Abstract
This study investigates structure-odor threshold relationships of aroma compounds using integrated S-curve analysis, molecular docking, and dynamics simulations. Molecular docking revealed odor thresholds were independent of binding energies but sensitive to structural variations, which altered receptor interaction pattern: eugenol formed hydrogen bonds with SER183, while its isomer isoeugenol preferentially bound TYR260. Similarly, phenylethyl alcohol established TYR278 hydrogen bonding absent in phenylethyl aldehyde. Molecular dynamics simulations identified hydrogen bond stability and receptor conformational flexibility as threshold determinants, exhibiting more stable hydrogen bonds and greater conformational flexibility displayed lower detection thresholds. These findings establish a predictive framework linking molecular structural features to odor thresholds while elucidating ligand-receptor interaction mechanisms, providing theoretical foundations for rational flavor design and sensory modulation strategies.
Keywords: Odor molecule, S-curve method, Molecular docking, Molecular dynamic simulation, Threshold
Graphical abstract
Highlights
-
•
S-curve method defines odor thresholds for 10 key food aroma molecules.
-
•
Molecular docking reveals binding mechanisms of odorants with receptors.
-
•
Dynamics link thresholds to hydrogen bonds and receptor flexibility.
1. Introduction
The capacity of humans to discern a multitude of volatile odors from food is largely attributed to the interaction between olfactory receptors in the olfactory epithelium and a spectrum of odorant molecules. This interaction facilitates the transduction of chemical signals into electrical impulses that are conveyed to the brain, enabling the recognition and differentiation of various scents (S. T. Zeng et al., 2024). While 391 olfactory receptors with physiological roles have been characterized in humans, the comprehensive understanding of their ligand interactions remains limited (J. Wang et al., 2024, Wang et al., 2024). Since the pioneering work of Buck and Axel in 1991 (Buck and Axel, 1991), which initiated the exploration of the sensing mechanisms and deorphanization of olfactory receptors, the number of olfactory receptors with identified ligands has only reached approximately 80 (de March et al., 2015). Consequently, the pursuit of key odorant molecules in food and the revelation of their binding modalities with olfactory receptors constitute an essential phase in deciphering the intricacies of olfactory perception.
In a comprehensive study conducted in 2014, Hofmann et al. assessed a vast array of approximately 10,000 food items, pinpointing approximately 230 pivotal odorants (Dunkel et al., 2014). They offered detailed characterizations of various aromatic profiles, including floral, sour, and fruity notes. Notably, among these identified odorants, there exists a subset with highly similar molecular structures, which humans are capable of discerning in terms of both intensity and typological distinctions. This observation underscores the notion that the interaction dynamics between structurally diverse odor molecules and olfactory receptors are markedly distinct, culminating in the nuanced olfactory perception by humans. Hofmann highlighted the case of ethyl vanillin, which exhibits a flavor intensity fourfold that of vanillin (Egawa et al., 2006). Consequently, the exploration of flavor intensities among structurally analogous odor molecules holds significant implications for the industrial manufacturing processes and the judicious application of food additives.
Molecular docking is a computational technique that identifies the optimal orientation and conformation of a ligand (odor molecule) when bound to a specific receptor (olfactory receptor), predicting the structure of the complex and the binding affinity (S. Zeng et al., 2023). Molecular dynamics (MD) simulations serve as a powerful tool for investigating the dynamic binding interactions between ligand molecules and receptor proteins. This approach enables quantitative assessment of binding stability and molecular recognition efficiency through key metrics including root mean square deviation (RMSD), root mean square fluctuation (RMSF), hydrogen bond, and radius of gyration (Rg). The application of MD simulations proves particularly crucial for elucidating the physiological mechanisms underlying odorant-olfactory receptor interactions, providing atomic-level insights into binding states and conformational adaptation processes that govern olfactory perception. (Hu et al., 2023).
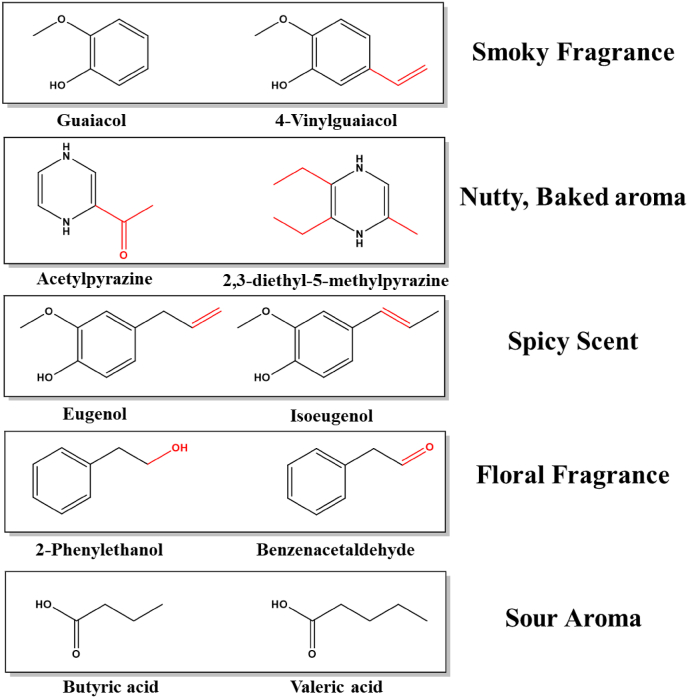
In order to further elucidate the binding mechanisms of odor molecules with their corresponding olfactory receptors, we have selected compounds known for their distinct scents and their ability to activate specific olfactory receptors. For instance, guaiacol and 4-vinylguaiacol, which possess a smoky fragrance, can activate the olfactory receptor OR10G4 (Mainland et al., 2013). 2-acetylpyrazine and 2,3-diethyl-5-methylpyrazine, known for their nutty, baked aroma, can activate OR5K1 (Marcinek et al., 2021). Eugenol and isoeugenol, recognized for their spicy scent, can activate OR5D18 (Zhou et al., 2023). 2-Phenylethanol and benzenacetaldehyde, with their floral fragrance, can activate OR2AG2 (Duroux et al., 2020). Lastly, butyric acid and valeric acid, which have a sour aroma, can activate OR51E1 (Xu and Pluznick, 2022). Furaneol (4-hydroxy-2,5-dimethyl-3(2H)-furanone), a ubiquitous flavor component in food systems, imparts characteristic caramel-like aroma notes (Dunkel et al., 2014). Despite its prevalence in olfactory environments where multiple aroma compounds are concurrently perceived, the sensory action between furaneol and odorants remains poorly characterized.
In this study, we employed the S-curve method to determine the odor thresholds of all target aroma compounds. Subsequently, molecular docking and molecular dynamics simulations were conducted to elucidate the binding mechanisms between odorant molecules and olfactory receptors. The findings provide theoretical support for understanding structure-threshold relationships in odorant molecules.
2. Materials and methods
2.1. Materials
The following high-purity chemicals were used in this study: Guaiacol (99 % purity), 2-Acetylpyrazine (98 % purity), 2,3-Diethyl-5-methylpyrazine (98 % purity), Eugenol (99 % purity), Isoeugenol (97 % purity), 2-Phenylethanol (99 % purity), Butyric Acid (99 % purity), and Valeric acid (99 % purity) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. 4-Vinylguaiacol (95 % purity) and Benzenacetaldehyde (95 % purity) were obtained from Shanghai Acmec Biochemical Co., Ltd. Furaneol was purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd.
2.2. Collection and structures of receptors
The olfactory receptors required for our study include OR2AG2 (UniProt ID: A6NM03), OR5K1 (UniProt ID: Q8NHB7), OR5D18 (UniProt ID: Q8NGL1), OR10G4 (UniProt ID: Q8NGN3), and OR51E1 (UniProt ID: Q8TCB6). These sequences were all retrieved from the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/). The database, a collaborative effort between EMBL-EBI and DeepMind, offers a comprehensive repository of predicted three-dimensional protein structures.
2.3. Sensory evaluation
The study involved a panel of seven trained and experienced experts (3 males, 4 females, and aged 22–32 years), all of whom were free from rhinitis and non-smokers, all participants had prior experience in sensory evaluation. Written informed consent was obtained from each participant, confirming their willingness to participate and authorize data usage. Panelists retained the right to withdraw from the trial at any time without providing any reason. This protocol was approved by the Ethics Committee of the College of Food Science and Technology at Henan University of Technology. All panel members were thoroughly informed about the details of the study. To prevent fatigue and carryover effects, the panel members were allowed a 5-min break between the evaluations of different samples.
The olfactory thresholds were measured using the three-alternative forced-choice (3-AFC) procedure (Cometto-Muñiz and Abraham, 2016). Three different concentrations of odorant were prepared by diluting it with ethanol, starting from the highest concentration to the lowest, with a dilution factor of 2 for each step, and diluted a total of 10 times. In each set of three samples, the diluted odorant sample was randomly numbered along with two ethanol samples and presented to a professional evaluator for olfactory assessment to distinguish the different samples. The correct response rate was adjusted using the correction factor to detect the probability of correct detection. Subsequently, an S-curve fitting was performed according to the following formula:
In this context, x represents the logarithm of the concentration, x0 represents the logarithm of the threshold value, is the slope of the S-curve, and the concentration corresponding to the corrected detection probability P = 0.5 is considered the measured threshold value.
2.4. Molecular docking
The protein underwent the following processing using AutoDockTools 1.5.6 software: first, the odorant receptor protein and small molecule ligand PDB files were added. Then, hydrogen atoms were introduced and Gasteiger charges were computed, resulting in output saved as a.pdbqt file. The docking box size was set, and docking sites were adjusted accordingly. All flexible bonds of the small molecule ligand were designated as rotatable. The receptor protein was rigidly configured for docking, utilizing the genetic algorithm. Docking outcomes were obtained through the execution of AutoGrid4 and AutoDock4, which disclosed binding energies, subsequently exported. Ultimately, the protein-ligand complexes were exported and stored as.pdb files using the freely available PyMOL software.
2.5. Molecular dynamics simulation
All molecular dynamics (MD) simulations were carried out employing the GROMACS package (version 2023.2) coupled with the CHARMM36m force field. Simulation boxes were constructed utilizing CHARMM-GUI. For all protein-ligand complex, the PPM 2.0 functionality was employed to align the transmembrane helices of the protein structure and insert them into a bilayer composed of 75 % palmitoyl-oleoyl-phosphatidylcholine (POPC) and 25 % deprotonated cholesteryl hemisuccinate (CHSD), with CHSD placed around the G-protein-coupled receptor (GPCR) structure. Solvation was performed using the TIP3P water model, supplemented with 0.15 M potassium chloride ions for neutralization, while maintaining a temperature of 310 K. Energy minimization and equilibration were conducted iteratively six times, each equilibration phase lasting 1 ns. Subsequent to equilibration, the final MD simulations were executed with a time step of 2 fs over a total simulation duration of 20 ns.
2.6. Statistical analysis
The protein-ligand complexes were visualized and analyzed using the freely available open-source software Avogadro and PyMOL. Sensory evaluation results were processed and graphed using the free open-source software SigmaPlot 15.0.
3. Results and discussion
3.1. Determination of key flavor component thresholds
Among the myriads of food odors, those reminiscent of smoky fragrance, toasted nuts, floral notes, spices, and sourness are particularly prominent (Dunkel et al., 2014). Consequently, we have selected guaiacol, 4-vinylguaiacol, acetylpyrazine, 2,3-diethyl-5-methylpyrazine, eugenol, isoeugenol, 2-phenylethanol, benzenacetaldehyde, n-butyric acid, and n-valeric acid as archetypal representatives of these five odor categories. Furthermore, these flavor constituents share notable structural homologies (Fig. 1).
Fig. 1.
The molecular structures of the 10 key flavor components.
Experimental results derived from the S-curve method demonstrated that the threshold value for guaiacol, which possesses a smoky aroma, is 6.58 μg/mL, whereas for 4-vinylguaiacol, it is 0.42 μg/mL (Fig. 2A). Prior literature has also indicated that compounds containing double bonds can enhance their aromatic intensity to a certain extent (Johnson et al., 2006; Miyazawa et al., 2020). The threshold value for 2-acetylpyrazine, which has a nutty roasted aroma, is 0.0018 μg/mL, while for 2,3-diethyl-5-methylpyrazine, it is 0.018 μg/mL (Fig. 2B). Eugenol, known for its spicy scent, has a threshold value of 0.026 μg/mL, and isoeugenol has a threshold value of 0.11 μg/mL (Fig. 2C). It is noteworthy that the structural difference between eugenol and isoeugenol is merely the rearrangement of the double bond, yet the threshold value of eugenol is only a quarter of that of isoeugenol. This phenomenon also indicates that even minor differences in the structure of odor components can lead to vastly different sensory experiences (Hasegawa et al., 2012; Laska, 2005). 2-phenylethanol, which has a floral scent, has a threshold value of 1.1 μg/mL, while benzaldehyde, also with a floral scent, has a threshold value of 0.57 μg/mL (Fig. 2D). From a structural chemistry perspective, while the lone electron pairs on the oxygen atom of aldehyde groups exhibit greater spatial accessibility, the reduced bond length of the C=O double bond (1.23 Å) compared to C-O single bonds (1.43 Å) diminishes its hydrogen-bonding propensity. (Wakabayashi et al., 2020; Z. Wang et al., 2024, Wang et al., 2024). This may be one of the factors that lead to different molecular thresholds for the two odors. Lastly, the threshold value for butyric acid, which has a sour aroma, is 9.67 μg/mL, and for valeric acid, it is 0.67 μg/mL (Fig. 2E). As previously mentioned, the case of vanillin and ethyl vanillin is very similar; an increase or decrease in carbon atoms can significantly affect the aroma threshold of odor molecules. This is because changes in the length of the carbon chain can affect the tightness of the binding between the ligand molecule and the receptor binding pocket, and secondly, carbon atoms may also influence the hydrophobic forces between the ligand and receptor (Barnum and Hong, 2022; Bauer et al., 2022; Niu et al., 2018). These complex factors ultimately lead to changes in the threshold values of odor molecules.
Fig. 2.
Olfactory threshold determination results for 10 key flavor compounds. (The solid line delineates the fitted curve, whereas the dashed line corresponds to the experimental curve.)
3.2. Molecular docking of key flavor component
Olfactory receptors are classified as G protein-coupled receptors (GPCRs), endowing them with a conserved protein structure characterized by seven transmembrane helices and a configuration of three intracellular and three extracellular loops (Ball et al., 2024). The amino acid sequences of these receptors exhibit notable variability, which underpins their functional diversity and confers specificity in the recognition of a broad spectrum of odorant molecules (Wu et al., 2024). The structural heterogeneity of odorants, encompassing variations in functional groups, carbon chain lengths, and chiralities, engenders a corresponding diversity in their binding conformations to olfactory receptors (Ben Khemis and Ben Lamine, 2021; Ben Khemis, Bouzid, Mechi and Ben Lamine, 2021). Consequently, the visualization of these receptor-odorant binding conformations is of paramount importance, serving as a fundamental prerequisite for elucidating the mechanisms of olfactory perception.
To uncover the binding modalities of pivotal food odorants with olfactory receptors, we utilized molecular docking methodologies to investigate the interactions between specific odorant molecules and their respective receptors. Specifically, we docked guaiacol and 4-vinylguaiacol with the olfactory receptor OR10G4; 2-acetylpyrazine and 2,3-diethyl-5-methylpyrazine with OR5K1; eugenol and isoeugenol with OR5D18; 2-phenylethanol and benzenacetaldehyde with OR2AG2; and n-butyric acid and n-pentanoic acid with OR51E1. The binding energies, as detailed in Table 1, spanned from −5.28 kcal/mol to −2.74 kcal/mol, suggesting a robust binding capacity between the odorant molecules and their receptors (Malmstrom and Watowich, 2011).
Table 1.
Molecular docking binding energies of 10 key flavor molecules.
| Olfactory Receptor | Ligand 1 | binding energy 1 (kcal/mol) | Ligand 2 | binding energy 2 (kcal/mol) |
|---|---|---|---|---|
| OR10G4 | Guaiacol | −4.15 | 4-Vinylguaiacol | −5.1 |
| OR5K1 | Acetylpyrazine | −3.84 | 2,3-Diethyl-5-Methylpyrazine | −4.78 |
| OR5D18 | Eugenol | −5.27 | Isoeugenol | −5.28 |
| OR2AG2 | 2-Phenylethanol | −4.30 | Benzenacetaldehyde | −4.31 |
| OR51E1 | Butyric acid | −2.74 | Valeric acid | −2.93 |
Notably, the binding energy of 4-vinylguaiacol to OR10G4 is considerably higher than that of guaiacol, potentially due to the hydrophobic effect conferred by the vinyl group at the terminal position, which may stabilize and tighten the binding of 4-vinylguaiacol within the receptor (Paudel et al., 2023). Interestingly, the presence of the vinyl group did not result in a change in the amino acid residues involved in the interaction with OR10G4, both guaiacol and 4-vinylguaiacol form hydrogen bonds with the HIS-104 and HIS-154 residues of OR10G4 (Fig. 3A). The binding energy of 2-acetylpyrazine with OR5K1 is −3.84 kcal/mol, which is markedly higher than the −4.78 kcal/mol observed for the binding of 2, 3-diethyl-5-methylpyrazine with the same receptor. It is postulated that the increased number of alkyl side chains in 2, 3-diethyl-5-methylpyrazine may enhance hydrophobic interactions, thus augmenting its binding affinity to OR5K1 (Saitoh et al., 2022). Furthermore, 2-acetylpyrazine engages in hydrogen bonding with the threonine residue at THR-109 of the olfactory receptor OR5K1. In contrast, 2,3-diethyl-5-methylpyrazine forms a hydrogen bond with the serine residue at SER-203 of the same receptor (Fig. 3B). This observation underscores the nuanced influence of molecular structure on receptor binding, highlighting the importance of hydrophobic interactions in modulating the affinity of odorant molecules for their respective olfactory receptors.
Fig. 3.
Molecular docking for 10 key flavor compounds. (A) Guaiacol and 4-Vinylguaiacol are depicted in complex with the olfactory receptor OR10G4. (B) Acetylpyrazine and 2,3-Diethyl-5-Methylpyrazine are shown docked with OR5K1. (C) Eugenol and Isoeugenol are illustrated interacting with OR5D18. (D) 2-Phenylethanol and Benzenacetaldehyde are portrayed in association with OR2AG2. (E) Butyric Acid and Valeric Acid are visualized in complex with OR51E1. Yellow dashed lines denote the hydrogen bonding interactions.
Eugenol and isoeugenol display nearly equivalent binding affinities for the olfactory receptor OR5D18, as do 2-phenylethanol and benzenacetaldehyde with OR2AG2. Repetitive molecular docking experiments have yielded congruent outcomes. However, for eugenol and isoeugenol, the repositioning of the double bond significantly modifies the binding conformation with OR5D18. Isoeugenol exhibits a shift in binding specificity, no longer interacting with the serine residue at SER-183 on the olfactory receptor OR5D18, but instead forming a hydrogen bond with the tyrosine residue at TYR-260 (Fig. 3C). Likewise, the docking results reveal that upon oxidation of the hydroxyl group of 2-phenylethanol to an aldehyde group, benzenacetaldehyde loses the capacity to form hydrogen bonds with TYR-278 on OR2AG2, concomitantly altering its binding conformation (Fig. 3D). These findings underscore the subtle yet critical impact of molecular structural alterations on receptor binding, emphasizing the pivotal role of hydrogen bonding and conformation in the interaction dynamics between odorant molecules and their corresponding olfactory receptors (Okamoto and Ando, 2024).
Structurally, butyric and valeric acids are distinguished by a single carbon atom; however, the binding energy of n-pentanoic acid with the olfactory receptor OR51E1 is 2.93 kcal/mol, whereas n-butyric acid exhibits a binding energy of 2.74 kcal/mol to OR51E1. There are still certain differences in the binding energy. Furthermore, the orientation of the carboxylic acid group in butyric acid is significantly reversed compared to that in valeric acid, resulting in entirely distinct binding conformations for these two odorant molecules with OR51E1. Butyric acid exhibits a preference for hydrogen bonding with the histidine residue at HIS-107 within the olfactory receptor OR51E1. In contrast, valeric acid demonstrates a propensity for forming hydrogen bonds with the glutamine residue at GLN-184 and the valine residue at VAL-198 of the same receptor (Fig. 3E). This observation underscores the profound influence of even minor structural variations on binding affinity and conformation, suggesting that subtle changes in molecular architecture can dramatically affect the interaction between odorant molecules and their corresponding olfactory receptors.
Our findings demonstrate that even minor structural variations among odorant molecules can precipitate substantial alterations in their binding modes with olfactory receptors. Likewise, the binding energy may vary due to modifications in side chains, even when the odorant molecules display analogous binding conformations. Furthermore, a clear correlation between binding energy and odor threshold is not evident, suggesting that the prediction of odor thresholds based on binding energy is fraught with inherent uncertainties. This study underscores the complexity of odorant-receptor interactions and highlights the limitations in using binding energy as a predictive metric for odor perception thresholds.
3.3. Molecular dynamic simulation of key flavor component
Following molecular docking, we performed a 20 ns molecular dynamics simulation of the complexes formed between key odorant molecules and olfactory receptors to investigate their binding patterns over time. RMSD (Root Mean Square Deviation) values were employed as a metric for the extent of conformational changes in proteins or ligands during the simulation, with lower values signifying greater stability and closer alignment to the initial conformation (Mahmoud et al., 2022). The RMSF (Root Mean Square Fluctuation) quantifies the average positional fluctuation of residues, with higher values indicating increased flexibility and dynamic motion of the respective atoms or residues (Iqbal et al., 2023). Hydrogen bonds (H-bonds) are crucial non-covalent interactions within protein-ligand complexes, and analysis of these interactions provides insights into the stability and dynamics of the complex. Molecular dynamics simulations elucidate the kinetics of hydrogen bond formation, persistence, and dissociation (Cao et al., 2022). Additionally, the Rg, a parameter indicative of a protein's three-dimensional configuration and size, reflects the compactness of the polypeptide chain. Variations in Rg throughout the simulation are indicative of conformational fluctuations and the degree of flexibility within the protein (Cetintas et al., 2023). Collectively, these metrics offer a comprehensive understanding of the dynamic behavior of protein-ligand interactions over the simulation timeframe, which is pivotal for functional protein studies.
Fig. 4A illustrates that the RMSD results demonstrate both guaiacol and 4-vinylguaiacol achieved equilibrium during the 20 ns molecular dynamics simulation. Notably, 4-vinylguaiacol exhibited significant fluctuations in ligand conformation between 6 and 8 ns, the fluctuation range exceeded 0.5 Å. The RMSF analysis indicated pronounced fluctuations in residues 50–60, 120–130, 230–240, and 260–270 during the binding interactions of guaiacol and 4-vinylguaiacol with OR10G4, the average fluctuation range is over 2 Å. These residues are predominantly situated in the ICL-1, TM-3, TM-6, and ECL-3 regions of the receptor. Additionally, 4-vinylguaiacol formed a greater number of hydrogen bonds with OR10G4, according to the statistical analysis of the raw data, 4-vinylguaiacol formed 251 hydrogen bonds, while guaiacol formed only 151 hydrogen bonds. The results clearly demonstrate that 4-vinylguaiacol significantly enhances the flexibility of OR10G4, with a fluctuation range exceeding 0.5 Å, leading to more pronounced overall conformational changes. In contrast, the maximum fluctuation range for guaiacol is only 0.3 Å(J. Wang et al., 2024, Wang et al., 2024). Consequently, the enhanced formation of hydrogen bonds and the increased flexibility of the protein conformation may be critical factors underlying the lower odor threshold of 4-vinylguaiacol.
Fig. 4.
Molecular dynamic simulation for 10 key flavor compounds. Each row of images, from left to right, represents the results for RMSD, RMSF, hydrogen bonds, and Rg, respectively.
The RMSD results revealed significant binding fluctuations for both 2-acetylpyrazine and 2,3-diethyl-5-methylpyrazine during the 5–15 ns timeframe, the fluctuation range exceeded 1 Å, with stabilization occurring only in the final 5 ns (Fig. 4B). Notably, residues 50–60, 80–90, 130–200, 220–230, and 260–280 exhibited pronounced fluctuations, the average fluctuation range is over 2 Å, primarily localized within the ICL-1, ICL-2, ECL-1, TM-4, ECL-2, and ECL-3 regions. Additionally, 2-acetylpyrazine formed a considerable number of hydrogen bonds with OR5K1 throughout the simulation (number is 147), while 2,3-diethyl-5-methylpyrazine began forming hydrogen bonds only around 18–20 ns (number is 11). The Rg analysis further indicated that both odorant molecules induced repeated contractions and conformational alterations in OR5K1 during the simulation, the maximum fluctuation range is close to 0.4 Å. Finally, in conjunction with sensory evaluation results, the odor threshold of 2-acetylpyrazine was found to be significantly lower than that of 2,3-diethyl-5-methylpyrazine, suggesting that the number of hydrogen bonds formed between odorant molecules and olfactory receptors may play a crucial role in influencing odor intensity.
The RMSD analysis indicated eugenol and isoeugenol achieved a state of stability in the latter half of the 15 ns simulation, with eugenol showing a marginally higher degree of ligand conformational change than isoeugenol (Fig. 4C). The RMSF results highlight considerable fluctuations in residues 260–270 throughout the molecular dynamics simulation, the average fluctuation range is over 3 Å, which are predominantly situated in the ECL-3 region. Additionally, eugenol established a greater number of hydrogen bonds during the initial 10 ns of the simulation (number is 197), whereas isoeugenol only formed an increased number of hydrogen bonds in the latter half of the simulation (number is 149). Eugenol also induces a higher level of conformational flexibility in OR5D18, with a fluctuation range approaching 0.6 Å, whereas the fluctuation range for isoeugenol is only 0.4 Å. By analogy with the case of 4-vinylguaiacol, the substantially lower odor threshold of eugenol relative to isoeugenol is likely due to the substantial hydrogen bonding in the early stages and the more flexible conformation of the receptor protein.
The RMSD data that revealed a significant fluctuation in 2-phenylethanol during the 15–20 ns interval, the conformational range change exceeds 1.5 Å, in contrast to the more stable binding of benzenacetaldehyde to OR2AG2 (Fig. 4D). Notably, residues 130–140, 230–240, and 260–270 showed enhanced fluctuation, the average fluctuation range is over 2 Å, with these residues predominantly situated in the ICL-2, TM-6, and ECL-3 regions. Additionally, compared with the 111 hydrogen bonds formed by 2-phenylethanol, benzaldehyde formed a greater number of hydrogen bonds with OR2AG2, amounting to 145. The Rg analysis further confirmed that both 2-phenylethanol and benzenacetaldehyde substantially augment the conformational flexibility of OR2AG2, the fluctuation range is consistently around 0.6 Å. Corroborating sensory evaluation outcomes, the increased hydrogen bonding by benzenacetaldehyde is a likely primary contributor to its lower odor threshold.
Compared with valeric acid, whose RMSD value stabilizes at 3 Å only after 5 ns, the RMSD value of butyric acid stabilizes at 2 Å, indicating that the RMSD data of butyric acid binding to OR51E1 is relatively stable (Fig. 4E). Specifically, butyric acid induces substantial fluctuations in residues 80–90, predominantly within the TM-2 region of OR51E1. In the case of valeric acid, the residues 170–200, located primarily in the ECL-2 region, exhibit more pronounced fluctuations, the average fluctuation range is over 3 Å. The analysis further reveals that butyric acid and valeric acid form a comparable number of hydrogen bonds, Butyric acid formed 106 hydrogen bonds, while valeric acid formed 104 hydrogen bonds. However, valeric acid significantly enhanced the overall conformational flexibility of OR51E1, with the receptor exhibiting recurring conformational contractions during the 20 ns simulation period, and a fluctuation range of approximately 0.6 Å. In contrast, the maximum fluctuation range for butyric acid is only 0.3 Å. These findings suggest that the lower odor threshold of valeric acid is likely intricately linked to the increased flexibility of the protein receptor, indicating a potential mechanistic correlation between conformational dynamics and olfactory perception.
Overall, our molecular dynamics simulations of 10 pivotal food odor molecules revealed interactions predominantly with ECL-2, ECL-3, ICL-2, and TM-6. Prior research correlates the conformational dynamics of these regions with the transition between the inactive and active states of olfactory receptors. In other words, these interaction sites potentially serve as critical loci that promote the expression of physiological roles within olfactory receptors (Choi et al., 2023; Pirona et al., 2024). A particularly noteworthy discovery is the correlation between the odor threshold of odor molecules and both the quantity of hydrogen bonds they form and the extent of flexibility they impart to olfactory receptors. The increase in the number of hydrogen bonds and the rotational radius is associated with a lower olfactory threshold. Conversely, a decrease in the number of hydrogen bonds and a smaller rotational radius leads to a higher olfactory threshold (Fig. 5). This finding could form a significant dataset for predicting odor thresholds and elucidating the mechanisms of odor molecules in future studies.
Fig. 5.
The image model indicates that an increase in the number of hydrogen bonds and the radius of gyration leads to a decrease in the threshold of flavor compounds (orange), whereas a decrease in the number of hydrogen bonds and the radius of gyration results in an increase in the threshold of flavor compounds (blue).
4. Conclusions
In this investigation, we employed S-curve method to determine odor thresholds for 10 key food odorants spanning five distinct olfactory categories. Subsequent integration of molecular docking and molecular dynamics simulations revealed critical correlations between these thresholds and binding mechanisms. Molecular docking demonstrated that minor structural variations in odorants induce substantial conformational changes in their binding patterns, where such conformational changes serve as critical modulators of threshold variations. Molecular dynamics simulations further established that odor thresholds correlate closely with both the hydrogen-bonding potential of odorant molecules and the conformational flexibility of receptor proteins, providing a theoretical foundation for predicting odor perception mechanisms. These findings collectively offer theoretical support for rational flavor engineering in food flavoring applications.
CRediT authorship contribution statement
Jingtao Wang: Writing – review & editing, Supervision. Chenglei Zhang: Writing – review & editing, Writing – original draft. Jiancai Qian: Investigation. Shan Wang: Writing – original draft. Wu Fan: Writing – review & editing, Methodology. Qingzhao Shi: Investigation. Jian Mao: Methodology. Jianping Xie: Investigation. Qidong Zhang: Supervision. Guobi Chai: Supervision, Methodology, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the State Key Program of National Natural Science Foundation of China (32130083), Young Elite Scientists Sponsorship Program by CAST (2017QNRC001), the Scientific and Technological Project of CNTC (110202401025XJ-07), and the scientific research program of innovation platform in State Tobacco Monopoly Administration (402021AWCX06).
Handling Editor: Professor A.G. Marangoni
Contributor Information
Qidong Zhang, Email: qdzhangcn@163.com.
Guobi Chai, Email: chaigb@zzu.edu.cn.
Data availability
Data will be made available on request.
References
- Ball L., Frey T., Haag F., Frank S., Hoffmann S., Laska M.…Krautwurst D. Geosmin, a food- and water-deteriorating sesquiterpenoid and ambivalent semiochemical, activates evolutionary conserved receptor OR11A1. J. Agric. Food Chem. 2024;72(28):15865–15874. doi: 10.1021/acs.jafc.4c01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnum G., Hong E.J. Olfactory coding. Curr. Biol. 2022;32(23):1296–1301. doi: 10.1016/j.cub.2022.10.067. [DOI] [PubMed] [Google Scholar]
- Bauer P., Ortner E., Buettner A. Influence of elongation and desaturation on chemosensory properties in acrylates and their corresponding 1-alken-3-ones. Anal. Bioanal. Chem. 2022;414(28):8009–8022. doi: 10.1007/s00216-022-04332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Khemis I., Ben Lamine A. Physico-chemical investigations of human olfactory receptors OR10G4 and OR2B11 activated by vanillin, ethyl vanillin, coumarin and quinoline molecules using statistical physics method. Int. J. Biol. Macromol. 2021;193(0):915–922. doi: 10.1016/j.ijbiomac.2021.10.155. [DOI] [PubMed] [Google Scholar]
- Ben Khemis I., Bouzid M., Mechi N., Ben Lamine A. Statistical physics modeling and interpretation of the adsorption of enantiomeric terpenes onto the human olfactory receptor OR1A1. Int. J. Biol. Macromol. 2021;171(0):428–434. doi: 10.1016/j.ijbiomac.2020.12.209. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Cao C., Deng C., Hu J., Zhou Y. Formation and molecular dynamics simulation of inclusion complex of large‐ring cyclodextrin and 4‐terpineol. Journal of food science. 2022;87(10):4609–4621. doi: 10.1111/1750-3841.16303. [DOI] [PubMed] [Google Scholar]
- Cetintas V.B., Duzgun Z., Akalin T., Ozgiray E., Dogan E., Yildirim Z.…Kosova B. Molecular dynamic simulation and functional analysis of pathogenic PTEN mutations in glioblastoma. J. Biomol. Struct. Dyn. 2023;41(21):11471–11483. doi: 10.1080/07391102.2022.2162582. [DOI] [PubMed] [Google Scholar]
- Choi C., Bae J., Kim S., Lee S., Kang H., Kim J.…Choi H.J. Understanding the molecular mechanisms of odorant binding and activation of the human OR52 family. Nat. Commun. 2023;14(1):8105–8119. doi: 10.1038/s41467-023-43983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cometto-Muñiz J.E., Abraham M.H. Dose–response functions for the olfactory, nasal trigeminal, and ocular trigeminal detectability of airborne chemicals by humans. Chem. Senses. 2016;41(1):3–14. doi: 10.1093/chemse/bjv060. [DOI] [PubMed] [Google Scholar]
- de March C.A., Ryu S., Sicard G., Moon C., Golebiowski J. Structure–odour relationships reviewed in the postgenomic era. Flavour Fragrance J. 2015;30(5):342–361. doi: 10.1002/ffj.3249. [DOI] [Google Scholar]
- Dunkel A., Steinhaus M., Kotthoff M., Nowak B., Krautwurst D., Schieberle P., Hofmann T. Nature's chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew. Chem. Int. Ed. 2014;53(28):7124–7143. doi: 10.1002/anie.201309508. [DOI] [PubMed] [Google Scholar]
- Duroux R., Mandeau A., Guiraudie-Capraz G., Quesnel Y., Loing E. A rose extract protects the skin against stress mediators: a potential role of olfactory receptors. Molecules. 2020;25(20):4743–4758. doi: 10.3390/molecules25204743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T., Kameyama A., Takeuchi H. Structural determination of vanillin, isovanillin and ethylvanillin by means of gas electron diffraction and theoretical calculations. J. Mol. Struct. 2006;794(1–3):92–102. doi: 10.1016/j.molstruc.2006.01.042. [DOI] [Google Scholar]
- Hasegawa T., Izumi H., Tajima Y., Yamada H. Structure-odor relationships of α-santalol derivatives with modified side chains. Molecules. 2012;17(2):2259–2270. doi: 10.3390/molecules17022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Zeng Z., Zhang J., Wu D., Li H., Geng F. Molecular dynamics simulation of the interaction of food proteins with small molecules. Food Chem. 2023;405 doi: 10.1016/j.foodchem.2022.134824. [DOI] [PubMed] [Google Scholar]
- Iqbal D., Alsaweed M., Jamal Q.M.S., Asad M.R., Rizvi S.M.D., Rizvi M.R.…Alyenbaawi H. Pharmacophore-based screening, molecular docking, and dynamic simulation of fungal metabolites as inhibitors of multi-targets in neurodegenerative disorders. Biomolecules. 2023;13(11):1613–1629. doi: 10.3390/biom13111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B.A., Ong J., Lee K., Ho S.L., Arguello S., Leon M. Effects of double and triple bonds on the spatial representations of odorants in the rat olfactory bulb. J. Comp. Neurol. 2006;500(4):720–733. doi: 10.1002/cne.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska M. Olfactory discrimination ability for aliphatic C6 alcohols as a function of presence, position, and configuration of a double bond. Chem. Senses. 2005;30(9):755–760. doi: 10.1093/chemse/bji067. [DOI] [PubMed] [Google Scholar]
- Mahmoud S.S.A., Elkaeed E.B., Alsfouk A.A., Abdelhafez E.M.N., Husain K. Molecular docking and dynamic simulation revealed the potential inhibitory activity of opioid compounds targeting the main protease of SARS-CoV-2. BioMed Res. Int. 2022;2022(0):1–12. doi: 10.1155/2022/1672031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland J.D., Keller A., Li Y.R., Zhou T., Trimmer C., Snyder L.L.…Matsunami H. The missense of smell: functional variability in the human odorant receptor repertoire. Nat. Neurosci. 2013;17(1):114–120. doi: 10.1038/nn.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom R.D., Watowich S.J. Using free energy of binding calculations to improve the accuracy of virtual screening predictions. J. Chem. Inf. Model. 2011;51(7):1648–1655. doi: 10.1021/ci200126v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinek P., Haag F., Geithe C., Krautwurst D. An evolutionary conserved olfactory receptor for foodborne and semiochemical alkylpyrazines. FASEB J. 2021;35(6) doi: 10.1096/fj.202100224R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa Y., Kawaguchi K., Katsuta R., Nukada T., Ishigami K. Analog synthesis of DAMASCENOLIDETM, an important aroma component of roses, and their odor properties. Biosci. Biotechnol. Biochem. 2020;84(8):1560–1569. doi: 10.1080/09168451.2020.1753498. [DOI] [PubMed] [Google Scholar]
- Niu Y., Yao Z., Xiao Z., Zhu G., Zhu J., Chen J. Sensory evaluation of the synergism among ester odorants in light aroma-type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res. Int. 2018;113(0):102–114. doi: 10.1016/j.foodres.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Okamoto C., Ando K. Molecular dynamics simulation analysis of structural dynamic cross correlation induced by odorant hydrogen-bonding in mouse eugenol ol- factory receptor. Biophysics and Physicobiology. 2024;21(1):1–10. doi: 10.2142/biophysico.bppb-v21.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel P., Choi J.S., Prajapati R., Seong S.H., Park S.E., Kang W.-C.…Jung H.A. In vitro human monoamine oxidase inhibition and human dopamine D4 receptor antagonist effect of natural flavonoids for neuroprotection. Int. J. Mol. Sci. 2023;24(21) doi: 10.3390/ijms242115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirona L., Ballabio F., Alfonso-Prieto M., Capelli R. Calcium-driven in silico inactivation of a human olfactory receptor. J. Chem. Inf. Model. 2024;64(8):2971–2978. doi: 10.1021/acs.jcim.4c00249. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Amezawa M., Horiuchi J., Nagumo Y., Yamamoto N., Kutsumura N.…Nagase H. Discovery of novel orexin receptor antagonists using a 1,3,5-trioxazatriquinane bearing multiple effective residues (TriMER) library. Eur. J. Med. Chem. 2022;240(0) doi: 10.1016/j.ejmech.2022.114505. [DOI] [PubMed] [Google Scholar]
- Wakabayashi M., Wakabayashi H., Riegel A.D., Eisenreich W., Engel K.-H. Analytical and sensory characterization of the stereoisomers of 3-mercaptocycloalkanones and 3-mercaptocycloalkanols. J. Agric. Food Chem. 2020;68(27):7184–7193. doi: 10.1021/acs.jafc.0c03113. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang D.Q., Huang M.Q., Sun B.G., Ren F.Z., Wu J.H.…Sun X.T. Decoding molecular mechanism underlying human olfactory receptor OR8D1 activation by sotolone enantiomers. J. Agric. Food Chem. 2024;72(10):5403–5415. doi: 10.1021/acs.jafc.3c09142. [DOI] [PubMed] [Google Scholar]
- Wang Z., Chang X., Hao W., Wang Y., Huang M., Sun B.…Zhao D. Study on interaction of aromatic substances and correlation between electroencephalogram correlates of odor perception in light flavor baijiu. J. Agric. Food Chem. 2024;72(29):16519–16529. doi: 10.1021/acs.jafc.4c02979. [DOI] [PubMed] [Google Scholar]
- Wu C., Xu M., Dong J., Cui W., Yuan S. The structure and function of olfactory receptors. Trends Pharmacol. Sci. 2024;45(3):268–280. doi: 10.1016/j.tips.2024.01.004. [DOI] [PubMed] [Google Scholar]
- Xu J., Pluznick J.L. Key amino acids alter activity and trafficking of a well-conserved olfactory receptor. Am. J. Physiol. Cell Physiol. 2022;322(6):1279–1288. doi: 10.1152/ajpcell.00440.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S., Zhang L., Li P., Pu D., Fu Y., Zheng R.…Zhang Y. Molecular mechanisms of caramel-like odorant-olfactory receptor interactions based on a computational chemistry approach. Food Res. Int. 2023;171 doi: 10.1016/j.foodres.2023.113063. [DOI] [PubMed] [Google Scholar]
- Zeng S.T., Zhang L.L., Zheng R.Y., Li P., Fu Y.J., Xi H.…Zhang Y.Y. Molecular recognition mechanisms of vanillin and high-throughput screening of its analogs based on olfactory receptors. Lwt-Food Science and Technology. 2024;202 doi: 10.1016/j.lwt.2024.116305. [DOI] [Google Scholar]
- Zhou C., Liu Y., Zhao G., Liu Z., Chen Q., Yue B.…Zhang X. Comparative analysis of olfactory receptor repertoires sheds light on the diet adaptation of the bamboo-eating giant panda based on the chromosome-level genome. Animals. 2023;13(6):979–994. doi: 10.3390/ani13060979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.