Abstract
Abstract
Ergothioneine (EGT) is a rare amino acid with potent antioxidant and anti-inflammatory properties, with a wide range of applications in food, cosmetics, and medicine. In the present study, Aspergillus oryzae, a common edible fungus, was engineered as an optimal host for EGT production. Moreover, two endogenous genes involved in EGT biosynthesis were characterized. The homolog AoEgt1 was shown to be localized in the vacuoles, whereas the homolog AoEgt2 was found in the peroxisomes. Overexpression of EGT biosynthetic genes from different organisms enhanced EGT production, yielding 15.17 mg EGT/g of dry weight. Using glucose as the carbon source and supplementing methionine (Met) as a precursor further increased EGT production to 20.03 mg EGT/g of dry weight, constituting an eight-fold increase compared to the wild-type strain. This study discusses the successful construction of a high-yielding A. oryzae strain for EGT biosynthesis, providing a novel strategy for efficient EGT synthesis.
Key points
• Two newly described homologs, AoEgt1 and AoEgt2, were identified in A. oryzae.
• AoEgt1 and AoEgt2 were found to contribute to EGT biosynthesis.
• EGT production was significantly increased by overexpression of Egt1 and Egt2.
• Glucose and Met supplementation in the medium increased EGT production.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00253-025-13505-2.
Keywords: Ergothioneine, Aspergillus oryzae, Neurospora crassa, Filamentous fungus, Agrobacterium transformation
Introduction
Ergothioneine (EGT) is a sulfur-containing amino acid derivative with unique antioxidant properties, and was first identified in Claviceps purpurea in 1909 (Han et al. 2021). Most plants and animals, including humans, cannot synthesize EGT, being mainly obtained from the diet (Pochini et al. 2019; Tschirka et al. 2018) and accumulates in metabolically active tissues, such as the liver, kidneys, and intestines (Tang et al. 2018). Through specific transporters, EGT can be delivered to sites of oxidative damage, reducing cellular oxidative stress (Deiana et al. 2004; Halliwell et al. 2018). In recent years, EGT has been classified as a “longevity vitamin” (Wijesekara and Xu 2024), highlighting its significance in human physiology. Previous studies have suggested its potential as a therapeutic agent for neurodegenerative disorders, demonstrating its ability to enhance hippocampal neurogenesis and mitigate age-related brain damage (Katsube et al. 2024; Nakamichi et al. 2016). Additionally, EGT exhibits protective effects against chronic diseases, such as cardiovascular disease and diabetes (Wu et al. 2021). In 2017, EGT has been recognized as a “Generally Recognized As Safe (GRAS)” substance (EFSA Panel on Dietetic Products, Nutrition and Allergies et al. 2017) and has become a multifunctional ingredient in nutraceuticals and cosmeceuticals. Its anti-aging properties, particularly in skin barrier repair and collagen synthesis, have also gained attention in recent years (Hseu et al. 2020; Liu et al. 2023; Tsay et al. 2021). However, despite its broad applications, microbial production of EGT remains inefficient (Takusagawa et al. 2019; Zhu et al. 2022).
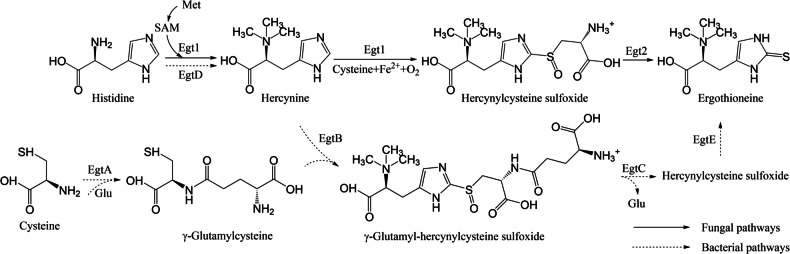
EGT is usually synthesized by bacteria and fungi via two different biosynthetic pathways (Fig. 1). The bacterial pathway involves the EGT biosynthetic genes EgtABCDE, which were first identified in Mycobacterium smegmatis (Seebeck 2010) and later observed in Methylobacterium (Alamgir et al. 2015) and Streptomyces species (Nakajima et al. 2015). The fungal pathway was first reported in Neurospora crassa (Bello et al. 2012; Hu et al. 2014) and differs from the bacterial pathway by requiring only two enzymes, bypassing γ-glutamylcysteine and avoiding competition with glutathione synthesis (Naowarojna et al. 2019). This simplified pathway makes fungi promising candidates for EGT biomanufacturing.
Fig. 1.
Two different biosynthetic pathways of ergothioneine (EGT). The EGT biosynthesis in N. crassa requires only two main enzymes (Egt1 and Egt2) (Bello et al. 2012; Hu et al. 2014); however, in Mycobacterium smegmatis, a gene cluster EgtABCDE is responsible for the biosynthesis of EGT (Seebeck 2010). Met, methionine; SAM, S-adenosylmethionine; Glu, glutamic acid
Several organisms have been engineered for EGT biosynthesis (Kamide et al. 2020; Kim et al. 2022). In bacterial systems, Escherichia coli has achieved EGT yields of 1.31 g EGT/L in liquid culture medium by enhancing cysteine (Cys) and S-adenosylmethionine (SAM) biosynthesis (Tanaka et al. 2019). In contrast, while certain fungi may contain up to 7 mg EGT/g of dry weight (Kalaras et al. 2017), the highest reported yield of Panus conchatus was only 148.79 mg EGT/L in liquid medium supplemented with histidine (His), methionine (Met), and Cys (Zhu et al. 2022). These findings highlight the challenge of obtaining high-yield EGT-producing fungal strains, underscoring the need for alternative strategies and more efficient hosts.
Aspergillus oryzae has emerged as an ideal host owing to its robust biosynthetic capacity and biological safety (Feng et al. 2021; Sun et al. 2024; Xiao et al. 2025). Co-expression of N. crassa Egt1 (NcEgt1) and Egt2 (NcEgt2) in A. oryzae previously yielded 231 mg EGT/kg of solid medium (Takusagawa et al. 2019). While the wild-type A. oryzae produces low EGT levels, recent bioinformatic analyses identified the homologs Egt1 and Egt2 in A. oryzae, confirming the presence of endogenous EGT biosynthetic genes in this species (Maini Rekdal et al. 2024).
In the present study, we constructed engineered A. oryzae strains containing both its endogenous genes and heterologous genes from N. crassa and Cordyceps militaris to enhance EGT production. Moreover, we optimized culture medium composition for a record EGT yield. These findings expand the current understanding about the potential of A. oryzae as a production platform and provide a valuable engineering strategy for improving EGT biosynthesis.
Materials and methods
Phylogenetic analysis
BLAST analyses of NcEgt1 (XP_956324.3) and NcEgt2 (XP_001728131.1) sequences were performed using NCBI. Phylogenetic tree construction was performed using MEGA 11.0 (Mega Limited, Auckland, New Zealand). The species included in the analysis are listed in Supplementary Table S1.
Microbial strains and culture media
Microbial strains in this study are listed in Table 1. E. coli DH5α (Tsingke Biotech, Beijing, China) and Agrobacterium tumefaciens AGL1 (TransGen Biotech, Beijing, China) were cultivated in Luria Bertani (LB) medium (0.1% tryptone, 0.5% yeast extract, 0.1% NaCl) supplemented with 50 µg/mL kanamycin (for E. coli) and 20 µg/mL rifampicin (for A. tumefaciens), followed by incubation at 37 °C and 30 °C, respectively. A. oryzae RIB40 and AoΔhisBΔpyrG were used as the control strain and background strain, respectively.
Table 1.
Strains used in this study
| Names | Descriptions | Sources |
|---|---|---|
| AoRIB40 | A wild-type strain of A. oryzae | Machida et al. (2005) |
| AoΔhisBΔpyrG | The dual histidine-uridine/uracil auxotrophic strain (AoΔhisBΔpyrG) constructed from AoΔpyrG | Thai et al. (2021) |
| pEX1H-GFP/AoΔhisBΔpyrG | AoΔhisBΔpyrG transformed with pEX1H-GFP | This study |
| pEX2B-Mcherry/AoΔhisBΔpyrG | AoΔhisBΔpyrG transformed with pEX2B-Mcherry | This study |
| pEX1H-AoEgt1-GFP/AoΔhisBΔpyrG | Random integration of AoEgt1 overexpression box from pEX1H-AoEgt1 in AoΔhisBΔpyrG | This study |
| pEX2B-AoEgt1-Mcherry/AoΔhisBΔpyrG | Random integration of AoEgt1 overexpression box from pEX2B-AoEgt1 in AoΔhisBΔpyrG | This study |
| pEX2B-AoEgt2-Mcherry/AoΔhisBΔpyrG | Random integration of AoEgt2 overexpression box from pEX2B-AoEgt2 in AoΔhisBΔpyrG | This study |
| pEX1H-AoEgt1-GFP-pEX2B-AoEgt2-Mcherry/AoΔhisBΔpyrG | Random integration of AoEgt1 and AoEgt2 overexpression boxes in AoΔhisBΔpyrG | This study |
| pEX1H-NcEgt1-GFP/AoΔhisBΔpyrG | Random integration of NcEgt1 overexpression box from pEX1H-NcEgt1 in AoΔhisBΔpyrG | This study |
| pEX2B-NcEgt1-Mcherry/AoΔhisBΔpyrG | Random integration of NcEgt1 overexpression box from pEX2B-NcEgt1 in AoΔhisBΔpyrG | This study |
| pEX2B-NcEgt2-Mcherry/AoΔhisBΔpyrG | Random integration of NcEgt2 overexpression box from pEX2B-NcEgt2 in AoΔhisBΔpyrG | This study |
| pEX1H-NcEgt1-GFP-pEX2B-NcEgt2-Mcherry/AoΔhisBΔpyrG | Random integration of NcEgt1 and NcEgt2 overexpression boxes in AoΔhisBΔpyrG | This study |
| pEX1H-NcEgt1-GFP-pEX2B-AoEgt2-Mcherry/AoΔhisBΔpyrG | Random integration of NcEgt1 and AoEgt2 overexpression boxes in AoΔhisBΔpyrG | This study |
| pEX1H-NcEgt1-GFP-pEX2B-CmEgt2-Mcherry/AoΔhisBΔpyrG | Random integration of NcEgt1 and CmEgt2 overexpression boxes in AoΔhisBΔpyrG | This study |
| pEX1H-AoVam3-GFP/AoΔhisBΔpyrG | Random integration of AoVam3 overexpression box from pEX1H-AoVam3 in AoΔhisBΔpyrG | This study |
| pEX1H-GFP-PTS1/AoΔhisBΔpyrG | Random integration of GFP-PTS1 overexpression box from pEX1H-GFP-PTS1 in AoΔhisBΔpyrG | This study |
A. tumefaciens-mediated transformation (ATMT) method was performed on induction medium (IM) (0.205% K2HPO4, 0.145% KH2PO4, 0.015% NaCl, 0.05% NH4SO4, 0.5% glycerol, 0.2% liquid/0.1% solid maltose, ddH2O to 90 mL, pH 5.8). Transformants were screened on Czapek-Dox (CD) medium (2% sucrose, 0.2% NaNO3, 0.1% KH2PO4, 0.05% MgSO4, 0.05% KCl, 0.05% NaCl, 0.002% FeSO4, pH 5.5). Dextrose peptone yeast extract (DPY) medium (2% sucrose, 1% peptone, 0.5% yeast extract, 0.5% KH2PO4, 0.05% MgSO4, pH 5.5) was supplemented with 0.1% uridine, 0.1% uracil, or 0.1% histidine for auxotrophic strain cultivation.
Construction of recombinant A. oryzae strains
DNA extraction was carried out using the fungal DNA midi kit (Beyotime Institute of Biotechnology, Nantong, China). Target genes AoEgt1 (XM_001727257) and AoEgt2 (XM_001821716) were amplified using the KOD FX enzyme (Toyobo, Shanghai, China) with A. oryzae genomic DNA as the template. The amplified fragments were ligated into the linearized pEX1H (Nguyen et al. 2016) or pEX2B (Nguyen et al. 2017) vectors using a one-step cloning kit (Vazyme, Nanjing, China), and subsequently transformed into E. coli DH5α for cloning and sequencing. The resulting plasmids pEX1H-AoEgt1-GFP and pEX2B-AoEgt2-Mcherry, were then transformed into A. tumefaciens AGL1 and further introduced into AoΔhisBΔpyrG using the ATMT method (Sun et al. 2019a, b). Transformants were selected on CD medium for further analysis. To generate dual-gene overexpression strains, single-gene transformants (AoEgt1 or AoEgt2) were used as background strains for the introduction of the second gene.
N. crassa genes NcEgt1 and NcEgt2 were chemically synthesized (Tongyong Biotech, Anhui, China), while the CmEgt2 gene (XM_006666800) was amplified via PCR using C. militaris genomic DNA. The resulting plasmids, i.e., pEX1H-NcEgt1-GFP, pEX2B-NcEgt2-Mcherry, and pEX2B-CmEgt2-Mcherry, were transformed into A. tumefaciens AGL1 and subsequently transformed into AoΔhisBΔpyrG as described above. DNA sequences of these genes are listed in Supplementary Information, while primer sequences are provided in Supplementary Table S2. Vector linearization and gene amplification results are shown in Supplementary Fig. S1.
Subcellular localization analysis
A. oryzae genes AoEgt1 and AoEgt2 were tagged at the 3′ ends with the sequences for Mcherry via pEX2B vectors (pEX2B-AoEgt1-Mcherry and pEX2B-AoEgt2-Mcherry), and transformed into AoΔhisBΔpyrG. The empty pEX2B vector was used as a control. The A. oryzae vacuolar protein AoVam3 (Shoji et al. 2006) was fused to the N-terminus of GFP (pEX1H-AoVam3-GFP), while the peroxisome targeting signal PTS1 (C-terminal tripeptide Ser-Arg-Leu) was fused to the C-terminus of GFP, generating pEX1H-GFP-PTS1 (Huang et al. 2022; Sun et al. 2019a, b). For co-localization analysis, AoVam3-GFP or GFP-PTS1 marker strains were first generated in AoΔhisBΔpyrG and then sequentially transformed with Mcherry-tagged AoEgt1 or AoEgt2. Fluorescence microscopy was performed using Leica DM6000 (Leica Microsystems, Wetzlar, Germany) for observation and imaging of samples.
Culture conditions
Strains were inoculated onto DPY solid medium and incubated at 30 °C for 5 days. Carbon source utilization was assessed in DPY medium supplemented with a single sugar 2% (w/v) solution, specifically sucrose, glucose, maltose, fructose, or glycerol. Amino acid supplementation was evaluated by supplementation with a single amino acid 0.1% (w/v) solution, specifically His, Cys, and Met. Control cultures were maintained in non-supplemented DPY medium. Optimization of Met concentration was conducted across a gradient from 0 to 1.0%.
Gene expression analysis
RNA extraction was performed using a fungal RNA kit (Jianshi Biotech, Beijing, China). cDNA was synthesized from 1 µg of total RNA using the HiScript III RT SuperMix for qPCR kit (Vazyme, Nanjing, China) (Supplementary Tables S3 to S4). qRT-PCR was performed using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) with SuperStar Blue Universal SYBR Master Mix (CWBIO, Taizhou, China) (Supplementary Tables S5 to S6). The housekeeping gene encoding histone H4 was used as the normalization control (Maruyama et al. 2002), and relative expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). Copy number detection was performed using genomic DNA following the same protocol described above. Primers for qRT-PCR are shown in Supplementary Table S7.
Detection and quantification of EGT
A. oryzae mycelia were freeze-dried under vacuum and ground into powder. EGT extraction was performed by suspending 100 mg of the sample in 5 mL of 70% ethanol (v/v), followed by incubation at 50 °C for 30 min. Extracts were centrifuged at 5000 rpm for 5 min, and the supernatant was filtered for high performance liquid chromatography (HPLC) analysis.
HPLC analysis was performed using a Waters Alliance e26952489 UV–visible detector system (Waters, Milford, CT, USA) equipped with a SymmetryShield™ RP18 column (4.6 × 250 mm, 5 µm; Waters, Wexford, Ireland). The UV detector was set at 257 nm. An aliquot of the sample (10 µL) was separated using a water/acetonitrile (95:5, v/v) mobile phase at a flow rate of 0.5 mL/min and a column temperature of 30 °C. A calibration curve was generated using an EGT standard (Aladdin, Shanghai, China).
Statistical analysis
Statistical analyses were performed using GraphPad Prime 8.0 (Dotmatics, San Diego, CA, USA). One-way ANOVA was used for comparisons among three or more groups. Data are presented as mean ± standard deviation. Software used for data analysis is listed in Table 2.
Table 2.
The relevant software used in this study
| Analysis software | Application | Web site |
|---|---|---|
| NCBI-Blast | Gene homology analysis | https://blast.ncbi.nlm.nih.gov/Blast.cgi |
| SignalP 4.1 | Prediction of signal peptide | https://services.healthtech.dtu.dk/service.php?SignalP-4.1 |
| PSORT II | Subcellular localization | https://www.genscript.com/psort.html |
| MEGA 11.0 | Phylogenetic analysis | https://www.megasoftware.net/ |
| GraphPad Prime 8.0 | Statistical analysis | https://www.graphpad.com |
| ChemDraw 14.0 | Chemical structure drawing | https://www.chemdraw.com.cn/ |
Results
Identification of EGT biosynthetic genes in A. oryzae
A BLASTP search using NcEgt1 or NcEgt2 as query sequences identified two evolutionarily conserved homologs in A. oryzae, designated AoEgt1 and AoEgt2. AoEgt1 was shown to contain a 5-histidine cysteine thiosynthase domain and a C-terminal methyltransferase domain, suggesting its potential involvement in two enzymatic steps of the EGT biosynthetic pathway. AoEgt2 was shown to harbor a pyridoxal phosphate (PLP)-dependent cysteine desulfurase domain. These findings indicate that, similar to N. crassa, A. oryzae possesses two key enzymes participating in EGT biosynthesis.
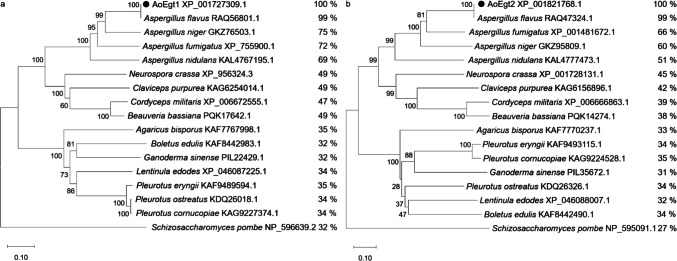
A phylogenetic tree was constructed based on AoEgt1 and AoEgt2 homologs (Fig. 2). AoEgt1 exhibited 69–99% sequence identity among Aspergillus species and 32–49% identity among other ascomycetous species. Additionally, it shared 49% sequence identity with NcEgt1 and clustered into two clades. A distinct clade composed of Boletus edulis, Ganoderma sinense, Lentinula edodes, and other edible basidiomycetes exhibited significantly lower sequence identities (32–35%). Schizosaccharomyces pombe formed a separate clade and was the most distantly related to A. oryzae (32% sequence identity). Similarly, AoEgt2 exhibited 99% sequence identity with Aspergillus flavus, while non-Aspergillus species showed less than 45% identity, underscoring the evolutionary conservation of these enzymes within the Aspergillus genus.
Fig. 2.
Phylogenetic tree analysis of Egt1 (a) and Egt2 (b). The two A. oryzae enzymes are marked by a black dot. The numbers on the right of the figures represent the results of sequence comparison results in % identity between A. oryzae and other fungi
Subcellular localization of EGT biosynthetic enzymes
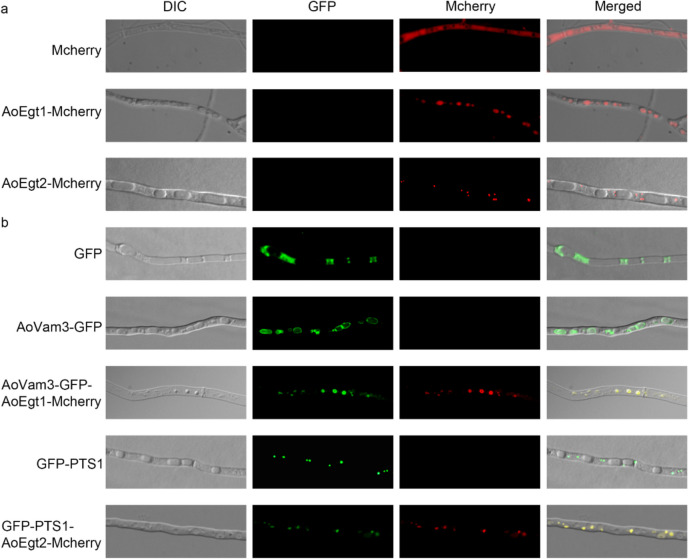
Bioinformatics analysis revealed no specific targeting signal sequence in the amino acid sequences of AoEgt1 and AoEgt2 (Supplementary Fig. S2). To determine their subcellular localization in A. oryzae, GFP and Mcherry were employed as reporter proteins. Fluorescence microscopy revealed that Mcherry-tagged AoEgt1 and AoEgt2 appeared as punctate structures of varying sizes (Fig. 3a). Based on reported organelle distribution and morphology in A. oryzae (Huang et al. 2022; Shoji et al. 2006; Sun et al. 2019a, b), these structures were hypothesized to be vacuoles or peroxisomes. To verify this, AoEgt1-Mcherry was co-expressed with the vacuolar marker AoVam3-GFP, while AoEgt2-Mcherry was co-expressed with the peroxisomal marker GFP-PTS1. As shown in Fig. 3b, the Mcherry fluorescence was found colocalized with AoVam3-GFP or GFP-PTS1 fluorescence, confirming that AoEgt1 is localized in vacuoles and AoEgt2 in peroxisomes.
Fig. 3.
Subcellular localization of AoEgt1 and AoEgt2 in A. oryzae. a Fluorescence observation of transformants under 40-fold microscope. From top to bottom: pEX2B-Mcherry/AoΔhisBΔpyrG, AoEgt1/AoΔhisBΔpyrG, and AoEgt2/AoΔhisBΔpyrG. b From top to bottom: The mycelium of AoΔhisBΔpyrG transformed with pEX1H-GFP, AoVam3-GFP, respectively; transformants of AoVam3-GFP co-transformed with AoEgt1-Mcherry, GFP-PTS1/AoΔhisBΔpyrG, and GFP-PTS1 co-transformed with AoEgt2-Mcherry. Left to right: differential interference contrast (DIC); GFP fluorescence image; Mcherry fluorescence image; DIC, GFP, and Mcherry merged image
A similar approach was used to construct expression plasmids pEX2B-NcEgt1-Mcherry, pEX2B-NcEgt2-Mcherry, and pEX2B-CmEgt2-Mcherry. PSORT analysis predicted that these proteins would be localized in the cytoplasm. To further determine their subcellular localization, these recombinant plasmids were co-transformed with pEX1H-GFP, respectively. As shown in Supplementary Fig. S3, the Mcherry fluorescence overlapped with pEX1H-GFP fluorescence, confirming the cytoplasmic localization of NcEgt1, NcEgt2, and CmEgt2.
Effect of Egt1/Egt2 overexpression on EGT biosynthesis
Co-expression of AoEgt1 and AoEgt2 resulted in a four-fold increase in EGT production (10.01 mg EGT/g of dry weight) compared to the wild-type strain (Fig. 4a). HPLC analysis confirmed a retention time of 5–6 min, consistent with the EGT standard (Fig. 4b). qRT-PCR analysis indicated that AoEgt1 expression increased 6.8-fold in the single overexpression strain and 5.7-fold in the co-overexpression strain relative to the control, while AoEgt2 expression increased 2.9-fold in the co-overexpression strain. Consistent with this, the copy number of AoEgt1 increased to 2.97 ± 0.39 and 3.03 ± 0.36 copies in the single overexpression and co-overexpression strains, while AoEgt2 increased to 2.61 ± 0.12 copies in the co-overexpression strain (Supplementary Fig. S4).
Fig. 4.
EGT detection in overexpressed strains. a The EGT contents of the wild-type and overexpressed strains. b HPLC analysis of EGT contents of A. oryzae strains. WT, the wild-type strain; DW, dry weight. Statistical analyses were performed using one-way ANOVA; the asterisk “**” indicates significant difference compared with the control group (P < 0.01)
To enhance EGT production, heterologous expression of NcEgt1 and NcEgt2 in A. oryzae was performed. Expression of NcEgt1 alone yielded 13.31 mg EGT/g of dry weight, a 5.5-fold increase compared to the wild-type strain. However, dual overexpression of NcEgt1 and NcEgt2 resulted in a slightly lower yield (12.22 mg EGT/g of dry weight), suggesting the need for optimized host-gene combinations. To further optimize EGT biosynthesis, the NcEgt1-overexpressing strain was combined with Egt2 homologs from other fungi, such as A. oryzae and C. militaris. Moreover, co-expression of NcEgt1 and CmEgt2 resulted in the highest yield (15.17 mg EGT/g of dry weight). Under liquid culture conditions, the wild-type strain produced 0.08 mg EGT/g of mycelium and 0.46 mg EGT/L of broth (Supplementary Fig. S5). While synthetase overexpression significantly increased EGT content (15-fold in mycelium and 7.5-fold in broth), the overall EGT yield was lower compared to solid medium, indicating that liquid conditions influence EGT biosynthesis in A. oryzae and significantly reduce metabolite production.
Carbon source for EGT production in A. oryzae
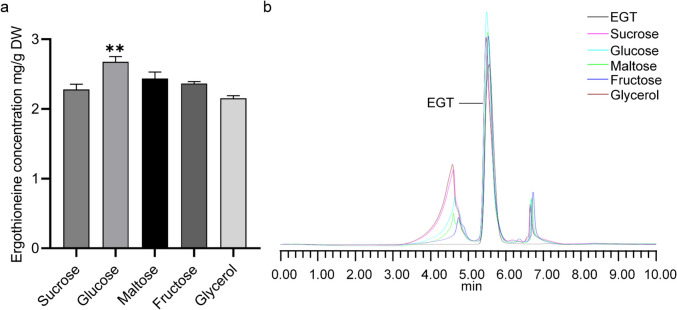
EGT production was evaluated in A. oryzae cultured with different carbon sources, including sucrose, glucose, maltose, fructose, and glycerol (Fig. 5b). As shown in Fig. 5a, glucose was the optimal carbon source for EGT biosynthesis, yielding 2.68 mg EGT/g of dry weight. No significant difference was observed among the other carbon sources, although sucrose and maltose yielded lower EGT levels compared to glucose, likely due to incomplete disaccharide utilization. Thus, glucose was selected for subsequent experiments.
Fig. 5.
The optimal carbon sources for EGT biosynthesis. a Comparison of different carbon sources for EGT biosynthesis. b HPLC analysis of EGT contents of A. oryzae with different carbon sources. DW, dry weight. Statistical analyses were performed using one-way ANOVA; the asterisk “**” indicates significant difference compared with the control group (P < 0.01)
Effect of amino acid supplementation on EGT contents
Since His, Cys, and Met serve as EGT biosynthetic precursors, culture media were supplemented with 0.1% of each amino acid to assess their effects on EGT content in A. oryzae. Precursor supplementation induced differential EGT yield improvements (Fig. 6a). His and Cys supplementation led to minor EGT increases, while Met supplementation significantly enhanced EGT production, achieving a two-fold increase compared to the control.
Fig. 6.
Effects of amino acid supplementation on EGT contents and fungal colony. a Effects of His, Cys, and Met on EGT contents. b Effects of different Met concentrations on EGT contents. c Effects of different Met concentrations on fungal colony. His, histidine; Cys, cysteine; Met, methionine; WT, the wild-type strain; DW, dry weight. Statistical analyses were performed using one-way ANOVA; the asterisk “**” indicates significant difference compared with the control group (P < 0.01)
Moreover, the effect of Met concentrations on EGT production was investigated. As shown in Fig. 6b, the highest EGT yield (5.12 mg EGT/g of dry weight) was observed at 0.8% Met supplementation. Notably, although Met promoted EGT biosynthesis, increasing its concentration inhibited the growth of A. oryzae, likely due to the toxic effects of excess amino acids (Fig. 6c).
Effect of Met supplementation on EGT contents in transgenic A. oryzae
Although EGT production increased in co-expressed strains compared to the parental strain, the increase was only modest (Fig. 4). Given that precursor shortages often limit metabolite production, media composition was optimized to maximize EGT yields. Met was still the best additive for EGT synthesis in overexpressed strains (Supplementary Fig. S6a). Interestingly, at 0.4% Met supplementation, the strain co-expressing NcEgt1-NcEgt2 exhibited higher EGT content compared to strains with inherently greater yields (Supplementary Fig. S6b–d). In contrast, the wild-type strain required 0.8% Met to achieve optimal productivity, thereby reducing precursor costs.
These findings demonstrate that the optimized treatment led to an impressive EGT production of up to 20.03 mg EGT/g of dry weight, an eight-fold increase compared to the wild-type strain (2.45 mg EGT/g of dry weight, Fig. 7). These results highlight the critical role of Met in flux regulation within this biosynthetic pathway and suggest a viable strategy for engineering strains with higher yields at reduced production costs.
Fig. 7.
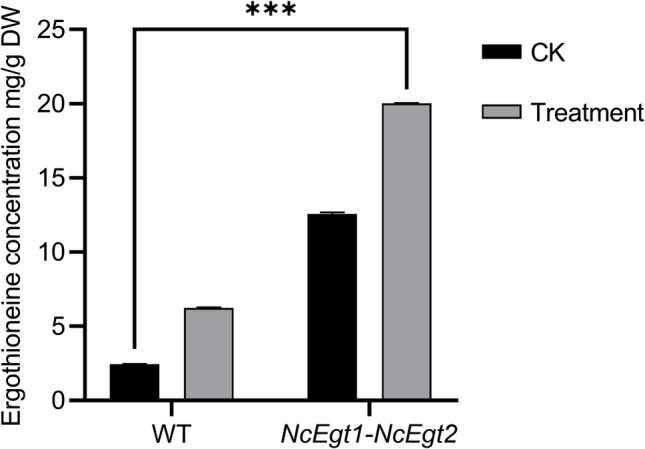
EGT contents in DPY medium supplemented with 0.4% Met (treatment) and based on DPY medium (CK). WT, the wild-type strain; DW, dry weight. Statistical analyses were performed using one-way ANOVA; the asterisk “***” indicates significant difference compared with the control group (P < 0.001)
Discussion
EGT is a stable antioxidant with the advantages of being natural, safe, and non-toxic compared to conventional alternatives. Currently, biosynthesis is the predominant approach for large-scale EGT production. In the present study, we identified two key enzymes involved in EGT biosynthesis in A. oryzae, designated AoEgt1 and AoEgt2.
To enhance EGT production in A. oryzae, we overexpressed these homologous genes within fungal pathways, including A. oryzae, N. crassa, and C. militaris. A previous study reported that heterologous expression of NcEgt1 promoted EGT biosynthesis in A. oryzae (Takusagawa et al. 2019). Consistently, our findings indicate that both AoEgt1 and NcEgt1 enhanced EGT production in A. oryzae. However, co-expression of NcEgt1 and NcEgt2 led to a slightly decrease in EGT yield, which may be attributed to feedback inhibition caused by the accumulation of hercynine following Egt1 gene integration (Pluskal et al. 2014).
Unlike the bacterial pathway, the fungal biosynthetic pathway is relatively simpler (Bello et al. 2012; Hu et al. 2014). Moreover, NcEgt1 utilizes Cys instead of γ-glutamylcysteine as a substrate, thereby circumventing competition with glutathione synthesis (Naowarojna et al. 2019). Furthermore, the fungal EGT biosynthetic pathway has demonstrated efficacy not only in fungi but also in bacteria. For instance, the transfer of two Trichoderma reesei genes involved in EGT biosynthesis into E. coli resulted in a yield of 4.34 g EGT/L in liquid medium (Chen et al. 2022), whereas an engineered E. coli strain utilizing M. smegmatis genes achieved only 1.31 g EGT/L in liquid medium (Tanaka et al. 2019), underscoring the high efficiency of the fungal pathway.
Amino acid supplementation plays a crucial role in regulating EGT content. Liu et al. (2024) identified Met deficiency as a major limiting factor in EGT biosynthesis. Consistent with this finding, our study demonstrated that, while His and Cys supplementation led to minor EGT increases, Met supplementation significantly enhanced EGT yield, reaching 4.51 mg EGT/g of dry weight, approximately double than that what was observed in the Met-deficient control medium. It is evident that increasing Met availability in the medium enhances EGT production. Specifically, Met facilitates SAM synthesis through its metabolic cycle while simultaneously generating S-adenosylhomocysteine and homocysteine. Homocysteine can be recycled back into both Met and SAM (Monné et al. 2022; Stipanuk 2020). Thereby, insufficient intracellular SAM supply also restricts EGT biosynthesis. To address this limitation, exogenous Met supplementation may serve as a viable strategy; furthermore, genetic engineering strategies to restore metabolic balance within the SAM cycle may potentiate EGT production. The efficacy of this strategy was demonstrated by Xiong et al. (2022).
The evolutionary conservation of EGT biosynthesis has been documented across diverse species (Bello et al. 2012; Maini Rekdal et al. 2024; Takusagawa et al. 2019). A. oryzae has emerged as a promising chassis for genetic engineering, as it not only exhibits native EGT productivity but also serves an ideal platform for the functional characterization of biosynthetic enzymes (Feng et al. 2021; Sun et al. 2024; Xiao et al. 2025). Subcellular localization analysis revealed that AoEgt1 is localized in vacuoles, whereas AoEgt2 is localized in peroxisomes, suggesting their potential involvement in intercellular nutrient transport (Shoji et al. 2006). Peroxisomes are rich in oxidases and catalases, which facilitate the detoxification of hydrogen peroxide and superoxide radicals generated within cells, thereby protecting cells from oxidative damage (Deori et al. 2018; Ganguli et al. 2019; Nordgren and Fransen 2014). Notably, N. crassa Egt homologs were localized in the cytoplasm, suggesting a potential link between cytoplasmic processes and EGT biosynthesis. This cytoplasmic localization may streamline EGT transport while reducing metabolic burden, thereby supporting sustained EGT production levels.
Future research will further explore the EGT biosynthetic pathway and investigate the genetic mechanisms underlying high EGT yields. Such studies could provide theoretical guidance for optimizing EGT biosynthesis, while integrating metabolic network models to regulate and optimize key enzymes expression levels, which could facilitate an efficient microbial cell factory for large-scale EGT production.
This study characterized two genes involved in the EGT biosynthetic pathway and identified the subcellular localization of key EGT biosynthetic enzymes. By integrating metabolic engineering strategies combining Egt homologs overexpression with medium optimization, we achieved an EGT yield of 20.03 mg EGT/g of dry weight under optimized conditions. These findings demonstrate that A. oryzae is a promising chassis for EGT production and provide new insights into its metabolism, thereby contributing to the development of functional fermented foods to meet market demands.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
RG and ZHH designed the manuscript. LHW conducted the study, analyzed the data, and drafted the manuscript. XQT, PHX, and YHD coordinated the research. The final manuscript was reviewed and approved by all authors.
Funding
This study was supported by National Natural Science Foundation of China (NSFC Grant NO. 32260009).
Declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Gao, Email: gaoruineau@163.com.
Zhihong Hu, Email: huzhihong426@163.com.
References
- Alamgir KM, Masuda S, Fujitani Y, Fukuda F, Tani A (2015) Production of ergothioneine by Methylobacterium species. Front Microbiol 6:1185. 10.3389/fmicb.2015.01185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello MH, Barrera-Perez V, Morin D, Epstein L (2012) The Neurospora crassa mutant NcΔEgt-1 identifies an ergothioneine biosynthetic gene and demonstrates that ergothioneine enhances conidial survival and protects against peroxide toxicity during conidial germination. Fungal Genet Biol 49:160–172. 10.1016/j.fgb.2011.12.007 [DOI] [PubMed] [Google Scholar]
- Chen ZH, He YZ, Wu XY, Wang L, Dong ZY, Chen XZ (2022) Toward more efficient ergothioneine production using the fungal ergothioneine biosynthetic pathway. Microb Cell Fact 21:76. 10.1186/s12934-022-01807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana M, Rosa A, Casu V, Piga R, Dessì MA, Aruoma OI (2004) L-Ergothioneine modulates oxidative damage in the kidney and liver of rats in vivo: studies upon the profile of polyunsaturated fatty acids. Clin Nutr 23:183–193. 10.1016/S0261-5614(03)00108-0 [DOI] [PubMed] [Google Scholar]
- Deori NM, Kale A, Maurya PK, Nagotu S (2018) Peroxisomes: role in cellular ageing and age related disorders. Biogerontology 19:303–324. 10.1007/s10522-018-9761-9 [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Turck D, Bresson JL, Burlingame B, Dean T, Fairweather-Tait S, Heinonen M, Hirsch-Ernst KI, Mangelsdorf I, McArdle HJ (2017) Statement on the safety of synthetic L-ergothioneine as a novel food–supplementary dietary exposure and safety assessment for infants and young children, pregnant and breastfeeding women. EFSA J 15:e05060. 10.2903/j.efsa.2017.5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Hauser M, Cox RJ, Skellam E (2021) Engineering Aspergillus oryzae for the heterologous expression of a bacterial modular polyketide synthase. J Fungi 7:1085. 10.3390/jof7121085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli G, Mukherjee U, Sonawane A (2019) Peroxisomes and oxidative stress: their implications in the modulation of cellular immunity during mycobacterial infection. Front Microbiol 10:424017. 10.3389/fmicb.2019.01121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Cheah IK, Tang RMY (2018) Ergothioneine–a diet-derived antioxidant with therapeutic potential. FEBS Lett 592:3357–3366. 10.1002/1873-3468.13123 [DOI] [PubMed] [Google Scholar]
- Han YW, Tang XY, Zhang YT, Hu XC, Ren LJ (2021) The current status of biotechnological production and the application of a novel antioxidant ergothioneine. Crit Rev Biotechnol 41:580–593. 10.1080/07388551.2020.1869692 [DOI] [PubMed] [Google Scholar]
- Hseu YC, Vudhya Gowrisankar Y, Chen XZ, Yang YC, Yang HL (2020) The antiaging activity of ergothioneine in UVA-irradiated human dermal fibroblasts via the inhibition of the AP-1 pathway and the activation of Nrf2-mediated antioxidant genes. Oxid Med Cell Longevity 2020:2576823. 10.1155/2020/2576823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Song H, Sae Her A, Bak DW, Naowarojna N, Elliott SJ, Qin L, Chen XP, Liu PH (2014) Bioinformatic and biochemical characterizations of C-S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway. Org Lett 16:5382–5385. 10.1021/ol502596z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Niu YL, Jin Q, Qin KH, Wang L, Shang YT, Zeng B, Hu ZH (2022) Identification of six thiolases and their effects on fatty acid and ergosterol biosynthesis in Aspergillus oryzae. Appl Environ Microbiol 88:e0237221. 10.1128/aem.02372-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaras MD, Richie JP, Calcagnotto A, Beelman RB (2017) Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chem 233:429–433. 10.1016/j.foodchem.2017.04.109 [DOI] [PubMed] [Google Scholar]
- Kamide T, Takusagawa S, Tanaka N, Ogasawara Y, Kawano Y, Ohtsu I, Satoh Y, Dairi T (2020) High production of ergothioneine in Escherichia coli using the sulfoxide synthase from Methylobacterium strains. J Agric Food Chem 68:6390–6394. 10.1021/acs.jafc.0c01846 [DOI] [PubMed] [Google Scholar]
- Katsube M, Ishimoto T, Fukushima Y, Kagami A, Shuto T, Kato Y (2024) Ergothioneine promotes longevity and healthy aging in male mice. GeroScience 46:3889–3909. 10.1007/s11357-024-01111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Jeong DW, Oh JW, Jeong HJ, Ko YJ, Park SE, Han SO (2022) Efficient synthesis of food-derived antioxidant L-ergothioneine by engineered Corynebacterium glutamicum. J Agric Food Chem 70:1516–1524. 10.1021/acs.jafc.1c07541 [DOI] [PubMed] [Google Scholar]
- Liu HM, Tang W, Wang XY, Jiang JJ, Zhang W, Wang W (2023) Safe and effective antioxidant: the biological mechanism and potential pathways of ergothioneine in the skin. Molecules 28:1648. 10.3390/molecules28041648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Xiang GD, Li LK, Liu T, Ke J, Xiong LB, Wei DZ, Wang FQ (2024) Engineering non-conventional yeast Rhodotorula toruloides for ergothioneine production. Biotechnol Biofuels Bioprod 17:65. 10.1186/s13068-024-02516-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto KI, Arima T, Akita O, Kashiwagi Y (2005) Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157–1161. 10.1038/nature04300 [DOI] [PubMed] [Google Scholar]
- Maini Rekdal V, van der Luijt CRB, Chen Y, Kakumanu R, Baidoo EEK, Petzold CJ, Cruz-Morales P, Keasling JD (2024) Edible mycelium bioengineered for enhanced nutritional value and sensory appeal using a modular synthetic biology toolkit. Nat Commun 15:2099. 10.1038/s41467-024-46314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama J, Nakajima H, Kitamoto K (2002) Observation of EGFP-visualized nuclei and distribution of vacuoles in Aspergillus oryzae arpA null mutant. FEMS Microbiol Lett 206:57–61. 10.1111/j.1574-6968.2002.tb10986.x [DOI] [PubMed] [Google Scholar]
- Monné M, Marobbio CMT, Agrimi G, Palmieri L, Palmieri F (2022) Mitochondrial transport and metabolism of the major methyl donor and versatile cofactor S-adenosylmethionine, and related diseases: a review. IUBMB Life 74:573–591. 10.1002/iub.2658 [DOI] [PubMed] [Google Scholar]
- Nakajima S, Satoh Y, Yanashima K, Matsui T, Dairi T (2015) Ergothioneine protects Streptomyces coelicolor A3 (2) from oxidative stresses. J Biosci Bioeng 120:294–298. 10.1016/j.jbiosc.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Nakayama K, Ishimoto T, Masuo Y, Wakayama T, Sekiguchi H, Sutoh K, Usumi K, Iseki S, Kato Y (2016) Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice. Brain Behav 6:e00477. 10.1002/brb3.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naowarojna N, Irani S, Hu WY, Cheng RH, Zhang L, Li XH, Chen JS, Zhang YJ, Liu PH (2019) Crystal structure of the ergothioneine sulfoxide synthase from Candidatus Chloracidobacterium thermophilum and structure-guided engineering to modulate its substrate selectivity. ACS Catal 9:6955–6961. 10.1021/acscatal.9b02054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Ho QN, Pham TH, Phan TN, Tran VT (2016) The construction and use of versatile binary vectors carrying pyrG auxotrophic marker and fluorescent reporter genes for Agrobacterium-mediated transformation of Aspergillus oryzae. World J Microbiol Biotechnol 32:204. 10.1007/s11274-016-2168-3 [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Ho QN, Do LTBX, Mai LTD, Pham DN, Tran HTT, Le DH, Nguyen HQ, Tran VT (2017) A new and efficient approach for construction of uridine/uracil auxotrophic mutants in the filamentous fungus Aspergillus oryzae using Agrobacterium tumefaciens-mediated transformation. World J Microbiol Biotechnol 33:107. 10.1007/s11274-017-2275-9 [DOI] [PubMed] [Google Scholar]
- Nordgren M, Fransen M (2014) Peroxisomal metabolism and oxidative stress. Biochimie 98:56–62. 10.1016/j.biochi.2013.07.026 [DOI] [PubMed] [Google Scholar]
- Pluskal T, Ueno M, Yanagida M (2014) Genetic and metabolomic dissection of the ergothioneine and selenoneine biosynthetic pathway in the fission yeast, S. pombe, and construction of an overproduction system. PLoS One 9:e105177. 10.1371/journal.pone.0105177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochini L, Galluccio M, Scalise M, Console L, Indiveri C (2019) OCTN: a small transporter subfamily with great relevance to human pathophysiology, drug discovery, and diagnostics. SLAS Discovery 24:89–110. 10.1177/247255521881282 [DOI] [PubMed] [Google Scholar]
- Seebeck FP (2010) In vitro reconstitution of mycobacterial ergothioneine biosynthesis. J Am Chem Soc 132:6632–6633. 10.1021/ja101721e [DOI] [PubMed] [Google Scholar]
- Shoji JY, Arioka M, Kitamoto K (2006) Vacuolar membrane dynamics in the filamentous fungus Aspergillus oryzae. Eukaryot Cell 5:411–421. 10.1128/ec.5.2.411-421.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH (2020) Metabolism of sulfur-containing amino acids: how the body copes with excess methionine, cysteine, and sulfide. J Nutr 150:2494S-2505S. 10.1093/jn/nxaa094 [DOI] [PubMed] [Google Scholar]
- Sun YL, Niu YL, He B, Ma L, Li GH, Tran VT, Zeng B, Hu ZH (2019a) A dual selection marker transformation system using Agrobacterium tumefaciens for the industrial Aspergillus oryzae 3.042. J Microbiol Biotechnol 29:230–234. 10.4014/jmb.1811.11027 [DOI] [PubMed] [Google Scholar]
- Sun YL, Niu YL, Huang H, He B, Ma L, Tu YY, Tran VT, Zeng B, Hu ZH (2019b) Mevalonate diphosphate decarboxylase MVD/Erg19 is required for ergosterol biosynthesis, growth, sporulation and stress tolerance in Aspergillus oryzae. Front Microbiol 10:1074. 10.3389/fmicb.2019.01074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZA, Wu YJ, Long SH, Feng S, Jia X, Hu Y, Ma MM, Liu JX, Zeng B (2024) Aspergillus oryzae as a cell factory: research and applications in industrial production. J Fungi 10:248. 10.3390/jof10040248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takusagawa S, Satoh Y, Ohtsu I, Dairi T (2019) Ergothioneine production with Aspergillus oryzae. Biosci Biotechnol Biochem 83:181–184. 10.1080/09168451.2018.1527210 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Kawano Y, Satoh Y, Dairi T, Ohtsu I (2019) Gram-scale fermentative production of ergothioneine driven by overproduction of cysteine in Escherichia coli. Sci Rep 9:1895. 10.1038/s41598-018-38382-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang RMY, Cheah IKM, Yew TSK, Halliwell B (2018) Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci Rep 8:1601. 10.1038/s41598-018-20021-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai HD, Nguyen BPT, Nguyen VM, Nguyen QH, Tran VT (2021) Development of a new Agrobacterium-mediated transformation system based on a dual auxotrophic approach in the filamentous fungus Aspergillus oryzae. World J Microbiol Biotechnol 37:92. 10.1007/s11274-021-03060-z [DOI] [PubMed] [Google Scholar]
- Tsay GJ, Lin SY, Li CY, Mau JL, Tsai SY (2021) Comparison of single and combined use of ergothioneine, ferulic acid, and glutathione as antioxidants for the prevention of ultraviolet B radiation-induced photoaging damage in human skin fibroblasts. Processes 9:1204. 10.3390/pr9071204 [Google Scholar]
- Tschirka J, Kreisor M, Betz J, Gründemann D (2018) Substrate selectivity check of the ergothioneine transporter. DMA 46:779–785. 10.1124/dmd.118.080440 [DOI] [PubMed] [Google Scholar]
- Wijesekara T, Xu BJ (2024) Occurrence, dietary sources, quantification and bioactivities of natural antioxidant ergothioneine–a longavity vitamin? Int J Food Sci Technol 59:5951–5963. 10.1111/ijfs.17414 [Google Scholar]
- Wu LY, Cheah IK, Chong JR, Chai YL, Tan JY, Hilal S, Vrooman H, Chen CP, Halliwell B, Lai MK (2021) Low plasma ergothioneine levels are associated with neurodegeneration and cerebrovascular disease in dementia. Free Radical Bio Med 177:201–211. 10.1016/j.freeradbiomed.2021.10.019 [DOI] [PubMed] [Google Scholar]
- Xiao ML, Wang Y, Yu L, Yan X, Zhu ZH, Tian E, Wang YM, Zou G, Zhou ZH, Wang PP (2025) Engineering industrial fungus Aspergillus oryzae for the sustainable biosynthesis of ergot alkaloids. Green Chem 27:438–449. 10.1039/D4GC04643A [Google Scholar]
- Xiong LB, Xie ZY, Ke J, Wang L, Gao B, Tao XY, Zhao M, Shen YL, Wei DZ, Wang FQ (2022) Engineering Mycolicibacterium neoaurum for the production of antioxidant ergothioneine. Food Bioeng 1:26–36. 10.1002/fbe2.12004 [Google Scholar]
- Zhu M, Han YW, Hu XC, Gong CB, Ren LJ (2022) Ergothioneine production by submerged fermentation of a medicinal mushroom Panus conchatus. Fermentation 8:431. 10.3390/fermentation8090431 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.