Abstract
Members of the tumor necrosis factor (TNF)-nerve growth factor (NGF) receptor family have been shown to be important costimulatory molecules for cellular activation. 4-1BB and Ox40 are two recently described members of this protein family which are expressed primarily on activated T cells. To gain insight into the signaling pathways employed by these factors, yeast two-hybrid library screens were performed with the cytoplasmic domains of 4-1BB and Ox40 as baits. TNF receptor-associated factor 2 (TRAF2) was identified as an interacting protein in both screens. The ability of both 4-1BB and Ox40 to interact with TRAF2 was confirmed in mammalian cells by coimmunoprecipitation studies. When the binding of the receptors to other TRAF proteins was investigated, 4-1BB and Ox40 displayed distinct binding patterns. While 4-1BB bound TRAF2 and TRAF1, Ox40 interacted with TRAF3 and TRAF2. Using deletion and alanine scanning analysis, we defined the elements in the cytoplasmic domains of both receptors that mediate these interactions. The 4-1BB receptor was found to have two independent stretches of acidic residues that can mediate association of the TRAF molecules. In contrast, a single TRAF binding domain was identified in the cytoplasmic tail of Ox40. The cytoplasmic domains of both receptors were shown to activate nuclear factor κB in a TRAF-dependent manner. Taken together, our results indicate that 4-1BB and Ox40 bind TRAF proteins to initiate a signaling cascade leading to activation of nuclear factor κB.
The tumor necrosis factor (TNF)-nerve growth factor (NGF) receptor superfamily is a growing receptor family that has been implicated in the activation of distinct signaling pathways which can mediate either apoptosis or cell survival (7, 19). Members of the family are defined by homology in their extracellular domains but have little sequence homology in their cytoplasmic tails. Yet, the cytoplasmic domains are important for the initiation of distinct signaling cascades (7, 19, 32).
4-1BB and Ox40 are two recently described TNF receptor-related proteins thought to play an important role in regulating T-cell-dependent immune responses (11, 12, 18, 21, 22). The expression of both receptors is restricted to activated T cells (3, 15, 16, 28). Transcription and translation of the 4-1BB and Ox40 genes are induced after primary activation of T lymphocytes by engagement of the T-cell receptor by peptide-major histocompatibility complex complexes and costimulatory signals or by mitogenic stimulation of the cells (3, 22). Murine 4-1BB and its human homolog ILA (receptor induced by lymphocyte activation) have been molecularly cloned (2, 27, 29, 35, 39). 4-1BB cross-linking in a secondary response can prolong the survival of activated T cells and enhance interleukin-2 production. Furthermore 4-1BB can provide costimulatory signals independent of CD28 (12, 22, 38). Ox40 was identified as a cell surface marker on activated rat CD4+ T cells. In mice and humans, it is expressed on CD4+ and CD8+ cells after activation (8, 30, 31). Human Ox40 was shown to promote adhesion of T lymphocytes to vascular endothelial cells (23). Ox40 expression is found on T cells specific for myelin basic protein in experimental autoimmune encephalomyelitis (44). An Ox40 immunotoxin led to specific depletion of these autoreactive lymphocytes and to amelioration of experimental autoimmune encephalomyelitis in rats (43). Besides this pathological effect, Ox40 augments the humoral immune response by interaction with its ligand on B cells (40). The interaction between Ox40 and its ligand Ox40L has been suggested to provide a costimulatory signal to T cells and additionally lead to proliferation and differentiation of B cells (18, 41).
Although a variety of physiological and pathological functions of these two members of the TNF-NGF receptor family have been identified, until now little has been known about their signal transduction pathways. To address this question, we used the cytoplasmic tails of 4-1BB and Ox40 (4-1BBCP and Ox40CP) as baits in yeast two-hybrid screens of a cDNA library derived from activated murine T cells. Both screens led to the independent isolation of TNF receptor-associated factor 2 (TRAF2), a member of the growing TRAF family of signal transducing molecules (10, 20, 25, 33, 34, 35, 37). This interaction was confirmed in mammalian cells. Moreover, both 4-1BB and Ox40 were found to bind additional members of the TRAF protein family. However, the TRAF binding sites in Ox40 and 4-1BB and their patterns of binding of TRAF proteins are not identical, suggesting that their signal transduction pathways may be subject to differential regulation. To investigate the signal transduction cascades triggered by 4-1BB and Ox40, both receptors were expressed as CD28 chimeric molecules in the human embryonic kidney cell line HEK293. Expression of the recombinant CD28–4-1BBCP and CD28-Ox40CP chimeras in HEK293 cells was found to activate NF-κB in a TRAF-dependent manner. This ability is comparable to that of CD30 and TNFR2, two other TNF receptor family members recently shown to activate nuclear factor κB (NF-κB) in a TRAF-dependent manner. Together, these data suggest that a conserved mechanism is used by a subgroup of the TNF-NGF receptor family to transduce signals in mammalian cells.
MATERIALS AND METHODS
Plasmids.
The yeast expression vectors pAS1 and pACT have been described previously (14). The Matchmaker mouse T-cell cDNA library was purchased from Clontech and is derived from a Kaplan T-lymphoma cell line. PCR was used to construct the wild type and all 5′ and 3′ deletions of the murine 4-1BB (m4-1BBCP) and Ox40 (mOx40CP) cytoplasmic domains. pAS1[m4-1BBCP] contains the coding sequence for the entire cytoplasmic domain of 4-1BB (amino acid residues 213 to 257). pAS1[mOx40CP] encodes the cytoplasmic domain of Ox40 (amino acid residues 237 to 272). The two cytoplasmic domains were amplified by PCR from cDNA of the murine T-cell line CTLL-2, using the oligonucleotide pairs sense (5′-ATCCATGGAGAAATGGATCAGGAAAAAATTCCC-3′)-antisense (5′-TAGGATCCGATAGTACATCACAGCTCATAGCC-3′) and sense (5′-ATCCATGGAGCGGAAGGCTTGGAGATTGCC-3′)-antisense (5′-TAGGATCCAAATCCACTCCTGTACTAATGC-3′), respectively. The cDNA was reverse transcribed from total RNA by using an oligo(dT)15 primer. The 5′ oligonucleotides added NcoI sites upstream of the coding sequences of the tails for the in-frame fusion to the GAL4 DNA binding domain of pAS1. All 3′ antisense oligonucleotides had BamHI sites downstream of the stop codons. The PCR products were then ligated into the NcoI and BamHI sites of pAS1. The C-terminal deletions of 4-1BB were constructed by PCR using two antisense oligonucleotides (5′-GTAGGATCCTCAAGCTGCTCCAGTGGTCTTC-3′ and 5′-GTAGGATCCTCACTGTGGACATCGGCAGCTACA-3′) that introduced in-frame stop codons after amino acids (aa) A234 and Q246. The N-terminal deletion was also made by PCR using a sense primer (5′-GGATCCATGGAGGCAGCTCAAGAGGAAGATGCTTG-3′) that created an NcoI site in frame with pAS1 upstream of the codon for A233 of 4-1BB. The internal deletion m4-1BBΔ236-238 and point mutations of mOx40CP were made by using the Chameleon mutagenesis system (Stratagene) according to the manufacturer’s protocol, with the sense primers 5′-ACTGGAGCAGCTCAAGCTTGTAGCTGCCGA-3′, 5′-AAACAGCTTCAGGGCGGCCGCCCAGGAGGAACACA-3′, 5′-CAGGACCCCGATCGCGGCCGCCCACACAGACGCAC-3′, and 5′-GATCCAGGAGGAAGCGGCCGCCGCACACTTTACTC-3′. To create XhoI sites upstream of the coding region of 4-1BB and Ox40, the sequences were reamplified by PCR using the sense primers 5′-ATACTCGAGAAAATGGATCAGGAAAAAATTCCC-3′ and 5′-ATACTCGAGACGGAAGGCTTGGAGATTGCC-3′, which introduced XhoI sites. These fragments were cloned in frame to the extracellular and transmembrane domains of CD28 into pcDNA3 (13). All constructs were verified by sequencing. Human TRAF1ΔN128, TRAF2, TRAF2ΔN171, TRAF3, TRAF3ΔN381, and TRAF3DN125, ΔC6 cDNAs have been described previously (13, 17). TRAF4 (CART1) was amplified from a human B-lymphoma cDNA library, using the sense primer 5′-ATACCATGGGTGGCTTCGACTACAAGTTCCTG-3′ and the antisense primer 5′-AATGTCGACCAGCCAGTGCCTGACTGAGGTCATG-3′. The murine TRAF5 cDNA was amplified by PCR from a C57 Black Kaplan T-lymphoma line V13 cDNA library by using the sense oligonucleotide 5′-ATAGGATCCTATGGCTCATTCGGAGGAGCAA-3′ and the antisense oligonucleotide 5′-GGATCCCTACAGATCCTCCAAGTCAGT-3′. The PCR fragment was cloned into the BamHI site of pACT and verified by sequencing.
Yeast strain, cell line, and antibodies.
The yeast two-hybrid experiments were performed in Saccharomyces cerevisiae Y153. For all transfection experiments, the human embryonic kidney cell line HEK293 (American Type Culture Collection) was used. Cell surface expression of CD28 chimeric proteins was analyzed by fluorescence-activated cell sorting using the phycoerythrin-conjugated monoclonal antibody (MAb) 37.51 (Pharmingen). For immunoprecipitation, the polyclonal rabbit anti-mouse CD28 antibody I-20 (Santa Cruz) was used. Western blot analysis was performed with a polyclonal rabbit anti-TRAF2 antibody (C-20; Santa Cruz).
Yeast two-hybrid screening.
Yeast two-hybrid library screening and analyses were performed as previously described (14). Briefly, Y153 was transformed with the appropriate pAS1 and pACT plasmid DNAs and subsequently plated on synthetic dextrose plates either lacking leucine, tryptophan, and histidine and containing 25 mM 3-aminotriazole (L−T−H− plates) or lacking leucine and tryptophan. After 3 days at 30°C, colonies from L−T−H− plates were transferred to Whatman filter paper and assayed for β-galactosidase activity. In general, β-galactosidase activity was apparent within 2 to 4 h, but the filters were allowed to incubate for at least 12 h at room temperature. In all assays, transformants were tested both for the ability to grow on L−T−H− plates and for β-galactosidase expression. Without exception, protein-protein interactions were considered to be positive only if both growth in the absence of histidine and expression of β-galactosidase were observed.
Immunoprecipitation assays.
HEK293 cells were transfected by CaPO4 precipitation of the appropriate plasmids according to standard protocols (42). In brief, 106 cells were seeded on 6-cm-diameter dishes. Six hours prior to transfection, the medium was changed. Ten micrograms of pcDNA3 containing the coding sequence of the various receptors were transfected in combination with 3 μg of a TRAF2 expression vector. Eight hours after transfection, the medium was changed again. Twenty-four hours later, the cells were lysed in lysis buffer (50 mM HEPES [pH 7.4], 100 mM NaCl, 1% Triton X-100, 10% glycerol, 10 mM NaF, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 8.5 μg of aprotinin per ml, 5.5 μg of leupeptin per ml). Lysates were incubated for 15 min on ice and then pelleted for 5 min at 15,000 rpm at 4°C. The supernatants were precleared by incubation with protein G-agarose beads. For the immunoprecipitation, lysates were incubated with a commercially available anti-CD28 antibody (I-20; Santa Cruz) at 4°C. Protein G-agarose beads were added 90 min later, and the mixture was incubated for a further 90 min. The CD28 chimera and interacting proteins were precipitated, washed three times in lysis buffer, and separated under reducing conditions on a sodium dodecyl sulfate–polyacrylamide gel. The presence of TRAF proteins in the immunoprecipitates was analyzed by Western blotting and chemiluminescence according to the manufacturer’s protocol (Amersham ECL system).
Luciferase assays.
Luciferase assays were performed as previously described (13). In brief, 5 × 105 HEK293 cells per well were seeded in six-well plates and transfected by standard CaPO4 precipitation with 40 ng each of a β-galactosidase plasmid as an internal transfection efficiency control and either a κB-responsive luciferase reporter plasmid containing two canonical κB sites (2×NF-κB promoter [26]) or a control plasmid lacking the κB sites. Cotransfected receptor and TRAF constructs are indicated in the figures. The amount of DNA for each transfection was kept constant at 2 μg per transfection by adding pcDNA3 plasmid. Cells were harvested 25 h after transfection and washed once in phosphate-buffered saline. After incubation in 0.5 ml of reporter lysis buffer (Promega) for 15 min at room temperature, the lysates were precipitated. Luciferase assays were performed with 20-μl aliquots of the supernatant and analyzed by luminometry. β-Galactosidase reactions were performed with the same lysates, and luciferase data were normalized to account for variations in transfection efficiency. For luciferase experiments, all transfections were done in triplicate, and the data shown are representative of at least three experiments.
BLAST database search.
cDNAs isolated in the yeast two-hybrid screens were sequenced by using standard dideoxynucleotide sequencing protocols. The sequences were analyzed by BLAST analysis using the National Center for Biotechnology Information database (4).
RESULTS
Yeast two-hybrid screening.
To identify proteins that interact with the cytoplasmic tails of murine 4-1BB (m4-1BBCP) or murine Ox40 (mOx40CP), we used the yeast two-hybrid system. The DNA sequences encoding the 45 aa of m4-1BBCP or the 36 aa of mOx40CP were isolated by reverse transcription-PCR from CTLL-2 cells. m4-1BBCP and mOx40CP were then cloned into the pAS1 expression vector, fusing the cytoplasmic tails of the receptors to the DNA binding domain of GAL4. The resulting vectors were used to screen a cDNA library prepared from activated murine T cells. With m4-1BBCP as bait, a screen of approximately 8 × 105 transformants yielded positive clones that grew on L−T−H− plates and expressed β-galactosidase. One of these clones interacted specifically with the 4-1BB fusion protein but not with the GAL4 DNA binding domain of pACT (data not shown). Sequence analysis and a subsequent BLAST search revealed that it encoded amino acid residues 182 to 501 of TRAF2. Approximately 2.4 × 105 transformants were analyzed in the mOx40CP library screen. Six positive clones that encoded TRAF2 were obtained; three of these were independent isolates (data not shown). These results suggest that the cytoplasmic domains of both 4-1BB and Ox40 can associate with TRAF2.
TRAF2 associates with 4-1BB and Ox40 in human HEK293 cells.
To confirm the specificity of the TRAF2 interaction with 4-1BB and Ox40 in cells, chimeric receptor constructs were made. The extracellular-plus-transmembrane domain of murine CD28 was fused to the cytoplasmic tail of murine 4-1BB or Ox40 (Fig. 1A). Each of these chimeric receptors was transfected transiently into HEK293 cells together with an expression vector coding for the C terminus including the TRAF domain of human TRAF2 (aa 87 to 501). Similar cell surface expression levels after transfection of the receptor constructs were confirmed by fluorescence-activated cell sorting analysis (data not shown). Immunoprecipitation with an anti-CD28 polyclonal Ab and subsequent Western blotting with an anti-TRAF2 antiserum confirmed the interaction of TRAF2 with the cytoplasmic tails of murine 4-1BB and Ox40 (Fig. 1B). In this biochemical assay, the cytoplasmic tails of 4-1BB and Ox40 showed a specific ability to interact with TRAF2 expressed after transfection of the recombinant DNA. Full-length CD28 was used as a negative control and did not precipitate TRAF2. These data confirm that TRAF2 can specifically associate with the cytoplasmic domains of both 4-1BB and Ox40.
FIG. 1.
TRAF2 binds to m4-1BBCP and mOx40CP in HEK293 cells. (A) Representation of the chimeric constructs used for the immunoprecipitation and luciferase experiments. The extracellular and transmembrane domains of murine CD28 were fused in frame to the entire cytoplasmic tail (aa 213 to 257) or a deletion mutant (aa 213 to 246) lacking the C-terminal 11 aa of murine 4-1BB or to the entire cytoplasmic domain of Ox40 (aa 237 to 272). (B) Coimmunoprecipitation of TRAF2 with m4-1BBCP and mOx40CP. The chimeric proteins were immunoprecipitated with an anti-CD28 serum after transfection of HEK293 cells. Immunoprecipitates were separated on sodium dodecyl sulfate–10% polyacrylamide gels under reducing conditions and analyzed by Western blotting with an anti-TRAF2 serum. The left panel shows the immunoprecipitates (IP); the right panel shows 5% of the cell lysates of 3 × 106 cells used for immunoprecipitation. The transfected receptor constructs are indicated above the lanes. Positions of truncated human TRAF2 protein and of immunoglobulin heavy and light chains (asterisks) are indicated.
TRAF2 is not the only TRAF protein that interacts with the cytoplasmic domains of 4-1BB and Ox40.
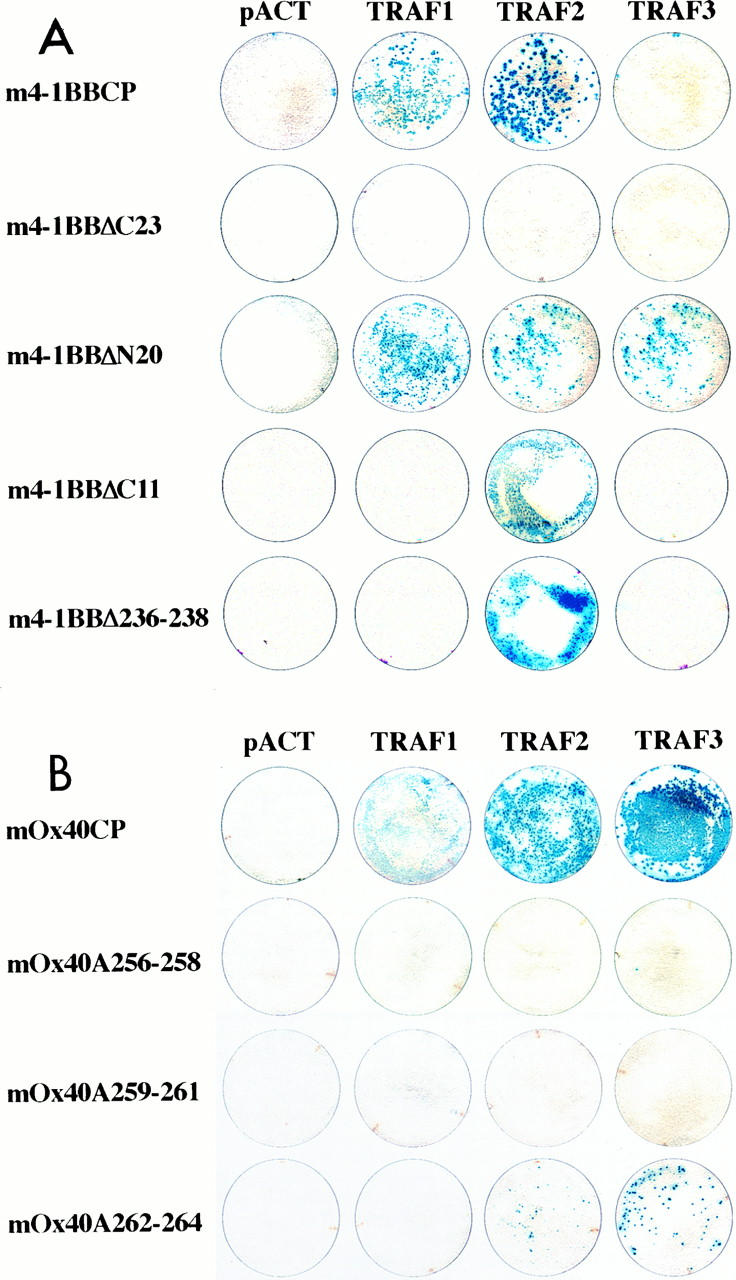
Although only TRAF2 was isolated in the yeast two-hybrid library screens, we were interested in determining whether additional members of the TRAF family could interact with the cytoplasmic tails of 4-1BB and Ox40. Therefore, the entire cytoplasmic domains of these receptors were used in directed yeast two-hybrid assays with TRAF1, TRAF2, TRAF3 (CRAF1, CD40bp), TRAF4 (CART1), and TRAF5. m4-1BBCP was found to associate with both TRAF1 and TRAF2 but had a higher affinity for TRAF2 (Fig. 2A). mOx40CP revealed its strongest interaction with TRAF3 but also bound to TRAF2 and showed a weak but reproducible binding to TRAF1 (Fig. 2B). Neither TRAF4 nor TRAF5 showed any interaction with 4-1BB or Ox40 (Fig. 3B and data not shown). These data suggest that 4-1BB and Ox40 have distinct binding patterns for members of the TRAF protein family and suggest that these receptors may have distinct signaling properties in activated T cells.
FIG. 2.
Directed yeast two-hybrid analyses using cytoplasmic domains of murine 4-1BB and Ox40 as baits. The TRAF molecules used for these experiments were cloned in frame to the GAL4 transactivation domain of pACT, and the designations of the resulting constructs are indicated. All filters were incubated for 12 h at room temperature to determine β-galactosidase activity. (A) Representative filters of the assays performed with m4-1BBCP and indicated mutants of this molecule as bait. (B) Representative filters of the yeast two-hybrid experiments done with the entire cytoplasmic tail of Ox40 (mOx40CP) and the indicated mutants as baits.
FIG. 3.
TRAF binding domains in the cytoplasmic tails of 4-1BB and Ox40 are conserved between species. (A) Alignment of the protein sequences of the cytoplasmic domains of 4-1BB and Ox40. The amino acid residues shown to be important for interaction of the TRAF molecules with either receptor are indicated by grey boxes. The tables summarize identical and similar amino acids in the cytoplasmic tails of the different species determined by BLAST analyses (4), showing percentages of similarity (top) and identity (bottom) between species of the amino acids in the grey boxes. The numbers in parentheses indicate the overall identity or similarity of the cytoplasmic tails between the various species. (B) Summary of yeast two-hybrid experiments. At least three experiments were performed for all analyses as described in the legend to Fig. 2. Shown is the level of β-galactosidase expression after 12 h at room temperature. +++, very strong signals; ++, strong signals; +, weak but significant interactions, −, no signal; n.d., not determined.
TRAF proteins interact with acidic amino acid residues in 4-1BB and Ox40.
To further define the distinct interaction patterns with members of the TRAF family, we mapped the binding domains of the TRAF molecules in the cytoplasmic tails of 4-1BB and Ox40. Deletion mutants of m4-1BBCP and Ala substitutions of certain amino acid residues of mOx40CP were cloned in frame to the GAL4 DNA binding domain of the pAS1 vector (Fig. 3B). These constructs were then used in directed yeast two-hybrid assays to determine their ability to bind TRAF1, TRAF2, and TRAF3.
N- and C-terminal deletion mutants of m4-1BBCP showed that the TRAF2 binding to m4-1BBCP is mediated by the C-terminal 23 aa of 4-1BB (Fig. 2A). This region of m4-1BBCP encodes two stretches of acidic amino acids. Deletion of the most C-terminal 11 aa caused a decrease in the ability of 4-1BBCP to bind TRAF2 and abolished TRAF1 binding, suggesting that the three glutamate residues (E247 to E249) might function in TRAF2 binding. An internal deletion of E236 to D238 also resulted in decreased m4-1BBCP interaction with TRAF2 (Fig. 2A). Although the two deletion mutants bind TRAF2 equally well, both have a lower affinity than the full-length cytoplasmic tail of 4-1BB. Both mutants are unable to interact with TRAF1 (Fig. 2A).
By computer analysis, we identified a region in Ox40 that is similar to the binding domains of TRAF2 in the cytoplasmic tails of CD30 and CD40. To determine the importance of the PIQEEHT (aa 257 to 263) region of mOx40CP for the interaction with TRAF2, a panel of alanine scanning mutants was constructed. Three stretches of amino acids, T256 to I258, Q259 to E261, and H262 to D264, of murine Ox40 were independently replaced with three alanine residues (Fig. 3B). These mutants were subsequently used in directed yeast two-hybrid assays. As shown in Fig. 2B, mutations of residues 256 to 258 and 259 to 261 to alanines abolished the ability of mOx40CP to associate with TRAF2 and TRAF3. However, mutations of residues 262 to 264 had only a minimal effect.
This mapping of the TRAF binding sites in the cytoplasmic tails of murine 4-1BB and murine Ox40 identified distinct motifs that are necessary for the interaction with TRAF proteins. Interestingly, the respective TRAF binding domains in the two receptors are highly conserved between species (Fig. 3A). The overall similarity between the cytoplasmic tails of murine 4-1BB and human 4-1BB (ILA) is 75.6%. The residues of the TRAF binding domains described in this report are 100% similar between mouse and human sequences and show 87.5% identity. mOx40CP reveals similarities of 94.4% to the cytoplasmic tail of rat Ox40 and 69.4% to the corresponding sequence of human Ox40. The residues shown to be necessary for TRAF binding of murine Ox40 are 100 and 88.9% similar to the corresponding rat and human sequences, respectively. The high homologies and the demonstrated binding of human TRAFs by murine 4-1BB and Ox40 suggest a similar binding pattern of TRAFs to the human homolog of 4-1BB (ILA) and the homologs of Ox40 in rats and humans. While the TRAF binding domains of the receptors show a high conservation between species, neither the QEEE/D motif of 4-1BB nor the PIQEE stretch of Ox40 was found in the cytoplasmic regions of any of the other described members of the TNF-NGF receptor superfamily.
TRAF2 mediates NF-κB activation induced by 4-1BB.
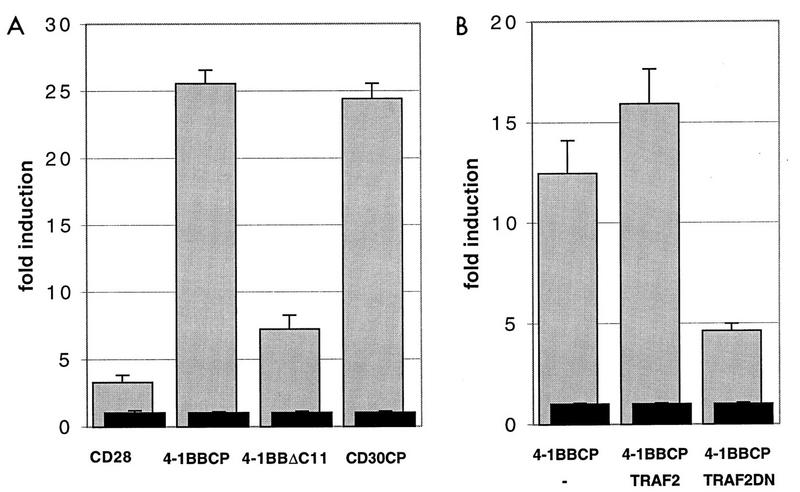
4-1BB and Ox40 have been described as costimulatory molecules for T-cell activation (11, 12, 22). Two other costimulatory receptors for lymphocytes, CD30 and CD40, have recently been shown to utilize TRAF proteins to activate NF-κB (13, 25, 36). Therefore, we examined the capability of 4-1BB to induce NF-κB-mediated gene expression. CD28 is a natural homodimer, and a chimeric CD28-CD30 construct was shown previously to induce NF-κB activation. This activation was shown to be dependent on the cytoplasmic tail of CD30 and its interaction with TRAF proteins (13). A chimeric CD28–m4-1BBCP construct was transiently transfected into HEK293 cells along with a reporter construct containing the firefly luciferase gene under the control of a 2×NF-κB promoter element. Overexpression of this chimeric construct led to a 10- to 20-fold induction of the transcription factor NF-κB in the HEK293 cells (Fig. 4A). As a positive control, a CD28-CD30 chimeric receptor was also transfected and examined for NF-κB activation (13). A full-length CD28 expression vector failed to induce NF-κB (Fig. 4A). Full-length CD4 which is expressed as a monomer and fusion proteins containing the extracellular and transmembrane domains of CD4 fused to the cytoplasmic domain of CD30 or 4-1BB also failed to induce NF-κB in the absence of cross-linking with a secondary antibody (data not shown). Interestingly, deletion of one of the two TRAF binding sites in the cytoplasmic tail of 4-1BB (m4-1BBΔC11) diminished NF-κB activation by more than 65%, suggesting that this effect is mediated at least in part by binding to TRAF proteins (Fig. 4A).
FIG. 4.
4-1BB induces NF-κB activation. (A) HEK293 cells were transfected with the chimeric CD28 constructs of a full-length CD28 expression vector, β-galactosidase expression constructs, and luciferase reporter constructs containing either two canonical NF-κB sites or a minimal promoter. Cells were harvested 25 h after transfection and analyzed for luciferase activity. The relative luciferase units were standardized to the β-galactosidase expression levels. The induction of the luciferase activity was calculated by dividing the relative luciferase units obtained after transfection of the reporter plasmid with the 2×NF-κB promoter by the relative luciferase units obtained after transfection of the reporter plasmid with the minimal promoter. The transfected DNAs are indicated. The error bars represent the standard deviations of triplicate transfections. Shown is one representative experiment of three independent transfection experiments. (B) HEK293 cells were transfected with a chimeric receptor construct of 4-1BB alone or cotransfected with full-length TRAF2 or a deletion mutant lacking most of the N-terminal ring finger (TRAF2DN) and assayed for NF-κB induction as described for panel A. Black bars represent the reporter construct with a minimal promoter; grey bars indicate transfections with the NF-κB reporter plasmid. The data are representative of three independent experiments.
To test this possibility further, full-length TRAF2 or a dominant negative TRAF2 (TRAF2DN) lacking the N-terminal 86 aa was cotransfected with the chimeric receptor construct. Overexpression of full-length TRAF2 augmented NF-κB activation by 4-1BBCP (Fig. 4B). TRAF2ΔN1-86 (TRAF2DN), which lacks most of the ring finger domain of TRAF2, still interacted with the receptor (Fig. 1B) but was unable to activate NF-κB (13, 36). Cotransfection of TRAF2DN and m4-1BBCP led to diminished NF-κB activation by the chimeric receptor molecule (Fig. 4B).
TRAF3 inhibits the TRAF2-dependent NF-κB activation triggered by Ox40.
TRAF2 was isolated in the yeast two-hybrid screens with both 4-1BBCP and Ox40CP. Therefore, we were interested in testing whether Ox40 also induces NF-κB. Overexpression of a chimeric CD28-Ox40CP fusion protein was found to be a potent inducer of NF-κB activation (Fig. 5A). Cotransfection of TRAF2 with the mOx40CP construct led to a significant enhancement of NF-κB activation (Fig. 5B). Since TRAF3 showed a strong interaction with Ox40 in the yeast two-hybrid system, its role in Ox40CP-induced NF-κB activation was also examined. HEK293 cells were transfected with full-length TRAF3 and Ox40CP. In contrast to the effects observed after cotransfection of TRAF2, TRAF3 cotransfection reproducibly inhibited the ability of Ox40CP to induce NF-κB activation (Fig. 5B). This inhibition was comparable to that observed when Ox40CP was cotransfected with the dominant negative TRAF2DN (Fig. 5C). Cotransfection of a TRAF3 mutant lacking the N-terminal ring finger also inhibited the ability of Ox40CP to activate NF-κB (Fig. 5C). These results suggest that the ability of Ox40CP to induce NF-κB can be differentially regulated by the relative abundance of TRAF2 and TRAF3.
FIG. 5.
TRAF proteins regulate Ox40-mediated NF-κB activation. HEK293 cells were transfected with DNAs as indicated. The inductions of NF-κB were calculated as described in the legend to Fig. 4A. The reporter construct with a minimal promoter is indicated by black bars; grey bars represent transfections with the NF-κB reporter plasmid. (A) HEK293 cells were transfected with a full-length CD28 expression vector or chimeric CD28 fusion proteins, and the induction of NF-κB was determined as described in the legend to Fig. 4A. (B) A CD28-Ox40CP fusion protein was expressed alone or in combination with full-length TRAF2 or TRAF3. Induction of NF-κB was calculated as described in the legend to Fig. 4A. Shown is the average induction of NF-κB obtained in three independent experiments that were all done as triplicate transfections. The error bars show the standard error of the mean for all transfections. (C) A CD28-fusion protein of Ox40CP was transfected alone or in combination with either the dominant negative TRAF2DN, full-length TRAF3, or an N-terminal deletion mutant of TRAF3 lacking aa 1 to 381 (TRAF3ΔN). The error bars represent the standard deviations of triplicate transfections. The experiment shown is representative of at least three independent transfection experiments.
DISCUSSION
In this report, we demonstrate that 4-1BB and Ox40, two members of the TNF-NGF receptor family, can use TRAF molecules to trigger cytoplasmic signal transduction cascades. Both m4-1BBCP and mOx40CP interacted with TRAF2 in a yeast two-hybrid system. The specificity of this interaction was confirmed by coimmunoprecipitation experiments in mammalian cells. These results indicate that the TRAF2 interaction with the cytoplasmic tails of 4-1BB and Ox40 is specific. We did not obtain any other members of the TRAF family in the yeast two-hybrid library screens, possibly because m4-1BBCP and mOx40CP bind only to TRAF2 or because other TRAF cDNAs are underrepresented in the mouse T-cell cDNA library used in these screens. Therefore, we performed directed yeast two-hybrid assays with TRAF1, TRAF2, TRAF3, TRAF4 (CART1), and TRAF5. Both receptors displayed measurable and distinct interactions with TRAF1, TRAF2, and TRAF3. This finding is consistent with observations made in our laboratory and others that different members of the TNF-NGF receptor superfamily have different binding specificities for TRAF proteins (9, 17, 20, 35). It is possible that the differential abilities of 4-1BB and Ox40 to bind TRAF proteins result in differential intracellular signaling events in vivo. Neither 4-1BB nor Ox40 was found to interact with TRAF4 or TRAF5 (Fig. 3B). However, this does not exclude the possibility that these two TRAF molecules or other members of this growing protein family are involved in signaling by 4-1BB and/or Ox40, since TRAF proteins are capable of binding to each other. For example, TRAF5 has been found to interact with TRAF2 in a yeast two-hybrid system (6).
Mutational analyses of the 4-1BB and Ox40 cytoplasmic domains revealed two independent TRAF binding sites in the cytoplasmic tail of 4-1BB and only one site in Ox40. This result suggests that the TRAF-dependent signaling complex assembled by 4-1BB in vivo may be dependent not only on the relative binding affinities of TRAF proteins for each of the two binding sites but also on the relative affinities of TRAF proteins for each other. Like previously identified TRAF binding sites, the TRAF binding sites in both 4-1BB and Ox40 receptors contain clusters of acidic amino acids. However, no consensus sequence for the specific binding of an individual TRAF protein is discernible from the data. Therefore, the in vivo binding of TRAF proteins to a specific receptor in the TNF receptor family may be influenced by their differential affinities for the receptors and the relative expression levels of the TRAF proteins. Furthermore, receptors like 4-1BB which contain two TRAF binding sites may facilitate the formation of specific heteromers of TRAF proteins.
Members of the TRAF family have been shown to be mediators of NF-κB activation in cells after binding to clustered TNF-NGF receptor family members (1, 5, 10, 13, 24, 25, 33, 34, 37). To investigate whether 4-1BB and Ox40 could also induce NF-κB in mammalian cells, we transfected HEK293 cells with the chimeric CD28–m4-1BBCP or CD28-mOx40CP receptors and found a 10- to 20-fold induction of luciferase expression after cotransfection with the 2×NF-κB construct (Fig. 4 and 5). These results suggest that NF-κB may function as a downstream mediator for at least some of the reported effects of 4-1BB and Ox40 in activated T lymphocytes. The cytoplasmic tails of both 4-1BB and Ox40 can induce the activation of NF-κB when expressed transiently as a dimer but not when expressed as a monomer. NF-κB activation by each receptor was shown to be dependent on TRAF proteins, and NF-κB activation was inhibited by a dominant negative TRAF2 (Fig. 4B and 5C). Interestingly, Ox40 was found by yeast two-hybrid analysis to bind most avidly to TRAF3. However, when TRAF3 was cotransfected with the Ox40 signaling construct, NF-κB activation was reproducibly repressed (Fig. 5B). Therefore, TRAF proteins are not interchangeable adapter molecules. TRAF3 appears either to act as an inhibitor of Ox40 signal transduction or to play a role in activating an alternative signal transduction pathway through the receptor. Thus, the signaling properties of Ox40 appear to be regulated by the relative levels of intracellular TRAF proteins.
This report describes the ability of the cytoplasmic domains of two members of the TNF-NGF receptor family, 4-1BB and Ox40, to bind to proteins of the TRAF family of intracellular adapter molecules. Multimerization of the cytoplasmic domains of 4-1BB and Ox40 in transfected cells can activate the transcription factor NF-κB in a TRAF-dependent manner. Interestingly, increased expression of individual TRAF proteins can either positively or negatively affect the ability of these receptors to induce NF-κB activation. These data suggest that both the differential binding affinity and relative abundance of individual TRAF proteins can influence the cellular response to receptor cross-linking. These results provide a potential explanation for the variable effects that have been observed when members of TNF-NGF receptor family are cross-linked on activated T cells.
ACKNOWLEDGMENTS
We thank C. Duckett, R. Gedrich, and J. Van Dongen for sharing reagents and unpublished results. D. Wang for help in figure preparation, and members of the Thompson laboratory for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, Yagita H, Okumura K, Inoue J, Watanabe T. Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NFκB activation. J Biol Chem. 1997;272:2042–2045. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 2.Alderson M R, Smith C A, Tough T W, Davis-Smith T, Armitage R J, Falk B, Roux E, Baker E, Sutherland G R, Din W S, Goodwin R G. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24:2219–2227. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 3.Al-Shamkhani A, Birkeland M L, Puklavec M, Brown M H, James W, Barclay A N. Ox40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the Ox40 ligand. Eur J Immunol. 1996;26:1695–1699. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Ansieau S, Scheffrahn I, Mosialos G, Brand H, Duyster J, Kaye K, Harada J, Dougall B, Hubinger G, Kieff E, Herrmann F, Leutz A, Gruss H J. Tumor necrosis factor receptor-associated factor (TRAF)-1, TRAF-2, and TRAF-3 interact in vivo with the CD30 cytoplasmic domain; TRAF-2 mediates CD30-induced nuclear factor κB activation. Proc Natl Acad Sci USA. 1996;93:14053–14058. doi: 10.1073/pnas.93.24.14053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Arch, R. H., and C. B. Thompson. Unpublished results.
- 7.Armitage R J. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol. 1994;6:407–413. doi: 10.1016/0952-7915(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 8.Birkeland M L, Copeland N G, Gilbert D J, Jenkins N A, Barclay A N. Gene structure and chromosomal localization of the mouse homologue of rat OX40 protein. Eur J Immunol. 1995;25:926–930. doi: 10.1002/eji.1830250410. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G, Cleary A M, Ye Z S, Hong D I, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 10.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD40L-mediated NF-κB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 11.DeBenedette M A, Chu N R, Pollok K E, Hurtado J, Wade W F, Kwon B S, Watts T H. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its up-regulation on M12 B lymphomas by cAMP. J Exp Med. 1995;181:985–992. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBenedette M A, Shahinian A, Mak T W, Watts T H. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 13.Duckett C S, Gedrich R W, Gilfillan M C, Thompson C B. Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17:1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn A E, Lee W-H, Elledge S J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 15.Dürkop H, Latza U, Himmelreich P, Stein H. Expression of the human Ox40 (hOX40) antigen in normal and neoplastic tissues. Br J Haematol. 1995;91:927–931. doi: 10.1111/j.1365-2141.1995.tb05413.x. [DOI] [PubMed] [Google Scholar]
- 16.Garni-Wagner B A, Lee Z H, Kim Y J, Wilde C, Kang C Y, Kwon B S. 4-1BB is expressed on CD45RAhiROhi transitional T cell in humans. Cell Immunol. 1996;169:91–98. doi: 10.1006/cimm.1996.0095. [DOI] [PubMed] [Google Scholar]
- 17.Gedrich R W, Gilfillan M C, Duckett C S, Van Dongen J L, Thompson C B. CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor-associated factor family of signal transducing proteins. J Biol Chem. 1996;271:12852–12858. doi: 10.1074/jbc.271.22.12852. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey W R, Fagnoni F F, Harara M A, Buck D, Engleman E G. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–762. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruss H J, Dower S K. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- 20.Hu H M, O’Rourke K, Boguski M S, Dixit V M. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J Biol Chem. 1994;269:30069–30072. [PubMed] [Google Scholar]
- 21.Hurtado J C, Kim S H, Pollok K E, Lee Z H, Kwon B S. Potential role of 4-1BB in T cell activation. Comparison with the costimulatory molecule CD28. J Immunol. 1995;155:3360–3367. [PubMed] [Google Scholar]
- 22.Hurtado J C, Kim Y J, Kwon B S. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- 23.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida T K, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 26.Klug C A, Gerety S K, Shah P C, Chen A-Y, Rice N R, Rosenberg N, Singh H. The v-abl tyrosine kinase negatively regulates NF-κB/Rel factors and blocks κ gene transcription in pre-B lymphocytes. Genes Dev. 1994;8:678–687. doi: 10.1101/gad.8.6.678. [DOI] [PubMed] [Google Scholar]
- 27.Kwon B S, Weissman S M. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon B S, Kestler D P, Eshhar Z, Oh K O, Wakulchik M. Expression characteristics of two potential T cell mediator genes. Cell Immunol. 1989;121:414–422. doi: 10.1016/0008-8749(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 29.Kwon B S, Kozak C A, Kim K K, Pickard R T. Genomic organization and chromosomal localization of the T-cell antigen 4-1BB. J Immunol. 1994;152:2256–2262. [PubMed] [Google Scholar]
- 30.Latza U, Dürkop H, Schnittger S, Ringeling J, Eitelbach F, Hummel M, Fonatsch C, Stein H. The human OX40 homolog: cDNA structure, expression and chromosomal assignment of the ACT35 antigen. Eur J Immunol. 1994;24:677–683. doi: 10.1002/eji.1830240329. [DOI] [PubMed] [Google Scholar]
- 31.Mallett S, Fossum S, Barclay A N. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers S M, Ross G M, Dostaler S M, Anderson M N, Weaver D F, Riopelle R J. Putative cytoplasmic amphiphilic domains in the nerve growth factor/tumour necrosis factor receptor superfamily. Biochim Biophys Acta. 1994;1196:21–28. doi: 10.1016/0005-2736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 33.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware C F, Yagita H, Okumura K. TRAF5, an activator of NF-κB and putative signal transducer for the lymphotoxin-β receptor. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 34.Regnier C H, Tomasetto C, Moog-Lutz C, Chenard M P, Wendling C, Basset P, Rio M C. Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family, which is expressed in breast carcinoma. J Biol Chem. 1995;270:25715–25721. doi: 10.1074/jbc.270.43.25715. [DOI] [PubMed] [Google Scholar]
- 35.Rothe M, Wong S C, Henzel W J, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 36.Rothe M, Sarma V, Dixit V M, Goeddel D V. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 37.Rothe M, Xiong J, Shu H B, Williamson K, Goddard A, Goeddel D V. I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc Natl Acad Sci USA. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz H, Blanco F J, von Kempis J, Valbracht J, Lotz M. ILA, a member of the human nerve growth factor/tumor necrosis factor receptor family, regulates T-lymphocyte proliferation and survival. Blood. 1996;87:2839–2845. [PubMed] [Google Scholar]
- 39.Schwarz H, Tuckwell J, Lotz M A. Receptor induced by lymphocyte activation (ILA): a new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene. 1993;134:295–298. doi: 10.1016/0378-1119(93)90110-o. [DOI] [PubMed] [Google Scholar]
- 40.Stuber E, Strober W. The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuber E, Neurath M, Calderhead D, Fell H P, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 42.van der Eb A J, Graham F L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65:826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg A D, Bourdette D N, Sullivan T J, Lemon M, Wallin J J, Maziarz R, Davey M, Palida F, Godfrey W, Engleman E, Fulton R J, Offner H, Vandenbark A A. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–189. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg A D, Lemon M, Jones A J, Vainiene M, Celnik B, Buenafe A C, Culbertson N, Bakke A, Vandenbark A A, Offner H. OX-40 antibody enhances for autoantigen specific V beta 8.2+ T cells within the spinal cord of Lewis rats with autoimmune encephalomyelitis. J Neurosci Res. 1996;43:42–49. doi: 10.1002/jnr.490430105. [DOI] [PubMed] [Google Scholar]