Abstract
Studies highlight the importance of social factors, such as race and socioeconomic status (SES), in disease management. Integrating these factors helps improve understanding of disease outcomes and the development of effective treatments. Thus, we aimed to systematically identify and review relevant studies exploring the relationship between SES and survival outcomes in GBM patients. An extensive exploration of academic databases, including Scopus, EMBASE, and PubMed, was undertaken, covering records from their inception until December 14, 2024. This search targeted specific keywords and their synonymous terms: glioblastoma, survival, and socioeconomic. We included 230,601 patients, with many individuals being diagnosed between the ages of 46 and 65. Notably, Female exhibited a higher risk (HR = 1.07, 95% CI: 1.05–1.09) of death compared to male, while African Americans demonstrated a higher risk than Caucasians (HR = 0.92, 95% CI: 0.88–0.97), alongside Hispanics (HR = 0.85, 95% CI: 0.72–0.99) and other races (HR = 0.78, 95% CI: 0.73–0.85). Similarly, unmarried individuals faced a higher risk (HR = 1.14, 95% CI: 1.09–1.20) compared to married counterparts. Noteworthy trends were observed in insurance, where private payers (HR = 1.11, 95% CI: 1.06–1.15) and government-based insurance (HR = 1.09, 95% CI: 1.00-1.19) showed increased risks compared to private insurance. However, associations in widowhood (HR = 2.45, 95% CI: 0.34–17.40), comorbidities (HR = 1.05, 95% CI: 0.93–1.18), median household income (MHI) (HR = 0.94, 95% CI: 0.85–1.05), and rural living (HR = 1.06, 95% CI: 0.98–1.16) were non-significant or inconclusive. These findings emphasize the complex interplay between socioeconomic factors and health risks, highlighting the necessity for tailored interventions to address health disparities across diverse demographic groups.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10143-025-03647-2.
Keywords: Socioeconomic status, Glioblastoma, Survival, Disparities
Introduction
Research focusing on the influence of socioeconomic factors on glioblastoma outcomes holds paramount importance due to the profound impact these determinants have on disease progression and patient outcomes. The intricate interplay between socioeconomic status (SES) and demographic patient characteristics has been extensively documented, revealing their consequential effects on treatment decisions and subsequent glioblastoma prognoses [6, 22]. Notably, adverse SES conditions have been closely associated with delays in the initiation of radiotherapy and poorer overall survival rates among newly diagnosed glioblastoma patients [12, 19].
Several studies have identified a positive association between higher SES and the incidence of glioblastoma multiforme (GBM), the most aggressive form of glioma. For example, Nilsson et al. (2018) reported a 1.14-fold increased risk of developing GBM among individuals in the highest SES quartile compared to those in the lowest [15]. Similarly, Plascak and Fisher (2013) found elevated GBM incidence rates among populations with higher SES [18]. Expanding on these findings, Gorenflo et al. (2023) demonstrated that higher area-level SES was not only linked to increased incidence but also to better survival outcomes in the United States, underscoring socioeconomic disparities in both disease burden and prognosis [7]. These disparities may be partially explained by confounding factors such as earlier diagnosis, access to specialized care, geographic advantages, greater health literacy, stronger psychosocial support, and improved treatment adherence—factors more commonly found among individuals with higher SES [24]. Properly adjusting for these variables is essential to accurately assess the independent effect of SES on GBM outcomes.
Studies highlight the importance of social factors, such as race and SES, in disease management. Integrating these factors helps improve understanding of disease outcomes and the development of effective treatments. By understanding the impact of socioeconomic factors on glioblastoma outcomes, healthcare practitioners can tailor interventions to mitigate disparities and elevate the overall standard of care for individuals grappling with this formidable manifestation of brain cancer. Thus, we aimed to systematically identify and review relevant studies exploring the relationship between SES and survival outcomes in GBM patients.
Materials and methods
Search strategy
An extensive exploration of academic databases, including Scopus, EMBASE, and PubMed, was undertaken, covering records from their inception until December 14, 2024. This search targeted specific keywords and their synonymous terms: glioblastoma, survival, and socioeconomic (see Table 1). The systematic review and meta-analysis adhered to the PRISMA guidelines, with rayyan.ai serving as the platform for study selection [16].
Table 1.
Search queries used to retrieve studies from the databases
| Database | Search terms |
|---|---|
| Scopus | ( ( ( ( glioblastoma AND multiforme ) OR ( glioblastoma ) ) OR ( high AND grade AND glioma ) ) OR ( grade AND iv AND glioma ) ) AND ( socioeconomic AND status ) |
| EMBASE | ((glioblastoma multiforme or Glioblastoma or high grade glioma or grade IV glioma) and socioeconomic status).mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword heading word, floating subheading word, candidate term word] |
| PubMed | (“social class“[MeSH Terms] OR (“social“[All Fields] AND “class“[All Fields]) OR “social class“[All Fields] OR (“socioeconomic“[All Fields] AND “status“[All Fields]) OR “socioeconomic status“[All Fields]) AND (“glioblastoma“[MeSH Terms] OR “glioblastoma“[All Fields] OR “glioblastomas“[All Fields] OR (“glioma“[MeSH Terms] OR “glioma“[All Fields] OR (“high“[All Fields] AND “grade“[All Fields] AND “glioma“[All Fields]) OR “high grade glioma“[All Fields]) OR ((“grade“[All Fields] OR “graded“[All Fields] OR “grades“[All Fields] OR “grading“[All Fields] OR “gradings“[All Fields]) AND (“ieee intell veh symp“[Journal] OR “iv“[All Fields]) AND (“glioma“[MeSH Terms] OR “glioma“[All Fields] OR “gliomas“[All Fields] OR “glioma s“[All Fields]))) |
Article selection process
Peer-reviewed studies examining SES as an independent variable influencing access to GBM care were included. Two independent reviewers (JH and SH) screened titles and abstracts for eligibility, followed by full-text screening of potentially relevant studies. Discrepancies were resolved by a third reviewer (JJ).
Eligibility criteria
Eligible studies were required to report survival data and include demographic information such as age, sex, ethnicity, and relevant socioeconomic indicators. We considered a range of study designs, including cohort, case-control, and cross-sectional studies, without restriction on year of publication. However, to ensure methodological rigor and the inclusion of primary data, we excluded case reports, reviews, editorials, commentaries, and letters without original research data. Only articles written in English or with an English translation were included.
Outcome of interests
The primary outcome of interest was overall survival (OS), assessed across various socioeconomic factors. These included sex (male vs. female), race (African American, Caucasian, Hispanic, and others), marital status (married, unmarried, widowed, or unknown), insurance type (private vs. government-based), and presence of comorbidities. Median household income (MHI) was analyzed by comparing lower- and higher-income brackets, and area of residence was assessed by contrasting urban and metropolitan settings. Because definitions of MHI and geographic classifications varied across studies, we did not impose a uniform cutoff. Instead, we harmonized data by categorizing income and geographic status using relative measures (e.g., tertiles or standardized classifications) based on each study’s definitions, ensuring comparability across studies.
Data extraction
For this systematic review and meta-analysis, the following information was extracted from each article: the title, link, source (including the database), year of publication, authors, country of study, journal name, study type/design, sample size, sampling method, inclusion and exclusion criteria, research question and primary aims, intervention details, classification of SES, the independent and dependent variables, confounders, population demographics (age, gender, sex, income, education, race/ethnicity, insurance coverage/type, comorbidities), primary outcome and its measurement, secondary outcomes and their measurement, period/time (duration and years), main results/conclusion, and GBM management details.
Quality assessment
In the assessment of observational studies’ quality, the researchers utilized the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool. This tool served as a methodological instrument for critically appraising the risk of bias in non-randomized studies, offering a structured framework for evaluating various domains of study design, conduct, and analysis. Through the ROBINS-I assessment, potential sources of bias, including confounding, selection bias, misclassification, and measurement error, among others, were systematically identified. By employing this tool, the researchers enhanced the validity and reliability of their assessments of observational studies, thereby ensuring the robustness and integrity of the evidence synthesized in the systematic review and meta-analysis.
Data analysis
The analysis utilized RStudio version 0.97.551, developed by RStudio, Inc. The metafor function package was employed to create graphics and perform quantitative measurements in this analysis. The pooled effect estimates were determined using hazard ratio (HR) and 95% confidence interval (CI). Heterogeneity across studies was assessed using the inconsistency index (I2), which ranged from 0 to 100%. An I2 value greater than 50% or a p-value less than 0.10 indicated statistically significant heterogeneity. The inverse-variance method with a random-effects model was employed for HR meta-analysis, regardless of the presence of heterogeneity. Publication bias was qualitatively assessed using funnel plot analysis, supplemented by a regression-based Egger’s test to evaluate the potential for small-study effects. Two-tailed p-values were used in the study, with statistical significance set at p < 0.05, except for heterogeneity where p < 0.10 was considered significant.
Results
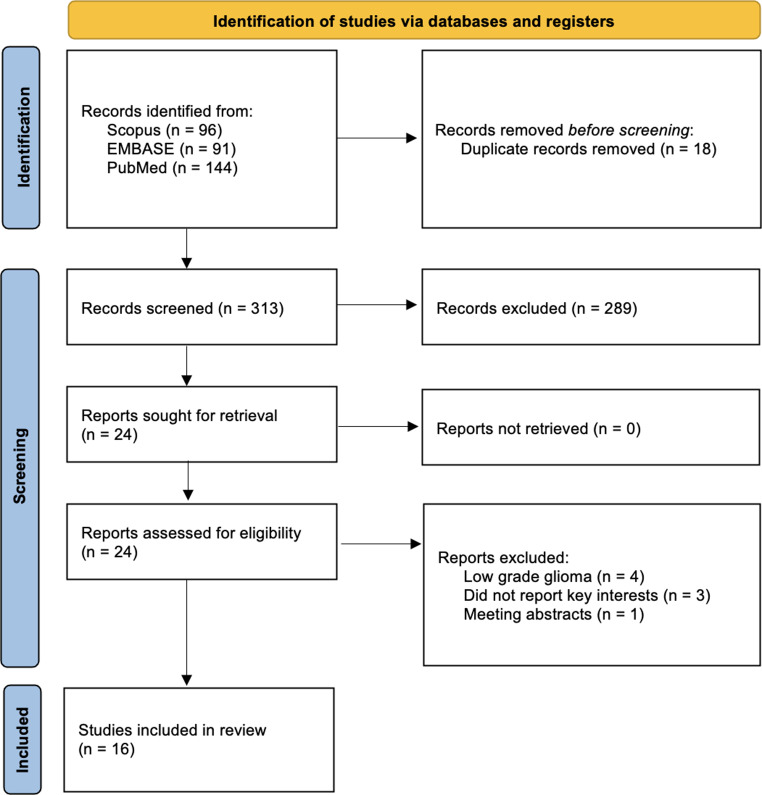
The PRISMA 2020 flow diagram (Fig. 1) outlines the systematic review process, focusing on searches conducted solely within databases and registers. In the first phase, a total of 331 records were identified from Scopus (n = 96), EMBASE (n = 91), and PubMed (n = 144). After removing 18 duplicate records, 313 unique records remained for screening. In the subsequent phase, Study Screening, 289 records were excluded based on title and abstract review. Of the remaining 24 reports, all were successfully retrieved and assessed for eligibility. Following full-text evaluation, 16 studies met the inclusion criteria and were included in the final review [1–3, 6, 9–14, 17, 19, 20, 22, 26]. The reasons for exclusion during this phase included four studies on low-grade glioma, two studies that did not report key interests, and one meeting abstract.
Fig. 1.
PRISMA diagram for study screening
The aggregated dataset encompasses a comprehensive analysis of demographic, clinical, and socioeconomic variables across multiple studies, with a cumulative patient population of 230,601 individuals from the USA, China, Italy, Brazil, Sweden, and the Philippines. The treatment modalities employed demonstrate significant variability, reflecting the heterogeneity of clinical decision-making. Specifically, biopsy was utilized in 18,812 cases, while surgical interventions were categorized into gross total resection (GTR), performed in 25,298 cases, and subtotal resection (STR), conducted in 22,352 cases. Additionally, a conservative management approach was adopted for 17,499 patients.
Concerning the total number of patients who received either radiation therapy alone, chemotherapy alone, or a combination of both, the data cannot be aggregated by the researcher due to some studies reporting overlapping information across groups. Nonetheless, the overall figures indicate that 118,188 patients underwent radiation therapy, 19,906 received chemotherapy, and 78,899 were treated with a combination of radiation and chemotherapy. Tumor localization data revealed a predominance of supratentorial tumors, accounting for 47,168 cases, alongside infratentorial tumors (n = 566) and overlapping tumor regions (n = 9,200). Laterality was also documented, with left-sided tumors (n = 453), right-sided tumors (n = 453), and bilateral tumors (n = 90) identified.
From a demographic perspective, the racial and ethnic composition of the cohort highlights substantial diversity. The majority of participants identified as Caucasian (n = 182,835), followed by African American (n = 11,563), Hispanic (n = 9,343), Asian (n = 3,749), and other racial or ethnic categories, which collectively accounted for 23,111 individuals. Insurance coverage patterns further illustrate disparities in healthcare access, with private insurance being the most prevalent form of coverage, utilized by 88,796 patients, while government-based insurance supported 61,407 individuals. Geographic distribution data revealed that the majority of patients resided in metropolitan areas (n = 85,804), with smaller proportions inhabiting urban areas (n = 34,275) and rural areas (n = 1,792). The categorization of living area and marital status deviates from conventional classifications such as rural, metropolitan, or urban designations, adopting instead alternative frameworks tailored to the specific contexts of each study. For instance, geographic distribution is delineated using region-specific criteria. In studies conducted in the United States, populations are classified by state, while in Italy, the population is segmented into three distinct regions—south, central, and north—reflecting regional distinctions unique to the country’s socio-geographic structure.
Marital status demonstrated that the majority of patients were married (n = 47,563), followed by those who were single (n = 7,360), widowed (n = 5,010), or had an unknown marital status (n = 892). It is important to note that marital status is operationalized differently across studies, with some investigations distinguishing between categories such as single, unmarried, and widowed, despite these groups sharing the common characteristic of being unpartnered. In light of these variations, the classifications are presented in their original form as reported by the respective studies. Additional details regarding patient characteristics are provided in Tables 2 and 3 for further reference and analysis.
Table 2.
Demographic attributes derived from incorporated studies investigating the influence of socioeconomic status on glioblastoma survival
| Study ID | Country | Data collection | Total cohort, n | Treatment received | Tumor details |
|---|---|---|---|---|---|
| Pollom 2018 | USA | 2010–2013 | 12,738 |
Biopsy = 2768 STR = 4700 GTR = 5270 Radiation and Chemotherapy = 12,738 |
n/r |
| Liu 2020 | USA | 2005–2016 | 28,952 |
Radiation = 21,690 Chemotherapy = 19,906 Surgery = 5516 |
Location Supratentorial = 22,630 Infratentorial = 365 Overlapping = 5957 |
| Tosoni 2021 | Italy | April 2017– December 2017 | 106 |
Biopsy = 8 STR = 74 GTR = 24 |
MGMT methylation Methylated = 47 Unmethylated = 54 |
| Estevez-Ordonez 2023 | USA | 2008–2019 | 995 |
Biopsy = 298 STR = 250 GTR– 447 Chemotherapy = 127 Radiation and Chemotherapy = 868 |
Location Supratentorial = 853 Infratentorial = 47 Other = 86 Laterality Right = 432 Left.= 447 Bilateral = 86 |
| Xie 2018 | USA | 2004–2015 | 30,767 |
Surgery = 22,835 Conservative = 7841 Unknown = 91 |
Laterality Unilateral = 50,178 Bilateral = 10,768 Paired = 568 |
| Chang 2005 | USA | 1988–2001 | 10,987 |
Biopsy = 2510 Surgery = 8071 Conservative = 71 Radiation = 8127 |
Location Supratentorial = 10,987 Site Frontal = 2521 Temporal = 2538 Parietal = 1887 Occipital = 474 Others = 3567 |
| Rong 2016 | USA | 2007–2012 | 16,690 |
Surgery = 10,382 Radiation = 8163 |
Tumor size ≤ 45 mm = 5891 > 45 mm = 5598 Unknown = 2176 |
| Lynch 2012 | Brazil | 1998–2008 | 66 |
STR = 28 GTR = 38 Radiation = 45 |
n/r |
| Barnholtz-Sloan 2007 | USA | June 1991– December 1999 | 1530 |
Biopsy = 145 Surgery = 318 Multiple treatment = 1067 |
Location Supratentorial = 1073 Other = 110 Overlapping = 347 |
| Hong 2022 | Philippines | 2015–2019 | 48 |
Biopsy = 5 STR = 27 GTR = 16 Radiation and chemotherapy = 7 Radiation = 11 |
Laterality Right = 21 Left = 23 Bilateral = 4 Location Frontal = 14 Parietal = 5 Temporal = 6 ≥ 2 lobe = 19 Multifocal = 4 |
| Hodges 2021 | USA | 2004–2014 | 103,652 |
Biopsy = 9306 GTR = 12,448 STR = 10,297 Unknown = 52,599 Conservative = 8827 Radiation = 65,128 Chemotherapy = 59,100 |
Focality Unifocal = 35,521 Multifocal = 7562 Unknown = 53,394 n/r |
| Marta 2021 | Brazil | 1999–2000 | 4511 |
Surgery = 622 Radiation = 162 Chemotherapy = 29 Surgery + Radiation = 319 Surgery + Chemotherapy = 57 Radiation + Chemotherapy = 158 Surgery + Radiation + Chemotherapy = 698 Other = 141 Conservative = 202 |
n/r |
| Bohn 2018 | USA | 2010–2014 | 3517 |
GTR = 1253 STR = 1657 Conservative = 558 |
Location Frontal = 1328 Temporal = 1201 Parietal = 747 Occipital = 197 |
| Pan 2015 | USA | 2000–2009 | 14,675 |
Biopsy = 3728 STR = 5185 GTR = 5762 Radiation = 14,675 |
Location Supratentorial = 11,625 Infratentorial = 154 Overlapping = 2896 |
| Bergqvist 2018 | Sweden | 2001–2013 | 1149 | Surgery + radiotherapy = 1149 | |
| Kasl 2016 | USA | 2000–2014 | 218 |
Biopsy = 44 STR = 134 GTR = 40 Radiation = 187 Chemotherapy = 171 |
Focality Unifocal = 184 Multifocal = 34 |
*GTR: gross total resection; MGMT: O6-methylguanine-DNA methyltransferase; n: number; n/r: not reported; STR: subtotal resection; USA: United States of America
Table 3.
Demographic overview categorized by socioeconomic status
| Study ID | Race | Household income | Insurance | Area of living | Marital status |
|---|---|---|---|---|---|
| Pollom 2018 |
Caucasian = 11,634; African American = 640; Other = 464 |
<$48,000 = 4,284; >$48,000 = 8,324; Unknown = 130 |
Private = 6858 Government-based = 5743 Unknown = 137 |
Metropolitan = 10,222 Urban = 20,179 Unknown = 437 |
- |
| Liu 2020 |
Caucasian = 23,101 Hispanic = 2872 African American = 1702 Asian = 1277 |
<$40,000 = 1388 $40,000–$60,000 = 8644 $60,000–$80,000 = 11,971 $80,000–$100,000 = 5552 >$100,000 = 1397 |
Private = 23,573 Not Insured = 760 Unknown = 4619 |
- | - |
| Tosoni 2021 | - |
< 36,152€ = 35 36,153 − 70,000€ = 44 70,001-100,000€ = 7 > 100,000€ = 20 |
Government-based = 106 |
North Italy = 76 Central Italy = 16 South Italy = 14 |
- |
| Estevez-Ordonez 2023 |
Caucasian = 857 African American = 117 Others = 21 |
Low = 294 Middle = 691 High = 10 |
Private = 686, Government-based = 273 Uninsured = 36 |
Metropolitan = 719 Micropolitan = 151 Rural = 38 Small town = 87 |
Married = 697 Widowed = 120 Single = 128 Unknown = 53 |
| Xie 2018 |
Caucasian = 27,557 African American = 1692 Others = 1441 Unknown = 57 |
Quartile 1 = 7685 Quartile 2 = 7663 Quartile 3 = 7705 Quartile 4 = 7712 |
Insured = 20,059 Government-based = 2453 Uninsured = 754 Unknown = 7501 |
Northeast = 5028 South = 6472 North Central = 2996 West = 16,271 |
Married = 20,076 Divorced = 2872 Widowed = 3550 Single = 4269 |
| Chang 2005 |
Caucasian = 10,032 African American = 486 Others = 454 Unknown = 15 |
- | - | - |
Married = 7396 Divorced = 888 Widowed = 1339 Single = 1076 Seperated = 48 |
| Rong 2016 |
African American = 806 Caucasian = 12,124 Other = 695 Unknown = 40 |
- |
Private = 11,591 Government-based = 1516 Uninsured = 558 |
Alaska = 8 California = 5654 Connecticut = 701 Georgia = 1383 Hawaii = 113 Iowa = 620 Kentucky = 830 Louisiana = 651 Michigan = 701 New Jersey1505 New Mexico = 244 Utah = 377 Washington = 878 |
Married = 8613 Not married = 4607 Unknown = 445 |
| Lynch 2012 | Low income = 66 | Government-based = 66 | - | - | |
| Barnholtz-Sloan 2007 |
Caucasian = 645 Hispanic = 53 African American = 18 Asian = 25 |
≤ $30,000 = 427 > $30,000 = 1084 |
Government-based = 1530 |
North East = 282 South = 86 Midwest = 499 West = 663 |
Married = 980 Not married = 529 |
| Hong 2022 | - |
Low = 8 Lower middle = 40 |
Government-based = 48 |
Urban = 21 Rural = 27 |
Married = 35 Widowed = 1 Single = 12 |
| Hodges 2021 |
Caucasian = 81,900 Hispanic = 4815 Asian = 1638 African American = 5124 |
- |
Private = 40,523 Government-based = 47,244 Uninsured = 3433 Unknown = 2277 |
Metropolitan = 73,567 Urban = 14,075 Rural = 1727 Unknown = 4108 |
- |
| Marta 2021 | - |
Private = 2436 Government-based = 2075 |
Metropolitan = 1296 Others = 3215 |
- | |
| Bohn 2018 |
Caucasian = 2890 Hispanic = 192 Asian = 186 African American = 205 |
- |
Private = 3029 Government-based = 283 Not insured = 97 |
- | - |
| Pan 2015 |
Caucasian = 11,881 African American = 773 Asian = 623 Hispanic = 1411 |
- | - |
Northeast = 2561 Midwest = 1521 South = 2943 West = 7650 |
Married = 9707 Single = 1875 Unmarried = 2699 Unknown = 394 |
| Bergqvist 2018 | - |
Low = 430 Middle = 478 Higher = 220 |
- | - | - |
| Kasl 2016 |
Caucasian = 205 Others = 13 |
Low = 65 Middle = 125 High = 28 |
Private = 100 Government-based = 118 |
- |
Married = 59 Not married = 159 |
According to the meta-analysis, females exhibited a higher risk (HR = 1.07, 95% CI: 1.05–1.09, p < 0.00001) of death compared to the males counterpart, while African Americans demonstrated a higher risk than Caucasians (HR = 0.92, 95% CI: 0.88–0.97, p = 0.0004), alongside Hispanics (HR = 0.85, 95% CI: 0.72–0.99, p = 0.04) and other races (HR = 0.78, 95% CI: 0.73–0.85, p < 0.00001). Similarly, unmarried individuals faced a higher risk (HR = 1.14, 95% CI: 1.09–1.20, p < 0.00001) compared to married counterparts. Noteworthy trends were observed in insurance, where private payers (HR = 1.11, 95% CI: 1.06–1.15, p < 0.00001) and government-based insurance (HR = 1.09, 95% CI: 1.00-1.19, p = 0.05) showed increased risks compared to private insurance. However, associations in widowhood (HR = 2.45, 95% CI: 0.34–17.40, p = 0.37), comorbidities (HR = 1.05, 95% CI: 0.93–1.18, p = 0.43), MHI (HR = 0.94, 95% CI: 0.85–1.05, p = 0.28), and rural living (HR = 1.06, 95% CI: 0.98–1.16, p = 0.16) were non-significant or inconclusive. Summary of meta-analysis was displayed in Table 4.
Table 4.
The analysis of socioeconomic variables and their association with risk factors
| Socioeconomic variables | Reference | HR [95% CI] | I2 | p-value | |
|---|---|---|---|---|---|
| Sex | Male | Female | 1.07 [1.05, 1.09] | 9% | < 0.00001 |
| Race | African American | Caucasian | 0.92 [0.88, 0.97] | 47% | 0.0004 |
| Hispanic | Caucasian | 0.85 [0.72, 0.99] | 95% | 0.04 | |
| Others | Caucasian | 0.78 [0.73, 0.85] | 68% | < 0.00001 | |
| Marital status | Unmarried | Married | 1.14 [1.09, 1.20] | 31% | < 0.00001 |
| Widow | Married | 2.45 [0.34, 17.40] | 70% | 0.37 | |
| Unknown | Not married | 0.87 [0.75, 1.01] | 63% | 0.07 | |
| Insurance | Private payer | Private insurance | 1.11 [1.06, 1.15] | 0% | < 0.00001 |
| Government based insurance | Private insurance | 1.09 [1, 1.19] | 66% | 0.05 | |
| Comorbid | With comorbid | No comorbid | 1.05 [0.93, 1.18] | 41% | 0.43 |
| MHI | Low income | High income | 0.94 [0.85, 1.05] | 72% | 0.28 |
| Area of living | Urban | Metropolitan | 1.07 [1.04, 1.10] | 0% | < 0.00001 |
| Rural | Metropolitan | 1.06 [0.98, 1.16] | 0% | 0.16 | |
The quality assessment using the ROBINS-I indicated a low-to-moderate risk of bias (Fig. 2). An asymmetrical distribution was observed for sex and MHI funnel plot, which analyzed the impact of those variables on survival outcomes in GBM patients (supplementary files).
Fig. 2.
ROBINS-I for study assessment
Discussion
The present study’s findings on socioeconomic variables and their association with health risk factors align in part with existing literature yet al.so introduce nuanced perspectives that warrant further exploration. For instance, the observation that lower MHI does not significantly alter risk (HR = 0.94 [0.85, 1.05], p = 0.28) contrasts with a substantial body of research underscoring the adverse health impacts of low socioeconomic status [6, 10]. This discrepancy might be attributed to the diverse composition of the study sample or the robust adjustment for confounders such as insurance type and race/ethnicity, which could mitigate the apparent impact of income. Similarly, the finding that racial or ethnic minorities exhibit lower HR compared to Caucasians diverges from conventional narratives about minority health disparities [27]. The adjusted model possibly accounts for overlapping disadvantages, thus unveiling a more complex relationship between race and health outcomes than previously understood.
Gender differences in health risks, highlighted by the elevated hazard ratio for males (HR = 1.07 [1.05, 1.09]), corroborate prior studies indicating increased vulnerabilities among men due to behavioral and social factors. This consistency across diverse populations affirms the significance of gender as a determinant of health, suggesting targeted interventions are necessary. Men may be less likely to seek timely medical attention, adhere to treatment protocols, or access supportive care—factors that are further exacerbated by SES-related barriers such as limited healthcare resources or financial strain [4, 25].
Meanwhile, marital status emerges as another critical factor, with unmarried individuals facing higher risks (HR = 1.14 [1.09, 1.20])—a trend supported by literature linking marriage to better health through mechanisms like social support. Married individuals are more likely to benefit from emotional encouragement, assistance with medical decision-making, and help navigating the healthcare system. These forms of support can lead to earlier diagnosis, greater treatment adherence, and improved overall outcomes [4, 25]. However, the wide CI observed for widows’ points to unique challenges within this subgroup, urging deeper investigation into how different marital transitions affect health risks [4, 25].
Insurance type and area of living further illustrate the intricate dynamics between access to resources and health outcomes. The modestly increased risks associated with private payer and government-based insurance relative to private insurance resonate with discussions on healthcare accessibility and quality, as patients with limited or less comprehensive insurance may face barriers such as delayed diagnosis, restricted treatment options, or reduced access to specialized care [5, 21]. Urban living presents a mixed picture; while urban residents face slightly elevated risks (HR = 1.07 [1.04, 1.10]), rural-urban differences appear less pronounced in this analysis, likely due to adjustments for socioeconomic factors. These synthesized insights highlight the layered interactions of demographic, socioeconomic, and environmental elements in shaping health outcomes [23]. Emphasizing diversity and intersectionality, the study enriches our understanding of health disparities and calls for tailored strategies addressing these multifaceted influences.
Study limitations and suggestion for future studies
A key limitation of this study is that, with fewer than 10 studies included in the meta-analysis, a meta-regression could not be performed, and even if attempted, the results would likely be insignificant due to insufficient data; therefore, future studies with larger sample sizes are encouraged to validate these findings. Secondly, the high heterogeneity observed across included analyses, largely due to variability in study designs, population characteristics, and definitions of socioeconomic variables. Additionally, comparing outcomes between high-income countries and low- and middle-income countries could provide valuable insights and should be considered in future research to address gaps in understanding disparities across diverse economic contexts. The disparities in conditions like GBM among racial and ethnic groups may stem not only from race itself but also from overlapping factors such as insurance coverage, income levels, and systemic inequities [8]. However, inconsistencies in categorizing variables like MHI—with differing income brackets preventing a comprehensive meta-analysis—introduce ambiguity into the results. Despite these challenges, the study included MHI findings, potentially complicating the interpretation of socioeconomic impacts on health outcomes.
For stakeholders and policymakers, addressing these disparities requires a multifaceted approach that considers intersecting socioeconomic, demographic, and geographic factors while acknowledging the study’s limitations. Broad policy initiatives, such as expanding insurance coverage, subsidizing healthcare for low-income households, and investing in preventive care and community-based support systems, can help mitigate systemic barriers faced by underserved populations. Policymakers should also prioritize comprehensive data collection efforts that account for intersectional identities and underrepresented groups, enabling more precise identification of at-risk populations.
Conclusions
In conclusion, our study explored socioeconomic variables’ connections to risk factors. Females exhibited slightly higher risks of death than males, while Caucasians had lower risks than African Americans, with Hispanics and other groups also showing decreased risks. Marital status notably impacted risk, with unmarried and widowed individuals at higher risk. Both private and government-based insurance correlated with increased risks. However, comorbidities and MHI had minimal effects on risk levels. These findings emphasize the complex interplay between socioeconomic factors and health risks, highlighting the necessity for tailored interventions to address health disparities across diverse demographic groups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed JH, WB, CB, FR, BS, DF, and PA. The first draft of the manuscript was written by JH, WB, CB, FR, BS, DF, and PA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barnholtz-Sloan JS, Maldonado JL, Williams VL, Curry WT, Rodkey EA, Barker FG, Sloan AE (2007) Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol 85:171–180. 10.1007/s11060-007-9405-4 [DOI] [PubMed] [Google Scholar]
- 2.Bergqvist J, Iderberg H, Mesterton J, Henriksson R (2018) The effects of clinical and sociodemographic factors on survival, resource use and lead times in patients with high-grade gliomas: a population-based register study. J Neurooncol 139:599–608. 10.1007/s11060-018-2899-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang SM, Barker FG (2005) Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer 104:1975–1984. 10.1002/cncr.21399 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Mathur MB, Case BW, VanderWeele TJ (2023) Marital transitions during earlier adulthood and subsequent health and well-being in mid- to late-life among female nurses: an outcome-wide analysis. Global Epidemiol 5:100099. 10.1016/j.gloepi.2023.100099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cyr ME, Etchin AG, Guthrie BJ, Benneyan JC (2019) Access to specialty healthcare in urban versus rural US populations: a systematic literature review. BMC Health Serv Res 19:974. 10.1186/s12913-019-4815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estevez-Ordonez D, Abdelrashid M, Coffee E, Laskay NMB, Atchley TJ, Chkheidze R, Fiveash JB, Markert JM, Lobbous M, Maveal BM, Burt Nabors L (2023) Racial and socioeconomic disparities in glioblastoma outcomes: A single-center, retrospective cohort study. Cancer 129:3010–3022. 10.1002/cncr.34881 [DOI] [PubMed] [Google Scholar]
- 7.Gorenflo MP, Shen A, Murphy ES, Cullen J, Yu JS (2023) Area-level socioeconomic status is positively correlated with glioblastoma incidence and prognosis in the united States. Front Oncol 13:1110473. 10.3389/fonc.2023.1110473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grochans S, Cybulska AM, Simińska D, Korbecki J, Kojder K, Chlubek D, Baranowska-Bosiacka I (2022) Epidemiology of glioblastoma Multiforme–Literature review. Cancers 14:2412. 10.3390/cancers14102412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges TR, Labak CM, Mahajan UV, Wright CH, Wright J, Cioffi G, Gittleman H, Herring EZ, Zhou X, Duncan K, Kruchko C, Sloan AE, Barnholtz-Sloan JS (2021) Impact of race on care, readmissions, and survival for patients with glioblastoma: an analysis of the National Cancer database. Neurooncol Adv 3:vdab040. 10.1093/noajnl/vdab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong MAC, Omar AT, Khu KJO (2022) Socioeconomic factors affecting survivorship of glioblastoma patients in the Philippines. J Clin Neurosci 98:89–95. 10.1016/j.jocn.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Kasl RA, Brinson PR, Chambless LB (2016) Socioeconomic status does not affect prognosis in patients with glioblastoma multiforme. Surg Neurol Int 7:S282–290. 10.4103/2152-7806.181985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu EK, Yu S, Sulman EP, Kurz SC (2020) Racial and socioeconomic disparities differentially affect overall and cause-specific survival in glioblastoma. J Neurooncol 149:55–64. 10.1007/s11060-020-03572-y [DOI] [PubMed] [Google Scholar]
- 13.Lynch JC, Welling L, Escosteguy C, Pereira AGL, Andrade R, Pereira C (2013) Socioeconomic and educational factors interference in the prognosis for glioblastoma multiform. Br J Neurosurg 27:80–83. 10.3109/02688697.2012.709551 [DOI] [PubMed] [Google Scholar]
- 14.Marta GN, Moraes FY, Feher O, Vellutini E, de AS, Pahl FH, Gomes M, de QT, Cardoso ACC, Neville IS, Hanna SA, Palhares DMF, Teixeira MJ, Maldaun MVC, Pereira AAL (2021) Social determinants of health and survival on Brazilian patients with glioblastoma: a retrospective analysis of a large populational database. Lancet Reg Health Am 4:100066. 10.1016/j.lana.2021.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson J, Holgersson G, Järås J, Bergström S, Bergqvist M (2018) The role of income in brain tumor patients: a descriptive register-based study: no correlation between patients’ income and development of brain cancer. Med Oncol 35:52. 10.1007/s12032-018-1108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan I-W, Ferguson SD, Lam S (2015) Patient and treatment factors associated with survival among adult glioblastoma patients: A USA population-based study from 2000–2010. J Clin Neurosci 22:1575–1581. 10.1016/j.jocn.2015.03.032 [DOI] [PubMed] [Google Scholar]
- 18.Plascak JJ, Fisher JL (2013) Area-based socioeconomic position and adult glioma: a hierarchical analysis of surveillance epidemiology and end results data. PLoS ONE 8:e60910. 10.1371/journal.pone.0060910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollom EL, Fujimoto DK, Han SS, Harris JP, Tharin SA, Soltys SG (2018) Newly diagnosed glioblastoma: adverse socioeconomic factors correlate with delay in radiotherapy initiation and worse overall survival. J Radiat Res 59:i11–i18. 10.1093/jrr/rrx103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong X, Yang W, Garzon-Muvdi T, Caplan JM, Hui X, Lim M, Huang J (2016) Influence of insurance status on survival of adults with glioblastoma multiforme: A population-based study. Cancer 122:3157–3165. 10.1002/cncr.30160 [DOI] [PubMed] [Google Scholar]
- 21.Scott ECS, Hoskin PJ (2024) Health inequalities in cancer care: a literature review of pathways to diagnosis in the united Kingdom. eClinicalMedicine 76:102864. 10.1016/j.eclinm.2024.102864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosoni A, Gatto L, Franceschi E, Di Nunno V, Lodi R, Mura A, Di Battista M, Bartolini S, Brandes AA (2021) Association between socioeconomic status and survival in glioblastoma: an Italian single-centre prospective observational study. Eur J Cancer 145:171–178. 10.1016/j.ejca.2020.12.027 [DOI] [PubMed] [Google Scholar]
- 23.Watts MJ, Kotsila P, Mortyn PG, Sarto I, Monteys V, Urzi Brancati C (2020) Influence of socio-economic, demographic and climate factors on the regional distribution of dengue in the united States and Mexico. Int J Health Geogr 19:44. 10.1186/s12942-020-00241-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wigertz A, Lönn S, Hall P, Feychting M (2010) Non-participant characteristics and the association between socioeconomic factors and brain tumour risk. J Epidemiol Community Health 64:736–743. 10.1136/jech.2008.085845 [DOI] [PubMed] [Google Scholar]
- 25.Wójcik G, Zawisza K, Jabłońska K, Grodzicki T, Tobiasz-Adamczyk B (2021) Transition out of marriage and its effects on health and health–Related quality of life among females and males. COURAGE and COURAGE-POLFUS–Population based Follow-Up study in Poland. Appl Res Qual Life 16:13–49. 10.1007/s11482-019-09742-z [Google Scholar]
- 26.Xie J-C, Yang S, Liu X-Y, Zhao Y-X (2018) Effect of marital status on survival in glioblastoma multiforme by demographics, education, economic factors, and insurance status. Cancer Med 7:3722–3742. 10.1002/cam4.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yearby R (2018) Racial disparities in health status and access to healthcare: the continuation of inequality in the united States due to structural racism. Am J Econ Sociol 77:1113–1152. 10.1111/ajes.12230 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.