Abstract
Sister chromatids in early mitotic cells are held together mainly by interactions between centromeres. The separation of sister chromatids at the transition between the metaphase and the anaphase stages of mitosis depends on the anaphase-promoting complex (APC), a 20S ubiquitin-ligase complex that targets proteins for destruction. A subunit of the APC, called APC-α in Xenopus (and whose homologs are APC-1, Cut4, BIME, and Tsg24), has recently been identified and shown to be required for entry into anaphase. We now show that the mammalian APC-α homolog, Tsg24, is a centromere-associated protein. While this protein is detected only during the prophase to the anaphase stages of mitosis in Chinese hamster cells, it is constitutively associated with the centromeres in murine cells. We show that there are two forms of this protein in mammalian cells, a soluble form associated with other components of the APC and a centromere-bound form. We also show that both the Tsg24 protein and the Cdc27 protein, another APC component, are bound to isolated mitotic chromosomes. These results therefore support a model in which the APC by ubiquitination of a centromere protein regulates the sister chromatid separation process.
Identification and characterization of proteins which regulate the transition from metaphase to anaphase are important objectives, as missegregation of chromosomes can result in the development of cancer, hereditary forms of disease, and birth defects (17, 33, 52). At the metaphase stage of mitosis, the kinetochore regions of the sister chromatids are connected in a bipolar fashion to two opposing spindle poles. The mechanical forces applied by the spindles on the sister chromatids and the cohesion that exists between the sister chromatids order the chromatids on the metaphase plate, a prerequisite for correct chromatid segregation (1, 55). To ensure that the sister chromatids are correctly segregated in mitotic cells, regulatory mechanisms that control the sister chromatid separation process exist. Components of a spindle assembly checkpoint that monitors the attachment of microtubules to the kinetochores have been described, e.g., the MAD2 protein (7, 35). Furthermore, structural as well as regulatory proteins that control the timing of sister chromatid cohesion and release exist (4, 21, 55, 60). Sister chromatids in metaphase cells are predominantly held together at their centromere regions, chromosomal regions which mainly consist of heterochromatic sequences and onto which the kinetochore assembles during mitosis (41). Heterochromatic domains have been shown to be important for pairing of meiotic chromatids in Drosophila melanogaster (10, 26), which suggests that these chromosomal domains could be of importance also for sister chromatid pairing in mitotic cells.
A number of conserved mammalian centromeric proteins have been characterized, although their roles in sister chromatid cohesion have not been elucidated (41). DNA topoisomerase II is known to be required for the resolution of interlockings occurring between sister chromatid DNA strands during mitosis, but it is not believed to be involved in the regulation of the sister chromatid separation process (4, 54). A putative regulator of DNA topoisomerase II has recently been identified in Drosophila and suggested to facilitate decatenation of sister chromatids at anaphase (3). Furthermore, the products of a number of Drosophila and yeast genes have been shown to regulate the sister chromatid separation process, although their exact roles during this process are not clear (8, 9, 15, 25, 40, 43, 47, 57).
Apart from DNA topoisomerase II, the activity of a ubiquitin-dependent proteolytic system is also required for the release of sister chromatid cohesion. A ubiquitin-ligase complex, termed the anaphase-promoting complex (APC) or cyclosome (30, 48), has been shown to control entry into anaphase and exit from mitosis by ubiquitination of a set of target proteins, thereby initiating a protein degradation program performed by the 26S proteasome (11, 18, 20, 29). The APC is a multisubunit protein complex (27, 30, 48), and four of its components have been characterized at the molecular level. Three of these (Cdc16, Cdc23, and Cdc27) belong to the tetratricopeptide repeat family and bind to each other (23, 30, 32). A fourth subunit of the APC (called APC-α in Xenopus, APC-1 in budding yeast, Cut4 in fission yeast, and Tsg24 in mouse) was recently identified and shown to be related to an Aspergillus nidulans mitotic checkpoint regulator, BIME (13, 38, 39, 44, 59, 61).
The best-characterized targets for the APC are the two mitotic cyclins A and B, which have been shown to contain a destruction box, an amino acid sequence motif required for ubiquitin-dependent proteolysis (16, 28). Experiments using mutated versions of cyclin B have shown that degradation of cyclin B by the APC is necessary for exit from mitosis (22, 50). The same experiments also revealed that proteins other than the mitotic cyclins have to be degraded in order for the cell to proceed beyond metaphase. Furthermore, when the known APC subunits, Cdc16, Cdc23, Cdc27, and APC-1, are inactivated in different model systems, cells arrest with a preanaphase phenotype (19, 23, 24, 32, 38, 42, 51, 59, 61). This strongly suggests that the APC regulates inhibitors of anaphase and/or proteins that physically promote cohesion between sister chromatids by a ubiquitin-dependent proteolytic mechanism. Three putative inhibitors of anaphase that appear to be directly regulated by the APC, the Pds1 and Ase1 proteins in budding yeast (25, 57, 58) and the Cut2 protein in fission yeast (15), have been described. The product of the Drosophila gene fizzy has been shown to be required for degradation of cyclins during mitosis, suggesting that it takes part in the same regulatory pathway as the APC (9, 43). At this point, however, no direct link between the sister chromatid cohesion process, the centromere region, and the APC has been established.
We now show that the Tsg24 protein, a mammalian APC component, is a centromere-associated protein which appears to have a transient function during mitosis. We identified two cellular forms of the Tsg24 protein in a set of biochemical experiments, a soluble form associated with other APC components and a centromere-associated form of this protein. We show that both the Tsg24 protein and the APC subunit Cdc27 are bound to isolated mitotic chromosomes. These results support a model in which the APC is directly involved in the ubiquitination and degradation of a centromeric protein required for sister chromatid cohesion.
MATERIALS AND METHODS
Antibodies.
The affinity-purified rabbit anti-mouse Tsg24 antibody has been characterized previously (44). Preimmune serum from rabbits injected with the Tsg24 protein was purified by the same procedure as was used for purification of the anti-Tsg24 antibody. The rabbit anti-human Cdc16p and Cdc27p antibodies recognize two subunits of the APC (51) and were a gift from P. Hieter. The human CREST autoantiserum recognizes a set of conserved human centromeric proteins (a gift from N. F. Rothfield and W. C. Earnshaw). The monoclonal anti-CTR453 antibody recognizes a centrosomal protein (2) and was a gift from M. Bornens. The anti-P1 antibody detects a murine protein involved in initiation of DNA replication (45). The monoclonal proliferating cell nuclear antigen (PCNA) antibody detects a subunit of DNA polymerase δ (32551A; Pharmingen). The anti-α-lamin antibody was a gift from S. Georgatos, the antipericentrin antibody was a gift from M. Kirschner, and the α-tubulin antibodies were purchased from Sigma.
Cell culture and indirect immunofluorescence microscopy.
Mouse Swiss-3T3 fibroblasts and Chinese hamster ovary (CHO) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), from GIBCO, containing 10% fetal calf serum (Sigma). Cells were plated at a low density and grown at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Cells were fixed in ice-cold methanol-acetone (50:50) for 5 min and preincubated with 3% bovine serum albumin prior to addition of the first antibody. Cells were also analyzed by two other methods, either by using paraformaldehyde-fixed cells or by adding the primary antibody to unfixed cells and then postfixing them (37). The primary antibodies were anti-Tsg24 (1:20), anti-Cdc16 (1:150), anti-CREST (1:500), anti-PCNA (1:500), and anti-CTR453 (1:1). The secondary antibodies were a fluorescein isothiocyanate-conjugated swine anti-rabbit immunoglobulin G (IgG) (diluted 1:50; Boehringer Mannheim) and a rhodamine-conjugated goat anti-mouse IgG (diluted 1:80; Boehringer Mannheim). The cells were also stained with 1 μg of Hoechst 33258 per ml for 15 s. The slides were mounted in a 78% glycerol mounting medium containing 1 mg of paraphenylene diamine per ml, examined with a Zeiss Axioscope microscope, and photographed with Kodak TMAX 400 film. To determine the expression of the Tsg24 protein at different stages of interphase, CHO cells were synchronized by a serum starvation method as described previously (45, 46). Briefly, CHO cells were cultured in DMEM including 0.1% fetal calf serum (Sigma) for 48 h. An equal number of cells were then processed at 2-h intervals following addition of DMEM including 10% fetal calf serum. The synchrony of the cell population was determined by incorporation of bromodeoxyuridine (BrdU) for 30 min prior to fixation of the cells. The cells were then stained with an anti-BrdU antibody (Amersham Corp.), and the fraction of BrdU-positive cells was determined: 0 h, 22%; 4 h, 23%; 12 h, 45%; 16 h, 60%; 20 h, 15%; and 24 h, 28%. Protein samples taken from the synchronized cells at different time points after serum addition were analyzed by immunoblotting with the anti-Tsg24 and the anti-α-lamin antibodies.
Immunoblotting.
Protein extracts were boiled in sodium dodecyl sulfate (SDS) reducing buffer (62.5 mM Tris-HCl [pH 6.8], 10% glycerol, 2.3% SDS, 10 mM dithiothreitol). The proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to an Immobilon-P membrane in transfer buffer (41 mM Tris, 192 mM glycine, 0.02% SDS [pH 8.3]) (31). The filters were incubated with the primary antibodies, anti-Tsg24 (1:150), anti-Cdc16 (1:250), anti-P1 (1:200), and anti-α-lamin (1:1,000), after which washing and detection were performed as described previously (45, 46).
Immunoprecipitation and preparation of nuclei.
Extracts from Swiss-3T3 or from CHO cells to be used in immunoprecipitation experiments were lysed in a mild lysis buffer (50 mM Tris [pH 7.5], 0.1 M NaCl, 1% Triton X-100) or radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 8.0], 0.15 M NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS) including protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg of pepstatin per ml, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml). The lysate was precleared with Sepharose-protein A beads (Pharmacia Biotech) and incubated with anti-Tsg24, anti-Cdc16, or anti-P1 antibodies and Sepharose-protein A beads, which were then washed in lysis buffer. The samples were boiled in sample buffer and analyzed by immunoblotting. Nuclei to be used for salt elution experiments were prepared as follows. Swiss-3T3 cells were immersed in a hypotonic buffer (10 mM HEPES [pH 7.8], 1.5 mM MgCl2, 0.5 mM dithiothreitol, 10 mM NaCl, 1.0 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml) and homogenized in a Dounce homogenizer. Nuclei were pelleted and incubated in a hypotonic buffer with increasing concentrations of NaCl (10 mM to 0.3 M). The salt eluates were concentrated by ultrafiltration (with a filter from Amicon) and boiled in sample buffer. The nuclei were also boiled in sample buffer.
Purification of mitotic chromosomes.
Mitotic chromosomes were prepared from CHO cells as described previously (56). Briefly, CHO cells were incubated overnight at 37°C in DMEM plus 10% newborn calf serum and 0.125 mg of Colcemid per ml. Mitotic cells were collected by squirting a stream of medium over them. Cells were pelleted and resuspended in swelling buffer {5 mM NaCl, 2 mM MgCl2, 5 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 0.5 mM EDTA (pH 7.2) with KOH} at 0°C for 10 min, thereafter repelleted, quickly resuspended in ice-cold lysis buffer (10 mM PIPES, 2 mM EDTA, 0.1% β-mercaptoethanol, 1 mM spermidine HCl, 0.5 mM spermine HCl [pH 7.2, with KOH], 0.1% digitonin [Sigma], 2 mg of α2-macroglobulin [Sigma] per ml), and homogenized in a Dounce homogenizer. Mitotic chromosomes stained by Hoechst 33258 were observed in a fluorescence microscope during the homogenization. When the majority of the mitotic chromosomes were seen to be separate from each other, the lysate was centrifuged briefly at low speed to remove unbroken cells and chromosome clusters. The supernatant was layered over a sucrose gradient consisting of 20 to 60% (wt/vol) sucrose in lysis buffer without the digitonin and centrifuged at 2,500 × g for 15 min. Chromosomes were collected from the side of the tube and boiled in Laemmli sample buffer for subsequent immunoblotting analysis.
RESULTS
The Tsg24 protein is transiently detected in the centromere regions of mitotic chromosomes in CHO cells.
The APC has been shown to regulate the sister chromatid separation process by selective protein ubiquitination. In order to identify the cellular locations at which the APC is active in mammalian cells, we have analyzed the cellular distribution of one of its protein subunits, Tsg24 (APC-α). We have developed an antibody against the protein encoded by the Tsg24 gene and shown that the affinity-purified antibody recognizes a 200-kDa protein expressed in mammalian and Xenopus cells (39, 44). The specificity of the anti-Tsg24 antibody has been further tested by expression of a full-length Tsg24 cDNA clone in vitro or in Schizosaccharomyces pombe. In both cases, a 200-kDa protein band, identical in size to that for the protein observed in mammalian extracts, was recognized by the anti-Tsg24 antibody (data not shown). We have now investigated the subcellular locations of the Tsg24 protein in three different mammalian cell types by indirect immunofluorescence microscopy methods.
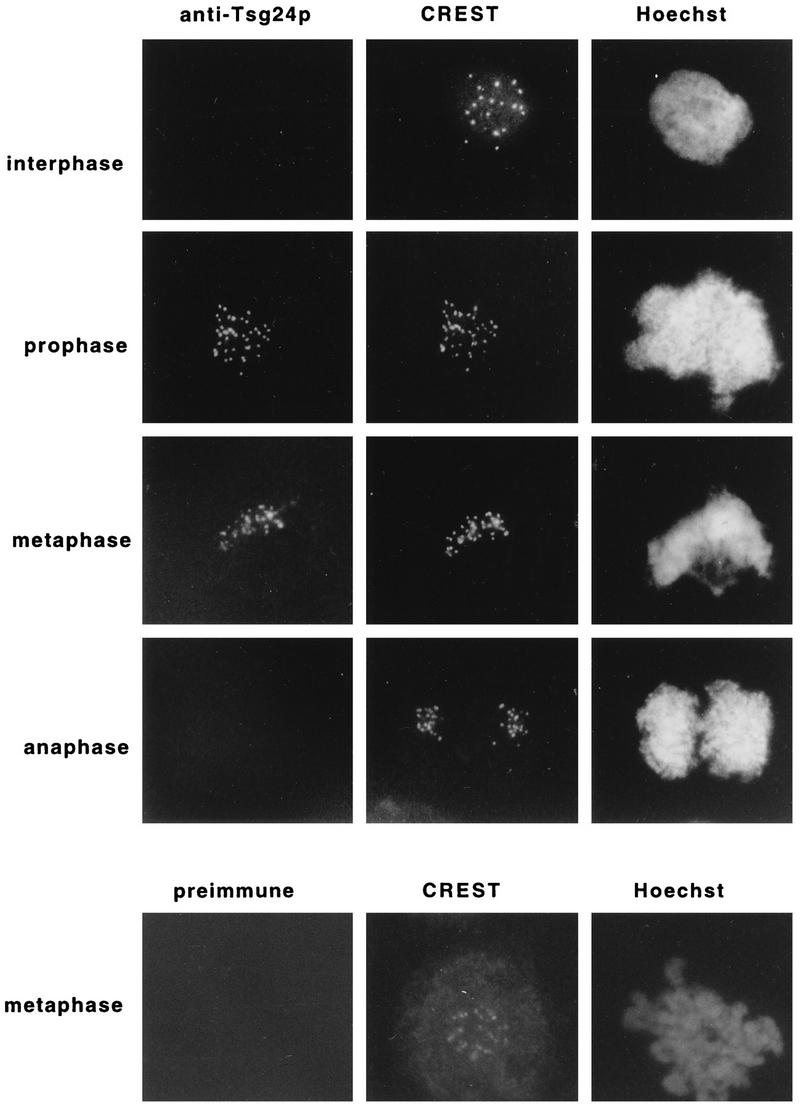
CHO cells were triple stained with the affinity-purified anti-Tsg24 antibody, with a CREST antiserum, and with Hoechst 33258 (which labels DNA) (Fig. 1). The CREST antiserum recognizes a group of conserved centromeric proteins expressed in both interphase and mitotic cells (41), and the pairs of dots labelled by the CREST antiserum in Fig. 1 correspond to the centromeres of each sister chromatid pair. The same pairs of dots were also labelled by the anti-Tsg24 antibody, showing that the Tsg24 protein is a centromere-associated protein. In contrast to the constitutive labelling pattern seen by the CREST antiserum, the anti-Tsg24 antibody gave only a centromeric signal during the prophase, metaphase, and early anaphase (data not shown) stages of mitosis (Fig. 1). In addition to the methanol-acetone fixation protocol used in the experiment described in the legend to Fig. 1, cells were also analyzed by two other methods (37), either by using paraformaldehyde-fixed cells or by adding the primary antibody to unfixed cells and then postfixing them (see Materials and Methods). In neither of these two methods did we detect the Tsg24 protein in interphase CHO cells (data not shown). The chromosomal colocalization of the Tsg24 protein and proteins required for centromere function, as well as the temporally restricted (prophase to early-anaphase) centromeric signal displayed by the anti-Tsg24 antibody, therefore suggests that the Tsg24 protein is involved in a regulatory activity taking place at the centromere during the metaphase-to-anaphase transition.
FIG. 1.
The Tsg24 protein transiently accumulates in the centromeric regions of mitotic chromosomes. Interphase or mitotic CHO cells were fixed with methanol-acetone (50:50 [vol/vol]) and triple stained with the anti-Tsg24 antibody (1:20) or preimmune serum (1:20), a CREST antiserum (1:500), and Hoechst 33258 in an indirect immunofluorescence microscopy experiment. The secondary antibodies were a fluorescein isothiocyanate-conjugated swine anti-rabbit IgG and a rhodamine-conjugated goat anti-human IgG. The cells were analyzed by indirect immunofluorescence microscopy.
The Tsg24 protein binds to murine centromeres in a cell cycle-independent manner.
We have also analyzed the cellular distributions of the Tsg24 protein in two murine cell lines, Swiss-3T3 and L cells, and compared them with the distribution of the CREST antigen. We found, as expected, that the anti-Tsg24 antibody labelled the centromeres in early mitotic murine cells (Fig. 2). Surprisingly, however, the anti-Tsg24 antibody also labelled the centromeric regions in late-anaphase as well as in interphase cells, i.e., the Tsg24 protein appears to be bound to the centromeric regions of murine chromosomes throughout the cell cycle (Fig. 2). To test this possibility, interphase Swiss-3T3 cells were also stained with a monoclonal antibody against PCNA (a subunit of DNA polymerase δ), which stains interphase cells differently depending on their cell cycle stage and DNA replication activity (5, 6) (Fig. 2). We found that irrespective of the PCNA pattern observed in interphase cells, the Tsg24 staining pattern remained the same, verifying that its association to centromeric heterochromatin is cell cycle independent (Fig. 2). Labelling of synchronized Swiss-3T3 cells with the anti-Tsg24 antibody confirmed that Tsg24 is bound to the centromeres in G1, S, and G2 cells (data not shown). The specificity of the antibody was also tested by microinjection of the anti-Tsg24 antibodies into Swiss-3T3 cells. We found that the microinjected anti-Tsg24 antibodies labelled the centromeric regions in a pattern indistinguishable from that seen in Fig. 2, whereas injected preimmune serum did not label any nuclear structures (data not shown).
FIG. 2.
The Tsg24 protein binds to murine centromeres in a cell cycle-independent manner. Mitotic or interphase murine Swiss-3T3 cells were fixed with methanol-acetone (50:50 [vol/vol]), triple stained with different combinations of antibodies, and analyzed by indirect immunofluorescence microscopy. Rows 1 and 2 show mitotic cells labelled with the anti-Tsg24 antibody (1:20), a CREST antiserum (1:500), and Hoechst 33258. Rows 3 to 5 show interphase cells and late-S-phase cells triple stained with the anti-Tsg24 antibody (1:20) or a preimmune serum, a monoclonal anti-PCNA antibody (1:500), and Hoechst 33258. The anti-PCNA antibody labels early- (e) and late-S-phase (l) cells. Late-S-phase cells were also analyzed at a higher magnification (rows 4 and 5). The secondary antibodies used were the same as in Fig. 1, except that a rhodamine-conjugated goat anti-mouse IgG was used to label the anti-PCNA antibody. Fixation of cells with paraformaldehyde gave identical results but weaker signals. Staining of murine L cells gave identical results.
To understand the basis for the different temporal patterns of accumulation of the Tsg24 protein in the centromeric regions of CHO and murine cells, we compared the expression of the Tsg24 protein in synchronized cell cultures by Western blot analysis. We have previously shown that the Tsg24 protein is constitutively expressed throughout the cell cycle in murine cells (44). The transient accumulation of the Tsg24 protein at the centromere in CHO cells suggested that the Tsg24 protein could be more selectively expressed in these cells. Analysis of the expression of the Tsg24 protein in synchronized CHO cells by Western blotting, however, did not support this hypothesis. In contrast, we found that the Tsg24 protein is also constitutively expressed throughout interphase in CHO cells (Fig. 3). The difference in temporal detection of the Tsg24 protein at the centromere in murine and CHO cells cannot therefore be explained by differential protein expression. Instead, this difference must be explained in some other way, e.g., because the Tsg24 antigen is not recognized by the anti-Tsg24 antibody during interphase and in late-mitotic CHO cells. Alternatively, the Tsg24 protein could have a more complex distribution pattern in murine centromeres than in CHO cells (see below).
FIG. 3.

The Tsg24 protein is uniformly expressed throughout the cell cycle. Protein extracts were prepared from a serum-deprived synchronized population of CHO cells, following the addition of serum to the cells. Equal numbers of cells were sampled at the indicated time points (0 to 24 h). The extracts were analyzed by immunoblotting with the anti-Tsg24 serum. To ensure that equal amounts of proteins had been loaded onto the gel at all time points, the same immunoblot was labelled in parallel with an anti-α-lamin antibody. m, mitotic extract.
The Tsg24 protein binds to the major satellite regions of murine centromeres.
Murine centromeres are known to be approximately 10 to 100 times larger than the corresponding chromosomal regions found in many other organisms. This difference is mainly attributed to the occurrence of large blocks of γ-satellite sequences (the major satellite sequences) in the murine centromeres (49, 53). The size of the γ-satellite sequences in the murine centromeres allows them to be directly visualized by Hoechst 33258 staining (34). Staining of interphase and mitotic murine cells with Hoechst 33258 can therefore be used to directly analyze the overlap between a putative centromeric protein and the centromere region by immunofluorescence microscopy. We found that the location of the Tsg24 protein perfectly overlaps with the location of centromeric regions in interphase and mitotic murine cells stained with Hoechst 33258 (Fig. 2). Furthermore, a comparison of the distribution of the Tsg24 protein in late-S-phase cells, i.e., in cells where centromeric heterochromatin is replicated (5, 14), showed that the distributions of Tsg24, PCNA, and centromeric heterochromatin in interphase cells were indistinguishable from each other (Fig. 2).
Interestingly, the centromeric regions in murine prophase/metaphase cells labelled by the CREST antiserum or by the anti-Tsg24 antibody differ considerably in size (Fig. 2). The CREST antiserum labels dots in murine cells distributed in a pairwise fashion, similar to what is seen in CHO cells (Fig. 1). This confirms that the CREST antibody stains the region in murine centromeres, the minor satellite region, that corresponds to the α-satellite region in human centromeres (41, 49, 53). In contrast, the size of the centromeric structures labelled by the anti-Tsg24 antibody in murine mitotic cells and their colocalization with regions labelled by Hoechst 33258 suggest that the Tsg24 protein binds to the major satellite sequences of murine centromeres. Whether the Tsg24 protein also binds to the regions of the murine centromeres that correspond to the α-satellite regions of CHO cells is not possible to determine, as the cell cycle-independent labelling of the murine γ-satellite sequences obscures the adjacent minor satellite regions.
Two APC subunits bind to different mitotic cellular structures.
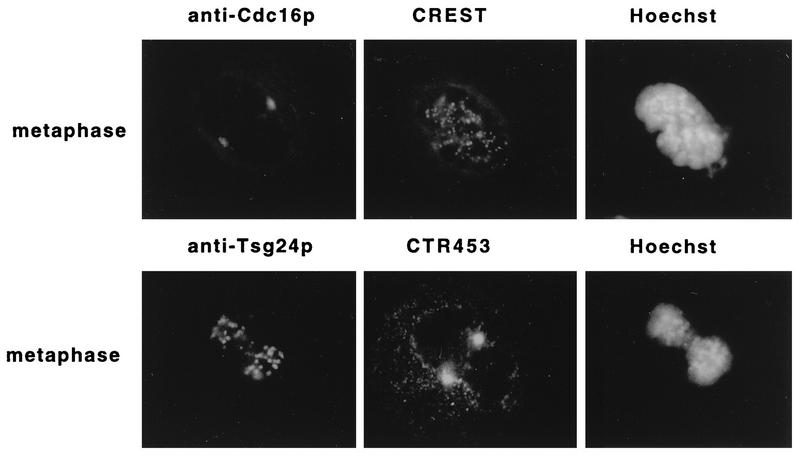
The data for localization of the Tsg24 protein to the centromere differ from the immunolocalization data for two other APC subunits, Cdc16 and Cdc27, which have been localized to the mitotic spindle and the centrosome (36, 51). To ensure that this difference in localization was not caused by the use of different fixation procedures or cell types, antibodies against the human Cdc16 and Cdc27 proteins (51) and against a mitotic spindle protein (CTR453) (2) were tested on mitotic Swiss-3T3 cells by different fixation protocols (methanol-acetone or paraformaldehyde). The antibodies against the Cdc16 protein, Cdc27 protein (data not shown), and the CTR453 antigen labelled the centrosomes but not the centromeric regions, whereas the anti-Tsg24 antibody and the CREST antiserum labelled the centromeric regions but not the centrosomes (Fig. 4). Similar comparative analysis of the distributions of these antigens in interphase cells revealed that the anti-Cdc16 and the anti-Cdc27 antibodies stained the centrosome in Swiss-3T3 cells (data not shown) (51), whereas the anti-Tsg24 antibodies labelled centromeric heterochromatin in Swiss-3T3 cells (Fig. 2). We therefore conclude that different components of the APC appear to be differently located in both interphase and mitotic cells.
FIG. 4.
The APC subunits Tsg24 and Cdc16 are differently located in mitotic cells. Mitotic Swiss-3T3 cells were fixed with methanol-acetone (50:50 [vol/vol]), triple stained with different combinations of antibodies, and analyzed by indirect immunofluorescence microscopy. Row 1 shows a metaphase cell labelled with the anti-Cdc16 antibody (1:150), a CREST antiserum (1:500), and Hoechst 33258. Row 2 shows a metaphase cell labelled with the anti-Tsg24 antibody (1:20), an antibody against centrosomal protein CTR453 (1:1), and Hoechst 33258. The secondary antibodies used were the same as for Fig. 1 and 2.
The mouse Tsg24 protein is part of a soluble APC.
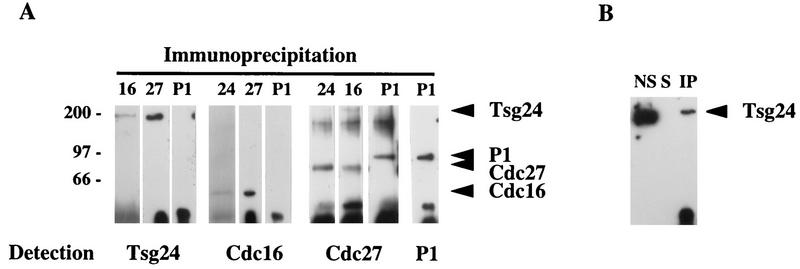
The finding that the Tsg24 and the Cdc16 and Cdc27 proteins are differently located in mammalian cells surprised us. Experimental data for both Xenopus and yeast have shown that the homologs of the Tsg24 and the Cdc16 and Cdc27 proteins in these organisms are part of the APC (39, 59, 61). To confirm that Tsg24 is also part of the APC in murine cells, we have immunoprecipitated proteins from asynchronously growing Swiss-3T3 cells by using antibodies against Tsg24 or against Cdc16 and Cdc27 (51). Immunoprecipitated Tsg24 complexes were found to contain both Cdc16 and Cdc27 (Fig. 5A). Conversely, immunoprecipitated Cdc16 and Cdc27 material contained Tsg24 (Fig. 5A). The replication protein P1 was used as a control in these experiments (45) and was not immunoprecipitated by Tsg24, Cdc16, or Cdc27 antibodies. We therefore conclude that the Tsg24 protein is part of a mammalian protein complex, most likely corresponding to the APC described for other organisms.
FIG. 5.
The APC subunits Tsg24, Cdc16, and Cdc27 are coimmunoprecipitated from mammalian cells. (A) Protein extracts were prepared from Swiss-3T3 cells and immunoprecipitated with anti-Tsg24, anti-Cdc16, anti-Cdc27, or anti-P1 antibodies. The immunoprecipitates were detected by Western blot analysis with the same antibodies. The material precipitated with the anti-Cdc16 antibodies included Cdc16, the anti-Tsg24 antibody precipitated Tsg24, the anti-Cdc27 precipitated Cdc27, and the anti-P1 antibody immunoprecipitated the P1 protein (only the anti-P1–P1 results are shown). The arrowheads indicate the protein bands precipitated. The filters were probed sequentially with different antibodies, and some of the anti-P1 antibody signal remained after washing, explaining the P1 band seen on the filters probed with the anti-Cdc27 antibody. The anti-P1 antibody detects a replication protein in murine cells and was used here as a negative control (45). Molecular masses (indicated at the left) are in kilodaltons. (B) In order to test the solubility of the Tsg24 protein in these experiments, protein extracts were solubilized either with RIPA buffer or with mild lysis buffer (data not shown). Three fractions were tested in parallel by Western blotting: proteins not solubilized by the extraction buffer (NS), proteins solubilized and immunoprecipitated with the anti-Tsg24 antibody (IP), and proteins solubilized but not immunoprecipitated with the anti-Tsg24 antibody (5).
The Tsg24 protein that is bound to the centromeres is, however, not likely to be part of a soluble fraction that can be immunoprecipitated. To test this, we carried out two different control experiments. In the first experiment, we analyzed how much of the total cellular Tsg24 protein was available for immunoprecipitation. We found that only a small fraction (less than 10%) of the Tsg24 protein was soluble in the RIPA buffer or the mild lysis buffer used in the immunoprecipitation experiment (see Materials and Methods) (Fig. 5B). Similarly, large fractions of the Cdc16 and the Cdc27 proteins were not soluble under these experimental conditions (data not shown). These results suggest that a large fraction of the Tsg24 protein is tightly bound to a cellular structure(s).
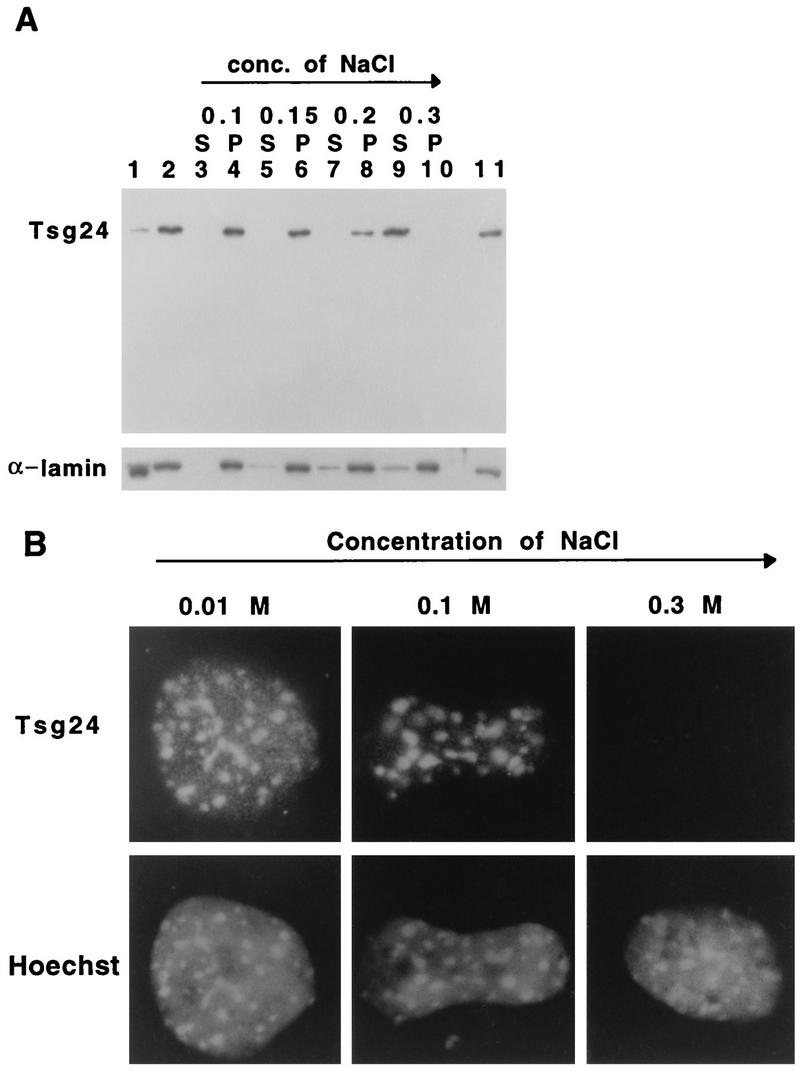
The solubility of the Tsg24 protein was also tested by a nuclear salt extraction procedure. Nuclei from asynchronously growing Swiss-3T3 cells were incubated in a hypotonic buffer with increasing concentrations of salt (0.1 to 0.3 M NaCl). The salt eluate (Fig. 6) and the nuclei (Fig. 6) were analyzed separately by Western blotting with the anti-Tsg24 or anti-α-lamin antibodies. Most of the Tsg24 protein was found in the nucleus and was resistant to elution by 0.1, 0.15, and 0.2 M NaCl, whereas the incubation of nuclei with 0.3 M NaCl removed most of the Tsg24 protein. In order to confirm that the eluted Tsg24 protein corresponds to the centromeric Tsg24 protein observed in interphase Swiss-3T3 cells by immunofluorescence microscopy, cells treated with increasing concentrations of salt were analyzed by indirect immunofluorescence microscopy (Fig. 6B). We found that cells treated with NaCl concentrations of 0.1 M still displayed a strong centromeric signal, whereas cells treated with 0.3 M NaCl had lost their centromeric signal entirely (Fig. 6B). The results of the Western blotting and the immunofluorescence microscopy experiments with the salt-extracted cells are in close agreement, suggesting that a large fraction of the Tsg24 protein is tightly bound to the centromere regions of interphase cells. It is also likely that this salt-resistant protein fraction corresponds to the protein fraction not solubilized in the immunoprecipitation experiments described above.
FIG. 6.
The binding of Tsg24 to the centromere is salt dependent. (A) Nuclei were prepared from asynchronously growing Swiss-3T3 cells and extracted with hypotonic buffers containing increasing NaCl concentrations (conc.). The proteins which had been eluted into a supernatant (S) were concentrated and subjected to Western blot analysis. The extracted nuclei, the pellet (P), were also analyzed by Western blotting. The Western blots were labelled with anti-Tsg24 and anti-α-lamin antibodies. Total cellular protein extract (lane 11), total cytoplasm (lane 1), and total nuclear proteins prior to extraction (lane 2) are also shown. (B) Nuclei extracted with different salt concentrations were centrifuged onto glass slides, fixed, and stained with the anti-Tsg24 antibody and Hoechst 33258.
The Tsg24 and the Cdc27 proteins are bound to purified mitotic chromosomes.
An important control for the immunofluorescence microscopy data would be to confirm by some other means that the Tsg24 protein is bound to mitotic chromosomes. One additional antibody has been raised against a different region of the Tsg24 protein. This antibody, however, produces only a very weak signal in Western blot applications, and subsequently no signal at all is detected in immunofluorescence microscopy. We have also tried to overexpress the Tsg24 protein fused to an epitope tag in murine cells. So far, however, this has not resulted in a detectable expression of this protein. Overexpression of the Tsg24 protein in budding yeast cells results in the accumulation of small degradation products, also possibly explaining the lack of detectable expression of this protein in mammalian cells. To circumvent these technical problems and to determine if the Tsg24 protein binds to mitotic chromosomes, we have instead used a biochemical procedure for isolating mitotic chromosomes from CHO cells (56). Antibodies against the Tsg24 protein, the P1 protein (a replication initiation protein bound to chromosomes during G1 of the cell cycle) (45), pericentrin (a centrosomal protein) (12), and α-tubulin (a mitotic spindle protein) were applied to a filter containing protein extracts prepared from mitotic cells or from chromosomes isolated from mitotic cells. It can be seen in Fig. 7 that the anti-Tsg24, the anti-P1, the α-tubulin, and the antipericentrin antibodies all reacted with proteins in the mitotic cell extract (Fig. 7), but only the Tsg24 protein was found in the protein extract prepared from isolated mitotic chromosomes (Fig. 7). This shows that the Tsg24 protein binds to both interphase (Fig. 6) and mitotic (Fig. 7) chromosomes and confirms the results of the immunofluorescence experiments described in the legends to Fig. 1 and 2.
FIG. 7.

The APC components Tsg24 and Cdc27 are bound to mitotic chromosomes. Protein extracts were prepared from mitotic CHO cells (m) and from isolated chromosomes prepared from mitotic CHO cells (pmc). The protein extracts were analyzed by immunoblotting with the anti-Tsg24, anti-P1, antipericentrin, anti-α-tubulin, and anti-Cdc27 antibodies. It is likely that the band with a unique molecular weight detected by the anti-Cdc27 antibody in extracts prepared from isolated mitotic cells is due to the artificial removal of posttranslational modifications (see the text) during the purification procedure, as this protein has been shown to be phosphorylated in mitotic cells (39).
The fact that neither pericentrin nor α-tubulin was found in the protein extract prepared from isolated mitotic chromosomes suggests that the centrosomal and mitotic spindle proteins do not contaminate this extract. This gave us an opportunity to test if the APC protein Cdc27 was part not only of the mitotic spindle (51) but also of the mitotic chromosomes. We therefore labelled the filters described in the legend to Fig. 7 with an antibody against the Cdc27 protein. We found that the Cdc27 protein, in contrast to pericentrin and α-tubulin, was included in the protein fraction prepared from isolated mitotic chromosomes (Fig. 7). This shows that a fraction of the Cdc27 protein, in contrast to what immunofluorescence microscopy data show, is bound to mitotic chromosomes. A similar Western blotting experiment was also carried out with the anti-Cdc16 antibody. In this case, however, only a very weak signal was observed in the protein extract prepared from purified mitotic chromosomes. We cannot explain the apparent lack of the Cdc16 protein in the protein fraction isolated from mitotic chromosomes, although this is consistent with the fact that the relative amount of the Cdc16 protein that is coimmunoprecipitated with the Tsg24 protein is much smaller than that of the Cdc27 protein. Therefore, the Cdc16 protein could be more loosely attached to the Tsg24 protein (and to the mitotic chromosomes), a feature which would affect its quantitative recovery in these experiments.
DISCUSSION
We have analyzed the cellular distribution of the Tsg24 protein, a murine APC subunit, and made several important observations. We found that the Tsg24 protein is a centromere-associated protein (Fig. 1, 2, 4, and 6B), which for the first time correlates the presumed activity of the APC as a regulator of sister chromatid cohesion with the chromosomal region responsible for sister chromatid cohesion. A centromeric location and function for the Tsg24 protein have been suggested by the preanaphase arrest phenotype observed in A. nidulans, Saccharomyces cerevisiae, and S. pombe mutants in which the gene corresponding to Tsg24 in these organisms has been inactivated (24, 38, 59, 61). It has been proposed that sister chromatids in metaphase cells are mainly held together at their centromeres by interactions maintained by DNA topoisomerase II and by a hypothetical protein(s) that may have a structural or regulatory role in the cohesion process (4, 21, 22). The localization of the Tsg24 protein to the centromere region in mammalian cells suggests that this protein is directly involved in the ubiquitination and degradation of a centromeric protein(s) required for sister chromatid cohesion in mitotic cells.
When the distributions of the Tsg24 protein in CHO cells and murine cells were compared by indirect immunofluorescence microscopy, a striking difference was observed. Whereas the anti-Tsg24 antibody only transiently labelled the centromeres in CHO cells during mitosis (Fig. 1), the murine centromeres were labelled at all stages of the cell cycle (Fig. 2). To determine if this could be a result of differential protein expression taking place in the different cell types, protein extracts were prepared from synchronized cells at different stages of their cell cycles. We could show, however, that this explanation was not correct, as the Tsg24 protein was constitutively expressed throughout the cell cycle in both CHO and murine cells (Fig. 3). The intranuclear localizations of the Tsg24 protein in CHO and murine cells were also compared. The Tsg24 protein was found to colocalize with the CREST protein, with the α-satellite region within the centromere regions of CHO cells and with the γ-satellite region in the murine centromeres (Fig. 1, 2, and 6). A direct correlation between the centromere-associated Tsg24 protein detected in immunofluorescence experiments and the 200-kDa Tsg24 protein identified by Western blotting methods was also established by a nuclear salt extraction method (Fig. 6). It was not possible to determine if the Tsg24 protein also transiently accumulated in the minor satellite regions of the centromeres in murine cells, as an antibody signal from this region would have been obscured by the constitutive binding of the Tsg24 protein to the closely situated γ-satellite regions. The most likely explanation for these apparently contradictory results is that the Tsg24 protein is constitutively bound to the centromere regions as seen in murine cells but that the antigen is temporally masked in CHO cells. We cannot, however, rule out the possibilities that the Tsg24 protein has a transient function during the prophase to the early anaphase stages of mitosis and that this function is revealed by the transient detection of this protein in the centromere regions of mitotic CHO cells. One obvious function could be to take part in the regulation of the sister chromatid separation process as part of the APC.
Sister chromatids in metaphase cells are predominantly held together at their centromere regions, chromosomal regions which mainly consist of heterochromatic sequences (41). Heterochromatic domains have been shown to be important for pairing of meiotic chromatids in Drosophila (10, 26), suggesting that these chromosomal domains could also be of importance for sister chromatid pairing in mitotic cells. An interesting observation from our experiments is the correlation between the relative sizes of the centromere regions in CHO and murine cells and the corresponding distributions of the Tsg24 protein (Fig. 1 and 2). It is likely that as the centromere region increases in size (as it has done in murine cells), the distribution of the machinery that regulates the separation of centromere regions at mitosis needs to be increased in parallel.
We were surprised to find that the Tsg24 protein and other subunits (Cdc16 and Cdc27) of the APC did not colocalize in mitotic cells (Fig. 4). We have tried several different fixation procedures, but we have not been able to detect a centromeric signal with the Cdc16 or the Cdc27 antibodies or a centrosomal/mitotic spindle signal with the Tsg24 antibodies. This result suggests that different APC components are bound to different nuclear structures. Alternatively, however, there are at least two technical explanations for these results. One such explanation is that the conditions used for immunofluorescence microscopy result in a loss of different APC subunits at different nuclear locations. A second explanation is that the epitopes are not recognized by the antibodies, perhaps as a result of an alternative orientation or conformation of the APC at different cellular locations. To test these alternative hypotheses, we have carried out two different types of biochemical experiments to complement the results of the immunofluorescence experiments. We first showed by immunoprecipitation experiments that the Tsg24 protein and the Cdc16 and Cdc27 proteins are part of a protein complex, which probably corresponds to the APC (Fig. 5), a result that is in agreement with data presented for other organisms. In a second experiment we isolated mitotic chromosomes from CHO cells and analyzed their protein compositions by Western blotting methods. We could show that both the Tsg24 protein and the Cdc27 protein were strongly bound to isolated mitotic chromosomes, whereas under the same experimental conditions, the centrosomal protein pericentrin and the mitotic spindle protein α-tubulin were not bound (Fig. 7). This shows that a fraction of the Cdc27 protein, in contrast to what immunofluorescence microscopy data show, is bound to mitotic chromosomes. Based on the fact that both the Cdc27 and the Tsg24 proteins are part of a soluble protein complex that regulates the sister chromatid separation process and that the Tsg24 protein has been shown to be a centromere-associated protein, we propose that these proteins together form a centromere-associated protein complex. At present, however, we have no direct evidence that the chromosome-associated form of Tsg24 and the Cdc27 protein have APC activity or are indeed in a complex on the chromosome. The possibility that these proteins, in addition to their accepted function in the APC, also have additional chromosome-associated functions cannot be excluded.
We have not been able either by immunofluorescence methods or by biochemical fractionation methods to show that the APC component Cdc16 is associated with mitotic chromosomes. This could be a result of a combination of technical problems or, alternatively, of the existence of subcomplexes containing a smaller number of APC subunits. These subcomplexes could themselves be functional or represent intermediates used for the transient assembly of the APC during mitosis. Evidence that the APC is a transient structure, assembled during the cell cycle, has been presented (59). The assembly and the disassembly of the APC appear to be regulated by the cyclic-AMP regulatory pathway acting on the Cut4 protein, the S. pombe protein corresponding to Tsg24 (59). As soon as antibodies against additional mammalian APC components become available, it should be possible to determine their cellular localization and to test the above predictions.
ACKNOWLEDGMENTS
We thank Katarina Gell for valuable technical assistance, Ö. Melefors for help with the immunoprecipitation experiments, and W. C. Earnshaw, N. F. Rothfield, M. Bornens, S. Georgatos, M. Kirschner, and P. Hieter for generously supplying us with control antibodies. We also thank W. Zachariae and K. Nasmyth for communicating unpublished information and G. Farrants, M. Sjöberg, W. Zachariae, and K. Nasmyth for reading the manuscript.
This work was supported by the The Swedish Cancer Society, Magnus Bergvalls Stiftelse, the Alex och Eva Wallström Stiftelse, and the Karolinska Institutet.
REFERENCES
- 1.Ault J G, Rieder C L. Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol. 1994;6:41–49. doi: 10.1016/0955-0674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 2.Bailly E, Doree E, Nurse P, Bornens M. p34-cdc2 is located in both nucleus and cytoplasm: part is centrosomally associated and enters vesicles at anaphase. EMBO J. 1989;8:3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat M A, Philp A V, Glover D M, Bellen H J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell. 1996;87:1103–1114. doi: 10.1016/s0092-8674(00)81804-8. [DOI] [PubMed] [Google Scholar]
- 4.Bickel S E, Orr-Weaver T L. Holding chromatids together to ensure they go their separate ways. Bioessays. 1996;18:293–300. doi: 10.1002/bies.950180407. [DOI] [PubMed] [Google Scholar]
- 5.Bravo R, Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celis J E, Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cell: subdivision of S phase. Proc Natl Acad Sci USA. 1985;82:3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R H, Waters J C, Salmon E D, Murray A W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea R J, Stratmann R, Lehner C F, John U P, Saint R. The three rows gene of Drosophila melanogaster encodes a novel protein that is required for chromosome disjunction during mitosis. Mol Biol Cell. 1993;4:1161–1174. doi: 10.1091/mbc.4.11.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson I A, Roth S, Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dernburg A F, Sedat J W, Hawley R S. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell. 1996;86:135–146. doi: 10.1016/s0092-8674(00)80084-7. [DOI] [PubMed] [Google Scholar]
- 11.Deshaies R J. The self-destructive personality of a cell cycle in transition. Curr Opin Cell Biol. 1995;7:781–789. doi: 10.1016/0955-0674(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 12.Doxsey S J, Stein P, Evans L, Calarco P D, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 13.Engle D B, Osmani S A, Osmani A H, Rosborough S, Xiang X, Morris N R. A negative regulator of mitosis in Aspergillus is a putative membrane-spanning protein. J Biol Chem. 1990;27:16132–16137. [PubMed] [Google Scholar]
- 14.Fox M H, Arndt-Jovin D J, Jovin T M, Baumann P H, Robert-Nicoud M. Spatial and temporal distribution of DNA replication sites localized by immunofluorescence and confocal microscopy in mouse fibroblasts. J Cell Sci. 1991;99:247–253. doi: 10.1242/jcs.99.2.247. [DOI] [PubMed] [Google Scholar]
- 15.Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- 16.Glotzer M, Murray A W, Kirschner M W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 17.Hartwell L H, Kastan M B. Cell cycle control and cancer. Science. 1994;266:1821–1827. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 18.Hilt W, Wolf D H. Proteasomes: destruction as a programme. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 19.Hirano T, Hiraoka Y, Yanagida M. A temperature-sensitive mutation of the Schizosaccharomyces pombe gene nuc2 that encodes a nuclear scaffold-like protein blocks spindle elongation in mitotic anaphase. J Cell Biol. 1994;106:1171–1183. doi: 10.1083/jcb.106.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochstrasser M. Ubiquitin, proteasome, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 21.Holloway S L. Sister chromatid separation in vivo and in vitro. Curr Opin Genet Dev. 1995;5:243–248. doi: 10.1016/0959-437x(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 22.Holloway S L, Glotzer M, King R W, Murray A W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- 23.Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–277. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 24.James S W, Mirabito M P, Scacheri P C, Morris N R. The Aspergillus nidulans bimE (blocked-in-mitosis) gene encodes multiple cell cycle functions involved in mitotic checkpoint control and mitosis. J Cell Sci. 1995;108:3485–3499. doi: 10.1242/jcs.108.11.3485. [DOI] [PubMed] [Google Scholar]
- 25.Juang Y L, Huang J, Peters J M, McLaughlin M E, Tai C Y, Pellman D. APC-mediated proteolysis of Ase1 and the morphogenesis of the mitotic spindle. Science. 1997;275:1311–1314. doi: 10.1126/science.275.5304.1311. [DOI] [PubMed] [Google Scholar]
- 26.Karpen G H, Le M-H, Le H. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science. 1996;273:118–122. doi: 10.1126/science.273.5271.118. [DOI] [PubMed] [Google Scholar]
- 27.King R W, Deshaies R J, Peters J M, Kirschner M W. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 28.King R W, Glotzer M, Kirschner M W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol Biol Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King R W, Jackson P K, Kirschner M W. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 30.King R W, Peters J-M, Tugendreich S, Rolfe M, Hieter P, Kirschner M W. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Lamb R R, Michaud W A, Sikorski R S, Hieter P A. Cdc16p, Cdc23p and Cdc27p form a complex essential for mitosis. EMBO J. 1994;13:4321–4328. doi: 10.1002/j.1460-2075.1994.tb06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasko D, Cavenee W, Nordenskjöld M. Loss of constitutional heterozygosity in human cancer. Annu Rev Genet. 1991;25:281–314. doi: 10.1146/annurev.ge.25.120191.001433. [DOI] [PubMed] [Google Scholar]
- 34.Leonhardt H, Page A W, Weier H-U, Bestor T H. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell. 1992;71:865–873. doi: 10.1016/0092-8674(92)90561-p. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 36.Mirabito P M, Morris N R. BimA, a TPR-containing protein required for mitosis, localizes to the spindle pole body in Aspergillus nidulans. J Cell Biol. 1993;120:959–968. doi: 10.1083/jcb.120.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicol L, Jeppesen P. Human autoimmune sera recognize a conserved 26 kD protein associated with mammalian heterochromatin that is homologous to heterochromatin protein 1 of Drosophila. Chromosome Res. 1994;2:245–253. doi: 10.1007/BF01553325. [DOI] [PubMed] [Google Scholar]
- 38.Osmani S A, Engle D B, Doonan J H, Morris N R. Spindle formation and chromatin condensation in cells blocked at interphase by mutation of a negative cell cycle control gene. Cell. 1988;52:241–251. doi: 10.1016/0092-8674(88)90513-2. [DOI] [PubMed] [Google Scholar]
- 39.Peters J-M, King R W, Höög C, Kirschner M W. Identification of BIME as a subunit of the anaphase promoting complex. Science. 1996;274:1199–1201. doi: 10.1126/science.274.5290.1199. [DOI] [PubMed] [Google Scholar]
- 40.Philp A V, Axton J M, Saunders R D C, Glover D M. Mutations in the Drosophila melanogaster gene three rows permit aspects of mitosis to continue in the absence of chromatid segregation. J Cell Biol. 1993;106:87–98. doi: 10.1242/jcs.106.1.87. [DOI] [PubMed] [Google Scholar]
- 41.Pluta A F, Mackay A M, Ainsztein A M, Goldberg I G, Earnshaw W C. The centromere: hub of chromosomal activities. Science. 1995;270:1591–1594. doi: 10.1126/science.270.5242.1591. [DOI] [PubMed] [Google Scholar]
- 42.Samejima I, Yanagida M. Bypassing anaphase in fission yeast cut9 mutation: requirement of cut9 to initiate anaphase. J Cell Biol. 1994;127:1655–1670. doi: 10.1083/jcb.127.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigrist S, Jacobs H, Stratmann R, Lehner C F. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starborg M, Brundell E, Gell K, Höög C. A novel murine gene encoding a 216-kDa protein is related to a mitotic checkpoint regulator previously identified in Aspergillus nidulans. J Biol Chem. 1994;269:24133–24137. [PubMed] [Google Scholar]
- 45.Starborg M, Brundell E, Gell K, Larsson C, White I, Daneholt B, Höög C. A murine replication protein accumulates temporarily in the heterochromatic regions of nuclei prior to initiation of DNA replication. J Cell Sci. 1995;108:927–934. doi: 10.1242/jcs.108.3.927. [DOI] [PubMed] [Google Scholar]
- 46.Starborg M, Brundell E, Gell K, Höög C. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci. 1996;109:143–153. doi: 10.1242/jcs.109.1.143. [DOI] [PubMed] [Google Scholar]
- 47.Stratmann R, Lehner C F. Separation of sister chromatids in mitosis requires the Drosophila pimples product, a protein degraded after the metaphase/anaphase transition. Cell. 1996;84:25–35. doi: 10.1016/s0092-8674(00)80990-3. [DOI] [PubMed] [Google Scholar]
- 48.Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca F C, Ruderman J V, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–198. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunkel C E, Coelho P A. The elusive centromere: sequence divergence and functional conservation. Curr Opin Genet Dev. 1995;5:756–767. doi: 10.1016/0959-437x(95)80008-s. [DOI] [PubMed] [Google Scholar]
- 50.Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase-to-anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 52.Van Den Berg D J, Francke U. Roberts syndrome: a review of 100 cases and a new rating system for severity. Am J Med Genet. 1993;47:1104–1123. doi: 10.1002/ajmg.1320470735. [DOI] [PubMed] [Google Scholar]
- 53.Vissel B, Choo K H. Mouse major (γ) satellite DNA is highly conserved and organized into extremely long tandem arrays: implications for recombination between nonhomologous chromosomes. Genomics. 1989;5:407–414. doi: 10.1016/0888-7543(89)90003-7. [DOI] [PubMed] [Google Scholar]
- 54.Warburton P E, Earnshaw W C. Untangling the role of DNA topoisomerase II in mitotic chromosome structure and function. Bioessays. 1997;19:97–99. doi: 10.1002/bies.950190203. [DOI] [PubMed] [Google Scholar]
- 55.Wells W A E. The spindle-assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]
- 56.Wordeman L. The isolation of functional mitotic organelles from tissue culture cells. In: Fantes P, Brooks R, editors. The cell cycle, a practical approach. Oxford, England: IRL Press; 1996. pp. 235–251. [Google Scholar]
- 57.Yamamoto A, Guacci V, Koshland D. Pds1p is required for faithful execution of anaphase in the yeast. J Cell Biol. 1996;133:85–98. doi: 10.1083/jcb.133.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto A, Guacci V, Koshland D. Pdsp1, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J Cell Biol. 1996;133:99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita Y M, Nakaseko Y, Samejima I, Kumada K, Yamada H, Michaelson D, Yanagida M. 20 S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature. 1996;384:276–279. doi: 10.1038/384276a0. [DOI] [PubMed] [Google Scholar]
- 60.Yanagida M. Frontier questions about sister chromatid separation in anaphase. Bioessays. 1995;17:519–526. doi: 10.1002/bies.950170608. [DOI] [PubMed] [Google Scholar]
- 61.Zachariae W, Shin T H, Galova M, Obermaier B, Nasmyth K. Identification of two novel subunits of the anaphase promoting complex of Saccharomyces cerevisiae. Science. 1996;274:1202–1204. doi: 10.1126/science.274.5290.1201. [DOI] [PubMed] [Google Scholar]