ABSTRACT
Coronaviruses (CoVs) can emerge from zoonotic sources and cause severe diseases in humans and animals. CoVs encode for a macrodomain (Mac1) that binds to and removes ADP-ribose from target proteins. SARS-CoV-2 Mac1 promotes virus replication in the presence of interferon (IFN) and blocks the production of IFN, although the mechanisms by which it mediates these functions remain unknown. Mac1 inhibitors could help elucidate these mechanisms and serve as therapeutic agents against CoV-induced diseases. We previously identified compound 4a (a.k.a. MCD-628), a pyrrolo-pyrimidine that inhibited Mac1 activity in vitro at low micromolar levels. Here, we determined the binding mode of 4a by crystallography, further defining its interaction with Mac1. However, 4a did not reduce CoV replication, which we hypothesized was due to its acidic side chain limiting permeability. To test this hypothesis, we developed several hydrophobic derivatives of 4a. We identified four compounds that both inhibited Mac1 in vitro and inhibited murine hepatitis virus (MHV) replication: 5a, 5c, 6d, and 6e. Furthermore, 5c and 6e inhibited SARS-CoV-2 replication only in the presence of IFNγ, similar to a Mac1 deletion virus. To confirm their specificity, we passed MHV in the presence of 5a to identify drug-resistant mutations and identified an alanine-to-threonine and glycine-to-valine double mutation in Mac1. Recombinant virus with these mutations had enhanced replication compared with the WT virus when treated with 5a, demonstrating the specificity of these compounds during infection. However, this virus is highly attenuated in vivo, indicating that drug resistance emerged at the expense of viral fitness.
IMPORTANCE
Coronaviruses (CoVs) present significant threats to human and animal health, as evidenced by recent outbreaks of MERS-CoV and SARS-CoV-2. CoVs encode for a highly conserved macrodomain protein (Mac1) that binds to and removes ADP-ribose from proteins, which promotes virus replication and blocks IFN production, although the exact mechanisms remain unclear. Inhibiting Mac1 could provide valuable insights into these mechanisms and offer new therapeutic avenues for CoV-induced diseases. We have identified several unique pyrrolo-pyrimidine-based compounds as Mac1 inhibitors. Notably, at least two of these compounds inhibited both murine hepatitis virus (MHV) and SARS-CoV-2 replication. Furthermore, we identified a drug-resistant mutation in Mac1, confirming target specificity during infection. However, this mutant is highly attenuated in mice, indicating that drug resistance appears to come at a fitness cost. These results emphasize the potential of Mac1 as a drug target and the promise of structure-based inhibitor design in combating CoV infections.
KEYWORDS: SARS-CoV2, COVID-19, coronavirus, murine hepatitis virus, COVID-19, nsp3 macrodomain, ADP-ribosylation
INTRODUCTION
CoVs are large, positive-sense RNA viruses that infect a wide variety of mammalian species, including humans. Some human CoVs (HCoVs), such as HCoV-OC43, HKU1, NL63, and HCoV-229E, are endemic and contribute to the common cold, whereas others, severe acute respiratory syndrome (SARS)-CoV, Middle East respiratory syndrome (MERS)-CoV, and SARS-CoV-2 have caused epidemic outbreaks of severe disease and human fatalities. The recent COVID-19 pandemic caused by SARS-CoV-2 resulted in the deaths of over 7 million people worldwide and had profound social and economic consequences (1). Beyond the devastating human toll, the pandemic disrupted healthcare systems, led to widespread economic downturns, and prompted significant changes in global public health infrastructure and policies (2). SARS-CoV-2 is now endemic in the human population and continues to cause severe disease in humans. Furthermore, many other CoVs have been identified in wildlife, posing a continuous threat of zoonotic transmission that could lead to additional epidemics (3). Thus, there is an urgent need for novel therapeutic interventions and further vaccine development.
CoVs evade the host’s innate immune response by encoding for multiple proteins that either repress the production of interferon (IFN) or directly inhibit IFN-stimulated genes (ISGs) (4, 5). Several PARP proteins are highly induced by IFN and are part of the antiviral response (6, 7). Most PARPs act as ADP-ribosyltransferases (ARTs) that add single (mono) or multiple (poly) units of ADP-ribose onto proteins (8). ADP-ribosylation can be reversed by several different classes of enzymes, including macrodomains (9). All CoVs, alphaviruses, Hepatitis E virus, and Rubella virus encode a macrodomain in their genome, indicating that a broad spectrum of positive-sense RNA viruses utilize macrodomains to reverse ADP-ribosylation during infection (10, 11). For CoVs, the conserved macrodomain is encoded within non-structural protein 3 (nsp3) and is called Mac1. Nsp3 has multiple domains and is the largest protein encoded by the CoV genome with a molecular mass of ~200 kD and contains multiple modular domains, including the papain-like protease domain (12). Mac1 binds to and removes ADP-ribose from protein, countering host PARPs (7, 13). Prior research has shown that murine hepatitis virus (MHV), SARS-CoV, MERS-CoV, and SARS-CoV-2 viruses engineered with point mutations that reduce Mac1 ADP-ribose binding or hydrolysis activity replicate poorly, lead to enhanced IFN and pro-inflammatory cytokine responses, and cause minimal disease in animal models of infection (14–22). Furthermore, recombinant alphaviruses with macrodomain mutations are also highly attenuated in cell culture and mice (23–25). Understanding the role of viral macrodomains in immune evasion and viral replication is crucial for developing effective therapeutic interventions and vaccines.
Interestingly, the complete deletion of SARS-CoV-2 Mac1 does not substantially impair viral replication in cell culture, which contrasts with other CoVs, such as MHV and MERS-CoV, where Mac1 deletion leads to unrecoverable viruses (20). However, SARS-CoV-2 Mac1-deletion and point mutant viruses exhibited increased sensitivity to IFN-γ, led to increased production of IFN and ISGs, and did not cause severe disease in mice (20–22). This indicates that SARS-CoV-2 Mac1 plays a critical role during infection, although its specific targets during infection and downstream consequences, such as its effect on the viral lifecycle, remain largely unknown. Mac1 inhibitors could thus be useful tools to help identify these targets and better understand how Mac1 directly promotes virus replication and pathogenesis. Furthermore, as Mac1 is completely conserved across all CoVs and is vital for viral pathogenesis, it could be a unique therapeutic target for SARS-CoV-2 and other potential pandemic CoVs (3).
Since the outbreak of SARS-CoV in 2003 and up to the start of the COVID-19 pandemic, several studies have determined the structure of Mac1 from multiple CoVs and alphaviruses, including SARS-CoV, 229E, Infectious Bronchitis Virus (IBV), MERS-CoV, HKU4, Chikungunya virus (CHIKV), and Venezuelan Equine Encephalitis virus (VEEV) (26–31). Much like macrodomains that had been discovered from species such as archaea (32), the viral macrodomains form an αβα sandwich-like structure with several β-sheets surrounded by α-helices on both sides, with a highly defined ADP-ribose binding pocket. Shortly after the pandemic began, several SARS-CoV-2 Mac1 structures were determined, which provided detailed atomic-level resolution of the SARS-CoV-2 Mac1 protein, facilitating drug-discovery efforts (13, 33–36).
Multiple groups have now identified Mac1 inhibitors through high-throughput screening and targeted drug development using these crystal structures (37–46). Through these efforts, several compounds with IC50 values between 0.4 and 10 μM have been discovered with high specificity in vitro (47). One of these studies utilized a unique crystallography-based fragment screen that identified several small molecules that bound to the ADP-ribose-binding pocket of Mac1 (37). These fragments served as promising starting points for further inhibitor development for multiple groups (38, 39, 41). Starting with a small pyrrolo-pyrimidine fragment with weak in vitro potency (IC50 of 180 μM), we synthesized a series of primary and secondary amino acid-based pyrrolo-pyrimidines to determine whether more potent Mac1 inhibitors could be developed. The previously described luminescent-based AlphaScreen (AS) assay was utilized to screen ~60 pyrrolo-pyrimidines for their ability to inhibit Mac1-ADP-ribose binding. Of these pyrrolo pyrimidines, we identified a tryptophanate (MCD-628) that inhibited SARS-CoV-2 Mac1-ADP-ribose binding with an IC50 of 6.1 μM. MCD-628 incubation with Mac1 also increased its thermal stability to nearly the same degree as ADP-ribose, indicating that it directly binds to Mac1. However, this compound contains a carboxylic acid group that suggests it would have poor permeability and be unlikely to inhibit virus replication. Many potent Mac1 inhibitors have acidic or highly polar moieties, likely limiting their ability to inhibit virus replication or pathogenesis (47). To date, only one Mac1 inhibitor has been published that represses CoV replication in cell culture (42). Thus, future Mac1 inhibitors must be designed to increase their inhibition of Mac1 biochemical functions in vitro and repress CoV replication in cell culture or animal model infections.
In this study, we solved a co-crystal structure of Mac1 with MCD-628 (termed 4a herein) and designed a novel series of pyrrolo-pyrimidine-based Mac1 inhibitors with increased lipophilicity to identify compounds that could both inhibit Mac1 in vitro and repress CoV replication in cell culture. We replaced the acidic moiety of our lead compound, MCD-628 (termed 4a herein), with several esters and amide couplings with hydrophobic pyridines. These modifications largely improved cell permeability while maintaining inhibitory activity in vitro. We identified four compounds that substantially repressed MHV replication in cell culture, including two that repressed MHV and SARS-CoV-2. Importantly, these compounds only suppressed SARS-CoV-2 in the presence of IFN-γ, in line with results demonstrating that Mac1-deleted or mutated SARS-CoV-2 viruses are highly sensitive to IFN-γ (20–22). Additionally, mutations conferring resistance to one of these inhibitors were identified, further confirming their target specificity. These findings demonstrate that Mac1 inhibitors can repress virus replication and offer a promising platform for developing Mac1 chemical probes and CoV antivirals.
RESULTS
Structural and biochemical analysis of pyrrolo-pyrimidine-based SARS-CoV-2 Mac1 inhibitors
Using a series of amino acid-based 7H-pyrrolo[2,3-d] pyrimidines, we previously created compound 4a (S), derived from tryptophan, that inhibited SARS-CoV-2 Mac1 binding to ADP-ribose with a 6.1 μM IC50 value (Fig. 1A and B) (41). 4a also inhibited Mac1 enzyme activity and bound to Mac1, as demonstrated by a thermal shift profile similar to Mac1’s natural ligand, ADP-ribose (41). Having established that 4a binds and inhibits Mac1 in vitro, we sought to get more insight into the mechanism by which 4a binds to Mac1 by determining the structure of 4a with Mac1. We solved the structure of 4a with Mac1 and refined it to 1.1 Å resolution (Fig. 1C and D; Table S1). In this structure, some of the key features are a hydrogen bond with D22 and the backbone of I23, multiple hydrogen bonds between the carboxylate with neighboring water molecules, and finally a hydrogen bond between the indole NH and L126 (Fig. 1C and D). F156, previously seen to form pi-stacking interactions with ADP-ribose and inhibitors, has considerable flexibility, and although it is next to the pyrrolo-pyrimidine and contributes to hydrophobic interactions, the geometry does not allow pi-stacking interactions. This co-crystal structure provides a strong starting point for the synthesis of additional Mac1 inhibitors.
Fig 1.
Crystal structure of 4a provides new insight into its interaction with Mac1. (A and B) Chemical synthesis plan to produce 4a-b (A), and the chemical structure of 4a (B). (C and D) Crystal structure of 4a in two different poses (PDB id. 9GUB). These poses include images where the pyrrolo-pyrimidine is oriented in the front left (C) or in the lower middle (D). Note that the carboxylate makes hydrogen bonds with three different water molecules, and the tryptophanate makes a hydrogen bond with the backbone of L126. The sigma-A weighted 2Fo-Fc electron density map is contoured at 1.0 σ. Waters are shown as red spheres, and hydrogen bonds are illustrated as black dashed lines.
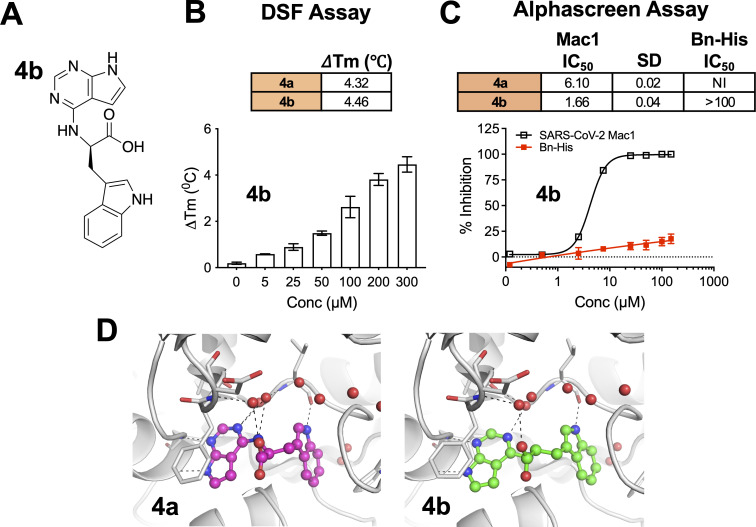
Next, we tested whether the enantiomer of 4a, 4b (R) (Fig. 2A), would also bind and inhibit SARS-CoV-2 Mac1. Indeed, 4b interacted with Mac1, as it had a similar thermal shift profile to that of 4a (Fig. 2B). Furthermore, 4b inhibited Mac1 binding to an ADP-ribosylated peptide in an AlphaScreen assay with an IC50 of 1.66 μM, with almost no inhibition of the Bn-His peptide control (Fig. 2C). We were able to reproduce the experimental binding mode of 4a by molecular modeling and subsequently demonstrated that 4b would interact with Mac1 in a similar manner (Fig. 2D).
Fig 2.
Compound 4b interacts with Mac1 and inhibits Mac1-ADP-ribose binding. (A) Chemical structure of compound 4b. (B) Compound 4b was incubated with SARS-CoV-2 Mac1 at increasing concentrations, and the thermal stability of SARS-CoV-2 Mac1 was determined by a DSF assay. The ΔTm is the average of five experimental replicates. n = 5. (C) Competition assays were used to demonstrate that 4a and 4b block the interaction between Mac1 and ADP-ribosylated peptides in the AS assay. The IC50 represents the average value of 2 independent experiments, each done with three experimental replicates. The graphs are from one experiment representative of two independent experiments. (D) Compounds 4a and 4b were docked into Mac1 using PDB:9GUB. Hydrogen bonds are illustrated as dashed lines.
Compounds 4a/4b have a negatively charged carboxylic acid moiety at physiological pH, which we hypothesized might preclude its ability to cross cellular membranes. To address this potential problem, we first replaced the carboxylic acid with methyl (5a/5b) and isopropyl (5c/5d) esters (Fig. 3A). Unexpectedly, the esters derived from 4b, 5b, and 5d did not demonstrate any significant inhibition of Mac1 in the AlphaScreen assay (data not shown), whereas the 4a derivatives, 5a and 5c, interacted with Mac1 by the thermal shift assay (Fig. 3B) and inhibited Mac1 in the AlphaScreen assay with IC50 values of 14.14 μM and 3.66 μM, respectively (Fig. 3C). Based on modeling, the additional carbon atoms on these molecules appear to protrude out of the binding pocket and have only minimal impact on the overall interaction of these compounds with Mac1 (Fig. 3D). Importantly, the addition of the esters dramatically increased the lipophilicity of these compounds, as the logD at pH 7.4 of these compounds went from −0.61 (4a) to 1.5 (5a) and 3.33 (5c), indicating that the ester-modified compounds are much more likely to cross cellular membranes and target Mac1 during infection.
Fig 3.
Compounds 5a and 5c interact with Mac1 and inhibit Mac1-ADP-ribose binding. (A) Modification of 4a-b to ester derivatives 5a and 5c. (B) Compounds 5a and 5c were incubated with SARS-CoV-2 Mac1 at increasing concentrations, and the thermal stability of SARS-CoV-2 Mac1 was determined by a DSF assay. The ΔTm is the average of five experimental replicates. n = 5. (C) Competition assays were used to demonstrate that 5a and 5c block the interaction between Mac1 and ADP-ribosylated peptides in the AS assay. The IC50 represents the average value of two independent experiments. n = 3 experimental replicates. (D) Compounds 5a and 5c were docked into Mac1 using PDB: 9GUB. Hydrogen bonds are illustrated as dashed lines. (E) The LogD values were experimentally determined using the shake-flask method for 4a, 5a, and 5c.
Pyrrolo-pyrimidine-based esters inhibit MHV-JHM (JHMV) replication
We next aimed to determine if these compounds could inhibit CoV replication. In this study, we evaluated the antiviral activity of Mac1 inhibitors in both JHMV and SARS-CoV-2 models. The deletion of Mac1 in SARS-CoV-2 led to only a modest growth defect (2-fold to 3-fold), unless cells were pre-treated with IFN-γ, suggesting that Mac1 is not a critical factor for SARS-CoV-2 replication in cell culture. However, Mac1 is critical for the replication of JHMV, as we were unable to recover JHMV with a deletion of Mac1, making JHMV a better model for initial testing of Mac1 inhibitors (19). To enable more efficient screening of compounds for impacts on virus replication, we replaced ORF4 of JHMV with nanoluciferase (JHMV-nluc) (Fig. S1), as the deletion of ORF4 does not affect JHMV replication or pathogenesis (48). JHMV-nluc replicated like WT virus (Fig. 4A) and, importantly, produced over 106 light units at peak replication (Fig. 4B). Next, we tested the ability of 4a, 4b, 5a, and 5c to inhibit JHMV-nluc replication in DBT cells at concentrations ranging from 25 to 200 μM, using GS-441524 (active metabolite of remdesivir) as a positive control for inhibition at concentrations comparable with those used for the Mac1 inhibitors. Although the reported EC50 for GS-441524 is ~0.84 μM, and experimental concentrations ranged in other studies have generally been between 0 and 20 μM, we opted for a higher concentration of GS-441524 in our experiments so that we could directly compare the activity of our compounds with this known standard (49). DBT cells are astrocytoma cell lines that are susceptible to JHMV. JHMV replication in DBT cells is highly dependent on Mac1 activity, as a D1329A mutant virus replicates very poorly in these cells (19). As expected, 4a and 4b did not affect JHMV replication, as opposed to GS-441524, which inhibited virus replication at all concentrations. In contrast, both 5a and 5c inhibited JHMV replication, with 5c being significantly more potent, having inhibited JHMV to nearly the same level as GS-441524 at 25 μM (Fig. 4C). Importantly, none of these molecules showed substantial cytotoxicity at the concentrations tested (Fig. S2A and B).
Fig 4.
Compounds 5a and 5c, but not 4a, inhibit MHV replication. (A) In total, 17 Cl-1 cells were infected with JHMV-WT and JHMV-nLuc viruses at an MOI = 0.1. Cells and supernatants were collected at indicated time points, and progeny virus was determined by plaque assay; (B) 17 Cl-1 cells were infected as described in A. Lysates were collected at indicated times, and luciferase activity was determined using a nano-Glo luciferase assay kit measured as per the manufacturer’s instructions. The results in A and B are from one experiment representative of two independent experiments. N = 3 biological replicates. (C) DBT cells were infected with JHMV-nLuc at an MOI = 0.1, and at one hpi, the indicated concentration of each compound was added to the media. Lysates were collected at 20 hpi, and luciferase activity was measured as described in B. (D and E) DBT cells were infected with JHMV-WT, and at 1 hpi, the indicated concentration of each compound was added to the media. Cells and supernatants were collected at 20 hpi, and progeny virus was measured by plaque assay. The results in C-E are from one experiment representative of two independent experiments. n = 3 biological replicates. (F) The combined average % JHMV-WT inhibition by 5c on DBT cells over two independent experiments. (G and H) L929 cells were infected with JHMV-WT, and at 1 hpi, the indicated concentration of each compound was added to the media. Cells and supernatants were collected at 20 hpi, and progeny virus was measured by plaque assay. The results in G-H are from one experiment representative of two independent experiments. n = 3 biological replicates. (I) The combined average % JHMV-WT inhibition by 5c on L929 cells over three independent experiments. The results in C, D, E, G, and H are from one experiment representative of three independent experiments. n = 3 biological replicates.
Next, we tested whether 5a or 5c would impact the production of infectious virus. Indeed, we found that both 5a and 5c inhibited the production of infectious virus following the infection of both DBT and L929 cells with JHMV (Fig. 4D and E). Compound 5a only inhibited virus production at 200 μM, whereas 5c inhibited virus replication with as little as 25 μM and decreased replication by ~1.5 and 3 logs at 50 μM on DBT and L929 cells, respectively. To better determine the EC50 for 5c, we tested its activity at concentrations from 0 to 50 μM on DBT, L292, and 17 Cl-1 cells (Fig. 4F and G; Fig. S3A). The inhibition of virus production at these concentrations was dose-dependent, and using these data, we determined that the EC50 for 5c on was ~10–20 μM, not substantially different from its IC50 of 3.66 μM (Fig. 4H and I; Fig. S3B).
Next, amide couplings were conducted with carboxylates 4a and 4b to create 25 additional compounds, many of which included highly hydrophobic side chains to increase the lipophilicity. Of these, five compounds demonstrated IC50 values of less than 10 μM in our initial screening and were named 6a–6e (Fig. 5A and data not shown). Compounds 6a and 6b contain a pyridine group attached to the amide, with the only difference being a chlorine atom on 6b. Compounds 6d (S) and 6e (R) are enantiomers and only differ from 6a in the position of the nitrogen on the pyridine. Finally, 6c has an amide group that replaces the carboxylate. Of these compounds, only 6e was derived from 4b, whereas the rest were derived from 4a. Following dose-response curves, we found that each of these five compounds had very similar IC50 values ranging from 4.0 to 8.4 μM in the AlphaScreen assay (Fig. 5B). They also had thermal shifts of 1–3°C in the DSF assay when incubated with the SARS-CoV-2 Mac1 protein, indicating a direct interaction with Mac1 (Fig. 5C). All of them had cLogD values between 1 and 3, indicating increased lipophilicity compared with the parent compound 4a (LogD −0.61) (Fig. 5D). Based on our modeling data, the position of each of these molecules in the binding pocket does not change significantly. The only major difference is the position of the pyridine for each molecule (Fig. 5E). For 6a, 6b, and 6d, the pyridine protrudes out from the pocket and into slightly different poses for each one. In contrast, the pyridine of 6e extends into the oxyanion subsite, which could explain its slightly greater inhibition of Mac1-ADP-ribose binding in the AlphaScreen assay.
Fig 5.
Group 6 compounds interact with Mac1 and inhibit Mac1-ADP-ribose binding. (A) Modification of 4a-b to several new derivatives, 6a–6e. 6a–6d are derivatives of 4a, whereas 6e is a derivative of 4b. (B) Competition assays were used to demonstrate that 6a–6e block the interaction between Mac1 and ADP-ribosylated peptides in the AS assay. (C) Compounds 6a–6e were incubated with SARS-CoV-2 Mac1 at increasing concentrations, and the thermal stability of SARS-CoV-2 Mac1 was determined by a DSF assay. Quantification data in B and C represent the average value of 2 independent experiments. (D) Predicted cLogD values of 6a–6e. (E) Compounds 6a–6e were docked into Mac1. Hydrogen bonds are illustrated as dashed lines.
Inhibition of JHMV replication by 6d and 6e
Similar to series 5, we next tested if compounds in series 6 could inhibit virus replication. Using JHMV-nLuc, our initial screening found that only 6d and 6e inhibited light production from MHV replication in a dose-dependent manner (Fig. 6A). Furthermore, 6d and 6e demonstrated no substantial impact on cell viability (Fig. S4A and B). We next performed dose-response curves for 6d and 6e on both DBT and L929 cells at concentrations ranging from 50 to 200 μM and found that the compounds inhibited MHV in a dose-dependent manner on both cells, with better activity on DBT cells (Fig. 6B and C). Using these results, we determined that the EC50 value for 6d and 6e on DBT cells was 77.1 and 53.5 μM, respectively (Fig. 6D and E).
Fig 6.
Compounds 6d and 6e inhibit JHMV replication. (A) DBT cells were infected with JHMV-nLuc at an MOI = 0.1, and at 1 hpi, the indicated concentration of each compound was added to the media. GS-441524 (active metabolite of remdesivir) was used as a positive control for inhibition at relative experimental concentrations. Lysates were collected at 20 hpi, and luciferase activity was measured. Luciferase activity was determined using a nano-Glo luciferase assay kit measured as per the manufacturer’s instructions. The results in A are from one experiment and are representative of two independent experiments. n = 3 biological replicates. (B) DBT cells were infected with JHMV-WT, and at one hpi, the indicated concentration of each compound was added to the media. Cells and supernatants were collected at 20 hpi, and progeny virus was measured by plaque assay. (C) L929 cells were infected with JHMV-WT, and at one hpi, the indicated concentration of each compound was added to the media. Cells and supernatants were collected at 20 hpi, and progeny virus was measured by plaque assay. The results in B-C are from one experiment representative of two independent experiments. n = 3 biological replicates. (D and E) The combined average % JHMV-WT inhibition by 6d and 6e, respectively, on DBT cells over two independent experiments.
Compounds 5c and 6e inhibit SARS-CoV-2 replication
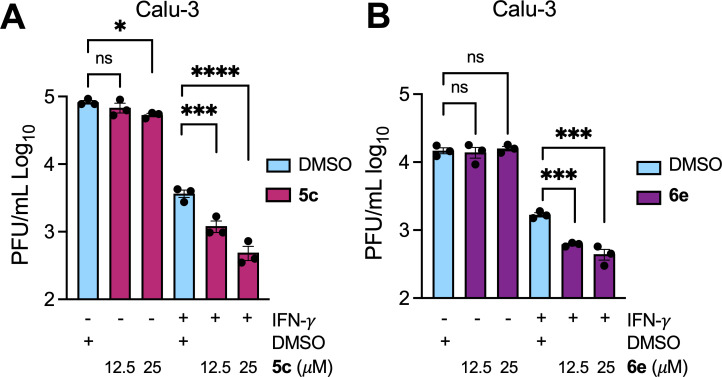
Having established antiviral activity against JHMV, we next wanted to determine if our pyrrolo-pyrimidine-based compounds could also inhibit SARS-CoV-2 replication. We hypothesized that our compounds would be more potent against SARS-CoV-2 as they were identified for their ability to inhibit the SARS-CoV-2 Mac1 protein, not the MHV Mac1 protein, in vitro (Fig. 3 and 5). Recently, we demonstrated that a full Mac1 deletion virus (SARS-CoV-2 ΔMac1) replicates normally in cell culture compared with the WT virus, except when cells are pre-treated with IFN-γ. IFN-γ induces the upregulation of PARP enzymes in epithelial cells, which leads to the PARP14-dependent production of IFN-induced cytoplasmic ADP-ribose bodies (ICABs), which can be eliminated by Mac1 protein expression (50–52). In the presence of 100 U of IFN-γ, SARS-CoV-2 ΔMac1 replicated ~10-fold worse than WT virus in Calu-3 cells, and this defect can be reversed by the addition of a PARP14 inhibitor, demonstrating that Mac1 is a viral countermeasure to PARP14 antiviral activity (20, 53). Importantly, we did not see the same phenotype when cells were pretreated with IFN-γ. Thus, we hypothesized that our Mac1 inhibitors would only inhibit SARS-CoV-2 if cells are pre-treated with IFN-γ. Hence, we pretreated Calu-3 cells with IFN-γ and then infected the cells in the presence or absence of 5c or 6e from 0 to 25 μM (Fig. 7). First, we confirmed that neither 5c nor 6e affected the viability of Calu-3 cells (Fig. S5). In the absence of IFN-γ, 6e did not reduce infectious virus production, and 5c only reduced viral titers ~ 2-fold at 25 μM. In contrast, each compound reduced infectious virus production in the presence of IFN-γ at both 12.5 and 25 μM (Fig. 7A and B); 5c reduced viral titers by 3-fold and 7.5-fold, whereas 6e reduced them by 2.7-fold and 3.8-fold at 12.5 and 25 μM, respectively, indicating that the EC50 for each compound would be no greater than 12.5 μM, again similar to their IC50 values. Furthermore, we also found that 5c and 6e did not inhibit MERS-CoV replication (Fig. S6). These results demonstrate that our pyrrolo-pyrimidine-based Mac1 inhibitors are more potent against SARS-CoV-2 than other CoVs, and the fact that they only inhibit virus production in the presence of IFN-γ strongly indicates that they specifically target Mac1 at the concentrations tested.
Fig 7.
Compounds 5c and 6e inhibit SARS-CoV-2 replication. (A and B) Calu-3 cells were mock or IFN-γ pre-treated (100 U) for 18 h, then were infected with SARS-CoV-2, and at 1 h post-infection (hpi), the indicated concentration of 5c (A) or 6e (B) was added to the media. Cells and supernatants were collected at 20 hpi, and progeny virus was measured by plaque assay. The results in A-B are from one experiment representative of 3 independent experiments. n = 3 biological replicates.
To further demonstrate the specificity of our compounds, we looked to identify drug-resistant mutations in JHMV. We used JHMV for these experiments to avoid any potential gain-of-function issues with the SARS-CoV-2 virus. We passaged three separate biological replicates of JHMV 3× in the presence of 150 μM 5a (5a1, 5a2, and 5a3) (Fig. 8A). At this concentration, 5a inhibits infectious virus production by ~0.5 logs so that we would get a suitable concentration of virus produced to continue passaging (Fig. 8B). Virus exposed to 5a became resistant by passage 2. We then took passage 3 virus and plaque-picked two separate biological replicates twice before sequencing the macrodomain from each isolate. In one of the plaque-picked viruses, we identified by Sanger sequencing two macrodomain mutations in neighboring residues, A1438T and G1439V (Fig. 8C). Remarkably, these are the same mutations that appeared in previous work with a different compound (42). Furthermore, we had engineered a recombinant virus with these mutations in a prior study evaluating different point mutants of Mac1, which demonstrated that this virus replicated at near WT levels in cell culture but was highly attenuated in mice (19). Notably, the A1438T/G1439V recombinant virus had increased replication compared with WT virus in the presence of 5a (Fig. 8D). This result demonstrates that these mutations confer some resistance to 5a and suggests that it targets Mac1. We also found that this virus had increased replication in the presence of 5c, although the increase was not statistically significant (Fig. 8D).
Fig 8.
Identification of a Mac1 drug-resistant mutation. (A) Cartoon depiction of the passaging method for creating the drug-resistant virus. Three separate wells of DBT cells were initially infected with 0.1 MOI JHMV in the presence of DMSO or 5a. Each well was then passaged by taking 100 µL of cells/supernatants from the prior passage and infecting a new well of DBT cells. Image was created using BioRender.com. (B) Cells and supernatants were collected at 18–20 hpi at each passage, and progeny virus was measured by plaque assay. (C) Progeny virus at passage three was sequenced, which identified a two amino acid A1439T/G1439V mutation. (D) DBT cells were infected with WT or A1438T/G1439V recombinant virus at an MOI of 0.1 in the presence of DMSO, 5a, or 5c. Cells and supernatants were collected at 20 hpi, and progeny virus was measured by plaque assay. The data in D is from one experiment representative of three independent experiments. n = 3 biological replicates.
In total, we developed a series of pyrrolo-pyrimidine-based compounds that inhibit Mac1 activity in vitro and also repress both MHV and SARS-CoV-2 replication in cell culture by specifically targeting Mac1.
DISCUSSION
Coronaviruses (CoVs) remain a global health threat, as demonstrated by the SARS-CoV-2 pandemic and earlier outbreaks of SARS-CoV and MERS-CoV. Over the past two decades, research on the conserved CoV macrodomain (Mac1) has found that it is critical for pathogenesis and promotes virus replication in the presence of interferon (IFN). The development of Mac1 inhibitors offers therapeutic potential and serves as a valuable strategy for probing the underlying mechanisms by which Mac1 promotes viral pathogenesis. In this study, we focused on improving previously developed Mac1 inhibitors as both chemical and antiviral agents (41).
Although reverse genetics has proven to be a powerful tool in understanding Mac1 biology, there are several limitations to CoV reverse genetic systems. First, they are not available to all researchers; deletion mutations are not always recoverable and may have undesired impacts on neighboring genes, point mutations may not fully attenuate the functions of the protein, and they do not allow for temporal evaluation of function. Developing molecular probes targeting SARS-CoV-2 Mac1 would help uncover Mac1’s biological functions and advance our understanding of CoV biology, particularly how CoVs evade the host immune response. Specifically, Mac1 targeting probes offer an additional method to investigate how ADP-ribosylation, a process reversed by Mac1, impacts the interaction between the virus and host immune responses and disease outcomes during CoV infection, both in vitro and in vivo. This is exemplified by previous studies where SARS-CoV-2 Mac1 deletion or mutation in animal models leads to attenuation of viral replication and enhanced interferon production, suggesting that the lack of Mac1 weakens the virus, whereas at the same time, it strengthens host defenses. This fitness tradeoff could emerge with the addition of our Mac1 inhibitors in vivo, warranting further exploration of our compounds in animal models, especially since we are aware of the immunological limitations of cell culture that does not fully recapitulate the complexity of the lung environment (20, 21). Additionally, it is crucial to consider that although our current in vitro and cell culture results are promising, further screening and optimization of Mac1 inhibitors are necessary to achieve a level of potency that is therapeutically relevant in vivo. Furthermore, moving these compounds into animal models will require additional studies to evaluate pharmacokinetics, toxicity, and immune-modulatory effects to provide a clearer picture of their potential for clinical application.
Previously, we expanded upon a prior fragment screen and identified several pyrrolo-pyrimidine-based compounds with IC50 values less than 25 μM. Pyrrolo-pyrimidine-based compounds are promising candidates as Mac1 inhibitors due to their molecular mimicry of adenine, which enables them to fit effectively into the ADP-ribose binding pocket of Mac1. This mimicry facilitates strong interactions within Mac1’s active site, making these compounds valuable starting points for inhibitor development (37, 41). The most potent pyrrolo-pyrimidine from our previous work was 4a (MCD-628), a tryptophanate. Furthermore, its enantiomer, 4b, had even more potent inhibitory activity against Mac1 with an IC50 below 2 μM. Here, we solved the crystal structure of 4a with Mac1, which revealed key interactions such as hydrogen bonds with the amino acids D22 and I23, nearby water molecules, and between the indole NH group and L126.
Although 4a inhibited Mac1 activity in vitro, its physicochemical properties, most notably a prominent carboxylic acid that contributed to its negative logD value, were significant impediments to its antiviral activity. Many of the published Mac1 inhibitors, including those we synthesized, contain polar or acidic moieties that could limit their cellular permeability, which is a key determinant in translating in vitro activity into cell culture and in vivo efficacy. To address this problem, those moieties were initially modified to methyl and isopropyl ester groups to improve the lipophilicity of the compounds, as demonstrated with derivatives 5a and 5c, which had substantially improved logD values compared with 4a. Despite the modest impact of these modifications on Mac1 inhibition, increasing the logD value correlated with their ability to inhibit virus replication. Interestingly, the 4a, but not the 4b-derived esters, inhibited Mac1 activity in vitro despite 4b being the more potent inhibitor. Based on the co-crystal structure of 4a and the docking model of 4b (Fig. 2), the esterification could create some steric clash with the pyrimidine in the active conformation binding to Mac1. This was also observed in amide derivatives 6a–6e, where only 6e derived from 4b was active and predicted to have a distinct binding mode from the enantiomer 6d (Fig. 6E).
To further explore the potential for replacing the carboxylate of 4a/4b with more hydrophobic molecules, we introduced several amide-coupled pyridines (series 6). None of these modifications substantially improved the IC50 of this series, as their IC50 values ranged from 4.04 µM (6c) to 8.37 µM (6a). Despite their ability to inhibit Mac1 in vitro, 6a–6c were unable to repress virus replication, whereas 6d and 6e were modest inhibitors of MHV replication, and 6e also inhibited SARS-CoV-2 in the presence of IFN-γ. The reason for this discrepancy is unclear, although it is noted that both 6d and 6e have nitrogen atoms in the 2 position of the pyridine ring, whereas 6a and 6b have the nitrogen in the four position. Regardless, structural insights from inhibitors such as 4a and 4b provide a foundation for developing next-generation inhibitors that can more effectively enter cells and demonstrate robust antiviral activity in vivo. Future strategies to optimize the Mac1 inhibitor antiviral activity will include (i) improving their pharmacokinetic properties, (ii) developing alternative delivery methods such as using nanoparticles to address the drug-delivery challenges, and (iii) structural modifications that enhance the compounds’ ability to penetrate deeper into the Mac1 binding pocket and increase binding affinity.
To demonstrate the specificity of our hit compounds for Mac1 during infection, we first tested both 5c and 6e for the inhibition of SARS-CoV-2 in the presence and absence of IFN-γ, as we have done previously (42). Each compound only inhibited virus replication in the presence of IFN-γ, which strongly indicates that these compounds target Mac1, as it is unlikely that there are other viral proteins where inhibition would demonstrate such stark differences between IFN-γ-treated and untreated cells. To further confirm specificity, we passed MHV in the presence of 5a with the goal of identifying drug-resistant mutations. We identified a resistant virus, and interestingly, it contained a two-amino-acid mutation in Mac1, A1438T/G1439V, which we observed previously after passaging MHV in the presence of a separate compound (42). Having the same mutation appear after passaging MHV in the presence of two different Mac1 inhibitors indicates that Mac1 is highly constrained in the ADP-ribose binding pocket and has limited options for developing resistance. We also previously reported that MHV A1438T/G1439V is highly attenuated in mice, indicating a fitness trade-off with the development of drug resistance against Mac1 targeting compounds. To better understand the fitness trade-offs with these mutations, we plan to further characterize the effect the A1438T/G1439V mutations have on Mac1 ADP-ribose binding and hydrolysis and perform a more substantial evaluation of the replication and drug resistance for both recombinant JHMV and SARS-CoV-2 viruses containing these mutations. Furthermore, we will generate additional drug-resistant mutant viruses and analyze any genetic changes both in Mac1 and across the genome. This will help identify novel resistance mechanisms and contribute to the optimization of Mac1-targeting compounds.
In summary, our findings underscore the potential of pyrrolo-pyrimidine-based compounds as both therapeutic agents and molecular probes, enabling more profound insights into the role of Mac1 in CoV biology. Although this study serves as proof of concept, it is limited to cell culture systems, which have significant immunological limitations and do not fully replicate the complexity of the lung environment. Future studies should be focused on improving these compounds and moving them into ex vivo or in vivo systems to investigate the broader biological impacts of these inhibitors. By refining these inhibitors through targeted structural modifications and addressing pharmacokinetic limitations, we will continue to enhance their efficacy and therapeutic profiles. As we further investigate the mechanisms of Mac1 and its impact on viral replication, we will enable the development of innovative strategies to mitigate the threat posed by highly pathogenic human coronaviruses and potentially other emerging pathogens.
MATERIALS AND METHODS
Chemistry
See supplemental methods.
Cell culture and reagents
Delayed brain tumor (DBT), L929, Vero E6, and HeLa cells expressing the MHV receptor carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) (HeLa-MHVR), and baby hamster kidney cells expressing CEACAM1 (BHK-MVR) (all cell lines gifts provided by Stanley Perlman, University of Iowa) were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, HEPES, sodium pyruvate, nonessential amino acids, and l-glutamine. Calu-3 cells (ATCC) were grown in MEM supplemented with 20% FBS. Human IFN-γ was purchased from R&D Systems. ADP-ribosylated and control peptides were purchased from Cambridge Peptides. Recombinant SARS-CoV-2 proteins were expressed with an N-terminal His-tag from a pET21a + expression vector and purified as previously described (13).
Crystallization, data collection, processing, and refinement
Protein crystallization was performed using the sitting-drop vapor-diffusion method in a Swissci 3D 96-well plate. The well solution contained the reported crystallization condition (35) but varied in PEG 3,000 concentration: 0.1 M CHES pH 9.5, 28-32% PEG (vol/vol) 3000; 100 nL of 0.8 mM SARS-CoV-2 Mac1 was mixed with 100 nL of the crystallization solution using Mosquito pipetting robot (TTP Labtech). Crystals grew within 24 h. Crystals from one droplet were then crushed, diluted in 100 µL reservoir solution, and used as seeding solution; 0.8 mM SARS-CoV-2 Mac1 and 5 mM inhibitor were mixed and incubated at RT for 30 min. Crystallization drops were set up by mixing 100 nL of SARS-CoV-2 Mac1 and ligand solution and 100 nL of the reservoir solution. Then streak seeding was performed. Crystallization plates were monitored at RT with IceBear (54), and co-crystals appeared within 24 h. For cryo-cooling, the crystals were soaked in 0.1 M CHES pH 9.5, 32% PEG (vol/vol) 3000 with 0.5 mM of the compound. X-ray diffraction data were collected on beamline BioMAX at MAX IV, Lund, Sweden. The data set was processed by the XDS program package via XDSGUI (Table S1) (55, 56).
The structures were solved with PHASER (57) by the method of molecular replacement by using SARS-CoV-2 Mac1 (PDB: 8TV6) as a search model. Model building and refinement were performed with Coot (58) and REFMAC5 (59), respectively (Table S1). The structures were visualized in PyMOL version 1.7.2.1 (Schrödinger).
Docking
The solved structure of 4a with Mac1 was prepared using the Schrödinger Protein Preparation Wizard (schrödinger.com), which adds hydrogens, predicts protonation status of titratable groups, optimizes hydrogen bonds, and then performs a constrained minimization. Only the water networks near the ligand were retained. Ligands were prepared using LigPrep and then docked into the receptor using Glide with XP precision (60, 61). The top-scoring models were refined using Prime mmGBSA, allowing flexibility of the ligand and any residue/water within 5 Å of the ligand (62, 63).
AlphaScreen (AS) assay
The AlphaScreen reactions were carried out in 384-well plates (Alphaplate, PerkinElmer, Waltham, MA) in a total volume of 40 µL in buffer containing 25 mM HEPES (pH 7.4), 100 mM NaCl, 0.5 mM TCEP, 0.1% BSA, and 0.05% CHAPS. All reagents were prepared as 4× stocks, and 10 µL of each reagent was added to a final volume of 40 µL. All compounds were transferred acoustically using ECHO 555 (Beckman Inc) and preincubated after mixing with purified His-tagged macrodomain protein (250 nM) for 30 min at RT, followed by the addition of a 10 amino acid biotinylated and ADP-ribosylated peptide [ARTK(Bio) QTARK (Aoa-RADP)S] (Cambridge peptides) (625 nM). After 1 h incubation at RT, streptavidin-coated donor beads (7.5 µg/mL) and nickel chelate acceptor beads (7.5 µg/mL) (PerkinElmer AlphaScreen Histidine Detection Kit) were added under low light conditions, and plates were shaken at 400 rpm for 60 min at RT protected from light. Plates were kept covered and protected from light at all steps and read on the BioTek plate reader using an AlphaScreen 680 excitation/570 emission filter set. For counter screening of the compounds, 25 nM biotinylated and hexahistidine-tagged linker peptide (Bn-His6) (PerkinElmer) was added to the compounds, followed by the addition of beads as described above. For data analysis, the percent inhibition was normalized to positive (DMSO+ labeled peptide) and negative (DMSO + macrodomain + peptide, no ADPr) controls. The IC50 values were calculated via four-parametric non-linear regression analysis, constraining bottom (=0), top (=100), & Hillslope (=1) for all curves.
Differential scanning fluorimetry (DSF)
Thermal shift assay with DSF involved the use of LightCycler 480 Instrument (Roche Diagnostics). In total, a 15 µL mixture containing 8 × SYPRO Orange (Invitrogen) and 10 µM macrodomain protein in buffer containing 20 mM HEPES-NaOH, pH 7.5, and various concentrations of ADP-ribose or hit compounds was mixed on ice in a 384-well PCR plate (Roche). Fluorescent signals were measured from 25 to 95°C in 0.2°C/30/S steps (excitation, 470–505 nm; detection, 540–700 nm). The main measurements were carried out in triplicate. Data evaluation and Tm determination involved the use of the Roche LightCycler 480 Protein Melting Analysis software, and data fitting calculations involved the use of single-site binding curve analysis on GraphPad Prism. The thermal shift (ΔTm) was calculated by subtracting the Tm values of the DMSO from the Tm values of the compounds.
Determination of LogD
In total, 0.11 mg of each compound was weighed out into 2 mL centrifuge tubes; 1 mL of octanol-saturated PBS or 0.1M HCl was added to each centrifuge tube and vortexed to dissolve. Then, 0.5–1.0 mL of the PBS-saturated octanol was added to the 0.5–1.0 mL octanol-saturated PBS supernatant and vortexed. Next, 100 μL of the octanol-saturated PBS phase from each centrifuge tube was added to separate UPLC vials; 100 μL of 50:50 MP H2O:ACN was added to each vial and vortexed. The remaining octanol-saturated PBS layer was removed from the vial using a micropipette and stored in a separate vial, and 100 μL of the PBS-saturated octanol layer from each centrifuge tube was added to separate UPLC vials. In total, 100 μL of 50:50 MP H2O:ACN was added to each vial and vortexed. These procedures were repeated in triplicate and then analyzed by UPLC/UV-VIS.
Cell viability assay
Delayed brain tumor (DBT), L929, and Calu3 Cellular metabolic activity was assessed using a CyQUANT MTT cell proliferation assay (Thermo Fisher Scientific) by following the manufacturer’s instructions.
Generation of recombinant pBAC-JHM constructs
Recombinant pBAC-JHMVIA constructs were created using Red recombination as previously described (17). For pBAC-JHMVIA-nLuc, the nano-luciferase gene was amplified with ends homologous to the 5’ and 3’ ends of ORF4 using the following primers:
F 5’-GGCAGCAAGTAGTTATGGCCCTCATCGGTCCCAAGACTACTATTGCTGCT GTCTTCACACTCGAAGATTTCG-3’
R 5’- GGCGTCACTCACAAGCCAAATCTCCATGTAGCTGGTGG TTACGCCAGAATGCGTTCGCACAGCCGCCAGCCGGTCA GCCAGTGTTACAACCAATTAAC-3’
The PCR product was recombined into pBAC-JHMVIA, replacing the ORF4 gene, creating pBAC-JHMVIA-nLuc (Fig. S1). pBAC-JHMVIA-A1438T/G1439V was previously described (19). BAC DNA was analyzed by restriction enzyme digest, PCR, and direct sequencing for isolation of correct clones.
Reconstitution of recombinant pBAC-JHMV-derived virus
Approximately 1 × 106 BHK-MVR cells were transfected with approximately 0.5–1 µg of pBAC-JHMVIA DNA and 1 µg of pcDNA-MHV-N plasmid using PolyJet (SignaGen) as a transfection reagent. Stocks of the resulting virus were created by infecting ∼1.5 × 107 17 Cl-1 cells at a multiplicity of infection (MOI) of 0.1 PFU/cell and collecting both the cells and supernatant at 16–20 hpi. The cells were freeze-thawed, and debris was removed prior to collecting virus stocks. Virus stocks were quantified by plaque assay on HeLa-MHVR cells and sequenced with the collection of infected 17 Cl-1s or DBT cells using TRIzol. RNA was isolated, and cDNA was prepared using MMLV-reverse transcriptase per the manufacturer’s instructions (Thermo Fisher Scientific). The nLuc gene sequence was amplified by PCR using the same primers as described above for sequencing BACs, and then, the resulting PCR products were sequenced by Sanger sequencing. The sequence was analyzed using DNA Star software.
Virus infection
DBT and L929 were infected at an MOI of 0.1 with MHV strains JHMVIA-WT or JHMVIA-nLuc. Calu-3 cells were infected at an MOI of 0.1 with recombinant SARS-CoV-2 (Wuhan strain). For Calu-3 cells, trypsin-TPCK (1 µg/mL) was added to the medium at the time of infection. All infections included a 1 h adsorption phase, except for Calu-3 cells, where the adsorption phase was increased to 2 h. Compounds were added after the adsorption phase. GS-441524 (MedChem Express) was used as a positive control. Infected cells and supernatants were collected at indicated time points, and titers were determined on HeLa-MHVR (MHV) or Vero E6 cells (SARS-CoV-2). Alternatively, luciferase values were determined using the Promega Dual-Glo luciferase assay system according to the manufacturer’s instructions (Promega). For IFN-γ pretreatment experiments, human IFN-γ was added to Calu-3 cells 18–20 h prior to infection and was maintained in culture media throughout the infection.
Identification of drug-resistant MHV mutant viruses
DBT cells were infected in triplicate as described above. After each infection, the viral titer from each individual well was determined and then passed to a new well of DBT cells. After three passages, two consecutive plaque picks were performed from two of the three individually passaged viral samples to collect individual isolates of MHV. RNA was isolated from these isolates using Trizol per manufacturer’s instructions. cDNA was prepared using MMLV-reverse transcriptase per the manufacturer’s instructions (Thermo Fisher Scientific), and PCR was performed using the following primers: Forward 5’-ggctgttgtggatggcaagca-3’ and Reverse 5’-gctttggtaccagcaacggag-3’. PCR products were sequenced by Sanger Sequencing (Azenta).
Statistical analysis
All statistical analyses were done using a multivariate t-test to assess differences in mean values between groups, and graphs are expressed as geometric means ± geometric standard deviations (SD) (virus titers) or ± standard errors of the means (SEM). All data were analyzed using GraphPad Prism software. Significant P values are denoted with asterisks: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001. Numbers above statistical marks indicate fold changes between groups.
ACKNOWLEDGMENTS
We thank Stanley Perlman for cell lines, Lisa Jenkins (NIH) for the HRMS data, the Oulu Structural Biology core facility, a member of Biocenter Finland, Instruct-ERIC Centre Finland, and FINStruct. ARF would like to thank funding from the NIH (R35GM138029), the NIH-funded Chemical Biology of Infectious Diseases (CBID) COBRE at the University of Kansas (P20GM113117), a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (#UL1TR002366), a J.R. and Inez Jay Award from the University of Kansas, a graduate student fellowship to JJP from the University of Kansas Madison and Lila Self graduate fellowship program, and an undergraduate research scholarship provided to NFS from the NIH funded Kansas IDeA Network of Biomedical Research Excellence (K-INBRE) (P20GM103418). DF would like to thank funding from the McDaniel College Student-Faculty Summer Research Fund, the Jean Richards Fund, the Schofield Fund, and the Scott and Natalie Dahne Fund. LL would like to thank funding from the Sidrid Jusélius Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conceptualization: JJP, LL, DVF, ARF. Data curation: JJP, MTHD, DC, DKJ, NFS, MF, AR, LL, DVF, ARF. Formal analysis: JJP, MTHD, DC, SP, MJH, DKJ, AR, LL, DVF, ARF. Funding acquisition: JJP, LL, DVF, ARF. Methodology: JJP, MTHD, DKJ, AR, LL, DVF, ARF. Investigation: JJP, MTHD, DC, LMS, IC, GC, DT, JP, NFS, JJOC, PS, MF, SP, DKJ, AR, LL, DVF, ARF. Project administration: LL, DVF, ARF. Resources: DKJ, AR, LL, DVF, ARF. Visualization: JJP, MTHD, DC, LMS, IC, GC, DT, JP, NFS, DKJ, MF, SP, MJH, AR, LL, DVF, ARF. Validation: JJP, MTHD, MJH, DKJ, AR, LL, DVF, ARF. Supervision: DKJ, AR, MJH, LL, DVF, ARF. Writing—original draft: JJP, ARF. Writing—review & editing: JJP, MTHD, DC, LMS, IC, GC, DT, JP, NS, JJOC, PS, MF, SP, MJH, DKJ, AR, LL, DVF, ARF.
Contributor Information
Lari Lehtiö, Email: lari.lehtio@oulu.fi.
Dana V. Ferraris, Email: dferraris@mcdaniel.edu.
Anthony R. Fehr, Email: arfehr@ku.edu.
Stacey Schultz-Cherry, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Volker Thiel, Universitat Bern, Bern, Switzerland.
DATA AVAILABILITY
Atomic coordinates and structure factors will be available at the Protein Data Bank with the id. 9GUB. Raw diffraction data will be available at fairdata.fi (https://doi.org/10.23729/c2152e19-38ec-4092-878d-f353358cbe5a). All other data will be available through FigShare at the time of publication 10.6084/m9.figshare.c.7558059.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.03865-24.
Supplemental methods, figures, and table.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. World Health Organization . 2025. Number of COVID-19 cases reported to WHO (cumulative total). Available from: https://data.who.int/dashboards/covid19/cases. Retrieved 16 Mar 2025.
- 2. World Health Organization . 2020. Impact of COVID-19 on people's livelihoods, their health and our food systems. World Health Organization. [Google Scholar]
- 3. Zhao J, Wan W, Yu K, Lemey P, Pettersson JH-O, Bi Y, Lu M, Li X, Chen Z, Zheng M, Yan G, Dai J, Li Y, Haerheng A, He N, Tu C, Suchard MA, Holmes EC, He W-T, Su S. 2024. Farmed fur animals harbour viruses with zoonotic spillover potential. Nature 634:228–233. doi: 10.1038/s41586-024-07901-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minkoff JM, tenOever B. 2023. Innate immune evasion strategies of SARS-CoV-2. Nat Rev Microbiol 21:178–194. doi: 10.1038/s41579-022-00839-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yao T, Foo C, Zheng G, Huang R, Li Q, Shen J, Wang Z. 2023. Insight into the mechanisms of coronaviruses evading host innate immunity. Biochim Biophys Acta Mol Basis Dis 1869:166671. doi: 10.1016/j.bbadis.2023.166671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckei L, Krieg S, Bütepage M, Lehmann A, Gross A, Lippok B, Grimm AR, Kümmerer BM, Rossetti G, Lüscher B, Verheugd P. 2017. The conserved macrodomains of the non-structural proteins of Chikungunya virus and other pathogenic positive strand RNA viruses function as mono-ADP-ribosylhydrolases. Sci Rep 7:41746. doi: 10.1038/srep41746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grunewald ME, Chen Y, Kuny C, Maejima T, Lease R, Ferraris D, Aikawa M, Sullivan CS, Perlman S, Fehr AR. 2019. The coronavirus macrodomain is required to prevent PARP-mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathog 15:e1007756. doi: 10.1371/journal.ppat.1007756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lüscher B, Ahel I, Altmeyer M, Ashworth A, Bai P, Chang P, Cohen M, Corda D, Dantzer F, Daugherty MD, et al. 2022. ADP-ribosyltransferases, an update on function and nomenclature. FEBS J 289:7399–7410. doi: 10.1111/febs.16142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rack JGM, Perina D, Ahel I. 2016. Macrodomains: structure, function, evolution, and catalytic activities. Annu Rev Biochem 85:431–454. doi: 10.1146/annurev-biochem-060815-014935 [DOI] [PubMed] [Google Scholar]
- 10. Fehr AR, Jankevicius G, Ahel I, Perlman S. 2018. Viral macrodomains: unique mediators of viral replication and pathogenesis. Trends Microbiol 26:598–610. doi: 10.1016/j.tim.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung AKL, McPherson RL, Griffin DE. 2018. Macrodomain ADP-ribosylhydrolase and the pathogenesis of infectious diseases. PLoS Pathog 14:e1006864. doi: 10.1371/journal.ppat.1006864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lei J, Kusov Y, Hilgenfeld R. 2018. Nsp3 of coronaviruses: structures and functions of a large multi-domain protein. Antiviral Res 149:58–74. doi: 10.1016/j.antiviral.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alhammad YMO, Kashipathy MM, Roy A, Gagné J-P, McDonald P, Gao P, Nonfoux L, Battaile KP, Johnson DK, Holmstrom ED, Poirier GG, Lovell S, Fehr AR. 2021. The SARS-CoV-2 conserved macrodomain is a mono-ADP-ribosylhydrolase. J Virol 95:e01969-20. doi: 10.1128/JVI.01969-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Putics A, Filipowicz W, Hall J, Gorbalenya AE, Ziebuhr J. 2005. ADP-ribose-1"-monophosphatase: a conserved coronavirus enzyme that is dispensable for viral replication in tissue culture. J Virol 79:12721–12731. doi: 10.1128/JVI.79.20.12721-12731.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eriksson KK, Cervantes-Barragán L, Ludewig B, Thiel V. 2008. Mouse hepatitis virus liver pathology is dependent on ADP-ribose-1’’-phosphatase, a viral function conserved in the alpha-like supergroup. J Virol 82:12325–12334. doi: 10.1128/JVI.02082-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuri T, Eriksson KK, Putics A, Züst R, Snijder EJ, Davidson AD, Siddell SG, Thiel V, Ziebuhr J, Weber F. 2011. The ADP-ribose-1’’-monophosphatase domains of severe acute respiratory syndrome coronavirus and human coronavirus 229E mediate resistance to antiviral interferon responses. J Gen Virol 92:1899–1905. doi: 10.1099/vir.0.031856-0 [DOI] [PubMed] [Google Scholar]
- 17. Fehr AR, Athmer J, Channappanavar R, Phillips JM, Meyerholz DK, Perlman S. 2015. The nsp3 macrodomain promotes virulence in mice with coronavirus-induced encephalitis. J Virol 89:1523–1536. doi: 10.1128/JVI.02596-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fehr AR, Channappanavar R, Jankevicius G, Fett C, Zhao J, Athmer J, Meyerholz DK, Ahel I, Perlman S. 2016. The conserved coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome coronavirus infection. MBio 7:e01721-16. doi: 10.1128/mBio.01721-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voth LS, O’Connor JJ, Kerr CM, Doerger E, Schwarting N, Sperstad P, Johnson DK, Fehr AR. 2021. Unique mutations in the murine hepatitis virus macrodomain differentially attenuate virus replication, indicating multiple roles for the macrodomain in coronavirus replication. J Virol 95:e0076621. doi: 10.1128/JVI.00766-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alhammad YM, Parthasarathy S, Ghimire R, Kerr CM, O’Connor JJ, Pfannenstiel JJ, Chanda D, Miller CA, Baumlin N, Salathe M, Unckless RL, Zuñiga S, Enjuanes L, More S, Channappanavar R, Fehr AR. 2023. SARS-CoV-2 Mac1 is required for IFN antagonism and efficient virus replication in cell culture and in mice. Proc Natl Acad Sci USA 120:e2302083120. doi: 10.1073/pnas.2302083120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taha TY, Suryawanshi RK, Chen IP, Correy GJ, McCavitt-Malvido M, O’Leary PC, Jogalekar MP, Diolaiti ME, Kimmerly GR, Tsou CL, Gascon R, Montano M, Martinez-Sobrido L, Krogan NJ, Ashworth A, Fraser JS, Ott M. 2023. A single inactivating amino acid change in the SARS-CoV-2 NSP3 Mac1 domain attenuates viral replication in vivo. PLoS Pathog 19:e1011614. doi: 10.1371/journal.ppat.1011614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerr CM, Pfannenstiel JJ, Alhammad YM, O’Connor JJ, Ghimire R, Shrestha R, Khattabi R, Saenjamsai P, Parthasarathy S, McDonald PR, Gao P, Johnson DK, More S, Roy A, Channappanavar R, Fehr AR. 2024. Mutation of a highly conserved isoleucine residue in loop 2 of several β-coronavirus macrodomains indicates that enhanced ADP-ribose binding is detrimental for replication. J Virol 98. doi: 10.1128/jvi.01313-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McPherson RL, Abraham R, Sreekumar E, Ong S-E, Cheng S-J, Baxter VK, Kistemaker HAV, Filippov DV, Griffin DE, Leung AKL. 2017. ADP-ribosylhydrolase activity of Chikungunya virus macrodomain is critical for virus replication and virulence. Proc Natl Acad Sci USA 114:1666–1671. doi: 10.1073/pnas.1621485114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abraham R, Hauer D, McPherson RL, Utt A, Kirby IT, Cohen MS, Merits A, Leung AKL, Griffin DE. 2018. ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc Natl Acad Sci USA 115:E10457–E10466. doi: 10.1073/pnas.1812130115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abraham R, McPherson RL, Dasovich M, Badiee M, Leung AKL, Griffin DE. 2020. Both ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain affect neurovirulence in mice. MBio 11:e03253-19. doi: 10.1128/mBio.03253-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saikatendu KS, Joseph JS, Subramanian V, Clayton T, Griffith M, Moy K, Velasquez J, Neuman BW, Buchmeier MJ, Stevens RC, Kuhn P. 2005. Structural basis of severe acute respiratory syndrome coronavirus ADP-ribose-1’’-phosphate dephosphorylation by a conserved domain of nsP3. Structure 13:1665–1675. doi: 10.1016/j.str.2005.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egloff MP, Malet H, Putics A, Heinonen M, Dutartre H, Frangeul A, Gruez A, Campanacci V, Cambillau C, Ziebuhr J, Ahola T, Canard B. 2006. Structural and functional basis for ADP-ribose and poly(ADP-ribose) binding by viral macro domains. J Virol 80:8493–8502. doi: 10.1128/JVI.00713-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu Y, Cong L, Chen C, Wei L, Zhao Q, Xu X, Ma Y, Bartlam M, Rao Z. 2009. Crystal structures of two coronavirus ADP-ribose-1’’-monophosphatases and their complexes with ADP-Ribose: a systematic structural analysis of the viral ADRP domain. J Virol 83:1083–1092. doi: 10.1128/JVI.01862-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malet H, Coutard B, Jamal S, Dutartre H, Papageorgiou N, Neuvonen M, Ahola T, Forrester N, Gould EA, Lafitte D, Ferron F, Lescar J, Gorbalenya AE, de Lamballerie X, Canard B. 2009. The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol 83:6534–6545. doi: 10.1128/JVI.00189-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammond RG, Schormann N, McPherson RL, Leung AKL, Deivanayagam CCS, Johnson MA. 2021. ADP-ribose and analogues bound to the deMARylating macrodomain from the bat coronavirus HKU4. Proc Natl Acad Sci USA 118:e2004500118. doi: 10.1073/pnas.2004500118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cho CC, Lin MH, Chuang CY, Hsu CH. 2016. Macro domain from Middle East respiratory syndrome coronavirus (MERS-CoV) is an efficient ADP-ribose binding module: crystal structure and biochemical studies. J Biol Chem 291:4894–4902. doi: 10.1074/jbc.M115.700542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allen MD, Buckle AM, Cordell SC, Löwe J, Bycroft M. 2003. The crystal structure of AF1521 a protein from Archaeoglobus fulgidus with homology to the non-histone domain of macroH2A. J Mol Biol 330:503–511. doi: 10.1016/s0022-2836(03)00473-x [DOI] [PubMed] [Google Scholar]
- 33. Frick DN, Virdi RS, Vuksanovic N, Dahal N, Silvaggi NR. 2020. Molecular basis for ADP-ribose binding to the Mac1 domain of SARS-CoV-2 nsp3. Biochemistry 59:2608–2615. doi: 10.1021/acs.biochem.0c00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin MH, Chang SC, Chiu YC, Jiang BC, Wu TH, Hsu CH. 2020. Structural, biophysical, and biochemical elucidation of the SARS-CoV-2 nonstructural protein 3 macro domain. ACS Infect Dis 6:2970–2978. doi: 10.1021/acsinfecdis.0c00441 [DOI] [PubMed] [Google Scholar]
- 35. Michalska K, Kim Y, Jedrzejczak R, Maltseva NI, Stols L, Endres M, Joachimiak A. 2020. Crystal structures of SARS-CoV-2 ADP-ribose phosphatase: from the apo form to ligand complexes. IUCrJ 7:814–824. doi: 10.1107/S2052252520009653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rack JGM, Zorzini V, Zhu Z, Schuller M, Ahel D, Ahel I. 2020. Viral macrodomains: a structural and evolutionary assessment of the pharmacological potential. Open Biol 10:200237. doi: 10.1098/rsob.200237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuller M, Correy GJ, Gahbauer S, Fearon D, Wu T, Díaz RE, Young ID, Carvalho Martins L, Smith DH, Schulze-Gahmen U, et al. 2021. Fragment binding to the Nsp3 macrodomain of SARS-CoV-2 identified through crystallographic screening and computational docking. Sci Adv 7:eabf8711. doi: 10.1126/sciadv.abf8711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schuller M, Zarganes-Tzitzikas T, Bennett J, De Cesco S, Fearon D, von Delft F, Fedorov O, Brennan PE, Ahel I. 2023. Discovery and development strategies for SARS-CoV-2 NSP3 macrodomain inhibitors. Pathogens 12:324. doi: 10.3390/pathogens12020324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gahbauer S, Correy GJ, Schuller M, Ferla MP, Doruk YU, Rachman M, Wu T, Diolaiti M, Wang S, Neitz RJ, Fearon D, Radchenko DS, Moroz YS, Irwin JJ, Renslo AR, Taylor JC, Gestwicki JE, von Delft F, Ashworth A, Ahel I, Shoichet BK, Fraser JS. 2023. Iterative computational design and crystallographic screening identifies potent inhibitors targeting the Nsp3 macrodomain of SARS-CoV-2. Proc Natl Acad Sci USA 120:e2212931120. doi: 10.1073/pnas.2212931120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roy A, Alhammad YM, McDonald P, Johnson DK, Zhuo J, Wazir S, Ferraris D, Lehtiö L, Leung AKL, Fehr AR. 2022. Discovery of compounds that inhibit SARS-CoV-2 Mac1-ADP-ribose binding by high-throughput screening. Antiviral Res 203:105344. doi: 10.1016/j.antiviral.2022.105344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherrill LM, Joya EE, Walker A, Roy A, Alhammad YM, Atobatele M, Wazir S, Abbas G, Keane P, Zhuo J, Leung AKL, Johnson DK, Lehtiö L, Fehr AR, Ferraris D. 2022. Design, synthesis and evaluation of inhibitors of the SARS-CoV-2 nsp3 macrodomain. Bioorg Med Chem 67:116788. doi: 10.1016/j.bmc.2022.116788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wazir S, Parviainen TAO, Pfannenstiel JJ, Duong MTH, Cluff D, Sowa ST, Galera-Prat A, Ferraris D, Maksimainen MM, Fehr AR, Heiskanen JP, Lehtiö L. 2024. Discovery of 2-amide-3-methylester thiophenes that target SARS-CoV-2 Mac1 and repress coronavirus replication, validating Mac1 as an antiviral target. J Med Chem 67:6519–6536. doi: 10.1021/acs.jmedchem.3c02451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brosey CA, Houl JH, Katsonis P, Balapiti-Modarage LPF, Bommagani S, Arvai A, Moiani D, Bacolla A, Link T, Warden LS, Lichtarge O, Jones DE, Ahmed Z, Tainer JA. 2021. Targeting SARS-CoV-2 Nsp3 macrodomain structure with insights from human poly(ADP-ribose) glycohydrolase (PARG) structures with inhibitors. Prog Biophys Mol Biol 163:171–186. doi: 10.1016/j.pbiomolbio.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsika AC, Fourkiotis NK, Charalampous P, Gallo A, Spyroulias GA. 2022. NMR study of macro domains (MDs) from betacoronavirus: backbone resonance assignments of SARS–CoV and MERS–CoV MDs in the free and the ADPr-bound state. Biomol NMR Assign 16:9–16. doi: 10.1007/s12104-021-10052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Correy GJ, Kneller DW, Phillips G, Pant S, Russi S, Cohen AE, Meigs G, Holton JM, Gahbauer S, Thompson MC, Ashworth A, Coates L, Kovalevsky A, Meilleur F, Fraser JS. 2022. The mechanisms of catalysis and ligand binding for the SARS-CoV-2 NSP3 macrodomain from neutron and x-ray diffraction at room temperature. Sci Adv 8:eabo5083. doi: 10.1126/sciadv.abo5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dasovich M, Zhuo J, Goodman JA, Thomas A, McPherson RL, Jayabalan AK, Busa VF, Cheng SJ, Murphy BA, Redinger KR, Alhammad YMO, Fehr AR, Tsukamoto T, Slusher BS, Bosch J, Wei H, Leung AKL. 2022. High-throughput activity assay for screening inhibitors of the SARS-CoV-2 Mac1 macrodomain. ACS Chem Biol 17:17–23. doi: 10.1021/acschembio.1c00721 [DOI] [PubMed] [Google Scholar]
- 47. O’Connor JJ, Ferraris D, Fehr AR. 2023. An update on the current state of SARS-CoV-2 Mac1 inhibitors. Pathogens 12:1221. doi: 10.3390/pathogens12101221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ontiveros E, Kuo L, Masters PS, Perlman S. 2001. Inactivation of expression of gene 4 of mouse hepatitis virus strain JHM does not affect virulence in the murine CNS. Virology (Auckl) 289:230–238. doi: 10.1006/viro.2001.1167 [DOI] [PubMed] [Google Scholar]
- 49. Pruijssers AJ, George AS, Schäfer A, Leist SR, Gralinksi LE, Dinnon KH III, Yount BL, Agostini ML, Stevens LJ, Chappell JD, et al. 2020. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep 32:107940. doi: 10.1016/j.celrep.2020.107940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribeiro VC, Russo LC, Hoch NC. 2024. PARP14 is regulated by the PARP9/DTX3L complex and promotes interferon γ-induced ADP-ribosylation. EMBO J 43:2908–2928. doi: 10.1038/s44318-024-00125-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Russo LC, Tomasin R, Matos IA, Manucci AC, Sowa ST, Dale K, Caldecott KW, Lehtiö L, Schechtman D, Meotti FC, Bruni-Cardoso A, Hoch NC. 2021. The SARS-CoV-2 Nsp3 macrodomain reverses PARP9/DTX3L-dependent ADP-ribosylation induced by interferon signaling. J Biol Chem 297:101041. doi: 10.1016/j.jbc.2021.101041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kar P, Chatrin C, Đukić N, Suyari O, Schuller M, Zhu K, Prokhorova E, Bigot N, Baretić D, Ahel J, Elsborg JD, Nielsen ML, Clausen T, Huet S, Niepel M, Sanyal S, Ahel D, Smith R, Ahel I. 2024. PARP14 and PARP9/DTX3L regulate interferon-induced ADP-ribosylation. EMBO J 43:2929–2953. doi: 10.1038/s44318-024-00126-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parthasarathy S, Saenjamsai P, Hao H, Ferkul A, Pfannenstiel JJ, Suder EL, Bejan DS, Chen Y, Schwarting N, Aikawa M, Muhlberger E, Orozco RC, Sullivan CS, Cohen MS, Davido DJ, Hume AJ, Fehr AR. 2024. PARP14 is pro- and anti-viral host factor that promotes IFN production and affects the replication of multiple viruses. bioRxiv:2024.04.26.591186. doi: 10.1101/2024.04.26.591186 [DOI] [Google Scholar]
- 54. Daniel E, Maksimainen MM, Smith N, Ratas V, Biterova E, Murthy SN, Rahman MT, Kiema T-R, Sridhar S, Cordara G, Dalwani S, Venkatesan R, Prilusky J, Dym O, Lehtiö L, Koski MK, Ashton AW, Sussman JL, Wierenga RK. 2021. IceBear: an intuitive and versatile web application for research-data tracking from crystallization experiment to PDB deposition. Acta Crystallogr D Struct Biol 77:151–163. doi: 10.1107/S2059798320015223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brehm W, Trivino J, Krahn JM, Uson I, Diederichs K. 2023. XDSGUI: a graphical user interface for XDS, SHELX and ARCIMBOLDO. J Appl Crystallogr 56:1585–1594. doi: 10.1107/S1600576723007057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kabsch W. 2010. XDS. Acta Crystallogr D Biol Crystallogr 66:125–132. doi: 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. doi: 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. doi: 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. 2011. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67:355–367. doi: 10.1107/S0907444911001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Friesner R.A, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. 2004. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749. doi: 10.1021/jm0306430 [DOI] [PubMed] [Google Scholar]
- 61. Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. 2006. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49:6177–6196. doi: 10.1021/jm051256o [DOI] [PubMed] [Google Scholar]
- 62. Jacobson MP, Friesner RA, Xiang Z, Honig B. 2002. On the role of the crystal environment in determining protein side-chain conformations. J Mol Biol 320:597–608. doi: 10.1016/s0022-2836(02)00470-9 [DOI] [PubMed] [Google Scholar]
- 63. Jacobson MP, Pincus DL, Rapp CS, Day TJF, Honig B, Shaw DE, Friesner RA. 2004. A hierarchical approach to all-atom protein loop prediction. Proteins 55:351–367. doi: 10.1002/prot.10613 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods, figures, and table.
Data Availability Statement
Atomic coordinates and structure factors will be available at the Protein Data Bank with the id. 9GUB. Raw diffraction data will be available at fairdata.fi (https://doi.org/10.23729/c2152e19-38ec-4092-878d-f353358cbe5a). All other data will be available through FigShare at the time of publication 10.6084/m9.figshare.c.7558059.