous cell extracts (as) supplemented (or not) with a 100-fold molar excess of unlabeled mE2FP4 or mut23P4 competitor oligonucleotides were included for comparison. The mE2FP4 competitor prevents formation of the Ets-specific complex, whereas mut23P4 competes for formation of E2F-specific complexes. (B) Supershift assays were conducted, in the presence of a 100-fold molar excess of unlabeled mE2FP4 oligonucleotide, using either antibodies (α) directed against proteins known to interact with E2F (p130, p107, and cyclins [cyc.] A and E) or recombinant proteins purified from E. coli (GST and GST-Rb fusion polypeptide). p.i., preimmune serum.
E2F is the generic name of DNA-binding heterodimers composed of a member of the E2F family and a member of the related DP family (25). In the course of the cell cycle, the transcriptional activity of E2F is differentially regulated through interactions with other proteins, in particular the product of the retinoblastoma tumor suppressor gene (pRB) and related proteins (p107 and p130), referred to as pocket proteins, as well as cyclins and cdks (24). To check that the E2F motif of promoter P4 is also the target of mitotic cycle-dependent E2F complexes, we performed gel retardation assays with the E2FP4 probe and crude extracts of synchronized FR3T3 cells harvested at different times after release from serum starvation (Fig. 5A, lanes 1 to 5). The specificity of the complexes observed under these conditions was ascertained in competition experiments with homologous and heterologous oligonucleotides (data not shown). We also performed immunoshift assays to identify the protein constituents of the resolved complexes (Fig. 5B). Extracts of serum-starved cells arrested in G0 and sustaining basal P4 activity gave rise to one major E2F-specific complex called E2 (Fig. 5A, lane 1). The E2 band was specifically supershifted upon addition of polyclonal antibodies reacting with pocket protein p130 (Fig. 5B, lane 3) and thus appears to correspond with a multiprotein complex containing at least this polypeptide in addition to E2F. P4 promoter activation at the G1/S transition was accompanied by the disappearance of the G1-specific E2 complex and the appearance of three more rapidly migrating complexes named E3, E4, and E5 (Fig. 5A, lane 2). These complexes were supershifted upon addition of bacterially expressed glutathione S-transferase (GST)–Rb fusion polypeptide to the extract (Fig. 5B, lane 8). GST alone had no effect (lane 9). GST-Rb carries the pocket region of the pRB protein (21) which specifically interacts with so-called free E2F (the E2F-DP heterodimer). Furthermore, E3-E5 complexes migrate, with respect to E1 and E2, as do the free E2F complexes of a well-characterized E2F-binding site with respect to the corresponding pocket protein-E2F complexes (44). We thus attributed formation of complexes E3, E4, and E5 to binding of free E2F. Later in the S phase, we detected the slowly migrating complex E1 in addition to the three just mentioned (Fig. 5A, lanes 3 and 4). E1 appears to be a multiprotein complex comprising E2F, cyclin A (Fig. 5B, lane 7), p107 (Fig. 5B, lane 5), and cdk2 (data not shown). All complexes faded when the cells moved into the G2 phase, i.e., when promoter P4 reverted to basal activity (Fig. 5A, lane 5). Hence, S-phase activation of P4 correlates with changes in the complex binding to the E2F site, from a G1-specific E2F-p130 complex to S-specific free E2F forms accompanied later by an E2F-cyclin A-p107-cdk2 complex.
CREs mediate P4 promoter repression in contact-inhibited cells.
As illustrated in Fig. 3B, all three mutants affected in one or both CRE motifs of promoter P4 showed hyperactivity in stable transfectants arrested in mid-G1 by contact inhibition. After dilution, this effect disappeared as the cells reentered the G1 phase of the mitotic cycle. Whether one CRE, the other, or both were mutated, the G1 effect was the same. To determine whether the CRE elements mediate promoter P4 repression in confluent cells, activation in growing cells, or both, we again performed transient transfection assays as described above for analysis of the E2F-like binding site, this time comparing the wild-type construct with ones carrying a mutated CRE. Since all three CRE mutants displayed similar regulation through the cell cycle, we limited our study to the P4mut04Luc construct.
As shown in Fig. 4B (P4mut04Luc panel), the CRE acted in cis both as a silencing element in contact-inhibited cells and as an activating element in growing cells released from confluence. CRE-mediated activation was also observed in growing cells released from serum starvation. In the latter case, the CRE contributed similarly to P4 activity during G1 and S (Fig. 4A, P4mut04Luc panel). This means that it has little to do with S-phase P4 induction (cf. Fig. 2B). Interestingly, the CRE appeared to play a different role in serum-starved cells than in contact-inhibited cells: in the former, CRE-mediated activation rather than repression was observed (Fig. 4A, P4mut04Luc panel). P4 silencing through the CREs thus appears unrelated to cell growth arrest per se but rather specific to contact inhibition. This was further ascertained by transiently transfecting asynchronous FR3T3 cell cultures with constructs driven by wild-type and CRE-mutant P4 promoters and measuring promoter activity at different cell densities during subsequent growth. In agreement with the activating role of CREB/ATF transcription factors in proliferating cells (see above and reference 36), the CRE mutant was two to three times weaker than the wild-type promoter in subconfluent cultures. As the cells reached confluence, the activity of the wild-type promoter progressively decreased, the CRE mutant being less affected and becoming up to 15 times more potent than the wild-type P4 (data not shown). Release from contact inhibition caused the CRE to switch from an inhibitory to an activating element (Fig. 4B, P4mut04Luc panel). This may explain why S-associated induction of promoter P4 is stronger in cells synchronized by contact inhibition than in cells synchronized by serum starvation (cf. Fig. 2B and 3B).
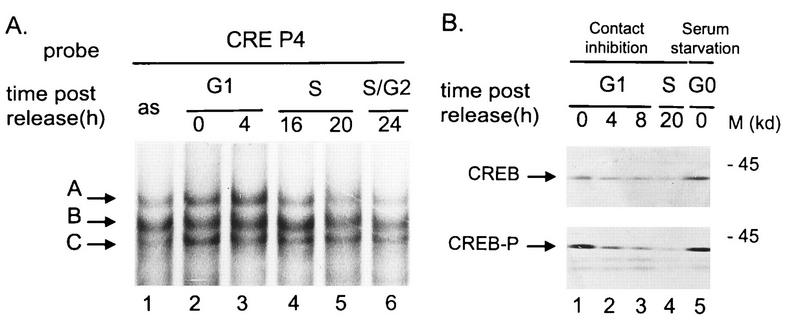
Due to their location within the left-hand terminal palindromic sequence of the parvoviral genome, the two CREs are mirror images of each other (36). Both interact with the same proteins of the ATF/CREB transcription factor family, forming three specific protein-DNA complexes (labeled A, B, and C in Fig. 6A) which can be visualized in gel retardation assays (36). The protein constituents of these three complexes have been analyzed by immunoshift assays and in vitro reconstitution experiments using ATF/CREB proteins purified from recombinant baculovirus-infected Spodoptera frugiperda SF9 cells. Complexes A and B result from binding of CREB1 homodimers and CREB1/ATF1 heterodimers, respectively, while C is a still elusive complex comprising ATF1 (36a). To detect possible cell cycle-dependent changes in protein-CRE interactions, we performed gel retardation assays with an oligonucleotide probe (CREP4 [Fig. 1C]) bearing the CRE a element of the P4 promoter, using FR3T3 whole-cell extracts prepared at different times after release from contact inhibition. Complexes A, B, and C were detected irrespective of the phase in which the cells were harvested (Fig. 6A, lanes 2 to 6). However, it is worth noting that the relative abundance of complex A was lower when the CREP4 oligonucleotide was incubated with S- and G2-specific extracts compared with G1 extracts. DNA binding of transcription factor CREB1 (responsible for the formation of complex A) requires phosphorylation of a serine at position 133 (12). Western blotting analysis of the above-mentioned cell extracts was performed to detect possible cell cycle variations in CREB1 production and/or phosphorylation. The total amount of CREB1 was found to decrease when cells reentered the mitotic cycle after contact inhibition (Fig. 6B, upper panel, lanes 1 to 4). Phosphorylated CREB1 was likewise down-regulated (Fig. 6B, lower panel, lanes 1 to 4), so that the ratio of phosphorylated to total CREB1 remained unchanged. Furthermore, serum-starved cells were similar to contact-inhibited cells with regard to their content in both total and phosphorylated CREB1 (Fig. 6B, lanes 5). We conclude that the CRE-mediated promoter P4 inhibition specific to contact-inhibited cells cannot be explained by the observed variation of CREB1 steady-state levels and does not correlate with a significant change in CREB1 phosphorylation.
FIG. 6.
FR3T3 cell proteins interacting with the P4 promoter CRE motif. (A) Extracts from asynchronous FR3T3 cultures (as) or from FR3T3 cells harvested at different time points after release from contact inhibition were incubated with 32P-end-labeled oligonucleotide CREP4 (Fig. 1). Specific DNA-protein complexes (arrows; see reference 36) were fractionated by electrophoretic mobility shift assays and revealed by autoradiography. The free probe ran out of the gel. (B) Extracts from FR3T3 cells released from contact inhibition (lanes 1 to 4) or blocked by serum starvation (lanes 5) were analyzed by Western blotting for their content of CREB1. Upper panel, total CREB1; lower panel, CREB1 phosphorylated on serine residue 133. The antibody specifically recognizing phosphorylated CREB1 did not react with CREBs when the extracts were pretreated with phage lambda phosphatase (data not shown). M, apparent molecular mass of proteins standards.
CKI p27KIP1 accumulates in FR3T3 cells grown to a high density and converts CRE motifs from activating to inhibitory elements.
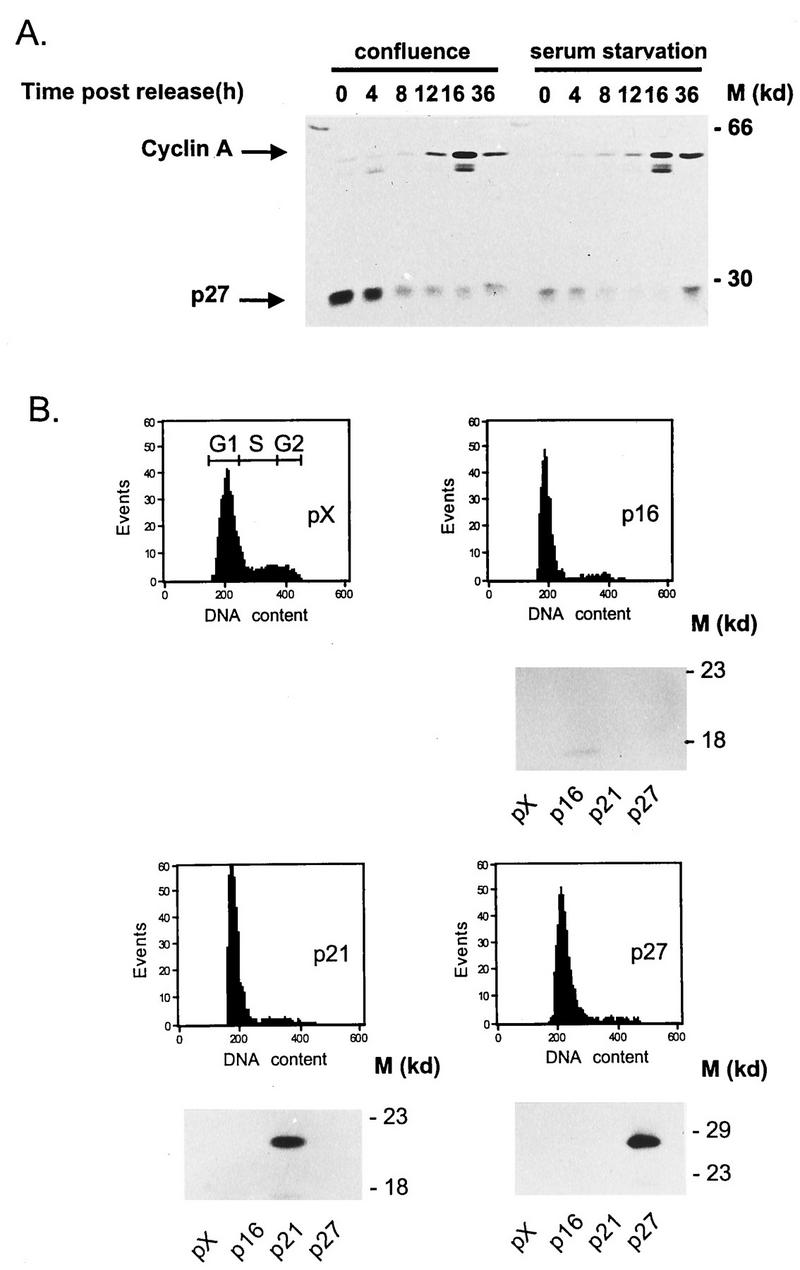
It is worth noting at this point that serum-starved and contact-inhibited cells are not arrested in exactly the same state. Growth arrest induced by serum starvation (best described as a G0 state) is accompanied by down-regulation of genes encoding proteins required for cell cycle progression (such as cyclins [61]) and by stabilization of the CKIs p16 and p21 (61). In contrast, growth suppression at high cell density is mainly due to failure of another CKI, p27, to be degraded (39). Similar stabilization of p27 has been found to correlate with cell growth arrest in mid-G1 following lovastatin treatment (20). Interestingly, we observed CRE-dependent inhibition of P4 promoter activity by lovastatin in the above-mentioned stable transfectants harboring the luciferase-encoding plasmid (data not shown). Furthermore, p27 was found to accumulate in confluent but not in serum-starved FR3T3 cells and to be quickly degraded upon cell release from contact inhibition, before the induction of cyclin A production that marks the S phase (Fig. 7A). These observations suggest that CRE-mediated inhibition of the P4 promoter in contact-inhibited cells could involve CKI p27.
FIG. 7.
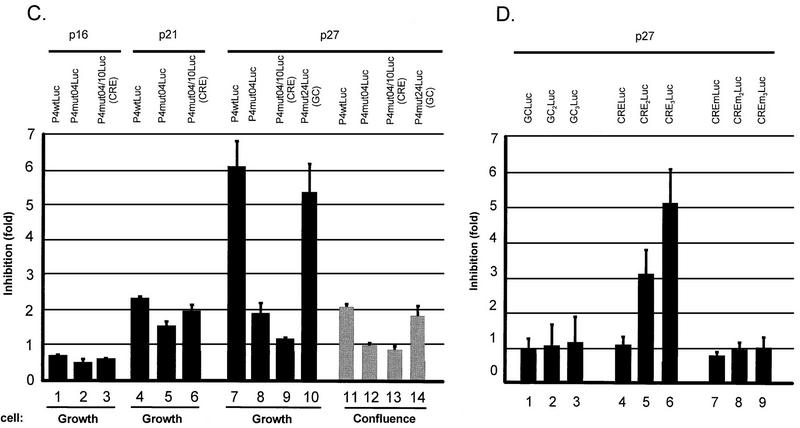
Influence of p27 overexpression on whole and minimal P4 promoter construct activities. (A) Whole protein extracts were prepared from FR3T3 cells at increasing times after release from confluence or serum starvation (see Fig. 2C and 3C for cell cycle progression). Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The upper and lower parts of the blot were incubated with antibodies directed against cyclin A and CKI p27, respectively. Immunocomplexes were revealed with the ECL detection system. M, apparent molecular masses of protein standards. (B) FACS analysis of FR3T3 cultures cotransfected with either of the CKI-expressing plasmid (p16, p21, or p27) or with the corresponding empty vector (pX). CKI overexpression was verified by Western blot analysis shown below the FACS profiles. (C) Growing or confluent FR3T3 cell cultures were cotransfected with each of the indicated P4Luc reporter plasmids (Fig. 1B) and either p16, p21, or p27 CKI-expressing vector. Transient expression assays were carried out at 48 h posttransfection and are presented as ratios of luciferase activities achieved in the presence of empty versus CKI-expressing vector (i.e., as factors of CKI-induced promoter repression). (D) Growing FR3T3 cell cultures were cotransfected with each of the indicated minimal promoter constructs (see Materials and Methods) and p27-expressing vector. Transient expression assays were carried out at 48 h posttransfection and are presented as ratios of luciferase activities achieved in the presence of empty versus p27-expressing vector (i.e., as factors of p27-induced promoter repression).
To test this possibility directly, we cotransfected asynchronous FR3T3 cells with a CKI (p16, p21, or p27)-encoding plasmid and a construct bearing a reporter gene driven by wild-type or mutant promoter P4. As shown in Fig. 7B, plasmid-driven overexpression of all tested CKIs could be detected by Western blotting and led to cell cycle arrest in G1. Furthermore, overexpression of CKI p27 resulted in P4 promoter inhibition (Fig. 7C, column 7). This inhibitory effect of p27 required the presence of two functional CRE motifs (Fig. 7C, columns 8 and 9) but did not depend on an intact proximal GC box, as shown by the behavior of the mut24 derivative, in which this box is replaced by a BglII site (Fig. 1A). This derivative proved as sensitive to p27 overexpression as the wild-type promoter (Fig. 7C, column 10). In contrast, overexpression of CKIs p21 and p16 had little or no effect on the activity of the P4 promoter, whether wild type or mutated (Fig. 7C, columns 1 to 6). The slight repression observed when p21 was overexpressed proved to be CRE independent (Fig. 7C, columns 5 and 6). The CRE motifs thus appear as specific targets for p27-induced inhibition of the P4 promoter. In cells grown to confluence, plasmid-driven p27 synthesis resulted only in limited (yet significant and CRE-mediated) suppression of promoter activity (Fig. 7C, columns 11 to 14). This result is in keeping with the high level of p27 naturally accumulated by contact-inhibited cells (see above).
To confirm the ability of the P4 CREs to mediate p27-induced promoter silencing, one to three copies of the CRE a′ motif were cloned in front of a minimal promoter consisting of the P4 TATA box and downstream sequences. Constructs containing at least two copies of the CRE proved sensitive to plasmid-driven p27 overexpression (Fig. 7D, columns 5 and 6), while a construct comprising only one CRE did not respond (Fig. 7D, column 4). Minimal promoters supplemented with one to three repeats of the same length but containing a GC box (Fig. 7D, columns 1 to 3) or a mutated (BglII substitution) CRE (Fig. 7D, columns 7 to 9) likewise failed to respond to p27. When present in at least two copies, the P4 CREs are thus both necessary and sufficient to mediate the repressive effect of p27, even outside the context of the full P4 promoter.
DISCUSSION
We have shown here that regulation of the P4 promoter of parvovirus MVMp is host cell cycle dependent. Analysis of a series of linker-scanning mutants of promoter P4 has enabled us to distinguish two components of this regulation: activation at the G1/S transition and repression specific to growth arrest at high density (contact inhibition). We have further identified cis-acting elements within the promoter that mediate these two processes. A binding site for the E2F transcription factor is the major determinant of S-associated P4 activation, while two CREs mediate promoter silencing in contact-inhibited cells.
The E2F-binding site is required for P4 promoter activation when host cells enter the S phase. Promoter P4 drives the transcription unit encoding the parvoviral NS proteins, particularly the major product NS1, essential to amplification and expression of viral DNA (10). Hence E2F-mediated S-phase induction of NS gene expression may contribute, along with the S-phase specificity of host factors directly involved in the viral life cycle, to the well-known dependence of parvovirus replication upon host cell proliferation (11). The present results indicate that destruction of the E2F motif leads to an 80% reduction of promoter P4 activity in the S phase, without affecting the basal expression measured in G1. Furthermore, data to be presented elsewhere substantiate the importance of the E2F-binding site for the parvoviral life cycle, showing that S-phase induction of promoter P4 is necessary for the burst of parvoviral DNA replication and gene expression, and that mutations in E2F that abolish S-phase activation of P4 inactivate the virus unless the NS proteins are provided in trans (11a). Thus, S-phase activation of promoter P4 provides a clue to the S phase dependence of parvovirus multiplication.
The generic name E2F designates heterodimeric transcription factors composed of two polypeptides, an E2F and a DP family member. Currently, five distinct E2F and three DP proteins have been characterized (25, 26, 33). The transcriptional activity of E2F-DP dimers, often referred to as free E2F, is regulated according to the point in the cell cycle by formation of higher-order complexes. In particular, interaction with members of the pocket protein family (pRB, p130, and p107) prevents E2F from activating responsive promoters. It can even lead to repression of some promoters (50). Binding of pocket proteins to E2F heterodimers (and hence the activity of the E2F transcription factor) is regulated by pocket protein phosphorylation, apparently catalyzed mainly by cell cycle-regulated cdks (14, 53). E2F proteins interact with cyclins and cdks either directly or through pocket proteins. After phosphorylation by cyclin-cdk complexes at the G1/S transition, pocket proteins can no longer bind to E2F proteins. This leads to the formation of transcriptionally active free E2F, known to transactivate target promoters at the G1/S transition (34). Accordingly, variations in P4 promoter activity throughout the cell cycle correlate with binding of different complexes to the E2F-binding site. During the G1 phase, the site is occupied by an E2F-p130 protein complex. In agreement with the ability of p130 to prevent E2F from activating transcription (56), only basal P4 activity is detected in G1. Yet contrary to some other promoters (55), P4 does not appear to be a target of p130-mediated repression. P4 activation at the G1/S transition is accompanied by replacement of the E2F-p130 complex by free E2F at the E2F-binding site, in keeping with the view that free dimers are the transcription-enhancing forms of E2F (4). Transient P4 hyperactivity during the S phase coincides with maintenance of free E2F at this site and the appearance of an additional higher-order complex containing p107, cyclin A, and cdk2. This complex is thought to be involved in the later inhibition of E2F-mediated transactivation (4). All of these complexes fade in late S and G2, when the P4 promoter reverts to its basal activity. Proteins p130 and p107 interact specifically with certain members of the E2F family, in particular E2F4 and E2F5 (4). Since these pocket proteins are present in some of the complexes formed with the P4 E2F-binding site, E2F4 and E2F5 are candidate constituents of these complexes.
We have further shown that CREs present within the P4 sequence mediate promoter down-regulation in contact-inhibited FR3T3 cells. This silencing effect is restricted to G1 arrest at high cell density. Interestingly, this repression was observed only in the presence of two functional CRE motifs. When contact-inhibited cells are diluted and allowed to reenter the mitotic cycle, the CREs switch to mediating promoter activation. In most situations, in fact, these CREs appear to mediate up-regulation of the P4 promoter, notably in cells arrested in G0/G1 by serum starvation or released from this block into the mitotic cycle and allowed to progress through the various phases. Contact inhibition thus represents a special situation in which the CRE-mediated effect on P4 promoter activity is negative rather than positive. Two regulatory pathways with opposite transcriptional effects thus appear to converge on the CREs of the P4 promoter, whose response clearly distinguishes contact inhibition from serum starvation.
Cell cycle progression is regulated by the sequential activation of different cyclins and cdks (37, 38). Inhibitors of specific cyclin-cdk complexes (the CKIs) play key roles in this regulatory network (15, 31). Serum starvation causes cycle arrest in the G0/G1 phase, mainly as a result of reduced expression of a variety of proteins including the D, E, and A cyclins (58). Release from serum starvation allows cyclin reexpression and subsequent cell cycle progression (41). In contrast, contact inhibition causes induction of various CKIs, notably p27KIP1, leading to cell cycle arrest in mid-G1 through inhibition of G1 cyclin-cdk complexes (31, 39). Release from contact inhibition is accompanied by degradation of p27KIP1, allowing reentry into the cell cycle. Hence, serum starvation and confluence cause growth arrest via different mechanisms. This may explain the opposite regulatory effects of CREs on the P4 promoter in these two situations. In keeping with this view, we have confirmed, first, that contact-inhibited FR3T3 cells have a much higher steady-state level of p27KIP1 than serum-starved or growing cultures. We have further shown more directly that plasmid-driven overexpression of p27KIP1 has an inhibitory effect on P4 promoter activity. This effect was striking in growing cultures but limited in contact-inhibited cells, which argues in favor of the view that it occurs naturally in the latter.
On the basis of sequence similarities, CKIs fall into one of two families. Proteins p16, p15, p18, and p19 belong to the INK4 family and specifically inhibit cyclin-cdk4 and cyclin-cdk6 complexes. Proteins p21, p27, and p57 belong to the CIP/KIP family and interfere with the activity of most cyclin-cdk complexes (47). The CIP/KIP inhibitors p21 and p27 carry out similar tasks in cell cycle control, inactivating the same cyclin-cdk complexes. They share a common N-terminal residue sequence believed to interact with the target complexes (30). They differ from the INK4 family CKIs in that they do not appear to require the retinoblastoma gene product pRB to inhibit cell-cycle progression (30). Experiments with p21 and p27 knockout mice suggest functional compensation between these CKIs in controlling cell proliferation during development (32). On the other hand, the activities of p21 and p27 do not overlap entirely, because their C-terminal sequences differ. Protein p21, but not p27, interacts with the proliferating cell nuclear antigen (PCNA) and inhibits PCNA-dependent DNA replication (29). The present data point to an additional functional difference between p21 and p27, i.e., the ability of the latter but not the former to convert CRE-binding transcriptional activators into repressors. Like promoter inhibition in confluent cells, this p27-mediated conversion was observed only when at least two CRE motifs were present.
In conclusion, the present work has uncovered two distinct cell cycle-related regulatory pathways affecting parvoviral gene expression. The first involves the E2F factor; it was known from other systems but is of special interest in parvovirus research because it imposes an early limitation on transcription, rendering progression of the viral life cycle dependent on entry of host cells into the S phase. The second is mediated by cis-acting CREs and appears specific to growth arrest by contact inhibition. It involves CRE-mediated promoter repression brought about by the CKI p27KIP1. This new function of p27 distinguishes it from p21, another member of the CIP/KIP kinase inhibitor family with which it shares several activities. The molecular mechanism by which p27KIP1 interferes with the activity of CREBs remains to be unraveled. The P4 CREs have recently been shown, in rat fibroblasts, to interact with transcription factors of the ATF/CREB family (36), factors known to be regulated by various kinases (36, 43). No correlation was observed between CRE-mediated repression and the phosphorylation state of CREB1. Given the multitude of proteins interacting with CREs and the cooperative action of the two CREs in p27-mediated repression, it could be interesting to identify new polypeptides binding to such CRE motif repeats. A worthy focus of future research might thus be the influence of kinase inhibitor p27 on the function of CRE-protein complexes and the phosphorylation of the protein constituents.
ACKNOWLEDGMENTS
We are grateful to Jan J. Cornelis and Pidder Jansen-Dürr for fruitful discussions.
This work was supported by the Commission of the European Communities. L.D. and F.F. are fellows, of the Commission of the European Communities and of the Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture, Belgium, respectively.
REFERENCES
- 1.Ahn J K, Gavin B J, Kumar G, Ward D C. Transcriptional analysis of minute virus of mice P4 promoter mutants. J Virol. 1989;63:5425–5439. doi: 10.1128/jvi.63.12.5425-5439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astell C R, Thomson M, Merchlinsky M, Ward D C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983;11:999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel I, Frederick M. Introduction of DNA into mammalian cells. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 9.7.12–9.7.18. [Google Scholar]
- 4.Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 5.Boeuf H, Reimund B, Jansen Durr P, Kedinger C. Differential activation of the E2F transcription factor by the adenovirus E1a and EIV products in F9 cells. Proc Natl Acad Sci USA. 1990;87:1782–1786. doi: 10.1073/pnas.87.5.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaney W G, Howard D R, Pollard J W, Sallustio S, Stanley P. High-frequency transfection of CHO cells using polybrene. Somatic Cell Mol Genet. 1986;12:237–244. doi: 10.1007/BF01570782. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou C F, Omary M B. Mitotic arrest with anti-microtubule agents or okadaic acid is associated with increased glycoprotein terminal GlcNAc’s. J Cell Sci. 1994;107:1833–1843. doi: 10.1242/jcs.107.7.1833. [DOI] [PubMed] [Google Scholar]
- 9.Christensen J, Cotmore S F, Tattersall P. Minute virus of mice transcriptional activator protein NS1 binds directly to the transactivation region of the viral P38 promoter in a strictly ATP-dependent manner. J Virol. 1995;69:5422–5430. doi: 10.1128/jvi.69.9.5422-5430.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotmore S F, Christensen J, Nüsch J P F, Tattersall P. The NS1 polypeptide of murin parvovirus minute virus of mice binds to DNA sequences containing the motif [ACCA]2-3. J Virol. 1995;69:1652–1660. doi: 10.1128/jvi.69.3.1652-1660.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 11a.Deleu, L., et al. Unpublished data.
- 12.Della Fazia M A, Servillo G, Sassone-Corsi P. Cyclic AMP signalling and cellular proliferation: regulation of CREB and CREM. FEBS Lett. 1997;410:22–24. doi: 10.1016/s0014-5793(97)00445-6. [DOI] [PubMed] [Google Scholar]
- 13.Desdouets C, Matesic G, Molina C A, Foulkes N S, Sassone Corsi P, Brechot C, Sobczak Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dynlacht B D, Flores O, Lees J A, Harlow E. Differential regulation of E2F trans-activation by cyclin/cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 15.Elledge S J, Winston J, Harper J W. A question of balance: the role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 1996;6:388–392. doi: 10.1016/0962-8924(96)10030-1. [DOI] [PubMed] [Google Scholar]
- 16.Faisst S, Perros M, Deleu L, Spruyt N, Rommelaere J. Mapping of upstream regulatory elements in the P4 promoter of parvovirus minute virus of mice. Virology. 1994;202:466–470. doi: 10.1006/viro.1994.1363. [DOI] [PubMed] [Google Scholar]
- 17.Fuks F, Deleu L, Dinsart C, Rommelaere J, Faisst S. ras oncogene-dependent activation of the P4 promoter of minute virus of mice through a proximal P4 element interacting with the Ets family of transcription factors. J Virol. 1996;70:1331–1339. doi: 10.1128/jvi.70.3.1331-1339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouilleux F, Sola B, Couette B, Foy H R. Cooperation between structural elements in hormono-regulated transcription from the mouse mammary tumor virus promoter. Nucleic Acids Res. 1991;19:1563–1569. doi: 10.1093/nar/19.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Z, Plaza S, Perros M, Cziepluch C, Rommelaere J, Cornelis J J. NF-Y controls transcription of the minute virus of mice P4 promoter through interactions with an unusual binding site. J Virol. 1994;69:239–246. doi: 10.1128/jvi.69.1.239-246.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hengst L, Reed S I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 21.Hiebert S W, Chellappan S P, Horowitz J M, Nevins J R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- 22.Kawai S, Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984;4:1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar V, Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988;55:145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- 24.La Thangue N B. DP and E2F proteins: components of a heterodimeric transcription factor implicated in cell cycle control. Curr Opin Cell Biol. 1994;6:443–450. doi: 10.1016/0955-0674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 25.La Thangue N B. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994;19:108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 26.La Thangue N B. E2F and the molecular mechanisms of early cell-cycle control. Biochem Soc Trans. 1996;24:54–59. doi: 10.1042/bst0240054. [DOI] [PubMed] [Google Scholar]
- 27.Lorson C, Burger L R, Mouw M, Pintel D J. Efficient transactivation of the minute virus of mice P38 promoter requires upstream binding of NS1. J Virol. 1996;70:834–842. doi: 10.1128/jvi.70.2.834-842.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luckow B, Schuetz G. CAT constructs with multiple unique restriction sites for functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987;15:8451–8462. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375:159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Castellanos C, Moreno S. Recent advances on cyclins, CDKs and CDK inhibitors. Trends Cell Biol. 1997;7:95–98. doi: 10.1016/S0962-8924(96)10055-6. [DOI] [PubMed] [Google Scholar]
- 31.Massague J, Polyak K. Mammalian antiproliferative signals and their targets. Curr Opin Genet Dev. 1995;5:91–96. doi: 10.1016/s0959-437x(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 32.Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto G P. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 33.Mudryj M, Hiebert S W, Nevins J R. A role for the adenovirus inducible E2F transcription factor in a proliferation dependent signal transduction pathway. EMBO J. 1990;9:2179–2184. doi: 10.1002/j.1460-2075.1990.tb07387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuman E, Flemington E K, Sellers W R, Kaelin W G. Transcription of the E2F-1 gene is rendered cell cycle dependent by E2F DNA-binding sites within its promoter. Mol Cell Biol. 1994;14:6607–6615. doi: 10.1128/mcb.14.10.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagano M, Durst M, Joswig S, Draetta G, Jansen-Dürr P. Binding of the human E2F transcription factor to the retinoblastoma protein but not to cyclin A is abolished in HPV-16-immortalized cells. Oncogene. 1992;7:1681–1686. [PubMed] [Google Scholar]
- 36.Perros M, Deleu L, Vanacker J M, Kherrouche Z, Spruyt N, Faisst S, Rommelaere J. Upstream CREs participate in the basal activity of minute virus of mice promoter P4 and in its stimulation in ras-transformed cells. J Virol. 1995;69:5506–5515. doi: 10.1128/jvi.69.9.5506-5515.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Perros, M., et al. Submitted for publication.
- 37.Pines J. Cyclins and cyclin-dependent kinases: theme and variations. Adv Cancer Res. 1995;66:181–212. doi: 10.1016/s0065-230x(08)60254-7. [DOI] [PubMed] [Google Scholar]
- 38.Pines J. Cyclins, CDKs and cancer. Semin Cancer Biol. 1995;6:63–72. doi: 10.1006/scbi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 39.Polyak K, Kato J Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 40.Polyak K, Lee M H, Erdjument Bromage H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 41.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhode S D. Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973;11:856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sassone-Corsi P. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- 44.Schulze A, Zerfass K, Spitkovsky D, Middendorp S, Berges J, Helin K, Jansen-Dürr P, Henglein B. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc Natl Acad Sci USA. 1995;92:11264–11268. doi: 10.1073/pnas.92.24.11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seed B, Sheen J Y. A simple phase-extraction assay for chloramphenicol acetyl-transferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 46.Shao Z H, Robbins P D. Differential regulation of E2F and Sp1-mediated transcription by G1 cyclins. Oncogene. 1995;10:221–228. [PubMed] [Google Scholar]
- 47.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 48.Shiyanov P, Bagchi S, Adami G, Kokontis J, Hay N, Arroyo M, Morozov A, Raychaudhuri P. p21 disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996;16:737–744. doi: 10.1128/mcb.16.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegl G, Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. II. Biochemical characteristics of virus replication. Arch Gesamte Virusforsch. 1973;40:119–127. doi: 10.1007/BF01242643. [DOI] [PubMed] [Google Scholar]
- 50.Smith E J, Nevins J R. The Rb-related p107 protein can suppress E2F function independently of binding to cyclin A/cdk2. Mol Cell Biol. 1995;15:338–344. doi: 10.1128/mcb.15.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Southern P J, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under the control of the SV40 early region promoter. J Mol Appl Genet. 1982;1:327–341. [PubMed] [Google Scholar]
- 52.Spegelaere P, van Hille B, Spruyt N, Faisst S, Cornelis J J, Rommelaere J. Initiation of transcription from the minute virus of mice P4 promoter is stimulated in rat cells expressing a c-Ha-ras oncogene. J Virol. 1991;65:4919–4928. doi: 10.1128/jvi.65.9.4919-4928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki Takahashi I, Kitagawa M, Saijo M, Higashi H, Ogino H, Matsumoto H, Taya Y, Nishimura S, Okuyama A. The interactions of E2F with pRB and with p107 are regulated via the phosphorylation of pRB and p107 by a cyclin-dependent kinase. Oncogene. 1995;10:1691–1698. [PubMed] [Google Scholar]
- 54.Tattersall P, Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983;46:944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tommasi S, Pfeifer G P. In vivo structure of the human cdc2 promoter: release of a p130–E2F-4 complex from sequences immediately upstream of the transcription initiation site coincides with induction of cdc2 expression. Mol Cell Biol. 1995;15:6901–6913. doi: 10.1128/mcb.15.12.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vairo G, Livingston D M, Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 57.Vanacker J M, Corbau R, Adelmant G, Perros M, Laudet V, Rommelaere J. Transactivation of a cellular promoter by the NS1 protein of the parvovirus minute virus of mice through a putative hormone-responsive element. J Virol. 1996;70:2369–2377. doi: 10.1128/jvi.70.4.2369-2377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Won K A, Xiong Y, Beach D, Gilman M Z. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc Natl Acad Sci USA. 1992;89:9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yee A S, Raychaudhuri P, Jakoi L, Nevins J R. The adenovirus-inducible factor E2F stimulates transcription after specific DNA binding. Mol Cell Biol. 1989;9:578–585. doi: 10.1128/mcb.9.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshizumi M, Hsieh C M, Zhou F, Tsai J C, Patterson C, Perrella M A, Lee M E. The ATF site mediates downregulation of the cyclin A gene during contact inhibition in vascular endothelial cells. Mol Cell Biol. 1995;15:3266–3272. doi: 10.1128/mcb.15.6.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zerfass K, Schulze A, Spitkovsky D, Friedman V, Henglein B, Jansen-Dürr P. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J Virol. 1995;69:6389–6399. doi: 10.1128/jvi.69.10.6389-6399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zerfass-Thome K, Schulze A, Zwerschke W, Vogt B, Helin K, Bartek J, Henglein B, Jansen-Dürr P. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol Cell Biol. 1997;17:407–415. doi: 10.1128/mcb.17.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]