Abstract
Background
Transcription factor 19 (TCF19) is an important member of the transcription factor family, but its role in cancer is not well understood. This study aims to clarify the function of TCF19 and explore its mechanisms across various cancers, with a specific focus on breast cancer, using bioinformatics analysis and histological validation.
Methods
Gene expression profiles and clinical data were obtained from sources like The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), and Genotype-Tissue Expression (GTEx) for bioinformatics analysis. Tumor–immune interactions were studied using the TISIDB database for immune-related insights. To confirm these findings, TCF19 expression was measured using quantitative RT-PCR, immunohistochemistry (IHC), and western blot assays. Additional validation was done with various experimental techniques, including CCK-8, colony-forming, transwell, and cell line-derived xenograft assays.
Results
TCF19 is significantly upregulated in many cancerous tissues, including breast cancer, as confirmed across multiple datasets and clinical samples. Higher TCF19 levels were associated with a poorer prognosis. Experimentally reducing TCF19 expression in MCF-7 cells impaired key cellular functions such as proliferation, invasion, migration, and tumor formation. Pathway enrichment analysis of TCGA samples revealed a strong link between TCF19 and the PLK1 pathway, a finding further supported by western blot results showing PLK1 downregulation following TCF19 silencing.
Conclusions
High TCF19 expression is closely linked to poorer outcomes, especially in breast cancer. These findings highlight TCF19 as a promising biomarker with broad potential applications in cancer diagnosis and prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02546-8.
Keywords: BRCA, Prognosis, TCF19, PLK1, Proliferation
Introduction
Cancer is a life-threatening disease that results from complex physiological and pathological processes. It imposes significant physical and emotional burdens on patients and families and adds economic and social strain [1]. The lack of accurate molecular markers for early diagnosis and prognosis contributes to high mortality, especially in less developed countries [2]. Breast cancer (BRCA) has now overtaken lung cancer as the most common malignancy worldwide, representing one-fourth of cancer cases and one-sixth of cancer-related deaths among women. Its incidence is widespread, with mortality rates higher than other cancers in half of the affected countries [3–5]. In China, breast cancer accounts for 12.2% of global cases and 9.6% of cancer-related deaths [6]. While the mortality rate for breast cancer has declined by 40% over the past three decades, this progress has slowed in recent years [7]. Breast cancer is complex, with diverse molecular features and subtypes, making it challenging to achieve consistent treatment outcomes. Some patients may not experience significant survival benefits despite existing therapies [8, 9].
Research has shown that transcription factor 19 (TCF19), a regulator in the late G1/S phase of the cell cycle, plays a role in cellular proliferation [10, 11]. Located on chromosome 6p21.2, TCF19 spans 5.69 kilobases [12]. A study by Du et al. revealed that TCF19 negatively regulates WWC1, enhancing the invasiveness and metastatic potential of colorectal cancer cells [13]. Additionally, overactivation of TCF19 has been associated with the progression of non-small cell lung cancer and liver cancer, often correlating with poor prognosis [12–14].
The exact role and mechanism of TCF19 in various cancers, particularly in breast cancer, are not fully understood. Different molecular phenotypes within breast cancer subtypes result in varied treatment responses, metastasis patterns, and prognostic outcomes. Molecular profiling has become essential in guiding precise treatment selection in breast cancer research [15–17]. Therefore, there is an urgent need to identify effective molecular markers to improve breast cancer treatment. Existing biomarkers often lack sensitivity, specificity, and practical utility, underscoring the need for more accurate prognostic markers for breast cancer.
This study aims to clarify the role and potential applications of TCF19 in breast cancer by using comprehensive bioinformatics analysis of sequencing data from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO). The study will also include clinical specimens and data to support the findings, which will be validated through in vitro functional studies.
Methods
Data collection and preprocessing
RNA sequencing data from the TCGA repository, consisting of 11,093 samples, was used as the primary data source. Additional datasets from the GEO database (GSE1456 and GSE9893 for survival validation, and GSE70947 and GSE93601 for expression level analysis) were included. RNA sequencing data in TPM format was preprocessed and normalized to log2 using the Toil method [18]. The GTEx database provided RNA data for normal tissues, and immune correlations were examined using the TISIDB platform.
All BRCA tissue samples were collected from patients at the Anhui Provincial Cancer Hospital who had undergone biopsy or surgery. Follow-up assessments continued until September 2022, using overall survival (OS) to indicate the time from diagnosis to death or any other endpoint. All specimens were collected and processed anonymously, in strict adherence to ethical guidelines and the principles of the Declaration of Helsinki.
Cell culture, transfection 0rocedure, and reagents
The MCF-10A (normal mammary epithelial cell line), MCF-7, and MDA-MB-231 (breast cancer cell lines) were obtained from the Shanghai Cell Bank. Cells were grown in DMEM with 10% FBS and incubated at 37 °C with 5% CO₂. The TCF19 knockdown vector was developed by Shanghai Generay Biotechnology Co., Ltd., fused with the pPACKH1 plasmid, and transfected into cells with sh1/2-TCF19/NC. After 3 days, virus particles were collected and used to infect cells via TUNDUX virus transducers. Positive cells were identified through puromycin screening.
Quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR) and immunohistochemistry (IHC)
Seven pairs of BRCA and adjacent non-tumor tissues were preserved in RNAlater reagent and dissolved in TRIzol to extract high-quality RNA. One microgram of RNA was reverse-transcribed using the Verso cDNA kit. Gene expression was analyzed using the 2−ΔΔCT method. Primer sequences were: TCF19 (forward: 5ʹ-CACTCAGACCCTCCGACTCT-3ʹ, reverse: 5ʹ-CGATCGTGTAGGTCCGAGAAG-3ʹ) and GAPDH (forward: 5ʹ-GGTGAAGGTCGGAGTCAACG-3ʹ, reverse: 5ʹ-CAAAGTGTCATGGATGHACC-3ʹ).
TCF19 expression was measured in paraffin-embedded tissue sections from Anhui Provincial Hospital. Sections were heated for antigen retrieval, treated with 3% hydrogen peroxide to inactivate enzymes, and then incubated with an anti-TCF19 antibody (Abcam, no. ab230005) at 37 °C for 1 h. For the secondary developing reagents, a labeled streptavidin–biotin kit (Dako, CA, USA) was employed. The final steps of the procedure were executed in strict accordance with the standardized manual, and the resultant staining outcomes were evaluated by two seasoned pathologists. For this study, immunohistochemical staining was carried out on 100 samples of qualified paraffin-embedded BRCA tissues. The staining intensity was assigned scores as follows: 3 for strong, 2 for moderate, and 1 for weak. The proportion of cells exhibiting positive staining was scored as 4 for > 75%, 3 for 35%–75%, 2 for 10%–35%, 1 for < 10%, and 0 for no positivity. By employing this approach, the staining index (SI) values were calculated for each patient by multiplying the intensity score by the percentage score. Based on the heterogeneity observed in the OS log-rank test statistic, the cutoff value distinguishing high and low expression of TCF19 was determined to be 6.
Cell proliferation assay (CCK-8)
To assess cell proliferation, the CCK-8 test kit (Abcam, Shanghai, China) was employed. In a 96-well plate, each well was seeded with a confluent monolayer of 2 × 103 cells. Subsequently, for the next 4 consecutive days, a volume of 100 μl of supplemented medium and 10 μl of CCK-8 reagents were added to each well. After 2 h of incubation at 37 °C in a light-restricted environment, the absorbance of each well was measured at 450 nm using a spectrophotometer equipped with a microplate reader. To ensure the robustness of the results, each sample was tested in triplicate wells, and the experiment was independently repeated three times.
Colony-forming assay
To evaluate the development of anchorage-independent colonies, we employed two-layered agar-coated six-well plates. As a base layer, a solution consisting of 0.5% agar was applied to the six-well plates and allowed to solidify at ambient temperature. Subsequently, a suspension of 1 × 103 cells, cultured in DMEM medium with a 0.35% agar solution, was carefully seeded onto the preformed foundation layer. The resulting plates were then incubated for 14 days at 37 °C in an atmosphere supplemented with 5% CO2. Following incubation, the samples were fixed with a 3.7% formaldehyde solution for 10 min, followed by staining with 0.1% crystal violet for 20 min at room temperature. Colony enumeration was conducted under optical microscopy at a magnification of 100×.
Transwell assay
Approximately 6 × 104 cells are seeded in the Transwells upper chamber (Corning-Costar) and cultured with fresh serum-free DMEM in the lower chamber with DMEM with 10% serum. After 24 h, wash the chamber with PBS and subsequently fix it with 4% methanol for 20 min. Finally, the migrated cells were stained with 0.1% crystal violet and counted.
Animal experiment
Experiments were conducted using female BALB/c mice (Vitalriver, Beijing, China), which were 4 weeks old and bred under sterile conditions. MCF-7 or MDA-MB-231 cells (3 × 106), transfected with sh1-TCF19 and the NC group, were suspended in DMEM. The injection site is unified at 0.3cm of the back of the axillary of the right forelimb. The mice were subsequently divided into two groups (n = 5). The nude mice were inoculated with MCF-7 or MDA-MB-231 cells transfected with sh1-TCF19 and the NC. The tumor volume was measured every 3 days. After 24 days, the mice were euthanized, and the tumor weight was recorded. The animal experimentation ethics committee of the University of Science and Technology in China approved the animal experiment.
Western blot
MCF-7 or MDA-MB-231 cells belonging to the sh1/2-TCF19 and NC groups were harvested from the culture medium. RIPA cell lysate was employed to extract proteins, followed by the addition of 5× loading buffer in a 1:4 ratio. Subsequently, the samples were subjected to a boiling water bath for 10 min, loaded onto a gel, and the proteins were separated by SDS-PAGE electrophoresis. The protein was then transferred to a PVDF membrane using the semi-dry transfer method. To block non-specific binding, a solution of 5% nonfat milk powder was utilized. Diluted anti-PLK1 antibody was carefully added dropwise and incubated overnight at 4 °C. After a subsequent 2-h incubation at room temperature, the protein was thoroughly washed three times with PBST. Finally, ECL was added to facilitate exposure and development, and Image J software was employed for band analysis. The antibodies employed in the western blot procedure were as follows: β-actin (Abcam, no. ab8227) and anti-PLK1 (Abcam, no. ab189139).
Statistical analysis
The R software (× 64, version 3.6.3) was employed for data normalization and statistical analysis. To evaluate the diagnostic value of TCF19, receiver operating characteristic (ROC) analysis was conducted using the “pROC” package. The expression levels of TCF19 in different groups were compared using the Mann–Whitney U test. Enrichment analysis was performed using the “cluster Profiler” package [19]. Single-factor and multi-factor statistics of TCGA BRCA data were analyzed using the Cox regression module of the “survival” package in R software. Correlation analysis was conducted using the Spearman method. Kaplan–Meier survival analysis and immune infiltration analysis were performed using the “survival” and “GSVA” packages [20–22]. A statistical significance level of P < 0.05 (* P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001) was considered significant.
Results
Up-regulation of TCF19 in tumor tissues
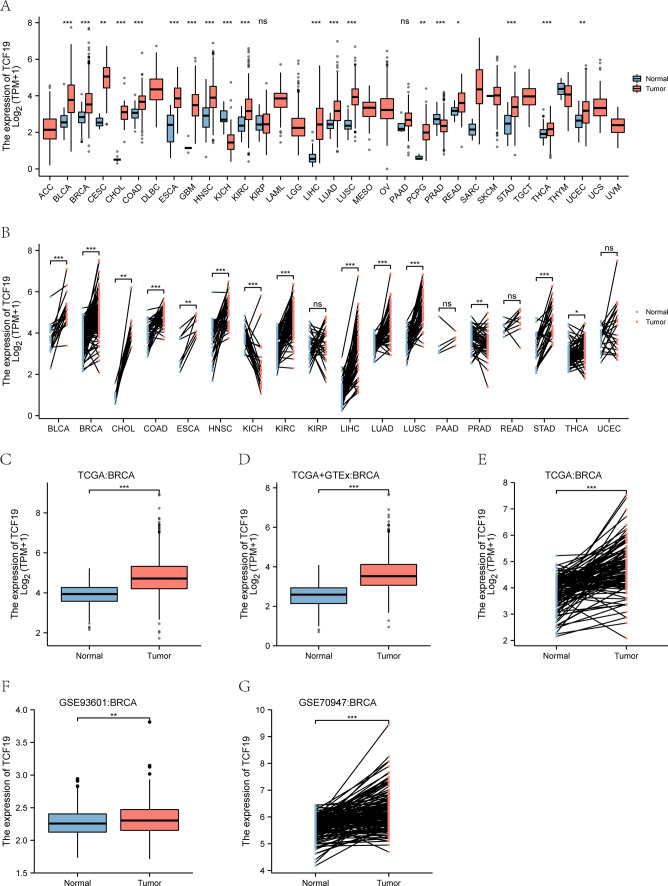
TCF19 is significantly up-regulated in multiple cancers, including bladder (BLCA), colon (COAD), lung (LUAD, LUSC), head and neck (HNSC), kidney (KIRC), rectum (READ), and stomach (STAD) (P < 0.001) (Fig. 1A). Its expression is higher in tumor tissues than in normal tissues, shown in both unpaired (Fig. 1B) and paired analyses (Fig. 1C). Analysis of TCGA and GTEx data indicates elevated TCF19 in BRCA compared to normal tissues (Fig. 1D–E), consistent with results from the GSE93601 (Fig. 1F) and GSE70947 (Fig. 1G) datasets.
Fig. 1.
The expression level of TCF19 in human cancer. A Pan-cancer analysis in TCGA cohort; B pan-cancer pair analysis in TCGA cohort; C expression in TCGA breast cancer tumor tissues and the normal; D expression in TCGA and GTEx of breast cancer; E expression in TCGA breast cancer paired samples; F expression of the GSE93601 dataset in BRCA; G expression of the GSE70947 dataset in BRCA (*P < 0.05, **P < 0.01, ***P < 0.001)
Diagnostic potential of TCF19 across cancers
Subsequent detailed analysis (Fig. 2A–K) reveals that TCF19 exhibits potential as a highly effective diagnostic marker in various types of cancer, including BLCA (AUC = 0.839, 95% CI 0.788–0.891), esophageal carcinoma (ESCA) (AUC = 0.934, 95% CI 0.874–0.995), head and neck squamous cell carcinoma (HNSC) (AUC = 0.864, 95% CI 0.815–0.913), kidney chromophobe (KICH) (AUC = 0.924, 95% CI 0.867–0.982), KIRC (AUC = 0.827, 95% CI 0.787–0.867), liver hepatocellular carcinoma (LIHC) (AUC = 0.964, 95% CI 0.94–0.983), LUAD (AUC = 0.838, 95% CI 0.804–0.872), LUSC (AUC = 0.972, 95% CI 0.958–0.987), stomach adenocarcinoma (STAD) (AUC = 0.853, 95% CI 0.791–0.915), and another subgroup of BLCA (AUC = 0.836, 95% CI 0.759–0.913). Additionally, the receiver operating characteristic (ROC) curve for the BRCA cohort GSE90947 individually demonstrates an AUC of 0.778 (95% CI 0.724–0.831) (Fig. 2L).
Fig. 2.
ROC curve of pan-cancer. A–K ROC curve of BLCA, BRCA, COAD, ESCA, HNSC, KICH, KIRC, LIHC, LUAD, LUSC, STAD; L ROC curve of the GSE70947 dataset in BRCA
The prognostic value of TCF19 in pan-cancer
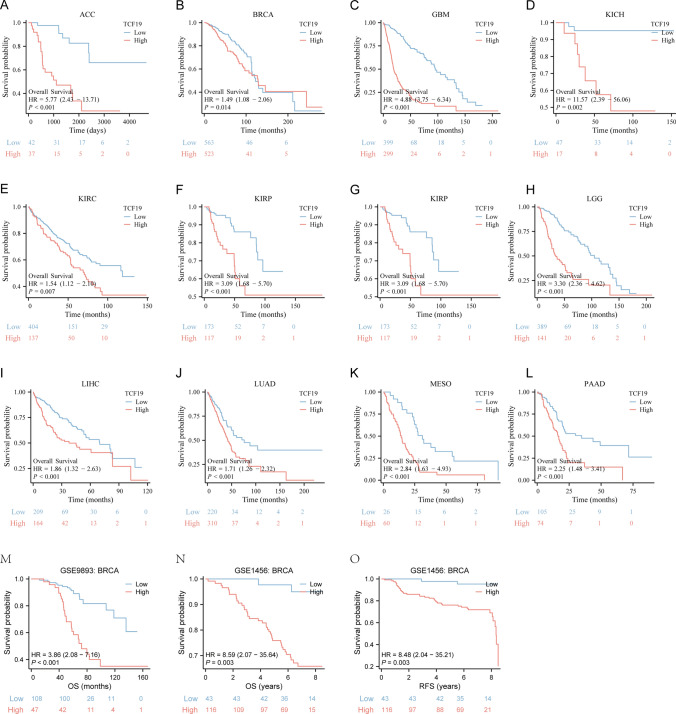
The OS of patients with up-regulated TCF19 expression exhibited a significantly shorter outcome in comparison to patients with high adrenocortical carcinoma (ACC) (P < 0.001), breast invasive carcinoma (BRCA) (P = 0.014), glioblastoma multiforme (GBM) (P < 0.001), KICH (P = 0.002), KIRC (P = 0.007), kidney renal papillary cell carcinoma (KIRP) (P < 0.001), acute myeloid leukemia (LAML) (P = 0.018), brain lower grade glioma (LGG) (P < 0.001), LIHC (P < 0.001), LUAD (P < 0.001), mesothelioma (MESO) (P < 0.001), and pancreatic adenocarcinoma (PAAD) (P < 0.001), as indicated by Fig. 3A–L. Subsequently, we compiled the microarray expression data and prognostic information from the GSE9893 and GSE1456 datasets. Consistent with the findings from the TCGA cohort analysis, patients categorized within the low-expression TCF19 group demonstrated a more favorable prognosis in comparison to those in the high-expression TCF19 group (Fig. 3M–O).
Fig. 3.
Kaplan–Meier survival curves comparing the high and low expression of TCF19 in different types of cancer in Kaplan–Meier Plotter. A–L Kaplan–Meier analysis of the association between TCF19 expression and OS in TCGA cohort; M OS analysis of GSE9893 in BRCA; N, O OS and RFS analysis of GSE1456 in BRCA
In BRCA samples from TCGA, multivariate analysis (Table 1) found that Age (HR = 2.107) and TCF19 expression (HR = 1.440) were independently linked to OS. N stage and age were also significant prognostic factors.
Table 1.
Univariate and multivariate analysis of the association of TCF19 with OS in BRCA (TCGA)
| Characteristics | Subtype | Total(N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| T stage | T1&T2 | 905 | Reference | |||
| T3&T4 | 174 | 1.608 (1.110–2.329) | 0.012 | 0.822 (0.461–1.467) | 0.507 | |
| N stage | N0 | 514 | Reference | |||
| N1 | 357 | 1.956 (1.329–2.879) | < 0.001 | 1.705 (1.093–2.659) | 0.019 | |
| N2 | 116 | 2.519 (1.482–4.281) | < 0.001 | 1.438 (0.600–3.449) | 0.415 | |
| N3 | 76 | 4.188 (2.316–7.574) | < 0.001 | 2.101 (0.880–5.013) | 0.094 | |
| M stage | M0 | 902 | Reference | |||
| M1 | 20 | 4.254 (2.468–7.334) | < 0.001 | 1.849 (0.861–3.972) | 0.115 | |
| PR status | Negative | 342 | Reference | |||
| Positive | 687 | 0.732 (0.523–1.024) | 0.068 | |||
| ER status | Negative | 240 | Reference | |||
| Positive | 792 | 0.712 (0.495–1.023) | 0.066 | |||
| HER2 status | Negative | 558 | Reference | |||
| Positive | 157 | 1.593 (0.973–2.609) | 0.064 | |||
| Pathologic stage | Stage I&Stage II | 799 | Reference | |||
| Stage III&Stage IV | 260 | 2.391 (1.703–3.355) | < 0.001 | 2.016 (0.943–4.310) | 0.071 | |
| Age | ≤ 60 | 601 | Reference | |||
| > 60 | 481 | 2.020 (1.465–2.784) | < 0.001 | 2.107 (1.460–3.041) | < 0.001 | |
| Race | White | 753 | Reference | |||
| Asian | 60 | 0.754 (0.239–2.383) | 0.631 | |||
| Black or African American | 180 | 1.151 (0.765–1.731) | 0.501 | |||
| Histological type | Infiltrating Ductal | 772 | Reference | |||
| Infiltratin Lobular | 205 | 0.827 (0.526–1.299) | 0.410 | |||
| TCF19 | Low | 540 | Reference | |||
| High | 542 | 1.398 (1.014–1.927) | 0.041 | 1.440 (1.005–2.064) | 0.047 | |
TCF19 is related to immune infiltration in pan-cancer
The immune infiltration analysis was conducted using the single-sample gene set enrichment analysis (ssGSEA) algorithm. Figure 4A demonstrates a positive correlation between the expression level of TCF19 and the presence of aDC and Th2 cells. Notably, in KIRC, MESO, and UVM, the level of immune cell infiltration showed a strong association with TCF19. In the ssGSEA analysis of BRCA (Fig. 4B), TCF19 exhibited an inverse relationship with the abundance of NK cells and mast cells, while displaying a positive correlation with the presence of aDC and Th2 cells.
Fig. 4.
Immune-related analysis. A TCGA immune cell infiltration analysis in pan-cancer (B) immune cell infiltration analysis of BRCA. C–E Correlation analysis between TCF19 and Immunoinhibitor, Immunostimulator and MHC molecule in the TISIDB database
The TISIDB website further analyzed the association between TCF19 and Immunoinhibitory, Immunostimulatory, and MHC molecules (Fig. 4C–E). In ESCA, LUSC, and uveal melanoma (UVM), TCF19 demonstrated a negative correlation with these immune-related molecules. Conversely, in BRCA, KIRC, and THCA, TCF19 exhibited a positive correlation with the same immune-related molecules.
Pathway enrichment analysis of TCF19 in BRCA
By employing the median expression level of TCF19 in BRCA samples from TCGA as a threshold, we identified differentially expressed genes (DEGs) that met the criteria of LogFC = 2 and P < 0.05, as illustrated in Fig. 5A. Subsequently, a comprehensive analysis combining gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) enrichment was performed to elucidate the biological implications of these DEGs. The results indicated significant enrichment in various pathways including trans splicing, via spliceosome pathway, mRNA trans splicing, SL addiction pathway, Cajal body pathway, Spliceosomal snRNP complex pathway, Bitter taste receptor activity pathway, Pre-mRNA 5ʹ-splice site binding pathway, Spliceosome pathway, and RNA transport pathway, as depicted in Fig. 5B. Furthermore, GSEA we observed robust associations between TCF19 and key pathways such as the S phase, Cell cycle, PLK1, Mitotic G1 Phase, and G1 S Transition by GSEA, as illustrated in Fig. 5C–G. Moreover, we performed Spearman correlation analysis using BRCA samples from TCGA, GSE93601, and GSE70947 datasets, revealing a strong correlation between PLK1 and TCF19, as depicted in Fig. 5H–J.
Fig. 5.
Pathway enrichment and correlation analysis in BRCA. A Differential genes in TCGA cohort. B KEGG and GO analyze the ways of the rank top. C–G Pathway enrichment by GSEA; H–J correlation between TCF19 and PLK1 gene of TCGA, GSE93601, and GSE70947
TCF19 expression level and cell function experiments
The mRNA expression of TCF19 in BRCA tissues exhibited a significant up-regulation (P < 0.05), as delineated in Fig. 6A. Notably, Further detection of the protein levels of TCF19 showed that it was highly expressed in tumor tissues (Fig. 6A, B), and the immunohistochemical staining yielded consistent results (Fig. 6C). The colony-forming assay provided compelling evidence that the inhibition of TCF19 led to a substantial decrease in the proliferation of MCF-7 and MDA-MB-231 cells (Fig. 6D). Furthermore, the CCK-8 assay revealed a significantly diminished cell proliferation capability in the knockdown TCF19 group compared to the NC group (Fig. 6E). Additionally, the transwell assay demonstrated a considerable suppression of the invasion and migration abilities of BRCA cells upon TCF19 knockdown, as shown in Fig. 6F. To strengthen the convincing power of the cell experiment, further testing showed that the protein levels of TCF19 and PLK1 in normal breast epithelial cells were lower than those in breast tumor cells (Fig. 6G).
Fig. 6.
Verification of the role of TCF19 in BRCA by biological assay. A–C TCF19 expression of tumor compared with normal by RT-qPCR, Western blot and IHC. D Colony-forming assays showing knockdown of TCF19 inhibited the BRCA cells proliferation. E CCK8 assays showing knockdown of TCF19 inhibited the BRCA cells proliferation. F Transwell assay showed that knockdown of TCF19 significantly inhibited the invasion and migration ability of BRCA cells compared to control. G Expression of TCF19 and PLK1 in cell lines (***P < 0.001)
TCF19 may inhibit tumor growth in vivo by regulating PLK1
To investigate the functional role of TCF19 in vivo, a tumorigenic assay was employed using nude mice. The mice were divided into two groups, namely the sh1-TCF19 group and the NC group, with five mice in each group. After 24 days, the mice were sacrificed, and the tumors were collected for analysis. Remarkably, the tumorigenicity assay conducted on nude mice demonstrated a marked decrease in tumorigenic ability upon TCF19 silencing compared to the control group. The tumors in the sh1-TCF19 group exhibited significantly smaller size, weight, and volume when compared to the control group, as depicted in Fig. 7A. Collectively, these findings suggest that TCF19 plays a pivotal role in promoting tumor growth in vivo and may function as a central gene in MCF-7 and MDA-MB-231 cells. Previous pathway enrichment and gene correlation studies have shown that PLK1 may be a target gene for TCF19 exertion, and we stained mouse tumor tissues and found that the expression level of PLK1 was also reduced in the TCF19 silencing group (Fig. 7B).
Fig. 7.
TCF19 may inhibit tumor growth in vivo by regulating PLK1. A, B Knockdown of TCF19 inhibits tumor growth in vivo. C Kaplan–Meier survival curves comparing the high and low expression of TCF19 in IHC. D Knockdown of TCF19 inhibits the expression of PLK1. (**P < 0.01, ***P < 0.001)
Our investigation into the prognostic implications of TCF19 using both TCGA and GEO datasets revealed a consistent pattern. IHC analysis further confirmed that elevated TCF19 expression was associated with an unfavorable prognosis in BRCA patients, with an HR of 2.87 and a significance level of P < 0.001 (Fig. 7C). To gain deeper insights into the clinical relevance of TCF19, we collected common clinical parameters from patients with available pathological specimens. Univariate and multivariate analyses were performed using these parameters. The outcomes yielded compelling evidence suggesting that TCF19 can be regarded as an independent prognostic predictor in BRCA patients, based on a cohort of clinical specimens (Table 2).
Table 2.
Univariate and multivariate analysis of the association of TCF19 with OS in clinical samples
| Characteristics | Subtype | Total(N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| TCF19 | Low | 44 | Reference | |||
| High | 56 | 2.867 (1.669–4.925) | < 0.001 | 3.254 (1.750–6.050) | < 0.001 | |
| Age | – | 100 | 0.971 (0.944–0.998) | 0.035 | 0.983 (0.954–1.012) | 0.249 |
| T stage | T1&T2 | 87 | Reference | |||
| T3&T4 | 13 | 0.554 (0.236–1.304) | 0.177 | |||
| N stage | N0 | 31 | Reference | |||
| N1 | 46 | 0.787 (0.439–1.412) | 0.422 | |||
| N2 | 14 | 0.558 (0.232–1.342) | 0.192 | |||
| N3 | 9 | 1.161 (0.435–3.102) | 0.766 | |||
| M stage | M0 | 89 | Reference | |||
| M1 | 11 | 1.936 (0.756–4.958) | 0.169 | |||
| Pathologic stage | I | 9 | Reference | |||
| II | 55 | 1.641 (0.643–4.193) | 0.300 | |||
| III | 25 | 0.827 (0.280–2.444) | 0.731 | |||
| IV | 11 | 2.588 (0.736–9.103) | 0.138 | |||
| ER | Negative | 51 | Reference | |||
| Positive | 49 | 1.019 (0.608–1.710) | 0.942 | |||
| PR | Negative | 60 | Reference | |||
| Positive | 40 | 1.397 (0.829–2.355) | 0.209 | |||
| HER-2 | Positive | 56 | Reference | |||
| Negative | 44 | 0.930 (0.555–1.558) | 0.783 | |||
| Ki67 (%) | – | 100 | 1.010 (0.999–1.022) | 0.076 | 1.010 (0.997–1.024) | 0.126 |
| PAM50 | HER-2(HR-) | 17 | Reference | |||
| HER-2(HR +) | 39 | 1.700 (0.764–3.783) | 0.193 | 1.725 (0.747–3.983) | 0.202 | |
| TNBC | 31 | 1.509 (0.668–3.412) | 0.323 | 1.448 (0.594–3.526) | 0.415 | |
| Luminal B | 10 | 0.493 (0.130–1.877) | 0.300 | 0.336 (0.085–1.336) | 0.121 | |
| Luminal A | 3 | 4.417 (1.162–16.790) | 0.029 | 3.241 (0.802–13.096) | 0.099 | |
Following an enrichment analysis of differentially expressed genes in the BRCA dataset of the TCGA cohort, a significant association between TCF19 and the PLK1 pathway was discovered. Furthermore, the results of Western blot analysis (depicted in Fig. 7D) demonstrated that the sh1-TCF19 group exhibited downregulation of PLK1 in comparison to the control group. Further analysis of the relationship between TCF19 and PLK1 in multiple TCGA databases, as shown in Supplementary Fig. 1, showed that the expression levels of TCF19 showed a significant positive correlation. Notably, the research conducted by Krautkramer et al. revealed the ability of TCF19 to promote the proliferation of insulinoma cells [23]. Additionally, a molecular correlation analysis encompassing TCGA, GSE93601, and GSE70947 samples unveiled a strong positive correlation between the expression levels of PLK1 and TCF19. These findings propose that TCF19 may contribute to tumorigenesis by modulating the PLK1 pathway. It is also important to emphasize that the PLK1 pathway has been extensively implicated in numerous cancers, including BRCA [24, 25], and plays a pivotal role in various biological processes, such as BRCA cell proliferation, drug resistance, and metastasis [26–28].
Discussion
Despite advancements in medical technology and treatment approaches, many patients with BRCA benefit, but a substantial number still face poor prognoses due to cancer’s complex nature and individual variability. Therefore, identifying simple yet effective biomarkers is essential to support critical clinical decision-making for these patients.
In this study, elevated TCF19 expression was identified as a key factor linked to poor prognosis. Research indicates TCF19’s role in cellular processes like proliferation, apoptosis, and invasion, and emerging evidence suggests its connection to tumorigenesis [11, 29]. Emerging studies have begun to uncover a functional connection between TCF19 and tumorigenesis [17, 30, 31]. However, there is no consensus on TCF19’s specific role across cancer types, and a pan-cancer analysis is lacking. To address this, we conducted an extensive investigation of TCF19 across multiple cancers, focusing on BRCA, analyzing gene expression, prognostic impact, immune infiltration, and signaling pathways, supported by biological experiments to validate our findings.
Our bioinformatics analysis across multiple independent cohorts highlights elevated TCF19 expression in many cancers compared to normal tissues. Previous studies, including Zhou et al. in lung cancer and Du et al. in colorectal cancer, also report TCF19 overexpression [12, 30]. TCF19 has prognostic significance, as shown by Kaplan–Meier analysis of TCGA data, where higher TCF19 levels correlate with shorter OS in several cancers, including BRCA. An independent GEO cohort supports these results, showing a link between high TCF19 expression and lower OS and DFS in BRCA patients. Multivariate analysis further identifies TCF19 as an independent prognostic factor for BRCA, underscoring its potential as a biomarker.
Standard treatments like surgery and chemoradiotherapy do not cover all BRCA cases. Identifying gene targets and immune checkpoints is valuable for predicting immunotherapy outcomes. This study examined the immunotherapeutic potential of TCF19 by analyzing its relationship with immune cell infiltration. We found an inverse correlation between TCF19 and NK and mast cell abundance, and a positive correlation with aDC and Th2 cells. Unlike T cells, NK cells can kill tumor cells without antigen sensitivity, and their reduction is linked to higher cancer risk and tumor proliferation, possibly due to immune evasion [32]. High TCF19 levels in BRCA correlate with poor prognosis and lower NK cell levels, suggesting potential for further exploration in NK cell therapy [33]. This study found that the high level of TCF19 in BRCA predicts poor prognosis, and the expression level is negatively correlated with NK cells, which has the potential to be further explored in detail in NK cell therapy for BRCA.
This study aimed to explore TCF19’s functional role in BRCA. RT-qPCR analysis confirmed significantly higher TCF19 expression in BRCA compared to healthy breast tissue, supported by IHC findings showing that lower TCF19 levels correspond with better prognosis, consistent with bioinformatics predictions. GSEA pathway enrichment identified the cell cycle as a key pathway associated with TCF19. Further CCK-8 and colony formation assays showed TCF19’s regulatory effect on BRCA cell proliferation, and Western blot experiments indicated TCF19 and PLK1 involvement. Together, these bioinformatics and experimental findings suggest TCF19 plays a critical role in BRCA pathogenesis.
Conclusions
This study focused on examining TCF19’s role in the development and progression of BRCA, using advanced bioinformatics and molecular biology techniques. We emphasized the interaction between TCF19 and PLK1, uncovering significant findings that provide a strong basis for future research. These insights suggest that TCF19 holds potential as a valuable biomarker in BRCA research, meriting further exploration and validation in future studies.
Supplementary Information
Supplementary Material 1. Supplementary Figure 1. The correlation between TCF19 and PLK1 in TCGA cancers.
Author contributions
JP and YS conceived, designed the study and revised the manuscript. HY, JW, QC, and YP performed the experiments and analyzed the data. HY, JW, XQ and QC collected clinical specimens and data. JW conducted the animal experiments. HY, JW, YS and JP prepared all the figures and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Funds of China (No. 82172775), National Key Clinical Specialty Construction Project 2021 Oncology (No. 2021GJLC01), Anhui Province Cancer Bioimmunotherapy Clinical Medical Research Center (No. 202101B10202005), Breast cancer precision tumor research and innovation team (No. 2022AH010077), Anhui Provincial Clinical Medical Research Transformation Special Project (No. 202304295107020055) and Beijing Science and Technology Innovation Medical Development Foundation Project (No. KC2021-JF-0167-23). Funders only provide financial support.
Data availability
The datasets analyzed during the current study are available for all projects in TCGA (https://portal.gdc.cancer.gov/), GSE93601, GSE70947, GSE9893, and GSE1456 from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), GTEx database (https://www.gtexportal.org/), TISIDB websites (http://cis.hku.hk/TISIDB/index.php). All raw data are available from the corresponding author.
Declarations
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Anhui Provincial Hospital (No. 2022-ZNW-02), and all patients gave informed consent. This study was reviewed and approved by the University of Science and Technology of China Animal Experimentation Ethics Committee (No. USTCACUC192401041). All procedures of animal study were performed according to the protocols approved by the Institutional Animal Ethics Care and Use Committee of the University of Science and Technology of China. According to the Ethics Committee, the maximum tumor size must not exceed 20 mm in any direction in an adult mouse, and in our experiment, the maximal tumor size remained within this limit.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haiyang Yu, Jingjing Wei and Qinhao Chen contributed equally to this work.
Contributor Information
Jing Pan, Email: jingpandoc@126.com.
Yubei Sun, Email: sunyubei@ustc.edu.cn.
References
- 1.Jokhadze N, Das A, Dizon DS. Global cancer statistics: a healthy population relies on population health. CA A Cancer J Clin. 2024;74(3):224–6. 10.3322/caac.21838. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan A. Improving access to global cancer services. Lancet. 2023;401(10385):1338–9. 10.1016/S0140-6736(23)00505-6. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 4.Bashar MDA, Begam N. Breast cancer surpasses lung cancer as the most commonly diagnosed cancer worldwide. Indian J Cancer. 2022;59(3):438–9. 10.4103/ijc.IJC_83_21. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. 2019;1152:1–7. 10.1007/978-3-030-20301-6_1. [DOI] [PubMed] [Google Scholar]
- 6.Lei S, Zheng R, Zhang S, et al. Breast cancer incidence and mortality in women in China: temporal trends and projections to 2030. Cancer Biol Med. 2021;18(3):900–9. 10.20892/j.issn.2095-3941.2020.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaquinto AN, Sung H, Newman LA, et al. Breast cancer statistics. CA A Cancer J Clin. 2024. 10.3322/caac.21863. (Published online October 2024). [Google Scholar]
- 8.Wang Y, Bi X, Luo Z, Wang H, Ismtula D, Guo C. Gelsolin: a comprehensive pan-cancer analysis of potential prognosis, diagnostic, and immune biomarkers. Front Genet. 2023;14:1093163. 10.3389/fgene.2023.1093163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Li Y, Jing Y, et al. Tubulin alpha-1b chain was identified as a prognosis and immune biomarker in pan-cancer combing with experimental validation in breast cancer. Sci Rep. 2024;14(1):8201. 10.1038/s41598-024-58982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rustamadji P, Wiyarta E, Anggreani I. Correlation of before and after invasive breast cancer neoadjuvant chemotherapy for NFkB, Cyclin D1, and survivin expression. Iran J Pathol. 2023;18(2):156–64. 10.30699/ijp.2023.562935.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G, Fontaine D, Lodh S, Blumer J, Roopra A, Davis D. TCF19 impacts a network of inflammatory and DNA damage response genes in the pancreatic β-cell. Metabolites. 2021;11(8):513. 10.3390/metabo11080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z, Chen G, Deng C, et al. TCF19 contributes to cell proliferation of non-small cell lung cancer by inhibiting FOXO1. Cell Biol Int. 2019;43(12):1416–24. 10.1002/cbin.11189. [DOI] [PubMed] [Google Scholar]
- 13.Du W, Huang Z, Luo L, et al. TCF19 aggravates the malignant progression of colorectal cancer by negatively regulating WWC1. Eur Rev Med Pharmacol Sci. 2020;24(2):655–63. [DOI] [PubMed] [Google Scholar]
- 14.Zeng CX, Fu SB, Feng WS, Zhao JY, Li FX, Gao P. TCF19 enhances cell proliferation in hepatocellular carcinoma by activating the ATK/FOXO1 signaling pathway. Neo. 2019;66(01):46–53. 10.4149/neo_2018_171227N845. [DOI] [PubMed] [Google Scholar]
- 15.Ilić I, Cvetković J, Ilić R, et al. Differences in histological subtypes of invasive lobular breast carcinoma according to immunohistochemical molecular classification. Diagnostics. 2024;14(6):660. 10.3390/diagnostics14060660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velazquez-Caldelas TE, Zamora-Fuentes JM, Hernandez-Lemus E. Coordinated inflammation and immune response transcriptional regulation in breast cancer molecular subtypes. Front Immunol. 2024;15:1357726. 10.3389/fimmu.2024.1357726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Zhang N, Zhang H, et al. Deciphering the TCF19/miR-199a-5p/SP1/LOXL2 pathway: implications for breast cancer metastasis and epithelial-mesenchymal transition. Cancer Lett. 2024;597: 216995. 10.1016/j.canlet.2024.216995. [DOI] [PubMed] [Google Scholar]
- 18.Vivian J, Rao AA, Nothaft FA, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol. 2017;35(4):314–6. 10.1038/nbt.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS J Integr Biol. 2012;16(5):284–7. 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinform. 2013;14(1):7. 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Lichtenberg T, Hoadley KA, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400-416.e11. 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–95. 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Krautkramer KA, Linnemann AK, Fontaine DA, et al. Tcf19 is a novel islet factor necessary for proliferation and survival in the INS-1 β-cell line. Am J Physiol Endocrinol Metab. 2013;305(5):E600–10. 10.1152/ajpendo.00147.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim J, Hwang YS, Yoon HR, et al. PLK1 phosphorylates RhoGDI1 and promotes cancer cell migration and invasion. Cancer Cell Int. 2024;24(1):73. 10.1186/s12935-024-03254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Zhang S, Wang F, He J, Jiang W, Zhang L. DLGAP5 promotes lung adenocarcinoma growth via upregulating PLK1 and serves as a therapeutic target. J Transl Med. 2024;22(1):209. 10.1186/s12967-024-04910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Wang J, Xie S, et al. Lasiokaurin regulates PLK1 to induce breast cancer cell G2/M phase block and apoptosis. J Cancer. 2024;15(8):2318–28. 10.7150/jca.93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanini E, Forster-Gross N, Bachmann F, et al. Dual TTK/PLK1 inhibition has potent anticancer activity in TNBC as monotherapy and in combination. Front Oncol. 2024;14:1447807. 10.3389/fonc.2024.1447807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreekumar S, Montaudon E, Klein D, et al. PLK1 inhibitor Onvansertib enhances the efficacy of alpelisib in PIK3CA-mutated HR-positive breast cancer resistant to palbociclib and endocrine therapy: preclinical insights. Cancers. 2024;16(19):3259. 10.3390/cancers16193259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondal P, Sen S, Klein BJ, et al. TCF19 promotes cell proliferation through binding to the histone H3K4me3 mark. Biochemistry. 2020;59(4):389–99. 10.1021/acs.biochem.9b00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian Y, Xin S, Wan Z, et al. TCF19 promotes cell proliferation and tumor formation in lung cancer by activating the Raf/MEK/ERK signaling pathway. Transl Oncol. 2024;45: 101978. 10.1016/j.tranon.2024.101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji P, Chang J, Wei X, et al. Genetic variants associated with expression of TCF19 contribute to the risk of head and neck cancer in Chinese population. J Med Genet. 2022;59(4):335–45. 10.1136/jmedgenet-2020-107410. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Gao H, Azhar MS, et al. Interleukin signaling in the regulation of natural killer cells biology in breast cancer. Front Immunol. 2024;15:1449441. 10.3389/fimmu.2024.1449441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz S, Klausz K, Albici AM, et al. Novel NKG2D-directed bispecific antibodies enhance antibody-mediated killing of malignant B cells by NK cells and T cells. Front Immunol. 2023;14:1227572. 10.3389/fimmu.2023.1227572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Supplementary Figure 1. The correlation between TCF19 and PLK1 in TCGA cancers.
Data Availability Statement
The datasets analyzed during the current study are available for all projects in TCGA (https://portal.gdc.cancer.gov/), GSE93601, GSE70947, GSE9893, and GSE1456 from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), GTEx database (https://www.gtexportal.org/), TISIDB websites (http://cis.hku.hk/TISIDB/index.php). All raw data are available from the corresponding author.