Abstract
The gene encoding human IAP-like protein (hILP) is one of several mammalian genes with sequence homology to the baculovirus inhibitor-of-apoptosis protein (iap) genes. Here we show that hILP can block apoptosis induced by a variety of extracellular stimuli, including UV light, chemotoxic drugs, and activation of the tumor necrosis factor and Fas receptors. hILP also protected against cell death induced by members of the caspase family, cysteine proteases which are thought to be the principal effectors of apoptosis. hILP and Bcl-xL were compared for their ability to affect several steps in the apoptotic pathway. Redistribution of cytochrome c from mitochondria, an early event in apoptosis, was not blocked by overexpression of hILP but was inhibited by Bcl-xL. In contrast, hILP, but not Bcl-xL, inhibited apoptosis induced by microinjection of cytochrome c. These data suggest that while Bcl-xL may control mitochondrial integrity, hILP can function downstream of mitochondrial events to inhibit apoptosis.
Programmed cell death (apoptosis) is an evolutionarily conserved cellular suicide process by which extraneous or damaged cells are eliminated from an organism (38, 68). Deregulation of the apoptotic pathway has been implicated in the pathogenesis of a wide variety of human diseases, including cancer, autoimmune diseases, virus infections, neurodegenerative diseases, and AIDS (65). Based largely on genetic studies with the nematode Caenorhabditis elegans (33), two mammalian gene families whose products play pivotal roles in the cell death pathway have been identified. The caspases (cysteine aspartic proteases), a family of at least 10 mammalian proteases with homology to the nematode ced-3 gene product (1), are activated during apoptosis (2, 8, 14, 26, 27, 58) and are thought to be the principal executors of the apoptotic process (28). The bcl-2 gene family encodes a group of CED-9-related proteins which are central regulators of the cellular apoptotic threshold (5). These proteins have been localized to the outer nuclear, endoplasmic reticular, and outer mitochondrial membranes (13, 41, 43, 51). In the nematode, genetic analysis has placed the ced-9 gene upstream of ced-3 function. Similarly, in several mammalian models of apoptosis, overexpression of Bcl-2 has been shown to inhibit the activation of cellular caspases, suggesting that caspases act downstream of Bcl-2 protein function (2, 8, 14, 58). More recent studies have suggested that Bcl-2 family members can function both downstream and upstream of caspase activity (60a). One potential Bcl-2-regulated event in the apoptotic process is the loss of mitochondrial membrane potential (56), followed by the release into the cytosol of at least two mitochondrial proteins, apoptosis-initiating factor (62) and cytochrome c (44). In cell-free systems, cytochrome c has been shown to activate caspases. Bcl-2 and related proteins can block mitochondrial disruption and the release of cytochrome c in response to a variety of apoptotic stimuli (40, 71).
Cellular apoptosis is a defense mechanism utilized by the host to eliminate virally infected cells. To counter the host apoptotic response, many DNA viruses encode proteins that interfere with key regulatory steps in the apoptotic pathway. These proteins include homologs of known cellular apoptotic modulators such as Bcl-2-related proteins (32, 50), soluble cytokine receptors (60), and antagonists of cytokine receptor-induced protein-protein interaction-mediated cell death signals (3, 35, 64). Two baculovirus antiapoptotic genes have been identified, the caspase inhibitor gene p35 and the iap (inhibitor-of-apoptosis) gene (17). Although iap genes were discovered first in insect virus genomes (19), cellular homologs have recently been identified in the genomes of insects, birds, and mammals (22, 25, 31, 42, 53, 55, 66). Certain baculovirus IAPs (Cp-IAP and Op-IAP) can functionally replace the baculovirus caspase inhibitor p35, as their identification was achieved by phenotypic rescue of p35-deficient virus (16, 18, 20).
Three bona fide mammalian IAP-related proteins, designated human IAP-like protein (hILP), c-IAP1, and c-IAP2, have been identified (15). These proteins contain two major elements: (i) an amino-terminal domain containing three imperfect repeats of an ∼65-amino-acid cysteine- and histidine-rich sequence termed the baculovirus IAP repeat (BIR) and (ii) a carboxy-terminal RING finger, a zinc-binding domain which has been identified in a number of proteins that function in cellular differentiation and proliferation (7, 74). In the mammalian IAP-related proteins the BIR and RING domains are separated by an amphipathic region of 120 to 170 residues, whose role has not been defined. This region is not found in the baculovirus IAPs. A fourth mammalian gene has also been identified, encoding a protein termed neuronal apoptosis-inhibitory protein (55) which possesses limited homology to the IAPs. Aside from the BIR domains, however, neuronal apoptosis-inhibitory protein does not resemble the classical IAPs described above, and so its relationship to the prototype IAPs is unclear.
hilp (25), also called xiap (42) and MIHA (66), is widely expressed in human tissues and encodes a 57-kDa cytoplasmic protein (25). hILP can impede apoptotic cell death induced by virus infection and by overexpression of caspases (25). c-IAP1 and c-IAP2 were identified as components of the type 2 tumor necrosis factor (TNF) receptor (TNFR2) signaling complex. These factors have been shown to exist in the cell associated with the signaling molecule, TRAF2 (53). Activation of TNFR2 by its ligand, TNF alpha (TNF-α), is thought to recruit the TRAF2–c-IAP complex to the cytoplasmic domain of TNFR2. While TRAF2 has been shown to bind directly to the TNFR2 cytoplasmic domain, the c-IAPs do not bind directly but are thought to be recruited through their association with TRAF2. The TRAF2–c-IAP1 heteromer has also been identified in the type 1 TNF receptor (TNFR1) signaling complex (59).
In this study we examined the properties of hILP. hILP was found to impede apoptotic cell death induced by a wide variety of extracellular stimuli. The BIR-containing domain was sufficient for this protection. hILP did not interact with any of the six known TRAF proteins, implying that its role may be distinct from those of the c-IAPs. Furthermore, redistribution of cytochrome c associated with the induction of apoptosis was blocked by the Bcl-2 family member Bcl-xL but not by hILP. In contrast, overexpression of hILP, but not Bcl-xL, protected cells from apoptotic cell death induced by the microinjection of cytochrome c. These findings demonstrate that Bcl-xL and hILP function upstream and downstream, respectively, of cytochrome c in the apoptotic cascade.
MATERIALS AND METHODS
Cells and transfections.
Human embryonic kidney 293 cells were grown at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. For transfections, 10-cm-diameter dishes were seeded with 5 × 105 cells. The medium was replaced the following day, and cells were transfected by the calcium phosphate procedure as previously described (24). MCF7 cells expressing the Fas receptor (MCF7F cells) (63) (a gift of V. Dixit) were grown in RPMI 1640 containing 10% fetal bovine serum, 200 μg of G418 per ml, and 100 μg of hygromycin per ml at 37°C in 5% CO2. For transfections, each well of a six-well plate was seeded with 2.5 × 105 cells, and 24 h after plating, the cells were transfected with Lipofectin (Life Technologies). A 1:5 ratio of pCMV-lacZ or pGreenLantern (pCMV-GLP; Life Technologies) to test expression vector was used to ensure that every β-galactosidase- or GreenLantern protein (GLP)-positive cell had also taken up the test expression vector. For apoptosis assays, MCF7 cells were treated with either anti-Fas (150 ng/ml) plus cycloheximide (1 μg/ml), recombinant human TNF-α (rhTNF-α) (40 ng/ml), cisplatin (20 μg/ml), or UV light (5 min on a 312-nm transilluminator [Fisherbiotech]). After 12 h, cells were fixed, stained for β-galactosidase expression, and scored for apoptosis based on morphology.
Plasmids.
The expression vectors encoding TRAF1, -2, and -3 have been described previously (24). The Myc epitope-tagged human TRAF4/CART1 expression vector was kindly provided by C. Rudin and J. Van Dongen. The murine TRAF5 vector was kindly provided by R. Arch; the amino terminus was modified to incorporate the Myc epitope tag (25) prior to subcloning into the pcDNA3 mammalian expression vector (Invitrogen). Human TRAF6 was obtained by PCR amplification from a human T-cell cDNA library followed by subcloning into the pFLAG-CMV-2 expression vector (Kodak).
The expression vector pEBB (11) was kindly provided by B. Mayer. The pEBB-FLAG expression vector was constructed by modification of the cloning sequences of pEBB to incorporate a FLAG epitope tag and translational termination codons in all three frames. The full-length hILP expression vector encodes the entire 497-amino-acid open reading frame of hILP subcloned in frame with the FLAG epitope of pEBB-FLAG. The hILP deletion plasmids were made in the same vector as follows. The ΔRing vector is a deletion construct encoding residues 1 to 449. The Δloop vector was constructed by PCR-mediated fusion, using Pfu polymerase, of residues 1 to 342 to residues 445 to 497. The 3×BIR vector encodes residues 1 to 399 and was a kind gift of R. Clem and J. M. Hardwick.
The pEBG mammalian glutathione S-transferase (GST) fusion vector (45) was a kind gift of B. Mayer. The open reading frames of hILP and c-IAP1 (kindly provided by R. Clem and J. M. Hardwick) were subcloned into pEBG in frame with the GST reading frame to generate pEBG-hILP and pEBG-c-IAP1, respectively. pCIneo-Bcl-xL was constructed by PCR subcloning of the Bcl-xL open reading frame into pCIneo (Promega). The open reading frames of caspase 2 and caspase 8 were subcloned by PCR, using Pfu polymerase, into pcDNA3. All constructs were verified by sequence analysis.
Precipitation and immunoblot analysis.
Cells were cotransfected with 5 μg each of TRAF expression vector and mammalian GST expression vector by the calcium phosphate procedure. Medium was replaced 24 h following transfection. Lysates were prepared 48 h after transfection as follows. Cells were washed once in phosphate-buffered saline and lysed for 10 min at room temperature in 1 ml of lysis buffer (25 mM HEPES [pH 7.9], 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 1 mM dithiothreitol, 0.1 mM phenylmethanesulfonyl fluoride, 1.0 μg of chymostatin per ml, 0.5 μg of pepstatin per ml, 0.5 μg of bestatin per ml, 0.5 μg of leupeptin per ml, 0.5 μg of aprotinin per ml, and 3 μg of antipain per ml). Lysates were clarified by microcentrifugation at 4°C for 15 min at 16,000 × g, and the protein concentrations in the supernatants were determined by using the Bradford reagent (Bio-Rad). For GST precipitation experiments, samples were incubated with 10 μl of a 50% slurry of glutathione agarose beads, equilibrated in lysis buffer and preblocked with bovine serum albumin, for at least 30 min at 4°C on a rotating platform. The beads were washed four times with 0.5 ml of lysis buffer at 4°C. Aliquots were separated by electrophoresis on sodium dodecyl sulfate–9.5% polyacrylamide gels and immunoblotted with commercial rabbit polyclonal antibodies to human TRAF1, TRAF2, or TRAF3 (Santa Cruz Biotechnologies) or with murine monoclonal antibodies to the Myc epitope (Pharmingen clone 9E10) or FLAG (Kodak M2). Blots were subsequently probed with horseradish peroxidase-conjugated anti-rabbit or anti-mouse monoclonal antibodies (Amersham) as required and developed with an enhanced chemiluminescence detection system (Amersham).
Microinjection.
Cell microinjection was performed on the stage of a Nikon Diaphot 300 inverted microscope with an Eppendorf pressure injector (model 5246) and micromanipulator (model 5171). Microinjection needles (about 0.1-μm inner diameter) were pulled from glass capillaries with a horizontal electrode puller (Sutter Instrument model P-97) and loaded with Eppendorf microloaders. Cells were plated on glass cellocate coverslips (Eppendorf) 24 h prior to transfection. Twenty-four to 48 hours after transfection, GLP-positive cells were chosen for injection. To identify injected cells, the injectate contained 0.3% dextran-conjugated Texas red (10,000 molecular weight, lysine fixable; Molecular Probes). Dye alone or dye plus cytochrome c was injected into the cytoplasm of 293 cells (pressure, 80 to 100 hPa; time, 0.3 s). Cells were switched into fresh medium immediately after injection. The concentration of cytochrome c (Sigma no. C7752 from horse heart) in the pipette was 3 mg/ml. The intracellular concentrations of microinjected proteins are estimated to represent a 10- to 100-fold dilution of the amount delivered into the cell. Minaschek et al. (46), using similar equipment, demonstrated that the injection parameters used in their study, (pressure, 100 hPa; time, 0.5 s) deliver about 0.05 pl into the cytosol of 3T3 cells. We estimate the volume of 293 cells to be about 5 pl, similar to that of 3T3 cells. Based on the approximate volume delivered per cell (0.05 to 0.5 pl), the concentration of cytochrome c delivered per cell is estimated to be 150 to 1,500 fg and equivalent to 2.4 to 24 μM. On average, 100 to 150 cells were injected. Two hours after injection, cells were scored for apoptosis, and 35-mm photographs were taken with a Nikon Diaphot 300 inverted microscope.
RESULTS
hILP inhibits apoptosis induced by diverse stimuli.
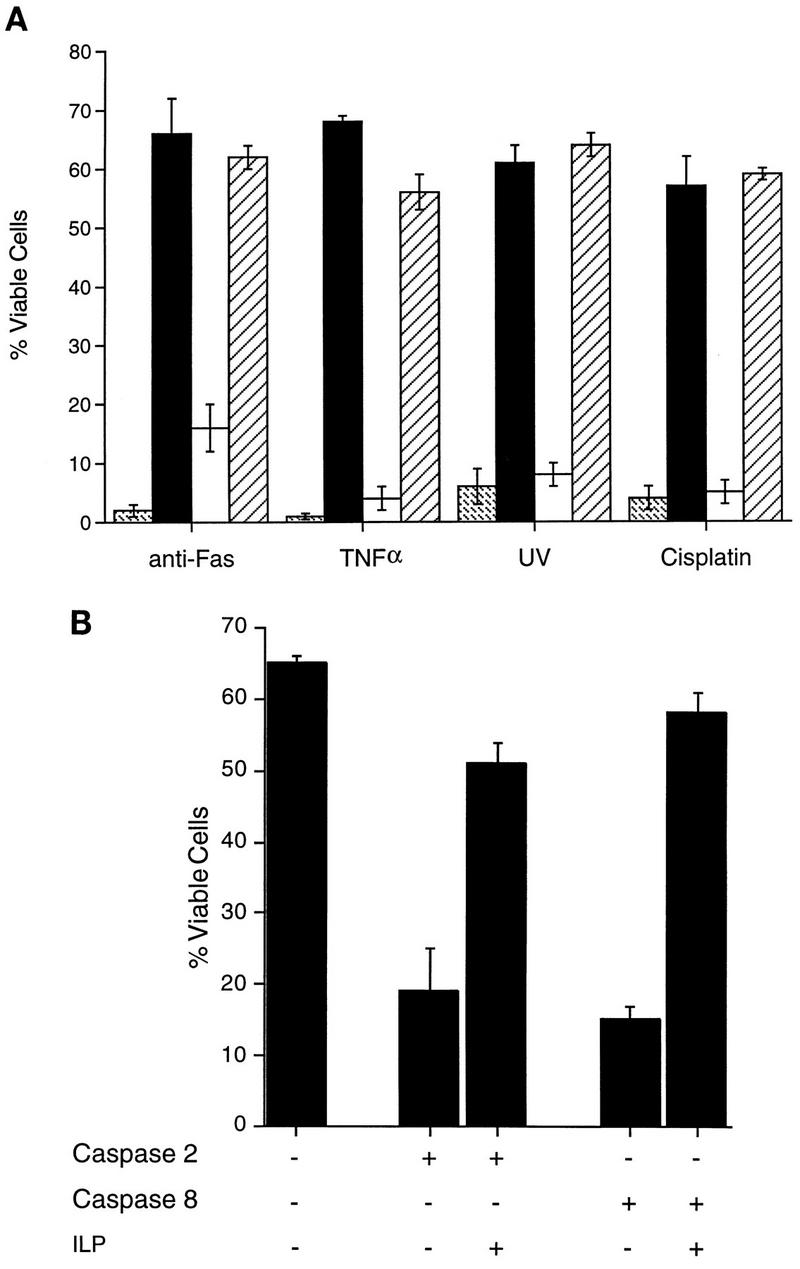
The ability of hILP to block different apoptotic stimuli was tested by transient transfection into the MCF7F human breast carcinoma cell line. These cells have previously been shown to undergo apoptosis when exposed to UV light or when treated with TNF-α or agonistic antibodies to Fas (3). An expression vector encoding FLAG epitope-tagged hILP was transfected, together with a LacZ expression vector, and cells were treated with TNF-α, anti-Fas, UV light, or the cytotoxic drug cisplatin. Twelve hours later, the cells were stained for β-galactosidase expression, and the viability of β-galactosidase-positive cells was determined by morphological examination under light microscopy as described previously (3). As shown in Fig. 1A, expression of hILP almost completely reversed the apoptotic effects of each of these stimuli. The degree of inhibition was comparable to that achieved by expression of the antiapoptotic Bcl-xL gene (Fig. 1A).
FIG. 1.
hILP blocks cell death induced by a variety of stimuli. (A) MCF7F cells were transfected with pEBB ( ), pEBB-hILP (▪), pCI (□), or pCI-Bcl-xL (▨). Twenty-four hours after transfection, cells were treated with fresh medium or medium supplemented with either anti-Fas antibody and cycloheximide, rhTNF-α, or cisplatin or were exposed to UV. After 12 h, cells were fixed, stained for β-galactosidase expression, and scored for apoptotic morphology. (B) MCF7F cells were transfected with pCMV-lacZ together with pcDNA3, pcDNA3-caspase 2, or pcDNA3-caspase 8 with or without cotransfection of pEBB-hILP expression vector. At 24 h after transfection, cells were fixed, stained for β-galactosidase expression, and scored for apoptotic morphology. Results are expressed as percent viable cells (number of flat blue cells/number of flat and round blue cells × 100). The data represent means ± standard deviations (n = 3).
), pEBB-hILP (▪), pCI (□), or pCI-Bcl-xL (▨). Twenty-four hours after transfection, cells were treated with fresh medium or medium supplemented with either anti-Fas antibody and cycloheximide, rhTNF-α, or cisplatin or were exposed to UV. After 12 h, cells were fixed, stained for β-galactosidase expression, and scored for apoptotic morphology. (B) MCF7F cells were transfected with pCMV-lacZ together with pcDNA3, pcDNA3-caspase 2, or pcDNA3-caspase 8 with or without cotransfection of pEBB-hILP expression vector. At 24 h after transfection, cells were fixed, stained for β-galactosidase expression, and scored for apoptotic morphology. Results are expressed as percent viable cells (number of flat blue cells/number of flat and round blue cells × 100). The data represent means ± standard deviations (n = 3).
Overexpression of hILP has previously been found to inhibit the induction of apoptosis by caspase 1 (25, 66). To determine whether this inhibition is limited to caspase 1, the ability of hILP to inhibit apoptosis induced by other caspases was examined. Caspases 2 and 8 have been implicated in apoptotic cell death by TNF-α and Fas (6, 23, 48). Therefore, MCF7F cells were transfected with expression vectors encoding caspase 2 or caspase 8, and the effects of hILP were examined by cotransfection with either the hILP expression vector or a vector control. As shown in Fig. 1B, the apoptotic cell death induced by either caspase 2 or caspase 8 was markedly impaired by expression of hILP.
The BIR domains of hILP are necessary for inhibition of apoptosis.
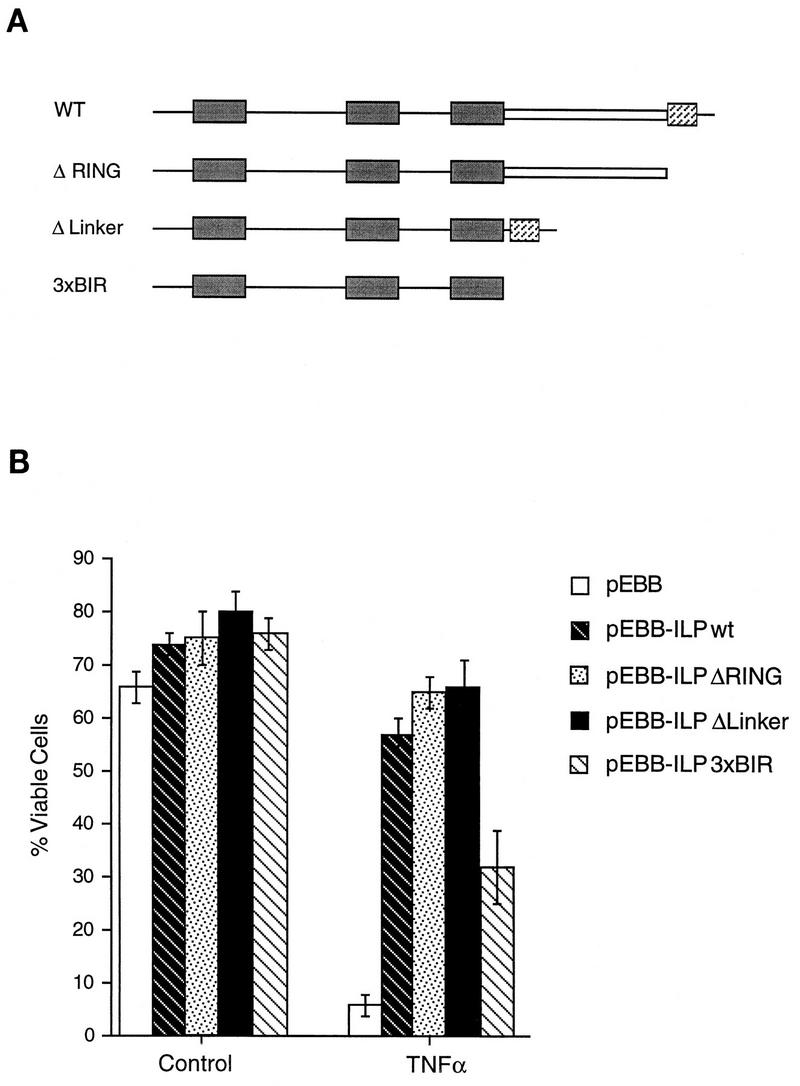
To define elements in hILP which are required for its protective effects, deletion mutants which lacked the RING finger, the linker region between the BIRs and the RING domain, or both the linker region and the RING finger were constructed, as summarized in Fig. 2A. These plasmids were transfected into MCF7F cells, which were subsequently stimulated with recombinant TNF-α. As shown in Fig. 2B, each of the deletion mutants still protected against TNF-α-induced death, indicating that the BIRs are sufficient for the protective effects of hILP. A deletion mutant lacking the BIRs was not expressed to detectable levels under the conditions used and so could not be assessed in this assay.
FIG. 2.
Deletion analysis of hILP. (A) Schematic diagram of hILP showing deletion mutants. BIR domains (solid boxes), the amphipathic region (open box), and the ring finger (hatched box) are shown. WT, wild type. (B) MCF7F cells were transfected with pCMV-lacZ and either pEBB, pEBB-hILP, or pEBB deletion mutants of hILP. After 24 h, medium was removed and replaced with either fresh medium alone or fresh medium plus rhTNF-α. Cells were incubated for 12 h, at which time cells were fixed, stained for β-galactosidase expression, and scored for apoptotic morphology. The expression of FLAG-tagged deletion constructs was confirmed by immunoblotting with anti-FLAG bioM5 (Kodak) (data not shown). Results are expressed as percent viable cells (number of flat blue cells/number of flat and round blue cells × 100). The data represent the means ± standard deviations (n = 3).
hILP does not possess the TRAF-binding properties of the c-IAPs.
The experiments described above revealed that the major elements required for the protective effects of hILP are the three amino-terminal BIR domains. Since the BIR domains of c-IAP1 mediate protein-protein interactions with TRAF proteins (53), the ability of hILP to interact with TRAFs was tested. Human embryonic kidney 293 cells were cotransfected with mammalian expression vectors encoding the six known TRAF proteins (10, 12, 34, 36, 37, 49, 52, 54) together with a mammalian expression vector encoding hILP fused to the GST protein (pEBG-hILP). Control transfections were also performed with either the parental GST vector alone (pEBG) or a GST-c-IAP1 chimera (pEBG-cIAP-1). Lysates were prepared from transfected cells, and complexes were coprecipitated by using glutathione agarose beads and examined by immunoblot analysis with anti-TRAF antibodies. hILP did not coprecipitate any of the six known TRAFs (Fig. 3). Under the same conditions, however, c-IAP1 coprecipitated with TRAF1 and TRAF2, as observed previously (53). High levels of GST-hILP chimeric protein were expressed, as observed by immunoblot analysis with a monoclonal antibody specific to GST (data not shown). These data suggest that unlike the c-IAPs, hILP does not directly associate with TRAFs.
FIG. 3.
hILP does not interact with TRAF proteins. 293 cells were cotransfected with the indicated mammalian GST expression vectors, together with expression vectors encoding each of the six indicated TRAF proteins. Lysates were precipitated by incubation with glutathione agarose beads, and TRAF proteins were identified by Western blot analysis as described in Materials and Methods. The expression of the GST chimeric proteins was also confirmed by using an anti-GST antibody (data not shown).
Effects of Bcl-xL and hILP on cytochrome c immunolocalization during apoptosis.
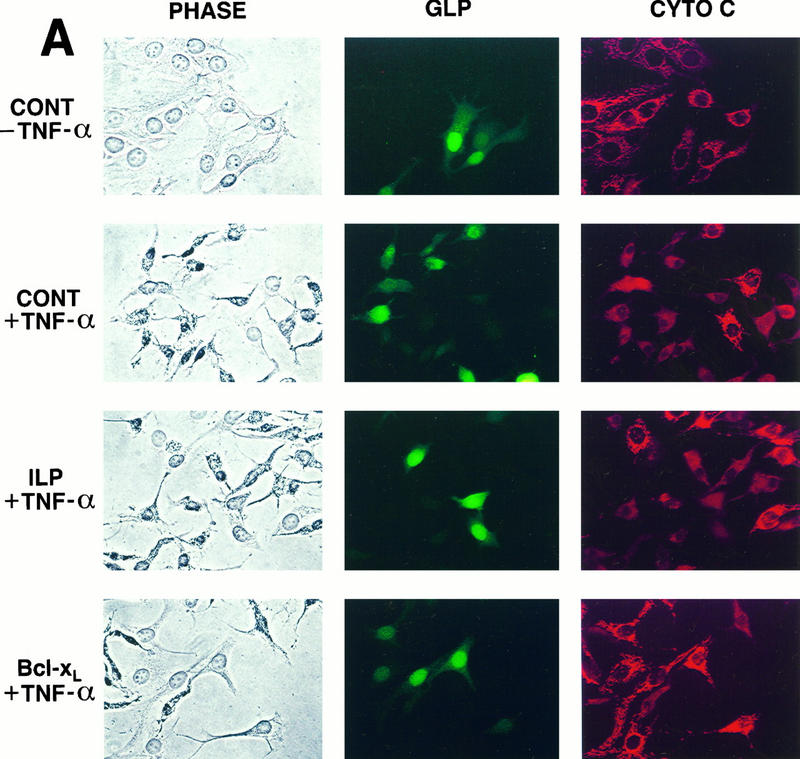
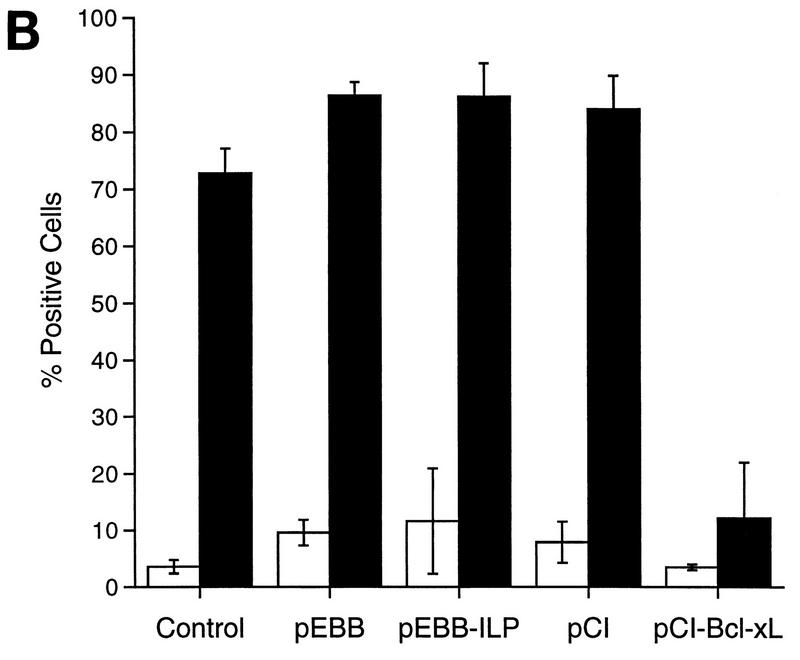
The induction of apoptosis by the engagement of cell surface receptors such as Fas or TNFR1 has been shown to trigger a number of morphological and biochemical changes in the cell. One of the earliest detectable markers during cell death is the loss of mitochondrial function (56). Furthermore, apoptotic cell death has recently been shown to correlate with the release of cytochrome c from the mitochondria into the cytosol, an event which is blocked by overexpression of Bcl-2 family members (40, 44). To determine whether hILP can block TNF-α-mediated release of cytochrome c, we monitored cytochrome c immunolocalization in cells that had been transfected with either hILP or Bcl-xL. As shown in Fig. 4A, TNF-α treatment induced a change in the cytochrome c staining pattern from a punctuate mitochondrial profile in untreated cells to a diffuse cytosolic distribution. Expression of hILP had no effect on the redistribution of cytochrome c after TNF-α treatment, while expression of Bcl-xL nearly completely blocked the redistribution. Quantitative analysis of these experiments is presented in Fig. 4B. These results suggest that hILP exerts its protective effects without affecting TNF-α-induced cytochrome c redistribution and that the mechanism by which hILP inhibits apoptosis is distinct from that utilized by Bcl-xL.
FIG. 4.
Bcl-xL, but not hILP, blocks the redistribution of cytochrome c. (A) MCF7 cells were transiently transfected with the indicated plasmids together with pCMV-GLP. Twenty-four hours after transfection, cells were treated with rhTNF-α and cycloheximide or with medium alone. Six hours after treatment, cells were fixed, immunostained for cytochrome c (CYTO C), mounted, and observed by phase-contrast and fluorescent microscopy. Representative fields are shown. Note the punctate staining of cytochrome c in the pEBB-transfected cells without TNF-α and the Bcl-xL-transfected cells treated with TNF. In contrast, note the diffuse cytochrome c staining in the pEBB- and ILP-transfected cells treated with TNF-α; nontransfected cells whose cytochrome c has not redistributed are included in these fields for comparison. CONT, pEBB transfected. (B) Quantitative analysis of cytochrome c redistribution. GLP-positive cells were scored for cytochrome c release, and the data were expressed as percent positive cells (number of GLP-positive cells with diffuse cytochrome c staining/total number of GLP-positive cells × 100). The data represent means ± standard deviations (n = 2). Open bars represent data collected from cells cultured in medium alone, while solid bars represent data collected from cells cultured in the presence of TNF-α.
hILP exerts its antiapoptotic effects at a point downstream of cytochrome c.
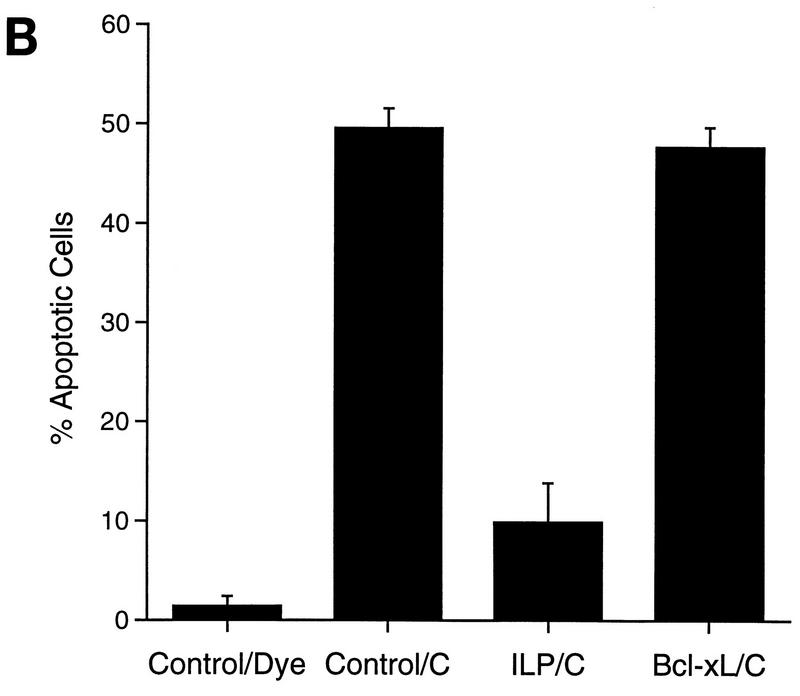
The inability of hILP to block the redistribution of cytochrome c suggests that the antiapoptotic function of hILP is localized to a point downstream of cytochrome c action. To examine whether hILP could inhibit cytochrome c-mediated death, 293 cells were transfected with an hILP or Bcl-xL expression vector, or with a vector control, and subsequently microinjected with cytochrome c. As shown in Fig. 5, microinjection of cytochrome c into control transfected cells induced a number of morphological changes consistent with apoptosis, including membrane blebbing, cell shrinkage, and nuclear condensation and fragmentation. Control microinjections did not produce these effects. However, microinjection of cytochrome c into cells which had been transfected with the hILP expression vector completely protected the cells from cytochrome c-induced apoptosis. In contrast, expression of Bcl-xL had little or no effect on the cytochrome c-induced changes. These findings indicate that while the induction of apoptosis by cytosolic cytochrome c is not subject to regulation by Bcl-xL, it is efficiently inhibited by hILP, suggesting that the protective effects of hILP are focused downstream of cytochrome c-mediated activation of caspases.
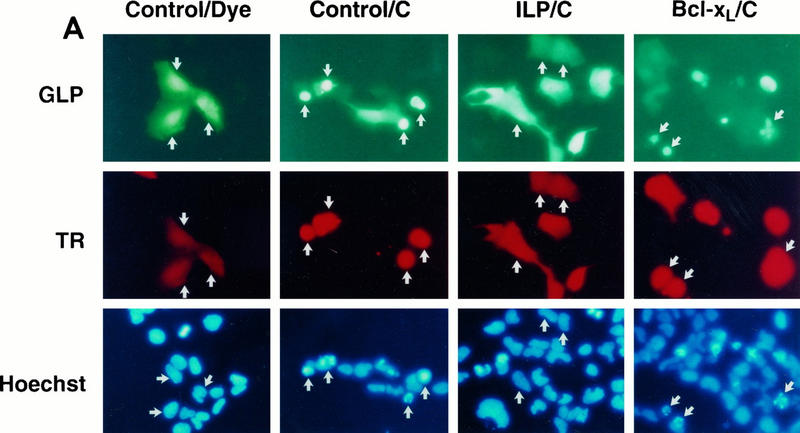
FIG. 5.
hILP, but not Bcl-xL, blocks cytochrome c-induced apoptosis. (A) 293 cells were transiently transfected with pCMV-GLP and either pEBB, pEBB-hILP, pCI, or pCI-Bcl-xL. After 24 to 48 h, GLP-positive cells were microinjected with Texas red dye alone (TR) or dye plus 3 mg of cytochrome c per ml (C). Two hours after injection, cells were incubated with Hoechst dye 33342, fixed, mounted, and observed by phase-contrast and fluorescent microscopy. Representative fields are shown. Note the condensed and fragmented nuclei in the cytochrome c-injected cells (red fluorescence) transfected with vector or Bcl-xL (green fluorescence) and the absence of such nuclei in the hILP-transfected cells. (B) Quantitative analysis of apoptosis induced by cytochrome c microinjection. GLP/Texas red-positive cells were scored for condensed (apoptotic) nuclei. Results are expressed as percent apoptotic cells (number of cells with apoptotic nuclei/number of TR-positive cells × 100). The data represent means ± standard deviations (n = 3).
DISCUSSION
Since the discovery of the first iap genes in insect viruses, the mode of action of these proteins in the apoptotic pathway has remained elusive. One potentially important clue to the role of the IAPs was provided by an understanding of the relationship between the baculovirus IAP and the baculovirus apoptosis inhibitor P35 (18). Although IAP and P35 possess no obvious sequence homology, in the context of the virus these proteins are functionally interchangeable, suggesting a redundant role in cell death. Since P35 is known to exert its effects by binding to and competitively inhibiting members of the caspase family of cysteine proteases (4, 9, 53, 70), IAPs might function in an analogous manner. In fact, a recent report indicates that hILP indeed functions to inhibit certain caspases (21). The mechanism of hILP inhibition of caspases may differ from that of P35, however, as we can easily detect cleavage of P35 by recombinant caspases (4) but have been unable to detect cleavage of hILP under similar conditions (3a). Similarly, the baculoviral IAP homolog Op-IAP has been shown to exert its effects at a point downstream in the apoptotic cascade, although it does not appear to affect active capases (44a), suggesting a mechanism distinct from that of p35.
Of the three human IAP-related proteins identified to date, hILP possesses the most potent antiapoptotic properties (25, 42, 53, 66). To gain insight into the mechanism of protection by hILP, we examined the ability of hILP to protect against apoptosis induced by a variety of apoptotic stimuli. As shown in Fig. 1, hILP efficiently blocks cell death induced by TNF-α, Fas, UV light, and genotoxic agents, indicating that hILP functions downstream of a point of convergence of these diverse stimuli. While the apoptotic events triggered by TNF-α and agonistic antibodies to Fas are cell surface receptor mediated, DNA damage induced by exposure to UV and cisplatin is thought to trigger apoptosis in response to these insults. The ability of hILP to inhibit apoptosis by all of these stimuli distinguishes it from other antiapoptotic proteins such as the viral DED-containing proteins E8 and MC159 and the endogenous DED-containing protein Flame-1, which target cell surface-associated activation of caspase 8 and are unable to inhibit UV-induced apoptosis (3, 61). Further, while the apoptotic effects of anti-Fas are not dependent on p53 (29), those of cisplatin are p53 dependent (67, 69). Thus, hILP functions downstream of stimulus-dependent pathways that initiate the apoptotic cascade.
Deletion analysis of hILP revealed that the BIR domains of hILP are sufficient to confer protection against these apoptotic stimuli (Fig. 2). This result is in accord with studies with Drosophila in which expression of the BIR domains of DIAP1 was sufficient to rescue cells from normally occurring cell death in the eye (31). The BIR domains of other members of the IAP family have been shown to play important roles in protein-protein interactions. For example, the BIR domains of the baculovirus IAP Op-IAP are required for interaction with the proapoptotic Drosophila protein Doom (30). Similarly, the BIRs of the two other known mammalian IAPs (c-IAP1 and c-IAP2) mediate the interactions with TRAF proteins (53). However, we did not detect any interaction between hILP and any of the six known TRAFs (Fig. 3). Although it is possible that hILP might block apoptosis through an interaction with an as-yet-unknown TRAF, it is more likely that hILP is not a TRAF binding protein and that the BIR domains mediate interactions with other proteins.
Recent reports have described the involvement of mitochondria in the cell death cascade (62, 72, 73). For example, mitochondrial dysfunction, including disruption of the electron transport chain, is a necessary event in the induction of apoptosis (57). Consistent with a critical role for mitochondria in apoptosis, it has been reported that cytochrome c is released from the mitochondria into the cytosol and that cytochrome c itself can effect apoptosis by inducing the activation of caspases (44). These findings imply a sequential order of events leading to the apoptotic death of the cell and also suggest a number of distinct levels of the cell death pathway which might be subject to regulation. Our observations that Bcl-xL and hILP have differential effects on cytochrome c release and cytochrome c-mediated apoptosis support this sequential ordering of the apoptotic pathway.
Recent findings have led to several potential mechanisms by which Bcl-xL may maintain mitochondrial integrity. The recent finding that Bcl-xL can insert into membranes and form ion channels (47) is consistent with a potential role for Bcl-xL in maintaining mitochondrial integrity by contributing to the maintenance of mitochondrial membrane potential. Alternatively, the report of Kharbanda et al. (39) demonstrating that Bcl-xL can bind directly to cytochrome c suggests a more direct role for Bcl-xL in maintaining cytochrome c in its mitochondrial compartment. Our observation that hILP is unable to inhibit the redistribution of cytochrome c suggests that it may function at a more downstream level in the cell death cascade. hILP, in contrast to Bcl-xL, was able to protect against death induced by microinjection of cytochrome c. One effect of cytosolic cytochrome c is the activation of downstream caspases (40, 71). The placement of hILP downstream of cytochrome c release suggests that hILP may function in the cell death pathway by controlling the processing of downstream caspases from zymogens or, by analogy with P35, by direct interaction with and inhibition of the mature protease. Further studies will be required to distinguish between these possibilities.
ACKNOWLEDGMENTS
We thank V. Dixit for MCF7F cells; J. Yuan for the caspase 2 cDNA; R. Arch, C. Rudin, and J. Van Dongen for providing TRAF4 and TRAF5 cDNAs; B. Mayer for providing pEBB and pEBG vectors; and R. Clem and J. M. Hardwick for providing IAP cDNAs. We thank A. Srinivasan, R. Gedrich, C. Rudin, and members of the Thompson and Tomaselli labs for insightful discussions. We also thank C. Mazur and L. Trout for assistance in the preparation of the manuscript.
This work was supported in part by research grant P01 DK49799 (to C.B.T.) from the National Institutes of Health. C.S.D. is a Special Fellow of the Leukemia Society of America.
REFERENCES
- 1.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong R, Aja T, Xiang J, Gaur S, Krebs J, Hoang K, Bai X, Korsmeyer S, Karanewsky D, Fritz L, Tomaselli K. Fas-induced activation of the cell death related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- 3a.Armstrong, R. C. Unpublished observations.
- 3.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G H, Senkevich T G, Alnemri E S, Moss B, Lenardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertin J, Mendrysa S M, LaCount D J, Gaur S, Krebs J F, Armstrong R C, Tomaselli K J, Friesen P D. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced Ced-3/ICE-like protease. J Virol. 1996;70:6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boise L H, Gottschalk A R, Quintans J, Thompson C B. Bcl-2 and Bcl-2-related proteins in apoptosis regulation. Curr Top Microbiol Immunol. 1995;200:107–121. doi: 10.1007/978-3-642-79437-7_8. [DOI] [PubMed] [Google Scholar]
- 6.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 7.Borden K L, Boddy M N, Lally J, O’Reilly N J, Martin S, Howe K, Solomon E, Freemont P S. The solution structure of the RING finger domain from the acute promyelocytic leukaemia proto-oncoprotein PML. EMBO J. 1995;14:1532–1541. doi: 10.1002/j.1460-2075.1995.tb07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulakia C A, Chen G, Ng F W H, Teodoro J G, Branton P E, Nicholson D W, Poirier G G, Shore G C. Bcl-2 and adenovirus E1B 19 kDA protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymerase. Oncogene. 1996;12:29–36. [PubMed] [Google Scholar]
- 9.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Mankovich J, Shi L, Greenberg A H, Miller L K, Wong W W. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 11.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD40L-mediated NF-κB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 12.Cheng G, Cleary A M, Ye Z S, Hong D I, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 13.Chen-Levy Z, Cleary M L. Membrane topology of the Bcl-2 proto-oncogenic protein demonstrated in vitro. J Biol Chem. 1990;265:4929–4933. [PubMed] [Google Scholar]
- 14.Chinnaiyan A M, Orth K, O’Rourke K, Duan H, Poirier G G, Dixit V M. Molecular ordering of the cell death pathway: Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 15.Clem R J, Duckett C S. The iap genes: unique arbitrators of cell death. Trends Cell Biol. 1997;7:337–339. doi: 10.1016/S0962-8924(97)01088-X. [DOI] [PubMed] [Google Scholar]
- 16.Clem R J, Fechheimer M, Miller L K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 17.Clem R J, Hardwick J M, Miller L K. Anti-apoptotic genes of baculoviruses. Cell Death Differ. 1996;3:9–16. [PubMed] [Google Scholar]
- 18.Clem R J, Miller L K. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clem R J, Miller L K. Induction and inhibition of apoptosis by insect viruses. In: Tomei L D, Cope F O, editors. Apoptosis II: the molecular basis of apoptosis in disease. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 89–110. [Google Scholar]
- 20.Crook N E, Clem R J, Miller L K. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deveraux Q L, Takahashi R, Salvesen G S, Reed J C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 22.Digby M R, Kimpton W G, York J J, Connick T E, Lowenthal J W. ITA, a vertebrate homologue of IAP that is expressed in T lymphocytes. DNA Cell Biol. 1996;15:981–988. doi: 10.1089/dna.1996.15.981. [DOI] [PubMed] [Google Scholar]
- 23.Duan H, Dixit V M. RAIDD is a new ‘death’ adaptor molecule. Nature. 1997;385:86–99. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- 24.Duckett C S, Gedrich R W, Gilfillan M C, Thompson C B. Induction of nuclear factor κB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17:1535–1542. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duckett C S, Nava V E, Gedrich R W, Clem R J, Van Dongen J L, Gilfillan M C, Shiels H, Hardwick J M, Thompson C B. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 26.Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 27.Fearnhead H O, Dinsdale D, Cohen G M. An interleukin-1-beta converting enzyme-like protease is a common mediator of apoptosis in thymocytes. FEBS Lett. 1995;375:283–288. doi: 10.1016/0014-5793(95)01228-7. [DOI] [PubMed] [Google Scholar]
- 28.Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs E J, McKenna K A, Bedi A. p53-dependent DNA damage-induced apoptosis requires Fas/APO-1-independent activation of CPP32β. Cancer Res. 1997;57:2550–2554. [PubMed] [Google Scholar]
- 30.Harvey A J, Bidwai A P, Miller L K. Doom, a product of the Drosophila mod (mdg4) gene, induces apoptosis and binds to baculovirus inhibitor of apoptosis proteins. Mol Cell Biol. 1997;17:2835–2843. doi: 10.1128/mcb.17.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hay B A, Wassarman D A, Rubin G M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 32.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hengartner M O, Horvitz H R. Programmed cell death in Caenorhabditis elegans. Curr Opin Genet Dev. 1994;4:581–586. doi: 10.1016/0959-437x(94)90076-f. [DOI] [PubMed] [Google Scholar]
- 34.Hu H M, O’Rourke K, Boguski M S, Dixit V M. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J Biol Chem. 1994;269:30069–30072. [PubMed] [Google Scholar]
- 35.Hu S, Vincenz C, Buller M, Dixit V M. A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- 36.Ishida T, Mizushima S-I, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J-I. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 37.Ishida T, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J-I. TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr J F, Wyllie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharbanda S, Pandey P, Schofield L, Isreals S, Roncinske R, Yoshida K, Bharti A, Yuan Z-M, Saxena S, Weichselbaum R, Nalin C, Kufe D. Role for Bcl-xL as an inhibitor of cytosolic cytochrome c accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 41.Krajewski S, Tanaka S, Takayama S, Schibler M J, Fenton W, Reed J C. Investigation of the subcellular distribution of the Bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 42.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda J-E, MacKenzie A, Korneluk R G. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 43.Lithgow T, van Driel R, Bertram J F, Strasser A. The protein product of the oncogene bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth Differ. 1994;5:411–417. [PubMed] [Google Scholar]
- 44.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 44a.Manji G A, Hozak R R, LaCount D J, Friesen P D. Baculovirus inhibitor of apoptosis functions at or upstream of the apoptotic suppressor P35 to prevent programmed cell death. J Virol. 1997;71:4509–4516. doi: 10.1128/jvi.71.6.4509-4516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer B J, Hirai H, Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr Biol. 1995;5:296–305. doi: 10.1016/s0960-9822(95)00060-1. [DOI] [PubMed] [Google Scholar]
- 46.Minaschek G, Bereiter-Hahn J, Berthold G. Quantitation of the volume of liquid injected into cells by means of pressure. Exp Cell Res. 1989;183:434–442. doi: 10.1016/0014-4827(89)90402-3. [DOI] [PubMed] [Google Scholar]
- 47.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Bcl-xL forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 48.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 49.Nakano H, Oshima H, Chung W, Williams-Abbott L, Ware C F, Yagita H, Okumura K. TRAF5, an activator of NF-κB and putative signal transducer for the lymphotoxin-β receptor. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 50.Neilan J G, Lu Z, Afonso C L, Kutish G F, Sussman M D, Rock D L. An African swine fever virus gene with similarity to the proto-oncogene bcl-2 and the Epstein-Barr virus gene BHRF1. J Virol. 1993;67:4391–4394. doi: 10.1128/jvi.67.7.4391-4394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen M, Millar D G, Yong V W, Korsmeyer S J, Shore G C. Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J Biol Chem. 1993;268:25265–25268. [PubMed] [Google Scholar]
- 52.Regnier C H, Tomasetto C, Moog-Lutz C, M.-P. C, Wendling C, Basset P, Rio M-C. Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family, which is expressed in breast carcinoma. J Biol Chem. 1995;270:25715–25721. doi: 10.1074/jbc.270.43.25715. [DOI] [PubMed] [Google Scholar]
- 53.Rothe M, Pan M-G, Henzel W J, Ayres T M, Goeddel D V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 54.Rothe M, Wong S C, Henzel W J, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 55.Roy N, Mahadevan M S, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, Salih M, Aubry H, Tamai K, Guan X, Ioannou P, Crawford T O, de Jong P J, Surh L, Ikeda J-E, Korneluk R G, MacKenzie A. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 56.Schulze-Osthoff K, Bakker A C, Vanhaesebroeck B, Beyaert R, Jacob W A, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 57.Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene. 1996;12:2251–2257. [PubMed] [Google Scholar]
- 59.Shu H-B, Takeuchi M, Goeddel D V. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith G L. Virus proteins that bind cytokines, chemokines or interferons. Curr Opin Immunol. 1996;8:467–471. doi: 10.1016/s0952-7915(96)80032-x. [DOI] [PubMed] [Google Scholar]
- 60a.Srinivasan, A., F. Li, A. Wong, L. Kodandapani, J. Robert Smidt, J. F. Krebs, L. C. Fritz, J. C. Wu, and K. J. Tomaselli. Bcl-xL functions downstream of caspase-8 to inhibit fas- and TNFR1-induced apoptosis of MCF7 breast carcinoma cells. J. Biol. Chem., in press. [DOI] [PubMed]
- 61.Srinivasula S M, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce C M, Litwack G, Tomaselli K J, Armstrong R C, Alnemri E S. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J Biol Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- 62.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tewari M, Beidler D R, Dixit V M. CrmA-inhibitable cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995;270:18738–18741. doi: 10.1074/jbc.270.32.18738. [DOI] [PubMed] [Google Scholar]
- 64.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 65.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 66.Uren A, Pakusch M, Hawkins C, Puls K L, Vaux D L. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vasey P A, Jones N A, Jenkins S, Dive C, Brown R. Cisplatin, camptothecin, and taxol sensitivities of cells with p53-associated multidrug resistance. Mol Pharmacol. 1996;50:1536–1540. [PubMed] [Google Scholar]
- 68.Vaux D L, Haecker G, Strasser A. An evolutionary perspective on apoptosis. Cell. 1994;76:777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 69.Vikhanskaya F, Clerico L, Valenti M, Stanzione M S, Broggini M, Parodi S, Russo P. Mechanism of resistance to cisplatin in a human ovarian-carcinoma cell line selected for resistance to doxorubicin: possible role of p53. Int J Cancer. 1997;72:155–159. doi: 10.1002/(sici)1097-0215(19970703)72:1<155::aid-ijc22>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 70.Xue D, Horvitz H R. Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature. 1995;377:248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 72.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin S A, Petit P X, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J L, Petit P X, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed cell death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou S, Yang Y, Scott M J, Pannuti A, Fehr K C, Eisen A, Koonin E V, Fouts D L, Wrightsman R, Manning J E. Male-specific lethal 2, a dosage compensation gene of Drosophila, undergoes sex-specific regulation and encodes a protein with a RING finger and a metallothionein-like cysteine cluster. EMBO J. 1995;14:2884–2895. doi: 10.1002/j.1460-2075.1995.tb07288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]