Abstract
It has been proposed that the functions of the cyclin-dependent kinase inhibitors p21Cip1/Waf1 and p27Kip1 are limited to cell cycle control at the G1/S-phase transition and in the maintenance of cellular quiescence. To test the validity of this hypothesis, p21 was expressed in a diverse panel of cell lines, thus isolating the effects of p21 activity from the pleiotropic effects of upstream signaling pathways that normally induce p21 expression. The data show that at physiological levels of accumulation, p21, in addition to its role in negatively regulating the G1/S transition, contributes to regulation of the G2/M transition. Both G1- and G2-arrested cells were observed in all cell types, with different preponderances. Preponderant G1 arrest in response to p21 expression correlated with the presence of functional pRb. G2 arrest was more prominent in pRb-negative cells. The arrest distribution did not correlate with the p53 status, and proliferating-cell nuclear antigen (PCNA) binding activity of p21 did not appear to be involved, since p27, which lacks a PCNA binding domain, produced similar arrest Bs. In addition, DNA endoreduplication occurred in pRb-negative but not in pRb-positive cells, suggesting that functional pRb is necessary to prevent DNA replication in p21 G2-arrested cells. These results suggest that the primary target of the Cip/Kip family of inhibitors leading to efficient G1 arrest as well as to blockade of DNA replication from either G1 or G2 phase is the pRb regulatory system. Finally, the tendency of Rb-negative cells to undergo endoreduplication cycles when p21 is expressed may have negative implications in the therapy of Rb-negative cancers with genotoxic agents that activate the p53/p21 pathway.
Progression through the cell cycle is mediated by a phylogenetically conserved family of protein kinases known as cyclin-dependent kinases (Cdks). Cdks are composed of a catalytic subunit and a requisite positive regulatory subunit termed a cyclin (56). The Cdk activities that govern cell cycle progression require coordination and regulation. In most cases, positive regulation is mediated at the level of cyclin accumulation (34, 47, 50). However, many aspects of cell cycle control require negative regulation of Cdks. Negative regulation of Cdk activity is achieved either by phosphorylation of the catalytic subunit or via the binding of Cdk inhibitory proteins known as CKIs (47). Increases in the levels of inhibitors which bind to cyclin-Cdk complexes render these complexes inactive. Two families of mammalian CKIs have been described: the INK4 inhibitors and the Cip/Kip inhibitors (for a review, see reference 65). INK4 inhibitors contain an ankyrin repeat motif and are specific for Cdk4 and Cdk6, the most divergent of the cell cycle-associated Cdks. There are four known members of the family: p15, p16, p18, and p19 (24, 26, 33, 65). Cip/Kip inhibitors target a broader spectrum of Cdks, including Cdk2, Cdk4 and Cdk6 and, possibly, Cdk1 (27). The family consists of three members: p21Cip1, p27Kip1, and p57Kip2 (65). All contain a conserved amino-terminal Cdk inhibitory domain of approximately 80 amino acids (39, 42, 73). The three-dimensional structure of the inhibitory domain of p27Kip1 bound to cyclin A-Cdk2 reveals a mechanism of inhibition where strong interactions between the inhibitor, the cyclin, and the Cdk allow deformation of and interference with the Cdk active site (62, 63).
CKIs have been implicated in negative regulation of the cell cycle by both internal and external signals (28, 65). Accumulation of p27 is associated with a quiescent or resting state in many cell types (30, 31, 57, 67). More significantly, p27-nullizygous mice exhibit hyperproliferative disorders consistent with an inability of a variety of cell types to cease proliferation on schedule (19, 37, 48). Treatment of cells with ionizing radiation is associated with p53-dependent accumulation of p21; p53 is a transcription factor mobilized by DNA damage that ultimately mediates a variety of cellular responses including cell cycle arrest (14, 16). Cells from p21-nullizygous mice are at least partially defective in G1 arrest in response to ionizing radiation (4, 11). p21 can also be induced by other stimuli, such as cytokines and cell adhesion events, and during cell growth and differentiation under both p53-dependent and p53-independent conditions (7, 10, 18, 25, 38, 41, 46, 68, 78, 79). Thus, Cip/Kip inhibitors appear to be effectors of cell cycle arrest in a wide variety of signaling contexts.
For the most part, the inferred target of CKI-mediated control has been the G1/S-phase transition. These conclusions have been based on ectopic expression of CKIs in transient transfections (27, 58, 73) and observations of cells from nullizygous mice. For example, transient transfection of p21 in human diploid fibroblasts led to an accumulation of G1 cells (27). Furthermore, whereas p21-nullizygous mouse embryo fibroblasts (MEFs) were partially defective in their G1 arrest response to ionizing radiation, they were apparently normal in their G2 arrest response (11). Nevertheless, both these experimental avenues have some limitations. Transient-transfection experiments lead to variable, uneven expression and do not permit follow-up over several cell cycles. Subjecting cells to ionizing radiation is likely to produce a number of responses in addition to induction of p21, thus, complicating the analysis of the specific role of p21.
In a different experimental approach, ectopic constitutive expression of a p21 transgene targeted to the mouse liver was reported to lead to stunted liver development (77). However, a direct evaluation of the cell cycle arrest distribution was not possible in that case.
To circumvent these problems, we sought to conditionally express CKIs at close to physiological levels in a variety of different transformed and nontransformed cell types. Specifically, p21 was placed under control of a tetracycline-repressible promoter in several cell lines. Additionally, recombinant p21- and p27-expressing adenoviruses were used to extend the study. These methods of conditional ectopic expression at approximately physiological levels allowed the determination of the effects of p21 and, to a lesser extent, p27 in a controlled experimental environment. Specifically, the cell cycle effects produced by these molecules could be evaluated largely removed from the potential noise generated by activation of pleiotropic upstream signaling pathways. Previous reports using similar approaches have shown that under these conditions cells ceased proliferation. However, a detailed analysis of cell cycle distribution was not reported, nor was the relationship to Rb status investigated (61, 74).
Our data suggest that in addition to the previously inferred role of p21 at the G1/S-phase transition, p21 (and p27) also has the capacity to arrest cells in G2. Our data also implicate the retinoblastoma protein, pRb, as an important factor in the cellular response to p21, particularly in the context of regulation of DNA replication.
MATERIALS AND METHODS
Plasmids and adenovirus constructs.
The human p21Cip1/Waf1 cDNA containing the full-length coding region of p21 was isolated by PCR, cloned into the pUHD10-3 plasmid (H. Bujard, Heidelberg, Germany) to allow tetracycline-regulated conditional expression, and verified by sequencing. The pBabe plasmid containing a puromycin resistance gene was used for cotransfection with the p21-containing plasmid. Adenovirus type 5 constructs with E1A/E1B deficiency, expressing p21 and p27 from a cytomegalovirus promoter, and empty control adenovirus were a gift from J. DeGregori and J. Nevins, Duke University, Durham, N.C., and J. Cogswell, Glaxo Wellcome.
Cell lines: growth conditions, transfection and selection procedures, adenovirus infection, and gamma irradiation procedure.
A549 human lung carcinoma cells and Kb human epidermoid carcinoma cells (Duncan Walker, Glaxo Wellcome), WI38 human diploid fibroblasts (American Type Culture Collection), and HaCat cells (N. Fusenig, Heidelberg, Germany) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a 37°C incubator with 5% CO2.
The human cervical carcinoma cell line HeLa tTA (H. Bujard, Heidelberg, Germany), the human osteosarcoma cell line Saos2 tTA (6), the human colon carcinoma cell line RKO tTA (Duncan Walker, Glaxo Wellcome), the human lung cancer cell line H1299 tTA (6), and the rat fibroblast cell line Rat1 tTA (60) were grown in DMEM–10% FBS in a 37°C incubator with 5% CO2. The medium was supplemented with 0.35 mg of G418 (Geneticin; Gibco) per ml, which is the maintenance dose for the Neor marker on the pUHD15-1 plasmid containing the tTA transactivator that had previously been stably transfected in these cells. Cells were cotransfected with the pUHD10-3 p21 plasmid and the puromycin resistance plasmid by the Lipofectamine procedure as specified by the manufacturer (GIBCO BRL). After cotransfection, cells that had been stably cotransfected were selected and maintained, with 2,000 and 1,000 ng/ml of puromycin (Calbiochem), respectively, in the presence of 2 μg of tetracycline per ml to suppress expression.
Individual clones obtained were split 1:2, grown in the presence or absence of tetracycline for 3 days, and then tested by Western blot analysis for inducible expression of p21 protein. Clones that had tightly controlled expression and induction of p21 up to desired levels were used for subsequent experiments (HeLa Tet p21-10.8, Saos2 Tet p21-32.4, RKO Tet p21-4, H1299 Tet p21-24.4, and Rat1 Tet p21-4).
MEFs from Rb+/− and Rb−/− mice were kindly provided by T. Jacks (Massachusetts Institute of Technology) and Jean Y. Wang (University of California San Diego), maintained in DMEM with 10% FBS at subconfluence, and used at passages 2 to 4. Experiments were carried out by comparing cells at identical passage numbers obtained from littermate embryos.
Adenoviruses produced in 293 cells were isolated from cell lysates as described previously (64). Virus doses were adjusted for each cell line to avoid cytotoxicity and to produce similar effects to those observed with the tetracycline system. Equivalent multiplicities of infection (MOIs) for the p21, p27, and control viruses were used in the experiments. Infection was done by replacing the medium for 2 h with DMEM–2% FBS containing virus, with gentle rocking of the flasks every 15 min. Then the medium was aspirated and replaced with the normal DMEM-10% FBS. The cells were harvested after 3 days. All the experiments were done at subconfluence; the cells were plated prior to infection at a starting density similar to that for the tetracycline-controlled induction experiments.
RKO cells were irradiated in situ in tissue culture plates with 4 Gy of γ-radiation from a 137Cs source at 3.8 Gy/min and harvested 5.5 h after irradiation, a time point chosen to have maximal levels of p21 present. MEFs were irradiated in situ in tissue culture plates with an initial dose of 4 Gy followed by a second irradiation with 2 Gy after 12 h, and the cells were harvested at 30 h for flow cytometric analysis.
Antibodies.
Anti-p21 antibodies were from Santa Cruz Biotechnology (C-19 and rabbit polyclonal antibodies, used for Western blots) and Pharmigen (15431E rabbit polyclonal antibodies, used for immunoprecipitations). Anti-cyclin D1 monoclonal antibodies (DCS-6 for Western blots, and DCS-11 for immunoprecipitations) were a generous gift from J. Bartek (Danish Cancer Society, Copenhagen, Denmark). Anti-cyclin E monoclonal antibodies (HE12 for Western blots, and HE 172 for immunoprecipitations) were originally obtained from Emma Lees and Ed Harlow (Massachusetts General Hospital Cancer Center, Boston, Mass.); a polyclonal antibody (M-20; Santa Cruz) was used for rodent cyclin E Western blotting. Cyclin A polyclonal antibody was described previously (30). Anti-cyclin B1 (monoclonal antibody, clone GNS1) and anti-pRb (polyclonal antibody, C-15) were from Santa Cruz. A polyclonal antibody (laboratory stock 8970) against the C-terminal 12 amino acids of human cdc2 (3, 13) was kindly provided by Clare McGowan (The Scripps Research Institute).
Immunoprecipitations and Western blots.
All experiments were done with subconfluent cells. Cells were plated at 2.5 × 103 cells/cm2 in medium with or without tetracycline, grown for 3 or 6 days, and then harvested by trypsinization. The cells were lysed in IP buffer (50 mM Tris [pH 7.5], 250 mM NaCl, 0.2% Nonidet P-40, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM NaF, 1 mM okadaic acid, 100 μg of phenylmethylsulfonyl fluoride per ml, 2 mg each of leupeptin, pepstatin, and aprotinin per ml) or by published procedures (43, 46) for the cyclin D1 kinase assay. Protein concentration was determined by the Bradford method (Bio-Rad). Lysates were precleared for 1 h and then immunoprecipitated with the appropriate antibody for 3 to 16 h at 4°C. Complexes bound to protein A+G-agarose (Santa Cruz) were washed four times with IP buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
For Western blot analyses, 0.2 mg of total-cell lysate and an equivalent amount of immunoprecipitation supernatant were resolved by SDS-PAGE on 6 to 12% gradient gels and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore). The blots were incubated with the primary antibody for 3 h and then with a horseradish peroxidase-conjugated secondary antibody (Promega, Chemicon) for 1 h. The bound antibodies were visualized by enhanced chemiluminescence (Pierce).
Kinase activity assays.
Kinase assays were performed with lysates prepared as described above. To measure cyclin E-, cyclin A-, and cyclin B-associated kinase activities, 0.2 mg of lysate protein was immunoprecipitated and analyzed as described previously (61) and histone H1 was used as a substrate. To measure the cyclin D1-associated kinase activity, Rb kinase assays were performed as described previously (40, 43) with the DCS-11 monoclonal antibody for immunoprecipitation from 0.2 mg of lysate and 1 μg of recombinant full-length Rb protein (QED Bioscience Inc.) as a substrate. The reaction products were separated by SDS-PAGE, and the gel was stained with Coomassie blue, dried, exposed to X-ray film, and quantitated with a PhosphorImager (Molecular Dynamics).
Flow cytometry analysis.
Cells were pulsed for 45 min prior to harvesting by adding bromodeoxyuridine (BrdU; Sigma) directly to the culture medium to a final concentration of 10 μM. The cells were then harvested, stained with propidium iodide and anti-BrdU fluorescein isothiocyanate (FITC)-conjugated antibody (Becton Dickinson) as specified by the manufacturer, and analyzed by flow cytometry on a FACScan station with Cell Quest software (Becton Dickinson).
FISH assay.
A Chromosome 8 specific centromeric probe labeled with Cy3 (Amersham) was used for the fluorescence in situ hybridization (FISH) assay as specified by the manufacturer. A Zeiss Axioskop microscope with a 63× oil immersion objective and a Hamamatsu 3CCD camera were used for image collection.
RESULTS
Regulated expression of p21 at physiologically relevant levels.
Using the tTA tetracycline-repressible expression system, we were able to establish conditional expression of p21 in a number of human cell lines, as well as in Rat1 fibroblasts (Table 1) (6, 60). In addition, our initial studies were extended to other cell types by using transient transduction of recombinant p21- and p27-expressing adenoviruses. To evaluate the contribution of pRb to the phenotype associated with p21 expression, both pRb-positive and pRb-negative cells were used (Table 1). The cells also differed in terms of their p53 status (Table 1). Note that although HeLa cells are technically pRb and p53 positive, pRb is inactive and p53 does not accumulate in these cells due to the activities of human papillomavirus oncoproteins E6 and E7.
TABLE 1.
Cell lines used in this study showing which were stably transfected with the tetracycline system and which were transduced with adenoviruses for p21 or p27
| Cell line | Rb status | p53 status | Tet p21 | Transduction ofa:
|
|
|---|---|---|---|---|---|
| Ad21 | Ad27 | ||||
| RKO (human colon carcinoma) | + | + | Yes | No | No |
| Rat1 (rat fibroblast) | + | + | Yes | No | No |
| A549 (human lung carcinoma) | + | + | No | Yes | No |
| Saos2 (human osteosarcoma) | − | − | Yes | Yes | Yes |
| HeLa (human cervical carcinoma) | − | − | Yes | Yes | Yes |
| Kb (human epidermoid carcinoma) | − | − | No | Yes | No |
| H1299 (human lung carcinoma) | + | − | Yes | Yes | Yes |
| HaCat (human keratinocyte) | + | − | No | Yes | No |
| SW480 (human colon carcinoma) | + | − | No | No | Yes |
| MEF Rb+/− (mouse embryo fibroblast) | + | + | No | Yes | Yes |
| MEF Rb−/− (mouse embryo fibroblast) | − | + | No | Yes | Yes |
Ad21 and Ad27, recombinant p21- and p27-expressing adenoviruses.
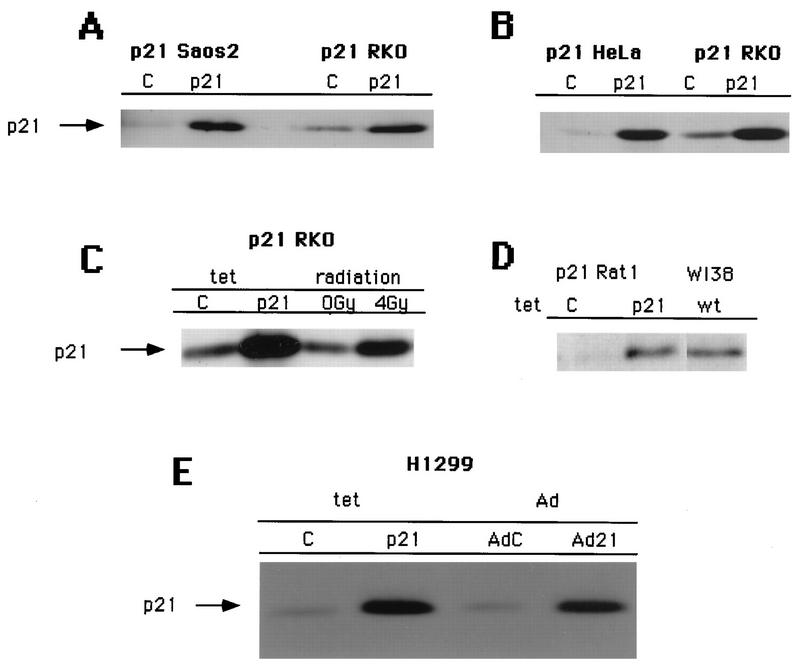
Stable transfectant clones that expressed p21 at similar levels upon removal of tetracycline from the growth medium were selected (Fig. 1A and B). Furthermore, levels of p21 accumulation were similar to those observed upon γ-irradiation of the same p53-positive RKO colon carcinoma cells (Fig. 1C) or in immortal Rat1 fibroblasts compared to senescing populations of human diploid fibroblasts (Fig. 1D).
FIG. 1.
Tetracycline-controlled expression of p21 in a panel of pRb-positive and pRb-negative cell lines. Shown are Western blots of total-cell lysates (0.2 mg of protein) with an antibody against p21 (C-19). Tetracycline-controlled p21 expression in uninduced cells (lanes C) and induced cells (lanes p21) is demonstrated. Equal numbers of cells were plated at low density (see Materials and Methods) and incubated for 3 days in medium with or without tetracycline. (A) Comparison of the levels of expression in Saos2 Tet p21 and RKO Tet p21 cells. (B) Comparison of the levels of expression in HeLa Tet p21 cells and RKO Tet p21 cells. (C) Comparison of the levels of expression in RKO Tet p21 cells and in RKO cells subjected to 0 or 4 Gy of ionizing radiation. (D) Comparison of the levels of expression in Rat1 Tet p21 cells and the levels in WI38 normal human diploid fibroblasts approaching senescence. (E) Comparison of the levels of expression in H1299 Tet p21 and adenovirus (Ad)-transduced H1299 cells (with an MOI of 200).
The doses of adenovirus used in our experiments were subsaturating in terms of the phenotype observed and were titrated down to MOIs that produced cell cycle effects and levels of expression comparable to those of the tetracycline-regulated expression system (Fig. 1E and data not shown).
Expression of p21 leads to cell cycle arrest.
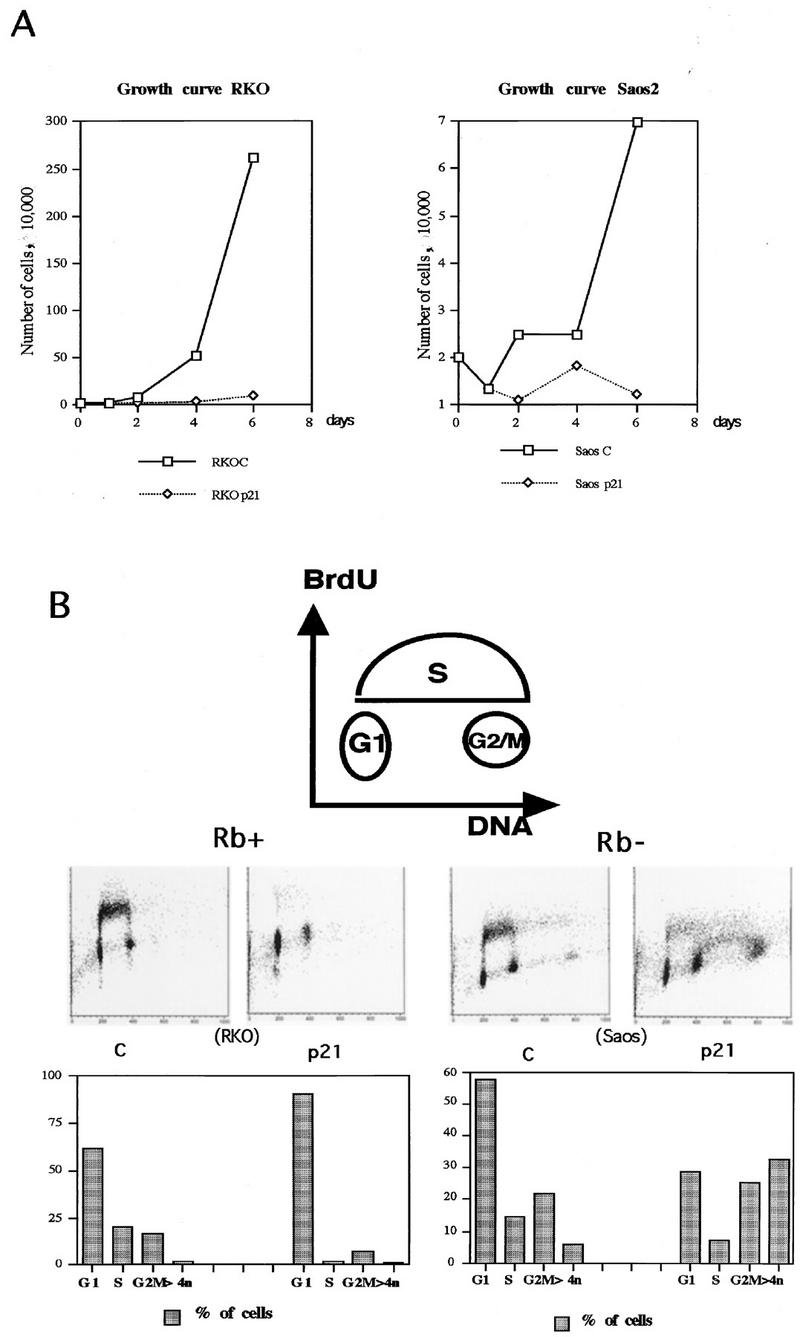
To assess the effects on cell growth in response to the expression of p21 and in the absence of other physiological perturbations, inducible clones were cultured for 6 days in the presence or absence of tetracycline. Proliferation was monitored by direct counting of cells. Examples of growth curves generated in this fashion for one pRb-positive (RKO) and one pRb-negative (Saos2) p21-inducible cell line are shown in Fig. 2A. Whereas cells cultured under p21-repressive conditions (presence of tetracycline) proliferate exponentially, cells induced for p21 rapidly cease proliferation.
FIG. 2.
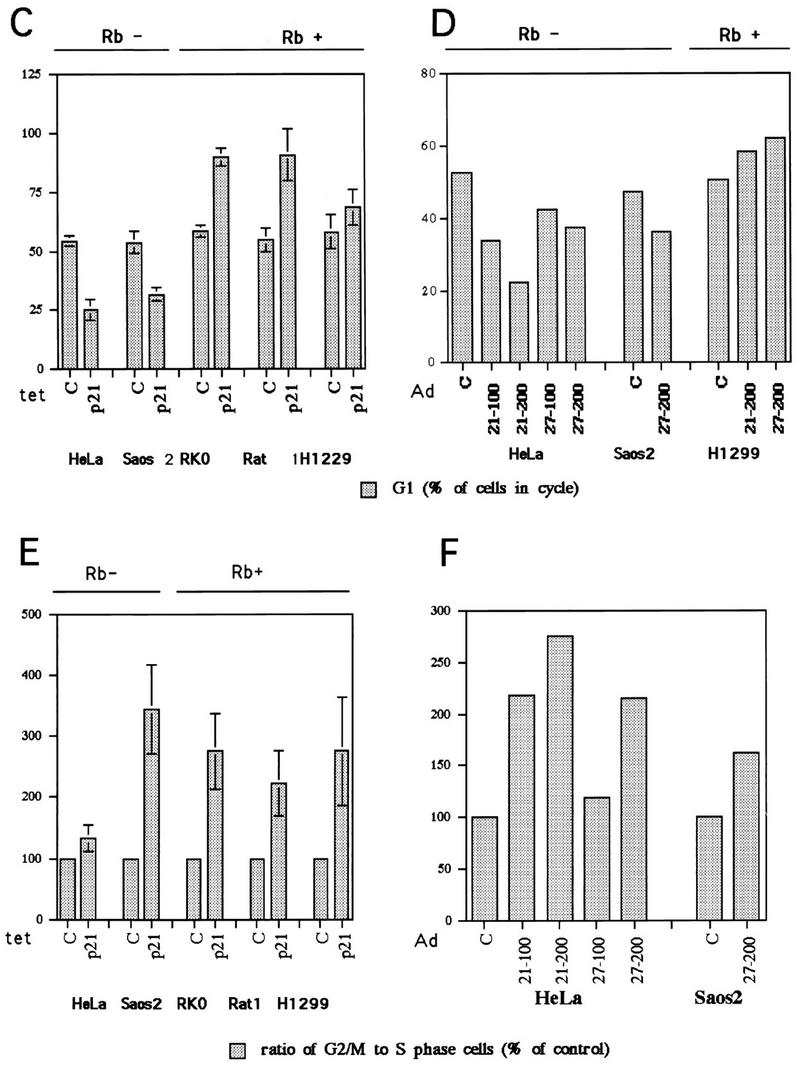
p21 expression arrests cells not only in G1 but also in G2. (A) Growth curves for the p21-transfected RKO and Saos2 cells. Cells were plated at equal densities in triplicate in six-well plates and grown for 6 days in the presence or absence of tetracycline. At the indicated times, the cells were harvested by trypsinization and counted with a hemocytometer. (B) Two-dimensional flow cytometry data of representative experiments illustrating the presence of both G1 and G2/M arrest in response to p21 expression, as well as the different relative preponderance of the two modes of arrest in pRb-positive (RKO) and pRb-negative (Saos2) cells. The cells were grown for 3 days with or without tetracycline. The DNA content measured by propidium iodide staining is shown on the x axis, and BrdU incorporation detected with an FITC-conjugated anti-BrdU antibody is shown on the y axis. The cartoon on top illustrates the distribution of cell cycle phases in such an analysis. The histograms under each flow cytometry plot depict the percentage of cells with G1 (2n), S, G2/M (4n), and more than 4n DNA content, respectively. Note the presence of a significant population of cells with greater than 4n DNA content in the pRb-negative Saos2 cells. (C and E) Quantitative evaluation of the cell cycle distribution following p21 expression in Rb-negative cells versus Rb-positive cells. The cells were grown for 3 days with or without tetracycline, and their cell cycle distribution was assessed by flow cytometry, as described in Materials and Methods. (C) G1 accumulation; (E) G2/M accumulation. The results are presented as an increase of the ratio of the number of cells with 4n or more DNA content to the number of cells with S-phase DNA content. (D and F) Comparison of the effects of p21 and p27 expression on cell cycle distribution, using adenovirus (Ad) transduction as described in Materials and Methods. MOIs of 100 and 200 were used, as indicated. Flow cytometry data were used for quantitation, as described for panels C and E.
To determine the specific effects on cell cycle progression mediated by p21 expression, flow cytometric analysis was performed on cultures maintained under inducing or noninducing conditions for 3 or 6 days. Before being harvested, the cells were pulse-labeled for 45 min with BrdU to mark the cells undergoing DNA replication. The cells were then fixed and stained with propidium iodide to detect total nuclear DNA and with anti-BrdU antibodies to detect nuclei that had incorporated BrdU during the labeling interval. Populations were then subjected to a two-dimensional analysis where the DNA content (propidium iodide staining) was plotted on the abscissa and BrdU incorporation (FITC anti-BrdU fluorescence) was plotted on the ordinate. This allows precise resolution of the population into G1, S and G2/M fractions as illustrated in the cartoon at the top of Fig. 2B. Analysis of the Rb-positive RKO cells in this fashion indicates that after 3 days of p21 induction, DNA replication had ceased and cells were arrested primarily with a G1 DNA content. However, it is clear from the raw data and the quantitation shown below as a histogram that there was also a persistent population of cells (approximately 10%) with a G2- or M-phase DNA content, although this fraction was reduced relative to what was observed in an asynchronous population. Since there was no evidence of mitotic cells in these populations based on visual observation, it is likely that these represent G2-arrested cells.
When a similar analysis was performed on Rb-negative Saos2 cells, a more complex pattern was observed. Unlike the RKO cells, Saos2 cells expressing p21 exhibited a reduction in the number of G1 cells relative to asynchronous controls. On the other hand, the percentage of cells with a G2/M DNA content was increased. Furthermore, although the percentage of cells in the S phase appeared to be reduced relative to asynchronous controls, a significant population of cells was still undergoing DNA replication, probably representing cell leakage through an inefficient G1 blockade. These results, which were reproducible in several different clones of each cell type, suggest that p21 has a reduced ability to mediate G1 arrest in Saos2 cells relative to RKO cells. Under such conditions, a higher proportion of cells arrest in G2. Another difference between the Saos2 and RKO cells expressing p21, which is discussed in greater detail below, is the accumulation of cells with a greater than 4n DNA content in the former (Fig. 2B; also see Fig. 5). Notably, there is a peak of cells with an 8n DNA content, as well as a second S-phase population between 4n and 8n, in the p21 expressing Saos2 cells.
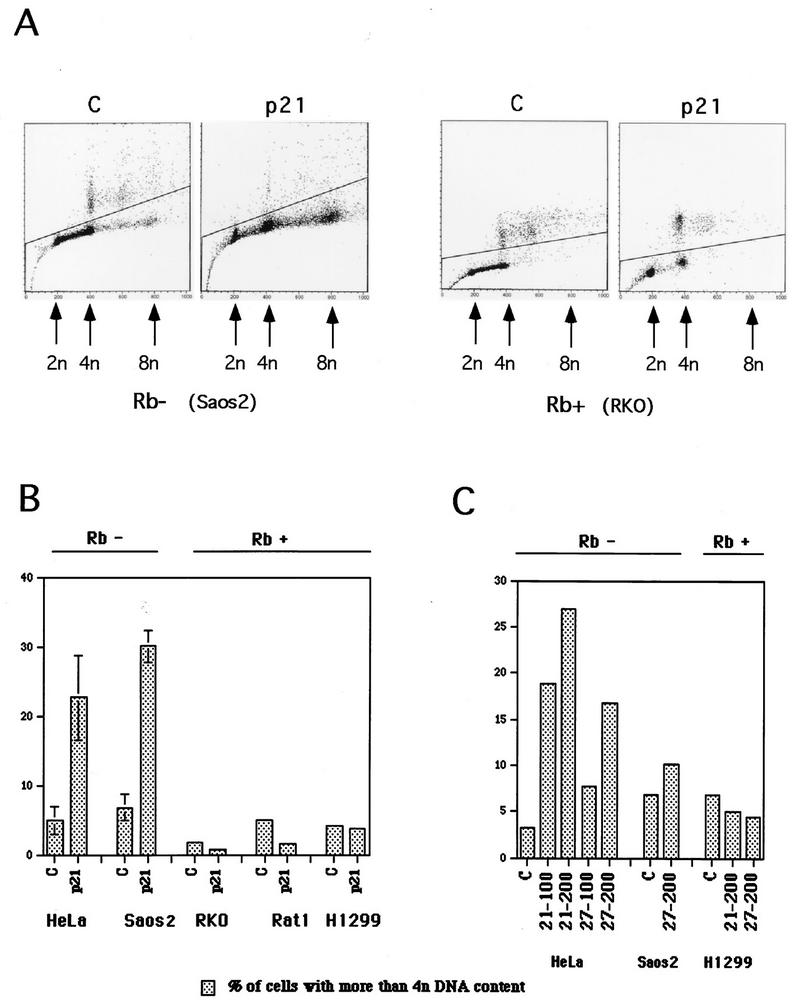
FIG. 5.
Endoreduplication occurs in pRb-negative cells, but is prevented in pRb-positive cells. (A) Flow cytometric data from representative experiments, illustrating endoreduplication in pRb-negative cells (Saos2 cells) upon p21 expression versus the absence of endoreduplication in pRb-positive cells (RKO cells). Experiments were done as described in the legend to Fig. 2 and in Materials and Methods. Plots of the propidium iodide fluorescence peak area (on abscissa), which measures the DNA content, versus the peak width (on the ordinate), which permits the gating out of clumped cells, are shown. (B) Quantitative evaluation of endoreduplication following p21 expression in pRb-negative and pRb-positive cells. Flow cytometric data were used for quantitation. (C) Comparison of the effects of p21 and p27 on endoreduplication, using adenovirus transduction, as described in Materials and Methods. MOIs of 100 and 200 were used, as indicated. Flow cytometry was used for quantitation.
p21-induced arrest patterns correlate with pRb function.
RKO cells have functional Rb, whereas Saos2 cells have an inactivating mutation of Rb. Therefore, the difference in response to p21 expression might be explained as a consequence of RKO cells having an intact pRb regulatory system and the lack of a pRb regulatory system in Saos2 cells. To test this hypothesis, other pRb-positive and -negative cell lines expressing p21 under tetracycline control were tested. Rat1 fibroblasts and H1299 human lung carcinoma cells are functionally pRb positive, whereas HeLa cells are functionally pRb negative. The two additional pRb-positive cell lines behaved essentially as did the RKO cells in response to p21 expression: cells arrested with an increased G1 population and a small but reproducible G2 population (Fig. 2B and C). To differentiate between a G2-arrested population and a residual cycling population, we compared the G2/M population (DNA content of 4n) to the S-phase population and expressed the result as a ratio (Fig. 2E). The S-phase population actively synthesizing DNA was considered a measure of residual cycling, and thus an increase in the ratio of G2/M- to S-phase cells was taken to be indicative of G2/M arrest or enrichment. Furthermore, no accumulation of cells with a greater than 4n DNA content was observed in these pRb-positive cells (Fig. 2B; also see Fig. 5). On the other hand, HeLa cells, which are functionally pRb negative due to the presence of the human papillomavirus E7 oncoprotein, exhibited a pattern similar to that observed for the Saos2 cells: arrested populations had decreased numbers of G1 cells compared to asynchronous populations (Fig. 2C) and increased numbers of cells with 4n and greater than 4n DNA content (see Fig. 5).
To extend the scope of this study, p21 was ectopically expressed in several additional pRb-positive and -negative cell lines by recombinant adenovirus transduction. A549 human lung carcinoma cells and HaCat human keratinocytes are pRb positive, whereas KB human epidermoid carcinoma cells are pRb negative. The A549 and HaCat pRb-positive cell lines arrested with an increase in the G1 population, whereas the KB cells exhibited a decrease in the G1 cell population and an increase in the number of cells with 4n or greater DNA content (Table 2). Thus, there was a strong positive correlation between a functional pRb regulatory system and a constellation of responses to p21 expression: tight G1 arrest for most cells, a small fraction of G2-arrested cells, and no evidence of increase in DNA content beyond 4n. Cells without functional pRb, on the other hand, inefficiently arrested in G1 and had a larger population arrested in G2 and an increase in the number of nuclei with greater than 4n DNA content. In contrast, there was no correlation between the p53 genotypes of these cell lines and any aspect of the arrest behavior.
TABLE 2.
G1 accumulation correlates with the presence of functional Rb and inversely correlates with endoreduplication
| Cell line | Rb status | p53 status | G1/S accumu- lation | DNA rerepli- cation |
|---|---|---|---|---|
| RKO (human colon carcinoma) | + | + | Yes | No |
| Rat1 (rat fibroblast) | + | + | Yes | No |
| A549 (human lung carcinoma) | + | + | Yes | No |
| Saos2 (human osteosarcoma) | − | − | No | Yes |
| HeLa (human cervical carcinoma) | − | − | No | Yes |
| Kb (human epidermoid carcinoma) | − | − | No | Yes |
| H1299 (human lung carcinoma) | + | − | Yes | No |
| HaCat (human keratinocyte) | + | − | Yes | No |
| SW480 (human colon carcinoma) | + | − | Yes | No |
| MEF Rb+/− (mouse embryo fibroblast) | + | + | Yes | No |
| MEF Rb−/− (mouse embryo fibroblasts) | − | + | No | Yes |
p27 confers a similar arrest phenotype to p21.
The p21 molecule contains a Cdk inhibition domain, as well as a domain that binds and inhibits the function of proliferating-cell nuclear antigen (PCNA), the processivity factor of DNA polymerase δ. To determine if any of the arrest characteristics mediated by p21 are based on interactions with PCNA, as well as to test if other members of the Cip/Kip inhibitor family can exert similar effects, parallel adenovirus transduction experiments were undertaken to express either p21 or p27, which contains a Cdk-inhibitory domain but no PCNA binding domain. pRb-negative HeLa and Saos2 cells transduced with recombinant p27-expressing adenovirus exhibited the same cell cycle arrest distribution as was observed with p21-expressing adenovirus as well as with tetracycline-regulated expression of p21: a decrease in the G1 population, an increase in the G2 population, and an increase in the population with greater than 4n DNA content (Fig. 2D and F). Similarly, Rb-positive H1299 cells behaved identically in their response to p21 and p27 expression (Fig. 2D and F). Furthermore, similar effects for p21 and p27 were observed in congenic MEFs (Table 2; see Fig. 7), as discussed below. Therefore, it is unlikely that interactions with PCNA account significantly for the p21-associated phenotypes observed.
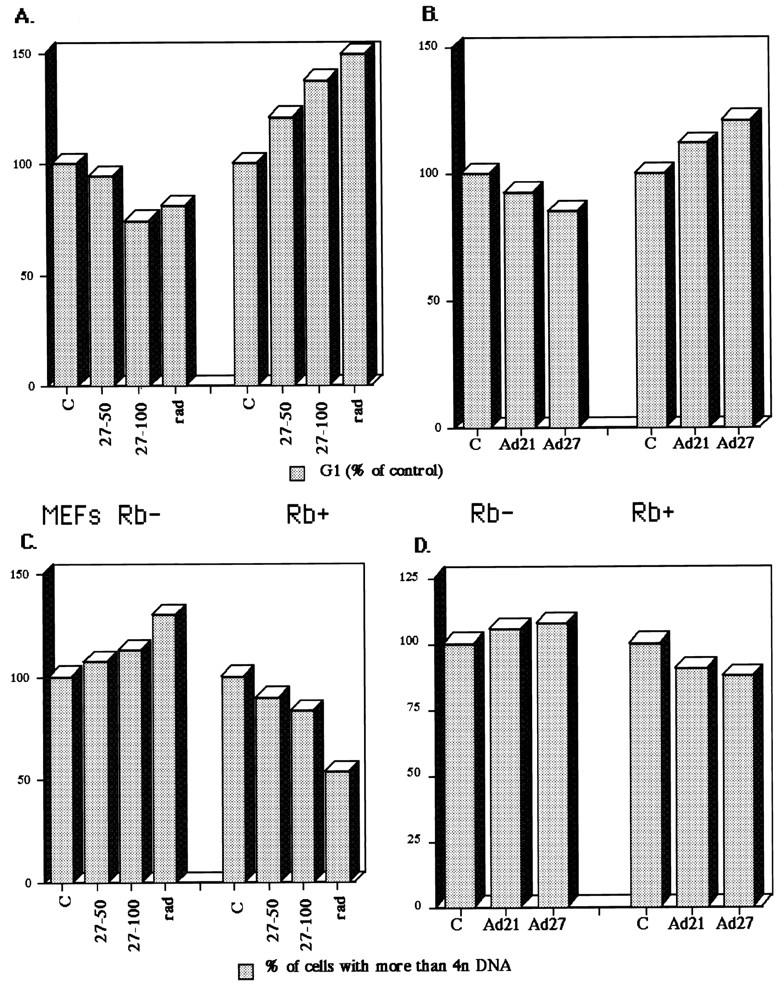
FIG. 7.
Effects of Cip/Kip expression and γ-irradiation on the cell cycle distribution of MEFs heterozygous (+/−) or homozygous (−/−) for Rb deletion. (A and C) Comparison of effects of γ-irradiation (rad) and p27 adenovirus on the cell cycle distribution in Rb−/− MEFs (Rb−) and Rb+/− MEFs (Rb+). The percentage of cells in G1 (A), and cells with more than 4n DNA content (C) were evaluated by flow cytometry as described in Materials and Methods. Control adenovirus (C) and p27 adenovirus at MOIs of 50 and 100 (27-50 and 27-100) were used as indicated and as described in Materials and Methods and plotted as a percentage of the control. γ-Irradiation consisted of a 4-Gy dose, followed by a 2-Gy dose at 12 h and harvesting at 30 h. (B and D) Similarity of the effects of p21 and p27 adenoviruses (Ad21 and Ad27) on the cell cycle distribution of Rb− and Rb+ MEFs. Equal doses (50 MOI) of the different adenoviruses were used.
Efficient G1 arrest in pRb-positive cells correlates with inhibition of cyclin D1-associated kinase.
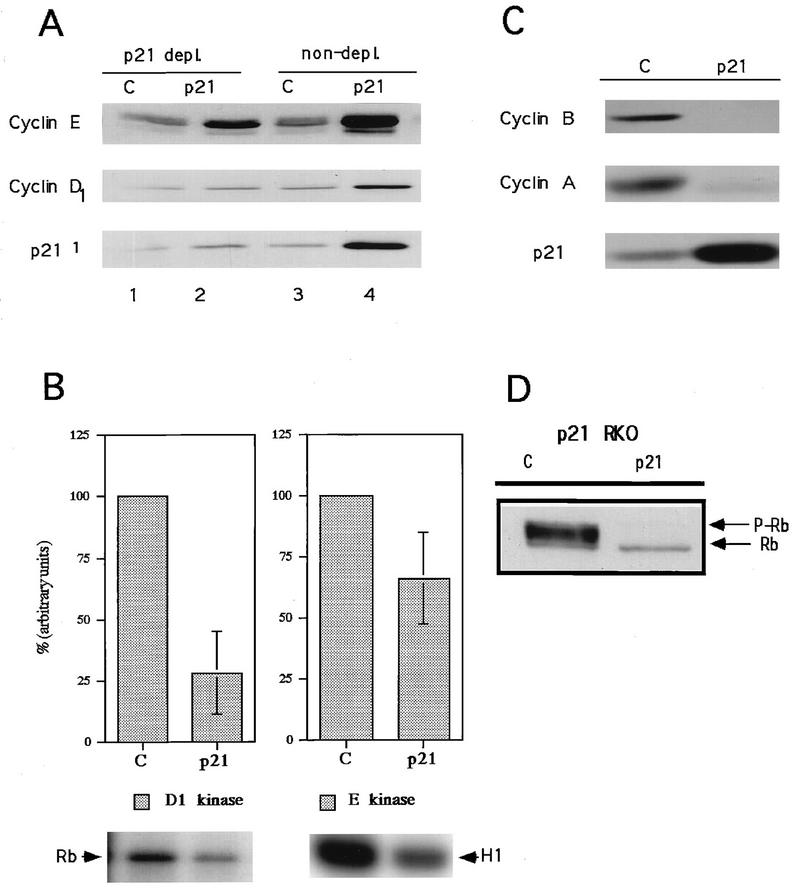
To investigate the mechanism that confers differential cell cycle arrest in pRb-positive and pRb-negative cells, the interactions between p21 and Cdks in RKO (pRb positive) and Saos2 (pRb negative) cells were investigated. Compared to asynchronous control cells, arrested RKO cells contained elevated levels of cyclins D1 and E (Fig. 3A). This may be due to cell cycle synchronization in G1, as well as to feedback control of cyclin expression or stability (8, 76). Cyclins A and B1, on the other hand, were virtually undetectable in the arrested cells, presumably due to accumulation of the majority of cells in G1, where these cyclins are not expressed (Fig. 3C).
FIG. 3.
Effects of p21 expression on cyclin-dependent kinase activities, cyclin protein levels and Rb phosphorylation in pRb-positive cells (RKO cells). (A) p21 association with cyclins D and E as analyzed by p21 immunodepletion followed by immunoblotting. Lanes 3 and 4, total-cell lysate; lanes 1 and 2, an equivalent amount of supernatant after one round of p21 immunoprecipitation. (B) p21-mediated changes in cyclin D1- and cyclin E-associated kinase activities. pRb was used as a substrate for cyclin D1-associated kinase, and histone H1 was used as a substrate for cyclin E-associated kinase. Quantitation was performed by PhosphorImager analysis. Data from three experiments are combined in the histograms as the mean ± standard error. A representative autoradiogram is shown below each histogram. (C) Changes in levels of cyclin A and B1 in response to p21 expression. (D) Western blot depicting the effects of p21 expression on pRb phosphorylation. Rb, hypophosphorylated form; P-Rb, hyperphosphorylated form.
Whereas G1 cyclin levels were increased in p21-arrested RKO cells, this was not reflected in associated kinase activities. Even though cyclin E levels increased severalfold, there was a decrease in the associated kinase activity (Fig. 3A and B). More significantly, an almost fourfold increase in cyclin D1 levels was associated with a fourfold decrease in the associated kinase activity (Fig. 3A and B). This inhibition of cyclin D1-associated Cdk4 (and/or Cdk6) correlated with a shift from hyperphosphorylation of pRb in asynchronous cells to exclusively hypophosphorylated pRb (Fig. 3D). A similar inhibition of Rb phosphorylation occurred in the other Rb-positive cell lines upon expression of either p21 or p27 (data not shown). Although the absolute inhibition of cyclin E-associated Cdk2 kinase was not great (approximately 35%), the specific activity of the kinase is perhaps a better indication of biological inhibition, since the accumulation of cyclin E is normally periodic through the cell cycle and the activity measured in asynchronous cultures is likely to be contributed by the small fraction of cells traversing the G1/S boundary whereas that measured in the G1-arrested cultures is likely to be contributed by all of the cells. Normalizing the absolute kinase activity to cyclin levels based on densitometric scanning of Western blots, a significant inhibition of cyclin E-associated kinase specific activity (greater than 85%) was observed. By using the same considerations, the specific activity of cyclin D1-associated kinase was found to be inhibited by approximately 95%.
To determine if association with and therefore inhibition by p21 can account for the inhibition of cyclin D1- and cyclin E-associated kinase, p21 was immunodepleted from lysates. A single round of immunodepletion removed 75 to 80% of the p21 from lysates of induced RKO cells (Fig. 3A), as measured by laser densitometry (data not shown). The immunodepletion likewise removed 60 to 70% of the cyclin E and 70 to 75% of the cyclin D1 from the same lysates (Fig. 3A and data not shown). Little cyclin D1 or cyclin E was immunodepleted from control cell lysates (Fig. 3A). Thus, at this level of resolution, interaction with p21 can account for the observed inhibition of cyclin D1- and cyclin E-associated kinase activities, the loss of pRb phosphorylation, and presumably the arrest of cells in G1.
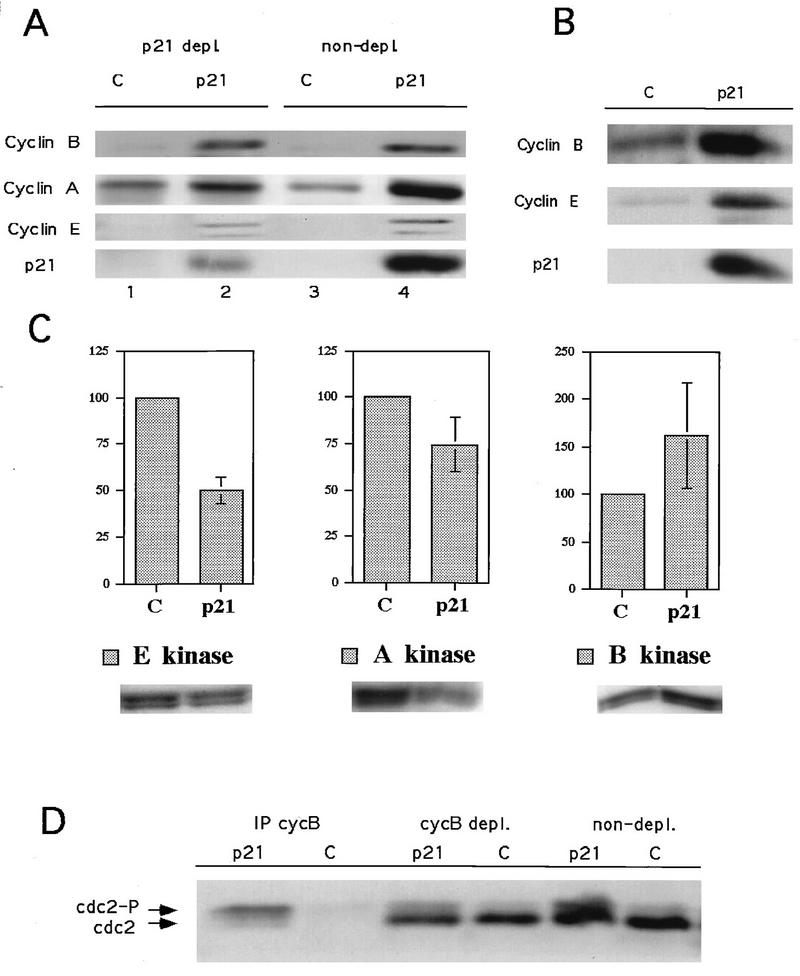
Cyclin levels in p21-induced Saos2 cells reflect the differential characteristics of the arrested population compared to RKO cells. Cyclin E levels were elevated in arrested cells relative to those in asynchronous cells, as with RKO cells, but the cyclin A and B1 levels were also elevated (Fig. 4A and B), consistent with a predominant population of G2-arrested cells. Cyclin D1 levels, which are low in Saos2 cells, were not analyzed. The absolute kinase activities associated with cyclin A and cyclin E were reduced modestly, while those associated with cyclin B1 levels were increased (Fig. 4C). However, when the considerations of cell cycle synchrony of the arrested populations and kinase specific activity were factored into the analysis, cyclin E- and cyclin A-associated kinase specific activities were found to be strongly inhibited, approximately 20- and 8-fold respectively; the cyclin B1-associated kinase specific activity did not appear to be as strongly inhibited, with an approximately 2-fold inhibition. However, it should be pointed out that pronounced cell cycle effects on kinase specific activities are anticipated with cyclin B/Cdc2, since the activity of this kinase, but not its accumulation, is limited to mitotic cells. Therefore, the specific activity of cyclin B/Cdc2 in the asynchronous control, which contains few mitotic cells, is expected to be low (see below). The reduction of cyclin A- and cyclin B1-associated kinase specific activities could account for the predominance of G2 arrest observed in these populations, whereas the inhibition of cyclin E-associated kinase could account for the small persistent G1-arrested population.
FIG. 4.
Effects of p21 expression on cyclin-dependent kinase activities and cyclin levels in Rb-negative cells (Saos2 cells). (A) p21 association with cyclin E and A but not cyclin B1. Lanes 3 and 4, total cell lysate; lanes 1 and 2, an equivalent amount of supernatant after one round of p21 immunoprecipitation. (B) Changes in cyclin levels in a parallel experiment; a higher exposure of the film was used to allow the visualization of the low levels present in the control cells. (C) Changes in relevant cyclin-dependent kinase activities, with histone H1 as a substrate. Data from three experiments are combined in the histograms as the mean ± standard error. A representative autoradiogram is shown below each histogram. (D) Western blot depicting the effects of p21 expression on cdc2 levels and phosphorylation status in total cell lysates (non-depl.), cyclin B depleted lysates (cycB depl.), and cyclin B immunoprecipitates (IP cycB). cdc2, dephosphorylated form; cdc2-P, hyperphosphorylated form. Experiments were done as described in Materials and Methods.
To determine if the effects on cyclin E-, A-, and B1-associated kinase activities could be a direct consequence of association with p21, p21 immunodepletion experiments were performed on extracts from p21-arrested Saos2 cells. A single cycle of p21 immunodepletion removed 75% of the p21 from these lysates (Fig. 4A and data not shown). p21 immunodepletion removed approximately 50% of the cyclin A and none of the cyclin B1 (Fig. 4A and data not shown). Thus, a significant fraction of cyclin A in arrested cells is associated with p21, potentially accounting for the observed inhibition of cyclin A-associated kinase activity. The lack of immunodepletion of cyclin B1 indicates that in these cells, cyclin B1 is not a direct target of p21-mediated inhibition. However, to investigate the molecular mechanism of cyclin B1/Cdc2 kinase inhibition, we analyzed the phosphorylation state of Cdc2 associated with cyclin B1 by immunoblotting cyclin B1 immunoprecipitates. Cdc2 bound to cyclin B1 in p21-arrested Saos2 cells was predominantly in the hyperphosphorylated state associated with inhibitory phosphorylation of T14 and Y15 (Fig. 4D). This observation is consistent with the relatively low specific activity of cyclin B/Cdc2 in these cells. However, as pointed out above, the specific activity of cyclin B/Cdc2 in asynchronous cells is low as well, since dephosphorylation of Cdc2 occurs only in mitotic cells. This is likely to account for the apparent modest (twofold) inhibition of cyclin B/Cdc2 specific activity observed in p21-arrested cells relative to asynchronous cells. The mechanism whereby p21 expression leads to inhibitory phosphorylation of Cdc2 remains to be determined but may be an indirect consequence of inhibition of cyclin A-associated kinase activity (see Discussion).
Expression of p21 in Rb-negative cells leads to endoreduplication in a significant fraction of the population.
As described above, one of the phenotypic consequences of p21 and p27 expression in pRb-negative but not pRb-positive cells is an accumulation of cells with a greater than 4n DNA content. To elucidate the basis of this phenomenon, flow cytometric data were subjected to an analysis designed to distinguish between individual nuclei having greater than 4n DNA content and cell aggregates. Multinuclear cells were ruled out, since they were not observed at significant levels based on microscopic scanning of arrested populations. Plots of fluorescence peak area (abscissa), which measures DNA content, versus peak width (ordinate), which permits the gating out of clumped cells, are shown in Fig. 5A. This gating indicates that the greater than 4n population produced by p21 expression in the Saos2 cells corresponds to an increased DNA content per cell and not to cell aggregation. Also, the accumulation of a distinct peak at a DNA content of 8n suggests that discrete rounds of DNA replication are responsible for this population (Fig. 5A). In contrast, all of the fluorescence corresponding to a DNA content of greater than 4n in RKO (Rb-positive) cells is associated with an increase in peak area and most probably corresponds to cell aggregates. Therefore, a subpopulation of Saos2 cells arrested in G2 by expression of p21 appear to undergo at least a round of DNA replication without mitosis (endoreduplication), resulting in cells of at least double the normal ploidy. RKO cells do not undergo endoreduplication in response to induction of p21.
To determine the generality of endoreduplication in pRb-negative cells, a panel of Rb-positive and -negative cells was analyzed as above, with either tetracycline-dependent or recombinant adenovirus-dependent expression of p21 or p27. Whereas tetracycline-dependent expression of p21 in pRb-positive Rat1 and H1299 cells did not stimulate endoreduplication cycles, it did so in functionally pRb-negative HeLa cells (Fig. 5B). When adenovirus transduction was used to express p21 or p27 in pRb-positive HaCat, A549, and SW480 cells and pRb-negative KB cells, similar results were obtained (Table 2). PCNA effects were ruled out as contributing to the endoreduplication phenotype, since transduction of p27-expressing adenoviruses into pRb-negative cells stimulated endoreduplication as effectively as did p21-expressing adenoviruses (Fig. 5C).
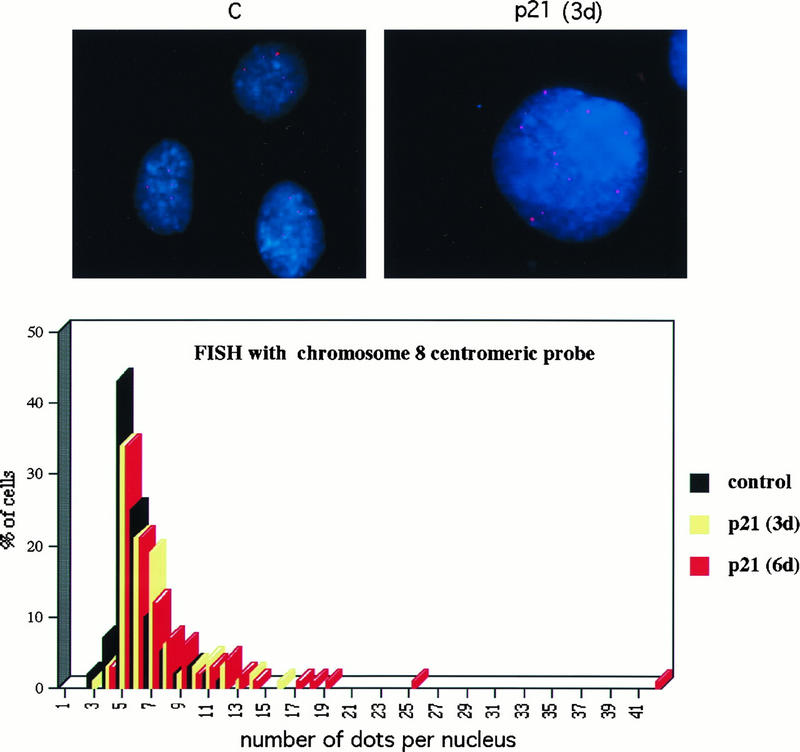
To confirm, by using an independent approach, that endoreduplication, leading to cells of increased ploidy, occurred in response to p21 expression, Saos2 cells were analyzed for an arbitrarily chosen specific chromosomal marker by FISH analysis before and after tetracycline-regulated induction of p21. Fixed nuclei were analyzed in the absence of p21 expression or after 3 or 6 days of p21 expression by using a human chromosome 8 centromeric probe. Micrographs of representative nuclei are shown at the top of Fig. 6. The pink dots represent positive signals produced by the presence of sequences homologous to the probe either on unreplicated chromosomes or on pairs of sister chromatids postreplication. Histograms produced by counting the signals from 100 random nuclei from each population are shown at the bottom of Fig. 6. As can be seen, the primary mode of each population is five or six dots, suggesting that the Saos2 genome contains sequences capable of hybridizing to the probe five or six times. Upon increasing the time of p21 expression, a second small mode appeared at 11 to 12 dots, consistent with a single round of endoreduplication and an integral increase in ploidy. By 6 days of incubation, a small but significant number of nuclei with more than 12 dots were observed, suggesting that additional endoreduplication cycles had occurred in a subpopulation of cells. This shift was congruent with the shift in DNA ploidy assessed by flow cytometry. Thus, based both on flow cytometric analysis and FISH analysis, p21 expression in Rb-negative cells stimulates endoreduplication.
FIG. 6.
(A) FISH analysis of Saos2 cells with a Chromosome 8 centromeric probe. Images were taken with a 63× oil immersion objective, as described in Materials and Methods. Preparations of nuclei were obtained by hypotonic lysis of cells. Chromatin is stained blue with 4′,6-diamidino-2-phenylindole (DAPI), and the Cy3 fluorescent centromeric probe hybridized to its target appears as pink dots. The left panel shows nuclei from uninduced control cells; the right panel shows an example of a nucleus with an increased number of dots after 3 days of p21 expression. (B) Scoring of a time course experiment. A total of 100 nuclei in each sample, examined with a 63× objective, were scored for the number of dots.
Experiments with MEFs.
The results reported above were obtained with cell lines derived primarily from tumors. Even though the Rb genotype could be determined, these cell lines are expected to be genetically heterogeneous. Our approach to circumventing the lack of congeneity in the analysis was to investigate several cell lines of each Rb genotype. In all nine cases investigated, the p21 (or p27) response phenotype correlated with the Rb genotype. This represents a reasonably high level of statistical significance. Nevertheless, to extend these studies to a genetically controlled format, we performed similar experiments on congenic Rb−/− and Rb+/− MEFs. When such MEFs were transduced with p21 and p27 adenoviruses, a dose-dependent response was observed (Fig. 7). For the Rb+/− MEFs, cells accumulated in G1 and there was a reduction in the population of cells with a greater than 4n DNA content (Fig. 7). Conversely, for the Rb−/− MEFs, there was a decrease in the number of cells arrested in G1 and an increase in the number of cells with a greater than 4n DNA content (Fig. 7). These results parallel exactly those obtained with Rb-positive and Rb-negative cell lines reported above. We could not easily measure the accumulation of G2-arrested cells in the Rb−/− MEFs exposed to p21 or p27, because even early-passage cells of such a genotype accumulate a significant polyploid population. Nevertheless, the reduction in G1-arrested cells coupled with a blockade of proliferation strongly implies a G2 arrest preceding endoreduplication, which we could monitor. Thus, in a clean genetic background, p21 and p27 confer predominantly an Rb-dependent G1 arrest, but in the absence of pRb, they confer a G2 arrest followed by endoreduplication, confirming that these different phenotypes segregate with the Rb genotype.
It might be argued that G2 arrest and endoreduplication correspond to an unnatural situation of forced p21 expression in an Rb-negative context. We used Rb+/− and Rb−/− MEFs to determine if natural stimuli that lead to induction of p21 might produce the same phenotypes. Therefore, Rb+/− and Rb−/− MEFs were subjected to γ-irradiation and, after 30 h, analyzed for cell cycle parameters. Whereas Rb+/− MEFs accumulated in G1 and exhibited a decrease in the number of cells with a DNA content of greater than 4n, Rb−/− MEFs showed a decrease in the number of G1 cells and an increase in the number of cells with a DNA content of greater than 4n (Fig. 7A and C). Therefore, γ-irradiation, which leads to induction of p21 via p53, confers the same phenotype as ectopic expression of p21 or p27. It is thus likely that G2 arrest followed by endoreduplication is a consequence that may be encountered when Rb-negative p53-positive tumors are subjected to genotoxic stresses (see Discussion).
DISCUSSION
Previous work has emphasized the G0/G1 regulatory roles of p21 and p27 (for reviews, see references 17 and 65). p21 has been implicated in G1 arrest following DNA damage (14, 16), in the response to cytokines and loss of substrate adhesion (7, 10, 18) and in maintenance of terminally differentiated cells in a nonproliferative state (25, 35, 36, 45, 46, 70). The data presented in this report show that p21 plays a role at the G2/M-phase transition as well. The reasons that such a late cell cycle role may not have been detected previously are that many experiments addressing the functions of p21 have been focused specifically on G1 responses and there is likely to be a redundancy in the mechanisms regulating the G2/M-phase transition. Nevertheless, human lymphoblastoid cells checkpoint arrested in G2 by DNA damage accumulated high levels of Cdk-bound p21 (2). In addition, p21 was reported to be induced upon ectopic p53 expression in tissue culture cells that resulted in both G1 and G2 arrest (1, 6). However, those experiments could not directly address the role of p21 in the arrest pattern.
Experiments with nullizygous mice indicate partial but not complete redundancy.
In the context of the many inferred roles of p21 alluded to above, it is surprising that p21-nullizygous mice are developmentally normal (4, 11). One might therefore conclude that many of the regulatory functions attributed to p21 are redundant with other regulatory mechanisms. However, the p53-dependent G1 arrest response to DNA damage is at least partially defective in fibroblasts from p21-nullizygous mice (4), supporting the idea that this is one of the critical functions of p21 that cannot be completely compensated by other modes of regulation. That p21-mediated Cdk inhibition may overlap with other regulatory mechanisms in a number of other experimental contexts is a likely explanation for why no evidence for a regulatory role for p21 at the G2/M phase transition has been forthcoming. The fact that fibroblasts from p21-nullizygous mice and p53-negative cell lines, in general, arrest in G2 in response to DNA damage made it difficult to assess the contribution of p21 induction in p53-positive cells. Other lines of evidence, however, support the possibility of a G2/M-phase regulatory role for p21. First, p53-positive tumor cells rendered p21 nullizygous by homologous recombination, although capable initially of arresting in G2 in response to DNA damage, could not maintain the arrest and ultimately initiated abnormal rounds of DNA replication and underwent apoptosis (75). Thus, although p21 was not required for the short-term G2 arrest response, it might be important for a stable long-term response. Second, cycling p21−/− MEFs were slightly accelerated into mitosis relative to wild-type MEFs, where p21 accumulated in the nucleus late in G2 and bound to cyclin A-Cdk2 complexes (15). This suggests that p21 has an effect on late cell cycle events, even in normal cycling cells.
Different responses to p21 of pRb-positive and pRb-negative cells.
Cycling pRb-positive cells arrested preponderantly in G1 upon Cip/Kip expression, with a small but persistent subpopulation in G2. On the other hand, pRb-negative cells arrested in a complementary fashion, primarily in G2 with a lesser proportion in G1 (Fig. 2B and C; Table 2). In the cell lines tested (nine in addition to the MEFs), there were no exceptions to the correlation between p21 (and p27)-induced phenotypes and Rb status. Therefore, even though this set represents an extremely genetically diverse pool, there can be no escape from the conclusion that the Rb status is a key determinant in the cellular response to Cip/Kip inhibitors. The consistent and reproducible effects observed across the spectrum of very different cell lines used, in addition to arguing for the generality of the paradigm described in this paper, assuages possible concerns about cell type specificity, tissue specificity (epithelial cells versus fibroblasts), and species specificity (human versus rodent).
The difference between pRb-positive and pRb-negative cells most probably reflects the relative sensitivities of potential p21 targets and the gradual increase of p21 levels upon induction, since we observed that p21 levels increased gradually over the course of several days (Fig. 8 and data not shown). Under the experimental conditions used, cyclin D1-associated kinase was clearly the most sensitive to p21-mediated inhibition. However, inhibition of cyclin D-associated kinases is likely to have regulatory consequences only in pRb-positive cells. It has been demonstrated that D-type cyclins are not essential in pRb-negative cells (40, 55). Therefore, the efficient G1 arrest of pRb-positive cells in the context of p21 expression most probably reflects the inhibition of cyclin D-associated kinases and concomitant block to pRb phosphorylation. Cyclin E-Cdk2 has also been shown to be essential for the G1/S phase transition (53; for a review, see reference 59). However, the inhibition of cyclin E-Cdk2 in response to p21 expression, although significant, was somewhat less complete than that observed for cyclin D-associated kinase. This may explain the less efficient G1 arrest observed in pRb-negative cells. The fact that some cells do arrest in G1, however, suggests that sufficient inhibition of cyclin E-Cdk2 occurs within a subpopulation, possibly due to cell-to-cell variation in p21 expression levels. We have not detected a significant correlation between wild-type cyclin E protein levels or the length of G1 with Rb status in our panel of cell lines (data not shown). However, our data are consistent with the idea that there is an altered G1 restriction point regulation in Rb-negative cells, as previously reported (32).
FIG. 8.
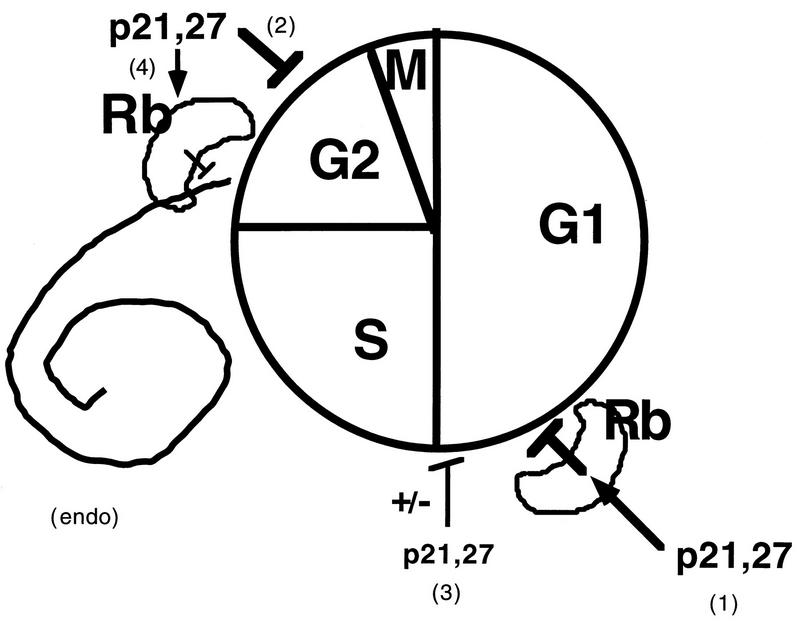
Model of the cell cycle effects of p21 (and p27). Based on differences between pRb-positive and pRb-negative cells, it appears that the presence of a functional Rb pathway is necessary for maximal G1 arrest (pathway 1). G1 arrest in the absence of a functional pRb pathway is inefficient (pathway 3). A second target of p21 action, present in all cell types but more apparent in pRb-negative cells, exists at the G2/M transition (pathway 2). Finally, p21, which probably contributes to preventing inappropriate DNA replication in G2-arrested cells in the presence of a functional pRb pathway (pathway 4), can actually lead to endoreduplication in pRb-negative cells (endo). The cell cycle distributions in our Rb+ and Rb− cells suggest that the Rb-dependent G1/S arrest (pathway 1) is the most sensitive to p21 and is triggered first, followed by the G2/M arrest (pathway 2) and possibly last by an Rb-independent G1/S arrest (pathway 3). Cycling cells will accumulate predominantly in the first of the arrests that comes into effect.
The predominant G2 arrest observed in pRb-negative cells and limited G2 arrest observed in pRb-positive cells is most likely due to p21-mediated inhibition of cyclin A-Cdk2. The dynamics of arrest in pRb-negative cells probably reflects the inefficiency of inhibition of cyclin E-Cdk2. Although cyclin A-Cdk2 also was not inhibited with high efficiency, based on an in vitro assay and the fact that p21-expressing cells were not apparently impaired for progression through the S phase, the degree of inhibition was probably sufficient to block entry into mitosis. Although cyclin A function has been shown to be required for progression both through S phase and into mitosis (5, 22, 54), the relative levels required have not yet been established. It is unlikely that inhibition of cyclin B1-associated kinase by p21 contributes to G2 arrest, since there was no evidence for significant amounts of cyclin B1 associated with p21. We have observed, nevertheless, that in p21-induced G2-arrested cells, cyclin B1-Cdk2 is inhibited by phosphorylation of Cdc2. Although the mechanism for this indirect inhibition by p21 is not yet known, a similar phenomenon has been observed in Xenopus egg extracts, where inhibition of Cdk2 leads to inhibitory phosphorylation of Cdc2 and concomitant G2 arrest (23). The residual G2 arrest observed in pRb-positive cells is most probably mechanistically related to that for which the rationale is provided above, except that efficient inhibition of cyclin D-associated kinases and presence of a pRb regulatory pathway ensure that most cells are arrested in G1, before inhibition of cyclin A-Cdk2 can become significant (Fig. 8). It is noteworthy that the previously described in vitro affinities of p21 for different cyclin-Cdk complexes (27) are consistent with our in vivo results.
pRb is necessary to block endoreduplication.
After arrest in G2, a significant subpopulation of pRb-negative cells responding to p21 or p27 expression underwent cycles of endoreduplicative DNA replication. Although a significant fraction of pRb-positive cells arrested in G2, endoreduplication was never observed. Two conclusions can be drawn from these observations. First, p21 can arrest cells in G2 in a physiological environment that is permissive for entering the S phase without an intervening mitosis. This would seem to be a violation of the normal safeguards that prevent cell cycle events from occurring out of order. Second, however, initiation of the S phase, whether from G1 or G2, requires neutralization of the inhibitory functions of pRb. This cannot happen in the presence of both pRb and p21.
Based on models in yeast cells, amphibian egg extracts, and Drosophila, two requirements must be met for DNA replication to be initiated at chromosomal origins (71, 72). First, origins need to be “licensed” after the prior round of replication is completed. In yeast and Drosophila, licensing requires strong downregulation of Cdk activities, which normally occurs at the end of mitosis but which may be mimicked by genetic manipulation or developmental regulation. Second, Cdk activities required for replication need to be activated. Based on these criteria, p21 could promote endoreduplication by partial inhibition of Cdks. The incomplete inhibition of cyclin E- and/or cyclin A-associated kinase activities may allow sufficient activity for initiation of replication but not sufficient to proceed to mitosis. The appropriate balance may not be met in every cell, since many apparently do not undergo endoreduplication. Additionally, licensing of origins may not be efficient, since endoreduplicative replication appears to proceed slowly in our system (see the differential BrdU incorporation levels in Fig. 2B).
That pRb appears to be capable of blocking endoreduplication in G2-arrested cells suggests a role in enforcing the order of cell cycle events. Whatever critical functions downstream of pRb are required for replication after passage through G1 appear to be required again if replication is to occur from G2. The regulation of pRb phosphorylation, in fact, may be an important G2 function of p21 in response to DNA damage. It is noteworthy that p21-nullizygous cells subjected to DNA damage underwent G2 arrest followed by an endoreduplicative cycle whereas isogenic controls that were wild type for p21 underwent stable G2 arrest (75). Thus, p21 accumulation, although not critical for G2 arrest, was important for blocking subsequent endoreduplication, possibly by inhibiting pRb phosphorylation. At first glance, these data would appear to be in conflict with conclusions drawn from our work, in that loss of p21 expression is associated with endoreduplication in one case but p21 expression promotes endoreduplication in the other. The apparent paradox, however, is resolved by proposing (i) that three criteria need to be met for endoreduplication to occur, i.e., G2 arrest, partial Cdk inhibition, and neutralization of pRb, and (ii) that the Rb genotype determines the role that p21 will play. These requirements, however, are general and therefore presumably can be met via different mechanistic routes. As such, p21 expression can contribute to endoreduplication in pRb-negative cells by inhibiting Cdks and conferring G2 arrest; pRb is not present and therefore does not require neutralization. On the other hand, it is likely that the dominant effect of p21 expression in pRb-positive cells is to collaborate with pRb to block endoreduplication by preventing the Cdk-dependent neutralization of pRb (Fig. 8). Thus, the presence of active pRb appears to be the critical determinant in the susceptibility of a cell to endoreduplication and the response that p21 expression elicits in this process.
G2 arrest and endoreduplication with endogenous induction of p21.
We have demonstrated by using Rb−/− MEFs that ionizing radiation causes G2 arrest and endoreduplication, analogous to exogenous expression of p21 and p27 in the same cells (Fig. 7). It has been demonstrated that ionizing radiation, by causing double-strand breaks in genomic DNA, leads to p53-dependent induction of p21 (14, 41, 49, 66). These experiments, therefore, allowed us to conclude that p21 expression is most probably responsible for this constellation of phenotypes in both instances.
p21 is also induced in fibroblasts as a consequence of clonal senescence (51, 69). It is therefore noteworthy that as Rb−/− MEFs approach senescence, they are particularly prone to become polyploid (33a). It is likely, therefore, that the accumulation of p21 in this Rb−/− context is promoting cycles of endoreduplication. We have demonstrated that this process can be accelerated by additional expression of p21 or p27 via adenovirus transduction (Fig. 7).
Finally, it has been reported that ectopic expression of the myogenic transcription factor, MyoD, in Rb−/− mouse myocytes but not in wild-type myocytes leads to G2 arrest and endoreduplication (52). Since MyoD promotes p53-independent induction of p21, it is likely that, here again, p21 expression in an Rb-negative context is leading to the phenotypic constellation that we observed via direct exogenous expression of p21 in Rb-negative cells. Thus, based on the phenotypic consequences of direct expression of p21 without collateral activation of upstream signaling pathways, as described in our studies, it can be concluded that aberrant responses to clonal senescence and to myogenic signaling in Rb−/− cells are due to the interaction between p21 expression and the Rb-negative genotype.
Implications for cancer therapy.
The observation that p21 can promote endoreduplication in pRb-negative contexts has potential practical implications for cancer therapy. For tumors that are pRb negative but p53 positive and therefore capable of inducing p21 in response DNA damage, endoreduplication and genetic destabilization may be a consequence of radiation therapy or chemotherapy. In a worst-case scenario, such genetic destabilization may lead, in some cells, to bypassing of normal arrest and apoptosis mechanisms and thus to enhanced malignancy. An example of such a situation is familial retinoblastoma, where one germ line copy of the Rb gene is mutated. Rb-negative cells occur at a relatively high frequency due to loss of heterozygosity at the RB locus and result in neoplasia, most notably in the retina (21). However, such patients are at a greatly increased risk of developing secondary tumors in surrounding mesenchymal tissue exposed to ionizing radiation therapy (9, 12, 29). It is possible that induction of p21 in pRb-negative cells within such tissues leads to endoreduplication, genetic instability, and, ultimately, secondary malignancy.
Functions of p21 at the G2/M transition.
It is possible, and indeed likely, that the roles of p21 in G2 arrest under physiological conditions are not completely redundant with those of other concurring mechanisms, especially with regard to the stability of long-term G2 arrest as well as prevention of DNA rereplication from occurring during such arrest (Fig. 8). Long-term arrest might be used by the cell for repair of massive DNA damage, facilitated by the presence of a ready template provided by the duplicated sister chromatid.
ACKNOWLEDGMENTS
We thank Joe Nevins and James DeGregori (Duke University) and John Cogswell (Glaxo Wellcome) for the gift of control p21 and p27 adenoviruses; Glen Nemerow and Dan von Segrenn (Scripps) for advice on adenovirus production, purification, and titer determination; Jean Wang and Laura Whittaker (UCSD) and Tyler Jacks (MIT) for MEFs; Nick Rhind for advice on irradiation and digital microscopy; and Peter Vogt, Paul Russell, Curt Wittenberg, Ben Cravatt, Kevin Sullivan, and Fred Jones for critical reading of the manuscript.
This work was supported by NIH grant GM46006 to S.I.R.
REFERENCES
- 1.Agarwal M L, Agarwal A, Taylor W R, Stark G R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beamish H, Williams R, Chen P, Lavin M F. Defect in multiple cell cycle checkpoints in ataxia-telangiectasia postirradiation. J Biol Chem. 1996;271:20486–20493. doi: 10.1074/jbc.271.34.20486. [DOI] [PubMed] [Google Scholar]
- 3.Blasina A, Paegle E S, McGowan C H. The role of inhibitory phosphorylation of cdc2 following DNA replication block and radiation-induced damage in human cells. Mol Biol Cell. 1997;8:1013–1023. doi: 10.1091/mbc.8.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso M, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Ko L J, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 7.Chin Y E, Kitagawa M, Su W C, You Z H, Iwamoto Y, Fu X Y. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 8.Clurman B E, Sheaff R J, Thress K, Groudine M, Roberts J M. Turnover of cyclin E by the ubiquitin-proteosome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]
- 9.Cordon-Cardo C. Mutation of cell cycle regulators: biological and clinical implications for human neoplasia. Am J Pathol. 1995;147:545–560. [PMC free article] [PubMed] [Google Scholar]
- 10.Datto M B, Li Y, Panus J F, Howe D J, Xiong Y, Wang X F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 12.Diatloff-Zito C, Turleau C, Cabanis M O, de Grouchy J. Low dose rate ionizing radiation induces increased growth capacities of d-deletion retinoblastoma skin fibroblasts. Carcinogenesis. 1984;5:1305–1310. doi: 10.1093/carcin/5.10.1305. [DOI] [PubMed] [Google Scholar]
- 13.Dulic V, Lees E, Reed S I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 14.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–23. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 15.Dulic V, Stein G H, Far D F, Reed S I. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role in the G2/M-phase transition? Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Deiry W, Harper J W, O’Connor P M, Velculescu V E, Canman C E, Jackman J, Pietenpol J A, Burrell M, Hill D E, Wang Y, Wiman K G, Mercer W E, Kastan M B, Kohn K W, Elledge S J, Kinzler K W, Vogelstein B. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 17.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 18.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 19.Fero M L, Rivkin M, Tasch M, Porter P, Carow C E, Firpo E, Polyak K, Tsai L H, Broudy V, Perlmutter R M, Kaushansky K, Roberts J M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 20.Gallant P, Fry A M, Nigg E A. Protein kinases in the control of mitosis: focus on nucleocytoplasmic trafficking. J Cell Sci Suppl. 1995;19:21–28. doi: 10.1242/jcs.1995.supplement_19.3. [DOI] [PubMed] [Google Scholar]
- 21.Gallie B L. Retinoblastoma gene mutations in human cancer. N Engl J Med. 1994;330:786–787. doi: 10.1056/NEJM199403173301112. [DOI] [PubMed] [Google Scholar]
- 22.Girard F, Strausfeld U, Fernandez A, Lamb N J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 23.Guadagno T M, Newport J W. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- 24.Guan K L, Jenkins C W, Li Y, Nichols M A, Wu X, O’Keefe C L, Matera A G, Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994;8:2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- 25.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 26.Hannon G, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 27.Harper J W, Elledge S J, Keyomarsi K, Dynlacht B, Tsai L H, Zhang P, Dobrowolski S, Bai C, Connell C L, Swindell E, Fox M P, Wei N. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper J W, Elledge S J. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins M M, Wilson L M, Burton H S, Potok M H, Winter D L, Marsden H B, Stovall M A. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst. 1996;88:270–278. doi: 10.1093/jnci/88.5.270. [DOI] [PubMed] [Google Scholar]
- 30.Hengst L, Dulic V, Slingerland J M, Lees E, Reed S I. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hengst L, Reed S I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 32.Herrera R E, Sah V P, Williams B O, Makela T P, Weinberg R A, Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996;16:2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirai H, Roussel M F, Kato J Y, Ashmun R A, Sherr C J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Jacks, T. Personal communication.
- 34.Jeffrey P D, Russo A A, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich N P. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 35.Jiang H, Lin J, Su Z Z, Collart F R, Huberman E, Fisher P B. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21 WAF1/CIP1 expression in the absence of p53. Oncogene. 1994;9:3397–3406. [PubMed] [Google Scholar]
- 36.Jiang H, Lin J, Su Z Z, Herlyn M, Kerbel R S, Weissman B E, Welch D R, Fisher P B. The melanoma differentiation-associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene. 1995;10:1855–1864. [PubMed] [Google Scholar]
- 37.Kiyokawa H, Kineman R D, Manova T K, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 38.Koyama H, Raines E W, Bornfeldt K E, Roberts J M, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 39.Lee M H, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 40.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacLeod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka S, Edwards M C, Bai C, Parker S, Zhang P, Baldini A, Harper J W, Elledge S J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 43.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Megyesi J, Udvarhelyi N, Safirstein R L, Price P M. The p53-independent activation of transcription of p21 WAF1/CIP1/SDI1 after acute renal failure. Am J Physiol. 1996;271:F1211–F1216. doi: 10.1152/ajprenal.1996.271.6.F1211. [DOI] [PubMed] [Google Scholar]
- 45.Missero C, Calautti E, Ekner R, Chin J, Tsai L-H, Livingston D M, Dotto G P. Involvement of the cell cycle inhibitor Cip1/WAF1 and the E1a-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto G P. The absence of p21Cip1/Waf1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 47.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y, Nakayama K. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 49.Nelson W G, Kastan M B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994;14:1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nigg E A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 51.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 52.Novitch B G, Mulligan G J, Jacks T, Lassar A B. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parry D, Bates S, Mann D J, Peters G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumor suppressor gene product. EMBO J. 1995;14:503–511. doi: 10.1002/j.1460-2075.1995.tb07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308:697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polyak K, Kato J Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 58.Polyak K, Lee M H, Erdjument B H, Koff A, Roberts J M, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 59.Reed S I. Cyclin E: in mid-cycle. Biochim Biophys Acta. 1996;1287:151–153. doi: 10.1016/0304-419x(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 60.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynisdottir I, Massague J. The subcellular location of p15INK4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 62.Russo A A, Jeffrey P D, Patten A K, Massague J, Pavletich N P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 63.Russo A A, Jeffrey P D, Pavletich N P. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 64.Schwarz J K, Bassing C H, Kovesdi I, Datto M B, Blazing M, George S, Wang X F, Nevins J R. Expression of the E2F1 transcription factor overcomes type beta transforming growth factor-mediated growth suppression. Proc Natl Acad Sci USA. 1995;92:483–487. doi: 10.1073/pnas.92.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 66.Slebos R J, Lee M H, Plunkett B S, Kessis T D, Williams B O, Jacks T, Hedrick L, Kastan M B, Cho K R. p53-dependent G1 arrest involves pRb-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1994;91:5320–5324. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slingerland J M, Hengst L, Pan C H, Alexander D, Stampfer M R, Reed S I. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor beta-arrested epithelial cells. Mol Cell Biol. 1994;14:3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith R C, Branellec D, Gorski D H, Guo K, Perlman H, Dedieu J F, Pastore C, Mahfoudi A, Denefle P, Isner J M, Walsh Kenneth. p21Cip1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 1997;11:1674–1689. doi: 10.1101/gad.11.13.1674. [DOI] [PubMed] [Google Scholar]
- 69.Stein G H, Dulic V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 70.Steinman R A, Hoffman B, Iro A, Guillouf C, Liebermann D A, El-Houseini M. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994;9:3389–3396. [PubMed] [Google Scholar]
- 71.Stern B, Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- 72.Stillman B. Cell cycle control of DNA replication. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- 73.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 74.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abbrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 75.Waldman T, Lengauer C, Kinzler K W, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 76.Won K A, Reed S I. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 1996;15:4182–4193. [PMC free article] [PubMed] [Google Scholar]
- 77.Wu H, Wade M, Krall L, Grisham J, Xiong Y, Van Dyke T. Targeted in vivo expression of the cyclin-dependent kinase inhibitor p21 halts hepatocyte cell-cycle progression, postnatal liver development and regeneration. Genes Dev. 1996;10:245–260. doi: 10.1101/gad.10.3.245. [DOI] [PubMed] [Google Scholar]
- 78.Zeng Y X, el-Deiry W S. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene. 1996;12:1557–1564. [PubMed] [Google Scholar]
- 79.Zhu X, Ohtsubo M, Enhmer R M, Roberts J M, Assoian R K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]