Abstract
Background and Objectives
Evidence regarding effectiveness of screening for emotional and cognitive problems after stroke is lacking. The primary aim of this study was to test the hypothesis that an intervention focusing on screening and care for emotional and cognitive problems after stroke would improve societal participation at 1 year. Second, we tested the hypotheses that this intervention would improve emotional and cognitive concerns, symptoms of anxiety, symptoms of depression, quality of life (QoL), self-efficacy, and disability.
Methods
This multicenter, patient-masked, cluster-randomized controlled trial assigned clusters (1:1) to intervention or usual care. Clusters were Dutch nonuniversity hospitals with a stroke unit. Ischemic stroke patients aged 18 years and older discharged home without inpatient or outpatient rehabilitation were included. The intervention was a consultation conducted by a specialized stroke nurse at the outpatient neurology clinics at 6 weeks after stroke and included screening for emotional and cognitive problems, screening for participation restrictions, self-management support, and, if needed, referral to rehabilitation services. The primary outcome was societal participation (Restrictions subscale of the Utrecht Scale for Evaluation of Rehabilitation—Participation [USER-P-R]) 1 year after stroke. Secondary outcomes were cognitive and emotional concerns (Checklist for Cognitive and Emotional Consequences following Stroke), symptoms of anxiety (Hospital Anxiety and Depression Scale—Anxiety subscale [HADS-A]), symptoms of depression (HADS—Depression subscale), QoL (5-level version of the EuroQoL 5-dimensional questionnaire [EQ-5D-5L], EuroQoL Visual Analog Scale [EQ-VAS], and Patient-Reported Outcome Measurement Information System [PROMIS-10]), self-efficacy (General Self-Efficacy Scale), and disability (modified Rankin Scale) at 3 and 12 months. Continuous outcome data were analyzed using a mixed model for repeated measures, and ordinal data were analyzed with an ordinal mixed-effects model.
Results
Between August 14, 2019, and May 11, 2022, a total of 531 patients were included in 12 clusters. The mean age was 70.6 ± 9.7 years, 40.0% were female, and the median NIH Stroke Scale score was 2 (2). Two hundred sixty-four patients were included in 6 hospitals providing the intervention and 267 patients in 6 hospitals providing usual care. Primary analysis demonstrated no difference in USER-P-R score at 1 year after stroke (mean difference [MD] 0.77; 95% CI −2.46 to 4.06; p = 0.652). Secondary outcome analyses at 3 months showed a MD between the intervention group and usual care group in HADS-A scores of −0.86 (95% CI −1.33 to −0.39), a MD in EQ-5D-5L index scores of 0.044 (95% CI 0.022–0.065), and a MD in EQ-VAS score of 2.90 (95% CI 0.69–5.10). Secondary outcome analyses at 1 year demonstrated a MD in EQ-5D-5L index scores of 0.043 (95% CI 0.021–0.064).
Discussion
Screening for emotional and cognitive problems did not improve societal participation at 1 year after stroke. The potential for improvement in anxiety and QoL warrants further investigation. Cost-effectiveness will be analyzed in our upcoming economic evaluation.
Trial Registration Information
Netherlands Trial Register: NL7295. Registered: September 25, 2018. First patient enrolled: August 14, 2019.
Classification of Evidence
This study provides Class III evidence that, in patients recovering from an ischemic stroke, active screening for emotional and cognitive problems at 6 weeks after stroke did not improve societal participation at 1 year.
Introduction
After stroke, many patients experience emotional and cognitive problems. These consequences occur not only with major stroke but also in patients with minor stroke.1 Improved treatment options for the acute phase have resulted in an increasing number of patients having minor instead of major disabilities.2,3 It is specially in these minor stroke patients that emotional and cognitive consequences negatively influence societal participation and quality of life (QoL).4-6 Guidelines, therefore, recommend screening for emotional and cognitive problems after stroke.7,8 Nonetheless, evidence supporting the effectiveness of screening and care for emotional and cognitive problems after stroke is limited.9 Previous studies emphasized the importance of closing this knowledge gap. It is important to note that patients listed emotional and cognitive problems as top research priorities.10-12
We examined the clinical effectiveness, cost-effectiveness, and feasibility of an intervention focusing on screening and care for emotional and cognitive problems, compared with usual care, in stroke patients discharged home directly without inpatient rehabilitation or follow-up treatment at an outpatient rehabilitation clinic.13 This article reports the results regarding its clinical effectiveness; cost-effectiveness and feasibility will be published separately. Our primary aim was to test the hypothesis that an intervention focusing on screening and care for emotional and cognitive problems would improve societal participation 1 year after stroke, as measured using the patient-reported Restrictions subscale of the Utrecht Scale for Evaluation of Rehabilitation at the Participation level (USER-P-R) questionnaire. Societal participation was chosen as the primary objective, as improving societal participation is a key treatment objective, and because societal participation includes clinically relevant outcomes such as work, social roles, and daily activities. Second, we tested the hypothesis that this intervention would (1) improve societal participation at 3 months and (2) improve emotional and cognitive concerns, symptoms of anxiety, symptoms of depression, QoL, self-efficacy, and disability at 3 months and 1 year after stroke.
Methods
Trial Design
The ECO-stroke trial was a Dutch multicenter, patient-masked, cluster-randomized controlled trial. Clusters were hospitals. A cluster design was chosen because we aimed to mirror clinical practice by recruiting health care providers for the trial intervention who performed stroke aftercare on a daily basis. Because most Dutch hospitals have 1 health care provider for stroke aftercare, a patient-level randomization was considered infeasible, as this would probably lead to contamination.
Standard Protocol Approvals, Registrations, and Patient Consents
The Medical Research Ethics Committees United at Nieuwegein determined that the ECO-stroke trial was not subject to the Dutch Medical Research Involving Human Subjects Act (WMO) (reference number: W18.169). For research not subject to the WMO in the Netherlands, approval is not obtained from a central ethics review committee, but from the local medical ethics committees of the hospitals involved. The trial protocol and amendments were consequently approved by the local medical ethics committees of all participating hospitals. Written informed consent was obtained from all participants in the study. The study was registered in the Netherlands Trial Register (NL7295) on September 25, 2018. Full details on the design of the study have been published elsewhere.13 The first and most recent signed copy of the protocol and the statistical analysis plan (SAP) are available as eSAPs 1 and 2.
Cluster Eligibility and Randomization
All Dutch hospitals with a neurology department were invited to participate. Hospitals intending to participate were assessed using a questionnaire and interviews (eTables 1 and 2). The following exclusion criteria were applied for hospital participation: (1) current stroke care including screening for emotional and cognitive problems using validated screening instruments for most of the patients and/or (2) current stroke care including long-term follow-up (≥6 months) with repeated emotional and cognitive screening, with or without validated screening instruments, for most of the patients. Eligible hospitals were randomized 1:1 using the web-based statistical software program Randomizer using block sizes of 2.14 Hospitals were randomized as unmatched pairs at the same time by an independent statistician, to prevent predictability due to fixed block sizes. The randomization sequence was unknown to the independent statistician and investigators.
Trial Participants
Eligible patients were aged 18 years or older, with a clinical diagnosis of ischemic stroke being discharged home without inpatient or outpatient rehabilitation. Exclusion criteria were serious comorbidity presumably interfering with the outcomes (e.g., a psychiatric disorder requiring supervision by a psychiatrist); serious comorbidity with a progressive course (e.g., cancer); mild cognitive impairment or dementia; a life expectancy <6 months; TIA defined as signs and symptoms <24 hours and not accompanied by ischemic lesions in the corresponding vascular territory on CT or MRI scan; hemorrhagic stroke; and inability to understand questionnaires or legal incompetence, on the basis of clinical judgment.
Interventions
In the intervention group, further to usual care, the intervention was planned at the outpatient neurology clinics 6 weeks after stroke. The intervention consisted of 1 face-to-face consultation lasting 1 hour, conducted by a specialized nurse, nurse practitioner, or physician assistant employed at the participating hospital and experienced in stroke aftercare, hereinafter referred to as “nurse.” The intervention included the following:
Screening for emotional and cognitive problems using the Checklist for Cognitive and Emotional Consequences following Stroke (CLCE-24), the Montreal Cognitive Assessment, and the Hospital Anxiety and Depression Scale (HADS)
Screening for restrictions of participation with the USER-P-R
Self-management support consisting of (i) individualized information provision, (ii) assessment of self-efficacy using the Dutch General Self-Efficacy Scale (GSES), (iii) shared decision making, and (iv) provision of contact details
The use of a decision tool for referral to rehabilitation or other services
The intervention has been described in detail in the published protocol.13 Patients in the control group received usual care. While stroke aftercare varied by hospital, it included at least one 30-minute consultation focused on assessing and treating cardiovascular risk factors, as well as addressing emotional and cognitive issues.
Procedures
Patients were screened for eligibility and asked to participate within 4 weeks after stroke by the treating health care professional or an investigator. An information letter was offered. If patients decided to participate, an informed consent form was signed.
Baseline characteristics were assessed within 6 weeks after stroke by reviewing medical charts, complemented by a telephone interview by a researcher (T0). The NIH Stroke Scale (NIHSS) was completed at the time of the index event by a treating physician. Questionnaires were completed at 6 weeks after stroke (T1) (simultaneously with the intervention), 3 months after stroke (T2), and 1 year after stroke (T3). At T2 and T3 for the intervention group and at all time points for the control group, patients received questionnaires by post or email. At T1, all patients in the intervention group received the questionnaire by post and brought these to the consultation, as some were used for the intervention. The modified Rankin Scale (mRS) score was recorded at discharge and at T2 and T3 by phone by a health care provider or an investigator. In the intervention group, the CLCE-24 at T1 was administered during the intervention. All other questionnaires are patient-reported outcome measures and were completed at T1, T2, and T3 by patients at home, without the support of any health care provider or investigator. If unanswered for 2 weeks, patients received up to 2 reminder calls.
Outcomes
The primary outcome was the level of societal participation measured with the USER-P-R at 1 year after stroke. USER-P-R is a patient-reported questionnaire measuring restrictions experienced on 11 subdomains of participation, that is, work (paid or unpaid) or education, household duties, outdoor mobility, sports, going out, day trips and other outdoor activities, leisure activities, relationship with partner, visits to family or friends, family or friends coming to visit, and contacting others by phone or computer.15,16 The USER-P-R measures the level of participation after an illness or a condition; the prestroke participation level was, therefore, not assessed. Items are converted to a 0–100 sum score, with higher scores indicating greater participation. The USER-P-R has demonstrated good psychometric properties in various populations, primarily Dutch, including those with stroke.15-19
Secondary outcomes were the level of societal participation (USER-P-R) at 3 months and emotional and cognitive concerns (CLCE-24 emotion subscore and cognition subscore), symptoms of depression (HADS-D), symptoms of anxiety (HADS-A), QoL (5-level version of the EuroQol 5-dimensional questionnaire [EQ-5D-5L], EQ Visual Analog Scale [EQ-VAS]), Patient-Reported Outcome Measurement Information System (PROMIS Global Mental Health and PROMIS Global Physical Health), self-efficacy (Dutch adaptation of the GSES), and disability (mRS), at 3 months and 1 year after stroke. The CLCE-24 measures the presence of 9 emotional and 13 cognitive concerns. Two blank items are included in case other problems are present.20 The 14-item HADS includes two 7-item subscales, 1 for anxiety (HADS-A) and 1 for depression (HADS-D), evaluating anxiety and depression on a 0–21 sum score for each subscale, with more signs producing a higher score.21 The EQ-5D-5L, assessing QoL across 5 domains, results in a 5-digit code that can be converted to an index score, which is a single summary figure with a maximum value of 1.22,23 The EQ-VAS measures QoL on a 0–100 scale. A higher QoL is represented by a higher EQ-5D-5L index score and a higher EQ-VAS score. The PROMIS Global-10 consists of 2 subscales assessing Global Mental Health and Global Physical Health (ranging from 0 to 20).24 The PROMIS Global Mental Health and Global Physical Health scores can be converted to a T-score in which 50 reflects the mean of a reference population and 10 is the matching SD. The 10-item GSES evaluates self-efficacy, resulting in a sum score from 10 (low level of self-efficacy) to 40 (high).25 The mRS measures disability from 0 (“no symptoms at all”) to 6 (“dead”).26
Masking
Researchers and health care providers were aware of treatment allocations. Patients were masked to the treatment allocation: patients gave written informed consent to receive usual care or usual care plus a structured assessment of stroke-related problems. After the last measurement had been completed, patients were informed about the treatment allocation. The patient-reported primary and secondary outcomes, except for the mRS, were completely masked because the questionnaires were completed by the patients without support from the research team. The mRS might have been influenced by unmasked researchers while the other outcomes were unaffected.
Sample Size Calculation
Because USER-P-R data for the target population were unavailable at the time of protocol development, the sample size calculation was based on calculation of Cohen d as the benchmark for assessing the relative magnitude of differences in participation between both groups. We assumed that a Cohen d of 0.35 would be clinically relevant. A total of 12 clusters, 6 for each group, encompassing 36 patients per cluster (432 patients in total) would be needed to achieve 80% power. This assumed an intracluster correlation between hospitals of 0.01, unequal clusters, and a 2-sided α of 5%.27 We expected an attrition rate of 15% at 12 months, and the planned sample size was increased to 258 patients per treatment group or 516 patients in total. During the trial's inclusion period, large variations in cluster sizes were observed. Therefore, the sample size calculation was adjusted for the harmonic mean, resulting in a required total of 531 patients. An overview of the actual number of patients per cluster and treatment allocation, along with the corresponding harmonic means and final power (80.75%), is provided in eTable 3.
Statistical Analysis
A SAP was finalized before database lock (eSAP 2). Baseline characteristics were summarized using simple descriptive statistics. Because of the cluster design, baseline differences between treatment groups were statistically tested using independent-samples t tests for normally distributed continuous variables, Mann-Whitney U tests for non-normally distributed continuous variables, and a χ2 test for binary and categorical data.
The primary outcome was the mean between-group difference in USER-P-R scores at 1 year after stroke. Because outcomes were assessed at multiple time points, a mixed model for repeated measures (MMRM) was used. The average treatment effect (least squares means) and the absolute treatment difference at 1 year were extracted from this model and are presented with their 95% CI and p value. The model included fixed terms for treatment allocation group, visit, treatment allocation, group-by-visit interaction, baseline USER-P-R score, age, NIHSS score, level of education (based on the Dutch classification system developed by Verhage), and baseline mood (HADS-D).28 The individual patients and hospitals were included as the random effects, thereby accounting for the intrapatient and intrahospital clustering. An unstructured covariance matrix was used as variance-covariance structure; p values were based on Satterthwaite corrected degrees of freedom. Missing data of the baseline covariates of stroke severity, level of education, and mood were imputed using mean imputation.
The secondary outcomes CLCE-24 cognition subscore, CLCE-24 emotion subscore, HADS-A score, HADS-D score, PROMIS Global Mental Health score, PROMIS Global Physical Health score, EQ-5D-5L score, EQ-VAS score, and GSES score were analyzed using the same model. The ordinal mRS scores were analyzed using the same statistical approach, applying an ordinal mixed-effects model with the same random and fixed effects. The results are described using common odds ratios and 95% CI.
All analyses were performed according to the modified intention-to-treat principle: patients were analyzed according to their randomized allocation and were required to have at least 1 postbaseline visit.29 Consequently, the primary analysis population included patients with at least 1 completed assessment of the primary outcome measure. When data on both follow-up measurements were missing, patients were excluded (eTable 4). The same applied to the secondary outcomes. Regarding missing data, a complete case analysis was performed as a sensitivity analysis, including patients with complete baseline data and at least 1 postbaseline measurement. In an additional sensitivity analysis, the outcome of USER-P-R at 1 year was analyzed according to a prespecified rewindowing schedule. The rewindowing schedule is explained in the SAP (eSAP 2). Secondary outcomes were considered exploratory and were not corrected for multiple testing. The aforementioned sensitivity analyses were prespecified in the SAP (eSAP 2), as well as exploratory subgroup analyses. In addition, 1 non-prespecified sensitivity analysis and 2 non-prespecified subgroup analyses were added afterward. In this sensitivity analysis, deceased patients were assigned the worst possible score rather than being treated as missing at random. The added subgroup analyses included 1 examining patients with baseline participation restrictions (USER-P-R score <90) and another comparing patients below and above the clinically relevant anxiety threshold (HADS-A score <8 vs HADS-A score ≥8). All statistical analyses, except linear and ordinal mixed-effects models, were performed in IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY). Linear and ordinal mixed-effects models were constructed in RStudio 2022.07.2, using the lme4 package.30,31
Data Availability
Deidentified participant data underlying the results presented in this article will be shared with investigators after a review committee's approval and a signed data-sharing agreement. Proposals can be submitted 9–36 months after publication.
Results
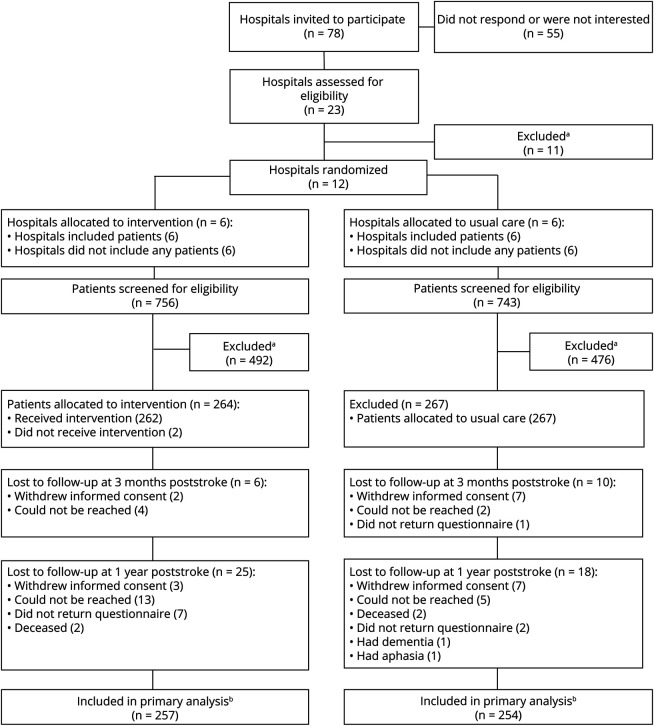
Between July 31, 2019, and November 25, 2020, 12 hospitals were included (Figure) and randomized (1:1). All hospitals were nonuniversity hospitals in the Netherlands with a stroke unit treating >100 stroke patients per year. An overview of the usual care before the start of the trial is given in eTables 1 and 2.
Figure. Study Profile.
(A) Reasons for exclusion are shown in eAppendix 1. (B) Patients with 1 postbaseline measurement were included in the primary analyses.
In the 6 intervention hospitals, 756 patients were screened for eligibility, whereas in the 6 usual care hospitals, 743 were screened. Between August 14, 2019, and May 11, 2022, 531 patients were included: 264 patients in the intervention group and 267 in the control group (Figure and eAppendix 1). The follow-up ended at July 3, 2023, when the last patient's 12-month measurement was completed. In the intervention clusters, 257 patients had at least 1 postbaseline measurement and were included in the primary analysis. In the usual care clusters, 254 patients were included in the primary analysis. An overview of missing data is given in eTable 4. Data were analyzed between October 9, 2023, and November 30, 2023.
Baseline characteristics are provided in Table 1. In the intervention group, a higher number of patients were treated with IV thrombolysis (IVT). Besides, the prevalence of hypercholesterolemia and the systolic and diastolic blood pressures in this group was also higher and the use of oral anticoagulants was lower. The mRS scores at discharge were worse in the intervention group. Baseline EQ-5D-5L index scores were lower in the intervention group. Mean USER-P-R scores at baseline were 82.5 (SD 19.5) in the intervention group and 82.4 (SD 19.9) in the control group.
Table 1.
Baseline Characteristics
| Intervention (n = 264) | Usual care (n = 267) | p Value | |
| Age, y, mean (SD) | 70.6 (9.9) | 70.6 (9.4) | 0.529 |
| Female sex, n (%) | 109 (41.3) | 106 (39.7) | 0.709 |
| Level of education based on the Dutch classification system developed by Verhage, n (%) | 0.037 | ||
| Low | 178 (67.4) | 182 (68.1) | |
| High | 77 (29.2) | 51 (19.1) | |
| Not otherwise specified | 2 (0.8) | 3 (1.1) | |
| NIHSS score at admission, median (IQR) | 2 (2) | 2 (2) | 0.346 |
| Treated with IVT, n (%) | 80 (30.3) | 56 (21.0) | 0.014 |
| Treated with EVT, n (%) | 8 (3.0) | 14 (5.2) | 0.201 |
| Duration of hospital admission, d, median (IQR) | 2 (2) | 2 (1) | 0.012 |
| mRS score at discharge, n (%) | 0.006 | ||
| 0 | 33 (12.5) | 38 (14.2) | |
| 1 | 124 (47.0) | 143 (53.6) | |
| 2 | 77 (29.2) | 82 (30.7) | |
| 3 | 20 (7.6) | 4 (1.5) | |
| 4 | 2 (0.8) | 0 (0.0) | |
| 5 | 0 (0.0) | 0 (0.0) | |
| Previous stroke, n (%) | 32 (12.1) | 41 (15.4) | 0.279 |
| Previous TIA, n (%) | 36 (13.6) | 27 (10.1) | 0.209 |
| Hypertension, n (%) | 152 (57.6) | 150 (56.2) | 0.745 |
| Hypercholesterolemia, n (%) | 192 (72.7) | 124 (46.4) | <0.001 |
| Diabetes mellitus, n (%) | 54 (20.5) | 56 (21.0) | 0.883 |
| Atrial fibrillation, n (%) | 23 (8.7) | 30 (11.2) | 0.332 |
| USER-P-R score, mean (SD) | 82.5 (19.5) | 82.4 (19.9) | 0.918 |
| CLCE-24 cognition subscore, mean (SD) | 2.3 (2.5) | 2.6 (3.0) | 0.737 |
| CLCE-24 emotion subscore, mean (SD) | 2.2 (2.0) | 2.4 (2.1) | 0.195 |
| HADS-A score, mean (SD) | 5.0 (4.3) | 4.8 (3.9) | 0.978 |
| HADS-D score, mean (SD) | 4.5 (3.9) | 4.0 (3.4) | 0.333 |
| EQ-5D-5L index score, mean (SD) | 0.792 (0.152) | 0.821 (0.151) | 0.033 |
| EQ-VAS score, mean (SD) | 72.7 (16.0) | 74.6 (14.9) | 0.168 |
| PROMIS Global Physical Health score, mean (SD) | 43.3 (6.2) | 43.1 (6.6) | 0.669 |
| PROMIS Global Mental Health score, mean (SD) | 45.5 (8.0) | 45.1 (7.9) | 0.534 |
| GSES score, mean (SD) | 33.4 (5.3) | 32.5 (5.6) | 0.137 |
| MoCA score, mean (SD) | 25.9 (3.1) | NA | NA |
| BMI, kg/m2, mean (SD) | 27.1 (4.8) | 27.2 (4.7) | 0.743 |
| Systolic blood pressure, mm Hg, mean (SD) | 147 (22) | 140 (20) | <0.001 |
| Diastolic blood pressure, mm Hg, mean (SD) | 83 (14) | 79 (11) | <0.001 |
| Cardiovascular drug use after the index event | |||
| Antiplatelet agents | 232 (87.9) | 222 (83.1) | 0.121 |
| Oral anticoagulants | 33 (12.5) | 50 (18.7) | 0.048 |
| Lipid-lowering agents | 244 (92.4) | 250 (93.6) | 0.584 |
| Antihypertensive agents | 160 (60.6) | 156 (58.4) | 0.609 |
Abbreviations: BMI = body mass index; CLCE-24 = Checklist for Cognitive and Emotional Consequences following Stroke; EVT = endovascular thrombectomy; EQ-5D-5L = 5-level version of the EuroQol 5-dimensional questionnaire; EQ-VAS = EQ Visual Analog Scale; GSES = General Self-Efficacy Scale; HADS-A = Hospital Anxiety and Depression Scale—Anxiety subscale; HADS-D = Hospital Anxiety and Depression Scale—Depression subscale; IQR = interquartile range; IVT = IV thrombolysis; mRS = modified Rankin Scale; MoCA = Montreal Cognitive Assessment; NA = not applicable; NIHSS = NIH Stroke Scale; PROMIS = Patient-Reported Outcome Measurement Information System; USER-P-R = Restrictions subscale of the Utrecht Scale for Evaluation of Rehabilitation—Participation.
Of 264 patients allocated to the intervention group, 262 (99.2%) attended the intervention. In 233 patients (88.3%), all screening instruments were completed. Regarding referrals after the intervention, 14 patients (5.3%) were referred to first-line therapies such as physical therapy, occupational therapy, or speech therapy and 20 patients (7.6%) were referred to outpatient rehabilitation treatment. A total of 68 patients (25.8%) received an additional follow-up appointment at the stroke aftercare outpatient clinic, and 91 patients (34.5%) were referred to primary nurse–led stroke care. Although all patients in the Netherlands receive follow-up care by their general practitioner, in 65 patients (24.6%), the general practitioner was asked to actively monitor or treat specific poststroke consequences.
Primary Outcome
The results of the MMRM are given in Table 2. At 1 year after stroke, the mean USER-P-R score did not differ significantly between the intervention group and the usual care group (mean difference [MD] 0.77; 95% CI −2.47 to 4.06; p = 0.652) (Table 3). The intracluster correlation for the primary outcome was 0.013.
Table 2.
Results of the MMRM Assessing the Effect of the Intervention on the Primary Outcome
| Effect type | Parameter | Estimate | SE | t Value | 95% CI |
| Fixed effects | Intercept (usual care at 3 mo) | 82.835 | 1.197 | 69.208 | 80.549 to 85.103 |
| Age, y | −0.245 | 0.061 | −4.009 | −0.362 to −0.122 | |
| Level of education | 2.475 | 1.373 | 1.803 | −0.189 to 5.169 | |
| NIHSS score | 0.218 | 0.219 | 0.999 | −0.202 to 0.653 | |
| Baseline HADS-D score | −0.665 | 0.188 | −3.534 | −1.041 to −0.304 | |
| Baseline USER-P-R score | 0.584 | 0.035 | 16.655 | 0.515 to 0.652 | |
| Visit at 1 y | 0.319 | 1.115 | 0.286 | −1.869 to 2.502 | |
| Intervention at 3 mo | 1.894 | 1.693 | 1.119 | −1.308 to 5.142 | |
| Interaction visit by intervention at 1 y | −1.121 | 1.580 | −0.709 | −4.211 to 1.983 | |
| Variance | |||||
| Random effects | Between-participant variance | 86.748 | |||
| Between-site variance | 2.155 | ||||
| Residual variance | 150.213 | ||||
Abbreviations: HADS-D = Hospital Anxiety and Depression Scale—Depression subscale; MMRM = mixed model for repeated measures; NIHSS = NIH Stroke Scale; USER-P-R = Restrictions subscale of the Utrecht Scale for Evaluation of Rehabilitation—Participation.
Table 3.
Primary Outcome and Secondary Outcomes
| Primary outcome at 1 y after stroke | Mean intervention | Mean usual care | Mean difference (95% CI) | p Value |
| USER-P-R score | 83.93 | 83.15 | 0.77 (−2.47 to 4.06) | 0.652 |
| Secondary outcomes at 3 mo after stroke | ||||
| USER-P-R score | 84.72 | 82.84 | 1.89 (−1.31 to 5.14) | |
| CLCE-24 cognition subscore | 2.34 | 2.54 | −0.21 (−0.59 to 0.18) | |
| CLCE-24 emotion subscore | 2.28 | 2.32 | −0.04 (−0.33 to 0.25) | |
| HADS-A subscore | 4.22 | 5.08 | −0.86 (−1.33 to −0.39) | |
| HADS-D subscore | 3.94 | 4.31 | −0.37 (−0.85 to 0.14) | |
| EQ-5D-5L index score | 0.842 | 0.799 | 0.044 (0.022 to 0.065) | |
| EQ-VAS score | 76.39 | 73.50 | 2.90 (0.69 to 5.10) | |
| PROMIS Global Physical Health T-score | 44.10 | 43.49 | 0.61 (−0.19 to 1.41) | |
| PROMIS Global Mental Health T-score | 45.68 | 45.65 | 0.03 (−1.03 to 1.09) | |
| GSES score | 33.80 | 32.84 | 0.97 (0.16 to 1.78) | |
| OR of treatment effect for unfavorable outcome (95% CI) | ||||
| mRS score | 1.96 | 1.99 | 1.13 (0.58 to 2.22) | |
| Secondary outcomes at 1 y after stroke | Mean difference (95% CI) | |||
| CLCE-24 cognition subscore | 2.81 | 2.84 | −0.03 (−0.42 to 0.36) | |
| CLCE-24 emotion subscore | 2.43 | 2.37 | 0.06 (−0.24 to 0.36) | |
| HADS-A subscore | 4.33 | 4.80 | −0.47 (−0.95 to 0.01) | |
| HADS-D subscore | 4.17 | 4.52 | −0.35 (−0.85 to 0.14) | |
| EQ-5D-5L index score | 0.831 | 0.788 | 0.043 (0.021 to 0.064) | |
| EQ-VAS score | 75.56 | 73.88 | 1.69 (−0.57 to 3.95) | |
| PROMIS Global Physical Health T-score | 43.83 | 43.32 | 0.51 (−0.31 to 1.32) | |
| PROMIS Global Mental Health T-score | 45.78 | 45.04 | 0.74 (−0.34 to 1.83) | |
| GSES score | 33.19 | 32.89 | 0.29 (−0.53 to 1.12) | |
| OR of treatment effect for unfavorable outcome (95% CI) | ||||
| mRS score | 1.79 | 1.97 | 0.78 (0.39 to 1.54) |
Abbreviations: CLCE-24 = Checklist for Cognitive and Emotional Consequences following Stroke; EQ-5D-5L = 5-level version of the EuroQol 5-dimensional questionnaire; EQ-VAS = EQ Visual Analog Scale; GSES = General Self-Efficacy Scale; HADS-A = Hospital Anxiety and Depression Scale—Anxiety subscale; HADS-D = Hospital Anxiety and Depression Scale—Depression subscale; mRS = modified Rankin Scale; OR = odds ratio; PROMIS = Patient-Reported Outcome Measurement Information System; USER-P-R = Restrictions subscale of the Utrecht Scale for Evaluation of Rehabilitation—Participation.
Secondary Outcomes at 3 Months and 1 Year
At 3 months after stroke, the MD in USER-P-R scores between the intervention group and the usual care group was 1.89 (95% CI −1.31 to 5.14) (Table 3). At 3 months after stroke, the MD in HADS-A scores between both groups was −0.86 (95% CI −1.33 to −0.39), the MD in EQ-5D-5L index scores was 0.044 (95% CI 0.022–0.065), the MD in EQ-VAS scores was 2.90 (95% CI 0.69–5.10), and the MD in GSES scores was 0.97 (95% CI 0.16–1.78).
At 1 year, the MD between the intervention and usual care group was 0.043 (95% CI 0.021–0.064) for the EQ-5D-5L index score (Table 2).
An overview of the results for all secondary outcomes is presented in Table 3.
Sensitivity Analyses
Complete case analyses at 3 months and 1 year and sensitivity analysis according to a rewindowing schedule demonstrated comparable results (eTables 5–8). In a repeated analysis where the worst possible score on the primary outcome was assigned to the 4 deceased patients at 1 year (1 in the intervention group and 3 in the usual care group), comparable results were observed (eTables 5–8).
Additional Subgroup Analyses
Prespecified additional exploratory subgroup analyses demonstrated comparable results regarding the primary outcome for all subgroups, as well as a non-prespecified additional secondary subgroup analysis for patients with participation restrictions at baseline, defined as a USER-P-R score <90 (eTable 9).
Classification of Evidence
This study provides Class III evidence that, in patients recovering from an ischemic stroke, active screening for emotional and cognitive problems 6 weeks after stroke did not improve societal participation at 1 year.
Discussion
In this multicenter, cluster-randomized controlled trial of stroke patients discharged home directly without inpatient rehabilitation or follow-up treatment at an outpatient rehabilitation clinic, a screening and care intervention for emotional and cognitive problems did not improve the primary outcome of societal participation at 1 year after stroke. Exploratory secondary outcome analyses suggest that patients receiving the intervention might experience fewer symptoms of anxiety at 3 months and an improved QoL at 3 months and 1 year after stroke.
Previous studies examining the effectiveness of screening and care for emotional and/or cognitive problems after stroke are scarce, especially regarding societal participation as an outcome.32-35 Moreover, previous studies are heterogeneous, which hampers comparison. These studies screened for a broad spectrum of stroke-related problems and did not consistently use validated instruments to screen for emotional or cognitive problems. One study offered 5 home visits with a follow-up of 18 months,33 another study offered frequent telephonic consultations during the first year,32 1 performed the intervention at 6 months,35 and 1 did not specify the timing and frequency of the assessment.34 An improvement in social activities was demonstrated in 1 previous study,33 but our finding that societal participation did not improve is in line with most of the previous research.32,34,35
Multiple considerations might explain why the primary outcome did not differ between treatment groups. First, because emotional and cognitive problems after stroke have increasingly drawn the attention of health care providers, the contrast may have been small. For example, current guidelines recommend screening and, in recent campaigns in the Netherlands, increased awareness of emotional and cognitive problems after brain injury.7,36,37 Second, societal participation is a complex outcome measure, influenced by various components, so the relationship between the intervention and societal participation might have been too indirect. Third, we developed an intervention consisting of a single consultation because we aimed to create an efficient intervention that does not require extensive resources, is easy to implement, and is cost-effective. However, this may have been insufficient to produce significant changes. Fourth, our intervention aimed to offer individualized education and patient-tailored follow-up care based on screening results. This level of patient-tailored care may have been too complex or time-consuming for a 1-hour consultation. Fifth, protocol adherence is a critical factor to consider. Most of the participants received the intervention (99.2%) and completed all screening instruments (88.3%), but adherence to the study protocol also encompasses additional elements, such as follow-up care. While many patients received an additional appointment at the stroke aftercare outpatient clinics or were referred to primary nurse–led stroke care, referral rates to first-line therapies and outpatient rehabilitation treatment were low. Although these referral rates might be appropriate for minor stroke patients, higher rates could potentially have improved the intervention's effectiveness. In a broader sense, low protocol fidelity may reduce the effectiveness. Therefore, in accordance with the Medical Research Council's guideline, a comprehensive process evaluation assessing protocol adherence will be published separately.13,38
At 3 months after stroke, exploratory secondary analyses suggest that patients experienced fewer symptoms of anxiety and higher QoL, similar to findings of a comparable intervention after cardiac arrest.39 These hypothesis-generating effects may have resulted from reassurance and information provision.40 Possibly, the act of screening itself, acknowledging the relationship between stroke and its potential emotional and cognitive consequences, while dedicating time and attention to these consequences, might have a reassuring impact. Previous studies examining screening interventions after stroke reported conflicting results.32,34,35,41-43 Two studies, 1 including weekly assessments of depression or anxiety and 1 examining a postdischarge program with structured assessments including mental health and cognition, demonstrated higher QoL and improvement in anxiety.41,42 One study did not demonstrate a change in QoL but found less anxiety after an outreach nursing support program.43 Other studies did not find enhanced QoL or a decrease in symptoms of anxiety after some form of screening.32,34,35 Comparing these results is complicated because the studies were heterogeneous regarding study population and timing, frequency, and content of the screening.
QoL was assessed using the EQ-5D-5L index score and the EQ-VAS score. Although the results from both measures pointed in the same direction, the effects measured with the EQ-VAS were smaller than those measured with the EQ-5D-5L. This may be explained by previous findings, showing that the EQ-VAS score is less responsive than the EQ-5D-5L index score in stroke patients.44
The effect sizes we found for both the measurement of symptoms of anxiety and the QoL are small and below minimally important differences.45,46 Nonetheless, our intervention does no harm and is cheap. Whether this small clinical effect is sufficient to justify implementation in practice will largely depend on the results of the cost-effectiveness analysis, which will be published separately.
The question might be raised whether minor stroke patients were the right target population. While emotional and cognitive problems are probably addressed during inpatient rehabilitation in patients with severe stroke, we believe that these problems might go unnoticed in patients with mild stroke who are discharged home without adequate follow-up care. Although improvements may be challenging to measure, we believe that this was the appropriate target group, because we have shown previously that emotional and cognitive problems occur just as frequently in patients discharged home compared with patients who were discharged to inpatient rehabilitation.4
Strengths of our trial include its randomized design and sufficient sample size. Besides, we used relevant patient-reported outcomes. In addition, health care providers who actually perform stroke aftercare on a daily basis were recruited to perform the intervention, thus adequately mirroring clinical practice. This increases its external validity and also improves future implementation processes.
The following limitations apply to this trial. First, the number of clusters in our trial was relatively small. Randomization leads to a more balanced baseline distribution with larger sample sizes. Because cluster randomization is performed at the cluster level, a smaller number of clusters increase the likelihood of unequal baseline distributions. Consequently, we ideally would have included a greater number of clusters with fewer patients per cluster, as baseline characteristics in our study were partly unevenly distributed. While more patients in the intervention group were treated with IVT, the mRS score at discharge was lower. In addition, vascular risk factors were more prevalent in the intervention group. We, therefore, assumed that these unevenly distributed baseline characteristics did not favor the intervention group but might even have led to underestimation of the true effect size. Next, patients were not informed about the cluster allocation during the course of the trial to maintain patient masking. Patients were generally unaware of the exact differences between usual care and the intervention and were thus masked to treatment allocation. Yet, patients might have presumed their treatment allocation, and it is possible that more patients in the intervention group anticipated receiving the intervention. Another limitation is the ceiling effect of the USER-P-R: 170 of 531 patients scored 100 at baseline. Nonetheless, subgroup analyses did not demonstrate a difference at 1 year in patients with participation restrictions at baseline (eTable 9). Hence, the lack of change of the primary outcome was probably not due to this ceiling effect. Another limitation is that the outcome assessors of the mRS were not masked to the treatment allocation. Furthermore, during this trial, we did not record how patients were treated after a referral, for example, with cognitive behavioral therapy or pharmacotherapy. Recording this information could have provided valuable insights and is recommended for future research. Finally, self-management was a component of our intervention, but the duration may have been too short for optimal self-management support. We incorporated key elements of self-management, namely education and shared decision making, for which some evidence of effectiveness exists.40,47 A more comprehensive self-management approach may have been more effective, as shown by a trial conducted after our study.47
The results of the ECO-stroke trial demonstrate that screening for emotional and cognitive problems in stroke patients discharged home directly without inpatient or outpatient rehabilitation did not improve societal participation 1 year after stroke. The intervention demonstrated a potential beneficial effect on anxiety at 3 months after stroke and on QoL at 3 months and at 1 year after stroke, which warrants further investigation. The cost-effectiveness of this intervention will be analyzed in our upcoming economic evaluation.
Acknowledgment
The authors thank all participants for their contributions to this study. The authors also thank nurse practitioner P. Zandbelt, MANP, and the patient association Hersenletsel.nl for their contribution to the development of the intervention, and R.J. de Haan for the initial conception of the statistics. The authors thank all specialized nurses, nurse practitioners, and physician assistants who performed the intervention.
Glossary
- CLCE-24
Checklist for Cognitive and Emotional Consequences following Stroke
- EQ-VAS
EuroQol Visual Analog Scale
- EQ-5D-5L
5-level version of the EuroQoL 5-dimensional questionnaire
- HADS
Hospital Anxiety and Depression Scale
- HADS-A
Hospital Anxiety and Depression Scale—Anxiety subscale
- HADS-D
Hospital Anxiety and Depression Scale—Depression subscale
- IVT
IV thrombolysis
- GSES
General Self-Efficacy Scale
- MD
mean difference
- MMRM
mixed model for repeated measures
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- PROMIS
Patient-Reported Outcome Measurement Information System
- QoL
quality of life
- SAP
statistical analysis plan
- USER-P-R
Restrictions subscale of the Utrecht Scale for Evaluation of Rehabilitation—Participation
- VAS
Visual Analog Scale
- WMO
Medical Research Involving Human Subjects Act
Appendix. Coinvestigators
| Name | Location | Role | Contributions |
| Lahcen L. Hani, MD, Drs | Department of Neurology, Noordwest Ziekenhuisgroep locatie Den Helder, Den Helder, the Netherlands | Local principal Investigator | Supervision and project administration of local site, review of the final manuscript |
| Anja A. Frinks | Department of Neurology, Noordwest Ziekenhuisgroep locatie Den Helder, Den Helder, the Netherlands | Health care professional performing the intervention, data collection | Data collection, review of the final manuscript |
| Paul H.P. Bienfait, MD | Department of Neurology, Gelre ziekenhuizen, Apeldoorn, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Marjon M.J. Bosman-Haverkamp, BSc | Department of Neurology, Gelre ziekenhuizen, Apeldoorn, the Netherlands | Data collection | Data collection, review of the final manuscript |
| Edwin E.W. Peters, MD | Department of Neurology, Admiraal de Ruyter Hospital, Vlissingen, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Ina I. Wassenaar | Department of Neurology, Admiraal de Ruyter Hospital, Vlissingen, the Netherlands | Health care professional performing the intervention, data collection | Data collection, review of the final manuscript |
| Walid W. Moudrous, MD, Drs | Department of Neurology, Maasstad Hospital, Rotterdam, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Elke E.E. Meier-Harleman | Department of Neurology, Maasstad Hospital, Rotterdam, the Netherlands | Data collection | Data collection, review of the final manuscript |
| Mirjam M. Janmaat, MD, Drs | Department of Neurology, BovenIJ ziekenhuis, Amsterdam, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Annet A. Verburg | Department of Neurology, BovenIJ ziekenhuis, Amsterdam, the Netherlands | Health care professional performing the intervention, data collection | Data collection, review of the final manuscript |
| Patricia P.H.A. Halkes, MD, Dr | Department of Neurology, Noordwest Ziekenhuisgroep locatie Alkmaar, Alkmaar, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Mariska V.M. Blankendaal | Department of Neurology, Noordwest Ziekenhuisgroep locatie Alkmaar, Alkmaar, the Netherlands | Data collection | Data collection, review of the final manuscript |
| Anita A.C.A. Veldt | Department of Neurology, Noordwest Ziekenhuisgroep locatie Alkmaar, Alkmaar, the Netherlands | Data collection | Data collection, review of the final manuscript |
| Tobien A.H.C.M.L. Schreuder, MD | Department of Neurology, Zuyderland Medisch Centrum, Heerlen, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Tiny H.W.E. Simons-Sporken, CRC | Department of Neurology, Zuyderland Medisch Centrum, Heerlen, the Netherlands | Data collection | Data collection, review of the final manuscript |
| Lisette E. Maasland, MD, PhD | Department of Neurology, Het Van Weel-Bethesda Ziekenhuis, Dirksland, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| José J.A. Franse | Department of Neurology, Het Van Weel-Bethesda Ziekenhuis, Dirksland, the Netherlands | Health care professional performing the intervention, data collection | Data collection, review of the final manuscript |
| Sanne S.M. Zinkstok, MD, PhD | Department of Neurology, Tergooi medical center, Hilversum, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Kitty C.M. Harrison-Hilhorst, M-ANP | Department of Neurology, Tergooi medical center, Hilversum, the Netherlands | Health care professional performing the intervention, data collection | Data collection, review of the final manuscript |
| Mary-Lou M.P.J. van Goor, MD, Dr | Department of Neurology, Laurentius ziekenhuis Roermond, Roermond, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Kirsten K.F.F. de Bock-Dumoulin | Department of Neurology, Laurentius ziekenhuis Roermond, Roermond, the Netherlands | Data collection | Data collection, review of the final manuscript |
| Delmar D.S.M. Molenaar, MD, Dr | Department of Neurology, Ziekenhuis Amstelland, Amstelveen, the Netherlands | Local principal investigator | Supervision and project administration of local site, review of the final manuscript |
| Irene I.M. Vliegenthart, MSc | Department of Neurology, Ziekenhuis Amstelland, Amstelveen, the Netherlands | Health care professional performing the intervention, data collection | Data collection, review of the final manuscript |
Author Contributions
J.P.L. Slenders: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. R.M. Van Den Berg-Vos: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. C.M. Van Heugten: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. J. Visser-Meily: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. R.P.A. van Eijk: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. M.A.A. Moonen: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. S. Godefrooij: major role in the acquisition of data; analysis or interpretation of data. V.I.H. Kwa: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data.
Study Funding
This study was funded by ZonMw (program Efficiency Studies; project number 843004122).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Moran GM, Fletcher B, Feltham MG, Calvert M, Sackley C, Marshall T. Fatigue, psychological and cognitive impairment following transient ischaemic attack and minor stroke: a systematic review. Eur J Neurol. 2014;21(10):1258-1267. doi: 10.1111/ene.12469 [DOI] [PubMed] [Google Scholar]
- 2.Balami JS, Sutherland BA, Edmunds LD, et al. A systematic review and meta-analysis of randomized controlled trials of endovascular thrombectomy compared with best medical treatment for acute ischemic stroke. Int J Stroke. 2015;10(8):1168-1178. doi: 10.1111/ijs.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slenders JPL, Verberne DPJ, Visser-Meily JMA, Van den Berg-Vos RM, Kwa VIH, van Heugten CM. Early cognitive and emotional outcome after stroke is independent of discharge destination. J Neurol. 2020;267(11):3354-3361. doi: 10.1007/s00415-020-09999-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Kemp J, Kruithof WJ, Nijboer TCW, van Bennekom CAM, van Heugten C, Visser-Meily JMA. Return to work after mild-to-moderate stroke: work satisfaction and predictive factors. Neuropsychol Rehabil. 2019;29(4):638-653. doi: 10.1080/09602011.2017.1313746 [DOI] [PubMed] [Google Scholar]
- 6.van Dijk EJ, de Leeuw FE. Recovery after stroke: more than just walking and talking again if you don't look for it, you won't find it. Eur J Neurol. 2012;19(2):189-190. doi: 10.1111/j.1468-1331.2011.03520.x [DOI] [PubMed] [Google Scholar]
- 7.Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98-e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 8.Holloway RG, Arnold RM, Creutzfeldt CJ, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(6):1887-1916. doi: 10.1161/STR.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 9.Quinn TJ, Richard E, Teuschl Y, et al. European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur Stroke J. 2021;6(3):I-xxxviii. doi: 10.1177/23969873211042192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin BL, Mei YX, Wang WN, et al. Unmet care needs of community-dwelling stroke survivors: a systematic review of quantitative studies. BMJ Open. 2021;11(4):e045560. doi: 10.1136/bmjopen-2020-045560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke--consensus from stroke survivors, caregivers, and health professionals. Int J Stroke. 2014;9(3):313-320. doi: 10.1111/j.1747-4949.2012.00942.x [DOI] [PubMed] [Google Scholar]
- 12.Weerasekara I, Baye J, Burke M, et al. What do stroke survivors' value about participating in research and what are the most important research problems related to stroke or transient ischemic attack (TIA)? A survey. BMC Med Res Methodol. 2021;21(1):209. doi: 10.1186/s12874-021-01390-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slenders JPL, Van den Berg-Vos RM, van Heugten CM, et al. Screening and patient-tailored care for emotional and cognitive problems compared to care as usual in patients discharged home after ischemic stroke (ECO-stroke): a protocol for a multicenter, patient-blinded, cluster randomized controlled trial. BMC Health Serv Res. 2020;20(1):1049. doi: 10.1186/s12913-020-05902-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randomizer: a randomization service for multicenter clinical trials [online]. Accessed May 4, 2020. randomizer.at/index.html.
- 15.Lee JH, Park JH, Kim YJ, Lee SH, Post MWM, Park HY. Validity and reliability of the Korean version of the Utrecht scale for evaluation of rehabilitation-participation. Occup Ther Int. 2017;2017:9452051. doi: 10.1155/2017/9452051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post MW, van der Zee CH, Hennink J, Schafrat CG, Visser-Meily JM, van Berlekom SB. Validity of the Utrecht scale for evaluation of rehabilitation-participation. Disabil Rehabil. 2012;34(6):478-485. doi: 10.3109/09638288.2011.608148 [DOI] [PubMed] [Google Scholar]
- 17.van der Zee CH, Kap A, Rambaran Mishre R, Schouten EJ, Post MW. Responsiveness of four participation measures to changes during and after outpatient rehabilitation. J Rehabil Med. 2011;43(11):1003-1009. doi: 10.2340/16501977-0879 [DOI] [PubMed] [Google Scholar]
- 18.de Graaf JA, Volkers EJ, Schepers VPM, Visser-Meily JMA, Post MWM. Validity of the Utrecht scale for evaluation of rehabilitation-participation restrictions scale in a hospital-based stroke population 3 months after stroke. Top Stroke Rehabil. 2022;29(7):516-525. doi: 10.1080/10749357.2021.1956047 [DOI] [PubMed] [Google Scholar]
- 19.van der Zee CH, Post MW, Brinkhof MW, Wagenaar RC. Comparison of the Utrecht Scale for Evaluation of Rehabilitation-Participation with the ICF Measure of Participation and Activities Screener and the WHO Disability Assessment Schedule II in persons with spinal cord injury. Arch Phys Med Rehabil. 2014;95(1):87-93. doi: 10.1016/j.apmr.2013.08.236 [DOI] [PubMed] [Google Scholar]
- 20.Van Heugten C, Rasquin S, Winkens I, Beusmans G, Verhey F. Checklist for cognitive and emotional consequences following stroke (CLCE-24): development, usability and quality of the self-report version. Clin Neurol Neurosurg. 2007;109(3):257-262. doi: 10.1016/j.clineuro.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Spinhoven P, Ormel J, Sloekers P, Kempen G, Speckens A, Van Hemert A. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363-370. doi: 10.1017/s0033291796004382 [DOI] [PubMed] [Google Scholar]
- 22.Wolfs CA, Dirksen CD, Kessels A, Willems DCM, Verhey FR, Severens JL. Performance of the EQ-5D and the EQ-5D+C in elderly patients with cognitive impairments. Health Qual Life Outcomes. 2007;5:33. doi: 10.1186/1477-7525-5-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devlin N, Parkin D, Janssen B. Methods for Analysing and Reporting EQ-5D Data. Springer Nature; 2020. [PubMed] [Google Scholar]
- 24.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873-880. doi: 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosscher RJ, Smit JH. Confirmatory factor analysis of the general self-efficacy scale. Behav Res Ther. 1998;36(3):339-343. doi: 10.1016/s0005-7967(98)00025-4 [DOI] [PubMed] [Google Scholar]
- 26.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091-1096. doi: 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 27.Donner A, Klar N. Statistical considerations in the design and analysis of community intervention trials. J Clin Epidemiol. 1996;49(4):435-439. doi: 10.1016/0895-4356(95)00511-0 [DOI] [PubMed] [Google Scholar]
- 28.Verhage F. Intelligence and Age: Study with Dutch People Aged 12-77. Van Gorcum; 1964. [Google Scholar]
- 29.Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ. 2010;340:c2697. doi: 10.1136/bmj.c2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accessed October 2023. cran.r-project.org/web/packages/lme4/lme4.pdf.
- 31.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. R-project.org/. [Google Scholar]
- 32.Rochette A, Korner-Bitensky N, Bishop D, et al. The YOU CALL-WE CALL randomized clinical trial: impact of a multimodal support intervention after a mild stroke. Circ Cardiovasc Qual Outcomes. 2013;6:674-679. doi: 10.1161/CIRCOUTCOMES.113.000375 [DOI] [PubMed] [Google Scholar]
- 33.Fens M, van Heugten CM, Beusmans G, Metsemakers J, Kester A, Limburg M. Effect of a stroke-specific follow-up care model on the quality of life of stroke patients and caregivers: a controlled trial. J Rehabil Med. 2014;46(1):7-15. doi: 10.2340/16501977-1239 [DOI] [PubMed] [Google Scholar]
- 34.Forster A, Young J, Chapman K, et al. Cluster randomized controlled trial: clinical and cost-effectiveness of a system of longer-term stroke care. Stroke. 2015;46(8):2212-2219. doi: 10.1161/STROKEAHA.115.008585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forster A, Young J, Green J, et al. Structured re-assessment system at 6 months after a disabling stroke: a randomised controlled trial with resource use and cost study. Age Ageing. 2009;38(5):576-583. doi: 10.1093/ageing/afp095 [DOI] [PubMed] [Google Scholar]
- 36.Accessed January 1, 2024. richtlijnendatabase.nl/richtlijn/herseninfarct_en_hersenbloeding/revalidatie_na_herseninfarct_-bloeding/cognitieve_problemen_na_herseninfarct.html.
- 37.Accessed May 5, 2024. stichtinghersenschudding.nl/2022/06/23/campagne-onzichtbare-gevolgen-doe-je-ook-mee/.
- 38.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical Research Council guidance. BMJ. 2015;350:h1258. doi: 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulaert VR, van Heugten CM, Winkens B, et al. Early neurologically-focused follow-up after cardiac arrest improves quality of life at one year: a randomised controlled trial. Int J Cardiol. 2015;193:8-16. doi: 10.1016/j.ijcard.2015.04.229 [DOI] [PubMed] [Google Scholar]
- 40.Crocker TF, Brown L, Lam N, Wray F, Knapp P, Forster A. Information provision for stroke survivors and their carers. Cochrane Database Syst Rev. 2021;11:CD001919. doi: 10.1002/14651858.CD001919.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kam Yuet Wong F, Wang SL, Ng SS, et al. Effects of a transitional home-based care program for stroke survivors in Harbin, China: a randomized controlled trial. Age Ageing. 2022;51(2):afac027. doi: 10.1093/ageing/afac027 [DOI] [PubMed] [Google Scholar]
- 42.Xu L, An L, Lin Q, Liu M. Early personalized care in stroke patients improves the cognitive function and promotes the recovery process of disease. Int J Clin Exp Med. 2020;13:2466-2473. [Google Scholar]
- 43.Boter H; HESTIA Study Group. Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke. 2004;35(12):2867-2872. doi: 10.1161/01.STR.0000147717.57531.e5 [DOI] [PubMed] [Google Scholar]
- 44.Golicki D, Niewada M, Karlińska A, et al. Comparing responsiveness of the EQ-5D-5L, EQ-5D-3L and EQ VAS in stroke patients. Qual Life Res. 2015;24(6):1555-1563. doi: 10.1007/s11136-014-0873-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P, Lin KC, Liing RJ, Wu CY, Chen CL, Chang KC. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Qual Life Res. 2016;25(6):1585-1596. doi: 10.1007/s11136-015-1196-z [DOI] [PubMed] [Google Scholar]
- 46.Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the minimal clinically important difference for the Hospital Anxiety and Depression Scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev. 2019;39(6):E6-E11. doi: 10.1097/HCR.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 47.Fu V, Weatherall M, McPherson K, et al. Taking Charge after Stroke: a randomized controlled trial of a person-centered, self-directed rehabilitation intervention. Int J Stroke. 2020;15(9):954-964. doi: 10.1177/1747493020915144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data underlying the results presented in this article will be shared with investigators after a review committee's approval and a signed data-sharing agreement. Proposals can be submitted 9–36 months after publication.