Abstract
Background
Frailty is a widely accepted predictor of health outcomes in patients including the critically ill. Biological age is also increasingly recognized as a determinant of chronic health outcomes. Whether these factors are independently predictive of mortality among the critically ill is unknown. We assessed whether biological age, measured as PhenoAge at Intensive Care Unit (ICU) admission, predicts mortality in critically ill patients independent of the Clinical Frailty Scale (CFS).
Methods
This single-center retrospective cohort study included adult patients with available CFS and PhenoAge data at admission to ICU, excluding patients with incomplete records for key variables. The Levine PhenoAge model was used to estimate each patient’s biological age (PhenoAge). PhenoAge was then calibrated to generate a regression residual to reflect excessive biological age unexplained by chronological age.
Results
Of the 1,073 critically ill adult patients analyzed, 117 died (10.9%) before hospital discharge. PhenoAge and CFS were significantly correlated (correlation coefficient, 0.235; P=0.001). PhenoAge (receiver operating characteristic curve [AUROC], 0.622) and its residuals (AUROC, 0.627) and CFS (AUROC, 0.601) were predictive of hospital mortality, with no significant differences in their ability to differentiate between survivors and non-survivors (paired comparison to CFS: P=0.586 and P=0.537, respectively). PhenoAge interacted with frailty in its effect on mortality (P=0.004) which was particularly prominent among those who were not clinically frail (CFS ≤3).
Conclusions
PhenoAge and CFS, both measured at ICU admission, independently predicted hospital mortality. PhenoAge showed a notable interaction with frailty, particularly in non-frail patients.
Keywords: aging, critical care outcomes, DNA methylation, frailty, prognosis
INTRODUCTION
Frailty is well recognized as an important predictor of short- and long-term health outcomes [1-3]. Increasing chronological age is associated with an increased risk of frailty; nonetheless, frailty can also occur in individuals who are not chronologically old for reasons that have not been fully established. Recent research has shown that biological (or phenotypical) age and chronological age can diverge substantially over time between different people, with those who age faster biologically considered to have accelerated biological aging [4]. The mechanistic bases for accelerated aging are likely multiple, and may include DNA methylation, spontaneous somatic mutations such as clonal hematopoiesis of indeterminate potential, mitochondrial dysfunction, and excessive endoplasmic reticulum stress [5,6]. Regardless of the pathogenic mechanisms that lead to developing accelerated aging, accelerated aging compared to chronological age, as measured by epigenetic biomarkers of DNA methylation, has been shown to be a strong predictor of long-term health outcomes, above and beyond a combination of risk factors including chronological age, body mass index, socioeconomic status, and comorbidities [7,8]. Both prevalence and change in frailty over a 3-year period have also been associated with accelerated biological (or epigenetic) aging [9].
Although accelerated biological aging has been accepted as a determinant of long-term health status, its impact on outcomes of patients in the acute care setting has only recently been recognized [10]. As frailty is important in determining outcomes among the critically ill, it is pivotal to determine whether frailty is related to accelerated biological aging in the acute care setting, and how these two factors interact to affect mortality outcomes. We hypothesized that biological age exceeding chronological age measured at intensive care unit (ICU) admission was associated with frailty, and that these two factors might interact and remain predictive of mortality among the critically ill despite adjustments for other important predictors. In this study, we assessed the relationship between biological aging and frailty data at ICU admission and determined their independent abilities to predict mortality among the critically ill.
MATERIALS AND METHODS
We followed the standard ethical and data protection procedures in accordance with the approving committees and with the Helsinki Declaration of 1975. This sub-study was approved by the Quality Improvement Unit and ICU Clinical Information System Administrative Committee at Fiona Stanley Hospital as a further analysis of an existing study (No. 49386), with written informed consent waived.
This single-center retrospective cohort study was a sub-study of a recently completed project that examined biological age as a predictor of mortality of the critically ill [10]. The study site is a tertiary teaching hospital in Perth, Western Australia; its 40-bed ICU admits critically ill patients of most major medical and surgical specialties including medical and surgical oncology, burns, cardiac surgery, and heart and lung transplantation. In this study, de-identified administrative health data for critically ill patients admitted to the ICU between June 2015 and June 2021 were extracted from the Clinical Information System MetaVision in March 2023 (Figure 1).
Figure 1.

Flowchart of study inclusions and exclusions. ICU: intensive care unit.
We calculated the biological age (or “PhenoAge”) of our study patients according to the equations described by Levine et al. [7,8,11] using data from the third National Health and Nutrition Examination Survey (NHANES) III and IV studies. In brief, nine blood biomarkers that are reflective of DNA methylation (C-reactive protein, glucose concentration, mean red blood cell volume, red blood cell distribution width, albumin concentration, creatinine concentration, lymphocyte percentage, alkaline phosphatase, and white cell count) were used in conjunction with chronological age to construct the Levine’s PhenoAge model. Because increasing chronological age can confound the accuracy of PhenoAge [7], we calculated the PhenoAge residuals by regressing PhenoAge on chronological age. Patients who had a positive regression residual were considered to have accelerated aging or PhenoAgeAccel. Accelerated aging occurs when one is biologically older than his/her chronological age according to DNA methylation biomarkers. Patients without prospectively collected frailty data and the full set of nine blood tests within 24 hours of ICU admission to estimate their PhenoAge were excluded. For patients who had the nine blood tests repeated within the first 24 hours of ICU admission, only those tests conducted closest to the ICU admission time were used for consistency.
We used the Clinical Frailty Scale (CFS) for assessing frailty [12]. According to the ability of the patients to perform their daily physical activities, CFS can range from 1 (very fit) to 9 (terminally ill with a life expectancy less than 6 months). These data were assessed and recorded prospectively on admission to the ICU for our study patients prior to initiation of this study. Both PhenoAge and CFS were assessed based on patient condition at ICU admission, potentially capturing a mix of chronic biological aging and acute physiological stress rather than habitual baseline. Only patients with recorded CFS and PhenoAge data were included to ensure reliable assessment of frailty and biological age, as these were the primary variables of interest. Patients without these data were excluded to avoid bias from incomplete records. We compared the median Acute Physiology and Chronic Health Evaluation (APACHE) II score in the study cohort with the master cohort including those without CFS to assess whether selection bias was substantial [10].
Statistical Analyses
First, we analyzed differences in characteristics between hospital survivors and non-survivors using univariable analyses, such as chi-square and Mann-Whitney tests. Area under the receiver operating characteristic curve (AUROC) was used to quantify the ability of the CFS and PhenoAge residuals for differentiating between survivors and non-survivors. We assessed the quantitative associations between PhenoAge residuals and CFS using Spearman correlation coefficients. Second, we assessed whether there were interactions between CFS and PhenoAge residuals in their associations with mortality using a two-way analysis of variance (ANOVA) test. Third, the ability of CFS and PhenoAge residuals for predicting mortality were assessed in a Cox proportional hazards regression model including an interaction term between PhenoAgeAccel and CFS if this was deemed necessary from the two-way ANOVA result. Finally, we repeated the Cox regression by adjusting for other covariates that are biologically plausible to contribute to an increase in mortality, including chronological age, the number of severe comorbidities (according to the APACHE II model definitions), diabetes mellitus and severe acute illness as evidenced by an APACHE II score greater than 15. Sensitivity analysis using a spline function to allow non-linearity of CFS was conducted to confirm robustness of our results.
The sample size of the current study was determined and limited by the availability of prospectively collected CFS data. With over 1,000 patients and mortality of over 10%, the current study would have 80% post hoc statistical power to confirm an AUROC >0.60 to predict mortality by the PhenoAge residuals or CFS. In this study, all data were analyzed using R software version 4.3.1, 2023 (R Foundation for Statistical Computing) [13] and MedCalc version 22.009, 2023 (MedCalc Software) [14], and a P-value <0.05 without Bonferroni adjustment was taken as significant.
RESULTS
Of 2,950 patients admitted to the ICU during the study period who had their PhenoAges computed, 1,073 (36.4%) also had prospectively collected CFS data of whom 117 (10.9%) died after admission. The median APACHE II score of the current cohort was 16 (which was not substantially different from that of the master cohort: median APACHE II score, 15) [10]. Similarly, patients with frailty data (n=1,877) and without frailty data were not significantly different in age (P=0.952), sex (P=0.370), or hospital mortality (with frailty data: 10.9% vs. 9.3%; P=0.152), suggesting that missing data did not introduce substantial systematic bias.
Chronological age was not significantly different between survivors and non-survivors (P=0.117). As expected, CFS was significantly higher among the non-survivors compared to survivors. The non-survivors were significantly older biologically, as measured by either crude PhenoAge (P=0.001) or regression-generated PhenoAge residuals (P=0.001), compared to survivors (Table 1, Figure 2). PhenoAge residuals were significantly correlated with CFS (correlation coefficient, 0.235; 95% CI, 0.177–0.290; P=0.001). PhenoAge (AUROC, 0.622; 95% CI, 0.568–0.676) and its residuals (AUROC, 0.627; 95% CI, 0.573–0.680) and CFS (AUROC, 0.601; 95% CI, 0.544–0.658) were all predictive of hospital mortality, and there were no significant differences in their abilities to differentiate between survivors and non-survivors (paired comparison to the CFS: P=0.586 and P=0.537, respectively). As expected, the median APACHE II score was significantly higher among the non-survivors compared to the survivors (25 vs. 15, respectively) and non-elective surgery and chronic respiratory diseases were more common among those who died after ICU admission.
Table 1.
Descriptive characteristics of the cohort and differences between hospital survivors and non-survivors
| Variable | All patients (n=1,073) | Survivor (n=956, 89.1%) | Non-survivor (n=117, 10.9%) | P-valued) |
|---|---|---|---|---|
| Chronological age (yr) | 63 (49 to 72) | 63 (49 to 72) | 65 (55 to 73) | 0.117 |
| Clinical Frailty Scalea) | 3 (3 to 4) | 3 (3 to 4) | 4 (3 to 6) | 0.001 |
| Male | 631 (58.8) | 562 (58.8) | 69 (59.0) | 0.999 |
| Body mass index (kg/m2) | 28 (24 to 33) | 28 (25 to 33) | 28 (24 to 32) | 0.388 |
| PhenoAge (yr) | 92.4 (77.4 to 109.4) | 91.5 (76.1 to 107.7) | 102.5 (87.2 to 121.0) | 0.001 |
| Crude difference between PhenoAge and chronological age (yr) | 29.3 (19.5 to 41.0) | 28.4 (19.3 to 40.1) | 36.3 (25.7 to 49.6) | 0.001 |
| PhenoAge residuals (yr) | –2.7 (–12.1 to 9.4) | –3.7 (–12.3 to 8.7) | 5.4 (–5.2 to 18.7) | 0.001 |
| PhenoAgeAccelb) | 478 (44.5) | 404 (42.3) | 74 (63.2) | 0.001 |
| Elective surgical admission | 307 (28.6) | 297 (31.1) | 10 (8.5) | 0.001 |
| Chronic respiratory disease | 56 (5.2) | 42 (4.4) | 14 (12.0) | 0.001 |
| Chronic cardiovascular diseasec) | 103 (9.6) | 88 (9.2) | 15 (12.8) | 0.277 |
| End-stage renal failurec) | 40 (3.7) | 36 (3.8) | 4 (3.4) | 0.999 |
| Cirrhosisc) | 25 (2.3) | 21 (2.2) | 4 (3.4) | 0.615 |
| Immune diseasec) | 25 (2.3) | 21 (2.2) | 4 (3.4) | 0.615 |
| Immunosuppressedc) | 88 (8.2) | 73 (7.6) | 15 (12.8) | 0.080 |
| Lymphomac) | 21 (2.0) | 18 (1.9) | 3 (2.6) | 0.882 |
| Leukemiac) | 25 (2.3) | 21 (2.2) | 4 (3.4) | 0.615 |
| Metastatic cancerc) | 64 (6.0) | 60 (6.3) | 4 (3.4) | 0.305 |
| AIDSc) | 1 (0.1) | 1 (0.1) | 0 | 0.999 |
| Diabetes mellitus | 245 (22.8) | 224 (23.4) | 21 (17.9) | 0.224 |
| Admission diagnosis | 0.001 | |||
| Cardiac surgery | 38 (3.5) | 35 (3.7) | 3 (2.6) | |
| Burns | 10 (0.9) | 8 (0.8) | 2 (1.7) | |
| Liver or pancreatic surgery | 136 (12.7) | 132 (13.8) | 4 (3.4) | |
| Upper gastrointestinal tract surgery | 72 (6.7) | 69 (7.2) | 3 (2.6) | |
| Vascular surgery | 16 (1.5) | 14 (1.5) | 2 (1.7) | |
| Urological surgery | 6 (0.6) | 6 (0.6) | 0 | |
| Pneumonia/aspiration | 102 (9.5) | 84 (8.8) | 18 (15.4) | |
| Sepsis | 299 (27.9) | 268 (28.0) | 31 (26.5) | |
| Cardiac arrest | 41 (3.8) | 25 (2.6) | 16 (13.7) | |
| Bowel obstruction/perforation | 43 (4.0) | 42 (4.4) | 0 (0.9) | |
| Drug overdoses/self-harm | 23 (2.1) | 23 (2.4) | 0 | |
| Stroke/coma/seizures | 59 (5.5) | 51 (5.3) | 8 (6.8) | |
| Obstructive airway or interstitial lung disease | 33 (3.1) | 30 (3.1) | 3 (2.6) | |
| Acute kidney injury | 21 (2.0) | 18 (1.9) | 3 (2.6) | |
| Cardiogenic shock/heart failure | 49 (4.6) | 37 (3.9) | 12 (10.3) | |
| Others | 55 (5.1) | 55 (5.8) | 50 (42.7) | |
| APACHE II score | 16 (11.0 to 21.0) | 15 (11.0 to 20.0) | 25 (19.0 to 31.0) | 0.001 |
| APACHE II score >15 | 544 (50.7) | 445 (46.5) | 99 (84.6) | 0.001 |
| Invasive ventilation within 24 hours of admission | 441 (41.1) | 357 (37.3) | 84 (71.8) | 0.001 |
| Requiring vasopressor within 24 hours of admission | 555 (51.7) | 461 (48.2) | 94 (80.3) | 0.001 |
| Requiring renal replacement therapy within 24 hours of admission | 95 (8.9) | 66 (6.9) | 29 (24.8) | 0.001 |
| ICU stay (day) | 3 (1 to 5) | 3 (1 to 5) | 4 (1 to 7) | 0.017 |
| Hospital stay (day) | 10 (7 to 19) | 11 (7 to 20) | 6 (2 to 15) | 0.001 |
Values are presented as median (interquartile range) or number (%).
AIDS: Acquired immunodeficiency syndrome; APACHE: Acute Physiology and Chronic Health Evaluation; ICU: intensive care unit.
Clinical Frailty Scale score is 1-9;
PhenoAgeAccel existed when one’s PhenoAge was older than the corresponding chronological defined by a positive PhenoAge residual after regressing PhenoAge of all study patients on their corresponding chronological age;
Comorbidities are defined according to the APACHE II model.
P-values were generated by Mann-Whitney or chi-square test.
Figure 2.
Error bar (indicating 95% CI) plots showing the difference in Clinical Frailty Scale and PhenoAge residuals between survivors and non-survivors.
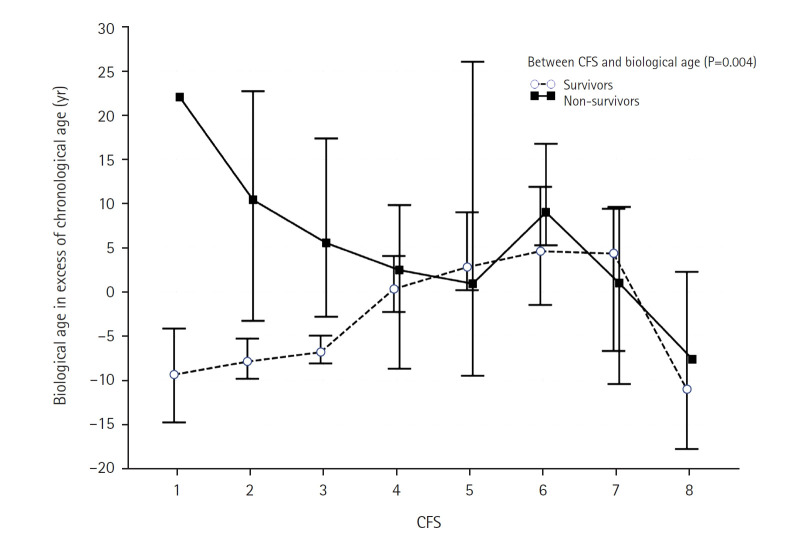
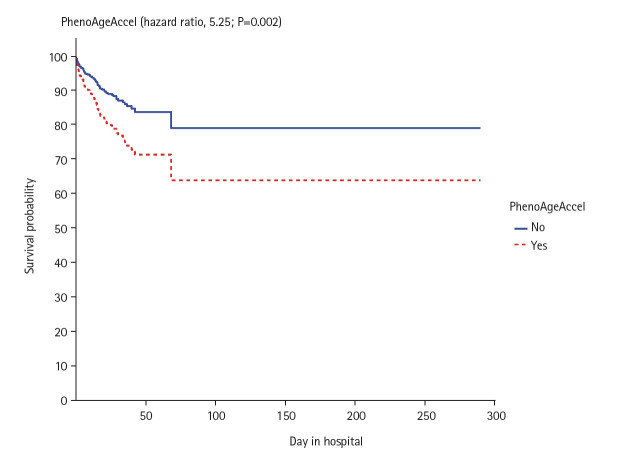
In the 2-way ANOVA analysis, we found that PhenoAge residuals had an interactive effect with CFS in relation to hospital mortality (P=0.004) (Figure 3), in particular for those who had CFS ≤3. The independent effects of CFS, PhenoAgeAccel, and the interactive term between these two factors on mortality were then analyzed by a Cox regression model, which showed that CFS, PhenoAgeAccel, and their interactive terms were all predictive of mortality. This was illustrated by differences in survival time between those with and without PhenoAgeAccel after adjusting for CFS and the interaction term between CFS and PhenoAgeAccel (Figure 4), and differences in survival time between those with different CFS after adjusted for PhenoAgeAccel and the interaction term between CFS and PhenoAgeAccel (Supplementary Figure 1).The significance of the CFS (hazard ratio [HR] per grade increment from CFS 1, 1.26; 95% CI, 1.05–1.50; P=0.011) and PhenoAgeAccel (HR, 3.17; 95% CI, 1.07–9.34; P=0.037) on mortality remained unchanged after adjustments for the number of severe comorbidities, diabetes mellitus, chronological age, and APACHE II score >15 (Supplementary Figure 2, Table 2). Using a spline function to allow non-linearity in CFS also did not change the statistical significance of the interaction term between PhenoAge and CFS.
Figure 3.
Differences in PhenoAge residuals between survivors and non-survivors stratified by Clinical Frailty Scale (CFS).
Figure 4.
Differences in survival time between those with and without PhenoAgeAccel after adjusted for Clinical Frailty Scale (CFS) and the interaction term between CFS and PhenoAgeAccel.
Table 2.
Cox proportional hazards regression models for assessing the independent significance of CFS and PhenoAgeAccel on hospital mortality of the critically ill (n=1,073)
| Predictor in the model | Parsimony Cox model |
P-value | Fully-adjusted Cox model |
P-value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| CFS (HR per grade increment) | 1.36 (1.15–1.62) | 0.001 | 1.26 (1.05–1.50) | 0.011 |
| PhenoAgeAccel | 5.25 (1.81–15.28) | 0.002 | 3.17 (1.07–9.34) | 0.037 |
| CFS×PhenoAgeAccel (interaction term) | 0.76 (0.60–0.96) | 0.023 | 0.81 (0.64–1.03) | 0.084 |
| Chronological age | - | - | 1.00 (0.99–1.02) | 0.594 |
| APACHE II score >15 | - | - | 3.89 (2.26–6.71) | 0.001 |
| No. of severe APACHE II comorbidities | - | - | 0.92 (0.70–1.19) | 0.515 |
| Diabetes mellitus | - | - | 0.57 (0.33–1.01) | 0.053 |
The c-index of the parsimony and fully adjusted models were 0.650 and 0.734, respectively.
CFS: Clinical Frailty Scale; HR: hazard ratio; APACHE: Acute Physiology and Chronic Health Evaluation.
DISCUSSION
In this study we found that PhenoAgeAccel and frailty, assessed at ICU admission, were correlated quantitatively and both predictive of mortality, with an interactive effect. PhenoAgeAccel measured at ICU admission may reflect a combination of chronic biological aging and acute physiological stress, although it remains unclear whether it captures long-term aging or acute illness effects alone. These results have clinical and research implications and require further discussion.
First, recent advances have elucidated possible mechanisms of aging and the importance of accelerated biological aging especially for those who are not old chronologically [5,6]. The Levine PhenoAge model is a second-generation machine-learning biological age estimation algorithm that has been validated to be predictive of long-term health outcomes. In this study, we found that frailty was correlated with PhenoAgeAccel, consistent with the hypothesis that frailty is a model of biological age [1]. We also extended the utility of the Levine PhenoAge model to patients who were admitted to the ICU by showing that PhenoAgeAccel remained predictive of mortality after adjustments for frailty as well as other important covariates. For instance, a non-frail patient (CFS ≤3) with elevated PhenoAge may warrant closer monitoring for mortality risk, despite apparent resilience. This result contrasted with the poor performance of chronological age in predicting mortality [15] and the findings of a smaller study (n=262) that compared DNA methylation biomarkers and frailty in stable patients [16]. If our results can be confirmed by other studies, use of biological age instead of chronological age for risk adjustment for the critically ill would appear justified. Our findings suggest that integrating PhenoAge into ICU risk stratification would improve triage decision-making and resource allocation or personalized interventions, such as targeted nutritional support. PhenoAge could also be used as an inclusion criterion or an outcome for interventional trials for critically ill patients. Furthermore, the stronger PhenoAge association we observed in non-frail patients may reflect acute physiological stress unmasking subclinical frailty, and further studies are needed to explore this hypothesis.
Second, a few recent studies showed that biological age can be conceptualized as a dynamic clock that can be dialed forward and backward by diseases and interventions, respectively [17]. Exercises and/or nutritional supplementations have been shown to be successful for rewinding the epigenetic or biological age clock of humans and primates within 4 weeks to 12 months [18-21]. While some studies suggest that biological age can be modified in chronic settings, whether PhenoAge in critically ill patients can be altered remains untested and requires further investigation.
Third, there are limitations to this study. This is a single-center observational study and only 36% of our patients who had their PhenoAge computed also had prospectively collected CFS data. Although the median APACHE II score was similar between those with and without the CFS data, selection bias is still possible. As such, confirmation of the external validity of our results by a prospective multicenter study is needed. CFS was assessed at ICU admission, potentially capturing acute rather than baseline frailty, while PhenoAge was applied in an acute context, differing from its chronic health design. As noted above, we also lacked serial data on our patients’ biological age and currently, there is no evidence to suggest that biological age of a critically ill patient can be dialed backward, let alone to be certain that reducing biological age in the critically ill can improve mortality. Additionally, spanning 2015–2021, the study period encompassed evolving ICU practices that may have influenced outcomes. Nonetheless, these are important research questions that deserve further investigation.
Finally, multiple biological age estimation models or epigenetic clocks are currently in use [22,23]. Whether some models are better suited for the critically ill than others remains uncertain, and demands comparative studies to determine. While PhenoAge calculation requires laboratory data (e.g., blood biomarkers), its real-time implementation in ICUs may be limited by processing time, but automated systems could mitigate this barrier. Although they are better than chronological age in predicting mortality, the AUROC values of the PhenoAge (0.622) and CFS (0.601) were still relatively low on their own, below typical clinical thresholds (e.g., 0.8), limiting their applicability to assist clinical decision-making [24].
In conclusion, the extent of being biologically older than one’s chronological age according to the Levine model was correlated significantly with frailty assessments based on a person’s ability to perform their daily activities. PhenoAge and CFS, both measured at ICU admission, independently predicted hospital mortality, with PhenoAge showing a notable interaction with frailty, particularly in non-frail patients. Further research is needed to determine whether PhenoAge in critical illness reflects modifiable factors amenable to intervention.
KEY MESSAGES
▪ PhenoAge, measured at intensive care unit admission using the Levine model, was significantly correlated with frailty (CFS) and may reflect a combination of chronic biological aging and acute physiological stress.
▪ PhenoAge and CFS independently predicted hospital mortality in critically ill patients, with PhenoAge’s effect most pronounced in patients who were not clinically frail (CFS ≤3).
▪ Further research is needed to explore whether PhenoAge in critical illness can be modified through interventions, as we did not evaluate such approaches in this study.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
ACKNOWLEDGMENTS
None.
AUTHOR CONTRIBUTIONS
Conceptualization: KMH. Methodology: KMH, NPA. Formal analysis: KMH, NPA. Data curation: KMH, NPA. Visualization: KMH, NPA. Project administration: NPA. Funding acquisition: KMH. Writing – original draft: KMH, NPA. Writing – review & editing: KMH, NPA. All authors read and agreed to the published version of the manuscript.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.000200.
Differences in survival time between those with different Clinical Frailty Scale (CFS) scores after adjustments for PhenoAgeAccel and the interaction term between CFS and PhenoAgeAccel.
Differences in survival time between those with and without PhenoAgeAccel after adjustments for Clinical Frailty Scale (CFS), the interaction term between CFS and PhenoAgeAccel, chronological age, number of severe comorbidities, diabetes mellitus and the Acute Physiology and Chronic Health Evaluation II score >15.
REFERENCES
- 1.Ji L, Jazwinski SM, Kim S. Frailty and biological age. Ann Geriatr Med Res. 2021;25:141–9. doi: 10.4235/agmr.21.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Geer L, Fredrikson M, Chew MS. Frailty is a stronger predictor of death in younger intensive care patients than in older patients: a prospective observational study. Ann Intensive Care. 2022;12:120. doi: 10.1186/s13613-022-01098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Na YS, Kim JH, Baek MS, Kim WY, Baek AR, Lee BY, et al. In-hospital mortality prediction using frailty scale and severity score in elderly patients with severe COVID-19. Acute Crit Care. 2022;37:303–11. doi: 10.4266/acc.2022.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott ML, Caspi A, Houts RM, Ambler A, Broadbent JM, Hancox RJ, et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat Aging. 2021;1:295–308. doi: 10.1038/s43587-021-00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachun D, Lu AT, Bick AG, Natarajan P, Weinstock J, Szeto MD, et al. Clonal hematopoiesis associated with epigenetic aging and clinical outcomes. Aging Cell. 2021;20:e13366. doi: 10.1111/acel.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor RC, Hetz C. Mastering organismal aging through the endoplasmic reticulum proteostasis network. Aging Cell. 2020;19:e13265. doi: 10.1111/acel.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2018;15:e1002718. doi: 10.1371/journal.pmed.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–91. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verschoor CP, Lin DT, Kobor MS, Mian O, Ma J, Pare G, et al. Epigenetic age is associated with baseline and 3-year change in frailty in the Canadian longitudinal study on aging. Clin Epigenetics. 2021;13:163. doi: 10.1186/s13148-021-01150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho KM, Morgan DJ, Johnstone M, Edibam C. Biological age is superior to chronological age in predicting hospital mortality of the critically ill. Intern Emerg Med. 2023;18:2019–28. doi: 10.1007/s11739-023-03397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. Correction: a new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2019;16:e1002760. doi: 10.1371/journal.pmed.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr. 2020;20:393. doi: 10.1186/s12877-020-01801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team . R Foundation; 2023. R: a language and environment for statistical computing [Internet] [cited 2025 Mar 20]. Available from: https://www.R-project.org/ [Google Scholar]
- 14.MedCalc Software . MedCalc Software; 2023. MedCalc Statistical Software for windows, version 22.009 [Internet] [cited 2025 Mar 20]. Available from https://www.medcalc.org. [Google Scholar]
- 15.Spiegelhalter D. How old are you, really?: communicating chronic risk through 'effective age' of your body and organs. BMC Med Inform Decis Mak. 2016;16:104. doi: 10.1186/s12911-016-0342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience. 2017;39:83–92. doi: 10.1007/s11357-017-9960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poganik JR, Zhang B, Baht GS, Tyshkovskiy A, Deik A, Kerepesi C, et al. Biological age is increased by stress and restored upon recovery. Cell Metab. 2023;35:807–20.e5. doi: 10.1016/j.cmet.2023.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohman T, Bains G, Cole S, Gharibvand L, Berk L, Lohman E. High-Intensity interval training reduces transcriptomic age: a randomized controlled trial. Aging Cell. 2023;22:e13841. doi: 10.1101/2022.12.22.22283853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald KN, Campbell T, Makarem S, Hodges R. Potential reversal of biological age in women following an 8-week methylation-supportive diet and lifestyle program: a case series. Aging (Albany NY) 2023;15:1833–9. doi: 10.18632/aging.204602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho E, Qualls C, Villareal DT. Effect of diet, exercise, or both on biological age and healthy aging in older adults with obesity: secondary analysis of a randomized controlled trial. J Nutr Health Aging. 2022;26:552–7. doi: 10.1007/s12603-022-1812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh P, Gollapalli K, Mangiola S, Schranner D, Yusuf MA, Chamoli M, et al. Taurine deficiency as a driver of aging. Science. 2023;380:eabn9257. doi: 10.1126/science.abn9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCrory C, Fiorito G, O'Halloran AM, Polidoro S, Vineis P, Kenny RA. Early life adversity and age acceleration at mid-life and older ages indexed using the next-generation GrimAge and Pace of Aging epigenetic clocks. Psychoneuroendocrinology. 2022;137:105643. doi: 10.1016/j.psyneuen.2021.105643. [DOI] [PubMed] [Google Scholar]
- 23.Duan R, Fu Q, Sun Y, Li Q. Epigenetic clock: a promising biomarker and practical tool in aging. Ageing Res Rev. 2022;81:101743. doi: 10.1016/j.arr.2022.101743. [DOI] [PubMed] [Google Scholar]
- 24.Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75:25–36. doi: 10.4097/kja.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences in survival time between those with different Clinical Frailty Scale (CFS) scores after adjustments for PhenoAgeAccel and the interaction term between CFS and PhenoAgeAccel.
Differences in survival time between those with and without PhenoAgeAccel after adjustments for Clinical Frailty Scale (CFS), the interaction term between CFS and PhenoAgeAccel, chronological age, number of severe comorbidities, diabetes mellitus and the Acute Physiology and Chronic Health Evaluation II score >15.