Abstract
Background and Objectives
Optical coherence tomography (OCT) has emerged as a valuable marker for assessing inflammation and neuroaxonal degeneration in multiple sclerosis (MS). Although traditional markers such as brain atrophy and axonal loss are crucial for monitoring MS progression, their clinical application can be limited by various factors. This meta-analysis of longitudinal studies aims to assess the predictive value of OCT-derived retinal layer thickness thresholds for monitoring and predicting MS disease progression and cognitive decline.
Methods
Our systematic review and meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. A comprehensive systematic search was performed using electronic databases (PubMed, Embase, Web of Science, and Google Scholar) for longitudinal studies using Spectral Domain-OCT (SD-OCT) to assess retinal layer thickness and its predictive value for MS progression. Data were extracted on study design, OCT measurements, disability progression definitions, and clinical outcomes. We analyzed hazard ratios (HR) and odds ratios (OR) for associations between OCT-measured thresholds and disability progression, including physical and cognitive deterioration.
Results
Our study included 14 longitudinal studies that met our inclusion criteria, 13 studies were included in our quantitative analysis, with a total of 3,683 participants. Baseline peripapillary retinal nerve fiber layer (pRNFL) thickness below 88 μm was significantly associated with increased risk of future disease progression and physical worsening measured by Expanded Disability Status Scale progression (HR = 2.376, p < 0.001; HR = 2.258, p < 0.001, respectively). The same was noted for ganglion cell-inner plexiform layer (GCIPL) thickness below 77 μm (HR = 2.751, p < 0.001 and HR = 2.66, p < 0.001, respectively). In addition, annualized rates of pRNFL thinning above 1.5 μm/y and GCIPL thinning above 1 μm/y also significantly predicted disease worsening (HR = 3.019, p = 0.005 and HR = 3.535, p < 0.001, respectively).
Discussion
OCT-derived retinal layer thresholds, specifically a pRNFL thickness of ≤88 μm and a GCIPL thickness of ≤77 μm, are significantly associated with an increased risk of future MS disability progression. Furthermore, annual thinning rates of pRNFL >1.5 μm/y and GCIPL >1 μm/y demonstrate greater predictive power and are more clinically relevant for identifying individuals at high risk of both physical and cognitive disability progression outcomes. Further research is needed to standardize OCT thresholds and improve clinical use in treatment planning.
Introduction
Multiple sclerosis (MS) is an autoimmune, inflammatory, and neurodegenerative disease of the CNS.1 The incidence and prevalence of MS are increasing worldwide, with an estimate that a new person is diagnosed with MS every 5 minutes.2 In MS, clinical deterioration exhibits a stronger correlation with neurodegeneration and axonal loss rather than demyelination. Multiple pathways, including oxidative stress, hypoxia, autoantibodies, and metabolic disturbances, have been implicated in the process of axonal loss.3 Numerous imaging brain volume segmentation methods show that brain atrophy and axonal loss are valuable markers for clinical disability, cognitive worsening, and markers of progression.4 However, these techniques are difficult to use and are currently not easily accessible in the clinical setting. Measurement of brain atrophy using brain volume loss on MRI is believed to reflect neurodegeneration in MS but suffers from technical and clinical applicability limitations and sensitivity-specificity issues. OCT is a noninvasive technique, relatively inexpensive, easy to perform in clinical practice, fast, and can generate reliable quantitative measures.5 Moreover, OCT measures are easier to standardize with no confounding interrater variability, enabling individual longitudinal monitoring.
Optical coherence tomography (OCT) uses patterns of infrared reflection to enable the accurate measurement of retinal layers, including the retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL).6 The peripapillary retinal nerve fiber layer (pRNFL) and macular ganglion cell-inner plexiform layer (GCIPL) have been recognized as valuable indicators of neurodegeneration in MS.7 Several studies have established a correlation between the thinning of retinal layers and the progression of disability clinical pictures of patients.6,8-10 However, the widespread adoption of OCT in MS clinical practice has been hampered by confounders such as age, sex, disease duration, and race, as well as the absence of clearly defined OCT threshold values for predicting disease progression.11
OCT has been widely used as an outcome measure in multiple MS clinical trials, assessing the efficacy of treatments such as fingolimod, erythropoietin, phenytoin, and PEGylated interferon beta-1a. These trials have used OCT metrics such as RNFL and pRNFL thickness to evaluate neuroprotection and disease progression (NCT01705236, NCT00355095, and NCT01337427). In addition to its role in clinical trials, OCT is used to detect early structural changes before clinical symptoms appear, with scans performed at baseline and follow-ups many months after treatment initiation to monitor drug effects and detect potential adverse changes (67, 68).
Our previous meta-analysis provided a comprehensive comparison of retinal layer thickness measures in individuals with various MS subtypes, highlighting a significant reduction in both pRNFL and GCIPL thickness,7 focused on cross-sectional OCT studies. Therefore, in our current meta-analysis summarizing findings from recent longitudinal studies, we aim to evaluate the statistical associations between OCT measurement and future disease progression, including physical and cognitive deterioration from prospective studies.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklists were used to instruct the management and the conduction of this systematic review and meta-analysis. The meta-analysis was preregistered on PROSPER (CRD42024536036).
Search Strategy
Electronic databases (PubMed, Embase, Web of Science, and Google Scholar)12 were used to conduct the electronic search. Only prospective studies published in English were included, and no date restriction was placed on this search. Both electronic and manual searching procedures were used in the studies' identification. The electronic databases and search terms specified below were used in electronic searches and applied by 2 independent reviewers. We reviewed the relevant articles' citations and simultaneously sought information from experts in the field. The search terms included keywords related to MS (“Multiple Sclerosis” [Mesh] OR “Multiple Sclerosis” OR “MS”), AND keywords related to SD-OCT (“Tomography, Optical Coherence” [Mesh] OR “Optical Coherence Tomography” OR “OCT” OR “SD-OCT”), AND keywords related to disease progression (: “Disease Progression” [Mesh] OR “Disease Progression” OR “Disease Exacerbation” OR “Clinical Deterioration” OR “Disability Progression” OR “Disability Worsening” OR “Disease Activity” OR “EDSS Progression”).
Literature Screening and Selection Criteria
The titles and abstracts of all articles initially found according to the selection criteria were independently evaluated by 2 independent reviewers (G.S. and J.A.). The following inclusion criteria were used: (1) original studies using SD-OCT in patients with MS to study the retinal layer thickness, (2) studies reporting associations of retinal layer thickness and disease progression, (3) studies using longitudinal study design, (4) diagnosis of MS based on McDonald criteria, (5) studies reporting risk of disability progression as the outcome (i.e., [OR] ratio, hazard ratio [HR], and relative risk [RR]), and (6) studies reporting Expanded Disability Status Scale (EDSS), MS Functional Composite and MS Severity Score as a component in the definition of “disability progression.”
However, all articles that did not use Spectral Domain-OCT were excluded. Studies that met all the selection criteria had their full texts retrieved by the same 2 independent authors.
Data Extraction
Two authors extracted the data independently, compared their results, and reached agreement. The extracted data included author, year of publication, study design, baseline population (age, number of male and female), disability progression definition, duration of follow-up, EDSS score (at baseline and different points of follow-up), symbol digit modalities test (SDMT) score (at baseline and different points of follow-up), disease duration, diagnostic criteria of MS, OCT device, OCT time points, OCT quality control, number of patients and control, measures of association (HR, RR, and OR) (with and without adjustment), cutoff values that were used cross-sectionally and longitudinally of each retinal layer, and history of prior ON.
Quality Assessment
Two independent reviewers assessed the quality of the studies using The Quality in Prognosis Studies (QUIPS) tool.13 The QUIPS tool, designed for prognostic factor studies, assesses bias across 6 domains: (1) study participation, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) study confounding, and (6) statistical analysis and reporting. The overall risk of bias is categorized as “low” when all 6 domains are assigned a low risk of bias. This comprehensive assessment framework ensures a thorough evaluation of the methodological quality of each study, providing a robust foundation for the reviewers to gauge the reliability and validity of the findings.
Data Synthesis and Analysis
For the data extracted from the included articles, a meta-analysis was performed using the inverse variance weighted method to combine summary measures using random-effects models to minimize the effect of between-study heterogeneity. The primary outcome of interest was overall disease progression, which includes both physical and cognitive decline. As secondary outcomes, we separately assessed physical disability, measured by EDSS progression, and cognitive deterioration. “High” and “low” baseline pRNFL or GCIPL were defined according to the cutoff chosen by each study. Subgroup analyses were also performed according to the different predictive cutoffs used in the articles for the primary outcome (pRNFL <88 µm, pRNFL <92–98 μm). For the annualized rate of change, different predictive cutoffs were used for annualized loss of GCIPL (alGCIPL) and annualized loss of pRNFL (aLpRNFL) (aLGCIPL >0.5–1 μm/y, aLGCIPL >1 μm/y, aLpRNFL >1.5–2 μm/y). The estimates of HRs or ORs were reported using a random-effects model or fixed model according to the heterogeneity. Heterogeneity was assessed using the Cochrane χ2 statistic and the I2 statistic (15). Publication bias was evaluated through funnel plots. Study-level characteristics included study design, number of total participants, and risk of bias as characteristics for assessment of heterogeneity. All the statistical tests were 2-sided, and statistical significance was defined as p being less than 0.05.
Data Availability
Data available on request from the corresponding author.
Results
Study Selection Process
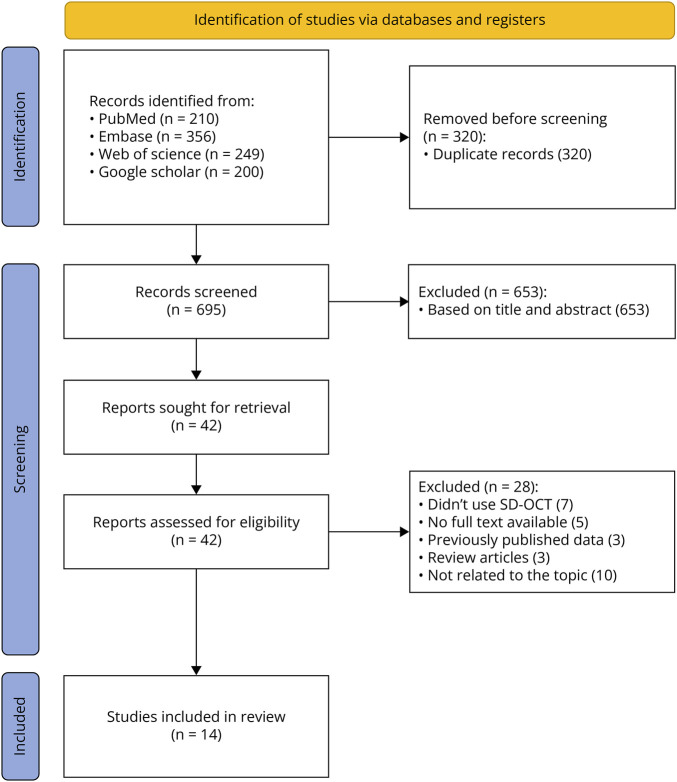
Figure 1 shows the PRISMA flow diagram for our meta-analysis. Initially, a total of 1,015 records were identified through database searches: 210 from PubMed, 356 from Embase, 249 from Web of Science, and 200 from Google Scholar. After removing 320 duplicate records, 695 records remained for screening. During the screening phase, 653 records were excluded based on title and abstract, leaving 42 reports sought for retrieval. These 42 reports were then assessed for eligibility. Based on the eligibility assessment, 28 reports were excluded for various reasons (specific reasons for exclusion are provided in Figure 1). Ultimately, 14 studies were included in the review.12,14-26
Figure 1. PRISMA Flowchart of Included Studies in the Systematic Review and Meta-analysis .
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Characteristics of Included Articles
Table 1 presents a summary of the sociodemographic characteristics of the participants included. The average follow-up duration ranged from 2 to 10 years with a mean of 4.72 years. A total of 3,942 participants, 72.99% women, and with a mean age of 35.61 years, were included. The mean disease duration was 4.5 years, and the mean EDSS score was 1.47 with a SD of 0.62. Although the majority of articles used a prospective longitudinal design, only one study24 used a retrospective longitudinal design. In OCT measurements, 6 articles used 87–88 μm as the cross-sectional cutoff for the pRNFL layer to predict future outcomes, while 3 articles used 1.5 μm/y as the longitudinal cutoff. Regarding the GCIPL layer, 9 articles used a cross-sectional cutoff of 77 μm, 4 articles used 70 μm, while 4 articles applied a longitudinal cutoff of 1 μm/y. The definition of disability worsening varied between articles, with the majority defining it as EDSS progression. However, 4 articles additionally required cognitive deterioration, measured with the SDMT, as a criterion. SD-OCT devices, namely Spectralis (Heidelberg Engineering) and/or Cirrus (Carl Zeiss Meditec) were used, with intervals between scans ranging from 1 year to ≥7 years for longitudinal assessments. Further clinical characteristics are detailed in eTable 1. A detailed summary of the inclusion and exclusion criteria of each included article is given in eTable2.
Table 1.
Sociodemographic and Clinical Characteristics of Included Articles
| Author/year | Study design | Follow-up duration | Participants | Disease course N (%) |
MS diagnostic criteria | Number of female N (%) | Mean age Mean (SD) y |
Baseline EDSS median (range) | SDMT baseline mean (SD) | Disease duration | Prior ON (%) | SD-OCT device |
| Alba-Arbalat et al. (2023)12 | Prospective | 10 y | 207 | CIS 17 (8), RRMS 174 (84), SPMS 14 (7), PPMS 2 (1) | McDonald criteria 2017 | 151 (73) | 40.9 (IQR 34.5–48.6) | 1.5 (1.0–2.5) | 3 (IQR 2–4) | 8.3 (2.4–14.1) mo | 20.5% | Spectralis SD-OCT device (Heyex 5.30 Heidelberg Engineering, Germany) |
| Berek et al. (2022)14 | Prospective | 6 y | 93 | RRMS 86 (92.5) SPMS 7 (7.5) |
McDonald criteria 2010 | 74 (79.6) | 35.8 (9.0) | 1.5 (0–6.0) | 54.9 (10.8) | 3.9 (0.1–30.7) y | 35.4% | Heidelberg Engineering, Heidelberg, Germany; software Heidelberg Eye Explorer software version 5.4.8.0) |
| Bsteh et al. (2022)19 | Retrospective | 6 y | 231 | RRMS 231 (100) | McDonald criteria 2017 | 171 (74.0) | 30.3 (8.1) | 0 (0–2.5) | NA | 45 (0–179) d | NA | OCT (Heidelberg Engineering, Heidelberg, Germany; software Heidelberg Eye Explorer software version 6.9a) |
| Bsteh et al. (2019)15 | Prospective | 3 y | 141 | RRMS 141 (100) | McDonald criteria 2010 | 107 (75.9) | 35.1 (9.4) | 1.5 (0.0–6.5) | 54.1 (10.2) | 7.1 (7.4) y | 20.6% | SD-OCT; Spectralis; Heidelberg Engineering |
| Bsteh et al. (2019)16 | Prospective | 3 y | 151 | RRMS 141 (100), 10 lost of follow-up | McDonald criteria 2010 | 119 (78.8) | 35.1 (9.4) | 1.5 (0.0–6.5) | 54.0 (10.1) | 5.8 (2.7) y | 19.9% | SD-OCT; Spectralis; Heidelberg Engineering |
| Bsteh et al. (2021)17 | Prospective | 3 y | 183 | RRMS 183 (100), 15 lost to follow-up | McDonald criteria 2010 | 135 (73.8) | 34.8 (9.2) | 1.5 (0.0–6.5) | 54.3 (10.1) | 5.3 (6.2) y | 20.8% | SD-OCT; Spectralis; Heidelberg Engineering |
| Bsteh et al. (2021)18 | Prospective | 4 y | 113 | RRMS 91 (80.5) | McDonald criteria 2010 | 91 (80.5) | 34.2 (8.6) | 1.0 (0.0–6.5) | 55.3 (9.8) | 7.0 (6.4) y | 19.5% | SD-OCT; Spectralis; Heidelberg Engineering |
| Bsteh et al. (2020)25 | Prospective | 4 y | 171 | RRMS 171 (100) | McDonald criteria 2010 | 125 (73.1) | 35.2 (9.6) | 1.5 (0–6.5) | 54.0 (10.3) | 6.1 (6.5) y | 21.1% | SD-OCT; Spectralis; Heidelberg Engineering |
| Cilingir et al. (2021)20 | Prospective | 34.1 mo (mean duration) | 137 | RRMS 137 (100) | McDonald criteria 2010 | 99 (72.3) | 33.16 (9.1) | 1.5 (1.0–2.0) | NA | 2.25 (1.54) y | 7.66% | SD-OCT; Spectralis; Heidelberg Engineering |
| Lambe et al. (2021)21 | Prospective | 10.4 y (median duration) | 117 | RRMS 92 (78.6), PMS 25 (21.3) | McDonald criteria 2005 | GCIPL ≥70 µm 57 (79) GCIPL <70 µm 33 (73) |
GCIPL ≥70 µm 43.13 (1.35) GCIPL <70 µm 42.52 (1.64) |
GCIPL ≥70 µm 2.0 (1.5–3.0) GCIPL <70 µm 3.0 (2.0–4.5) |
GCIPL ≥70 µm 31 (43) GCIPL <70 µm 23 (51) |
GCIPL ≥70 µm 6.92 (6.80) y GCIPL <70 µm 10.87 (8.93) y |
GCIPL ≥70 µm 43% GCIPL <70 µm 51% |
SD-OCT; Cirrus; Carl Zeiss Meditec |
| Lin et al. (2021)26 | Prospective | 23.9 mo (mean duration) | 78 | CIS 16 (21), RRMS 62 (79) | McDonald criteria 2017 | 50 (64.1) | 33.7 (7.4) | 1.5 (1.0–2.0) | NA | 12.1 (11.8–12.7) mo | NA | Spectral domain OCT (Heidelberg Engineering, Heidelberg, Germany) |
| Martinez-Lapiscina et al. (2016)22 | Prospective | 2 y (median) | 879 | CIS 70 (8), RRMS 668 (76), SPMS 79 (9), PPMS 62 (7) | McDonald criteria 2010 | 584 (66) | 40.6 (8.1) | 2.0 (1.5–3.5) | 6.5 (2.7–13.4) y | 32% | SD-OCT; Spectralis; Heidelberg Engineering or Cirrus; Carl Zeiss Meditec | |
| Schurz et al. (2021)24 | Retrospective | 2.9 y (mean duration) | 60 | RRMS 53 (88.3), SPMS 7 (11.7) | McDonald criteria | 40 (66.7) | 34.5 (11.2) | 1.0 (0.0–6.5) | 6.3 (7.2) y | 35.0% | SD-OCT; Spectralis; Heidelberg Engineering | |
| Wauschkuhn et al. (2023)23 | Prospective | 7 y | 1,381 | CIS 193 (14), RRMS 1188 (86) | McDonald criteria 2017 | 137 (68) | 32 (5) | 1.0 (0–2.0) | NA | 2.0 (1.5–4.0) mo | 35% | Spectral domain OCT (Heidelberg Engineering Spectralis OCT2) |
Abbreviations: CIS = clinically isolated syndrome; EDSS = Expanded Disability Status Scale; GCIPL = Ganglion cell-inner plexiform layer; IQR = interquartile range; NA = not available; OCT = optical coherence tomography; PPMS = primary progressive MS; RRMS = relapsing-remitting MS; SDMT = symbol digit modalities test; SD-OCT = spectral-domain optical coherence tomography; SPMS = secondary progressive MS.
Different OCT Thresholds and Disease Progression
Cross-Sectional Cutoffs Association With Disease Progression
The analysis of different retinal layer thresholds revealed a significant association with the prediction of disease progression, defined as physical disability progression (measured by EDSS scores) and/or cognitive deterioration. Table 2 presents that baseline pRNFL measurements below 87–88 μm yielded a significant HR of 2.376 (95% CI 1.884–2.995, p < 0.001, eFigure 1), while measurements between 92 and 100 μm showed a lower significant pooled HR of 1.831 (95% CI 1.430–2.345, p < 0.01, eFigure 1). The heterogeneity was very low (I2 approximately null); therefore, a fixed-effects model was used. As for the GCIPL layer, a baseline thickness threshold below 77 μm was associated with a pooled HR of 2.751 for disease progression (95% CI 1.983–3.816, p < 0.001, eFigure 2), 70 μm was associated with a lower pooled HR of 2.097 (95% CI 1.417–3.104, p < 0.001, eFigure 2), and high vs low baseline thickness comparison within the range of 70–77 μm yielded a significant pooled HR of 2.461 (95% CI 1.914 to 3.164, p < 0001, eFigure 3). Again, the heterogeneity was very low (I2 approximately null); therefore, a fixed-effects model was used. In addition, a baseline pRNFL thickness below 87–88 μm was associated with higher odds of future disease progression, with an OR of 3.006 (95% CI 1.77–5.108, p = 0.043, eFigure 4), with moderate heterogeneity (I2 = 68%). Similarly, for the GCIPL baseline thickness, a high vs low baseline thickness comparison within the range of 70–77 μm yielded a significant pooled OR of 5.811 (95% CI 2.554–13.223, p < 0.001, eFigure 5), with no observed heterogeneity between studies (I2 = 0%), so a fixed-effects model was used.
Table 2.
Summary of the Hazard Ratio for Disease Progression (Physical Disability Progression and/or Cognitive Deterioration) of Different Cutoff Values of pRNFL and GCIPL Baseline Measures
| Retinal layer thresholds | Cutoff chosen (μm) | Hazard ratio and 95% CI | p Value | I2 test | Egger test |
| Cross-sectional predictive cutoff | |||||
| pRNFL | |||||
| Baseline pRNFL | 87–88 μm | 2.376 (1.884–2.995) | <0.001 | 0% | 0.038 |
| Baseline pRNFL | 92–100 μm | 1.831 (1.430–2.345) | <0.01 | 0% | 0.033 |
| GCIPL | |||||
| High vs low baseline thickness | 70–77 μm | 2.461 (1.914–3.164) | <0.001 | 0% | 0.96 |
| Baseline GCIPL | 77 μm | 2.751 (1.983–3.816) | <0.001 | 0% | 0.79 |
| Baseline GCIPL | 70 μm | 2.097 (1.417–3.104) | <0.001 | 0% | 0.23 |
Abbreviations: CI = confidence interval; GCIPL = macular ganglion cell/inner plexiform layer; pRNFL = peripapillary retinal nerve fiber layer; μm/y: micrometer per year.
Association of Annualized Rate of Change in Retinal Layer and Disease Progression
An annualized rate of change in GCIPL thickness of more than 1 μm/y showed a pooled HR of 3.307 for disease progression (95% CI 1.697–6.446, p < 0.001, eFigure 6), and a cutoff between 0.5 and 1 μm/y revealed a higher pooled HR of 3.535 (95% CI 2.112–5.917, p < 0.001, eFigure 7). As for the pRNFL layer, annualized rates of change between 1.5 and 2 μm/y also predicted disease progression, with a pooled HR of 3.019 (95% CI 1.389–6.561, p = 0.005, eFigure 8), as presented in Table 3. Notably, the heterogeneity tests showed low I2 values across all analyses, indicating minimal variation between articles, so a fixed-effects model was used.
Table 3.
Summary of the Hazard Ratio for Disease Progression (Physical Disability Progression and/or Cognitive Deterioration) of Different Cutoff Values of pRNFL and GCIPL Annualized Rate of Change
| Retinal layer thresholds | Cutoff chosen (μm) | Hazard ratio and 95% CI | p Value | I2 test | Egger test |
| Annualized rate of change | |||||
| aLGCIPL | |||||
| aLGCIPL | 0.5–1 μm/y | 3.535 (2.112–5.917) | <0.001 | 0% | 0.34 |
| aLGCIPL | 1 μm/y | 3.307 (1.697–6.446) | <0.001 | 42% | — |
| aLpRNFL | |||||
| aLpRNFL | 1.5–2 μm/y | 3.019 (1.389–6.561) | 0.005 | 35% | — |
Abbreviations: alGCIPL = annualized loss of GCIPL; aLpRNFL = annualized loss of pRNFL; CI = confidence interval; μm/y = micrometer per year.
An annualized rate of GCIPL thinning more than 1 μm/y was associated with higher odds of disease progression, with a pooled OR of 13.083 (95% CI 7.278–23.517, p < 0.001, eFigure 9), and moderate heterogeneity (I2 = 54%), while the annualized rate of change in pRNFL more than 1–1.5 μm/y exhibited a lower pooled OR of 6.752 (95% CI 1.131–40.323, p = 0.036, eFigure 10) with a notably elevated level of heterogeneity (I2 = 90%); therefore, a random-effects model was used, as given in Table 4.
Table 4.
Summary of the Odds Ratio for Disease Progression of Different Predictive Cutoff Values of pRNFL and GCIPL Baseline and Annualized Rate of Change in Thickness
| Retinal layer thresholds | Cutoff (μm) | Odds ratio and 95% CI | p Value | I2 test |
| Cross-sectional predictive cutoff | ||||
| pRNFL layer | ||||
| Baseline pRNFL | 87–88 μm | 3.006 (1.77–5.108) | 0.043 | 68% |
| GCIPL layer | ||||
| High vs low baseline thickness | 70–77 μm | 5.811 (2.554–13.223)) | <0.001 | 0% |
| Annualized rate of change | ||||
| aLGCIPL | ||||
| aLGCIPL | 1 μm/y | 13.083 (7.278–23.517) | <0.001 | 54% |
| aLpRNFL | ||||
| aLpRNFL | 1–1.5 μm/y | 6.752 (1.131–40.323) | 0.036 | 90% |
Abbreviations: alGCIPL = annualized loss of GCIPL; aLpRNFL = annualized loss of Prnfl; CI = confidence interval; GCIPL = macular ganglion cell/inner plexiform layer; pRNFL = peripapillary retinal nerve fiber layer; μm/y = micrometer per year.
OCT Thresholds and Physical Disability Progression (EDSS Progression)
Different thresholds for retinal layers showed a significant pooled HR for predicting the worsening of physical disability, as measured by reaching EDSS milestones. When comparing the high vs low baseline thickness of the pRNFL layer, a cutoff between 87 and 100 μm was predictive of EDSS progression, associated with a significant HR of 2.028 (95% CI 1.699–2.42, p < 0.001, eFigure 11). Similarly, for baseline pRNFL measurements below 87–88 μm, the HR increased significantly to 2.258 (95% CI 1.752–2.909, p < 0.001, eFigure 12). The heterogeneity was very low in both (I2 = 0%); therefore, a fixed-effects model was used.
A baseline thickness predictive threshold in the GCIPL layer between 70 and 77 μm was associated with progression of EDSS, with a HR of 2.519 (95% CI 1.905–3.328, p < 0.001, eFigure 13), and no observed heterogeneity (I2 = 0%). In addition, baseline GCIPL measurements below 77 μm yielded a higher significant HR of 2.66 (95% CI 1.815–3.915, p < 0.001, eFigure 14), while measurements below 70 μm were associated with an HR of 2.493 (95% CI 1.48–4.19, p = 0.001, eFigure 15), as given in Table 5. For these thresholds, the heterogeneity was very low, and therefore, the fixed-effects model was used.
Table 5.
Summary of the Hazard Ratios for Physical Disability Worsening (Specifically EDSS Progression) of Different Predictive Cutoff Values of pRNFL and GCIPL Baseline
| Retinal layer thresholds | Cutoff chosen | Hazard ratio and 95% CI | p Value | I2 test | Egger test |
| Cross-sectional predictive cutoff | |||||
| pRNFL | |||||
| High vs low baseline thickness | 87–100 μm | 2.028 (1.699–2.42) | <0.001 | 0% | 0.016 |
| Baseline pRNFL | 87–88 μm | 2.258 (1.752–2.909) | <0.001 | 0% | 0.042 |
| GCIPL | |||||
| High vs low baseline thickness | 70–77 μm | 2.519 (1.905–3.328) | <0.001 | 0% | 0.76 |
| Baseline GCIPL | 77 μm | 2.66 (1.815–3.915) | <0.001 | 15% | 0.69 |
| Baseline GCIPL | 70 μm | 2.493 (1.48–4.19) | 0.001 | 0% | — |
Abbreviations: alGCIPL = annualized loss of GCIPL; aLpRNFL = annualized loss of pRNFL; CI = confidence interval; GCIPL = macular ganglion cell/inner plexiform layer; EDSS = Expanded Disability Status Scale; pRNFL = peripapillary retinal nerve fiber layer; μm/y = micrometer per year.
OCT Predictive Thresholds and Future Relapse Risk
Certain OCT measures may also serve as predictors for future relapse in patients with MS.
Bsteh et al. (2022) reported that neither a pRNFL thickness of less than 88 μm nor a GCIPL thickness of less than 77 μm was significantly associated with relapse risk (aHR = 1.3, p = 0.116; aHR = 1.4, p = 0.098, respectively).19
Similarity, Wauschkuhn et al.23 reported that cross-sectional GCIPL cutoffs (<70 μm and <77 μm) were not significantly associated with relapse risk (aHR = 1.08, p = 0.73 and aHR = 1.09, p = 0.77, respectively). However, longitudinal measurements by Bsteh et al. (2021) further explored the predictive value of changes in pRNFL and GCIPL thickness over time. They found that a reduction in pRNFL thickness of ≥2 μm/y did not significantly increase relapse risk in either the first (aHR = 2.1, p = 0.363) or second year (aHR = 2.2, p = 0.143). However, a GCIPL reduction of >0.5 μm/y was significantly associated with higher relapse risk in both the first year (aHR = 1.9, p = 0.031) and the second year (aHR = 2, p = 0.027).18
Risk of Bias Assessment
The risk of bias across studies was evaluated using the Quality in Prognosis Studies (QUIPS) tool and is detailed in the supplementary material (eTable 3). Most studies showed low risk in most domains, with moderate risk primarily noted in Study Attrition and Study Confounding. Overall, these assessments offer valuable insights into each study's methodological quality and potential biases, with an overall risk of bias generally classified as low.
Publication Bias and Sensitivity Analysis
For disease progression, the sensitivity analyses of different pRNFL cross-sectional predictive cutoffs revealed that no study significantly influenced the overall significance of the results. The visual inspection of the funnel plot showed asymmetry and a shift to a positive in the high vs low meta-analysis, 88 µm threshold meta-analysis, and a threshold between 92 and 98 μm meta-analysis, indicating asymmetry around the combined effect size (eFigure 16). The quantitative assessments by the Egger test showed the same results of a significant publication bias in those 3 comparisons (p = 0.017, 0.038–0.033, respectively), indicating potential publication bias (Table 2). To address this, we used the trim and fill analysis of Duval and Tweedie.27 This analysis suggested that even in the absence of publication bias, the effect size would still be significant (eFigure 17). The recalculated HR was 1.845 (95% CI 1.59–2.13) for the high vs low meta-analysis, 2.18 (95% CI 1.77–2.68) for the 88 µm threshold meta-analysis, and 1.68 (95% CI 1.34–2.11) for 92–98 µm threshold meta-analysis indicating a sustained high level of significance postadjustment. However, the GCIPL sensitivity analyses revealed that no study significantly affected the overall significance of the results. Examination of the funnel plot and Egger test did not indicate a publication bias (Table 2, and eFigure 18, eFigure 19).
Regarding physical disability progression, the Egger test and funnel plots showed a potential publication bias for some comparisons, such as high vs low baseline thickness for pRNFL (p = 0.016) and baseline pRNFL <88 μm (p = 0.042) (eFigure 20, eFigure 21, respectively), although no significant bias was observed for GCIPL layer thresholds (eFigure 22). To address this, we used the trim and fill analysis of Duval and Tweedie. The readjusted HR was 1.847 (95% CI 1.57–2.16) for the high vs low meta-analysis, 2.04 (95% CI 1.63–2.56) for the 88 µm threshold meta-analysis showing a sustained high level of significance postadjustment.
Discussion
Our pooled analysis, which included 14 studies with a total of 3,693 participants, showed a significant correlation between the baseline thickness of pRNFL and GCIPL, as well as their annualized rates of change, with the progression of the disease. Higher HRs and ORs for disease progression, as measured by EDSS progression and cognitive deterioration, were associated with lower baseline pRNFL and GCIPL thicknesses, as summarized in Figure 2. We found similar correlations with annualized rates of change, suggesting that these OCT measures could serve as reliable biomarkers for predicting disease progression and a sensitive tool to detect treatment effects during clinical trials.
Figure 2. Associations of pRNFL and GCIPL Thickness With Risk of Disease Progression and Sustained EDSS Worsening.
This schematic illustrates the significant predictive correlations between retinal layer thicknesses (pRNFL and GCIPL) and the risk of disease progression, defined as physical disability worsening and/or cognitive deterioration (significant decline of cognitive function measured by the SDMT score). Physical disability worsening defined by an increase in EDSS steps from a baseline score as defined in eTable 2. Thinner layers are linked to higher hazard ratios (HR), indicating an increased risk of disease progression. GCIPL = macular ganglion cell/inner plexiform layer; pRNFL = peripapillary retinal nerve fiber layer; SDMT = symbol digit modalities test. Created in BioRender. Ismail, A. (2025) BioRender.com/z53u456.
A growing body of evidence supports the use of OCT thresholds as valuable prognostic markers in the clinical management of MS. Our findings are in line with a recent systematic review of 8 studies that reported similar results.28 In addition, 2 recent meta-analyses demonstrated a significant negative correlation coefficient between pRNFL and GCIPL measurements and EDSS worsening, as well as a positive correlation coefficient between pRNFL and cognitive performance.29,30 However, no significant association was found between GCIPL and cognitive performance.
The cross-sectional predictive analysis revealed that the baseline pRNFL threshold of 87–88 μm was the most commonly used across the included studies and showed the highest risk estimate, supporting its relevance as a cutoff threshold in clinical assessments to predict disease progression, including physical (measured by EDSS progression) and cognitive deterioration. In addition, Bsteh et al. (2021) reported that this cutoff demonstrated a high specificity of 72.3% and a sensitivity of 46%, with an area under the curve of 0.64.31 Moreover, baseline GCIPL thresholds below 77 μm or below 70 μm showed a higher predictive value. This is in line with the literature showing that a GCIPL thinner than 70 μm is predictive of disease progression, whereas values above 77 μm are associated with a more stable disease course,21,23 also 77 μm cutoff showed a specificity of 77% and a sensitivity of 48.4%.31 Our findings indicate that GCIPL thinning presents a higher risk estimate for overall disease progression compared with pRNFL measures. This finding is consistent with previous meta-analyses that have highlighted the advantages of GCIPL in detecting early brain atrophy.7 The enhanced specificity of GCIPL for MS-associated neuroaxonal damage, along with its superior correlation between structure and function, allows it to identify neuroaxonal damage significantly sooner than pRNFL scans. Moreover, GCIPL has the largest range to assess thinning rates and is less prone to potential confounders such as a floor effect, which might be present in the analysis of pRNFL at an advanced stage of the disease.31 The effect of previous or current optic neuritis (ON) also seems to be less pronounced in GCIPL than in pRNFL. Given that the macula, which contains the thickest retinal ganglion cell layer complex, is significantly affected by MS-related damage, GCIPL emerges as a valuable biomarker for assessing neurodegeneration within the visual pathway.32
Both cross-sectional pRNFL and GCIPL thicknesses were predictive of future physical worsening, but GCIPL thickness demonstrated a stronger effect compared with pRNFL. This finding is consistent with recent meta-analyses, which reported a higher correlation between GCIPL and EDSS compared with pRNFL.29 This highlights the potential for using GCIPL as a more sensitive marker in clinical practice for predicting physical disability worsening in patients with MS. The specificity of GCIPL for physical disability aligns with previous research that suggests that the macular region, given its dense concentration of retinal ganglion cells, may be directly affected by neurodegenerative processes affecting physical function.33,34
Similarly, the qualitative summary showed that both cutoffs demonstrated a predictive value for forecasting cognitive decline, with pRNFL thickness showing a stronger effect. This is consistent with previous meta-analysis that identified a significant correlation between RNFL thickness and performance on cognitive tests, including the SDMT, Paced Auditory Serial Addition Test, and Word List Generation, but they did not find a significant correlation between GCIPL thickness and cognitive performance.30 This lack of association may be explained by a faster rate of GCIPL thinning, potentially leading to a floor effect before cognitive impairment becomes evident.35,36
Longitudinal analysis further supported the utility of OCT measures in monitoring disease progression over time, with the advantage of having the patient as his or her own control subject over time. Annualized rates of change in pRNFL and GCIPL thickness were significant predictors of future disease progression. Specifically, a threshold of annualized loss of 1.5–2 μm/y of pRNFL and more than 1 μm/y loss in GCIPL was associated with larger effect sizes than the cross-sectional thresholds to predict EDSS progression and cognitive decline. In addition, a pRNFL threshold of 1.5–2 demonstrated high specificity (90%–95%) and sensitivity (58%–76.1%).15 Similarly, the GCIPL threshold exhibited a specificity of 86% and a sensitivity of 78%.18 These findings support that longitudinal cutoffs provide a better predictive value compared with cross-sectional cutoffs. This finding is in line with the literature, where longitudinal GCIPL loss was associated with disease progression, independent of relapse activity, failure of immunotherapy, active MS lesions within cerebral MRI, and brain atrophy.35,37-43
Our analysis demonstrated that the annual thinning rate yielded larger effect sizes than cross-sectional measures, emphasizing the superiority of longitudinal monitoring in predicting disease progression, and ability to track disease activity and treatment responses over time. In particular, longitudinal GCIPL measurements emerged as the most robust biomarker for disease progression. Consistent with previous studies that showed that the rate of retinal layer thinning over time is equivalent in eyes with and without prior optic neuritis after 6 months, despite a persistent absolute difference in cross-sectional measures.44-46 This integrated approach allows for more personalized and effective MS management. Baseline OCT values that suggest a higher risk of disability can prompt early initiation of more aggressive therapies, while rapid retinal layer thinning signaling the need for treatment adjustments or switching to more effective disease-modifying therapies.
Owing to the limited research on OCT threshold measures as a prognostic tool for disease progression in MS, our systematic review and meta-analysis have some limitations, primarily due to the small number of eligible studies. After applying the inclusion and exclusion criteria, only 14 studies were included, most of which focused on relapsing-remitting MS (RRMS). As a result, the applicability of our findings to primary and secondary progressive MS is limited and our conclusions mainly pertain to the RRMS population. In addition, the meta-analyses were conducted on HRs derived from different cutoffs, which may introduce variability in the results. Furthermore, due to the very limited number of studies that adjusted for disease-modifying therapies (DMTs), we were unable to perform meta-regression or subgroup analyses based on different DMTs.
Nevertheless, our study has valuable implications for both clinical practice and future research. OCT measures could be integrated into routine assessments to enhance the prediction and monitoring of disease progression in patients with MS. Our findings also highlight the need for standardized OCT protocols and thresholds to improve consistency across clinical settings.
Future research should focus on validating these thresholds in larger, more diverse MS populations to confirm their broader applicability. In addition, further subgroup analyses are needed to account for different DMT types and clinical settings. Incorporating OCT measures into randomized clinical trials will be essential to establishing their prognostic value and their role in guiding therapeutic decision-making.
In conclusion, although baseline pRNFL thickness of ≤88 μm and baseline GCIPL thickness of ≤77 μm are associated with an increased risk of future disease progression, annual thinning rates greater than 1.5 μm/y for pRNFL and 1 μm/y for GCIPL seem to have greater predictive power, making them more clinically relevant for identifying individuals at high risk for clinical deterioration. Additional research is needed to investigate how changes in retinal layer thickness, and the rates of these changes, can serve as predictors for the progression of physical and cognitive disabilities over time.
Author Contributions
N.K. El Ayoubi: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. A. Ismail: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. G. Sader: drafting/revision of the manuscript for content, including medical writing for content. N. Abi Chakra: drafting/revision of the manuscript for content, including medical writing for content; study concept or design. J. El Ahdab: drafting/revision of the manuscript for content, including medical writing for content. J. Abboud: drafting/revision of the manuscript for content, including medical writing for content. S.J. Khoury: drafting/revision of the manuscript for content, including medical writing for content; study concept or design.
Glossary
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- GCIPL
ganglion cell-inner plexiform layer
- HR
hazard ratio
- MS
multiple sclerosis
- OCT
optical coherence tomography
- OR
odds ratios
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- pRNFL
peripapillary retinal nerve fiber layer
- QUIPS
Quality in Prognosis Studies
- RNFL
retinal nerve fiber layer
- RRMS
relapsing-remitting MS
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Magyari M, Sorensen PS. The changing course of multiple sclerosis: rising incidence, change in geographic distribution, disease course, and prognosis. Curr Opin Neurol. 2019;32(3):320-326. doi: 10.1097/WCO.0000000000000695 [DOI] [PubMed] [Google Scholar]
- 2.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816-1821. doi: 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin MC, Douglas JN, Meyers L, Lee S, Shin Y, Gardner LA. Neurodegeneration in multiple sclerosis involves multiple pathogenic mechanisms. Degener Neurol Neuromuscul Dis. 2014;4:49-63. doi: 10.2147/DNND.S54391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavazzi E, Zivadinov R, Dwyer MG, et al. MRI biomarkers of disease progression and conversion to secondary-progressive multiple sclerosis. Expert Rev Neurother. 2020;20(8):821-834. doi: 10.1080/14737175.2020.1757435 [DOI] [PubMed] [Google Scholar]
- 5.Ontaneda D, Fox RJ. Imaging as an outcome measure in multiple sclerosis. Neurotherapeutics. 2017;14(1):24-34. doi: 10.1007/s13311-016-0479-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso R, Gonzalez-Moron D, Garcea O. Optical coherence tomography as a biomarker of neurodegeneration in multiple sclerosis: a review. Mult Scler Relat Disord. 2018;22:77-82. doi: 10.1016/j.msard.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 7.El Ayoubi NK, Ismail A, Fahd F, Younes L, Chakra NA, Khoury SJ. Retinal optical coherence tomography measures in multiple sclerosis: a systematic review and meta-analysis. Ann Clin Transl Neurol. 2024;11(9):2236-2253. doi: 10.1002/acn3.52165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80(1):47-54. doi: 10.1212/WNL.0b013e31827b1a1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abalo-Lojo JM, Limeres CC, Gómez MA, et al. Retinal nerve fiber layer thickness, brain atrophy, and disability in multiple sclerosis patients. J Neuroophthalmol. 2014;34(1):23-28. doi: 10.1097/WNO.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 10.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801-813. doi: 10.1002/ana.24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambe J, Saidha S, Bermel RA. Optical coherence tomography and multiple sclerosis: Update on clinical application and role in clinical trials. Mult Scler. 2020;26(6):624-639. doi: 10.1177/1352458519872751 [DOI] [PubMed] [Google Scholar]
- 12.Alba-Arbalat S, Solana E, Lopez-Soley E, et al. Predictive value of retinal atrophy for cognitive decline across disease duration in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2024;95(5):419-425. doi: 10.1136/jnnp-2023-332332 [DOI] [PubMed] [Google Scholar]
- 13.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280-286. doi: 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 14.Berek K, Hegen H, Hocher J, et al. Retinal layer thinning as a biomarker of long-term disability progression in multiple sclerosis. Mult Scler. 2022;28(12):1871-1880. doi: 10.1177/13524585221097566 [DOI] [PubMed] [Google Scholar]
- 15.Bsteh G, Hegen H, Teuchner B, et al. Peripapillary retinal nerve fibre layer thinning rate as a biomarker discriminating stable and progressing relapsing-remitting multiple sclerosis. Eur J Neurol. 2019;26(6):865-871. doi: 10.1111/ene.13897 [DOI] [PubMed] [Google Scholar]
- 16.Bsteh G, Hegen H, Teuchner B, et al. Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult Scler. 2019;25(2):196-203. doi: 10.1177/1352458517740216 [DOI] [PubMed] [Google Scholar]
- 17.Bsteh G, Berek K, Hegen H, et al. Macular ganglion cell-inner plexiform layer thinning as a biomarker of disability progression in relapsing multiple sclerosis. Mult Scler. 2021;27(5):684-694. doi: 10.1177/1352458520935724 [DOI] [PubMed] [Google Scholar]
- 18.Bsteh G, Hegen H, Altmann P, et al. Retinal layer thinning predicts treatment failure in relapsing multiple sclerosis. Eur J Neurol. 2021;28(6):2037-2045. doi: 10.1111/ene.14829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bsteh G, Hegen H, Altmann P, et al. Retinal layer thickness predicts disability accumulation in early relapsing multiple sclerosis. Eur J Neurol. 2023;30(4):1025-1034. doi: 10.1111/ene.15718 [DOI] [PubMed] [Google Scholar]
- 20.Cilingir V, Batur M. First measured retinal nerve fiber layer thickness in RRMS can be used as a biomarker for the course of the disease: threshold value discussions. J Neurol. 2021;268(8):2858-2865. doi: 10.1007/s00415-021-10469-x [DOI] [PubMed] [Google Scholar]
- 21.Lambe J, Fitzgerald KC, Murphy OC, et al. Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology. 2021;96(16):e2058-e2069. doi: 10.1212/WNL.0000000000011788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574-584. doi: 10.1016/S1474-4422(16)00068-5 [DOI] [PubMed] [Google Scholar]
- 23.Wauschkuhn J, Solorza Buenrostro G, Aly L, et al. Retinal ganglion cell loss is associated with future disability worsening in early relapsing-remitting multiple sclerosis. Eur J Neurol. 2023;30(4):982-990. doi: 10.1111/ene.15681 [DOI] [PubMed] [Google Scholar]
- 24.Schurz N, Sariaslani L, Altmann P, et al. Evaluation of retinal layer thickness parameters as biomarkers in a real-world multiple sclerosis cohort. Eye Brain. 2021;13:59-69. doi: 10.2147/EB.S295610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bsteh G, Hegen H, Altmann P, et al. Retinal layer thinning is reflecting disability progression independent of relapse activity in multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6(4):2055217320966344. doi: 10.1177/2055217320966344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin TY, Vitkova V, Asseyer S, et al. Increased serum neurofilament light and thin ganglion cell–inner plexiform layer are additive risk factors for disease activity in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1051. doi: 10.1212/NXI.0000000000001051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 28.Swinnen S, De Wit D, Van Cleemput L, Cassiman C, Dubois B. Optical coherence tomography as a prognostic tool for disability progression in MS: a systematic review. J Neurol. 2023;270(2):1178-1186. doi: 10.1007/s00415-022-11474-4 [DOI] [PubMed] [Google Scholar]
- 29.Mirmosayyeb O, Yazdan Panah M, Mokary Y, et al. Optical coherence tomography (OCT) measurements and disability in multiple sclerosis (MS): a systematic review and meta-analysis. J Neurol Sci. 2023;454:120847. doi: 10.1016/j.jns.2023.120847 [DOI] [PubMed] [Google Scholar]
- 30.Mirmosayyeb O, Zivadinov R, Weinstock-Guttman B, Benedict RHB, Jakimovski D. Optical coherence tomography (OCT) measurements and cognitive performance in multiple sclerosis: a systematic review and meta-analysis. J Neurol. 2023;270(3):1266-1285. doi: 10.1007/s00415-022-11449-5 [DOI] [PubMed] [Google Scholar]
- 31.Graves JS. Optical coherence tomography in multiple sclerosis. Semin Neurol. 2019;39(6):711-717. doi: 10.1055/s-0039-1700528 [DOI] [PubMed] [Google Scholar]
- 32.Lee TH, Ji YS, Park SW, Heo H. Retinal ganglion cell and axonal loss in optic neuritis: risk factors and visual functions. Eye. 2017;31(3):467-474. doi: 10.1038/eye.2016.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saidha S, Sotirchos ES, Oh J, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70(1):34-43. doi: 10.1001/jamaneurol.2013.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knier B, Leppenetier G, Wetzlmair C, et al. Association of retinal architecture, intrathecal immunity, and clinical course in multiple sclerosis. JAMA Neurol. 2017;74(7):847-856. doi: 10.1001/jamaneurol.2017.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul F, Calabresi PA, Barkhof F, et al. Optical coherence tomography in multiple sclerosis: a 3-year prospective multicenter study. Ann Clin Transl Neurol. 2021;8(12):2235-2251. doi: 10.1002/acn3.51473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulido-Valdeolivas I, Andorrà M, Gómez-Andrés D, et al. Retinal and brain damage during multiple sclerosis course: inflammatory activity is a key factor in the first 5 years. Sci Rep. 2020;10(1):13333. doi: 10.1038/s41598-020-70255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciccarelli O, Barkhof F, Calabrese M, et al. Using the progression independent of relapse activity framework to unveil the pathobiological foundations of multiple sclerosis. Neurology. 2024;103(1):e209444. doi: 10.1212/WNL.0000000000209444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baetge SJ, Dietrich M, Filser M, et al. Association of retinal layer thickness with cognition in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1018. doi: 10.1212/NXI.0000000000001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisa M, Croese T, Dalla Costa G, et al. Subclinical anterior optic pathway involvement in early multiple sclerosis and clinically isolated syndromes. Brain J Neurol. 2021;144(3):848-862. doi: 10.1093/brain/awaa458 [DOI] [PubMed] [Google Scholar]
- 40.Oh J, Sotirchos ES, Saidha S, et al. Relationships between quantitative spinal cord MRI and retinal layers in multiple sclerosis. Neurology. 2015;84(7):720-728. doi: 10.1212/WNL.0000000000001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stellmann JP, Cetin H, Young KL, et al. Pattern of gray matter volumes related to retinal thickness and its association with cognitive function in relapsing-remitting MS. Brain Behav. 2017;7(2):e00614. doi: 10.1002/brb3.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vidal-Jordana A, Pareto D, Cabello S, et al. Optical coherence tomography measures correlate with brain and spinal cord atrophy and multiple sclerosis disease-related disability. Eur J Neurol. 2020;27(11):2225-2232. doi: 10.1111/ene.14421 [DOI] [PubMed] [Google Scholar]
- 43.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801-813. doi: 10.1002/ana.24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Britze J, Pihl-Jensen G, Frederiksen JL. Retinal ganglion cell analysis in multiple sclerosis and optic neuritis: a systematic review and meta-analysis. J Neurol. 2017;264(9):1837-1853. doi: 10.1007/s00415-017-8531-y [DOI] [PubMed] [Google Scholar]
- 45.Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. 2016;22(5):641-648. doi: 10.1177/1352458515598020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77(3):517-528. doi: 10.1002/ana.24351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the corresponding author.