Abstract
The transcription factor NF-κB is normally sequestered in the cytoplasm by members of the IκB family, including IκBα, IκBβ, and the recently cloned IκBɛ. Upon cellular activation, these inhibitors are rapidly phosphorylated on two amino-terminal serines, ubiquitinated, and degraded by the 26S proteasome, releasing a functional NF-κB. To determine the importance of IκBβ in NF-κB regulation in T cells, we generated transgenic mice expressing a constitutively active IκBβ mutant (mIκBβ) under the control of the lck promoter. The transgene contains the two critical N-terminal serine residues mutated to alanines and therefore no longer susceptible to degradation upon cell activation. mIκBβ is unable to totally displace IκBα from RelA-containing complexes, thus allowing a transient activation of NF-κB upon T-cell stimulation. However, mIκBβ completely blocks NF-κB activity after IκBα degradation. In addition, as a consequence of this inhibition, ikba expression is down regulated, along with that of other NF-κB-regulated genes. These transgenic mice have a significant reduction in the peripheral T-cell population, especially CD8+ cells. The remaining T cells have impaired proliferation in response to phorbol 12-myristate 13-acetate plus phytohemagglutinin or calcium ionophore but not to anti-CD3/anti-CD28 costimulation. As a result of these alterations, transgenic animals present defects in immune responses such as delayed-type hypersensitivity and the generation of specific antibodies against T-cell-dependent antigens. These results show that in nonstimulated T cells, IκBβ cannot efficiently displace IκBα bound to RelA-containing complexes and that persistent NF-κB activity is required for proper T-cell responses in vivo.
NF-κB plays an essential role in the transcriptional regulation of genes involved in the early onset of immune and inflammatory responses such as major histocompatibility complex class I, immunoglobulin κ light chain, interleukins, granulocyte-macrophage colony-stimulating factor, beta interferon, and T-cell receptor β chain among others (for reviews, see references 4, 6, 28, 31, 37, 44, 47, 61, 66, and 70). The mammalian members of this family can be grouped into two classes: class I members, which include NF-κB1 (p105/p50) and NF-κB2 (p100/p52), are transcribed as precursors (p105 and p100) and then proteolytically processed to yield the DNA binding subunits p50 and p52, respectively, and class II members, which include RelA (p65), c-Rel, and RelB, are not proteolytically processed and contain a transcriptional activation domain in their C termini. Members of these two groups form homo- and heterodimers through a highly conserved ∼300-amino-acid region, the Rel homology domain, that contains the DNA binding and dimerization domains and the nuclear localization signal and is required for the interaction with the IκB proteins.
In most cell types, NF-κB is maintained inactive in the cytoplasm complexed with the IκB proteins. These proteins contain ankyrin repeats, which are necessary for the interaction with Rel/NF-κB complexes. Members of this family include IκBα, IκBβ, and the recently cloned IκBɛ, which not only retain the NF-κB complexes in the cytoplasm but also inhibit their DNA binding and transactivation capacity (6, 62, 69). The IκB family also includes Bcl-3, the product of the proto-oncogene bcl-3, which is nuclear and has been shown to act as an inhibitor or activator of NF-κB transcriptional activity, depending on the complexes involved (7, 12, 15, 29, 30, 33, 51, 67, 75, 77). In addition, the C-terminal portions of the precursors p105 and p100 contain ankyrin repeats and function as IκBs. The existence of these C-terminal polypeptides as independent IκBs (IκBγ and IκBδ, respectively) has been demonstrated only in certain cell types (16, 26, 34, 46, 49, 50, 55, 56, 63). Two IκB-like proteins have recently been cloned (IκB-R and IκB-L); however, their relevance to NF-κB regulation has not been established (1, 53).
NF-κB is activated by a wide variety of agents, including cytokines like tumor necrosis factor alpha (TNF-α), tumor promoters like phorbol 12-myristate 13-acetate (PMA), interleukin-1 (IL-1), lipopolysaccharide (LPS), and many viruses. This activation leads to the inducible degradation of the inhibitors and concomitant migration of active NF-κB to the nucleus (reviewed in references 6, 7, 28, 30, 66, and 70). Since IκBα was the first molecule cloned, most of the characterization of IκB function has been done with it. Treatment with various stimuli leads to the phosphorylation of two critical N-terminal serine residues at positions 32 and 36 (13, 14, 20, 23, 64, 68, 76). Once phosphorylated, this molecule is ubiquitinated on at least two amino-terminal lysines, becoming a target for degradation by the ubiquitin-26S proteasome pathway (2, 5, 19, 57). Recently a component of a protein kinase complex was molecularly cloned and identified as a serine threonine kinase that specifically phosphorylates the two amino-terminal serines of IκB (24, 54). It is not clear if this subunit forms part of a previously described kinase complex whose activity depends on ubiquitination (20). In addition, IκBα contains at the C-terminus PEST sequences, which are also required for its degradation (76).
Similar to IκBα, IκBβ is degraded upon stimulation and contains the conserved N-terminal serines and lysines together with the PEST sequences at the C terminus, suggesting that these molecules are controlled by similar mechanisms (67). In support of this concept, mutations of serines 19 and 23 to alanines in IκBβ create a dominant-negative form that is no longer degraded upon induction (23, 32, 45). The significance of the presence of more than one type of IκB molecule is unclear. Recent studies postulate a model in which agents that promote persistent NF-κB activity induce both IκBα and IκBβ degradation. Then the newly synthesized, unphosphorylated IκBβ acts as a chaperone of NF-κB, blocking the inhibitory effect of IκBα and maintaining NF-κB activity even after IκBα resynthesis (65). In addition, studies performed with IκBα null mutant mice suggest that IκBα would be the molecule in charge of the postinductional repression of NF-κB activity (9, 35).
We report here that the expression of a constitutively active IκBβ mutant in T cells of transgenic mice completely inhibits the persistent activation of RelA/p50 in response to PMA-phytohemagglutinin (PHA) stimulation. This inhibition results in impaired induction of some previously reported NF-κB-regulated genes including ikba. mIκBβ transgenic mice have reduced numbers of peripheral T cells, which do not proliferate in response to selected mitogenic stimuli. As a consequence, these animals have defective T-cell-dependent immune responses such as delayed-type hypersensitivity (DTH) and the production of antibodies specific to T-cell-dependent antigens.
MATERIALS AND METHODS
Generation and analysis of transgenic mice.
The coding region of the mouse IκBβ cDNA was obtained by PCR of WEHI 231 cells with specific oligonucleotides (67). The constitutively active IκBβ carrying alanines instead of serines 19 and 23 was generated by the method of Kunkel et al. (39). The mutated cDNA was then cloned into plasmid pTLC, described previously (74). Briefly, the cDNA was placed under the control of 3.2 kb of the mouse proximal lck promoter (43), followed by the β-globin initiation signal to maximize translation efficiency. A 2.1-kb stretch of human growth hormone sequence was placed downstream of the cDNA to confer stability to the mRNA transcript, and a 2.1-kb fragment encompassing the human CD2 gene locus control region (41) was inserted at the 3′ end. Generation of the transgenic mice and PCR genotyping of tail DNA were performed as described previously (52); briefly, the transgene was microinjected into (C57BL/6J × DBA/2)F1 eggs (The Jackson Laboratory) and the eggs were transferred to the oviduct of ICR (Sprague-Dawley) foster mothers. Northern blots were performed with 20 μg of total RNA from the thymus of the different lines (Trizol method; GIBCO-BRL). The ikbb cDNA was also cloned in a pET19b vector (Novagen); the histidine-tagged IκBβ was bacterially produced, purified, and used to generate rabbit polyclonal antibodies, which were used throughout this study.
EMSA and Western blot analysis.
Thymic single-cell suspensions were prepared from 7-week-old mice by standard procedures (21) with RPMI 1640 containing 10% heat-inactivated fetal calf serum (FCS). The cells were stimulated with mouse TNF-α (5 ng/ml; Genzyme Diagnostics) or with PMA (20 ng/ml; Sigma) plus PHA (1 μg/ml; Sigma) for 30, 60, and 180 min at 37°C. Cytoplasmic and nuclear extracts were prepared as previously reported (58). Nuclear extracts (2 μg) were preincubated for 10 min at 20°C with poly(dI-dC) (3 μg; Pharmacia) in a buffer containing 20 mM HEPES (pH 7.9), 60 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol, and 17% glycerol in a final volume of 25 μl. Then 2 × 104 cpm of 32P-labeled palindromic κB (25) or, as a loading control, an oligonucleotide containing the octamer motif (62) was added, and the mixture was incubated for another 20 min at 20°C. When appropriate, nuclear extracts were preincubated with antibodies before the addition of the probe. The complexes were separated on 5.5% native polyacrylamide gels. For the splenic T-cell electrophoretic mobility shift assay (EMSA), cells were purified on murine T-cell enrichment columns (R&D Systems), stimulated with PMA-PHA, and processed as mentioned above. Nuclear extracts (2 μg) were preincubated for 10 min at 20°C with poly(dI-dC) (4 μg) in a buffer containing 10 mM Tris-HCl (pH, 7.5), 50 mM NaCl, 1 mM EDTA, and 5% glycerol in a final volume of 25 μl. The corresponding cytoplasmic extracts (34 μg) were separated by polyacrylamide gel electrophoresis (12.5% polyacrylamide), transferred to nitrocellulose, and probed with anti-IκBα, anti-IκBβ, and anti-lactate dehydrogenase (anti-LDH) antibodies after successive stripping of the membranes. Whole-cell extracts from 7-week-old mouse thymus were prepared as described previously (42) and probed with anti-RelA or anti-p50 antibodies.
Cell labeling and immunoprecipitation.
Isolated thymocytes (4 × 107) were labeled for 4 h at 37°C in 10 ml of methionine-free Dulbecco’s modified Eagle’s medium containing 10% dialyzed FCS and 0.5 mCi of [35S]methionine per ml (1,000 Ci/mmol; Amersham). Immunoprecipitations (with 6 × 106 cells per point) were performed as described previously (38) with minor modifications. Briefly, the cells were resuspended in radioimmunoprecipitation assay buffer without sodium dodecyl sulfate and cleared with preimmune serum and protein A-Sepharose. After the first immunoprecipitation was carried out with anti-RelA antibody under nondenaturing conditions, the supernatants were analyzed for the presence of free IκBα and IκBβ. The RelA-immunocomplexes were denatured, diluted fourfold, and sequentially precipitated with anti-RelA, anti-IκBα, and anti-IκBβ antibodies. Samples were analyzed by polyacrylamide gel electrophoresis followed by fluorography.
Reverse transcriptase PCR analysis.
Thymocytes from control and transgenic mice were isolated, and 5 × 107 cells/time point were incubated for 30, 60, and 180 min with PMA (20 ng/ml) plus PHA (1 μg/ml). Total RNA was extracted (Trizol method), and first-strand cDNA synthesis was performed with 2 μg of RNA as specified by the manufacturer of Superscript II RNase H reverse transcriptase (GIBCO-BRL). Amplification conditions with a Perkin-Elmer thermal cycler were 94°C for 2 min, 60°C for 2 min, and 72°C for 45 s (1 cycle) followed by 94°C for 30 s, 60°C for 30 s, and 72°C for 45 s (30 cycles). The sequences of the primers were as follows: for nfkb1, 5′-GCA CCG TAA CAG CAG GAC CCA AGG ACA-3′ and 5′-CCC GTC ACA CAT CCT GCT GTT CTG TCC ATT CT-3′; for IκBα, 5′-CAG GAC TGG GCC ATG GAG GG-3′ and 5′-TGG CCG TTG TAG TTG GTG GC-3′; for ICAM-1, 5′-CCG CTT CCG CTA CCA TCA CCG TGT ATT C-3′ and 5′-GCC TTC CAG GGA GCA AAA CAA CTT CTG C-3′; and for β-actin, 5′-CCA CCA GAC AAC ACT GTG TTG GCA T-3′ and 5′-AGA GGT ATC CTG ACC CTG AAG TAC C-3′. The primers for TNF-α and IL-6 were obtained from Stratagene. Labeled amplified products were electrophoretically separated in 6% native polyacrylamide–bisacrylamide gels (40:1) and then subjected to autoradiography.
Flow cytometry.
Flow cytometry was done with a Coulter Epics Profile II flow cytometer and cell sorter. Thymocytes and splenocytes were prepared by standard procedures (21), and 5 × 105 cells/reaction were incubated with fluorescein isothiocyanate- or phycoerythrin-labeled antibodies (1 μg each) for 30 min on ice, washed twice with 2% FCS in phosphate-buffered saline, and resuspended in 250 μl of 1% formaldehyde in PBS. Anti-mouse CD4 and CD8 antibodies were obtained from GIBCO-BRL. An average of 104 cells were recorded in each case.
T-cell proliferation assays.
Splenic T cells were purified on murine T-cell enrichment columns, and 105 cells were stimulated with PMA (2 ng/ml) in the presence of PHA (1 μg/ml) or the calcium ionophore A23187 (1 μM) or by being cultured in wells previously coated with anti-CD3 and anti-CD28 antibodies (Pharmigen). Cell proliferation was measured as [3H]thymidine incorporation after incubation of 100 μl of cultures in a 96-well microtiter plate for 72 h with 0.5 μCi of [3H]thymidine (Amersham) per well.
T-cell-dependent immune response analyses.
DTH reaction assays were performed with five mice per genotype per condition (naive or sensitized), as previously described (22), with 0.5% fluorescein isothiocyanate in acetone-dibutyl pthalate (1:1) as a hapten solution. For histopathological analysis, the treated ears were fixed by immersion in 10% buffered formalin, embedded in paraffin blocks, processed by routine methods, sectioned at a thickness of 4 to 6 μm, stained with hematoxylin and eosin, and examined by light microscopy. The sections were graded for severity of edema and cellular infiltrate without knowledge of the group as follows: 0, none; 1, minimal; 2, mild; 3, moderate; 4, marked.
For the production of specific antibodies against T-cell-dependent antigen, 6-week-old mice (three per genotype) were immunized by intraperitoneal injection of 100 μg of keyhole limpet hemocyanin (KLH; Calbiochem) coupled to (4-hydroxy-3-nitrophenyl)acetate (NP; Biosearch Technologies, Inc.) precipitated in alum. NP-KLH conjugates at ratios of 17:1 and alum precipitates were prepared as described previously (18, 40). Serum samples were collected prior to immunization and at 7-day intervals after immunization for 3 weeks. Levels of NP-specific immunoglobulin G1 (IgG1) were determined by enzyme-linked immunosorbent assay (ELISA) with NP-bovine serum albumin (BSA) (17:1) as a capture agent and goat anti-mouse isotype-specific serum directly conjugated to horseradish peroxidase (Southern Biotechnology).
RESULTS
Generation of transgenic mice expressing a constitutively active IκBβ in the thymus.
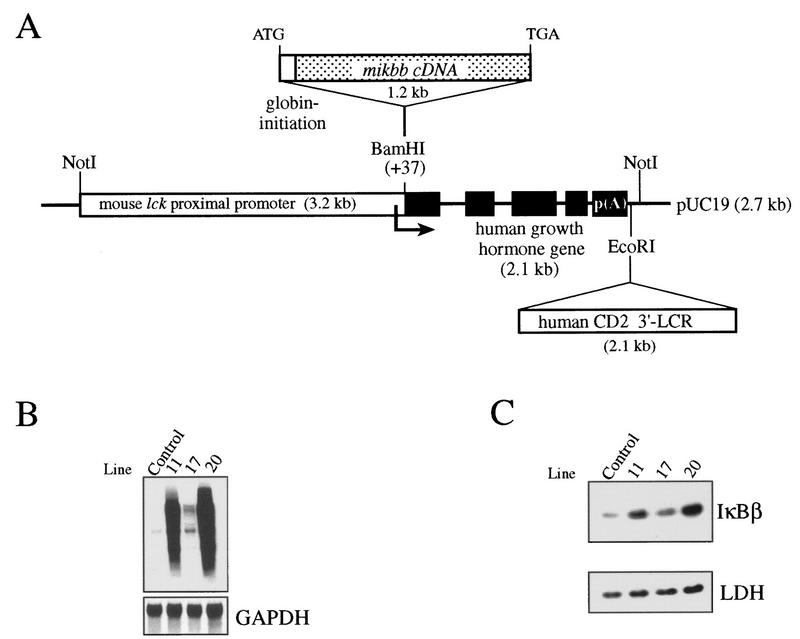
Previous studies with transfected cells have shown the importance of serines 19 and 23 in the inducible degradation of IκBβ (23, 32, 45). To assess the role of IκBβ in NF-κB regulation and its importance for T-cell function in vivo, we have altered the cellular IκB balance by expressing in mouse T cells a constitutively active IκBβ mutant (mIκBβ) bearing both serines (at positions 19 and 23) mutated to alanines and therefore resistant to inducible degradation. For this purpose, the mIκBβ cDNA was placed under the control of the mouse lck proximal promoter and the CD2 3′-locus control region, which confers copy number-dependent and integration site-independent expression of the transgene to all thymocyte subsets including the early stages of T-cell development and extends its expression to peripheral T cells (Fig. 1A) (3, 17, 52, 73). Several independent mouse lines expressing the transgenic transcript and protein in the thymus were obtained (Fig. 1B and C). Lines 11 and 20 expressed the highest level of the protein and were therefore used for the present study (Fig. 1C). The reported results correspond to line 20 and are representative of both lines.
FIG. 1.
(A) Scheme of the mutated ikbb transgene. The mouse ikbb cDNA with the serines 19 and 23 (67) mutated to alanines was placed under the control of the mouse proximal lck promoter. Human growth hormone gene sequences and the human CD2 gene locus control region were added to confer transcript stability and to confer copy number-dependent and position-independent expression of the transgene, respectively. Generation of transgenic mice and PCR genotyping of tail DNA were performed as described previously (52). (B) Northern blot analysis of the different transgenic lines. Total RNA (20 μg) prepared from the thymus of control mice and different transgenic mice was hybridized with the ikbb or the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA. (C) Cytoplasmic extracts (34 μg) prepared from thymocytes of the corresponding mice were analyzed with anti-IκBβ and anti-LDH antibodies.
The constitutively active IκBβ blocks the persistent NF-κB DNA binding activity.
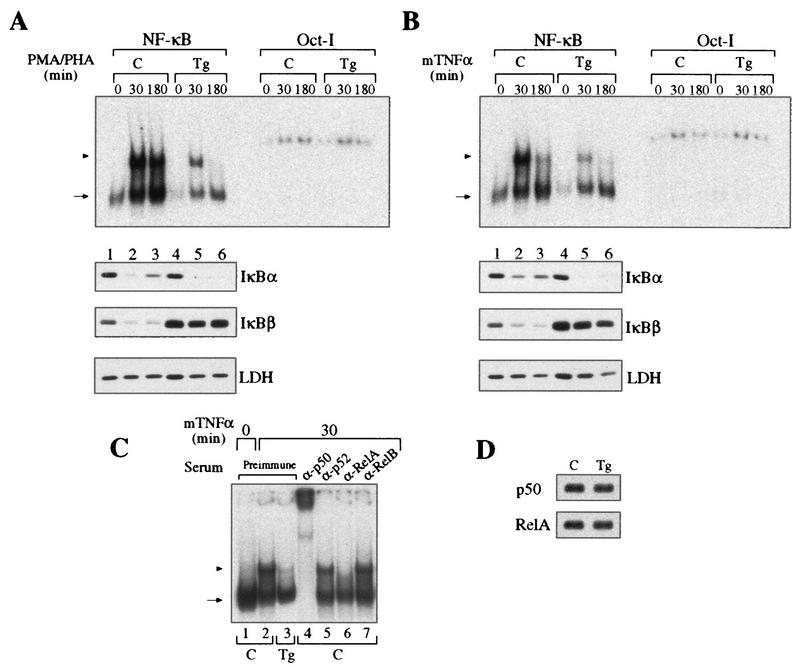
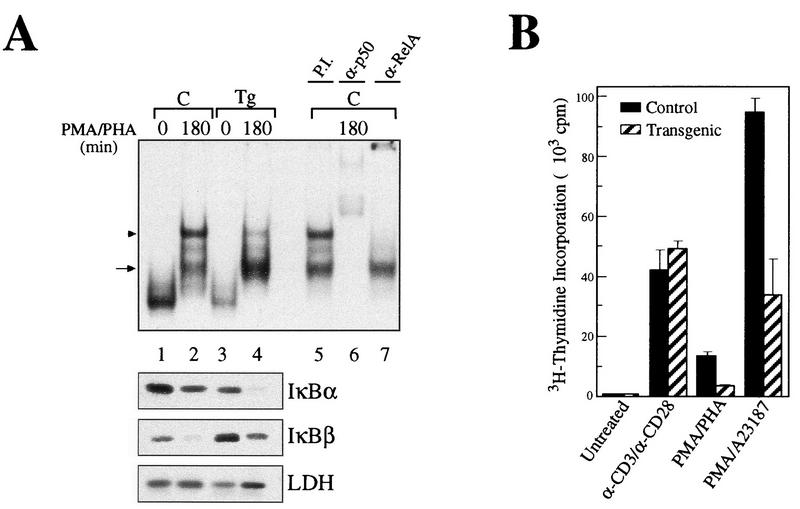
Control and transgenic thymocytes were stimulated with PMA-PHA or TNF-α, and their NF-κB DNA binding activities were analyzed by EMSA with a κB palindromic oligonucleotide (Fig. 2A and B). Extracts from resting thymocytes expressing mIκBβ have lower basal κB DNA binding activity than do controls (Fig. 2A and B, compare lanes 1 and 4), suggesting an effect of IκBβ on p50 homodimers. After 30 min of stimulation with either PMA-PHA or TNF-α, transgenic thymocytes, unlike control thymocytes, presented a marked reduction in the inducible NF-κB DNA binding activity (compare lanes 2 and 5), which was more than 95% inhibited after 3 h (compare lanes 3 and 6). After 6 h of stimulation with PMA-PHA, control extracts maintained the same level of inducible κB binding activity as at 30 min, whereas it was completely absent in extracts from transgenic thymocytes (data not shown). The inducible NF-κB activity inhibited by mIκBβ was composed mainly of p50/RelA heterodimers, as concluded from the addition of specific antibodies to the reaction mixtures (Fig. 2C). The protein levels of p50 and RelA were comparable in whole-cell extracts from control and transgenic resting thymocytes (Fig. 2D). Western blot analysis of the corresponding cytoplasmic extracts showed that the typical reappearance of IκBα observed after longer times of stimulation in control cells was absent in transgenic thymocytes (Fig. 2A and B, compare lanes 3 and 6). In addition, and in contrast to the wild-type IκBβ, mIκBβ was resistant to degradation induced by both stimuli (compare IκBβ, lanes 1 to 3 and lanes 4 to 6). These results demonstrate that although the expression of mIκBβ only partially blocks the initial NF-κB activation, it completely inhibits the persistent one. Furthermore, the transient peak of NF-κB activity is insufficient to induce IκBα resynthesis.
FIG. 2.
Expression of the constitutively active IκBβ inhibits the persistent NF-κB activity. (A and B) EMSA with a palindromic κB and nuclear extracts from control (C, lanes 1 to 3) or transgenic (Tg, lanes 4 to 6) thymocytes treated with PMA-PHA (A) or mouse TNF-α (B) for the indicated periods. The arrowhead indicates p50/RelA heterodimers, and the arrow indicates p50 homodimers. The corresponding cytoplasmic extracts were analyzed by Western blotting with antibodies against IκBα, IκBβ, and LDH. EMSA with Oct-I oligonucleotide was used as a loading control. (C) Determination of the composition of the NF-κB complexes inhibited by mIκBβ by preincubation of the 30-min TNF-α-stimulated extracts with the indicated antibodies. (D) Western blot analysis of whole-cell extracts from control and transgenic thymuses with anti-p50 and anti-RelA antibodies.
A significant fraction of IκBα is complexed to RelA in unstimulated mIκBβ transgenic thymocytes.
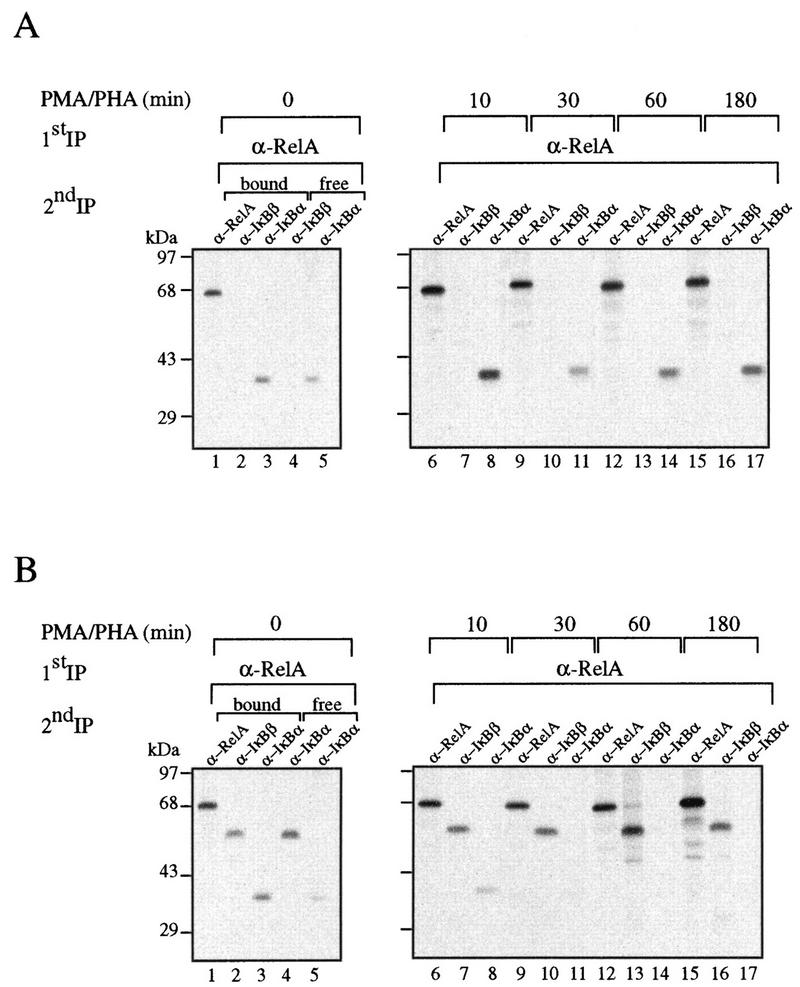
The initial induction of NF-κB activity, together with the presence of virtually normal levels of IκBα in unstimulated transgenic thymocytes, prompted us to investigate the interaction between RelA and both IκBα and IκBβ during the course of the induction. Control and transgenic thymocytes were metabolically labeled with [35S]methionine and incubated for different periods with PMA-PHA. The extracts were first immunoprecipitated with anti-RelA antibodies, and the remaining supernatants were sequentially precipitated with anti-IκBα and anti-IκBβ antibodies. The RelA-containing immune complexes were denatured and sequentially precipitated with anti-RelA, anti-IκBα, or anti-IκBβ antibodies. In unstimulated control cells, RelA was exclusively complexed to IκBα (Fig. 3A, compare lanes 2 and 3). Treatment of these cells with PMA-PHA promoted the rapid degradation of IκBα (lane 11), and its levels started to recover 1 h after stimulation (lanes 14 and 17). Surprisingly, in transgenic cells, regardless of the excess of mIκBβ, most of the IκBα was still bound to RelA (Fig. 3B, lanes 2 and 3). As in control cells, treatment with PMA-PHA induced a rapid degradation of IκBα; however, in transgenic cells, IκBα did not reappear after longer times of stimulation (lanes 14 and 17). We have not detected IκBα in PMA-PHA-stimulated transgenic thymocytes for as long as 6 h after treatment (data not shown). These results indicate that even in the presence of an excess of mIκBβ, a significant fraction of RelA remains complexed to IκBα. Upon stimulation, IκBα is rapidly degraded, releasing the NF-κB complexes responsible for the peak of induction observed in the transgenic cells. After longer times and despite the absence of IκBα, the NF-κB persistent DNA binding activity is inhibited (Fig. 2A and B); therefore, we conclude that mIκBβ can replace IκBα bound to RelA only after signal-induced IκBα degradation.
FIG. 3.
The constitutively active IκBβ is unable to completely displace IκBα bound to RelA. Control (A) and transgenic (B) thymocytes labeled with [35S]methionine for 4 h were treated with PMA-PHA for the indicated periods. The cell extracts were first immunoprecipitated with anti-RelA antibodies, and the RelA-containing immune complexes were denatured and sequentially precipitated with anti-RelA, anti-IκBα, or anti-IκBβ antibodies. The supernatants of the first precipitations were sequentially precipitated with anti-IκBα and anti-IκBβ antibodies.
mIκBβ blocks the expression of some NF-κB-regulated genes.
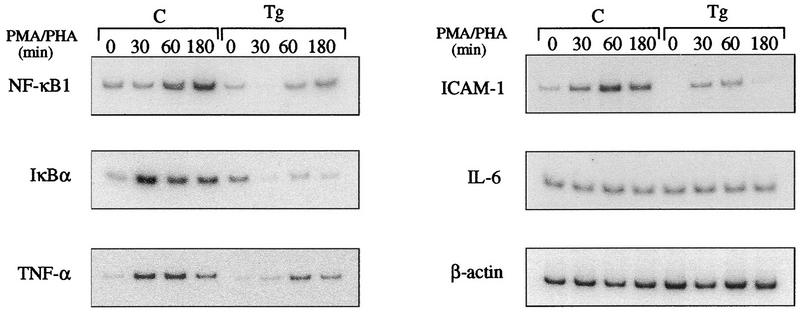
ikba is positively controlled by NF-κB at the transcriptional level (52, 59); therefore, the failure of ikba to reappear in transgenic thymocytes after stimulation could be the result of inhibited NF-κB activity. Another possibility is that the newly synthesized IκBα is not able to displace mIκBβ complexed to NF-κB and therefore is rapidly degraded in the free form. To determine the cause of the failure of IκBα to reappear, thymocytes of control and transgenic mice were stimulated with PMA-PHA and analyzed by reverse transcriptase PCR with primers specific for IκBα and other NF-κB-regulated genes. In contrast to control cells, induction of ikba expression following PMA-PHA stimulation was dramatically reduced in transgenic thymocytes (Fig. 4). Expression of nfkb1, TNF-α, and ICAM-1 are NF-κB dependent since they are also down regulated following stimulation of transgenic thymocytes. The expression of all these genes was significantly increased following stimulation of control cells, while the expression of β-actin, used as an internal control, remained constant (Fig. 4). It is worthwhile noting that the induced, and not the basal, expression of ikba and nfkb1 is affected, since the levels of both proteins were comparable in nonstimulated control and transgenic thymocytes (Fig. 2A, B, and D). On the other hand, IL-6 expression was not affected in stimulated control and transgenic cells, suggesting that, at least in thymocytes, IL-6 is not regulated by NF-κB. These results demonstrate that the failure of IκBα protein to reappear in transgenic thymocytes is due to a decreased gene expression as a consequence of the inhibition of NF-κB activity. In addition, the expression of other NF-κB-regulated genes such as nfkb1, TNF-α, and ICAM-1 is affected by the mIκBβ inhibitory effect on NF-κB activity.
FIG. 4.
Effect of the constitutively active IκBβ on the expression of NF-κB-regulated genes. Total RNA prepared from control (C) or transgenic (Tg) thymocytes stimulated for different periods with PMA-PHA was reverse transcribed, and the cDNAs were amplified by semiquantitative PCR with specific primers (see Materials and Methods).
Animals expressing mIκBβ have a reduced number of peripheral T cells.
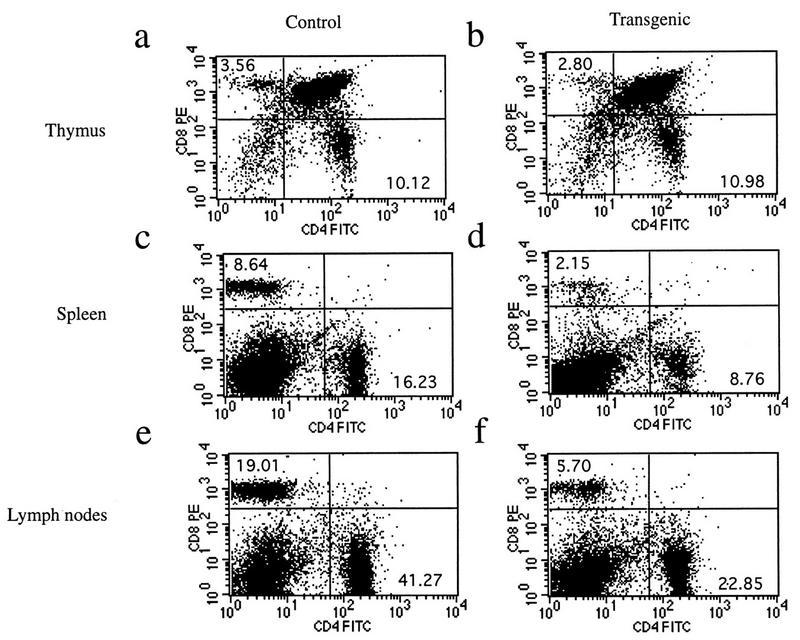
Transgenic animals expressing mIκBβ in T cells breed and develop normally. Histopathological analyses of the thymus, spleen, and lymph node from transgenic mice revealed no abnormalities, with the exception of a decrease in the staining with immunospecific markers (CD4 and CD8) of T-cell areas in both spleen and lymph nodes (data not shown). Fluorescence-activated cell sorter analysis of thymocytes from control and transgenic mice showed that they had comparable ratios of single- and double-positive CD4+/CD8+ cells (Fig. 5a and b). However, CD4+ and CD8+ cell populations from the spleen (Fig. 5c and d) and lymph nodes (Fig. 5e and f) of transgenic animals were dramatically reduced, particularly in the CD8+ compartment. Previous reports with some Rel/NF-κB family null mutant mice, such as nfkb1−/− and c-rel−/−, show impaired T-cell proliferation (36, 60). In addition, it was previously reported that the ligation to the CD28 receptor initiates a potent costimulatory signal that leads to the degradation of IκBβ and the persistent activation of NF-κB (32). To confirm the expression of mIκBβ in transgenic peripheral T cells, we performed EMSA and Western blot analysis of the corresponding extracts. As shown in Fig. 6A, mIκBβ is expressed in peripheral T cells and blocks the inducible NF-κB activity (compare lanes 2 and 4) composed mainly of RelA/p50 heterodimers (compare lanes 5, 6, and 7). The complexes observed in unstimulated extracts are supershifted only by anti-p50 antibodies (data not shown), indicating that they correspond to p50 homodimers. The shift observed in their mobility after stimulation is probably the result of biochemical modifications or a change in their composition. Once we had confirmed the existence of mIκBβ expression in peripheral T cells, we decided to investigate whether the proliferative responses of these cells were altered. To do this, splenic T cells were stimulated with different stimuli for 72 h in the presence of [3H]thymidine. As shown in Fig. 6B, T cells from mIκBβ transgenic mice were able to proliferate similarly to controls when costimulated with anti-CD3 and anti-CD28 antibodies. However, reduced T-cell proliferative responses were detected following stimulation with PMA-PHA or PMA combined with the calcium ionophore A23187. The normal response observed even in the presence of a nondegradable IκBβ indicates that the transient, but not the persistent, NF-κB activity is required for the CD28 costimulation pathway. These results indicate that mice expressing a constitutively active IκBβ have a reduced peripheral T-cell population with impaired T-cell proliferation in response to some but not all mitogenic signals.
FIG. 5.
Transgenic animals show a reduction in the peripheral T-cell compartment. Flow-cytometric analysis of 7-week-old control and transgenic mice is shown. Cell suspensions from thymus (a and b), spleen (c and d), and lymph nodes (e and f) were analyzed for the expression of CD4 and CD8 surface markers. Numbers in each quadrant represent subpopulation percentages. These plots are representative of several experiments. The total cell counts in the thymus, spleen, and lymph nodes of control and transgenic mice are as follows: thymus, (9.6 ± 4.4) × 107 (control) and (14.1 ± 5.7) × 107 (transgenic) (n = 25 animals per genotype); spleen, (7.2 ± 1.1) × 107 and (7.5 ± 2.1) × 107 (n = 14); lymph nodes, (6.0 ± 1.4) × 107 and (4.7 ± 2.0) × 107 (n = 2).
FIG. 6.
Splenic T cells from transgenic animals show impaired proliferation in response to some mitogenic signals. (A) EMSA with a palindromic κB and nuclear extracts from control (C, lanes 1 and 2) or transgenic (Tg, lanes 3 and 4) splenic T cells treated with PMA-PHA for 180 min. Preincubations with preimmune serum (P.I., lane 5) or with the respective antibodies (lanes 6 and 7) allowed the identification of the complexes as p50/RelA heterodimers (arrowhead) and p50-containing complexes, possibly homodimers (arrow). The corresponding cytoplasmic extracts were analyzed by Western blotting with antibodies against IκBα, IκBβ, and LDH. (B) Splenic T cells were stimulated with anti-CD3 plus anti-CD28, PMA-PHA, or PMA-A23187 over a period of 3 days in the presence of 0.5 μCi of [3H]thymidine per 100 μl. Each column represent the results of six independent measurements.
The T-cell-dependent immune response is affected in mice expressing mIκBβ.
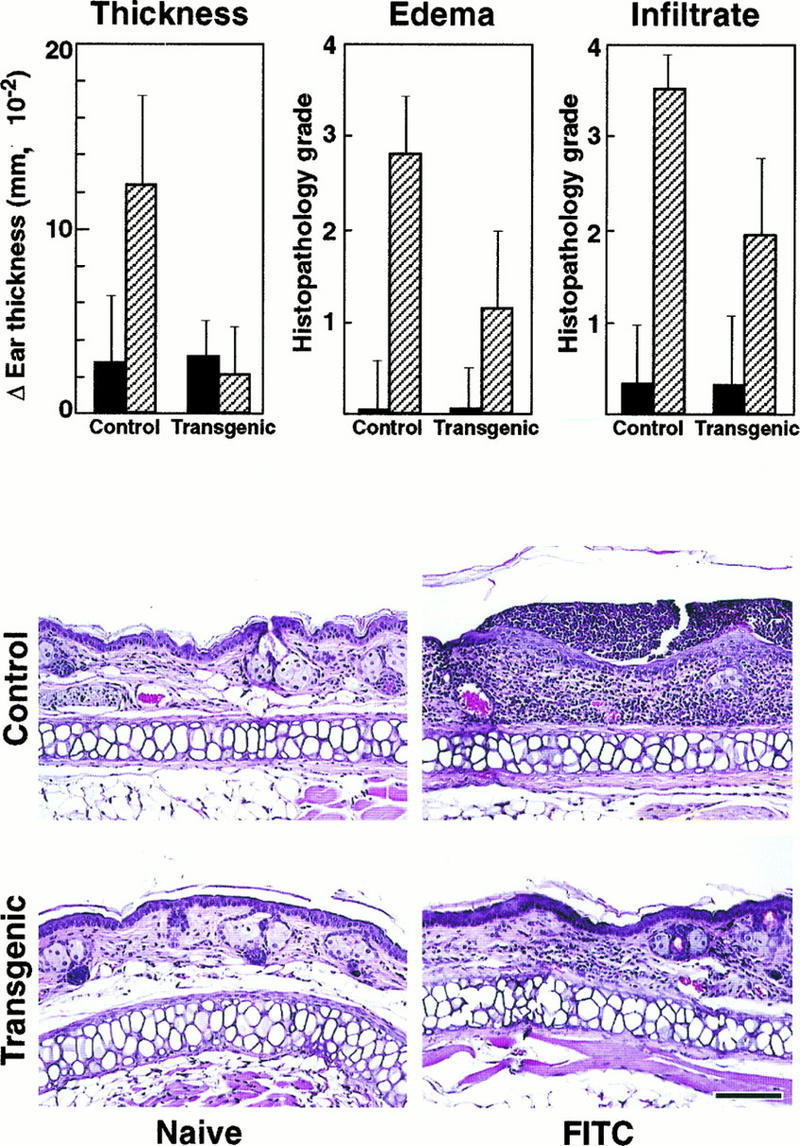
Since the mIκBβ transgenic animals have defects in both T-cell number and in vitro function, we investigated whether they might have impaired immune responses such as the DTH reaction or T-cell-dependent specific antigen antibody production. The first assay involves a contact sensitivity reaction which depends on an intact in vivo function of antigen-specific CD4+ T helper type 1 (Th1) cells (48, 72). For this, four groups of five wild-type and transgenic mice were exposed to a hapten solution (sensitized) or to the solvent (naive). After 1 week of afferent phase, the baseline ear thickness was measured and the ears of all mice were treated epicutaneously with the hapten solution. After 24 h, the ear thickness was measured, the mice were sacrificed, and the ears were prepared for histopathologic grading (Fig. 7).
FIG. 7.
Impaired DTH reaction in mIκBβ transgenic mice. Changes in ear thickness and histopathologic scores for severity of edema and cellular infiltrates in sensitized transgenic mice were significantly lower than in sensitized controls (top panels). Naive control and transgenic mice were histopathologically unremarkable (left middle and bottom panels). Ear lesions in sensitized controls were characterized by a moderate edema and marked transdermal cellular infiltrate (right middle panel); ear lesions in sensitized transgenic mice were similar in nature but significantly less severe (right bottom panel). Sections were graded for severity of edema and cellular infiltrate without knowledge of treatment group as follows: 0, none; 1, minimal; 2, mild; 3, moderate; and 4, marked. Bar, 100 μm.
Naive control and naive transgenic mouse ears were histopathologically nonremarkable (Fig. 7). Sensitized control mice responded strongly to the exogenous antigen as measured in terms of change in ear thickness, edema, and cellular infiltrates (Fig. 7). Ear lesions in sensitized control mice were characterized histopathologically by a moderate dermal edema, a marked mixed cellular infiltrate that extended from the superficial epidermis into the deep dermis, and multifocal microabscesses consisting of discrete neutrophilic aggregates within the epidermis (Fig. 7). In contrast, ear thickness, edema, and cellular infiltrates were significantly reduced in mIκBβ mice in comparison with sensitized controls (Fig. 7).
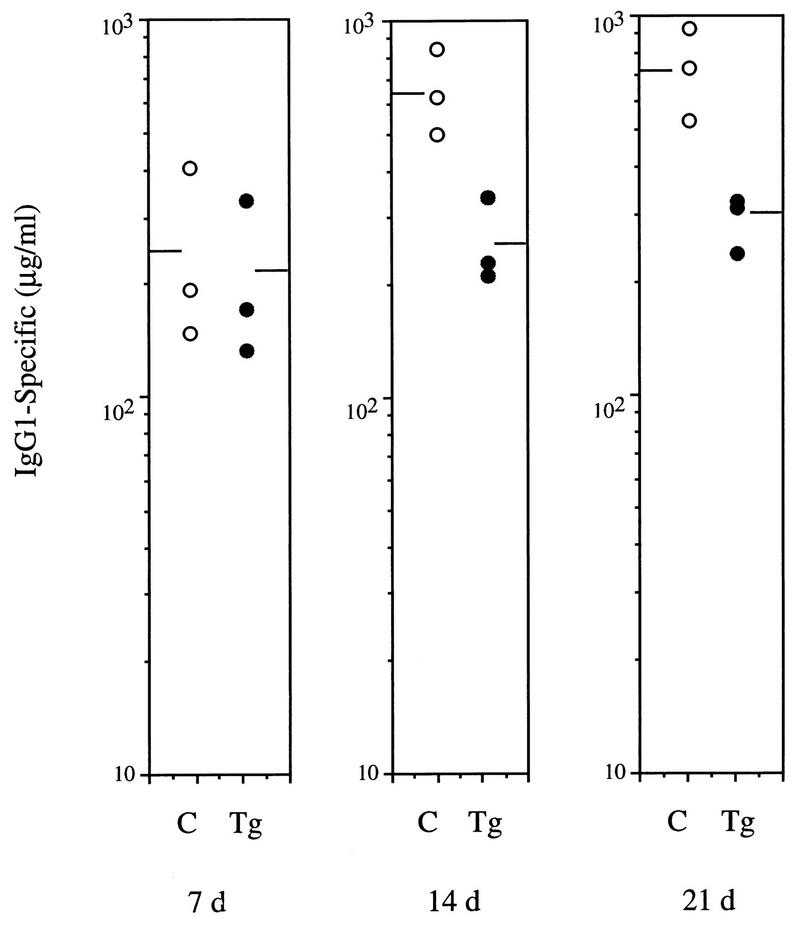
To assess the humoral response to T-cell-dependent antigens, control and transgenic animals were immunized with NP-KLH. The production of anti-NP IgG1 antibodies was determined by ELISA on serum samples obtained 7, 14, and 21 days after immunization. On day 7 after immunization, both control and transgenic mice had comparable levels of specific-NP IgG1; however, serum from transgenic animals obtained on days 14 and 21 showed approximately threefold-lower levels of specific Igs than did serum from control mice (Fig. 8). These data confirm that animals expressing mIκBβ in T cells present impaired immune responses that could be the result of a direct effect of the lack of persistent NF-κB activity on T-cell function and/or a consequence of the altered development of mature T cells observed in these animals.
FIG. 8.
Altered humoral immune response to the T-cell-dependent antigen NP-KLH. Control (C) and transgenic (Tg) mice (three animals per group) were immunized with NP-KLH, and serum samples were collected after 7, 14, and 21 days. Levels of NP-specific IgG1 were determined by ELISA. The levels of NP-specific antibodies in unchallenged controls were below the limits of detection.
DISCUSSION
The Rel/NF-κB/IκB system is induced in response to a variety of challenges in different cells and organisms. Different signal transduction pathways converge in one of the earliest events in Rel/NF-κB regulation, the inducible degradation of the inhibitor molecules that trap the Rel/NF-κB transcription factor in the cytoplasm. In this context, the existence of more than one member in this family of inhibitors invites speculation on the possibility of specific roles for each molecule. The results obtained by the IκBβ gain-of-function approach, i.e., the expression of a constitutively active IκBβ in transgenic mice as presented in this report, provide some answers to essentially two questions. (i) Can IκBβ functionally displace IκBα? (ii) If so, what are the implications of the inhibition of NF-κB activity in T-cell function?
The expression of a constitutively active IκBβ molecule in T cells has no significant effects on the general health of the mice. Stimulation of control and transgenic thymocytes with several agents reveals that only the persistent NF-κB activity, especially in response to PMA-PHA, is totally inhibited by the expression of an IκBβ resistant to inducible degradation. The same observations were made for IL-1 and for anti-CD3/anti-CD28 costimulation (data not shown). Extracts from unstimulated transgenic cells show a modest reduction in the DNA binding of p50 homodimers in comparison with control cells, and this inhibition is not due to differences in the basal protein levels. In stimulated cells, the major inhibitory effect of mIκBβ is on the DNA binding of p50/RelA heterodimers. It is interesting that thymocyte stimulation with PMA-PHA or TNF-α promotes the rapid degradation of both IκBα and IκBβ. In contrast to what has been reported for Jurkat cells (67), we found that TNF-α was clearly able to induce degradation of the endogenous IκBβ in primary T cells, indicating that there must be cell-type-specific differences that affect the involvement of IκBβ in different signal transduction pathways. Surprisingly, analysis of the interaction of RelA with IκBα and IκBβ during the course of stimulation with PMA-PHA shows that even in the presence of an excess of mIκBβ with respect to IκBα (approximately threefold by Western blot analysis [Fig. 2A and B]), the latter is still complexed to RelA. This suggests possible differences in the affinities of the two IκBs for RelA. This observation is supported by the recently reported expression in T cells of transgenic mice of a constitutively active form of IκBα (ΔN), which, like mIκBβ, is also resistant to signal-induced degradation (11). In this case, in contrast to our observations, there was a dramatic decrease in the amount of endogenous IκBα bound to RelA, indicating that the mutant form of IκBα efficiently competes the endogenous wild-type protein (11).
A model has recently been proposed (65), in which after the stimulus-induced degradation of both IκBs, the binding of the newly synthesized unphosphorylated IκBβ to NF-κB blocks the inhibitory effect of the newly synthesized IκBα. This event occurs without masking the nuclear localization signal or preventing the binding of NF-κB to DNA, thereby allowing for persistent NF-κB activity. We observed inhibition of NF-κB DNA binding in cells expressing mIκBβ, suggesting that the IκBβ that accumulated after 3 h of stimulation with PMA-PHA should be phosphorylated. Despite our efforts, we were unable to detect shifts in mIκBβ migration after phosphatase treatment as previously reported (reference 65 and data not shown). The inhibition of the persistent NF-κB activity by mIκBβ resulted in a down regulation of the induced expression of several NF-κB-regulated genes including IκBα, NF-κB1, TNF-α, and ICAM-1. IL-6, on the other hand, was not affected, suggesting that at least in thymocytes, IL-6 expression does not require a persistent NF-κB activity.
Transgenic mice have a marked reduction in the number of T cells, especially CD8+ cells, in peripheral lymphoid organs. Interestingly, this asymmetry in the susceptibility of CD4+ and CD8+ single-positive cells to the inhibition of NF-κB has also been observed in transgenic mice expressing the constitutively active IκBα (11) and in transgenic mice overexpressing a wild-type human IκBα under the regulation of the human β-globin promoter and the 3′ locus control element of the human CD2 gene (27). In contrast to mIκBβ, the transgenic expression of both forms of IκBα promotes a decrease in the thymic CD8+ population, also compromising the CD4+ population in the case of the overexpression of the wild-type IκBα (11, 27). These data suggest that the transient peak of NF-κB activity observed in thymocytes expressing mIκBβ would be sufficient for the activation of the pathways involved in either the maturation or the survival of CD8+ single-positive cells in the thymus. We then determined whether the remaining T cells also have impaired proliferative responses, like the T cells of mice lacking some of the Rel/NF-κB members (36, 60). We found that transgenic splenic T cells were able to respond to costimulation with anti-CD3/anti-CD28 antibodies similarly to the response seen in control cells, suggesting that the transient peak of NF-κB activity was sufficient to trigger this response. On the other hand, transgenic T cells had impaired proliferation in response to PMA-PHA or PMA-A23187. Recent reports documented the role of NF-κB in the prevention of apoptosis in different experimental models, such as RelA-deficient hepatocytes and fibroblasts (8, 10), human fibroblasts or Jurkat lymphomas infected with retrovirus encoding a dominant-negative IκBα containing both N- and C-terminal mutations (69), and a stable human fibrosarcoma cell line expressing the IκBα counterpart of mIκBβ (71). Moreover, the overexpression of the constitutively active IκBα has been shown to enhance apoptosis of both CD4+ and CD8+ in response to primary T-cell receptor stimulation (11).
The T-cell-dependent immune responses, such as DTH, are affected in mIκBβ transgenic animals. In contact hypersensitivity reactions, Langerhans cells migrate to the regional lymph node, where they present the hapten, recruiting and activating antigen-specific T cells. These activated T cells may be responsible for the ear swelling in mice and contact dermatitis in humans (for a review, see reference 48). The significant reduction in the ear inflammation observed in the transgenic mice reflects the reduction in the number of T cells in lymph nodes and possibly the impaired activation of the remaining cells. In humoral T-cell-dependent responses, the antigen induces switching in conjunction with contact-dependent signals from T cells and signals from cytokines; mice expressing mIκBβ showed lower levels of specific IgG1, reflecting the alterations in T-cell function.
Finally, this report shows that although IκBβ did not completely displace IκBα from RelA complexes in unstimulated T cells, it did block the persistent NF-κB activity after IκBα-induced degradation. In contrast to individual Rel/NF-κB member knockout approaches, where more that one cell type is affected, the IκBβ gain-of-function approach allowed us to address the importance of the persistent NF-κB activity in the proper function of T cells in immune responses. Our findings, together with the results of similar studies (11, 27), strongly support the involvement of NF-κB transcriptional activity in T-lineage development. Future studies, involving null mutations of the newly cloned IκB proteins, IκBβ, IκBɛ, IκB-R, and IκB-L, will be essential for understanding the functional roles of the individual members of this family of proteins in the regulation of NF-κB.
ACKNOWLEDGMENTS
We are grateful to Carol S. Ryan, Mavis Swerdel, Alice Lee, Sergio Lira, all the staff in Veterinary Sciences at BMS, and Debra S. Barton for their excellent technical assistance. We also thank Kenneth Class for flow cytometry, Willy Kratil for artwork, and James K. Loy for photoimaging. We also thank Daniel Carrasco, Violetta Iotsova, Jorge Caamaño, Rolf-Peter Ryseck, and Elizabeth Galbreath for valuable comments on the manuscript.
REFERENCES
- 1.Albertella M R, Campbell R D. Characterization of a novel gene in the human major histocompatibility complex that encodes a potential new member of the IkB family of proteins. Hum Mol Genet. 1994;3:793–799. doi: 10.1093/hmg/3.5.793. [DOI] [PubMed] [Google Scholar]
- 2.Alkalay I, Yaron A, Hatzubai A, Jung S, Avraham A, Gerlitz O, Pashut-Lavon I, Ben-Neriah Y. In vivo stimulation of IκB phosphorylation is not sufficient to activate NF-κB. Mol Cell Biol. 1995;15:1294–1301. doi: 10.1128/mcb.15.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J M, Forbush K A, Perlmutter R M. Functional dissection of the lck proximal promoter. Mol Cell Biol. 1992;12:2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 5.Baldi L, Brown K, Franzoso G, Siebenlist U. Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of IkBa. J Biol Chem. 1996;271:376–379. doi: 10.1074/jbc.271.1.376. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin A S. The NF-κB and IκB proteins, new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Baldwin A S., Jr The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 8.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 9.Beg A A, Sha W C, Bronson R T, Baltimore D. Constitutive NF-κB activation, enhanced granulopoiesis, and neonatal lethality in IκBα-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 10.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–169. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 11.Boothby M R, Mora A L, Scherer D C, Brockman J A, Ballard D W. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-κB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U. The oncoprotein Bcl-3 directly transactivates through κB motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 13.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 15.Caamano J H, Perez P, Lira S A, Bravo R. Constitutive expression of Bcl-3 in thymocytes increases the DNA binding of NF-κB (p50) homodimers in vivo. Mol Cell Biol. 1996;16:1342–1348. doi: 10.1128/mcb.16.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capobianco A J, Chang D, Mosialos G, Gilmore T D. p105, the NF-κB p50 precursor protein, is one of the cellular proteins complexed with the v-Rel oncoprotein in transformed chicken spleen cells. J Virol. 1992;66:3758–3767. doi: 10.1128/jvi.66.6.3758-3767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrasco D, Rizzo C A, Dorfman K, Bravo R. The v-rel oncogene promotes malignant T-cell leukemia/lymphoma in transgenic mice. EMBO J. 1996;15:3640–3650. [PMC free article] [PubMed] [Google Scholar]
- 18.Chase M W. Production of antiserum. Methods Immunol Immunochem. 1967;1:197–209. [Google Scholar]
- 19.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of the IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 21.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Isolation and fractionation of mononuclear cell populations. In: Sons J W, editor. Current protocols in immunology. New York, N.Y: Greene Publishing Associates & Wiley-Interscience; 1992. pp. 3.1.1–3.1.5. [Google Scholar]
- 22.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strover W. In vivo assays for mouse lymphocyte function. In: Sons J W, editor. Current protocols in immunology. New York, N.Y: Greene Publishing Associates & Wiley-Interscience; 1992. pp. 4.2.1–4.2.5. [Google Scholar]
- 23.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Didonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 25.Dobrzanski P, Ryseck R-P, Bravo R. Both N- and C-terminal domains of RelB are required for full transactivation: role of the N-terminal leucine zipper-like motif. Mol Cell Biol. 1993;13:1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrzanski P, Ryseck R-P, Bravo R. Specific inhibition of RelB/p52 transcriptional activity by the C-terminal domain of p100. Oncogene. 1995;10:1003–1007. [PubMed] [Google Scholar]
- 27.Esslinger C W, Wilson A, Sordat B, Beermann F, Jongeneel V. Abnormal T lymphocyte development induced by targeted overexpression of I-kappa-B-alpha. J Immunol. 1997;158:5075–5078. [PubMed] [Google Scholar]
- 28.Finco T S, Baldwin A S. Mechanistic aspects of NF-κB regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 29.Fujita T, Nolan G P, Liou H-C, Scott M L, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-κB p50 homodimers. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore T D, Morin P J. The IκB proteins: members of a multifunctional family. Trends Genet. 1993;9:427–433. doi: 10.1016/0168-9525(93)90106-r. [DOI] [PubMed] [Google Scholar]
- 31.Grilli M, Chiu J-S, Lenardo M J. NF-κB and Rel—participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 32.Harhaj E W, Maggirwar S B, Good L, Sun S-C. CD28 mediates a potent costimulatory signal for rapid degradation of IκBβ which is associated with accelerated activation of various NF-κB/Rel heterodimers. Mol Cell Biol. 1996;16:6736–6743. doi: 10.1128/mcb.16.12.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haskill S, Beg A A, Tompkins S M, Morris J S, Yurochko A D, Sampson-Johanne A, Mondal K, Ralph P, Baldwin A S. Characterization of an immediate early gene induced in adherent monocytes that encodes IκB-like activity. Cell. 1991;65:1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- 34.Inoue J-I, Kerr L D, Kakizuka A, Verma I M. IκBγ, a 70 kd protein identical to the C-terminal half of p110 NF-κB: a new member of the IκB family. Cell. 1992;68:1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 35.Klement J F, Rice N R, Car B D, Abbondanzo S J, Powers G D, Bhatt H, Chen C-H, Rosen C A, Stewart C L. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Köntgen F, Grumont R J, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 37.Kopp E B, Ghosh S. NF-κB and Rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 38.Kovary K, Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991;11:2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 40.Lalor P A, Nossal G J V, Sanderson R D, McHeyzer-Williams M G. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl) acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur J Immunol. 1992;22:3001–3011. doi: 10.1002/eji.1830221136. [DOI] [PubMed] [Google Scholar]
- 41.Lang G, Mamalaki C, Greenberg D, Yannoutsos N, Kioussis D. Deletion analysis of the human CD2 gene locus control region in transgenic mice. Nucleic Acids Res. 1991;19:5851–5856. doi: 10.1093/nar/19.21.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lernbecher T, Müller U, Wirth T. Distinct NF-κB/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993;365:767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- 43.Lewis D B, Yu C C, Forbush K A, Carpenter J, Sato T A, Grossman A, Liggitt D H, Perlmutter R M. Interleukin 4 expressed in situ selectively alters thymocyte development. J Exp Med. 1991;173:89–100. doi: 10.1084/jem.173.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liou H-C, Baltimore D. Regulation of the NF-κB/rel transcription factor and κB inhibitor system. Curr Opin Cell Biol. 1993;5:477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 45.McKinsey T A, Brockman J A, Scherer D C, Al-Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by the Tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercurio F, DiDonato J A, Rosette C, Karin M. p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes Dev. 1993;7:705–718. doi: 10.1101/gad.7.4.705. [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto S, Verma I M. Rel/NF-κB story. Adv Cancer Res. 1995;66:255–292. [PubMed] [Google Scholar]
- 48.Muller H K, Bucana C, Kripke M L. Antigen presentation in the skin: modulation by u.v. radiation and chemical carcinogens. Semin Immunol. 1992;4:205–215. [PubMed] [Google Scholar]
- 49.Naumann M, Nieters A, Hatada E N, Scheidereit H C. NF-κB precursor p100 inhibits nuclear translocation and DNA binding of NF-κB/rel-factors. Oncogene. 1993;8:2275–2281. [PubMed] [Google Scholar]
- 50.Naumann M, Wulczyn F G, Scheidereit C. The NF-κB precursor p105 and the proto-oncogene product Bcl-3 are IκB molecules and control nuclear translocation of NF-κB. EMBO J. 1993;12:213–222. doi: 10.1002/j.1460-2075.1993.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nolan G P, Fujita K, Bhatia K, Huppi C, Lio H-C, Scott M L, Baltimore D. The bcl-3 proto-oncogene encodes a nuclear IκB-like molecule that preferentially interacts with NF-κB p50 and p52 in a phosphorylation-dependent manner. Mol Cell Biol. 1993;13:3557–3566. doi: 10.1128/mcb.13.6.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez P, Lira S A, Bravo R. Overexpression of RelA in transgenic mouse thymocytes: specific increase on levels of the inhibitor IκBα. Mol Cell Biol. 1995;15:3523–3530. doi: 10.1128/mcb.15.7.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray P, Zhang D-H, Elias J A, Ray A. Cloning of a differentially expressed IκB-related protein. J Biol Chem. 1995;270:10680–10685. doi: 10.1074/jbc.270.18.10680. [DOI] [PubMed] [Google Scholar]
- 54.Régnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 55.Rice N R, MacKichan M L, Israel A. The precursor of NF-κB p50 has IκB-like functions. Cell. 1992;71:243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- 56.Scheinman R I, Beg A A, Baldwin A S J. NF-κB p100 (Lyt-10) is a component of H2TF1 and can function as an IκB-like molecule. Mol Cell Biol. 1993;13:6089–6101. doi: 10.1128/mcb.13.10.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Signal-induced degradation of IκBα requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with “mini-extracts,” prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott M L, Fujita T, Liou H-C, Nolan G P, Baltimore D. The p65 subunit of NF-κB regulates IκB by two distinct mechanisms. Genes Dev. 1993;7:1266–1276. doi: 10.1101/gad.7.7a.1266. [DOI] [PubMed] [Google Scholar]
- 60.Sha W C, Liou H-C, Tuomanen E I, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 61.Siebenlist U, Franzoso G, Brown K. Structure, regulation, and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 62.Singh H, Sen R, Baltimore D, Sharp P A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986;319:154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- 63.Sun S-C, Ganchi P A, Beraud C, Ballard D W, Greene W C. Autoregulation of the NF-κB transactivator RelA (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc Natl Acad Sci USA. 1994;91:1346–1350. doi: 10.1073/pnas.91.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun S C, Elwood J, Greene W C. Both amino- and carboxy-terminal sequences within IκBα regulate its inducible differentiation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 67.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. IκBβ regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 68.Traenckner E B-T, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 70.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 71.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 72.Weih F, Carrasco D, Durham S K, Barton D S, Rizzo C A, Ryseck R-P, Lira S A, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 73.Weih F, Lira S A, Bravo R. Overexpression of RelB in transgenic mice does not affect IκBα levels: differential regulation of RelA and RelB by the inhibitor protein. Oncogene. 1996;12:445–459. [PubMed] [Google Scholar]
- 74.Weih F, Ryseck R-P, Chen L, Bravo R. Apoptosis of nur77/N10-transgenic thymocytes involves the Fas/Fas ligand pathway. Proc Natl Acad Sci USA. 1996;93:5533–5538. doi: 10.1073/pnas.93.11.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whiteside S T, Epinat J-C, Rice N R, Israël A. I kappa B epsilon, a novel member of the IκB family, controls RelA and cRel NF-κB activity. EMBO J. 1997;16:1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whiteside S T, Ernst M K, LeBail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wulczyn F G, Naumann M, Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-κB. Nature. 1992;358:597–599. doi: 10.1038/358597a0. [DOI] [PubMed] [Google Scholar]