Abstract
Non-small cell lung cancer (NSCLC) accounts for the majority of lung cancer cases and remains the leading cause of cancer-related mortality worldwide. Firstly, this review explores the limitations of conventional therapies, chemotherapy, radiotherapy, and surgery, focusing on the development of drug resistance and significant toxicity that often hinder their efficacy. Thereafter, advancements in targeted therapies, such as immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs), are discussed, highlighting their impact on improving outcomes for patients with specific genetic mutations, including c-ros oncogene 1 receptor tyrosine kinase (ROS1), anaplastic lymphoma kinase (ALK), and epidermal growth factor receptor (EGFR). Additionally, the emergence of novel immunotherapies and phytochemicals is examined, emphasizing their potential to overcome therapeutic resistance, particularly in advanced-stage diseases. The review also delves into the role of next-generation sequencing (NGS) in enabling personalized treatment approaches and explores the clinical potential of innovative agents, such as bispecific T-cell engagers (BiTEs) and antibody-drug conjugates (ADCs). Finally, we address the socioeconomic barriers that limit the accessibility of these therapies in low-resource settings and propose future research directions aimed at improving the long-term efficacy and accessibility of these treatments.
Keywords: Lung cancer, Targeted therapy, Drug resistance, Immune checkpoint inhibitors (ICIs), Next-generation sequencing (NGS)
Graphical abstract
Highlights
-
•
A comprehensive review of lung cancer therapies, including chemotherapy, targeted therapy, and immunotherapy.
-
•
In-depth exploration of drug resistance mechanisms, cellular plasticity, and critical signaling pathways (EGFR, ALK, and ROS1).
-
•
Summary of emerging therapies, including antibody-drug conjugates (ADCs) and phytochemicals, to address drug resistance.
-
•
Emphasis on the role of next-generation sequencing (NGS) and molecular profiling in advancing personalized treatment approaches for lung cancer.
1. Introduction
Lung cancer, a complex and multifactorial disease, is characterized by the uncontrolled rapid proliferation of abnormal cells in lung tissues, which ultimately results in the formation of malignant tumors [1]. Lung cancer is classified histopathologically into two primary categories: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), with NSCLC accounting for approximately 85% of all cases [2]. NSCLC can be further divided into subtypes with different molecular and histological profiles, such as large-cell lung cancer, lung adenocarcinoma, and squamous-cell carcinoma [3]. This heterogeneity within NSCLC poses significant challenges in developing effective targeted therapies, as each subtype may exhibit differential responses to treatment due to unique genetic mutations and tumor microenvironmental factors. Approximately 18% of all cancer deaths are caused by lung cancer, which is the world's leading cause of cancer-related mortality, according to a comprehensive analysis of current epidemiological data. In 2020, over 1.8 million new lung cancer cases were reported worldwide, accounting for 11.4% of all newly diagnosed cancers [4]. Lung cancer continues to pose a significant global health challenge, characterized by elevated mortality rates primarily attributed to late-stage diagnosis and the emergence of resistance to existing treatments. While current therapies such as chemotherapy, radiotherapy, and immunotherapy have provided some clinical benefits, they often fail due to complex resistance mechanisms within tumor cells. Recent research has emphasized the importance of understanding the molecular pathways in lung cancer progression to develop more effective treatments [5]. Specifically, long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) are key mediators of sensitivity and resistance to therapies, such as chemotherapy based on cisplatin [6]. Overcoming chemotherapy resistance is one of the central challenges in the treatment of lung cancer. Studies have shown that certain miRNAs, such as miR-21, contribute to cisplatin resistance by downregulating pro-apoptotic genes, leading to tumor survival and progression [7]. Additionally, lncRNAs, including exosomal lncRNAs, have been implicated in altering the tumor microenvironment (TME), thus promoting drug resistance and tumor progression [8].
We consider several key clinical and molecular criteria to evaluate the effectiveness of these emerging therapies. Clinically, therapies are assessed based on their impact on objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) in clinical trials. These endpoints are critical for determining the real-world efficacy of the therapies in extending patient survival and preventing disease progression. Toxicity profiles, including the severity and frequency of adverse events, are also important for balancing efficacy with safety. At the molecular level, therapies are evaluated based on their ability to target specific genetic mutations or pathways, such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and c-ros oncogene 1 receptor tyrosine kinase (ROS1) mutations. Additionally, the mechanisms by which these therapies overcome drug resistance, whether through direct inhibition of oncogenic drivers or modulation of the TME, are crucial for determining long-term effectiveness. The ability of emerging therapies to circumvent resistance mechanisms, particularly in the context of immune evasion and acquired mutations, is a significant focus of ongoing research.
In men, lung cancer remains the leading cause of cancer-related deaths, while in women, it ranks second in mortality and third in incidence. Gender-based disparities in lung cancer incidence and mortality are noteworthy. These gender differences are largely attributable to historical patterns of tobacco consumption, which remains the primary risk factor for lung cancer development [4]. Cellular plasticity plays a crucial role in developing drug resistance in lung cancer. The ability of cancer cells to transition between different phenotypic states, such as epithelial-mesenchymal transition (EMT), contributes to the heterogeneity within tumors and allows subpopulations of cells to evade therapeutic interventions. EMT has been linked to resistance mechanisms against both chemotherapy and targeted therapies by promoting cancer cell survival, invasion, and metastasis. Additionally, the TME and its interactions with immune cells and fibroblasts further promote cellular plasticity, contributing to drug resistance. Recent studies have shown that targeting key regulators of cellular plasticity, such as SRY-related HMG-box gene 11 (SOX11), can restore sensitivity to therapies and improve patient outcomes.
Molecular studies have identified several critical oncogenes and tumor suppressors in lung cancer pathogenesis. SOX11, a transcription factor, has garnered considerable attention for its dual role in tumorigenesis across various cancers, including lung cancer. Depending on the cellular context, SOX11 acts as an oncogene or tumor suppressor. In certain settings, SOX11 promotes tumor cell proliferation, contributing to cancer progression, while in others, it inhibits cellular growth and prevents tumorigenesis [9]. The multifaceted role of SOX11 in regulating cellular plasticity and modulating the TME may also contribute to therapeutic resistance, making it a potential therapeutic target for advanced lung cancers. Recent preclinical studies have highlighted the potential of SOX11 inhibition in enhancing the efficacy of conventional chemotherapeutics and immune checkpoint inhibitors (ICIs), particularly in resistant tumors. N-Myc downstream-regulated gene 2 (NDRG2) is another important tumor suppressor implicated in lung cancer and other malignancies. NDRG2 downregulation is associated with increased tumor aggressiveness, poor prognosis, and enhanced metastatic potential in several cancer types, including NSCLC [10]. Mechanistically, NDRG2 exerts its tumor-suppressive effects by inhibiting cancer cell proliferation, inducing apoptosis, and suppressing metastatic pathways. Identifying NDRG2 as a prognostic marker holds promise for personalized medicine approaches in lung cancer, where molecular profiling could guide therapeutic decisions. Targeting NDRG2-mediated pathways may offer a novel strategy for mitigating tumor progression and metastasis in aggressive lung cancer phenotypes. Molecular insights into the role of oxidative stress, apoptosis, and DNA damage response pathways have revealed that novel compounds like curcumin derivatives can modulate these pathways, offering therapeutic benefits for overcoming resistance. It has been shown that curcumin regulates various signaling cascades, such as the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) and mitogen-activated protein kinase (MAPK) pathways, which are essential for lung cancer cell survival and proliferation [5].
The most significant multifactorial risk factor for NSCLC, which accounts for over 80% of lung cancer cases, is cigarette smoking. Smoking introduces a variety of carcinogens, including polycyclic aromatic hydrocarbons and nitrosamines, which induce genetic mutations that drive tumorigenesis [11]. In addition to smoking, genetic predisposition, occupational exposure to carcinogens such as asbestos, and environmental factors like radon and particulate matter (PM) significantly contribute to lung cancer risk. Pulmonary comorbidities, such as chronic obstructive pulmonary disease (COPD), further exacerbate the risk of lung cancer development [[12], [13], [14]]. Recent global trends in lung cancer incidence and mortality exhibit significant geographic variation, primarily influenced by differences in tobacco consumption patterns and regulatory measures. In 2018, the World Health Organization (WHO) reported that lung cancer death rates were three to four times higher in transitional economies compared to transitioned ones. This gap is anticipated to grow, as 80% of smokers worldwide aged 15 and above live in low- and middle-income countries (LMICs), where tobacco control regulations are less strict [15]. While advancements in lung cancer therapies, such as targeted treatments and immunotherapies, have significantly improved patient outcomes, their accessibility remains highly dependent on socioeconomic factors. In low- and middle-income regions, several barriers limit the adoption of these novel therapies. High treatment costs, lack of access to molecular diagnostics, and underdeveloped healthcare infrastructure present major challenges. For example, targeted therapies that require molecular profiling, such as those targeting EGFR or ALK mutations, may not be widely available in these regions due to the high cost of diagnostic tests and treatments. Furthermore, disparities in healthcare funding, regional regulatory approvals, and limited availability of clinical trials exacerbate the unequal distribution of these advanced therapies. Addressing these socioeconomic disparities is crucial to ensuring equitable access to life-saving treatments.
Additionally, Turner estimated that in 2017, 14% of global lung cancer deaths were linked to outdoor ambient fine PM (PM 2.5) air pollution, with substantial regional differences ranging from 4.71% in the United States to 20.6% in China. These findings underscore the global public health burden of lung cancer and the need for comprehensive strategies to address both smoking cessation and environmental carcinogen exposure [16]. Lung cancer incidence and mortality closely mirror each other due to the disease's high fatality rate, reflecting the maturity of the global tobacco epidemic [17]. While the epidemic is less pronounced among women, its impact continues to grow, with increasing lung cancer rates among women attributed to rising smoking prevalence in many countries [17]. Interestingly, recent epidemiological data suggest that lung cancer incidence in women may soon surpass that in men, a trend potentially linked to gender-specific smoking behaviors and differences in susceptibility to environmental carcinogens [18]. This emerging pattern necessitates targeted public health interventions to curb tobacco use and reduce lung cancer incidence, particularly in vulnerable populations.
This review aims to provide a comprehensive and updated analysis of the evolving therapeutic landscape for lung cancer, focusing on both conventional and novel treatment strategies. The paper is structured as follows. (1) Overview of current treatment strategies: This section summarizes the traditional approaches to lung cancer treatment, including surgery, chemotherapy, and radiotherapy. It critically assesses their effectiveness and the challenges of tumor resistance mechanisms, especially in late-stage patients. (2) Targeted therapies and resistance mechanisms: We explore the advances in targeted therapies, particularly focusing on tyrosine kinase inhibitors (TKIs) such as those targeting EGFR, ALK, and ROS1 mutations. This section highlights how molecular profiling has enabled more precise treatments and discusses emerging resistance pathways that limit long-term efficacy. (3) Immunotherapies: This section covers the advancements in immunotherapies, including ICIs like programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors. We discuss the successes of these therapies in certain subtypes of NSCLC and their limitations, particularly regarding immune-related adverse effects and treatment resistance. (4) Phytochemicals and natural compounds: In this novel section, we delve into the role of phytochemicals and natural compounds in cancer therapy. We evaluate recent studies demonstrating their potential in overcoming drug resistance and improving the efficacy of conventional chemotherapy through oxidative stress modulation and apoptosis induction. (5) Antibody-drug conjugates (ADCs) and bispecific T-cell engagers (BiTEs): We explored the potential role of BiTEs and ADCs in treating lung cancer. These therapies offer a promising approach to overcoming treatment resistance by harnessing the specificity of monoclonal antibodies (mAbs) while delivering potent cytotoxic agents. (6) Clinical implications and future directions: The final section discusses the clinical implications of these therapeutic advances, emphasizing the importance of molecular profiling for personalized medicine. We also highlight future directions, including the potential of liquid biopsies and other emerging technologies for dynamic, real-time tumor monitoring. By providing an in-depth discussion of established and novel therapeutic modalities, this review offers a comprehensive resource for clinicians and researchers aiming to improve outcomes for lung cancer patients.
2. Limitations of traditional therapies
Traditional therapies for lung cancer primarily include surgery, radiotherapy, and chemotherapy (Fig. 1). The medical treatment for stage I or II NSCLC is surgical tumor excision with adjuvant treatment [19]. On the other hand, chemotherapy or radiotherapy becomes the preferred form of treatment when the disease reaches stages III or IV [20,21]. Nevertheless, most traditional chemotherapeutic agents possess the same drawbacks, and hence, in cancer patients, chemotherapy has limited efficacy. These drawbacks include a lack of specificity against the target, poor bioavailability, and the emergence of drug resistance [22].
Fig. 1.
Comprehensive overview of lung cancer treatment modalities: surgical resection, chemotherapy, radiation therapy, targeted therapy, and immunotherapy. This presents the primary treatment strategies for lung cancer, highlighting the roles of surgery, chemotherapy, radiation therapy, targeted therapy, and immunotherapy. At the center, the lung with a tumor represents the focal point of these therapeutic approaches. Surrounding icons symbolize each of the key treatment modalities, indicating the multimodal approach often required for effective lung cancer management.
Over the previous decade, targeted therapy and immunotherapy have revolutionized lung cancer treatment. Recent research has also revealed that ICIs as a monotherapy are now an attractive option and a first-line treatment for advanced lung cancer, which plays one of the most successful examples in immunotherapy development against malignant tumors with an enormous profit. These ICIs have changed the standard for lung cancer care and offered new hope to metastatic patients or locally advanced disease [23]. Additionally, recent studies have shown that the introduction immunotherapy using immune checkpoint inhibitors (e.g., anti-programmed cell death (PD-1) and anti-PD-L1) has revolutionized the treatment of NSCLC, which has better survival outcomes along Saggese [24]. Similarly, targeted therapies have joined the standards with which patients diagnosed with NSCLC are now treated [24]. Targeted therapies have also been the standard of care for individuals with NSCLC, adding another option to chemotherapy [25] . One of the most important breakthroughs has been made with molecularly targeted therapies that have led to the development of inhibitors such as receptor tyrosine kinase (RTK) directed against mutations in EGFR and ALK mutations [26]. Moreover, the chemoimmunotherapy regimen improved response rates in early lung cancer [27]. This review will briefly overview anti-cancer drugs and phytochemicals recently studied for lung cancer treatment. In osteosarcoma, the combination of cisplatin and ursolic acid has demonstrated a synergistic effect by reducing drug resistance through a multistep mechanism, including ferritinophagy. Drawing parallels to lung cancer, this suggests combining conventional chemotherapeutics such as cisplatin with phytochemicals may similarly modulate oxidative stress and reduce resistance, enhancing therapeutic efficacy [28].
3. Targeted therapies
In the previous twenty years, targeted therapy has had a major influence in managing NSCLC by directly targeting molecular abnormalities of cancer cells [29,30] (Fig. 2). Table 1 provides a detailed overview of key targeted therapies for lung cancer, including efficacy metrics, associated toxicities, and potential combination strategies. This has certainly taken individualized therapy to a higher level where, inversely, the selection of potential targeted therapies is personalized based on imaging biomarkers and mutation-driven research [31]. Moreover, the broad use of high throughput technologies such as next-generation sequencing (NGS) has been essential to discovering these actionable mutations. Reverse transcription polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) allowed the selection of patient populations for EGFR and ALK alterations on which RTK inhibitors have shown clinical benefit, especially in lung adenocarcinoma [32,33]. The inclusion of targeted alterations not previously appreciated in an established NSCLC is demonstrated by using NGS to detect the clonal evolution process [34]. In order to precisely identify molecular alterations that drive tumor growth, NGS has emerged as a transformative tool in the treatment of lung cancer. By revealing actionable mutations in genes such as EGFR, ALK, ROS1, and mesenchymal-epithelial transition factor (MET), NGS has paved the way for the application of targeted therapies, thereby individualizing treatment approaches. Additionally, NGS allows the detection of emerging resistance mutations, which might inform therapy adjustments, particularly in patients who initially respond to therapy but subsequently acquire resistance. In clinical practice, using NGS in lung cancer has shifted the paradigm toward a more dynamic, personalized treatment model, where real-time molecular profiling informs therapeutic decisions. This has enhanced the efficacy of treatments and reduced unnecessary toxicities associated with non-targeted therapies. Integrating NGS into routine clinical workflows is crucial for optimizing patient outcomes, particularly in identifying rare mutations and tailoring therapy to specific molecular subtypes. NGS also facilitates liquid biopsies, a minimally invasive approach to monitor tumor evolution and treatment response. As lung cancer is highly heterogeneous, the ability to detect and track molecular changes in circulating tumor DNA (ctDNA) provides clinicians with real-time insights, allowing for more timely and personalized interventions. In lung cancer patients, using NGS-guided therapy has significantly improved OS and PFS. Developing advanced algorithms and machine learning techniques to interpret complex NGS data further strengthens its role in clinical decision-making. The ongoing integration of NGS into routine clinical practice will be essential for fully unlocking the benefits of personalized medicine in lung cancer treatment. Comprehensive molecular profiling has enhanced access to targeted therapies, underscoring the importance of precision medicine in reshaping the therapeutic landscape of NSCLC [35]. The more aggressive era of precision medicine in lung cancer has given rise to promising targeted therapies that either work around resistance mechanisms or improve outcomes directly. Therapeutic targets and molecular pathways in lung cancer, including approved and investigational targeted therapies, are at the forefront of ongoing research and clinical trials, driving advancements in treatment strategies. While novel therapies such as ICIs and targeted therapies have demonstrated superior efficacy in specific subsets of lung cancer patients, they are not without significant side effects. Immune-related adverse events (irAEs), such as pneumonitis, colitis, and dermatitis, can occur with immunotherapies, requiring careful monitoring and management. In contrast, traditional chemotherapy regimens are typically associated with systemic toxicities, including myelosuppression, nausea, and hair loss. Targeted therapies, such as TKIs, offer a more precise approach but can still cause significant toxicities, including skin rash, diarrhea, and, in some cases, interstitial lung disease. Comparatively, the side effect profiles of novel therapies often reflect immune modulation and targeted molecular inhibition, distinct from the broader cytotoxic effects of chemotherapy.
Fig. 2.
Targeted oncogenes and approved therapeutics in lung cancer: key genetic mutations and downstream signaling pathways with corresponding inhibitors. The figure illustrates critical oncogenes involved in the pathogenesis of lung cancer together with the downstream signaling pathways they are linked to, such as phosphatidylinositol 3-kinase (PI3K)-protein kinase B (AKT), Janus kinase (JAK)-signal transducer and activator of transcription (STAT), and rat sarcoma (RAS)-rapidly accelerated fibrosarcoma (RAF)-mitogen-activated protein kinase kinase (MEK)-extracellular signal-regulated kinase (ERK). At the top of the figure, the approved targeted therapies for each mutation are listed, such as Adagrasib and Sotorasib for Kirsten rat sarcoma viral oncogene homologue (KRAS), Crizotinib and Lorlatinib for reactive oxygen species (ROS), and Gefitinib, Erlotinib, and Osimertinib for receptor tyrosine kinase (RTK). The lower part of the figure depicts the downstream molecular pathways affected by these mutations and targeted by the respective drugs. ALK: anaplastic lymphoma kinase; MET: mesenchymal-epithelial transition factor; TORC: target of rapamycin complex; PLC: phospholipase C; IP3: inositol 1,4,5‑trisphosphate; DAG: diacylglycerol; PKC: protein kinase C.
Table 1.
Summary of key targeted therapies for lung cancer: efficacy, toxicities, and combination strategies.
| Therapy type | Drug name | Target (Mutation/pathway) | Efficacy (overall survival (OS), progression-free survival (PFS), objective response rate (ORR), hazard ratio (HR)) | Detailed toxicities | Combination strategies/notes |
|---|---|---|---|---|---|
| Kirsten rat sarcoma viral oncogene homologue (KRAS) inhibitors | Sotorasib | KRAS G12C | OS: 12.5months, PFS: 6.8months, ORR: 37.1%, HR for death: 0.69 |
Grade 3–4 diarrhea (9%), elevated liver enzymes (11%), fatigue (7%) | Potential for combination with immune checkpoint inhibitors (ICIs) to enhance efficacy in resistant tumors |
| Adagrasib | KRAS G12C | OS: 14.1 months, PFS: 7.3 months, ORR: 42%, HR for death: 0.65 |
Grade 3–4 nausea (7%), elevated alanine aminotransferase/aspartate aminotransferase (ALT/AST) (15%), fatigue (9%) | Being tested in combination with src homology region 2 domain-containing phosphatase (SHP2) inhibitors and chemotherapy to overcome resistance | |
| Epidermal growth factor receptor (EGFR) inhibitors | Osimertinib | EGFR T790 M, L858R | OS: 38.6 months, PFS: 18.9 months, ORR: 80%, HR for progression: 0.46 |
Grade 3–4 diarrhea (4%), interstitial lung disease (2%), rash (10%) | Combination with chemotherapy in EGFR-mutant non-small cell lung cancer (NSCLC) shows increased survival in clinical trials |
| Erlotinib | EGFR exon 19 deletion | OS: 26.8 months, PFS: 13.1 months, ORR: 58%, HR for progression: 0.63 |
Grade 3–4 rash (6%), diarrhea (5%), fatigue (7%) | Trials combining with anti-angiogenic agents such as bevacizumab show enhanced PFS | |
| Anaplastic lymphoma kinase (ALK) inhibitors | Alectinib | ALK fusion | OS: 34.8 months, PFS: 34.8 months, ORR: 83%, HR for progression: 0.50 |
Grade 3–4 constipation (4%), fatigue (5%), myalgia (7%) | Combination with programmed death-ligand 1 (PD-L1) inhibitors is being explored to enhance response in resistant ALK-positive cases |
| Brigatinib | ALK fusion | OS: 33.3 months, PFS: 25.3 months, ORR: 74%, HR for progression: 0.62 |
Grade 3–4 hypertension (9%), hyperglycemia (6%), fatigue (7%) | Dual therapy with mammalian target of rapamycin (mTOR) inhibitors under study to improve outcomes in ALK-positive patients | |
| Mesenchymal-epithelial transition factor (MET) inhibitors | Capmatinib | MET exon14 (METex14) skipping | OS: 12.4 months, PFS: 5.5 months, ORR: 40.6%, HR for progression: 0.64 |
Grade 3–4 peripheral edema (8%), nausea (7%), elevated ALT/AST (12%) | Combination with EGFR inhibitors or ICIs is under investigation to overcome resistance |
| Programmed death-1 (PD-1) inhibitors | Pembrolizumab | PD-1 | OS: 30.0 months, PFS: 9.3 months, ORR: 45%, HR for progression: 0.68 |
Grade 3–4 pneumonitis (4%), fatigue (12%), pruritus (6%) | Shows synergistic effects when combined with chemotherapy and other ICIs (e.g., cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors) |
| Nivolumab | PD-1 | OS: 29.0 months, PFS: 6.7 months, ORR: 19%, HR for progression: 0.73 |
Grade 3–4 fatigue (9%), diarrhea (7%), rash (5%) | Combination with ipilimumab (CTLA-4 inhibitor) shows enhanced efficacy in NSCLC but also higher rates of adverse events | |
| CTLA-4 inhibitors | Ipilimumab | CTLA-4 | OS: 16.3 months, PFS: 4.5 months, ORR: 10%, HR for progression: 0.82 |
Grade 3–4 colitis (6%), rash (5%), endocrinopathies (4%) | Frequently combined with PD-1 inhibitors like nivolumab to improve response rates in advanced NSCLC |
OS: the median duration in months that patients survive after treatment. PFS: the median time in months before the cancer progresses. ORR: the percentage of patients whose cancer shrinks or disappears after treatment. HR: a metric indicating the risk of death or progression; lower values suggest better outcomes. Detailed toxicities: specific adverse events are reported by grade (severity), with common severe toxicities included for each therapy.
3.1. Kirsten rat sarcoma viral oncogene homologue (KRAS) inhibitors
The majority of RTK, including EGFR, ALK, and MET, use the KRAS protein, a tiny guanine triphosphatase (GTPase) encoded by the KRAS proto-oncogene, as a binary switch in signal transduction to regulate many cellular processes [36,37]. The rat sarcoma (RAS) family member frequently mutated in lung cancer is the KRAS [38]. KRAS inhibitors like sotorasib (AMG510) and adagrasib (MRTX849) have recently emerged as potentially effective therapies for the treatment of NSCLC with KRAS mutations, particularly the KRAS G12C mutation [39,40]. Thus, these inhibitors are a breakthrough for the era of precision medicine, thereby making them a new therapeutic avenue in patients harboring such selective genetic alteration [39,41,42]. Sotorasib is recognized as a clinically effective targeted agent (specially selected that displays limited efficacy) that has been proven to be a melody of testimonies due to its endogenous role proposed for V38A and R41Q. Additionally, both adagrasib and sotorasib, based on clinical trial data, were effective against NSCLC, where Sotorasib became the first Food and Drug Administration (FDA)-approved drug launched targeting KRAS G12C mutations [43]. Sotorasib (Lumakras, Amgen) monotherapy was approved as a second-line treatment for metastatic or locally advanced KRAS G12C-mutant NSCLC in 2021. However, an on-label KRAS G12C-selective inhibitor, adagrasib (Mirati Therapeutics), has also been approved by the FDA for use in previously attended patients with PD-L1-negative metastatic or locally advanced NSCLC and whose tumors have positive KAR2 mutation status. Both adagrasib and sotorasib have shown some activity in pretreated metastatic NSCLC patients [44,45]. Studies demonstrate the potential of these agents to address this difficult-to-treat subset, which represents a paradigm shift for the therapy of KRAS-mutated lung cancer [44,46]. Additionally, studies found that it was being investigated to treat KRAS-mutant lung cancer and other agents, such as src homology region 2 domain-containing phosphatase (SHP)2 inhibitors [47,48]. Studies have demonstrated that combining sotorasib with an investigational SHP2 inhibitor was generally well tolerated and had anti-tumor activity across multiple tumor types among individuals for whom no other targeted therapies were indicated [49].
3.2. ROS inhibitors
The ROS-1 gene was identified as a product of the RNA UR2 avian sarcoma virus in the 1980s [50]. Several signaling pathways linked to proliferation, differentiation, cell growth, and survival are largely activated by ROS-1. ROS-1 rearrangement results in the deregulation of kinase activity in the protein and the aberrant activation of signaling pathways by creating phosphotyrosine-recruitment sites in the terminal tail of ROS, mediated by extracellular signal-regulated kinase 1/2 (ERK1/2), insulin receptor substrate 1 (IRS-1), SHP1/SHP2, AKT, the PI3K pathway, signal transducers, MAPKs, signal transducer and activator of transcription 3 (STAT3), and VAV guanine nucleotide exchange factor 3 (VAV3) [51]. Studies have demonstrated different ROS inhibitors that are effective in NSCLC. The first ROS1 inhibitor drug approved by the FDA was crizotinib for the treatment of advanced ROS1-rearranged lung cancer and is still the gold standard first-line treatment for ROS1-rearranged NSCLC [52,53]. Crizotinib is used in patients with ROS1-positive NSCLC because preclinical research has demonstrated that it inhibits ROS1 [54]. Early results in patients with ROS1-rearranged NSCLC treated with crizotinib are promising [55]. Initially, crizotinib is active. However, resistance mechanisms may be acquired, reinforcing the need for highly potent ROS1 inhibitors [56]. Another study revealed that lung cancer cell lines ABC-20 with CD74-ROS1 and HCC78 containing SLC34A2 showed resistance to crizotinib [37]. On the other hand, we found that a dual inhibitor of c-met and PI3K was not good enough for inhibition of HCC78R cells; however, combined treatment with an AXL inhibitor, gilteritinib or cabozantinib had a greater effect [57]. Research Studies have also recently verified the efficacy of lorlatinib in ROS-positive lung cancer [58].
3.3. ALK inhibitors
ALK is an RTK family member encoded by the ALK gene on chromosome 2p23 [59]. A chromosomal inversion event causes a segment of the ALK gene to fuse with the echinoderm microtubule-associated protein-like 4 (EML4) gene in a tiny percentage of NSCLC tumors. Oncogene addiction is the resultant state caused by constitutively activating and transforming EML4-ALK fusion protein [60]. EML4-ALK fusion and other ALK rearrangements are seen in 3%–7% of NSCLC patients, and they are associated with younger ages, histories of mild or never smoking, and histology of adenocarcinoma [60,61]. After initiating crizotinib therapy for patients with ALK-positive lung cancer, the majority of these patients experience a relapse a few years later [62,63]. Studies observed that lung cancer patients with ALK-positive NSCLC experience relapse in the central nervous system (CNS), which is not being treated by traditional medications [64] .
Alectinib is the second-generation ALK inhibitor and has shown remarkable efficacy in treating NSCLC patients. Its toxicity is typically well-tolerated compared to other anticancer drugs [65]. In 2015, crizotinib was replaced by the highly selective ALK inhibitor alectinib for those patients whose conditions had progressed or who could not tolerate crizotinib [66]. Because alectinib is considered an essential drug for ALK rearrangement lung carcinoma, patients with lung adenocarcinoma and drug-induced interstitial pulmonary disease might be able to use it [67]. Alectinib has also been reported to have potent anti-tumor activity in NSCLC with EML4-ALK rearrangement of genes [68]. According to Yoshida et al. [69], alectinib is considered a new standard therapy frontline option for treatment-naïve ALK-positive NSCLC. Still, its use sometimes raises resistance concerns. It opens doors to alternative approaches like cytotoxic chemotherapy or second-generation ALK inhibitory agents such as ceritinib if the tumor resists previously used targeted therapeutics. A study demonstrated that alectinib treatment is viable and favorable for patients with ALK rearrangement-positive pulmonary adenocarcinomas [70]. Alectinib has especially presented impressive results in the cohort of ALK-positive NSCLC compared to crizotinib [71]. Haemolytic anemia is a side effect of alectinib, the gold standard treatment for NSCLC with ALK rearrangement, which selectively inhibits the action of ALK tyrosine kinases [72]. A French study suggested that alectinib improves clinical outcomes over crizotinib and reduces costs resulting from drug inefficiency [73]. This shows that alectinib is a selective and more potent second-generation ALK-TKI with superior anti-tumor activity. Besides its better activity, this drug shows less toxicity in advanced ALK-rearranged NSCLC patients [74].
In addition to alectinib, next-generation ALK inhibitors, especially ceritinib, ensartinib, brigatinib, and lorlatinib, have also been recently introduced into the medical practice for the positive treatment of NSCLC with an activating rearrangement involving the gene encoding ALK [75]. Brigatinib also demonstrated very good efficacy when given to those patients who failed cceritinib [76]. In ALK-positive NSCLC, brigatinib is considered one of the important therapies since it has also demonstrated potent activity against multiple ALK gene-acquired resistance mutations [77]. Similarly, studies have also revealed the efficacy of ensartinib in NSCLC since it successfully suppressed the MET exon14 (METex14) mutations both in vivo and in vitro [78]. Ensartinib reduces the adhesion, migration, and invasion of NSCLC cells. This action is linked to the production of phosphorylated AKT (p-AKT), the ERK signaling pathway, and matrix metalloproteinase (MMP)-2 and MMP-9 being downregulated [79]. In a phase III randomized clinical trial research in NSCLC patients with prior ensartinib, crizotinib was generally well tolerated and established good clinical activity [80,81]. Lorlatinib is a third-generation tyrosine kinase inhibitor that targets ALK [82]. According to studies, lorlatinib is the preferred first-line treatment for patients with advanced ALK-rearranged NSCLC since it is more effective than alectinib and ceritinib [83,84]. These drugs have successfully overcome resistance mechanisms and improved patient quality of life, particularly in ALK-positive NSCLC patients. This makes them potent in both first- and second-line treatment of patients, paving the way for precision therapies for NSCLC with rearranged ALK.
3.4. B-rapidly accelerated fibrosarcoma (RAF) inhibitors
Homonymous genes encode three kinases known as RAF enzymes, A-RAF, B-RAF, and C-RAF, essential for mediating cellular signal transduction in mammalian cells [85]. One of the most frequently repeated cancer-related genes in cancers is the BRAF protein encoded by the BRAF gene [86]. The RAF-mitogen-activated protein kinase kinase (MEK)-ERK signaling is a three-tiered kinase cascade. ARAF is part of the RAF-MEK-ERK signaling cascade [87]. It has been studied that BRAF inhibitors, dabrafenib and trametinib, are highly effective in treating lung cancer and the variant that features the BRAF V600E mutations. These agents work by inhibiting certain genetic mutations that drive the growth of the cancer, thus allowing highly targeted therapy. The combination of trametinib and dabrafenib has exhibited rapid and profound reactions in patients with the BRAF V600E-mutated lung. This combination, which has also been used as a model treatment against this specific mutation, has proven successful in noticeable tumor reduction and increased adenocarcinoma survival [88]. In addition, it has been established that dabrafenib and trametinib demonstrate a very high survival rate in patients with the BRAF V600E mutation. Hence, relevant agencies have considered and approved them for treating metastatic NSCLC with BRAF V600E mutations [89]. In another study, it was shown that the same combination provided immense clinical benefits to the patients with no substantial toxic effects in a long-term perspective [90]. Combining these anticancer drugs has proved highly efficient and can represent targeted therapies that provide clinically significant benefits for better outcomes.
3.5. MET inhibitors
MET aberrations have been extensively analyzed in various oncogenic processes and have been proven as a powerful facilitator in the invasion of tumor, angiogenesis, and metastasis [91]. In NSCLC, MET dysregulation is usually driven by one of the three mechanisms: gene amplification, METex14 mutations, or protein overexpression [92]. Comprehending MET dysregulations as the main driver in the acquired resistance setting and the emergence of novel MET inhibitors has unveiled novel therapeutic opportunities [93]. MET inhibitors work by interfering with an enzyme (MET RTK), which modifies its expression status in several types of cancers, including NSCLC, with MET changes. Capmatinib and tepotinib are the Hallmark MET inhibitors used in NSCLC with METex14 skipping mutations [94]. It has been reported that “capmatinib is approved for first-line therapy for adult patients with metastatic or advanced NSCLC exhibiting a METex14 skipping alteration” [95]. In clinical studies, capmatinib has exhibited clinically significant efficacy and a controllable safety profile [96]. The selective MET inhibitor tepotinib has also shown efficacy in NSCLC patients with METex14 skipping mutations when used with capmatinib. Clinically, it has demonstrated robust responses and tolerability in clinical studies [[96], [97], [98]]. The potential spectrum of action in these more selective MET inhibitors, like tepotinib and capmatinib, could establish the new standard treatment under a first-line setting regarding NSCLC patients who have developed aberrant METex14 skipping mutations [99]. Overall, capmatinib and tepotinib as MET kinase inhibitors exhibit excellent antitumor activities against NSCLC patients with an in-frame deletion of the specific exon 14 regions within MET such that enhancing targeted therapies against actioning on inhibiting transcription activation pathway may benefit patient survival.
3.6. RTK inhibitors
EGFR is the most well-established biomarker in NSCLC due to its multiple regulatory effects on proliferation and invasion [100,101]. TKIs against EGFR (EGFR-TKIs) are the standard treatment for patients with advanced EGFR-mutant NSCLC. EGFR-TKIs are small molecules that inhibit the activity of EGFR and downstream pathways by binding to irreversible EGFR; thus, they have shown potential for treatment as they prevent this activation [102]. The most frequent EGFR mutations, Del19 and L858R, are found in 12%–47% of lung tumors with adenocarcinoma histology. These mutations activate ligand-independent EGFR and validate predictive biomarkers for treating NSCLC [103]. Several clinical trials of patients treated with first-generation EGFR reversible inhibitors, such as gefitinib and erlotinib, reported using RTK inhibitors in NSCLC despite resistance mechanisms inhibiting their usage in cancer treatments. Patients' survival increased by 50% as a result of this treatment, compared with chemotherapy. Also, afatinib and dacomitinib, the second-generation RTK inhibitors, showed a higher affinity for inhibiting the EGFR kinase domain. A recent study also demonstrated that the survival of patients treated with afatinib is almost doubled compared to chemotherapy [104]. Similarly, scientific studies also demonstrated longer patient survival with dacomitinib than gefitinib [105,106]. However, it has been noted that side effects, including severe nausea, skin irritation, vomiting, gastrointestinal toxicity (constipation, diarrhea), and ulceration, are more frequently severe with dacomitinib and afatinib. Studies have also shown that a secondary mutation of the EGFR kinase domain is the most typical mechanism of resistance to first- and second-generation drugs. Osimertinib, a third-generation EGFR-RTK inhibitor, demonstrated more affinity for mutated receptors. Osimertinib significantly improved patient survival compared to the first- and second-generation drugs with a favorable tolerability profile [105,107,108].
The treatment with gefitinib, afatinib, erlotinib, or osimertinib as first-line therapy has recently been recommended by the European Society for Medical Oncology for lung cancers with an EGFR-activating mutation [105]. Along with gefitinib, chemotherapeutic drugs like pemetrexed or carboplatin have also been recommended as first-line therapy for NSCLC. Recent studies analyzed the anticancer effect of bevacizumab and erlotinib combination compared to erlotinib monotherapy in NSCLC. In individuals with EGFR + NSCLC, it was demonstrated that combination therapy improved both OS and PFS [109,110]. The combined therapy targeting vascular endothelial growth factor receptor (VEGFR) and EGFR appears to function synergistically due to the interaction between their signaling pathways, as anti-angiogenic therapy causes vascular normalization, which can raise the intra-tumor concentration of RTK inhibitor [111]. Osimertinib is recommended as a second-line treatment following systemic progression when a T790 M mutation in exon 20 is detected [105]. However, the guidelines suggest immunotherapy (atezolizumab) or platinum-based chemotherapy with or without bevacizumab in the absence of this mutation [105]. Novel combination therapies have been the subject of numerous early-phase clinical trials. For instance, osimertinib and gefitinib have not been the subject of many active trials [112,113]. Furthermore, multiple other investigations are assessing the efficacy of new third-generation EGFR RTK inhibitors (rociletinib, avitinib, and nazartinib), non-selective RTK inhibitors (anlotinib, momelotinib, and sorafenib), or RTK inhibitors (capmatinib and tepotinib), Janus kinase 2 (JAK2) inhibitors (momelotinib and pacritinib), and the AXL RTK inhibitor (gilteritinib). Although these ongoing studies should yield encouraging outcomes, it should be highlighted that most methods combining RTK inhibitors have not succeeded in clinical phases recently due to ineffectiveness or tolerability [114].
3.7. Addressing long-term efficacy and resistance mechanisms in targeted lung cancer therapies
In the landscape of targeted therapies for lung cancer, a significant gap in current research concerns the long-term efficacy and durability of these treatments. The emergence of resistance remains a formidable barrier, even though TKIs and ICIs have revolutionized the management of lung cancer by offering substantial clinical benefits. Resistance mechanisms frequently undermine these therapies' sustained efficacy, particularly those driven by secondary mutations, genetic heterogeneity, epigenetic modifications, and TME alterations. Tumor plasticity and the ability of cancer cells to evade targeted inhibition through clonal evolution or adaptive resistance further complicate treatment outcomes over time. Despite the impressive short-term outcomes of these therapies, as evidenced by improved PFS and ORR, there remains a dearth of long-term studies evaluating OS and patient outcomes beyond five years. This is especially critical in understanding the chronic implications of targeted therapies, including cumulative toxicities, acquired resistance, and long-term quality of life in patients subjected to prolonged treatment. The potential for chronic toxicity, ranging from cardiovascular and gastrointestinal complications to irAEs in immunotherapy-treated patients, requires closer investigation to ensure that the benefits of prolonged therapy do not come at the cost of significant harm. To address these gaps, future research must prioritize a deeper exploration of resistance's molecular underpinnings, including cancer stem cells (CSCs)' contributions, epigenetic reprogramming, and the tumor-immune landscape. Through technologies such as liquid biopsy, comprehensive molecular profiling will be pivotal in tracking tumor evolution and the emergence of resistance mechanisms in real time. Moreover, the design of long-term clinical trials that incorporate both efficacy and safety endpoints is essential. Studies focusing on novel combination therapies, which target multiple pathways or integrate immune modulation with targeted inhibition, offer promise in extending therapeutic durability and overcoming resistance. When coupled with real-time molecular monitoring, these strategies can increase long-term survival and maintain an acceptable quality of life for lung cancer patients.
3.8. Immunotherapy
Immunotherapy, particularly ICIs, has become an innovative approach for treating lung cancer, opening novel therapies, and improving outcomes (Fig. 3). The first approved immunotherapy for lung cancer was Nivolumab, followed by atezolizumab, durvalumab, and pembrolizumab [115]. These ICIs have transformed the field of advanced NSCLC, rewriting treatment guidelines and providing hope to patients [23]. Immunotherapy, which is effective in all levels of the disease, is also suitable for treating NSCLC, as it is being used frequently and it is still working [28]. A monoclonoal antibody (pembrolizumab) has improved OS compared to the traditional platinum-based chemotherapy in patients with NSCLC with more than 50% of PD-L1 expression [116]. Immunotherapy drugs are now approved for both the first and second-line treatments of metastatic NSCLC, and the successes in extensive-stage SCLC demonstrate conclusively that immunotherapies can be an effective way to treat these conditions [117]. In SCLC, CSCs are critical contributors to the development of chemoresistance. These CSCs not only exhibit resistance to conventional chemotherapy but also pose challenges to immunotherapy. Recent research suggests that targeting CSCs in SCLC through immunotherapy and molecular targeting may be key to overcoming resistance [118]. Reducing the self-renewal capacity of these stem cells may significantly improve the effectiveness of existing treatments. Additionally, CSCs have been implicated in driving resistance to both chemotherapy and immunotherapy, particularly in SCLC. The recurrence and metastasis of lung cancer are facilitated by CSCs' ability to self-renew and differentiate into multiple tumor cell types. The development of therapies specifically targeting CSCs, such as anti-CD133 antibodies or inhibitors of the Notch and Wnt signaling pathways, holds potential for overcoming chemoresistance in lung cancer [119].
Fig. 3.
Mechanism of immune checkpoint inhibition in cancer therapy: programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade leading to T-cell activation and tumor cell death. On the left side of the figure, immune checkpoints like PD-1/PD-L1 and CTLA-4 are shown to block T-cell activation in the tumor microenvironment (TME), allowing cancer cells to avoid being detected by the immune system. The right side of the figure shows how anti-PD-1 and anti-CTLA-4 antibodies, such as immune checkpoint inhibitors (ICIs), disrupt this interaction, allowing T-cells to be activated and mount an immune attack against tumor cells, leading to cell death. APC: antigen presenting cell.
Immunotherapy is an innovative strategy that boosts the body's immune system to identify and eliminate cancer cells [120]. ICIs, represented by the PD-1 inhibitor (pembrolizumab and nivolumab) and PD-L1 inhibiter (durvalumav and atezolizmab), have substantially transformed advanced NSCLC treatment (Fig. 3). Today, they are the standard of care for many metastatic malignancies and provide a major clinical benefit in NSCLC treatment. For example, in the treatment of NSCLC, neoadjuvant therapy is one such context where PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors are used to combat cancer cells by leveraging body immunity. ICIs are expectedly superior in terms of practically transforming lung cancer treatments to individually selective and organ-oriented selections since they improve prognosis integrity and their stand quality [[121], [122], [123], [124]].
3.9. PD-1/PD-L1 inhibitors
Treatment for lung cancer, especially NSCLC, has significantly improved because of PD-1/PD-L1 inhibitors such as atezolizumab, pembrolizumab, and nivolumab. These inhibitors improve the immune system's ability to recognize and eliminate cancer cells from the body by specifically targeting the PD-1 receptor or its ligand, PD-L1. Studies have shown that PD-1/PD-L1 inhibitors are effective in treating advanced NSCLC, improving patient survival and quality of life through the use of individualized and targeted therapy approaches [125,126].
PD-1/PD-L1 inhibitors are used in NSCLC treatment and have shown encouraging results [127]; however, it is important to note that these inhibitors can also cause immune-related side effects, so careful monitoring must be ensured [126]. It has been shown that PD-1/PD-L1 inhibitors combined with chemotherapy or other targeted therapy can enhance treatment responses synergistically [128]. This multimodal strategy aims to boost the immune response against cancer cells and reduce adverse effects [128].
3.10. CTLA-4 inhibitors
Ipilimumab and other CTLA-4 antagonists showed probably the most noticeable guarantee for patients by conceivable treatment of lung disease, especially NSCLC. These inhibitors improve the body's ability to detect and eliminate cancer cells by focusing on CTLA-4, a checkpoint protein that modulates immune responses. Ipilimumab, a fully human monoclonal antibody that targets CTLA-4, has been shown by Wolchok and colleagues to increase antitumor immune responses and improve survival in patients with metastatic cancers, including lung cancer [129]. When ipilimumab is used with other medications like radiation or chemotherapy, Wilkins et al. [130] report encouraging evidence of improved treatment responses and patient outcomes. It has been shown that combining ipilimumab with other ICIs, such as PD-1 inhibitors like nivolumab, has a synergistic effect for patients with lung cancer. Additionally, it has been demonstrated that combined therapy enhances anti-tumor immunity and decreases tumor cell escape from the immune system [130]. Also, clinical trials on advanced lung cancer that combined ipilimumab with chemotherapy showed pronounced benefits in improving anti-tumor responses and prolonging survival [131]. Results have displayed the encouraging effects on patients and therapeutic responses when ipilimumab is combined with other drugs such as radiation or chemotherapy [131].
4. Recent advancements in combination therapy
In lung cancer treatment, immunotherapy combined with targeted therapies like molecularly targeted drug treatments has shown promising results. Some studies suggest that giving immunotherapy and targeted drugs together may help patients with lung cancer respond better and live longer. The combination of targeted therapy and immunotherapy gives a comprehensive treatment strategy for lung cancer heterogeneity [118]. This combined regimen is better from a prognostic viewpoint for NSCLC patients [116]. Immunotherapy combined with targeted agents like EGFR or ALK inhibitors improved the clinical outcomes of patients with NSCLC, according to Osmani [31]. This approach, which uses immunotherapy with the immune system and targeted medicines to target specific genetic alterations, can lead to a more personalized and efficient treatment plan. Recent studies have also emphasized the role of oxidative stress in modulating platelet activity, which contributes to cancer progression by promoting angiogenesis and facilitating tumor metastasis through interactions with tumor cells [132]. Targeting the interplay between oxidative stress and platelet function presents a potential therapeutic avenue in lung cancer. Due to their antioxidant properties, phytochemicals may also play a role in modulating this interaction, further enhancing the effectiveness of anti-metastatic therapies. It has been reported that a combination of immunotherapy and anti-angiogenic drugs cooperate to inhibit lung cancer tumorigenesis [23]. The ultimate goal of this multimodal strategy is to remodel the TME and enhance immunogenicity for cancer cell death. While significant progress has been made with targeted therapies and immunotherapy, these approaches are not without limitations. Resistance mechanisms, particularly the emergence of secondary mutations in genes such as EGFR and ALK, continue to pose significant challenges in lung cancer treatment. Furthermore, treatment strategies are complicated by the heterogeneity of lung cancer, particularly between different histological subtypes such as adenocarcinoma and squamous cell carcinoma. A more in-depth understanding of tumor microenvironmental factors and tumor heterogeneity is essential for developing more effective combination therapies. Additionally, while in patients with high PD-L1 expression, ICIs have shown exceptional efficacy, their efficacy remains limited in patients with low or no PD-L1 expression, constituting a significant portion of NSCLC patients [133]. A promising way to overcome this limitation is to combine PD-L1 inhibitors with other targeted medicines, such as TKIs or anti-angiogenic agents.
4.1. ADCs
ADCs have emerged as a novel therapeutic strategy for lung cancer by enabling targeted delivery of cytotoxic drugs directly to malignant cells (Fig. 4). These include the cytotoxic medicines bonded to mAbs that selectively direct powerful anticancer agents right at tumor cells without harming healthy tissues [134]. Recently, a different class of compounds has started to be used as ADCs for treating drug resistance in EGFR-mutant lung adenocarcinoma, a type of lung cancer where conventional TKIs fails [135]. These studies showed that ADCs could be used as a targeted therapy for lung cancer, causing cell cycle arrest and interfering with cell proliferation [136]. In addition, the encouraging results of early-phase trials for NSCLC with ADCs targeting specific antigens like human epidermal growth factor receptor (HER)2, HER3, and trophoblast cell surface antigen 2 (TROP2) highlight a trend toward increasing popularity and effectiveness of using ADCs as treatments for lung cancers [137]. Sacituzumab govitecan (SG) is a hydrolyzable linker connecting an anti-Trop-2-directed antibody and a topoisomerase I inhibitory drug. The FDA has approved SG for the treatment of metastatic triple-negative breast cancer (TNBC), hormone receptor (HR)-positive, HER2-negative, and breast cancer. For metastatic urothelial cancer, SG received accelerated approval. In combination with platinum-based chemotherapy agents, one study investigated the efficacy of SG in treating SCLC. The study demonstrated synergistic growth-inhibitory effects when SG was coupled with either carboplatin or cisplatin in vitro. In combination with carboplatin, SG had significant anti-tumor activity in SCLC tumor-bearing mice and animals when administered to animals with SCLC tumors [138]. In this manner, ADC development paved the way for tailored approaches to thoracic malignancies, which are landmark achievements in the era of precision oncology among lung cancer patients [139]. By combining the cell-killing properties of anticancer drugs and the specificity associated with mAbs, ADCs have proven extremely potent and can potentially improve lung cancer therapeutics.
Fig. 4.
Mechanism of antibody-drug conjugate (ADC) in cancer therapy: targeted binding, internalization, and induction of apoptosis in tumor cells. The process by which ADCs specifically target cancer cells is depicted in the figure. The ADC attaches itself to a tumor-associated antigen on the surface of cancer cells. The ADC is absorbed into an early endosome through endocytosis upon binding. Following trafficking of the ADC to the lysosome, the cytotoxic chemical is released, leading the cancer cell to undergo programmed cell death or apoptosis.
4.2. BiTEs
As for lung cancer, especially NSCLC, BiTEs have emerged as powerful therapeutics. These immune checkpoints have been specifically developed to bind with a tumor-associated antigen and CD3 of T-cells simultaneously, which helps in establishing cross-linkage between cancer cells' surface and the T-cell membrane that further supports efficient T-cell-induced killing of cancerous load [140]. In the context of lung cancer, BiTEs provide a new approach to activation immunotherapeutic technique that mobilizes T cell's cytotoxic activity against tumor cells and may thus overcome the suppressive nature of an immune-suppressed environment often found in tumors [141]. To our knowledge, Tarlatamab is currently being tested in clinical trials for SCLC patients. It is a bispecific T-cell engager with an extended half-life that targets 3 (DLL3) [142]. In preclinical models of SCLC, they also found that AMG 757, a half-life extended DLL3-targeted bispecific T-cell engager, was highly potent [143]. In addition, the technology of new BiTE formats, namely nanoparticles, proves that advancement in this field continues to make the T cells for cancer immunotherapy more effective and selective [144]. Thus, these studies prove the possibility of BiTEs to be applied for lung cancer targeting and other cancer types. With the ability to activate T cells and specifically target cancer cells, these agents can potentially improve outcomes for lung cancer patients.
4.3. Clinical implications
From a clinical perspective, these advancements in targeted therapies and immunotherapy have several important implications. The routine use of molecular profiling in lung cancer patients is essential for identifying actionable mutations like EGFR, ALK, and ROS1, which guide personalized treatment decisions. The advent of NGS has transformed clinical practice by allowing oncologists to tailor therapies based on specific genetic alterations. For patients with advanced NSCLC, the use of ICIs, particularly in combination with chemotherapy or anti-angiogenic agents, has become the standard of care. These combination regimens have demonstrated improved PFS and OS, but they also require careful patient monitoring to manage irAEs, such as colitis, pneumonitis, and dermatitis, which can sometimes be life-threatening. The clinical adoption of these therapies has also underscored the importance of managing treatment-related toxicities, particularly with prolonged use of ICIs. Recent guidelines recommend close monitoring for signs of immune-related toxicity and early intervention with corticosteroids or immunosuppressive agents in severe cases. Moreover, the development of liquid biopsy techniques offers a promising non-invasive alternative for detecting genetic mutations and monitoring treatment responses, allowing for dynamic adjustments to therapy based on real-time tumor evolution [145]. This innovation could enhance patient outcomes by enabling more precise and timely interventions.
The rapid advancements in targeted therapies, immunotherapies, and molecular diagnostics have significantly transformed the management of lung cancer, particularly improving patient outcomes for those with specific genetic alterations such as EGFR, ALK, and ROS1 mutations. However, these therapies also present challenges in clinical practice, including the management of irAEs and ensuring equitable access to these treatments across diverse populations. Clinical studies, such as recent trials involving EGFR inhibitors like osimertinib and afatinib, have shown that while these therapies can extend PFS, the management of side effects such as skin toxicity and diarrhea remains a significant clinical hurdle [146,147]. Translational research continues to be crucial in understanding the mechanisms of resistance and developing combination therapies that can extend the therapeutic window while minimizing toxicities. Ongoing clinical trials and real-world studies continue to explore the long-term efficacy and safety of these emerging therapies across diverse patient populations. The inclusion of a broad demographic representation in these studies is essential to understand differential treatment responses and tailor therapies to various genetic and environmental backgrounds. Additionally, the integration of NGS into routine clinical practice facilitates real-time molecular monitoring, enabling clinicians to adapt treatment strategies in response to tumor evolution and resistance [146,147]. Future research should focus on optimizing these therapeutic approaches, particularly in managing chronic toxicities and improving patient quality of life. Large-scale prospective cohort studies and randomized controlled trials (RCTs) are needed to validate the long-term benefits and safety of combination therapies and novel agents such as BiTEs and ADCs.
4.4. Phytochemicals against lung cancer
Phytochemicals are bioactive compounds present in fruits, vegetables, grains, and other plant materials and have been extensively studied due to their health benefits and the potential to treat different diseases [148]. Compared to conventional therapies like chemotherapy and radiation, phytochemicals represent a novel class of natural anticancer agents that act through distinct mechanisms. Unlike traditional therapies that broadly target rapidly dividing cells, phytochemicals often exert their effects through specific molecular pathways, modulating oxidative stress, inflammation, apoptosis, and cell cycle regulation. For example, compounds like curcumin and resveratrol influence key signaling cascades, such as the PI3K/AKT and MAPK pathways, which are critical for cancer cell survival. These phytochemicals can induce apoptosis, inhibit angiogenesis, and suppress metastasis by targeting multiple pathways in tumor growth and progression. Phytochemicals possess various chemical entities, including alkaloids, flavonoids, glycosides, polyphenols, steroidal saponins, terpenoids, organosulphur compounds, essential oils, and vitamins [149]. Studies have demonstrated the role of phytochemicals in treating lung cancer, suggesting these medicinal plant-derived phytochemicals as potential options for anticancer drugs used in preclinical and clinical research studies [150]. Phytochemicals, such as curcumin, berberine (BBR), and gambogic acid (GA), are currently under investigation in clinical settings for their potential to enhance the efficacy of conventional treatments. Unlike chemotherapy, associated with systemic toxicity, phytochemicals typically exhibit lower toxicity profiles, making them promising candidates for combination therapies. For example, curcumin has been shown to sensitize lung cancer cells to cisplatin, thereby overcoming drug resistance. Additionally, phytochemicals like GA can specifically target molecular pathways, such as the pentose phosphate pathway (PPP), thereby selectively inhibiting tumor cell proliferation while sparing healthy cells. This specificity contrasts with the broader cytotoxic effects of conventional therapies, which often affect both cancerous and healthy tissues. In this review, we have also extensively studied phytochemicals with more focus on targeting lung cancer (both SCLC and NSCLC) by alkaloids, flavonoids, terpenoids, and essential oils. The details about phytochemicals, their mechanism of action, and assays performed to determine their efficacy have been provided in Table 2.
Table 2.
Recent phytochemicals targeting lung cancer.
| Classification | Compound name | Source | Chemical structure | Lungs anti-cancer mechanism | Assay method |
|---|---|---|---|---|---|
| Alkaloids | Renieramycin M | Blue sponge Xestospongia sp. |  |
Reniermycin-induced lung cancer cell apoptosis mediated by p53. Myeloid cell leukemia-1 (MCL-1) and B-cell lymphoma 2 (BCL-2) apoptotic factors are suppressed by it with reduction of aoikis, metastasis invasiveness & tumor formation of cancer cells by suppressing anchorage-independent cell growth. | Cell viability and apoptosis assay, wound closure assay, anoikis assay, Western blotting, colony formation assay, and matrigel invasion assay |
| Neferine | Nelumbo nucifera (Lotus) |  |
Reactive oxygen species (ROS) production, activation of mitogen-activated protein kinases (MAPKs), lipids peroxidation, and decreased matrix metalloproteinase (MMP) & Ca2+ depletion were produced by Neferine. Bcl12 and nuclear factor-kappa B (NF-κB) suppression and Bad, Bax, C cytochrome, and caspases were up-regulated by this alkaloid. Neferine induces p51, p53, and G1 phases and arrests the cell cycle. Apoptosis was induced by all these processes occurring simultaneously. | Cell proliferation and clonogenic assay, cytotoxicity, cell cycle analysis, Western blot analysis, and DNA fragmentation analysis were performed in A549 cells, and cell antioxidant status, ROS, and MM potential were accessed to confirm anti-cancer activity. | |
| Noscapine | – |  |
Noscapine showed reductions in the expression of phosphorylated protein kinase B (p-AKT), survivin, poly(adenosine diphosphate (ADP)-ribose) polymerase (PARP), BCL-2, AKT, and cyclin D1 and increases in the expression of Bax, p53, p21, caspases 3, 8, and 9, cleaved caspases 3, 8, and 9, and cleaved PARP. It activates multiple mechanisms, which ultimately leads to apoptotic cell death. | In-vivo (murine xenograft model) and in-vitro (A549 and H460 lung cancer cell) methods were performed to investigate the lung anti-cancer activity of the noscapine and cisplatin combination. | |
| Piperlongumine (PL) alkaloid | Piper longum Linn. |  |
PL's pro-apoptotic effects were strengthened by the elevated expression of cleaved PARP, Bax, and cancer stem cell (CSC) marker CD44, along with decreased messenger RNA (mRNA) levels of the epithelial-mesenchymal transition (EMT) regulator Twist1, and lower levels of phosphorylated mammalian target of rapamycin (p-mTOR) and the adaptor protein Ruk/CIN85. In vitro experiments indicate that PL can inhibit cell survival, migration, and invasion, while in vivo studies confirm its capacity to reduce tumor growth and metastasis. | Lewis lung carcinoma (LLC) cell line model was used to access the anti-metastatic potential of PL. Cell viability, cell migration, cell invasion, quantitative polymerase chain reaction (qPCR), and Western blot analysis were performed to confirm lung anti-cancer activity. | |
| Globospiramine | Voacanga globose |  |
Potential cytotoxic and anti-proliferative activities on lung cancer cells A549 were observed by inhibiting the MAPK14 (p38α) pathway and inducing caspase-dependent apoptosis in A549 cells. | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, caspase-Glo® 3/7 apoptosis assay, tetramethylrhodamine methyl ester (TMRM) staining, cell event TM caspase 3/7, and Western blot analysis were performed. | |
| Pancracine | Amaryllidaceae plants |  |
Apoptosis in MOLT-4 cells was induced by the high activity of caspases triggered by paracrine. This was facilitated by increased phosphorylation of p53 at Ser392 and p38 MAPK at Thr180/Tyr182. Additionally, there was an upregulation of p27, a simultaneous downregulation of phosphorylated Rb at Ser807/811, and a decrease in p-AKT at Thr308. | Trypan blue assay and the OrxCEL Ligence system were used to explore cell viability and proliferation. The impact of alkaloids on the cell cycle was evaluated by flow cytometry. Annexin V/PI was performed for apoptosis. Western blotting was performed to detect proteins triggering growth arrest or apoptosis. | |
| Solanidine | Plants of Solnanceae family |  |
Apoptosis and anti-angiogenic effects result from solanidine inhibiting the hypoxia-induced DNA damage response (DDR) proteins pATMser1981 and pHIF-1αser696. Regression of angiogenesis is a component of the anti-cancer impact in lung cancer, and it can be attributed to reduced expression of angiogenic mediators, including vascular endothelial growth factor (VEGF), MMP-2, and MMP-9, as well as inflammatory cytokines like interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α). | Comet assay, immunoblotting (IB), quantitative reverse transcription (qRT)-PCR, immunohistochemistry (IHC), immunofluorescence (IF) and enzyme-linked immunosorbent assay (ELISA), gelatin zymography, LLC model matte gel assays, in-vivo Dalton's lymphoma ascites (DLA) tumor model assays, and corneal neovascularization assay were performed to access anti-cancer activity of alkaloids. | |
| Protopine | Fumaria agrarian |  |
Two lung cancer cell lines, NCI-H460 and NCI-H23, are susceptible to protopine's cytotoxic and anti-proliferative effects. However, NCL-H460 is more sensitive to the anti-cancer effects of protopine than NCL-H23 on human lung cancer cell lines. | Cytotoxic and anti-proliferative assays on two lung cancer cell lines, NCI-H460 and NCI-H23, were performed to assess lung anti-cancer activity. | |
| Gloriosine | – |  |
Autophagic cell death induces cell cycle arrest through Yes-associated protein (YAP) transcriptional activity negative regulation in non-small cell lung cancer (NSCLC) by Gloriosine. The Hippo signaling pathway retains the YAP protein in the cytoplasm, preventing its movement into the nucleus and thereby inhibiting the expression of downstream target genes. This inhibition of YAP localization suppresses the AKT/mTOR pathway, ultimately triggering autophagy-dependent cell death. | Autophagy/Hippo signaling pathway was induced in A549 cells. A spheroid formation assay was performed to reveal the lung anti-cancer activity of alkaloids. | |
| Berberine (BBR) | Coptis chinensis Franch |  |
The growth and progression of cancer are associated with TF Sp1 and PDPK1 expression reduction by BBR. Via the miR-19a/TF/MAPK axis, cell cycle regulation and phosphatidylinositol 3-kinase (PI3K)/AKT pathway modification cause apoptosis. This mechanism activates the ROS/adenosine monophosphate-activated protein kinase (AMPK) pathway. Lipogenesis and cell proliferation are inhibited by BBR. In addition, BBR induces the permeabilization of the mitochondrial membrane and activates caspase 9 and caspase 3. | – | |
| Vincamine | Vinca minor lesser or dwarf periwinkle | 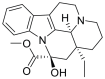 |
Vincamine lowered MMP, which releases cytochrome C and caspase 3-dependent apoptosis. Vincamine depleted ions in cancer cells and quenched hydroxyl free radicals. | The anticancer potential of vincamine was determined by molecular assays using A549 cells. In-silico studies predicted the anti-proliferative activity of vincamine. | |
| Sanguinarine | Sanguinaria canadensis | 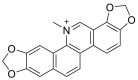 |
Sanguinarine targets epidermal growth factor receptor (EGFR) in NSCLC via H-bonding and hydrophobic interactions. | Molecular docking using Autodock vina studies confirms the anti-cancer potential of this alkaloid. | |
| Nitidum alkaloid C | Zanthoxylum nitidum | Structure is not available because this is not a discrete structure. | p53 was activated, CDK4/6 was suppressed, and the cell cycle was arrested by Nitidum alkaloid. Modulation of EMT suppresses the proliferation. Up-regulation of Nitidum alkaloids also causes E-cadherin and down-regulation of N-cadherin along with suppression of the EGFR/AKT/mTOR signaling pathway in A549 cells. | Sulforhodamine B (SRB) assay was used to evaluate the anti-proliferative activity of alkaloids on A549 human cancer cell lines. | |
| Flavonoids | Acacetin | Silver birch and Damiana |  |
The NSCLC (A549 & H460) metastasis, proliferation, and invasiveness were inhibited by Acacetin in a dose-dependent manner. This flavonoid inhibits the cell cycle G2/M phases and suppresses anti-apoptotic proteins BCL-2, cyclin B1 and D, and PDL1. Acacetin increased the expression of Bak protein, p53, and miR-34a. Thus, by regulating microRNA (miRNA), miR-34a, acacetin induced apoptosis of NSCLC. | To confirm NSCLC activity, several assays were conducted, including flow cytometry, PCR analysis, invasion, wound healing, and assays for cell viability, cell cycle, and apoptosis. |
| Tangeretin |
Citrus peels are tangerines, mandarins, grapefruits, and oranges |
 |
Signal transducer and activator of transcription (STAT)-3, phosphorylated STAT-3 (p-STAT-3), Janus kinase (JAK), and phosphorylated JAK (p-JAK) expression are suppressed by tangeretin, which decreases the metastasis of lung cancer along with increased caspase 3 expression, which leads to apoptosis. JAK/STAT-3, NF-κB/intercellular adhesion molecule 1 (ICAM-1), and caspase 3 signaling were changed by tangeretin, which shows promising potential to combat LC. | Immunohistochemical investigation and Western blot analysis of caspase 3 expression and NF-κB were performed to access tangeretin anticancer potential in urethane-induced lung cancer in BALB/c mice model. | |
| Myricetin |
Phaseolus vulgari, Brassica oleracea, Capsicum annuum, and Allium sativum |
 |
Sub-G1 phase aggregation was induced by myricetin, which blocks the entry of cells in the S-phase and thus leads to apoptosis. ROS and MMP alternations occur in A549 cells. ↑ p53 expressions and ↓EGFR expressions take place, which causes arrest of the cell cycle and cytotoxicity. |
MTT assay, cell cycle analysis, (AO) and (EtBr) Staining, DNA fragmentation assay, MMP measurement, and reverse transcription polymerase chain reaction (RT-PCR) were performed to confirm anti-cancer activity. |
|
| Eriodictyol | Eriodictyon californicum |  |
Eriodictyol regulates the BCL-2/Bax pathway by arresting cell cycle G2/M phases and apoptosis induction in the cell. mTOR/PI3K/AKT signaling pathway and MMP were inhibited concentration-dependent. BCL-2 and Bax expression was also suppressed. | MTT assay, flow cytometry, comet, and Western blot analysis were performed. | |
| Wogonin | Scutellaria baicalensis |  |
Wogonin significantly raised the levels of Bad and cleaved caspase 3 with reduced expression of BCL-2 in a concentration-dependent manner. PIK-3 signaling pathways and ErbB4may be inhibited by wogonin, which inhibits the metastasis and invasion of lung cancer cells. | Network pharmacology and Western blot analysis was used to examine the key targets of wogonin. Analysis was also done using the Kyoto Encyclopedia of Genes and Genomes and ontology functional analysis. | |
| Rutin | Citrus reticulata and Citrus limon |  |
Rutin demonstrates anti-cancer activity against lung cancer. | Various in-vitro tests were performed to confirm lung anti-cancer properties. | |
| Glycosides | GA | Garcinia hanburyi HooK.F. |  |
Gambogic acid (GA), a natural xanthonoid extracted from Garcinia hanburyi, has been widely studied for its potent anticancer activities. Structurally, GA contains a highly conjugated system, contributing to its ability to induce ROS generation, which leads to cancer cell apoptosis. GA exerts its anticancer effects by inhibiting key pathways, including the 6-phosphogluconate dehydrogenase (6PGD)-driven pentose phosphate pathway (PPP), thus reducing nucleotide biosynthesis and tumor growth. Furthermore, GA has shown specificity in targeting the PPP pathway, leading to decreased cancer cell proliferation, specifically in NSCLC models. | In this study, the antiproliferative effects of GA on NSCLC were evaluated using CCK-8 viability assays, demonstrating a dose-dependent inhibition of cell growth. Flow cytometry analysis was further utilized to assess apoptosis, showing that GA treatment increased apoptotic markers, such as cleaved caspase 3, in A549 cells. Moreover, in vivo, xenograft models confirmed the tumor-suppressing activity of GA, with significant reductions in tumor volume observed in mice treated with GA compared to controls. Chemical probing and ABPP technology were used to access GA protein targets. An LC mouse model was used to determine lung anti-cancer activity. |
| Hesperidin glycoside | Citrus specie |  |
The cell viability, invasion, and migration of HeLa cells were suppressed in a time and dose-dependent manner by HG1 and HG2. DNA fragmentation and acceleration of nuclear condensation cause the apoptosis and suppression of metastasis of lung cancer cells. | Trypan blue and MTS assays for cell viability of MRC5 and A549 cells were performed. Cancer cell migration suppression was investigated by scratch assay. Matrigel assay was used to investigate cancer cell invasion inhibition. | |
| Cyanidin 3-O-glucoside | Lonicera caerulea L. |  |
C3G reduced tumor growth, increased apoptosis of large cell carcinoma (LCC) and IL-1β, TNF-α, C-reactive protein, IL-6, NF-κB mRNA, and C3G reduced cyclooxygenase-2 (COX-2) and cytokines levels. C3G causes increased blockage of NF-κB kinase α mRNA, suppression of metastasis, transforming growth factor beta (TGF-β), CD44, EGFR, and VEGF. All these mechanisms ultimately lead to the termination of LCC tumorigenesis. | The anti-cancer effects of C3G on lung large cell carcinoma in the H661 lung LCC lines xenografted into BALB/c nude mice were investigated by histopathological observations, IL-6 analysis, serum CRP analysis, qPCR, and Western blotting. | |
| Aloe emodin 3-O-glucoside | Aloe barbadensis M. or Rheum palmatum L. |  |
AE3G suppressed mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) and AKT signaling pathways and BCL-2 expression. NCI-H460 cells, NCI-H1299 cells, and NSCLC cells by using AE3G, growth significantly decreased. It also causes the expression of Bax and cytochrome C release. AE3G suppresses MMP-2 and MMP-9 expression by mRNA. MMP inhibition, cleavage of caspases 9,8, and 3, PARP, and DNA fragmentation are also augmented by AE3G. | The NSCLC models used in this study included A549 cells, as well as other human NSCLC lines (NCI-H460 cells and NCI-H1299 cells) and xenograft mice of the BALB/c nu/nu variety that carried A549 cells. We studied cell migration, MMP inhibition, DNA fragmentation, and apoptotic gene expression using Western blotting, wound healing assay, JC-1 staining, or terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) staining. | |
| Malayoside | Antiaris toxicaria Lesch |  |
NCI-H460 cells apoptosis, Nur77 expression, and mitochondrial localization were induced by Malayoside by activating the MAPK-Nur77 signaling pathway, including ERK and p38 phosphorylation. Thus, Malayoside is responsible for the apoptosis of human NSCLC. | To confirm anti-NSCLC activity, assays for cell proliferation, cell cycle apoptosis analysis, PCR, and Western blot analysis were carried out. In-vivo, NCI-H460 subcutaneous tumor mice were used to investigate anti-tumor activity. | |
| Hyperoside | Hypericum perforatum |  |
In T790M-positive NSCLC cells, glycoside inhibited cell proliferation and triggered apoptosis. Additionally, hyperoside elevated the forkhead box protein O1 (FoxO1) expression while reducing the long non-coding RNA (lncRNA) colon cancer-associated transcript 1 (CCAT1) expression. | Anti-cancer activity was investigated by in-vitro (cell viability and apoptosis assays, western blot analysis, immunohistochemistry, RT-qPCR) and in-vivo experiments. | |
| Ouabain | Acokanthera schimperi and Strophanthus gratus |  |
Ouabain-induced DNA ds breaks, ROS generation, and apoptosis. Ouabain effectively suppressed STAT3 expression and phosphorylation to block STAT3-mediated transcription and downstream target proteins without needing Na+/K+ -ATPase. | Cell viability assay, clonogenic survival assay, flow cytometry analysis (RT-PCR), small interfering RNA (siRNA) transfection (ROS) measurement, and Western blotting were performed to confirm anti-cancer activity. | |
| Saponins | Zygo-albuside D | Zygophyllum album |  |
Zygo-albuside D induces the up-regulation of proapoptotic genes and suppresses the anti-apoptotic genes. This saponin causes CDK-2 and BCL-2 inhibition, which ultimately leads to the apoptosis of non-small cell lung carcinoma (A549 cells). | MTT assay (for cytotoxicity), Annexin V/propidium iodide (PI) staining flow cytometry to investigate the apoptotic effect, RT-PCR, and CDK-2 inhibition assay were performed to investigate A549 anti-cancer activity. |
| Aspiletrein A | Aspidistra letreae |  |
p-AKT/AKT signaling pathway was inhibited by Aspiletrein A, which poses an anti-metastatic effect, anti-proliferative effect, and anti-invasive effects on tumor growth of lungs. Moreover, AA posed significant cytotoxic effects on H460, H23, and A549 human lung cancer cells. | The ant-tumor MTT, wound healing, and trans well invasion assays investigated the ant-tumor activity of AA. Western blotting was used to investigate the suppression of AKT and BCL-2. | |
| Aescin | Aesculus hippocastanum L. |  |
LC proliferation was blocked by aescin significantly. AKT, mTOR, MEK, and ERK were downregulated. In the presence of aescin, AKT-mTOR down-regulations induce cytotoxic autophagy-mediated apoptosis. By suppressing HIF-1α and VEGF gene expressions, mobility and invasion were blocked. Additionally, it also successfully monitors the expression of the EGFR gene. | Both in vitro and in vivo analyses were performed to ascertain the anti-cancer activity of aescin. | |
| Trilliumosides K and L | Trillium govanianum |  |
Trilliumoside K exhibited a significant cytotoxic effect against the A-549 (Lung). Trilliumosides L. inhibited A-549 cell line migration and colony-forming ability in vitro. Additionally, ↑ ROS and ↑ MMP were noted. BCL-2 was down-regulated, while Bax was up-regulated. | – | |
| Essential Oils (EOs) | Citral, camphene, and valerianol |
Lemongrass (Cymbopogon citrates Stapf) |  |
Apoptosis and cycle arrest in A549 cells were induced by Ha Loc leaf essential oil (HLL). (caspase 3, BCL-2, and Bax) HLL altered the regulatory proteins of the apoptotic process HLL. | Flow cytometric analysis, fluorescent nuclear staining, and western blot analysis were performed to assess anti-cancer activity. |
| Octanoic acid and hexanoic acid | Morinda citrifolia |  |
M. citrifolia EOs induced cytotoxicity in A549 cells. ↓ LC growth was observed. The degradation of cellular enzymes increases the amount of apoptosis-mediated cell death. | An in-vitro cytotoxic assay was performed to observe the cytotoxicity against A549 lung cancer cells. | |
| Bicyclogermacrene, germacrene D, and E-caryophyllene | Myrcia splendens |  |
High cytotoxic activity in the THP-1, A549, and B16-F10 tumor cells was observed by M. splendens EOs. Colony formation migratory capacity of A549 cells was also inhibited. Apoptotic morphological changes were also observed. | MTT assay, clonogenic assay, and wound healing assay were performed to assess the anti-cancer activity of EOs. | |
| β-caryophyllene oxide (CPO) | Ediblemedicinal plants |  |
Carcinogenesis and proliferation in A54 cells were inhibited. ↓The activity of Ki67 and proliferating cell nuclear antigen (PCNA) was observed. P21, P53, and caspases 3, 9, and 7 expressions were ↑ with ↓ expression of BCL-2. The arrest of S and G2/M phases take place. Glutathione (GSH) and glutathione peroxidase (GPx) levels ↑ and 4-hydroxynonenal (4-HNE) levels was ↓ by EOs. | Cytotoxic analysis, DNA damage evaluation, flow cytometric analysis, and apoptotic analysis were performed to assess the lung anti-cancer activity of EOs. | |
| Eucalyptol and ledol | Eucalyptus tereticornis |  |
Anti-proliferative activity and free radical scavenging were observed in adeno carcinomic human alveolar basal epithelial A549 cancer cell line. | In-vitro 2,2-diphenylpicrylhydrazyl (DPPH) and MTT assays were performed to analyze anti-oxidative and lung anti-cancer activity. | |
| Linalool and linalyl acetate | Lavandula officinalis |  |
Linalyl acetate (LA)EO decreases genotoxicity and deleterious histopathological effects in dose-dependent order. On HepG2 and A549 cell lines, LAEO has a highly cytotoxic effect that results in 100% of these cell's death. | Micronucleus, chromosomal aberration, comet assay, histopathological studies, and cell viability assays were performed to assess genotoxicity. | |
| Viridiflorol | Aromatic plants |  |
Viridifloral inhibits cell viability in a concentration-dependent manner. It induces high early apoptosis and cytotoxicity in A549 cells. | MTT and Annexin V assays, cell viability, and apoptotic assessment were performed to access cancer cell responses. | |
| Artemisia ketone and camphor | Santolina chameacyparissus (Santo) |  |
These oils target DNA and oncogenic proteins in the LCC and induce apoptosis in A549 cells, thus exerting cytotoxicity. It suppressed cell motility in A549 cells. Santo did not change the E-cadherin /vimentin mRNA ratio but decreased Ezrin expression. A549 cells expressed ezrin protein, and the knockdown of Ezrin suppressed cell motility. | siRNA transfection, Western blot, immunofluorescence (IF) and microscopy, cell viability, flow cytometry, and scratch assay were performed to analyze lung anti-cancer activity. | |
| Linalool and 1,8-cineole | Present in over 200 species of plants |  |
G0/G1 and/or G2/M cell cycle phases were arrested and oils inhibited cell proliferation. ROS production and MMP depolarization were produced by linalool. Cell migration and cell growth were inhibited by linalool. Both oils demonstrate ant-proliferative, anti-metastatic, and sensitizer properties for lung cancer therapy. | Cell viability analysis, cell cycle analysis, cell death, detection of apoptosis in situ, determination of ROS and MMP, wound healing assay, and western blot analysis were performed to analyze the anti-cancer activity of these oils. | |
| Terpenoids | Myrcene | Plants such as hops, lemongrass, bay leaves, and cannabis |  |
ROS and cell death were triggered by myrcene in a dose-dependent manner. Cell integrity was altered, and DNA damage in A549 lung adenocarcinoma cells was produced. Caspase-3 activity was ↑ and MMP was ↓. Apoptotic cell death was induced in A549 lung adenocarcinoma cells by mitochondria-mediated cell death signaling and oxidative stress induction. | Cytotoxic analysis, cell cycle analysis, the cellular organelle integrity assay, colony formation assay, MMP assay, caspases activation, Cytochrome release assay, and γH2AX assay were performed to assess myrcene lungs anti-cancer activity. |
| Nimbolide (NB) | Flowers and leaves of neem tree | 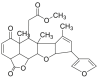 |
NB inhibited A549 human NSCLC cell colony formation. ROS production was increased by NB, which leads to endoplasmic reticulum (ER) stress, damage of DNA, and eventually, induction of apoptosis in NSCLC cells. | Cell viability assay, cell apoptotic assay, Colony formation analysis, ROS measurement, IF assay, comet, Western blot, PT-PCR, and siRNA assay were performed. | |
| 3β-acetoxy-1β, 4α-dihydroxy-11αH-eudesman-12, 6α-olide | Achillea millefolium | – | Achillea millefolium A significantly increased p53, Bax, caspases 3/8/12, and GRP78 proteins in NCI-H292 cells, triggering clear apoptosis via endogenous and exogenous pathways. | Annexin V-fluorescein isothiocyanate (FITC)/PI stain, TUNEL method, and Western blotting analysis were performed to assess lung anti-cancer activity. |
−: no data.
5. Conclusion
Clinical practice has shifted paradigm due to the rapid evolution of molecularly targeted therapies and immunotherapies for lung cancer. For patients with advanced lung cancer, these therapies may result in an increased chance of survival and a better quality of life. However, the clinical efficacy of these therapies is highly dependent on early and accurate molecular diagnosis and effective management of associated toxicities. As new therapeutic combinations are explored, the challenge remains in ensuring that these advanced treatments are accessible and affordable to all patients, regardless of their geographical or socio-economic status. In the past few years, novel therapies for lung cancer have been developed, offering new hope and better outcomes. Some of these have been enabled by the era of precision medicine (genomic and proteomic-based evaluations that provide in-vitro cancer testing). Meanwhile, molecularly targeted therapies are those like EGFR and ALK inhibitors, which attach to cancer cells bearing specific mutations that have produced significant response rates. The era of ICIs with the PD-1 and PDL1 agents has also offered a promising horizon by up-regulating our internal defense system to better fight against cancer cell lines. Especially when combined with other targeted therapies or traditional treatments, these immunotherapies can pack a big punch - sometimes, even restoring resistance to deepen their total effect. The new strategies, such as ADCs and epigenetic therapies, also update our armamentarium against lung carcinomas in more specific, more potent methods. Not only do these strides in treatment save lives, but they also deliver patients a better quality of life with few to none of the toxic side effects seen in many conventional treatments. In conclusion, these new therapeutic agents offer a paradigm change in lung cancer care by affording more individualized and less toxic approaches to treatment.
The clinical landscape of lung cancer treatment has significantly advanced with the development of targeted therapies, immunotherapies, and personalized medicine approaches. These therapies have shown increased efficacy, especially in patients with specific genetic alterations such as EGFR, ALK, ROS1, and MET mutations. The quality of life and patient survival rates have increased with ICIs targeting PD-1, PD-L1, and CTLA-4, especially when used with chemotherapy or other targeted therapies. However, managing irAEs is crucial for patient safety during these treatments. The increasing use of molecular profiling, liquid biopsy techniques, and NGS has transformed the diagnostic and treatment decision-making processes, allowing for more precise and personalized therapeutic regimens. As research progresses, further integration of novel agents such as ADCs, BiTEs, and phytochemicals holds promise for improving therapeutic outcomes. The challenge moving forward lies in ensuring equitable access to these advanced therapies across diverse patient populations, particularly in low- and middle-income regions, and in optimizing management strategies to mitigate adverse effects while enhancing efficacy. Future research must continue exploring these emerging therapies and their combinations to address resistance mechanisms and improve the long-term prognosis for lung cancer patients. In addition to improving our understanding of the molecular underpinnings of lung cancer, the integration of NGS in clinical practice has made it possible to develop highly personalized treatment regimens. The ability to tailor therapies based on specific genetic alterations, coupled with the real-time monitoring of tumor dynamics through liquid biopsy, positions NGS as a cornerstone of precision medicine in lung cancer. As more targeted therapies become available, the role of NGS in guiding therapeutic decisions will continue to expand, ultimately improving patient outcomes and minimizing treatment-related toxicities. In the rapidly evolving landscape of lung cancer therapy, the challenge remains to balance innovation with the rigorous assessment of safety and efficacy. While novel therapies, such as targeted and immunotherapies, show promising clinical outcomes, their rapid development often precedes comprehensive evaluations across diverse patient populations. This raises concerns about long-term safety, unforeseen side effects, and varied responses due to genetic, environmental, or socioeconomic factors. To address these challenges, ongoing clinical trials must include diverse cohorts to evaluate the differential responses in subpopulations, such as those defined by race, ethnicity, comorbidities, and socioeconomic status. Moreover, regulatory pathways should continue to prioritize adaptive trial designs, such as basket trials and umbrella trials, which enable faster evaluation of multiple therapies or biomarker-specific populations while maintaining rigorous safety protocols. These approaches help facilitate the early identification of adverse events and resistance mechanisms. Parallel efforts in post-marketing surveillance are critical to continuously assess long-term efficacy and safety in real-world settings. Integrating these strategies ensures that while the development of new therapies progresses swiftly, patient safety and efficacy in diverse populations remain central to clinical practice.
In preclinical and clinical studies, numerous phytochemicals have been evaluated for potential antitumor activity. Currently, a significant number of such phytochemicals are being used as cancer therapy after complete clinical trials. Hence, clinical research assessed the effectiveness of a few phytochemicals, leading them to drug formulation for NSCLC remedial utilization. Phytochemicals could prove a vital element of future public health strategies for the treatment and prevention of cancer. Moreover, the innovative clinical trial paradigm will enable the effective evaluation of new agents and novel combinations for an enhanced appreciation of molecular heterogeneity, leading to ultimately better outcomes. The success of emerging lung cancer therapies hinges not only on their efficacy but also on their accessibility. Socioeconomic factors, including healthcare funding and infrastructure, continue to limit the widespread adoption of novel treatments, particularly in low-income regions. Addressing these challenges through international cooperation, cost-reduction strategies, and expanded access to diagnostics will ensure that all patients, regardless of socioeconomic status, benefit from the latest advancements in lung cancer therapy. Moreover, detailed mechanistic studies and specific clinical trials are necessary to delineate the relevance of phytochemicals in human health and cancer contexts, including NSCLC. The long-term efficacy of targeted therapies in lung cancer remains an open question, with resistance mechanisms presenting a significant challenge to sustained treatment success. Future research should prioritize understanding the evolution of resistance over time and developing novel combination strategies to prolong the therapeutic window. Long-term clinical trials that assess both survival and quality of life, combined with real-time molecular profiling, will be essential for advancing personalized treatment approaches and addressing the limitations of current therapies.
CRediT authorship contribution statement
Caiyu Jiang: Writing – original draft. Shenglong Xie: Writing – original draft. Kegang Jia: Methodology. Gang Feng: Writing – review & editing. Xudong Ren: Writing – review & editing. Youyu Wang: Writing – review & editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Gang Feng, Email: fenggang@med.uestc.edu.cn.
Xudong Ren, Email: renxudong@med.uestc.edu.cn.
Youyu Wang, Email: wangyouyu@med.uestc.edu.cn.
References
- 1.Risch A., Plass C. Lung cancer epigenetics and genetics. Int. J. Cancer. 2008;123:1–7. doi: 10.1002/ijc.23605. [DOI] [PubMed] [Google Scholar]
- 2.Stefani A., Piro G., Schietroma F., et al. Unweaving the mitotic spindle: A focus on Aurora kinase inhibitors in lung cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelaziz H.M., Gaber M., Abd-Elwakil M.M., et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafizadeh M., Najafi M., Makvandi P., et al. Versatile role of curcumin and its derivatives in lung cancer therapy. J. Cell. Physiol. 2020;235:9241–9268. doi: 10.1002/jcp.29819. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafizadeh M., Zarrabi A., Hushmandi K., et al. Lung cancer cells and their sensitivity/resistance to cisplatin chemotherapy: Role of microRNAs and upstream mediators. Cell. Signal. 2021;78 doi: 10.1016/j.cellsig.2020.109871. [DOI] [PubMed] [Google Scholar]
- 7.Abadi A.J., Zarrabi A., Gholami M.H., et al. Small in size, but large in action: microRNAs as potential modulators of PTEN in breast and lung cancers. Biomolecules. 2021;11:304. doi: 10.3390/biom11020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Entezari M., Ghanbarirad M., Taheriazam A., et al. Long non-coding RNAs and exosomal lncRNAs: Potential functions in lung cancer progression, drug resistance and tumor microenvironment remodeling. Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.112963. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z., Jiang S., Lu C., et al. SOX11: Friend or foe in tumor prevention and carcinogenesis? Ther. Adv. Med. Oncol. 2019;11 doi: 10.1177/1758835919853449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W., Yang Y., Fan C., et al. Clinical and pathological significance of N-Myc downstream-regulated gene 2 (NDRG2) in diverse human cancers. Apoptosis. 2016;21:675–682. doi: 10.1007/s10495-016-1244-3. [DOI] [PubMed] [Google Scholar]
- 11.Cao M., Chen W. Epidemiology of lung cancer in China, Thorac. Cancer. 2019;10:3–7. doi: 10.1111/1759-7714.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Li W.X., Bai C., et al. Particulate matter-induced epigenetic changes and lung cancer. Clin. Respir. J. 2017;11:539–546. doi: 10.1111/crj.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samet J.M., Avila-Tang E., Boffetta P., et al. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009;15:5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eapen M.S., Hansbro P.M., Larsson-Callerfelt A.K., et al. Chronic obstructive pulmonary disease (COPD) and its association with lung cancer: Molecular mechanism and therapeutic targets, Austin J. Pulm. Respir. Med. 2021;8:1074. [Google Scholar]
- 15.World Health Organization. WHO global report on trends in prevalence of tobacco smoking 2000-2025, 2nd ed. Geneva: World Health Organization. https://iris.who.int/handle/10665/272694. (Accessed 29 May 2018).
- 16.Turner M.C., Andersen Z.J., Baccarelli A., et al. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020;70:460–479. doi: 10.3322/caac.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thun M., Peto R., Boreham J., et al. Stages of the cigarette epidemic on entering its second century. Tob. Control. 2012;21:96–101. doi: 10.1136/tobaccocontrol-2011-050294. [DOI] [PubMed] [Google Scholar]
- 18.Lortet-Tieulent J., Renteria E., Sharp L., et al. Convergence of decreasing male and increasing female incidence rates in major tobacco-related cancers in Europe in 1988–2010. Eur. J. Cancer. 2015;51:1144–1163. doi: 10.1016/j.ejca.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Naylor E.C. Adjuvant therapy for stage I and II non-small cell lung cancer. Surg. Oncol. Clin. N. Am. 2016;25:585–599. doi: 10.1016/j.soc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu L.N., Larkins E., Sinha A.K., et al. FDA approval summary: Atezolizumab as adjuvant treatment following surgical resection and platinum-based chemotherapy for stage II to IIIA NSCLC. Clin. Cancer Res. 2023;29:2973–2978. doi: 10.1158/1078-0432.CCR-22-3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wathoni N., Puluhulawa L.E., Joni I.M., et al. Monoclonal antibody as a targeting mediator for nanoparticle targeted delivery system for lung cancer. Drug Deliv. 2022;29:2959–2970. doi: 10.1080/10717544.2022.2120566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X., Yu Z., Feng L., et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021;268 doi: 10.1016/j.carbpol.2021.118237. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q., Su C., Zhou C. Recent advances in immunotherapy for lung cancer. Cancer Innov. 2023;2:18–24. doi: 10.1002/cai2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saggese P., Martinez C.A., Tran L.M., et al. Genotoxic treatment enhances immune response in a genetic model of lung cancer. Cancers (Basel) 2021;13:3595. doi: 10.3390/cancers13143595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasaka M., Gadgeel S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer, Expert Rev. Anticancer Ther. 2018;18:63–70. doi: 10.1080/14737140.2018.1409624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pottier C., Fresnais M., Gilon M., et al. Tyrosine kinase inhibitors in cancer: Breakthrough and challenges of targeted therapy. Cancers (Basel) 2020;12:731. doi: 10.3390/cancers12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crinò L., Weder W., van Meerbeeck J., et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;21:v103–v115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 28.Tang Z., Dong H., Li T., et al. The synergistic reducing drug resistance effect of cisplatin and ursolic acid on osteosarcoma through a multistep mechanism involving ferritinophagy. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/5192271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imyanitov E.N., Iyevleva A.G., Levchenko E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103194. [DOI] [PubMed] [Google Scholar]
- 30.Yuan M., Huang L., Chen J., et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019;4:61. doi: 10.1038/s41392-019-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osmani L., Askin F., Gabrielson E., et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018;52:103–109. doi: 10.1016/j.semcancer.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCutcheon J.N., Giaccone G. Next-generation sequencing: Targeting targeted therapies. Clin. Cancer Res. 2015;21:3584–3585. doi: 10.1158/1078-0432.CCR-15-0407. [DOI] [PubMed] [Google Scholar]
- 33.Muthusamy B., Patil P.D., Pennell N.A. Perioperative systemic therapy for resectable non-small cell lung cancer. J. Natl. Compr. Canc. Netw. 2022;20:953–961. doi: 10.6004/jnccn.2022.7021. [DOI] [PubMed] [Google Scholar]
- 34.Sabari J.K., Santini F., Bergagnini I., et al. Changing the therapeutic landscape in non-small cell lung cancers: The evolution of comprehensive molecular profiling improves access to therapy. Curr. Oncol. Rep. 2017;19:24. doi: 10.1007/s11912-017-0587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Politi K., Herbst R.S. Lung cancer in the era of precision medicine. Clin. Cancer Res. 2015;21:2213–2220. doi: 10.1158/1078-0432.CCR-14-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández A., Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tímár J. The clinical relevance of KRAS gene mutation in non-small-cell lung cancer. Curr. Opin. Oncol. 2014;26:138–144. doi: 10.1097/CCO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 38.Westcott P.M.K., To M.D. The genetics and biology of KRAS in lung cancer, Chin. J. Cancer. 2013;32:63–70. doi: 10.5732/cjc.012.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jänne P.A., Riely G.J., Gadgeel S.M., et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N. Engl. J. Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 40.Palma G., Khurshid F., Lu K., et al. Selective KRAS G12C inhibitors in non-small cell lung cancer: Chemistry, concurrent pathway alterations, and clinical outcomes. NPJ Precis. Oncol. 2021;5:98. doi: 10.1038/s41698-021-00237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceddia S., Landi L., Cappuzzo F. KRAS-mutant non-small-cell lung cancer: From past efforts to future challenges. Int. J. Mol. Sci. 2022;23:9391. doi: 10.3390/ijms23169391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia X., Liu M., Cheng Z. Sotorasib as first-line therapy in patients with advanced non-small cell lung cancer with KRAS gene mutations combined with brain metastases: A case report. AME Case Rep. 2024;8:48. doi: 10.21037/acr-23-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan S., Wiegand J., Zhang P., et al. BCL-XL PROTAC degrader DT2216 synergizes with sotorasib in preclinical models of KRASG12C-mutated cancers. J. Hematol. Oncol. 2022;15:23. doi: 10.1186/s13045-022-01241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inno A., Marchetti F., Valerio M., et al. Activity of sotorasib against brain metastases from NSCLC harboring KRAS p.G12C mutation: A case report. Drug Target Insights. 2023;17:90–91. doi: 10.33393/dti.2023.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Sullivan É., Keogh A., Henderson B., et al. Treatment strategies for KRAS-mutated non-small-cell lung cancer. Cancers (Basel) 2023;15:1635. doi: 10.3390/cancers15061635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee C., Yi J., Park J., et al. Hedgehog signalling is involved in acquired resistance to KRASG12C inhibitors in lung cancer cells. Cell Death Dis. 2024;15:56. doi: 10.1038/s41419-024-06436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bungaro M., Novello S., Passiglia F. Targeting KRASp.G12C mutation in advanced non-small cell lung cancer: A new era has begun. Curr. Treat. Options Oncol. 2022;23:1699–1720. doi: 10.1007/s11864-022-01033-4. [DOI] [PubMed] [Google Scholar]
- 48.Cascetta P., Marinello A., Lazzari C., et al. KRAS in NSCLC: State of the art and future perspectives. Cancers (Basel) 2022;14:5430. doi: 10.3390/cancers14215430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santarpia M., Ciappina G., Spagnolo C.C., et al. Targeted therapies for KRAS-mutant non-small cell lung cancer: From preclinical studies to clinical development-a narrative review, Transl. Lung Cancer Res. 2023;12:346–368. doi: 10.21037/tlcr-22-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balduzzi P.C., Notter M.F., Morgan H.R., et al. Some biological properties of two new avian sarcoma viruses. J. Virol. 1981;40:268–275. doi: 10.1128/jvi.40.1.268-275.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charest A., Wilker E.W., McLaughlin M.E., et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res. 2006;66:7473–7481. doi: 10.1158/0008-5472.CAN-06-1193. [DOI] [PubMed] [Google Scholar]
- 52.D’Angelo A., Sobhani N., Chapman R., et al. Focus on ROS1-positive non-small cell lung cancer (NSCLC): Crizotinib, resistance mechanisms and the newer generation of targeted therapies. Cancers (Basel) 2020;12:3293. doi: 10.3390/cancers12113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sehgal K., Patell R., Rangachari D., et al. Targeting ROS1 rearrangements in non-small cell lung cancer with crizotinib and other kinase inhibitors. Transl. Cancer Res. 2018;7:S779–S786. doi: 10.21037/tcr.2018.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cargnelutti M., Corso S., Pergolizzi M., et al. Activation of RAS family members confers resistance to ROS1 targeting drugs. Oncotarget. 2015;6:5182–5194. doi: 10.18632/oncotarget.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogura H., Nagatake-Kobayashi Y., Adachi J., et al. TKI-addicted ROS1-rearranged cells are destined to survival or death by the intensity of ROS1 kinase activity. Sci. Rep. 2017;7:5519. doi: 10.1038/s41598-017-05736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou H.Y., Li Q., Engstrom L.D., et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc. Natl. Acad. Sci. USA. 2015;112:3493–3498. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato Y., Ninomiya K., Ohashi K., et al. Combined effect of cabozantinib and gefitinib in crizotinib-resistant lung tumors harboring ROS1 fusions. Cancer Sci. 2018;109:3149–3158. doi: 10.1111/cas.13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duruisseaux M. Lorlatinib: A new treatment option for ROS1-positive lung cancer. Lancet Oncol. 2019;20:1622–1623. doi: 10.1016/S1470-2045(19)30716-8. [DOI] [PubMed] [Google Scholar]
- 59.Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 60.Soda M., Choi Y.L., Enomoto M., et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 61.Shaw A.T., Yeap B.Y., Mino-Kenudson M., et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camidge D.R., Bang Y.J., Kwak E.L., et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi Y.L., Soda M., Yamashita Y., et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N. Engl. J. Med. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 64.Otterson G.A., Riely G.J., Shaw A.T., et al. Clinical characteristics of ALK+ NSCLC patients (pts) treated with crizotinib beyond disease progression (PD): Potential implications for management. J. Clin. Oncol. 2012;30:7600. [Google Scholar]
- 65.Yamamoto Y., Okamoto I., Otsubo K., et al. Severe acute interstitial lung disease in a patient with anaplastic lymphoma kinase rearrangement-positive non-small cell lung cancer treated with alectinib. Invest. New Drugs. 2015;33:1148–1150. doi: 10.1007/s10637-015-0284-9. [DOI] [PubMed] [Google Scholar]
- 66.Chahin M., Krishnan N., Matthews-Hew T., et al. Metastatic anaplastic lymphoma kinase rearrangement-positive adenocarcinoma of occult primary mimicking ovarian cancer. Cureus. 2020;12 doi: 10.7759/cureus.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nitawaki T., Sakata Y., Kawamura K., et al. Case report: Continued treatment with alectinib is possible for patients with lung adenocarcinoma with drug-induced interstitial lung disease. BMC Pulm. Med. 2017;17:173. doi: 10.1186/s12890-017-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tani T., Yasuda H., Hamamoto J., et al. Activation of EGFR bypass signaling by TGFα overexpression induces acquired resistance to alectinib in ALK-translocated lung cancer cells. Mol. Cancer Ther. 2016;15:162–171. doi: 10.1158/1535-7163.MCT-15-0084. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida H., Kim Y.H., Ozasa H., et al. Efficacy of ceritinib after alectinib for ALK-positive non-small cell lung cancer. In Vivo. 2018;32:1587–1590. doi: 10.21873/invivo.11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu R., Shi Z., Duan T., et al. Feasibility and safety of neoadjuvant alectinib in pulmonary invasive mucinous adenocarcinoma with ALK rearrangement: Case report and literature review. Onco Targets Ther. 2021;14:5107–5113. doi: 10.2147/OTT.S334213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Camidge D.R., Kim H.R., Ahn M.J., et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 72.Misawa K., Nakamichi S., Iida H., et al. Alectinib-induced severe hemolytic Anemia in a patient with ALK-positive non-small cell lung cancer: A case report. Onco Targets Ther. 2023;16:65–69. doi: 10.2147/OTT.S398375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sivignon M., Monnier R., Tehard B., et al. Cost-effectiveness of alectinib compared to crizotinib for the treatment of first-line ALK+ advanced non-small-cell lung cancer in France. PLoS One. 2020;15 doi: 10.1371/journal.pone.0226196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwama E., Goto Y., Murakami H., et al. Survival analysis for patients with ALK rearrangement-positive non-small cell lung cancer and a poor performance status treated with alectinib: Updated results of lung oncology group in Kyushu 1401. Oncologist. 2020;25 doi: 10.1634/theoncologist.2019-0728. 306–e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spagnuolo A., Maione P., Gridelli C. Evolution in the treatment landscape of non-small cell lung cancer with ALK gene alterations: From the first- to third-generation of ALK inhibitors. Expert Opin. Emerg. Drugs. 2018;23:231–241. doi: 10.1080/14728214.2018.1527902. [DOI] [PubMed] [Google Scholar]
- 76.Jain R.K., Chen H. Spotlight on brigatinib and its potential in the treatment of patients with metastatic ALK-positive non-small cell lung cancer who are resistant or intolerant to crizotinib. Lung Cancer (Auckl) 2017;8:169–177. doi: 10.2147/LCTT.S126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ali R., Arshad J., Palacio S., et al. Brigatinib for ALK-positive metastatic non-small-cell lung cancer: Design, development and place in therapy. Drug Des. Devel. Ther. 2019;13:569–580. doi: 10.2147/DDDT.S147499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xia Y., Jin R., Li M., et al. Potent antitumor activity of ensartinib in MET exon 14 skipping-mutated non-small cell lung cancer. Cancer Lett. 2023;561 doi: 10.1016/j.canlet.2023.216140. [DOI] [PubMed] [Google Scholar]
- 79.Zhang B., Qiao T., Gao C. Effects and mechanism of ensartinib (X-396) on the adhesion and metastasis of non-small cell lung cancer cells. Pharmazie. 2019;74:543–546. doi: 10.1691/ph.2019.9461. [DOI] [PubMed] [Google Scholar]
- 80.Horn L., Infante J.R., Reckamp K.L., et al. Ensartinib (X-396) in ALK-positive non-small cell lung cancer: Results from a first-in-human phase I/II, multicenter study. Clin. Cancer Res. 2018;24:2771–2779. doi: 10.1158/1078-0432.CCR-17-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y., Zhou J., Zhou J., et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: A multicentre, phase 2 trial. Lancet Respir. Med. 2020;8:45–53. doi: 10.1016/S2213-2600(19)30252-8. [DOI] [PubMed] [Google Scholar]
- 82.Waqar S.N., Morgensztern D. Lorlatinib: A new-generation drug for ALK-positive NSCLC. Lancet Oncol. 2018;19:1555–1557. doi: 10.1016/S1470-2045(18)30789-7. [DOI] [PubMed] [Google Scholar]
- 83.El Darsa H., Abdel-Rahman O., Sangha R. Pharmacological and clinical properties of lorlatinib in the treatment of ALK-rearranged advanced non-small cell lung cancer. Expert Opin. Pharmacother. 2020;21:1547–1554. doi: 10.1080/14656566.2020.1774552. [DOI] [PubMed] [Google Scholar]
- 84.Nagasaka M., Ou S.I. Lorlatinib should be considered as the preferred first-line option in patients with advanced ALK-rearranged NSCLC. J. Thorac. Oncol. 2021;16:532–536. doi: 10.1016/j.jtho.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 85.Zebisch A., Troppmair J. Back to the roots: The remarkable RAF oncogene story. Cell. Mol. Life Sci. 2006;63:1314–1330. doi: 10.1007/s00018-006-6005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malumbres M., Barbacid M. RAS oncogenes: The first 30 years. Nat. Rev. Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 87.Avruch J., Khokhlatchev A., Kyriakis J.M., et al. Ras activation of the raf kinase: Tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog. Horm. Res. 2001;56:127–155. doi: 10.1210/rp.56.1.127. [DOI] [PubMed] [Google Scholar]
- 88.Ota T., Okabayashi A., Fukuoka M. Rapid and dramatic responses to dabrafenib and trametinib in BRAF V600E-mutated lung adenocarcinoma. Respirol. Case Rep. 2021;9 doi: 10.1002/rcr2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin J.E., An H.J., Park H.S., et al. Efficacy of dabrafenib/trametinib in pancreatic ductal adenocarcinoma with BRAF NVTAP deletion: A case report. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.976450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding H., Zhuang Z., Xie J., et al. Durable clinical response of advanced lung adenocarcinoma harboring EGFR-19del/T790M/BRAFV600E mutations after treating with osimertinib and dabrafenib plus trametinib: A case report. Onco Targets Ther. 2020;13:7933–7939. doi: 10.2147/OTT.S240775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frampton G.M., Ali S.M., Rosenzweig M., et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 92.Remon J., Hendriks L.E.L., Mountzios G., et al. MET alterations in NSCLC-current perspectives and future challenges. J. Thorac. Oncol. 2023;18:419–435. doi: 10.1016/j.jtho.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 93.Socinski M.A., Pennell N.A., Davies K.D. MET exon 14 skipping mutations in non-small-cell lung cancer: An overview of biology, clinical outcomes, and testing considerations. JCO Precis. Oncol. 2021;5 doi: 10.1200/PO.20.00516. PO.20.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mathieu L.N., Larkins E., Akinboro O., et al. FDA approval summary: Capmatinib and tepotinib for the treatment of metastatic NSCLC harboring MET exon 14 skipping mutations or alterations. Clin. Cancer Res. 2022;28:249–254. doi: 10.1158/1078-0432.CCR-21-1566. [DOI] [PubMed] [Google Scholar]
- 95.Grande E., Giovannini M., Marriere E., et al. Effect of capmatinib on the pharmacokinetics of digoxin and rosuvastatin administered as a 2-drug cocktail in patients with MET-dysregulated advanced solid tumours: A phase I, multicentre, open-label, single-sequence drug-drug interaction study. Br. J. Clin. Pharmacol. 2021;87:2867–2878. doi: 10.1111/bcp.14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi W., Park S.Y., Lee Y., et al. The clinical impact of capmatinib in the treatment of advanced non-small cell lung cancer with MET exon 14 skipping mutation or gene amplification. Cancer Res. Treat. 2021;53:1024–1032. doi: 10.4143/crt.2020.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Falchook G.S., Kurzrock R., Amin H.M., et al. First-in-man phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin. Cancer Res. 2020;26:1237–1246. doi: 10.1158/1078-0432.CCR-19-2860. [DOI] [PubMed] [Google Scholar]
- 98.Le X., Paz-Ares L.G., Van Meerbeeck J., et al. Tepotinib in patients (pts) with advanced non-small cell lung cancer (NSCLC) with MET amplification (METamp) J. Clin. Oncol. 2021;39:9021. [Google Scholar]
- 99.Grodkiewicz M., Kozieł P., Chmielewska I., et al. Optimizing treatment strategies for a MET exon 14 skipping mutation in non-small-cell lung cancer: A case report of sequential immunotherapy and targeted therapy and literature review. Oncol. Clin. Pract. 2023;19:448–453. [Google Scholar]
- 100.Herbst R.S., Heymach J.V., Lippman S.M. Lung cancer. N. Engl. J. Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hynes N.E., Lane H.A. Correction: ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5:580. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 102.Recondo G., Facchinetti F., Olaussen K.A., et al. Making the first move in EGFR-driven or ALK-driven NSCLC: First-generation or next-generation TKI? Nat. Rev. Clin. Oncol. 2018;15:694–708. doi: 10.1038/s41571-018-0081-4. [DOI] [PubMed] [Google Scholar]
- 103.Konduri K., Gallant J.N., Chae Y.K., et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discov. 2016;6:601–611. doi: 10.1158/2159-8290.CD-16-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sequist L.V., Yang J.C., Yamamoto N., et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 105.Planchard D., Popat S., Kerr K., et al. Correction to: “metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up”. Ann. Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 106.Wu Y.-L., Cheng Y., Zhou X., et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 107.Hochmair M.J., Morabito A., Hao D., et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: Final analysis of the GioTag study. Future Oncol. 2020;16:2799–2808. doi: 10.2217/fon-2020-0740. [DOI] [PubMed] [Google Scholar]
- 108.Morgillo F., Della Corte C.M., Fasano M., et al. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open. 2016;1 doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Motta-Guerrero R., Leon Garrido-Lecca A., Failoc-Rojas V.E., et al. Effectiveness and safety of the bevacizumab and erlotinib combination versus erlotinib alone in EGFR mutant metastatic non-small-cell lung cancer: Systematic review and meta-analysis. Front. Oncol. 2024;13 doi: 10.3389/fonc.2023.1335373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sakharkar P., Kurup S. Comparing efficacy of erlotinib and bevacizumab combination with erlotinib monotherapy in patients with advanced non-small cell lung cancer (NSCLC): A systematic review and meta-analysis. Diseases. 2023;11:146. doi: 10.3390/diseases11040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pennell N.A., Jr Lynch T.J. Combined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLC. Oncologist. 2009;14:399–411. doi: 10.1634/theoncologist.2008-0276. [DOI] [PubMed] [Google Scholar]
- 112.National Institutes of Health (NIH) Clinical trials. https://clinicaltrials.gov/
- 113.Zhang N., Liang C., Song W., et al. Antitumor activity of histone deacetylase inhibitor chidamide alone or in combination with epidermal growth factor receptor tyrosine kinase inhibitor icotinib in NSCLC. J. Cancer. 2019;10:1275–1287. doi: 10.7150/jca.28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Z., Tam K.Y. Combination strategies using EGFR-TKi in NSCLC therapy: Learning from the gap between pre-clinical results and clinical outcomes. Int. J. Biol. Sci. 2018;14:204–216. doi: 10.7150/ijbs.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Citra Wahyudin H.U., Afriani A., Anggrainy F., et al. Immunotherapy in lung cancer: A narrative literature review. Biosci. Med. J. Biomed. Transl. Res. 2023;7:3024–3030. [Google Scholar]
- 116.Song C., Liu W., Wan Y., et al. Editorial: Immunotherapy and multimodality therapy for lung cancer. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1372513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mohindra N. Current state of immunotherapy: Chipping away at the tip of the iceberg. J. Cancer Immunol. Ther. 2018;1:1–2. [Google Scholar]
- 118.Guo W., Qiao T., Li T. The role of stem cells in small-cell lung cancer: Evidence from chemoresistance to immunotherapy. Semin. Cancer Biol. 2022;87:160–169. doi: 10.1016/j.semcancer.2022.11.006. [DOI] [PubMed] [Google Scholar]
- 119.MacDonagh L., Gray S.G., Breen E., et al. Lung cancer stem cells: The root of resistance. Cancer Lett. 2016;372:147–156. doi: 10.1016/j.canlet.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 120.Cho J.H. Immunotherapy for non-small-cell lung cancer: Current status and future obstacles. Immune Netw. 2017;17:378–391. doi: 10.4110/in.2017.17.6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Akazawa Y., Yoshikawa A., Kanazu M., et al. Non-small cell lung cancer with tumor proportion score > 90% could increase the risk of severe immune-related adverse events in first-line treatments with immune checkpoint inhibitors: A retrospective single-center study. Thorac. Cancer. 2022;13:2450–2458. doi: 10.1111/1759-7714.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Belluomini L., Fiorica F., Frassoldati A. Immune checkpoint inhibitors and radiotherapy in NSCLC patients: Not just a fluke. Oncol. Ther. 2019;7:83–91. doi: 10.1007/s40487-019-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakamura T., Takeyasu Y., Yoshida T., et al. End-of-life impact of concurrent diabetes mellitus and adrenal insufficiency as immune-related adverse events in an advanced non-small cell lung cancer patient. Thorac. Cancer. 2022;13:3073–3075. doi: 10.1111/1759-7714.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Niki M., Nakaya A., Kurata T., et al. Pembrolizumab-induced autoimmune encephalitis in a patient with advanced non-small cell lung cancer: A case report. Mol. Clin. Oncol. 2019;10:267–269. doi: 10.3892/mco.2018.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weiss S.A., Wolchok J.D., Sznol M. Immunotherapy of melanoma: Facts and hopes. Clin. Cancer Res. 2019;25:5191–5201. doi: 10.1158/1078-0432.CCR-18-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J., Yuan R., Song W., et al. PD-1, PD-L1 (B7-H1) and tumor-site immune modulation therapy: The historical perspective. J. Hematol. Oncol. 2017;10:34. doi: 10.1186/s13045-017-0403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ekinci F., Erdogan A.P., Yildirim S., et al. Inflammatory prognostic index in metastatic renal carcinoma treated with nivolumab. J. Coll. Physicians Surg. Pak. 2022;32:1288–1294. doi: 10.29271/jcpsp.2022.10.1288. [DOI] [PubMed] [Google Scholar]
- 128.Facchinetti F., Veneziani M., Buti S., et al. Clinical and hematologic parameters address the outcomes of non-small-cell lung cancer patients treated with nivolumab. Immunotherapy. 2018;10:681–694. doi: 10.2217/imt-2017-0175. [DOI] [PubMed] [Google Scholar]
- 129.Wolchok J.D., Kluger H.M., Callahan M.K., et al. Safety and clinical activity of nivolumab (anti-PD-1, BMS-936558, ONO-4538) in combination with ipilimumab in patients (pts) with advanced melanoma (MEL) J. Clin. Oncol. 2013;31:9012. [Google Scholar]
- 130.Wilkins A., McDonald F., Harrington K., et al. Radiotherapy enhances responses of lung cancer to CTLA-4 blockade. J. Immunother. Cancer. 2019;7:64. doi: 10.1186/s40425-019-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang B., Dang J., Ba D., et al. Potential function of CTLA-4 in the tumourigenic capacity of melanoma stem cells. Oncol. Lett. 2018;16:6163–6170. doi: 10.3892/ol.2018.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang X., Yu S., Li X., et al. Research progress on the interaction between oxidative stress and platelets: Another avenue for cancer? Pharmacol. Res. 2023;191 doi: 10.1016/j.phrs.2023.106777. [DOI] [PubMed] [Google Scholar]
- 133.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 134.Challita-Eid P.M., Satpayev D., Yang P., et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76:3003–3013. doi: 10.1158/0008-5472.CAN-15-1313. [DOI] [PubMed] [Google Scholar]
- 135.Yamaguchi M., Hirai S., Sumi T., et al. Angiotensin-converting enzyme 2 is a potential therapeutic target for EGFR-mutant lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2017;487:613–618. doi: 10.1016/j.bbrc.2017.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang X., Zhou L., Jian Y., et al. Effectiveness of antibody-drug conjugate (ADC): Results of in vitro and in vivo studies. Med. Sci. Monit. 2018;24:1408–1416. doi: 10.12659/MSM.908971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Merle G., Friedlaender A., Desai A., et al. Antibody drug conjugates in lung cancer. Cancer J. 2022;28:429–435. doi: 10.1097/PPO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 138.Cardillo T.M., Zalath M.B., Arrojo R., et al. Sacituzumab govitecan plus platinum-based chemotherapy mediates significant antitumor effects in triple-negative breast, urinary bladder, and small-cell lung carcinomas. Oncotarget. 2024;15:144–158. doi: 10.18632/oncotarget.28559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie H., Adjei A.A. Antibody-drug conjugates for the therapy of thoracic malignancies. J. Thorac. Oncol. 2019;14:358–376. doi: 10.1016/j.jtho.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 140.Tian Z., Liu M., Zhang Y., et al. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021;14:75. doi: 10.1186/s13045-021-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mohan N., Ayinde S., Peng H., et al. Structural and functional characterization of IgG- and non-IgG-based T-cell-engaging bispecific antibodies. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1376096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paz-Ares L., Champiat S., Lai W.V., et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent small-cell lung cancer: An open-label, phase I study. J. Clin. Oncol. 2023;41:2893–2903. doi: 10.1200/JCO.22.02823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Giffin M.J., Cooke K., Lobenhofer E.K., et al. AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer. Clin. Cancer Res. 2021;27:1526–1537. doi: 10.1158/1078-0432.CCR-20-2845. [DOI] [PubMed] [Google Scholar]
- 144.Alhallak K., Sun J., Wasden K., et al. Nanoparticle T-cell engagers as a modular platform for cancer immunotherapy. Leukemia. 2021;35:2346–2357. doi: 10.1038/s41375-021-01127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Diaz L.A., Jr., Bardelli A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Araghi M., Mannani R., Heidarnejad Maleki A., et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023;23:162. doi: 10.1186/s12935-023-02990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Majeed U., Manochakian R., Zhao Y., et al. Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J. Hematol. Oncol. 2021;14:108. doi: 10.1186/s13045-021-01121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alam M., Ali S., Ashraf G.M., et al. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022;379 doi: 10.1016/j.foodchem.2022.132135. [DOI] [PubMed] [Google Scholar]
- 149.Koche D., Shirsat R., Kawale M. An overview of major classes of phytochemicals: their types and role in disease prevention. Hislopia J. 2016;9:1–11. [Google Scholar]
- 150.Iqbal J., Abbasi B.A., Mahmood T., et al. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017;7:1129–1150. [Google Scholar]







