Abstract
In medial prefrontal cortex (mPFC), fast-spiking parvalbumin (PV) interneurons regulate excitability and microcircuit oscillatory activity important for cognition. Although PV interneurons inhibit pyramidal neurons, they themselves express δ subunits of GABAA receptors important for slow inhibition. However, the specific contribution of δ-containing GABAA receptors to the function of PV interneurons in mPFC is unclear. We explored cellular, synaptic, and local-circuit activity in PV interneurons and pyramidal neurons in mouse mPFC after selectively deleting δ subunits in PV interneurons (cKO mice). In current-clamp recordings, cKO PV interneurons exhibited a higher frequency of action potentials and higher input resistance than wild type (WT) PV interneurons. Picrotoxin increased firing and GABA decreased firing in WT PV interneurons but not in cKO PV interneurons. The δ-preferring agonist THIP reduced spontaneous inhibitory postsynaptic currents disproportionately in WT pyramidal neurons compared with cKO pyramidal neurons. In WT slices, depolarizing the network with 400 nM kainate increased firing of pyramidal neurons but had little effect on PV interneuron firing. In contrast, kainate application in cKO slices preferentially activated PV interneurons rather than pyramidal neurons. At the population level, kainate induced broadband increases in local field potentials in WT but not cKO slices. These results on cells and network activity can be understood through increased excitability of cKO PV interneurons. In summary, our study demonstrates that δ-containing GABAA receptors in mPFC PV interneurons play a crucial role in regulating their excitability and the phasic inhibition of pyramidal neurons, elucidating intricate mechanisms governing cortical circuitry.
Keywords: δ-containing GABAA receptors, medial prefrontal cortex, neuronal excitability, parvalbumin interneurons
Graphical Abstract
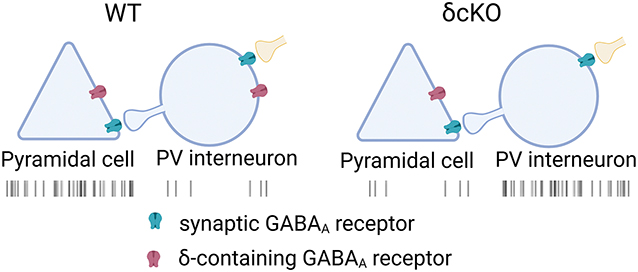
NEW & NOTEWORTHY
We reveal the critical role of δ-containing GABAA receptors in parvalbumin interneurons in the medial prefrontal cortex, important for human neuropsychiatric disorders. We demonstrate these receptors’ importance in regulating neuronal excitability and network dynamics. δ-containing receptors act as a brake on interneuron activity, maintaining the excitation-inhibition balance in cortical circuits. Our findings provide insights into how disruptions in inhibitory signaling alter network function through a receptor subtype that is a target of neurotherapeutics.
INTRODUCTION
The medial prefrontal cortex (mPFC) participates in higher-order cognitive functions, including decision-making, working memory, attention, and behavioral flexibility (1,2). Dysfunction of the mPFC has been implicated in various neuropsychiatric disorders, including schizophrenia, depression, and addiction (3–5). This underscores the importance of understanding the neural circuits and mechanisms underlying mPFC function. Among the cell types in mPFC, parvalbumin (PV) interneurons, a subclass of GABAergic neurons found throughout the cerebral cortex, are essential in regulating the activity and network dynamics of the mPFC (6–10).
PV interneurons in the mPFC provide powerful inhibitory inputs onto the soma and proximal dendrites of pyramidal neurons, the main excitatory output neurons of the cortex. This perisomatic inhibition is essential for regulating the firing rate and timing of action potentials in pyramidal neurons, thereby gating the flow of information within the mPFC and between the mPFC and other brain regions (11–13). Additionally, PV interneurons are primarily involved in the generation and synchronization of gamma-frequency brain oscillations in the mPFC (11,14,15). These high-frequency oscillations are thought to play a crucial role in coordinating neuronal activity and facilitating communication between different brain regions during cognitive processes.
Interestingly, PV interneurons themselves express GABAA receptors and receive GABAergic inhibition from other interneurons (16,17). GABAA receptors are heteropentameric ion channels, with subunit combinations that confer distinct functional and pharmacological properties (18). Among these, the δ subunit is of interest because δ-containing GABAA receptors are primarily localized to extrasynaptic regions and mediate tonic inhibition (19–21), as well as slow phasic inhibition (22–25). These receptors are expressed by only select neuronal types and are combined with specific subunits for distinct functions. In principal neurons, δ subunits preferentially pair with α4 (forebrain) or with α6 (cerebellum) subunits. However, in interneurons, δ subunits pair with α1 subunits, resulting in distinct receptor functional properties (26). Previous studies have shown that δ-containing GABAA receptors in PV interneurons contribute to tonic inhibition as well as high frequency oscillations in hippocampus and basal amygdala (27–30). However, in mPFC, the specific role of δ-containing GABAA receptors in regulating the excitability and output of PV interneurons, and consequently their influence on pyramidal neuron activity and network oscillations, remains largely unknown. Understanding the function of these receptors on PV interneurons is important because δ-containing GABAA receptors are thought to be a target of emerging neurotherapeutics such as neurosteroids for postpartum depression, for sleep aids, and for epilepsy treatment (31–35). Understanding cell-type specific contributions could aid the development of more selective therapeutics. The subtle, sustained inhibition mediated by these receptors combined with cell-type specificity may allow development of therapeutics with greater efficacy and fewer side effects.
In this study, we selectively deleted δ-containing GABAA receptors in PV interneurons using a conditional knockout (cKO) mouse model. Through electrophysiological recordings, pharmacological manipulations, and gene expression analysis, we directly investigated the impact of these receptors on the intrinsic properties and synaptic output of PV interneurons. This approach allowed us to dissect the contribution of inhibition mediated by δ-containing GABAA receptors to the overall excitability and function of PV interneurons and principal neurons within the mPFC microcircuitry. We find evidence that δ-containing GABAA receptors are important for basal excitability of PV interneurons, which checks the excitability of pyramidal neurons across a broad spectrum of oscillatory activity in response to stimulation.
MATERIALS AND METHODS
Ethical Approval
All procedures were carried out in accordance with protocol (22–0344) approved by the Institutional Animal Care and Use Committee at Washington University.
Mice
Male and female, Ai14::PVCre (Ai14, Jackson lab, #007914; PVCre, Jackson Lab, #017320), PVCre, and Gabrd floxed::PVCre (36) mice from 4 – 8 weeks old were used. For some experiments in which only WT PV interneurons were recorded, we used Ai14::PVCre reporter mice. Alternatively, in experiments with conditional Gabrd deletion (cKO), pAAV-FLEX-GFP (Addgene, #28304) was administered by retro-orbital sinus injection to label PV cells in Gabrd floxed∷PVCre mice or PVCre littermates (27).
Slice preparation
Mice were subjected to isoflurane anesthesia and subsequently decapitated. A Leica VT1200 vibratome was employed to prepare 300-μm thick coronal brain slices. During the slicing procedure, the slices were kept in an ice-cold, modified NMDG-HEPES recovery artificial cerebrospinal fluid (aCSF) solution containing (in mM): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2, and 10 MgSO4 (300 mOsm; pH 7.3–7.4). Following slicing, the slices recovered in the modified NMDG-HEPES recovery aCSF at 32°C. A Na+-rich spike-in solution (4 ml, 2 M) was added to the recovery aCSF to gradually increase Na+ concentration, which improved recording success rate (Ting et al., 2018). After the recovery period, the slices were maintained in a modified HEPES holding aCSF (composition in mM: 92 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 2 CaCl2, and 2 MgSO4; 300 mOsm; pH 7.3–7.4) for a minimum of 1 hour at 25°C prior to conducting experimental recordings. All drugs were sourced from Sigma-Aldrich, unless otherwise specified.
Whole-cell patch-clamp recording
For the duration of the recording, slices were placed in a recording chamber and continuously perfused (2 ml/min, 32°C) with oxygenated, regular aCSF containing (in mM): 125 NaCl, 25 glucose, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, and 1 MgCl2 (310 mOsM), equilibrated with a mixture of 95% oxygen and 5% CO2. Somatic whole-cell recordings were obtained from visually identified layer 2/3 mPFC neurons using IR-DIC microscopy (Nikon FN1 microscope, Photometrics Prime camera). Whole-cell recordings were obtained using borosilicate glass pipettes (World Precision Instruments) with an open tip resistance ranging from 3 to 6 MΩ. The recordings were conducted using a MultiClamp 700B amplifier (Molecular Devices), a Digidata 1550 16-bit A/D converter, and pClamp 10.4 software (Molecular Devices). After the initial break-in, a 5-minute stabilization period was observed before commencing the recordings.
Measurement of action potentials
To capture action potentials, neurons were recorded in current-clamp mode using pipettes containing a K-gluconate internal solution. This solution was composed of (in mM): 140 K-gluconate, 4 MgCl2, 10 HEPES, 0.4 EGTA, 4 MgATP, 0.3 NaGTP, and 10 phosphocreatine, with osmolarity of 290 mOsm and pH adjusted to 7.25 using KOH. To assess membrane properties and evoke action potentials, step currents starting from −50 pA were injected for 500 ms, with increments of 50 pA. To confirm the involvement of GABAA receptors, the non-competitive antagonist picrotoxin (PTX, 100 μM) or agonist GABA (5 μM) was bath applied for 5 min. All current-clamp recordings were done in episodic mode at 50 kHz sampling rate and 10 kHz filter cutoff frequency using an eight-pole Bessel filter.
Measurement of spontaneous inhibitory and excitatory postsynaptic currents (sIPSCs and sEPSCs)
Phasic currents were recorded in voltage-clamp mode using pipettes filled with Cs-methanesulfonate (in mM: 130 Cs-methanesulfonate, 4 NaCl, 0.5 CaCl2, 10 HEPES, 5 EGTA, 5 QX-314, 0.5 NaGTP, and 2 MgATP; pH was adjusted to 7.3 with CsOH; 290 mOsm). To record isolated sIPSCs, neurons were held at 0 mV (see Fig. 3). To record sIPSCs and sEPSCs simultaneously, neurons were held at −40 mV (see Supplemental Figure S1).
Figure 3. THIP decreased the frequency of sIPSCs in WT pyramidal neuron but not in cKO pyramidal neurons.

A, B, Representative WT pyramidal neuron (A) and cKO pyramidal neuron (B) held at 0 mV at baseline (top) or 1 μM THIP (bottom). C-E, sIPSC characteristics of WT pyramidal neurons (N = 15) and cKO pyramidal neurons (N = 17). C, sIPSC frequency. Two-way ANOVA revealed a significant genotype × THIP interaction (F(1,30) = 5.640, P = 0.024), but no main effect of genotype (F(1,30) = 2.664, P = 0.113). Baseline frequencies were similar between WT and cKO (Sidak’s test, P = 0.709). D, sIPSC peak amplitude. Two-way ANOVA showed no genotype × THIP interaction (F(1,30) = 1.027, P = 0.319) or genotype effect (F(1,30) = 0.029, P = 0.867). Baseline amplitudes were similar between groups (Sidak’s test, P = 0.873). E, sIPSC weighted decay τ (τw). Two-way ANOVA showed no genotype × THIP interaction (F(1,30) = 2.083, P = 0.159) or genotype effect (F(1,30) = 0.791, P = 0.381). Baseline decay was similar between groups (Sidak’s test, P = 0.985).
In some experiments, we pharmacologically isolated sIPSCs of PV interneurons by maintaining a voltage of −70 mV with a whole-cell pipette solution of cesium chloride (in mM: 130 CsCl, 10 HEPES, 5 EGTA, 2 MgATP, 0.5 NaGTP, and 4 QX-314; pH adjusted to 7.3 with CsOH; 290 mOsm). The selective AMPA receptor antagonist NBQX (10 μM, Tocris Bioscience) and NMDA receptor antagonist D-APV (50 μM, Tocris Bioscience) were added in the aCSF to inhibit ionotropic glutamate receptors. All phasic current recordings were performed in gap-free mode at 5 kHz sampling rate and filtered at 2 kHz using an eight-pole Bessel filter.
Measurement of kainate-induced action potentials
To induce network activity, 400 nM kainate in aCSF was perfused to slices. Kainate-induced tonic currents were measured by whole-cell recordings at −70 mV. Cesium chloride (same as above) was used as internal solution. After bath application of kainate for 5 min, 10 μM NBQX was applied to inhibit kainate-induced tonic currents. For cell-attached recordings, aCSF was used as pipette solution. The number of action potentials before application of kainate and 10 min after kainate was measured and evaluated. Cell-attached recordings were performed in gap-free mode with sampling rate at 50 kHz and filtered at 10 kHz. The Chi square test was performed to compare the fraction of neurons exhibiting action potentials between WT and cKO.
Measurement of local field potentials
As a measure of local-circuit activity, local field potential (LFP) recordings were conducted on 400 μm brain slices. Pipettes were filled with aCSF and placed at 100 μm depth in mPFC layer 2/3. LFP recordings were conducted in gap-free mode with sampling rate at 10 kHz and filter cutoff frequency between 2 Hz to 1 kHz using a bandpass filter. After 5 min baseline recordings, 400 nM kainate in aCSF was perfused for 10 min. The LFP recordings during the one-minute period before application of kainate and 10th minute after kainate were used for analysis. The power spectral density of the signal was estimated using the pspectrum function in MATLAB (version R2023a). The frequency range (59–61 Hz) was notch-filtered to remove the 60 Hz noise.
Bulk RNA-seq and differential expression analysis
RNA-seq was performed on two cohorts (n=12 mice total; 3 WT and 3 cKO per cohort). Mice were deeply anesthetized with isoflurane until unresponsive to tail pinch. Brains were rapidly removed, and tissue samples of frontal cortex were obtained and frozen at −80 °C until use. Total RNA was isolated from cortex tissue using RNeasy Plus Mini Kit (QIAGEN GmbH) in accordance with the manufacturer’s protocols. The integrity of total RNA was validated by an Agilent bioanalyzer. RNA samples that passed quality control were analyzed for sequencing by the Genome Technology Access Center at the McDonnell Genome Institute, Washington University School of Medicine. RNA samples were prepared according to the library kit manufacturer’s protocol, indexed, pooled, and sequenced on an Illumina NovaSeq X Plus. Basecalls and demultiplexing were performed using Illumina’s DRAGEN and BCLconvert version 4.2.4 software. RNA-seq reads were aligned to the Ensembl release 101 primary assembly using STAR version 2.7.9a (37). Gene counts were obtained using Subread:featureCount version 2.0.3 (38). The ribosomal fraction, known junction saturation, and read distribution over known gene models were quantified using RSeQC version 4.0 (39).
Gene counts were imported into R/Bioconductor package EdgeR (40) and TMM normalization size factors were calculated to adjust for differences in library size. Ribosomal genes and genes not expressed in at least one sample were excluded from further analysis. The TMM size factors and count matrix were then imported into R/Bioconductor package Limma (41). Weighted likelihoods based on the observed mean-variance relationship of every gene and sample were calculated for all samples with Limma’s voomWithQualityWeights (42). Differential expression analysis was performed to analyze differences between conditions. Results were filtered for genes with false discovery rate ≤ 0.05.
Statistical analyses
The number of action potentials was measured in Clampfit using peak detection algorithms. The membrane resistance was determined by measuring the steady-state voltage change in response to a −50 pA current. sIPSCs and sEPSCs were detected and analyzed as previously described using Clampfit algorithms (27). Data were analyzed and graphed using GraphPad Prism (version 9.0.0 for Windows, GraphPad Software). Paired t test or ANOVA as appropriate was conducted with Graphpad Prism. Summary data were presented as mean ± SEM. P values are indicated in the figure.
RESULTS
Deleting δ-containing GABAA receptors augmented PV interneuron excitability.
In the central nervous system, δ-containing GABAA receptors primarily mediate tonic inhibition (21,43,44). THIP, a δ-receptor preferring agonist, induces tonic currents in principal neurons of mouse hippocampus and neocortex (45). Previously, we demonstrated that THIP elicited tonic currents in WT mPFC PV interneurons, predominantly mediated by δ-containing GABAA receptors. These currents were greatly reduced in PV interneurons from PVCre mice crossed with δ-floxed mice (cKOs), also used in the present study (14). These studies functionally validated the selectivity of the PV-Cre mouse line and the efficacy of the gene deletion. Considering that genetic removal of δ receptors diminished tonic inhibition in response to the δ-preferring agonist, we hypothesized that the absence of δ-containing receptors might elevate the excitability of PV interneurons. We conducted current-clamp recordings and quantified the number of action potentials in response to a series of step-current injections in both WT and cKO PV interneurons (Fig. 1A, B). Notably, cKO PV interneurons exhibited significantly more action potentials compared to WT PV interneurons (Fig. 1C). Additionally, the input resistance of cKO PV interneurons was significantly elevated compared to that of WT PV interneurons (Fig. 1D). Consistent with a role for GABAA receptors in this altered excitability, 100 μM PTX increased firing in response to current injection in WT PV interneurons (Fig. 1E, G) but not in cKO PV interneurons (Fig. 1F, H). Input resistance in WT PV was unchanged after PTX (baseline: 166.4 ± 8.361 MΩ vs. PTX: 172.2 ± 8.813 MΩ, paired t test, p = 0.122, N = 11). These results suggest a role for GABAA conductance in the excitability difference between WT and cKO PV interneurons.
Figure 1. cKO PV interneurons showed altered neuronal excitability from WT in mPFC.

A, B, Representative action potential patterns of WT (A) and cKO (B) PV interneurons with a 500 ms, 400 pA depolarizing current injection. C, Average number of action potentials in WT (N = 15) and cKO (N = 16) PV interneurons elicited over 500 ms at the indicated current amplitudes. Two-way ANOVA (F(1,29) = 15.78, P = 0.0004) indicated a difference between WT and cKO PV interneurons. D, Summary of membrane input resistance for WT and cKO PV interneurons. Unpaired t test revealed differences between groups (p = 0.0041). E, F, Representative action potential patterns of WT baseline, WT PTX, cKO baseline, and cKO PTX. 300 pA current was injected to elicit action potentials. G, Average number of action potentials in WT PV interneurons (baseline vs. 100 μM PTX, N = 11) elicited at the indicated current amplitudes. Two-way ANOVA showed a drug effect on number of action potentials (F(1, 10) = 15.44, P = 0.0028). H, Average number of action potentials in cKO PV interneurons (baseline vs. 100 μM PTX, N = 7) elicited at the indicated current amplitudes. Two-way ANOVA showed no drug effect on number of action potentials (F(1, 6) = 1.233, P = 0.3093). P values are indicated in the figure.
If δ-containing receptors participate in PV interneuron excitability, we expect a difference in responsiveness of WT and cKO PV interneurons to the natural transmitter GABA, in addition to the previously demonstrated differential response to PTX (Fig.1). In WT PV interneurons, 5 μM GABA decreased the number of spikes in response to depolarizing current injection (Fig. 2A, C). On the contrary, GABA did not alter the number of action potentials in cKO PV interneurons (Fig. 2B, D). Surprisingly, these changes occurred in the absence of a detectable decrease in the input resistance at −70 mV in either genotype, suggesting excitability measures are more sensitive (WT baseline: 138.4 ± 4.657 MΩ vs. WT GABA: 138.1 ± 6.412 MΩ, paired t test, p = 0.949, N = 10; cKO baseline: 173.9 ± 7.549 MΩ vs. cKO GABA: 180.2 ± 10.04 MΩ, paired t test, p = 0.169, N = 9).
Figure 2. GABA reduced action potentials in WT but not cKO PV interneurons.

A, B, Representative action potential patterns of WT baseline (A1), WT GABA (A2), cKO baseline (B1), and cKO GABA (B2) with a 500 ms, 300pA current injection. The red dashed box indicates the region highlighted in the inset, which shows individual action potentials at higher temporal resolution. C, Average number of action potentials in WT PV interneurons (baseline vs. 5 μM GABA, N = 10) elicited at the indicated current amplitudes. Two-way ANOVA showed a drug effect on number of action potentials (F(1, 9) = 13.26, P = 0.005). D, Average number of action potentials in cKO PV interneurons (baseline vs. 5 μM GABA, N = 9) elicited at the indicated current amplitudes. Two-way ANOVA showed no drug effect on number of action potentials (F(1, 8) = 0.494, P = 0.502). P values are indicated in the figure.
GABAergic PV interneurons release the inhibitory neurotransmitter GABA onto pyramidal neurons (46). Therefore, altering the excitability of PV interneurons might be expected to alter transmitter release detected by layer 2/3 pyramidal neurons. To test this, we applied 1 μM THIP to slices while recording sIPSCs in WT and cKO pyramidal neurons (Fig. 3A, B). Two-way ANOVA revealed a significant interaction between genotype and THIP treatment (F (1, 30) = 5.64, P = 0.024). Although THIP markedly reduced sIPSC frequency in WT pyramidal neurons, it had minimal effect in cKO pyramidal neurons (Fig. 3C). Neither the peak amplitude nor the kinetics of sIPSCs was altered by THIP in WT or cKO recordings (Fig. 3D, E). The lack of effect on sIPSC amplitude in the context of the frequency decrease could be explained if many of these events in both genotypes are univesicular. In line with previous findings (47), THIP also increased the ratio of EPSC frequency to that of IPSCs in WT layer 2/3 pyramidal neurons by reducing the frequency of sIPSCs while increasing the frequency of sEPSCs (Supplemental Fig. S1).
To more fully understand the network impact of δ subunit loss, we also explored effects of the manipulation on afferent inhibition in the PV interneurons themselves. We reasoned that PV interneurons receive input from non-PV interneurons (17), which are not δ bearing (36) and thus should not be affected by THIP application. Indeed, THIP had no effect on the frequency or decay of sIPSCs in PV interneurons of either WT or cKO slices (Fig. 4). THIP did increase the amplitude of sIPSCs in WT PV interneurons (Fig. 4D), likely because of increased baseline noise masking smaller amplitude events, as shown in the cumulative probability distribution of sIPSC amplitudes (Supplemental Fig. S2). Indeed, THIP significantly increased the standard deviation of the holding current in WT PV interneurons (2.869 ± 0.212) more than in cKO PV interneurons (0.953 ± 0.32) (unpaired t test, P = 0.0001, also see (14)). The effect on amplitude was not observed in cKO cells.
Figure 4. THIP had no effect on the frequency of sIPSCs in WT or cKO PV interneurons.

A, Representative trace of a WT PV interneuron held at −70 mV at baseline (top) or in the presence of 1 μM THIP (bottom). B, Representative traces of a cKO PV interneuron held at −70 mV at baseline (top) or in the presence of 1 μM THIP (bottom). C-E, sIPSC characteristics of WT PV interneurons (N = 10) and cKO PV interneurons (N = 11). C, frequency of sIPSC. Two-way ANOVA showed no interaction between genotype and THIP (F(1,19) = 0.034, P = 0.855). D, Peak amplitude of sIPSC. Two-way ANOVA showed an interaction between genotype and THIP (F(1,19) = 5.928, P = 0.025). THIP increased the peak amplitude of sIPSC in WT PV interneurons but not cKO PV interneurons (Sidak’s test, P = 0.022 and 0.819, respectively). E, weighted decay τ (τw) of sIPSC. Two-way ANOVA showed no interaction between genotype and THIP (F(1,19) = 0.115, P = 0.738). P values are indicated in the figure.
Deleting δ subunits in PV interneurons altered the excitability of pyramidal neurons and network activity.
To investigate whether the altered phasic inhibition of pyramidal neurons would impact the excitability of pyramidal neurons, we applied a low concentration (400 nM) of kainate to excite the network. Kainate activates kainate receptors, a subset of ionotropic glutamate receptors (48) but also is a non-desensitizing AMPA receptor agonist (49) and may activate a small percentage of these receptors (50,51). Indeed, 400 nM kainate induced steady tonic current, sensitive to 10 μM NBQX, in layer 2/3 pyramidal neurons (0.436 ± 0.134 pA/pF, N = 6) and in PV interneurons (0.447 ± 0.106 pA/pF, N = 4) (Supplemental Figure S3). In cell-attached recordings of pyramidal neurons, kainate induced robust firing in WT but not in cKO pyramidal neurons (Fig. 5A–C).
Figure 5. Kainate-induced neuronal excitability was altered in cKO.

Spike raster plots show cell-attached patch recordings from separate recording sessions aligned by kainate application. Therefore, firing rates but not network synchronization can be inferred from the rasters. A, B, Spike raster plot of pyramidal neuron firing in response to kainate application, compared to baseline, in WT (A) and cKO (B) mice. C, Quantification of the fraction of pyramidal neurons exhibiting action potentials (APs) or no APs during kainate application in WT and cKO conditions. More pyramidal neurons exhibited APs in the WT condition upon kainate treatment (chi square test, P = 0.0306). D, E, Spike raster plot of PV interneuron firing in response to kainate application, compared to baseline, in WT (D) and cKO (E) mice. F, Fraction of PV interneurons exhibiting APs or no APs during kainate application in WT and cKO conditions. More PV interneurons exhibited APs in the cKO condition upon kainate treatment (chi square test, P = 0.007). P values are indicated in the figure.
We hypothesized that the reduction of kainate effects on cKO pyramidal neurons may result from the higher excitability of cKO PV interneurons and increased GABA output to these principal neurons (Figs. 1–3). To test this, we recorded kainate-induced action potentials from cell-attached recordings of PV interneurons (Fig. 5D, E) and observed reciprocal effects compared with pyramidal neurons; WT PV cells showed little responsiveness to kainate, but cKO PV neurons exhibited strong responsivity to kainate. These results demonstrate the strong influence of δ-containing GABAA on two major cell types of the cortex.
We explored the implications of these excitability differences on local network function. In the mPFC, PV interneurons play a crucial role in generating brain oscillations. To investigate how deletion of δ-containing receptors would affect oscillations, we conducted local field recordings in mPFC layer 2/3 during application of kainate. As predicted by previous work (52), kainate increased gamma power (30–100 Hz band; 2.4 ± 0.4 (10−3mV2/Hz) vs. kainate: 5.2 ± 0.6 (10−3mV2/Hz), paired t test, p = 0.0058). However, our results showed that kainate induced broad oscillations in WT slices across frequencies (Fig. 6A,B, E). In contrast, kainate failed to induce broadband power increases in cKO cortex (Fig. 6C, D, E). These results show that that a single receptor class in one group of interneurons has a strong impact on overall network function.
Figure 6. Kainate induced broadband oscillations in mPFC layer 2/3 of WT but not cKO mice.

A, Representative LFP traces recording from mPFC layer 2/3 in a WT mouse slice at baseline (top) and 10 min after application of kainate (bottom). B, Power spectral density plot showing the frequency composition of the LFP signal at baseline (black) and after kainate application (red) in the WT slice. Kainate induced a broadband increase in oscillatory power across multiple frequencies. C, Representative LFP traces from a cKO mouse slice at baseline (top) and 10 min after kainate application (bottom). D, Power spectral density plot for the cKO slice, showing little change in oscillatory power with kainate application compared to baseline across most frequencies. E, Quantification of oscillatory power from 15 – 80 Hz during baseline and kainate application in WT and cKO slices. Two-way ANOVA showed an interaction between genotype and kainate (F(1,18) = 12.56, P = 0.002), as well as main effects of genotype (F(1,18) = 15.76, P < 0.001) and drug (F(1,18) = 17.72, P < 0.001). Post-hoc tests showed that kainate increased the broadband power in WT (Sidak’s test, P < 0.001) but not cKO slices (Sidak’s test, P = 0.86). The vertical gray bars in panel B and D indicate the frequency range (59–61 Hz) that was notch-filtered to remove 60 Hz noise. P values are indicated in the figure.
Our findings demonstrate that deletion of δ-containing GABAA receptors in PV interneurons alters excitability of both PV interneurons and pyramidal neurons through acute effects on GABA inhibition, revealed by antagonist and agonist application. However, our results leave open the possibility that gene deletion may have indirect consequences on non-GABAergic influences on cellular or network excitability, either cell-autonomously on PV interneurons or through indirect effects on other cells in the circuit. Thus, we sought to preliminarily investigate whether deletion of δ subunits in PV interneurons influences cortical gene expression, potentially contributing to observed excitability differences. We performed bulk RNA-seq analysis on six mice in each genotype. Based on a false discovery rate criterion of 0.05 and expression difference cutoff of 2-fold, no genes were differentially expressed between the WT and cKO groups. Supplemental Table S1 shows the detailed results for top transcripts. These hypothesis-generating results do not allow us to exclude conclusively a contribution of secondary transcriptional changes to excitability differences. Nevertheless, no immediate gene candidates emerged to explain cortical excitability differences in response to the cKO manipulation.
DISCUSSION
In this study, we investigated the role of δ-containing GABAA receptors in PV interneurons of the mPFC and their impact on neuronal excitability, network oscillations, and overall circuit function. Using a Gabrd cKO mouse line, we uncovered a crucial role for these receptors in regulating the excitability of PV interneurons and, consequently, the inhibition exerted by these interneurons on pyramidal neurons. The impact of these specialized receptors has been unclear, especially given the prominence of more conventional GABAA receptors (e.g., those containing a γ2 subunit) at synapses (53), demonstrated herein by the lack of impact of δ loss on phasic inhibition in PV interneurons (Figure 4).
Our results demonstrate that the absence of δ-containing GABAA receptors leads to increased excitability of PV interneurons, as evidenced by their heightened firing rates and elevated input resistance compared to WT PV interneurons. This increased excitability can be attributed to the loss of tonic inhibition mediated by δ-containing GABAA receptors, which normally dampens the excitability of PV interneurons. The observed effects of the GABAA receptor antagonist PTX and the δ-preferring agonist THIP further support the involvement of these receptors in regulating PV interneuron excitability. Notably, the loss of δ occurs in the context of unaltered phasic transmission IPSCs, Figures 3,4) (14), supporting the idea that slow, sustained inhibitory signaling in a critical minority of cells can have a major impact on network function.
Notably, the increased excitability of PV interneurons in the cKO mice had profound consequences for the inhibitory control exerted by these interneurons on pyramidal neurons. The frequency of sIPSCs in WT pyramidal neurons was reduced upon THIP application, indicating decreased release of GABA from PV interneurons. Conversely, THIP failed to alter sIPSC frequency in cKO pyramidal neurons, likely due to the heightened excitability and aberrant inhibitory output of PV interneurons lacking δ-containing GABAA receptors.
The altered excitability of PV interneurons and the consequent dysregulation of inhibitory control over pyramidal neurons had significant implications for overall network dynamics in the mPFC. We found that kainate-induced oscillations, which are known to depend on the activity of PV interneurons (30,54), were reduced in the cKO cortex compared to WT slices (Figure 6). This observation highlights the critical role of δ-containing GABAA receptors in shaping the oscillatory activity of the mPFC. mPFC oscillatory, activity, in turn, is crucial for coordinating neuronal activity and facilitating communication between brain regions during cognitive processes (55). We acknowledge the limitations of the preparation chosen to study oscillations in our work. Although the ex vivo slice preparation allowed us to draw conclusions about intrinsic cortical network oscillatory behavior free of subcortical influences and long-range cortical interactions, it is possible that the results would differ in intact animals because of these extrinsic influences. Specifically, kainate-mediated changes did not result in increased synchronization of pyramidal cell firing (Figure 5) or selective changes to gamma oscillations (Figure 6). Such differences from in vivo observations might result from overstimulation of PV interneurons, which has been shown to disrupt gamma oscillations in hippocampus and amygdala (28,56).
We reasoned that altering GABAergic inhibition in PV interneurons over the course of development could result in transcriptional differences that participate in excitability changes. Such changes could help explain the apparent differences between pharmacological and genetic removal of δ-containing receptors on excitability (Figure 1). This was an unexpected result suggesting the possibility that genetic deletion has additional effects on excitability beyond GABAergic inhibition. As a preliminary approach, we performed an unbiased bulk transcriptional analysis of cortex. Our analysis, meant to detect broad transcriptional changes that might affect overall excitability evident in Figures 5 and 6, did not reveal any genes differentially expressed in cKO cortex. It is possible, as hypothesized, that the deletion of δ-containing GABAA receptors in PV interneurons primarily affects their functional properties and connectivity without inducing substantial transcriptional changes. Alternatively, the bulk approach used in this study may have missed cell type-specific transcriptional changes. PV interneurons constitute a small proportion of the total cell population in the cortex, so cell-autonomous transcriptional changes may be missed in favor of transcriptional profiles of other cell types in the bulk tissue sample. To address these limitations, future studies could employ single-cell RNA-seq techniques (57), which enable the profiling of gene expression at the individual cell level and allow for the identification of cell-type specific transcriptional changes. It is also possible that non-transcriptional changes in response to δ deletion could contribute to cell or network excitability differences. These could be addressed with future proteomic studies.
The findings of this study have important implications for understanding neural mechanisms underlying cognitive functions mediated by the mPFC, such as working memory, attention, and decision-making, specific functions of mPFC. Dysregulation of PV interneuron function and disruption of mPFC network dynamics have been implicated in various neuropsychiatric disorders, including schizophrenia and autism spectrum disorders (5). Our results provide insights into the specific contribution of δ-containing GABAA receptors in shaping the excitability and inhibitory output of PV interneurons in mPFC. Because the activity of PV interneurons has been linked to disorders as diverse as autism, depression, anxiety disorders, epilepsy, and schizophrenia (58–61), targeting the sustained inhibition of PV interneurons with δ selective pharmacology and/or gene therapy directed at PV inhibition could offer novel therapeutic interventions for these disorders (34,62). Because of our focus on δ-containing receptors, our work does not directly address the role of synaptic inhibition in the excitability of PV interneurons. We acknowledge the important influence of phasic inhibition from multiple afferent sources (63).
Our work can be placed in the broader context of the role of tonic inhibition in PV interneurons previously studied in hippocampus and amygdala. Earlier work found that removing the δ subunit from PV interneurons in the hippocampus increased gamma oscillation frequency but not power (28). In the amygdala, δ-containing GABAA receptors in PV interneurons respond to neurosteroids, enhancing tonic inhibition and modulating network oscillations (14).
Although our study utilized acute slice preparations, other work from our group is exploring the impact of chronic alterations in δ-containing GABAA receptor expression on PV interneuron function and mPFC network dynamics and behavior in vivo (14), where sleep oscillations are affected with minimum impact on behavioral metrics. Additional extensions of the present work will be important to rational development of drugs that target δ-containing GABAA receptors, of current interest neuropsychiatry (34,62,64).
In conclusion, our findings highlight the pivotal role of δ-containing GABAA receptors in regulating the excitability and inhibitory output of PV interneurons in the mPFC, thereby shaping the balance between excitation and inhibition necessary for proper network function and cognitive processing. Because the activity of cortical PV interneurons is believed to be dysfunctional and contribute to schizophrenia, anxiety disorders, epilepsy and others, our observations contribute to understanding one pathway of potential intervention for therapeutic benefit.
SUPPLEMENTAL MATERIAL
Supplemental Fig. S1, S2, S3, and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.27122301.v1
ACKNOWLEDGMENTS
The authors thank members of the Taylor Family Institute for Innovative Psychiatric Research for discussion and input. We thank Dr. Jamie Maguire for a gift of the floxed δ mice and Dr. Yanbo Yu for preliminary help on differential gene expression.
GRANTS
The work was funded by NIMH grants R01 MH123748 and P50 MH122379 (SM and CFZ) and F30 MH126548 (PML).
Footnotes
DISCLOSURES
CFZ is a member of the Scientific Advisory Board for Sage Therapeutics and holds equity in Sage Therapeutics. Sage Therapeutics had no role in the design or interpretation of the experiments herein. The remaining authors declare no competing financial interests.
DATA AVAILABILITY
Full results are available at Figshare (https://doi.org/10.6084/m9.figshare.26026741.v1).
REFERENCES
- 1.Jobson DD, Hase Y, Clarkson AN, Kalaria RN. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 2021. Jul;3(3):fcab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011. Feb;15(2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006. Jul;1071:67–79. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs DS, Moghaddam B. Medial prefrontal cortex encoding of stress and anxiety. Int Rev Neurobiol. 2021. Mar 19;158:29–55. [DOI] [PubMed] [Google Scholar]
- 5.Xu P, Chen A, Li Y, Xing X, Lu H. Medial prefrontal cortex in neurological diseases. Physiol Genomics. 2019. Sep 1;51(9):432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binette AN, Liu J, Bayer H, Crayton KL, Melissari L, Sweck SO, et al. Parvalbumin-Positive Interneurons in the Medial Prefrontal Cortex Regulate Stress-Induced Fear Extinction Impairments in Male and Female Rats. J Neurosci. 2023. May 31;43(22):4162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson B, Glick C, Huguenard JR. Prefrontal PV interneurons facilitate attention and are linked to attentional dysfunction in a mouse model of absence epilepsy. Elife [Internet]. 2023. Apr 4;12. Available from: 10.7554/eLife.78349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S-S, Mack NR, Shu Y, Gao W-J. Prefrontal GABAergic Interneurons Gate Long-Range Afferents to Regulate Prefrontal Cortex-Associated Complex Behaviors. Front Neural Circuits. 2021. Jul 12;15:716408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M. Prefrontal Parvalbumin Neurons in Control of Attention. Cell. 2016. Jan 14;164(1–2):208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady ES, Griffiths J, Andrianova L, Bielska MH, Saito T, Saido TC, et al. Alterations to parvalbumin-expressing interneuron function and associated network oscillations in the hippocampal - medial prefrontal cortex circuit during natural sleep in AppNL-G-F/NL-G-F mice. Neurobiol Dis. 2023. Jun 15;182:106151. [DOI] [PubMed] [Google Scholar]
- 11.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009. Jun 4;459(7247):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013. Jun 20;498(7454):363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014. Jan 2;505(7481):92–6. [DOI] [PubMed] [Google Scholar]
- 14.Lambert PM, Salvatore SV, Lu X, Shu H-J, Benz A, Rensing N, et al. A role for δ subunit-containing GABA A receptors on parvalbumin positive neurons in maintaining electrocortical signatures of sleep states. bioRxiv [Internet]. 2024. Mar 29; Available from: 10.1101/2024.03.25.586604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlén M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012. May;17(5):537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ. Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev. 2017. Oct 1;97(4):1619–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013. Aug;16(8):1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigel E, Steinmann ME. Structure, Function, and Modulation of GABAA Receptors *. J Biol Chem. 2012. Nov 23;287(48):40224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic Localization of δ Subunit-Containing GABAA Receptors and Their Activation by GABA Spillover in the Mouse Dentate Gyrus. J Neurosci. 2003. Nov 19;23(33):10650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002. Aug;136(7):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glykys J, Mann EO, Mody I. Which GABAA Receptor Subunits Are Necessary for Tonic Inhibition in the Hippocampus? J Neurosci. 2008. Feb 6;28(6):1421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu H-J, Lu X, Bracamontes J, Steinbach JH, Zorumski CF, Mennerick S. Pharmacological and Biophysical Characteristics of Picrotoxin-Resistant, δSubunit-Containing GABAA Receptors. Front Synaptic Neurosci. 2021. Nov 18;13:763411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun M-Y, Shu H-J, Benz A, Bracamontes J, Akk G, Zorumski CF, et al. Chemogenetic Isolation Reveals Synaptic Contribution of δ GABAA Receptors in Mouse Dentate Granule Neurons. J Neurosci. 2018. Sep 19;38(38):8128–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Z, McGee TP, Houston CM, Brickley SG. The contribution of delta subunit-containing GABAA receptors to phasic and tonic conductance changes in cerebellum, thalamus and neocortex. Front Neural Circuits. 2013;7:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herd MB, Brown AR, Lambert JJ, Belelli D. Extrasynaptic GABA(A) receptors couple presynaptic activity to postsynaptic inhibition in the somatosensory thalamus. Journal of Neuroscience. 2013;33(37):14850–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milenkovic I, Vasiljevic M, Maurer D, Höger H, Klausberger T, Sieghart W. The parvalbumin-positive interneurons in the mouse dentate gyrus express GABAA receptor subunits α1, β2, and δ along their extrasynaptic cell membrane. Neuroscience. 2013. Dec 19;254:80–96. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Lambert P, Benz A, Zorumski CF, Mennerick SJ. Allopregnanolone Effects on Inhibition in Hippocampal Parvalbumin Interneurons. eNeuro [Internet]. 2023. Mar;10(3). Available from: 10.1523/ENEURO.0392-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonoudiou P, Colmers PLW, Walton NL, Weiss GL, Smith AC, Nguyen DP, et al. Allopregnanolone Mediates Affective Switching Through Modulation of Oscillatory States in the Basolateral Amygdala. Biol Psychiatry. 2022. Feb 1;91(3):283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, Proddutur A, Elgammal FS, Ito T, Santhakumar V. Status epilepticus enhances tonic GABA currents and depolarizes GABA reversal potential in dentate fast-spiking basket cells. J Neurophysiol. 2013. Apr;109(7):1746–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferando I, Mody I. Altered gamma oscillations during pregnancy through loss of δ subunit-containing GABA(A) receptors on parvalbumin interneurons. Front Neural Circuits. 2013. Sep 17;7:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017. Jul 29;390(10093):480–9. [DOI] [PubMed] [Google Scholar]
- 32.Walton N, Maguire J. Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiology of Stress. 2019. Nov;11:100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy DS. Neurosteroid replacement therapy for catamenial epilepsy, postpartum depression and neuroendocrine disorders in women [Internet]. Vol. 34, Journal of Neuroendocrinology. John Wiley & Sons, Ltd; 2022. p. e13028. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/jne.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whissell PD, Lecker I, Wang D-S, Yu J, Orser BA. Altered expression of δGABAA receptors in health and disease. Neuropharmacology. 2015;88:24–35. [DOI] [PubMed] [Google Scholar]
- 35.Hoy SM. Ganaxolone: A review in epileptic seizures associated with cyclin-dependent kinase-like 5 deficiency disorder. Paediatr Drugs [Internet]. 2025. Jan 10 [cited 2025 Jan 15]; Available from: https://pubmed.ncbi.nlm.nih.gov/39792341/ [DOI] [PubMed] [Google Scholar]
- 36.Lee V, Maguire J. Impact of inhibitory constraint of interneurons on neuronal excitability. J Neurophysiol. 2013. Dec;110(11):2520–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013. Jan 1;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014. Apr 1;30(7):923–30. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012. Aug 15;28(16):2184–5. [DOI] [PubMed] [Google Scholar]
- 40.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010. Jan 1;26(1):139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015. Apr 20;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu R, Holik AZ, Su S, Jansz N, Chen K, Leong HS, et al. Why weight? Modelling sample and observational level variability improves power in RNA-seq analyses. Nucleic Acids Res. 2015. Sep 3;43(15):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003. Nov 25;100(24):14439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu X, Zorumski CF, Mennerick S. Lack of Neurosteroid Selectivity at δ vs. γ2-Containing GABAA Receptors in Dentate Granule Neurons. Front Mol Neurosci. 2020. Jan 23;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006. Aug;16(8):1134–41. [DOI] [PubMed] [Google Scholar]
- 46.Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014. Aug 1;345(6196):1255263. [DOI] [PubMed] [Google Scholar]
- 47.Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J Neurophysiol. 2007. Mar;97(3):2293–300. [DOI] [PubMed] [Google Scholar]
- 48.Hadzic M, Jack A, Wahle P. Ionotropic glutamate receptors: Which ones, when, and where in the mammalian neocortex. J Comp Neurol. 2017. Mar 1;525(4):976–1033. [DOI] [PubMed] [Google Scholar]
- 49.Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995. Jan;14(1):185–9. [DOI] [PubMed] [Google Scholar]
- 50.Jiang L, Xu J, Nedergaard M, Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001. May;30(2):503–13. [DOI] [PubMed] [Google Scholar]
- 51.Cherubini E, Rovira C, Ben-Ari Y, Nistri A. Effects of kainate on the excitability of rat hippocampal neurones. Epilepsy Res. 1990. Jan-Feb;5(1):18–27. [DOI] [PubMed] [Google Scholar]
- 52.Gloveli T, Dugladze T, Rotstein HG, Traub RD, Monyer H, Heinemann U, et al. Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proc Natl Acad Sci U S A. 2005. Sep 13;102(37):13295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2(8):795–816. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FEN, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007. Feb 15;53(4):591–604. [DOI] [PubMed] [Google Scholar]
- 55.Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol. 2011. Jun 1;21(3):475–85. [DOI] [PubMed] [Google Scholar]
- 56.Antonoudiou P, Tan YL, Kontou G, Upton AL, Mann EO. Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations. J Neurosci. 2020. Sep 30;40(40):7668–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018. Aug 7;50(8):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh S, Topolnik L. Inhibitory circuits in fear memory and fear-related disorders. Front Neural Circuits. 2023. Mar 23;17:1122314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes H, Brady LJ, Schoonover KE. GABAergic dysfunction in postmortem dorsolateral prefrontal cortex: implications for cognitive deficits in schizophrenia and affective disorders. Front Cell Neurosci. 2024. Sep 24;18:1440834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juarez P, Martínez Cerdeño V. Parvalbumin and parvalbumin chandelier interneurons in autism and other psychiatric disorders. Front Psychiatry. 2022. Oct 12;13:913550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X, Lachance M, Rossignol E. Involvement of cortical fast-spiking parvalbumin-positive basket cells in epilepsy. Prog Brain Res. 2016. Jun 7;226:81–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson SM. Modulators of GABAA receptor-mediated inhibition in the treatment of neuropsychiatric disorders: past, present, and future. Neuropsychopharmacology. 2024. Jan;49(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Druga R, Salaj M, Al-Redouan A. Parvalbumin - positive neurons in the neocortex: A review. Physiol Res. 2023. Jul 31;72(Suppl 2):S173–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maguire JL, Mennerick S. Neurosteroids: mechanistic considerations and clinical prospects. Neuropsychopharmacology [Internet]. 2023. Jun 27; Available from: 10.1038/s41386-023-01626-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Full results are available at Figshare (https://doi.org/10.6084/m9.figshare.26026741.v1).


