ABSTRACT
Epigallocatechin gallate (EGCG), the major catechin in green tea, is of considerable interest principally due to its proposed antihypertensive and cardioprotective properties. New research shows that EGCG can help relax the circulation of blood vessels, reduce arterial stiffness of arteries, and promote antioxidant activity promotion, which results in lowering blood pressure (BP) and better‐improving heart health. It also affects signaling pathways related to nitric oxide (NO) production, inflammation, and oxidative stress, which are crucial for vascular homeostasis. Although animal research and clinical trials demonstrate that regular intake of EGCG significantly decreases BP and improves lipid profiles, further studies are needed to confirm these benefits in diverse populations. This review highlights the relevant biological data supporting these effects and the mechanisms by which EGCG impacts cardiovascular health. This review provides a new perspective on the many favorable effects of EGCG, such as its potential role in cardiovascular disease prevention, essential hypertension (HTN), and atherosclerosis. These results point to the need for more clinical trials aimed at determining whether EGCG may be used as a natural approach to reducing HTN and its cardiovascular complications through dietary interventions to enhance public health.
Keywords: cardioprotective, cardiovascular, epigallocatechin, green tea, herbal medicine, hypertension, phytotherapy
Abbreviations
- BP
blood pressure
- EGCG
epigallocatechin gallate
- HTN
hypertension
- MAP
mean arterial pressure
- MI
myocardial infarction
- NO
nitric oxide
- NOS
nitric oxide synthase
1. Introduction
Hypertension (HTN), often termed the “silent killer,” is a significant risk factor for cardiovascular diseases, which remain one of the leading causes of morbidity and mortality worldwide [1]. As the global prevalence of HTN continues to rise [2], there is growing interest in dietary and lifestyle interventions that may provide effective management and prevention strategies. Among these, epigallocatechin gallate (EGCG), a major catechin found in green tea, has emerged as a promising candidate due to its potential anti‐hypertensive and cardioprotective effects [2, 3, 4].
Numerous studies have highlighted EGCG's ability to modulate various physiological processes that contribute to cardiovascular health. Its antioxidant properties help combat oxidative stress, a key player in endothelial dysfunction and vascular inflammation [5, 6]. Additionally, EGCG has been shown to improve endothelial function, reduce arterial stiffness, and influence signaling pathways that regulate blood pressure (BP). These mechanisms suggest that EGCG could serve as a natural therapeutic agent for managing HTN and promoting heart health [5, 6, 7, 8].
This review seeks to consolidate current biological evidence supporting EGCG's antihypertensive and cardioprotective properties. It aims to provide new insights into EGCG's potential as a dietary intervention for HTN and cardiovascular disease prevention by clarifying its mechanisms of action and reviewing pertinent clinical and preclinical studies. Finally, studying EGCG's therapeutic properties may contribute to the development of effective natural strategies for regulating cardiovascular health.
2. Method
A comprehensive literature review was conducted to discover studies on EGCG. The searches were done from January 2000 to October 2024 using PubMed, Scopus, Web of Science, and Google Scholar. The search strategy used was (“Epigallocatechin gallate” OR “EGCG” OR “green tea”) AND (“hypertension” OR “blood pressure”) AND (“cardiovascular health” OR “heart disease” OR “vascular function”). Filters were used to combine English peer‐reviewed studies with clinical, preclinical, in vitro, and in vivo studies. Only articles with specific inclusion criteria were selected, such as RCTs, observational and clinical studies, and animal studies that investigated the effects of EGCG on cardiovascular parameters such as BP, endothelial function, and lipid metabolism. Only articles that complied with the above criteria and reported quantitative and qualitative cardiovascular outcomes were considered. Non‐peer‐reviewed articles, studies without a cardiovascular focus, and studies with unclear methodology and outcome measures were excluded.
The study selection process had three steps. Two authors, A.B. and R.E., independently examined the titles and abstracts to identify potentially relevant articles. The full‐text articles were then reviewed to determine relevancy and fulfill the inclusion criteria. The discrepancies were resolved through consensus or with the assistance of a third reviewer, M.P. The PRISMA flow diagram included information on the number of records identified, screened, and excluded.
The reviewers used a standardized form to extract data that was independent of them and included study characteristics such as author and year, population, intervention, dosage details, and results. Key outcomes included changes in BP, endothelial function, and lipid profiles. A narrative synthesis was used to compare the extracted data.
The results were given in tables summarizing study characteristics and findings, with additional figures depicting patterns in EGCG's cardiovascular effects. There was also a narrative synthesis that explained the trends and potential clinical applications of EGCG in cardiovascular health management.
3. EGCG
EGCG, a potent polyphenol primarily found in green tea, has shown significant therapeutic potential across various diseases due to its antioxidant, anti‐inflammatory, and anti‐carcinogenic properties [9, 10].
Green tea polyphenols have been shown to extend the lifespan of mice, flies, and nematodes [11, 12]. EGCG has neuroprotective effects [13, 14] and can prevent the progression of many types of tumors, such as endometrial adenocarcinoma, hepatocellular carcinoma, and lung cancer [15, 16, 17]. EGCG is beneficial in assuaging P. gingivalis‐induced periodontitis due to its anti‐inflammatory effects [18]. Treatment with EGCG can improve endothelial function [19], lower triglycerides (TGs), total cholesterol (TC), low‐density, and very low‐density lipoprotein cholesterol (LDL‐C) fractions, and increase high‐density lipoprotein cholesterol (HDL‐C) and leptin [20, 21]. For heart failure with diastolic failure and preserved systolic function, ECGC is of important interest [22]. It has been revealed that it can also decrease the size of myocardial infarctions (MI) and enhance the ultrastructure of cardiomyocytes [23].
4. EGCG and Its Effects on Various Pathologies
It has been shown to have promising effects in the treatment and prevention of non‐communicable chronic diseases [24], enhancing BP modulation [25] and decreasing the risk of diabetes [26]. Furthermore, EGCG improves lipid profile and blood glucose and has antioxidant, anti‐inflammatory [27], anti‐carcinogenic [9], and anti‐atherosclerotic effects [28]. EGCG attenuates the level of TGs [29], cholesterol, LDL [24], and free fatty acids concentrations [30]. It may also decline lipid absorption from the intestine, which is associated with improved liver TG concentrations and insulin resistance [31]. EGCG improves oxidative stress [32] and increases lipid oxidation [33], which are key obesity‐related factors. It controls appetite by modulating hormones [34] or delaying gastric chymus emptying [34]. Thus, EGCG may reduce body weight and pertinent diseases [35, 36].
EGCG affects stress [37]. It can attenuate corticosterone levels to provide hypnotic and anxiolytic effects [38]. It decreases anxiogenic behavior, improves the quality of sleep [39], and increases sleep time without any specific side effects [40]. In patients with MS, where the interleukin 6 (IL‐6) level is high, EGCG attenuates the levels of IL‐6 and improves anxiety and functional capacity [41]. Additionally, it reduces the risk of CVDs in patients with MS [41]. It has been demonstrated that EGCG has neuroprotective effects that decrease the severity of autoimmune encephalomyelitis by attenuating demyelination and brain inflammation damage [42].
The modulatory activity of EGCG on connective tissues has been widely investigated. It has been studied that EGCG has the ability to treat unexplained infertility and shrink uterine fibroids as one of the major causes of idiopathic infertility [43]. Moreover, EGCG has beneficial effects on bone‐related metabolic and differentiation processes [44]. EGCG can improve the healing of femoral bone defects [51] and is demonstrated to regulate the activities of several cells, which promotes the healing of bone fractures [45]. EGCG promotes wound healing because of its antioxidant, anti‐inflammatory, and antiangiogenic properties. It decreases scare thickness and positively affects elasticity, hydration, and erythema; thus, it can be useful in scare management [46]. In addition, EGCG solution, as prophylactic skin care, significantly decreases the severity and incidence of radiation‐induced dermatitis [47].
The potentialities of EGCG in various conditions have been widely studied. It enhances the efficacy of epidural catheter analgesia management in patients with multiple rib fractures [48] and some drugs, such as nintedanib [49], Lisinopril [50], and fexofenadine [51]. EGCG has also been demonstrated to inhibit the development and metastasis of different cancers, such as colon and prostate malignancies [52, 53, 54].
5. EGCG and HTN: Mechanisms of Actions
Typically, HTN arises from a combination of multiple factors, including environmental factors and genetic predisposition [55]. The conventional pathophysiology of HTN involves increased sympathetic nervous system activity, triggering the activation of the renin‐angiotensin‐aldosterone system, vascular endothelial dysfunction, insulin resistance, and the dysregulation of neurohormonal factors [56, 57]. Regrettably, even with the ongoing advancement of antihypertensive medications and the emergence of novel surgical techniques, effectively managing HTN remains considerably unsatisfactory [58]. This issue could be attributed, at least in part, to the current drugs not adequately targeting the relevant mechanisms and the counter‐regulatory responses triggered by these treatments, leading to a reduction in their ability to lower BP. As a result, there is a pressing need for innovative treatment approaches [57].
The renin‐angiotensin system (RAS) regulates the balance of sodium and BP by employing coordinated mechanisms within the central nervous system (CNS), kidney, and cardiovascular system [59, 60]. This system's effects are brought about by the conversion of angiotensinogen into angiotensin I through the action of renin, followed by the angiotensin‐converting enzyme (ACE) cleaving angiotensin I to produce angiotensin II (Ang II). As the ultimate effector of this system, Ang II activates angiotensin II type 1 receptors (AT1R) found in the CNS, kidneys, and blood vessels, leading to an increase in sympathetic activity, reabsorption of the sodium, and vasoconstriction [59].
EGCG exhibits mighty antioxidant and anti‐inflammatory properties, making it a recommended supplement for cardiac health. Studies have demonstrated that the molecules extracted from green tea have BP‐lowering effects, including lowered systolic BP and diastolic BP, −1.17 and −1.24 mm Hg [61]. In vivo, investigations have shown that the impact of EGCG on decreasing BP by 8.7% depends on both the duration and dosage of administration beyond 2 weeks [62] and almost reduced mean BP by about 8%–10% [63]. Preliminary research suggests that EGCG may act as a potential blocker by suppressing the activities of renin and ACE in both in vitro and in silico studies [64, 65]. In a 2022 study, EGCG progressively repressed the increase of systolic BP by ‐23, −17, −13, and −11 mmHg (reductions of 11.6%, 8.7%, 6.7%, and 5.9%) following 4, 3, 2, and 1 weeks of supplementation. These impressions on reducing BP were crucially correlated with intrarenal alterations in the Agtr2, Ace, and Ren transcriptional levels [3].
The pathophysiology of essential HTN goes beyond a simple increase in BP; it involves an accurate equilibrium between vasoconstrictors and vasodilators, which play a crucial role in this condition [66]. Disruption of this balance contributes to endothelial dysfunction, causing an extreme release of substances that have vasoconstrictive properties [66, 67, 68]. Another significant factor in the development of EH is the heightened stress of oxidation and changes in the total antioxidant capacity (TAC), commonly observed in various CVDs [69, 70, 71, 72, 73].
In recent years, it has become evident that oxidative stress plays a role in high BP by causing inflammation, kidney dysfunction, and vascular constriction [74]. It has been demonstrated that mitochondria, a notable origin of O2·− radicals, experience impaired functioning in HTN, illustrating its novel role in this disease [75, 76]. Research has shown that HTN and endothelial dysfunction are linked to the molecular process in which the dysfunction of mitochondrial deacetylase Sirtuin (Sirt)3 leads to hyperacetylation and so inactivation of SOD2, a pivotal antioxidant found in mitochondria [77]. Investigations on human and animal models with EH have revealed a significant reduction in Sirt3 expression and activity in association with HTN [77].
EGCG has been shown to have potent anti‐inflammatory and antioxidant features, making it useful for cardiovascular health [78]. The polyphenols found in green tea act as scavengers for reactive oxygen species (ROS), producing phenolic radicals that are more stable [79]. The potential of EGCG to scavenge radicals has been extensively studied, primarily due to its high concentration in green tea and the D and B ring containing galloyl group [79]. Through electron paramagnetic resonance (EPR) spectroscopy, it has been observed that EGCG interacts with O2–, resulting in the D‐ring oxidation [80]. Additionally, EPR studies have indicated that EGCG can also scavenge O2– and OH [81]. Alvarez‐Cilleros et al. have indicated that EGCG can prevent dysfunction of the endothelium in human umbilical vein endothelial cells (HUVECs) by reducing the generation of ROS and inhibiting stress‐related pathways [82].
Nitric oxide (NO) production via the endothelial isoform of nitric oxide synthase (eNOS) in the endothelial cells (ECs) plays a pivotal role in regulating vascular tone and systemic hemodynamic [83]. When NO reaches vascular smooth muscle (VSM), it facilitates the production of cyclic guanylate monophosphate (cGMP) through guanylate cyclase [84, 85]. Consequently, cGMP provokes smooth muscle relaxation and governs inflammation and cellular proliferation within the vessel wall [86]. To elaborate, diffusion of NO through the vascular smooth muscle cells (VSMCs) became a stimulant for activating the cGMP‐ protein kinase G (PKG) axis. This activation, in turn, triggers Ca2+‐activated potassium channels, leading to hyperpolarization of the membrane and inhibition of Ca2+ influx extracellularly and/or release of Ca2+ from the endoplasmic reticulum, ultimately causing vasodilation. Additionally, activation of the PKG reduces VSMC contractility by phosphorylation of myosin light chain phosphatase (MLCP) [87, 88]
EGCG exhibits various effects, including the modulation of Ca2+ channels and the reduction of ROS generation almost about 26%–30% [89]. Interestingly, studies conducted by Campos‐Toimil et al. in 2007 demonstrated that EGCG‐induced relaxation may have a biphasic nature. Initially, EGCG was found to trigger an influx of Ca2+ into smooth muscle cells of the aorta in rats through nonselective cation channels with Ca2+ permeability and voltage‐operated Ca2+ channels. EGCG's primary mode of action involves activating the phosphatidylinositol 3‐kinase (PI3K)‐Akt‐NO/cGMP pathway [90]. Additionally, EGCG inhibits PDE activity, leading to increased cyclic nucleotide levels of approximately 33%–88% [91]. In a study in 2022, it was demonstrated that EGCG therapy ameliorates endothelial dysfunction. The researchers hypothesized that EGCG treatment may elevate plasma NO levels, contributing to the detected rise in endothelium‐dependent relaxation in response to acetylcholine. This resulted in BP declining approximately 40% in comparison with angiotensin II‐infused hypertensive mice with no treatment [8]. However, among Male non‐smokers, 40–65 years old, with 28< BMI <38, EGCG decreased diastolic BP (mean change: placebo −0.058 vs. EGCG −2.68 mmHg) [92], and another randomized clinical trial by Wilasrusmee et al. discovered that EGCG can reduce SBP, DBP, and mean arterial pressure (MAP) by about 120.93–115.5, 81.87–77.85, and 94.89–90.57 mmHg, respectively [93].
Figure 1 demonstrates how EGCG decreases the risk of atherosclerosis by prohibiting Notch receptor activity induced by oxidized LDL. EGCG can decrease the ROS level in mitochondria and stabilize the potential of the mitochondrial membrane, therefore attenuating cell swelling and endothelial cell apoptosis. EGCG and catechin can increase the eNOS, preserving endothelial function. EGCG can limit oxidative stress by regulating the p38 MAPK and ERK1/2 pathways. The main mechanism involves the activation of PI3K by EGCG through a yet‐to‐be‐identified receptor site localized on the cell membrane or in the cytoplasm, which stimulates the NO/cGMP signaling cascade and, in turn, elicits vasorelaxation.
FIGURE 1.
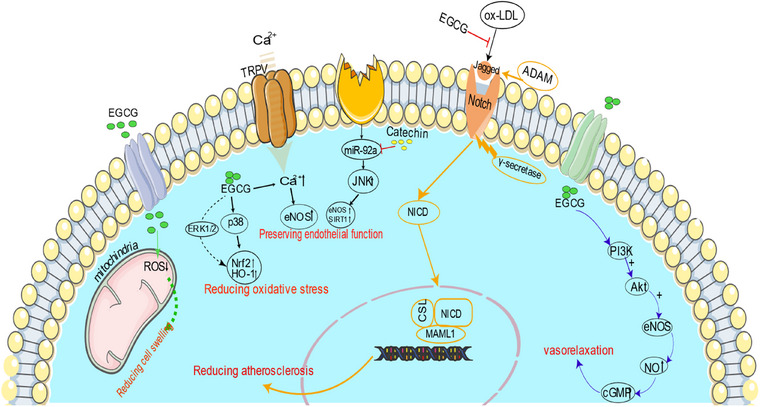
Protective mechanisms of Epigallocatechin‐3‐gallate (EGCG) against atherosclerosis through oxidative stress reduction and endothelial function preservation. Akt indicates α serine/threonine‐protein kinase; HO‐1, heme oxygenase‐1; NICD, Notch intracellular domain; Nrf, nuclear factor E2‐related factor; PI3K, phosphatidylinositol‐3‐kinase; TRPV, transient receptor potential vanilloid type.
6. EGCG and Other Vascular Diseases
6.1. Atherosclerosis
Atherosclerosis is a complex, chronic inflammatory condition affecting medium and large arteries driven by lipid accumulation. Key contributors to its progression include ECs, white blood cells, and smooth muscle cells within the artery walls. The most severe outcomes of atherosclerosis, like heart attack and stroke, result from the occurrence of additional blood clot formation [94]. Although atherosclerosis is typically associated with advancing age, it is noteworthy to mention that it is becoming more prevalent in younger individuals due to modern lifestyles. Nonetheless, it is commonly considered an ailment of the elderly [95]. Atherosclerosis follows a complex pathogenesis encompassing three significant factors: inflammation, lipid metabolism alteration, and endothelial lining damage [96].
Several studies have elucidated the potential benefits of EGCG, including anti‐inflammatory, antioxidant, and lipid‐lowering activities [25, 97]. Atherosclerosis‐related inflammation involves bioactive lipids, signaling pathways, proinflammatory cytokines, and adhesion molecules [98]. Signaling pathways such as NLRP3 inflammasome, Notch and Wnt signaling pathways, and toll‐like receptors are implicated in atherosclerosis development and regression [99]. Activation of Type II ECs through stress‐related stimuli like tumor necrosis factor‐α (TNF‐α) triggers the nuclear factor‐κB (NF‐κB) signaling pathway, a pivotal regulator of inflammatory responses [100]. In a study by Reddy et al., the inhibitory effect of EGCG on NF‐κB activation in ECs was investigated. HCAECs were stimulated with TNF‐α for an hour and treated with EGCG at the specific concentrations. The results showed that ECGG effectively suppressed NF‐κB transcriptional activity in TNF‐α stimulated HCAECs [100].
Furthermore, T cells secrete various inflammatory cytokines, significantly contributing to the progression of atherosclerosis. The impact of EGCG on cytokine production by human peripheral T cells was examined using a non‐toxic dosage range (0.1e20 mM) stimulation with P/I induced human T‐cell activation, EGCG dose‐dependently inhibited the production of INFg, TNF‐a, IL‐4, and IL‐2 in response to P/I stimulation [101]. Additionally, EGCG treatment was shown to reduce circulating levels of interferon‐γ monocyte chemoattractant protein‐1, interleukin‐6, and TNF‐α levels in apolipoprotein E‐deficient mice [102].
Altered lipid metabolism serves as a risk factor for the development and progression of atherosclerosis. Dyslipidemia plays a significant role in initiating atherosclerosis, where the accumulation of oxidized LDL triggers the disease onset. Oxidative stress on the Endothelium leads to the formation of oxidized cholesterol, named oxysterols. Both types of diabetes mellitus (DM) can induce or accelerate atherosclerosis development, with elevated glucose levels, dyslipidemia, and other metabolic changes closely implicated in atherosclerosis pathogenesis at various stages [103]. The Retention of LDL particles contributes to an increased risk of fatty streak development and atherosclerosis progression [104]. Although lipid‐lowering therapies have been effective in treating atherosclerosis, CVDs remain the leading global cause of death [105].
In many studies, promising EGCG influences on lipid profile include reducing the levels of TC, TG, LDL‐C, and VLDL by approximately 75%–80%, 7%–8%, 10%–11%, and 6%–7%, respectively, and increasing the level of HDL‐C approximately about 50% with a high dose of EGCG in comparison with a model group during 10 weeks and diabetes indicators have been demonstrated [106, 107]. In a study by Niu et al., EGCG was found to potentially lower TC, TG, LDL‐C, and serum glucose (GLU) levels. In addition, it increased NO and HDL‐C levels, particularly during the later stage of the experiment [97]. In a study by Wang et al., EGCG administration was found to decrease atherosclerotic plaque formation in mice by increasing anti‐inflammatory cytokine and interleukin‐10 levels and reducing pro‐inflammatory cytokine, IL‐6 and TNF‐α levels. Furthermore, EGCG modulated high‐fat‐induced dyslipidemia, evidenced by reducing TC, TG, and LDL‐C levels by approximately 3%, 10%, and 2%–3%, respectively, and increasing HDL‐C levels by about 15% [102].
The endothelium is responsible for generating vasodilator (NO) and vasoconstrictor (endothelin) molecules. An imbalance in the production of these vasoactive substances results in the deterioration of endothelial function, known as endothelial dysfunction [27, 108]. The healthy functioning of the endothelium involves maintaining dynamic vascular tone, facilitating angiogenesis, regulating hemostasis, and serving as an antioxidant, anti‐inflammatory, and antithrombotic barrier. Vascular endothelial dysfunction manifests as compromised vasodilation, increased oxidative stress, chronic inflammation, elevated leukocyte adhesion and permeability, as well as endothelial cell aging [109]. Initiated by endothelial injury, the accumulation and infiltration of the modified LDL particles within the subendothelial space transpire [103].
Previous reports exhibited the protective feature of EGCG against CVDs through preserving endothelial function via anti‐inflammatory and anti‐oxidative influences, besides inducing NO production [110, 111]. Similarly, other studies showed that EGCG‐induced vasorelaxation primarily relies on a NO‐dependent mechanism, as evidenced by experiments involving the inhibition of endothelial NO production [112, 113, 114]. In a study by Kim et al. EGCG reduced intracellular lipid accumulation in aortic ECs by promoting the co‐localization of lipid droplets, LDs, and autolysosomes [115]. Furthermore, EGCG treatment showed promise in increasing fibrous cap thickness and reducing atherosclerotic lesions by about 25%, and also decreasing levels of TC, TG, and LDL‐C by approximately 50%, 30%, 30%–33%, respectively, and increasing the level of HDL‐C about 66% [116]. Moreover, compared to the control group, the EGCG‐treated groups exhibited decreased expression levels of VEGFA and MMP‐2 in cardiac tissues, indicating a potential inhibitory effect of ECGC on atherosclerosis, including reducing the levels of TC, TG, LDL‐C, approximately 25%, 33%, 30% respectively, and increased the level of HDL‐C approximately about 50% with a high dose of EGCG in comparison with model group in mice with coronary heart disease [117].
6.2. Aortic Aneurysm
Abdominal aortic aneurysm (AAA) is a life‐threatening vascular disease. This disease is characterized by an irreversible localized dilation in the abdominal aorta; the aortic wall diameter is ≥3.0 cm or at least 50% greater than its normal diameter [118, 119]. Male sex, advanced age, tobacco use, obesity, genetic predisposition, older age, and family history are known as AAA risk factors [120, 121]. This disease affects 4%–8% of the male population over 65 years old, 4–6 times more than the female population. This dilation can exceed abruptly and end up in a ruptured AAA. Therefore, only 50% of these cases can make it to a medical center alive; among these patients, only 50% go through an emergent surgical repair [122, 123]. Rupture, as the most common vital consequence of aortic aneurysm, is in charge of 60% of the mortality rate [133]. In total, AAA annually results in 150 000–200 000 deaths globally and is considered a public health concern [123]. AAA pathophysiology is defined as a multifactorial process that consists of inflammatory responses, matrix metalloproteinase (MMP) activation, oxidative stress, intraluminal thrombus, smooth muscle apoptosis, and extracellular matrix (ECM) degeneration in the latest studies [124, 125, 126]. As a result, there is no FDA‐approved, safe, and effective medication available for this disease so far, and the only accepted management is an invasive endovascular intervention [127]. Preventive surgery is indicated in patients with an aortic wall diameter ≥5.5, asymptomatic patients with an AAA >4 cm that has grown >1 cm in 1 year, and symptomatic patients with any diameter [128]. Intervention is indicated when the risk of rupture takes over the risk of the surgery itself [123, 129], so a reliable treatment is needed for patients with aneurism that is not susceptible to rupture to decrease the aortic wall diameter rise or to decrease the risk of aortic aneurysm rupture [129, 130]. ECM degeneration is known as a mechanism that weakens the aorta wall and leads to aortic aneurysms [131]. The degeneration of elastin, one of the main ECM components in charge of vasodilation and rupture resistance in arteries, has a clarified role in aneurysm generation and progress [132]. Therefore, many studies have shown that it is a target for medications. In the following paragraph, we will look at some studies investigating the effect of Epigallocatechin on elastin degeneration and AAAs. Sinha et al. showed that polyphenols such as epigallocatechin have elastin regenerative effects on rat's primary aortic smooth muscle cells by investigating it in an in vitro environment. They reported that the group of cultured cells that were exposed to polyphenols gained more insoluble cross‐linked elastin in their structure [133]. Ellis and co‐workers reported that EGCG enhances elastin formation by increasing elastin gene expression and replicating mRNA half‐life in induced pluripotent stem cell‐derived VSMCs for tissue engineering [134]. Jin et al. published their study on the EGCG effect on stabilizing elastin fibers in pericardium cells in an in vitro environment, and they showed no broken elastic fibers in cells exposed to EGCG [135].
Setozaki et al. showed that EGCG intake can decrease the aorta's wall diameter in rats with abdominal aortic diameter induced by focal elastase injection in the aorta's wall. They compared two groups of rats. The intervention group took EGCG 2 weeks before the induction and continued for 2 weeks after induction. The aorta wall diameter was increased after induction in both groups, but in the intervention group, it was not as large as in the control group. However, it was almost smaller in the EGCG group, about 20%, in comparison with the control group (2.3 vs. 2.9 mm). The Intima layer was thinner in the intervention group, yet the media layer was thicker. Elastin composition was significantly higher in the intervention group before the intervention, and there was no noticeable difference in elastin decrease in both groups, but after the second day, elastin composition increased in the intervention group significantly. They reported that no adverse effect was detected in the intervention group, including no stenosis and no thickness in carotid arteries or coronary arteries [136]. This study was confirmed by David Tilson in a commentary letter. He reported that the results of their previous experiments and the Setozaki study support each other and are not controversial [137]. Another murine study was performed and reported in 2006 by Ro and colleagues; they reported significant differences in AAA dilation between two groups of rats that were/were not treated by AneuMastat. Furthermore, untreated mice had a significantly larger aorta diameter at sacrifice relative to their starting size (768 vs. 532 µm, p = 0.001). Additionally, no significant increase in aorta size was seen within treated or Sham mouses (550 vs. 580 µm and 478 vs. 477 µm). Aneumastat is a polyphenol‐rich EGCG that can be used as a preventive compound [138]. Another study by Tyrie et al. examined aneumastat as a preventive medication for AAA. They reported that although there was a significant decrease in the prevalence of AAA in mice taking aneumastat, AAAs (1.5× normal diameter) have been identified in 55% of the AngII group and 20% of the AngII AneuMastat(R) group. There was no histopathologic difference in aortic aneurysms between the two groups [139]. Since EGCG is one of the grape‐seed polyphenol (GSP) components, looking at the study that Ma et al. performed in 2020 is not out of grace. They examined the effect of GSP on AAA development. They demonstrated that GSP intake significantly inhibited AAA formation in male mice [140]. Another study was published 3 years ahead of Ma's study by Wang et al. which was quite similar. In this study, grape seed polyphenol administration also inhibited AAA development in rats [141].
6.3. Coronary Artery Disease (CAD)
CAD is a common heart condition recognized by stable angina, unstable angina, MI, or sudden cardiac death [142]. CAD develops due to plaque formation within the intima of the coronary artery walls. This occurs through the buildup of lipids and immune cells in the artery's subendothelium. The process triggers an inflammatory response in the vascular endothelium, leading to endothelial dysfunction, which is the primary driver of both atherosclerosis and CAD [143, 144–147].
The benefits of EGEC are categorized into three parts: Its effects on preventing and reversing endothelial dysfunction due to control plaque formation, its benefits on managing CAD‐related risk factors such as HTN, hyperlipidemia, or DM, and finally, its positive effects in treating patients with CAD [148, 149, 150].
Polyphenols such as EGCG enhance endothelial function and induce vascular relaxation, resulting in protective influences on the development and progression of CVDs [151]. Following MI, irreversible microscopic changes occur in the myocardium due to hypoxia [152, 153]. Although rapid reperfusion can help reverse the negative effect of hypoxia, several studies demonstrate that reperfusion could damage the myocardium through many pathways [154, 155, 156]. One of the main molecules involved in this process is ROS [155]. Other causes are immediate cellular PH correction and intracellular Ca+ increases [154]. Patients who have experienced acute myocardial ischemia and are candidates for reperfusion treatment might have some myocardial reperfusion injuries, such as cardiac dysfunction, an increase in myocardial infarct size, and cardiac fibrosis due to myocardial apoptosis.
It has been seen that the administration of EGCG in these patients reduces the reperfusion damage with different molecular pathways, of which the most important ones are tyrosine kinases and the SIRT1 signaling pathway in diabetic rats [157, 158]. In addition, in an experimental study on isoproterenol‐induced MI rats by Othman et al., EGCG exhibited a significant positive effect, especially if it was used as an early intervention in MI, by preserving redox balance and applying anti‐apoptotic character [26].
Myocardial apoptosis is a process that occurs in MI. Amongst numerous active molecules in apoptosis, P53 and bcl‐2 are shown to play a crucial role. The EGCG has an inhibitory effect on both of them; thereby, it has a positive influence against apoptosis. Thus, EGCG ameliorates cardiac hypertrophy, also the heart weight index (HWI) and left ventricular weight index (LVWI) were raised by 55.8% and 72.3%, respectively [159], and cardiac injury via reperfusion in MI in rats [159, 160]. Also, it has been demonstrated that EGCG positively affects the PI3K/Akt signaling pathway, leading to reduced ischemic reperfusion cardiac injury [161]. Furthermore, EGCG has a cardioprotective effect against reperfusion damage by inhibiting STAT‐1 activation [162]. EGCG positively impacts serum lipid profile as a major cardiovascular risk factor and improves the antioxidant system and myocardial fiber morphology to protect patients with a high hypercholesterolemia diet against hypercholesteremia‐related cardiovascular abnormalities [163]. Several studies suggest that EGCG is a complementary treatment in managing DM, another risk factor for cardiovascular disease by several molecular pathways [164, 165, 166], and EGCG exhibited protective effects against cardiovascular events in type 2 diabetes patients prone to CAD [26].
EGCG inhibits the development and progression of cancer by reducing angiogenesis, cell proliferation, invasion, and metastasis, enhancing apoptosis, and overcoming chemoresistance by regulating the activity of associated molecules. It also increases plasma and intracellular drug concentration and enhances drug efficacy.
7. Conclusions
Table 1 represents the cardioprotective potentials of EGCG reported by various studies. Figure 2 summarizes the effect of EGCG on cardiovascular health.
TABLE 1.
Cardioprotective potentials of EGCG reported by various studies.
| Disease | Dose | Target (s) | Effect (s) | Model | Cell line/animal | Ref. |
|---|---|---|---|---|---|---|
| HTN | 50, 250, 500 or 1000 mg/kg |
Atgr2, Ace2, Agtr2, Ace, and Mas1 Ren mRNA |
Activated the intrarenal counter‐regulatory axis (ACE/AngII/AT2R) |
In vivo (animal) | Rat | [3] |
| 50 mg/kg | BH4, cGMP, eNOS, NOx‐2 | Antioxidant effects | In vivo (animal) | C57BL/6J mice | [8] | |
| 10 mg/kg | Oatp1a5, Oct1, P‐gp | Reduced nadolol bioavailability | In vivo (animal) | Rat | [167] | |
| 1 mM | KCNQ5 | Hyperpolarization of smooth muscle and vasodilation leading to mesenteric artery relaxation | In vitro (animal) in vivo (animal) |
Stages V and VI Xenopus laevis oocytes Rat |
[168] | |
| 50 mg/kg/12 h | MDA, S100A4 | Reduced urine volume, proteinuria, kidney fibrosis, oxidative, and inflammatory damage | In vivo (animal) | Dahl/SS rats | [169] | |
| 300 mg/kg | miRNA‐126a‐3p, miRNA‐150‐5p, SP1, AT1R | Upregulated miRNAs, which are mainly involved in vascular smooth cell or endothelial cell apoptosis, proliferation, and migration, angiogenic pathways like MAPK and PI3K/Akt/eNOS | In vivo (animal) | SD rats | [170] | |
|
200 mg/kg 1–100 µM |
PI3K, NO, adiponectin |
Stimulated endothelial production of NO, which is dependent on activation of PI3K, thus reduced BP. Enhanced the ability of insulin to mediate vasorelaxation Improved insulin sensitivity and adiponectin levels Mimicked vasodilator actions of insulin |
In vivo (animal) in vitro (animal) | WKY, male SHR‐mesenteric vascular beds isolated from SHR | [171] | |
| 50, 100 or 200 mg/kg/d | MFN‐2, KLF‐4/MFN‐2/p‐Erk1/2 signaling |
Inhibited pulmonary artery smooth muscle cells Promoted mitochondrial fusion and inhibited proliferation |
In vivo (animal) | Male SD rats | [172] | |
| 20 mg/h. | NF‐kB, GAD67, IL‐1b and TH | Restored the balance between the excitatory and inhibitory neurotransmitters | In vivo (animal) | Male normotensive WKY rats | [173] | |
| 458 mmol/L | PAH, TH, DBH, PNMT, SOD1, HDAC7, Noxa1 | Altered epigenetic regulators antioxidant effect | In vivo (animal) | WKY rats | [174] | |
| 50 µM |
MAPK, MMP‐2, NF‐κB, Spm–Cer–S1P, SPHK, ERK1/2 SMase |
Anti‐proliferative effects | In vitro (animal) | Bovine pulmonary artery smooth muscle cells | [175] | |
|
2.5 µM, 75 mg/kg |
HO‐1, p38 MAPK, Nrf‐2 |
Anti‐atherogenic effects | In vitro (human) in vivo (animal) | Human aortic endothelial cells, C57BL/6J mice | [176] | |
| 10 µM | NF‐κB, TNF‐α, ICAM, VCAM, CCL2 | Anti‐inflammatory effects | In vitro (human) | HCAECs | [100] | |
| 300 mg/day | Kisspeptin, NO |
Stimulated production of NO from endothelium Downregulated level of kisspeptin as a probable vasoactive substance |
Human (clinical) | — | [25] | |
| 800 mg/day | — | Anti‐hypertensive effects (reduced mean SBP: −2.85, DBP: −2.68 from baseline | Human (clinical) | — | [92] | |
| 300 mg/day | — | Anti‐hypertensive effects (reduced approximately mean SBP: −4.2%, DBP: −4.9%, MAP: −4.5% from baseline | Human (clinical) | — | [93] | |
| Cardiac hypertrophy | 10–100 µM | ANP, BNP, IKKβ/NF‐κB signaling, ERKs, p38, JNKs, AP‐1, c‐Fos, c‐Jun |
Blocked EGFR transactivation and its downstream events Attenuated NADPH oxidase |
In vitro (animal) in vivo (animal) |
Neonatal ventricular myocytes Male SD rats |
[177] |
| 50 mg/kg | ANP, MDA, GPx, SOD, CAT, NF‐κB AP‐1, MMP‐9 MMP‐2 |
Attenuated pressure overload‐induced LV hypertrophy Antioxidant effect |
In vivo (animal) | Male SD rats | [178] | |
| Stroke | — | — |
Delayed the stroke onset by 10% of the average lifespan Lowered the rate of increase in SBP and DBP Decreased nighttime and daytime HR |
In vivo (animal) | Malignant Stroke‐prone spontaneously hypertensive rats | [179] |
| 10–50 µM |
JNK signaling c‐jun mRNA |
Inhibited Ang II‐stimulated VSMC hypertrophy | In vitro (animal) | Aortic smooth muscle cells of SD rats | [180] | |
| Pre‐eclampsia | 100 mg | — | Enhanced the efficacy of nifedipine in pregnancy‐mediated severe pre‐eclampsia patients | Human (clinical) | Pregnant women | [181] |
| AAA | 20 mg/day (EGCG solution | TNF‐α, IL‐1β, MMP‐1, MMP‐9 |
Preserved the aortic thickness and elastin content of the medial layer via elastin regeneration Anti‐inflammatory effect |
In vivo (animal) | Male rats | [136] |
| CaCl2‐induced AAA | 250 mg/kg | Elastin, collagen fibrils |
Prevented aortic aneurysmal dilation Anti‐inflammatory effect |
In vivo (animal) | C57BL6 mice | [138] |
| AngII‐induced AAA | — | — | Reduced aortic aneurysm incidence | In vivo (animal) | C57BL6 mice | [139] |
FIGURE 2.
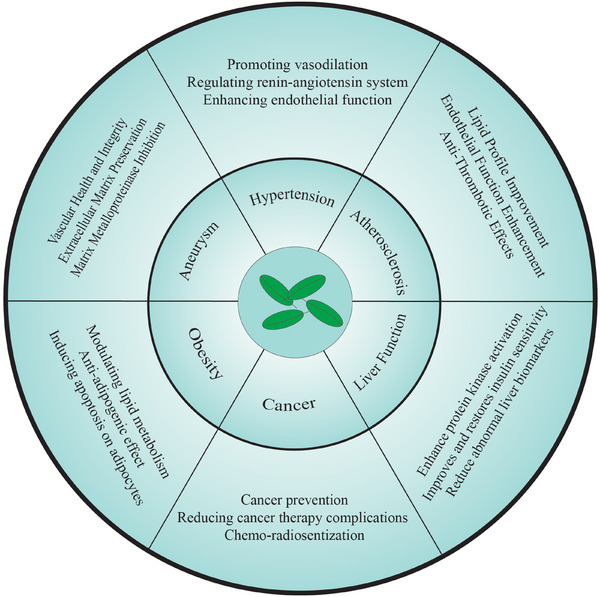
Cardioprotective effects of EGCG on cardiovascular health.
In conclusion, EGCG possesses impressive anti‐hypertensive and cardioprotective properties encompassing a range of complex biological pathways. The level of research into its antioxidant capacity, ability to modify NO synthesis, and its role in inhibiting critical components of the renin‐angiotensin system are well documented. These actions increase vascular function, decrease oxidative stress, and lower BP. In addition to its BP‐lowering effects, EGCG has been demonstrated to suppress pathological changes in the heart, including cardiac hypertrophy, fibrosis, and inflammation, which are commonly observed in chronic HTN and cardiovascular diseases. The ability of this compound to target cellular signaling pathways associated with oxidative stress, inflammation, and fibrosis further supports its broad‐spectrum therapeutic activities. Although these positive preclinical and early clinical results are highly promising, further research is warranted to clarify the mechanisms of action and to evaluate the long‐term efficacy and safety of EGCG in humans, particularly in patients with HTN or other cardiovascular conditions. This emerging body of research points to the promise of natural compounds such as EGCG for developing novel therapeutic and preventive strategies for cardiovascular disease.
Author Contributions
M.H.P. developed the idea and supervised the project. R.E., A.B., F.T.I., M.K., M.S.S., M.H.P., M.Y., A.B., F.Q.T., J.S., F.H., and A.R. contributed to the primary drafting of the manuscript. M.K. reviewed and revised the manuscript. All authors critically read and approved the final manuscript.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Reza Eshraghi, Ashkan Bahrami, Faraz Tayyar Iravanlou, and Mehdi Karimi contributed equally to this work.
Funding: The authors received no specific funding for this work.
Contributor Information
Mehdi Karimi, Email: Karimi9010@gmail.com.
Mohammad Hossein Pourhanifeh, Email: mhph.lord.1996@gmail.com.
Data Availability Statement
No new data were generated.
References
- 1. Staessen J. A., Wang J., Bianchi G., and Birkenhäger W. H., “Essential Hypertension,” Lancet 361, no. 9369 (2003): 1629–1641. [DOI] [PubMed] [Google Scholar]
- 2. Mills K. T., Bundy J. D., Kelly T. N., et al., “Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population‐Based Studies From 90 Countries,” Circulation 134, no. 6 (2016): 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parn K. W., Ling W. C., Chin J. H., and Lee S. K., “Safety and Efficacy of Dietary Epigallocatechin Gallate Supplementation in Attenuating Hypertension via Its Modulatory Activities on the Intrarenal Renin‐Angiotensin System in Spontaneously Hypertensive Rats,” Nutrients 14, no. 21 (2022): 4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James A., Wang K., and Wang Y., “Therapeutic Activity of Green Tea Epigallocatechin‐3‐Gallate on Metabolic Diseases and Non‐Alcoholic Fatty Liver Diseases: The Current Updates,” Nutrients 15, no. 13 (2023): 3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babu P. V. and Liu D., “Green Tea Catechins and Cardiovascular Health: An Update,” Current Medicinal Chemistry 15, no. 18 (2008): 1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng J., Chen Y., Wang J., et al., “EGCG Protects Vascular Endothelial Cells From Oxidative Stress‐Induced Damage by Targeting the Autophagy‐Dependent PI3K‐AKT‐mTOR Pathway,” Annals of Translational Medicine 8, no. 5 (2020): 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei X.‐Y., Zeng Y.‐F., Guo Q.‐H., et al., “Cardioprotective Effect of Epigallocatechin Gallate in Myocardial Ischemia/Reperfusion Injury and Myocardial Infarction: A Meta‐Analysis in Preclinical Animal Studies,” Scientific Reports 13, no. 1 (2023): 14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mohd Sabri N. A., Lee S. K., Murugan D. D., and Ling W. C., “Epigallocatechin Gallate (EGCG) Alleviates Vascular Dysfunction in Angiotensin II‐Infused Hypertensive Mice by Modulating Oxidative Stress and eNOS,” Scientific Reports 12, no. 1 (2022): 17633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Min K. J. and Kwon T. K., “Anticancer Effects and Molecular Mechanisms of Epigallocatechin‐3‐Gallate,” Integrative Medicine Research 3, no. 1 (2014): 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Granja A., Frias I., Neves A. R., Pinheiro M., and Reis S., “Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems,” BioMed Research International 2017 (2017): 5813793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unno K., Pervin M., Taguchi K., Konishi T., and Nakamura Y., “Green Tea Catechins Trigger Immediate‐Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening,” Molecules (Basel, Switzerland) 25, no. 7 (2020): 1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez T., Schriner S. E., Okoro M., et al., “Green Tea Polyphenols Extend the Lifespan of Male Drosophila Melanogaster While Impairing Reproductive Fitness,” Journal of Medicinal Food 17, no. 12 (2014): 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ehrnhoefer D. E., Bieschke J., Boeddrich A., et al., “EGCG Redirects Amyloidogenic Polypeptides Into Unstructured, Off‐Pathway Oligomers,” Nature Structural & Molecular Biology 15, no. 6 (2008): 558–566. [DOI] [PubMed] [Google Scholar]
- 14. Afzal M., Safer A. M., and Menon M., “Green Tea Polyphenols and Their Potential Role in Health and Disease,” Inflammopharmacology 23, no. 4 (2015): 151–161. [DOI] [PubMed] [Google Scholar]
- 15. Wang H., Bian S., and Yang C. S., “Green Tea Polyphenol EGCG Suppresses Lung Cancer Cell Growth Through Upregulating miR‐210 Expression Caused by Stabilizing HIF‐1α,” Carcinogenesis 32, no. 12 (2011): 1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roomi M. W., Roomi N. W., Kalinovsky T., Niedzwiecki A., and Rath M., “Micronutrient Synergy in the Fight Against Hepatocellular Carcinoma,” Cancers (Basel) 4, no. 2 (2012): 323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mei X., Wu Y. Y., Mao X., and Tu Y. Y., “Antagonism of Phenanthrene Cytotoxicity for Human Embryo Lung Fibroblast Cell Line HFL‐I by Green Tea Polyphenols,” Environmental Pollution 159, no. 1 (2011): 164–168. [DOI] [PubMed] [Google Scholar]
- 18. Cai Y., Chen Z., Liu H., Xuan Y., Wang X., and Luan Q., “Green Tea Epigallocatechin‐3‐Gallate Alleviates Porphyromonas Gingivalis‐Induced Periodontitis in Mice,” International Immunopharmacology 29, no. 2 (2015): 839–845. [DOI] [PubMed] [Google Scholar]
- 19. Widlansky M. E., Hamburg N. M., Anter E., et al., “Acute EGCG Supplementation Reverses Endothelial Dysfunction in Patients With Coronary Artery Disease,” Journal of the American College of Nutrition 26, no. 2 (2007): 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu X., Pan J., and Zhou X., “Amelioration of Lipid Profile and Level of Antioxidant Activities by Epigallocatechin‐Gallate in a Rat Model of Atherogenesis,” Heart, Lung and Circulation 23, no. 12 (2014): 1194–1201. [DOI] [PubMed] [Google Scholar]
- 21. Huang L. H., Liu C. Y., Wang L. Y., Huang C. J., and Hsu C. H., “Effects of Green Tea Extract on Overweight and Obese Women With High Levels of Low Density‐Lipoprotein‐Cholesterol (LDL‐C): A Randomised, Double‐Blind, and Cross‐Over Placebo‐Controlled Clinical Trial,” BMC Complementary and Alternative Medicine [Electronic Resource] 18, no. 1 (2018): 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y., Karim M. R., Wang B., and Peng J., “Effects of Green Tea (‐)‐Epigallocatechin‐3‐Gallate (EGCG) on Cardiac Function—A Review of the Therapeutic Mechanism and Potentials,” Mini ‐ Reviews in Medicinal Chemistry 22, no. 18 (2022): 2371–2382. [DOI] [PubMed] [Google Scholar]
- 23. Tu Q., Jiang Q., Xu M., et al., “EGCG Decreases Myocardial Infarction in Both I/R and MIRI Rats Through Reducing Intracellular Ca2+ and Increasing TnT Levels in Cardiomyocytes,” Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University 30, no. 6 (2021): 607–616. [DOI] [PubMed] [Google Scholar]
- 24. Chen I. J., Liu C. Y., Chiu J. P., and Hsu C. H., “Therapeutic Effect of High‐Dose Green Tea Extract on Weight Reduction: A Randomized, Double‐Blind, Placebo‐Controlled Clinical Trial,” Clinical Nutrition 35, no. 3 (2016): 592–599. [DOI] [PubMed] [Google Scholar]
- 25. Chatree S., Sitticharoon C., Maikaew P., et al., “Epigallocatechin Gallate Decreases Plasma Triglyceride, Blood Pressure, and Serum Kisspeptin in Obese Human Subjects,” Experimental Biology and Medicine (Maywood, NJ) 246, no. 2 (2021): 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Othman A. I., El‐Sawi M. R., El‐Missiry M. A., and Abukhalil M. H., “Epigallocatechin‐3‐Gallate Protects Against Diabetic Cardiomyopathy Through Modulating the Cardiometabolic Risk Factors, Oxidative Stress, Inflammation, Cell Death and Fibrosis in Streptozotocin‐Nicotinamide‐Induced Diabetic Rats,” Biomedicine & Pharmacotherapy 94 (2017): 362–373. [DOI] [PubMed] [Google Scholar]
- 27. Sun H.‐J., Wu Z.‐Y., Nie X.‐W., and Bian J.‐S., “Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide,” Frontiers in Pharmacology 10 (2020): 1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yin J., Huang F., Yi Y., Yin L., and Peng D., “EGCG Attenuates Atherosclerosis Through the Jagged‐1/Notch Pathway,” International Journal of Molecular Medicine 37, no. 2 (2016): 398–406. [DOI] [PubMed] [Google Scholar]
- 29. de Morais Junior A. C., Schincaglia R. M., Passarelli M., Pimentel G. D., and Mota J. F., “Acute Epigallocatechin‐3‐Gallate Supplementation Alters Postprandial Lipids After a Fast‐Food Meal in Healthy Young Women: A Randomized, Double‐Blind, Placebo‐Controlled Crossover Study,” Nutrients 12, no. 9 (2020): 2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan H., Li Y., Ling F., et al., “The Phytochemical Epigallocatechin Gallate Prolongs the Lifespan by Improving Lipid Metabolism, Reducing Inflammation and Oxidative Stress in High‐Fat Diet‐Fed Obese Rats,” Aging Cell 19, no. 9 (2020): e13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y. K., Cheung C., Reuhl K. R., et al., “Effects of Green Tea Polyphenol (‐)‐Epigallocatechin‐3‐Gallate on Newly Developed High‐Fat/Western‐Style Diet‐Induced Obesity and Metabolic Syndrome in Mice,” Journal of Agricultural and Food Chemistry 59, no. 21 (2011): 11862–11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cazzola R. and Rondanelli M., “N‐Oleoyl‐Phosphatidyl‐Ethanolamine and Epigallo Catechin‐3‐Gallate Mitigate Oxidative Stress in Overweight and Class I Obese People on a Low‐Calorie Diet,” Journal of Medicinal Food 23, no. 3 (2020): 319–325. [DOI] [PubMed] [Google Scholar]
- 33. Willems M. E. T., Şahin M. A., and Cook M. D., “Matcha Green Tea Drinks Enhance Fat Oxidation During Brisk Walking in Females,” International Journal of Sport Nutrition and Exercise Metabolism 28, no. 5 (2018): 536–541. [DOI] [PubMed] [Google Scholar]
- 34. Fernandes R. C., Araújo V. A., Giglio B. M., et al., “Acute Epigallocatechin 3 Gallate (egcg) Supplementation Delays Gastric Emptying in Healthy Women: A Randomized, Double‐Blind, Placebo‐Controlled Crossover Study,” Nutrients 10, no. 8 (2018): 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S. N., Kwon H. J., Akindehin S., Jeong H. W., and Lee Y. H., “Effects of Epigallocatechin‐3‐Gallate on Autophagic Lipolysis in Adipocytes,” Nutrients 9, no. 7 (2017): 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoshitomi R., Yamamoto M., Kumazoe M., et al., “The Combined Effect of Green Tea and α‐Glucosyl Hesperidin in Preventing Obesity: A Randomized Placebo‐Controlled Clinical Trial,” Scientific Reports 11, no. 1 (2021): 19067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unno K., Furushima D., Hamamoto S., et al., “Stress‐Reducing Function of Matcha Green Tea in Animal Experiments and Clinical Trials,” Nutrients 10, no. 10 (2018): 1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vignes M., Maurice T., Lanté F., et al., “Anxiolytic Properties of Green Tea Polyphenol (‐)‐Epigallocatechin Gallate (EGCG),” Brain Research 1110, no. 1 (2006): 102–115. [DOI] [PubMed] [Google Scholar]
- 39. Caro D. C., Rivera D. E., Ocampo Y., Franco L. A., and Salas R. D., “Pharmacological Evaluation of Mentha Spicata L. and Plantago Major L., Medicinal Plants Used to Treat Anxiety and Insomnia in Colombian Caribbean Coast,” Evidence‐Based Complementary and Alternative Medicine 2018 (2018): 5921514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tubbs A. S., Kennedy K. E. R., Alfonso‐Miller P., Wills C. C. A., and Grandner M. A., “A Randomized, Double‐Blind, Placebo‐Controlled Trial of a Polyphenol Botanical Blend on Sleep and Daytime Functioning,” International Journal of Environmental Research and Public Health 18, no. 6 (2021): 3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benlloch M., Cuerda Ballester M., Drehmer E., et al., “Possible Reduction of Cardiac Risk After Supplementation With Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients With Multiple Sclerosis. A Pilot Study,” Nutrients 12, no. 12 (2020): 3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J., Ren Z., Xu Y., Xiao S., Meydani S. N., and Wu D., “Epigallocatechin‐3‐Gallate Ameliorates Experimental Autoimmune Encephalomyelitis by Altering Balance Among CD4+ T‐Cell Subsets,” American Journal of Pathology 180, no. 1 (2012): 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siblini H., Al‐Hendy A., Segars J., et al., “Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive‐Aged Women,” Nutrients 15, no. 2 (2023): 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shen C. L., Yeh J. K., Cao J. J., and Wang J. S., “Green Tea and Bone Metabolism,” Nutrition Research 29, no. 7 (2009): 437–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Madhurakkat Perikamana S. K., Lee S. M., Lee J., et al., “Oxidative Epigallocatechin Gallate Coating on Polymeric Substrates for Bone Tissue Regeneration,” Macromolecular Bioscience 19, no. 4 (2019): e1800392. [DOI] [PubMed] [Google Scholar]
- 46. Ud‐Din S., Foden P., Mazhari M., et al., “A Double‐Blind, Randomized Trial Shows the Role of Zonal Priming and Direct Topical Application of Epigallocatechin‐3‐Gallate in the Modulation of Cutaneous Scarring in Human Skin,” Journal of Investigative Dermatology 139, no. 8 (2019): 1680–1690.e16. [DOI] [PubMed] [Google Scholar]
- 47. Zhao H., Zhu W., Zhao X., et al., “Efficacy of Epigallocatechin‐3‐Gallate in Preventing Dermatitis in Patients With Breast Cancer Receiving Postoperative Radiotherapy: A Double‐Blind, Placebo‐Controlled, Phase 2 Randomized Clinical Trial,” JAMA Dermatology 158, no. 7 (2022): 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang L., Liu W., You H., Chen Z., Xu L., and He H., “Assessing the Analgesic Efficacy of Oral Epigallocatechin‐3‐Gallate on Epidural Catheter Analgesia in Patients After Surgical Stabilisation of Multiple Rib Fractures: A Prospective Double‐Blind, Placebo‐Controlled Clinical Trial,” Pharmaceutical Biology 58, no. 1 (2020): 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veerman G. D. M., van der Werff S. C., Koolen S. L. W., et al., “The Influence of Green Tea Extract on Nintedanib's Bioavailability in Patients With Pulmonary Fibrosis,” Biomedicine & Pharmacotherapy 151 (2022): 113101. [DOI] [PubMed] [Google Scholar]
- 50. Misaka S., Ono Y., Uchida A., et al., “Impact of Green Tea Catechin Ingestion on the Pharmacokinetics of Lisinopril in Healthy Volunteers,” Clinical and Translational Science 14, no. 2 (2021): 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Misaka S., Ono Y., Taudte R. V., et al., “Exposure of Fexofenadine, but Not Pseudoephedrine, Is Markedly Decreased by Green Tea Extract in Healthy Volunteers,” Clinical Pharmacology & Therapeutics 112, no. 3 (2022): 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hao X., Xiao H., Ju J., Lee M. J., Lambert J. D., and Yang C. S., “Green Tea Polyphenols Inhibit Colorectal Tumorigenesis in Azoxymethane‐Treated F344 Rats,” Nutrition and Cancer 69, no. 4 (2017): 623–631. [DOI] [PubMed] [Google Scholar]
- 53. Luo K. L., Luo J. H., and Yu Y. P., “(‐)‐Epigallocatechin‐3‐Gallate Induces Du145 Prostate Cancer Cell Death via Downregulation of Inhibitor of DNA Binding 2, a Dominant Negative Helix‐Loop‐Helix Protein,” Cancer Science 101, no. 3 (2010): 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fujiki H., Sueoka E., Watanabe T., and Suganuma M., “Primary Cancer Prevention by Green Tea, and Tertiary Cancer Prevention by the Combination of Green Tea Catechins and Anticancer Compounds,” Journal of Cancer Prevention 20, no. 1 (2015): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sekar D., Shilpa B. R., and Das A. J., “Relevance of microRNA 21 in Different Types of Hypertension,” Current Hypertension Reports 19, no. 7 (2017): 57. [DOI] [PubMed] [Google Scholar]
- 56. McMaster W. G., Kirabo A., Madhur M. S., and Harrison D. G., “Inflammation, Immunity, and Hypertensive End‐Organ Damage,” Circulation Research 116, no. 6 (2015): 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pugh D. and Dhaun N., “Hypertension and Vascular Inflammation: Another Piece of the Genetic Puzzle,” Hypertension 77, no. 1 (2021): 190–192. [DOI] [PubMed] [Google Scholar]
- 58. Wang Z., Chen Z., Zhang L., et al., “Status of Hypertension in China: Results From the China Hypertension Survey, 2012–2015,” Circulation 137, no. 22 (2018): 2344–2356. [DOI] [PubMed] [Google Scholar]
- 59. Sparks M. A., Crowley S. D., Gurley S. B., Mirotsou M., and Coffman T. M., “Classical Renin‐Angiotensin System in Kidney Physiology,” Comprehensive Physiology 4, no. 3 (2014): 1201–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oliveira‐Paula G. H., Pereira S. C., Tanus‐Santos J. E., and Lacchini R., “Pharmacogenomics and Hypertension: Current Insights,” Pharmacogenomics and Personalized Medicine 12 (2019): 341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu R., Yang K., Ding J., and Chen G., “Effect of Green Tea Supplementation on Blood Pressure: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials,” Medicine 99, no. 6 (2020): e19047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tan H. J., Ling W. C., Chua A. L., and Lee S. K., “Oral Epigallocatechin Gallate Reduces Intestinal Nadolol Absorption via Modulation of Oatp1a5 and Oct1 Transcriptional Levels in Spontaneously Hypertensive Rats,” Phytomedicine 90 (2021): 153623. [DOI] [PubMed] [Google Scholar]
- 63. Zhang Q., Hu L., Chen L., et al., “(‐)‐Epigallocatechin‐3‐Gallate, the Major Green Tea Catechin, Regulates the Desensitization of β1 Adrenoceptor via GRK2 in Experimental Heart Failure,” Inflammopharmacology 26, no. 4 (2018): 1081–1091. [DOI] [PubMed] [Google Scholar]
- 64. Ke Z., Su Z., Zhang X., et al., “Discovery of a Potent Angiotensin Converting Enzyme Inhibitor via Virtual Screening,” Bioorganic & Medicinal Chemistry Letters 27, no. 16 (2017): 3688–3692. [DOI] [PubMed] [Google Scholar]
- 65. Li F., Takahashi Y., and Yamaki K., “Inhibitory Effect of Catechin‐Related Compounds on Renin Activity,” Biomedical Research 34, no. 3 (2013): 167–171. [DOI] [PubMed] [Google Scholar]
- 66. Olczak K. J., Taylor‐Bateman V., Nicholls H. L., Traylor M., Cabrera C. P., and Munroe P. B., “Hypertension Genetics Past, Present and Future Applications,” Journal of Internal Medicine 290, no. 6 (2021): 1130–1152. [DOI] [PubMed] [Google Scholar]
- 67. Konukoglu D. and Uzun H., “Endothelial Dysfunction and Hypertension,” Advances in Experimental Medicine and Biology 956 (2017): 511–540. [DOI] [PubMed] [Google Scholar]
- 68. Masaki T. and Sawamura T., “Endothelin and Endothelial Dysfunction,” Proceedings of The Japan Academy Series B‐Physical and Biological Sciences 82, no. 1 (2006): 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. González J., Valls N., Brito R., and Rodrigo R., “Essential Hypertension and Oxidative Stress: New Insights,” World Journal of Cardiology 6, no. 6 (2014): 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu Y., Ding Y., Ramprasath T., and Zou M. H., “Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension,” Antioxidants & Redox Signaling 34, no. 9 (2021): 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Senoner T. and Dichtl W., “Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target?” Nutrients 11, no. 9 (2019): 2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sorriento D., De Luca N., Trimarco B., and Iaccarino G., “The Antioxidant Therapy: New Insights in the Treatment of Hypertension,” Frontiers in Physiology 9 (2018): 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kattoor A. J., Pothineni N. V. K., Palagiri D., and Mehta J. L., “Oxidative Stress in Atherosclerosis,” Current Atherosclerosis Reports 19, no. 11 (2017): 42. [DOI] [PubMed] [Google Scholar]
- 74. Harrison D. G., Marvar P. J., and Titze J. M., “Vascular Inflammatory Cells in Hypertension,” Frontiers in Physiology 3 (2012): 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dikalova A. E., Bikineyeva A. T., Budzyn K., et al., “Therapeutic Targeting of Mitochondrial Superoxide in Hypertension,” Circulation Research 107, no. 1 (2010): 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dikalov S. I., Nazarewicz R. R., Bikineyeva A., et al., “Nox2‐Induced Production of Mitochondrial Superoxide in Angiotensin II‐Mediated Endothelial Oxidative Stress and Hypertension,” Antioxidants & Redox Signaling 20, no. 2 (2014): 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dikalova A. E., Itani H. A., Nazarewicz R. R., et al., “Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension,” Circulation Research 121, no. 5 (2017): 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pervin M., Unno K., Takagaki A., Isemura M., and Nakamura Y., “Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites,” International Journal of Molecular Sciences 20, no. 15 (2019): 3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Forester S. C. and Lambert J. D., “The Role of Antioxidant Versus Pro‐Oxidant Effects of Green Tea Polyphenols in Cancer Prevention,” Molecular Nutrition & Food Research 55, no. 6 (2011): 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Severino J. F., Goodman B. A., Kay C. W., et al., “Free Radicals Generated During Oxidation of Green Tea Polyphenols: Electron Paramagnetic Resonance Spectroscopy Combined With Density Functional Theory Calculations,” Free Radical Biology and Medicine 46, no. 8 (2009): 1076–1088. [DOI] [PubMed] [Google Scholar]
- 81. Shi X., Ye J., Leonard S. S., et al., “Antioxidant Properties of (‐)‐Epicatechin‐3‐Gallate and Its Inhibition of Cr(VI)‐Induced DNA Damage and Cr(IV)‐ or TPA‐Stimulated NF‐kappaB Activation,” Molecular and Cellular Biochemistry 206, no. 1‐2 (2000): 125–132. [DOI] [PubMed] [Google Scholar]
- 82. Álvarez‐Cilleros D., Ramos S., Goya L., and Martín M., “Colonic Metabolites From Flavanols Stimulate Nitric Oxide Production in Human Endothelial Cells and Protect Against Oxidative Stress‐Induced Toxicity and Endothelial Dysfunction,” Food and Chemical Toxicology 115 (2018): 88–97. [DOI] [PubMed] [Google Scholar]
- 83. Farah C., Michel L. Y. M., and Balligand J. L., “Nitric Oxide Signalling in Cardiovascular Health and Disease,” Nature Reviews Cardiology 15, no. 5 (2018): 292–316. [DOI] [PubMed] [Google Scholar]
- 84. Cary S. P., Winger J. A., and Marletta M. A., “Tonic and Acute Nitric Oxide Signaling Through Soluble Guanylate Cyclase Is Mediated by Nonheme Nitric Oxide, ATP, and GTP,” PNAS 102, no. 37 (2005): 13064–13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Denninger J. W. and Marletta M. A., “Guanylate Cyclase and the .NO/cGMP Signaling Pathway,” Biochimica Et Biophysica Acta 1411, no. 2‐3 (1999): 334–350. [DOI] [PubMed] [Google Scholar]
- 86. Klinger J. R., “The Nitric Oxide/cGMP Signaling Pathway in Pulmonary Hypertension,” Clinics in Chest Medicine 28, no. 1 (2007): 143–167. ix. [DOI] [PubMed] [Google Scholar]
- 87. Gao Y., Chen T., and Raj J. U., “Endothelial and Smooth Muscle Cell Interactions in the Pathobiology of Pulmonary Hypertension,” American Journal of Respiratory Cell and Molecular Biology 54, no. 4 (2016): 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Puzserova A. and Bernatova I., “Blood Pressure Regulation in Stress: Focus on Nitric Oxide‐Dependent Mechanisms,” Physiological Research 65, no. Suppl 3 (2016): S309–S342. [DOI] [PubMed] [Google Scholar]
- 89. Liu P. L., Liu J. T., Kuo H. F., Chong I. W., and Hsieh C. C., “Epigallocatechin Gallate Attenuates Proliferation and Oxidative Stress in Human Vascular Smooth Muscle Cells Induced by Interleukin‐1β via Heme Oxygenase‐1,” Mediators of Inflammation (2014): 523684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Romano M. R. and Lograno M. D., “Epigallocatechin‐3‐Gallate Relaxes the Isolated Bovine Ophthalmic Artery: Involvement of Phosphoinositide 3‐Kinase‐Akt‐Nitric Oxide/cGMP Signalling Pathway,” European Journal of Pharmacology 608, no. 1‐3 (2009): 48–53. [DOI] [PubMed] [Google Scholar]
- 91. Alvarez E., Campos‐Toimil M., Justiniano‐Basaran H., Lugnier C., and Orallo F., “Study of the Mechanisms Involved in the Vasorelaxation Induced by (‐)‐Epigallocatechin‐3‐Gallate in Rat Aorta,” British Journal of Pharmacology 147, no. 3 (2006): 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brown A. L., Lane J., Coverly J., et al., “Effects of Dietary Supplementation With the Green Tea Polyphenol Epigallocatechin‐3‐Gallate on Insulin Resistance and Associated Metabolic Risk Factors: Randomized Controlled Trial,” British Journal of Nutrition 101, no. 6 (2009): 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wilasrusmee K. T., Sitticharoon C., Keadkraichaiwat I., et al., “Epigallocatechin Gallate Enhances Sympathetic Heart Rate Variability and Decreases Blood Pressure in Obese Subjects: A Randomized Control Trial,” Scientific Reports 14, no. 1 (2024): 21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Falk E., “Pathogenesis of Atherosclerosis,” Journal of the American College of Cardiology 47, no. Suppl 8 (2006): C7–12. [DOI] [PubMed] [Google Scholar]
- 95. Tohirova J. and Shernazarov F., “Atherosclerosis: Causes, Symptoms, Diagnosis, Treatment and Prevention,” Science and Innovation 1, no. D5 (2022): 7–12. [Google Scholar]
- 96. Poznyak A. V., Bharadwaj D., Prasad G., Grechko A. V., Sazonova M. A., and Orekhov A. N., “Renin‐Angiotensin System in Pathogenesis of Atherosclerosis and Treatment of CVD,” International Journal of Molecular Sciences 22, no. 13 (2021): 6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Niu Y., Na L., Feng R., et al., “The Phytochemical, EGCG, Extends Lifespan by Reducing Liver and Kidney Function Damage and Improving Age‐Associated Inflammation and Oxidative Stress in Healthy Rats,” Aging Cell 12, no. 6 (2013): 1041–1049. [DOI] [PubMed] [Google Scholar]
- 98. Zhu Y., Xian X., Wang Z., et al., “Research Progress on the Relationship Between Atherosclerosis and Inflammation,” Biomolecules 8, no. 3 (2018): 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kong P., Cui Z. Y., Huang X. F., Zhang D. D., Guo R. J., and Han M., “Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention,” Signal Transduction and Targeted Therapy 7, no. 1 (2022): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Reddy A. T., Lakshmi S. P., Maruthi Prasad E., Varadacharyulu N. C., and Kodidhela L. D., “Epigallocatechin Gallate Suppresses Inflammation in Human Coronary Artery Endothelial Cells by Inhibiting NF‐κB,” Life Sciences 258 (2020): 118136. [DOI] [PubMed] [Google Scholar]
- 101. Huang S. C., Kao Y. H., Shih S. F., et al., “Epigallocatechin‐3‐Gallate Exhibits Immunomodulatory Effects in Human Primary T Cells,” Biochemical and Biophysical Research Communications 550 (2021): 70–76. [DOI] [PubMed] [Google Scholar]
- 102. Wang Q., Zhang J., Li Y., et al., “Green Tea Polyphenol Epigallocatechin‐3‐Gallate Increases Atherosclerotic Plaque Stability in Apolipoprotein E‐Deficient Mice Fed a High‐Fat Diet,” Kardiologia Polska (Polish Heart Journal) 76, no. 8 (2018): 1263–1270. [DOI] [PubMed] [Google Scholar]
- 103. Poznyak A., Grechko A. V., Poggio P., Myasoedova V. A., Alfieri V., and Orekhov A. N., “The Diabetes Mellitus‐Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation,” International Journal of Molecular Sciences 21, no. 5 (2020): 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Khatana C., Saini N. K., Chakrabarti S., et al., “Mechanistic Insights Into the Oxidized Low‐Density Lipoprotein‐Induced Atherosclerosis,” Oxidative Medicine and Cellular Longevity 2020 (2020): 5245308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen W., Schilperoort M., Cao Y., Shi J., Tabas I., and Tao W., “Macrophage‐Targeted Nanomedicine for the Diagnosis and Treatment of Atherosclerosis,” Nature Reviews Cardiology 19, no. 4 (2022): 228–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang C. S., Wang X., Lu G., and Picinich S. C., “Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and human Relevance,” Nature Reviews Cancer 9, no. 6 (2009): 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li W., Zhu C., Liu T., et al., “Epigallocatechin‐3‐Gallate Ameliorates Glucolipid Metabolism and Oxidative Stress in Type 2 Diabetic Rats,” Diabetes & Vascular Disease Research 17, no. 6 (2020): 1479164120966998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Medina‐Leyte D. J., Domínguez‐Pérez M., Mercado I., Villarreal‐Molina M. T., and Jacobo‐Albavera L., “Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review,” Applied Sciences 10, no. 3 (2020): 938. [Google Scholar]
- 109. Xu S., Ilyas I., Little P. J., et al., “Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies,” Pharmacological Reviews 73, no. 3 (2021): 924–967. [DOI] [PubMed] [Google Scholar]
- 110. Eng Q. Y., Thanikachalam P. V., and Ramamurthy S., “Molecular Understanding of Epigallocatechin Gallate (EGCG) in Cardiovascular and Metabolic Diseases,” Journal of Ethnopharmacology 210 (2018): 296–310. [DOI] [PubMed] [Google Scholar]
- 111. Wang Z. M., Gao W., Wang H., et al., “Green Tea Polyphenol Epigallocatechin‐3‐Gallate Inhibits TNF‐α‐Induced Production of Monocyte Chemoattractant Protein‐1 in human Umbilical Vein Endothelial Cells,” Cellular Physiology and Biochemistry 33, no. 5 (2014): 1349–1358. [DOI] [PubMed] [Google Scholar]
- 112. Lorenz M., Wessler S., Follmann E., et al., “A Constituent of Green Tea, Epigallocatechin‐3‐Gallate, Activates Endothelial Nitric Oxide Synthase by a Phosphatidylinositol‐3‐OH‐kinase‐, cAMP‐Dependent Protein Kinase‐, and Akt‐Dependent Pathway and Leads to Endothelial‐Dependent Vasorelaxation,” Journal of Biological Chemistry 279, no. 7 (2004): 6190–6195. [DOI] [PubMed] [Google Scholar]
- 113. Kim J. A., Formoso G., Li Y., et al., “Epigallocatechin Gallate, a Green Tea Polyphenol, Mediates NO‐Dependent Vasodilation Using Signaling Pathways in Vascular Endothelium Requiring Reactive Oxygen Species and Fyn,” Journal of Biological Chemistry 282, no. 18 (2007): 13736–13745. [DOI] [PubMed] [Google Scholar]
- 114. Aggio A., Grassi D., Onori E., et al., “Endothelium/Nitric Oxide Mechanism Mediates Vasorelaxation and Counteracts Vasoconstriction Induced by Low Concentration of Flavanols,” European Journal of Nutrition 52, no. 1 (2013): 263–272. [DOI] [PubMed] [Google Scholar]
- 115. Kim H.‐S., Montana V., Jang H.‐J., Parpura V., and Kim J.‐A., “Epigallocatechin Gallate (EGCG) Stimulates Autophagy in Vascular Endothelial Cells: A Potential Role for Reducing Lipid Accumulation*♦,” Journal of Biological Chemistry 288, no. 31 (2013): 22693–22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang W., Zhang Z.‐Z., Wu Y., et al., “(–)‐Epigallocatechin‐3‐Gallate Ameliorates Atherosclerosis and Modulates Hepatic Lipid Metabolic Gene Expression in Apolipoprotein E Knockout Mice: Involvement of TTC39B,” Frontiers in Pharmacology 9 (2018): 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huang X., Chu Y., Ren H., and Pang X., “Antioxidation Function of EGCG by Activating Nrf2/HO‐1 Pathway in Mice With Coronary Heart Disease,” Contrast Media & Molecular Imaging 2022 (2022): 8639139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Amioka N. and Miyoshi T., “Fibrates: A Possible Treatment Option for Patients With Abdominal Aortic Aneurysm?,” Biomolecules 12, no. 1 (2022): 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cameron S. J. and Russell H. M., “Antithrombotic Therapy in Abdominal Aortic Aneurysm: Beneficial or Detrimental?,” Blood, The Journal of the American Society of Hematology 132, no. 25 (2018): 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. DeRoo E., Stranz A., Yang H., Hsieh M., Se C., and Zhou T., “Endothelial Dysfunction in the Pathogenesis of Abdominal Aortic Aneurysm,” Biomolecules 12, no. 4 (2022): 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Golledge J., “Abdominal Aortic Aneurysm: Update on Pathogenesis and Medical Treatments,” Nature Reviews Cardiology 16, no. 4 (2019): 225–242. [DOI] [PubMed] [Google Scholar]
- 122. Weaver L. M., Loftin C. D., and Zhan C.‐G., “Development of Pharmacotherapies for Abdominal Aortic Aneurysms,” Biomedicine & Pharmacotherapy 153 (2022): 113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Puertas‐Umbert L., Almendra‐Pegueros R., Jimenez‐Altayo F., et al., “Novel Pharmacological Approaches in Abdominal Aortic Aneurysm,” Clinical Science 137, no. 15 (2023): 1167–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yuan Z., Lu Y., Wei J., Wu J., Yang J., and Cai Z., “Abdominal Aortic Aneurysm: Roles of Inflammatory Cells,” Frontiers in Immunology 11 (2021): 609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Piacentini L., Werba J. P., Bono E., et al., “Genome‐Wide Expression Profiling Unveils Autoimmune Response Signatures in the Perivascular Adipose Tissue of Abdominal Aortic Aneurysm,” Arteriosclerosis, Thrombosis, and Vascular Biology 39, no. 2 (2019): 237–249. [DOI] [PubMed] [Google Scholar]
- 126. Sakalihasan N., Michel J.‐B., Katsargyris A., et al., “Abdominal Aortic Aneurysms,” Nature Reviews Disease Primers 4, no. 1 (2018): 34. [DOI] [PubMed] [Google Scholar]
- 127. Liu J., Liu M., Feng J., et al., “Alpha‐Ketoglutarate Ameliorates Abdominal Aortic Aneurysm via Inhibiting PXDN/HOCL/ERK Signaling Pathways,” Journal of Translational Medicine 20, no. 1 (2022): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kyriacou H., Mostafa A. M., Sumal A. S., Hellawell H. N., and Boyle J. R., “Abdominal Aortic Aneurysms Part Two: Surgical Management, Postoperative Complications and Surveillance,” Journal of Perioperative Practice 31, no. 9 (2021): 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Golledge J., Moxon J., Singh T., Bown M., Mani K., and Wanhainen A., “Lack of an Effective Drug Therapy for Abdominal Aortic Aneurysm,” Journal of Internal Medicine 288, no. 1 (2020): 6–22. [DOI] [PubMed] [Google Scholar]
- 130. Golledge J., Thanigaimani S., Powell J. T., and Tsao P. S., “Pathogenesis and Management of Abdominal Aortic Aneurysm,” European Heart Journal 44, no. 29 (2023): 2682–2697, 10.1093/eurheartj/ehad386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wang X., Lane B. A., Eberth J. F., Lessner S. M., and Vyavahare N. R., “Gold Nanoparticles That Target Degraded Elastin Improve Imaging and Rupture Prediction in an AngII Mediated Mouse Model of Abdominal Aortic Aneurysm,” Theranostics 9, no. 14 (2019): 4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Qian G., Adeyanju O., Olajuyin A., and Guo X., “Abdominal Aortic Aneurysm Formation With a Focus on Vascular Smooth Muscle Cells,” Life 12, no. 2 (2022): 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sinha A., Nosoudi N., and Vyavahare N., “Elasto‐Regenerative Properties of Polyphenols,” Biochemical and Biophysical Research Communications 444, no. 2 (2014): 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ellis M. W., Riaz M., Huang Y., et al., “Epigallocatechin Gallate Facilitates Extracellular Elastin fiber Formation in Induced Pluripotent Stem Cell Derived Vascular Smooth Muscle Cells for Tissue Engineering,” Journal of Molecular and Cellular Cardiology 163 (2022): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jin W., Guo G., Chen L., Lei Y., and Wang Y., “Elastin Stabilization Through Polyphenol and Ferric Chloride Combined Treatment for the Enhancement of Bioprosthetic Heart Valve Anticalcification,” Artificial Organs 42, no. 11 (2018): 1062–1069. [DOI] [PubMed] [Google Scholar]
- 136. Setozaki S., Minakata K., Masumoto H., et al., “Prevention of Abdominal Aortic Aneurysm Progression by Oral Administration of Green Tea Polyphenol in a Rat Model,” Journal of Vascular Surgery 65, no. 6 (2017): 1803–1812.e2. [DOI] [PubMed] [Google Scholar]
- 137. Tilson M. D., “Epigallocatechin Gallate for Medical Management of the Abdominal Aortic Aneurysm,” Journal of Vascular Surgery 73, no. 4 (2021): 1471. [DOI] [PubMed] [Google Scholar]
- 138. Ro C. Y. and Fukumoto R., “AneuMastat (R) Prevents Aneurysm Formation in a Murine Model of Abdominal Aortic Aneurysm (AAA),” Journal of the American College of Surgeons 203, no. 3 (2006): S103. [Google Scholar]
- 139. Tyrie L. S., Fujikura K., Luo J., et al., “AneuMastat Reduces Aneurysm Incidence in the Angiotensin II (AngII)‐Induced Model of Abdominal Aortic Aneurysm (AAA) in the Wildtype C57BL6 Mouse,” Journal of the American College of Surgeons 205, no. 3 (2007): S111. [Google Scholar]
- 140. MA D., Shen H. T., Zhao J. J., et al., “Grape‐Seed Polyphenols Inhibit AAA in Mice via Regulation of Macrophage Polarization,” Bratislava Medical Journal/Bratislavske Lekarske Listy 121, no. 9 (2020): 680–685, 10.4149/BLL_2020_106. [DOI] [PubMed] [Google Scholar]
- 141. Wang C., Wang Y., Yu M., et al., “Grape‐Seed Polyphenols Play a Protective Role in Elastase‐Induced Abdominal Aortic Aneurysm in Mice,” Scientific Reports 7, no. 1 (2017): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Álvarez‐Álvarez M. M., Zanetti D., Carreras‐Torres R., Moral P., and Athanasiadis G., “A Survey of Sub‐Saharan Gene Flow Into the Mediterranean at Risk Loci for Coronary Artery Disease,” European Journal of Human Genetics 25, no. 4 (2017): 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Shao C., Wang J., Tian J., and Tang Y.‐D., “Coronary Artery Disease: From Mechanism to Clinical Practice,” Coronary Artery Disease: Therapeutics and Drug Discovery 1177 (2020): 1–36. [DOI] [PubMed] [Google Scholar]
- 144. Gao W., Liu H., Yuan J., et al., “Exosomes Derived From Mature Dendritic Cells Increase Endothelial Inflammation and Atherosclerosis via Membrane TNF‐α Mediated NF‐κB Pathway,” Journal of Cellular and Molecular Medicine 20, no. 12 (2016): 2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Herrero‐Fernandez B., Gomez‐Bris R., Somovilla‐Crespo B., and Gonzalez‐Granado J. M., “Immunobiology of Atherosclerosis: A Complex Net of Interactions,” International Journal of Molecular Sciences 20, no. 21 (2019): 5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Marchio P., Guerra‐Ojeda S., Vila J. M., Aldasoro M., Victor V. M., and Mauricio M. D., “Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation,” Oxidative Medicine and Cellular Longevity 2019 (2019): 8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Medina‐Leyte D. J., Zepeda‐García O., Domínguez‐Pérez M., González‐Garrido A., Villarreal‐Molina T., and Jacobo‐Albavera L., “Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches,” International Journal of Molecular Sciences 22, no. 8 (2021): 3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Babu P. V. A., Si H., and Liu D., “Epigallocatechin Gallate Reduces Vascular Inflammation in db/db Mice Possibly Through an NF‐κ B‐Mediated Mechanism,” Molecular Nutrition & Food Research 56, no. 9 (2012): 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dludla P. V., Nkambule B. B., Mazibuko‐Mbeje S. E., et al., “Tea Consumption and Its Effects on Primary and Secondary Prevention of Coronary Artery Disease: Qualitative Synthesis of Evidence From Randomized Controlled Trials,” Clinical Nutrition ESPEN 41 (2021): 77–87. [DOI] [PubMed] [Google Scholar]
- 150. Reiter C. E., Kim J.‐A., and Quon M. J., “Green Tea Polyphenol Epigallocatechin Gallate Reduces Endothelin‐1 Expression and Secretion in Vascular Endothelial Cells: Roles for AMP‐Activated Protein Kinase, Akt, and FOXO1,” Endocrinology 151, no. 1 (2010): 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yamagata K., “Polyphenols Regulate Endothelial Functions and Reduce the Risk of Cardiovascular Disease,” Current Pharmaceutical Design 25, no. 22 (2019): 2443–2458. [DOI] [PubMed] [Google Scholar]
- 152. Zhang Y., Wen W., and Liu H., “The Role of Immune Cells in Cardiac Remodeling After Myocardial Infarction,” Journal of Cardiovascular Pharmacology 76, no. 4 (2020): 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chen B.‐H., An D.‐A., He J., et al., “Myocardial Extracellular Volume Fraction Radiomics Analysis for Differentiation of Reversible Versus Irreversible Myocardial Damage and Prediction of Left Ventricular Adverse Remodeling After ST‐Elevation Myocardial Infarction,” European Radiology 31 (2021): 504–514. [DOI] [PubMed] [Google Scholar]
- 154. Wu M.‐Y., Yiang G.‐T., Liao W.‐T., et al., “Current Mechanistic Concepts in Ischemia and Reperfusion Injury,” Cellular Physiology and Biochemistry 46, no. 4 (2018): 1650–1667. [DOI] [PubMed] [Google Scholar]
- 155. Zhou T., Chuang C.‐C., and Zuo L., “Molecular Characterization of Reactive Oxygen Species in Myocardial Ischemia‐Reperfusion Injury,” BioMed Research International 2015 (2015): 864946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Raedschelders K., Ansley D. M., and Chen D. D., “The Cellular and Molecular Origin of Reactive Oxygen Species Generation During Myocardial Ischemia and Reperfusion,” Pharmacology & Therapeutics 133, no. 2 (2012): 230–255. [DOI] [PubMed] [Google Scholar]
- 157. Stangl V., Dreger H., Stangl K., and Lorenz M., “Molecular Targets of Tea Polyphenols in the Cardiovascular System,” Cardiovascular Research 73, no. 2 (2007): 348–358. [DOI] [PubMed] [Google Scholar]
- 158. Wu Y., Xia Z.‐Y., Zhao B., et al., “(‐)‐Epigallocatechin‐3‐Gallate Attenuates Myocardial Injury Induced by Ischemia/Reperfusion in Diabetic Rats and in H9c2 Cells Under Hyperglycemic Conditions,” International Journal of Molecular Medicine 40, no. 2 (2017): 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sheng R., Gu Z.‐L., Xie M.‐L., Zhou W.‐X., and Guo C.‐Y., “EGCG Inhibits Cardiomyocyte Apoptosis in Pressure Overload‐Induced Cardiac Hypertrophy and Protects Cardiomyocytes From Oxidative Stress in Rats,” Acta Pharmacologica Sinica 28, no. 2 (2007): 191–201. [DOI] [PubMed] [Google Scholar]
- 160. Piao C. S., Kim D.‐S., Ha K.‐C., Kim H.‐R., Chae H.‐J., and Chae S.‐W., “The Protective Effect of Epigallocatechin‐3 Gallate on Ischemia/Reperfusion Injury in Isolated Rat Hearts: An Ex Vivo Approach,” Korean Journal of Physiology & Pharmacology 15, no. 5 (2011): 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Xuan F. and Jian J., “Epigallocatechin Gallate Exerts Protective Effects Against Myocardial Ischemia/Reperfusion Injury Through the PI3K/Akt Pathway‐Mediated Inhibition of Apoptosis and the Restoration of the Autophagic Flux,” International Journal of Molecular Medicine 38, no. 1 (2016): 328–336. [DOI] [PubMed] [Google Scholar]
- 162. Townsend P. A., Scarabelli T. M., Pasini E., et al., “Epigallocatechin‐3‐Gallate Inhibits STAT‐1 Activation and Protects Cardiac Myocytes From Ischemia/Reperfusion‐Induced Apoptosis,” FASEB Journal 18, no. 13 (2004): 1621–1623. [DOI] [PubMed] [Google Scholar]
- 163. Zhong W., Huan X. D., Cao Q., and Yang J., “Cardioprotective Effect of Epigallocatechin‐3‐Gallate Against Myocardial Infarction in Hypercholesterolemic Rats,” Experimental and Therapeutic Medicine 9, no. 2 (2015): 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164. Oršolić N., Sirovina D., Gajski G., Garaj‐Vrhovac V., Jembrek M. J., and Kosalec I., “Assessment of DNA Damage and Lipid Peroxidation in Diabetic Mice: Effects of Propolis and Epigallocatechin Gallate (EGCG),” Mutation Research/Genetic Toxicology and Environmental Mutagenesis 757, no. 1 (2013): 36–44. [DOI] [PubMed] [Google Scholar]
- 165. Chakrawarti L., Agrawal R., Dang S., Gupta S., and Gabrani R., “Therapeutic Effects of EGCG: A Patent Review,” Expert Opinion on Therapeutic Patents 26, no. 8 (2016): 907–916. [DOI] [PubMed] [Google Scholar]
- 166. Wan C., Ouyang J., Li M., Rengasamy K. R., and Liu Z., “Effects of Green Tea Polyphenol Extract and Epigallocatechin‐3‐O‐Gallate on Diabetes Mellitus and Diabetic Complications: Recent Advances,” Critical Reviews in Food Science and Nutrition (2022): 1–29. [DOI] [PubMed] [Google Scholar]
- 167. Schloss J., Ryan K., and Steel A., “Corrigendum to a Randomised, Double‐Blind, Placebo‐Controlled Clinical Trial Found That a Novel Herbal Formula UROX® BEDTIME BUDDY Assisted Children for the Treatment of Nocturnal Enuresis. Phytomedicine (2021) 153783,” Phytomedicine 99 (2022): 153783, 10.1016/j.phymed.2021.153783. [DOI] [PubMed] [Google Scholar]
- 168. Redford K. E., Rognant S., Jepps T. A., and Abbott G. W., “KCNQ5 Potassium Channel Activation Underlies Vasodilation by Tea,” Cellular Physiology and Biochemistry 55, no. S3 (2021): 46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Luo D., Xu J., Chen X., et al., “(‐)‐Epigallocatechin‐3‐Gallate (EGCG) Attenuates Salt‐Induced Hypertension and Renal Injury in Dahl Salt‐Sensitive Rats,” Scientific Reports 10, no. 1 (2020): 4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Qian B. J., Tian C. C., Ling X. H., et al., “miRNA‐150‐5p Associate With Antihypertensive Effect of Epigallocatechin‐3‐Gallate Revealed by Aorta miRNome Analysis of Spontaneously Hypertensive Rat,” Life Sciences 203 (2018): 193–202. [DOI] [PubMed] [Google Scholar]
- 171. Potenza M. A., Marasciulo F. L., Tarquinio M., et al., “EGCG, a Green Tea Polyphenol, Improves Endothelial Function and Insulin Sensitivity, Reduces Blood Pressure, and Protects Against Myocardial I/R Injury in SHR,” American Journal of Physiology. Endocrinology and Metabolism 292, no. 5 (2007): E1378–E1387. [DOI] [PubMed] [Google Scholar]
- 172. Zhu T. T., Zhang W. F., Luo P., et al., “Epigallocatechin‐3‐Gallate Ameliorates Hypoxia‐Induced Pulmonary Vascular Remodeling by Promoting Mitofusin‐2‐Mediated Mitochondrial Fusion,” European Journal of Pharmacology 809 (2017): 42–51. [DOI] [PubMed] [Google Scholar]
- 173. Kanlaya R. and Thongboonkerd V., “Protective Effects of Epigallocatechin‐3‐Gallate From Green Tea in Various Kidney Diseases,” Advances in Nutrition 10, no. 1 (2019): 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lamothe J., Khurana S., Tharmalingam S., et al., “Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids,” Antioxidants (Basel) 10, no. 4 (2021): 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chowdhury A., Sarkar J., Chakraborti T., and Chakraborti S., “Role of Spm‐Cer‐S1P Signalling Pathway in MMP‐2 Mediated U46619‐Induced Proliferation of Pulmonary Artery Smooth Muscle Cells: Protective Role of Epigallocatechin‐3‐Gallate,” Cell Biochemistry and Function 33, no. 7 (2015): 463–477. [DOI] [PubMed] [Google Scholar]
- 176. Pullikotil P., Chen H., Muniyappa R., et al., “Epigallocatechin Gallate Induces Expression of Heme Oxygenase‐1 in Endothelial Cells via p38 MAPK and Nrf‐2 That Suppresses Proinflammatory Actions of TNF‐α,” Journal of Nutritional Biochemistry 23, no. 9 (2012): 1134–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Li H. L., Huang Y., Zhang C. N., et al., “Epigallocathechin‐3 Gallate Inhibits Cardiac Hypertrophy Through Blocking Reactive Oxidative Species‐Dependent and ‐Independent Signal Pathways,” Free Radical Biology and Medicine 40, no. 10 (2006): 1756–1775. [DOI] [PubMed] [Google Scholar]
- 178. Chen D. D., Dong Y. G., Liu D., and He J. G., “Epigallocatechin‐3‐Gallate Attenuates Cardiac Hypertrophy in Hypertensive Rats in Part by Modulation of Mitogen‐Activated Protein Kinase Signals,” Clinical and Experimental Pharmacology & Physiology 36, no. 9 (2009): 925–932. [DOI] [PubMed] [Google Scholar]
- 179. Ikeda M., Suzuki C., Umegaki K., Saito K., Tabuchi M., and Tomita T., “Preventive Effects of Green Tea Catechins on Spontaneous Stroke in Rats,” Medical Science Monitor 13, no. 2 (2007): Br40–Br45. [PubMed] [Google Scholar]
- 180. Zheng Y., Song H. J., Kim C. H., et al., “Inhibitory Effect of Epigallocatechin 3‐O‐Gallate on Vascular Smooth Muscle Cell Hypertrophy Induced by Angiotensin II,” Journal of Cardiovascular Pharmacology 43, no. 2 (2004): 200–208. [DOI] [PubMed] [Google Scholar]
- 181. Shi D. D., Guo J. J., Zhou L., and Wang N., “Epigallocatechin Gallate Enhances Treatment Efficacy of Oral Nifedipine Against Pregnancy‐Induced Severe Pre‐Eclampsia: A Double‐Blind, Randomized and Placebo‐Controlled Clinical Study,” Journal of Clinical Pharmacy and Therapeutics 43, no. 1 (2018): 21–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated.


