Abstract
In recent years, a major focus in the field of tissue engineering has been the search for a suitable biomaterial for clinical applications. Researchers have sought to optimize natural, synthetic, and hybrid options, with an aim to enhance biological, chemical, physical, and mechanical properties. In the past decade, silk fibroin has emerged as a promising approach due to its suitable properties. Specifically, the chemical modification of silk fibroin with methacrylate agents, namely glycidyl methacrylate, methacrylic anhydride, and gelatin methacryloyl, confers the material with improved biophysical properties. This review presents an in-depth overview of silk fibroin’s structure and suitable properties, silk fibroin methacrylate synthesis and characterization techniques, and applications of silk fibroin in bone and cartilage, skin, and nerve tissue engineering. Challenges include a limited understanding of methacrylate agents on specific cell types, which can be addressed by further in vivo investigations utilizing biomaterial compounds to confer tissue-specific needs. We conclude with our perspective of the present limitations and future trends of the methacrylated SF platform.
Keywords: Silk fibroin, methacrylation, glycidyl methacrylate, methacrylic anhydride, gelatin methacryloyl, tissue engineering


1. Introduction
In recent years, tissue engineering has emerged as an innovative approach to producing patient-specific tissues for clinical applications. Specifically, tissue engineering relies on biocompatible scaffolds seeded with cells and growth factors to maintain, improve, and restore damaged tissues. Various tissue engineering scaffolds are fabricated via natural, synthetic, or hybrid polymers, resulting in a wide range of structures and properties. Natural polymers generally mimic the components of the extracellular matrix (ECM) and are inherently bioactive, but these materials have inferior structural stability and mechanical properties. , On the other hand, synthetic polymers such as polyesters, polyurethanes, electronically conducting polymers, and others exhibit superior properties including tunability and strength. However, synthetic compounds support minimal cell growth and have restricted flexibility, representing a major challenge for clinical translation. Thus, synthesizing an optimal hybrid alternative that combines natural polymers’ bioactivity with synthesized polymers’ properties represents a significant goal for researchers in the field to improve in vivo applicability.
Silk fibroin (SF) is a suitable biomaterial for tissue engineering due to many of its physicochemical properties. One common form of silk fibroin used in research applications comes from the domesticated Bombyx mori (B. mori) species, which has long been used in the textile and medical industries. B. mori cocoon fibers are constituted of silk fibers with an internal fibroin core and external sericin shell. SF, a protein with a semicrystalline structure, functions in a load-bearing capacity. Sericin is an amorphous, water-soluble globular protein that acts as a gumming agent for the fibroin filaments. After a degumming procedure, sericin-free SF fibers have been shown to exhibit a higher molecular weight, increased mechanical strength, and a faster degradation rate. By modifying SF and combining it with other polymers, researchers can fabricate hybrid structures with enhanced cellular behavior. This article will cover in detail the structures and properties of SF, describe the production of SF methacrylate, and present a comprehensive review of its tissue engineering applications to date.
2. Silk Fibroin Structures and Properties
2.1. Structures
SF is composed of a heavy chain (391 kDa) and a light chain (25 kDa) linked together by a covalent disulfide bond, with a P25 glycoprotein (30 kDa) attached to this heavy and light complex, shown in Figure . The heavy chain, light chain, and P25 glycoprotein are assembled in a 6:6:1 molar ratio. The heavy chain consists of two exons (67 and 1570 bp) and one intron (971 bp). The amino acid sequence of the heavy chain is a 5,263-residue polypeptide chain (45.9% glycine, 30.3% alanine, 12.1% serine, 5.3% tyrosine, 1.8% valine, and 4.7% other 15 types) with 12 low-complexity crystalline domains. These crystalline domains are composed of subdomains of repeated Gly-X dipeptide motifs (the X being alanine, serine, threonine, and valine). Each subdomain forms a β-strand, which is connected to the next β-strand by a four residue β-turn. This results in a secondary structure of antiparallel β-sheets that contain a polar side and exhibit N-to-C directionality, forming a two-layered solenoid structure. , The light chain has a standard amino acid composition with a nonrepetitive sequence, including seven tryptic and four chymotryptic peptides. This chain plays a marginal role in the fiber as compared to the heavy chain. The P25 glycoprotein, which contains asparagine-linked oligosaccharide chains, is hydrophobically associated with the heavy and light complex.
1.
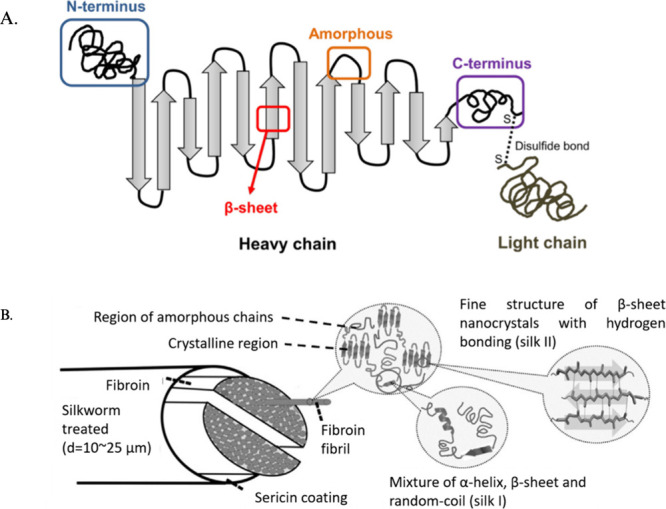
An illustration of the silk fibroin (SF) structure. (A) SF heavy and light chain linked by a disulfide bond with antiparallel β-sheets and N-to-C directionality. (B) SF fibril structure with silk I and silk II structures in the polypeptide chain. Reproduced with permission from ref . Available under a CC-BY 4.0 license. Copyright 2021 Multidisciplinary Digital Publishing Institute (MDPI).
The two crystalline forms of SF are silk I and silk II. Silk I exhibits a zigzag structure, while silk II has an antiparallel β-sheet structure. It has been shown that silk II has a higher stability than silk I, measured by comparing the two forms’ crystal melting temperatures. As such, there have been many efforts to convert silk I to silk II, such as through methanol or potassium phosphate treatment. A third crystalline form of SF (silk III), which includes hexagonal packing of silk molecules in a 3-fold helical chain conformation, has also been observed To date, there is little literature surrounding the advantages of silk III aside from its helical structure. Altogether, SF’s unique structure contributes to its superior properties compared to many other biomaterials.
2.2. Properties
First, SF exhibits stronger mechanical properties than many other natural and synthetic alternatives. Specifically, SF has a modulus of 15 to 17 GPa, ultimate tensile strength of 610 to 690 MPa, and breakage strain of up to 16%. As such, SF scaffolds are suitable for load-bearing soft tissues. Next, SF is highly biocompatible with native tissues, as it has been shown to be biologically inert and compatible. For this reason, SF is approved by the FDA to be used in sutures, which suggests its applicability for in vivo implantation. Additionally, SF is a biodegradable and bioabsorbable polymer. Following enzymatic degradation of SF, the resulting amino acids and peptides are more easily absorbed in vivo. However, because of native silk fibers’ high secondary β-sheet content, degradation is slower vis-a-vis regenerated SF solutions. To address this limitation, researchers have fabricated regenerated SF through various dissolution techniques to alter its secondary structure and produce different solubility degrees. To date, dissolution techniques include salt solutions (e.g., 9.3 M LiBr or LiSCN), , Ajisawa’s reagent (CaCl2/C2H5OH/H2O), alcohol treatment (e.g., methanol or ethanol), and more. All water-based dissolution processes include a dialysis step against deionized water or a buffer. , Previous research has shown that lower β-sheet structures with a smaller content of silk II lead to faster degradation. Hence, enhancing the biodegradability and bioabsorbability of SF will improve its clinical applicability in patient tissues.
3. Silk Fibroin Methacrylation
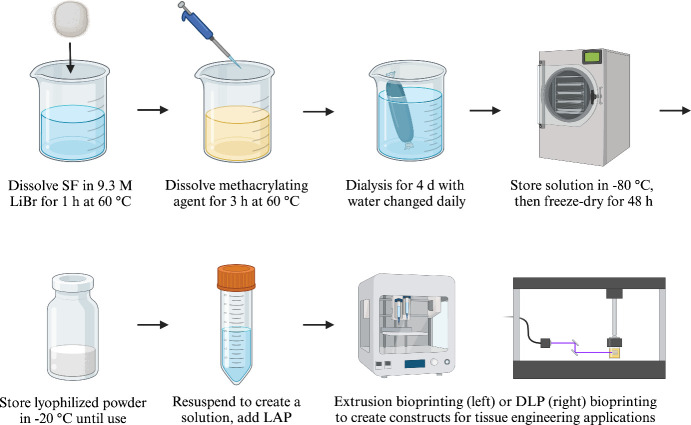
Recent studies have validated the technique of modifying SF with a methacrylate agent, which enhances many of its structures and properties. As illustrated in Figure , this process can be achieved through dissolving SF and a methacrylate agent under heat, dialysis with dialysis membranes while replacing distilled water daily, and lyophilization under −80 °C to form a freeze-dried powder until further use. Previous literature has noted the importance of dialysis periods in the SF methacrylation reaction in removing unreacted chemical residues. These include unreacted chemical cross-linkers or aqueous chemicals such as LiBr. Generally, the end of the dialysis process is defined by the conductivity of the dialysis water dropping below 10 μS. One study by Hong et al. in 2020 proposed a further standardization in dialysis periods for methacrylated SF hydrogels translated into clinical applications. Using dialysis membranes with a molecular weight cutoff size of 12–14 kDa and changing water three times daily, Hong observed an ideal dialysis period at 7 days, which yielded signals for FT-IR results, functional amine groups, and increasing cell proliferation. Upon resuspending the powder to create a methacrylated SF solution, a physical photo-cross-linking step takes place that consists of a photoinitiator such as lithium phenyl (2,4,6-trimethylbenzoyl) phosphinate (LAP) absorbing energy from light radiation, creating free radicals that attack the active groups on the polymer. This process introduces functional groups that form cross-links between the polymer’s chains, resulting in a hydrogel. Below, we will introduce applications of three common methacrylate agents that have been used thus far in studies, namely glycidyl methacrylate (GMA), methacrylic anhydride (MA), and gelatin methacryloyl (GelMA), which is made through the synthesis of gelatin with MA. A comparison of SF’s methacrylation with GMA and MA is depicted in Figure .
2.
A schematic representation of the synthesis protocol for silk fibroin (SF) methacrylation. This procedure consists of dissolving SF and the methacrylate agent, dialysis, lyophilization, and resuspension for tissue engineering applications such as extrusion bioprinting and digital light processing (DLP) bioprinting. Created with BioRender.com.
3.

A comparison of (A) glycidyl methacrylate (GMA) and (B) methacrylic anhydride (MA) in chemically modifying silk fibroin (SF). The SF-GelMA reaction is similar to the SF-MA reaction. Created with BioRender.com.
3.1. Silk Fibroin Modified with Glycidyl Methacrylate
One of the most common ways to modify SF uses GMA (C7H10O3), an ester of methacrylic acid and glycidyl. By varying the amount of GMA introduced to SF, which results in varying degrees of methacrylation, several groups have successfully demonstrated a range of hydrogel properties for tissue engineering applications.
In 2018, Kim’s group was one of the first to successfully modify SF with GMA to create a printable bioink. Specifically, the Kim group substituted the primary amines of SF with methacrylate by incorporating various molar ratios of GMA into a SF solution. (Methacrylation was confirmed by a 1H NMR characterization, which showed the presence of methacrylate vinyl groups at δ = 6.2–6 and 5.8–5.6 ppm, as well as a methyl group of GMA δ = 1.8 ppm.) The SF-based bioink was then UV-cross-linked and incorporated with LAP to form a hydrogel with remarkable mechanical and rheological properties. By varying the amount of GMA added, Kim et al. found that an increase in GMA concentration corresponded to a higher degree of methacrylation, enhancing the hydrogel’s properties. (An optimal GMA concentration was observed from 282 to 424 nM.) From a 30% SF-GMA bioink, Kim et al. produced organ structures including the ear, brain, trachea, heart, lung, and blood vessels, all with high resolution and stability. Additionally, this group was also able to encapsulate cells inside the hydrogels with notable cytocompatibility, shown by strong cell viability and cell-to-cell interactions. Recent studies have used protocols largely similar to that in Kim’s study, as well as investigating other factors such as additional biomaterial additions and reactional variables. Because different tissue types require different conditions, future studies should consider finding optimal methacrylate agent concentrations for corresponding mechanical properties that best suit each tissue environment.
The Kim group performed a follow-up study in 2021 that demonstrated a wider range of applications, including cartilage, skin, and nerve tissue engineering, which will be discussed in a later section of this review paper.
In addition to the work by Kim et al., other groups have also presented various applications of SF with GMA. For example, Bucciarelli et al. characterized a porous sponge scaffold using SF-GMA in 2019. The group used a mixer to create air bubbles as a template for pores, ensuring cell attachment, proliferation, and neovascularization in the scaffold for soft tissues. By changing the SF-GMA composition, Bucciarelli et al. were able to adjust various sponge properties (e.g., porosity, dissolution, water absorption, cell viability, etc.). Porosity may be helpful for regenerating tissue types requiring significant diffusion and vascularization properties, such as bone, cartilage, skin, muscle, nerve, and vasculature. SF in bone and cartilage tissue engineering is well-studied, but applications to the latter tissue types require further validation.
Bae et al. fabricated SF-GMA ultrathin fibers through electrospinning and dual cross-linking in 2020. Treatment with an ethanol solution insolubilized the fibers and induced a structural transformation from random coils to β-sheets. Increasing the cross-linking density of the fibers also improved water resistance, which may be helpful in exploring the cytocompatibility of SF-GMA for future applications in mimicking the ECM. To improve ECM characterization with the SF platform, researchers can seek to incorporate collagen, elastin, adhesive proteins, and relevant growth factors to best simulate the in vivo environment. Approaches with SF-GelMA may be best suited in this regard for gelatin’s similarities with collagen in contributing to tissue stability and structure.
3.2. Silk Fibroin Modified with Methacrylic Anhydride
Another approach to creating methacrylated SF can be achieved with the addition of MA (C8H10O3), an organic compound derived from methacrylic acid. MA has been widely utilized to chemically modify gelatin to create GelMA, conferring the gelatin with photo-cross-linking properties. Newer studies have borrowed from this synthesis protocol to incorporate MA in SF, instead of gelatin. For example, an early study by Bessonov et al. synthesized a SF-MA solution for hydrogel scaffolds in 2019. Bessonov’s group fabricated various structures including films, micropatterns, pads, and macroporous sponges. The hydrogels were biocompatible and exhibited stronger mechanical properties (e.g., Young’s modulus) than a pure-SF hydrogel. Interestingly, when hexafluoroisopropanol was used instead of formic acid to prepare the SF-MA solution, Bessonov et al. observed a higher storage modulus and increased cell spreading in the resulting hydrogels. Bessonov’s work represents a foundation for the SF-MA platform, which future studies can expand upon for more creating more complex construct geometries and translating to in vivo applications.
More recently, in 2023, Wu’s group produced a hydrogel using a 4% (w/v) SF-MA solution mixed with LAP, followed by chemical cross-linking, physical cross-linking, and β-sheet formation. The resulting hydrogel possessed a homogeneous porous structure, as well as suitable mechanical characteristics, biodegradability, and biocompatibility. Similar to Kim et al.’s 2018 study with GMA, Wu et al. found that adjusting MA content in the SF-MA hydrogel led to varying physical and biological characteristics. Specifically, a higher degree of methacrylation was observed with increasing concentrations of MA added, as shown by a 1H NMR test which revealed two peaks at δ = 6.31 and 5.86 ppm, as well as a peak in the 2 ppm range indicating the presence of MA. Other characterizations included FTIR spectroscopy to observe the SF-MA hydrogel’s secondary structure, X-ray diffraction to examine crystalline structures, scanning electron microscope (SEM) for morphology, as well as swelling ratio, rheology, mechanical, and in vitro degradation testing for physical properties. As aforementioned, as in SF-GMA, the varied concentrations and corresponding characteristics for SF-MA noted by Wu et al. may be worth analyzing in the context of different tissue types’ requirements.
There are key differences between the usage of MA and GMA for SF methacrylation. Specifically, Kim et al.’s 2018 study noted crystallization of SF with SF-MA, likely due to a reaction byproduct, methacrylic acid, reducing the solution’s pH. The protonated (and thereby neutralized) carboxyl group may consequently reduce hydrophilicity, decrease charge repulsion, and accelerate hydrophobic interactions, all of which contribute to cross-links and gelation. In other words, ionizing SF’s free amino groups hinders the reaction with MA. On the other hand, the use of GMA resulted in a stable reaction with no acidic byproduct. Additionally, GMA reacts with the vinyl, hydroxyl, and carboxyl groups on SF, furthering methacrylation and maximizing cross-linking density. In addition to pH differences, future studies should investigate other factors necessary to standardize the SF-GMA and SF-MA reactions to better assess their hydrogels’ respective properties.
3.3. Silk Fibroin Modified with Gelatin Methacryloyl
A third methacrylate agent used to modify SF is GelMA. GelMA is prepared by covalently bonding naturally derived polymer gelatin with methacrylic groups. MA is most common for GelMA synthesis, and the degree of methacrylation can be varied by altering the ratio of MA to gelatin. In addition to adjusting MA and gelatin concentration, Shirahama et al.’s 2016 study further optimized GelMA synthesis by varying the reaction’s carbonate-bicarbonate buffer molarity, pH, temperature, and time. Specifically, this group found optimal conditions using a 0.1 mL/g ratio of MA to gelatin, 10–20% gelation concentration, 0.25 M carbonate-bicarbonate buffer, pH 9, and 35–50 °C reaction temperature, which produced near-complete substitution within 1 h.
By applying GelMA to modify SF, Xiao et al. characterized a SF-GelMA-based “interpenetrating polymer network” hydrogel in 2011. The hydrogel was created by first preparing a 6 wt % GelMA, 0.5–2 wt % SF, and photoinitiator (2-hydroxy-1-[4-(hydroxyethoxy)-phenyl]-2-methyl-l-propanone) solution, then subjecting it to vigorous stirring, UV exposure, and methanol treatment. Xiao’s group observed a low swelling ratio, high Young’s modulus, high resistance of collagenase degradation, and dense network structure. Furthermore, they also fabricated porous microscaffolds, suggesting SF-GelMA to be a suitable biomaterial.
In addition to SF with GelMA, other researchers have demonstrated the possible incorporation of other novel methacrylate agents such as hyaluronic acid methacryloyl (HAMA) in fabricating SF-GelMA. A study by Li’s group in 2022 produced a bioprintable hydrogel prepared by modifying GelMA and HAMA with SF. This hydrogel possessed higher adhesion strength than a similar GelMA/HAMA hydrogel without the incorporation of SF. Li’s group also found increased cell actin expression and improved cell adhesion. Upon using the hydrogel to 3D print a cell-laden porous scaffold structure, this group observed structural stability, cell morphology, and cell proliferation, indicating GelMA’s applicability for tissue engineering cell-laden structures.
Most recently, in 2023, Shi et al. presented a novel approach using liquid nitrogen to obtain a composite hydrogel based on SF, GMA, and GelMA. Mechanical testing found that the addition of GelMA enhanced mechanical properties, and the composite hydrogel also showed cytocompatibility and biocompatibility. Additionally, the implantation of composite hydrogel scaffolds into mice demonstrated in vivo structural stability and angiogenesis. These results showcase the integrability of SF-GelMA hydrogel-based constructs for in vivo studies. Shi’s validation of the methacrylated SF platform with additional biomaterial compounds is relevant for future studies to consider more novel mixtures. Further applications of methacrylated SF specific to tissue type are discussed in a later section.
4. Methacrylation Characterization
Researchers use a range of characterization methods to determine the success of SF methacrylation. The most common of these include nuclear magnetic resonance (NMR) spectroscopy and Fourier-transform infrared (FTIR) spectroscopy, as well as SEM imaging for visual confirmation. A standard method to determine the success of methacrylation is the degree of methacrylation (DoM) through NMR peak integration, discussed in depth later. Additionally, because methacrylation is associated with improved physical characteristics, studies investigating cross-linked methacrylated SF to construct hydrogels can aim toward improving mechanical, chemical, and biological properties.
4.1. Spectroscopy and Imaging
Of the different types of NMR, proton (1H) NMR is the most prevalent type of NMR used for characterizing SF methacrylation. There are several relevant peak signals in the 1H NMR spectra for methacrylated SF. Kim et al. demonstrated the signal of SF’s aromatic acids (δ = 7.5–6.9 ppm) to be characteristic of SF presence and used it to normalize the spectrum. Kim’s group also observed that the signal from lysine methylene (δ = 2.95–2.8 ppm) was reduced by SF methacrylation and calculated DoM as follows:
The methacrylate vinyl group (δ = 6.2–6 ppm and δ = 5.8–5.6 ppm) is enhanced by SF methacrylation and can be used as an alternate method to find DoM. For SF modified with GelMA, studies have verified methylene group peaks (δ = 5.6 ppm and δ = 5.3 ppm) to be MA-characteristic peaks. Another relevant signal for SF methacrylation is the methyl group (δ = 1.8 ppm), which increases with greater methacrylate agent amounts. Together, an evaluation of the lysine methylene, methacrylate vinyl, and methyl group signals can validate the success of SF methacrylation.
FTIR is another spectroscopy method used to identify specific chemical groups in methacrylated SF, allowing for the combination of spectral and spatial visualization of the sample. , FTIR peaks of methacrylated SF can be analyzed with respect to the methacrylate agent. For GMA, Kim’s group observed wagging of the CHOH (1238 cm–1), CH2 (1156 cm–1), and RR’C = CH2 (951 cm–1) groups in the spectra of SF-GMA samples. For MA, Wu’s group proposed that the peaks of MA may be obscured by SF’s larger molecular weight. Additionally, for GelMA samples, Farasatkia’s group showed peaks related to vibrations of the C–N–H bond (1570–1550 cm–1) and stretching of the N–H bond (3400–3200 cm–1). These and other peaks characteristic of methacrylate agents may confirm substitution by methacrylate functional groups, representative of SF methacrylation.
SEM imaging can be used to visualize methacrylated SF hydrogels’ internal structure three-dimensionally and at high magnifications. Methacrylated SF hydrogels generally reveal fibrous structure and porous architecture. In particular, Chen et al. reported on the effect of methacrylation on physical properties, wherein higher degrees of methacrylation result in stiffer, more durable, and smaller pored hydrogels. Thus, an evaluation of methacrylated SF’s hydrogel architecture can provide information on its successful methacrylation and physical properties.
4.2. Physical Testing
Because methacrylation improves the hydrogel’s physical properties, compressive and tensile strength have been shown to increase as the methacrylate agent increases. An additional test for surgical applications of methacrylated SF hydrogels is the suture retention (also known as suture pull-out) test, although this has not been well characterized for methacrylated SF. , Future experiments using methacrylated SF hydrogels in surgical applications may include characterizing suture retention of in vitro or in vivo anastomoses depending on application.
Standard rheological analyses for methacrylated SF hydrogels include storage modulus (G’), loss modulus (G”), and loss factor (tan δ). Methacrylated SF hydrogels exhibit higher G’ and G”, while tan δ seems to be marginally dependent on methacrylation. Notably, Kim’s group showed that methacrylated SF hydrogels show viscous properties against high shear stress (G” surpasses G’ at around 200 Pa). Specifically, this group observed typical gel properties at a low shear stress of 0.5 Pa and fluidic properties at a higher shear stress of 300 Pa45. These results suggest that tailoring shear stress may be an important factor for fine-tuning the structural properties of methacrylated SF hydrogels for different purposes.
Like other hydrogels, methacrylated SF hydrogels can be assessed for their water retention capabilities. This test is particularly relevant to in vivo experiments using methacrylated SF, as hydrogels with improved water retention capabilities are desirable for the absorption of water, blood, and other nutrients. To this extent, multiple groups including Kim et al. and Zhou et al. have found a reduction in swelling capacities with increasing methacrylation content, which is likely due to increasing cross-linking density. , Future experiments may attempt to further reduce swelling in methacrylated SF hydrogels by attempting even higher methacrylation degrees, among other factors.
Another important property for utilizing methacrylated SF in cell cultivation experiments is degradation. In terms of hydrolytic degradation, Kim’s group observed slowed hydrolytic degradation rates with increased methacrylation degrees. For enzymatic degradation, Atila’s group demonstrated improved enzymatic degradation of methacrylated SF hydrogels compared to that of pure SF hydrogels. These degradation properties are likely attributed to the increased cross-linking density that accompanies methacrylation and are worth investigating for in vitro or in vivo applications of methacrylated SF hydrogels.
Biocompatibility analyses can provide key insight into the behavior of methacrylate SF hydrogels for in vitro and in vivo studies. Various cell types can be assessed through tests for cellular adhesion, cell proliferation (i.e., cell viability assay), and 3D cellular encapsulation. Studies by Xiao et al. and Barroso et al. have observed high cell viability for all hydrogels of various methacrylate agents (e.g., GMA and GelMA), indicating that methacrylation does not induce cytotoxicity, which represents a promising direction for further tissue studies. ,
5. Tissue Engineering Applications
Using methacrylated SF solutions, researchers can fabricate various scaffold structures with different techniques. These techniques include the aforementioned cross-linking and 3D bioprinting, ,, as well as other strategies like electrospinning, porogens, and micropatterning. Tissue engineering structures produced by these techniques include hydrogels and constructs, artificial fibers and mats, sponges, and micropatterned films, all with improved physical properties attributed to methacrylation-induced cross-links. Several studies in the past four years have demonstrated the application of methacrylated SF tissue-engineered structures in bone and cartilage (Table ), skin (Table ), and nerve (Table ) systems.
1. A Summarization of Recent Methacrylated SF-Based Approaches in Bone and Cartilage Tissue Engineering.
| Biomaterials | Seeded Cells | Fabrication Method | Construct Type | Results |
|---|---|---|---|---|
| SF, GMA | Human, rat-derived chondrocytes | 3D DLP bioprinting | Hydrogel | Increasing cell viability and proliferation up to 14 days; differentiation of chondrogenesis and neo-cartilage formation after 4 weeks; in vivo transplantation into mice |
| SF, GMA | Human turbinate-derived stem cells, human chondrocytes | 4D DLP bioprinting | Trachea mimetic tissue | Controlled curvature of constructs; integration with host trachea; accurate formation of epithelium and cartilage at predicted sites |
| SF, GMA, PEGDA | Porcine chondrocytes | 3D extrusion-based bioprinting | Cartilage constructs | Porous internal structure; up to 0.7% shear strain; 60% uniaxial compressive strain; suitable rheological, mechanical; tailorable degradation rate up to 28 days; 95% cell viability at day 14; neocartilage formation; collagen type II and aggrecan expression |
| SF, GMA | Mouse preosteoblasts | 3D DLP bioprinting | Scaffold | In 15% SF-MA scaffolds: higher cell proliferation; better-defined morphology in progressively increased calcium deposition |
| SF, GMA, HAMA | Rat bone marrow mesenchymal stem cells, TGF-β1, E7 peptide | UV photocross-linking | Scaffold | Sequentially controlled release of bioactive molecules; synergistically induced cell recruitment and chondrogenic differentiation 12 weeks postop |
| SF, GelMA, GMA | Rat bone marrow mesenchymal stem cells | 3D extrusion bioprinting | Hydrogel and scaffold | Shape fidelity during bioprinting; tunable mechanical properties; biocompatibility, cell viability, proliferation, and morphology after 21 days; 60–70% degradation ratios after 60 days of immersion |
2. A Summarization of Recent Methacrylated SF-Based Approaches in Skin Tissue Engineering.
| Biomaterials | Seeded Cells | Fabrication Method | Construct Type | Results |
|---|---|---|---|---|
| SF, GMA, methacrylated chitosan | Mouse fibroblasts | UV photocross-linking | Hydrogel | Tannic acid-reinforced hydrogels: 5-fold mechanical performance increase; up to 51 kPa adhesiveness; antioxidative properties; antimicrobial properties; promoted wound healing up to day 14 in mice skin defects |
| SF, GMA, GelMA | Human adipose-derived stem cells, platelet-rich plasma | UV photocross-linking | Hydrogel | Increased rheological and mechanical properties (G’ almost 1 order of magnitude higher than G”); cell proliferation and migration up to day 14 |
| SF, GMA | Prussian blue nanozymes, VEGF, Polymyxin | Differential hydrogel loading into mold | Microneedle patch | Biocompatibility with internal organs (confirmed with HE staining); drug-sustained release up to 8 days; antioxidant, pro-angiogenesis, antibacterial properties |
| SF, GMA, Gel-GMA | Human keratinocytes, mouse fibroblasts, human umbilical vascular endothelial cells | 3D DLP bioprinting | Skin model | Higher storage modulus of Gel-GMA and elasticity of SF-GMA; higher wound healing rate and cell proliferation at appropriate skin layers up to day 14 |
3. A Summarization of Recent Methacrylated SF-Based Approaches in Nerve Tissue Engineering.
| Biomaterials | Seeded Cells | Fabrication Method | Construct Type | Results |
|---|---|---|---|---|
| SF, MA | Human neuroblastoma cells | UV photocross-linking, film casting | Scaffold | Increased mechanical stiffness, water stability, rigidity (to 480 kPa), contact angle (to 70.8°); cell differentiation and adhesion for 12 days |
| SF, GMA, GO | Mouse neuroblast cells | 3D DLP bioprinting | Hydrogel | Improved hydrogel mechanical, electroconductive (up to 6.5 S/m), neurogenic (expression of neuronal proteins) properties; cell proliferation, viability for 5 days |
| SF, MA | Rat Schwann cells | Electrospun fiber films, UV photocross-linking | Scaffold | Oriented axonal growth, Schwann cell myelination, motor function recovery (using sciatic nerve function index) 12 weeks postop |
| SF, GMA | Rat PC12 cells | UV photocross-linking | Hydrogel | Promoted cell growth, function, and regeneration; inhibited inflammatory reaction and oxidative stress 7 days after spinal cord injury |
| SF, GMA, LM-AC | Rat neural stem cells | UV photocross-linking | Hydrogel | In situ cell adhesion, cell growth, neural regeneration; repair of complete transection spinal cord injury after 7 days |
| SF, GMA, BP, GA | Mouse macrophages, human neuroblastoma cells | UV photocross-linking | Hydrogel | Cell differentiation and proliferation; inhibited inflammation, promoted conductivity (up to 0.4 S/m) |
5.1. Bone and Cartilage Tissue Engineering
In 2020, Hong et al. created a SF-based composite hydrogel using the methacrylated SF approach. Specifically, they synthesized a human or rabbit-derived chondrocyte-laden SF-GMA bioink hydrogel via 3D digital light processing (DLP) bioprinting. The results showed cell viability, proliferation, and chondrogenic differentiation, and Hong also demonstrated an in vivo application by successfully transplanting the hydrogel into a partially defective rabbit trachea.
Building off of the 3D DLP method, a 2020 study by the Kim group expanded upon the work by Hong et al. to demonstrate the successful 4D DLP bioprinting technique of the earlier SF-GMA bioink. Kim et al. printed a trachea mimetic tissue using human turbinate-derived stem cells and human chondrocytes to target the tracheal mucous ring and hyaline cartilage ring, respectively. After 8 weeks of implantation into a rabbit trachea, they observed integration with the native tissues and the formation of both epithelium and cartilage at predicted sites.
Expanding upon their 2020 experiment, this new study involved the implantation of a DLP-printed SF hydrogel into rabbit. This experiment tested the advantages of both 3D and 4D bioprinting, instead of solely relying on 4D like in their 2020 experiment. The 2021 report offered further information on fine-tuning the bioink’s physicochemical properties, as well as combining it with other novel compounds such as graphene oxide. The positive results of the studies by Hong et al. and Kim et al. suggest the potential to use SF-based constructs for further in vivo cartilage tissue engineering studies.
A different bioprinting application was performed by Bandyopadhyay et al. in 2021. This study used porcine cells instead of rabbit cells, which was used in the previous studies. Building on previous methacrylated SF approaches, Bandyopadhyay’s group mixed porcine chondrocytes with a SF-GMA and polyethylene glycol diacrylate (PEGDA) solution, showcasing a different hybrid bioink compared to the aforementioned experiments. This bioink was used to create cartilage constructs through 3D extrusion-based bioprinting. These constructs had a porous internal structure, rheological, mechanical, and degradation properties befitting of cartilage regeneration. Like Hong’s and Kim’s groups, Bandyopadhyay’s group also observed cytocompatibility, neocartilage formation, and collagen type II and aggrecan expression in this experiment, all of which indicated promise for cartilage tissue repair and regeneration. The next step for the SF-GMA-PEGDA composite should consider in vivo implantation into relevant animal models.
In 2022, Rajput’s group synthesized and 3D DLP bioprinted a photocurable SF-GMA hydrogel that showed bone tissue-like viscoelastic behavior using yet a different species of cells, namely mice. In contrast with cartilage tissue engineering applications using chondrocytes, Rajput encapsulated mouse pre-osteoblasts in the hydrogels with high cell viability, proliferation, morphology, and cytoskeleton organization. Rajput et al. also observed increased calcium deposition, indicating the hydrogel’s ability to drive osteogenesis. In addition to osteoblasts, researchers may seek to incorporate other bone cell types including osteoclasts and bone lining cells.
More recently, Mao et al. developed a novel SF-based strategy with the controlled sequential release of bioactive molecules to promote in situ cartilage regeneration in 2023. Mao et al. used a different hybrid bioink than previously reported: a SF-based scaffold with SF-GMA and HAMA coatings created directly through UV photo-cross-linking of the SF-based solutions. Transforming growth factor-β1 (TGF-β1) was loaded into the SF scaffold and bone marrow mesenchymal stem cells (BMSCs)-specific-affinity peptide (E7) in the SF-GMA and HAMA coatings. Both were successfully released in a sequential and controlled fashion, synergistically inducing the recruitment and chondrogenic differentiation of rat BMSCs. This demonstrates yet another species–in addition to rabbits, mice, and pigs–which is compatible with methacrylation technology. Mao et al. also successfully implanted the scaffold into the defected knee joint of a rabbit and observed in situ cartilage regeneration. This technique using the sequentially controlled release shows promise for a more refined approach to cartilage regeneration in clinical treatments. The cross-species implantation of rat BMSCs into rabbits is indeed a notable model; it may be interesting to explore the integration of animal methacrylated SF models into human platforms as a next step toward eventual clinical tissue engineering applications.
A 2023 experiment done by Yang et al. proposed a hybrid SF-GMA-GelMA bioink for in vivo applications in rats. In this experiment, Yang’s group used synthesized hybrid bioinks with different degrees of methacrylation and then created scaffolds of varying shapes through extrusion bioprinting to assess rheology. For in vitro evaluation, rats were executed and subcutaneously implanted with the hydrogels cultured with rat BMSCs. Yang et al. observed optimal printability and biocompatibility results from the bioink with 5% GelMA and 10% 242 mM SF-GMA, confirming the effect of methacrylation on hydrogel properties. These results are promising for further experimentation with blending methacrylate agents for refining hydrogel properties.
5.2. Skin Tissue Engineering
In 2020, He et al. synthesized a tannic acid-reinforced blend hydrogel composed of methacrylated chitosan and SF-GMA. Like the previously mentioned study by Yang et al., this experiment incorporated two different methacrylate agents for improved properties. Specifically, the use of tannic acid was shown to enhance the hydrogels’ mechanical performance, adhesiveness, and antioxidative properties. Additionally, it conferred hydrogels with antimicrobial properties against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) as well as cytocompatibility with mouse fibroblasts. Notably, these properties enabled the hydrogels to promote wound healing in rats’ infected skin defects, which has broad implications for the use of tannic acid in SF approaches for in vivo skin tissue engineering.
One 2021 study by Lu et al. demonstrated a SF-GMA-GelMA composite loaded with adipose-derived stem cells (ADSCs) and platelet-rich plasma (PRP) for pressure ulcers on the skin. This 2021 study’s incorporation of GelMA with GMA is unique in the interaction of two methacrylate agents instead of one, which was commonly seen in previous studies. This group observed optimal properties at a 1:1 SF-GMA to GelMA ratio, specifically with respect to rheological, mechanical, and biological properties. Notably, cell proliferation and migration of ADSCs were validated, which is particularly relevant to tissue repair in wound healing. To build upon Lu’s work, future studies may utilize this platform for broader wound healing applications than pressure ulcers, including diabetic wounds, infected wounds, or burn wounds. Outside of wound healing, future research may also seek to apply the regenerative properties of ADSCs with SF-GMA-GelMA to bone, cardiac, and other degenerative tissue systems.
An interesting study conducted by Guan et al. in 2022 proposed a multifunctional SF-GMA microneedle patch using a mouse model to specifically promote the healing of diabetic wounds, which are common and difficult-to-treat complications in diabetic patients. Guan’s group produced their patch by differentially loading two hydrogels into a mold. The patch was encapsulated with Prussian blue nanozymes, VEGF, and Polymyxin, and possessed biocompatibility, drug-sustained release, antioxidant, pro-angiogenesis, and antibacterial properties. These results show the promise of a new therapeutic approach to improve diabetic wound healing through a new platform using a microneedle patch.
In 2023, Choi et al. demonstrated a comprehensive approach of seeding multiple cell types onto a SF-GMA with gelatin (Gel-GMA) 3D DLP bioprinting model. Specifically, the group utilized keratinocytes, fibroblasts, and vascular endothelial cells to constitute the epidermis and dermis. Choi et al. reported the bioprinted skin model with varied thicknesses to exhibit strong mechanical properties and cell proliferation at the appropriate skin layers. One next step for researchers may be to apply the SF-GMA and Gel-GMA composite platform onto the hypodermis layer along with this layer’s characteristic cells of adipocytes. The incorporation of adipocytes may combine the setup by Lu’s group as discussed earlier.
5.3. Nerve Tissue Engineering
Moysenovich et al. demonstrated cytocompatibility of a uniquely designed SF-MA scaffold with human neuroblastoma cells (SH-SY5Y) in a 2020 study. Specifically, Moysenovich’s design utilized the platform’s UV photo-cross-linking properties to increase rigidity to 480 kPa and contact angle to 70.8°, among other properties. Following cell growth, spontaneous phosphorylation of Src and Akt protein kinases was notably observed. Together, the validation of neuronal differentiation and neural cell adhesion confirms this geometry for neural tissue engineering applications and encourages future studies to further experiment with different scaffold mechanics. Potential areas to consider alongside rigidity and angle include scaffold porosity, fiber directionality, and surface topography.
Also in 2020, Ajiteru et al. presented a composite hydrogel for 3D DLP bioprinting with superior mechanical, electroconductive, and neurogenic properties than pure SF. This composite hydrogel was fabricated through a covalent reduction of graphene oxide (GO)a monomolecular layer of graphite that has oxygen-containing functionalitiesby SF-GMA. Previously, combining reduced GO with biomaterials was limited by its hydrophobicity and chemical stability. However, Ajiteru’s group demonstrated the successful incorporation of reduced GO with SF and observed mouse neuroblast proliferation and viability in addition to the previously mentioned properties, showing the promise of using a composite methacrylated SF and GO copolymer biomaterial for neural tissue engineering. In particular, the electroconductive properties exhibited in Ajiteru’s studies are relevant to neural tissue engineering approaches. Biomaterials in addition to GO may be investigated to confer increased electroconductivity to methacrylated SF.
In 2022, Chen et al. developed a SF-MA scaffold with Arg-Gly-Asp signals. Oriented axonal growth and Schwann cell myelination were observed, as well as in vivo motor function recovery in a rat sciatic model. The incorporation of peptides is an interesting approach for increased cell growth that is worth applying in other periphery nerves such as the arm or facial nerves, as well as motor and sensory nerves beyond the peripheral nerve.
Zhou et al. conducted their 2022 study synthesizing a SF-GMA hydrogel treated with basic fibroblast growth factor (bFGF) for neuronal cell recovery. Zhou’s UV photo-cross-linked hydrogel promoted nerve axon regeneration, inhibited glial cell proliferation, and improved neuronal mitochondrial function in rats. Upon inducing spinal cord injury, the PC12 cell-laden hydrogel was observed to inhibit inflammatory reaction and oxidative stress. Together, these results demonstrate the effect of SF-GMA treated with bFGF for improving nerve function repair. Further studies may seek to expand upon bFGF with other growth factors to induce cell recovery in the methacrylated SF platform.
In 2023, Liu et al. fabricated their UV photo-cross-linked hydrogel composed of a novel GMA-SF and laminin-acrylate (LM-AC) composite. The incorporation of LM-AC was particularly interesting as a glycoprotein that interacts with SF-GMA’s hydrogel network. However, Liu did not observe statistical differences between the SF-GMA and SF-GMA/LM-AC’s mechanical properties, cell adhesion, or cell proliferation. Perhaps other glycoproteins may be worth investigating to better interact with methacrylated SF’s hydrogel network. Nevertheless, Liu’s group showed in situ adhesion between the hydrogel and two transected stumps of a rat spinal cord, even under spinal cord stretching. This network provided a favorable microenvironment for neural stem cell growth; notably, Liu et al. observed promoted neural regeneration in the repair of complete transection SCI over 8 weeks, which led to hind limb locomotion recovery. In addition to the spinal cord, researchers should expand this protocol to nerves and brain tissues.
Most recently in 2024, Zhang et al. integrated black phosphorus (BP) and glycyrrhizic acid (GA) with SF-GMA for an injectable UV photo-cross-linked hydrogel to promote neuronal regeneration after spinal cord injury. Using mouse macrophages and human SH-SY5Y cells, this study validated neural cell differentiation and proliferation, confirming a repairing effect. Among other findings, Zhang’s group demonstrated inhibited inflammation and promoted conductivity, which accelerated the recovery process of the injury site in an in vivo mouse model. Moving forward, the finding on inflammation in particular is worth exploring in other potential areas for neuritis around the body such as peripheral tissues, where a consideration of Chen’s aforementioned work will be noteworthy as well.
6. Current Limitations and Future Directions
Current studies have attempted a narrow range of methacrylate agents, methacrylation degrees, and biomaterial compounds in addition to methacrylated SF.
It is worth considering alternative methacrylate agents. Methyl methacrylate (MMA) may be a promising choice but has been poorly studied with only one recent study in 2020 synthesizing poly(methyl methacrylate) (PMMA) and SF composite mats seeded with human dental stem cells. PMMA is traditionally used for bone tissue engineering for its strength, however, it lacks bioactivity, biointegration, and biodegradability. Because these biological drawbacks are precisely countered by silk fibroin, future studies may consider more diverse cell types to expand the scope of PMMA-SF to other tissue types. Another potential methacrylate agent is 2-isocyanatoethyl methacrylate (IEM). However, no recent studies have investigated this approach for larger-scale tissue engineering applications beyond the hydrogel scale. The study by Kim et al. in 2018 synthesizing silk fibroin with IEM may be a helpful platform to expand upon.
Another area for improvement in future work is incorporating more diverse blends of biomaterial compounds–whether natural, synthetic, or hybrid. Many current approaches use a simple blend of silk fibroin and methacrylate agent. To better target tissue-specific demands for ultimate translational applications, future studies should seek additional compounds: nonbiological compounds (e.g., polymers, bioceramics, metals, and composites) that allow the patient’s own cells to promote bone regeneration for bone tissue engineering as well as conductive biomaterials (e.g., conductive polymers, carbon nanomaterials, and conductive inorganic nanomaterials) to facilitate wound healing for skin tissue engineering and to promote electrical conductivity for nerve tissue engineering.
Moreover, current research utilizing methacrylated SF is limited to a small handful of tissue types, namely the bone and cartilage, skin, and nerve. Moving forward, experiments with methacrylated SF in more tissue types such as the cardiac and muscular systems will provide key insight for advances on a more comprehensive scale. To achieve this, modifying the methacrylated SF platform with tissue-specific components to assimilate conditions of these tissue types should also be explored.
7. Conclusion
Despite these limitations, methacrylated SF is only a recently emerging field in the past decade, with more potential to be fine-tuned by researchers specializing in different tissue engineering niches. Overall, this platform represents a promising direction for tissue engineering purposes due to its biocompatibility and structural integrity. By modifying SF with a methacrylate agent such as GMA, MA, or Gel-MA, researchers have been able to improve its integrability for in vitro and in vivo studies. Other studies have incorporated other biomaterials and produced a range of tissue engineering constructs, ranging from hydrogels, fibers, sponges, and films, to other large-scale scaffolds. Researchers have characterized diverse approaches in improving the methacrylated SF-based constructs’ applicability, demonstrating the implications of methacrylated SF for tissue engineering purposes. Altogether, further studies may seek to incorporate a combination of methacrylation and tissue engineering approaches, with the ultimate goal of biomedical innovation and clinical translation in the field of healthcare.
The authors declare no competing financial interest.
References
- Howard D., Buttery L. D., Shakesheff K. M., Roberts S. J.. Tissue engineering: strategies, stem cells and scaffolds. J. Anat. 2008;213(1):66–72. doi: 10.1111/j.1469-7580.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah S., Chen X.. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Applied Materials Today. 2020;20:100656. doi: 10.1016/j.apmt.2020.100656. [DOI] [Google Scholar]
- Amini S., Salehi H., Setayeshmehr M., Ghorbani M.. Natural and synthetic polymeric scaffolds used in peripheral nerve tissue engineering: Advantages and disadvantages. Polym. Adv. Technol. 2021;32(6):2267–2289. doi: 10.1002/pat.5263. [DOI] [Google Scholar]
- Asadi N., Del Bakhshayesh A. R., Davaran S., Akbarzadeh A.. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020;242:122528. doi: 10.1016/j.matchemphys.2019.122528. [DOI] [Google Scholar]
- Bucciarelli A., Motta A.. Use of Bombyx mori silk fibroin in tissue engineering: From cocoons to medical devices, challenges, and future perspectives. Biomaterials Advances. 2022;139:212982. doi: 10.1016/j.bioadv.2022.212982. [DOI] [PubMed] [Google Scholar]
- Sun, W. ; Gregory, D. A. ; Tomeh, M. A. ; Zhao, X. . Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22 (3), 1499 10.3390/ijms22031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangi A., Jajpura L.. The biopolymer sericin: Extraction and applications. J. Text Sci. Eng. 2015;5(1):1–5. doi: 10.4172/2165-8064.1000188. [DOI] [Google Scholar]
- Wang L., Luo Z., Zhang Q., Guan Y., Cai J., You R., Li X.. Effect of degumming methods on the degradation behavior of silk fibroin biomaterials. Fibers Polym. 2019;20:45–50. doi: 10.1007/s12221-019-8658-9. [DOI] [Google Scholar]
- Zafar M. S., Belton D. J., Hanby B., Kaplan D. L., Perry C. C.. Functional material features of Bombyx mori silk light versus heavy chain proteins. Biomacromolecules. 2015;16(2):606–614. doi: 10.1021/bm501667j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C. Z., Confalonieri F., Medina N., Zivanovic Y., Esnault C., Yang T., Jacquet M., Janin J., Duguet M., Perasso R.. et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000;28(12):2413–2419. doi: 10.1093/nar/28.12.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S., Dhyani V., Kaur T., Singh N.. Spatiotemporal Control over Cell Proliferation and Differentiation for Tissue Engineering and Regenerative Medicine Applications Using Silk Fibroin Scaffolds. ACS Appl. Bio Mater. 2020;3(6):3476–3493. doi: 10.1021/acsabm.0c00305. [DOI] [PubMed] [Google Scholar]
- Zhou C. Z., Confalonieri F., Jacquet M., Perasso R., Li Z. G., Janin J.. Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins. 2001;44(2):119–122. doi: 10.1002/prot.1078. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Kikuchi Y., Takagi T., Kikuchi A., Oyama F., Shimura K., Mizuno S.. Primary structure of the silk fibroin light chain determined by cDNA sequencing and peptide analysis. J. Mol. Biol. 1989;210(1):127–139. doi: 10.1016/0022-2836(89)90295-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Inoue S., Mizuno S.. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the HL complex of silk fibroin produced by Bombyx mori. Insect biochemistry and molecular biology. 1999;29(3):269–276. doi: 10.1016/S0965-1748(98)00135-0. [DOI] [PubMed] [Google Scholar]
- Asakura, T. Structure of Silk I (Molecules 2021, 26 (12), 3706 10.3390/molecules26123706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Y. ; Wang, H. ; Wei, K. ; Yang, Y. ; Zheng, R. Y. ; Kim, I. S. ; Zhang, K. Q. . A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18 (3), 237 10.3390/ijms18030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebe P., Partlow B. P., Kaplan D. L., Wurm A., Zhuravlev E., Schick C.. Silk I and Silk II studied by fast scanning calorimetry. Acta Biomater. 2017;55:323–332. doi: 10.1016/j.actbio.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Valluzzi R., Gido S. P., Muller W., Kaplan D. L.. Orientation of silk III at the air-water interface. Int. J. Biol. Macromol. 1999;24(2–3):237–242. doi: 10.1016/S0141-8130(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Vepari C., Kaplan D. L.. Silk as a biomaterial. Prog. Polym. Sci. 2007;32(8–9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademolqorani S., Tavanai H., Chronakis I. S., Boisen A., Ajalloueian F.. The determinant role of fabrication technique in final characteristics of scaffolds for tissue engineering applications: A focus on silk fibroin-based scaffolds. Materials Science and Engineering: C. 2021;122:111867. doi: 10.1016/j.msec.2021.111867. [DOI] [PubMed] [Google Scholar]
- Holland C., Numata K., Rnjak-Kovacina J., Seib F. P.. The biomedical use of silk: past, present, future. Adv. Healthcare Mater. 2019;8(1):1800465. doi: 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
- Cao Y., Wang B.. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009;10(4):1514–1524. doi: 10.3390/ijms10041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizabal A., Costa C. M., Saiz P. G., Gonzalez B., Pérez-Álvarez L., Fernández de Luis R., Garcia A., Vilas-Vilela J. L., Lanceros-Méndez S.. Processing Strategies to Obtain Highly Porous Silk Fibroin Structures with Tailored Microstructure and Molecular Characteristics and Their Applicability in Water Remediation. J. Hazard Mater. 2021;403:123675. doi: 10.1016/j.jhazmat.2020.123675. [DOI] [PubMed] [Google Scholar]
- Wöltje, M. ; Kölbel, A. ; Aibibu, D. ; Cherif, C. . A Fast and Reliable Process to Fabricate Regenerated Silk Fibroin Solution from Degummed Silk in 4 h. Int. J. Mol. Sci. 2021, 22 (19), 10565 10.3390/ijms221910565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johari N., Moroni L., Samadikuchaksaraei A.. Tuning the conformation and mechanical properties of silk fibroin hydrogels. Eur. Polym. J. 2020;134:109842. doi: 10.1016/j.eurpolymj.2020.109842. [DOI] [Google Scholar]
- Wang H.-Y., Zhang Y.-Q., Wei Z.-G.. Characterization of undegraded and degraded silk fibroin and its significant impact on the properties of the resulting silk biomaterials. Int. J. Biol. Macromol. 2021;176:578–588. doi: 10.1016/j.ijbiomac.2021.02.100. [DOI] [PubMed] [Google Scholar]
- Santi S., Mancini I., Dirè S., Callone E., Speranza G., Pugno N., Migliaresi C., Motta A.. A bio-inspired multifunctionalized silk fibroin. ACS Biomaterials Science & Engineering. 2021;7(2):507–516. doi: 10.1021/acsbiomaterials.0c01567. [DOI] [PubMed] [Google Scholar]
- Lu Q., Zhang B., Li M., Zuo B., Kaplan D. L., Huang Y., Zhu H.. Degradation mechanism and control of silk fibroin. Biomacromolecules. 2011;12(4):1080–1086. doi: 10.1021/bm101422j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Yeon Y. K., Lee J. M., Chao J. R., Lee Y. J., Seo Y. B., Sultan M. T., Lee O. J., Lee J. S., Yoon S. I.. et al. Publisher Correction: Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018;9(1):2350. doi: 10.1038/s41467-018-04517-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H., Lee O. J., Lee Y. J., Lee J. S., Ajiteru O., Lee H., Suh Y. J., Sultan M. T., Kim S. H., Park C. H.. Cytocompatibility of modified silk fibroin with glycidyl methacrylate for tissue engineering and biomedical applications. Biomolecules. 2021;11(1):35. doi: 10.3390/biom11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel M., Baumann K., Groll J., Gbureck U.. Simultaneous structuring and mineralization of silk fibroin scaffolds. J. Tissue Eng. 2018;9:2041731418788509. doi: 10.1177/2041731418788509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Lei J., Chen M., Sun Y., Jianwen H., Li S., Gang L., Zhang M., Yixin S., Zhang F.. et al. Synthesis and Characterization of Photo-Cross-Linkable Silk Fibroin Methacryloyl Hydrogel for Biomedical Applications. ACS Omega. 2023;8(34):30888–30897. doi: 10.1021/acsomega.3c01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Hong H., Ajiteru O., Sultan M. T., Lee Y. J., Lee J. S., Lee O. J., Lee H., Park H. S., Choi K. Y.. et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat. Protoc. 2021;16(12):5484–5532. doi: 10.1038/s41596-021-00622-1. [DOI] [PubMed] [Google Scholar]
- Bucciarelli A., Muthukumar T., Kim J. S., Kim W. K., Quaranta A., Maniglio D., Khang G., Motta A.. Preparation and Statistical Characterization of Tunable Porous Sponge Scaffolds using UV Cross-linking of Methacrylate-Modified Silk Fibroin. ACS Biomater Sci. Eng. 2019;5(12):6374–6388. doi: 10.1021/acsbiomaterials.9b00814. [DOI] [PubMed] [Google Scholar]
- Bae S. B., Kim M. H., Park W. H.. Electrospinning and dual crosslinking of water-soluble silk fibroin modified with glycidyl methacrylate. Polym. Degrad. Stab. 2020;179:109304. doi: 10.1016/j.polymdegradstab.2020.109304. [DOI] [Google Scholar]
- Yue K., Trujillo-de Santiago G., Alvarez M. M., Tamayol A., Annabi N., Khademhosseini A.. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessonov I. V., Rochev Y. A., Arkhipova A. Y., Kopitsyna M. N., Bagrov D. V., Karpushkin E. A., Bibikova T. N., Moysenovich A. M., Soldatenko A. S., Nikishin I. I.. Fabrication of hydrogel scaffolds via photocrosslinking of methacrylated silk fibroin. Biomedical Materials. 2019;14(3):034102. doi: 10.1088/1748-605X/ab04e0. [DOI] [PubMed] [Google Scholar]
- Lee B. H., Shirahama H., Cho N.-J., Tan L. P.. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Adv. 2015;5(128):106094–106097. doi: 10.1039/C5RA22028A. [DOI] [Google Scholar]
- Bupphathong, S. ; Quiroz, C. ; Huang, W. ; Chung, P. F. ; Tao, H. Y. ; Lin, C. H. . Gelatin Methacrylate Hydrogel for Tissue Engineering Applications-A Review on Material Modifications. Pharmaceuticals (Basel) 2022, 15 (2), 171 10.3390/ph15020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Wang Y., Ferracci G., Zheng J., Cho N. J., Lee B. H.. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019;9(1):6863. doi: 10.1038/s41598-019-42186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahama H., Lee B. H., Tan L. P., Cho N. J.. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016;6:31036. doi: 10.1038/srep31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., He J., Nichol J. W., Wang L., Hutson C. B., Wang B., Du Y., Fan H., Khademhosseini A.. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta biomaterialia. 2011;7(6):2384–2393. doi: 10.1016/j.actbio.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Huang C., Liu H., Han X., Wang Z., Huang J., Yan Y., Wang Z.. A Silk Fibroin Methacryloyl-Modified Hydrogel Promoting Cell Adhesion for Customized 3D Cell-Laden Structures. ACS Applied Polymer Materials. 2022;4(10):7014–7024. doi: 10.1021/acsapm.2c00952. [DOI] [Google Scholar]
- Shi X., Wang X., Shen W., Yue W.. Biocompatibility of silk methacrylate/gelatin-methacryloyl composite hydrogel and its feasibility as a vascular tissue engineering scaffold. Biochem. Biophys. Res. Commun. 2023;650:62–72. doi: 10.1016/j.bbrc.2023.01.097. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim J., Choi J., Park Y., Ki C.. Characterization of silk hydrogel formed with hydrolyzed silk fibroin-methacrylate via photopolymerization. Polymer. 2018;153:232–240. doi: 10.1016/j.polymer.2018.08.019. [DOI] [Google Scholar]
- Yang J., Li Z., Li S., Zhang Q., Zhou X., He C.. Tunable metacrylated silk fibroin-based hybrid bioinks for the bioprinting of tissue engineering scaffolds. Biomaterials Science. 2023;11(5):1895–1909. doi: 10.1039/D2BM01978G. [DOI] [PubMed] [Google Scholar]
- He X., Liu X., Yang J., Du H., Chai N., Sha Z., Geng M., Zhou X., He C.. Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing. Carbohydr. Polym. 2020;247:116689. doi: 10.1016/j.carbpol.2020.116689. [DOI] [PubMed] [Google Scholar]
- Ling S., Qi Z., Knight D. P., Shao Z., Chen X.. FTIR imaging, a useful method for studying the compatibility of silk fibroin-based polymer blends. Polym. Chem. 2013;4(21):5401–5406. doi: 10.1039/c3py00508a. [DOI] [Google Scholar]
- Farasatkia A., Kharaziha M., Ashrafizadeh F., Salehi S.. Transparent silk/gelatin methacrylate (GelMA) fibrillar film for corneal regeneration. Materials Science and Engineering: C. 2021;120:111744. doi: 10.1016/j.msec.2020.111744. [DOI] [PubMed] [Google Scholar]
- Atila D., Hasirci V., Tezcaner A.. Coaxial electrospinning of composite mats comprised of core/shell poly(methyl methacrylate)/silk fibroin fibers for tissue engineering applications. J. Mech Behav Biomed Mater. 2022;128:105105. doi: 10.1016/j.jmbbm.2022.105105. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Lin R. Z., Qi H., Yang Y., Bae H., Melero-Martin J. M., Khademhosseini A.. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct Mater. 2012;22(10):2027–2039. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritani S., Kaneko J., Ito D., Morito M., Ishizawa T., Akamatsu N., Tanaka M., Iida T., Tanaka T., Tanaka R.. Silk fibroin vascular graft: a promising tissue-engineered scaffold material for abdominal venous system replacement. Sci. Rep. 2020;10(1):21041. doi: 10.1038/s41598-020-78020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catto V., Farè S., Cattaneo I., Figliuzzi M., Alessandrino A., Freddi G., Remuzzi A., Tanzi M. C.. Small diameter electrospun silk fibroin vascular grafts: Mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Materials Science and Engineering: C. 2015;54:101–111. doi: 10.1016/j.msec.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Ghaznavi A. M., Kokai L. E., Lovett M. L., Kaplan D. L., Marra K. G.. Silk fibroin conduits: a cellular and functional assessment of peripheral nerve repair. Annals of plastic surgery. 2011;66(3):273–279. doi: 10.1097/SAP.0b013e3181e6cff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liang K., Zhao S., Zhang C., Li J., Yang H., Liu X., Yin X., Chen D., Xu W.. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018;108:383–390. doi: 10.1016/j.ijbiomac.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Xiao W., Li J., Qu X., Wang L., Tan Y., Li K., Li H., Yue X., Li B., Liao X.. Cell-laden interpenetrating network hydrogels formed from methacrylated gelatin and silk fibroin via a combination of sonication and photocrosslinking approaches. Materials Science and Engineering: C. 2019;99:57–67. doi: 10.1016/j.msec.2019.01.079. [DOI] [PubMed] [Google Scholar]
- Barroso I. A., Man K., Villapun V. M., Cox S. C., Ghag A. K.. Methacrylated silk fibroin hydrogels: pH as a tool to control functionality. ACS Biomaterials Science & Engineering. 2021;7(10):4779–4791. doi: 10.1021/acsbiomaterials.1c00791. [DOI] [PubMed] [Google Scholar]
- Farokhi M., Aleemardani M., Solouk A., Mirzadeh H., Teuschl A. H., Redl H.. Crosslinking strategies for silk fibroin hydrogels: promising biomedical materials. Biomed Mater. 2021;16(2):022004. doi: 10.1088/1748-605X/abb615. [DOI] [PubMed] [Google Scholar]
- Wei L., Wu S., Kuss M., Jiang X., Sun R., Reid P., Qin X., Duan B.. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioact Mater. 2019;4:256–260. doi: 10.1016/j.bioactmat.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Mo X., Huang C., He C., Wang H.. Electrospun scaffolds from silk fibroin and their cellular compatibility. J. Biomed Mater. Res. A. 2010;93(3):976–983. doi: 10.1002/jbm.a.32497. [DOI] [PubMed] [Google Scholar]
- Nazarov R., Jin H.-J., Kaplan D. L.. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- Sun W., Taylor C. S., Zhang Y., Gregory D. A., Tomeh M. A., Haycock J. W., Smith P. J., Wang F., Xia Q., Zhao X.. Cell guidance on peptide micropatterned silk fibroin scaffolds. J. Colloid Interface Sci. 2021;603:380–390. doi: 10.1016/j.jcis.2021.06.086. [DOI] [PubMed] [Google Scholar]
- Floren, M. ; Migliaresi, C. ; Motta, A. . Processing Techniques and Applications of Silk Hydrogels in Bioengineering. J. Funct Biomater 2016, 7 (3), 26 10.3390/jfb7030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutipakdeevong J., Ruktanonchai U. R., Supaphol P.. Process optimization of electrospun silk fibroin fiber mat for accelerated wound healing. J. Appl. Polym. Sci. 2013;130(5):3634–3644. doi: 10.1002/app.39611. [DOI] [Google Scholar]
- Tamada Y.. New process to form a silk fibroin porous 3-D structure. Biomacromolecules. 2005;6(6):3100–3106. doi: 10.1021/bm050431f. [DOI] [PubMed] [Google Scholar]
- Farasatkia A., Kharaziha M.. Robust and double-layer micro-patterned bioadhesive based on silk nanofibril/GelMA-alginate for stroma tissue engineering. Int. J. Biol. Macromol. 2021;183:1013–1025. doi: 10.1016/j.ijbiomac.2021.05.048. [DOI] [PubMed] [Google Scholar]
- Hong H., Seo Y. B., Kim D. Y., Lee J. S., Lee Y. J., Lee H., Ajiteru O., Sultan M. T., Lee O. J., Kim S. H.. et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Seo Y. B., Yeon Y. K., Lee Y. J., Park H. S., Sultan M. T., Lee J. M., Lee J. S., Lee O. J., Hong H.. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials. 2020;260:120281. doi: 10.1016/j.biomaterials.2020.120281. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A., Mandal B. B., Bhardwaj N.. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J. Biomed Mater. Res. A. 2022;110(4):884–898. doi: 10.1002/jbm.a.37336. [DOI] [PubMed] [Google Scholar]
- Rajput M., Mondal P., Yadav P., Chatterjee K.. Light-based 3D bioprinting of bone tissue scaffolds with tunable mechanical properties and architecture from photocurable silk fibroin. Int. J. Biol. Macromol. 2022;202:644–656. doi: 10.1016/j.ijbiomac.2022.01.081. [DOI] [PubMed] [Google Scholar]
- Mao Z., Bi X., Wu C., Zheng Y., Shu X., Wu S., Guan J., Ritchie R. O.. A Cell-Free Silk Fibroin Biomaterial Strategy Promotes In Situ Cartilage Regeneration Via Programmed Releases of Bioactive Molecules. Adv. Healthc Mater. 2023;12(1):e2201588. doi: 10.1002/adhm.202201588. [DOI] [PubMed] [Google Scholar]
- Lu K., Li K., Zhang M., Fang Z., Wu P., Feng L., Deng K., Yu C., Deng Y., Xiao Y.. Adipose-derived stem cells (ADSCs) and platelet-rich plasma (PRP) loaded gelatin/silk fibroin hydrogels for improving healing in a murine pressure ulcer model. Chemical Engineering Journal. 2021;424:130429. doi: 10.1016/j.cej.2021.130429. [DOI] [Google Scholar]
- Guan G., Zhang Q., Jiang Z., Liu J., Wan J., Jin P., Lv Q.. Multifunctional Silk Fibroin Methacryloyl Microneedle for Diabetic Wound Healing. Small. 2022;18(51):e2203064. doi: 10.1002/smll.202203064. [DOI] [PubMed] [Google Scholar]
- Choi K. Y., Ajiteru O., Hong H., Suh Y. J., Sultan M. T., Lee H., Lee J. S., Lee Y. J., Lee O. J., Kim S. H.. A digital light processing 3D-printed artificial skin model and full-thickness wound models using silk fibroin bioink. Acta Biomaterialia. 2023;164:159–174. doi: 10.1016/j.actbio.2023.04.034. [DOI] [PubMed] [Google Scholar]
- Lin D., Li M., Wang L., Cheng J., Yang Y., Wang H., Ye J., Liu Y.. Multifunctional hydrogel based on silk fibroin promotes tissue repair and regeneration. Adv. Funct. Mater. 2024;34(39):2405255. doi: 10.1002/adfm.202405255. [DOI] [Google Scholar]
- Moysenovich A. M., Tatarskiy V. V., Yastrebova M. A., Bessonov I. V., Arkhipova A. Y., Kolosov A. S., Khamidullina A. I., Bogush V. G., Debabov V. G., Shaitan K. V.. Akt and Src mediate the photocrosslinked fibroin-induced neural differentiation. Neuroreport. 2020;31(10):770–775. doi: 10.1097/WNR.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajiteru O., Sultan M. T., Lee Y. J., Seo Y. B., Hong H., Lee J. S., Lee H., Suh Y. J., Ju H. W., Lee O. J.. et al. A 3D Printable Electroconductive Biocomposite Bioink Based on Silk Fibroin-Conjugated Graphene Oxide. Nano Lett. 2020;20(9):6873–6883. doi: 10.1021/acs.nanolett.0c02986. [DOI] [PubMed] [Google Scholar]
- Chen X., Tang X., Wang Y., Gu X., Huang T., Yang Y., Ling J.. Silk-inspired fiber implant with multi-cues enhanced bionic microenvironment for promoting peripheral nerve repair. Biomaterials Advances. 2022;135:112674. doi: 10.1016/j.msec.2022.112674. [DOI] [PubMed] [Google Scholar]
- Zhou L., Wang Z., Chen D., Lin J., Li W., Guo S., Wu R., Zhao X., Lin T., Chen G.. An injectable and photocurable methacrylate-silk fibroin hydrogel loaded with bFGF for spinal cord regeneration. Materials & Design. 2022;217:110670. doi: 10.1016/j.matdes.2022.110670. [DOI] [Google Scholar]
- Liu Y., Zhang Z., Zhang Y., Luo B., Liu X., Cao Y., Pei R.. Construction of adhesive and bioactive silk fibroin hydrogel for treatment of spinal cord injury. Acta Biomaterialia. 2023;158:178–189. doi: 10.1016/j.actbio.2022.12.048. [DOI] [PubMed] [Google Scholar]
- Zhang B., Wang W., Gao P., Li X., Chen L., Lin Z., Chen H., Liang W., Kong Z., Lin D.. Injectable, Electroconductive, Free Radical Scavenging Silk Fibroin/Black Phosphorus/Glycyrrhizic Acid Nanocomposite Hydrogel for Enhancing Spinal Cord Repair. Adv. Healthcare Mater. 2024;13:2304300. doi: 10.1002/adhm.202304300. [DOI] [PubMed] [Google Scholar]
- Ray S. C.. Application and uses of graphene oxide and reduced graphene oxide. Applications of graphene and graphene-oxide based nanomaterials. 2015;6(8):39–55. doi: 10.1016/B978-0-323-37521-4.00002-9. [DOI] [Google Scholar]
- Atila D., Hasirci V., Tezcaner A.. Coaxial electrospinning of composite mats comprised of core/shell poly (methyl methacrylate)/silk fibroin fibers for tissue engineering applications. Journal of the Mechanical Behavior of Biomedical Materials. 2022;128:105105. doi: 10.1016/j.jmbbm.2022.105105. [DOI] [PubMed] [Google Scholar]
- Ramanathan S., Lin Y.-C., Thirumurugan S., Hu C.-C., Duann Y.-F., Chung R.-J.. Poly (methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers. 2024;16(3):367. doi: 10.3390/polym16030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koons G. L., Diba M., Mikos A. G.. Materials design for bone-tissue engineering. Nature Reviews Materials. 2020;5(8):584–603. doi: 10.1038/s41578-020-0204-2. [DOI] [Google Scholar]
- Yu R., Zhang H., Guo B.. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nano-micro letters. 2022;14:1–46. doi: 10.1007/s40820-021-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblado L. R., Martínez-Ramos C., Pradas M. M.. Biomaterials for neural tissue engineering. Frontiers in Nanotechnology. 2021;3:643507. doi: 10.3389/fnano.2021.643507. [DOI] [Google Scholar]