Abstract
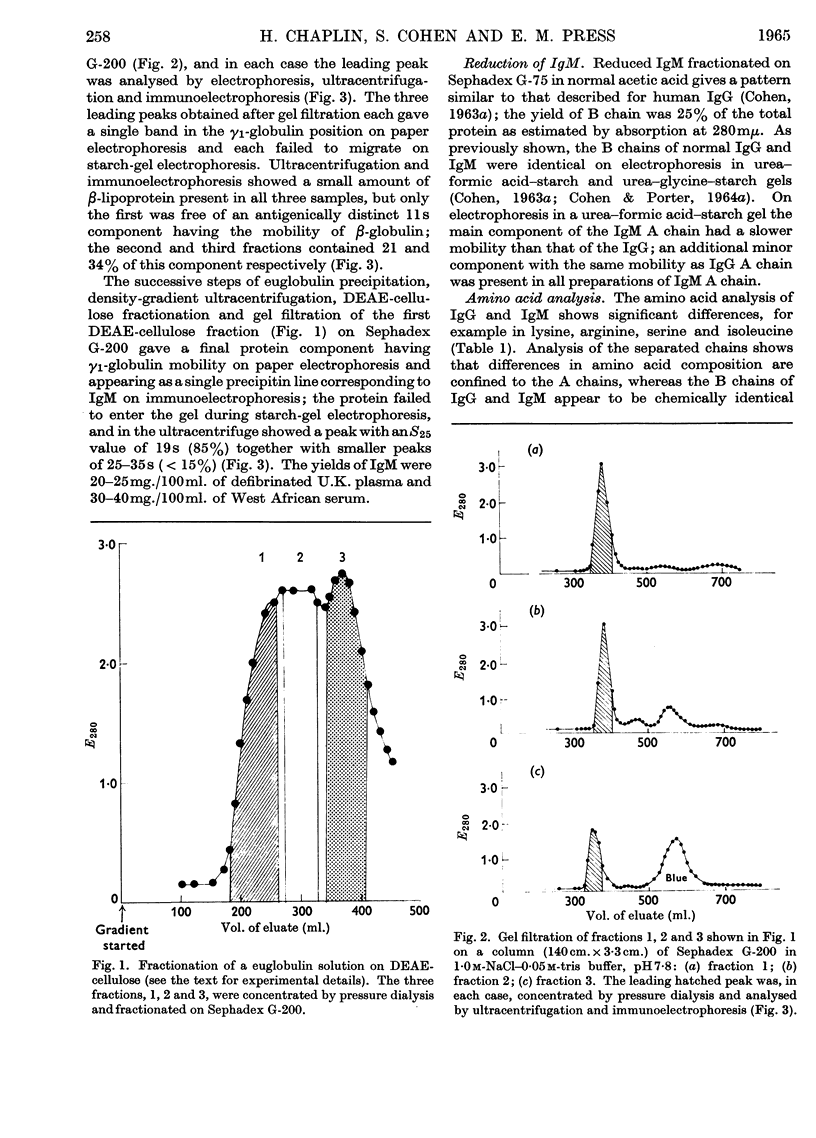
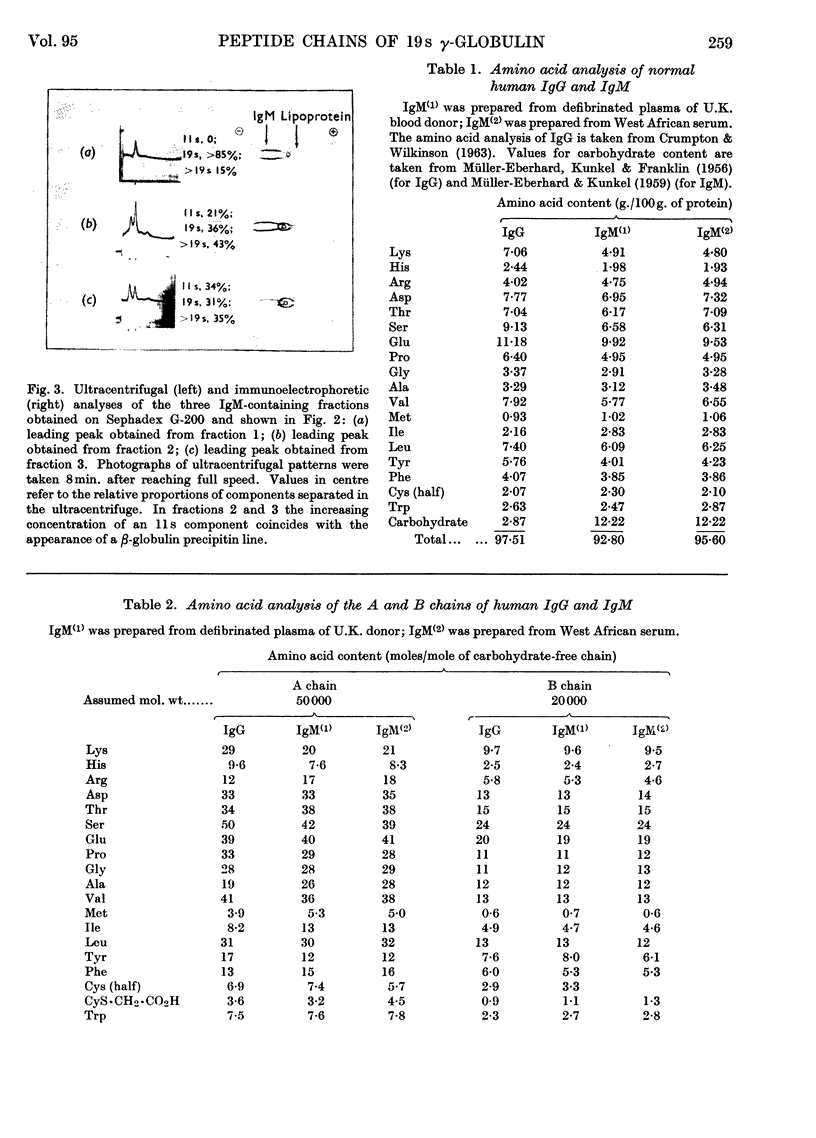
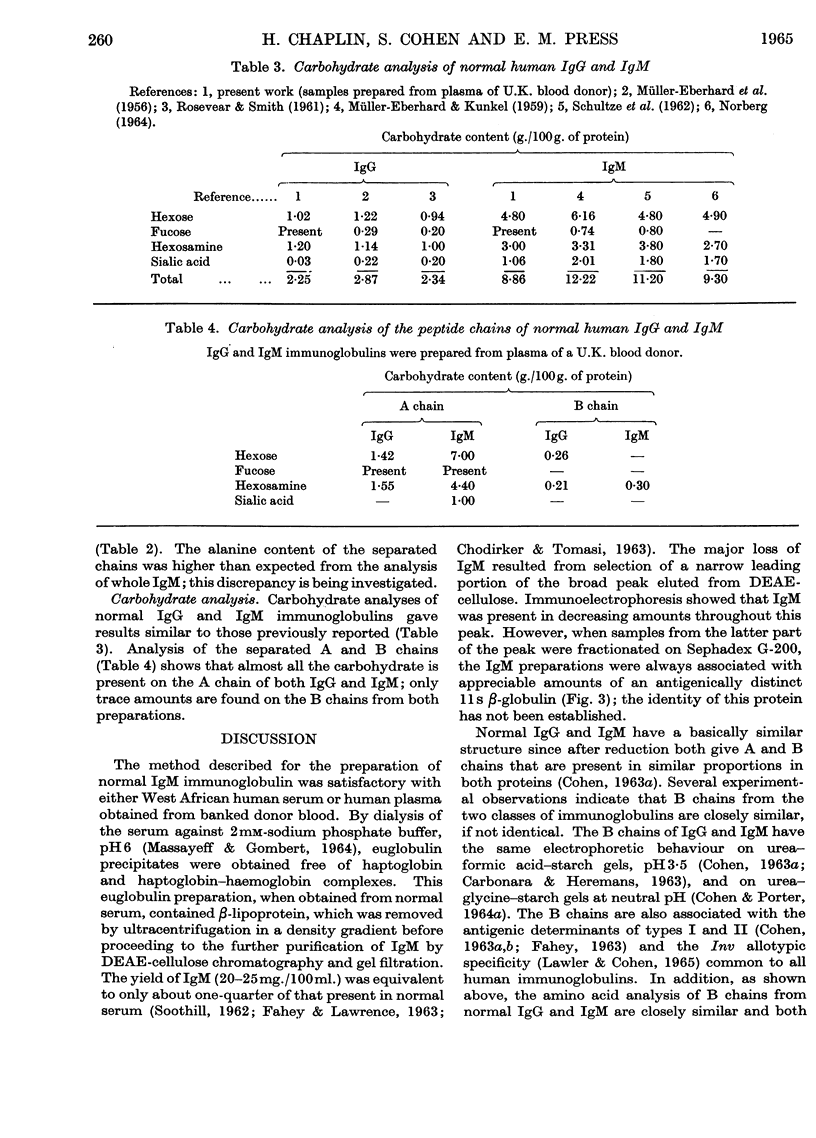
1. A method is described for preparing pure samples of 19s γ-globulin (IgM) from normal human serum by using successive steps of dialysis, density-gradient ultracentrifugation, chromatography on DEAE-cellulose, and gel filtration on Sephadex G-200. The yield of IgM (20–25mg./100ml. of serum) was equivalent to about one-quarter of that present in normal serum. 2. Analysis of the separated peptide chains of normal IgM and IgG (7s γ-globulin) showed considerable differences in the amino acid composition of A chains from the two proteins; their respective B chains, on the other hand, were similar in composition. The carbohydrate of both proteins is confined almost entirely to the A chains; the IgM A chain contains about four times as much carbohydrate as the IgG A chain. 3. These findings support the view that the different classes of human immunoglobulin have B chains that are identical and A chains that are chemically distinct.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARBONARA A. O., HEREMANS J. F. Subunits of normal and pathological gama-1A-globulins. (beta-2A-globulins). Arch Biochem Biophys. 1963 Jul;102:137–143. doi: 10.1016/0003-9861(63)90331-x. [DOI] [PubMed] [Google Scholar]
- CHODIRKER W. B., TOMASI T. B., Jr GAMMA-GLOBULINS: QUANTITATIVE RELATIONSHIPS IN HUMAN SERUM AND NONVASCULAR FLUIDS. Science. 1963 Nov 22;142(3595):1080–1081. doi: 10.1126/science.142.3595.1080. [DOI] [PubMed] [Google Scholar]
- COHEN S. PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL AND PATHOLOGICAL HUMAN GAMMA GLOBULINS. Biochem J. 1963 Nov;89:334–341. doi: 10.1042/bj0890334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. Properties of the separated chains of human gamma-globulin. Nature. 1963 Jan 19;197:253–255. doi: 10.1038/197253a0. [DOI] [PubMed] [Google Scholar]
- CRUMPTON M. J., WILKINSON J. M. AMINO ACID COMPOSITIONS OF HUMAN AND RABBIT GAMMA-GLOBULINS AND OF THE FRAGMENTS PRODUCED BY REDUCTION. Biochem J. 1963 Aug;88:228–234. doi: 10.1042/bj0880228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibnall A. C., Rees M. W., Williams E. F. The total nitrogen content of egg albumin and other proteins. Biochem J. 1943 Sep;37(3):354–359. doi: 10.1042/bj0370354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Porter R. R. Heterogeneity of the peptide chains of gamma-globulin. Biochem J. 1964 Feb;90(2):278–284. doi: 10.1042/bj0900278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Human serum macroglobulins and dissociation units. I. Physicochemical properties. J Biol Chem. 1958 Apr;231(2):1107–1118. [PubMed] [Google Scholar]
- EDELMAN G. M., POULIK M. D. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. doi: 10.1084/jem.113.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY F. L., LAWRENCE M. E. QUANTITATIVE DETERMINATION OF 6.6 S GAMMA-GLOBULINS, BETA-2A-GLOBULINS AND GAMMA-1-MACROGLOBULINS IN HUMAN SERUM. J Immunol. 1963 Nov;91:597–603. [PubMed] [Google Scholar]
- FAHEY J. L. STRUCTURAL BASIS FOR THE DIFFERENCES BETWEEN TYPE I AND TYPE II HUMAN GAMMA-GLOBULIN MOLECULES. J Immunol. 1963 Oct;91:448–459. [PubMed] [Google Scholar]
- FIREMAN P., VANNIER W. E., GOODMAN H. C. IMMUNOCHEMICAL STUDIES OF HUMAN SERUM FRACTIONATED BY GEL FILTRATION WITH SEPHADEX G-200. Proc Soc Exp Biol Med. 1964 Apr;115:845–849. doi: 10.3181/00379727-115-29054. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C., KUNKEL H. G., MULLER-EBERHARD H. J. Two types of gamma-globulin differing in carbohydrate content. Proc Soc Exp Biol Med. 1956 Oct;93(1):146–150. doi: 10.3181/00379727-93-22690. [DOI] [PubMed] [Google Scholar]
- JOHANSEN P. G., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 2. The hexose, hexosamine, acetyl and amide-nitrogen content of hen's-egg albumin. Biochem J. 1960 Nov;77:239–247. doi: 10.1042/bj0770239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A. The induction and repression of amino acid oxidation in Pseudomonas fluorescens. Biochem J. 1964 Jul;92(1):1–8. doi: 10.1042/bj0920001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLANDER J., BENGTSSON S., PHILIPSON L. FRACTIONATION OF HUMAN PLASMA MACROGLOBULINS BY GEL FILTRATION ON PEARL-CONDENSED AGAR. Proc Soc Exp Biol Med. 1964 Apr;115:861–865. doi: 10.3181/00379727-115-29058. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Ultracentrifugal characteristics and carbohydrate content of macromolecular gamma-globulins. Clin Chim Acta. 1959 Mar;4(2):252–258. doi: 10.1016/0009-8981(59)90138-x. [DOI] [PubMed] [Google Scholar]
- McDougall E. I., Deutsch H. F. A comparison of the native and monomer forms of macroglobulin from pathological human serum with particular reference to electrophoretic mobility differences. Biochem J. 1964 Jan;90(1):163–170. doi: 10.1042/bj0900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORBERG R. CARBOHYDRATE CONTENT OF NORMAL 19 S GLOBULINS. Clin Chim Acta. 1964 Jan;9:89–90. doi: 10.1016/0009-8981(64)90051-8. [DOI] [PubMed] [Google Scholar]
- ROSEVEAR J. W., SMITH E. L. Glycopeptides. I. Isolation and properties of glycopeptides from a fraction of human gamma-globulin. J Biol Chem. 1961 Feb;236:425–435. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SCHULTZE H. E., HAUPT H., HEIDE K., MOESCHLIN G., SCHMIDTBERGER R., SCHWICK G. [Studies on gamma macroglobulin in human serum]. Z Naturforsch B. 1962 May;17B:313–322. [PubMed] [Google Scholar]
- SOBER H. A., PETERSON E. A. Protein chromatography on ion exchange cellulose. Fed Proc. 1958 Dec;17(4):1116–1126. [PubMed] [Google Scholar]
- SOOTHILL J. F. Estimation of eight serum proteins by a gel diffusion precipitin technique. J Lab Clin Med. 1962 May;59:859–870. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]



