Abstract
Using small-angle x-ray scattering (SAXS), we obtained direct experimental evidence on the structure of hydrated polyatomic anions, with hydration effects starkly different from those of cations (J. Chem. Phys. 2011, 134, 064513). We propose that the size and charge density of the naked ions do not sufficiently account for the differences in the SAXS curves. For cations, the ion-ion contribution gives a prominent first-order diffraction peak, whereas for anions, the low-Q enhancement in the SAXS curves indicates density inhomogeneities as a result of ion-water interactions.
Introduction
Ion hydration is closely related to a wide range of chemical and biological processes. Based on the nature of the ions and the interaction between the hydrated ions and water, some clear trends are observed such as the empirical Hofmeister series, which is associated with protein folding, protein crystallization and related phenomena such as colloidal assembly and enzyme activity1–3. Ion size and charge density have been shown to correlate with how strongly the ion binds to, and the electrostatic ordering of, surrounding water molecules4,5. However, direct experimental evidence for explicit long-range effects of ions on the hydrogen-bond structure of liquid water is still scant6–9.
Results obtained from x-ray spectroscopy and scattering techniques on various aqueous salt solutions10,11 yield direct experimental evidence of specific ion effects on the structure of water. Important structural information in the first and second hydration shells of an ion can be deduced from x-ray absorption and scattering spectra such as x-ray Raman scattering (XRS)10, but such techniques do not probe structures beyond the close proximity of excited molecules. Complementary to x-ray spectroscopy, small-angle x-ray scattering (SAXS) is a technique which is highly sensitive to the presence of long-range density inhomogeneities in liquid samples12–15. SAXS measures the intensity of x-ray photons that are elastically scattered at small angles, and is plotted as a function of the scattering momentum transfer Q, defined as Q = 4π sin θ/𝜆. Through an enhancement of the structure factor in the region of low Q it can reliably identify small deviations from the average electron density due to instantaneous aggregation structures. The long-range pair-correlation function between various particles has an exponential decay at large r due to such aggregations that give rise to a low-Q enhancement. If the solution is homogeneous beyond the few nearest neighbor interactions the low-Q structure factor would approach Q=0 as a straight line12.
In a previous study of a cation series with the same counter ion Cl- 10, we note the following important observations on cation hydration: Na+ induces weakened and distorted hydrogen bonds in the solution, but the overall density of aqueous solutions of NaCl resembles pure water; in stark contrast, solutions of multivalent cations such as Mg2+ and Al3+ exhibit a first-order diffraction peak in the intermediate Q-region in the SAXS curves10. The position of this small peak is concentration dependent, and is related to the distance between the solvated cations10. This is indicative of the contribution from a long-range, electrostatic cation-cation interaction on the overall line shape of the SAXS curves. Analysis of the molecular form factors of solvated cations suggests that Mg2+ and Al3+ form well-defined, quasi-lattice hydration shells, where water in the first hydration shell has a higher local density than bulk water10. The contrasting behavior of Na+ compared to Mg2+ and Al3+ suggests that ion size and charge density critically affect how the ions restructure water molecules around them.
This naturally leads to the question of whether hydrated anions with different charge states exhibit the differences that were observed for cations. Here we report the results from a SAXS study of aqueous solutions of sodium salts with polyatomic anions that are similar in size but with varying charge: perchlorate (ClO4-), sulfate (SO42-), and phosphate (PO43-). Details of the experiment can be found in Ref10.
Results and Discussion
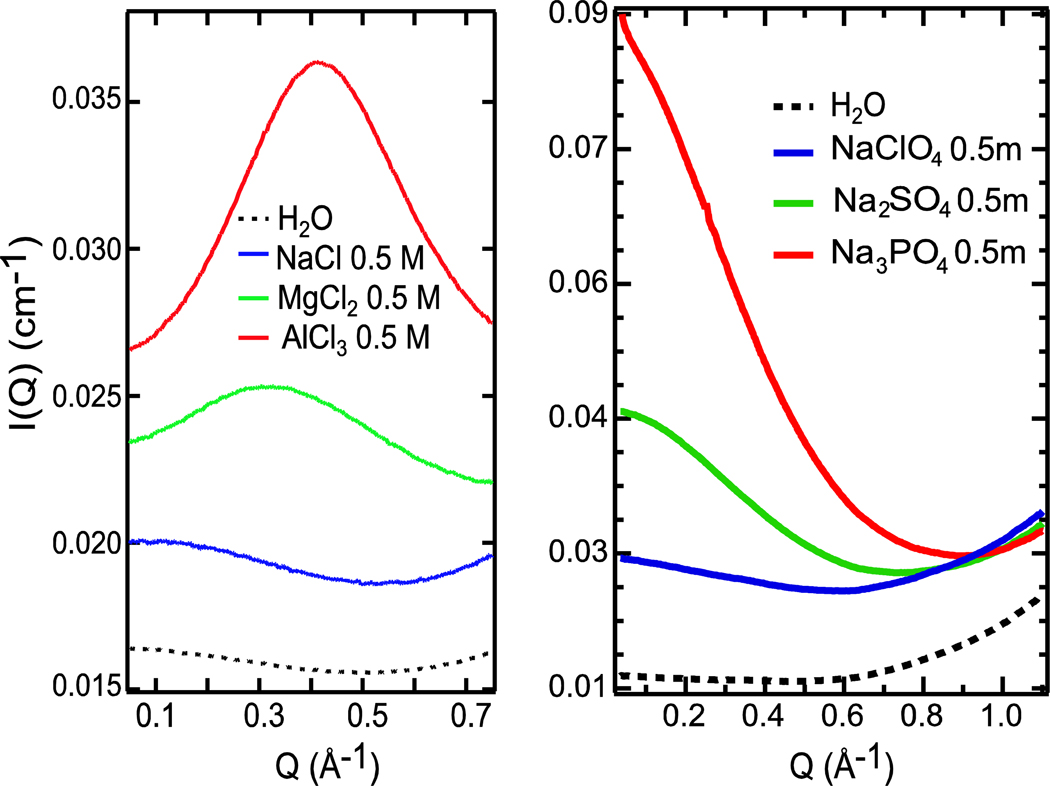
The SAXS scattering curves of 0.5 m (moles per solute/mass of solvent) NaClO4, Na2SO4 and Na3PO4 are compared with the cation series in similar concentrations (Figure 1). The line shape of the scattering curve of NaClO4 looks very similar to that of NaCl. Although the sodium concentration varies in the anion series, the effect of sodium on the low-Q region of aqueous solutions is rather small10. The scattering curves of the higher charge-density Na2SO4 and Na3PO4 have progressively more enhanced intensity at low Q indicative of large density inhomogeneities due to the formation of water with a different density in the hydration shells. However, the distinct first-order diffraction peaks that appeared in the intermediate Q-region in the SAXS curves of Mg2+ and Al3+ are not present in those of the anion species with equal, but negative charge. This indicates that there is no well-defined distance between the anions that changes with concentration, as seen for the Mg2+ and Al3+ cations10. Clearly there is an important asymmetry in terms of long-range ordering in the liquid between highly charged cations and anions. For Mg2+ and Al3+ cations such an ordering in terms of an equal distance was observed at low concentrations down to 50 Å ion-ion separation.10
Figure 1.
SAXS curves of 0.5 M NaCl, MgCl2, AlCl3 (left)10 and 0.5 m NaClO4, Na2SO4, Na3PO4 (right). Note the different concentration units between the cation and anion series that results only in less than 5% difference in solute concentrations.
We further examined the same concentration series (0.1 – 0.7 m) for the aqueous solutions of these salts (Figure 2). There is no formal procedure on how to separate the bulk water scattering signal from the regions around the ions. However, here we assume that the bulk water contribution is not significantly altered compared to pure water. After subtracting the water signal from the total scattering intensity and dividing the difference with the concentration, the difference between perchlorate and the higher-charged sulfate and phosphate became more apparent (Figure 3). For perchlorate solutions, the difference in scattering intensity between the solution and pure water is rather small making it challenging to separate contributions from around the ions with respect to the surrounding solution and we have therefore left out a detailed analysis. The scattering of the more highly charged sulfate and phosphate anions, after removing water scattering, overlap fairly well and the resulting Lorentzian line shape may be interpreted as the scattering of one individual structure unit10. In the cation case, this SAXS form factor deviates at low Q due to the repulsive cation-cation interactions. A similar phenomenon was not observed for the sulfate and phosphate solutions.
Figure 2.
SAXS curves of 0.1, 0.25, 0.5, 0.7 m (from bottom to top) NaClO4, Na2SO4, Na3PO4 (from left to right). Dotted line is SAXS curve of pure water. Note that the scales of I(Q) in the plots are different.
Figure 3.
From left to right the low-Q structure factor of NaClO4, Na2SO4, Na3PO4 with bulk water signal subtracted and normalized by concentration (to 1 M). Note the different scales on the I(Q) axes.
In order to estimate the size of the hydration shells around the ions as a region that has a density that differs from the surrounding liquid we use simple scattering theory as typically applied to estimate the size of nanoparticles and described in reference 10. Using the homogeneous spherical model (approximating all hydrated ions as spheres with radius R) and fitting the unit structure scattering P(Q) = [3/(QR)3]2[sin(QR) – QR cos (QR)]2, we obtain the radius of a single hydrated structure to be 2.90 Å for SO42-, and 3.64 Å for PO43-. Compared with the cation case (2.71 Å for Mg2+ and 3.18 Å for Al3+ 10), there is not a significant difference in terms of sizes for the hydrated ions. This indicates that size alone does not account for the difference in the scattering behavior in terms of the lack of a first-order diffraction peak for the anions. We note that polyatomic anions cannot be adequately described using the point-charge hard-sphere model as commonly applied in the case of cations. Instead, the charge distribution within the ions contributes to how they interact with the surrounding water molecules. The central atom (Cl, S or P) of the anion, bearing a partial positive charge, is isolated from the surrounding solvent, and is thus not directly involved in the solvation process. The charge on these polyatomic anions is therefore quite diffuse. Obviously, the higher the overall ionic charge, the higher the partial negative charge is on the oxygen atoms. This is why sulfate and phosphate have a much stronger attractive interaction with water causing higher density in the hydration shell than does perchlorate. Note the increase in size between the sulfate and phosphate ion hydration layers mirroring a similar relative change between Mg2+ and Al3+ hydration layers indicating that the water layers are affected further out into the solution with increasing charge.
We can only speculate on the origin of this asymmetry between the cations and anions in terms of the long-range interaction. That the size of the hydration layer in terms of difference in density in comparison to the bulk water is slightly larger than for the cations could be understood from the larger size of the polyatomic anions themselves. If the delocalized nature of the charge in the anions is also responsible for the fact that less ordering occurs in comparison to the highly charged cations in solution is an open question. There can also be important differences in the way that the local water interacts with the two classes of ions that further leads to long-range effects through the hydrogen bonding network of the water between the ions. We hope that these new experimental data will stimulate more theoretical studies based on models that include long-range interactions in aqueous solutions.
ACKNOWLEDGMENT
We acknowledge the National Science Foundation (US) (Grant No. CHE-0809324), Office Basic Energy Sciences (BES) through SSRL, the Department of Energy through the SLAC Laboratory Directed Research and Development Program and the Air Force Office of Scientific Research through the MURI program under AFOSR Award No. FA9550-10-1-0572. We also acknowledge the support of the SSRL Structural Molecular Biology group funded by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Grant and the U.S. Department of Energy, Office of Biological and Environmental Research.
REFERENCES
- (1).Hofmeister F Arch. Exp. Pathol. Pharmakol 1888, 24, 247. [Google Scholar]
- (2).Collins KD; Neilson GW; Enderby JE Biophys. Chem 2007, 128, 95. [DOI] [PubMed] [Google Scholar]
- (3).Zhang Y; Cremer PS Annu. Rev. Phys. Chem 2010, 61, 63. [DOI] [PubMed] [Google Scholar]
- (4).Collins KD Proc. Natl. Acad. Sci 1995, 92, 5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hribar B; Southall NT; Vlachy V; Dill KA J. Am. Chem. Soc 2002, 124, 12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Omta AW; Kropman MF; Woutersen S; Bakker HJ Science 2003, 301, 347. [DOI] [PubMed] [Google Scholar]
- (7).Kropman MF; Bakker HJ J. Am. Chem. Soc 2004, 126, 9135. [DOI] [PubMed] [Google Scholar]
- (8).Tielrooij KJ; Garcia-Araez N; Bonn M; Bakker HJ Science 2010, 328, 1006. [DOI] [PubMed] [Google Scholar]
- (9).Irudayam SJ; Henchman RH J. Chem. Phys 2012, 137, 034508. [DOI] [PubMed] [Google Scholar]
- (10).Waluyo I; Huang C; Nordlund D; Bergmann U; Weiss TM; Pettersson LGM; Nilsson AJ Chem. Phys 2011, 134, 064513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Waluyo I; Huang C; Nordlund D; Weiss TM; Pettersson LGM; Nilsson AJ Chem. Phys 2011, 134, 224507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Huang C; Wikfeldt KT; Tokushima T; Nordlund D; Harada Y; Bergmann U; Niebur M; Weiss T; Horikawa Y; Leetmaa M; Ljungberg MP; Takahashi O; Lenz A; Ojamäe L; Lyubartsev AP; Shin S; Pettersson LGM; Nilsson A Proc. Natl. Acad. Sci 2009, 106, 15214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Nishikawa K; Hayashi H; Iijima TJ Phys. Chem 1989, 93, 6559. [Google Scholar]
- (14).Nishikawa K; Kodera Y; Iijima TJ Phys. Chem 1987, 91, 3694. [Google Scholar]
- (15).Hayashi H; Nishikawa K; Iijima TJ Phys. Chem 1990, 94, 8334. [Google Scholar]