Abstract
Cancer-related cognitive impairment (CRCI) affects a significant proportion of cancer patients and survivors, impacting their memory, focus, mood, and quality of life. While non-pharmacological interventions have shown effectiveness in managing this condition, some studies have also explored various pharmacological agents, including donepezil, a reversible acetylcholinesterase inhibitor. This compound demonstrated beneficial effects on cognition in Alzheimer’s disease; however, its effectiveness in CRCI remains unknown. This systematic review and meta-analysis aims to evaluate the efficacy of donepezil in improving cognitive outcomes in cancer patients. We conducted a search of PubMed/Medline, Embase, Cochrane Library, ClinicalTrials.gov, and the International Clinical Trials Registry Platform. Both randomized controlled trials and observational studies were included. We extracted data on cognitive outcomes of donepezil treatment, including the Hopkins Verbal Learning Test–Revised (HVLT-R), Trail Making Test (TMT), and Controlled Oral Word Association Test (COWA), as well as reported adverse events (AEs). Out of 896 identified records, nine studies involving 837 patients met the inclusion criteria, including five RCTs and four observational studies. Donepezil failed to demonstrate significant improvements in cognitive outcomes in HVLT-R, TMT, and COWA. Fatigue, measured as a secondary outcome, also showed no significant improvement. AEs, such as insomnia and headache, were more prevalent in adults than children. Donepezil did not increase the risk of AEs compared to placebo. This meta-analysis found that donepezil does not provide cognitive improvements for CRCI patients. Given the complex and multifactorial nature of CRCI, the lack of efficacy suggests that donepezil may not address the underlying mechanisms of the condition.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-025-01708-w.
Keywords: Cancer-related cognitive impairment, Donepezil, Cancer patients, Cognitive well-being, Cancer survivorship
Introduction
Cancer-related cognitive impairment (CRCI) is a common condition that affects cancer patients both during and after oncologic treatment. The prevalence of CRCI varies across the studies ranging between 13% and 75% depending on the type of cancer and phase of the treatment [1–6]. The International Cancer and Cognition Task Force (ICCTF) defines CRCI as cognitive changes or impairments that occur in cancer patients and has established guidelines to help researchers investigate this heterogenic condition [5]. CRCI is multi-causal, influenced by demographics, genetic and psychological factors, as well as the disease itself or the treatment [1]. These neurological deficits can occur in patients with brain tumors or central nervous system (CNS) metastases, due to the lesion location, but similar cognitive symptoms are also present in non-CNS cancers, such as breast, colon, lung, and other tumors [2–4, 7–9].
The mechanism behind CRCI is still under investigation, with proposed causes including inflammatory cytokines, tumor-derived extracellular vesicles, or immune infiltration to the brain [10]. Additionally, pharmacological agents used for cancer treatment may induce neurotoxicity by increasing blood–brain barrier permeability, triggering neuroinflammation, oxidative stress and impairing neurotransmission [11]. As a result, cancer patients often report problems with memory, focus and multitasking, along with reduced confidence, and suffer from potential economic impacts due to work-related difficulties [12]. Patients often adapt various coping strategies, including nutritional products, alternative medicine treatments, implementing healthy lifestyle practices or physical and mental exercise [13]. To date, only cognitive training has shown effectiveness in improving memory performance in cancer survivors [14, 15]. Currently, no pharmacological agent is recommended for CRCI, and guidelines prioritize non-pharmacological interventions including psychoeducation, cognitive rehabilitation and physical activity [16].
However, non-pharmacological interventions also have limitations, such as the need for trained personnel to supervise cognitive training or its implementation, the long duration required for noticeable effects or considerable time commitment from the patient and often their caregivers [17, 18]. Therefore, pharmacological treatments are also being explored for this condition. Various pharmacological approaches have already been tested, including memantine, methylphenidate and donepezil—the subject of this review [19].
Donepezil, a reversible acetylcholinesterase inhibitor, has been widely studied in Alzheimer's disease (AD) demonstrating efficacy in improving both cognitive and functional outcomes [20]. It is considered safe and well-tolerable with side effects such as headache, insomnia or diarrhea being consistent with its cholinergic mechanism [20–23]. Donepezil’s long half-life of 70 h and high bioavailability allows once-a-day dosing, making it convenient for patients [24–26]. It has also been investigated in other conditions such as traumatic brain injury, mild cognitive impairment, stroke and cancer-related cognitive impairment [27–29]. Promising results from animal studies and the treatment of other conditions have led to the proposal of donepezil as an possible pharmacological treatment for CRCI [11, 30, 31]. In a mouse model, donepezil reduced deficits caused by chemotherapy and improved glucose metabolism in the brain measured by positron emission tomography [32, 33]. In rats, it alleviated doxorubicin-induced cognitive impairment, possibly due to reduced neuroinflammation and oxidative stress without affecting cancer treatment efficacy [34].
Observational studies have suggested effectiveness of donepezil in CRCI; however, the evidence from placebo-controlled randomized clinical trials (RCTs) has been limited, making it challenging to draw definitive conclusions [35–42]. Recently, Rapp et al. published the largest study to date on donepezil in cancer patients, nearly doubling the sample size of previous RCTs [43]. The negative results from this study have raised questions about the feasibility of donepezil for CRCI [43]. These inconsistencies highlighted the need for a comprehensive evaluation of available data to clarify the effectiveness of donepezil in this context, therefore validating the need of this study. We conducted a systematic review and meta-analysis to consolidate the findings of all relevant studies and provide robust evidence to validate the usage of donepezil in CRCI. Additionally, our analysis underscores the challenges in identifying effective pharmacological interventions for CRCI.
Methods
Data sources
This systematic review and meta-analysis is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 2020 version [44]. The research question was structured using the population (P), intervention (I), comparison (C), outcome (O), time-to-follow-up (T) (PICOT) framework, a validated tool for defining clinical research questions [45, 46]. In brief, P—describes the patient population or the problem, I—type of intervention, drug or approach toward the problem, C—specifies how the outcomes will be measured, what will the comparison, O—includes investigated outcomes, and T—specifies time of intervention duration of follow-up [45, 46]. We searched PubMed/Medline, Embase, Cochrane Library, clinicaltrials.gov and International Clinical Trials Registry Platform (ICTRP). To visualize the search process, we used interactive tool for PRISMA diagram generation [47]. This systematic review and meta-analysis was registered in PROSPERO (CRD42024570391).
Study selection
The detailed search strategy is presented in Table 1S. The inclusion criteria followed our research question according to the PICOT framework and comprised: (P)—patients with cancer or after oncological treatment, (I)—Donepezil treatment with no dose restriction, (C)—placebo or no comparison group, (O)—Primary outcomes included cognitive function assessed via standardized tests. Secondary outcomes encompassed treatment-related adverse events (AE) such as: fatigue, insomnia, headache, pain, nausea, vomiting, diarrhea and dizziness, (T)—time-to-follow-up with no restriction. We included both RCTs and observational studies due to small number of available trials. Exclusion criteria were set as follows: (1) review articles, (2) case reports, (3) conference abstracts, (4) animal studies, (5) studies in languages other than English, and (6) studies with unavailable data or unpublished results.
Data extraction and quality assessment
Studies identified in the search were initially screened by title and abstracts. If studies met the inclusion criteria, a full-text review was conducted. Both screening and full-text analysis were performed independently by two investigators (MF, MG), with a third reviewer (SM) resolving conflicts and making final decisions. Screening and study selection was performed using Rayyan platform to facilitate work and tract progress of each investigator [48]. Data extraction included: author, year, number of participants, follow-up duration, donepezil duration, cancer type, previous treatment and relevant outcomes reported at least across 3 studies such as: results of cognitive tests reported at (HVLT, TMT, COWA and other), fatigue score and AE. The results of cognitive tests were extracted as mean values and their standard deviations (SD) in placebo and experimental groups. AEs were assessed as the number of patients experiencing specific conditions throughout the study. One researcher extracted data (MF), which was then reviewed by other investigators (MG, SM), resolving inconsistencies through discussion. For the cognitive outcomes we chose 24-week treatment timepoint as it was the only timepoint shared across at least 3 RCTs. For the observational studies and the analysis of the AE, we analyzed the incidence number at the end of the study.
Special considerations
Correa et al. reported their data as Z-score compared with published normative values according to the age and education [49]. Since individual patient data were not seek and baseline demographics of participants landed in between middle-aged and older-aged normative values groups, we estimated baseline mean values according to the average points between those two groups. Post-treatment results were calculated based on these estimated means; hence, caution is advised when interpreting the findings from Correa’s 2016, study. Two studies were described by more than one record—Castellino et al. [37, 50] and Rapp et al. [39, 40]. For these studies, we extracted results from both sources with preference of the published article in case of duplication.
Risk of bias assessment
We assessed risk of bias using two complementary tools [51, 52]. For randomized trials, we employed the Cochrane Risk of Bias 2 (RoB 2) tool, which evaluates five domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of the reported result. Each domain was rated as low risk, some concerns, or high risk, with an overall judgment derived qualitatively (no numerical score was calculated). For non-randomized studies, the ROBINS-I tool (Risk Of Bias In Non-randomized Studies of Interventions) was used. This tool examines seven domains: bias due to confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result, with each domain judged from low to critical risk of bias. An overall risk of bias judgment was then derived from these domain-level assessments, again without assigning a numerical score.
Statistical analysis
Statistical analysis was conducted using R Studio [53] and Review Manager (RevMan) version 5.4, The Cochrane Collaboration. The RevMan5 calculator was used to transform standard errors and confidence intervals to standard deviations. Heterogeneity among studies was evaluated using the I2 and Tau2 statistics, and the Cochran Q-test was employed to determine whether the observed heterogeneity was statistically significant (with a threshold of p < 0.10). Regardless of the level of heterogeneity, a random-effects model was applied, following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [54]. A meta-analysis was performed for all outcomes that were reported by at least three studies. All results regarding the efficacy of donepezil in improving cognitive outcomes or reducing fatigue compared to placebo were analyzed based on data from randomized controlled trials (RCTs) using random-effects model. A random‐effects model was used due to anticipated heterogeneity in study populations, including different cancer types or age of participants [55]. The prevalence of AE was evaluated using a single-arm meta-analysis, incorporating data from both RCTs and observational studies to provide a more comprehensive assessment. For this analysis, we extracted data on the number of patients experiencing specific AEs. Additionally, subgroup analysis was performed for the prevalence of AE in a single-arm meta-analysis, as incorporation of observational studies allowed us to stratify the patients into groups based on their demographic characteristics. Subgroup analysis was conducted using generalized linear mixed model [56].
Continuous outcomes were reported as either mean difference (MD) or standardized mean difference (SMD). SMD was used only when outcomes were measured on different scales, enabling the pooling of results across studies. To report the prevalence of selected outcomes in the single-arm meta-analysis, prevalence was expressed as the number of events per 100 individuals. The pooled effect estimates were tested for statistical significance using a Z-test, with all outcomes reported with 95% confidence intervals and a p value of < 0.05 considered significant. Subgroup analyses were performed using Q-test for subgroup differences [57].
Sensitivity assessment
For each cognitive outcome, both fixed-effect and random-effects models were considered, as detailed in Table 2S. Given the differences in participant characteristics across studies, the main manuscript reports results based on the random-effects model. In addition, for each cognitive outcome, we performed leave-one-out sensitivity analyses using both random-effects (Table 3S) and fixed-effect models (Table 4S) to assess the robustness of our findings. Publication bias was not assessed due to small number of included studies (< 10) [58].
Results
Study search and study characteristics
The initial search across databases resulted in 896 results, of which 170 were duplicates. After screening by title and/or abstracts we retrieved 44 articles for full-text review. We identified 11 records, describing 9 studies that met the inclusion criteria and therefore were incorporated into this systematic review [35–43, 50, 59]. The literature search process is presented on PRISMA diagram in Fig. 1. The majority of those studies were conducted in the USA, while one was an international study. We included five RCTs [39–43, 59] and four observational studies [35–38]. The sample size of included studies ranged from 11 to 276. Risk of bias assessment revealed that, while RCTs presented relatively low risk of bias, the observational studies due to considerable dropout rate, open label and single-arm design were recognized with at least moderate risk. The study by Correa et al. presented critical risk of bias due to severe deviation from the intended intervention and dropout rate [38] (Figure 1S).
Fig. 1.
PRISMA flow diagram of study screening and selection’
Participant characteristics
The pooled population of this study was 837, out of which 77.15% were women. Overall, 55,36% of all patients were given donepezil. Included studies varied in different types of cancer and age of participants. Two studies were conducted on breast cancer patients [41, 43], five on brain cancer patients [36–40] and two included survivors of various types of cancer [35, 42]. Table 1 provides details on studies conducted primarily in patients with CNS tumors, while Table 2 presents studies investigating other malignancies. Given the limited number of randomized controlled trials (RCTs) in each group and the exploratory nature of this study, we combined both groups for pooled analysis. Additionally, two studies examined donepezil in pediatric populations, accounting for 9.8% of all patients included in the analysis. This distinction between adult and pediatric populations was further incorporated into the subgroup analysis. The dose of the treatment varies from 3 to 10 mg daily, often escalated progressively during the intervention.
Table 1.
Characteristics of included studies, * median
| Study ID | Country | Number of participants | Design | Treatment | Males-to-females ratio (%/%) | Mean age (SD or range) | Duration | Cancer type | Previous treatment | Summarized effects of donepezil treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Rapp 2015, Naughton 2017 | USA | 198 | Placebo-controlled RTC | Single daily 5 mg for 6 weeks, escalated to single daily 10 mg for 18 weeks | 46.5/53.5 | 55.1 (19–84) | Up to 24 weeks | Brain | Partial or whole-brain irradiation ≥ 6 months before enrollment | Improved health-related quality of life (HRQL) in patients with more severe baseline cognitive symptoms compared to placebo, whereas decreased functional well-being and increased fatigue observed in those with fewer symptoms relative to placebo |
| Shaw 2006 | USA | 35 | Observational | Single daily 5 mg for 6 weeks, escalated to single daily 10 mg for 18 weeks | N/A | 45 | Up to 30 weeks | Brain | Partial or whole-brain irradiation ≥ 6 months before enrollment | Improved cognitive function, mood, and health-related quality of life (HRQL) |
|
Castellino 2012 |
USA | 11 | Observational | Single daily 5 mg for 6 weeks, escalated to single daily 10 mg for patients > = 35 kg | 54.5/45.5 | 11.1 (9.3–17.3) | Up to 36 weeks | Brain | Partial or whole-brain irradiation ≥ 6 months before enrollment | Well-tolerated with observed improvements in executive function and memory in pediatric brain tumor survivors |
| Correa 2016 | USA | 15 | Observational | Single daily 5 mg for 4 weeks, escalated to single daily 10 mg for 20 weeks | 66.7/33.3 | 58.9 (14.1) | Up to 24 weeks | Brain | Completion of RT and/or chemotherapy 6 months before enrollment | Well-tolerated and associated with improvements in attention, graphomotor speed, visual delayed recall, and social well-being; however, self-reported quality of life improvement did not correlate with cognitive test performance, suggesting potential influence from factors such as mood or fatigue |
| NCT00688376 | International: USA, Argentina, Australia, Canada, Chile, France, Germany, Netherlands, Spain, United Kingdom | 71 | Placebo-controlled RTC | Single daily 3 mg for 3 weeks escalated incrementally in 3 weeks intervals to single daily 5 or 10 mg depending on the body weight | 49.3/50.7 | 11.9(2.95) | 12 weeks | Brain | Completion of RT/chemotherapy 12 months before enrollment | No significant impact on attention, executive function, or reaction time was observed compared to placebo in pediatric cancer survivors with attention impairment |
Table 2.
Characteristics of included studies, *median
| Study ID | Country | Number of participants | Design | Treatment | Males-to-females ratio (%/%) | Mean age (SD or range) | Duration | Cancer type | Previous treatment | Summarized effects of donepezil treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Rapp 2024 | USA | 276 | Placebo-controlled RTC | Single daily 5 mg for 6 weeks, escalated to single daily 10 mg for 18 weeks | 0/100 | 57.1 (10.5) | Up to 24 weeks | Breast |
≥ 4 cycles of adjuvant/neoadjuvant cytotoxic chemotherapy 1–5 years before enrollment |
No significant effect on memory, cognitive function, or subjective cognitive function compared to placebo |
| Lawrance 2015 | USA | 62 | Placebo-controlled RTC | Single daily 5 mg for 6 weeks, escalated to single daily 10 mg for 18 weeks | 0/100 | 55.8 (39–79) | Up to 36 weeks | Breast |
> 4 cycles of adjuvant/neoadjuvant cytotoxic chemotherapy 1–5 years before enrollment |
Improved verbal memory and executive function in patients with poorer baseline cognitive performance compared to placebo |
| Bruera 2003 | USA | 27 | Observational | Single daily 5 mg | 33.3/66.7 | 52*(24–75) | 1 week | Hematological, gastrointestinal, lung, head and neck, breast | N/A, during study patients were given oral morphine-equivalent daily dose of 180 mg per day | Significantly improved sedation, fatigue, well-being, anxiety, and constipation in cancer patients receiving opioids |
| Bruera 2007 | USA | 142 | Placebo-controlled RTC | Single daily 5 mg | 35.2/64.8 | 56 (12.2) | 1 week | Breast, gynecologic, gastrointestinal, lung, and others | N/A | Not superior to placebo in the management of cancer-related fatigue |
Outcomes reported in the studies
In our analysis, we primarily focused on the effectiveness of donepezil in improving cognitive outcomes for cancer patients. ICCTF recommends several cognitive tests to assess CRCI, including Hopkins Verbal Learning Test—Revised (HVLT-R), Trail Making Test (TMT) and Controlled Oral Word Association (COWA) [5]. Unfortunately, only three RCTs reported results from these tests [39, 41, 43], which strongly limited our analysis due to the small sample size. Donepezil, in our analysis, failed to demonstrate significant improvement in any of these cognitive assessments.
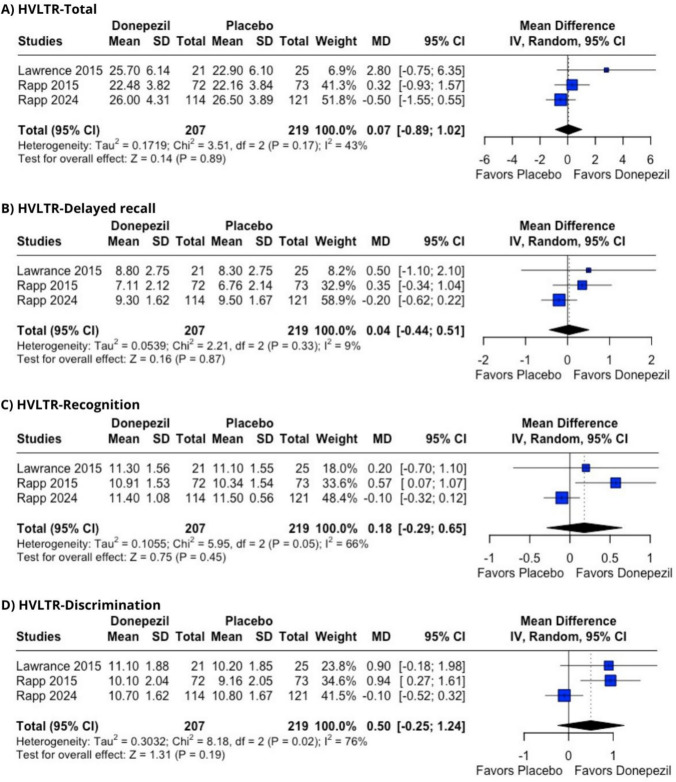
Among the tests, the HVLT-R is widely used to assess memory and verbal learning in brain disorder populations [60]. This test consists of six individual forms, which can be graded separately. In this study, we report only the total score, delayed recall, recognition, and discrimination results, as these were the only data available from the placebo-controlled studies. The pooled results showed no significant improvement across any of the domains: (1) HVLT-R total score (MD = 0.07 [− 0.89; 1.02], p = 0.89, I2 = 43%), (2) HVLT-R delayed recall (MD = 0.04 [− 0.44; 0.51], p = 0.87, I2 = 9%), (3) HVLT-R recognition (MD = 0.18 [− 0.29; 0.65], p = 0.45, I2 = 66%), (4) HVLT-R discrimination (MD = 0.50 [− 0.25; 1,24], p = 0.19, I2 = 76%) (Figure 2A–D).
Fig. 2.
Hopkins Verbal Learning Test results for donepezil versus placebo for CRCI. A total score. B Delayed recall. C recognition. D discrimination. Pooled mean difference and 95% confidence interval were calculated using random effects
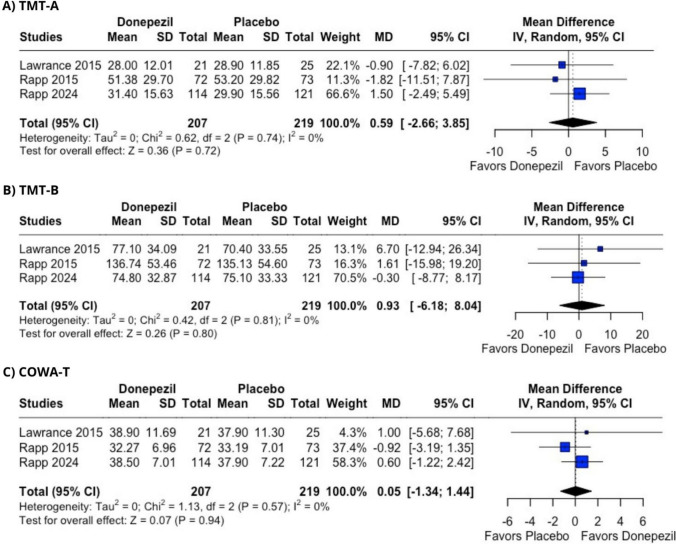
As part of our broader evaluation of cognitive function, we also considered the TMT, which measures processing speed and executive functions. It consists of two parts: A and B. Part A involves connecting labeled circles in numerical order, while Part B requires alternating between numbers and letters while organizing them in both numerically and alphabetically. Patient’s cognition is evaluated based on the completion time for each part [61]. In the cancer patients involved in this study, donepezil showed no improvement compared to placebo in their TMT completion times based on pooled results from three reports [39, 41, 43]: Part A (MD = 0.59 [− 2.66; 3.85], p = 0.72, I2 = 0%) and Part B (MD = 0.93 [− 6.18; 8.04], p = 0.80, I2 = 0%) (Figure 3A and B).
Fig. 3.
Cognitive tests assessing donepezil effectiveness in CRCI in comparison with placebo. A Trail Making test, part A. B Trail Making test, part B. C Controlled Oral Word Association Test. Pooled mean difference and 95% confidence interval were calculated using random-effects model
COWA test is used for assessing phonemic fluency. In this test, patients name as many words as possible starting with a chosen letter, commonly F, A, or S [62, 63]. This is often referred to as COWAT-FAS and is typically combined with the HVLT-R for improved diagnostic accuracy. Consistent with the findings from the HVLT-R and TMT, donepezil also did not demonstrate effectiveness in the COWAT versus the placebo group (MD = 0.05 [− 1.34; 1.44], p = 0.94, I2 = 0%) (Figure 3C). Another outcome evaluated was improvement in fatigue scores, which was reported by four studies [40–43]. Study by Rapp et al. reported it using patient-reported outcomes measurement information system fatigue (PROMIS-F) form [43, 64], while other studies commonly utilized the functional assessment of chronic illness therapy-fatigue scale (FACIT-F) [40–42, 65]. Due to the use of different scales for this outcome, we utilized the standardized mean difference (SMD) to assess the effect of the treatment. Those scales in addition to measuring intensity of fatigue assess its effects on daily life, providing additional information compared with prevalence [66, 67].
Donepezil administration failed to show a more beneficial effect on fatigue than placebo, and the data were homogeneous across the studies (SMD = − 0.05 [− 0.22; 0.12], p = 0.57, I2 = 0%) (Figure 2S).
For all cognitive outcomes, we performed sensitivity analysis and directly compared the pooled effects using either fixed- or random-effect model (Table 2S). No differences in terms of sensitivity were noted. Additionally, we performed leave-one-out analysis using both random-effects (Table 3S) and fixed-effect models (Table 4S). These analyses revealed that upon exclusion of the study by Rapp et al. [43], significant increase in HVLTR-Recognition and HVLTR-Discrimination scores was noted. This analysis revealed that the results of the pooled analysis were sensitive to the exclusion of individual studies, supporting the need for further research.
Fortunately, despite the lack of effectiveness in improving patients’ mental state, donepezil did not lead to a higher prevalence of serious or non-serious AEs. We first assessed serious side effects reported in the RCTs [39, 41, 43], such as insomnia, headache, and pain. We report these AEs as risk difference between donepezil and placebo groups, Fig. S3. Donepezil did not contribute to an increased prevalence of serious AEs: insomnia (RR = 1.77 [0.55; 5.62], p = 0.336, I2 = 0%), headache (RR = 0.99 [0.23; 4.32], p = 0.993, I2 = 0%), pain (RR = 1.43 [0.23; 8.95], p = 0.704, I2 = 0%).
To combine findings from RCTs and observational studies, we conducted a single-arm meta-analysis of non-serious AEs, presenting the data as prevalence.
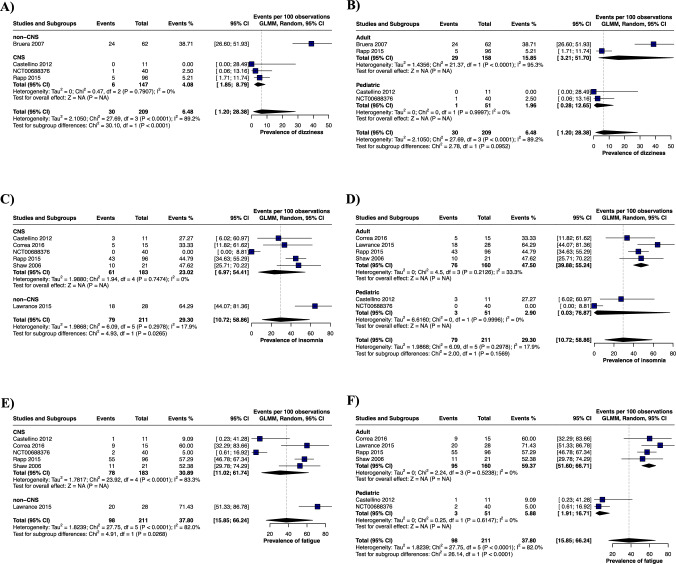
Due to significant heterogeneity in the pooled analysis, subgroup analyses were conducted by malignancy type (CNS tumor vs. non-CNS tumor) and mean participant age. However, it is important to note that these subgroups include a small number of studies and limited pooled sample size. First, stratification by malignancy type revealed a significantly lower prevalence of dizziness in the CNS tumor group compared with non-CNS tumor patients reported by Bruera et al. [42]. (4.08% in CNS vs. 38.71% in non-CNS; Fig. 4A). Additionally, the CNS tumor group exhibited lower prevalences of insomnia (23.02% in CNS vs. 64.29% in non-CNS) and fatigue (30.89% in CNS vs. 71.43% in non-CNS) compared with the study by Lawrence et al. [41] (Fig. 4C and E). When stratified by age, the pediatric population showed a significantly lower prevalence of fatigue compared with adults (5.88% in children vs. 59.37% in adults; Figure 4F), although no differences were noted in dizziness or insomnia between age groups (Fig. 4B and D). Next, we evaluated the prevalence of headache and found no differences across tumor sites or age groups (Fig. S4A and B). Additionally, as part of our analysis, we assessed gastrointestinal symptoms, including nausea, vomiting, and diarrhea. While no differences were observed for nausea and diarrhea when stratified by tumor site (Fig. S4G) or by age (Fig. S4D, F, and H), the prevalence of vomiting was significantly lower in the CNS tumor group compared with non-CNS tumor patients (8.84% in CNS vs. 25% in non-CNS; Fig. S4E). Finally, no differences in the prevalence of anorexia were detected between the subgroups (Fig. S4I and J).
Fig. 4.
Prevalence of neurological adverse effects during donepezil treatment for CRCI subgroups based on their cancer type (CNS and non-CNS) and age of participants (adult and pediatric population). A Subgroup analysis of the prevalence of dizziness based on cancer type, B Subgroup analysis of the prevalence of dizziness based on age of participants, C Subgroup analysis of the prevalence of insomnia based on cancer type, D Subgroup analysis of the prevalence of insomnia based on age of participants, E Subgroup analysis of the prevalence of fatigue based on cancer type, F Subgroup analysis of the prevalence of fatigue based on age of participants. The results are presented as the number of events per 100 observations with 95% confidence interval
Discussion
Despite its effectiveness in various conditions associated with cognitive impairment such as AD [68], Parkinson’s disease [69] or mild cognitive impairment (MCI) [70], the utilization of donepezil in CRCI has only been speculated in clinical recommendations [11, 30, 31]. While cholinergic enhancement effectively increases cognitive performance in MCI [71], its effectiveness in CRCI has not yet been validated. Our analysis shows that, based on available evidence, donepezil fails to improve cognitive profile of cancer patients. CRCI can also vary depending on the tumor. While CRCI most commonly describes non-CNS tumors, the cognitive decline is one of the most common symptoms also in the CNS malignancies or brain metastases, reaching the prevalence of up to 90% [72, 73]. This condition has multifactorial etiology resulting from tumor site, direct neurotoxic effect of oncologic drugs, or from a systemic AE from the treatment [10, 11]. In the CNS the cognitive decline can be a result of a direct mass effect of the tumor resulting in tissue narcosis and edema, or arise as a result of surgical interventions [74]. Chemotherapy and radiotherapy also have been proven to affect neurocognitive functions in CNS tumor patients even without disease progression [75, 76]. Similarly, in head and neck cancer, these treatments can affect the central nervous system and lead to cognitive deficits [77, 78]. In colorectal cancer, cognitive decline has been observed, with similar reductions in function between those receiving chemotherapy and those who did not [9]. Moreover, a meta-analysis in breast cancer patients reported that chemotherapy was associated with declines in attention, concentration, and executive function, although the effect was mild despite reaching statistical significance [79]. Notably, the cognitive impairment is more pronounced when comparing cancer patients receiving chemotherapy with healthy controls than when compared with patients undergoing non-systemic treatments. These findings suggest that it may be valuable to compare the effectiveness of donepezil in cancer patients with that in cancer-free individuals who exhibit cognitive impairment. However, it is important to recognize that the degree of cognitive decline may depend on multiple factors, including age, baseline cognitive reserve, and co-occurring symptoms [80, 81].
Across studies, educational status and baseline depression have been linked with declines in neurocognitive function [77]. Additionally, co-occurring conditions like depression, fatigue, and impaired emotional functioning further contribute to the severity of cognitive impairment [82, 83]. In preclinical models, donepezil has been shown to improve vascular function via acetylcholine-dependent vasodilation [84] and reduce neuroinflammation by decreasing microglial activation and lowering TNFα and IL-1α levels [85]. Donepezil treatment also demonstrated effectiveness in preventing postoperative cognitive decline in a mouse model of isoflurane-induced spatial memory impairment. This effect could rely on increased choline acetylase level [86]. Since CRCI origin is not primarily cholinergic in nature, inhibition of acetylcholinesterase by donepezil might not be sufficient to improve patients’ cognition. Additionally, differences in genetic profiles, especially polymorphisms of cytochromes CYP2D6 and CYP3A4, can potentially influence donepezil plasma concentration leading to reduced efficacy or increased side effects in some individuals [87–89]. Cancer survivors often are also under chronic pharmacological treatment for pain or other comorbidities, which might potentially interact with donepezil [90, 91]. Exclusion criteria varied across studies included in this analysis. Common exclusions included major neurologic disorders (e.g., epilepsy in Correa et al. [38]. and Rapp et al. [43]; multiple sclerosis and traumatic brain injury in Lawrence et al. [41]), dementia (Rapp et al. [43], Lawrence et al. [41]), and active cancer progression. Severe depression led to exclusion in two studies (Rapp et al. [43]; Lawrence et al.[41]), whereas mild or treated depression was accepted in others (Rapp et al. [39]; Bruera et al. [35]). Stable comorbidities were generally permitted. For instance, diabetes and hypothyroidism were allowed in pediatric populations (NCT00688376; Castellino et al. [37]), controlled epilepsy was accepted (Correa et al. [38]), and treated depression, insomnia, or anxiety were allowed in studies such as Rapp et al. [43] and Lawrence et al. [41]. This variability indicates that donepezil may not be universally effective for CRCI and may be limited by the condition’s complex nature.
Several other pharmacological treatments also have been tested in CRCI, including memantine, methylphenidate and melatonin [92–94]. In a study by Nakamura et al. investigating the effects of memantine for CRCI prevention, memantine did not demonstrate significant improvements across a range of cognitive tests. However, in the COWA test, approximately 40% of participants showed an improvement greater than 0.5 SD, while in the HVLT-R total and delayed recall tests, these values were 48.9% and 51.1%, respectively. Similar to our analysis, the participants in their study varied in cancer stage, histological type, and oncological treatment [93]. Regarding cancer-related fatigue, methylphenidate demonstrated improvements in the functional assessment of cancer therapy-fatigue (FACT-F), with more pronounced effects observed with prolonged intervention. However, no significant effect was noted on cognition as measured by the Mini-Mental State Examination (MMSE). Unfortunately, the number of studies assessing methylphenidate’s impact on cognition remains limited [94]. Promising results, however, come from a study investigating the effects of melatonin in breast cancer patients. This treatment led to improvements in TMT A and B, COWA, and other cognitive measures. However, the cognitive benefits observed in this study may have been largely influenced by improvements in sleep quality and depressive symptoms [92]. The considerable variability in patient demographics, comorbidities, treatment mechanisms, and the complex interplay between CRCI and conditions such as depression and sleep disturbances make direct comparisons between different studies challenging.
Additionally, investigating CRCI has been difficult for both researchers and clinicians as there are no clear guidelines on how to diagnose or define it. According to ICCTF, to be diagnosed with CRCI patients should present either > 1.5 SD worse results on two or more measurements, or > 2SD on a single test compared with normative values for their age and demographics [5]. Recommended tests may include neuroimaging, neuropsychological tests or self-reported cognitive impairment [5, 95]. Cognitive impairment was a core criterion for study participation across trials, typically defined by both self-reported cognitive complaints or objective impairment on standardized tests. For example, Rapp et al. [43] required subjective cognitive concerns confirmed by an HVLT-R score of ≤ 7, while Lawrence et al. [41] used a FACT-Cog PCI score of < 63. In pediatric studies, attention or school-related difficulties were required and verified through neuropsychological testing. Notably, two studies by Bruera et al. [35, 42] excluded participants with cognitive dysfunction by requiring a normal MMSE score to minimize confounding factors in the assessment of fatigue or sedation. Correa et al. [38] required an MMSE score within the 18–27 range but allowed inclusion of patients with self-reported cognitive symptoms after brain tumor treatment. Considering those participant criteria, as well as the fact that baseline characteristics of some of the included studies, based on relatively large SD of cognitive measurements, show that only the fraction of participants actually presented CRCI at the beginning of the study [39, 43]. Based on the normative results for the population reflected by the mean age of the included patients, we cautiously suggest that there is an indication that a notable proportion of these patients also failed to meet the ICCTF criteria for CRCI [41, 96]. Taking that into consideration, there is a probability that the beneficial effect of donepezil in cognitively impaired patients might be masked by lack of improvement in patients with no CRCI. Future studies should conduct more detailed screening process prior to enrolling patients to the study to ensure investigating the population of interest. Finally, studies by Shaw et al. and Correa et al. reported beneficial effects of donepezil [36, 38]. These studies included brain tumor patients, most of whom had undergone radiotherapy. However, both studies were non-randomized and had small sample sizes, including only 24 and 15 patients, respectively. Notably, the patient populations in these studies were similar, suggesting that donepezil might be particularly beneficial for irradiated brain tumor patients.
Unique subgroup of cancer patients experiencing CRCI are childhood cancer survivors. Pediatric cancers contribute to around 200 thousand cancer cases globally and approximately every one in 260 children will be diagnosed with cancer before the age of 20 [97, 98]. Fortunately, more than 80% of young patients become long-term survivors after they successfully complete the treatment [99]. As it appears, however, tumor leave long lasting burden on those patients, which can contribute to various disabilities in their adult life such as reduced task efficiency, memory issues, or worse overall well-being [100, 101]. CRCI may affect their education and professional development, eventually contributing to reduced financial security [101]. Those reasons highlight the need for effective therapeutic approaches to prevent future complications. Unfortunately, included studies conducted on pediatric population differed in reported outcomes; therefore, pooling the results in single meta-analysis was unfeasible. The only pediatric RCT identified by our search strategy also reported lack of the effect on the cognition measured with Test of Variables of Attention (NCT00688376). In observational studies, donepezil improved some of the cognitive measurements (Delis–Kaplan Executive Function Scale and Wide Range Assessment of Memory and Learning, 2nd Edition) and was generally well tolerated [37]. To address differences between adult and pediatric populations, we conducted a subgroup analysis of AEs based on participant age. Our findings suggest that AEs during donepezil treatment are less common in younger patients compared to adults. However, it is important to note that the pediatric sample size was relatively small, limiting the statistical significance of many outcomes. Additional studies, preferably RCTs designed in accordance with ICCTF recommendations, are needed to draw definitive conclusions.
In addition to its potential cognitive benefits, donepezil is also being tested for chemotherapy-induced peripheral neuropathy (CIPN) [102]. Similar to CRCI, CIPN can occur as a response to cancer treatment, with nearly 70% of patients reporting it immediately after chemotherapy [103]. However, unlike CRCI, CIPN often resolves over time, with prevalence decreasing by half within 3 months of treatment completion [103]. In mouse model, donepezil has been shown to alleviate CIPN-related allodynia and reduce peripheral nerve degeneration, demonstrating its neuroprotective properties [104]. Additionally, in a rat model, donepezil also showed to reduce neuropathic pain and also abolished social withdrawal associated with CIPN [105]. An ongoing clinical trial is evaluating the efficacy of donepezil in treating CIPN, with secondary outcomes including anxiety, depression, quality of life scores and pain management [102]. This trial may offer evidence supporting the use of donepezil in other cancer-related conditions, despite its limited success in improving CRCI.
Our analysis had several limitations; first, the pooled population of patients was relatively small, which may have limited the statistical power and contributed to the overall lack of observed effect. To address this issue, we included both randomized controlled trials and observational studies in our analysis. The selected studies were assessed for potential risk of bias using the Cochrane tools, RoB 2 and ROBINS-I. Notably, there was considerable heterogeneity in some of the reported outcomes, which could have arisen from a differences in the baseline characteristics of the study populations. Due to small number of included studies, we combined studies investigating CNS and non-CNS tumors, which resulted in confounding effect of different patients’ population. Due to the limited number of studies, we could only perform a restricted subgroup analysis based on different tumor sites. Additionally, the long-term follow-up data were limited; therefore, meta-regression analysis of the relationship between the duration of the treatment and its efficacy was not implementable. AEs were also assessed by pooling the results from RCTs and observational trials. Where feasible, we stratified the outcomes into other subcategories, which in some cases improved the heterogeneity metrics. However, due to the large heterogeneity in outcomes and the limited sample size, additional studies with similar stratification are necessary to draw more robust and coherent conclusions. Additionally, many of the included studies relied on self-reported cognitive impairment for participant recruitment, which may not always align with objectively measured CRCI [106]. Taken that into account, some participants at baseline might not have been cognitively impaired; therefore, treatment of this group could have been unsuccessful. Most cognitive tests focus on mean performance levels, treating cognitive abilities as stable, trait-like characteristics of the individual. However, emerging research suggests that intraindividual variability (e.g., fluctuations in performance over time) may hold clinical relevance. It was demonstrated that this measurement could be sensitive indicator of cognitive function in breast cancer patients [107]. To address this, future studies should include meticulous diagnostic process to enroll participants that will benefit the most from the therapy. Tailoring donepezil therapy for patients with severe cognitive decline might yield more reliable results.
Conclusion
This systematic review and meta-analysis provides summary and evidence that donepezil treatment fails to improve the cognitive outcomes in cancer patients. Based on analysis of pooled number of 837 patients, we present lack of support for donepezil utilization in CRCI. The included studies varied considerably in patient demographics, cancer types, and baseline cognitive status. Notably, the inclusion of potentially cognitively normal patients in some studies may have masked the true therapeutic effect of donepezil in those with genuine CRCI. Furthermore, the robust evidence from well-designed RCTs represented only a fraction of the overall patient data, with many studies limited by small sample sizes. Consequently, while current evidence does not support the routine use of donepezil for CRCI, further research—especially well-designed RCTs in more homogeneous populations with confirmed cognitive impairment, is warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
MF contributed to conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, visualization, and writing—review and editing. MG contributed to investigation, writing—original draft, and writing—review and editing. SM contributed to investigation and writing—review and editing. CBP contributed to writing—review and editing and supervision. AY contributed to writing—review and editing and supervision.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
No ethical approval and consent to participate were required as this study was based on previously published data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sandra Moska and Mariola Gimła have contributed equally to this work.
References
- 1.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–13. 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt JE, Beckjord E, Bovbjerg DH, et al. Prevalence of perceived cognitive dysfunction in survivors of a wide range of cancers: results from the 2010 LIVESTRONG survey. J Cancer Surviv. 2016;10:302–11. 10.1007/s11764-015-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regier NG, Naik AD, Mulligan EA, et al. Cancer-related cognitive impairment and associated factors in a sample of older male oral-digestive cancer survivors. Psychooncology. 2019;28:1551–8. 10.1002/pon.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mir N, MacLennan P, Al-Obaidi M, et al. Patient-reported cognitive complaints in older adults with gastrointestinal malignancies at diagnosis—results from the cancer & aging resilience evaluation (CARE) study. J Geriatr Oncol. 2020;11:982–8. 10.1016/j.jgo.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–8. 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan ND, Dasari S, Rodriguez JL, et al. Post-treatment neurocognition and psychosocial care among breast cancer survivors. Am J Prev Med. 2015;49:S498–508. 10.1016/j.amepre.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pendergrass JC, Targum SD, Harrison JE. Cognitive impairment associated with cancer: a brief review. Innov Clin Neurosci. 2018;15:36–44 (PMID: 29497579). [PMC free article] [PubMed] [Google Scholar]
- 8.Simó M, Root JC, Vaquero L, et al. Cognitive and brain structural changes in a lung cancer population. J Thorac Oncol. 2015;10:38–45. 10.1097/JTO.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33:4085–92. 10.1200/JCO.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson B, Marks DL. Pretreatment cancer-related cognitive impairment—mechanisms and outlook. Cancers. 2019;11:687. 10.3390/cancers11050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming B, Edison P, Kenny L. Cognitive impairment after cancer treatment: mechanisms, clinical characterization, and management. BMJ. 2023. 10.1136/bmj-2022-071726. [DOI] [PubMed] [Google Scholar]
- 12.Bolton G, Isaacs A. Women’s experiences of cancer-related cognitive impairment, its impact on daily life and care received for it following treatment for breast cancer. Psychol Health Med. 2018;23:1261–74. 10.1080/13548506.2018.1500023. [DOI] [PubMed] [Google Scholar]
- 13.Selamat MH, Loh SY, Mackenzie L, Vardy J. Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PLoS ONE. 2014;9: e108002. 10.1371/journal.pone.0108002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135:799–809. 10.1007/s10549-012-2210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miotto EC, Savage CR, Evans JJ, et al. Semantic strategy training increases memory performance and brain activity in patients with prefrontal cortex lesions. Clin Neurol Neurosurg. 2013;115:309–16. 10.1016/j.clineuro.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Duivon M, Lange M, Binarelli G, et al. Improve the management of cancer-related cognitive impairment in clinical settings: a European Delphi study. J Cancer Surviv. 2023. 10.1007/s11764-023-01436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauth MR, Sullivan G, Blevins D, et al. Employing external facilitation to implement cognitive behavioral therapy in VA clinics: a pilot study. Implement Sci. 2010;5:75. 10.1186/1748-5908-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Wang H, Xing Y, et al. Dose–response relationship between computerized cognitive training and cognitive improvement. npj Digit Med. 2024;7:214. 10.1038/s41746-024-01210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wartena R, Brandsma D, Belderbos J. Are memantine, methylphenidate and donepezil effective in sparing cognitive functioning after brain irradiation? J Cancer Metastasis Treat. 2018. 10.20517/2394-4722.2018.66. [Google Scholar]
- 20.Gao Y, Liu Y, Li Y. Safety and efficacy of acetylcholinesterase inhibitors for Alzheimer’s disease: a systematic review and meta-analysis. Adv Clin Exp Med. 2024;33:1179. 10.17219/acem/176051. [DOI] [PubMed] [Google Scholar]
- 21.Jackson S, Ham RJ, Wilkinson D. The safety and tolerability of donepezil in patients with Alzheimer’s disease. Br J Clin Pharmacol. 2004;58:1–8. 10.1111/j.1365-2125.2004.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marucci G, Buccioni M, Ben DD, et al. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021;190: 108352. 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 23.Dunn NR, Pearce GL, Shakir SAW. Adverse effects associated with the use of donepezil in general practice in England. J Psychopharmacol (Oxf). 2000;14:406–8. 10.1177/026988110001400410. [DOI] [PubMed] [Google Scholar]
- 24.Moghul S, Wilkinson D. Use of acetylcholinesterase inhibitors in Alzheimer’s disease. Expert Rev Neurother. 2001;1:61–9. 10.1586/14737175.1.1.61. [DOI] [PubMed] [Google Scholar]
- 25.Shigeta M, Homma A. Donepezil for Alzheimer’s disease: pharmacodynamic, pharmacokinetic, and clinical profiles. CNS Drug Rev. 2001;7:353–68. 10.1111/j.1527-3458.2001.tb00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tariot PN, Braeckman R, Oh C. Comparison of steady-state pharmacokinetics of donepezil transdermal delivery system with oral donepezil. J Alzheimers Dis. 2022;90:161–72. 10.3233/JAD-220530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang WH, Park YH, Ohn SH, et al. Neural correlates of donepezil-induced cognitive improvement in patients with right hemisphere stroke: a pilot study. Neuropsychol Rehabil. 2011;21:502–14. 10.1080/09602011.2011.582708. [DOI] [PubMed] [Google Scholar]
- 28.Miller AL, Evanson NK, Taylor JM. Use of donepezil for neurocognitive recovery after brain injury in adult and pediatric populations: a scoping review. Neural Regen Res. 2024;19:1685–95. 10.4103/1673-5374.389628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jelic V, Darreh-Shori T. Donepezil: a review of pharmacological characteristics and role in the management of Alzheimer disease. Clin Med Insights Ther. 2010. 10.4137/CMT.S5410. [Google Scholar]
- 30.Von Ah D, Jansen CE, Allen DH. Evidence-based interventions for cancer- and treatment-related cognitive impairment. Clin J Oncol Nurs. 2014;18:17–25. 10.1188/14.CJON.S3.17-25. [DOI] [PubMed] [Google Scholar]
- 31.Karschnia P, Parsons MW, Dietrich J. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol. 2019;20:e92–102. 10.1016/S1470-2045(18)30938-0. [DOI] [PubMed] [Google Scholar]
- 32.Winocur G, Binns MA, Tannock I. Donepezil reduces cognitive impairment associated with anti-cancer drugs in a mouse model. Neuropharmacology. 2011;61:1222–8. 10.1016/j.neuropharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Lim I, Joung HY, Yu AR, et al. PET evidence of the effect of donepezil on cognitive performance in an animal model of chemobrain. BioMed Res Int. 2016. 10.1155/2016/6945415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ongnok B, Khuanjing T, Chunchai T, et al. Donepezil protects against doxorubicin-induced chemobrain in rats via attenuation of inflammation and oxidative stress without interfering with doxorubicin efficacy. Neurotherapeutics. 2021;18:2107–25. 10.1007/s13311-023-01347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruera E, Strasser F, Shen L, et al. The effect of donepezil on sedation and other symptoms in patients receiving opioids for cancer pain: a pilot study. J Pain Symptom Manag. 2003;26:1049–54. 10.1016/s0885-3924(03)00332-4. [DOI] [PubMed] [Google Scholar]
- 36.Shaw EG, Rosdhal R, D’Agostino RB Jr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–20. 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 37.Castellino SM, Tooze JA, Flowers L, et al. Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study. Pediatr Blood Cancer. 2012;59:540–7. 10.1002/pbc.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correa DD, Kryza-Lacombe M, Baser RE, et al. Cognitive effects of donepezil therapy in patients with brain tumors: a pilot study. J Neurooncol. 2016;127:313–9. 10.1007/s11060-015-2035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33:1653–9. 10.1200/JCO.2014.58.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naughton MJ, Douglas Case L, Peiffer A, et al. Quality of life of irradiated brain tumor survivors treated with donepezil or placebo: results of the WFU CCOP research base protocol 91105. Neuro-Oncol Pract. 2018;5:114–21. 10.1093/nop/npx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv Res Pract. 2016;10:176–84. 10.1007/s11764-015-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25:3475–81. 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 43.Rapp SR, Dressler EV, Brown WM, et al. Phase III randomized, placebo-controlled clinical trial of donepezil for treatment of cognitive impairment in breast cancer survivors after adjuvant chemotherapy (WF-97116). J Clin Oncol Off J Am Soc Clin Oncol. 2024. 10.1200/JCO.23.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseini M-S, Jahanshahlou F, Akbarzadeh MA, et al. Formulating research questions for evidence-based studies. J Med Surg Public Health. 2024;2: 100046. 10.1016/j.glmedi.2023.100046. [Google Scholar]
- 46.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12-13 (PMID: 7582737). [PubMed] [Google Scholar]
- 47.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis campbell systematic reviews. Campbell Syst Rev. 2022;18: e1230. 10.1002/cl2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test—revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. 10.1076/clin.12.1.43.1726. [Google Scholar]
- 50.Castellino SM, Donepezil in treating young patients with primary brain tumors previously treated with radiation therapy to the brain, NCT00452868, Updated August 8, 2018. https://clinicaltrials.gov/study/NCT00452868.
- 51.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 53.R Core Team (2024). _R: a language and environment for statistical computing_. R Foundation for Statistical Computing, Vienna, Austria. <https://www.R-project.org/>.
- 54.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
- 55.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 56.Lin L, Chu H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology. 2020;31:713–7. 10.1097/EDE.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McHugh ML. The chi-square test of independence. Biochem Med. 2013. 10.11613/BM.2013.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lau J, Ioannidis JPA, Terrin N, et al. The case of the misleading funnel plot. BMJ. 2006;333:597–600. 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59._ Efficacy and safety of donepezil hydrochloride in preadolescent and adolescent children with attention impairment following cancer treatment, NCT00688376, Updated December 14, 2021, https://clinicaltrials.gov/study/NCT00688376
- 60.Belkonen S. Hopkins verbal learning test. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. New York: Springer; 2011. 10.1007/978-0-387-79948-3_1127. [Google Scholar]
- 61.Guo Y. A selective review of the ability for variants of the trail making test to assess cognitive impairment. Appl Neuropsychol Adult. 2022;29:1634–45. 10.1080/23279095.2021.1887870. [DOI] [PubMed] [Google Scholar]
- 62.Bauer K, Malek-Ahmadi M. Meta-analysis of controlled oral word association test (COWAT) FAS performance in amnestic mild cognitive impairment and cognitively unimpaired older adults. Appl Neuropsychol Adult. 2023;30:424–30. 10.1080/23279095.2021.1952590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malek-Ahmadi M, Small BJ, Raj A. The diagnostic value of controlled oral word association test-FAS and category fluency in single-domain amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2011;32:235–40. 10.1159/000334525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terwee CB, Elsman EB, Roorda LD. Towards standardization of fatigue measurement: psychometric properties and reference values of the PROMIS Fatigue item bank in the dutch general population. Res Methods Med Health Sci. 2022;3:86–98. 10.1177/26320843221089628. [Google Scholar]
- 65.Montan I, Löwe B, Cella D, et al. General population norms for the functional assessment of chronic illness therapy (FACIT)-fatigue scale. Value Health. 2018;21:1313–21. 10.1016/j.jval.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the functional assessment of cancer therapy (FACT) measurement system. J Pain Symptom Manag. 1997;13:63–74. 10.1016/S0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 67.Ameringer S, Elswick RK, Menzies V, et al. Psychometric evaluation of the patient-reported outcomes measurement information system fatigue-short form across diverse populations. Nurs Res. 2016;65:279–89. 10.1097/NNR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li D-D, Zhang Y-H, Zhang W, Zhao P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front Neurosci. 2019;13:472. 10.3389/fnins.2019.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baba T, Takeda A, Murakami A, et al. Effect of donepezil for dementia prevention in Parkinson’s disease with severe hyposmia (The DASH-PD study): a randomized long-term placebo-controlled trial. eClinicalMedicine. 2022;51:101571. 10.1016/j.eclinm.2022.101571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Lian S, Zhang Y, Zhao Q. Efficacy and safety of donepezil for mild cognitive impairment: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2022;213: 107134. 10.1016/j.clineuro.2022.107134. [DOI] [PubMed] [Google Scholar]
- 71.Risacher SL, Wang Y, Wishart HA, et al. Cholinergic enhancement of brain activation in mild cognitive impairment during episodic memory encoding. Front Psychiatry. 2013;4:105. 10.3389/fpsyt.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–65. 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 73.Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47:324–34. 10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 74.Parsons MW, Dietrich J. Assessment and management of cognitive symptoms in patients with brain tumors. Am Soc Clin Oncol Educ Book. 2021;41:E90–9. 10.1200/EDBK_320813. [DOI] [PubMed] [Google Scholar]
- 75.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–75. 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 76.Prust MJ, Jafari-Khouzani K, Kalpathy-Cramer J, et al. Standard chemoradiation for glioblastoma results in progressive brain volume loss. Neurology. 2015;85:683–91. 10.1212/WNL.0000000000001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zer A, Pond GR, Razak ARA, et al. Association of neurocognitive deficits with radiotherapy or chemoradiotherapy for patients with head and neck cancer. JAMA Otolaryngol Neck Surg. 2017. 10.1001/jamaoto.2017.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Voon NS, Abdul Manan H, Yahya N. Cognitive decline following radiotherapy of head and neck cancer: systematic review and meta-analysis of MRI correlates. Cancers. 2021;13:6191. 10.3390/cancers13246191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernstein LJ, McCreath GA, Komeylian Z, Rich JB. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis. Neurosci Biobehav Rev. 2017;83:417–28. 10.1016/j.neubiorev.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 80.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–40. 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oppegaard KR, Mayo SJ, Armstrong TS, et al. An evaluation of the multifactorial model of cancer-related cognitive impairment. Nurs Res. 2023. 10.1097/NNR.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayo SJ, Edelstein K, Atenafu EG, et al. Cognitive symptoms across diverse cancers. JAMA Netw Open. 2024;7: e2430833. 10.1001/jamanetworkopen.2024.30833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oerlemans S, Schagen SB, Van Den Hurk CJ, et al. Self-perceived cognitive functioning and quality of life among cancer survivors: results from the PROFILES registry. J Cancer Surviv. 2022;16:303–13. 10.1007/s11764-021-01023-9. [DOI] [PubMed] [Google Scholar]
- 84.Pellegrini C, D’Antongiovanni V, Fornai M, et al. Donepezil improves vascular function in a mouse model of Alzheimer’s disease. Pharmacol Res Perspect. 2021;9: e00871. 10.1002/prp2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo HB, Cheng YF, Wu JG, et al. Donepezil improves learning and memory deficits in APP/PS1 mice by inhibition of microglial activation. Neuroscience. 2015;290:530–42. 10.1016/j.neuroscience.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 86.Su D, Zhao Y, Wang B, et al. Isoflurane-induced spatial memory impairment in mice is prevented by the acetylcholinesterase inhibitor donepezil. PLoS ONE. 2011;6: e27632. 10.1371/journal.pone.0027632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Varsaldi F, Miglio G, Scordo MG, et al. Impact of the CYP2D6 polymorphism on steady-state plasma concentrations and clinical outcome of donepezil in Alzheimer’s disease patients. Eur J Clin Pharmacol. 2006;62:721–6. 10.1007/s00228-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 88.Kagawa Y, Yamamoto Y, Ueno A, et al. Impact of CYP2D6, CYP3A5, and ABCB1 polymorphisms on plasma concentrations of donepezil and its metabolite in patients with Alzheimer disease. Ther Drug Monit. 2021;43:429–35. 10.1097/FTD.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 89.Yaowaluk T, Senanarong V, Limwongse C, et al. Influence of CYP2D6, CYP3A5, ABCB1, APOE polymorphisms and nongenetic factors on donepezil treatment in patients with Alzheimer’s disease and vascular dementia. Pharmacogenom Pers Med. 2019;12:209–24. 10.2147/PGPM.S211259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glare P, Aubrey K, Gulati A, et al. Pharmacologic management of persistent pain in cancer survivors. Drugs. 2022;82:275–91. 10.1007/s40265-022-01675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang C, Deng L, Karr MA, et al. Chronic comorbid conditions among adult cancer survivors in the United States: results from the National Health Interview Survey, 2002–2018. Cancer. 2022;128:828–38. 10.1002/cncr.33981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palmer ACS, Zortea M, Souza A, et al. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: a randomized, double-blind, placebo-controlled trial. PLoS ONE. 2020;15: e0231379. 10.1371/journal.pone.0231379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakamura ZM, Deal AM, Park EM, et al. A phase II single-arm trial of memantine for prevention of cognitive decline during chemotherapy in patients with early breast cancer: feasibility, tolerability, acceptability, and preliminary effects. Cancer Med. 2023;12:8172–83. 10.1002/cam4.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gong S, Sheng P, Jin H, et al. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLoS ONE. 2014;9: e84391. 10.1371/journal.pone.0084391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lv L, Mao S, Dong H, et al. Pathogenesis, assessments, and management of chemotherapy-related cognitive impairment (CRCI): an updated literature review. J Oncol. 2020;2020:1–11. 10.1155/2020/3942439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryan J, Woods RL, Murray AM, et al. Normative performance of older individuals on the Hopkins Verbal Learning Test-Revised (HVLT-R) according to ethno-racial group, gender, age and education level. Clin Neuropsychol. 2021;35:1174–90. 10.1080/13854046.2020.1730444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang J, Chan SC, Ngai CH, et al. Global incidence, mortality and temporal trends of cancer in children: a joinpoint regression analysis. Cancer Med. 2023;12:1903–11. 10.1002/cam4.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 99.Lupo PJ, Spector LG. Cancer progress and priorities: childhood cancer. Cancer Epidemiol Biomarkers Prev. 2020;29:1081–94. 10.1158/1055-9965.EPI-19-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the childhood cancer survivor study. Neuropsychology. 2009;23:705–17. 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma S, Brunet J. Young adults’ lived experiences with cancer-related cognitive impairment: an exploratory qualitative study. Curr Oncol. 2023;30:5593–614. 10.3390/curroncol30060422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kerckhove N, Tougeron D, Lepage C, et al. Efficacy of donepezil for the treatment of oxaliplatin-induced peripheral neuropathy: DONEPEZOX, a protocol of a proof of concept, randomised, triple-blinded and multicentre trial. BMC Cancer. 2022;22:742. 10.1186/s12885-022-09806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–70. 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 104.Kawashiri T, Shimizu S, Shigematsu N, et al. Donepezil ameliorates oxaliplatin-induced peripheral neuropathy via a neuroprotective effect. J Pharmacol Sci. 2019;140:291–4. 10.1016/j.jphs.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 105.Ferrier J, Bayet-Robert M, Dalmann R, et al. Cholinergic neurotransmission in the posterior insular cortex is altered in preclinical models of neuropathic pain: key role of muscarinic M2 receptors in donepezil-induced antinociception. J Neurosci. 2015;35:16418–30. 10.1523/JNEUROSCI.1537-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henneghan AM, Van Dyk K, Kaufmann T, et al. Measuring self-reported cancer-related cognitive impairment: recommendations from the cancer neuroscience initiative working group. JNCI J Natl Cancer Inst. 2021;113:1625–33. 10.1093/jnci/djab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao C, Rich JB, Tirona K, Bernstein LJ. Intraindividual variability in reaction time before and after neoadjuvant chemotherapy in women diagnosed with breast cancer. Psychooncology. 2017;26:2261–8. 10.1002/pon.4351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.