Abstract
Piroplasmosis is a major tick-borne disease that causes significant economic losses in livestock production across various regions. The present study aimed to provide a comprehensive assessment of the prevalence of bovine piroplasmosis in Gansu and Qinghai provinces, China, offering crucial baseline data for the development of effective control and prevention strategies. A total of 736 bovine blood samples were collected from Tianshui, Pingliang, and Lanzhou in Gansu, and Xining and Haidong in Qinghai. These samples were analyzed using PCR with universal Piroplasma primers targeting the 18S rRNA gene. The overall prevalence of bovine Piroplasma infection was found to be 25.54% (188/736). Three Theileria species were identified, including T. orientalis (14.27%, 105/736), T. annulata (10.73%, 79/736), and T. sinensis (0.54%, 4/736). No cases of Babesia or mixed infections were detected in this study. Notably, T. sinensis was reported for the first time in Pingliang, highlighting its potential expansion in the region. In conclusion, bovine piroplasmosis remains prevalent in both Gansu and Qinghai provinces, with T. orientalis being the predominant species. These findings underscore the need for strengthened surveillance and improved strategies for the prevention and control of piroplasmosis in the region.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00436-025-08491-3.
Keywords: Molecular prevalence, Theileriosis, Piroplasm, Bovine
Introduction
Piroplasms are protozoan parasites belonging to the Apicomplexa phylum, Sporozoa class, and Piroplasmida order. Among the most notable species are Babesia (family Babesiidae) and Theileria (family Theileriidae), both of which are tick-borne pathogens of significant concern. These parasites primarily infect red blood cells, lymphocytes, or macrophages in the host, leading to piroplasmosis, a disease that results in substantial economic losses to the global livestock industry. Clinically, the disease is characterized by initial symptoms of lethargy, anorexia, anemia, and persistent fever (ranging from 39 to 42 °C). As the disease progresses, affected animals may experience further complications, including loss of appetite, cessation of rumination, diarrhea, and the presence of blood or mucus in the feces. In severe cases, piroplasmosis can be fatal. Livestock that recover from the infection often become asymptomatic carriers, harboring the parasite for life and acting as reservoirs for continued transmission.
Theileria species primarily cause diseases in animals, with a global distribution that significantly impacts livestock production. These parasites are especially prevalent in tropical and subtropical regions, where they commonly infect ruminants, particularly cattle (Nazifi et al. 2009). In China, T. annulata, T. orientalis, and T. sinensis have been reported in cattle infected with Theileria spp. (Sun et al. 2020). In yaks, T. orientalis, T. sinensis have been documented (Lan et al. 2020; Li et al. 2020). Among these, T. annulata is the most pathogenic species, with a high mortality rate, and it affects cattle of all age groups (Brühlmann et al. 2024). In contrast, infections with T. orientalis and T. sinensis are generally less severe but are common in China (Luan et al. 2023; Zhang et al. 2024). The outbreaks of these infections in cattle are often mediated by Ixodidae ticks, which transmit the parasite through blood feeding. Ticks are the sole and indispensable vectors for bovine piroplasma transmission (Ceylan et al. 2024).
Gansu and Qinghai provinces, with their complex and varied landscapes, provide favorable geographic conditions for tick survival. Previous studies have documented the coexistence of 35 tick species (excluding unidentified species) in Gansu Province, including 9 species of Ixodes, 9 species of Haemaphysalis, 5 species of Dermacentor, 4 species of Hyalomma, 4 species of Rhipicephalus, 1 species of Ornithodoros, and 2 species of Argas (Wang et al. 1980). In Qinghai Province, 31 species of ticks from 6 genera and 2 families have been identified, with Haemaphysalis qinghaiensis and Dermacentor nuttalli being the dominant species, found in most areas (Han et al. 2016). In the Xining, Haidong, and Haibei regions, the primary endemic ticks are Haemaphysalis qinghaiensis, Haemaphysalis longicornis, and Dermacentor nuttalli. H. longicornis is the main vector for T. orientalis, while T. sinensis is transmitted by H. qinghaiensis (Wang et al. 2021; Sun et al. 2024), and Hyalomma anatolicum is the primary vector for T. annulata (Abbasi et al. 2024).
The spread of bovine piroplasma in Gansu and Qinghai provinces is facilitated by a diverse array of vector species, with grazing farms being at higher risk for tick-cattle contact. Ticks can easily enter cattle pens along with livestock, thus increasing the potential for disease transmission. Bovine piroplasma infection is a significant parasitic disease that poses a threat to both cattle and yak farming industries (Musinguzi 2017). Therefore, regular surveillance of piroplasma infections in key livestock areas, particularly in the northwestern regions of China, is critical. In this study, we collected cattle blood samples from five regions in Gansu and Qinghai provinces between 2023 and 2024. Nested PCR was utilized to detect the 18S rRNA gene of piroplasma, and the infection status was evaluated. Additionally, the tick species harboring piroplasma were identified, and their genetic variations were analyzed. This research aims to provide essential data to inform the scientific prevention and control of piroplasma in cattle in northwestern China.
Materials and methods
Study area and sample collection
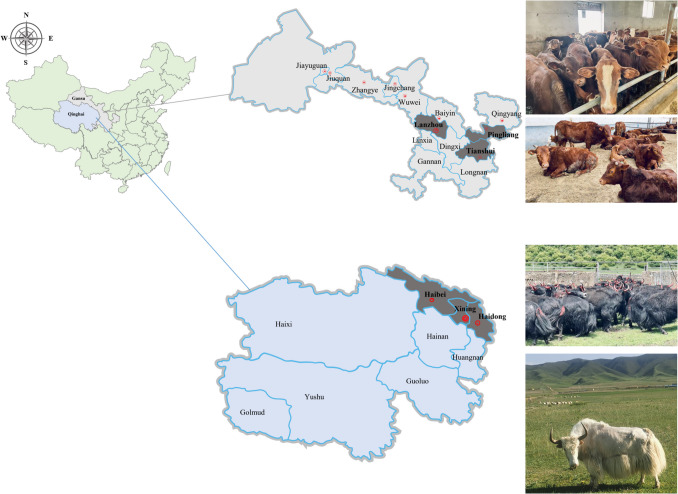
From March 2023 to May 2024, a total of 736 blood samples were randomly collected from cattle or yaks in farms located in Tianshui (Gansu), Pingliang (Gansu), Lanzhou (Gansu) city, Xining (Qinghai), Haibei (Qinghai) and Haidong (Qinghai) district, Gansu and Qinghai Province, China (Fig. 1). Blood samples were collected into EDTA-coated vacutainer tubes and transported in iceboxes to the laboratory of Vectors and Vector-Borne Diseases (VVBD), Lanzhou Veterinary Research Institute (LVRI), Chinese Academy of Agricultural Sciences (CAAS). Genomic DNA was extracted from 200 μl of each blood sample using a commercial DNA extraction kit, according to the manufacturer’s instructions (Qiagen DNA Blood Mini-Kit, Germany). DNA samples were stored at − 20 °C until use.
Fig. 1.
Geographical distribution of the sampled cities in Gansu and Qinghai Provinces, Northwest China
DNA extraction and nested PCR amplification of piroplasms 18S rRNA gene
A nested PCR (nPCR) was conducted to detect the presence of piroplasms in the blood samples, according to a report by Yang et al. (2014). In the first round of PCR, a set of primers reported previously (Piro1-S: 5’ CTTGACGGTAGGGTATTGGC- 3’, Piro3-AS:5’-CCTTCCTTTAAGTGATAAGGTTCAC- 3’) was used to amplify a 1400 bp gene fragment of 18S rRNA. In the second round of reactions, 2 μl of the products of the first-round reactions was used as a template. Minor modifications were made to the primers, according to 18S rRNA gene sequences of canine piroplasms available in GenBank, to amplify a 407 bp fragment, Piro-A: 5’-ATTACCCAATMCBGACACVGKG- 3’ and Piro-B: 5’-TTAAATACGAATGCCCCCAAC- 3’ (Olmeda et al. 1997). The PCR parameters and composition of the PCR mixture have been described previously (Yang et al. 2014). Briefly, reactions were conducted in a total of 25 μl, including 2.5 μl of 10 × PCR buffer, 2.0 μl of dNTP (2.5 mM each), 1.25U of Taq DNA polymerase (TaKaRa, Dalian, China), 2 μl of template DNA, 1 μl of each primer (10 pmol), and 16.25 μl of double distilled water. The PCR reaction was started with a one-step initial denaturation at 95 °C for 3 min, which was followed by 35 cycles of denaturation at 95 °C for 30 s, 55 °C for 30 s, and extension at 72 °C for 90 s, with a final extension step at 72 °C for 5 min. Positive and negative controls were included in all the PCR tests. PCR products were subjected to 1.5% agarose gel (AddGene, Watertown, MA, USA) electrophoresis and visualized by staining with SYBR Safe DNA Gel Stain (Termo Fisher Scientifc).
DNA sequencing and phylogenetic analysis
The PCR products of Piroplasma-positive samples from different sampling sites were sent to Sangon Biotech (Shanghai) Co., Ltd., and the accuracy of the sequence was verified by bidirectional Sanger sequencing. Species of Piroplasma were identified via the BLASTn tool provided by NCBI GenBank. The representative sequences successfully sequenced in this study were aligned with reference sequences downloaded from NCBI GenBank using ClustalW in MEGA7 (http://www.megasoftware.net/). The phylogenetic tree was constructed via the neighbor-joining (NJ) method. Evolutionary distances were calculated using the Kimura 2-parameter model, and tree reliability was assessed by bootstrap analysis with 1000 replicates. No trimming was applied after alignment, as all sequences were of comparable length (~ 407 bp) corresponding to the nested PCR product.
Statistical analysis
The difference in Theileria spp. prevalence among different locations and the order of animals was analyzed with the chi-square (χ2) test, using SPSS 20.0 (IBM, Chicago, IL, USA). The difference was considered statistically significant when p < 0.05. Ninety-five percent confidence intervals (95% CIs) and odds ratios (ORs) were also calculated to explore the strengths of association between Theileria spp. positivity and each factor.
Results
Prevalence of piroplasma in cattle
From a total of 736 samples across Gansu and Qinghai, including cattle and yaks, a prevalence of 25.54% (188/736) was observed for Theileria spp. and no Babesia spp., as determined by PCR analysis. Specifically, among the 217 cattle samples from Gansu, 75.12% (163/217) were found to be infected, with T. orientalis showing the highest prevalence at 98 cases, followed by T. annulata with 61 cases and T. sinensis with 4 cases. In contrast, the 519 yak samples from Qinghai showed a much lower infection rate of 4.82% (25/519), with T. annulata being the most prevalent, found in 18 cases, followed by T. orientalis in 7 cases.
When broken down by time periods, from March to June 2023 in Gansu, cattle from the Tianshui and Pingliang regions exhibited infection rates ranging from 65.05 to 100%, with T. orientalis and T. annulata being the predominant species (Table 1). In Qinghai, yaks in Xining, Haidong, Haibei, and across the province from March 2023 to June 2024 showed a marked difference in infection rates, with the highest being observed in Qinghai’s Haibei region, which had a prevalence rate of 6.55%, whereas Datong and the other areas showed even lower positivity rates, around 2.04%. Statistical analysis revealed significant variations in infection susceptibility between regions and hosts, with notable seasonal differences, as well as differences between years (2023 vs 2024) and Theileria species (Table 2).
Table 1.
Infection of bovine Theileria spp. in Gansu and Qinghai of China
| Time | Location | Host | No. of examined | No. of positive | Prevalence (%) | OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| 2023.3 | Gansu-Tianshui | Cattle | 22 | 17 (T. orientalis 15, T. annulata 2) | 77.27 | 59.8–94.8 | |
| Gansu-Pingliang | Cattle | 45 | 42 (T. orientalis 25, T. annulata 15, T. sinensi 2) | 93.33 | 86.0–100.6 | ||
| Gansu | Cattle | 67 | 59 (T. orientalis 40, T. annulata 17, T. sinensi 2) | 88.06 | 80.3–95.8 | 0.057 | |
| 2023.4 | Gansu-Tianshui | Cattle | 25 | 25 (T. orientalis 13, T. annulata 12) | 100 | 100–100 | |
| Gansu-Pingliang | Cattle | 11 | 10 (T. orientalis 6, T. annulata 3, T. sinensi 1) | 90.9 | 73.9–107.9 | ||
| Gansu | Cattle | 36 | 35 (T. orientalis 19, T. annulata 15, T. sinensi 1) | 97.22 | 91.9–100 | 0.126 | |
| 2023.5 | Gansu- Pingliang | Cattle | 103 | 67 (T. orientalis 39, T. annulata 27, T. sinensi 1) | 65.05 | 55.8–33.3 | |
| Gansu-Lanzhou | Cattle | 11 | 2 (T. annulata 2) | 18.18 | 0–41.0 | ||
| Gansu | Cattle | 114 | 69 (T. orientalis 39, T. annulata 29, T. sinensi 1) | 60.53 | 51.6–69.5 | 0.003 | |
| Total | Gansu | Cattle | 217 | 163 (T. orientalis 98, T. annulata 61, T. sinensi 4) | 75.12 | 20.7–29.4 | |
| 2023.6 | Qinghai-Xining | Yaks | 94 | 6 (T. orientalis 2, T. annulata 4) | 6.38 | 1.4–11.3 | |
| Qinghai-Haibei | Yaks | 229 | 15 (T. orientalis 4, T. annulata 11) | 6.55 | 3.3–9.85 | ||
| Qinghai | Yaks | 323 | 21 (T. orientalis 6, T. annulata 15) | 6.5 | 3.8–9.2 | 0.956 | |
| 2024.3–2024.6 | Qinghai-Haidong | Yaks | 196 | 4 (T. orientalis 1, T. annulata 3) | 2.04 | 0.1–4.0 | |
| Total | Qinghai | Yaks | 519 | 25 (T. orientalis 7, T. annulata 18) | 4.82 | 3.0–6.7 | |
| Total | Gansu and Qinghai | Cattle and Yaks | 736 | 188 (T. orientalis 105, T. annulata 79, T. sinensi 4) | 25.54 | 22.4–28.7 |
Table 2.
Factors associated with the prevalence of Theileria spp. in cattle and yaks in Gansu and Qinghai of China
| Characteristics | No. of examined | No. of positive | Prevalence (%) |
|---|---|---|---|
| Cattle types | |||
| Cattle | 217 | 163 | 75.12 |
| Yaks | 519 | 25 | 4.82 |
| p-value | 0.000 | ||
| Location | |||
| Gansu | 217 | 163 | 75.12 |
| Qinghai | 519 | 25 | 4.82 |
| p-value | 0.000 | ||
| Tianshui | 47 | 42 | 89.36 |
| Pinagliang | 159 | 119 | 74.84 |
| Lanzhou | 11 | 2 | 18.18 |
| p-value | 0.000 | ||
| Xining | 94 | 6 | 6.38 |
| Haidong | 196 | 4 | 2.04 |
| Haibei | 229 | 15 | 6.55 |
| p-value | 0.071 | ||
| Month (Gansu) | |||
| March | 67 | 59 | 88.06 |
| April | 36 | 35 | 97.22 |
| May | 114 | 69 | 60.53 |
| p-value | 0.001 | ||
| Year (Qinghai) | |||
| 2023 | 323 | 21 | 6.5 |
| 2024 | 196 | 4 | 2.04 |
| p-value | 0.021 | ||
| Theileria spp. species | |||
| T. orientalis | 736 | 105 | 14.27 |
| T. annulata | 736 | 79 | 10.73 |
| T. sinensi | 736 | 4 | 0.54 |
| p-value | 0.000 | ||
Phylogenetic tree
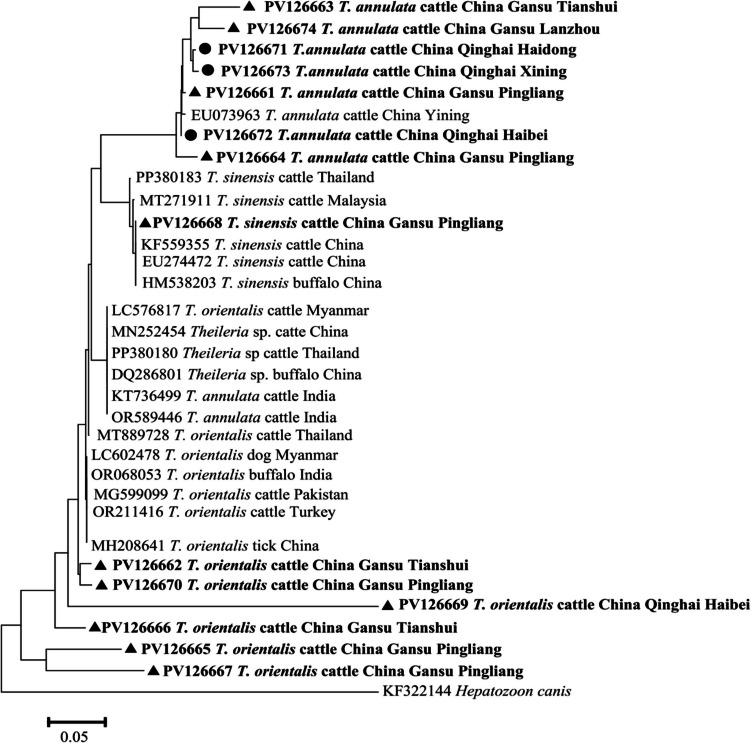
The phylogenetic analyses, utilizing the 18S rRNA gene, delineated distinct evolutionary lineages within Theileria. The Hepatozoon canis strain (KF322144) was used as an outgroup. The tree was constructed on the basis of neighbor-joining phylogeny and 1000 bootstrap replicates via MEGA 7. The gene sequences of Theileria were observed to segregate into three primary clades: T. orientalis, T. annulata, and T. sinensis. In Gansu and Qinghai, T. orientalis was found to have six haplotypes (PV126662, PV126670, PV126669, PV126666, PV126665, PV126667), which were closely related to T. orientalis from ticks in China (MH208641), cattle in Turkey (OR211416), cattle in Pakistan (MG599099), cattle in Thailand (MT889728), buffalo in India (OR068053), and a dog in Myanmar (LC602478). For T. sinensis, all isolates, including those from Pingliang cattle (PV126668) as well as cattle from Malaysia (MT271911), Thailand (PP380183) and China (KF559355, EU274472, HM538203), formed a single cohesive group. T. annulata, which clustered together with isolates from cattle in Yining, Xinjiang of China (EU073963). From phylogenetic analysis, T. orientalis and T. sinensis are closely related, whereas T. annulata is more distantly related and grouped with T. orientalis (Fig. 2).
Fig. 2.
Phylogenetic relationships among nucleotide sequences of barcode regions of 18S rRNA of Theileria spp. The neighbor-joining method was used to construct the trees by the Kimura- 2-parameter model. The numbers on the branches are percent bootstrapping values from 1000 replicates, with values > 50% shown in the tree. Each sequence is identified by its species, hosts, accession number, and country. Theileria species identified in the present study are indicated in bold type. ▲: Black triangles indicate sequences from Gansu Province (Lanzhou, Tianshui, and Pingliang); • : Black circles indicate sequences from Qinghai Province (Xining, Haidong, and Haibei)
Discussion
Theileria spp. and Babesia spp. are common tick-borne protozoan parasites with complex and poorly understood pathogenicity, which is influenced by multiple factors.
Theileria spp. has been identified in many animal hosts, such as cattle, buffaloes, sheep, goats, deer, yaks, takins, camels, giraffes, horses, dogs, cats, and rodents (Schnittger et al. 2022). Previous studies have reviewed the pooled prevalence of Theileria spp. in cattle in China, which was found to be 32.4%, as identified using a random effects model. Among the three cattle species, dairy cows had the lowest prevalence (21.5%) (Chen et al. 2022). The prevalence of T. annulata (22.2%) and T. orientalis (26.2%) was higher compared to other species of Theileria (T. luwenshuni: 0.9%, T. orientalis: 33%, T. ovis: 0.21%, T. sinensis: 20.2%, T. uilenbergi: 6.2%, Others: 0.9%) (Watts et al. 2016).
In the present study, we demonstrated the molecular detection based on the 18S rRNA gene in samples from beef cattle and yaks in Gansu and Qinghai provinces in the northwestern part of China, and Theileria spp. is widespread in cattle and yaks in Gansu and Qinghai provinces. Only three species were detected, which is consistent with the findings on piroplasms infection in cattle from Xinjiang with a similar survey period (Zhang et al. 2024). However, the infection rate in this study was much lower than that in Kashi, Xinjiang. The dominant species of proplasmosis in Qinghai and Gansu was T. orientalis (14.27%), followed by T. annulata (10.73%). The infection rate of T. sinensis was very low, only 0.54%, and the presence of T. sinensis was reported for the first time in Pingliang areas; therefore, its spread in cattle still needs further investigation. In contrast, the dominant species of Theileria spp. in Xinjiang cattle is T. annulata, and the infection rate of T. sinensis in Xinjiang cattle is lower than that in Gansu beef cattle. This may be due to T. sinensis being first discovered in Gansu Province (Bai et al. 2002). Additionally, the infection rate of piroplasms in yaks (4.82%) was much lower compared to that in beef cattle (75.12%), and T. sinensis was not detected in yaks from Qinghai province.
The geographical and climatic differences between Gansu and Qinghai provinces play a crucial role in shaping the transmission dynamics of Theileria spp. Both provinces are located in northwestern China, bordering Xinjiang and Tibet, yet they exhibit distinct climatic and topographical characteristics. Qinghai, characterized by its typical plateau climate, serves as the primary breeding region for yaks in China. In contrast, Gansu’s climate is influenced by multiple factors, resulting in diverse climatic conditions that support a well-developed beef cattle industry, shaped by both cultural and environmental factors. The plateau environment in Qinghai provides an optimal habitat for yaks; however, compared to the more variable climate in Gansu, cattle in Gansu are exposed to a broader range of environmental influences, which may contribute to regional differences in infection rates. Both provinces experience distinct seasonal variations, with sunshine duration generally exceeding the national average. The complex geographical landscape, combined with high biodiversity, provides a suitable habitat for ticks, thereby facilitating the transmission of tick-borne diseases such as piroplasmosis (Esteve-Gassent et al. 2016). The findings of this study suggest that climate and environmental conditions influence disease prevalence, as demonstrated by the notable disparity in infection rates between yaks and beef cattle. Yaks exhibited significantly lower infection rates than beef cattle, which may be attributed to differences in ecological behavior, feeding environment, and immune status. As a species native to high-altitude regions such as Qinghai, yaks may have evolved stronger natural immunity compared to beef cattle (Hu et al. 2021). Additionally, their relatively isolated habitat could limit their exposure to tick vectors, thereby reducing the likelihood of pathogen transmission. Interestingly, during our sampling, no ticks were observed on yaks, suggesting that environmental factors such as altitude and climate in Qinghai may influence the seasonal activity patterns of ticks (Ma et al. 2024). This may result in a temporal mismatch between peak tick activity and our sampling period, potentially explaining the absence of ticks despite the detection of Theileria spp. in yak blood samples. This finding suggests that infections may have resulted from prior exposure to ticks during active transmission seasons. Furthermore, yak samples from Datong Hui and Tu autonomous county in Xining were collected from a yak sire breeding facility—not a general livestock farm—operating under a closed management system, where, in principle, animals are only exported and no new individuals are introduced. The detection of Theileria in these animals raises the possibility that some yaks were already carrying the pathogen before entering the breeding facility. This highlights the need to strengthen pre-entry screening and biosecurity measures to prevent the introduction and persistence of tick-borne pathogens within such controlled environments. Similarly, in Gansu, no ticks were detected on beef cattle, yet Theileria infection was still present. This suggests that these animals, like the yaks, may have acquired the infection before their arrival at the farm, further emphasizing the necessity of rigorous pre-introduction screening protocols to minimize disease transmission risks. These findings underscore the importance of considering not only active vector populations but also historical pathogen exposure and pre-farm disease status in understanding the epidemiology of Theileria infections. Future studies should focus on characterizing seasonal fluctuations in tick activity, evaluating the persistence of Theileria infections in livestock, and assessing the effectiveness of pre-entry screening and quarantine measures. A deeper understanding of these factors will be crucial for refining disease control strategies, particularly in high-altitude or climatically diverse regions where vector surveillance and management present unique challenges.
In our study, the results showed that T. orientalis and T. annulata were common in cattle in Gansu and Qinghai provinces, with T. orientalis being the most widespread. Theileria orientalis typically presents as a mild or asymptomatic infection, especially in immunologically healthy cattle (Wang et al. 2020). Although it can cause mild clinical symptoms such as increased body temperature, anemia, and loss of appetite, it generally does not result in serious illness or death (Onizawa and Jenkins 2024). In some cases, the infection may be subclinical and difficult to diagnose (Ghaemi et al. 2012). Unlike T. annulata, clinical manifestation with T. orientalis infection is due to its effects on erythrocytes, with the infection sometimes designated as Theileria associated bovine anemia (TABA) (Lakew et al. 2023). Treatment of T. orientalis is usually effective with antiparasitic drugs like oxytetracycline and imidocarb (Imadox), and recovery is generally rapid (Emery 2021). Buparvaquone (BPQ) did not demonstrate efficacy in clearing subclinical T. orientalis infection, but it is effective for T. annulata (Mhadhbi et al. 2010; Bastos et al. 2024). In contrast, T. annulata is more pathogenic and can cause acute or chronic illness, including high fever, jaundice, severe anemia, weight loss, and, in severe cases, death (Agina et al. 2020). As the cause of tropical theileriosis, particularly in the Middle East, Africa, and parts of Asia, T. annulata triggers a more intense immune response and often results in significant immune escape (Gharbi et al. 2020; Nene and Morrison 2016). The higher parasitic load in the host body leads to more severe clinical symptoms (Tajeri et al. 2025). For T. annulata, due to its greater pathogenicity, more treatment protocols, including vaccination and strict vector control, may be necessary to prevent large outbreaks (Woods et al. 2021). Theileria sinensis generally exhibits low pathogenicity and is typically associated with mild clinical symptoms such as low fever, mild anemia, and loss of appetite (Agina et al. 2021). Severe clinical symptoms and mortality are rare. Theileria sinensis may be considered a cause of subclinical infection, particularly in immunologically healthy cattle (Liu et al. 2010). While it is less pathogenic than T. annulata, its presence in cattle herds can still affect livestock productivity. At present, no studies have reported specific drugs for it, but it can be speculated that other drugs that are effective against Theileria spp. may also have significant effects on it. Despite its mild pathogenicity, regular tick control measures are essential to prevent the spread of T. sinensis, especially in areas with high Theileria prevalence. While T. orientalis and T. sinensis are generally less pathogenic than T. annulata, the latter remains a more significant threat to livestock health due to its higher pathogenicity and more complex immune interactions (Chen et al. 2022). However, all three species necessitate regular monitoring and control strategies to manage their spread and impact on livestock farming.
Therefore, effective control measures against these three pathogens are essential. In order to reduce the impact of T. annulata on livestock production, farmers should adopt a comprehensive control strategy. The following measures are included: (1) Development of vaccines against T. orientalis and T. annulata to reduce the risk of infection in cattle (Agina et al. 2020). (2) Controlling tick populations is key to reducing the spread of T. annulata. Regular use of effective insecticides can help reduce tick transmission rates (Merino et al. 2013). (3) Through phased screening, infected cattle are detected in a timely manner and isolated, and treated to reduce the risk of the outbreak spreading. Regular epidemiological research is essential to improve our understanding of bovine piroplasm infections and to develop effective strategies for disease control and prevention in Chinese cattle herds. In this study, the 18S rRNA gene was used for molecular detection, which demonstrated its effectiveness in the detection of T. annulata. Therefore, it is recommended to conduct regular molecular testing in high-risk areas to detect pathogens in time and take effective prevention and control measures.
This study mainly focused on beef cattle and yak samples from Gansu and Qinghai provinces, and the sample sources were relatively limited. The uneven sample sizes of different varieties and different regions may affect the comprehensiveness and representativeness of the results. There was also a lack of testing for other pathogens, and we failed to detect Babesia or co-infections of pathogens, possibly due to limitations in sample collection and testing methods. Future studies could be combined with the detection of other pathogens to explore the interactions between different pathogens.
The genetic diversity and population structure of T. annulata are worthy of further study. In particular, for the genetic variants of T. sinensis and T. orientalis, the transmission dynamics can be understood through large-scale molecular epidemiological investigations. It is more important to further study the role of ticks in the transmission of T. annulata and explore the ability of ticks to carry different pathogens and their impact on infection rates in epidemic areas. Future studies should also pay attention to the differences in immune response between yaks and beef cattle, especially the immune tolerance and immune escape mechanism after Theileria infection, so as to provide theoretical support for the development of vaccines. This study provided important molecular epidemiological data on the distribution and genetic diversity of T. orientalis in Gansu and Qinghai provinces, revealed the dominant position of T. orientalis in these two provinces, and suggested strengthening tick control and pathogen detection. In order to reduce the impact of T. annulata on livestock production, it is urgent to take effective prevention and control measures and strengthen the monitoring and early diagnosis of relevant animal populations.
Conclusion
The findings of this study reveal that bovine piroplasmosis is widespread in the regions of Tianshui and Pingliang in Gansu, as well as in Xining, Haidong, and Haibei in Qinghai, as evidenced by PCR amplification of the 18S rRNA gene. This investigation enhances our understanding of the geographic distribution and genetic diversity of piroplasma infections in both beef cattle and yaks in northwestern China. Among the detected species, T. orientalis was the most prevalent, followed by T. annulata and T. sinensis. To reduce the economic impact of bovine piroplasma on livestock farming, it is essential for farmers to adopt integrated control measures. These measures should include not only effective treatment protocols for infected animals but also strategies to reduce tick infestations. The strategic use of acaricides is crucial in controlling tick populations and, thereby, limiting the transmission of piroplasmosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 1 (PNG 2.07 MB)
Supplementary Material 2 (PNG 694 KB)
Supplementary Material 3 (ZIP 30.3 MB)
Supplementary Material 4 (ZIP 14.1 MB)
Acknowledgements
The author would like to thank all the teachers and classmates who participated in the study and all the grassroots veterinarian staff who contributed to the sample collection.
Author contributions
Yijun Chai: Completed the manuscript, collected experimental samples, analyzed the experimental data, consulted the literature, and performed the overall experiment. Jin Che: Discussed and modified the manuscript, consulted the literature, and collected experimental samples. Shuaiyang Zhao: collected experimental samples. Guiquan Guan: Undertook funding, designed the experiments, and reviewed and edited the manuscript. Jinming Wang: Collected experimental samples, assisted in completing the experiment and manuscript. Hong Yin: Collected the experimental samples and overall design and supervision of the experiment. All authors read and approved the final manuscript.
Funding
The study was financially supported by NSFC (№31972701), the National Key Research and Development Program of China (2024YFD180010X), the Science Fund for Creative Research Groups (22 JR5RA024), and the Special Project (22 CX8 NA011) of Gansu Province, the Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP- 2021-LVRI), NBCITS (CARS- 37), and the National Parasitic Resources Center (NPRC- 2019 - 194 - 30).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Not applicable.
Consent to participate
Written informed consent was obtained from the cattle owner.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jinming Wang, Email: wjm0403@caas.cn.
Guiquan Guan, Email: guanguiquan@caas.cn.
Hong Yin, Email: yinhong@caas.cn.
References
- Abbasi AM, Nasir S, Bajwa AA, Akbar H, Artigas-Jerónimo S, Muñoz-Hernández C, Sánchez-Sánchez M, Moraga-Fernández A, de Mera IGF, de la Fuente J, Rashid MI (2024) De novo assembly of sialotranscriptome of Hyalomma anatolicum and insights into expression dynamics in response to Theileria annulata infection. Exp Appl Acarol 93(4):887-906. 10.1007/s10493-024-00962-z [DOI] [PubMed]
- Agina OA, Shaari MR, Isa NMM, Ajat M, Zamri-Saad M, Mazlan M, Muhamad AS, Kassim AA, Ha LC, Rusli FH, Masaud D, Hamzah H (2021) Molecular detection of Theileria species, Anaplasma species, Candidatus Mycoplasma haemobos, Trypanosoma evansi and first evidence of Theileria sinensis-associated bovine anaemia in crossbred Kedah-Kelantan x Brahman cattle. BMC Vet Res 17(1):246. 10.1186/s12917-021-02902- [DOI] [PMC free article] [PubMed]
- Agina OA, Shaari MR, Isa NMM, Ajat M, Zamri-Saad M, Hamzah H (2020) Clinical pathology, immunopathology and advanced vaccine technology in bovine theileriosis: a review. Pathogens 9(9):697. 10.3390/pathogens9090697 [DOI] [PMC free article] [PubMed]
- Bai Q, Liu GY, Yin H, Zhao QZ, Liu DK, Ren JX, Li X (2002) Theileria sinensis sp nov: a new species of bovine Theileria - molecular taxonomic studies. Acta Veterinaria Et Zootechnica Sinica 02:185–190 [Google Scholar]
- Bastos RG, Hassan A, Onzere CK, Herndon DR, Villarino NF, Laughery JM, Fry LM (2024) Transient efficacy of buparvaquone against the US isolate of Theileria orientalis Ikeda genotype in sub-clinically infected cattle. Front Vet Sci 11:1421710. 10.3389/fvets.2024.1421710 [DOI] [PMC free article] [PubMed]
- Brühlmann F, Perry C, Griessen C, Gunasekera K, Reymond J-L, Naguleswaran A, Rottenberg S, Woods K, Olias P (2024) TurboID mapping reveals the exportome of secreted intrinsically disordered proteins in the transforming parasite Theileria annulata. mBio 15(6): e0341223. 10.1128/mbio.03412-23 [DOI] [PMC free article] [PubMed]
- Ceylan O, Ma Z, Ceylan C, Culha MH, Galon EM, Ji S, Li H, Zafar I, Mohanta UK, Xuan X, Sevinc F (2024) Wide bovine tick-borne pathogen spectrum: predominancy of Theileria annulata and the first molecular detection of Ehrlichia minasensis in Turkey. Vet Res Commun 48(2):1037-1059. 10.1007/s11259-023-10266-z [DOI] [PubMed]
- Chen Y, Chen YY, Liu G, Lyu C, Hu Y, An Q, Qiu HY, Zhao Q, Wang CR (2022) Prevalence of Theileria in cattle in China: a systematic review and meta-analysis. Microb Pathog 162:105369. 10.1016/j.micpath.2021.105369 [DOI] [PubMed]
- Emery DL (2021) Approaches to Integrated Parasite Management (IPM) for Theileria orientalis with an emphasis on immunity. Pathogens 10(9):1153. 10.3390/pathogens10091153 [DOI] [PMC free article] [PubMed]
- Esteve-Gassent MD, Castro-Arellano I, Feria-Arroyo TP, Patino R, Li AY, Medina RF, de León AA, Rodríguez-Vivas RI (2016) Translating ecology, physiology, biochemistry and population genetics research to meet the challenge of tick and tick-borne diseases in North America. Arch Insect Biochem Physiol 92:38-64. 10.1002/arch.21327 [DOI] [PMC free article] [PubMed]
- Ghaemi P, Hoghooghi-Rad N, Shayan P, Eckert B (2012) Detection of Theileria orientalis in Iran by semi-nested PCR. Parasitol Res 110(2):527-31. 10.1007/s00436-011-2517-y [DOI] [PubMed]
- Gharbi M, Darghouth MA, Elati K, Al-Hosary AAT, Ayadi O, Salih DA, El Hussein AM, Mhadhbi M, Khamassi Khbou M, Hassan SM, Obara I, Ahmed LS, Ahmed J (2020) Current status of tropical theileriosis in Northern Africa: a review of recent epidemiological investigations and implications for control. Transbound Emerg 67(Suppl 1):8-25. 10.1111/tbed.13312 [DOI] [PubMed]
- Han R, Liu Z, Niu Q, Yang J, Guo K, Chen Z, Luo J, Yin H (2016) Analysis of tick species diversity in Qinghai region. Youth Committee of Parasitology, Zoological Society of China. In Proceedings of the 10th National Symposium for Young Researchers in Parasitology. State Key Laboratory of Veterinary Etiological Biology, Gansu Provincial Key Laboratory of Parasitology, Key Laboratory of Animal Diseases of the Ministry of Agriculture, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, p 89
- Hu C, Ding L, Jiang C, Ma C, Liu B, Li D, Degen AA (2021) Effects of management, dietary intake, and genotype on rumen morphology, fermentation, and microbiota, and on meat quality in yaks and cattle. Front Nutr 8:755255. 10.3389/fnut.2021.755255 [DOI] [PMC free article] [PubMed]
- Lakew BT, Eastwood S, Walkden-Brown SW (2023) Epidemiology and transmission of Theileria orientalis in Australasia. Pathogens 12(10):1187. 10.3390/pathogens12101187 [DOI] [PMC free article] [PubMed]
- Lan L, Li Y, Yang D (2020) Investigation of piroplsma infection in yaks in Ganzi prefecture, Sichuan province. China Animal Husbandry and Veterinary Medicine. 47(05):1514–1522 [Google Scholar]
- Li J, Jian Y, Jia L, Galon EM, Benedicto B, Wang G, Cai Q, Liu M, Li Y, Ji S, Tumwebaze MA, Ma L, Xuan X (2020) Molecular characterization of tick-borne bacteria and protozoans in yaks (Bos grunniens), Tibetan sheep (Ovis aries) and Bactrian camels (Camelus bactrianus) in the Qinghai-Tibetan Plateau Area, China. Ticks Tick Borne Dis 11(5):101466. 10.1016/j.ttbdis.2020.101466 [DOI] [PubMed]
- Liu A, Guan G, Liu Z, Liu J, Leblanc N, Li Y, Gao J, Ma M, Niu Q, Ren Q, Bai Q, Yin H, Luo J (2010) Detecting and differentiating Theileria sergenti and Theileria sinensis in cattle and yaks by PCR based on major piroplasm surface protein (MPSP). Exp Parasitol 126(4):476-81. 10.1016/j.exppara.2010.05.024 [DOI] [PubMed]
- Luan Y, Gou J, Zhong D, Ma L, Yin C, Shu M, Liu G, Lin Q (2023) The tick-borne pathogens: an overview of China’s situation. Acta Parasitol 68(1):1-20. 10.1007/s11686-023-00658-1 [DOI] [PMC free article] [PubMed]
- Ma R, Li C, Gao A, Jiang N, Li J, Hu W, Feng X (2024) Tick species diversity and potential distribution alternation of dominant ticks under different climate scenarios in Xinjiang, China. PLoS Negl Trop Dis 18(4): e0012108. 10.1371/journal.pntd.0012108 [DOI] [PMC free article] [PubMed]
- Merino O, Alberdi P, Pérez de la Lastra JM, de la Fuente J (2013) Tick vaccines and the control of tick-borne pathogens. Front Cell Infect Microbiol 3:30. 10.3389/fcimb.2013.00030 [DOI] [PMC free article] [PubMed]
- Mhadhbi M, Naouach A, Boumiza A, Chaabani MF, BenAbderazzak S, Darghouth MA (2010) In vivo evidence for the resistance of Theileria annulata to buparvaquone. Vet Parasitol 169(3-4):241-7. 10.1016/j.vetpar.2010.01.013 [DOI] [PubMed]
- Musinguzi SP (2017) Molecular epidemiological studies on trypanosomosis and piroplasmosis among livestock in southern Africa and central Asia. Doctoral Thesis, Graduate School of Animal Husbandry, Obihiro University of Agriculture and Veterinary Medicine.
- Nazifi S, Razavi SM, Esmailnejad Z, Gheisari H (2009) Study on acute phase proteins (haptoglobin, serum amyloid A, fibrinogen, and ceruloplasmin) changes and their diagnostic values in bovine tropical theileriosis. Parasitol Res 105(1):41-6. 10.1007/s00436-009-1360-x [DOI] [PubMed]
- Nene V, Morrison WI (2016) Approaches to vaccination against Theileria parva and Theileria annulata. Parasite Immunol 38(12):724-734. 10.1111/pim.12388 [DOI] [PMC free article] [PubMed]
- Olmeda AS, Armstrong PM, Rosenthal BM, Valladares B, del Castillo A, de Armas F, Miguelez M, González A, Rodríguez Rodríguez JA, Spielman A, Telford SR 3rd (1997) A subtropical case of human babesiosis. Acta Trop 67(3):229-34. 10.1016/s0001-706x(97)00045-4 [DOI] [PubMed]
- Onizawa E, Jenkins C (2024) Epidemiology, clinical signs, and risk factors associated with theileriosis in Australian Cattle (2006-2022). Pathogens 13(3):253. 10.3390/pathogens13030253 [DOI] [PMC free article] [PubMed]
- Schnittger L, Ganzinelli S, Bhoora R, Omondi D, Nijhof AM, Florin-Christensen M (2022) The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: species compilation, molecular phylogeny, and evolutionary insights. Parasitol Res 121(5):1207-1245. 10.1007/s00436-022-07424-8 [DOI] [PubMed]
- Sun M, Guan G, Liu Z, Wang J, Wang D, Wang S, Ma C, Cheng S, Yin H, Luo J (2020) Molecular survey and genetic diversity of Babesia spp. and Theileria spp. in cattle in Gansu Province, China. Acta Parasitol 65(2):422-429. 10.2478/s11686-020-00179-1 [DOI] [PubMed]
- Sun TB, Chen LF, Zou YX, Wu CY, Guo XY, Zhang XQ, Wang QY (2024) Epidemiological investigation and genetic evolution analysis of Bovine piroplasma in Hebei Province. Chinese Journal of Preventive Veterinary Medicine. 46(05):529–534 [Google Scholar]
- Tajeri S, de Laté PL, Hemmink JD, Vrettou C, Langsley G, Morrison WI (2025) Theileria annulata infects B-cells in sheep, which display lower dissemination potential compared to T. lestoquardi-infected ovine B-cells. Ticks Tick Borne Dis 16(2):102443. 10.1016/j.ttbdis.2025.102443 [DOI] [PubMed]
- Wang J, Yang J, Gao S, Liu A, Rashid M, Li Y, Liu Z, Liu J, Liu G, Luo J, Guan G, Yin H (2020) Rapid detection and differentiation of Theileria annulata, T. orientalis and T. sinensis using high-resolution melting analysis. Ticks Tick Borne Dis 11(1):101312. 10.1016/j.ttbdis.2019.101312 [DOI] [PubMed]
- Wang X, Li S, Yang L, Shen X (1980) Ticks of Gansu. J Lanzhou Univ. 10.13885/j.issn.0455-2059.1980.03.011
- Wang Y, Wang B, Zhang Q, Li Y, Yang Z, Han S, Yuan G, Wang S, He H (2021) The common occurrence of Theileria ovis in Tibetan sheep and the first report of Theileria sinensis in yaks from southern Qinghai, China. Acta Parasitol 66(4):1177-1185. 10.1007/s11686-021-00381-9 [DOI] [PubMed]
- Watts JG, Playford MC, Hickey KL (2016) Theileria orientalis: a review. N Z Vet J 64:3-9. 10.1080/00480169.2015.1064792 [DOI] [PubMed]
- Woods K, Perry C, Brühlmann F, Olias P (2021) Theileria’s strategies and effector mechanisms for host cell transformation: from invasion to immortalization. Front Cell Dev Biol 9:662805. 10.3389/fcell.2021.662805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Li YQ, Liu ZJ, Liu JL, Guan GQ, Chen Z, Luo JX, Wang XL, Yin H (2014) Molecular evidence for piroplasms in wild Reeves’ muntjac (Muntiacus reevesi) in China. Parasitol Int 63(5):713-6. 10.1016/j.parint.2014.06.002 [DOI] [PubMed]
- Zhang Y, Chen X, Zhang Y, Pu N, Zhao W, Wang Z, Sun Y, Jia C, Bo X (2024) An epidemiological survey of bovine piroplasmosis in Kashgar, Xinjiang, China. Parasitol Res 123(12):415. 10.1007/s00436-024-08439-z [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1 (PNG 2.07 MB)
Supplementary Material 2 (PNG 694 KB)
Supplementary Material 3 (ZIP 30.3 MB)
Supplementary Material 4 (ZIP 14.1 MB)
Data Availability Statement
No datasets were generated or analysed during the current study.