Abstract
Background and Objectives
Detecting neural surface antibodies (NSAbs) is essential for diagnosing autoimmune encephalitis. The recommended diagnostic strategy involves initial screening with tissue-based assays (TBAs), followed by confirmation with cell-based assays (CBAs). While specialized centers use in-house TBAs, many clinical laboratories depend on commercial TBAs, whose accuracy is yet to be fully assessed.
Methods
We selected 92 CSF and 99 serum samples from patients with autoimmune encephalitis and NSAbs confirmed by in-house TBAs and CBAs (20 samples each for AMPAR, GABAAR, GABABR, IgLON5, LGI1, NMDAR, and CASPR2; 19 for mGluR5; 17 for DPPX; and 15 for mGluR1 antibodies), along with 50 CSF and 50 serum samples from negative controls. We assessed the performance of a commercial indirect immunofluorescence (IIF)-TBA (EUROIMMUN). Slides were evaluated as “positive” or “negative” by 2 experienced investigators and 2 less experienced raters. Discordant results were re-evaluated through interrater discussion and assessed using Cohen's kappa.
Results
The experienced raters agreed on 94% (133/142) of CSF and 88% (131/149) of serum classifications (Cohen's kappa = 0.87 and 0.75, respectively, p < 0.001). Among CSF samples, 75% (106/142) were correctly identified while 19% (27/142) were misclassified (13 false positives, 14 false negatives). Among serum samples, 66% (98/149) were correctly identified while 22% (33/149) were misclassified (11 false positives, 22 false negatives). The poorest performance was seen in detecting NMDAR, GABAAR, and mGluR5 Abs, which were not identified in 5 of 10, 6 of 10, and 5 of 9 serum samples and in 4 of 10, 5 of 10, and 5 of 10 CSF samples, respectively. The overall sensitivity of the commercial IIF-TBA was 84% for CSF and 76% for serum while the specificity was 72% for CSF and 73% for serum. Less experienced raters correctly identified 69% (98/142) of CSF samples and 73% (109/149) of serum samples and misclassified 13% (18/142) of CSF samples and 11% (16/149) of serum samples, and 18% (26/142) of CSF samples and 16% (24/149) of serum samples remained discordant.
Discussion
The diagnostic performance of EUROIMMUN IIF-TBA in detecting NSAbs in autoimmune encephalitis is suboptimal. NMDAR antibodies, among the most common NSAbs, can be missed in 50% of cases. This commercial TBA should not be used alone as a screening method nor as a confirmatory technique for NSAbs.
Introduction
Autoimmune encephalitides are a group of disorders associated with antibodies against neural (neuronal or glial) cell-surface antigens (NSAbs).1 The detection of NSAbs in a patient's serum and CSF is essential for diagnosing these disorders, as it confirms the suspicions raised by clinical features and paraclinical investigations.2 Detection methods include immunohistochemistry (IHC) or immunofluorescence (IIF) on rodent brain tissue (tissue-based assays, TBAs), followed by confirmation of antigen specificity using cell-based assays (CBAs).3,4 Combination of these techniques enhances diagnostic accuracy,5-8 but this dual approach is rarely used in clinical laboratories, which primarily rely on CBAs or less frequently on commercially available TBAs. While some studies have evaluated the sensitivity and specificity of commercial CBAs for NSAbs detection,8 the accuracy of commercial TBAs remains undetermined. The aim of this study was to assess the performance of a commercially available IIF-TBA for neural surface antibody testing.
Methods
Patients, Inclusion Criteria, and Sample Selection
Patients' serum and CSF samples were obtained from the Neuroimmunology Collection (SCII number C0000051) at IDIBAPS-Hospital Clínic of Barcelona, Spain. This collection includes clinical data and samples from over 20,000 patients with suspected autoimmune neurologic disorders, referred between 2007 and 2024.
From this collection, we selected 191 samples from 148 patients with autoimmune encephalitis and NSAbs. This included 92 CSF samples (10 samples each for AMPAR, CASPR2, GABAAR, GABABR, IgLON5, LGI1, mGluR5, and NMDAR; 7 for DPPX; and 5 for mGluR1 antibodies) and 99 serum samples (10 samples each for AMPAR, CASPR2, DPPX, GABAAR, GABABR, IgLON5, LGI1, mGluR1, and NMDAR and 9 for mGluR5 antibodies). Patients' samples were included if they met the following criteria: (1) detection of NSAbs confirmed by 2 techniques: in-house IHC showing a characteristic pattern of reactivity with rat brain9 (CSF dilution 1:2, serum dilution 1:200) and CBA confirming the specific antibody and (2) neurologic syndrome consistent with the specific antibody. Samples with multiple antibodies, including intracellular neuronal antibodies and non-neuron–specific antibodies, were excluded.
In addition, we included 50 serum and 50 CSF samples (unpaired) from 100 patients with suspected autoimmune encephalitis who tested negative for in-house TBA and CBA (negative controls). Serum and CSF samples were stored at −20°C during the study period and at −80°C for long-term storage.
Detection of Neural Surface Antibodies by Commercial IIF-TBA
A commercial IIF-TBA (EUROIMMUN—ref. FA 111a-1010-3), consisting of 2 biochips per field with fixed sections of rat hippocampus and cerebellum (both gray and white matter), was used to test all samples. Following the manufacturer's instructions, serum (dilution 1:10) and CSF (1:1) samples were incubated with the tissue at room temperature for 30 minutes. Reactivity was detected using a fluorescent anti-human antibody provided in the kit.
The results were evaluated using a Zeiss Axio Imager M2 fluorescent microscope (Carl Zeiss, Jena, Germany) by 2 expert independent raters (F.G. and J.D.) and 2 less experienced raters (C.P. and C.M.), all of whom were blinded to the in-house IHC-TBA results and clinical information. Each rater scored reactivity for neuropil staining as either “positive” or “negative.” The expert raters discussed any discrepancies in their evaluations; if they reached an agreement, the result was deemed “concordant.” If no agreement was reached, the result was considered “discordant.” The complete study algorithm for assessment by the experienced raters is shown in Figure 1. No interrater discussion was performed between the less experienced raters.
Figure 1. Algorithm of the Study.
CBA = cell-based assay; IIF = indirect immunofluorescence; NSAbs = neural surface antibodies; TBA = tissue-based assay.
Statistical Analysis
Categorical variables were expressed as proportions and percentages. The Cohen's kappa coefficient was used to evaluate the level of agreement on positive/negative results between the 2 expert raters and between the 2 less experienced raters. Strength of agreement was considered according to the kappa index value, as reported10 (<0.40 was considered poor concordance, 0.41–0.57 fair, 0.58–0.75 good, and >0.75 excellent). The percentages of true/false-positive/negative results were calculated separately for serum and CSF samples. Sensitivity and specificity were determined after excluding samples with discordant results and were expressed as percentages with 95% confidence intervals. Statistical analyses were conducted using IBM SPSS Statistics 20.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board of the Hospital Clínic, Barcelona (Reg. HCB/2023/1183). All patients or their proxies provided written informed consent for the storage and use of serum, CSF, and clinical information for research purposes.
Data Availability
Anonymized participant data can be shared with qualified investigators on request to the corresponding author.
Results
Clinical Syndromes, Rater Concordance, and Performance of the Commercial IIF-TBA in CSF Samples
The demographics, clinical syndromes, and associated tumors of the 148 patients with NSAbs were consistent with the expected features for each antibody, as summarized in eTable 1.
Regarding CSF samples, the 2 experienced raters agreed on the negative/positive results in 69% (98/142) of cases (Cohen's kappa = 0.40, p < 0.001). After re-evaluating the discordant samples, they reached a consensus for 94% (133/142) of the samples, showing excellent agreement (Cohen's kappa = 0.87, p < 0.001), with 9 samples remaining discordant. Overall, using the commercial IIF-TBA, 75% (106/142) of the CSF samples were correctly identified (true positive/negative), 19% (27/142) were misclassified (false positive/negative), and 6% (9/142) remained discordant (Figure 2).
Figure 2. Pie Charts Showing the Percentages of True/False-Positive/Negative Results in CSF (A) and Serum (B) Samples Using EUROIMMUN IIF-TBA.
IIF = indirect immunofluorescence; NSAb+ = neural surface antibody positive; NC = negative control; TBA = tissue-based assay.
When considering only the antibody-positive samples, 78% (72/92) were correctly identified as positives, 15% (14/92) were misclassified as false negatives, and 7% (6/92) remained discordant (Figure 2).
Among the negative control CSF samples, 68% (34/50) were correctly identified as negatives, 26% (13/50) were misclassified as false positives, and 6% (3/50) remained discordant (Figure 2).
The overall sensitivity of the commercial IIF-TBA for CSF samples was 84% (95% CI 74.2%–90.8%), and its specificity was 72% (95% CI 57.4%–84.4%).
Less experienced raters agreed in 82% (116/142) of the cases (Cohen's kappa = 0.53, p < 0.001; no interrater discussion was performed), correctly identifying 69% (98/142) and misclassifying 13% (18/142). 18% (26/142) of the samples remained discordant (not shown).
Rater Concordance and Performance Evaluation of the Commercial IIF-TBA in Serum Samples
For serum samples, the 2 experienced raters initially agreed in 64% (96/149) of cases (Cohen's kappa = 0.32, p < 0.001). After re-evaluating the discordant samples, they reached a consensus for 88% (131/149) of the samples, showing good agreement (Cohen's kappa = 0.75, p < 0.001), with 18 samples remaining discordant.
Overall, using the commercial IIF-TBA, 66% (98/149) of the serum samples were correctly identified as true positives/negatives, 22% (33/149) were misclassified as false positives/negatives, and 12% (18/149) remained discordant (Figure 2). When considering only the antibody-positive samples, 70% (69/99) were classified as true positives, 22% (22/99) were misclassified as false negatives, and 8% (8/99) remained discordant (Figure 2).
Among the negative control serum samples, 58% (29/50) were correctly identified as negatives, 22% (11/50) were misclassified as false positives, and 20% (10/50) remained discordant (Figure 2).
The overall sensitivity of the commercial IIF-TBA for serum samples was 76% (95% CI 65.7%–84.2%), and its specificity was 73% (95% CI 56.1%–85.4%).
Less experienced raters agreed in 84% (125/149) of the cases (Cohen's kappa = 0.63, p < 0.001; no interrater discussion was performed), correctly identifying 73% (109/149) and misclassifying 11% (16/149). 16% (24/149) of the samples remained discordant (not shown).
Performance of the Commercial IIF-TBA in Detecting Specific Neural Antibodies
The antibody-specific pattern of hippocampal and cerebellar staining typically observed with in-house TBAs was not always detectable using the commercial IIF-TBA (Figure 3). Therefore, this section focuses on the positive/negative results for each antibody-positive sample (Figure 4), without considering the specific pattern of reactivity.
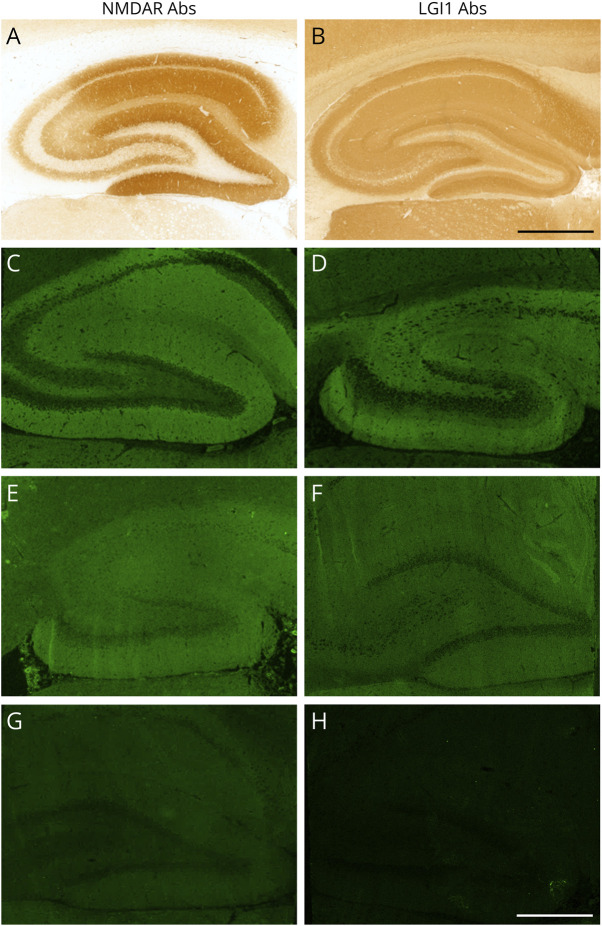
Figure 3. Examples of NMDAR and LGI1 Antibody Staining on In-House IHC and Commercial IIF-TBA.
Staining patterns of samples positive for NMDAR (left column) and LGI1 (right column) antibodies on rat hippocampus with in-house IHC and IIF-TBA. Characteristic staining patterns of NMDAR (A) and LGI1 (B) antibodies with in-house IHC. In some cases, the same recognizable typical staining pattern was observed on IIF-TBA (C, D), whereas in others, the sample was labeled as “positive” although a typical staining pattern was not observed (E, F). Examples of NMDAR (G) and LGI1 (H) antibody–positive samples with negative staining with IIF-TBA. Scale bar = 500 μm. Abs = antibodies; IHC = immunohistochemistry; IIF = indirect immunofluorescence; LGI1 = leucine-rich glioma inactivated 1; NMDAR = N-methyl-d-aspartate receptor; TBA = tissue-based assay.
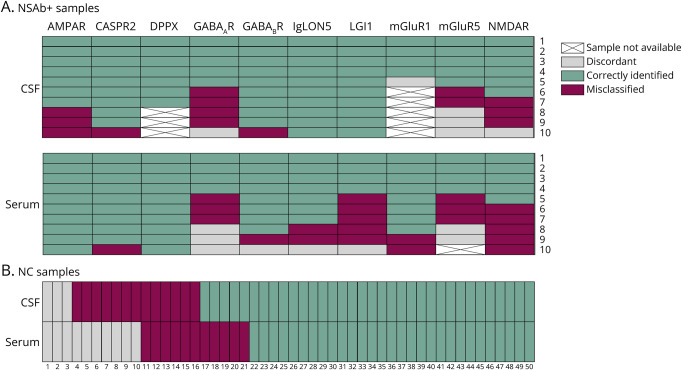
Figure 4. Heatmaps Showing the Detailed Results for Each Sample Tested With the Commercial IIF-TBA.
CSF (A, upper plot) and serum (A, lower plot) positive samples are grouped into different columns based on the specific antibody. Each sample is represented with a different color according to the results after the interrater agreement between the 2 expert raters (in green: true positive; in purple: false negative; in gray: discordant samples). CSF (B, top row) and serum (B, bottom row) negative controls (unpaired samples) are represented with different colors according to the results after the interrater agreement between the 2 expert raters (in green: true negative; in purple: false positive; in gray: discordant samples). NC = negative control; NSAb+ = neural surface antibody positive.
The detection of certain antibodies by the 2 experienced raters was optimal for both CSF and serum samples. For instance, DPPX antibodies were identified as positive in all samples: 7 of 7 CSF and 10 of 10 serum samples. Similarly, high performance was observed for CASPR2, GABABR, and mGluR1 antibodies, which were correctly identified in 9 of 10, 9 of 10, and 4 of 5 CSF samples and in 9 of 10, 8 of 10, and 8 of 10 serum samples, respectively.
However, the performance varied for other antibodies between serum and CSF. For example, IgLON5 and LGI1 antibodies were identified in all CSF samples (10/10), but only in 7 of 10 (IgLON5) and 4 of 10 (LGI1) serum samples. Conversely, AMPAR antibodies were better identified in serum (10/10) than in CSF (7/10).
The lowest performance was observed for NMDAR, GABAAR, and mGluR5 antibodies, which were correctly identified in 6 of 10, 5 of 10, and 5 of 10 CSF samples and in 5 of 10, 4 of 10, and 4 of 9 serum samples, respectively.
Discussion
This study evaluated the diagnostic yield of a commercial IIF-TBA in detecting NSAbs. We included 191 samples from patients with autoimmune encephalitis, each showing neuropil staining on in-house IHC, and 100 control samples that tested negative on in-house IHC. Our findings revealed that the overall sensitivity of the commercial TBA was 84% for CSF and 76% for serum samples. The implications of false-negative results in the clinical context of autoimmune encephalitides are significant. When used as a screening method, a negative test result may discourage further diagnostic assessments, such as CBA, potentially leading to the exclusion of a diagnosis, especially when clinical information is incomplete or not available. This scenario has important consequences. First, patients with autoimmune encephalitis may not receive timely immunotherapy, potentially resulting in worse outcomes.11 Second, if autoimmune encephalitis has a paraneoplastic etiology, as frequently occurring in patients with AMPAR, GABABR, and mGluR5 antibodies,12 an underlying tumor could be missed if not properly investigated.
We found that the performance of the commercial IIF-TBA varied not only with the type of sample (serum vs CSF) but also according to the antibody specificity. Although our study included a comprehensive variety of samples from patients with different autoimmune encephalitides, these diseases have varying prevalence in the general population, with NMDAR and LGI1 encephalitis being the most common.13,14 We found that 60% of CSF and 50% of serum samples positive for NMDAR antibodies and 100% of CSF and 40% of serum samples positive for LGI1 antibodies were detectable by the commercial TBA. Practically, this means that if 100 CSF samples from patients with LGI1 and NMDAR encephalitis were tested, around 50% of patients with NMDAR encephalitis would not be diagnosed. Conversely, if serum samples were used, around half of the patients with NMDAR or LGI1 encephalitis would be undiagnosed. Therefore, although the overall rate of false negatives in our cohort was approximately 20% (15% for CSF and 22% for serum), the percentage of missed diagnoses in real-life applications may be even higher, considering the greater prevalence of NMDAR and LGI1 encephalitis compared with other autoimmune encephalitides.
Regarding the assay readout, an important issue was that the typical pattern of brain reactivity observed with neural antibodies on in-house IHC was not consistently recognizable on the commercial IIF-TBA (Figure 3).9 This lack of characteristic immunostaining contributed to the rate of false-negative results and complicated the interpretation of true-negative samples. We found that the specificity of the commercial IIF-TBA was 72% for CSF and 73% for serum samples, indicating that approximately 25% of negative samples were misclassified.
Several factors may account for the discrepancies between the performances of the commercial IIF-TBA and in-house IHC: (1) Low neural autoantibody titers may not be detected by the commercial IIF-TBA, as suggested in a previous study.15 However, we did not observe significant differences in staining intensity on in-house IHC between true-positive and false-negative samples by the commercial IIF-TBA (data not shown). (2) The inclusion of selected brain regions (cerebellum and hippocampus only) in the commercial IIF-TBA mosaics, compared with the whole rodent brain in in-house IHC, may limit the diagnostic evaluation. (3) Antibody epitope specificity may be affected by the tissue processing and fixation of certain antigens, contributing to false-negative results.
In conclusion, our study suggests that the commercial IIF-TBA has limitations in detecting antibodies against neural surface antigens and should not be recommended as a screening test (or as confirmatory test) for autoimmune encephalitis. In this clinical setting, specific confirmatory tests (CBA for NSAbs) become the primary diagnostic tools. However, given that previous studies have identified challenges with some of these tests,8,16 we recommend referring patient samples to specialized neuroimmunology laboratories where in-house diagnostic techniques (TBAs and CBAs) are available. This is particularly important in cases of high clinical suspicion despite a negative result on the commercial IIF-TBA, especially if the commercial CBA is negative or results are incongruent with the clinical syndrome.
Acknowledgment
The authors thank EUROIMMUN for providing the TBA kits.
Glossary
- CBA
cell-based assay
- IHC
immunohistochemistry
- IIF
immunofluorescence
- NSAbs
neural surface antibodies
- TBA
tissue-based assay
Footnotes
Author Contributions
C. Papi: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. C. Milano: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. L. Arlettaz: drafting/revision of the manuscript for content, including medical writing for content. P. Businaro: drafting/revision of the manuscript for content, including medical writing for content. L. Marmolejo: drafting/revision of the manuscript for content, including medical writing for content. L. Naranjo: drafting/revision of the manuscript for content, including medical writing for content. J. Planagumà: drafting/revision of the manuscript for content, including medical writing for content. E. Martinez-Hernandez: drafting/revision of the manuscript for content, including medical writing for content. T. Armangue: drafting/revision of the manuscript for content, including medical writing for content. M. Guasp: drafting/revision of the manuscript for content, including medical writing for content. R.R. García: drafting/revision of the manuscript for content, including medical writing for content. E. Aguilar: drafting/revision of the manuscript for content, including medical writing for content. M. Gastaldi: drafting/revision of the manuscript for content, including medical writing for content. R. Iorio: drafting/revision of the manuscript for content, including medical writing for content. C. Gaig: drafting/revision of the manuscript for content, including medical writing for content. A. Saiz: drafting/revision of the manuscript for content, including medical writing for content. L. Sabater: drafting/revision of the manuscript for content, including medical writing for content. F. Graus: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. J.O. Dalmau: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. M. Spatola: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data.
Study Funding
The authors report no targeted funding.
Disclosure
C. Papi receives research support from Spanish National Health Institute Carlos III (FIS grant PI23/01366) and 2023 EAN Research Training Fellowship. C. Milano receives research support from Spanish National Health Institute Carlos III co-funded by the European Union (Rio-Hortega grant CM24/00055). L. Marmolejo receives research support from Spanish National Health Institute Carlos III (predoctoral research grant FI24/00021). M. Spatola receives research support from La Caixa Foundation (Junior Leader) and Spanish National Health Institute Carlos III (ISCIII) and co-funded by the European Union (FIS grant PI23/01366). J. Dalmau receives research support from CaixaResearch Health 2022 (HR22-00221), Spanish National Health Institute Carlos III (ISCIII) and co-funded by the European Union (FIS grant PI23/00858), Cellex Foundation, Fundació Clínic per a la Recerca Biomèdica (FCRB) Programa Multidisciplinar de Recerca, Generalitat de Catalunya Department of Health (SLT028/23/000071), Edmond J. Safra Foundation. He receives royalties from Euroimmun for the use of NMDA as an antibody test. He received a licensing fee from Euroimmun for the use of GABAB receptor, GABAA receptor, DPPX and IgLON5 as autoantibody tests. He has received a research grant from Sage Therapeutics. All the other authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378(9):840-851. doi: 10.1056/NEJMra1708712 [DOI] [PubMed] [Google Scholar]
- 2.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Höftberger R, Dalmau J, Graus F. Clinical neuropathology practice guide 5-2012: updated guideline for the diagnosis of antineuronal antibodies. Clin Neuropathol. 2012;31(5):337-341. doi: 10.5414/np300545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Graus F. Diagnostic criteria for autoimmune encephalitis: utility and pitfalls for antibody-negative disease. Lancet Neurol. 2023;22(6):529-540. doi: 10.1016/S1474-4422(23)00083-2 [DOI] [PubMed] [Google Scholar]
- 5.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-177. doi: 10.1016/s1474-4422(13)70282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spatola M, Petit-Pedrol M, Simabukuro MM, et al. Investigations in GABAA receptor antibody-associated encephalitis. Neurology. 2017;88(11):1012-1020. doi: 10.1212/WNL.0000000000003713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerstens J, Schreurs MWJ, de Vries JM, et al. Autoimmune encephalitis and paraneoplastic neurologic syndromes: a nationwide study on epidemiology and antibody testing performance. Neurol Neuroimmunol Neuroinflamm. 2024;11(6):e200318. doi: 10.1212/nxi.0000000000200318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-García R, Muñoz-Sánchez G, Naranjo L, et al. Limitations of a commercial assay as diagnostic test of autoimmune encephalitis. Front Immunol. 2021;12:691536. doi: 10.3389/fimmu.2021.691536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97(2):839-887. doi: 10.1152/physrev.00010.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. 1981;86(2):127-137. [PubMed] [Google Scholar]
- 11.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165. doi: 10.1016/s1474-4422(12)70310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graus F, Vogrig A, Muñiz-Castrillo S, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1014. doi: 10.1212/NXI.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760-772. doi: 10.1016/S1474-4422(18)30244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuliani L, Marangoni S, De Gaspari P, et al. Epidemiology of neuronal surface antibody-mediated autoimmune encephalitis and antibody-based diagnostics. J Neuroimmunol. 2021;357:577598. doi: 10.1016/j.jneuroim.2021.577598 [DOI] [PubMed] [Google Scholar]
- 15.Nagata N, Kanazawa N, Mitsuhata T, et al. Neuronal surface antigen-specific immunostaining pattern on a rat brain immunohistochemistry in autoimmune encephalitis. Front Immunol. 2022;13:1066830. doi: 10.3389/fimmu.2022.1066830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz-Sánchez G, Planagumà J, Naranjo L, et al. The diagnosis of anti-LGI1 encephalitis varies with the type of immunodetection assay and sample examined. Front Immunol. 2022;13:1069368. doi: 10.3389/fimmu.2022.1069368 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized participant data can be shared with qualified investigators on request to the corresponding author.