Abstract
Key message
This study first identifies the SbC1, an R2R3-MYB transcription factor, specifically function as the key positive regulator for anthocyanin biosynthesis in sorghum coleoptiles.
Abstract
Anthocyanins are pivotal in plant growth, development, and responses to biotic and abiotic stresses. However, the molecular mechanisms underlying anthocyanin biosynthesis in sorghum, one of the major cereal crops worldwide, remain largely unexplored. Here, through genome-wide association study (GWAS), virus induced gene silencing (VIGS) experiment and haplotype analysis, we identified a key R2R3-MYB gene, SbC1, that specifically regulates anthocyanin accumulation in sorghum coleoptiles but not in grain. Further transcriptomic analysis of the coleoptiles of the cultivars HYZ (SbC1) and QKY (sbc1-a mutant allele) demonstrated the positive regulatory role of SbC1 in anthocyanin biosynthesis genes. The SbC1 protein predominantly localizes within the cell nucleus, where it interacts with Tan1. The interaction between SbC1 and Tan1 was confirmed through split-luciferase (Split-LUC), yeast two-hybrid (Y2H), and coimmunoprecipitation (Co-IP) assays. Comparative genomic analysis suggested that the R2R3-MYB transcription factors responsible for anthocyanin biosynthesis exhibit a similar molecular genetic basis in the parallel evolution of organ decoloration across different cereals. Addationnaly, overexpression of SbC1 in the rice Osc1 mutant complete rescue the anthocyanin accumulation defection and enhanced drought resistance compared with the control. In summary, for the first time, we identified the key transcription factor that specifically governs anthocyanin biosynthesis in sorghum coleoptiles. This discovery represents a significant breakthrough in understanding the molecular mechanisms of anthocyanin accumulation in sorghum and offers valuable genetic resources for plant breeding and biotechnology.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00122-025-04930-y.
Introduction
Anthocyanins are water-soluble pigments that impart red, blue, and purple hues to plants and possess significant antioxidant properties (Grotewold 2006; Das et al. 2012; Saigo et al. 2020). These properties increase plant tolerance to various biotic and abiotic stresses, such as intense light, drought, and low temperatures, thereby improving adaptability (Das et al. 2012; An et al. 2020a, 2020b). Moreover, anthocyanin accumulation in plant organs (e.g., stigmas, apiculi, coleoptiles, stems, roots, leaves, glumes and grains) serves as an efficient visual genetic marker, facilitating rapid assessment in functional gene research and crop breeding. For example, purple endosperm and coleoptiles are widely used as stable genetic markers in maize and wheat haploid breeding (Chen et al. 2022; Kumar et al. 2022).
The anthocyanin biosynthetic pathway has been extensively characterized, and structural genes involved in this pathway have been successfully isolated from a wide range of plant species (Dixon et al. 2013; Zhang et al. 2014). The genes in the anthocyanin synthesis pathway are regulated primarily by MBW complexes, which consist of R2R3-MYB transcription factors from subgroups 5 and 6, basic helix-loop-helix (bHLH) transcription factors from subgroup IIIf, and WD-repeat proteins, or are regulated solely by MYB transcription factors from subgroup 7 (Zhang et al. 2014; Xu et al. 2015; Cappellini et al. 2021). The MYB transcription factor in the MBW ternary complex directly binds to the promoters of structural genes such as chalcone synthase (CHS), dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS), and glutathione S-transferase (GST), regulating the spatiotemporal expression of these genes and inducing anthocyanin accumulation in various tissues and organs (Xu et al. 2015; Wang et al. 2020; He et al. 2023a).
A transcription factor featuring a conserved R2R3-MYB domain regulates anthocyanin biosynthesis in crops such as maize and rice. In maize, the gene encoding the transcription factor is represented by ZmC1 (colorless 1) and its duplicate ZmPL1 (purple leaf 1), and its homologue in rice is OsC1. The ZmC1 gene in maize was the first identified R2R3-MYB gene that regulates anthocyanin accumulation in plants. It specifically controls pigment accumulation in the aleurone and scutellum of maize kernels without affecting pigment accumulation in other plant parts (Paz-Ares et al. 1987; Chatham et al. 2019). Another transcription factor, ZmPL1, determines pigment accumulation in the vegetative and reproductive organs of maize, such as leaves, glumes, and anthers (Zhang et al. 2000; Petroni et al. 2014; Chatham et al. 2019). The regulatory genes involved in anthocyanin biosynthesis in rice were identified by comparison with the sequences of known maize orthologues, genome-wide association studies (GWASs), and transcriptomic analysis (Zheng et al. 2019, 2021). At least nine anthocyanin regulators that positively control the colouration of plant organs, including two R2R3-MYBs (OsC1 and OsMYB3/Oskala3), have been identified in rice (Sakamoto et al. 2001; Fan et al. 2008; Zheng et al. 2019, 2021; Kim et al. 2021; Meng et al. 2021; Xia et al. 2021; Yang et al. 2021). OsC1 is responsible for pigmentation in vegetative and reproductive organs, including the leaf sheath, apiculus, hull, and stigma (Fan et al. 2008; Sun et al. 2018; Du et al. 2022; Jiang et al. 2024). OsMYB3/Oskala3 is a key regulator that determines pigment accumulation in rice pericarps (Kim et al. 2021; Zheng et al. 2021). In other cereal crops, such as foxtail millet and wheat, the C1 homologue genes SiC1 and TaC1 regulate anthocyanin accumulation in vegetative organs/tissues, respectively (Jiang et al. 2018; Li et al. 2022).
Sorghum, the fifth major cereal crop globally, is known for its diverse plant, glume, and grain colours, which are attributed to various flavonoids, such as anthocyanins and tannins (proanthocyanins). These compounds not only promote human health and reduce damage caused by birds but also play crucial roles in the formation of aromatic substances in sorghum distilled liquor (Dykes 2019; Zhang et al. 2023; Ding et al. 2024). Research on the genetic mechanisms underlying anthocyanin and tannin biosynthesis in sorghum has been a significant focus globally (Wu et al. 2012, 2019; Dykes 2019; Xie et al. 2019; Zhang et al. 2023; Ding et al. 2024). Numerous studies have reported quantitative trait loci (QTLs) for traits related to anthocyanin and tannin synthesis in sorghum, such as grain colour, tannin content, and plant colour, using both biparental QTL mapping and GWAS to identify significant trait-associated loci (Wu et al. 2012, 2019; Rhodes et al. 2014; Girma et al. 2019; Mace et al. 2019; Xie et al. 2019; Yang et al. 2024). Boddu et al. performed fine mapping of QTLs controlling sorghum grain colour and identified the MYB transcription factor Y1 as a candidate gene that regulates pigment deposition and 3-deoxyanthocyanidin accumulation, affecting grain colour and mould resistance (Boddu et al. 2005; Nida et al. 2021; Tao et al. 2021a, b). Tannin1 (Tan1) and Tannin2 (Tan2) encode a WD40 protein and a bHLH protein, respectively, and have been confirmed as pivotal genes involved in anthocyanin and tannin biosynthesis (Wu et al. 2012, 2019). Recent studies have shown that introducing the sorghum MYB transcription factor SbTT2 into maize activates the anthocyanin and tannin biosynthesis pathways (Lu et al. 2023). Despite extensive researches have been done, the number of identified genes for sorghum anthocyanin and tannin biosynthesis remains limited, and studies on the molecular mechanisms of key candidate genes are scarce.
The coleoptile is a key protective tissue in monocot seedlings (Inada et al. 2002). In sorghum, anthocyanin accumulation within the coleoptile neutralizes reactive oxygen species, reducing oxidative damage. This enhances seedling survival under drought, UV radiation, salinity, and other stress conditions, improving stress tolerance and adaptability (Yang et al. 2024). The objective of this study is to identify key regulatory genes governing anthocyanin accumulation in sorghum through GWAS and to elucidate their molecular mechanisms.In this study, we discovered that the SbC1 gene plays a crucial role in regulating anthocyanin accumulation in sorghum coleoptiles by performing GWAS, collinearity analysis and virus induced gene silencing (VIGS) experiment. Haplotype analysis revealed that the allelic variant sbc1-a of the SbC1 gene is associated with anthocyanin synthesis in the coleoptile but does not affect anthocyanin or tannin synthesis in the grain. Additionally, we found that SbC1 interacts with Tan1 and that SbC1 regulates the expression of structural genes, such as F3H, DFR, and ANS, involved in anthocyanin biosynthesis, thereby controlling anthocyanin accumulation in sorghum coleoptiles. Overexpression of SbC1 in a rice OsC1 mutant restored anthocyanin accumulation in tissues such as leaf sheaths, glumes, and stigmas, and significantly enhanced drought resistance. Collectively, our study has identified a pivotal gene positively regulates anthocyanin biosynthesis specifically in sorghum coleoptile, thereby providing a molecular basis and candidate gene for breeding stress-resistant varieties.
Materials and methods
Plant materials and phenotypic assessment
A collection of 242 sorghum germplasm lines, primarily comprising of landrace cultivars, modern cultivars, and advanced breeding materials sourced from China, were utilized in this study (Table S1). These resources were procured from the Center for Crop Germplasm Resources, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences (Beijing, China), and the Institute of Upland Crops, Guizhou Academy of Agricultural Sciences (Guiyang, China). In 2022, a phenotypic evaluation trial was conducted in Guiyang, Guizhou, China, to examine the coleoptile colour of the population. Germination assays were performed in Petri dishes, with seeds treated with a 1% hydrogen peroxide solution for 16 h, followed by rinsing with sterile water and culture on water-saturated filter paper. Coleoptile colour was assessed and recorded during the 7- to 10-day postgermination period: green was denoted as 1, and red was denoted as 2.
Anthocyanin content determination
The method of Wang et al. (2022) was used to measure total anthocyanin content. A 0.1 g leaf sheath from 2-week-old sorghum seedlings was placed in a 1.5 ml centrifuge tube with 800 μl of 0.1% HCl in methanol. The sample was ground and incubated at 4 °C for 1 h, with vortexing every 15 min (3–5 times). After centrifuging at 12,000 g for 8 min at 4 °C, the supernatant was collected. The residue was re-extracted three more times with the same solvent.The supernatants were combined and filtered through a 0.22 μm membrane into a new 10 ml tube. A 200 μl aliquot was diluted tenfold, and absorbance was measured at 530 nm and 657 nm. Total anthocyanin content was calculated using the formula:Total anthocyanin content = (A530—0.25 * A657) / M, where M is the fresh weight of the sample (g). Each sample was analyzed in triplicate.
GWAS
Genomic resequencing and SNP discovery were performed on 242 sorghum germplasm lines on the basis of our previous research (Zhang et al. 2023). The reference genome sequence and annotation files of sorghum (BTx623, v3.1.1) (McCormick et al. 2017) were acquired from the Phytozome database (https://phytozome-next.jgi.doe.gov/) (Goodstein et al. 2012). Quality control was executed on the raw reads via fastp software (v0.20.0) (Chen et al. 2018), resulting in clean reads. Subsequent alignment with the reference genome was performed via the BWA-MEM algorithm within BWA software (v0.7–17) (Li and Durbin 2009). SNPs for each genotype were identified and filtered via the SelectVariants and VariantFiltration tools within GATK4 software (v3.8.1) (McKenna et al. 2010), followed by further filtering with VCFtools software (v0.1.17) (Danecek et al. 2011) under the following criteria: retaining only biallelic variants, minimum allele frequency > 5%, and missing rate < 20%, ultimately resulting in 2,015,850 high-quality SNPs. The GWAS for coleoptile colour was executed employing the GLM and Blink statistical methods within GAPIT software (v3.0) (Wang et al. 2021). In the mixed linear model, the first three principal components derived from the whole-genome SNPs were designated as fixed effects to account for population structure, with random effects clustered on the basis of the genetic relationships among all genotypes. The genome-wide significance threshold was determined via the adjusted Bonferroni correction threshold, set at P = -log10 (0.05/2,015,850) = 7.6.
Identification of candidate genes
LD analysis and inference of haplotype blocks surrounding peak SNPs were conducted with LD BlockShow software (v1.39) (Dong et al. 2021). On the basis of pairwise R^2 values among SNPs within the localized Manhattan block region on chromosome 10, genomic regions encompassing candidate genes were delineated through LD, aiming to identify genes potentially influencing the traits of interest. The genomic sequence of HYZ v1.0 was retrieved from the China National GeneBank at https://db.cngb.org/search/project/CNP0002968/ (Ding et al. 2024). Collinearity relationships among the sorghum genomes BTx623 and HYZ within the candidate intervals were analysed via minimap2 software (v2.26) (Li 2021), and visual representations were generated in conjunction with the genomic annotation results via NGenomeSyn software (v1.41) (He et al. 2023 , c).
Phylogenetic analysis and comparative mapping
The protein sequence of the sorghum SbC1 gene (SbHYZ.10G81400) was derived from the HYZ V1.0 reference genome. Protein sequences homologous to SbC1 from other Gramineae crops were retrieved from the Phytozome database, followed by conserved domain analysis using the InterPro database. Multiple sequence alignments were performed via MAFFT software (v7.48) (Katoh et al. 2022). Phylogenetic trees were constructed via the maximum likelihood method, with MEGA software (v11.0) (Kumar et al. 2018), using 1000 bootstrap replicates. Genomic datasets for maize (B73, v4.0) (Wei et al 2009), rice (Nipponbare, v7.0) (Ouyang et al. 2007), wheat (Chinese Spring, v2.1) (Zhu et al. 2021), millet (Yugu1, v2.2) (Bennetzen et al. 2012), and sorghum (HYZ, v1.0) were acquired from Phytozome. The collinearity relationships of gene sequences across diverse species genomes were analysed via Jcvi software (v1.2.4) (Tang et al. 2024), and visual representations of the collinearity maps were generated via R software (v4.21) in conjunction with genomic annotation. Genome comparisons were conducted using protein sequences based on the Diamond software (v2.1.7.161) (Buchfink et al. 2015).
Haplotype and population genetics analysis
Using the genome sequence of the S. bicolor variety HYZ as a reference and resequencing data from 479 global sorghum germplasm samples (Boatwright et al. 2022; Zhang et al. 2023) (Table S7), polymorphic SNP loci within the SbC1 gene and its promoter sequence (2000 bp) were identified. Coupled with the geographical origin of the materials and the phenotypic traits of coleoptile colour, haplotype and haplotype network analyses of the candidate genes were conducted via CandiHAP software (v1.30) (Li et al. 2023). The phylogenetic tree of the SbC1 gene was constructed with vcF2Dis software (v1.50) (https://github.com/BGI-shenzhen/VCF2Dis). Amplification and sequencing of the SbC1 gene were executed through Sanger sequencing technology, with the assembled sequences analysed using DNASTAR Lasergene software (v11.0) (Burland 1999). Sequence alignment was performed using MAFFT software to ascertain the accuracy of critical variant loci.
Cloning, vector construction and transformation
The primers used were designed using Premier software (v5.0) (Lalitha 2000) (Table S8), and the full-length CDS of the SbC1 gene was amplified from cDNA derived from the red coleoptile of the sorghum variety HYZ. After amplification, the gene was cloned and inserted into the temporary vector PUC57 and, after sequencing verification, was ligated into the pCAMBIA1300 vector with the maize ubiquitin promoter (UBI), resulting in the UBI construct (Figure S1). This construct was subsequently introduced into Agrobacterium tumefaciens strain EHA105, and positive colonies were selected after transformation into rice variety 9311, following the Agrobacterium-mediated mature seed transformation protocol (Toki et al. 2006). Transgenic rice lines were screened on Murashige and Skoog medium supplemented with 75 mg/L hygromycin. DNA was extracted from the rice leaf samples, and the overexpression lines were identified via PCR via specific primers (Table S8). The T2 generation of positive rice lines was utilized for phenotypic analysis and anthocyanin quantification.
VIGS assay
The VIGS procedure was modified according to our previous report (He et al. 2023b). Sorghum seeds were sterilized (10% NaClO, 5 min; 75% ethanol, 3 min), rinsed (sterile ddH2O, 5 ×), and germinated (filter paper, 2 days). A 300-bp SbC1 fragment was amplified by PCR from HYZ seedling cDNA using specific primers (Table S8), digested (Kpn I, Nco I), and subsequently cloned and inserted into pTRV2, which yielded the TRV2-SbC1 construct. The pTRV1, pTRV2, and TRV2-SbC1 constructs were subsequently transformed into A. tumefaciens GV3101. For VIGS, Agrobacterium combinations (pTRV1 + pTRV2 or pTRV1 + TRV2-SbC1) were prepared (1:1 ratio) and used for seed inoculation. Inoculation involved vacuum infiltration (100 μM AS, 3.3 mM Cys, 5 mg L^-1 Tween 20; ~ 95 kPa, 2 h). Inoculated plants were cultured (18 °C, 16 h light/8 h dark cycle).
Subcellular localization of SbC1
To determine the subcellular localization of SbC1, the CDSs of SbC1 and Tan1 were individually cloned and inserted into the pCAMBIA1300-GFP vector. The agrobacteria with the GFP fusion plasmids and NLS-mCherry (NLS, nuclear localization sequence) were coinfiltrated into the leaves of tobacco (Nicotiana benthamiana) plants. At 3 days after infection, fluorescence signals were observed via a Leica SP5 confocal microscope (Leica, Wetzlar, Germany).
BiFC assay
For the interaction between SbC1 and Tan1 in planta, the CDSs of SbC1 and Tan1 were cloned and inserted into the pXY106 (nYFP) and pXY104 (cYFP) vectors, respectively. The resulting plasmids, along with the empty vectors pXY104 and pXY106, were introduced into A. tumefaciens strain GV3101, followed by transient expression in tobacco leaves over a 3-day period with the specified combinations. The yellow fluorescent protein (YFP) fluorescence signal was detected via a Leica SP5 confocal microscope.
Split-LUC assay
The Split-LUC assay was performed as previously described (Zhou et al. 2018). First, the CDS of SbC1 and sbc1-a were cloned and inserted into the pCAMBIA1300-nLUC vector, whereas the CDS of Tan1 and scb1-a were cloned and inserted into the pCAMBIA1300-cLUC vector. The resulting plasmids were transiently coinfiltrated into tobacco leaves for 3 days with the indicated combinations. The leaves were briefly immersed in luciferin buffer for 2 min, and LUC signals were detected via a chemiluminescence apparatus (Tanon, 5200, Shanghai, China).
Y2H assays
To confirm the interaction between SbC1 and Tan1, the CDS of Tan1 was cloned and inserted into the pGADT7 vector, whereas the CDS of SbC1 was cloned and inserted into the pGBKT7 vector. Plasmids with various AD/BD combinations were transformed into the yeast strain AH109 according to the standard procedure. The resulting yeast transformants were then plated onto minimal SC/Trp-Leu medium or SC/Trp-Leu-His-Ade medium and grown for 3 days at 28 °C.
Co-IP assays and immunoblot analysis
Co-IP assays were performed as previously described (He et al. 2023a). To confirm the interaction between SbC1 and Tan1 in planta, the CDS of SbC1 was cloned and inserted into the pCAMBIA1300-GFP vector, whereas the CDS of Tan1 was cloned and inserted into the pET101-Flag vector. The resulting plasmids, as well as the pCAMBIA1300-GFP empty vectors, were transiently expressed in tobacco leaves for 3 days with the indicated combinations. Total proteins were extracted with IP buffer (10 mM Tris–HCl [pH 7.5], 150 mM NaCl, 2 mM EDTA, 1 mM MG132, 0.5% [v/v] Nonidet P-40) and incubated with anti-Flag antibody-conjugated agarose (Medical Biological Laboratories, M185-10, Nagoya, Japan). Proteins were then probed by immunoblot analysis using anti-Flag (Medical Biological Laboratories, M185-7) and anti-GFP (TransGen Biotech, HT801, Beijing, China) antibodies.
Transcriptomic analysis
Following haplotype analysis, two sorghum varieties bearing the SbC1 allele, HYZ (SbC1) and QKY (sbc1-a), were delineated from 242 sorghum germplasms. These varieties exhibit closely related phylogenetic relationships. RNA was extracted from the sheaths of three-leaf-stage plants via the TRIzol method. Upon quality validation, RNA samples were sequenced via the Illumina NovaSeq 6000 platform (Illumina, San Diego, California, USA). The raw data were processed via fastp software to filter out low-quality reads, thereby obtaining high-quality clean reads. These clean reads were aligned against the sorghum HYZ reference genome via HISAT2 software (Version 2.2.1) (Kim et al. 2019), enabling the acquisition of genomic location and sequence feature information specific to the sequenced samples. The fragments per kilobase of transcript per million mapped reads (FPKM) values were calculated, and gene and transcript expression levels were assessed. Differential gene expression analysis was performed via the EdgeR package (v3.20) (Robinson et al. 2010), with the selection criteria |log2-fold change|> 1 and P value < 0.05. DEGs were annotated, and pathway enrichment analysis was conducted via the GO and KEGG databases.
qRT-PCR
Total RNA was extracted from the sheaths of sorghum varieties HYZ and QKY via the RNAprep Pure Plant Kit (TIANGEN, Beijing, China). cDNA synthesis was performed via the PrimeScriptTM RT Reagent Kit with gDNA Eraser (TaKaRa, Kusatsu, Japan) according to the manufacturer's instructions. qRT‒PCR was carried out using PowerUp SYBR Green Mix (Thermo Fisher Scientific, Waltham, MA, USA). The reaction mixture comprised 5 μl of PowerUp SYBR Green reagent, 1.0 μl of cDNA template, 0.25 μl of each primer (10 μM), and 3.5 μl of nuclease-free water. The qRT‒PCR was performed on an ABI7500 Real-Time PCR System (Thermo Fisher Scientific) following the manufacturer’s guidelines, with the sorghum tubulin alpha gene (Sobic.001G107200) serving as the reference gene. Relative gene expression levels were calculated via the 2-ΔΔCt method (Livak and Schmittgen 2001 ), and the sequences of the primers used for qRT‒PCR are provided in Table S8.
Drought tolerance assays
Seeds from the transgenic rice lines, wild-type rice, HYQ, and QKY were used as experimental materials and were germinated in an artificial climate chamber under long-day conditions (28 °C, 16 h light/8 h dark). Two weeks after seedling emergence, seedlings of uniform height sorghum and rice seedlings were subjected to drought stress via 20% PEG-6000. Two weeks after the onset of stress, phenotypes were observed, and data on the fresh weight and survival rates of the plants before and after drought stress were collected.
Data processing and statistical analysis
Descriptive statistical analysis and Student's t-test were performed using SPSS software (v22.0), with a significance level set at P < 0.05.
Results
Identification of candidate genes regulating sorghum coleoptile colouration
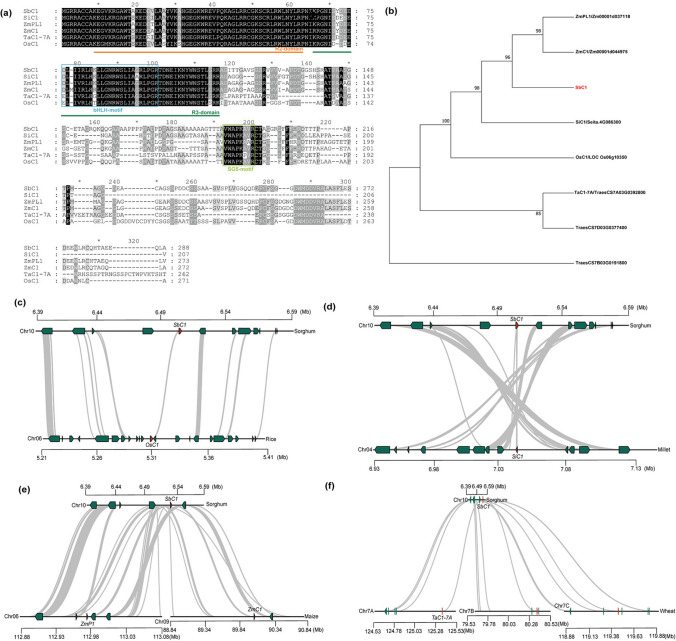
The coleoptile plays a critical role in facilitating the emergence of sorghum grains from the soil. The accumulation of anthocyanins in the coleoptile provides protective effects on seedlings against diverse stresses.To identify quantitative trait loci (QTLs) for the colouration of sorghum coleoptiles, we investigated the coleoptile colour of 242 sorghum germplasm resources at the seedling stage, specifically between 7 and 10 days after germination. Of these, 133 varieties presented a red coleoptile phenotype, whereas 109 presented a green coleoptile phenotype (Fig. 1a and Table S1). Consistent with the differences in colour, the average total anthocyanin content of red coleoptile sorghum accessions (0.65 mg/g) was significantly greater than that of green coleoptile sorghum accessions (0.05 mg/g) (Fig. 1a). In general, the accumulation degree of pigments of sorghum coleoptiles was positively associated with the total anthocyanin content. By performing a GWAS, we identified a single QTL region significantly associated with coleoptile colour, located on chromosome 10 between 6.41 and 6.45 Mb. (Fig. 1b and Table S2). Linkage disequilibrium (LD) heatmap further revealed that the SNP associated with coleoptile color showed strong linkage with adjacent SNPs within this QTL region (Fig. 1b). On the basis of the genomic annotation information for the reference genome BTx623 (V3.1.1), we identified three candidate genes (Sobic.010G077250, Sobic.010G077275, and Sobic.010G077300) within 50 kb vicinity of the significant single-nucleotide polymorphism (SNP) site. Protein sequence homology analysis of these three genes revealed no similarity with previously cloned anthocyanin synthesis-related genes in Arabidopsis, maize, and rice (Table S3). Considering the possible lack of annotation on the anthocyanin biosynthesis pathway in the reference genome variety BTx623 (green coleoptile and white grain), we conducted a microcolinearity analysis by comparing the candidate region sequences of BTx623 with the genome sequences of the sorghum cultivar HongYingZi (HYZ, red coleoptile and brown grain) (Ding et al. 2024). This led to the identification of a candidate gene, SbiHYZ.10G081400, in the HYZ genome (Fig. 1c). Notably, this gene was absent in the BTx623 genome and is hereafter referred to as SbC1 (Sorghum bicolor purple coleoptile) (Fig. 1c). The annotation analysis revealed that the protein sequence of SbC1 encodes an R2R3-MYB transcription factor (Figure S2).
Fig. 1.
Genome-wide association analysis of coleoptile colour in 242 sorghum accessions. a Coleoptile colour of green (left) and red (right) sorghum accessions; bars = 1 cm. Total anthocyanin content in red- and green-coleoptile sorghum accessions. b Manhattan plots showing the results of the GWAS of green- and red-coleoptile sorghum accessions. The peak regions on chromosome 10 are displayed in conjunction with the linkage disequilibrium blocks. The lines indicate the significance threshold value (P < 2.48 E-08). c Collinearity analysis was performed on the candidate region of chromosome 10 in the sorghum genomes HYZ (version v1.0) and BTx623 (version v3.1.1). The collinearity intervals are 7.37–7.39 Mb and 6.41–6.43 Mb
SbC1 positively regulates anthocyanin accumulation in sorghum coleoptiles
By using a gene expression database to analyse expression across various tissues and developmental stages of the sorghum cultivar HYZ (Ding et al. 2024), we observed that the expression of the SbC1 gene peaked in the stem/coleoptile tissues during the five-leaf stage, which coincided with the stage when coleoptile colouration became prominent (Figure S3). To investigate whether SbC1 is responsible for the accumulation of anthocyanins in sorghum coleoptiles, we examined the phenotypes of 479 sorghum accessions for which resequencing data was available. By utilizing the sorghum HYZ genome as a reference for haplotype analysis, we identified a total of 19 SNP sites within the SbC1 gene, encompassing 14 in the promoter region, two in exons and two in introns, and one in the 3'-untranslated region (3'UTR) (Table S4). Among these, a critical SNP variation characterized by a ‘C’ to ‘T’ mutation at the 124th (Chr10:7,387,103) base of the first exon of SbC1 was identified. SNPC124T resulted in the conversion of the glutamine-encoding codon CAG with the stop codon TAG, leading to premature termination of translation and yielding a truncated protein that lacks normal functionality (Fig. 2a). After observing the phenotypes of 37 accessions harbouring this specific mutation with the SNPC124T genotype, we found that all 37 accessions presented green pigmentation in their coleoptiles, which indicated that SbC1 might be a critical regulator of anthocyanin accumulation in sorghum coleoptiles. Consequently, the genotype that affects anthocyanin accumulation in coleoptiles carrying SNPC124T was designated the sbc1-a allele (Figure S4a). The identification of the sbc1-a allele was further supported by Sanger sequencing (Fig. 2b).
Fig. 2.
Natural variation in Sbc1 impacts anthocyanin accumulation in sorghum coleoptiles. a Important natural variation in Sbc1. Codon change from a glutamine (Gln) codon to a premature stop codon. b Alignment of the Sbc1 nucleotide sequence to the sbc1-a sequence is shown. c Phenotypes of sorghum seedlings resulting from VIGS. d Quantitative analysis of Sbc1 gene expression and anthocyanin content in sorghum coleoptiles following VIGS. The error bars represent means ± SDs (n = 3). Different lowercase letters indicate a significant difference at P < 0.05 based on a t-test
To determine the impact of SbC1 mutation on anthocyanin accumulation in other sorghum tissues, we investigated the genotypic and phenotypic data of sorghum germplasm resources (Zhang et al. 2023). The phenotypes of sorghum varieties harbouring SbC1 and sbc1-a alleles were specifically observed. During seed maturation, the pericarp and seed coat of sbc1-a variant grains presented red colouration that was similar to that observed in HYZ (Figure S4b and c). Furthermore, analysis of anthocyanin contents confirmed the presence of anthocyanins in both the pericarp and seed coat of the variety QingKeYang (QKY, a genetically similar line of HYZ with the sbc1-a allele) (Figure S3d). Additionally, 29 out of 37 varieties carrying the sbc1-a allele presented the same pericarp and testa colour as the QKY variety did (Table S5). Taken together, the findings from these tissues and those from coleoptiles suggest that allelic variation in the SbC1 gene specifically affects anthocyanin accumulation in sorghum coleoptiles.
To elucidate the functional role of the SbC1 gene, we conducted a virus-induced gene silencing (VIGS) experiment using HYZ (with the SbC1 genotype) as the experimental material. At approximately 2 days postinoculation, we observed notable lightening of the colouration of the coleoptiles in the experimental group (pTRV: SbC1) compared with that in the control group (pTRV: 00) (Fig. 2c). Additionally, we measured the transcript levels of SbC1 and the anthocyanin content in the coleoptiles in both the experimental and control groups. The results demonstrated successful knockdown of SbC1 expression in the coleoptiles within the experimental group, which corresponded with a significant reduction in anthocyanin content (experimental: 0.17 mg/g vs control: 0.69 mg/g) (Fig. 2d), implying that downregulation of SbC1 expression contributes to the lightening of coleoptile colour in HYZ seedlings.
The sbc1-a allele is found exclusively in Chinese varieties that might have been introduced from Africa and other regions of Asia
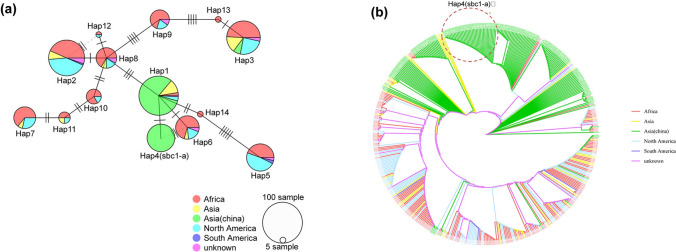
To investigate the sequence diversity of SbC1 and determine the distribution of the sbc1-a allele in the sorghum population, a haplotype network analysis was conducted, accounting for the geographic origin of 479 global sorghum resources. The analysis revealed 14 haplotypes (with variety numbers > 5) with distinct geographic distribution patterns (Fig. 3a). Most haplotypes exhibited a wide distribution across diverse geographic populations, except for Hap4, which occurred exclusively in Chinese sorghum resources carrying SNPC124T (sbc1-a). Compared with the other populations, the African sorghum population presented higher levels of haplotype and nucleotide diversity. The haplotypes Hap2, Hap3, and Hap5 were found to be the most common in the North American sorghum population, whereas Hap1 and Hap4 were predominantly observed in Chinese germplasms, which exhibited close genetic relatedness with only the SNPC124T mutant (Fig. 3a), suggesting the potential derivation of Hap4 from Hap1. Hap6, which is found mainly in Africa, North America, and India, was genetically classified into another subgroup that exhibited a closer genetic relationship to Hap1 (Fig. 3a). Thus, the haplotype Hap1 might have been introduced to China through the regions harbouring Hap1 and Hap6. Additionally, phylogenetic analysis of SbC1 gene diversity supported these findings, classifying different regional sorghum varieties into distinct branches, each fixing different haplotypes, with sbc1-a-carrying varieties clustered on a separate branch (Fig. 3b).
Fig. 3.
Haplotype network analysis of SbC1 from 479 sorghum accessions. a Haplotype network analysis of SbC1. Haplotypes of SbC1 are represented by circles. The size of each circle is proportional to the frequency of the corresponding haplotype. Lines between haplotypes represent the mutations, with each line representing one mutation; N ≥ 5. b Phylogenetic tree of SbC1 and its promoter region (2 kb)
Comparative analysis of C1 locus in cereals
The annotation analysis revealed that the nucleotide sequence of SbC1 encodes an R2R3-MYB transcription factor. To identify the function of SbC1, we first aligned its amino acid sequence with those of other identified anthocyanin regulators in Poaceae crops. The results revealed that the protein sequence of SbC1 is gene codes for homologs to those of ZmC1, ZmPL1, OsC1, SiC1, and TaC1-7A, containing the canonical bHLH-binding motif [D/E]Lx2[R/K]x3Lx6Lx3R in its R2R3 domain in the N-terminal region and the characteristic motif sequence for subgroup 5 at its C-terminal region (Fig. 4a). The subsequent phylogenetic analysis revealed that the protein sequence of SbC1 clustered together with those of ZmC1 and ZmPL1, indicating the homologous relationship of SbC1 with ZmC1 and ZmPL1 (Fig. 4b). A subcellular location analysis of the SbC1 protein revealed that, similar to ZmC1 and ZmPL1, the SbC1 protein was located in the nucleus, suggesting its potential function as a transcription factor (Figure S5). Comparative mapping results indicate that the chromosomal region containing the SbC1 gene in sorghum corresponds to five highly conserved chromosomal segments, located on chromosome 4 in foxtail millet, chromosome 6 in rice, chromosomes 6 and 9 in maize, and chromosomes 7A, 7B, and 7D in wheat (Table S6). Genome micro-collinearity analysis also revealed significant differences in genome size and gene numbers among cereal species, yet their genomes exhibited evident collinearity at the chromosomal level, with conserved gene markers and gene order (Fig. 4c-f). These findings are consistent with previous studies (Lai et al. 2004; Li et al. 2022).
Fig. 4.
Comparative genomic analysis of the C1 locus across different cereal species. a Protein sequence alignment of SbC1 with the homologous genes ZmC1, ZmPL1, OsC1, SiC1, and TaC1-7A, which revealed conserved motifs, including the bHLH binding motif and subgroup 5 characteristic motif. b Phylogenetic tree showing the evolutionary relationship between SbC1 and its homologues in other cereal species, indicating SbC1’s close relationship with ZmC1 and ZmPL1. c–f Synteny analysis of the C1 locus across different cereal species, illustrating the conserved genomic regions surrounding the C1 locus in sorghum compared with rice (c), foxtail millet (d), maize (e), and wheat (f). The grey lines represent conserved syntenic blocks, highlighting the evolutionary conservation of the C1 locus among these species
The SbC1 protein physically interacts with Tan1
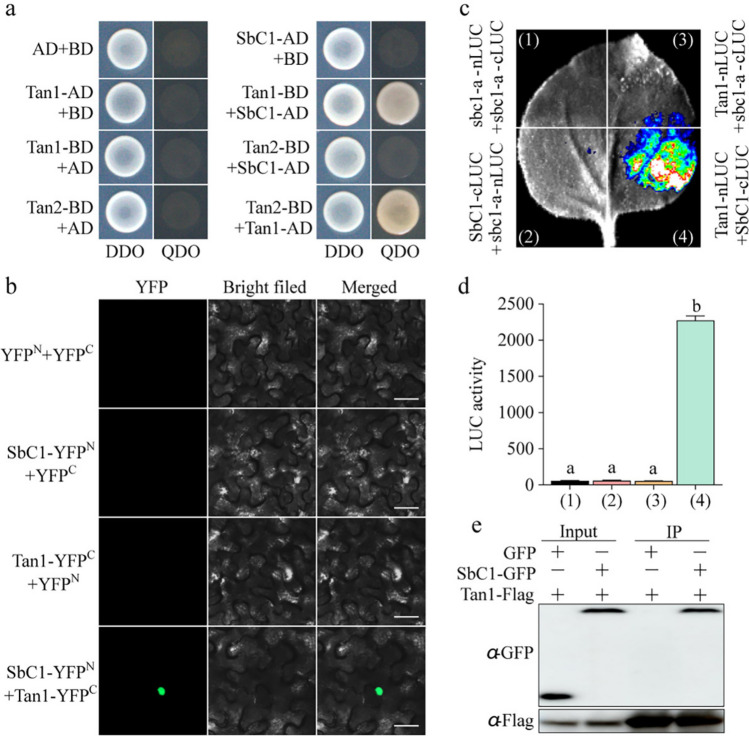
Previous studies have demonstrated that Tan1 (a homologue of Arabidopsis TTG1) and Tan2 (a homologue of Arabidopsis TT8) are responsible for condensed tannin accumulation in sorghum grains (Wu et al. 2012, 2019). In Arabidopsis, TTG1 and TT8 play critical roles as members of the MBW complex in regulating both anthocyanin and proanthocyanin accumulation (Gonzalez et al. 2008; Shi and Xie 2014; Xu et al. 2015). To investigate whether SbC1 recruits the identified Tan1 and Tan2 to regulate anthocyanin accumulation in sorghum coleoptiles, we performed a yeast two-hybrid (Y2H) assay. As expected, physical interactions between SbC1 and Tan2 were not observed in yeast (Fig. 5a). However, the SbC1 protein physically interacted with Tan1 in the yeast system (Fig. 5a). We subsequently performed a bimolecular fluorescence complementation (BiFC) assay to confirm the interaction between SbC1 and Tan1 in tobacco. Only tobacco leaves cotransformed with the SbC1-nYEP and Tan1-cYFP vectors presented a GFP signal localized within the cell nucleus (Fig. 5b). Furthermore, we performed a Split-LUC assay in tobacco to confirm the interactions of SbC1 and Tan1. The robust fluorescence signal was detected between SbC1-cLUC and Tan1-nLUC (sbc1-a as the negative control) (Fig. 5c-d). Additionally, a Co-IP assay was conducted to investigate the interaction between SbC1 and Tan1 in tobacco plants. Flag-Tan1 and SbC1-GFP or GFP proteins were transiently expressed in tobacco leaves, followed by immunoprecipitation of Flag-Tan1 using anti-Flag antibody-conjugated agarose. SbC1 and its interacting proteins were identified by immunoblot analysis with anti-Flag and anti-GFP antibodies. The result demonstrated that SbC1-GFP but not GFP itself can be coimmunoprecipitated by Tan1, implying that SbC1 interacts with Tan1 in planta (Fig. 5e). To investigate the potential involvement of Tan1 in anthocyanin accumulation in sorghum coleoptiles, we determined the expression pattern of Tan1 in different sorghum tissues. The results revealed that Tan1 also presented a relatively high expression level in sorghum coleoptiles (Figure S5a). Considering that SbC1 can physically interact with Tan1, Tan1 may also participate in the regulation of anthocyanin accumulation in sorghum coleoptiles through interactions with SbC1.
Fig. 5.
SbC1 physically interacts with Tan1 in the nucleus. a Yeast two-hybrid (Y2H) assay for the interaction between SbC1 and Tan1. Yeast transformants were plated on minimal SC-Trp/Leu medium (DDO) or SC-Trp/Leu/His/Ade (QDO) medium, and photographs were taken after the transformants had grown for 3 days at 28 ℃. The interaction between Tan1-AD and Tan2-BD as positive control. b Bimolecular fluorescence complementation (BiFC) assay. Various versions of SbC1 that interact with Tan1 were fused with the N- or C-terminus of yellow fluorescent protein (YFP) and transiently expressed in tobacco leaves for 3 days. The YFP fluorescence signal was detected via a Leica SP5 confocal microscope. bars = 100 mm. c Split-luciferase complementation (SLC) assay showing that SbC1 interacts with Tan1 in tobacco leaves. Protein sbc1-a was used as the negative control. d Quantification of LUC activity shown in (b). The error bars represent means ± SDs (n = 3). Different lowercase letters indicate a significant difference at P < 0.05 on the basis of one-way analysis of variance (ANOVA). e Coimmunoprecipitation assay showing the physical interaction between SbC1 and Tan1 in vivo. CoIP was conducted with Flag-Trap beads, and protein complexes were probed by immunoblot analysis with anti-GFP and anti-Flag antibodies
Transcriptome analysis revealed genes that were differentially expressed between HYZ and QKY
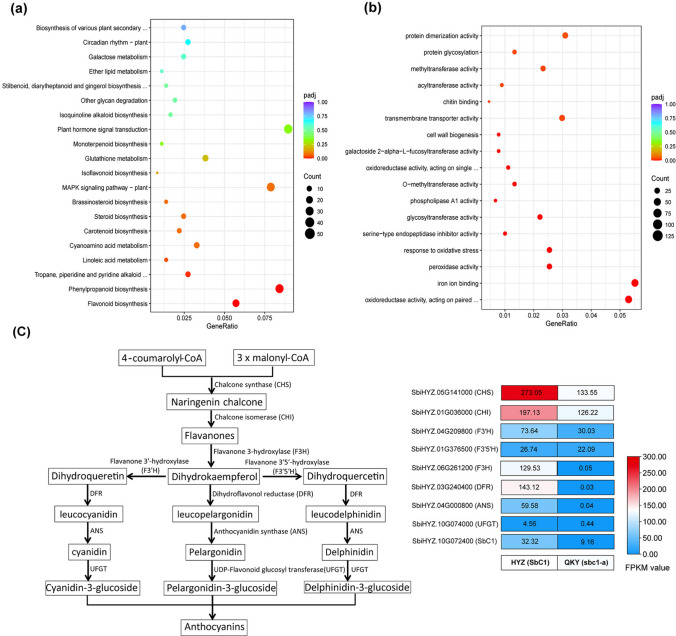
To elucidate the regulatory mechanism underlying colour pigmentation in sorghum coleoptiles, we conducted transcriptomic analyses on RNA samples extracted from the coleoptiles of two genetically similar landraces, HYZ (SbC1), with a red coleoptile, and QKY (sbc1-a), with a green coleoptile (Sequencing results revealed that QKY and HYZ share 99.5% of their genetic loci across 2,015,850 polymorphic SNP sites, with no genetic variation detected in any anthocyanin biosynthesis-related genes, except for the C1 gene). After data filtration, the clean reads were mapped to the reference genome of T2T-HYZ (Ding et al. 2024). In total, we identified 1573 differentially expressed genes (DEGs), comprising 906 upregulated and 667 downregulated genes. These DEGs were mapped to 98 KEGG pathways. Enrichment analysis revealed that allelic variation in SbC1 primarily influenced genes involved in phenylpropanoid biosynthesis, MPPK signal transduction, and flavonoid biosynthesis (Fig. 6a). The GO analysis revealed that the identified DEGs were associated primarily with functions related to oxidoreductase activity, iron ion binding, and peroxidase activity (Fig. 6b). Further analysis of differences in the expression of genes between the two varieties revealed a significant upregulation of genes involved in the early stage of anthocyanin biosynthesis (CHS, CHI, F3H, F3ʹH), as well as those involved in the late stage of anthocyanin synthesis (DRF, ANS, UFGT) specifically in the red coleoptiles of HYZ (Fig. 6c). To validate the reliability of the transcriptomic data, qRT-PCR was conducted on 8 genes involved in anthocyanin biosynthesis, revealing strong concordance with the RNA-seq results (Figure S7). Notably, the expression levels of F3H, DFR, ANS, and UFGT were negligible (FPKM < 0.1) (Fig. 6c) in QKY, suggesting that SbC1 might regulate anthocyanin biosynthesis in sorghum coleoptiles by controlling the expression of anthocyanin biosynthesis genes, especially F3H, DFR, ANS and UFGT.
Fig. 6.
RNA-seq analysis results. a Significantly enriched Kyoto Encyclopedia of Genes and Genomes pathways associated with the DEGs from HYZ (SbC1) vs. QKY (sbc1-a). b Significantly enriched Gene Ontology terms associated with DEGs from HYZ (SbC1) vs. QKY (sbc1-a). c Anthocyanin biosynthesis pathway and expression patterns of related genes in HYZ (SbC1) and QKY (sbc1-a)
SbC1 can complement the anthocyanin deficiency phenotype of the rice OsC1 mutant
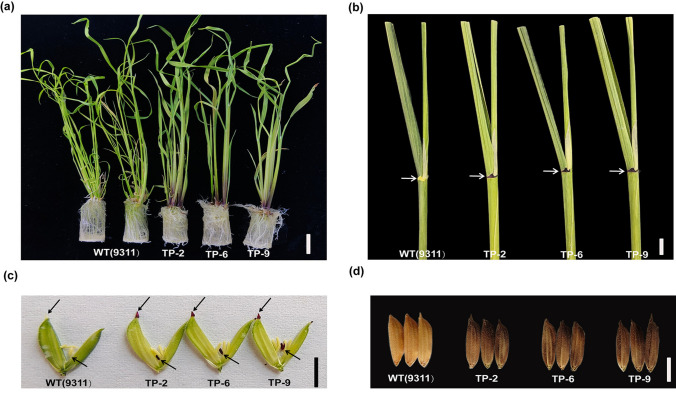
A previous study reported that the rice variety 9311 has green coleoptiles and leaf sheaths due to the null mutation of OsC1 (Qiao et al. 2021). To further verify the function of SbC1 in anthocyanin accumulation and investigate the conservation of gene functions among homologous genes, we introduced the coding sequences (CDSs) driven by a 35S promoter into the rice variety 9311. Both T2 transgenic and 9311 plants were cultivated under controlled conditions in a greenhouse, and phenotypic identification revealed noticeable pigmentation in various vegetative tissues, including the leaf sheath, ligule, stigma, apiculus, and hull apex, of the transgenic rice compared with those of the wild-type plants (Fig. 7a-d), indicating that SbC1 plays an analogous function with OsC1 in terms of positive regulate anthocyanin accumulation.
Fig. 7.
Functional assessment of SbC1 verified by overexpression in rice (9311) lines. a Overexpression of SbC1 in rice 9311 lines; bars = 1 cm. b Leaf phenotype comparison between the wild-type and transgenic lines; bars = 1 cm. c–d Spikelet and seed comparison between the wild-type and transgenic lines; bars = 1 cm
SbC1 improves drought tolerance in rice and sorghum
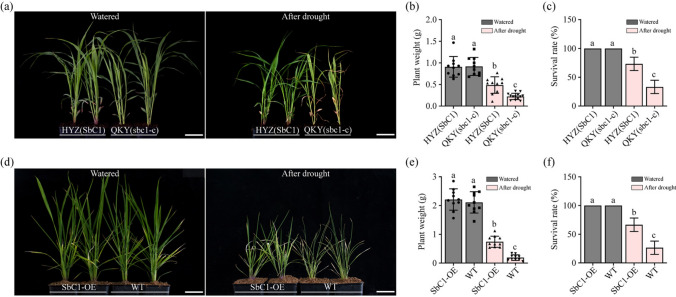
Numerous studies have demonstrated that the accumulation of plant anthocyanins can be induced by various stresses, serving as a defensive mechanism and augmenting plant biotic stress tolerance, such as by increasing drought resistance (Zhang et al. 2014; Huang et al. 2019; An et al. 2020a, 2020b; Liao et al. 2022). Considering the substantial deposition of anthocyanins in various organs (Fig. 7a-d) resulting from the high expression of SbC1 in transgenic rice, we hypothesized that these transgenic lines may exhibit robust resistance against multiple biotic stresses. To investigate the potential of SbC1 overexpression to increase drought resistance in rice, we exposed 21-day-old transgenic and mutant rice plants to drought stress conditions. The results revealed no statistically significant differences in the growth or physical characteristics of the plants under standard irrigation with water (Fig. 8a). However, the fresh weight and survival rates of the transgenic lines overexpressing SbC1 were greater than those of the mutants treated with PEG6000 (to simulate drought stress) (Fig. 8b, c). We subsequently conducted a similar drought stress treatment on the sorghum varieties HYZ and QKY. The fresh weight and survival rates of HYZ in the presence of functional SbC1, as depicted in Fig. 8d, were markedly greater than those of QKY, which is similar to the outcomes observed in transgenic rice (Fig. 8e, f). Taken together, these findings provide evidence for the potential of SbC1 to enhance drought tolerance in both rice and sorghum, highlighting its pivotal role in increasing plant resilience under drought stress.
Fig. 8.
Drought tolerance analyses. a Phenotypes of HYZ and QKY plants in response to drought stress. bars = 2 cm. b–c Plant weight and survival rates of HYZ and QKY. c Phenotypes of 9311 (WT) and SbC1-overexpressing plants in response to drought stress. e–f Weight and survival rates of 9311 (WT) and SbC1-overexpressing plants. bars = 2 cm. The error bars represent means ± SDs (n = 3). Different lowercase letters indicate a significant difference at P < 0.05 based on a t-test
Discussion
SbC1 is a candidate gene in the Rs2 locus that controls coleoptile colour
Pigmentation of the coleoptile represents a readily distinguishable phenotypic trait that can be used to determine the homogeneity or specificity of varieties (Khlestkina et al. 2002). Early studies utilized molecular markers such as RFLP, AFLP, and SSR to develop genetic linkage maps for this trait, pinpointing the QTLs controlling sorghum coleoptile colour as Rs1 and Rs2. Within the R931945-2–2/IS 8525 genetic population, Rs1 was mapped between 108.1 and 109.5 centimorgans (cM) on chromosome 6 (Mace and Jordan 2010), corresponding to a physical location between 52.20 and 56.61 Mb in the BTx623 genome (v3.1.1) (Mace et al. 2019). Further research indicated that Rs1 might also influence overall sorghum plant colouration (Boyles et al. 2017). Rs2 was genetically mapped between 32.1 and 35.3 cM on chromosome 10, corresponding to a physical location between 3.28 and 5.95 Mb on the BTx623 genome (v3.1.1) (Mace and Jordan 2010).
In this study, we conducted a GWAS using 242 Chinese sorghum germplasms and successfully mapped the genomic region associated with coleoptile colour to chromosome 10, which was consistent with the previously identified Rs2 locus. VIGS experiments further substantiated the pivotal role of SbC1 in controlling sorghum coleoptile pigmentation. Comparative genomic analysis with other sorghum genomes (HYZ) identified SbC1, a gene absent from the BTx623 genome (v3.1.1). Moreover, genomic sequence analysis revealed that SbC1 was incorrectly annotated in version v3.1.1, which hindered the accurate identification of this key gene through homology analysis (Li et al. 2022). The SbC1 gene in the updated BTx623 reference genome (v5.1) (https://phytozome-next.jgi.doe.gov/info/Sbicolor_v5_1) is annotated as Sobic.010G077237. Comparative analysis between the SbC1 genes from HYZ and BTx623 revealed sequence variations in both intronic and exonic regions, leading to alterations in the amino acid sequences (Figure S8). Whether these variations impact the function of the SbC1 gene requires further experimental validation.
The loss of anthocyanins in cereals might share the same genetic basis
During the domestication and improvement of various cereals, many traits have undergone parallel changes (Huang et al. 2022), such as grain size (Tao et al. 2020, 2021a, 2021b). One significant observation across these major cereals is the loss of anthocyanin accumulation in plant organs, including anthers, seeds, leaves, stems, and roots. However, whether these parallel trait changes occur via a common genetic mechanism remains largely unexplored (Li et al. 2022).
R2R3-MYB transcription factors are pivotal in anthocyanin synthesis, with numerous R2R3-MYBs identified as positive regulators of anthocyanin biosynthesis in cereals. ZmC1, the first cloned MYB transcription factor-encoding gene in maize, regulates anthocyanin biosynthesis in the grain aleurone layer (Cone et al. 1993). In this study, we identified the SbC1 (R2R3-MYB) gene as a key activator that promotes anthocyanin biosynthesis in sorghum coleoptiles, with nucleotide mutations in its exons impacting gene function. ZmC1, ZmPl1, OsC1, SiC1, and TaC1-7A, are orthologs of SbC1. Previous studies have shown that these genes have undergone various mutations during the domestication, diversification, and crop improvement processes, leading to the loss of gene function and subsequently affecting the accumulation of anthocyanins in the vegetative and floral organs of these crops (Morohashi et al. 2012; Jiang et al. 2018; Du et al. 2022; Li et al. 2022). Recent studies also identified another ortholog of SbC1, the R2R3-MYB transcription factor TdRCA1, which specifically regulates anthocyanin biosynthesis in the coleoptile of wild emmer wheat (Li et al. 2024). In this study, heterologous expression of SbC1 in the rice OsC1 mutant variety 9311 led to anthocyanin accumulation in various tissues, such as the leaf sheath, ligule, hull apex, and stigma, indicating that SbC1 and OsC1 share a conserved regulatory mechanism in anthocyanin biosynthesis. All these findings suggest that the c1 genes responsible for anthocyanin production played a key role in the loss of pigmentation across different cereal species.
The purple apiculus trait controlled by OsC1 is common in wild rice but rare in cultivars (Saitoh et al. 2004; Choudhury et al. 2014). However, certain traits for crop improvement, such as grain texture and flavour, governed by genes such as waxy and BADH2, exhibit allelic diversity across various landraces and cultivars, reflecting regional preferences (Olsen and Purugganan 2002; Kovach et al. 2009). Similar phenomena were observed in our study, and the allelic diversity of SbC1 revealed signatures of selection in Chinese sorghum but not in improved varieties in Asia and North America.
SbC1 specifically promotes coleoptile anthocyanin accumulation by activating the expression of anthocyanin biosynthesis genes
Classic genetic analyses has shown that anthocyanin and tannin synthesis in sorghum grains is controlled by a pair of recessive epistatic genes (B1/Tan1 and B2/Tan2) (Smith et al. 2000). In this study, through VIGS, haplotype analysis, and phenotypic investigation, we confirmed that allelic variation in the SbC1 gene affects anthocyanin accumulation in coleoptiles but not in grains. MYB transcription factors typically determine the specific activation of target genes (Li 2014; Liu et al. 2015; Allan and Espley 2018). In this study, we found that allelic variation in SbC1 affects the expression of structural genes related to anthocyanin biosynthesis, such as F3H, DFR, ANS, and UFGT. These findings are consistent with those of previous studies, suggesting that SbC1 may directly bind to the promoters of structural genes related to anthocyanin biosynthesis, activating their transcription and thereby regulating anthocyanin accumulation in coleoptiles. Therefore, future research should focus on exploring how SbC1 can be utilized in breeding programs to enhance anthocyanin accumulation and improve stress resistance in sorghum and other cereal crops.
The components of the MBW complex responsible for anthocyanin accumulation in sorghum coleoptiles remain elusive
The MBW ternary complex, comprising MYB, bHLH, and WD40 transcription factors, is critical in the biosynthesis of anthocyanins and proanthocyanidins across various plant species(Dixon et al. 2013). The bHLH transcription factor plays a crucial role in facilitating the assembly of transcriptional complexes at the promoters of anthocyanin biosynthetic genes through its interaction with the R3 region of its R2R3-MYB partner (Xu et al. 2015; LaFountain and Yuan 2021). It is widely accepted that WD40 proteins function primarily as scaffold proteins that facilitate interactions between bHLH proteins and various MYB proteins to regulate anthocyanin accumulation in different tissues rather than directly interacting with MYB proteins (Gonzalez et al. 2008; An et al. 2012; Xu et al. 2015; Gao et al. 2018; Liu et al. 2021). However, recent studies have reported direct interactions between WD40 and MYB proteins. For example, RcTTG1 interacts with RcMYB1, OsTTG1 with OsC1, FaLWD1 with FaMYB5, and RhWD40 with RhMYB114a and RhMYB3b (Yang et al. 2021; Jiang et al. 2023; Yan et al. 2023; He et al. 2023a). In this study, we conducted an in-depth investigation of the interactions between WD40, bHLH, and MYB transcription factors, with a particular focus on their regulatory roles in anthocyanin accumulation within sorghum coleoptiles and grains.
In our study, via Y2H, BiFC, and Co-IP assays, we determined that the sorghum WD40 protein Tan1 interacts with SbC1. However, SbC1 did not interact with Tan2, a bHLH transcription factor previously reported to be involved in sorghum proanthocyanin accumulation. Our group and Wu et al. reported that the Tan2 gene regulates anthocyanin and tannin biosynthesis in sorghum grain (Wu et al. 2019; Zhang et al. 2023). Analysis of gene expression databases across different tissues and stages of tanninsorghum revealed that Tan2 is not expressed in coleoptiles at the five-leaf stage, when coleoptile colouration is most prominent, but is highly expressed during grain development (Figure S5b). Thus, we hypothesize that Tan2 is not involved in regulating anthocyanin synthesis in coleoptiles but regulate the synthesis of anthocyanins and proanthocyanidins in grains through interactions with Tan1.
In maize, ZmPac1 is essential for anthocyanin accumulation in the aleurone layer and scutellum, whereas anthocyanin synthesis in the pericarp and other tissues is unaffected in ZmPac1 mutants (Carey et al. 2004; Chatham et al. 2019). In rice, a mutation in OsTTG1 (Tan1 homologue) significantly reduced anthocyanin accumulation in all tissues (Yang et al. 2021), indicating that regulatory genes for anthocyanin biosynthesis are relatively conserved among different Gramineae species, although their regulatory mechanisms exhibit specificity. Intriguingly, studies by Wu et al. (2019) and phenotypic data from 479 global sorghum germplasm resources revealed that functional variation in Tan1 affects grain anthocyanin and tannin biosynthesis but not coleoptile anthocyanin biosynthesis (Figure S9). These findings suggest the existence of other functionally redundant WD40 proteins involved in anthocyanin biosynthesis in coleoptiles.
The application of SbC1 in stress resistance
Anthocyanins provide protection to crops under various biotic and abiotic stresses (Das et al. 2012; An et al. 2020a, 2020b). For example, anthocyanins in wheat seeds and coleoptiles promote root and shoot elongation under osmotic stress conditions (Shoeva et al. 2017). In this study, compared with wild-type rice, transgenic rice demonstrated higher fresh weight and survival rates after drought stress. Similarly, the sorghum HYZ (carrying the SbC1) showed advantages in drought tolerance over the QKY (carrying the sbc1-a). This enhanced drought tolerance is likely due to the formation of an anthocyanin-rich flavonoid barrier in the coleoptiles of seedlings, which mitigates damage caused by drought stress. Given that transgenic rice lines continuously express the SbC1 gene during the mature stage and exhibit pigmented plants, it is possible that they may also have stress resistance at later developmental stages; however, this still requires further validation through field trials. Similarly, overexpression of TdRCA1, a homolog of SbC1, not only increases anthocyanin accumulation in wheat coleoptiles but also significantly enhances seedling resistance to Fusarium crown rot (Li et al. 2024). These results suggest that the accumulation of anthocyanins in coleoptiles may enhance the ability of seedlings to cope with stress conditions, and also indicate the potential application of the SbC1 gene in improving crop stress resistance.
In summary, Through GWAS, VIGS experiments, and haplotype analysis, we successfully identified an R2R3-MYB transcription factor, SbC1, specifically positive regulates anthocyanin accumulation in sorghum coleoptile. Transcriptome analysis further demonstrated the activation of anthocyanin biosynthesis genes by SbC1. Additionally, comparative genomic analysis and transformation experiments have proved the analogous function of SbC1 to OsC1 in terms of regulating anthocyanin biosynthesis. Additionally, the interaction between SbC1 and Tan1 was also demonstrated using different strategies. Recently, Yang et al. identified a bHLH transcription factor, SbPLSH1, that is closely associated with anthocyanin accumulation in the sorghum leaf sheath (Yang et al. 2024). Whether SbPLSH1 interacts with SbC1 or whether other unidentified bHLH members participate in coleoptile anthocyanin accumulation remains to be further elucidated. In future studies, we will focus on the identification and function analysis of other genes that regulate sorghum anthocyanin and tannin accumulation to comprehensively revealing the regulatory roles of these genes in coleoptile and other tissues pigmentation in sorghum.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
YD, RW and JH contributed to original writing the manuscript. YD, JH, JX, NC, XG, JG, JY and WL contributed to performed experiments, data analysis and interpretation. YZ, SL and YD designed and supervise the research, provided funding and review manuscripts. All authors read and approved the final version of the manuscript submitted for publication.
Funding
This work was supported by the National Natural Science Foundation of China (32160459), the Guizhou Natural Science Foundation of China (QKHJC[2023]YB169), the Innovation Capacity Building Project of Guizhou Scientific Institutions (QKFQ[2022]007), the Guizhou Academy of Agricultural Sciences Projects [National Science Foundation by Guizhou Academy of Agricultural Sciences (2022) 03, Guizhou Agricultural Germplasm Resources (2023) 06], and the Innovative Capacity Building of Breeding Research Infrastructure in Guizhou Province (QKFQ[2022]014). Guizhou provincial key laboratory of biotechnology breeding for special minor cereals (QKHPT[2025]026).
Data availability
The raw sequence data presented in this paper were previously published (Boatwright et al. 2022; Zhang et al. 2023) and have been deposited in both the China National GeneBank (CNP0002968) and the European Variant Archive (PRJEB51985).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanqing Ding, Ruoruo Wang and Jiaxian He have contributed equally to this work.
Contributor Information
Shengjun Li, Email: li_sj@qibebt.ac.cn.
Liyi Zhang, Email: 570129883@qq.com.
References
- Allan AC, Espley RV (2018) MYBs drive novel consumer traits in fruits and vegetables. Trends Plant Sci 23:693–705. 10.1016/j.tplants.2018.06.001 [DOI] [PubMed] [Google Scholar]
- An XH, Tian Y, Chen KQ, Wang XF, Hao YJ (2012) The apple WD40 protein MdTTG1 interacts with bHLH but not MYB proteins to regulate anthocyanin accumulation. J Plant Physiol 169:710–717. 10.1016/j.jplph.2012.01.015 [DOI] [PubMed] [Google Scholar]
- An JP, Wang XF, Zhang XW, Xu HF, Bi SQ, You CX, Hao YJ (2020) An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol J 18:337–353. 10.1111/pbi.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JP, Zhang XW, Bi SQ, You CX, Wang XF, Hao YJ (2020) The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple. Plant J 101:573–589. 10.1111/tpj.14555 [DOI] [PubMed] [Google Scholar]
- Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, Estep M, Feng L, Vaughn JN, Grimwood J, Jenkins J, Barry K, Lindquist E, Hellsten U, Deshpande S, Wang X, Wu X, Mitros T, Triplett J, Yang X, Ye C-Y, Mauro-Herrera M, Wang L, Li P, Sharma M, Sharma R, Ronald PC, Panaud O, Kellogg EA, Brutnell TP, Doust AN, Tuskan GA, Rokhsar D, Devos KM (2012) Reference genome sequence of the model plant Setaria. Nat Biotechnol 30(6):555–561. 10.1038/nbt.2196 [DOI] [PubMed] [Google Scholar]
- Boatwright JL, Sapkota S, Jin H, Schnable JC, Brenton Z, Boyles R, Kresovich S (2022) Sorghum association panel whole-genome sequencing establishes cornerstone resource for dissecting genomic diversity. Plant J 111:888–904. 10.1111/tpj.15853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddu J, Svabek C, Ibraheem F, Jones AD, Chopra S (2005) Characterization of a deletion allele of a sorghum Myb gene yellow seed1 showing loss of 3-deoxyflavonoids. Plant Sci 169:542–552. 10.1016/j.plantsci.2005.05.007 [Google Scholar]
- Boyles RE, Pfeiffer BK, Cooper EA, Zielinski KJ, Myers MT, Rooney WL, Kresovich S (2017) Quantitative trait loci mapping of agronomic and yield traits in two grain sorghum biparental families. Crop Sci 57:2443–2456. 10.2135/cropsci2016.12.0988 [Google Scholar]
- Buchfink B, Xie C, Huson DH (2015) Fast and sensitive protein alignment using DIAMOND. Nat Methods 12(1):59–60. 10.1038/nmeth.3176 [DOI] [PubMed] [Google Scholar]
- Burland, T. G. (1999). DNASTAR’s Lasergene sequence analysis software. In S. Misener & S. A. Krawetz (Eds.), Bioinformatics Methods and Protocols (71–91). Humana Press. 10.1385/1-59259-192-2:71 [DOI] [PubMed]
- Cappellini F, Marinelli A, Toccaceli M, Tonelli C, Petroni K (2021) Anthocyanins: from mechanisms of regulation in plants to health benefits in foods. Front Plant Sci 12:748049. 10.3389/fpls.2021.748049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CC, Strahle JT, Selinger DA, Chandler VL (2004) Mutations in the pale aleurone color1 regulatory gene of the Zea mays anthocyanin pathway have distinct phenotypes relative to the functionally similar TRANSPARENT TESTA GLABRA1 gene in Arabidopsis thaliana. The Plant Cell 16:450–464. 10.1105/tpc.018796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham LA, Paulsmeyer M, Juvik JA (2019) Prospects for economical natural colorants: insights from maize. Theor Appl Genet 132:2927–2946. 10.1007/s00122-019-03414-0 [DOI] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34(17):i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu X, Li S, Liu C, Zhang Y, Luo L, Miao L, Yang W, Xiao Z, Zhong Y, Li J, Chen R, Chen S (2022) Co-expression of transcription factors ZmC1 and ZmR2 establishes an efficient and accurate haploid embryo identification system in maize. Plant J 111:1296–1307. 10.1111/tpj.15888 [DOI] [PubMed] [Google Scholar]
- Choudhury BI, Khan ML, Dayanandan S (2014) Patterns of nucleotide diversity and phenotypes of two domestication related genes (OsC1 and Wx) in indigenous rice varieties in Northeast India. BMC Genetics 15:71. 10.1186/1471-2156-15-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone KC, Cocciolone SM, Burr FA, Burr B (1993) Maize anthocyanin regulatory gene pl is a duplicate of c1 that functions in the plant. Plant Cell 5:1795–1805. 10.1105/tpc.5.12.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R, 1000 Genomes Project Analysis Group (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158. 10.1093/bioinformatics/btr330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PK, Shin DH, Choi SB, Park YI (2012) Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol Cells 34:501–507. 10.1007/s10059-012-0151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Wang Y, Xu J, Jiang F, Li W, Zhang Q, Yang L, Zhao Z, Cheng B, Cao N, Gao X, Zhang X, Zou G, Yang F, Zhang L (2024) A telomere-to-telomere genome assembly of Hongyingzi, a sorghum cultivar used for Chinese Baijiu production. Crop J 12:635–640. 10.1016/j.cj.2024.02.011 [Google Scholar]
- Dixon RA, Liu C, Jun JH (2013) Metabolic engineering of anthocyanins and condensed tannins in plants. Curr Opin Biotechnol 24:329–335. 10.1016/j.copbio.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Dong SS, He WM, Ji JJ, Zhang C, Guo Y, Yang TL (2021) LDBlockShow: a fast and convenient tool for visualizing linkage disequilibrium and haplotype blocks based on variant call format files. Brief Bioinform 22(4):bbaa227. 10.1093/bib/bbaa227 [DOI] [PubMed] [Google Scholar]
- Du S, Wang Z, Chen Y, Tan Y, Li X, Zhu W, He G, Lei K, Guo L, Zhang Y (2022) Coleoptile purple line regulated by A-P gene system is a valuable marker trait for seed purity identification in hybrid rice. Rice Sci 29:451–461. 10.1016/j.rsci.2022.07.005 [Google Scholar]
- Dykes L (2019) Sorghum phytochemicals and their potential impact on human health. In: Zhao Zuo-Yu, Dahlberg Jeff (eds) Sorghum: methods and protocols. Springer New York, New York, NY, pp 121–140. 10.1007/978-1-4939-9039-9_9 [DOI] [PubMed] [Google Scholar]
- Fan FJ, Fan YY, Du JH, Zhuang JY (2008) Fine mapping of C (chromogen for anthocyanin) gene in rice. Rice Sci 15:1–6. 10.1016/s1672-6308(08)60012-8 [Google Scholar]
- Gao Y, Liu J, Chen Y, Tang H, Wang Y, He Y, Ou Y, Sun X, Wang S, Yao Y (2018) Tomato SlAN11 regulates flavonoid biosynthesis and seed dormancy by interaction with bHLH proteins but not with MYB proteins. Hortic Res 5:27. 10.1038/s41438-018-0032-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girma G, Nida H, Seyoum A, Mekonen M, Nega A, Lule D, Dessalegn K, Bekele A, Gebreyohannes A, Adeyanju A, Tirfessa A, Ayana G, Taddese T, Mekbib F, Belete K, Tesso T, Ejeta G, Mengiste T (2019) A large-scale genome-wide association analyses of ethiopian sorghum landrace collection reveal loci associated with important traits. Front Plant Sci 10:691. 10.3389/fpls.2019.00691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827. 10.1111/j.1365-313X.2007.03373.x [DOI] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40(D1):1178–1186. 10.1093/nar/gkr944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57:761–780. 10.1146/annurev.arplant.57.032905.105248 [DOI] [PubMed] [Google Scholar]
- He G, Zhang R, Jiang S, Wang H, Ming F (2023) The MYB transcription factor RcMYB1 plays a central role in rose anthocyanin biosynthesis. Hortic Res 10:uhad080. 10.1093/hr/uhad080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Zhao X, Xu Y, Wang Y, Zhang Z, Xiao L, Hudson M, Hu R, Li S (2023) An efficient virus-induced gene silencing (VIGS) system for gene functional studies in Miscanthus. GCB Bioenergy 15:805–820. 10.1111/gcbb.13051 [Google Scholar]
- He W, Yang J, Jing Y et al (2023) NGenomeSyn: an easy-to-use and flexible tool for publication-ready visualization of syntenic relationships across multiple genomes. Bioinformatics 39(3):btad121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Yuan Y, Tang Z, Huang Y, Kang C, Deng X, Xu Q (2019) Retrotransposon promoter of Ruby1 controls both light- and cold-induced accumulation of anthocyanins in blood orange. Plant Cell Environ 42:3092–3104. 10.1111/pce.13609 [DOI] [PubMed] [Google Scholar]
- Huang X, Huang S, Han B, Li J (2022) The integrated genomics of crop domestication and breeding. Cell 185:2828–2839. 10.1016/j.cell.2022.04.036 [DOI] [PubMed] [Google Scholar]
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T (2002) Three-dimensional progression of programmed death in the rice coleoptile. Int Rev Cytol 218:221–258. 10.1016/S0074-7696(02)18014-4 [DOI] [PubMed] [Google Scholar]
- Jiang W, Liu T, Nan W, Jeewani DC, Niu Y, Li C, Wang Y, Shi X, Wang C, Wang J, Li Y, Gao X, Wang Z (2018) Two transcription factors TaPpm1 and TaPpb1 co-regulate anthocyanin biosynthesis in purple pericarps of wheat. J Exp Bot 69:2555–2567. 10.1093/jxb/ery101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yue M, Liu Y, Zhang N, Lin Y, Zhang Y, Wang Y, Li M, Luo Y, Zhang Y, Wang X, Chen Q, Tang H (2023) A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol J 21:1140–1158. 10.1111/pbi.14024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Lyu S, Yu H, Zhang J, Sun B, Liu Q, Mao X, Chen P, Pan D, Chen W, Fan Z, Li C (2024) Transcription factor encoding gene OsC1 regulates leaf sheath color through anthocyanidin metabolism in Oryza rufipogon and Oryza sativa. BMC Plant Biol 24:147. 10.1186/s12870-024-04823-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlestkina EK, Pestsova EG, Röder MS, Börner A (2002) Molecular mapping, phenotypic expression and geographical distribution of genes determining anthocyanin pigmentation of coleoptiles in wheat (Triticum aestivum L.). Theor Appl Genet 104:632–637. 10.1007/s00122-001-0788-x [DOI] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL (2019) Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37(8):907–915. 10.1038/s41587-019-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Yang J, Ha SH, Kim JK, Lee JY, Lim SH (2021) An OsKala3, R2R3 MYB TF, is a common key player for black rice pericarp as main partner of an OsKala4, bHLH TF. Front Plant Sci 12:765049. 10.3389/fpls.2021.765049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach MJ, Calingacion MN, Fitzgerald MA, McCouch SR (2009) The origin and evolution of fragrance in rice (Oryza sativa L.). Proceedings of the National Academy of Sciences of the United States of America 106:14444–14449. 10.1073/pnas.0904077106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lin H, Li Q, Ruan Y, Cousins D, Li F, Gao S, Jackson K, Wen J, Murray JD, Xu P (2022) Anthocyanin pigmentation as a quantitative visual marker for arbuscular mycorrhizal fungal colonization of Medicago truncatula roots. New Phytol 236:1988–1998. 10.1111/nph.18504 [DOI] [PubMed] [Google Scholar]
- LaFountain AM, Yuan YW (2021) Repressors of anthocyanin biosynthesis. New Phytol 231:933–949. 10.1111/nph.17397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JS, Ma JX, Swigonova Z (2004) Gene loss and movement in the maize genome. Genome Res 14:1924–1931. 10.1101/gr.2332504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha S (2000) Primer premier 5. Biotech Softw Internet Rep 1:270–272 [Google Scholar]
- Li S (2014) Transcriptional control of flavonoid biosynthesis: fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal & Behav 9:e27522. 10.4161/psb.27522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H (2021) New strategies to improve minimap2 alignment accuracy. Bioinformatics 37(15):4572–4574. 10.1093/bioinformatics/btab705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14):1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fang X, Lin Z (2022) Convergent loss of anthocyanin pigments is controlled by the same MYB gene in cereals. J Exp Bot 73:6089–6102. 10.1093/jxb/erac270 [DOI] [PubMed] [Google Scholar]
- Li X, Shi Z, Gao J, Wang X, Guo K (2023) CandiHap: a haplotype analysis toolkit for natural variation study. Mol Breeding 43(3):21. 10.1007/s11032-023-01368-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang C, Xu X, Su Y, Gao Y, Yang J, Xie C, Ma J (2024) A MYB family transcription factor TdRCA1 from wild emmer wheat regulates anthocyanin biosynthesis in coleoptile. Theor Appl Genet 137:208. 10.1007/s00122-024-04723-9 [DOI] [PubMed] [Google Scholar]
- Liao HS, Yang CC, Hsieh MH (2022) Nitrogen deficiency- and sucrose-induced anthocyanin biosynthesis is modulated by HISTONE DEACETYLASE15 in Arabidopsis. J Exp Bot 73:3726–3742. 10.1093/jxb/erac067 [DOI] [PubMed] [Google Scholar]
- Liu J, Osbourn A, Ma P (2015) MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol Plant 8:689–708. 10.1016/j.molp.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Liu X, Duan J, Huo D, Li Q, Wang Q, Zhang Y, Niu L, Luo J (2021) The Paeonia qiui R2R3-MYB transcription factor PqMYB113 positively regulates anthocyanin accumulation in Arabidopsis thaliana and Tobacco. Front Plant Sci 12:810990. 10.3389/fpls.2021.810990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu N, Jun JH, Li Y, Dixon RA (2023) An unconventional proanthocyanidin pathway in maize. Nat Commun 14:4349. 10.1038/s41467-023-40014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace ES, Jordan DR (2010) Location of major effect genes in sorghum (Sorghum bicolor (L.) Moench). Theor Appl Genet 121:1339–1356. 10.1007/s00122-010-1392-8 [DOI] [PubMed] [Google Scholar]
- Mace E, Innes D, Hunt C, Wang X, Tao Y, Baxter J, Hassall M, Hathorn A, Jordan D (2019) The Sorghum QTL Atlas: a powerful tool for trait dissection, comparative genomics and crop improvement. Theor Appl Genet 132:751–766. 10.1007/s00122-018-3212-5 [DOI] [PubMed] [Google Scholar]
- McCormick RF, Truong SK, Sreedasyam A, Jenkins J, Shu S, Sims D, Kennedy M, Amirebrahimi M, Weers BD, McKinley B, Mattison A, Morishige DT, Grimwood J, Schmutz J, Mullet JE (2017) The Sorghum bicolor reference genome: improved assembly, gene annotations, a transcriptome atlas, and signatures of genome organization. Plant J 93(2):338–354. 10.1111/tpj.13781 [DOI] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20(9):1297–1303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Qi C, Wang C, Wang S, Zhou C, Ren Y, Cheng Z, Zhang X, Guo X, Zhao Z, Wang J, Lin Q, Zhu S, Wang H, Wang Z, Lei C, Wan J (2021) Determinant factors and regulatory systems for anthocyanin biosynthesis in rice apiculi and stigmas. Rice 14:37. 10.1186/s12284-021-00480-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K, Casas MI, Ferreyra MLF, Ferreyra LF, Mejía-Guerra MK, Pourcel L, Yilmaz A, Feller A, Carvalho B, Emiliani J, Rodriguez E, Pellegrinet S, McMullen M, Casati P, Grotewold E (2012) A genome-wide regulatory framework identifies maize pericarp color1 controlled genes. The Plant Cell 24:2745–2764. 10.1105/tpc.112.098004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nida H, Girma G, Mekonen M, Tirfessa A, Seyoum A, Bejiga T, Birhanu C, Dessalegn K, Senbetay T, Ayana G, Tesso T, Ejeta G, Mengiste T (2021) Genome-wide association analysis reveals seed protein loci as determinants of variations in grain mold resistance in sorghum. Theor Appl Genet 134:1167–1184. 10.1007/s00122-020-03762-2 [DOI] [PubMed] [Google Scholar]
- Olsen KM, Purugganan MD (2002) Molecular evidence on the origin and evolution of glutinous rice. Genetics 162:941–950. 10.1093/genetics/162.2.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, Orvis J, Haas B, Wortman J, Buell CR (2007) The TIGR rice genome annotation resource: improvements and new features. Nucl Acids Res 35:D883–D887. 10.1093/nar/gkl976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H (1987) The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J 6:3553–3558. 10.1002/j.1460-2075.1987.tb02684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroni K, Pilu R, Tonelli C (2014) Anthocyanins in corn: a wealth of genes for human health. Planta 240:901–911. 10.1007/s00425-014-2131-1 [DOI] [PubMed] [Google Scholar]
- Qiao W, Wang Y, Xu R, Yang Z, Sun Y, Su L, Zhang L, Wang J, Huang J, Zheng X, Liu S, Tian Y, Chen L, Liu X, Lan J, Yang Q (2021) A functional chromogen gene C from wild rice is involved in a different anthocyanin biosynthesis pathway in Indica and Japonica. Theor Appl Genet 134:1531–1543. 10.1007/s00122-021-03787-1 [DOI] [PubMed] [Google Scholar]
- Rhodes DH, Hoffmann L, Rooney WL, Ramu P, Morris GP, Kresovich S (2014) Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L) Moench] germplasm. J Agric Food Chem 62:10916–10927. 10.1021/jf503651t [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigo T, Wang T, Watanabe M, Tohge T (2020) Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr Opin Plant Biol 55:93–99. 10.1016/j.pbi.2020.04.001 [DOI] [PubMed] [Google Scholar]
- Saitoh K, Onishi K, Mikami I, Thidar K, Sano Y (2004) Allelic diversification at the C (OsC1) locus of wild and cultivated rice: nucleotide changes associated with phenotypes. Genetics 168:997–1007. 10.1534/genetics.103.018390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto W, Ohmori T, Kageyama K, Miyazaki C, Saito A, Murata M, Noda K, Maekawa M (2001) The Purple leaf (Pl) locus of rice: the Pl(w) allele has a complex organization and includes two genes encoding basic helix-loop-helix proteins involved in anthocyanin biosynthesis. Plant Cell Physiol 42:982–991. 10.1093/pcp/pce128 [DOI] [PubMed] [Google Scholar]
- Shi MZ, Xie DY (2014) Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent PatS Biotechnol 8:47–60. 10.2174/1872208307666131218123538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeva OY, Gordeeva EI, Arbuzova VS, Khlestkina EK (2017) Anthocyanins participate in protection of wheat seedlings from osmotic stress. Cer Res Commun 45(1):47–56. 10.1556/0806.44.2016.044 [Google Scholar]
- Smith CW, Frederiksen RA, Smith WC (2000) Sorghum: Origin, History, Technology and Production. Wiley, New York, NY [Google Scholar]
- Sun X, Zhang Z, Chen C, Wu W, Ren N, Jiang C, Yu J, Zhao Y, Zheng X, Yang Q, Zhang H, Li J, Li Z (2018) The C-S-A gene system regulates hull pigmentation and reveals evolution of anthocyanin biosynthesis pathway in rice. J Exp Bot 69:1485–1498. 10.1093/jxb/ery001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Krishnakumar V, Zeng X, Xu Z, Taranto A, Lomas JS, Zhang Y, Huang Y, Wang Y, Yim WC, Zhang J, Zhang X (2024) JCVI: A versatile toolkit for comparative genomics analysis. Imeta 3(4):e211. 10.1002/imt2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Zhao X, Wang X, Hathorn A, Hunt C, Cruickshank AW, van Oosterom EJ, Godwin ID, Mace ES, Jordan DR (2020) Large-scale GWAS in sorghum reveals common genetic control of grain size among cereals. Plant Biotechnol J 18(4):1093–1105. 10.1111/pbi.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Luo H, Xu J, Cruickshank A, Zhao X, Teng F, Hathorn A, Wu X, Liu Y, Shatte T, Jordan D, Jing H, Mace E (2021) Extensive variation within the pan-genome of cultivated and wild sorghum. Nat Plants 7(6):766–773. 10.1038/s41477-021-00925-x [DOI] [PubMed] [Google Scholar]
- Tao Y, Trusov Y, Zhao X, Wang X, Cruickshank AW, Hunt C, van Oosterom EJ, Hathorn A, Liu G, Godwin ID, Botella JR, Mace ES, Jordan DR (2021) Manipulating assimilate availability provides insight into the genes controlling grain size in sorghum. Plant J 108(1):231–243. 10.1111/tpj.15437 [DOI] [PubMed] [Google Scholar]
- Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47(6):969–976. 10.1111/j.1365-313X.2006.02836.x [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Z (2021) GAPIT version 3: boosting power and accuracy for genomic association and prediction. Genomics Proteomics Bioinform 19(4):629–640. 10.1016/j.gpb.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ji W, Liu Y, Zhou P, Meng Y, Zhang P, Wen J, Mysore KS, Zhai J, Young ND, Tian Z, Niu L, Lin H (2020) The antagonistic MYB paralogs RH1 and RH2 govern anthocyanin leaf markings in Medicago truncatula. New Phytol 229:3330–3344. 10.1111/nph.17097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Lu N, Liu C, Dixon RA, Wu Q, Mao Y, Yang Y, Zheng X, He L, Zhao B, Zhang F, Yang S, Chen H, Jun JH, Li Y, Liu C, Liu Y, Chen J (2022) MtGSTF7, a TT19-like GST gene, is essential for accumulation of anthocyanins, but not proanthocyanins in Medicago truncatula. J Exp Bot 73(12):4129–4146. 10.1093/jxb/erac112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Coe E, Nelson W, Zhang J, Zhou S, He R, Schaeffer M, Collura K, Kudrna D, Faga BP, Wissotski M, Golser W, Rock SM, Graves TA, Fulton RS, Coe E, Schnable PS, Schwartz DC, Ware D, Clifton SW, Wilson RK, Wing RA (2009) The physical and genetic framework of the maize B73 genome. PLoS Genet 5(11):e1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li X, Xiang W, Zhu C, Lin Z, Wu Y, Li J, Pandravada S, Ridder DD, Bai G, Wang ML, Trick HN, Bean SR, Tuinstra MR, Tesso TT, Yu J (2012) Presence of tannins in sorghum grains is conditioned by different natural alleles of Tannin1. Proceedings of the National Academy of Sciences of the United States of America 109:10281–10286. 10.1073/pnas.1201700109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Guo T, Mu Q, Wang J, Li X, Wu Y, Tian B, Wang ML, Bai G, Perumal R, Trick HN, Bean SR, Dweikat IM, Tuinstra MR, Morris G, Tesso TT, Yu J, Li X (2019) Allelochemicals targeted to balance competing selections in African agroecosystems. Nature Plants 5:1229–1236. 10.1038/s41477-019-0563-0 [DOI] [PubMed] [Google Scholar]
- Xia D, Zhou H, Wang Y, Li P, Fu P, Wu B, He Y (2021) How rice organs are colored: the genetic basis of anthocyanin biosynthesis in rice. Crop J 9:598–608. 10.1016/j.cj.2021.03.013 [Google Scholar]
- Xie P, Shi J, Tang S, Chen C, Khan A, Zhang F, Xiong Y, Li C, He W, Wang G, Lei F, Wu Y, Xie Q (2019) Control of bird feeding behavior by tannin1 through modulating the biosynthesis of polyphenols and fatty acid-derived volatiles in sorghum. Mol Plant 12:1315–1324. 10.1016/j.molp.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Xu W, Dubos C, Lepiniec L (2015) Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 20:176–185. 10.1016/j.tplants.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Yan Y, Zhao J, Lin S, Li M, Liu J, Raymond O, Vergne P, Kong W, Wu Q, Zhang X, Bao M, Bendahmane M, Fu X (2023) Light-mediated anthocyanin biosynthesis in rose petals involves a balanced regulatory module comprising transcription factors RhHY5, RhMYB114a, and RhMYB3b. J Exp Bot 74:5783–5804. 10.1093/jxb/erad253 [DOI] [PubMed] [Google Scholar]
- Yang X, Wang J, Xia X, Zhang Z, He J, Nong B, Luo T, Feng R, Wu Y, Pan Y, Xiong F, Zeng Y, Chen C, Guo H, Xu Z, Li D, Deng G (2021) OsTTG1, a WD40 repeat gene, regulates anthocyanin biosynthesis in rice. Plant J 107:198–214. 10.1111/tpj.15285 [DOI] [PubMed] [Google Scholar]
- Yang P, Bai Y, Zhao D, Cui J, Yang W, Gao Y, Zhang J, Wang Z, Wang M, Xue W, Chang J (2024) Identification and functional marker development of SbPLSH1 conferring purple leaf sheath in sorghum. Theor Appl Genet 137:137. 10.1007/s00122-024-04623-y [DOI] [PubMed] [Google Scholar]
- Zhang P, Chopra S, Peterson T (2000) A segmental gene duplication generated differentially expressed myb-homologous genes in maize. Plant Cell 12:2311–2322. 10.1105/tpc.12.12.2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelli E, Martin C (2014) Engineering anthocyanin biosynthesis in plants. Curr Protoc Plant Biol 19:81–90. 10.1016/j.pbi.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Zhang L, Xu J, Ding Y, Cao N, Gao X, Feng Z, Li K, Cheng B, Zhou L, Ren M, Tao Y, Zou G (2023) GWAS of grain color and tannin content in Chinese sorghum based on whole-genome sequencing. Theor Appl Genet 136:77. 10.1007/s00122-023-04307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wu H, Zhu H, Huang C, Liu C, Chang Y, Kong Z, Zhou Z, Wang G, Lin Y, Chen H (2019) Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves. New Phytol 223:705–721. 10.1111/nph.158076 [DOI] [PubMed] [Google Scholar]
- Zheng J, Wu H, Zhao M, Yang Z, Zhou Z, Guo Y, Lin Y, Chen H (2021) OsMYB3 is a R2R3-MYB gene responsible for anthocyanin biosynthesis in black rice. Mol Breed 41:51. 10.1007/s11032-021-01244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Bi G, Zhou JM (2018) Luciferase complementation assay for protein-protein interactions in plants. Curr Protoc Plant Biol 3:42–50. 10.1002/cppb.200666 [DOI] [PubMed] [Google Scholar]
- Zhu T, Wang L, Rimbert H, Rodriguez JC, Deal KR, De Oliveira R, Choulet F, Keeble-Gagnère G, Tibbits J, Rogers J, Eversole K (2021) Optical maps refine the bread wheat Triticum aestivum cv. Chinese Spring genome assembly. Plant J 107(1):303–314. 10.1111/tpj.15289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data presented in this paper were previously published (Boatwright et al. 2022; Zhang et al. 2023) and have been deposited in both the China National GeneBank (CNP0002968) and the European Variant Archive (PRJEB51985).