Abstract
The eukaryotic nucleolus contains a large number of small RNA molecules (snoRNAs) which, in the form of small nucleolar ribonucleoprotein complexes (snoRNPs), are involved in the processing and modification of pre-rRNA. The most abundant and one of the best-conserved snoRNAs is the U3 RNA. So far, only one human U3 snoRNA-associated protein, fibrillarin, has been characterized. Previously, the U3 snoRNPwas purified from CHO cells, and three proteins of 15, 50, and 55 kDa were found to copurify with the U3 snoRNA (B. Lübben, C. Marshallsay, N. Rottmann, and R. Lührmann, Nucleic Acids Res. 21:5377–5385, 1993). Here we report the cDNA cloning and characterization of the human U3 snoRNP-associated 55-kDa protein. The isolated cDNA codes for a novel nucleolar protein which is specifically associated with the U3 snoRNA. This protein, referred to as hU3-55k, is the first characterized U3 snoRNP-specific protein from humans. hU3-55k is a new member of the family of WD-40 repeat proteins and is conserved throughout evolution. It appears that the C-terminal end of hU3-55k is required for nucleolar localization and U3 snoRNA binding.
Eukaryotic cells contain a large number of small nucleolar RNAs (snoRNAs) which, in the form of small nucleolar ribonucleoprotein complexes (snoRNPs), are involved in the various steps of ribosome synthesis (reviewed in references 30 and 46). Several snoRNAs have previously been shown to be required for pre-rRNA processing (12, 30, 46), and a large set of snoRNAs is involved in ribose methylation and pseudouridylation of rRNA (8, 15, 24, 33, 34, 49). snoRNAs are heterogeneous in size, structural elements, and protein association. They are produced by two biosynthetic pathways. Most snoRNAs are encoded within the pre-mRNA introns of ribosomal or nucleolar proteins. The processing of such pre- mRNAs via endo- and exonucleolytic cleavages results in the generation of mature noncapped snoRNAs (30). Other snoRNAs, e.g., U3 snoRNA, are transcribed from independent genes and typically possess a modified 5′ terminus, usually a 5′ trimethylguanosine (TMG) cap (30).
snoRNPs can be divided into four groups, which appear to be functionally distinct (1, 46). Methylation guide snoRNPs direct the site-specific formation of 2′-O-methyl groups in mature rRNA. All snoRNAs of this class contain two conserved sequence elements, referred to as box C and box D (30), and contain an extended region (10 to 21 nucleotides) of base complementarity to mature rRNA (8, 24, 34, 49). Members of the second group of snoRNAs, which encompasses U3, U8, U14, and U22 snoRNAs, also contain the conserved box C and D elements and are involved in pre-rRNA processing reactions (reference 46 and references therein). All box C- and D-containing snoRNAs, including methylation guide snoRNAs, are associated with the conserved nucleolar protein fibrillarin, which thus is a common snoRNP component (30). Members of the third class of snoRNAs lack the box C and D elements but share another conserved sequence element, referred to as the ACA box (1). Such snoRNAs have been implicated in the site-specific synthesis of pseudouridine in rRNA (15, 33). The last group of snoRNAs consists of only one snoRNA, RNase MRP. RNase MRP is an endoribonuclease involved in the processing of pre-rRNA at site A3 in the internal transcribed spacer 1 (27).
Although many snoRNAs have been identified in a wide range of eukaryotes, very few snoRNP proteins have been identified so far. For yeast, containing more than 50 snoRNAs, only eight snoRNA-associated proteins have previously been described (1, 30). So far, only two human snoRNP proteins have been characterized, fibrillarin (30) and hPop1, a component of the human RNase MRP particle (28).
U3 snoRNA, one of the most conserved snoRNAs, is the most abundant snoRNA in cells. All reported U3 snoRNA sequences contain five evolutionarily conserved sequence elements, the A, B, C, C′, and D boxes (20, 31, 47, 48). The presence of an intact box C sequence has previously been shown to be essential for the efficient binding of fibrillarin to U3 snoRNA (2). U3 snoRNA is not exported to the cytoplasm but is retained in the nuclei of cells. Mature U3 snoRNA contains a trimethylguanosine cap structure at its 5′ end. The box D sequence element is required for efficient nuclear hypermethylation of U3 snoRNA both in vivo and in vitro (44, 45).
Eukaryotic rRNA is transcribed as a large 47S precursor and subsequently cleaved in a series of complex processing steps to generate mature 18S, 5.8S, and 28S rRNA species. U3 snoRNP is required for correct processing of the 18S rRNA and is involved in cleavages at site A0 in the 5′ external transcribed spacer, site A1 at the 5′ external transcribed spacer/18S boundary, and site A2 within internal transcribed spacer 1 (3, 13, 19, 23) (reviewed in references 30 and 46). Furthermore, a recent report suggests that U3 snoRNA facilitates the correct folding of the 18S rRNA (18).
As for other snoRNPs, our knowledge about the protein composition of U3 snoRNP is still very limited. Human U3 snoRNP is reported to contain at least six proteins with molecular masses of 74, 59, 36 (fibrillarin), 30, 13, and 12.5 kDa (35). Of these proteins, only the common snoRNP protein fibrillarin has been characterized. In yeast, besides fibrillarin, only one U3 snoRNP-associated protein, SOF1, has previously been characterized (21). SOF1 is an essential 56-kDa nucleolar protein that contains seven so-called Gβ repeat units or WD-40 repeats, which are thought to play a role in protein-protein interactions (21, 32). SOF1 is a specific U3 snoRNP protein, since it is not associated with other snoRNAs.
Previously, the U3 snoRNP particle was purified from CHO cells by anti-m3G-immunoaffinity and mono Q anion-exchange chromatography (26). Three proteins of 55, 50, and 15 kDa copurified with U3 snoRNA. These proteins may represent core U3 snoRNP proteins whose binding mediates the association of other proteins, such as fibrillarin, which are lost during high-salt purification (26). By using a rabbit antiserum raised against the 55-kDa protein, the binding site of this protein on U3 snoRNA was localized. Stable binding of the 55-kDa protein requires sequences located between nucleotides 97 and 204 of human U3 snoRNA, including the conserved box B and C sequence elements (26).
In this report, we describe the cDNA cloning and characterization of the human U3 snoRNP-associated 55-kDa protein. We show that the isolated cDNA codes for a novel nucleolar protein which is associated with U3 snoRNA. This protein, referred to as hU3-55k, is a specific U3 snoRNP component from which the C-terminal end appears to be required for U3 snoRNA binding and nucleolar localization.
MATERIALS AND METHODS
Peptide sequence analysis.
Purified 55-kDa protein for sequence analysis was isolated from CHO cells by anti-m3G-immunoaffinity and mono Q anion-exchange chromatography as previously described (26). A protein preparation containing the 55-kDa protein was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose. The 55-kDa band was identified by Ponceau S staining, excised, and subjected to trypsin digestion and microsequencing.
cDNA cloning of hU3-55k.
The peptides obtained from the 55-kDa protein, AFEEDQVAGRLK and VWNVAEN, were used to design the following two degenerate oligonucleotide primers: 55-1 [5′-GCN-TT(C/T)-GA(A/G)-GA(A/G)-GA(C/T)-CA(A/G)-GT-3′] and 55-2 [5′-TGG-AA(C/T)-GTN-GCN-GA(A/G)-AA-3′]. A human teratocarcinoma cDNA library constructed in the λgt11 vector (42) was screened with these oligonucleotides by standard techniques (38). Clones that hybridized with both oligonucleotides were subcloned in the EcoRI site of vector pGEM-3Zf(+) (Promega) and sequenced by the dideoxynucleotide chain termination method (39).
Sequence analysis revealed that the clones obtained were not full length; three EST sequences (HS892, HS081224, and HS705108) extended further to the 3′ end of the cDNA. To clone the missing 3′ end of hU3-55k cDNA, PCR primers (5′-CTT-CTC-TGT-GAC-ATC-CCC-CTG-GTG-3′ and 5′-CGC-AAG-CTT-CAA-AGA-GGG-TGG-GGC-ATA-GC-3′) were designed on the basis of the EST sequences and used to amplify by PCR the 3′ end of the cDNA from total HeLa cell RNA. A full-length clone was constructed by subcloning the EcoRI-HindIII PCR fragment behind hU3-55k cDNA in pGEM-3Zf(+).
In vitro transcription and translation.
In vitro transcription was performed with T7 RNA polymerase and full-length hU3-55k cDNA cloned in vector pGEM-3Zf(+) essentially as previously described (40). In vitro translation of hU3-55k was performed by incubating T7 mRNA with [35S]methionine (ICN) and wheat germ extract essentially as previously described (40). In vitro-translated hU3-55k protein was immunoprecipitated in IPP100 (100 mM NaCl, 10 mM Tris-Cl [pH 8.0], 2 mM MgCl2, 0.05% Nonidet P-40 [NP-40]) with 50 μl of rabbit anti-55-kDa antiserum or 50 μl of normal rabbit serum coupled to protein A agarose beads as previously described (28).
Transfection constructs.
To obtain a construct in which hU3-55k cDNA is cloned in frame behind the GFP coding sequence, hU3-55k cDNA was mutated by PCR to introduce an NdeI site at the start codon and a BamHI site directly behind the stop codon. The PCR product was cloned in the PCR-II vector (Invitrogen), digested with BamHI, and ligated in pEGFP-C1 vector (Clontech) which was digested with BglII, resulting in a plasmid, GFP-55k, containing the green fluorescent protein (GFP) and hU3-55k cDNAs fused in frame.
Vesicular stomatitis virus (VSV)-tagged cDNAs were constructed as follows. A VSV tag was added to the 5′ and 3′ ends of hU3-55k cDNA by PCR with oligonucleotides encoding the VSV tag. Full-length hU3-55k cDNA containing a 5′ VSV tag (MEIYTDIEMNRLGK) was cloned in the NheI/EcoRI sites of the pCI-neo vector (Promega), resulting in construct VSV-55k. An hU3-55k cDNA ending at the internal EcoRI site (missing the 3′ end) was subcloned in the EcoRI site of pCI-neo and digested with NheI and BglII to remove a small fragment containing the translational start ATG. VSV-55k was digested with NheI and BglII to release a fragment containing the 5′ VSV tag and translational start ATG, which was then cloned into NheI/BglII-digested plasmid pCI-neo with the 3′-end-shortened hU3-55k cDNA, resulting in construct VSV-55kΔC. This construct lacks the coding sequence for the last 17 C-terminal amino acids. The stop codon of full-length hU3-55k is not present in this construct, and the encoded protein is terminated by the first in-frame stop codon in the pCI-neo vector, giving rise to an additional 15 amino acids after the hU3-55k protein sequence.
Full-length hU3-55k cDNA containing a 3′ VSV tag (YTDIEMNRLGK-stop) was cloned in the EcoRI/SalI sites of pCI-neo, giving rise to construct 55k-VSV. Subsequently, 55k-VSV was partially digested with StyI, made blunt with Klenow polymerase, and digested with SalI to release a fragment of 1,370 bp which codes for a hU3-55k deletion mutant containing a C-terminal VSV tag and starting at amino acid 45. This fragment was cloned in the EcoRI (made blunt with Klenow polymerase) and SalI sites of pCI-neo, resulting in construct 55kΔN-VSV. A nontagged full-length hU3-55k cDNA was subcloned in the EcoRI site of pCI-neo, resulting in control construct 55k. The integrity of each construct was checked by sequence analysis.
Transient transfection of HeLa cells.
HeLa cell monolayers were grown to 80% confluence by standard tissue culture techniques, and subsequently 3 × 106 cells were transfected with 10 μg of the corresponding DNAs in a total volume of 400 μl of Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. Electroporation was performed at 296 V and a capacity of 950 μFa with a Gene Pulser II (Bio-Rad). After electroporation, cells were resuspended in 10 ml of Dulbecco’s modified Eagle’s medium with 10% fetal calf serum and grown on coverslips. Two days later, cells were washed twice with phosphate-buffered saline (PBS) and subsequently examined by using a fluorescence microscope with fluorescein isothiocyanate (FITC) adjustment or cells were fixed with methanol-acetone (5 min at −20°C) and used for immunofluorescence assays.
Immunofluorescence.
Indirect immunofluorescence assays with monoclonal antifibrillarin (72B9 [37]) and anti-U2B" (4G3 [17]) antibodies was performed with GFP-55k-transfected HeLa cells. Fixed cells were incubated with monoclonal antibodies diluted in PBS (1:1) for 1 h at room temperature, washed in PBS, and subsequently incubated with goat anti-mouse antibodies coupled to Texas Red (diluted 1:50 in PBS) for 1 h at room temperature. Cells were mounted with 50% PBS–glycerol and visualized by confocal microscopy.
HeLa cells transfected with VSV-tagged constructs were incubated with monoclonal anti-VSV antibodies (Boehringer; diluted 1:150 in PBS), followed by incubation with goat anti-mouse antibodies coupled to FITC (diluted 1:50 in PBS).
Preparation of cell extracts and immunoprecipitation.
HeLa cells were transiently transfected with VSV-tagged hU3-55k constructs and subsequently grown for 2 days. Cells were washed twice with PBS, resuspended in 1 ml of buffer A (25 mM Tris-Cl [pH 7.5], 100 mM KCl, 1 mM dithioerythritol, 2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 0.05% NP-40), and sonicated with a Branson microtip (three times for 20 s each). Insoluble materials were pelleted (12,000 × g, 15 min), and supernatants were used directly for immunoprecipitation. Monoclonal anti-VSV, antifibrillarin, and anti-U2B" antibodies were coupled to 20 μl of a 50% suspension of protein A agarose beads (Biozym) in IPP500 (500 mM NaCl, 10 mM Tris-Cl [pH 8.0], 0.05% NP-40) by incubation for 2 h at room temperature. Beads were washed twice with IPP500 and once with buffer B (10 mM Tris [pH 8.0], 100 mM KCl, 5 mM MgCl2, 0.05% NP-40), and 200 μl of extract was added and incubated for 2 h at 4°C. Beads were washed three times with buffer B, and coprecipitating RNAs were isolated by phenol-chloroform extraction and ethanol precipitation. RNAs were separated on a 7% denaturing polyacrylamide gel and blotted onto a Hybond-N membrane (Amersham). Northern blot hybridizations with antisense riboprobes specific for human U3, U8, and U2 RNAs were performed as previously described (52). For 3′-end labeling, coprecipitating RNAs were labeled with [32P]pCp and T4 RNA ligase as previously described (51) and resolved on an 8% denaturing polyacrylamide gel.
Nucleotide sequence accession number.
The hU3-55k cDNA sequence has been deposited in the GenBank, EMBL, and DDBJ databases under accession no. AJ001340.
RESULTS
Identification of U3-55k peptide sequences.
As described previously (26), the U3 snoRNP particle was purified from CHO cells via anti-m3G-immunoaffinity chromatography and mono Q anion-exchange chromatography. U3 snoRNA copurified with at least three proteins, with molecular masses of 55, 50, and 15 kDa. A protein preparation containing the 55-kDa protein was fractionated by SDS-PAGE and blotted onto nitrocellulose, and the region containing the 55-kDa protein was excised. The nitrocellulose-bound 55-kDa protein was subjected to trypsin digestion, and the resulting peptides were sequenced. Two peptide sequences of 12 and 7 amino acids in length, AFEEDQVAGRLK and VWNVAEN, were obtained.
Cloning of hU3-55k cDNA.
The peptide sequences obtained from the purified U3 snoRNP-associated 55-kDa protein from CHO cells were employed in designing two degenerate oligonucleotides (see Materials and Methods) that were used to screen a human teratocarcinoma cDNA library. Although these oligonucleotides were derived from hamster sequences, we speculated that as for other sn(o)RNP proteins (e.g., fibrillarin), the U3-55k sequence is highly conserved among mammals and chose a human cDNA library for screening. Several clones that hybridized with both oligonucleotides were selected, strongly suggesting that cDNAs coding for a protein containing both peptide sequences were found. Subcloning and sequence analysis of these clones (∼1.4 kb in length) revealed an open reading frame (ORF) starting at nucleotide 35 and extending to the 3′ end of the cDNA coding for 458 amino acids. No in-frame stop codon was present at the C-terminal end of the ORF, suggesting that the cloned cDNA was missing the 3′ end.
A comparison of this cDNA sequence with the nucleic acid sequences in databases revealed that three human EST sequences (HS705108, HS081224, and HS892) overlapped with our cDNA (overlap starting at nucleotides 1227, 1235, and 1250, respectively) and extended further to the 3′ end. An oligonucleotide was designed on the basis of these EST sequences and used to amplify the complete 3′ end from HeLa cell RNA by reverse transcription-PCR. An internal EcoRI restriction site close to the 3′ end of the cDNA appeared to be responsible for finding only 3′-truncated cDNAs during screening of the teratocarcinoma cDNA library.
A complete cDNA (1,521 bp) was constructed by combining the teratocarcinoma cDNA with the 3′ end obtained by reverse transcription-PCR. The combined cDNA encodes a protein of 475 amino acids, with a predicted molecular mass of 51.8 kDa and a pI of 7.8. The cDNA and deduced amino acid sequences are shown in Fig. 1A. The size of the cDNA was confirmed by Northern analysis of HeLa cell poly(A)+ RNA, which revealed an ∼1.7-kb mRNA (data not shown). The polypeptide encoded by the cDNA, hU3-55k, does contain both peptide sequences derived from the 55-kDa U3 snoRNP protein isolated from CHO cells. The first peptide sequence can be found from amino acids 102 to 113, and the second peptide sequence can be found from amino acids 267 to 273 (Fig. 1A).
FIG. 1.
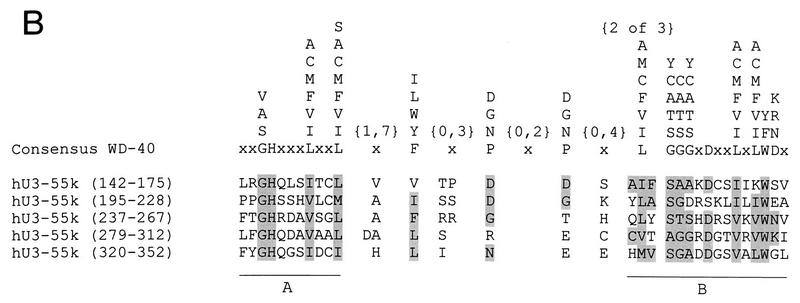
cDNA and deduced amino acid sequences of hU3-55k. (A) The obtained peptide sequences of the purified 55-kDa protein from CHO cells are shown in boldface. The putative bipartite NLS is double underlined, and the glutamic acid-rich region in the N-terminal part of the protein is shown in bold italics. The five WD-40 repeat regions are underlined and numbered. (B) The WD-40 repeat regions of hU3-55k were aligned manually with the consensus WD-40 repeat sequence (32). Amino acids which correspond to the consensus sequence are shaded. Parts A and B of WD-40 repeats are indicated.
To test whether the hU3-55k cDNA indeed represents the human homolog of the copurifying U3-55k protein from CHO cells, hU3-55k was translated in vitro and the resulting protein was immunoprecipitated with a rabbit antiserum generated against the purified 55-kDa CHO protein. This rabbit serum has been shown previously to recognize the native CHO and human 55-kDa proteins (26). The in vitro-translated hU3-55k protein was indeed immunoprecipitated by this rabbit antiserum (Fig. 2, lane 2), confirming that the protein encoded by hU3-55k cDNA is similar to the purified CHO protein. Serum from a nonimmunized rabbit was not able to immunoprecipitate the hU3-55k protein (Fig. 2, lane 3).
FIG. 2.
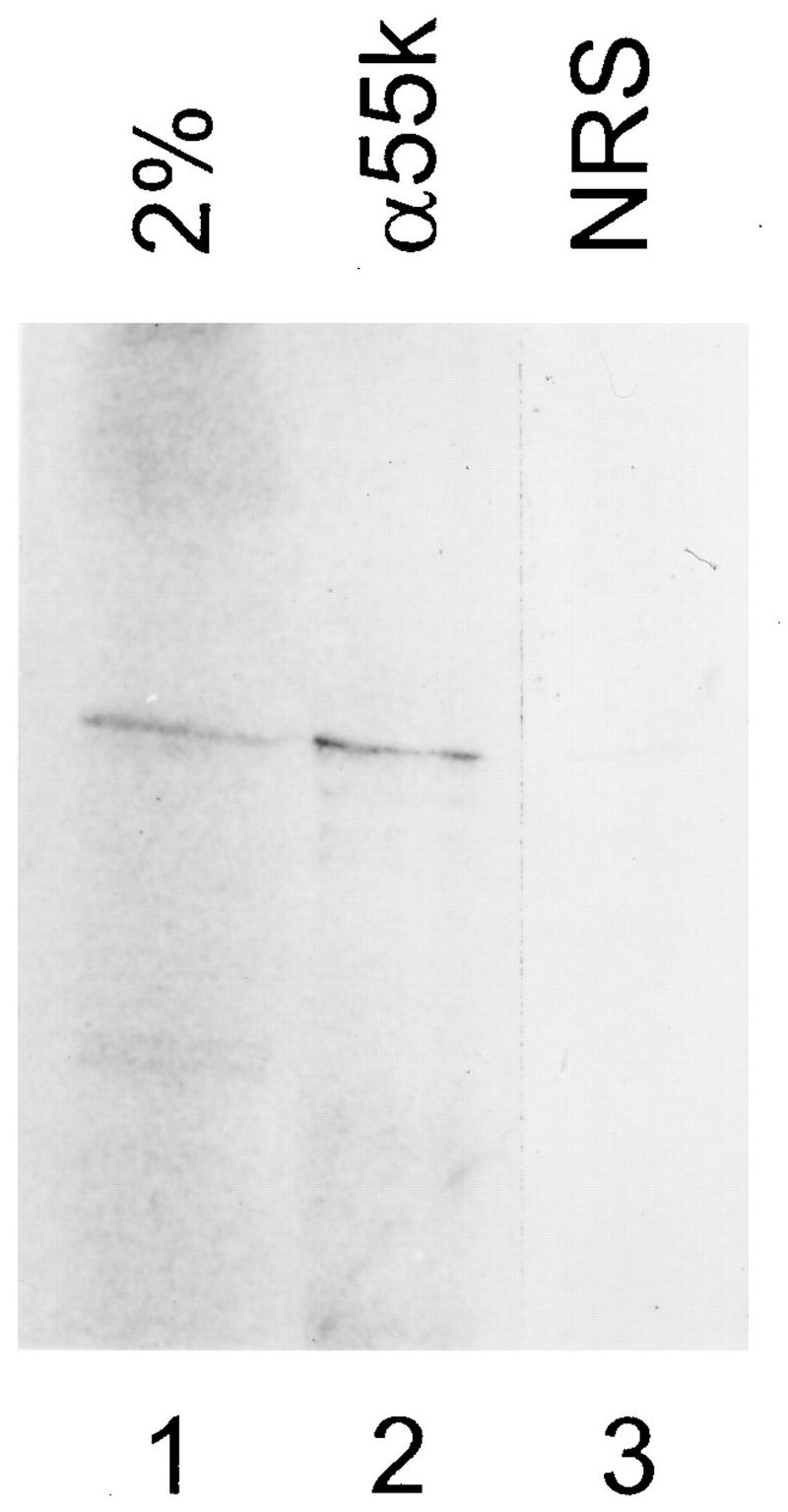
hU3-55k is similar to the CHO U3 55-kDa protein. [35S]methionine-labeled hU3-55k protein was generated by in vitro translation. After immunoprecipitation with rabbit antibodies, proteins from immunoprecipitates were analyzed by SDS-PAGE and visualized by autoradiography. Lane 1, input lysate corresponding to 2% of the amount used for immunoprecipitation; lane 2, protein immunoprecipitated by rabbit anti-55-kDa antibodies (α55k) (26); lane 3, control precipitation with serum from a nonimmunized rabbit (normal rabbit serum [NRS]).
hU3-55k is a member of the family of WD-40 repeat proteins.
A comparison of the hU3-55k protein sequence with the protein sequences in databases showed that hU3-55k is a novel human protein. A putative bipartite nuclear localization signal can be identified at the N terminus of hU3-55k (amino acids 8 to 40). Furthermore, a search for known protein motifs revealed the presence of five so-called WD-40 repeats or Gβ repeat units between amino acid positions 142 and 352 of hU3-55k (Fig. 1A). A WD-40 repeat consists of two conserved elements, A and B, separated by regions variable in both sequence and length (32, 50). The most characteristic features of a WD-40 repeat are the GH residues in part A and the WD residues in part B. The number of WD-40 repeats in a particular protein may vary from four to eight repeating units, spanning either the entire length of the protein or the N-terminal, C-terminal, or central part of it. A multiple alignment of the hU3-55k WD-40 repeat units and the WD-40 consensus sequence is depicted in Fig. 1B. Another interesting feature of hU3-55k is the glutamic acid-rich stretch between amino acids 64 and 73. A similar stretch of glutamic acid residues is present in a number of nucleolar and nonnucleolar proteins and may be involved in protein-protein interactions.
In addition to identifying protein sequence motifs, the database search with the hU3-55k protein sequence revealed two putative yeast homologs. Along with the SOF1 protein (21), which is partially homologous to hU3-55k (17% identity and 42% similarity), there is yeast polypeptide with a higher degree of homology to hU3-55k. This Saccharomyces cerevisiae protein, which is encoded by the eighth ORF of cosmid 9659 (U40829), is 33% identical and 58% similar to hU3-55k, and like hU3-55k, it contains a glutamic acid-rich region in the N-terminal part of the protein, a feature lacking in the SOF1 protein sequence. We therefore conclude that the polypeptide encoded by the yeast U40829 ORF represents the true homolog of hU3-55k. This finding implies that the yeast U3 snoRNP particle contains two related proteins, with each one containing several WD-40 repeat units. An alignment of hU3-55k and putative yeast homologs is shown in Fig. 3. The sequence conservation of hU3-55k suggests that this protein serves an important evolutionarily conserved function in the cell.
FIG. 3.
Multiple alignment of hU3-55k and putative yeast homologs. A multiple alignment of hU3-55k and putative yeast homologues SOF1 and S. cerevisiae cosmid U40829 was created by using the Boxshade World Wide Web server starting with an alignment created by the Pileup program from the Genetics Computer Group package. Amino acids absolutely conserved are marked by black boxes, and amino acids with conserved characters are marked by gray boxes. Dots indicate gaps.
hU3-55k is a specific U3 snoRNP component.
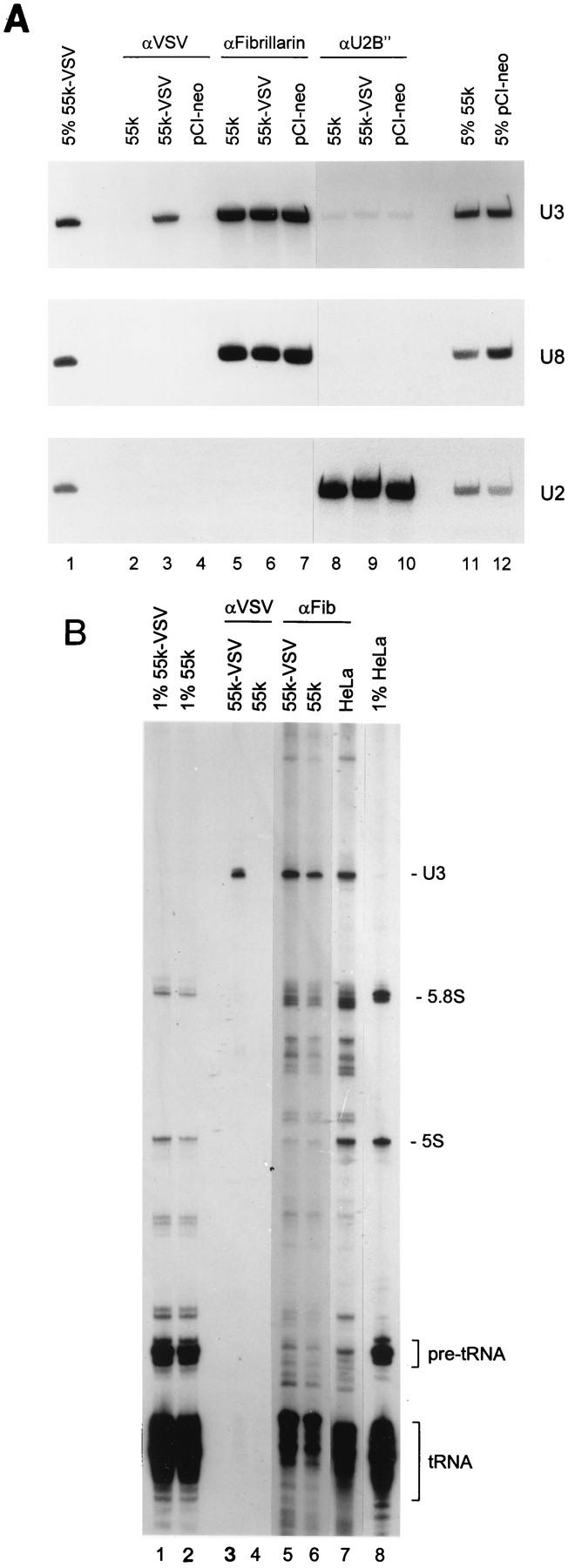
To determine whether the hU3-55k protein is a component of the human U3 snoRNP particle, immunoprecipitation experiments were performed with a tagged hU3-55k protein. hU3-55k cDNA with a 3′ VSV tag sequence (25) was cloned into the mammalian expression vector pCI-neo and expressed in transiently transfected HeLa cells. As controls, a construct containing hU3-55k cDNA without the VSV tag and the pCI-neo vector without insert were used. Two days after transfection, cells were lysed and the resulting total cell extract was used for immunoprecipitation with anti-VSV, antifibrillarin, and anti-U2B" monoclonal antibodies. RNAs were extracted from immunoprecipitates, supernatants, and total cell extracts; fractionated by gel electrophoresis; and analyzed by Northern blot hybridization with probes specific for U3 snoRNA, U8 snoRNA, and U2 snRNA.
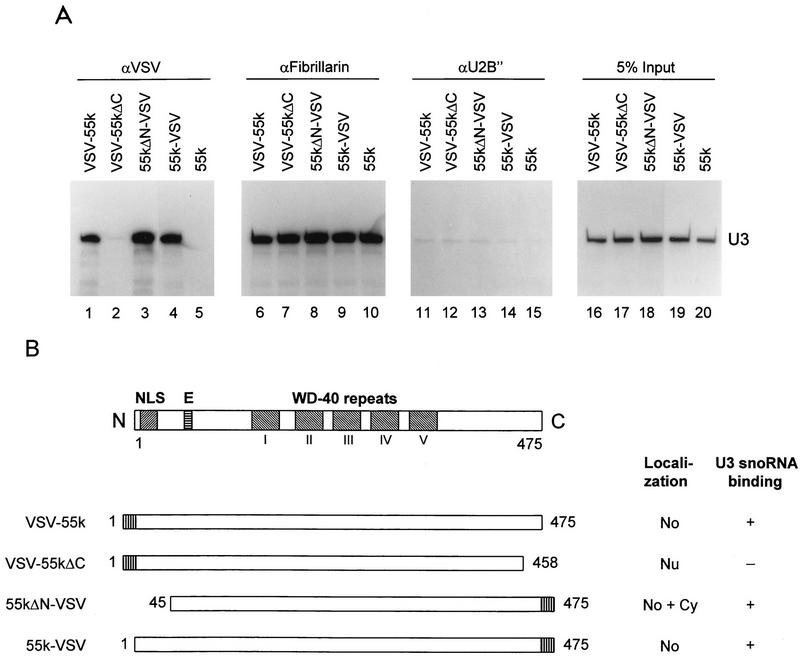
As is shown in Fig. 4A, lane 3, U3 snoRNA was coprecipitated by anti-VSV antibodies from cell extract containing VSV-tagged hU3-55k protein (55k-VSV), showing that the hU3-55k protein indeed is able to associate with the U3 snoRNP particle. The specificity of this result was established by the lack of U3 snoRNA coprecipitation with (i) anti-VSV antibodies for extracts from control cells (nontagged 55-kDa protein [55k] and pCI-neo vector) (Fig. 4A, lanes 2 and 4, respectively) and (ii) anti-U2B" antibodies (Fig. 4A, lanes 8 through 10). U3 snoRNPs were immunoprecipitated from all three extracts (55k-VSV, 55k, and pCI-neo) by antifibrillarin antibodies (Fig. 4A, lanes 5 through 7). No U2 snRNA could be detected in anti-VSV or antifibrillarin precipitates.
FIG. 4.
hU3-55k is a specific U3 snoRNP component. (A) 55k-VSV, 55k, and pCI-neo were transiently expressed in HeLa cells. Two days after transfection, cells were lysed and used for immunoprecipitation with anti-VSV (αVSV), antifibrillarin (αFibrillarin), and anti-U2B" (αU2B") antibodies. RNAs were extracted from immunoprecipitates (lanes 2 through 10) and total cell extracts (lanes 1, 11, and 12), resolved by PAGE, and transferred to a Northern blot. Specific antisense RNA probes were used to detect human U3, U8, and U2 sn(o)RNAs, as indicated on the right. Lanes 2 through 4, anti-VSV immunoprecipitations; lanes 5 through 7, antifibrillarin immunoprecipitations; lanes 8 through 10, anti-U2B" immunoprecipitations; lanes 1, 11, and 12, RNA isolated from 5% of indicated total cell extracts used for immunoprecipitations. (B) 55k-VSV and 55k were transiently expressed in HeLa cells. Two days after transfection, cells were lysed and used for immunoprecipitation with anti-VSV and antifibrillarin (αFib) antibodies. RNAs were extracted from immunoprecipitates and total cell extracts, 3′ end labeled with [32P]pCp, and resolved on an 8% denaturing polyacrylamide gel. Lanes 3 through 4, anti-VSV immunoprecipitations; lanes 5 through 7, antifibrillarin immunoprecipitations; lanes 1, 2, and 8, RNA isolated from 1% of indicated total cell extracts used for immunoprecipitations.
To determine whether the hU3-55k protein is a specific U3 snoRNP protein or a common snoRNP protein (like fibrillarin), the same blot was hybridized with a number of snoRNA probes. Neither U8 snoRNA (Fig. 4A) nor U13 snoRNA (data not shown) was detectably coprecipitated with anti-VSV antibodies from extract containing the 55k-VSV protein, indicating that the hU3-55k protein is not able to associate with these snoRNPs. Three classes of snoRNAs other than box C and D snoRNAs, such as U3 and U8, exist; they are methylation guide snoRNAs (e.g., U24), ACA box-containing snoRNAs (e.g., U17), and RNase MRP RNA (1, 46). To investigate whether the hU3-55k protein may be a component of these other classes of snoRNPs, the Northern blot of the immunoprecipitations was hybridized with U24, U17, and RNase MRP RNA probes (data not shown). In all of these cases, anti-VSV antibodies failed to detectably coprecipitate the respective RNAs from extract containing the 55k-VSV protein, indicating that hU3-55k is indeed not associated with these other classes of snoRNAs.
To further establish hU3-55k as a specific U3 snoRNP component, coprecipitating RNAs from cell extracts containing 55k-VSV and 55k (nontagged) proteins were 3′ end labeled with [32P]pCp and fractionated on an 8% polyacrylamide gel. As shown in Fig. 4B, lane 3, anti-VSV antibodies were able to specifically coprecipitate U3 snoRNA from cell extract containing the VSV-tagged hU3-55k protein. In contrast, antifibrillarin antibodies were able to immunoprecipitate a large number of snoRNAs from cell extracts containing 55k-VSV and 55k proteins and from nontransfected HeLa cells (Fig. 4B, lanes 5 through 7, respectively). Taken together, these results strongly suggest that the hU3-55k protein is specifically associated with U3 snoRNP.
hU3-55k is localized in the nucleolus.
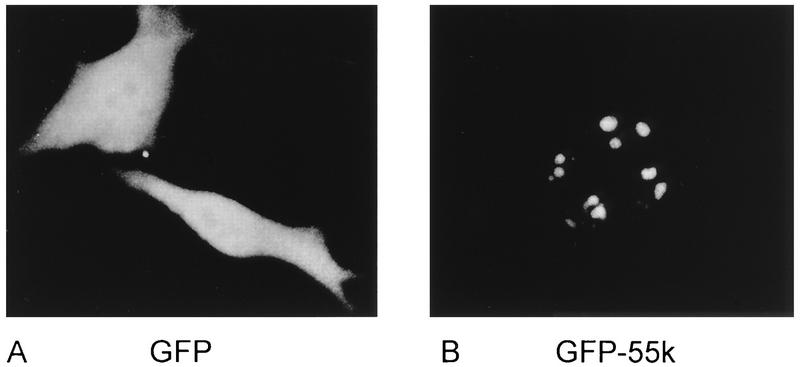
To investigate the subcellular localization of hU3-55k, a plasmid in which hU3-55k cDNA was fused to the GFP sequence in the mammalian expression vector pEGFP (9) was constructed. The resulting construct (GFP-55k) and pEGFP as a control were used to transiently transfect HeLa cells, and 2 days after transfection, the localization of the GFP-55k fusion protein was determined via direct fluorescence microscopy, i.e., in vivo with nonfixed cells. GFP alone gave a strong fluorescence distributed throughout the HeLa cell (Fig. 5A), indicating that GFP is uniformly distributed over the cell and does not localize to a specific compartment of a HeLa cell. Expression of the GFP-55k fusion protein, however, resulted in strong nucleolar fluorescence (Fig. 5B), strongly suggesting that hU3-55k (and thus GFP-55k) accumulates in the nucleolus of a HeLa cell. Cells with a relatively high level of GFP-55k expression showed in addition to nucleolar fluorescence weak or moderate nucleoplasmic fluorescence, suggesting that when the nucleolus is saturated with GFP-55k, the remaining GFP-55k molecules reside in the nucleoplasm.
FIG. 5.
Nucleolar accumulation of GFP-55k. GFP (A) and GFP-55k (B) were transiently expressed in HeLa cells. Two days after transfection, GFP and GFP-55k localization was examined by fluorescence microscopy in vivo with nonfixed cells.
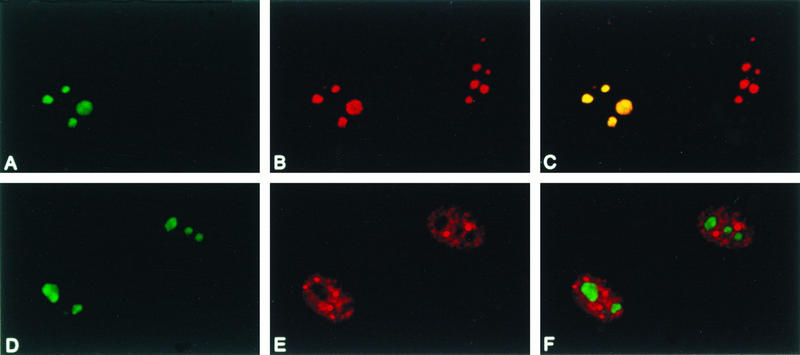
To more precisely assess the nucleolar localization of hU3-55k, GFP-55k-transfected cells were fixed with methanol-acetone 2 days after transfection and immunostained with a monoclonal antifibrillarin antibody. Fibrillarin is associated with a number of snoRNAs, including U3 snoRNA, and is localized to the dense fibrillar compartment of the nucleolus (37). As is shown in Fig. 6A through C, GFP-55k gave a somewhat more diffuse fluorescence of the nucleolus than did fibrillarin, which gave a more clumpy staining pattern of the nucleolus. However, superimposition of the two images showed that GFP-55k largely colocalized with fibrillarin, which might have been partially due to the fact that both fibrillarin and hU3-55k are associated with U3 snoRNA and therefore present in the same particle.
FIG. 6.
Colocalization of GFP-55k. GFP-55k was transiently expressed in HeLa cells. Two days after transfection, cells were fixed with methanol-acetone and incubated with antifibrillarin antibodies (A through C) or anti-U2B" antibodies (D through F), followed by incubation with Texas Red-labeled goat anti-mouse antibodies, and visualized by confocal microscopy. (A and D) GFP-55k localization (green); (B and E) fibrillarin localization and U2B" localization, respectively (red); (C and F) superimposition of panels A and B and panels D and E, respectively, with regions of colocalization shown in yellow.
Many snRNAs, snoRNAs, and their associated proteins, including fibrillarin, are present in a nuclear organelle termed the coiled body (5, 36). To determine whether hU3-55k can also be found in this nuclear organelle, GFP-55k-transfected cells were immunostained with a monoclonal antibody against U2B", a component of the U2 snRNP particle, which exhibited strong staining of coiled bodies and weaker speckled nucleoplasmic staining (Fig. 6E). As shown in Fig. 6D through F, GFP-55k did not accumulate in coiled bodies.
hU3-55k elements essential for nucleolar localization and U3 snoRNA binding.
The data discussed above show that the hU3-55k protein is a nucleolar protein which is specifically associated with U3 snoRNA. To determine which parts of hU3-55k are essential for nucleolar localization and U3 snoRNA binding, VSV-tagged deletion mutants of hU3-55k were constructed and expressed in HeLa cells by transient transfection. Two days after transfection, cells were lysed and the resulting total cell extracts were analyzed. The expression of tagged mutant proteins was checked by Western blotting of total cell extracts with anti-VSV antibodies. Only two of the eight mutants we used showed detectable expression of hU3-55k protein on a Western blot (data not shown). In particular, mutants from which larger parts of the hU3-55k protein were deleted could not be detected by anti-VSV antibodies. These mutant proteins are probably not expressed very well or are rapidly degraded.
The cellular localization and U3 snoRNA binding capacities of the two hU3-55k deletion mutants that had detectable protein expression were investigated. In the first mutant, 55kΔN-VSV, the N-terminal 44 amino acids of hU3-55k are deleted and a C-terminal VSV tag is added. This mutant protein thus lacks the putative bipartite nuclear localization signal at positions 8 to 40. The second mutant, VSV-55kΔC, lacks the C-terminal 17 amino acids of hU3-55k and contains an N-terminal VSV tag. The stop codon of full-length hU3-55k is lost in this construct, and the resulting protein is terminated by the first in-frame stop codon in the pCI-neo vector, giving rise to an additional 15 amino acids after the hU3-55k protein sequence.
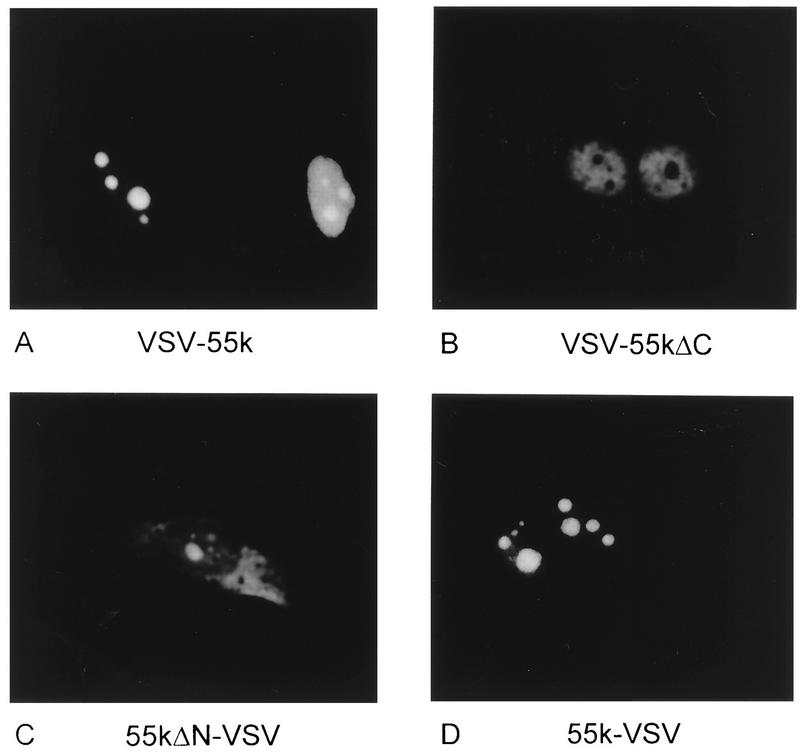
The localization of full-length and mutant proteins was investigated by immunofluorescence assays of transiently transfected cells with anti-VSV antibodies. Nontransfected HeLa cells and cells transfected with the nontagged hU3-55k construct (55k) gave weak background staining of whole cells (data not shown). Full-length hU3-55k proteins containing N- and C-terminal VSV tags, VSV-55k and 55k-VSV, respectively, exhibited strong nucleolar staining (Fig. 7A and D). As described above for GFP-55k, cells with relatively high levels of 55k-VSV and VSV-55k expression showed not only nucleolar staining but also nucleoplasmic staining, whereas no protein was detected in the cytoplasm (Fig. 7A). The mutant hU3-55k protein in which the putative nuclear localization signal is deleted, 55kΔN-VSV, showed strong nucleolar staining. Cells with a relatively high expression level of this protein showed both nucleolar staining and cytoplasmic fluorescence, but no staining was observed in the nucleoplasm of these cells (Fig. 7C). The second mutant, VSV-55kΔC, showed nuclear accumulation, but no staining was found in the nucleoli of these cells (Fig. 7B). In cells with a relatively high expression level of VSV-55kΔC, this localization pattern remained unchanged.
FIG. 7.
Elements required for nucleolar accumulation of hU3-55k. VSV-55k (A), VSV-55kΔC (B), 55kΔN-VSV (C), and 55k-VSV (D) were transiently expressed in HeLa cells. Two days after transfection, cells were fixed with methanol-acetone, incubated with anti-VSV antibodies and subsequently with FITC-conjugated goat anti-mouse antibodies, and visualized by fluorescence microscopy.
To investigate whether these mutant hU3-55k proteins are still able to associate with U3 snoRNA, we performed immunoprecipitation experiments with extracts from transfected HeLa cells. As shown in Fig. 8A, both full-length hU3-55k proteins, 55k-VSV and VSV-55k, and the N-terminal deletion mutant, 55kΔN-VSV, were able to associate with U3 snoRNA (Fig. 8A, lanes 1, 3, and 4). In contrast, the C-terminal deletion mutant, VSV-55kΔC, seemed to have lost the ability to associate with U3 snoRNA (Fig. 8A, lane 2). As a control, U3 snoRNPs were immunoprecipitated from all cell extracts by antifibrillarin antibodies (Fig. 8A, lanes 6 through 10), whereas no U3 snoRNA could be detected in anti-U2B" immunoprecipitates (Fig. 8A, lanes 11 through 15). A deletion of only 17 C-terminal amino acids of hU3-55k thus appears to have a drastic effect on U3 snoRNA binding and nucleolar localization. However, 44 N-terminal amino acids can be removed without significant loss of U3 snoRNA binding capacities and a deletion of the putative nuclear localization signal has only a limited effect on nucleolar localization.
FIG. 8.
hU3-55k elements required for U3 snoRNA binding. (A) VSV-55k, VSV-55kΔC, 55kΔN-VSV, 55k-VSV, and 55k were transiently expressed in HeLa cells. Two days after transfection, cells were lysed and used for immunoprecipitations with anti-VSV (αVSV), antifibrillarin (αFibrillarin), and anti-U2B" (αU2B") antibodies. RNAs were extracted from immunoprecipitates (lanes 1 through 15) and total cell extracts (lanes 16 through 20), resolved by PAGE, and transferred to a Northern blot. Precipitated U3 snoRNA was detected by hybridization with an antisense U3 probe. Lanes 1 through 5, anti-VSV immunoprecipitations; lanes 6 through 10, antifibrillarin immunoprecipitations; lanes 11 through 15, anti-U2B" immunoprecipitations; lanes 16 through 20, RNA isolated from 5% of indicated total cell extracts used for immunoprecipitation. (B) Schematic representations of the VSV-tagged proteins used for transfection studies and a summary of their abilities to accumulate in the nucleolus and to associate with U3 snoRNA. Amino acid numbers are indicated, and different domains of hU3-55k (bipartite NLS, glutamic acid-rich region [E], and WD-40 repeats) are boxed. No, nucleolar accumulation; Nu, nuclear accumulation; Cy, cytoplasmic accumulation; +, binding; −, no binding.
DISCUSSION
By anti-m3G-immunoaffinity and mono Q anion-exchange chromatography, Lübben et al. (26) previously identified three proteins of 55, 50, and 15 kDa from CHO cells which copurified with U3 snoRNA. In this report, we have described the isolation and characterization of a cDNA encoding the human U3 snoRNP-associated 55-kDa protein, hU3-55k. Immunoprecipitation of in vitro-translated hU3-55k with a rabbit serum raised against the purified 55-kDa protein from CHO cells (26) confirmed that the cloned human protein is similar to the purified CHO protein and that this protein is conserved between humans and rodents.
A sequence analysis of the hU3-55k protein revealed that this protein is a new member of the family of WD-40 repeat proteins. This group of proteins is characterized by four to eight conserved repeating units that usually end with WD, which were initially found in the β-subunit of heterotrimeric GTP-binding proteins (G proteins) and therefore have also been designated Gβ repeats or β-transducin repeats (11, 14, 32, 50). WD-40 repeats are found in a large variety of eukaryotic proteins. In general, proteins of this group seem to be involved in the regulation of cellular functions, such as cell division, cell fate determination, gene transcription, transmembrane signalling, mRNA modification, and vesicle function (32). Several WD-40 repeat proteins form multiprotein complexes, possibly interacting with other proteins via their WD-40 repeat regions. For example, they have a role in assembling macromolecules for mRNA splicing (PRP4) and modification (CstF) (4, 6, 10, 43). It can be imagined that hU3-55k has a similar function, a role in the assembly of the multiprotein complex in the processing of pre-rRNA.
The consensus sequence of the WD-40 repeat (Fig. 1B) indicates that this repeat may fold with a variable loop preceding the GH, followed by a β-strand–turn–β-strand–turn–β-strand and ending with WD, thus forming a small β-structure (32). This most likely not very stable structure could be stabilized by contact with other WD-40 repeats, leading to the formation of intramolecular dimers or tetramers (32). Interestingly, not only hU3-55k but also the SOF1 protein, a component of yeast U3 snoRNP, is a member of the WD-40 repeat family (21). However, the 56-kDa SOF1 protein is probably not the yeast homolog of hU3-55k. An alignment of hU3-55k with sequences in the EMBL and GenBank databases revealed that another yeast protein, the eight ORF of cosmid 9659, is more homologous to hU3-55k. This finding implies that at least in yeast, U3 snoRNP possesses two related proteins that contain several WD-40 repeat units; as mentioned above, these proteins may even form heterodimers. It will be interesting to find out if this is also the case in mammals. A candidate for a second WD-40 repeat family protein with a size similar to those of hU3-55k and SOF1 is the copurifying 50-kDa U3 snoRNP protein from CHO cells (21).
To confirm that hU3-55k is a component of the U3 snoRNP, we expressed a VSV-tagged version of hU3-55k in HeLa cells and after cell lysis immunoprecipitations with anti-VSV antibodies were performed. The results of these experiments showed that hU3-55k is indeed a component of U3 snoRNP. We could not detect other snoRNAs, such as U8, U13, U17, U24, or RNase MRP RNA, in immunoprecipitates by Northern blot hybridization. Since these RNAs are typical examples of the various snoRNA classes, these results strongly suggest that the hU3-55k protein is a specific U3 snoRNP protein. In addition, we were not able to detect snoRNAs other than U3 in the anti-VSV immunoprecipitate after 3′-end labeling with [32P]pCp. In contrast, the immunoprecipitate obtained with antifibrillarin antibodies contained a large number of different snoRNAs. These results corroborate our conclusion that hU3-55k is specifically associated with U3 snoRNA.
Lübben et al. (26) proposed that the 55-kDa protein is a core U3 snoRNP protein and may bind directly to the RNA. With a rabbit antiserum raised against the 55-kDa protein, they showed that stable association of the 55-kDa protein with the U3 snoRNP requires sequences located between nucleotides 97 and 204 of human U3 snoRNA, including the conserved box B and C sequence elements. However, in addition to the WD-40 repeat units and the putative bipartite NLS sequence in the N-terminal part of the protein, no known RNA-binding or other protein motifs could be found in hU3-55k. Additional experiments have to be performed to find out whether hU3-55k is bound directly to the RNA and which parts of the protein are involved in the binding to U3 snoRNP.
By using a fusion protein of GFP with hU3-55k (GFP-55k), we showed that hU3-55k localizes to the nucleolus of a HeLa cell. An immunofluorescence assay of GFP-55k-transfected cells with antifibrillarin antibodies showed that hU3-55k largely colocalized with fibrillarin in the nucleolus, as expected for a U3 snoRNP component. We could not detect any GFP-55k in the cytoplasm, but in cells with a relatively high level of GFP-55k expression, the protein was also present in the nucleoplasm of HeLa cells. It has been proposed that nucleolar accumulation is a two-step process. First, a nucleolar protein is transported from the cytoplasm to the nucleoplasm due to its NLS, and then one or several functional domains that interact specifically with other nucleolar components allow it to accumulate within the nucleolus (16, 41, 53). The subcellular localization of GFP-55k is in agreement with such a two-step process, with U3 snoRNP carrying the site of interaction with the hU3-55k protein.
Most nucleoplasmic snRNPs reveal a speckled pattern in immunofluorescence assays and appear to concentrate in coiled bodies, a putative storage compartment for snRNPs (5). Coiled bodies also contain fibrillarin (36), but the presence of U3 snoRNA is still discernible. Although in most previous studies U3 snoRNA was not found in coiled bodies (7, 29). Jimenez-Garcia et al. (22) reported a low level of U3 snoRNA in coiled bodies. An immunofluorescence assay of GFP-55k-expressing cells with anti-U2B" antibodies revealed that GFP-55k did not localize to coiled bodies. These results indicate that there are no or only very few GFP-55k-bound U3 snoRNP particles in coiled bodies and that the fibrillarin present in coiled bodies represents probably free (non-U3 snoRNP-bound) protein or is associated with a different subset of U3 snoRNP particles.
We found that sequences in the C-terminal end of hU3-55k are required for nucleolar localization and most likely U3 snoRNA binding. In contrast, a deletion of the first 44 amino acids from hU3-55k, which removes the putative NLS, had no dramatic effect on nucleolar localization and U3 snoRNA binding. 55kΔN-VSV still accumulated in the nucleolus and was able to associate with U3 snoRNA. However, in contrast to the results for full-length hU3-55k, HeLa cells that showed a relatively high expression level of the mutant protein also accumulated 55kΔN-VSV in the cytoplasm and no nucleoplasmic accumulation was observed. This suggests that only when 55kΔN-VSV can associate with U3 snoRNA is it retained in the nucleolus and that the overexpressed mutant protein is not able to stay in or enter the nucleus by itself, possibly due to deletion of the putative NLS. How does this protein enter the nucleolus? A possible explanation is that the mutant protein enters the nucleus with another U3 snoRNP protein or a transport protein, binds to U3 snoRNA, and then is retained in the nucleolus. Another possibility is that a second NLS in hU3-55k becomes functionally active in the mutant protein and can mediate transport to the nucleus.
A deletion of 17 C-terminal amino acids of hU3-55k had a more severe effect on nucleolar localization and U3 snoRNA binding. VSV-55kΔC localized to the nucleoplasm of HeLa cells and could not be detected in the nucleolus. In addition, this mutant seemed to be unable to associate with U3 snoRNA. These results are in accord with the idea that binding to U3 snoRNP is essential for nucleolar localization. Further experiments are needed to establish whether certain C-terminal amino acids are required for U3 snoRNP binding or whether a C-terminal-end deletion induces a conformational change in the hU3-55k protein which leads to the loss of U3 snoRNA binding capacities and therefore the loss of nucleolar retention.
This study is a further step in resolving the complexity of the human U3 snoRNP particle. Detailed knowledge of interactions among the components of U3 snoRNP and of the functions of snoRNP proteins is the next goal in the endeavor to understand the rRNA processing steps in which U3 snoRNP is involved.
ACKNOWLEDGMENTS
We thank G. J. M. Pruijn for helpful discussions and critical reading of the manuscript and J. M. H. Raats and A. van der Kemp for advice on tissue culture and immunofluorescence techniques. We are grateful to B. Lübben for isolation of the 55-kDa protein from CHO cells, K. M. Pollard and E. M. Tan for providing antifibrillarin (72B9) antibodies, and T. Kiss for providing plasmids of human U8, U13, and U24 snoRNAs.
This work was supported in part by the Netherlands Foundation for Chemical Research (SON) with financial aid from the Netherlands Organization for Scientific Research (NWO).
REFERENCES
- 1.Balakin A G, Smith L, Fournier M J. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. doi: 10.1016/s0092-8674(00)80156-7. [DOI] [PubMed] [Google Scholar]
- 2.Baserga S J, Yang X D, Steitz J A. An intact box C sequence in the U3 snRNA is required for binding of fibrillarin, the protein common to the major family of nucleolar snRNPs. EMBO J. 1991;10:2645–2651. doi: 10.1002/j.1460-2075.1991.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrame M, Tollervey D. Identification and functional analysis of two U3 binding sites on yeast pre-ribosomal RNA. EMBO J. 1992;11:1531–1542. doi: 10.1002/j.1460-2075.1992.tb05198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorn S P, Soltyk A, Beggs J D, Friesen J D. PRP4 (RNA4) from Saccharomyces cerevisiae: its gene product is associated with the U4/U6 small nuclear ribonucleoprotein particle. Mol Cell Biol. 1989;9:3698–3709. doi: 10.1128/mcb.9.9.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohmann K, Ferreira J, Santama N, Weis K, Lamond A I. Molecular analysis of the coiled body. J Cell Sci Suppl. 1995;19:107–113. doi: 10.1242/jcs.1995.supplement_19.16. [DOI] [PubMed] [Google Scholar]
- 6.Bordonne R, Banroques J, Abelson J, Guthrie C. Domains of yeast U4 spliceosomal RNA required for PRP4 protein binding, snRNP-snRNP interactions, and pre-mRNA splicing in vivo. Genes Dev. 1990;4:1185–1196. doi: 10.1101/gad.4.7.1185. [DOI] [PubMed] [Google Scholar]
- 7.Carmo-Fonseca M, Ferreira J, Lamond A I. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis—evidence that the coiled body is a kinetic nuclear structure. J Cell Biol. 1993;120:841–852. doi: 10.1083/jcb.120.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavaille J, Nicoloso M, Bachellerie J P. Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature. 1996;383:732–735. doi: 10.1038/383732a0. [DOI] [PubMed] [Google Scholar]
- 9.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple M A, Petersen-Bjorn S, Friesen J D, Beggs J D. The product of the PRP4 gene of S. cerevisiae shows homology to beta subunits of G proteins. Cell. 1989;58:811–812. doi: 10.1016/0092-8674(89)90930-6. [DOI] [PubMed] [Google Scholar]
- 11.Duronio R J, Gordon J I, Boguski M S. Comparative analysis of the beta transducin family with identification of several new members including PWP1, a nonessential gene of Saccharomyces cerevisiae that is divergently transcribed from NMT1. Proteins. 1992;13:41–56. doi: 10.1002/prot.340130105. [DOI] [PubMed] [Google Scholar]
- 12.Eichler D C, Craig N. Processing of eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1994;49:197–239. doi: 10.1016/s0079-6603(08)60051-3. [DOI] [PubMed] [Google Scholar]
- 13.Enright C A, Maxwell E S, Sollner-Webb B. 5′ETS rRNA processing facilitated by four small RNAs: U14, E3, U17, and U3. RNA. 1996;2:1094–1099. [PMC free article] [PubMed] [Google Scholar]
- 14.Fong H K, Hurley J B, Hopkins R S, Miake-Lye R, Johnson M S, Doolittle R F, Simon M I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci USA. 1986;83:2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganot P, Bortolin M L, Kiss T. Site specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell. 1997;89:799–809. doi: 10.1016/s0092-8674(00)80263-9. [DOI] [PubMed] [Google Scholar]
- 16.Girard J P, Bagni C, Caizergues-Ferrer M, Amalric F, Lapeyre B. Identification of a segment of the small nucleolar ribonucleoprotein-associated protein GAR1 that is sufficient for nucleolar accumulation. J Biol Chem. 1994;269:18499–18506. [PubMed] [Google Scholar]
- 17.Habets W J, Hoet M H, De Jong B A, Van der Kemp A, van Venrooij W J. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J Immunol. 1989;143:2560–2566. [PubMed] [Google Scholar]
- 18.Hughes J M. Functional base-pairing interaction between highly conserved elements of U3 small nucleolar RNA and the small ribosomal subunit RNA. J Mol Biol. 1996;259:645–654. doi: 10.1006/jmbi.1996.0346. [DOI] [PubMed] [Google Scholar]
- 19.Hughes J M, Ares M J. Depletion of U3 small nucleolar RNA inhibits cleavage in the 5′ external transcribed spacer of yeast pre-ribosomal RNA and impairs formation of 18S ribosomal RNA. EMBO J. 1991;10:4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes J M, Konings D A, Cesareni G. The yeast homologue of U3 snRNA. EMBO J. 1987;6:2145–2155. doi: 10.1002/j.1460-2075.1987.tb02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen R, Tollervey D, Hurt E C. A U3 snoRNP protein with homology to splicing factor PRP4 and G beta domains is required for ribosomal RNA processing. EMBO J. 1993;12:2549–2558. doi: 10.1002/j.1460-2075.1993.tb05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimenez-Garcia L F, Segura Valdez M L, Ochs R L, Rothblum L I, Hannan R, Spector D L. Nucleologenesis: U3 snRNA-containing prenucleolar bodies move to sites of active pre-rRNA transcription after mitosis. Mol Biol Cell. 1994;5:955–966. doi: 10.1091/mbc.5.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kass S, Tyc K, Steitz J A, Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990;60:897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- 24.Kiss-Laszlo Z, Henry Y, Bachellerie J P, Caizergues-Ferrer M, Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell. 1996;85:1077–1088. doi: 10.1016/s0092-8674(00)81308-2. [DOI] [PubMed] [Google Scholar]
- 25.Kreis T E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lübben B, Marshallsay C, Rottmann N, Lührmann R. Isolation of U3 snoRNP from CHO cells: a novel 55 kDa protein binds to the central part of U3 snoRNA. Nucleic Acids Res. 1993;21:5377–5385. doi: 10.1093/nar/21.23.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lygerou Z, Allmang C, Tollervey D, Seraphin B. Accurate processing of a eukaryotic precursor ribosomal RNA by ribonuclease MRP in vitro. Science. 1996;272:268–270. doi: 10.1126/science.272.5259.268. [DOI] [PubMed] [Google Scholar]
- 28.Lygerou Z, Pluk H, van Venrooij W J, Seraphin B. hPop1: an autoantigenic protein subunit shared by the human RNase P and RNase MRP ribonucleoproteins. EMBO J. 1996;15:5936–5948. [PMC free article] [PubMed] [Google Scholar]
- 29.Matera A G, Tycowski K T, Steitz J A, Ward D C. Organization of small nucleolar ribonucleoproteins (snoRNPs) by fluorescence in situ hybridization and immunocytochemistry. Mol Biol Cell. 1994;5:1289–1299. doi: 10.1091/mbc.5.12.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maxwell E S, Fournier M J. The small nucleolar RNAs. Annu Rev Biochem. 1995;64:897–934. doi: 10.1146/annurev.bi.64.070195.004341. [DOI] [PubMed] [Google Scholar]
- 31.Myslinski E, Segault V, Branlant C. An intron in the genes for U3 small nucleolar RNAs of the yeast Saccharomyces cerevisiae. Science. 1990;247:1213–1216. doi: 10.1126/science.1690452. [DOI] [PubMed] [Google Scholar]
- 32.Neer E J, Schmidt C J, Nambudripad R, Smith T F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- 33.Ni J W, Tien A L, Fournier M J. Small nucleolar RNAs direct site specific synthesis of pseudouridine in ribosomal RNA. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 34.Nicoloso M, Qu L H, Michot B, Bachellerie J P. Intron-encoded, antisense small nucleolar RNAs: the characterization of nine novel species points to their direct role as guides for the 2′-O-ribose methylation of rRNAs. J Mol Biol. 1996;260:178–195. doi: 10.1006/jmbi.1996.0391. [DOI] [PubMed] [Google Scholar]
- 35.Parker K A, Steitz J A. Structural analysis of the human U3 ribonucleoprotein particle reveals a conserved sequence available for base pairing with pre-rRNA. Mol Cell Biol. 1987;7:2899–2913. doi: 10.1128/mcb.7.8.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raska I, Andrade L E, Ochs R L, Chan E K, Chang C M, Roos G, Tan E M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991;195:27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- 37.Reimer G, Pollard K M, Penning C A, Ochs R L, Lischwe M A, Busch H, Tan E M. Monoclonal autoantibody from a (New Zealand black × New Zealand white)F1 mouse and some human scleroderma sera target an Mr 34,000 nucleolar protein of the U3 RNP particle. Arthritis Rheum. 1987;30:793–800. doi: 10.1002/art.1780300709. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherly D, Boelens W, van Venrooij W J, Dathan N A, Hamm J, Mattaj I W. Identification of the RNA binding segment of human U1 A protein and definition of its binding site on U1 snRNA. EMBO J. 1989;8:4163–4170. doi: 10.1002/j.1460-2075.1989.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt-Zachmann M S, Nigg E A. Protein localization to the nucleolus: a search for targeting domains in nucleoli. J Cell Sci. 1993;105:799–806. doi: 10.1242/jcs.105.3.799. [DOI] [PubMed] [Google Scholar]
- 42.Sillekens P T, Habets W J, Beijer R P, van Venrooij W J. cDNA cloning of the human U1 snRNA-associated A protein: extensive homology between U1 and U2 snRNP-specific proteins. EMBO J. 1987;6:3841–3848. doi: 10.1002/j.1460-2075.1987.tb02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takagaki Y, Manley J L. A human polyadenylation factor is a G protein beta-subunit homologue. J Biol Chem. 1992;267:23471–23474. [PubMed] [Google Scholar]
- 44.Terns M P, Dahlberg J E. Retention and 5′ cap trimethylation of U3 snRNA in the nucleus. Science. 1994;264:959–961. doi: 10.1126/science.8178154. [DOI] [PubMed] [Google Scholar]
- 45.Terns M P, Grimm C, Lund E, Dahlberg J E. A common maturation pathway for small nucleolar RNAs. EMBO J. 1995;14:4860–4871. doi: 10.1002/j.1460-2075.1995.tb00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tollervey D. Trans acting factors in ribosome synthesis. Exp Cell Res. 1996;229:226–232. doi: 10.1006/excr.1996.0364. [DOI] [PubMed] [Google Scholar]
- 47.Tyc K, Steitz J A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989;8:3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tycowski K T, Shu M D, Steitz J A. A small nucleolar RNA is processed from an intron of the human gene encoding ribosomal protein S3. Genes Dev. 1993;7:1176–1190. doi: 10.1101/gad.7.7a.1176. [DOI] [PubMed] [Google Scholar]
- 49.Tycowski K T, Smith C M, Shu M D, Steitz J A. A small nucleolar RNA requirement for site-specific ribose methylation of rRNA in Xenopus. Proc Natl Acad Sci USA. 1996;93:14480–14485. doi: 10.1073/pnas.93.25.14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Voorn L, Ploegh H L. The WD-40 repeat. FEBS Lett. 1992;307:131–134. doi: 10.1016/0014-5793(92)80751-2. [DOI] [PubMed] [Google Scholar]
- 51.van Gelder C W, Thijssen J P, Klaassen E C, Sturchler C, Krol A, van Venrooij W J, Pruijn G J. Common structural features of the Ro RNP associated hY1 and hY5 RNAs. Nucleic Acids Res. 1994;22:2498–2506. doi: 10.1093/nar/22.13.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verheijen R, Wiik A, De Jong B A, Hoier-Madsen M, Ullman S, Halberg P, van Venrooij W J. Screening for autoantibodies to the nucleolar U3- and Th(7-2) ribonucleoproteins in patients’ sera using antisense riboprobes. J Immunol Methods. 1994;169:173–182. doi: 10.1016/0022-1759(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 53.Yan C, Melese T. Multiple regions of NSR1 are sufficient for accumulation of a fusion protein within the nucleolus. J Cell Biol. 1993;123:1081–1091. doi: 10.1083/jcb.123.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]