Abstract
The ligand-activated aromatic hydrocarbon receptor (AHR) dimerizes with the AHR nuclear translocator (ARNT) to form a functional complex that transactivates expression of the cytochrome P-450 CYP1A1 gene and other genes in the dioxin-inducible [Ah] gene battery. Previous work from this laboratory has shown that the activity of the CYP1A1 enzyme negatively regulates this process. To study the relationship between CYP1A1 activity and Ah receptor activation we used CYP1A1-deficient mouse hepatoma c37 cells and CYP1A1- and AHR-deficient African green monkey kidney CV-1 cells. Using gel mobility shift and luciferase reporter gene expression assays, we found that c37 cells that had not been exposed to exogenous Ah receptor ligands already contained transcriptionally active AHR-ARNT complexes, a finding that we also observed in wild-type Hepa-1 cells treated with Ellipticine, a CYP1A1 inhibitor. In CV-1 cells, transient expression of AHR and ARNT leads to high levels of AHR–ARNT-dependent luciferase gene expression even in the absence of an agonist. Using a green fluorescent protein-tagged AHR, we showed that elevated reporter gene expression correlates with constitutive nuclear localization of the AHR. Transcriptional activation of the luciferase reporter gene observed in CV-1 cells is significantly decreased by (i) expression of a functional CYP1A1 enzyme, (ii) competition with chimeric or truncated AHR proteins containing the AHR ligand-binding domain, and (iii) treatment with the AHR antagonist α-naphthoflavone. These results suggest that a CYP1A1 substrate, which accumulates in cells lacking CYP1A1 enzymatic activity, is an AHR ligand responsible for endogenous activation of the Ah receptor.
Polycyclic aromatic hydrocarbons, halogenated aromatic hydrocarbons, and other, structurally related planar organochlorinated compounds elicit many adverse biological effects, including immunosuppression, teratogenesis, tumor promotion, hormonal disregulation, and cardiovascular disease (4, 8, 14, 46, 51). All of these seemingly unrelated biological effects are believed to be mediated by the sustained activation of the aromatic hydrocarbon receptor (AHR) and the subsequent perturbation of cellular homeostasis. AHR is a ligand-dependent basic helix-loop-helix (bHLH) transcription factor belonging to the bHLH/PAS gene family (5, 12). AHR and other genes in this family are expressed very early in embryogenesis (1, 2, 25, 49) and appear to be involved in important developmental functions such as neurogenesis (13, 42), tracheal morphogenesis (28, 73), regulation of circadian rhythm (29, 35, 58), and response to hypoxia (68).
Among the genes identified in the bHLH/PAS family, AHR appears to be the only member that requires ligand binding for activation. Ligand activation allows the cytosolic AHR to translocate into the nucleus and to dimerize with the AHR nuclear translocator (ARNT) (59), another member of the bHLH/PAS gene family. Heterodimeric AHR-ARNT complexes function as transcriptional activators by binding to consensus sequences termed AhRE (also known as DRE or XRE) in the regulatory domains of numerous genes (15, 32, 47). Genes transcriptionally regulated by AHR-ARNT complexes encode several foreign chemical-metabolizing enzymes including, the cytochrome P-450 enzymes CYP1A1, CYP1A2, and CYP1B1 (61, 64), NAD(P)H-menadione oxidoreductase (NMO1), UDP glucuronosyltransferase (UGT1A6), glutathione transferase (GSTA1), and a tumor-specific aldehyde dehydrogenase (ALDH3c, Ahd4) (44). In addition, transcription of plasminogen activator inhibitor II, interleukin-1β (65), and protooncogenes c-jun and jun D can also be activated by the AHR-ARNT complex (26, 55). Furthermore, due to competition with dimerization partners, a ligand-activated AHR may be involved in down-regulation of ARNT-ARNT homodimer-dependent transcriptional responses (66), or, conversely, AHR–ARNT-dependent transactivation may be inhibited by activation of the hypoxia response (22). Thus, it appears that AHR plays an important role not only in the regulation of xenobiotic metabolism but also in the maintenance of homeostatic functions.
Autoregulation of endogenous substrates by metabolizing enzymes is an important mechanism to maintain homeostasis in biological systems (43). Among the cytochrome P-450 enzymes induced by the AHR-ARNT complex, the clearest example is the transcriptional activation of the Cyp1a1 gene. In variant mouse hepatoma cell lines lacking functional CYP1A1 enzyme activity, transcription rates of Cyp1a1 and of several other genes in the [Ah] gene battery are remarkably elevated, such that mRNA levels in the untreated variant cell lines are comparable to those in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated wild-type Hepa-1 cells (21, 24, 56, 57, 60, 67). Stable expression of murine CYP1A1 or human CYP1A2 cDNAs in these cell lines represses deregulated CYP1A1 mRNA levels and restores TCDD inducibility (56, 57). It has been suggested that an endogenous CYP1A1 substrate is somehow involved in transcriptional regulation of the [Ah] gene battery. It has been proposed that this putative substrate could be an endogenous AHR ligand that is rendered inactive by metabolism or, alternatively, that metabolism could convert the substrate into a repressor of AHR-dependent transcription. In the present study, we have examined these two alternative hypotheses. The results suggest that a putative endogenous CYP1A1 substrate up-regulates gene transcription through activation of the AHR-ARNT complex. This putative endogenous CYPA1 substrate functions as an AHR activator and thus may represent an endogenous ligand for the AHR.
(This research was submitted by C.-Y. Chang in partial fulfillment of the requirements for the degree of Doctor of Philosophy from the University of Cincinnati.)
MATERIALS AND METHODS
Cell lines, transfections, and growth conditions.
The mouse Hepa-1 hepatoma cell line (3), its variants c37 (24, 33), CX4, CH3.3, and CPr (56, 57), and African green monkey adult kidney CV-1 cells (31) were cultured in α-minimum essential medium (Gibco BRL) supplemented with 5% fetal bovine serum and 1% antibiotic-antimycotic (Gibco BRL) in a humidified 5% CO2 atmosphere. Derivations and pertinent phenotypes of these cell lines are shown in Table 1. Duplicate or triplicate transient transfection experiments were performed by using standard calcium phosphate techniques (23) on cells grown either in 25-cm2 tissue culture flasks or on 12-well plates. In all experiments, to control for variations in transfection efficiency, cells were also cotransfected with plasmid pCMVβgal (ClonTech). To express AHR and ARNT, pcDNAI/B6AHR and pcDNAIneo/mARNT (see below) were included in all transfections with CV-1 cells. CV-1 cells express detectable levels of immunoreactive ARNT protein, but inclusion of pcDNAIneo/mARNT in the transfection causes a large increase of AHR–ARNT-dependent luciferase reporter expression. Since Hepa-1 cells already express endogenous AHR and ARNT, expression plasmids for these two proteins were not included in transfections with these cells unless otherwise specified. Twelve to 16 h after transfection, cells were washed three times with phosphate-buffered saline (PBS) and changed to fresh medium. TCDD (10 nM), control vehicle (dimethyl sulfoxide [DMSO]), or, in some experiments, α-naphthoflavone (ANF) (1 μM) were added to the cells 8 h later. Luciferase and β-galactosidase activities were measured 14 to 16 h after treatment, according to the manufacturer’s instructions (Promega). Cells grown in 25-cm2 flasks were transfected and washed with PBS as described above. Three to 4 h later, cells were trypsinized and seeded into 12-well or 24-well plates. After the cells had adhered to the plates, medium containing appropriate chemicals was added, and luciferase and β-galactosidase activities were measured 14 to 16 h after treatment. For experiments using the alkaline phosphatase reporter construct pGREII-oct-AF, fresh medium containing the appropriate chemicals was added to the cells 48 h after transfection, and alkaline phosphatase secreted into the medium was measured for the interval between 48 and 64 h after transfection. Alkaline phosphatase activity was assayed by a colorimetric method, with p-nitrophenyl phosphate as a substrate and measurement of the increase of absorbance at 405 nm as described previously (70), and was normalized to the cotransfected β-galactosidase activity. Alternatively, alkaline phosphatase activity was measured with a chemiluminescent assay system from Tropix, Inc., according to the manufacturer’s specifications. All experiments were repeated a minimum of three independent times, and the results shown are averages ± standard deviations (SD).
TABLE 1.
Pertinent phenotypes of cell lines used in these experiments
| Cell line | Derivation | Phenotype | Reference |
|---|---|---|---|
| Hepa-1 | C57BL/6 mouse hepatoma | AHR+ ARNT+ CYP1A1+; wild-type | 3 |
| c37 | Hepa-1 | AHR+ ARNT+ CYP1A1−; two nonsense mutations in the Cyp1a1 gene | 24, 33 |
| CX4 | c37 | AHR+ ARNT+ CYP1A1+; c37 stable transfectant of plasmid-encoded wild-type mouse CYP1A1 cDNA | 56, 57 |
| CH3.3 | c37 | AHR+ ARNT+ CYP1A1− CYP1A2+; c37 stable transfectant of plasmidencoded wild-type human CYP1A2 cDNA | 56, 57 |
| CPr | c37 | AHR+ ARNT+ CYP1A1−; c37 stable transfectant of a plasmid with enhancerless mouse CYP1A1 cDNA | 56, 57 |
| CV-1 | African green monkey kidney | AHR− ARNT+a CYP1A1− | 31 |
ARNT+ phenotype is weakly expressed in CV-1 cells.
A wild-type Hepa-1 cell line with a stably integrated pAhRDtkLuc3 reporter gene was generated by cotransfecting pAhRDtkLuc3 (see below) and pIND (Invitrogen), a plasmid conferring resistance to G418, into wild-type Hepa-1 cells. G418-resistant colonies were analyzed for luciferase expression in the presence or absence of TCDD treatment. Colonies that showed TCDD inducibility were chosen as reporter cell lines for analysis of AHR–ARNT-dependent gene activation. Medium containing 10 or 100 nM Ellipticine (Sigma), DMSO vehicle control, or 10 nM TCDD was added to semiconfluent cells, and luciferase activity was assayed 12 h after treatment and normalized to the amount of total protein in the cell lysate, as measured by the Bradford assay (Bio-Rad).
Plasmid constructs.
The Ah receptor expression plasmids pcDNAI/B6AHR and pcDNAI/D2AHR were cloned into the BamHI and XbaI sites of the pcDNAI/Amp vector (Invitrogen) with reverse transcription (RT)–PCR-amplified cDNA fragments from lymphocytes of C57BL/6J and DBA/2 mice, respectively. The amplified product contained 2,638 bases, beginning 39 nucleotides upstream from the ATG codon and ending 181 nucleotides past the termination codon in the Ahrb-1 allele, as previously described (7). Amino acid changes in the expression plasmids were generated by standard recombinant DNA techniques with the appropriate cDNA fragments obtained by RT-PCR from lymphocytes of DBA/2 mice (7). Unique restriction sites in the AHR cDNA were used to generate chimeric constructs pR, pNMR, pNM, pPNMR, pP, pVPNM, pVP, and pV by gradually replacing corresponding DNA fragments in pcDNAI/B6AHR with DNA fragments from pcDNAI/D2AHR. Relevant polymorphisms (7) in these constructs were confirmed by DNA sequencing, and expression of the encoded AHR proteins was verified by coupled in vitro transcription-translation. Complete details of the construction of these chimeric plasmids are available upon request. Reporter construct p1646P1Luc2 was generated by subcloning the Cyp1a1 5′-regulatory sequences from −1646 to +57 (20, 21) into the pGL2 basic vector (Promega). Complementary oligonucleotides encoding one copy of the AhRE sequence 5′ TCGAACTCACGCAACT 3′ (26) were annealed and ligated into the MluI site of the pGL2 promoter vector (Promega) to generate the pAhRESV40Luc2 reporter construct. pAhRDtkLuc2 was constructed by ligation of the mouse Cyp1a1 AhRD enhancer (coordinates −1100 to −896), containing three different AhRE motifs (20, 45), into a modified pGL2 basic vector that contains the herpes simplex virus type 1 thymidine kinase (tk) minimum promoter from −79 to +53 (39), from which the SP1-binding site was removed. pAhRDtkLuc3 and pAhRDSV40Luc3 were similarly constructed by cloning the AhRD sequences next to the tk or simian virus 40 (SV40) minimum promoter, respectively, in the pGL3 basic vector. The mouse ARNT expression plasmid pcDNAIneo/mARNT was generously provided by O. Hankinson (54). The chimeric construct τDBD/DR83-593, containing the AHR ligand-binding region (amino acids 83 to 593) fused to the glucocorticoid receptor DNA-binding and transactivation domains, and the alkaline phosphatase reporter pGREII-oct-AF were gifts from L. Poellinger and M. Whitelaw (70, 71). Plasmid pAhP1S, expressing full-length mouse CYP1A1, has been described previously (57). pAhP1short is a derivative of pAhP1S containing an insertion of 3 stop codons in the three frames at amino acid 429 which thus codes for a truncated, nonfunctional form of the CYP1A1 protein. Plasmid pcDNAI/B6AhR-GFP, encoding a fusion protein of the AHR and the green fluorescent protein (GFP) from Aequorea victoria, was generated as follows: GFP coding sequences were excised from pAlpha+GFP (Maxigene) and cloned into the EcoNI site at the 3′ end of the B6AHR coding sequences in pcDNAI/B6AHR, interposing between the two coding sequences a (Gly-Ala)5 linker, as described for the glucocorticoid receptor-GFP fusion protein (27). pAHRΔ495-805 was derived from pcDNAI/B6AHR by deleting the C-terminal 311 amino acids; this plasmid encodes an AHR protein which is capable of ligand binding and dimerization with ARNT but lacks transactivation activity.
In vitro transcription-translation.
One microgram each of pcDNAI/B6AHR-GFP and pcDNAIneo/mARNT were used in a 50-μl in vitro transcription-translation reaction with the TNT-coupled reticulocyte lysate system from Promega, according the manufacturer’s directions. Reaction mixtures were incubated at 30°C for 2 h, and translated proteins were kept at −20°C until use.
Gel mobility shift assays.
Cells were grown on 15-cm plates to subconfluence and treated with DMSO vehicle or 10 nM TCDD for 1 h. Nuclear extracts were prepared as described previously (6, 9) and kept at −70°C until use. For gel mobility shift assays, 20 μg of nuclear protein extract was mixed with 1 μg of poly(dI-dC)–poly(dI-dC) in binding buffer containing 0.12 M KCl, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, and 20 mM HEPES (pH 7.8) for 15 min at room temperature. One-tenth of a nanogram of 32P-labeled probe was added to the reaction mix and incubated for an additional 15 min at room temperature. Bromophenol blue was added before the reaction mixture was loaded onto a 4% nondenaturing polyacrylamide gel. The gel was run at 200 V for 2 h and visualized by autoradiagraphy. Quantitation of bound probe was done with the ImageQuaNT algorithm of the Storm 860 PhosphorImager (Molecular Dynamics). The mXRE-1 probe was generated as described previously (59), with [32P]dCTP as the labeled precursor.
For experiments using in vitro-translated proteins, 5 μl of the pcDNAI/B6AHR-GFP and 5 μl of the pcDNAIneo/mARNT translation reaction mixtures were combined and incubated with 0.1% DMSO or 100 nM TCDD at 30°C for 1 h. Reaction mixtures were used for gel mobility shift assays according to the protocols described above.
Western blots.
Nuclear and cytosolic extracts from Hepa-1, c37, and CX4 cells were analyzed for the presence of Ah receptor as described previously (53), by using a specific anti-AHR antibody, a generous gift of R. Pollenz. Chemiluminescent images were quantitated by Scan Analysis software (Biosoft). For Western blot analysis of CYP1A1 expression, CV-1 cells were harvested in 1× PBS 40 h after transfection, pelleted by centrifugation, and washed once with PBS. Cell pellets were resuspended in buffer A (10 mM Tris-HCl, 1 mM EDTA, 2 mM dithiothreitol [pH 7.5]) and homogenized with a Wheaton tissue grinder. Cell homogenates were spun down at 600 × g to clear out the nuclear pellet and then subjected to centrifugation at 105,000 × g for 1 h to collect the microsomal fraction. Microsomal pellets were dissolved in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 2% 2-mercaptoethanol, 10% glycerol). Twenty micrograms of the microsome-enriched fractions were separated by SDS–12% polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane for Western blotting. The membrane was incubated with BLOTTO (5% fat-free milk powder in TTBS [150 mM NaCl, 50 mM Tris, 0.2% Tween 20 {pH 7.5}]) for 2.5 h at room temperature and then incubated with primary antibody αP450c (rabbit anti-mouse CYP1A1; a gift from S. Kimura) diluted 1:5,000 in BLOTTO for 2 h at room temperature. The blot was washed 4 times with TTBS+ (300 mM NaCl, 50 mM Tris, 0.5% Tween 20 [pH 7.5]) and incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit secondary antibody for 1 h. The blot was washed and developed with the Amersham ECL detection system.
Fluorescence microscopy.
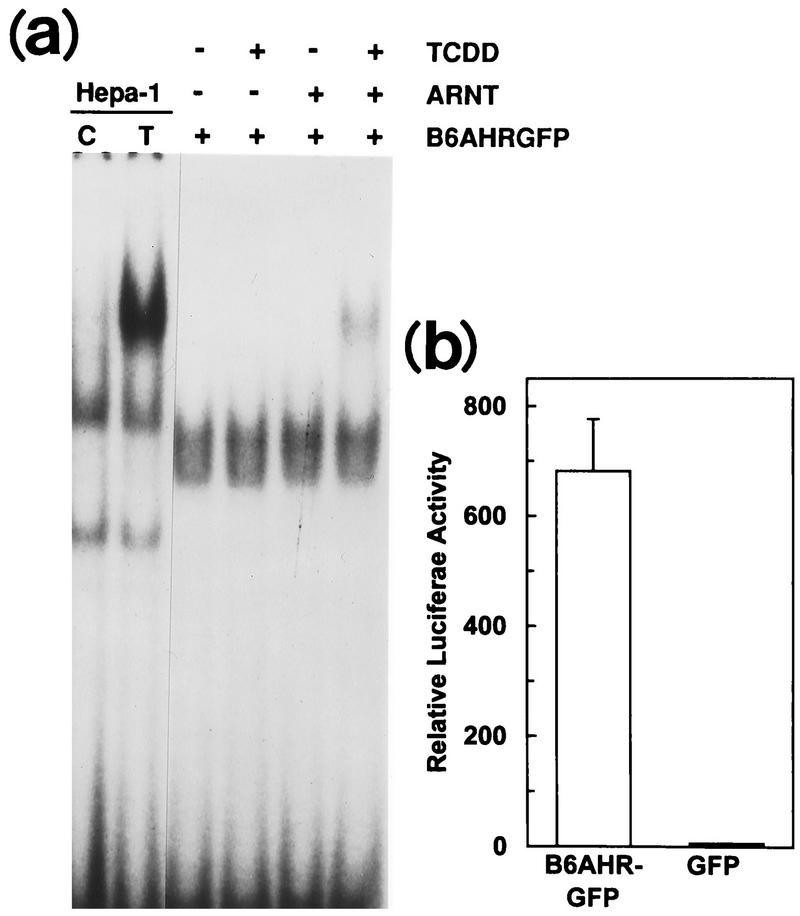
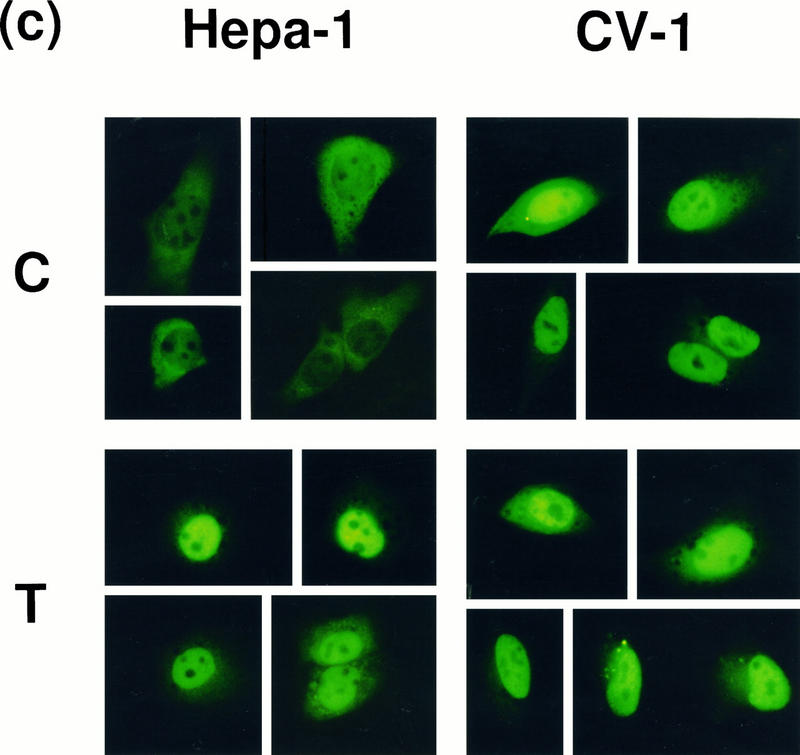
Hepa-1 or CV-1 cells were seeded onto coverslips and cotransfected with pcDNAI/B6AhR-GFP and pcDNAIneo/mARNT. Approximately 48 h after transfection, cells were treated with DMSO vehicle or 10 nM TCDD for 2 h. The coverslips were washed 3 times with PBS and fixed in 2% paraformaldehyde–PBS for 30 min at room temperature. After fixing, the coverslips were washed 3 times in PBS, mounted on microscope slides with 80% glycerol–PBS, and sealed with rubber cement. A Leitz Laborlux S microscope equipped with 3λ-Ploemopak incident fluorescent light was used to visualize cellular localization of the expressed GFP proteins with an excitation wavelength of 450 to 490 nm and an emission wavelength of 515 nm. Approximately 40 to 50 highly fluorescent cells were observed per coverslip of transfected cells. Transfection experiments were repeated 3 or 4 times; shown in Fig. 5c are typical representatives of each cell line and treatment.
FIG. 5.
Cellular localization of transfected AHR in CV-1 and Hepa-1 cells. (a) Gel mobility shift assays using in vitro-translated B6AHR-GFP and mARNT. Translated proteins were incubated with 0.1% DMSO (−) or 100 nM TCDD (+) for 1 h at 30°C before the gel shift reaction. Nuclear extracts from DMSO-treated (C) and TCDD-treated (T) Hepa-1 cells were also included in the gel shift assays as controls. (b) Reporter gene pAhRDtkLuc3 expression in CV-1 cells transiently transfected with pcDNAI/B6AHR-GFP or pAlpha+GFP. Control plasmid pCMVβgal and the mARNT expression plasmid pcDNAIneo/mARNT were also included in the transfection. (c) Subcellular localization of the AHR-GFP fusion protein. Hepa-1 and CV-1 cells grown on coverslips were cotransfected with pcDNAIneo/mARNT and pcDNAI/B6AHR-GFP and treated with either DMSO vehicle control (C) or 10 nM TCDD (T) for 2 h. Cells were fixed with 2% paraformaldehyde, and the expressed fusion proteins were visualized by fluorescence microscopy at 450 to 490 nm and 515 nm as excitation and emission wavelengths, respectively. Five representative cells from each transfection are shown for each cell line and treatment.
RESULTS
CYP1A1 autoregulates its own transcription through an AHR-ARNT complex.
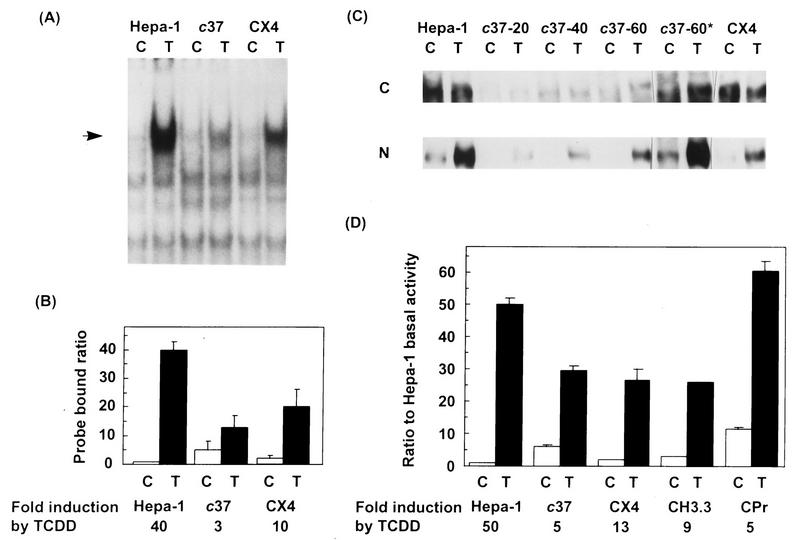
We used electrophoretic mobility shift assays to determine whether activated AHR-ARNT complexes were present in unstimulated CYP1A1-deficient cells. Nuclear protein extracts from wild-type Hepa-1, CYP1A1-deficient c37 cells, and CX4 cells, a c37 stable transfectant line that expresses a plasmid-encoded wild-type CYP1A1 enzyme, were analyzed with a 32P-labeled AhRE probe. Control nuclear extracts from Hepa-1 cells showed high TCDD-dependent recruitment of AhRE-binding complexes to the nucleus, whereas c37 extracts showed greatly reduced levels of these complexes. Expression of CYP1A1 in CX4 cells restored a wild-type-like phenotype, with extensive TCDD-induced nuclear translocation (Fig. 1A). Quantitation of several such gel shift experiments showed that CYP1A1 deficiency in c37 cells resulted in two separate effects. On the one hand, AHR-ARNT nuclear activation in untreated cells was fivefold higher than that observed in control Hepa-1 cells (Fig. 1B). On the other hand, TCDD-induced activation was reduced to one-third of the level observed in TCDD-treated Hepa-1 cells. As a result, overall TCDD-dependent induction, approximately 40-fold in Hepa-1 cells, was reduced to only 3-fold in c37 cells. CX4 cells partly restored the wild-type phenotype, with significantly lower uninduced and higher TCDD-induced levels of AHR-ARNT complexes. Western blot analysis of nuclear and cytosolic extracts from these cell lines (Fig. 1C) indicated that the amount of AHR in c37 cells was extremely low compared to Hepa-1 and CX4 cells. Results from three Western blots were quantitated, showing that c37 cells had only 3% of the AHR levels found in Hepa-1 cells and that CX4 cells had an almost complete restoration of the wild-type phenotype (Table 2); however, compared to Hepa-1 and CX4, a significantly larger proportion of the AHR in untreated c37 cells was localized to the nucleus (Table 2).
FIG. 1.
Detection of active AHR-ARNT complexes and of AHR in wild-type and variant mouse hepatoma cell lines. (A) Electrophoretic mobility shift assays. Hepa-1, c37, and CX4 cells were grown to subconfluence and treated with 10 nM TCDD (T) or with DMSO vehicle (C) for 1 h. Nuclear extracts were prepared, and gel mobility shift assays were performed as described in Materials and Methods. The arrow indicates the shifted AHR-ARNT complex. (B) Quantitation of mobility shift data. Three separate gel shift assays were quantitated by PhosphorImaging. The ordinate represents the ratio of probe bound by each of the nuclear extracts tested to probe bound by the untreated Hepa-1 nuclear extract and thus is a measure of changes relative to constitutive levels in wild-type cells. Indicated in the abscissa is the fold induction by TCDD for each cell line tested. (C) Western blot analysis of AHR. Cytosolic (C) and nuclear (N) extracts from Hepa-1, c37, and CX4 cells were separated in 7.5% acrylamide gels, transferred to polyvinylidene difluoride and analyzed for the presence of AHR with an AHR-specific antibody. For Hepa-1 and CX4, 20 μg of cytosolic or nuclear extract was used; for c37, 20, 40, and 60 μg of each extract was used, as indicated over the lanes. c37-60* is a 20-fold longer exposure of the lane denoted by c37-60. (D) Transient transfection assays. Hepa-1 (wild type), c37 (Hepa-1 derivative lacking CYP1A1 enzymatic activity), CX4 and CH3.3 (c37 stable transfectants expressing mouse CYP1A1 and human CYP1A2, respectively), and CPr (c37 stable transfectant carrying enhancerless mouse CYP1A1 cDNA) were transiently transfected with pAhRDtkLuc3 and pCMVβgal and treated with 10 nM TCDD or DMSO vehicle 14 to 16 h after transfection. Luciferase and β-galactosidase assays were performed as described in Materials and Methods. The ordinate represents the ratio of normalized luciferase activity to activity in untreated Hepa-1 cells. Fold induction by TCDD is the ratio of luciferase activity in TCDD-treated to untreated samples in the same transfection group.
TABLE 2.
Relative amounts and distributions of AHR in untreated cellsa
| Cell line | % AHR in nuclear extract (avg ± SD) | Relative amt of AHR (%) |
|---|---|---|
| Hepa-1 | 4.7 ± 0.3 | 100 |
| c37 | 6.1 ± 1.2b | 3 |
| CX4 | 3.2 ± 0.2 | 59 |
Values were calculated from the data in Fig. 1C.
Significantly different from the value for Hepa-1 cells (P = 0.02 [by a two-tailed test]).
We also measured the expression of pAhRDtkLuc3, a luciferase reporter plasmid driven by the AhRD enhancer (20, 45), in transient transfection assays in CYP1A1-deficient and wild-type Hepa-1 cells (cell lines and phenotypes are shown in Table 1). In addition to Hepa-1, c37, and CX4 cells, we also tested CH3.3 cells, derived from c37, which overexpress a plasmid encoding the human CYP1A2 enzyme, and CPr cells, also a c37 variant, which are stably transfected with a plasmid containing mouse CYP1A1 cDNA fused to an enhancerless SV40 promoter. CPr cells were used as a control, since absence of the AhRD enhancer prevents these cells from expressing detectable levels of CYP1A1 enzyme (56, 57). In good agreement with the gel shift data, basal luciferase expression in untreated CYP1A1-deficient cells was significantly higher than in wild-type Hepa-1 cells (Fig. 1D), and expression levels in TCDD-treated cells were within a twofold range for all cell lines regardless of CYP1A1 functional status. As a consequence, treatment with TCDD caused only a 5-fold induction in c37 and CPr, whereas similar treatment in wild-type Hepa-1 cells caused a 50-fold induction; hence, we surmise that TCDD treatment in these CYP1A1-deficient cell lines results in low induction values because these cells already have elevated constitutive levels of luciferase expression. In contrast, CX4 and CH3.3, the two c37 variants expressing CYP1A1 and CYP1A2 enzymes, respectively, showed significantly lower basal luciferase activity, resulting in an elevation to 13- and 9-fold induction, respectively, by TCDD (Fig. 1D). To test whether inhibition of CYP1A1 enzymatic activity in wild-type Hepa-1 cells would also increase basal levels of AHR–ARNT-dependent gene expression, we used Ellipticine, a suicide inhibitor of CYP1A1 activity (34) that, at the low doses used in our experiments, is not a mouse AHR agonist (17). Treatment with 10 or 100 nM Ellipticine significantly induced AHR–ARNT-driven luciferase gene expression, to levels approaching those observed in TCDD-treated cells (Table 3). These results suggest that activation of AHR-ARNT complexes can take place as a result of the interaction between AHR and a CYP1A1 substrate. Both wild-type and c37 cell lines have little CYP1B1 expression, which is detectable only by RT-PCR (10a); hence, the possible role of this putative compound as a CYP1B1 substrate remains to be elucidated.
TABLE 3.
Induction of AHR–ARNT-dependent reporter gene expression by Ellipticine in Hepa-1 cells stably transfected with pAhRDtkLuc3
| Treatment | Luciferase activity/μg of protein (avg ± SD) | Pa |
|---|---|---|
| 0.1% DMSO | 11.9 ± 1.3 | |
| 10 nM Ellipticine | 21.3 ± 0.0 | >0.05 |
| 100 nM Ellipticine | 41.5 ± 2.1 | <0.0001 |
| 10 nM TCDD | 66.8 ± 9.6 | <0.0001 |
Bonferroni P value for one-way analysis of variance of comparisons to DMSO-treated control.
Expression of AHR in CV-1 cells results in a dose-dependent increase in reporter gene expression in the absence of exogenous ligand.
It has been reported that transient transfection of a plasmid encoding AHR into CV-1 cells, which lack endogenous AHR expression (16), results in high levels of reporter gene expression in the absence of an exogenous AHR ligand and that addition of an exogenous ligand such as TCDD causes only a two- to threefold induction (16, 30, 37, 38). These findings, which were attributed to nonphysiological conditions resulting from overexpression of transfected plasmids, resemble our results in CYP1A1-deficient hepatoma cells; however, since CV-1 cells lack CYP1A1 activity, these results might be explained equally well by the accumulation of an endogenous CYP1A substrate capable of activating the formation of AHR-ARNT complexes.
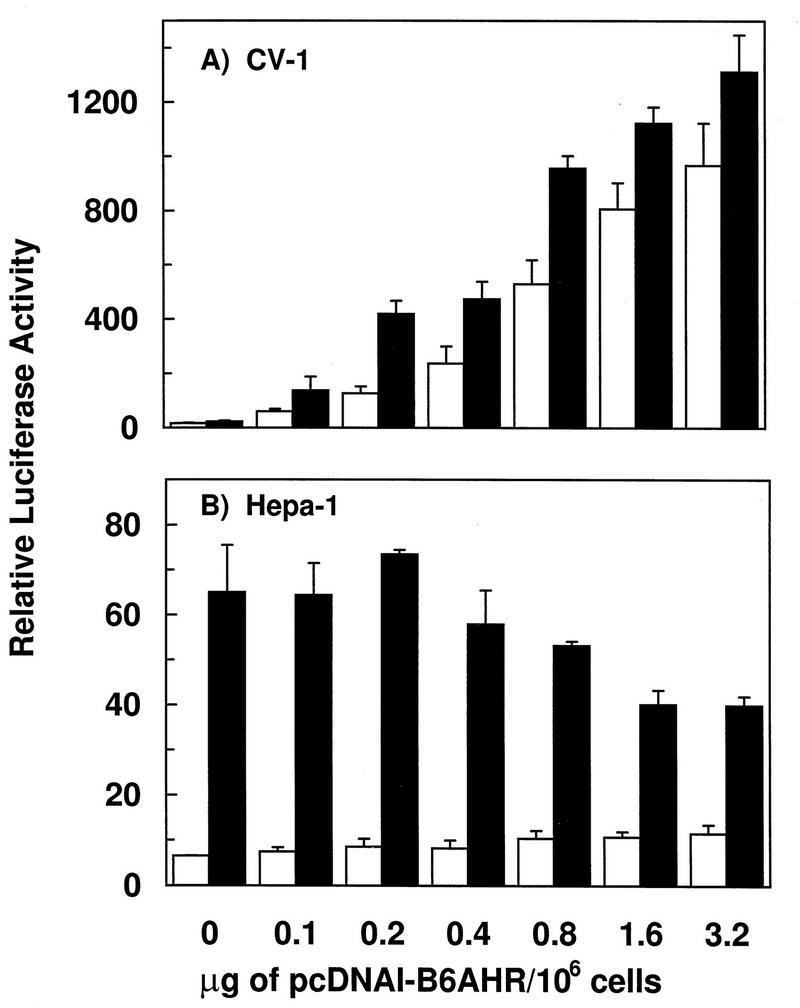
To test this possibility, we determined the effect of transfecting CV-1 cells with decreasing amounts of the expression plasmid pcDNAI/B6AHR. In the absence of TCDD treatment, decreasing amounts of this plasmid led to a dose-dependent decrease in luciferase expression, yet TCDD treatment caused less than a 2.5-fold induction, regardless of the amount of pcDNAI/B6AHR used in the transfection (Fig. 2A). By comparison, transfection of increasing amounts of pcDNAI/B6AHR into Hepa-1 cells, which express AHR endogenously, did not lead to significant changes in basal reporter gene expression levels, regardless of plasmid dose, and led only to a minor reduction in TCDD-induced levels at the higher plasmid doses (Fig. 2B). Since Hepa-1 cells express AHR endogenously, we cannot rule out experimentally the formal possibility that overexpression did not take place in the Hepa-1 cell line. We feel, however, that this possibility is rather unlikely, since other expression plasmids (e.g. β-galactosidase reporters) controlled by the same cytomegalovirus promoter used for AHR expression show clear dose-dependent expression effects.
FIG. 2.
AHR dose-dependent reporter gene expression in CV-1 (A) and Hepa-1 (B) cells. Transient transfections contained fixed amounts of pAhRDSV40Luc3 reporter gene, pCMVβgal, and pcDNAIneo/mARNT, and increasing amounts of pcDNAI/B6AHR, as shown on the abscissa. Twenty-four hours after transfection, cells were treated with DMSO vehicle control or 10 nM TCDD. Luciferase and β-galactosidase activities were measured 14 to 16 h after treatment. The ordinate represents luciferase activity normalized to β-galactosidase activity.
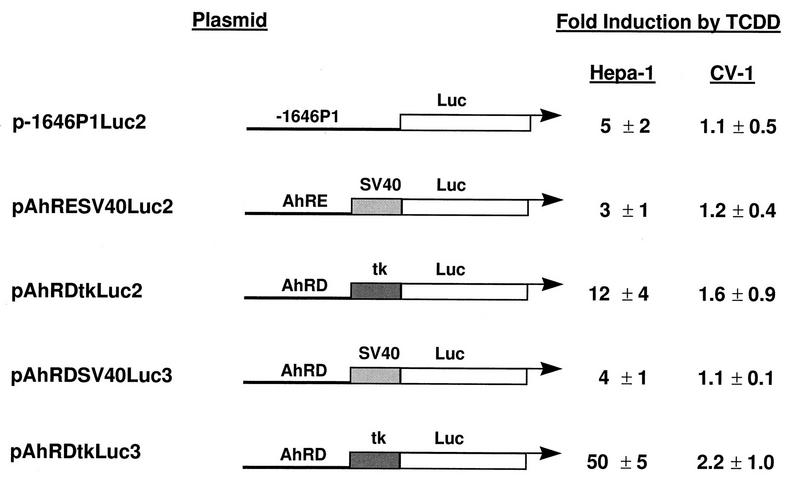
To rule out the possibility that the results observed were due to unknown peculiarities of one or another promoter-enhancer combination in the reporter constructs, we tested several such combinations. The combinations tested contained either the −1646 to +57 regulatory region of the mouse Cyp1a1 gene, the AhRD enhancer region fused to either an SV40 or a tk minimal promoter, or one copy of the AhRE motif fused to the SV40 minimal promoter. Similar patterns of AHR-dependent gene expression were observed regardless of the reporter constructs used. Basal (vehicle-treated) activities were always higher in CV-1 cells than in Hepa-1 cells transfected in parallel, and, depending on the particular enhancer-promoter combination used, TCDD treatment induced luciferase expression to a much higher extent in Hepa-1 cells than in CV-1 cells (Fig. 3), ruling out promoter-enhancer variations as the explanation for the results.
FIG. 3.
Effects of different promoter-enhancer combinations on TCDD induction of reporter gene expression in Hepa-1 and CV-1 cells. The different DNA sequence motifs used to construct the reporter plasmids shown are described in detail in Materials and Methods. Fold induction by TCDD is the ratio of the relative luciferase activities in TCDD-treated versus DMSO-treated samples.
CYP1A1 regulates activation of AHR-ARNT transcriptional complexes.
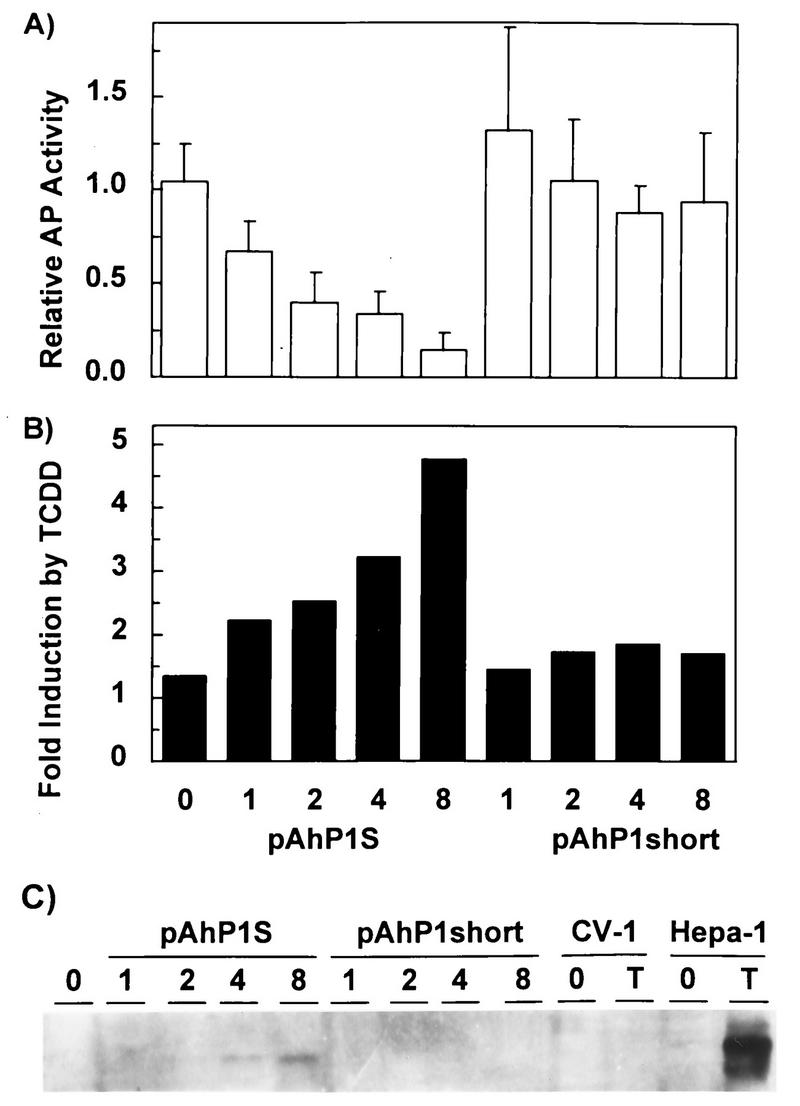
Since CV-1 cells do not have detectable CYP1A1 activity (26), a likely explanation for the results described above is that, as in c37 cells, an endogenous CYP1A1 substrate that accumulates in CV-1 cells due to the lack of CYP1A1 enzyme activity is also a putative AHR ligand. To test this hypothesis, we determined the effect of a coexpressing pAhP1S, an expression plasmid encoding full-length CYP1A1 cDNA, on basal AHR-dependent reporter expression. The experimental design was to examine whether the presence of a functional CYP1A1 enzyme would suppress the high constitutive levels of reporter gene expression. In addition to the control plasmid pCMVβgal, five additional plasmids were simultaneously transfected into the cells: (i) pcDNAI/B6AHR and pcDNAIneo/mARNT, needed to activate CYP1A1 expression from pAhP1S, since expression of this plasmid is controlled by the AhRD enhancer element (56); (ii) τDBD/DR83-593, a chimeric plasmid carrying the glucocorticoid receptor DNA-binding and transactivation domains fused to the AHR ligand-binding domain (70). This chimeric AHR, when activated by AHR ligands, transactivates genes containing glucocorticoid response elements (GREs) and thus serves as a sensor of changes in levels of putative AHR ligand (CYP1A substrate) resulting from CYP1A1 enzymatic activity); and (iii) pGREII-oct-AF, a GRE-containing reporter construct that responds to activation of the chimeric AHR by expressing secreted alkaline phosphatase (71).
If our hypothesis was correct, we would expect that increased doses of pAhP1S would lead to higher CYP1A1 activity and to decreased levels of the putative ligand. The overall effect would be detected as a dose-dependent decrease in alkaline phosphatase activity. This prediction was indeed correct. Expression of CYP1A1 caused a dose-dependent decrease in alkaline phosphatase expression (Fig. 4A) and a concomitant increase in TCDD inducibility (Fig. 4B). In contrast, expression of pAhP1short, a control plasmid encoding a truncated form of CYP1A1 lacking enzymatic activity, had no effect on basal or TCDD-induced reporter gene expression (Fig. 4A and B). A Western blot with antibodies against mouse CYP1A1 verified that pAhP1S, but not pAhP1short, expressed increasing amounts of full-length CYP1A1 in the transfected cells in a dose-dependent manner (Fig. 4C).
FIG. 4.
Inhibition by CYP1A1 of constitutive activation of AHR-ARNT complexes. CV-1 cells were cotransfected with the chimeric AHR construct τDBD/DR83-593, its reporter gene pGREII-oct-AF, the pCMVβgal control plasmid, and increasing amounts of pAhP1S or pAhP1short. To activate transcription of CYP1A1 from pAhP1S (encoding a functional enzyme) and pAhP1short (encoding a truncated, nonfunctional enzyme), we also cotransfected fixed amounts of pcDNAI/B6AHR and pcDNAIneo/mARNT in all experiments. (A) Medium was changed 40 h after transfection, and alkaline phosphatase (AP) and β-galactosidase activities were measured 14 to 16 h later. Relative AP activity is expressed as the ratio of AP to β-galactosidase activity. The doses, in micrograms, of plasmid per 106 cells of pAhP1S or pAhP1short are indicated on the abscissa. (B) Fold induction by TCDD, measured as the ratio of the relative AP activities in TCDD-treated versus DMSO-treated samples, is plotted as a function of plasmid dose. (C) Western blot analysis of CYP1A1 expressed in the transfected cells. Doses of pAhP1S or pAhP1short (in micrograms of plasmid per 106 cells) are indicated over the corresponding lanes. 0 and T, control and TCDD-treated untransfected CV-1 cells and Hepa-1 cells, respectively. Five micrograms of TCDD-treated Hepa-1 microsomal protein extract and 20 μg of all other microsomal extracts were separated by electrophoresis in 12% polyacrylamide gels.
Transfected AHR is targeted to the nucleus of CV-1 cells.
The transient transfection data discussed above suggests that the AHR may be constitutively activated in CV-1 cells and, therefore, that it may be targeted to the nucleus even in the absence of an exogenous ligand. To visualize the cellular localization of the transfected AHR in CV-1 cells and to compare it with its localization in control Hepa-1 cells, we made use of a plasmid expressing a GFP-tagged AHR protein. GFP, the green fluorescent protein from A. victoria, is an autofluorescent protein that can be directly observed by fluorescence microscopy at 450 to 490 nm and 515 nm as excitation and emission wavelengths, respectively. GFP fusion chimeras have been used extensively to visualize protein trafficking in cells (see reference 27 and references therein). Gel shift and transient transfection assays were used to verify that the functional characteristics of AHR were maintained by the AHR-GFP fusion protein. Indeed, AHR-GFP showed ligand-dependent dimerization with ARNT and DNA binding (Fig. 5a), as well as activation of luciferase expression in a transient transfection assay (Fig. 5b). In the absence of TCDD treatment, Hepa-1 cells transiently transfected with pcDNAI/B6AHR-GFP showed cytosolic localization of the vast majority of the chimeric AHR-GFP protein (Fig. 5c, upper left panel), whereas after TCDD treatment the majority of AHR-GFP was found in the nucleus (Fig. 5c, lower left panel). In contrast, CV-1 cells transfected with the same plasmid showed similar patterns of localization in untreated and TCDD-treated cells, with the majority of the AHR-GFP protein expressed in untreated cells already localized to the nucleus (Fig. 5c, right two panels), thus suggesting the constitutive presence of an AHR ligand or activator in these cells.
Inhibition of transactivation by wild-type AHR with chimeric and truncated AHR proteins.
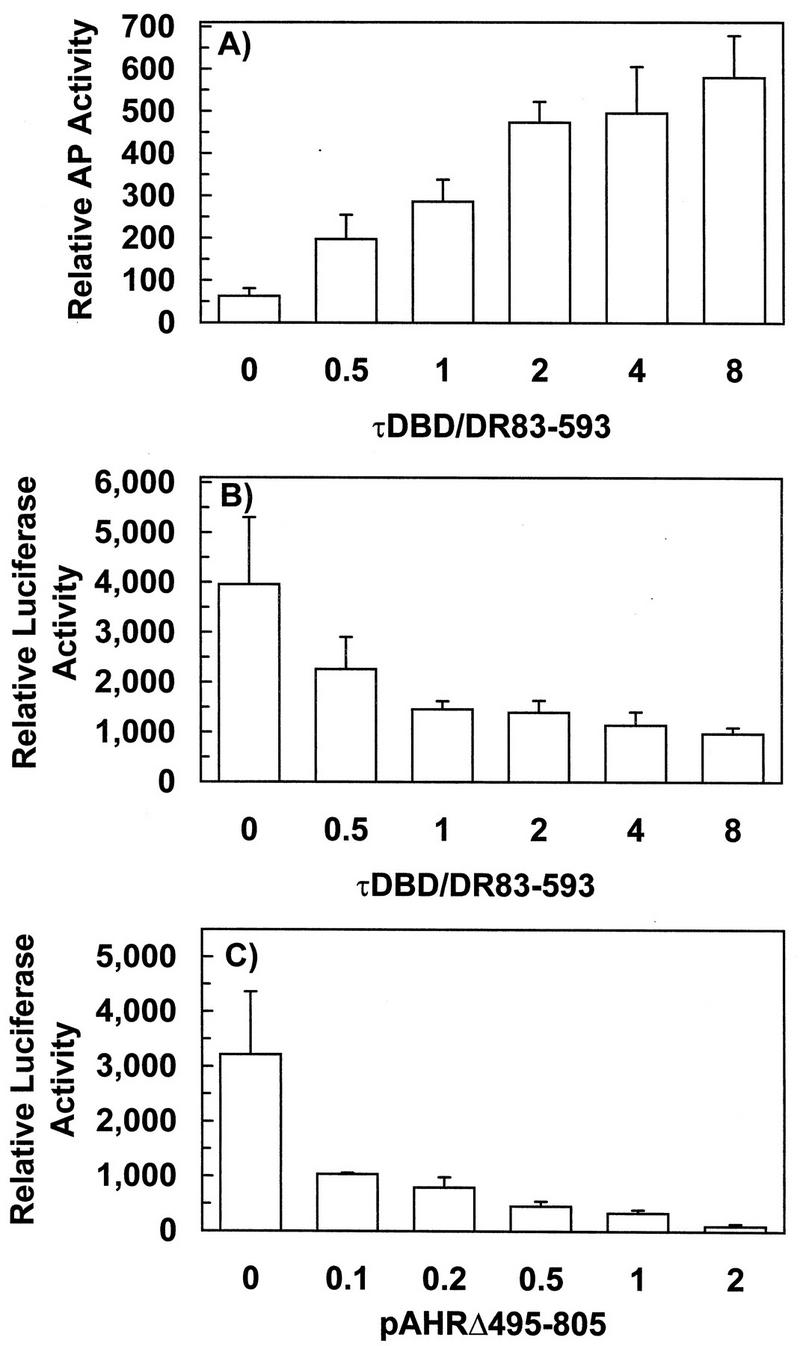
The previous results support the view that an endogenous CYP1A1 substrate possibly also functions as an AHR ligand. If this is the case, chimeric and truncated AHR containing the ligand-binding domain should compete with wild-type AHR for this putative endogenous ligand. To test this hypothesis, CV-1 cells were cotransfected with a fixed amount of pcDNAI/B6AHR and with increasing amounts of the chimeric τDBD/DR83-593 used in previous experiments along with the two reporter plasmids pAhRDSV40Luc3 and pGREII-oct-AF. Competition for ligand between wild-type and chimeric AHR in these experiments was monitored by the changes in expression of the two reporter genes. As the dose of τDBD/DR83-593 increased, expression of its corresponding reporter gene pGREII-oct-AF also increased, while expression of the reporter in pAhRDSV40Luc3, responsive to wild-type AHR, concomitantly decreased (Fig. 6B), suggesting that the chimeric Ah receptor in τDBD/DR83-593 and wild-type AHR compete for a common activator.
FIG. 6.
Competition of wild-type and chimeric or truncated AHR proteins for endogenous ligand. CV-1 cells were cotransfected with reporter constructs pAhRDSV40Luc3, pGREII-oct-AF, pCMVβgal, a fixed amount of pcDNAI/B6AHR, and increasing amounts of τDBD/DR83-593, as indicated on the abscissa (in micrograms per 106 cells). Secreted alkaline phosphatase (AP) activity (A) and luciferase activity (B) were assayed from the medium and cell lysate, respectively, of the same transfected cells and normalized to β-galactosidase activity. (C) Increasing amounts (in micrograms per 106 cells) of pAHRΔ495-805 expressing a truncated AHR were cotransfected into CV-1 cells with pcDNAI/B6AHR, pcDNAIneo/mARNT, pCMVβgal, and the reporter plasmid pAhRDtkLuc3. Luciferase and β-galactosidase activities were measured and calculated as described above.
To test the hypothesis further we used a truncated AHR, encoded by pAHRΔ495-805, as a competitor. This AHR mutant contains only the N-terminal 494 amino acids, including the bHLH and the ligand-binding domains but not the transactivating domain. Cotransfection of increasing amounts of this plasmid also caused a dose-dependent decrease of luciferase expression (Fig. 6C). This truncated AHR could also function as a dominant negative mutant preventing wild-type AHR from interacting with ARNT (10, 16), and it could be argued that its effect on luciferase expression was solely due to sequestration of ARNT. This is unlikely, however, because the chimeric AHR, τDBD/DR83-593, also blocks wild-type AHR function and yet does not dimerize with ARNT. It is more likely that activity of the truncated AHR is the result of additive dominant negative and ligand competition effects. Chimeric and truncated AHR have in common the ligand-binding domain, and both require ligand for activation, suggesting that this domain is responsible for their effect in blocking wild-type AHR function. Trivial explanations for these results, such as depletion of common elements of the transcription machinery, are possible, although unlikely, since expression of β-galactosidase, which shares the same elements and is used as a normalization factor, is unaffected by the increased doses of competitor plasmid. These results, therefore, are in better agreement with the conclusion that elevated, constitutive AHR–ARNT-dependent gene expression in CV-1 cells is due to activation of AHR, most likely by an endogenous CYP1A1 substrate that is also an AHR ligand. We cannot rule out, however, the possibility that other mechanisms might also be involved.
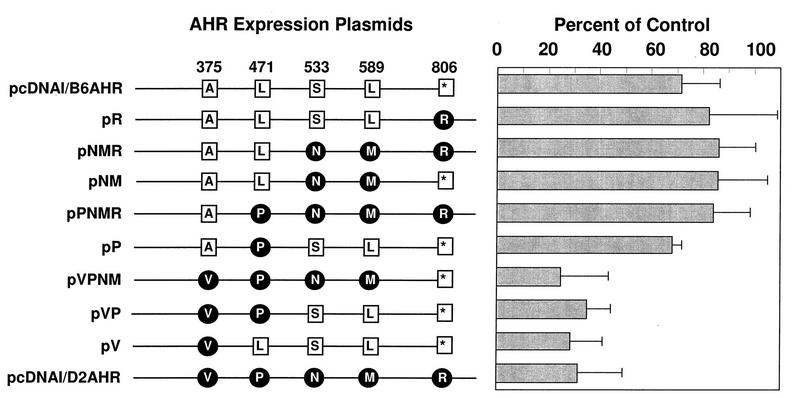
High- and low-ligand-affinity AHR variants show different affinities for the endogenous ligand.
ANF inhibits AHR–ARNT-dependent gene expression by acting as an AHR antagonist that blocks the binding of xenobiotic Ah receptor ligands (18, 40, 41, 72). In preliminary experiments using expression plasmids of the two AHR ligand affinity variants encoded by the Ahrb-1 and Ahrd alleles (48, 50), we observed that ANF blocked reporter plasmid expression directed by the low-affinity Ahrd allele to a greater extent than that of the high-affinity Ahrb-1 allele (data not shown). Five-amino-acid differences have been found between these two alleles (7), of which an A375V change is responsible for the high-versus low-affinity polymorphism (11, 52). If this ligand affinity polymorphism were also true for the putative endogenous ligand, we would expect that the observed differences in the ability of ANF to block Ahrb-1- and Ahrd-directed reporter expression would also depend on the presence of Ala or Val at position 375. To test this hypothesis, we constructed chimeric combinations of the two Ah receptor proteins by replacing corresponding DNA fragments in the pcDNAI/B6AHR and pcDNAI/D2AHR plasmids, which encode the AHRB-1 and AHRD proteins, respectively. We used these constructs, as well as the parental plasmids, for transient transfection experiments and determined the effect of ANF treatment on luciferase expression. In agreement with our preliminary observations, ANF treatment blocked the low-affinity AHR, encoded by pcDNAI/D2AHR, three to four times more efficiently (20 to 30%, compared to 70 to 80% of control values) than the high-affinity AHR, encoded by pcDNAI/B6AHR (Fig. 7). In addition, experiments with the chimeric combinations of the two Ah receptor proteins demonstrated that the presence of Val at position 375 resulted in extensive inhibition by ANF (Fig. 7), thus confirming our hypothesis that the same amino acid change responsible for the differences in ligand-binding affinities (11, 52) was responsible for the differences in ANF-dependent inhibition. The fact that a point mutation known to affect ligand affinity is the main determinant for ANF competition is compelling evidence that the effect of ANF is due to competition with an endogenous ligand present in CV-1 cells.
FIG. 7.
Differential antagonism by ANF of transactivation by high- and low-affinity AHR variants. CV-1 cells were transfected with pAhRDtkLuc3, pCMVβgal, pcNDAIneo/mARNT, and different pcDNAI/AHR chimeric constructs. The positions of amino acid differences between Ahrb-1 and Ahrd alleles, encoded by plasmids pcDNAI/B6AHR and pcDNAI/D2AHR, respectively, are shown at the top of the diagram on the left. Amino acids are represented by their one-letter codes. The star denotes the termination codon. Amino acid differences between Ahrb-1- and Ahrd-encoded proteins are shown in black lettering on an open square (Ahrb-1 allele) or white lettering on a filled circle (Ahrd allele). All chimeric constructs were generated by replacing segments from the cloned AHRB-1 cDNA with the corresponding regions from the cloned AHRD cDNA. Transfected CV-1 cells were treated with DMSO vehicle control or with 1 μM ANF (a dose predetermined in preliminary experiments) at 24 h after transfection. Luciferase and β-galactosidase activities were measured 14 to 16 h after treatment. The ordinate represents the ratio of luciferase activity in ANF-treated cells to that in control cells.
DISCUSSION
The data presented in this study show that constitutive activation of AHR-ARNT transcriptional complexes is regulated by the level of CYP1A enzymatic activity. In the absence of CYP1A enzymes, Hepa-1 variant c37, lacking CYP1A1 activity, and AHR- and CYP1A1-negative CV-1 cell lines show constitutive levels of AHR–ARNT-dependent gene expression approaching 20 to 80% of those found following treatment of Hepa-1 cells with TCDD, the most potent AHR ligand. In addition, inhibition of CYP1A1 enzymatic activity in wild-type Hepa-1 cells also leads to activation of AHR-ARNT in the absence of exogenous ligand. In both c37 and CV-1 cell lines, introduction of a CYP1A1 expression plasmid down-regulates the high levels of AHR-ARNT activation. These results suggest that an endogenous CYP1A1 substrate is responsible for the constitutive activation of AHR–ARNT-dependent gene expression.
Autoregulatory transcriptional derepression has been shown to take place for Cyp1a1 and other genes of the [Ah] battery (57). The present results extend this observation and show that derepression of these genes is due to endogenous activation of the AHR-ARNT transcriptional complex. The experiments represented in Fig. 1 indicate that in the absence of CYP1A1 activity, a greater proportion of the Ah receptor in untreated c37 cells is activated and hence translocated to the nucleus. Activated nuclear Ah receptor undergoes proteolytic degradation at a much higher rate than the cytosolic form (19, 53), which suggests that the low levels of AHR found in c37 cells are possibly due to resetting of steady-state levels resulting from constitutive nuclear translocation and subsequent degradation. Overexpression of CYP1A1 or CYP1A2 restores partially, but not completely, TCDD inducibility in CYP1A1-deficient cell lines, suggesting that CYP1A activity is not the only factor that controls constitutive AHR-ARNT activation. CV-1 cells, which do not express AHR, show a much higher degree of derepression than c37 cells; this suggests that other downstream genes regulated by AHR are also silent in this cell line and that these genes may also contribute to the autoregulatory mechanism. Alternatively, the balance between the rates of endogenous ligand synthesis and metabolism may be widely different in different cell lines, leading to different constitutive levels of AHR-ARNT activation. These two alternatives are not mutually exclusive.
Several lines of evidence presented in this article are consistent with the conclusion that this putative CYP1A1 substrate is also an endogenous Ah receptor ligand. First, its presence stimulates AHR–ARNT-dependent transactivation of reporter genes. Second, it promotes the DNA-binding activity of AHR-ARNT complexes. Third, it induces AHR nuclear translocation. Fourth, it requires the ligand-binding domain of AHR for its activity. Last, its effect is antagonized by ANF and shows functional characteristics for polymorphic AHR proteins similar to exogenous ligands. Formal proof that the compound involved is in fact a ligand of AHR must await purification and structural analysis.
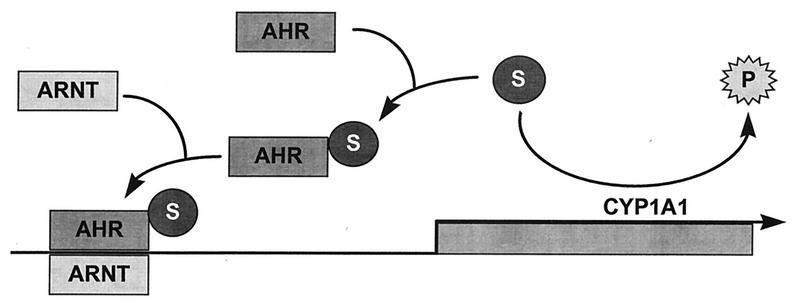
Figure 8 summarizes our current working hypothesis on the regulation of AHR–ARNT-dependent gene expression. The endogenous CYP1A1 substrate activates AHR in the absence of exogenous ligands, but the substrate is to some degree transformed into an inactive product by the functional CYP1A1 enzyme. Thus, in cells expressing CYP1A1, the substrate is maintained at a low concentration and AHR is relatively inactive. In cell lines lacking CYP1A1 activity, the substrate accumulates and AHR is constantly activated and able to dimerize with ARNT and to transactivate target genes.
FIG. 8.
Schematic representation of the current working hypothesis. CYP1A1 enzymatic activity converts the endogenous CYP1A1 substrate (S) to an inactive product (P). Substrate-bound AHR dimerizes with ARNT, and the AHR-ARNT complex transactivates Cyp1a1 and other target genes.
Nuclear localization of AHR has been reported for HeLa cells in the absence of an exogenous ligand (63). Similar results have been observed in the developing mouse embryo (1). Recently, stable transfection of AHR expression plasmids into receptorless cells has revealed that this protein plays an important role in the control of cell cycle progression and that no exogenous ligands are needed for this function (36, 69), further suggesting the existence of an endogenous AHR ligand. Other members of the bHLH/PAS gene family are believed to participate in early embryonic development, a stage in which cells continuously face decision checkpoints for proliferation, differentiation, or apoptosis. Activation of AHR by an endogenous ligand might be a critical event at any of these decision checkpoints. TCDD and other exogenous ligands may derail normal homeostasis by perturbing the balance in signal transduction pathways relevant to these decisions. In fact, TCDD is a powerful teratogen (8, 62).
ACKNOWLEDGMENTS
We are grateful to O. Hankinson (University of California, Los Angeles) for providing pcDNAIneo/mARNT and for originally supplying the c37 cell line. We thank M. L. Whitelaw and L. Poellinger (Karolinska Institute, Stockholm, Sweden) for the τDBD/DR83-593 and pGREII-oct-AF plasmids, S. Kimura (NCI) for the anti-CYP1A1 antibody, R. S. Pollenz (University of South Carolina) for the anti-AHR antibody, G. L. Hager (NCI) for suggesting the use of a (Gly-Ala)5 linker on the AHR-GFP fusion protein, S. Wert (Children’s Hospital Research Foundation, Cincinnati, Ohio) for help and advice with fluorescence microscopy, and S. Eltom and C. Jefcoate (University of Wisconsin) for communicating their results before publication. We also thank M. Carty, M. J. Carvan, T. P. Dalton, and D. W. Nebert for critical reading of the manuscript and for many suggestions throughout the course of this work.
This work was supported by grants NIEHS ES06273 and NIEHS P30 ES06096 and by a predoctoral fellowship to C.-Y.C. from the Pharmaceutical Research and Manufacturers of America Foundation.
REFERENCES
- 1.Abbott B D, Birnbaum L S, Perdew G H. Developmental expression of two members of a new class of transcription factors. I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–143. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- 2.Abbott B D, Perdew G H, Birnbaum L S. Ah receptor in embryonic mouse palate and effects of TCDD on receptor expression. Toxicol Appl Pharmacol. 1994;126:16–25. doi: 10.1006/taap.1994.1085. [DOI] [PubMed] [Google Scholar]
- 3.Bernard H P, Darlington G J, Ruddle F H. Expression of liver phenotypes in cultured mouse hepatoma cells: synthesis and secretion of serum albumin. Dev Biol. 1973;35:83–96. doi: 10.1016/0012-1606(73)90008-0. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum L S. Endocrine effects of prenatal exposure to PCBs, dioxins, and other xenobiotics: implications for policy and future research. Environ Health Perspect. 1994;102:676–679. doi: 10.1289/ehp.94102676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burbach K M, Poland A, Bradfield C A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrier F, Owens R A, Nebert D W, Puga A. Dioxin-dependent activation of murine Cyp1a-1 transcription requires protein kinase C-dependent phosphorylation. Mol Cell Biol. 1992;12:1856–1863. doi: 10.1128/mcb.12.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C-Y, Smith D R, Prasad V S, Sidman C L, Nebert D W, Puga A. Ten nucleotide differences, five of which cause amino acid changes, are associated with the Ah receptor locus polymorphism of C57BL/6 and DBA/2 mice. Pharmacogenetics. 1993;3:312–321. doi: 10.1097/00008571-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Couture L A, Abbott B D, Birnbaum L S. A critical review of the developmental toxicity and teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin: recent advances toward understanding the mechanism. Teratology. 1990;42:619–627. doi: 10.1002/tera.1420420606. [DOI] [PubMed] [Google Scholar]
- 9.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolwick K M, Swanson H I, Bradfield C A. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci USA. 1993;90:8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Elton, S., and C. R. Jefcoate. Personal communication.
- 11.Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem. 1994;269:27337–27343. [PubMed] [Google Scholar]
- 12.Ema M, Sogawa K, Watanabe N, Chujoh Y, Matsushita N, Gotoh O, Funae Y, Fujii-Kuriyama Y. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992;184:246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- 13.Ema M, Suzuki M, Morita M, Hirose K, Sogawa K, Matsuda Y, Gotoh O, Saijoh Y, Fujii H, Hamada H, Fujii-Kuriyama Y. cDNA cloning of a murine homologue of Drosophila single-minded, its mRNA expression in mouse development, and chromosome localization. Biochem Biophys Res Commun. 1996;218:588–594. doi: 10.1006/bbrc.1996.0104. [DOI] [PubMed] [Google Scholar]
- 14.Flesch-Janys D, Berger J, Gurn P, Manz A, Nagel S, Waltsgott H, Dwyer J H. Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. Am J Epidemiol. 1995;142:1165–1175. doi: 10.1093/oxfordjournals.aje.a117575. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa-Sehara A, Yamane M, Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic-responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci USA. 1988;85:5859–5863. doi: 10.1073/pnas.85.16.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukunaga B N, Probst M R, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995;270:29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 17.Gasiewicz T A, Kende A S, Rucci G, Whitney B, Willey J J. Analysis of structural requirements for Ah receptor antagonist activity: ellipticines, flavones, and related compounds. Biochem Pharmacol. 1996;52:1787–1803. doi: 10.1016/s0006-2952(96)00600-4. [DOI] [PubMed] [Google Scholar]
- 18.Gasiewicz T A, Rucci G. Alpha-naphthoflavone acts as an antagonist of 2,3,7,8-tetrachlorodibenzo-p-dioxin by forming an inactive complex with the Ah receptor. Mol Pharmacol. 1991;40:607–612. [PubMed] [Google Scholar]
- 19.Giannone J V, Okey A B, Harper P A. Characterization of polyclonal antibodies to the aromatic hydrocarbon receptor. Can J Physiol Pharmacol. 1995;73:7–17. doi: 10.1139/y95-002. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez F J, Kimura S, Nebert D W. Comparison of the flanking regions and introns of the mouse 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible cytochrome P1-450 and P2-450 genes. J Biol Chem. 1985;260:5040–5049. [PubMed] [Google Scholar]
- 21.Gonzalez F J, Nebert D W. Autoregulation plus upstream positive and negative control regions associated with transcriptional activation of the mouse P1-450 gene. Nucleic Acids Res. 1985;13:7269–7288. doi: 10.1093/nar/13.20.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gradin K, McGuire J, Wenger R H, Kvietikova I, Whitelaw M L, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham F L, Van der Erb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson O, Andersen R D, Birren B, Sander F, Negishi M, Nebert D W. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 1985;260:1790–1795. [PubMed] [Google Scholar]
- 25.Hirose K, Morita M, Ema M, Mimura J, Hamada H, Fujii H, Saijo Y, Gotoh O, Sogawa K, Fujii-Kuriyama Y. cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS factor (Arnt2) with close sequence similarity to the aryl hydrocarbon receptor nuclear translocator (Arnt) Mol Cell Biol. 1996;16:1706–1713. doi: 10.1128/mcb.16.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffer A, Chang C-Y, Puga A. Dioxin induces transcription of fos and jun genes by Ah receptor-dependent and -independent pathways. Toxicol Appl Pharmacol. 1996;141:238–247. doi: 10.1006/taap.1996.0280. [DOI] [PubMed] [Google Scholar]
- 27.Htun H, Barsony J, Renyi I, Gould D L, Hager G L. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci USA. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isaac D D, Andrew D J. Tubulogenesis in Drosophila: a requirement for the trachealess gene product. Genes Dev. 1996;10:103–117. doi: 10.1101/gad.10.1.103. [DOI] [PubMed] [Google Scholar]
- 29.Jackson F R, Bargiello T A, Yun S H, Young M W. Product of the per locus of Drosophila shares homology with proteoglycan. Nature. 1986;320:185–188. doi: 10.1038/320185a0. [DOI] [PubMed] [Google Scholar]
- 30.Jain S, Dolwick K M, Schmidt J V, Bradfield C A. Potent transactivation domain of the Ah receptor and the Ah receptor nuclear translocator map to their carboxyl termini. J Biol Chem. 1994;269:31518–31524. [PubMed] [Google Scholar]
- 31.Jensen F C, Girardi A J, Gilden R V, Koprowski H. Infection of human and simian tissue cultures with Rous sarcoma virus. Proc Natl Acad Sci USA. 1964;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones P B C, Durrin L K, Galeazzi D R, Whitlock J P., Jr Control of cytochrome P1-450 gene expression: analysis of a dioxin-responsive enhancer system. Proc Natl Acad Sci USA. 1986;83:2802–2806. doi: 10.1073/pnas.83.9.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura S, Smith H H, Hankinson O, Nebert D W. Analysis of two benzo[a]pyrene-resistant mutants of the mouse hepatoma Hepa-1 P1-450 via cDNA expression in yeast. EMBO J. 1987;6:1929–1933. doi: 10.1002/j.1460-2075.1987.tb02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesca P, Rafidinarivo E, Lecointe P, Mansuy D. A class of strong inhibitors of microsomal monooxygenases: the ellipticines. Chem Biol Interact. 1979;24:189–198. doi: 10.1016/0009-2797(79)90007-3. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Zwiebel L J, Hinton D, Benzer S, Hall J C, Rosbash R. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci. 1992;12:2735–2744. doi: 10.1523/JNEUROSCI.12-07-02735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Q, Whitlock J P., Jr The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol. 1996;16:2144–2150. doi: 10.1128/mcb.16.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason G G, Witte A M, Whitelaw M L, Antonsson C, McGuire J, Wilhelmsson A, Poellinger L, Gustaffson J-Å. Purification of the DNA binding form of dioxin receptor. Role of the Arnt cofactor in regulation of dioxin receptor function. J Biol Chem. 1994;269:4438–4449. [PubMed] [Google Scholar]
- 38.Matsushita N, Sogawa K, Ema M, Yoshida A, Fujii-Kuriyama Y. A factor binding to the xenobiotic responsive element (XRE) of P-4501a1 gene consists of at least two helix-loop-helix proteins, Ah receptor and arnt. J Biol Chem. 1993;268:21002–21006. [PubMed] [Google Scholar]
- 39.McKnight S L, Gavis E R, Kingsbury R, Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981;25:385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- 40.Merchant M, Arellano L, Safe S. The mechanism of action of a-naphthoflavone as an inhibitor of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1A1 gene expression. Arch Biochem Biophys. 1990;281:84–89. doi: 10.1016/0003-9861(90)90416-v. [DOI] [PubMed] [Google Scholar]
- 41.Merchant M, Morrison V, Santostefano M, Safe S. Mechanism of action of aryl hydrocarbon receptor antagonists: inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1A1 gene expression. Arch Biochem Biophys. 1992;298:389–394. doi: 10.1016/0003-9861(92)90426-w. [DOI] [PubMed] [Google Scholar]
- 42.Nambu J R, Lewis J O, Wharton K A J, Crews S T. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991;67:1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- 43.Nebert D W. Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Mol Endocrinol. 1991;5:1203–1214. doi: 10.1210/mend-5-9-1203. [DOI] [PubMed] [Google Scholar]
- 44.Nebert D W, Gonzalez F J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- 45.Nebert D W, Jones J E. Regulation of the mammalian cytochrome P1450 (CYP1A1) gene. Int J Biochem. 1989;21:243–252. doi: 10.1016/0020-711x(89)90182-1. [DOI] [PubMed] [Google Scholar]
- 46.Nebert D W, Puga A, Vasiliou V. Role of the Ah receptor and dioxin inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann NY Acad Sci. 1993;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- 47.Neuhold L A, Shirayoshi Y, Ozato K, Jones J E, Nebert D W. Regulation of mouse Cyp1a-1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989;9:2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okey A B, Vella L M, Harper P A. Detection and characterization of a low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1-450 by 3-methylcholanthrene. Mol Pharmacol. 1989;35:823–830. [PubMed] [Google Scholar]
- 49.Peters J M, Wiley L M. Evidence that murine preimplantation embryos express aryl hydrocarbon receptor. Toxicol Appl Pharmacol. 1995;134:214–221. doi: 10.1006/taap.1995.1186. [DOI] [PubMed] [Google Scholar]
- 50.Poland A, Glover E, Kende A S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- 51.Poland A, Knutson J C. 2,3,7,8,-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanisms of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 52.Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol. 1994;46:915–921. [PubMed] [Google Scholar]
- 53.Pollenz R S. The aryl-hydrocarbon receptor, but not the aryl-hydrocarbon receptor nuclear translocator protein, is rapidly depleted in hepatic and non-hepatic culture cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1996;49:391–398. [PubMed] [Google Scholar]
- 54.Probst M R, Reisz-Porszasz S, Agbunag R V, Ong M S, Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993;44:511–518. [PubMed] [Google Scholar]
- 55.Puga A, Nebert D W, Carrier F. Dioxin induces expression of c-fos and c-jun proto-oncogenes and a large increase in transcription factor AP-1. DNA Cell Biol. 1992;11:269–281. doi: 10.1089/dna.1992.11.269. [DOI] [PubMed] [Google Scholar]
- 56.Puga A, RayChaudhuri B, Salata K, Zhang Y-H, Nebert D W. Stable expression of mouse Cyp1a-1 and human CYP1A-2 cDNAs transfected into mouse hepatoma cells lacking detectable P450 enzyme activity. DNA Cell Biol. 1990;9:425–436. doi: 10.1089/dna.1990.9.425. [DOI] [PubMed] [Google Scholar]
- 57.RayChaudhuri B, Nebert D W, Puga A. The murine Cyp1a-1 gene negatively autoregulates its own transcription and that of other members of the aromatic hydrocarbon-responsive [Ah] gene battery. Mol Endocrinol. 1990;4:1773–1781. doi: 10.1210/mend-4-12-1773. [DOI] [PubMed] [Google Scholar]
- 58.Reddy P, Jacquier A C, Abovich N, Peterson G, Rosbash M. The period clock locus of D. melanogaster codes for a proteoglycan. Cell. 1986;46:53–61. doi: 10.1016/0092-8674(86)90859-7. [DOI] [PubMed] [Google Scholar]
- 59.Reyes H, Reiz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 60.Robertson J A, Hankinson O, Nebert D W. Autoregulation plus positive and negative elements controlling transcription of the genes in the [Ah] battery. Chem Scr. 1987;27A:83–87. [Google Scholar]
- 61.Savas U, Bhattacharyya K K, Christou M, Alexander D L, Jefcoate C R. Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem. 1994;269:14905–14911. [PubMed] [Google Scholar]
- 62.Silkworth J B, Cutler D S, Antrim L, Houston D, Tumasonis C, Kaminsky L S. Teratology of 2,3,7,8-tetrachlorodibenzo-p-dioxin in a complex environmental mixture from the Love Canal. Fundam Appl Toxicol. 1989;13:1–15. doi: 10.1016/0272-0590(89)90302-3. [DOI] [PubMed] [Google Scholar]
- 63.Singh S S, Hord N G, Perdew G H. Characterization of the activated form of the aryl hydrocarbon receptor in the nucleus of HeLa cells in the absence of exogenous ligand. Arch Biochem Biophys. 1996;329:47–55. doi: 10.1006/abbi.1996.0190. [DOI] [PubMed] [Google Scholar]
- 64.Sutter T R, Tang Y M, Hayes C L, Wo Y Y, Jabs E W, Li X, Yin H, Cody C W, Greenlee W F. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994;269:13092–13099. [PubMed] [Google Scholar]
- 65.Sutter T R, Guzman K, Dold K M, Greenlee W F. Targets for dioxin: genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science. 1991;254:415–418. doi: 10.1126/science.1925598. [DOI] [PubMed] [Google Scholar]
- 66.Swanson H I, Chan W K, Bradfield C A. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J Biol Chem. 1995;270:26292–26302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- 67.Vasiliou V, Puga A, Nebert D W. Negative regulation of the murine cytosolic aldehyde dehydrogenase-3 (Aldh-3c) gene by functional CYP1A1 and CYP1A2 proteins. Biochem Biophys Res Commun. 1992;187:413–419. doi: 10.1016/s0006-291x(05)81508-6. [DOI] [PubMed] [Google Scholar]
- 68.Wang G L, Jiang B-H, Rue E A, Semenza G L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weib C, Kolluri S K, Kiefer F, Gottlicher M. Complementation of Ah receptor deficiency in hepatoma cells: negative feedback regulation and cell cycle control by the Ah receptor. Exp Cell Res. 1996;226:154–163. doi: 10.1006/excr.1996.0214. [DOI] [PubMed] [Google Scholar]
- 70.Whitelaw M L, Gottlicher M, Gustaffson J-Å, Poellinger L. Definition of a novel ligand binding domain of a nuclear bHLH receptor: co-localization of ligand and hsp90 binding activities within the regulable inactivation domain of the dioxin receptor. EMBO J. 1993;12:4169–4179. doi: 10.1002/j.1460-2075.1993.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitelaw M L, Gustaffson J-Å, Poellinger L. Identification of transactivation and repression functions of the dioxin receptor and its basic helix-loop-helix/PAS partner factor Arnt: inducible versus constitutive modes of regulation. Mol Cell Biol. 1994;14:8343–8355. doi: 10.1128/mcb.14.12.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilhelmsson A, Whitelaw M L, Gustaffson J-Å, Poellinger L. Agonistic and antagonistic effects of alpha-naphthoflavone on dioxin receptor function. Role of the basic region helix-loop-helix dioxin receptor partner factor Arnt. J Biol Chem. 1994;269:19028–19033. [PubMed] [Google Scholar]
- 73.Wilk R, Weizman I, Shilo B. trachealess encodes a bHLH-PAS protein that is an inducer of tracheal cell fates in Drosophila. Genes Dev. 1996;10:93–102. doi: 10.1101/gad.10.1.93. [DOI] [PubMed] [Google Scholar]