Abstract
Cell cycle arrest in G1 in response to ionizing radiation or senescence is believed to be provoked by inactivation of G1 cyclin-cyclin-dependent kinases (Cdks) by the Cdk inhibitor p21Cip1/Waf1/Sdi1. We provide evidence that in addition to exerting negative control of the G1/S phase transition, p21 may play a role at the onset of mitosis. In nontransformed fibroblasts, p21 transiently reaccumulates in the nucleus near the G2/M-phase boundary, concomitant with cyclin B1 nuclear translocation, and associates with a fraction of cyclin A-Cdk and cyclin B1-Cdk complexes. Premitotic nuclear accumulation of cyclin B1 is not detectable in cells with low p21 levels, such as fibroblasts expressing the viral human papillomavirus type 16 E6 oncoprotein, which functionally inactivates p53, or in tumor-derived cells. Moreover, synchronized E6-expressing fibroblasts show accelerated entry into mitosis compared to wild-type cells and exhibit higher cyclin A- and cyclin B1-associated kinase activities. Finally, primary embryonic fibroblasts derived from p21−/− mice have significantly reduced numbers of premitotic cells with nuclear cyclin B1. These data suggest that p21 promotes a transient pause late in G2 that may contribute to the implementation of late cell cycle checkpoint controls.
Progression through the phases of the cell cycle is driven by the periodic activation of several cyclin-dependent kinases (Cdks). The activity of Cdk complexes is tightly regulated by a variety of mechanisms, such as periodic cyclin accumulation and degradation, nuclear localization, phosphorylation of Cdks, and association with a number of different Cdk inhibitors (CKIs) (31). These inhibitors were originally recovered from inactive cyclin-Cdk complexes isolated from quiescent cells and cells arrested in G1 by γ irradiation or incubation with transforming growth factor β or cyclic AMP, as well as from senescent fibroblasts (reviewed in reference 36). Their primary targets appeared to be Cdks associated with G1 cyclins (D-type cyclins and cyclin E), rate-limiting regulators of the G1/S-phase transition (23, 24, 30, 32). A model has therefore gained acceptance whereby activity of cyclin D1- and cyclin E-associated kinases and hence S-phase entry are inhibited when the cells are exposed to conditions that result in accumulation of CKIs (36). Cell cycle arrest in G1 caused by DNA damage or cellular senescence is, at least in part, mediated by p53-dependent accumulation of p21Cip1/Waf1/Sdi1 (p21) (reviewed in reference 36). Ectopic expression of p21 induces G1 arrest (17, 22), whereas the presence of p21 antisense RNA in quiescent cells promotes S phase (21). Consistent with its role as a G1 regulator, p21 was shown to accumulate in the nucleus during G1 phase, whereas both p21 mRNA and protein levels decline before S phase (11, 21).
However, several observations raise the possibility that p21 has roles at other stages of the cell cycle. First, p21 mRNA in human fibroblasts shows bimodal periodicity, with peaks in G1 and G2/M (19). Second, p21 protein was detected in a variety of different cyclin-Cdk complexes in nontransformed fibroblasts, including those not associated with the G1/S-phase transition (17, 40, 42). Whereas its association with cyclin D1 did not seem to vary during the cell cycle, p21 was shown to be present in cyclin A and cyclin B complexes only during the latter phases of the cell cycle, suggesting a functional interaction with cyclin A- and cyclin B-associated kinases (19).
To gain additional insight into the cell cycle roles of p21, we analyzed its cell cycle-dependent subcellular localization by immunofluorescence microscopy in nontransformed cells. This approach allowed the analysis of individual and unperturbed cells and, by simultaneous staining of proteins in pairs, was able to provide accurate information on relative subcellular localization and periodicity of each. In conjunction with biochemical analysis of cyclin complexes in extracts from synchronized cells, our immunolocalization experiments suggest that p21 plays a role during the G2/M-phase transition.
MATERIALS AND METHODS
Cell lines, synchronizations, and cell cycle analyses.
Normal human diploid foreskin fibroblasts (HDF; cell line Hs68) and human fetal lung fibroblasts (cell line IMR-90) were obtained from the American Type Culture Collection (Rockville, Md.). They were grown in Dulbecco modified Eagle medium (GIBCO) and in minimal essential medium–F-12 (50:50; GIBCO), respectively, supplemented with 10% fetal calf serum (FCS; GIBCO and BioWhittaker), 2 mM l-glutamine, 50 U of penicillin per ml, and 50 mg of streptomycin per ml. IMR-90 fibroblasts expressing human papillomavirus type 16 (HPV16) E6 oncogenes were generously provided by J. Shay (The University of Texas SW Medical Center, Dallas) (35). Human mammary epithelial cells were grown in supplemented MCDB 170 medium (Clonetics Corporation) as described previously (37). Mouse embryonic fibroblasts (MEFs) from p21−/− mice were provided generously by J. Brugarolas and T. Jacks (Massachusetts Institute of Technology, Cambridge, Mass.) at passage 3. The cells were grown in Dulbecco modified Eagle medium supplemented with 10% FCS (BioWhittaker), 2 mM l-glutamine, 50 U of penicillin per ml, and 50 mg of streptomycin per ml.
Normal HDF were synchronized at the G1/S phase boundary with aphidicolin by using a modification of the protocol described by Sewing et al. (34). Cells were arrested in G0 by serum deprivation for 3 to 4 days, and after serum (15% FCS) stimulation for 12 to 15 h, aphidicolin (5 μg/ml; Sigma) was added for another 24 h. The cells were released into the cell cycle by extensive washing with phosphate-buffered saline (PBS) and medium. Mitotic cells started to accumulate 10 h after release from the block, reaching the maximum after 12 h, as estimated by microscopic observation. For some experiments, IMR-90 fibroblasts were also arrested by serum deprivation for 3 to 5 days, after which they were stimulated with 20% FCS until appearance of mitotic cells (36 to 40 h). By a similar protocol (14), serum-starved cells were stimulated with 15% FCS for 10 h and arrested at the G1/S-phase boundary by addition of 1 mM hydroxyurea for 18 to 20 h. The cells were released into cell cycle by extensive washing with PBS (twice) and medium containing 10% FCS (twice). By this protocol, 15 to 20% mitotic cells (Hs68) were usually observed 8 h after release.
For fluorescence-activated cell sorting (FACS) analysis, cells were harvested by trypsinization, washed in PBS, and resuspended in 0.1% sodium citrate with 10% dimethyl sulfoxide. Propidium iodide-stained cells were analyzed in a fluorescence-activated cell sorter (FACScan; Becton Dickinson). The percentage of cells in different phases of cell cycle was determined by using the CellFit program.
Immunoprecipitations, immunoblot analyses, and kinase assays.
Preparation of cell extracts and the conditions for immunoprecipitation, histone H1 kinase assays, and immunoblotting have been described previously (9, 10). To conserve a limited amount of cell lysates (as the signal of p21 in cyclin immunocomplexes was relatively weak for some experiments, we needed large amount of extracts [100 to 500 μg of protein]), we usually carried out sequential immunoprecipitations of different cyclin complexes.
p21 depletion was carried out by incubating total-cell extracts (usually 200 μg) with saturating amounts of p21-specific antibodies (2 h), whereas the mock samples were incubated with protein A-Sepharose beads only. Upon treatment, aliquots of supernatant (40 to 50 μg) were removed for Western blot analysis, and the remaining extract was used for sequential immunoprecipitation using indicated cyclin-specific antibodies. As secondary antibodies, we used anti-mouse (Promega) and anti-rabbit (Promega) immunoglobulin G (IgG)-horseradish peroxidase conjugates. For Western blot analysis of immunocomplexes, when both immunoprecipitating and detecting antibodies were from the same origin, horseradish peroxidase-conjugated ImmunoPure protein A/G (Pierce) was used. In these cases, immunocomplexes immobilized on protein A-Sepharose bead samples were not boiled but only incubated in Laemmli buffer at 37°C (15 min). The proteins were visualized by using the ECL (enhanced chemiluminescence) detection system (Amersham). When mentioned, the ECL-revealed immunoblots were quantified by densitometry, using a Shimadzu CS-930 scanner (Kyoto, Japan).
Immunofluorescence.
Exponentially growing cells were plated on glass coverslips (Erie Scientific, Portsmouth, N.H.) treated (optionally) with Cell-Tak (Collaborative Biomedical Products, Bedford, Mass.) according to the manufacturer’s instructions. The cells were fixed either in cold methanol (−20°C, 10 min) or in 3.7% paraformaldehyde in PBS for 15 to 30 min at room temperature. Paraformaldehyde-fixed cells were optionally quenched in 50 mM ammonium chloride (5 min, 0.1 M in PBS) and permeabilized with 0.2% Triton X-100 in PBS for 10 min. All subsequent solutions were prepared in PBS with 0.1% Tween (PBS-T). In some cases, prior to fixation the cells were pulse-labeled for 15 min with bromodeoxyuridine (BrdU; 1:1,000 dilution; Amersham) according to the manufacturer’s instructions. Incubations with primary and secondary antibodies were carried in humidified chamber at room temperature (60 min). All antibody dilutions were prepared in 5% FCS in PBS-T. Anti-cyclin A, anti-cyclin B1, anti-cyclin D1, and anti-p21 antibodies were used at 1:100 dilution; the anti-p21 monoclonal antibody was used at 10 μg/ml; anti-cyclin E, anti-cyclin D1, and anti-cyclin A hybridoma supernatants were used nondiluted. Secondary antibodies used in this study included fluorescein-conjugated goat anti-rabbit IgG (1:150 dilution; Cappel), Texas red-conjugated goat anti-mouse IgG (1:500 dilution; Molecular Probes, Inc.), and biotinylated goat anti-mouse or anti-rabbit IgG (1:200 dilution; Pierce). The biotinylated antibodies were detected by incubation with Texas red- or fluorescein-conjugated streptavidin (1:1,000 dilution; Molecular Probes). For BrdU staining, after staining for the first antigen, cells were incubated for 15 min with 2 N HCl, subsequently washed with PBS, and then incubated for 1 h with a solution of mouse anti-BrdU monoclonal antibody and nuclease (undiluted; Amersham), followed by 30-min incubation with anti-mouse Texas red-conjugated antibody (1:500 dilution; Molecular Probes).
The cells were mounted on glass slides with Mowiol (Calbiochem, La Jolla, Calif.) containing N-propyl gallate. Specimens were examined and photographed on a Nikon (Diaphot) microscope using 40× and 63× lenses. Micrographs were taken on Kodak Tmax 400 (for the black-and-white prints) and on Kodak Ektachrome 400 and Fujichrome Provia 1600 (for the colored slides and prints) films. Exposure times were always kept constant for the indicated antigen.
Antibodies.
The following primary antibodies were used: anti-cyclin A, three different rabbit antisera (reference 27 and S. I. Reed) and hybridoma BF683 supernatant (E. Lees and E. Harlow, Massachusetts General Hospital, Boston); anti-cyclin B1, rabbit antiserum (C. McGowan and P. Russel, The Scripps Research Institute, La Jolla, Calif.) and mouse monoclonal IgG (sc-245; Santa Cruz Biotechnology, Santa Cruz, Calif.); anti-cyclin D1, affinity-purified antibody (8) and hybridoma supernatant HD45 (E. Lees and E. Harlow); anti-cyclin E, affinity-purified antibody and two different hybridoma supernatants, HE-172 (9) and HE12 (12); anti-Cdc2, rabbit antiserum against C-terminal peptides (C. McGowan and P. Russel) and mouse monoclonal IgG (sc-054 [Santa Cruz] and C12720 [Transduction Laboratories, Lexington, Ky.); anti-Cdk2, rabbit antiserum against C-terminal peptide (sc-163; Santa Cruz) and mouse monoclonal IgG (C18520; Transduction Laboratories); anti-PSTAIRE (a common motif shared by many Cdks), monoclonal antibody raised against the peptide (41); anti-Cdk4, rabbit antiserum (sc-601; Santa Cruz); anti-p21Cip1, rabbit antiserum generated against bacterially produced p21 (S. I. Reed), mouse monoclonal IgG (C24420; Transduction Laboratories), and rabbit polyclonal antibodies (sc-397; Santa Cruz); and anti-p27Kip1, mouse monoclonal IgG (K25020; Transduction Laboratories).
RESULTS
p21 colocalizes in the nucleus with cyclin A and cyclin B1 in nontransformed cells.
To assess the cell cycle-dependent nuclear distribution of p21 in unperturbed nontransformed cells by indirect immunofluorescence, we used anti-p21 antibodies in conjunction with antibodies specific for different cyclins whose subcellular localization during the cell cycle has been well documented (5, 24, 25, 27). These experiments were carried out in exponentially growing cultures of normal human fibroblast cell lines, Hs68 and IMR-90, and human breast epithelial cells, BE184. As expected, p21 was predominantly localized in the nucleus of G1 cells, as shown by double immunolocalization with G1 cyclins (cyclin E and cyclin D1), whereas it was virtually absent in S-phase cells, as defined by BrdU incorporation or by the presence of cyclin A (Fig. 1A and B; Table 1). Interestingly, a significant population of the cyclin E-positive cells showed a very low p21 signal (Table 1), suggesting that during most of G1 cyclin E-Cdk2 complexes may be inhibited to some degree by the presence of p21, but near the onset of S phase, degradation (or delocalization) of p21 could promote the rapid and concerted activation of cyclin E-associated kinase. Similarly, p21 was absent in some cells showing strong cyclin D1 nuclear accumulation.
FIG. 1.
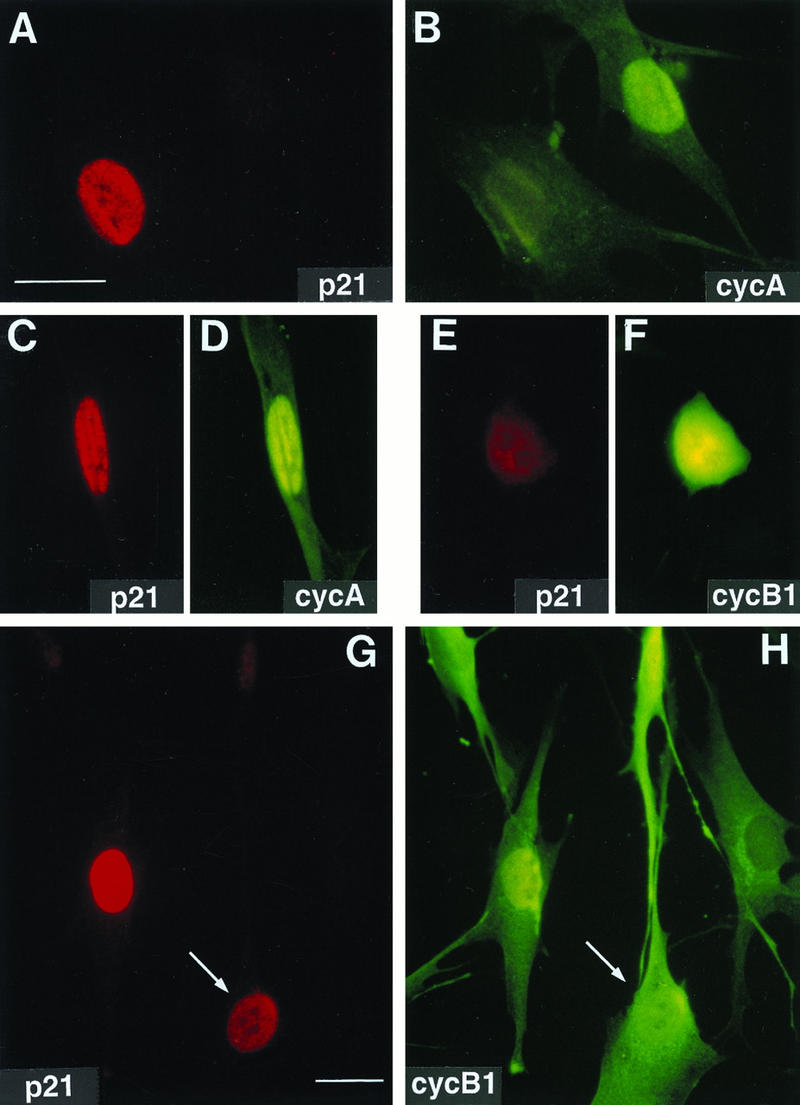
Nuclear colocalization of p21 with cyclin A and cyclin B1 in exponentially growing normal human fibroblasts. Asynchronous normal HDF (Hs68) were fixed in paraformaldehyde and simultaneously stained with mouse monoclonal anti-p21 (red; Texas red) and with rabbit polyclonal anti-cyclin A or anti-cyclin B1 (green; fluorescein) antibodies as described in Materials and Methods. Representative micrographs of cells in G1 phase (A and B), S phase (A, B, G, and H), and G2 phase (C, D, G, and H), and mitosis (E and F) are shown. Cyclin A accumulates in the nucleus in the beginning of the S phase, whereas cyclin B1 accumulates during late S phase and in G2 in the cytoplasm and enters the nucleus at the onset of mitosis (27). A cell with both cytoplasmic and nuclear cyclin B1 accumulation is marked with an arrow (G and H). Quantitation of localization experiments is shown in Tables 1 and 2. Exposure times for given antigen were constant for all micrographs. Bars, 10 μm.
TABLE 1.
Nuclear colocalization of p21 with G1 (D1 and E) and S-phase (A) cyclins
| p21 | % of cells exhibitinga:
|
|||||
|---|---|---|---|---|---|---|
| Cyclin D1
|
Cyclin Eb
|
Cyclin A
|
||||
| + | − | + | −/+ | + | − | |
| + | 37 | 11 | 41 | 34 | 6 | 48 |
| − | 24 | 28 | 25 | 29 | 17 | |
Percentage of cells exhibiting the presence (+) or absence (−) of cyclin versus p21 in the nucleus. The data shown are for Hs68 HDF, but similar results were obtained with IMR-90 HDF and human breast epithelial cells.
Only cells labeled with anti-cyclin E were scored. To distinguish cells which stained strongly with cyclin E-specific antibody from the situations in which cyclin E was visible but the signal was considerably fainter, we used the +/− symbol.
Surprisingly, we also observed a small but significant population of cells (5 to 7% of fibroblasts and 10% of breast epithelial cells) with strong nuclear staining for both cyclin A and p21, suggesting a later cell cycle stage with p21 nuclear accumulation (Fig. 1C and D; Table 1). To identify this stage more precisely, we carried out double immunostaining using a cyclin B1-specific antibody. Cyclin B1 is a mitotic cyclin that accumulates in the cytosol during late S phase and G2 and enters the nucleus at the onset of mitosis (4, 13, 27). As shown in Fig. 1G and H, p21 was undetectable in cells that exhibited an exclusively cytoplasmic distribution of cyclin B1 (i.e., late S phase) as well as in a majority of cells that had begun to accumulate nuclear cyclin B1 (early G2). However, many cells (1 to 2% of the total population), showing predominant nuclear and cytoplasmic or exclusively nuclear cyclin B1, also exhibited high levels of nuclear p21 (Fig. 1G and H; statistical analysis is presented in Table 2). The cyclin B1 localization pattern, the presence of visible nucleoli, and the absence of mitotic chromosome condensation, confirmed by counterstaining of DNA with Hoechst dye (see below), place the time of nuclear p21 accumulation as late in G2. The p21 signal becomes weak again with ongoing DNA condensation at early prophase and remains virtually absent during mitosis (Fig. 1E and F). This late-G2-specific colocalization of cyclin B1 and p21 is confirmed by localization experiments using synchronized fibroblasts (see below). Interestingly, premitotic nuclear accumulation of cyclin B1 prior to chromatin condensation could not be detected in transformed cells, such as tumor-derived HeLa cells (27), or in fibroblasts expressing the HPV16 E6 oncoprotein (see below). All of these cell lines express extremely low levels of p21.
TABLE 2.
Colocalization of p21 with cyclin B1a
| p21 | % of cells with indicated cyclin B staining patternb
|
||
|---|---|---|---|
| Cyt | CN | Nuc | |
| Present | 0 | 22 | 6 |
| Absent | 38 | 26 | 7 |
To pinpoint the timing of p21 reaccumulation into the nucleus in late stages of the cell cycle, we scored only cyclin B1-positive cells. The data shown are for Hs68 HDF, but similar results were obtained with IMR-90 HDF.
Cyclin B1-specific staining patterns (cf. Fig. 1H) were cytoplasmic (Cyt; late S and early G2 phase), cytoplasmic and nuclear (CN; mid-G2), and predominantly or exclusively nuclear (Nuc; late G2 and early stages of prophase).
Accumulation of p21 in late G2 leads to increasing association with cyclin-Cdk complexes.
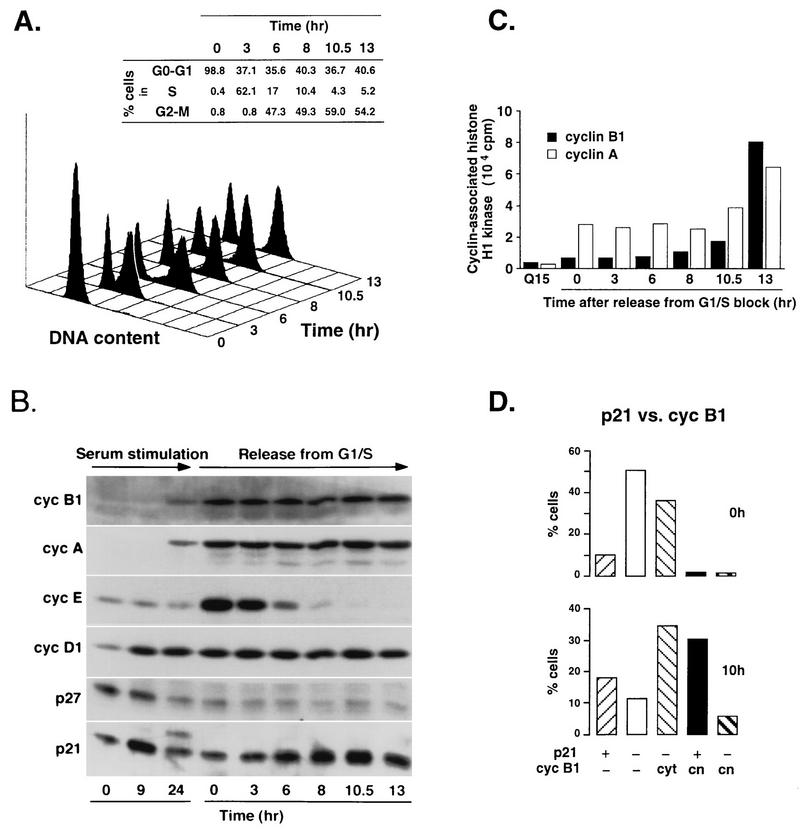
In an attempt to corroborate results obtained by immunofluorescence using asynchronously growing cells and to confirm that nuclear accumulation of p21 indeed occurs in G2, we analyzed both protein fluctuations and nuclear accumulation of p21 during the cell cycle in fibroblasts that were synchronized at the G1/S boundary by either an aphidicolin or a hydroxyurea block/release protocol (Materials and Methods). We chose this synchronization procedure because it allows a more precise analysis of late stages of the cell cycle, particularly between S phase and mitosis. After release from the G1/S block, cells were harvested at the indicated time intervals and the lysates were analyzed for total protein content or used for immunoprecipitation assays as described in Materials and Methods. The degree of cell cycle synchrony was monitored by FACS analysis, showing that a large population of cells reached G2 and/or M phase 10 to 13 h after release from the block (Fig. 2A). We also analyzed extracts prepared from quiescent (0 h), mid-G1 (9 h), and S-phase-enriched (24 h) cells obtained by serum stimulation of serum-starved cells. Immunoblot analyses of whole cell lysates for cyclin D1, cyclin E, cyclin A, and cyclin B1 during the same time course are shown in Fig. 2B. The beginning of mitosis was confirmed by a sharp rise of cyclin B1-associated kinase activity 13 h after release from the aphidicolin block (Fig. 2C). In contrast to another G1-phase-specific CKI, p27Kip1, whose levels did not change after a decrease in late G1, p21 levels steadily increased after release from the block, attaining a maximum at the G2/M boundary (10 h [Fig. 2B]). These results are in agreement with the previously reported pattern of p21 mRNA accumulation (19). The slower-migrating p21-specific bands observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of both total cell extracts and cyclin immunocomplexes (Fig. 2B and 3) probably represent phosphorylated p21 isoforms described previously (42). The role of p21 phosphorylation is unknown, but it has been shown that cyclin A-Cdk-p21 complexes prepared in vitro at subsaturating inhibitor concentrations are active and, in the presence of ATP, contain predominantly phosphorylated p21 (42). Of note, this migration pattern could not be reproduced in some experiments described below, probably because the anti-p21 antibodies used in these experiments could not readily recognize phosphorylated p21 isoforms.
FIG. 2.
Reaccumulation of p21 at G2/M-phase boundary in synchronized normal human fibroblasts. (A) FACS analysis of synchronized Hs68 fibroblasts at different times after release from an aphidicolin block. The percentage of cells in different phases of the cell cycle was determined by using the CellFit program (Materials and Methods). (B) Immunoblot analysis of cell extracts. Fibroblasts were synchronized either in G0 by serum starvation for 72 h, followed by serum stimulation for 9 and 24 h, or at the G1/S boundary, by a combination of serum stimulation (12 h) and aphidicolin block (20 h). Total-cell extracts were prepared from cells at the indicated times after serum stimulation or release from the block, analyzed by SDS-PAGE (8.5% gel for cyclins [cyc] B1, A, E, and D1; 12% gel for p27 and p21), and immunoblotted with specific antibodies against cyclins and CKIs. (C) Cyclin A- and cyclin B1-associated histone H1 kinase activities. Kinase activity of the cyclin A and cyclin B1 complexes immunoprecipitated from cell extracts described above was tested by using histone H1 as the substrate as described in Materials and Methods. Cells in late G1 (quiescent cells serum stimulated for 15 h) were used as a negative control. (D) Colocalization of p21 and cyclin B1 in cells synchronized by aphidicolin block at the G1/S boundary. Total populations of arrested cells (0 h [G1/S boundary]) and the cells after a release from the block (10 h [G2/M boundary]) were analyzed as described for Table 2. At least 500 cells were scored for each time point. cyt, cytoplasmic; cn, cytoplasmic and/or nuclear.
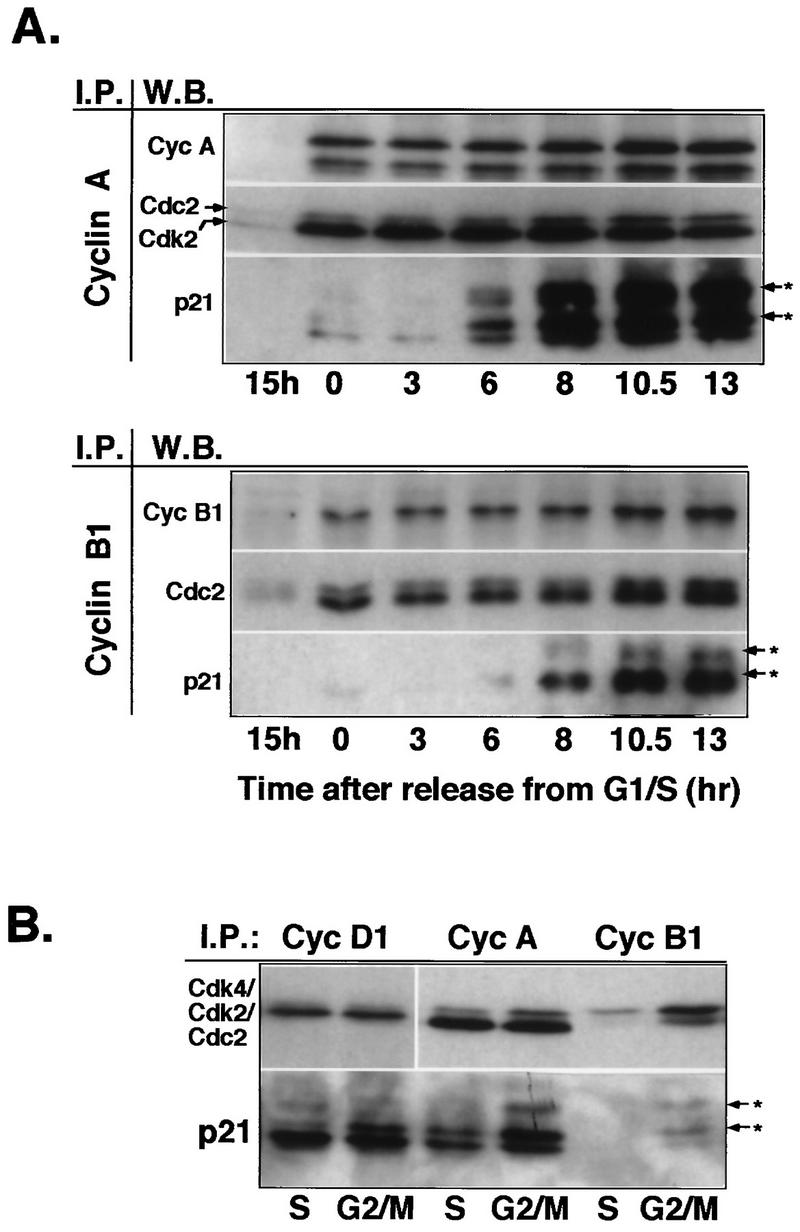
FIG. 3.
Increasing association of p21 with cyclin A and cyclin B1 in G2/M-phase cells. (A) Western blot analysis of cyclin complexes from lysates of synchronized Hs68 cells. Cyclin (Cyc) A and cyclin B1 complexes were immunoprecipitated (I.P.) from total lysates prepared from cells stimulated with serum for 15 h and cells released from aphidicolin block at the indicated time points. Immune complexes were separated on SDS–12% polyacrylamide gels, transferred to an Immobilon membrane, and detected by using the indicated antibodies (W.B. [Western blotting]) by ECL. Note that cyclin B1 immunoblots had to be exposed much longer than cyclin A immunoblots. (B) Comparative analysis of cyclin D1, cyclin A, and cyclin B1 immunocomplexes isolated from S-phase (pooled 0-, 3-, and 6-h time points)- and G2/M-phase (pooled 10.5- and 13-h time points)-enriched cell lysates. The resulting immunocomplexes were resolved on the same SDS–12% polyacrylamide gel. Immunoblots were probed with either Cdk-specific antibodies (anti-Cdk4, anti-PSTAIRE for Cdk2 and Cdc2) or anti-p21, as indicated. Arrows with asterisks indicate differently phosphorylated species of p21.
Conclusive evidence that nuclear reaccumulation of p21 is indeed a genuine G2 event was obtained by immunofluorescence analysis of synchronized normal fibroblasts. Whereas several hours after release from the aphidicolin block (S phase) relatively few cells stained positively for p21 (10 to 15%) and none of these were cyclin B1 positive, 10 h later (late G2 phase) an increasing population showed nuclear colocalization of p21 and cyclin B1 (Fig. 2D; see also Fig. 5C). The presence of p21-positive, cyclin B1-negative cells after aphidicolin (or hydroxyurea) block may explain the relatively high p21 levels observed in lysates prepared from cells at the G1/S boundary (Fig. 2B). These are likely to correspond to remaining mid-to-late G1 cells that had not yet reached the block point.
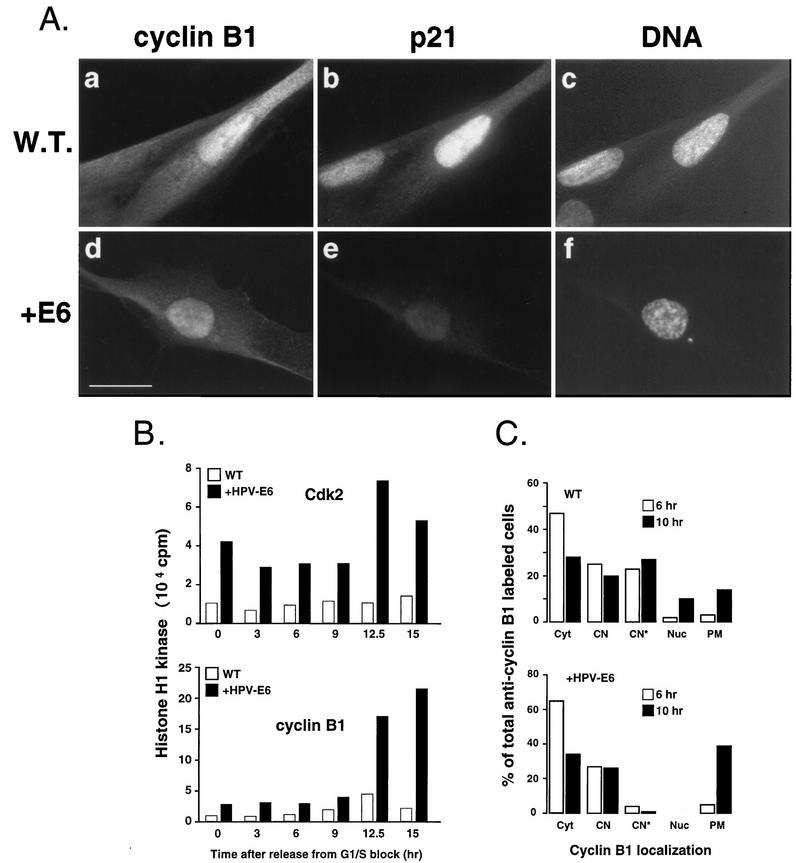
FIG. 5.
Deregulated G2/M-phase transition in p53-deficient cells. (A) Nuclear localization of cyclin B1 in wild-type cells (W.T.; IMR-90) and fibroblasts expressing low levels of p21 owing to expression of HPV16 E6 (+E6) as described in Materials and Methods. Formalin-fixed cells were stained with cyclin B1- (fluorescein; a and d) and p21Cip1-specific (Texas red; b and e) antibodies. Nuclei were counterstained with Hoechst 33258 to assess the state of DNA condensation (c and f). Experimental conditions were the same as those described for Fig. 1. Note the absence of nucleoli and the signs of DNA condensation in E6 cells accumulating nuclear cyclin B1. Bar, 10 μm. (B) Cyclin B1- and Cdk2-associated histone H1 kinase activity from aliquots prepared from synchronized wild-type and E6 fibroblasts. Cells were synchronized at the G1/S-phase boundary by aphidicolin block as described in the legend to Fig. 2. (C) Accelerated entry into mitosis of p53− p21− cells. The late stages of the cell cycle in synchronized wild-type and E6 cultures were analyzed based on subcellular distribution of cyclin B1. Cells were released from G1/S-phase block (hydroxyurea) for 6 and 10 h as described in Materials and Methods. Note that only cells accumulating cytoplasmic and nuclear cyclin B1 were scored. The following cyclin B1-specific staining patterns were distinguished: Cyt, cytoplasmic (like in Fig. 1H); CN, cytoplasmic and nuclear, no visible nucleoli (like in Fig. 1H); CN*, cytoplasmic and nuclear, with visible nucleoli (like in Fig. 1H); Nuc, predominantly nuclear with visible nucleoli (like in Fig. 1H and in 3A); PM, prophase and metaphase (like in Fig. 1F).
To determine whether p21 accumulating during late G2 targets specific cyclin-Cdk complexes, we analyzed by Western blot cyclin immunocomplexes isolated from the lysates prepared from cells in late G1 (15 h after serum stimulation) or at different times after release from the aphidicolin block (G1/S boundary). Resulting immunoblots presented in Fig. 3A showed increasing association of p21 with cyclin A and, to a much lesser extent, with cyclin B1 complexes as the cells progressed toward mitosis concomitant with its nuclear accumulation. In agreement with immunoblot analysis of p21 in whole extracts (Fig. 2B), much of the p21 in these complexes appeared to be phosphorylated (Fig. 3A). We also observed increasing association between cyclin D1 and p21 during the same time course (data not shown). At this point, it is important to emphasize that the presence of cyclin D1 during late stages of the cell cycle is not solely due to the presence of contaminating G1 cells, as this cyclin also accumulates into nucleus after S phase (7a). To compare the relative p21 levels in different cyclin complexes, we analyzed by Western blotting cyclin D1, cyclin A, and cyclin B1 immunoprecipitates from the equivalent amount of extracts prepared from S-phase-enriched (pooled 0-, 3-, and 6-h time points) or G2/M-phase-enriched (pooled 10.5- and 13-h time points) cells. Whereas p21 forms equally abundant complexes with cyclins D1 and A, there seems to be very little p21 complexed with cyclin B1 (Fig. 3B), which is in agreement with results showing that p21 is a poor inhibitor of cyclin B1-Cdc2 complexes (15, 17). We further addressed this issue in greater detail in experiments presented below (see Fig. 4). These results may be interpreted as indicative of association of p21 with cyclin A- and cyclin B1-Cdk complexes during mitosis, as indeed was proposed in reference 19. However, in light of our immunofluorescence results showing the absence of p21 in mitotic cells, it is more likely that association with cyclin A and cyclin B1 occurs before mitosis.
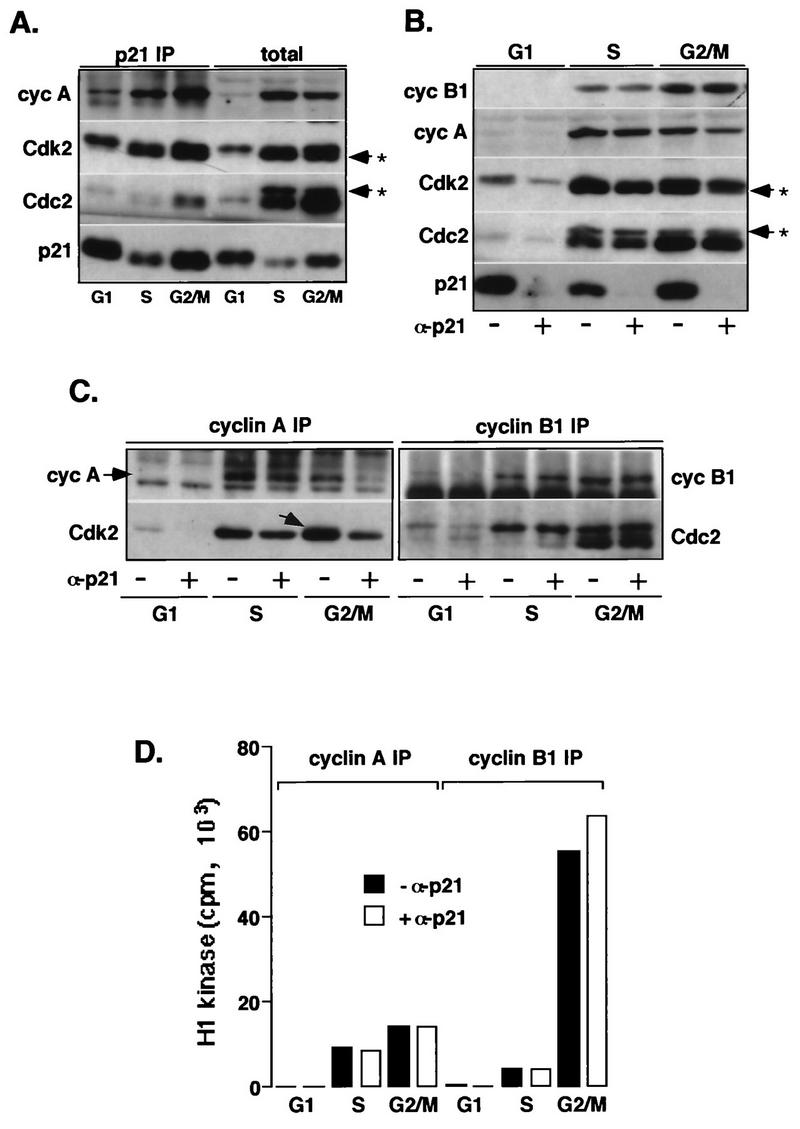
FIG. 4.
p21-associated cyclin (cyc)-Cdk2 complexes are inactive. Extracts prepared from normal fibroblasts (Hs68) synchronized in G1, S, and G2/M phases are depleted of p21. (A) Western blot analysis of p21 immunoprecipitates (IP) from the different cell extracts (200 μg) and total proteins in the corresponding lysates (40 μg). (B) Western blot analysis of total proteins in cell extracts (40 μg) depleted (+ α-p21) or not depleted (− α-p21) of p21. (C) Western blot analysis of cyclin A and cyclin B1 immunocomplexes isolated from the p21-depleted and mock-depleted extracts (150 μg). (D) Cyclin A- or B1-associated histone H1 kinase activity. In these experiments, cyclin A and cyclin B1 immunocomplexes, assayed for kinase activity by using histone H1 as a substrate, were separated by SDS-PAGE (11% gel), transferred onto an Immobilon membrane and simultaneously analyzed for the presence of cyclins and Cdks by Western blotting, and exposed to reveal histone H1-associated radioactivity. In addition, Coomassie blue-stained histone H1 bands remaining on the gel (about 50%) were excised, and associated radioactivity was analyzed by Cerenkov counting. The immunoblots were probed with indicated antibodies, except that in cyclin B1 immunoprecipitates, Cdc2 was also detected by using an anti-PSTAIRE monoclonal antibody. The extent of removal of cyclins or Cdks upon p21 depletion was evaluated by densitometric scanning of immunoblots. Note that in G2/M cells, cyclin A increasingly associates with unphosphorylated Cdk2 (indicated by an arrow in panel C). Arrows with asterisks in panels A and B indicate phosphorylated Cdk species.
p21-bound cyclin A-Cdk2 complexes are inactive.
The experiments reported above revealed an increasing association between p21 and cyclin-Cdk complexes after S phase, but they did not provide quantitation of the occupancy of these complexes with p21 and whether they are inhibited. To address this question, extracts prepared from normal fibroblasts synchronized in G1, S, and G2/M phases were depleted for p21 by incubation with p21-specific antibodies. As shown in Fig. 4B, this treatment resulted in nearly complete depletion of p21. Western blot analysis of p21 immunoprecipitates isolated from these extracts confirmed that p21 predominantly forms complexes with cyclin A, cyclin D1 (not shown), Cdk4 (not shown), and Cdk2 and that very little of cyclin B1 or Cdc2 is p21 bound (Fig. 4A). Although also observed in earlier phases of the cell cycle, this association significantly increased as cells approached the G2/M-phase boundary where p21 depletion removed approximately 50 to 70% of total cyclin A-Cdk2 complexes, as estimated by densitometric scanning of immunoblots (Fig. 4B and C). In addition, unlike the situation during S phase, when cyclin A associates almost exclusively with Thr160-phosphorylated Cdk2, cyclin A complexes from G2/M cells contained also high levels (30%) of unphosphorylated Cdk2 (these complexes are drastically reduced upon p21 depletion). This finding suggests that in addition to directly inhibiting Cdk2 kinase activity, p21 may inactivate cyclin A-Cdk2 by preventing Cdk-activating kinase (CAK)-mediated phosphorylation of Cdk2 as has been reported for p27Kip1 (28). In contrast to cyclin A and Cdk2, p21 depletion did not affect total cyclin B1 and Cdc2 levels in lysates, nor did it diminish the levels of cyclin B1-Cdc2 complexes (Fig. 4B). Moreover, cyclin A-Cdc2 complexes did not appear to be significantly affected by p21 depletion (data not shown), which is consistent with results showing that very little Cdc2 could be detected in p21 immunocomplexes (Fig. 4A). Thus, Cdk2 and Cdk4 appear to be the major p21 targets at the G2/M-phase boundary.
Interestingly, even though a significant fraction of cyclin A-Cdk2 is bound to p21 (Fig. 4C), the removal of p21-bound cyclin-Cdk complexes did not affect total cyclin A-associated histone H1 kinase activity (Fig. 4D), suggesting that these complexes are inactive. As expected, cyclin B1-associated H1 kinase activity was not depleted (Fig. 4D). Note that the remaining 30 to 50% of cyclin A-associated Cdk2 retains as much kinase activity as the total cyclin A-Cdk2 in undepleted extracts (Fig. 4C and D). Consistent with these results, we also could not detect significant levels of p21-associated histone H1 kinase activity by using two different p21-specific antibodies (data not shown) even though these complexes contained both Cdk2 phosphorylation isoforms (Fig. 4A). These results are in apparent conflict with other published data suggesting that most of the Cdk2-associated histone H1 kinase is associated with p21 (17, 42). However, aside from the fact that different p21-specific antibodies were used in these experiments, we cannot provide an explanation for this discrepancy. Nevertheless, our immunofluorescence results suggest that interactions between cyclin-Cdk complexes and p21 during the cell cycle are likely to be dynamic and may not be reliably understood exclusively from immunoblot or immunoprecipitation data.
p53-dependent accumulation of p21 in G2 affects G2-M-phase progression.
To address the biological significance of p21 accumulation during G2, we monitored late cell cycle events in nontransformed fibroblasts with perturbed expression of p21. For that purpose, we used normal HDF (IMR-90) that express low levels of p21 owing to the presence of the HPV16 E6 oncoprotein (35), which promotes degradation of p53 (33). We have previously shown that HPV16 E6-expressing (E6) fibroblasts failed to arrest in G1 upon exposure to ionizing radiation (IR), due to inefficient induction of p21 (9). Despite very low p21 levels, parameters of cell cycle progression such as S-phase entry, kinetics of cyclin accumulation, and pRb phosphorylation were indistinguishable in serum-stimulated E6 fibroblasts relative to wild-type cells (data not shown), suggesting that absence of p21 does not significantly interfere with progression through the early stages of the cell cycle. However, microscopic observation indicated that, in E6 cells, late stages of the cell cycle may be altered. Specifically, we noticed a higher number of mitotic cells in exponentially growing cultures, which could be the result of a faster rate of mitotic entry (resulting in a shorter G2 interval) combined with a prolonged duration of mitosis. Indeed, indirect immunofluorescence analysis revealed that exponentially growing E6 cultures lacked cells that accumulate nuclear cyclin B1 before the onset of early prophase events: all cells with apparent nuclear cyclin B1 showed signs of DNA condensation (compare wild-type cells with E6 cells in Fig. 5A). To test whether the lack of p21 during G2 in these cells might alter the activation state of Cdks late in the cell cycle and hence the timing of mitosis, we compared the kinetics of Cdk2- and cyclin B1-associated kinase activities between synchronized wild-type and E6 cells. Even though we could not observe a difference in timing of mitotic elevation of cyclin B1-associated kinase activity between wild-type and E6 cells (probably due to imprecision of our synchronization technique), both cyclin A-Cdk2- and cyclin B1-Cdc2-associated kinase activities were significantly higher in E6 cell extracts throughout the time course (Fig. 5B), possibly accounting for rapid initiation of mitotic events observed by immunofluorescence analysis. Elevated Cdk2 kinase activity at the time of Cdc2 kinase activation is likely a consequence of low p21 levels. Moreover, whereas wild-type cells showed a decline 15 h after the release (with a peak at the 12.5-h time point), cyclin B1-associated kinase activity persisted in E6 cells, consistent with the observed accumulation of mitotic cells (Fig. 5B).
To address more precisely the difference in kinetics of mitotic entry between wild-type and E6 cells, we measured the rate of cyclin B1 accumulation and nuclear localization and appearance of mitotic cells 6 (late S phase) and 10 (G2/M) h after release from G1/S-phase block imposed by treatment with hydroxyurea. We scored only the cells that had entered S phase after release (as apparent from cyclin B1 accumulation), in order to eliminate cells permanently arrested by serum starvation or drug treatment. As shown in Fig. 5C, 10 h after release from the block, there were nearly three times more mitotic cells (including cells with early signs of DNA condensation) in E6 cultures as in wild-type cultures. In contrast, premitotic cells accumulating nuclear cyclin B1, which were frequent in wild-type culture at this time point, could not be observed in E6 cultures. Instead, all cells showing predominant nuclear cyclin B1 localization lacked nucleoli and exhibited signs of DNA condensation (Fig. 5A, panels d to f). Hence, an apparent acceleration of entry into prophase in E6 cells may be related to the absence of a premitotic stage with nuclear accumulation of cyclin B1, the occurrence of which may depend on p21 (or active p53). The parallel observation that premitotic nuclear localization of cyclin B1 could not be detected in HeLa cells, expressing low p21 levels owing to the presence of the HPV18 E6 oncoprotein, supports this hypothesis (data not shown) (13, 27).
Although E6 function appears to be limited to p53 (and by extension p21), results need to be interpreted cautiously due to the potential for pleiotropic non-p21-related effects. However, taken together, our immunofluorescence and biochemical results suggest a correlation between elevated expression of p21 in G2, accumulation of late G2 cells, and deceleration of mitotic entry.
p21−/− MEFs are defective in cyclin B1 nuclear accumulation.
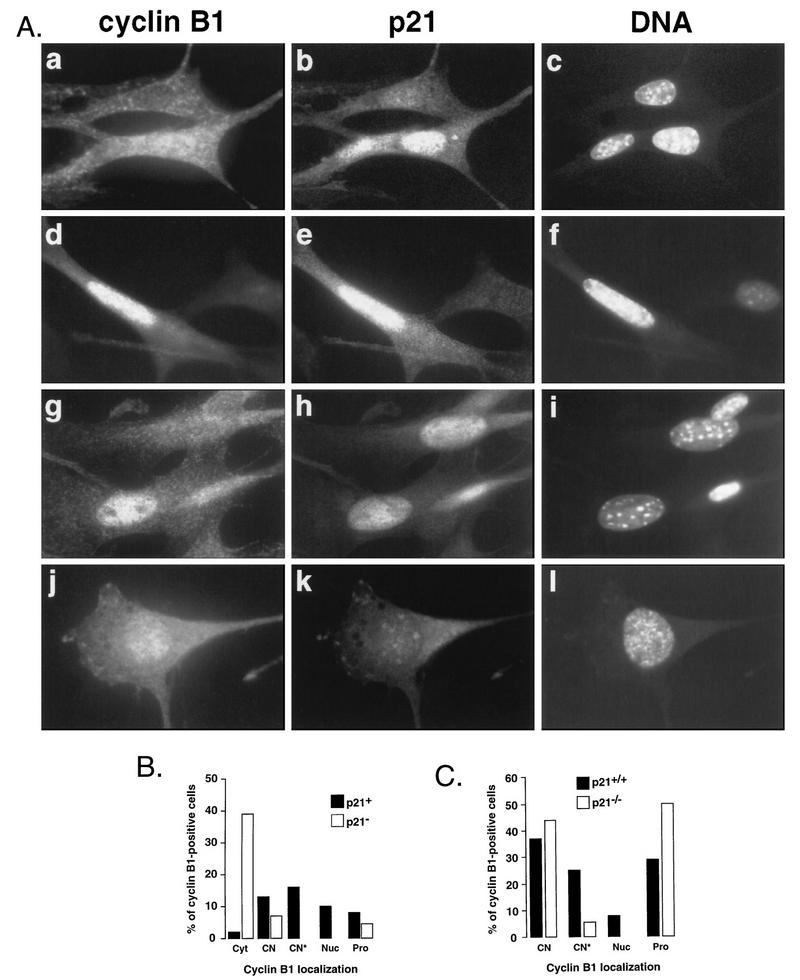
Although highly suggestive, the foregoing results do not discriminate genetically between a dependency on p21 and a dependency on p53 in the implementation or maintenance of a late G2 pause. To address this issue, we analyzed MEFs derived from p21−/− mice (6). Although p21−/− mice are viable, indicating that p21 is not essential for cell cycle progression, p21−/− MEFs were found to be deficient in the ability to arrest in G1 in response to DNA damage and to exhibit abnormal growth properties at later passages (6, 7). To examine whether p21−/− MEFs exhibit alterations in their patterns of cyclin B1 nuclear translocation relative to mitotic events, we analyzed exponentially growing cultures of wild-type and 21−/− MEFs (between passages 4 and 6) by immunofluorescence microscopy. Micrographs presented in Fig. 6A show typical cyclin B1 nuclear staining patterns in wild-type (panels a to i) and p21−/− (panels j to l) MEFs. In a fashion similar to that for HDF, in wild-type MEFs, premitotic nuclear translocation of cyclin B1 correlated with nuclear reaccumulation of p21 (Fig. 6A and B), but in contrast to the case for HDF, p21 could be also detected in some cells exhibiting early signs of DNA condensation (Fig. 6A, panels d to f). This might be explained by the fact that DNA condensation in mouse fibroblasts is more easily detected than in human cells. However, nuclear envelope breakdown was invariably associated with the loss of p21 signal, confirming our observation in human fibroblasts. Quantitative analysis comparing cyclin B1 localization patterns between asynchronously growing wild-type and p21−/− MEFs clearly shows that p21−/− populations contained many fewer cells showing nuclear cyclin B1 (Fig. 6C), and all exhibited clear evidence of mitotic DNA condensation (Fig. 6A, panels j to l). Moreover, p21−/− populations had greater numbers of prophase cells. Note that this analysis comprised only the cells exhibiting significant nuclear cyclin B1 staining, as the cytoplasmic antibody background was elevated as compared to flatter human fibroblasts, rendering scoring of cytoplasmic staining difficult. These data confirm those obtained in assays using human fibroblasts expressing HPV16 E6 and are consistent with a p21-dependent pause in late G2. In agreement with the pattern observed with p21−/− MEFs, we did not observe premitotic cyclin B1 nuclear accumulation in mouse fibroblasts mutated for p53 (data not shown).
FIG. 6.
p21−/− MEFs do not accumulate nuclear cyclin B1 before mitosis. (A) Nuclear colocalization of cyclin B1 and p21 in wild-type (a to i) and p21−/− (j to l) MEFs. The nuclei were counterstained with Hoechst 33258 (DNA) to assess the state of DNA condensation. Representative cells exhibiting cytoplasmic and nuclear (a) and nuclear (d and g) cyclin B1 localization are shown. Micrograph I shows a p21−/− cell with nuclear cyclin B1 with ongoing DNA condensation. (B) Quantitative analysis of p21-cyclin B1 colocalization in exponentially growing MEFs. Note that only cyclin B1-positive cells were scored. We distinguished cells with apparent accumulation of cyclin B1 in both in the cytoplasm and the nucleus without (CN) and with (CN*) visible nucleoli, predominantly in the nucleus (Nuc) as well as those in different stages of prophase (Pro). (C) Quantitative analysis showing cyclin B1 localization in wild-type (W.T.) and p21−/− MEFs. Only cells showing nuclear signal were scored.
In MEFs, p21 associates with only a subpopulation of cyclin A-Cdk2 complexes.
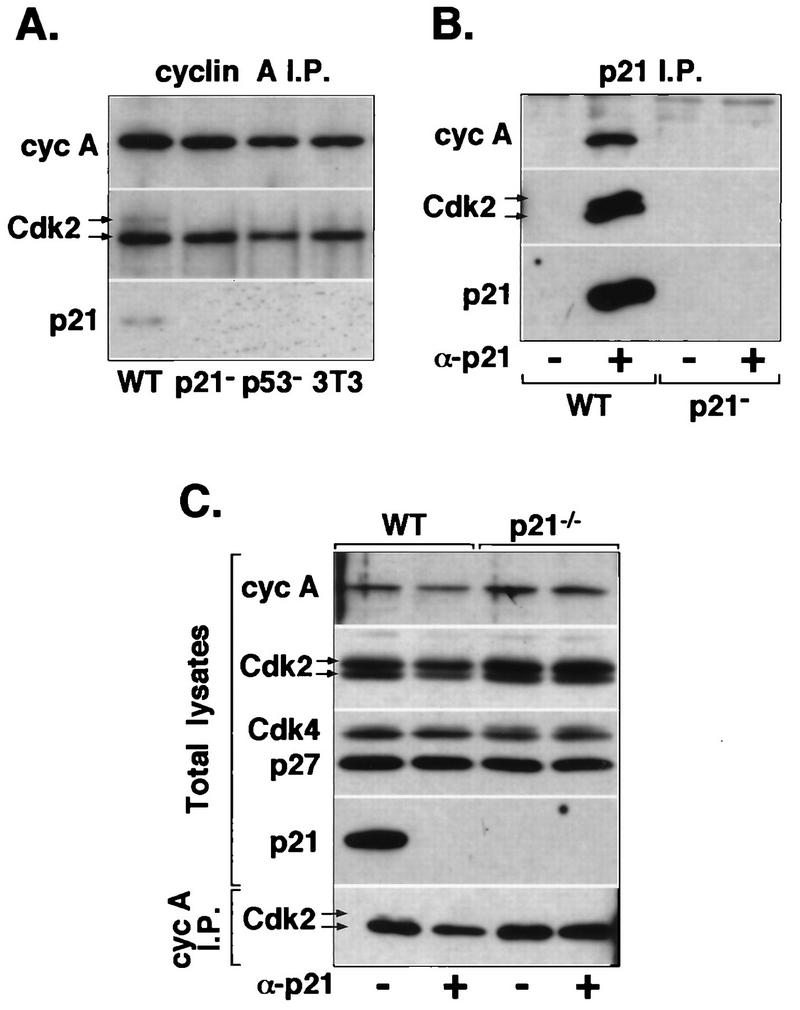
As p21 has been reported to be present in the majority of Cdk2 complexes in HDF (17, 42), we examined whether its absence in primary murine cells might affect the dynamics of cyclin A-Cdk2 complex formation and stability. Western blot analysis of cyclin A immunocomplexes isolated from cell lysates prepared from exponentially growing MEF cultures showed that neither cyclin A expression nor its association with Cdk2 is perturbed in p21−/− or p53−/− cells (Fig. 7A). Moreover, as shown by Western blot analysis of whole-cell lysates, complete immunodepletion of p21 from the extracts prepared from wild-type MEFs removed only a portion of total cyclin A and Cdk2 (25% based on densitometric scanning) and almost 50% of cyclin A-Cdk2 complexes, but the levels of Cdk4 were not significantly changed (Fig. 7B and C). These results are consistent with immunofluorescence observations suggesting that association of p21 with cyclin A-Cdk2 is confined to only a brief segment of the cell cycle (presumably when p21 is nuclear) and that p21 does not associate with the majority of Cdk2 as was previously suggested (17, 42). In addition, these results are in complete agreement with those obtained in HDF (Fig. 4). Furthermore, Western blot analysis of cyclin A immunocomplexes revealed that in wild-type cells, but not in p21−/− or p53−/− cells, a fraction of cyclin A is in association with inactive Cdk2 lacking phosphorylation of Thr160 (Fig. 7A). Unphosphorylated Cdk2 was also observed in cyclin A complexes from normal human fibroblasts at the G2/M-phase transition (Fig. 4C) but could not be detected in E6 cells (not shown). Since it has been shown that p21 can block phosphorylation of Cdk2 on Thr160 by CAK (3), we take this as evidence that p21 associates with and inactivates cyclin A-Cdk2 in vivo and that complexes with p21 detected in lysates are not merely an in vitro artifact. Consistent with this interpretation, p21 immune complexes are enriched for unphosphorylated Cdk2 (Fig. 7B). Finally, we found no evidence for elevated expression of another CKI, p27Kip1, in p21−/− MEFs, indicating that these cells do not compensate the lack of p21 with deregulated expression of p27 (Fig. 7C).
FIG. 7.
p21 association with cyclin A-Cdk2 complexes in MEFs. (A) Immunoprecipitates (I.P.) of cyclin (cyc) A immunocomplexes were isolated from extracts prepared from exponentially growing p21+ (wild-type p21+/+ [WT]; MEF and NIH 3T3) and p21− (p21−/− and p53−/− MEF) cells and were analyzed by immunoblotting for the presence of Cdk2 and p21Cip1. All fibroblasts were at passage 4. (B and C) Depletion of p21 in MEF extracts. Whole-cell extracts prepared from wild-type and p21−/− MEFs were incubated with p21-specific antibodies (+ α-p21) or protein A-Sepharose beads (− α-p21). (B) Western blot analysis of p21 immunoprecipitates tested for the presence of cyclin A and Cdk2. (C) Immunoblot analysis of aliquots of the p21-depleted or mock-depleted extracts for the presence of cyclin A, Cdk2, Cdk4, p27Kip1, and p21. The lower part of panel C shows immunoblot analysis (probed with anti-Cdk2) of cyclin A immunoprecipitates prepared from the same p21-depleted extracts. Arrows indicate two forms of Cdk2; the lower form represents Thr160-phosphorylated Cdk2.
DISCUSSION
The experiments presented in this report were aimed at understanding the role of the CKI p21 during the cell cycle of nontransformed cells. p21 is transcriptionally regulated by wild-type p53, and its elevated levels in response to DNA damage or cellular senescence lead to inactivation of G1 Cdks conferring the G1 arrest (reviewed in reference 36). Due to its nuclear accumulation during G1 and absence in S phase, it has been assumed that p21 acts primarily as a negative cell cycle regulator of the G1/S-phase transition. However, the pattern of expression of p21 mRNA during the cell cycle, with peaks in G1 and G2/M (19), and the presence of p21 in quaternary complexes with a cyclin, a Cdk, and the proliferating cell nuclear antigen (43) have suggested a more complex role for this CKI (36). Moreover, it has been reported that the majority of Cdk2 molecules in nontransformed fibroblasts are complexed with p21 (17) and that cyclin A and cyclin B1 associate with p21 late in the cell cycle, when they are presumed to execute their cell cycle functions (19). Thus, it has been suggested that p21 may serve either as a cyclin-Cdk assembly factor (42) or as an inhibitory buffer setting a threshold for kinase activation (17). However, the apparent absence of p21 from the nucleus during S phase when cyclin A and Cdk2 are nuclear, as well as the lack of impairment of cyclin-Cdk formation or function in cells expressing low p21 levels, is in obvious contradiction with some of these interpretations.
Double-immunostaining experiments allowed us to follow the dynamics of p21 through the cell cycle of asynchronously growing nontransformed cells with a high degree of precision, thus avoiding many of the artifacts inherent to synchronization procedures. In conjunction with biochemical analyses of cyclin complexes in synchronized cells, our results provide the following evidence supporting a possible role for p21 at the G2/M-phase transition. (i) p21, which is absent from the nucleus in S-phase cells, transiently reenters the nucleus during late G2 phase, concomitantly with nuclear translocation of cyclin B1, but is again undetectable during mitosis. (ii) Reaccumulation of p21 protein after S phase correlates with an increasing association with cyclin A-, cyclin D1-, and, to a much lesser extent, cyclin B1-Cdk complexes. (iii) At the G2/M-phase boundary, nearly half of cyclin A-Cdk2 complexes are p21 bound and are inactive. (iv) The presence and duration of this specific late G2 cell cycle stage (late G2 pause), characterized by nuclear translocation of cyclin B1 but no evidence of mitotic events, correlate with p21 expression and its nuclear accumulation. This cell cycle stage is missing in cells with low p21 levels or lacking p21, such as HPV16 E6 oncoprotein-expressing primary fibroblasts, p53− cells, or MEFs derived from p21−/− mice. (v) E6 fibroblasts, synchronized at the G1/S-phase boundary, enter into mitosis more rapidly than control fibroblasts. (vi) p21 immunodepletion experiments in MEFs showed that only a fraction of cyclin A-Cdk2 associates with p21, consistent with cyclin A-Cdk2 being available for p21 binding only during late G1 and G2, when both proteins colocalize in the nucleus.
What is the biological significance of the nuclear accumulation of p21 and association with cyclin-Cdk complexes at the onset of mitosis? Our results suggest that cyclin A-Cdk2 may be the primary post-S-phase target of p21 in normal fibroblasts and p21 may thereby either directly inhibit the active kinase or prevent its activation by CAK (29), as indicated by the p21-dependent elevated presence of unphosphorylated Cdk2 associated with cyclin A. In addition, association with p21 may block interaction of substrates with cyclin A-Cdk2 complexes, as proposed by Adams et al. (1). Taken together, these observations suggest that inactivation of cyclin A-Cdk2 may be part of a p21-dependent intrinsic mitotic attenuation mechanism (Fig. 8). Guadagno and Newport recently demonstrated that in Xenopus egg extracts, Cdk2 acts as a positive regulator of activation of cyclin B-Cdc2 complexes and that p21, by inactivating Cdk2, blocks progression into mitosis (16). Thus, by analogy, p21-mediated inhibition of cyclin A-Cdk2 in somatic cells may produce a pause or delay prior to entry into mitosis. This stage is virtually absent in the cells with low p21 levels, where premitotic nuclear accumulation of cyclin B1 could not be observed (see also reference 27). Presumably, in such cells, cyclin B1-Cdc2 kinase activity is sufficiently high at the time of nuclear translocation to initiate mitosis. One possible role for this p21-induced pause is to facilitate the integration of critical G2 checkpoint signals that regulate entry into mitosis through modulating the activity of cyclin A-Cdk2 (and cyclin B1-Cdc2?) complexes via other mechanisms (Fig. 8). Even though previous reports do not discuss G2 arrest occurring upon ectopic expression of p21, cells arrested with a G2/M DNA content are apparent upon close scrutiny of those experiments (17). Furthermore, observations that heterologous expression of p21 in the fission yeast resulted in predominantly G2 arrest (38), that regulated ectopic expression of p53, which induces p21, conferred cell cycle arrest in both G1 and G2 (2), and that regulated ectopic expression of p21 itself in some mammalian cell lines conferred predominantly G2 arrest (21a) provide additional in vivo evidence for p21 as a potential negative regulator of the G2/M-phase transition.
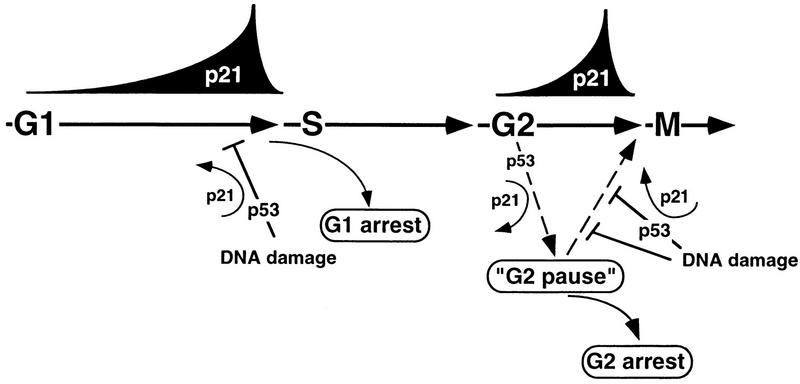
FIG. 8.
Model: p21 involvement in G2/M checkpoint control? In nontransformed (p53+) cells, nuclear accumulation of p21 and binding to cyclin-Cdk complexes during G1 and before mitosis (late G2) may facilitate checkpoint implementation at the G1/S-phase and G2/M-phase transitions. In G2, a p21-induced pause might potentiate the integration of G2 checkpoint signals that regulate entry into mitosis through modulating the activity of cyclin A-Cdk2 and cyclin B1-Cdc2 complexes. Alternatively, increasing association of p21 with Cdk complexes at both transition points may sensitize these kinases to respond to further increases of p21 resulting from DNA damage. Whereas it seems that p53 activity (and p21 accumulation) is required for DNA damage-induced G1 arrest, p53 (and p21) may be only a part of a redundant mechanism regulating G2 arrest.
In addition, p21 might be a direct mediator of DNA damage checkpoint control at both the G1/S and G2/M transitions (Fig. 8). Its cell cycle-regulated association with Cdk complexes in G2 may sensitize these kinases to respond to further increases of p21, resulting from DNA damage. These mechanisms would be redundant with G2 arrest mechanisms that are clearly not p21 dependent. Such a situation has been demonstrated for DNA damage-mediated G1 arrest, where cells from p21 nullizygous mice are only partially defective (6, 7), indicating the functional presence of redundant mechanisms or even another p21-like protein(s) (20). However, a G2 checkpoint role for p21 need not be completely redundant. The best evidence to date for a role for p21 in G2 checkpoint regulation is that although DNA damage conferred a transient G2/M arrest in p21−/− tumor cells, these cells eventually underwent apoptotic death after executing abnormal DNA replication events (39). Thus, p21-mediated inhibition of cyclin-Cdk complexes might contribute to long-term DNA damage-induced G2 arrest even though p21 is clearly not essential for the immediate G2 checkpoint response; e.g., p53-deficient cells that fail to arrest in G1 upon IR, such as early-passage NHF1-E6 fibroblasts, low-passage fibroblasts from individuals with Li-Fraumeni syndrome, and low-passage cells from p53- or p21-deficient mouse embryos, readily arrested in G2 following exposure to γ rays without detectable p21 induction (6, 18, 26). Although inhibitory phosphorylation of Cdc2 and down-regulation of cyclin B1 levels have been implicated, the detailed mechanisms whereby this regulation is carried out remain to be elucidated. Furthermore, the long-term effects of DNA damage on these cells without p21 have not been described. Therefore, a critical G2 checkpoint role for p21 in this context cannot be ruled out. Moreover, it has been reported that fibroblasts derived from patients with the familial ataxia-telangiectasia syndrome, immortal Li-Fraumeni syndrome fibroblasts, as well as late-passage E6 fibroblasts showed an attenuated G2 checkpoint response (26). Whereas these results may be interpreted as indicating that wild-type p53 function is directly required for a short-term G2 checkpoint response to IR, a more likely explanation is that lack of p53 function diminishes the capacity for IR-induced mitotic delay over the long term by promoting genetic instability and mutations. Thus, a completely redundant or an adjuvant role for p53-induced p21 in the implementation or maintenance of G2 checkpoint control is a possibility that needs to be thoroughly explored.
ACKNOWLEDGMENTS
We thank James Brugarolas and Tyler Jacks (Cambridge, Mass.) for p21−/− MEFs, Jacques Piette (IGM, Montepellier, France) for p53− primary fibroblasts, Clare McGowan and Paul Russell (Scripps Research Institute, La Jolla, Calif.) for anti-cyclin B1 antibodies, Martha Henze and Ludger Hengst (Scripps Research Institute) for anti-p21 antibody, Emma Lees and Ed Harlow (MGH, Boston, Mass.) for monoclonal cyclin D1, cyclin E, and cyclin A antibodies, Jerry Shay (University of Texas SW Medical Center, Dallas) for HPV16 E6-transduced IMR-90 cells, and Patrick Turowski (CRBM, Montpellier, France) for supplying some synchronized Hs68 cells. V.D. extends thanks to all members of Jacques Pouysségur’s laboratory (Centre de Biochimie, Nice, France) for their hospitality and for many discussions in the course of this work and to Marcel Dorée, Daniel Fisher, and Annick Péléraux (CRBM) for their helpful comments on the manuscript. Finally, special thanks go to Anne Brunet (Centre de Biochimie, Nice, France) for constant encouragement and for critically reading the original versions of the manuscript.
This work was partially supported by Association pour la Recherche sur le Cancer grant ARC-6852 to V.D. and Public Health Service grant GM46004 to S.I.R.
REFERENCES
- 1.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W G., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal M L, Agarwal A, Taylor W R, Stark G R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aprelikova O, Xiong Y, Liu E T. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995;270:18195–18197. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- 4.Bailly E, Pines J, Hunter T, Bornens M. Cytoplasmic accumulation of cyclin B1 in human cells: association with a detergent-resistant compartment and with the centrosome. J Cell Sci. 1992;101:529–545. doi: 10.1242/jcs.101.3.529. [DOI] [PubMed] [Google Scholar]
- 5.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 6.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 7.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21Cip1/Waf1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 7a.Dulić, V. Unpublished data.
- 8.Dulić V, Drullinger L F, Lees E, Reed S I, Stein G H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci USA. 1993;90:11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulić V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 10.Dulić V, Lees E, Reed S I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 11.El-Deiry W S, Harper J W, O’Connor P M, Velculescu V E, Canman C E, Jackman J, Pietenpol J A, Burrell M, Hill D E, Wang Y, Wiman K G, Mercer W E, Kastan M B, Kohn K W, Elledge S J, Kinzler K W, Vogelstein B. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 12.Faha B, Harlow E, Lees E. The adenovirus E1A-associated kinase consists of cyclin E-p33cdk2 and cyclin A-p33cdk2. J Virol. 1993;67:2456–2465. doi: 10.1128/jvi.67.5.2456-2465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girard F, Fernandez A, Lamb N. Delayed cyclin A and B1 degradation in non-transformed mammalian cells. J Cell Sci. 1995;108:2599–2608. doi: 10.1242/jcs.108.7.2599. [DOI] [PubMed] [Google Scholar]
- 14.Girard F, Strausfeld U, Fernandez A, Lamb N J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 15.Gu Y, Turck C W, Morgan D O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366:707–10. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- 16.Guadagno T M, Newport J W. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- 17.Harper J W, Elledge S J, Keyomarsi K, Dynlacht B, Tsai L H, Zhang P, Dobrowolski S, Bai C, Connell C L, Swindell E, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levedakou E N, Kaufmann W K, Alcorta D A, Galloway D A, Paules R S. p21CIP1 is not required for the early G2 checkpoint response to ionizing radiation. Cancer Res. 1995;55:2500–2502. [PubMed] [Google Scholar]
- 19.Li Y, Jenkins C W, Nichols M A, Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994;9:2261–2268. [PubMed] [Google Scholar]
- 20.Mazars R G, Jat P S. Expression of p24, a novel p21Waf1/Cip1/Sdi1-related protein, correlates with measurement of the finite proliferative potential of rodent embryo fibroblasts. Proc Natl Acad Sci USA. 1997;94:151–156. doi: 10.1073/pnas.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi M, Adami G R, Robetorye R S, Noda A, Venable S F, Dimitrov D, Pereira S O, Smith J R. Exit from G0 and entry into the cell cycle of cells expressing p21Sdi1 antisense RNA. Proc Natl Acad Sci USA. 1995;92:4352–4356. doi: 10.1073/pnas.92.10.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Niculescu A B, III, Chen X, Smeets M, Hengst L, Prives C, Reed S I. Effects of p21Cip1/Waf1 at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noda A, Ning Y, Venable S F, Pereira S O, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagano M, Theodoras A M, Tam S W, Draetta G F. Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev. 1994;8424:1627–1639. doi: 10.1101/gad.8.14.1627. [DOI] [PubMed] [Google Scholar]
- 26.Paules R S, Levedakou E N, Wilson S J, Innes C L, Rhodes N, Tlsty T D, Galloway D A, Donehower L A, Tainsky M A, Kaufmann W K. Defective G2 checkpoint function in cells from individuals with familial cancer syndromes. Cancer Res. 1995;55:1763–1773. [PubMed] [Google Scholar]
- 27.Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polyak K, Lee M H, Erdjument B H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 29.Poon R Y, Hunter T. Dephosphorylation of Cdk2 Thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin. Science. 1995;270:90–93. doi: 10.1126/science.270.5233.90. [DOI] [PubMed] [Google Scholar]
- 30.Quelle D E, Ashmun R A, Shurtleff S A, Kato J-Y, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 31.Reed S I, Bailly E, Dulić V, Hengst L, Resnitzky D, Slingerland J. G1 control in mammalian cells. J Cell Sci Suppl. 1994;18:69–73. doi: 10.1242/jcs.1994.supplement_18.10. [DOI] [PubMed] [Google Scholar]
- 32.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 34.Sewing A, Burger C, Brusselbach S, Schalk C, Lucibello F C, Muller R. Human cyclin D1 encodes a labile nuclear protein whose synthesis is directly induced by growth factors and suppressed by cyclic AMP. J Cell Sci. 1993;104:545–555. doi: 10.1242/jcs.104.2.545. [DOI] [PubMed] [Google Scholar]
- 35.Shay J W, Wright W E, Brasiskyte D, Van der Haegen B. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993;8:1407–1413. [PubMed] [Google Scholar]
- 36.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 37.Slingerland J M, Hengst L, Pan C H, Alexander D, Stampfer M R, Reed S I. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor beta-arrested epithelial cells. Mol Cell Biol. 1994;14:3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tournier S, Leroy D, Goubin F, Ducommun B, Hyams J S. Heterologous expression of the human cyclin-dependent kinase inhibitor p21Cip1 in the fission yeast, Schizosaccharomyces pombe reveals a role for PCNA in the chk1+ cell cycle checkpoint pathway. Mol Biol Cell. 1996;7:651–662. doi: 10.1091/mbc.7.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldman T, Lengauer C, Kinzler K W, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 40.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita M, Fukada S, Yoshikuni M, Bulet P, Hirai T, Yamaguchi A, Lou Y H, Zhao Z, Nagahama Y. Purification and characterization of maturation-promoting factor in fish. Dev Biol. 1992;147:8–15. doi: 10.1016/0012-1606(92)90259-j. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Hannon G J, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]