Abstract
Impaired blood flow and elevated reactive oxygen species (ROS) concentrations, generated primarily from NADPH oxidase (NOX), indicate risk for cardiovascular disease (CVD). Creatine monohydrate (CM) may reduce CVD risk by lowering ROS concentrations and increasing skeletal muscle microvascular blood flow (SMBF). To determine if NOX-derived ROS impairs SMBF and whether five days of CM supplementation reduces in-vivo ROS concentrations and improves SMBF. Seven individuals had two microdialysis probes placed (control (CON) and apocynin (APO): NOX inhibitor) in skeletal muscle to measure in-vivo ROS (Hydrogen Peroxide (H2O2)) concentrations and SMBF (ethanol outflow/inflow ratio, inversely related to blood flow) at rest and four hours post-meal consumption. Procedures were performed before (PRE) and after (POST) five days of CM supplementation (20 g/day). Dialysate H2O2 concentrations were lower in the APO probe compared to CON from 120–140 min (APO: 1.19 ± 0.39 µM; CON: 2.04 ± 0.95 µM, p = 0.039), 140–160 min (APO: 1.17 ± 0.37 µM; CON: 2.06 ± 0.98 µM, p = 0.034) and 160–180 min post meal ingestion (p ≤ 0.05). APO perfusion increased SMBF at 20–40 min, 120–140 min (APO: 0.61 ± 0.13; CON: 0.75 ± 0.09 µM, p = 0.048), 140–160 min (APO: 0.61 ± 0.12 µM; CON: 0.76 ± 0.14 µM, p = 0.046), 160–180 min, and 180–200 min post meal (p ≤ 0.05). Ethanol outflow/inflow ratio was lower (higher SMBF) POST CM supplementation compared to PRE CM supplementation at 0–20 min (p = 0.036) and 20–40 min (p = 0.049) following HC/HF meal consumption. Inhibition of NOX-derived ROS increased SMBF, suggesting that NOX activity may impair blood flow regulation over the duration of baseline and post-prandial time points. Further, CM supplementation could be an effective strategy for enhancing postprandial blood flow.
Keywords: Reactive oxygen species, Oxidative stress, Vascular function, Ergogenic supplement
Introduction
Cardiovascular disease (CVD) continues to persist as the preeminent cause of mortality within the United States [16, 29]. One of the earliest manifestations of CVD is vascular dysfunction which is characterized by the impaired function of the endothelial cells that line the inner surface of blood vessels [27]. The dysfunction of the endothelial cells can ultimately lead to compromised blood flow and increased risk for CVD. One of the key contributing mechanisms to the development and progression of endothelial dysfunction and disruptions in blood flow is the presence of excess reactive oxygen species (ROS) [37]. ROS are highly unstable molecules that when in excess, overpower the body’s natural antioxidant defenses and induce a state of oxidative stress. The consumption of meals that are high in carbohydrates (HC) or high in fats (HF) has been shown to elicit a pronounced increase in circulating ROS concentrations that can be detrimental to endothelial function and blood flow [15]. Furthermore, a prominent source of ROS generation in the vasculature is derived from the enzymatic activity of nicotinamide adenine dinucleotide phosphate oxidases (NOX), a group of multi-subunit transmembrane proteins [3, 24]. The overproduction of ROS concentrations by NOX triggers oxidative stress and thereby compromises endothelial function [19]. However, the effects of NOX-generated ROS concentrations on in-vivo skeletal muscle microvascular blood flow (SMBF) following a HC or HF meal are unclear.
Given the pivotal role that ROS plays in the pathogenesis and progression of endothelial dysfunction and CVD, there is an urgent need to identify novel potential interventions that can attenuate ROS concentrations and enhance blood flow. Recent evidence has shown that creatine monohydrate (CM), a popular ergogenic aid, may lower ROS concentrations and improve vascular function [1, 20, 30]. However, limited well-controlled clinical trials in humans have been conducted to investigate this issue. This shortage of studies investigating ROS concentrations can be largely attributed to the inherent challenge of directly measuring ROS concentrations in humans, with investigators frequently resorting to indirect biomarkers of lipid peroxidation in urine or plasma or in-vitro assessments of ROS. Nonetheless, a novel microdialysis technique has been developed in which in-vivo production of ROS concentrations can be measured in real-time. Therefore, the primary aim of the current study was to determine if NOX-derived ROS impairs SMBF at rest and in response to a HC or HF meal. Furthermore, we aimed to investigate whether five days of CM supplementation can reduce in-vivo ROS concentrations and increase SMBF at rest and in response to a HC or HF meal.
Methods
All procedures were approved by the Florida State University Institutional Review Board.
Participants
Young, healthy individuals from the greater Tallahassee, Florida area, and not taking any medications that affected central or peripheral circulation were recruited for this study. Participants were non-smokers, vapers, or users of chewing tobacco. For at least four weeks before study initiation, participants were asked to refrain from taking any CM supplementation, or antioxidant supplementation. Exclusion criteria were the presence of metabolic or cardiovascular disease, gastrointestinal disorders, or any condition that could potentially hinder metabolism or the delivery and transportation of oxygen. All participants provided written informed consent as approved by the Florida State University Institutional Review Board. Study visits were conducted on the Florida State University campus (IRB approval number: STUDY00000764. This pilot study, conducted from May 2022 to August 2023, concluded due to funding limitations. No harms or unintended effects were observed for either HC or HF meal consumption or CM supplementation. This study was conducted as part of the first author’s doctoral dissertation at Florida State University and adheres to the university's guidelines for publishing thesis-derived research [2].
Baseline visit and anthropometrics
At a baseline visit, height was measured with a stadiometer, and body mass with a digital electronic scale with body mass index (BMI) calculated as body mass in kilograms divided by height in meters squared (kg/m2). Dual-energy x-ray absorptiometry (DXA; Discovery W, Hologic Inc., Bedford, MA) was used to determine body fat percentage, fat mass, and lean mass.
Microdialysis protocol
Three days following the baseline visit, participants were asked to attend a microdialysis testing visit, following an overnight fast. Upon arrival at the laboratory, participants were asked to lie down in a supine position in a hospital bed. Utilizing sterile techniques and local, topical ethyl chloride anesthesia, two microdialysis probes with a 20 kDa membrane cut-off (CMA: Harvard Bioscience, Inc) were inserted in the same leg at least 3 cm apart into the vastus lateralis muscle of the quadriceps femoris muscle group. The two microdialysis probes were continuously perfused at a speed of 2.0 μL a minute with a control solution (CON) containing a 0.9% sodium chloride solution, 5 mM ethanol, 100 µM Amplex Ultrared (AMP), 1.0 U/mL horseradish peroxidase (HRP), and 10 U/mL superoxide dismutase (SOD). These compounds allow for the measurement of local SMBF and H2O2 concentrations. In addition, one probe was perfused with 1 mM of a local NOX inhibitor (apocynin: APO) to assess the NOX contributions to skeletal muscle ROS production. Ethanol enables the assessment of SMBF through the measurement of the perfusate (inflow) and dialysate (outflow) ethanol concentrations and the calculation of the ethanol outflow/inflow ratio, which is inversely related to blood flow. The addition of SOD to the microdialysis probes perfusate stimulates the conversion of the superoxide that passes into the microdialysis probe to H2O2, thereby allowing for the assessment of combined superoxide and H2O2 concentrations. The continuous perfusion of the microdialysis probes throughout the entire duration of the visit was controlled by two micro-infusion pumps (CMA 107, CMA/Microdialysis, Solna, Sweden). Before sample collection, probes were perfused for a 60-min equilibration period to allow for tissue recovery from probe insertion. Dialysate samples were then subsequently collected from each probe in 20-min increments in capped 150 µl polyethylene collection vials that were wrapped in aluminum foil to protect from light exposure. Following each sample collection, the fluorescence of the outflowing dialysate sample was immediately measured using a TD-700 laboratory fluorometer (Turner Designs, Sunnyvale, CA, USA) to assess in-vivo ROS concentrations (H2O2). The conversion of relative fluorescence units to H2O2 was achieved by reference to an H2O2 standard curve. Following at least 60 min of rest, the first four participants were randomized to receive a 600 kcal HC meal (150 g of glucose; 75-g glucose drink (AZER SCIENTIFIC INC, Morgantown, Pennsylvania) and jellybeans (Jelly Belly, Fairfield, CA)) or a 600 kcal HF meal (66 g of fat; heavy whipping cream (Publix, Lakeland, FL)). Participants were blinded to the treatment (HC or HF meal) until meal consumption. The last three participants were not randomized and just provided the HC meal. Samples of dialysate were collected in 20-min intervals over 4 h following food consumption.
CM supplementation
Upon the completion of the baseline experimental visit (PRE), participants were given 100 g of CM tasteless powder, with instructions to ingest 20 g mixed with water daily for five days (10 g in the morning and 10 g in the evening). The loading phase of CM (20 g per day for 5 days) has been demonstrated as a successful tactic to achieve muscle creatine saturation [18]. Following the five-day supplementation period, participants immediately underwent a follow-up microdialysis experiment (POST) during which they were administered the same HC or HF meal they had received in the first visit (parallel arm study design). An APO perfused microdialysis probe was not included during the POST CM-supplemented visit due to limited funding and the absence of prior evidence linking CM supplementation to NOX inhibition, as the study aimed to first determine whether CM alone could reduce ROS concentrations. For the CM supplementation, all participants were aware that they were receiving the supplement, and no placebo was used.
Statistical analysis
Statistical analyses were performed with Statistical Analysis System (SAS) (v.9.3; Institute, Cary, NC, USA). The randomization of participants was done by the principal investigator to receive either a HC or HF meal using SAS PROC PLAN procedure. The initial four participants in the study were properly randomized, and early analysis revealed a slight difference in time to peak ROS concentrations (H2O2) in the HC meal (~ 2–3 h) compared to the HF meal (≥ 4 h). To reduce participant burden, the subsequent three participants were not randomized and instead directly provided the HC meal. Normality for variables was assessed by using the proc univariate procedure to evaluate skewness. Marginal models were used to determine the main effect of APO and visit (CM supplementation) on H2O2 concentrations and SMBF (ethanal outflow/inflow ratio) with α set at 0.05. Post hoc analyses were adjusted for multiple comparisons using the Bonferroni adjustment. Missing data were classified as missing at random, and the maximum likelihood method was used to compute maximum likelihood estimates. Correlation analysis (PROC CORR) was performed to analyze the association between the change PRE to POST CM supplementation in H2O2 concentrations and SMBF in the CON to APO probes. Study outcomes were analyzed regardless of HF or HC meal due to the low sample size to achieve power for a given meal type. Data are reported as mean ± standard deviation (SD). All figures were constructed in GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
Seven participants (n = 7; 3 males, 4 females, 26 ± 4 years, 27.1 ± 5.4 kg/m2, 30.8 ± 9% body fat percentage) completed the study with 0% attrition rate. Five participants received the HC meal, and two participants received the HF meal. The only difference between groups on study outcomes was higher SMBF at 200–220 min after-HF meal consumption (HF: 0.61 ± 0.02) compared to HC (HC: 0.77 ± 0.12; p = 0.007) in the CON probe during the PRE supplementation visit. Due to the limited sample size, all data were combined to give a sample size of 7.
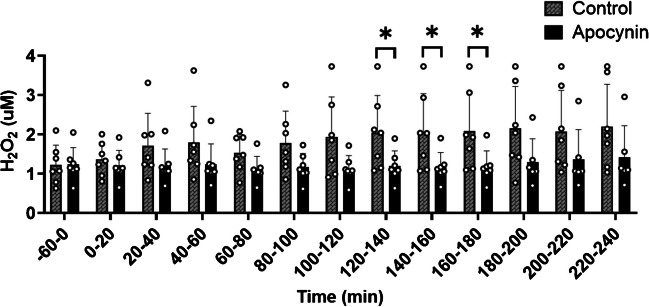
Dialysate H2O2 concentrations in response to Apocynin
At baseline (−60–0 min) APO did not affect dialysate H2O2 concentrations (p = 0.959) (Fig. 1). Following meal consumption, dialysate H2O2 concentrations were lower in the APO perfused probe compared to CON probe at 120–140 min (p = 0.039), 140–160 min (p = 0.034), and 160–180 min (p = 0.031).
Fig. 1.
Hydrogen peroxide (H2O2) concentrations at baseline and following a high carbohydrate (HC) or high fat (HF) meal. Baseline dialysate measurements were taken from −60–0 min, followed by subsequent measures every 20 min for up to four hours after consuming an HC or HF meal (n = 7). Marginal models were used to determine the main effect of APO. Post hoc analyses were adjusted for multiple comparisons using Bonferroni adjustment. * Indicates a significance between the CON probe and APO probes (p < 0.05). Abbreviations: H2O2, hydrogen peroxide; min: minutes
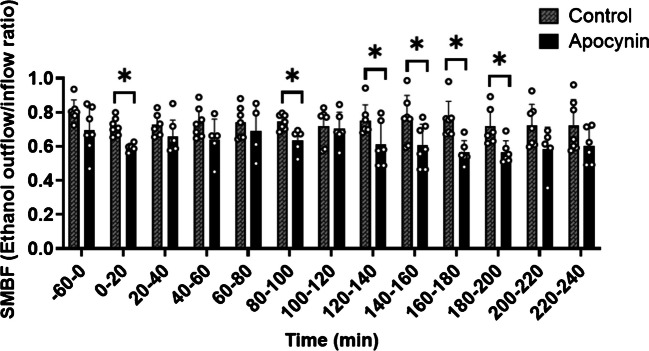
Ethanol outflow/inflow ratio from APO compared to CON probe
At baseline (−60–0 min), SMBF was not different between the APO and CON probe (p = 0.063) (Fig. 2). Following meal consumption, the ethanol outflow/inflow ratio was lower (indicative of higher SMBF) in APO than in CON at 0–20 min (p = 0.001), 80–100 min (p = 0.007), 120–140 min (p = 0.048), 140–160 min (p = 0.046), 160–180 min (p = 0.003), and 180–200 min (p = 0.017) (Fig. 2).
Fig. 2.
Skeletal muscle blood flow (SMBF) at baseline and following a high carbohydrate (HC) or high fat (HF) meal. Baseline dialysate measurements were taken from −60–0 min, followed by subsequent measures every 20 min for up to four hours after consuming an HC or HF meal (n = 7). Marginal models were used to determine the main effect of APO. Post hoc analyses were adjusted for multiple comparisons using Bonferroni adjustment. * Indicates a significance between the CON probe and APO probes (p < 0.05). Abbreviations: SMBF: skeletal muscle blood flow; min: minutes
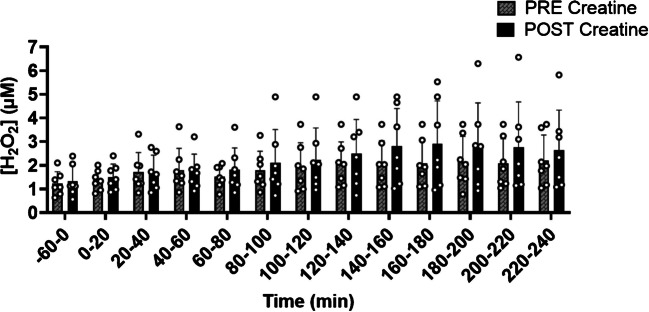
Dialysate H2O2 concentrations PRE and POST CM supplementation
Following CM supplementation, basal dialysate H2O2 concentrations were not different compared to PRE supplementation. Following the consumption of a HC or HF meal, no difference in dialysate H2O2 concentrations were seen at any time point following the consumption of a HC or HF meal (p ≥ 0.05) (Fig. 3).
Fig. 3.
Hydrogen peroxide (H2O2) concentrations at baseline and following high carbohydrate (HC) or high fat (HF) meal before (PRE) and after (POST) creatine monohydrate (CM). Baseline dialysate measurements were taken from −60–0 min, followed by subsequent measures every 20 min for up to four hours after consuming an HC or HF meal (n = 7). Marginal models were used to determine the main effect of the visit (CM supplementation). Post hoc analyses were adjusted for multiple comparisons using Bonferroni adjustment. * Indicates a significance between PRE and POST creatine monohydrate supplementation (p < 0.05). Abbreviations: PRE, before 5 days of creatine monohydrate supplementation; POST, after five days of creatine monohydrate supplementation; H2O2, hydrogen peroxide; min: minutes
Ethanol outflow/inflow ratio from PRE to POST CM supplementation in the CON probe
Ethanol outflow/inflow ratio was lower (higher SMBF) after CM supplementation compared to before CM supplementation at 0–20 min (p = 0.036) and 20–40 min (p = 0.049) post-HC or HF meal consumption (Fig. 4).
Fig. 4.
Ethanol outflow/inflow ratio at baseline and following high carbohydrate (HC) or high fat (HF) meal before (PRE) and after (POST) creatine monohydrate (CM). Baseline dialysate measurements were taken from −60–0 min, followed by subsequent measures every 20 min for up to four hours after consuming an HC or HF meal (n = 7). Marginal models were used to determine the main effect of the visit (CM supplementation). Post hoc analyses were adjusted for multiple comparisons using Bonferroni adjustment. * Indicates a significance between PRE and POST CM supplementation probes (p < 0.05). Abbreviations: PRE, before five days of creatine monohydrate supplementation; POST, after five days of creatine monohydrate supplementation; SMBF: skeletal muscle blood flow; min: minutes
Correlations between PRE and POST CM supplementation
Fat mass as determined by DXA was positively associated with the change in H2O2 concentrations between POST and PRE-CM supplementation at baseline (−60–0 min) (r2 = 0.86, p = 0.002) (Fig. 5), 0–20 min (r2 = 0.86, p = 0.002), 20–40 min (r2 = 0.71, p = 0.036) post-HC or HF meal consumption. Fat mass as determined by DXA was associated with a greater change in the ethanol outflow/inflow ratio between POST and PRE-CM supplementation at 20–40 min (r2 = 0.98, p = 0.001), 60–80 min (r2 = 0.77, p = 0.049), 80–100 min (r2 = 0.74, p = 0.01), 180–200 min (r2 = 0.92, p = 0.002).
Fig. 5.

Correlation between changes in fasted Hydrogen Peroxide (H2O2) (POST – PRE) and fat mass before (PRE) and after (POST) creatine monohydrate (CM). Correlation analysis (PROC CORR) was performed to analyze the association between the change from PRE five days of creatine monohydrate to POST five days of creatine monohydrate supplementation in H2O2 concentrations in the control probe compared to fat mass (r2 = −0.86, p = 0.002; n = 7). Abbreviations: H2O2, hydrogen peroxide; kg: kilograms
Discussion
The major findings of the pilot study was that 1) Dialysate H2O2 concentrations were lower in the APO perfused probe compared to the CON probe between 120–180 min after the consumption of a HC or HF meal, which coincided the higher SMBF as measured using the APO perfusion compared to the CON condition, 2) five days of CM supplementation did not affect local skeletal muscle (dialysate) H2O2 concentrations but enhanced SMBF, as indicated by the reduced ethanol outflow/inflow ratio, between 0–40 min after the consumption of a HC or HF meal, and 3) higher total body fat mass was associated with a greater change in H2O2 concentrations following CM supplementation at baseline (−60–0 min), and between 0–40 min following HC or HF meal consumption.
In the current study, there were significantly lower dialysate H2O2 concentrations in APO than in CON between 120–180 min following the consumption of a HC or HF meal. During this time frame, there was an increase seen in SMBF in the APO perfused probe compared to the CON probe. Thus, these data are evidence that NOX-produced ROS in the muscle might hamper SMBF following the consumption of a HC or HF meal. Evidence to the contrary could be that there was not always an elevated dialysate H2O2 with a higher blood flow measured around the control probe compared to the APO probe (0–20 min, 80–100 min, and 180–200 min); however, elevated blood flow could have artificially lowered the dialysate H2O2 concentrations by removing the H2O2 from the immediate vicinity of the microdialysis probe, thus resulting in underestimation of the dialysate H2O2 concentrations. NOX activity in-vitro has been shown to be elevated in response to hyperglycemia. Indeed, when exposed to high glucose, human aortic endothelial cells had increased expression of NOX and a NOX-associated protein (p47phox: a protein needed for the normal function of NOX), which led to increased production of ROS concentrations that were subsequently inhibited by APO administration [38]. The consumption of HC or HF meals tends to induce a state of heightened oxidative stress. Conversely, a period of fasting effectively decreased concentrations of ROS and suppressed the expression of p47phox [8, 11, 22]. Fisher-Wellman and colleagues specifically showed that meals that are HF result in the greatest increases in plasma H2O2 from 2–6 h post meal compared to HC, high protein, or a mixed macronutrient meal of equal caloric value [11]. However, in the current study this was not observed, potentially due to the limited sample size or differences in the methodological approaches to detect H2O2 concentrations (i.e., plasma versus dialysate/interstitial fluid) (data not shown). Furthermore, the dietary composition of a specific macronutrient can modulate the effects on NOX activation. In a study by Carnevale and colleagues, the inclusion of extra virgin olive oil in a Mediterranean diet significantly diminished NOX activity compared to a control diet in healthy individuals as shown by reduced extracellular levels of soluble NOX2-derived peptide [5]. In both the present study and the investigation conducted by Fisher-Wellman and colleagues, heavy whipping cream was employed, which is comprised of approximately 70% saturated fat in terms of total fat content [11]. However, Fisher-Wellman and colleagues used a percentage of body mass to make each meal between 720–1200 kcal which is markedly higher than the 600-kcal used for the current study. Therefore, it appears that the consumption of saturated fat may be particularly conducive to the activation of NOX with monosaturated fat mitigating the elevation in NOX-produced ROS from meal consumption.
In the current study, the higher SMBF around the APO probe compared to the CON probe was only observed following the administration of the HC or HF meal, and not at rest. This finding is consistent with that of La Favor and colleagues, who reported no significant differences in resting SMBF with the perfusion of APO in young sedentary males and females, both before and after an eight-week exercise intervention [19]. Hence, these findings suggest that skeletal muscle NOX activity may be relatively low under fasted, basal conditions, but the administration of a HC or HF meal serves as a potent stimulus for skeletal muscle NOX activity. This finding holds clinical implications, particularly for individuals whose dietary habits are typically characterized by the frequent consumption of HC or HF meals, as it is known that these meals can lead to endothelial dysfunction acutely and over time [12, 13, 31, 36, 40]. While endothelial function was not assessed in the current study, La Favor and colleagues found that APO perfusion restored compromised acetylcholine-stimulated blood flow in individuals with obesity [19]. The authors concluded that this outcome implies the critical involvement of ROS produced by NOX in the onset of endothelial dysfunction in the skeletal muscle microvasculature in individuals with obesity. While in the present study there were no significant correlations between fat mass and the change in H2O2 in the CON as compared to the APO probe at PRE, there is evidence showing that NOX plays a more substantial impact in obese individuals. Patel and colleagues demonstrated that individuals with obesity exhibited a marked increase in p47phox in the blood following the ingestion of a HF/HC meal over 3 h, whereas normal-weight individuals did not exhibit any significant changes in p47phox levels under the same conditions [28]. The authors concluded that individuals with obesity demonstrate a reduced ability to handle oxidative and inflammatory stress compared to those with a normal body mass. This disparity poses a particular threat to individuals with overweight/obesity, as amplified oxidative stress and inflammation could further impair blood flow, thereby escalating the severity of their condition. Furthermore, disruptions in skeletal muscle glucose uptake can hold deleterious implications, given that enhancements in SMBF serve to augment postprandial glucose uptake [32]. Such a disturbance might precipitate poor glucoregulatory control and prompt an elevated risk for type-2 diabetes [33].
A growing body of literature has begun to shed light on the potential of CM supplementation to lower ROS concentrations and enhance blood flow. Lawler and colleagues demonstrated the potent antioxidant properties of CM, as evidenced by its capacity to scavenge ionized radicals in a controlled cellular environment, a finding that was replicated in both animal and human cell lines that had undergone oxidative damage [20, 35]. Similarly, in humans, CM supplementation resulted in a marked reduction in post-exercise oxidative stress as measured in the blood and urine [30]. Notably, in the current study, H2O2 concentrations at any time point, while not statistically significant, tended to be higher after CM supplementation. This difference in findings could be due to the stressor applied to stimulate ROS concentrations (e.g., exercise, HC, or HF meal consumption). It is possible that the HC or HF meal used in this study did not elicit a robust or sustained enough increase in ROS to detect a significant antioxidant effect of CM. Compared to exercise-induced oxidative stress [30], the meal-induced ROS response may be more modest or transient, and more pronounced or prolonged oxidative challenges may be required to fully reveal CM’s antioxidant effects [20, 35]. This upward shift in ROS concentrations with CM supplementation may reflect a transient increase in mitochondrial respiration and electron transport chain activity following CM loading, leading to a short-lived elevation in ROS production in response to nutrient intake [7]. Alternatively, this pattern may reflect methodological limitations of microdialysis, including low probe recovery efficiency or restricted tissue diffusion, which may hinder the accurate detection of subtle, localized ROS changes [19]. Despite no change in H2O2 concentrations, five days of CM supplementation did enhance SMBF between 0–40 min after the consumption of a HC or HF meal. Arceiro and colleagues found that CM combined with resistance training increased peripheral blood flow compared to just CM supplementation alone [1]. Systemic arterial stiffness was attenuated following fatiguing isokinetic exercise in young men supplemented with CM supplementation [34]. Furthermore, Moraes and colleagues reported that CM supplementation improved microvascular blood flow after post-occlusion reactive hyperemia. Indeed, in the current study, an external stressor (i.e., a HC or HF meal) was needed to show improvements in blood flow with CM supplementation [9]. Thus, the application of an external stimulus, such as exercise, occlusion, or meal consumption, might enhance the effect of CM supplementation on blood flow [9, 34]. However, following three weeks of CM supplementation, individuals who chronically consumed a vegan diet exhibited a notable enhancement in baseline skin functional capillary density as well as endothelium-dependent capillary recruitment [39]. Given that creatine is present in meat and fish sources, individuals adhering to vegetarian or vegan dietary patterns possess markedly diminished creatine reserves compared to their omnivorous counterparts [17]. Therefore, individuals who follow a vegetarian or vegan diet likely derive more pronounced benefits from CM supplementation.
The effect of CM supplementation to increase SMBF following a HC or HF meal for 40 min in the current study is particularly beneficial as it has been seen that the consumption of a HC or HF meal can lead to a decrease in SMBF, a phenomenon that is deleterious due to its link to CVD [25]. Recent evidence shows that short-term high-calorie, HF feeding can blunt postprandial microvascular blood flow without affecting macrovascular blood flow or glucose levels, suggesting that impaired SMBF is an early and sensitive marker of metabolic dysfunction [4]. Given the prevalence of frequent HC and HF meals in modern dietary patterns, especially among individuals with overweight or obesity, strategies that can mitigate postprandial SMBF decrements are clinically meaningful [21, 33]. Furthermore, correlations in the current data set show that CM supplementation exhibits a greater change in lowering H2O2 concentrations at baseline (−60 – 0 min) and between 0–40 min following HC or HF meal consumption in individuals with greater fat mass. Those with greater fat mass may have more room for improvement in ROS concentrations and blood flow and thus may benefit the most from CM supplementation. However, population-specific clinical trials that involve individuals at risk for CVD should be conducted to see the possible benefits of CM supplementation. The data in the current study lends credibility to the hypothesis that CM may harbor vascular protective attributes [7].
Limitations
Despite the limited sample size in this single-blind pilot study, the current findings suggest a possible effect of NOX-produced ROS and CM on in-vivo SMBF. In addition, the absence of a standardized meal on the preceding night of each study visit led to potential variations in caloric intake and duration of fasting before microdialysis procedures although all participants were studied after at least 12 h of fasting. The caloric intake of the HC or HF meal was identical for each individual instead of being based on a percentage of body weight or total daily energy expenditure, which may have impacted results [11]. Furthermore, the study was done on a relatively healthy population with only one individual having a BMI in the obese range. A more diverse range of individuals with overweight and obesity may yield different results. In addition, direct perfusion of CM into skeletal muscle via microdialysis would have allowed for a more precise assessment of local vascular responses and ROS concentrations, independent of systemic absorption or metabolism. Future studies should use localized delivery to better clarify CM’s mechanistic effects on microvascular function and oxidative stress at the tissue level. Another limitation was the absence of blood draws, which restricted the ability to assess postprandial concentrations of insulin or other vasodilatory agents and, consequently, limited interpretation of the mechanisms underlying CM-induced enhancements in skeletal muscle blood flow. Finally, prior research has demonstrated that APO also acts as an antioxidant under certain conditions, thus constraining the ability to conclude that APO exclusively acts through NOX inhibition [6, 14]. While there are several NOX inhibitors, APO remains one of the most widely used NOX inhibitors and repeated evidence suggests that it predominately inhibits ROS concentrations generated by NOX [10, 19, 23, 26]. All ROS assessments obtained were transformed from units of fluorescence to H2O2 based on a standard curve of H2O2. By doing so, any plausible H2O2 scavenging influence of APO, or any potential interference of APO on the AMP, is negated with the use of the standard curve [19]. Further, APO perfusion inhibits multiple NOX isoforms, making it challenging to distinguish the individual effects of the different NOX isoforms on the study outcomes.
Conclusions
In conclusion, this study suggests that NOX inhibition may increase SMBF over the duration of baseline and the post-prandial period following the administration of a HC or HF meal, suggesting a role for NOX-derived ROS in the regulation of SMBF. Furthermore, while five days of CM supplementation did not affect in-vivo H2O2 concentrations, it did improve SMBF following the administration of a HC or HF meal. Together these findings suggest that NOX-produced ROS and CM supplementation may be a potential target and treatment, respectively, for reducing risk factors associated with endothelial dysfunction and CVD risk related to very HC or HF intake. However, further studies are needed in individuals with overweight or obesity to fully assess the effects of NOX-produced ROS and CM supplementation on a clinically relevant population. Further, such studies should consider a mixed macronutrient meal that more accurately reflects real-world dietary patterns.
Acknowledgements
Acknowledgments: We sincerely thank all of the participants that were in the study and Glanbia Nutritionals for providing the creatine monohydrate. We acknowledge the following members of the lab for their technical contributions: John C. DeCaro, Sequoia D. Ernst, Riley K. Hart, Ashley Jancura, and Connor Krassel.
Abbreviations
- APO
Apocynin
- CM
Creatine Monohydrate
- CON
Control
- CVD
Cardiovascular disease
- H2O2
Hydrogen peroxide
- NOX
Nicotinamide Adenine Dinucleotide Phosphate Oxidase
- ROS
Reactive Oxygen Species
- SMBF
Skeletal muscle microvascular blood flow
Author contributions
Conceptualization, P.A.B., H.E.C., & R.C.H.; methodology, P.A.B and H.E.C.; investigation, P.A.B., H.E.C., C.A.M., & M.M.A; writing – original draft, P.A.B.; writing – reviewing & editing P.A.B., H.E.C., C.A.M., M.M.A, R.C.H; supervision, R.C.H.
Funding
Glanbia Nutritionals provided creatine monohydrate (CM) used for this study.
Data availability
Availability of data and material: The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Florida State University Institutional Review Board. Participants signed informed consent before participating in the study.
Consent for publication
Participants signed informed consent regarding publishing their data.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arciero PJ et al (2001) Comparison of creatine ingestion and resistance training on energy expenditure and limb blood flow. Metab - Clin Exp 50(12):1429–1434. 10.1053/meta.2001.28159 [DOI] [PubMed] [Google Scholar]
- 2.Baker PA (2024) Creatine Monohydrate Supplementation Effects on Oxidative Stress and Blood Flow in Older Adults With Overweight/Obesity. The Florida State University [Google Scholar]
- 3.Bedard K, Krause K-H (2007) The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol Rev 87(1):245–313. 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- 4.Brayner B et al (2024) Short-term high-calorie high-fat feeding induces hyperinsulinemia and blunts skeletal muscle microvascular blood flow in healthy humans. Am J Physiol-Endocrinol Metab 327(1):E42–E54. 10.1152/ajpendo.00070.2024 [DOI] [PubMed] [Google Scholar]
- 5.Carnevale R et al (2014) Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 235(2):649–658. 10.1016/j.atherosclerosis.2014.05.954 [DOI] [PubMed] [Google Scholar]
- 6.Castor LRG et al (2010) Pro-oxidant activity of apocynin radical. Free Radic Biol Med 48(12):1636–1643. 10.1016/j.freeradbiomed.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Clarke H et al (2021) The Potential Role of Creatine in Vascular Health. Nutrients. 13(3):857. 10.3390/nu13030857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandona P et al (2001) RAPID COMMUNICATION: Inhibitory Effect of a Two Day Fast on Reactive Oxygen Species (ROS) Generation by Leucocytes and Plasma Ortho-Tyrosine and Meta-Tyrosine Concentrations. J Clin Endocrinol Metab 86(6):2899–2902. 10.1210/jcem.86.6.7745 [DOI] [PubMed] [Google Scholar]
- 9.de Moraes R et al (2014) Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr J 13(1):115. 10.1186/1475-2891-13-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuPont JJ et al (2014) NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am J Physiol-Ren Physiol 306(12):F1499–F1506. 10.1152/ajprenal.00058.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher-Wellman KH, Bloomer RJ (2010) Exacerbated postprandial oxidative stress induced by the acute intake of a lipid meal compared to isoenergetically administered carbohydrate, protein, and mixed meals in young. Healthy Men J Am Coll Nutr 29(4):373–381. 10.1080/07315724.2010.10719854 [DOI] [PubMed] [Google Scholar]
- 12.Guzik TJ et al (2002) Mechanisms of increased vascular superoxide production in human diabetes mellitus. Circulation 105(14):1656–1662. 10.1161/01.CIR.0000012748.58444.08 [DOI] [PubMed] [Google Scholar]
- 13.Guzik TJ et al (2006) Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26(2):333–339. 10.1161/01.ATV.0000196651.64776.51 [DOI] [PubMed] [Google Scholar]
- 14.Heumüller S et al (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51(2):211–217. 10.1161/HYPERTENSIONAHA.107.100214 [DOI] [PubMed] [Google Scholar]
- 15.Inoguchi T et al (2000) High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49(11):1939–1945. 10.2337/diabetes.49.11.1939 [DOI] [PubMed] [Google Scholar]
- 16.Joseph P et al (2017) Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ Res 121(6):677–694. 10.1161/CIRCRESAHA.117.308903 [DOI] [PubMed] [Google Scholar]
- 17.Kaviani M et al (2020) Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int J Environ Res Public Health 17(9):3041. 10.3390/ijerph17093041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreider RB et al (2017) International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 14(1):18. 10.1186/s12970-017-0173-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Favor JD et al (2016) Microvascular endothelial dysfunction in sedentary, obese humans is mediated by NADPH oxidase. Arterioscler Thromb Vasc Biol 36(12):2412–2420. 10.1161/ATVBAHA.116.308339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawler JM et al (2002) Direct Antioxidant Properties of Creatine. Biochem Biophys Res Commun 290(1):47–52. 10.1006/bbrc.2001.6164 [DOI] [PubMed] [Google Scholar]
- 21.Mah E, Bruno RS (2012) Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res 32(10):727–740. 10.1016/j.nutres.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 22.McCarthy CG et al (2013) High-fat feeding, but not strenuous exercise, increases blood oxidative stress in trained men. Appl Physiol Nutr Metab 38(1):33–41. 10.1139/apnm-2012-0222 [DOI] [PubMed] [Google Scholar]
- 23.Medow MS et al (2011) Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 111(1):20–26. 10.1152/japplphysiol.01448.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meza CA et al (2019) Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int J Mol Sci 20(15):3775. 10.3390/ijms20153775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ormazabal V et al (2018) Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 17(1):122. 10.1186/s12933-018-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park Y et al (2012) Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J Physiol 590(17):4255–4268. 10.1113/jphysiol.2012.234856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park K-H, Park WJ (2015) Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci 30(9):1213–1225. 10.3346/jkms.2015.30.9.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel C et al (2007) Prolonged reactive oxygen species generation and nuclear factor-κB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 92(11):4476–4479. 10.1210/jc.2007-0778 [DOI] [PubMed] [Google Scholar]
- 29.Powell-Wiley TM et al (2021) Obesity and cardiovascular disease: a scientific statement from the american heart association. Circulation 143(21):e984–e1010. 10.1161/CIR.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahimi R (2011) Creatine Supplementation Decreases Oxidative DNA Damage and Lipid Peroxidation Induced by a Single Bout of Resistance Exercise. J Strength Cond Res 25(12):3448. 10.1519/JSC.0b013e3182162f2b [DOI] [PubMed] [Google Scholar]
- 31.Roberts CK et al (2005) A high-fat, refined-carbohydrate diet induces endothelial dysfunction and oxidant/antioxidant imbalance and depresses NOS protein expression. J Appl Physiol 98(1):203–210. 10.1152/japplphysiol.00463.2004 [DOI] [PubMed] [Google Scholar]
- 32.Roberts-Thomson KM et al (2020) Postprandial microvascular blood flow in skeletal muscle: Similarities and disparities to the hyperinsulinaemic-euglycaemic clamp. Clin Exp Pharmacol Physiol 47(4):725–737. 10.1111/1440-1681.13237 [DOI] [PubMed] [Google Scholar]
- 33.Russell RD et al (2022) Impaired postprandial skeletal muscle vascular responses to a mixed meal challenge in normoglycaemic people with a parent with type 2 diabetes. Diabetologia 65(1):216–225. 10.1007/s00125-021-05572-7 [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Gonzalez MA et al (2011) Creatine supplementation attenuates hemodynamic and arterial stiffness responses following an acute bout of isokinetic exercise. Eur J Appl Physiol 111(9):1965–1971. 10.1007/s00421-011-1832-4 [DOI] [PubMed] [Google Scholar]
- 35.Sestili P et al (2011) Creatine as an antioxidant. Amino Acids 40(5):1385–1396. 10.1007/s00726-011-0875-5 [DOI] [PubMed] [Google Scholar]
- 36.Silver AE et al (2007) Overweight and Obese Humans Demonstrate Increased Vascular Endothelial NAD(P)H Oxidase-p47phox Expression and Evidence of Endothelial Oxidative Stress. Circulation 115(5):627–637. 10.1161/CIRCULATIONAHA.106.657486 [DOI] [PubMed] [Google Scholar]
- 37.Sugamura K, Keaney John F (2011) Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51(5):978–992. 10.1016/j.freeradbiomed.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taye A et al (2010) Effect of apocynin on NADPH oxidase-mediated oxidative stress-LOX-1-eNOS pathway in human endothelial cells exposed to high glucose. Eur J Pharmacol 627(1):42–48. 10.1016/j.ejphar.2009.10.045 [DOI] [PubMed] [Google Scholar]
- 39.Van Bavel D et al (2019) Effects of dietary supplementation with creatine on homocysteinemia and systemic microvascular endothelial function in individuals adhering to vegan diets. Fundam Clin Pharmacol 33(4):428–440. 10.1111/fcp.12442 [DOI] [PubMed] [Google Scholar]
- 40.Vogel RA et al (1997) Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 79(3):350–354. 10.1016/S0002-9149(96)00760-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and material: The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.